Abstract
Despite gains in knowledge of the intrinsic signals governing cancer progression, effective clinical management of cancer remains a challenge. Drug resistance and relapse, pose the greatest barriers to cancer care, and are often driven by the co-option of stem cell programs by subpopulations of aggressive cancer cells. Here, we focus on the role of the microenvironment in the acquisition and/ or maintenance of stem cell states in cancer in the context of resistance and metastasis. We further discuss the role of cancer stem cells in immune evasion through the course of metastasis, dormancy, and relapse. Understanding the niche in which cancer stem cells live and the signals that sustain them may lead to new strategies that target them by disrupting microenvironmental support.
Stem Cell Signals in Cancer
Despite advances in cancer treatment and management, a large fraction of patients with both metastatic and local disease still face primary or acquired resistance to therapy and eventually succumb to disease. To develop more effective therapeutic strategies, there is a great need to define the mechanisms underlying both resistance and metastatic progression. One central mechanism by which cells acquire these malignant features is the activation of developmental signaling pathways. Classic stem cell signals such as Oct4, Sox2, Wnt, or Notch are often aberrantly upregulated within cancer cells and drive the ability of tumors to self-renew and propagate in vivo [1–4]. Functionally, the activation of stem cell signals can be enriched in cancer stem cells (CSCs) and is associated with resistance to standard therapies and the induction of an epithelial-to-mesenchymal transition (EMT) [2–5]. Thus, stem cell signals drive core malignant features of progressive disease and relapse and represent a critical target for therapy (Figure 1). On the basis of this premise, inhibitors of classic stem signals have been developed, yielding important clinical successes [6]. Hedgehog (Hh) pathway inhibitors [95,96], for example, have been approved for the treatment of basal cell carcinoma [7,8] and acute myeloid leukemia [9]. While intrinsic signals have been a predominant focus of prior work, how the microenvironment may influence the stem cell state in cancer is less explored. Growing evidence supports a role for the tumor microenvironment (TME) in supporting stem cell fate, suggesting that direct targeting of stem cell signals alone may not be sufficient to eradicate CSCs [2–4]. Defining the microenvironmental signals that support stemness may enable new strategies that leverage TME modulation to ablate CSCs, block tumor progression, and improve responses to current therapies. Here, we explore the diverse microenvironmental signals that support stem cell fate in context of cancer therapy resistance and metastasis.
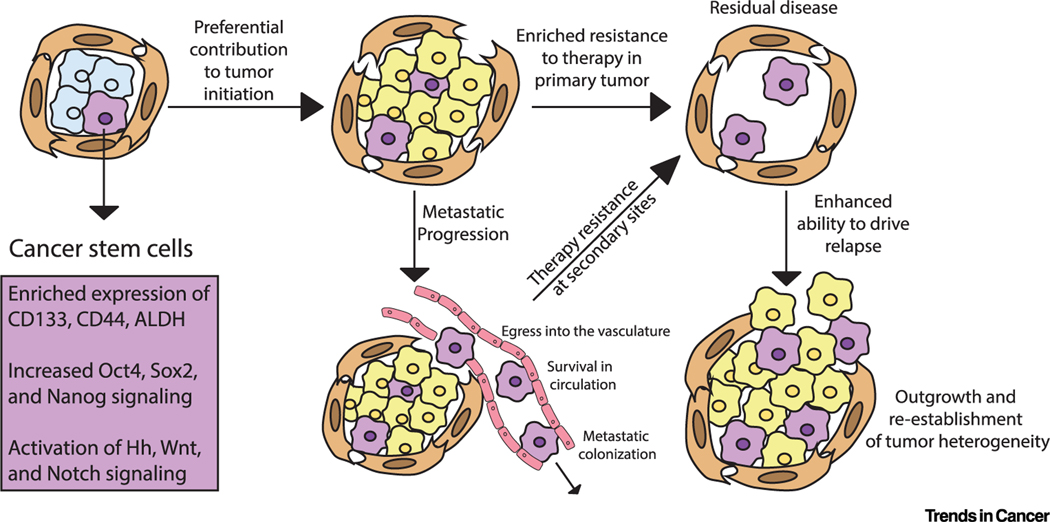
Figure 1. Cancer Cells Enriched for Stem Cell Programs Drive Features of Progressive Disease.
Within the tumor bulk, rare subpopulations of cancer cells are often enriched for the expression of CD133, CD44, and ALDH and the activation of classical development transcription factors and signals such as Oct4, Sox2, Nanog, Hedgehog (Hh), Notch, and Wnt. These cells, enriched for stem cell signals, preferentially contribute to tumor initiation, metastatic progression, and therapy resistance, driving relapse.
The Microenvironment in Therapy Resistance
As cancers progress, transformed cells can remodel the microenvironment to their advantage, often fueling inflammation and protumorigenic microenvironmental crosstalk [10,11]. Signals from the dysregulated immune and stromal environment can then feed into the activation of stem cell signals, which are commonly associated with enhanced resistance in the face of cytotoxic and targeted therapies [2–4,10,11].
Immune Signals Activate Developmental Pathways and Fuel Resistance to Conventional Therapies
Cancers are frequently associated with an inflammatory response [12,13], triggered by inflammatory cues released by activated stromal and cancer cells in response to necrosis at the center of the tumor. However, cancer cells can also direct infiltrating immune cells toward immunosuppressive and protumorigenic states [14]. The activation or polarization of cells, including tumor-associated macrophages (TAMs) [15,16], tumor-associated neutrophils (TANs) [17], myeloid-derived suppressor cells (MDSCs) [18], B cells [19], and subsets of T lymphocytes such as regulatory T cells [14], can both suppress adaptive immunity and directly signal to cancer cells, impacting cell fate and survival (Figure 2).
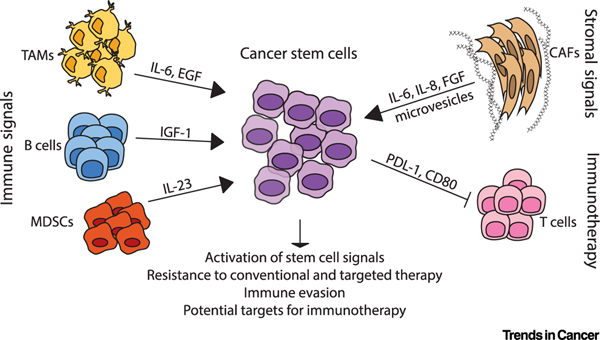
Figure 2. Stromal and Immune Signals Activate Stem Cell Signals and Enhance Therapeutic Resistance.
The tumor microenvironment is composed of stromal cells such as cancer-associated fibroblasts (CAFs) and immune cell populations, including tumor-associated macrophages (TAMs), myeloid-derived suppressor cells (MDSCs), and T and B lymphocytes. These cells release signaling molecules that can activate stem cell programs within cancer cells. Both immune cells and fibroblasts can provide inflammatory cytokines (such as interleukin [IL]-6, IL-23, and IL-8) and growth factors (epidermal growth factor [EGF], insulin-like growth factor [IGF]-1, fibroblast growth factor [FGF]) that activate classical stem cell signals and drive resistance to both conventional and targeted therapies. Cancer stem cells can also resist immune-mediated killing by upregulating programmed death-ligand 1 (PD-L1) and CD80, perhaps making this aggressive subfraction an appealing target for immunotherapy.
Growth factors and cytokines released by immune cells can activate downstream stem cell pathways, enhancing self-renewal and fueling therapy resistance [6,12,20]. Thus, disruption of immune signaling may be a promising strategy to block self-renewal and improve therapeutic sensitivity. In general, the role of tumor-associated myeloid populations has received the most attention in terms of understanding protumorigenic immune cell function [15,16]. Recent studies have shown that depletion of myeloid populations can sensitize tumors to conventional therapies, including chemotherapy in pancreatic cancer [21,22] and androgen deprivation therapy in prostate cancer [23]. In pancreatic cancer, TAMs were found to support CSC function through the activation of STAT3, a central effector pathway implicated in cell survival [22]. Macrophage depletion effectively reduced the CSC fraction, sensitizing them to chemotherapy in vivo [22], and dual-targeting of TANs in combination with TAMs increased chemosensitivity even further in preclinical models [21]. In prostate cancer, MDSC-derived interleukin (IL)-23 was similarly found to activate STAT3 in cancer cells, and MDSC depletion or treatment with an anti–IL-23 blocking antibody was sufficient to block or delay emergence of resistance to androgen deprivation therapy in autochthonous models [23]. In addition to myeloid cells, B cells have also recently been implicated in resistance to targeted therapy. In the context of melanoma, tumor-associated B cells promoted the induction of CD20+, CD133+, and CD271+ melanoma stem cell populations through the secretion of insulin-like growth factor (IGF) 1, driving resistance to Braf and mitogen-activated protein kinase kinase (MEK) inhibitors [24]. In a small, completed, single-arm Phase II trial (NCT01376713i), B cell depletion showed some clinical activity in patients with end-stage metastatic melanoma whose disease had progressed on targeted therapy [24]. Of the ten therapy-resistant patients who were enrolled in B cell depletion therapy, eight patients showed a clinical response as evaluated by Response Evaluation Criteria in Solid Tumors (RECIST) and immune-related response criteria (ir-RC) or the induction of necrotic tumor mass. Thus, blocking protumorigenic immune signaling represents a potential strategy for improving responsiveness to therapy, in some cases through direct disruption of stem cell signals or populations.
Stem Cell Signals and Response to Immunotherapy
In addition to conventional therapies, immunotherapy has emerged as a breakthrough strategy for cancer treatment [25]. Cancer cells often evade adaptive immune-mediated killing by upregulating immune-inhibitory cell surface signals such as programmed death-ligand 1 (PD-L1). Immune checkpoint therapy leverages blocking antibodies against these inhibitory molecules to derepress the immune response to the tumor [26–28]. Though many patients have seen durable responses to checkpoint blockade, clinical sensitivity is varied, and many patients exhibit primary or acquired resistance [29]. Response to immunotherapy can hinge on the expression of surface molecules, antigenicity, and T cell infiltration and function [29]. Modulation of these factors by CSCs may determine sensitivity to immunotherapy [30]. In some cases, CSCs have been found to preferentially upregulate the adaptive immune checkpoint PD-L1 [31,32], suggesting that checkpoint blockade may be an effective strategy for eradicating this subfraction. CSCs in pancreatic cancer also upregulate CD47 (the ‘don’t eat me’ signal) to evade innate immune killing, making them a relevant target for CD47 blocking antibodies [33]. While enriched expression of these immunosuppressive signals in stem/progenitor cells indicates that they would respond to diverse immunotherapy strategies, in some cases, stem cell fate has actually been associated with resistance to immunotherapy. For example, tumor-intrinsic upregulation of Wnt/β-catenin in melanoma has been associated with reduced T cell infiltration and poor clinical response to immunotherapy [34]. CSCs have also been found to drive resistance to adoptive T cell transfer, where patient-derived T cells are engineered to target a cancer antigen and drive an immune response upon retransplant. In squamous cell carcinoma, a population of CSCs preferentially evaded T cells through expression of CD80, driving T cell exhaustion and relapse [35]. These findings suggest that the efficacy of immunotherapy in targeting stemlike cancer cells may vary by tissue and the specific strategy being used. Given the recent emergence of immunotherapy, we are only just beginning to understand how these clinical strategies are affected by heterogeneous cancer cell populations; defining whether and how aggressive CSC populations may evade immunotherapy or drive resistance will be a critical area for future study. Interestingly, recent work in our laboratory has shown that CSCs in pancreatic cancer upregulate and functionally depend on the cell-intrinsic activation of inflammatory/immune networks, suggesting that immune-targeted therapies may carry the risk of inadvertently triggering the growth of CSCs and in turn driving tumor propagation [36]. Thus, as efforts to improve responses to immunotherapy across cancers intensify, delineating the direct impact of immunotherapy on non-immune cancer cells in general and CSCs in particular will be essential to ensuring the ultimate success of these approaches.
Cancer-Associated Fibroblasts Drive Therapy Resistance
Just as tumors can remodel the immune landscape, they can also condition the behavior of resident stromal cells. The stroma encompasses the extracellular matrix (ECM) and cell populations that maintain tissue integrity. Stromal cells are tissue specific and range from fibroblasts and stellate cells to endothelial cells, adipocytes, and neurons. Stromal cells are key players in orchestrating tissue repair and can enforce appropriate cell fate under homeostatic conditions [37]. However, perturbations in stromal signaling can promote malignant features of cancer through the activation of stem/developmental signals. Although we have focused on major cellular aspects of the stroma below, it should be noted that structural parameters of the ECM such as stiffness can also directly impact cancer cell fate [38].
During tumor development, surrounding stromal cells are often driven toward a dysregulated wound-healing state associated with protumorigenic function [39]. While we are only now beginning to understand how distinct stromal cell populations contribute differentially to therapy resistance, fibroblasts have been the most widely studied in this context. Studies across diverse tissues have used coculture and cotransplantation experiments to demonstrate that cancer-associated fibroblasts (CAFs) generally exert a protective influence on cancer cells in the context of both cytotoxic and targeted therapy [40,41]. Activated by signals in the TME, CAFs are characterized by enhanced ECM remodeling and changes in extracellular signaling, producing cytokines and growth factors that are co-opted by cancer cells to drive self-renewal (Figure 2) [39]. In some cases, CAF-mediated therapy resistance has been directly associated with the activation of stem cell signals [41]. For example, recent work has shown that activated Hh signaling in CAFs promotes stemness in breast cancer cells through fibroblast growth factor (FGF) 5. Hh pathway inhibition in aggressive triple-negative breast cancer blocked fibroblast activation, reducing aldehyde dehydrogenase 1 (ALDH1) expression in cancer cells and improving sensitivity to docetaxel in vivo [42]. A small, single-arm Phase I clinical trial (NCT02027376ii) completed in 2018 suggested that this combination of Hh pathway inhibitor and chemotherapy may be useful in treating patients with Hh-high triple-negative breast cancer [42]. Of the 12 therapy-resistant metastatic patients enrolled, two patients exhibited stable disease and one patient had a complete response to combination therapy by RECIST criteria [42]. CAFs in breast cancer have also been shown to drive resistance to hormone therapy through transfer of microvesicles containing miR-221. Uptake of CAF-derived microvesicles activated Notch signaling and contributed to the expansion of CD133HighERLo therapy-resistant cancer cells [43]. Thus, fibroblast–cancer crosstalk appears to be a general mechanism by which cancer cells acquire stem cell properties and resistance in the context of diverse therapeutic strategies.
Paradoxical Role for CAFs in Tumor Progression
Mounting evidence supports a role for the stroma in promoting stemness and resistance; however, studies in pancreatic ductal adenocarcinoma (PDAC) have challenged this concept [41]. Although PDAC is notorious for its dysplastic stroma and stromal signaling was thought to be protumorigenic, ablation of stromal signaling quite strikingly enhanced disease progression in genetically engineered mouse models, in one case driving an unexpected expansion of the stem-enriched fraction with no improvement in sensitivity to chemotherapy [41]. This was driven by enhanced immune suppression, highlighting the complexity of the TME and the importance of using immune-competent models. These studies suggest that a bulk ablation strategy with regard to the stroma may not be effective. Emerging technologies such as single-cell sequencing have clarified the extent of heterogeneity among stromal cells [44], and recent work has begun to dissect distinct stromal populations that can restrain tumorigenic function [45,46], signal to the immune system [47,48], and mediate responses to therapy [49]. As stromal subsets are further defined, the specific populations that support stem cell fate may become clear and thus identify novel therapeutic targets. For example, a distinct population of stem cell– supportive CD10+GPR77+ CAFs was recently identified in breast cancer [50]. Treatment of patient-derived xenografts with a GPR77-neutralizing antibody reduced the ALDH+ stem fraction and enhanced chemotherapy-induced apoptosis [50]. Thus, targeting distinct stromal– stem signaling pathways may be an effective approach to ablating the stem cell fraction and improving therapeutic sensitivity.
Endothelial Cells in Stemness and Therapy Resistance
In addition to fibroblasts, endothelial cells are an important element of the stroma to consider in the context of therapeutic resistance. As demands of advancing tumors outstrip the supply of the native vasculature, cancer cells secrete proangiogenic factors to promote the outgrowth of aberrant neovasculature [13]. Paracrine signals and direct interactions with endothelial cells in this niche promote therapy resistance [10]. In leukemia and glioblastoma in particular, stem cell populations are preferentially located proximal to blood vessels in the perivascular niche, suggesting that the vasculature supports stem fate and survival [10,51]. Our own work in leukemia has shown that CD98-mediated adhesion is required for long-term interactions between leukemia stem cells and vascular endothelial cells and is critical to leukemic propagation in vivo. Treatment with an anti-CD98 blocking antibody resulted in CSC depletion, impaired leukemia growth, and improved survival [52]. Similarly, we have shown that the adhesive signal syndecan-1 can support therapy resistance in aggressive leukemias. Syndecan-1 inhibition led to sensitization of blast crisis chronic myeloid leukemia to tyrosine kinase inhibitor therapy [92]. Endothelium derived miRNA has also been shown to support leukemia stem cell resistance to tyrosine kinase inhibitor therapy in chronic myeloid leukemia. miR-126 secreted by endothelial cells enhanced leukemia stem cell quiescence and self-renewal through mitogen-activated protein kinase (MAPK)/extracellular signal-regulated kinase (ERK) pathway activation [53], driving resistance to tyrosine kinase inhibitor treatment. Treatment with an miR-126 inhibitor in combination with a tyrosine kinase inhibitor depleted leukemia stem cell content, demonstrating the exciting potential of leveraging therapies that combinatorially target both CSCs and their endothelial support.
The Microenvironment in Metastasis
The same microenvironmental signals that drive stemness in the context of therapy resistance often also promote invasion and metastasis. Metastatic progression is a core contributor to overall cancer mortality [54] and is often closely associated with the activation of stem programs [2–5]. Metastasis can be thought of as occurring in several steps: the acquisition of invasive potential at the primary site and egress into the bloodstream, colonization and survival at the metastatic site, followed by eventual outgrowth and progression [54]. The fact that cancer cells have to survive harsh conditions at each step selects for cells with both heightened invasiveness and the ability to self-renew. Given that features of metastasis-initiating cells, though not completely congruent, overlap substantially with functionally defined CSC populations [5], interactions with the microenvironment at both primary and secondary sites can prime cancer cells for metastatic success by promoting the stem cell state.
Immune Signals in Promoting Initiation of Metastasis
Inflammatory signals produced by immune cells in the primary tumor can prime cancer cells for metastasis by convergently activating stem cell signals concomitant with programs that drive invasion or survival. Both myeloid and lymphoid cells secrete a wide array of cytokines, such as IL-1β, IL-6, IL-8, IL-23, and CCL2; these regulate E-cadherin/vimentin expression and drive the activation of developmental pathways (such as NF-κB, JAK/STAT, Wnt) and classical EMT-associated transcription factors (such as Zeb1, Snail1, and Slug) [55], pathways convergently associated with enhanced invasive capacity (Figure 3). For example, infiltrating TAMs have been shown to secrete Wnt ligands and activate Wnt signaling in breast cancer cells, driving loss of E-cadherin junctions and early dissemination [56]. Targeted depletion of these infiltrating TAMs with a colony-stimulating factor 1 receptor (CSF1R) blocking antibody reduced both early dissemination of cancer cells and late metastatic burden in this model [56], suggesting that TAM depletion could be a clinical strategy to block early metastatic progression.
Figure 3. Microenvironmental Signals at the Primary Tumor Condition Invasion and Metastasis.
Signaling molecules such as cytokines from immune cells and fibroblasts within the primary tumor site condition cancer cells for metastasis by activating developmental programs, such as Wnt, STAT, and Nf-kB, and classical transcription factors often associated with epithelial-to-mesenchymal transition (EMT), such as Zeb1, Snail, and Slug. Activation of developmental and EMT programs by these microenvironmental signals can promote invasion and extravasation from the primary tumor site into the vasculature. Remodeling of the extracellular matrix (ECM) by stromal and immune cells can also promote invasion. Abbreviations: TAM, tumor-associated macrophage; CAF, cancer-associated fibroblast.
Immune Cells at the Premetastatic Niche
Through the course of tumor development, signals from the primary site can prime distant sites and create a permissive premetastatic niche [57]. Shifts in the immune environment within this niche can then promote the survival and growth of metastasizing cancer cells. TAMs [58], MDSCs [59], and neutrophils [60] have all been implicated in boosting survival within the metastatic niche (Figure 4). In mouse models, primary breast tumor development was found to drive neutrophil accumulation in the lungs. Neutrophil conditioning of breast cancer cells boosted the proliferation and frequency of a functionally defined metastasis-initiating population within the lung. Further, genetic depletion of neutrophils reduced spontaneous lung metastases without impacting primary tumor burden [60], thus demonstrating the ability of immune signals to preferentially promote the expansion of aggressive metastasis-initiating cells at a secondary site [60]. Interestingly, inflammation can also mediate the outgrowth of dormant metastatic cells [61]. Inflammation at secondary sites can drive the infiltration of neutrophils, which produce neutrophil extracellular traps. These secreted webs of DNA and protein, meant to trap and kill bacteria, can actually drive laminin remodeling at metastatic sites and promote the outgrowth of tumor cells and consequent disease progression [62].
Figure 4. Microenvironmental Signals Promote Survival and Outgrowth at Metastatic Sites Through the Activation of Stem Cell Programs.
After migration from the primary tumor site, cancer cells must colonize the new, hostile tissue. Cancer cells can signal to neighboring immune cells and fibroblasts to prime and condition the metastatic niche. These microenvironmental cells, such as tumor-associated macrophages (TAMs), tumor-associated neutrophils (TANs), and cancer-associated fibroblasts (CAFs), in turn provide signals that promote the survival and self-renewal of cancer cells through the activation of stem cell pathways. TAM-secreted cytokines and neutrophil extracellular traps (NETs) produced by TANs mediate inflammation in the niche and promote survival and metastatic colonization. Microenvironmental signals can also promote metastatic outgrowth by modulating cancer stem cell metabolism or enhancing survival in the context of therapy. Cancer cells enriched for stem cell signals may also avoid immune-mediated killing by innate natural killer (NK) cells or cytotoxic CD8+ T cells at the metastatic site by downregulating NK ligands or upregulating immune checkpoints.
Immune Evasion Mechanisms in CSCs
Aside from invasiveness and survival, a crucial requirement for cancer outgrowth is the ability to evade immune-mediated killing [13]. In homeostatic conditions, quiescent normal stem cells evade immune surveillance [63], suggesting that stem cell fate in cancer may be associated with similar mechanisms of immune evasion [6,30,64]. In support of this idea, cancer cells have been shown to exploit a function of the polycomb repressive complex 2 conserved in stem cells to epigenetically downregulate the antigen-presenting complex MHC class I [65]. Activation of the developmental signal and oncogene Myc can similarly upregulate CD47 (the ‘don’t eat me’ signal) and PD-L1 (the ‘don’t kill me’ signal), reducing intratumoral T cell and macrophage infiltration in vivo [66]. Cancer-initiating cells also evade innate immune killing in some cases by downregulating natural killer (NK) ligands. In fact, absence of NKG2D ligands (NK ligands) can be used to isolate a functional stem cell fraction in leukemia [67]. These mechanisms of immune evasion may permit disseminated stemlike cancer cells to propagate at metastatic sites [68]. This idea has found support in breast cancer, where CSCs have been shown to downregulate NK ligands, enabling metastatic outgrowth [69]. Further, the achievement of clinical responses to checkpoint blockade in metastatic disease suggest that aggressive metastatic cells evade T cells at distant sites [28]. However, the specific mechanisms by which stem cell fate is tied to immune evasion has not been explored extensively in the context of metastasis. Interestingly, a recent study demonstrated that latent tumor cells selected from lung and breast cancer cell line metastases in fact simultaneously downregulate Wnt signaling and NK ligands during quiescence, leading to reduced NK killing of dormant cells [70]. Thus, stem cell fate may in some cases be decoupled from mechanisms of immune evasion to support metastasis.
Stromal Cells in Promoting Initiation of Metastasis
Paralleling their role in therapy resistance, stromal cells have also been shown to promote the invasive capacity and metastatic success of cancer cells. Activated stromal cells can support invasion through remodeling of the ECM and collagen structure, through adhesive interactions [41,71,72], or through direct extracellular signals (Figure 3) [41,73]. CAF coculture or conditioning of cancer cells can activate the transcription of stem and EMT programs through paracrine signaling molecules such as transforming growth factor (TGF)-β [74–76] or IL-6 [77]. Mesenchymal stem cells (MSCs), another type of stromal cell, have recently been shown to promote stemness, invasion, and consequent metastasis of pancreatic cancer cells through secretion of granulocytemacrophage colony-stimulating factor (GM-CSF) [78]. Interestingly, CAFs have been detected in the circulation of patients with metastatic breast cancer, suggesting that stromal cells from the primary tumor may also directly interact with CSCs in the circulation and promote colonization of the premetastatic niche [79].
Stromal Signals and Metastatic Colonization
Stromal cells can be a crucial source of support for the survival of cancer cells at a hostile secondary site. In order to survive, cancer cells remodel the stromal niche, driving signaling crosstalk that enhances stemness and metastatic success [80]. In return, extracellular fibroblast signals and interactions with the ECM activate stem/developmental signals such as Wnt, Notch, and Stat3 to promote the survival of metastasis-initiating cells during colonization (Figure 4) [81]. Signaling from endothelial cells at secondary sites can also be an important mediator of colonization. Adhesive interactions between cancer cells and the endothelium are crucial determinants of cancer cell intravasation and extravasation [54,80]; however, these interactions may also directly activate stem cell signals. For example, the cell surface molecule E-selectin, expressed on endothelial cells, is required for endothelial adhesion and bone metastasis of breast cancer lines in vivo. However, rather than regulating retention of cancer cells at the secondary site, this interaction appears to drive Wnt activation in cancer cells, concomitant with a mesenchymal-to-epithelial transition in vitro. This suggests a mechanism by which endothelial adhesion may decouple EMT programs from stemness, promoting metastatic colonization in the bone [82].
Stromal Signals in Dormancy and Regrowth of Metastatic Cells
Upon arrival at a secondary site, disseminated tumor cells must survive but may remain dormant and resist therapy for indeterminate periods of time, eventually regrowing to drive progression [83]. The signals governing metastatic outgrowth of these dormant cells are not well understood but could have important therapeutic implications in blocking the progression of systemic disease. Interactions with stromal populations and ECM molecules [84] can mediate ultimate regrowth of dormant disseminated tumor cells (DTCs). In breast cancer, CAFs have been shown to regulate the outgrowth of dormant metastatic CSCs by modulating their metabolism [85].
Patient-derived metastatic breast CSCs preferentially take up mitochondrial DNA from CAFsecreted extracellular vesicles [85]. Use of this CAF-derived mitochondrial DNA is specifically associated with the ability of CSCs to enhance oxidative phosphorylation. Upregulation of this metabolic pathway has been shown to drive proliferation and metastatic progression in the context of hormone therapy [85]. Hepatic stellate cells have similarly been shown to drive oxidative phosphorylation, a metabolic dependency of CSCs in pancreatic cancer metastases [86]. Juxtacrine and paracrine signals from endothelial cells also play a role in the survival and outgrowth of dormant metastatic cells. Chemotherapy selects for localization of metastatic breast cancer cells in the bone marrow perivascular niche, where endothelial adhesion activates integrin signaling, protecting them from chemotherapy-induced apoptosis [87]. Treatment with integrinblocking antibodies in transplant models both reduced disseminated tumor cell burden in the bone marrow and improved metastasis-free survival in the context of chemotherapy, suggesting that targeting endothelial–tumor cell crosstalk may provide new avenues for addressing dormant metastatic disease [87]. These results suggest that shifts in stromal signaling can directly support quiescent metastatic cells and trigger their proliferation, thus protecting them from therapy and driving an eventual relapse.
Concluding Remarks and Future Perspectives
The body of work outlined here demonstrates a clear role for the TME in the extrinsic regulation of stem cell fate in resistance and metastasis. These diverse cell populations and signaling mechanisms within the microenvironment present many new opportunities for indirect targeting of CSC features with the goal of improving therapeutic response and enabling more durable remissions. Still, the microenvironment is complex, and many of its cellular and non-cellular components remain understudied. Although we have focused on major stromal and immune cell types here, the role of cell populations such as neurons, adipocytes, and microbes remain to be studied in greater detail. Further, despite the advances discussed, studying TME–CSC crosstalk in a physiologic context remains challenging (see Outstanding Questions). In recent years, organoid techniques have greatly expanded our ability to dissect cellular crosstalk by providing more relevant in vitro models [88,89]. As microenvironmental elements are incorporated into these models, they will likely serve as critical tools with which to probe the elaborate extrinsic signals governing cell fate [90,91]. Nevertheless, given the complexity of the TME, delineating microenvironmental crosstalk in vivo will likely prove more useful in identifying novel signaling axes that drive stem cell fate in cancer. Intravital imaging presents an emergent strategy for interrogating interactions between microenvironmental cells and heterogeneous cancer cells in their native niche [92]. Live imaging is also extraordinarily powerful in delineating in high resolution both the spatial and temporal nature of cellular interactions and behavior [36,92,93], shedding light on dynamic processes such as development, tumor growth, metastasis, and immune infiltration [94]. Ultimately, our ability to successfully integrate the molecular and cellular understanding of oncogenesis with a systems view of the dynamics of cancer growth, will be essential for fundamentally changing our ability to effectively treat and manage this disease.
Highlights
Resistance to therapy and metastatic progression are two critical drivers of poor clinical outcome across cancers.
Aggressive subpopulations of cells that are enriched for stem cell signals, sometimes referred to as ‘cancer stem cells,’ are thought to be crucial drivers of these malignant features of disease.
Although a great deal of work in the field has focused on targeting the intrinsic dependencies of these aggressive cells, recent studies suggest that extrinsic microenvironmental signals are also crucial drivers of stem cell fate in cancer.
Defining the microenvironmental signals that support stem cell fate may point us toward new strategies that leverage microenvironmental modulation to ablate cancer stem cell populations and improve disease outcomes.
Outstanding Questions
What specific immune and stromal signals support stem cell fate in cancer, and how can we target these signals therapeutically?
How does stem cell fate mediate resistance or sensitivity to immunotherapy in different cancers? Will we be able to effectively target cancer stem cell populations using immunotherapy strategies?
Are there unique subsets of immune or stromal populations that support stem cell fate at both the primary tumor and metastatic sites? Can we develop strategies to ablate or inhibit these subsets to sensitize them to therapy and mitigate metastatic progression?
How do microenvironmental signals regulate the dormancy and later regrowth of disseminated cancer cells?
As in vitro models become more complex, will we be able to develop high-throughput screens to identify novel microenvironmental drivers of stem cell fate that model in vivo dependencies?
Acknowledgments
L.P.F. received support from National Institutes of Health (NIH) grant T32 GM007752 and NIH Ruth L. Kirschstein National Research Service Individual Award F31 CA247489. E.D. received support from NIH grant T32 GM007752. T.R. was supported by NIH grant R35 CA197699.
Footnotes
References
- 1.Reya T. et al. (2001) Stem cells, cancer, and cancer stem cells. Nature 414, 105–111 [DOI] [PubMed] [Google Scholar]
- 2.Batlle E and Clevers H (2017) Cancer stem cells revisited. Nat. Med. 23, 1124–1134 [DOI] [PubMed] [Google Scholar]
- 3.Nassar D and Blanpain C (2016) Cancer stem cells: basic concepts and therapeutic implications. Annu. Rev. Pathol. Mech. Dis. 11, 47–76 [DOI] [PubMed] [Google Scholar]
- 4.Lytle NK et al. (2018) Stem cell fate in cancer growth, progression and therapy resistance. Nat. Rev. Cancer 18, 669–680 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Oskarsson T. et al. (2014) Metastatic stem cells: sources, niches, and vital pathways. Cell Stem Cell 14, 306–321 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Clara JA et al. (2020) Targeting signalling pathways and the immune microenvironment of cancer stem cells – a clinical update. Nat. Rev. Clin. Oncol. 17, 204–232 [DOI] [PubMed] [Google Scholar]
- 7.Basset-Séguin N. et al. (2017) Vismodegib in patients with advanced basal cell carcinoma: primary analysis of STEVIE, an international, open-label trial. Eur. J. Cancer 86, 334–348 [DOI] [PubMed] [Google Scholar]
- 8.Lear JT et al. (2018) Long-term efficacy and safety of sonidegib in patients with locally advanced and metastatic basal cell carcinoma: 30-month analysis of the randomized phase 2 BOLT study. J. Eur. Acad. Dermatol. Venereol. 32, 372–381 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cortes JE et al. (2018) Glasdegib in combination with cytarabine and daunorubicin in patients with AML or high-risk MDS: phase 2 study results. Am. J. Hematol. 93, 1301–1310 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Prager BC et al. (2019) Cancer stem cells: the architects of the tumor ecosystem. Cell Stem Cell 24, 41–53 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Plaks V. et al. (2015) The cancer stem cell niche: how essential is the niche in regulating stemness of tumor cells? Cell Stem Cell 16, 225–238 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Grivennikov SI et al. (2010) Immunity, inflammation, and cancer. Cell 140, 883–899 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hanahan D and Weinberg RA (2011) Hallmarks of cancer: the next generation. Cell 144, 646–674 [DOI] [PubMed] [Google Scholar]
- 14.Gonzalez H. et al. (2018) Roles of the immune system in cancer: from tumor initiation to metastatic progression. Genes Dev. 32, 1267–1284 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mantovani A. et al. (2017) Tumour-associated macrophages as treatment targets in oncology. Nat. Rev. Clin. Oncol. 14, 399–416 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Aras S and Raza Zaidi M (2017) TAMeless traitors: macrophages in cancer progression and metastasis. Br. J. Cancer 117, 1583–1591 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wu L. et al. (2019) Tumor-associated neutrophils in cancer: going pro. Cancers (Basel) 11, 564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Veglia F. et al. (2018) Myeloid-derived suppressor cells coming of age review-article. Nat. Immunol. 19, 108–119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tsou P. et al. (2016) The emerging role of b cells in tumor immunity. Cancer Res. 76, 5591–5601 [DOI] [PubMed] [Google Scholar]
- 20.Sica A. et al. (2017) Tumor-associated myeloid cells as guiding forces of cancer cell stemness. Cancer Immunol. Immunother. 66, 1025–1036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nywening TM et al. (2018) Targeting both tumour-associated CXCR2+ neutrophils and CCR2+ macrophages disrupts myeloid recruitment and improves chemotherapeutic responses in pancreatic ductal adenocarcinoma. Gut 67, 1112–1123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mitchem JB et al. (2013) Targeting tumor-infiltrating macrophages decreases tumor-initiating cells, relieves immunosuppression, and improves chemotherapeutic responses. Cancer Res. 73, 1128–1141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Calcinotto A. et al. (2018) IL-23 secreted by myeloid cells drives castration-resistant prostate cancer. Nature 559, 363–369 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Somasundaram R. et al. (2017) Tumor-associated B-cells induce tumor heterogeneity and therapy resistance. Nat. Commun. 8, 607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sharma P and Allison JP (2015) The future of immune checkpoint therapy. Science 348, 56–61 [DOI] [PubMed] [Google Scholar]
- 26.Kruger S. et al. (2019) Advances in cancer immunotherapy 2019 – latest trends. J. Exp. Clin. Cancer Res. 38, 268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wei SC et al. (2018) Fundamental mechanisms of immune checkpoint blockade therapy. Cancer Discov. 8, 1069–1086 [DOI] [PubMed] [Google Scholar]
- 28.Ribas A and Wolchok JD (2018) Cancer immunotherapy using checkpoint blockade. Science 359, 1350–1355 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sharma P. et al. (2017) Primary, adaptive, and acquired resistance to cancer immunotherapy. Cell 168, 707–723 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Maccalli C. et al. (2018) The role of cancer stem cells in the modulation of anti-tumor immune responses. Semin. Cancer Biol. 53, 189–200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hsu JM et al. (2018) STT3-dependent PD-L1 accumulation on cancer stem cells promotes immune evasion. Nat. Commun. 9, 1908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lee Y. et al. (2016) CD44+ cells in head and neck squamous cell carcinoma suppress T-cell-mediated immunity by selective constitutive and inducible expression of PD-L1. Clin. Cancer Res. 22, 3571–3581 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cioffi M. et al. (2015) Cancer therapy: preclinical inhibition of CD47 effectively targets pancreatic cancer stem cells via dual mechanisms. Clin. Cancer Res. 21, 2325–2337 [DOI] [PubMed] [Google Scholar]
- 34.Spranger S. et al. (2015) Melanoma-intrinsic β-catenin signalling prevents anti-tumour immunity. Nature 523, 231–235 [DOI] [PubMed] [Google Scholar]
- 35.Miao Y. et al. (2019) Adaptive immune resistance emerges from tumor-initiating stem cells. Cell 177, 1172–1186.e14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lytle NK et al. (2019) A multiscale map of the stem cell state in pancreatic adenocarcinoma. Cell 177, 572–586.e22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Roberts KJ et al. (2017) The stromal niche for epithelial stem cells: a template for regeneration and a brake on malignancy. Cancer Cell 32, 404–410 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Oudin MJ and Weaver VM (2016) Physical and chemical gradients in the tumor microenvironment regulate tumor cell invasion, migration, and metastasis. Cold Spring Harb. Symp. Quant. Biol. 81, 189–205 [DOI] [PubMed] [Google Scholar]
- 39.Kalluri R (2016) The biology and function of fibroblasts in cancer. Nat. Rev. Cancer 16, 582–598 [DOI] [PubMed] [Google Scholar]
- 40.Valkenburg KC et al. (2018) Targeting the tumour stroma to improve cancer therapy. Nat. Rev. Clin. Oncol. 15, 366–381 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gieniec KA et al. (2019) Cancer-associated fibroblasts–heroes or villains? Br. J. Cancer 121, 293–302 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Cazet AS et al. (2018) Targeting stromal remodeling and cancer stem cell plasticity overcomes chemoresistance in triple negative breast cancer. Nat. Commun. 9, 2897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sansone P. et al. (2017) Evolution of cancer stem-like cells in endocrine-resistant metastatic breast cancers is mediated by stromal microvesicles. Cancer Res. 77, 1927–1941 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bartoschek M. et al. (2018) Spatially and functionally distinct subclasses of breast cancer-associated fibroblasts revealed by single cell RNA sequencing. Nat. Commun. 9, 5150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Mizutani Y. et al. (2019) Meflin-positive cancer-associated fibroblasts inhibit pancreatic carcinogenesis. Cancer Res. 79, 5367–5381 [DOI] [PubMed] [Google Scholar]
- 46.Djurec M. et al. (2018) Saa3 is a key mediator of the protumorigenic properties of cancer-associated fibroblasts in pancreatic tumors. Proc. Natl. Acad. Sci. U. S. A. 115, E1147–E1156 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Elyada E. et al. (2019) Cross-species single-cell analysis of pancreatic ductal adenocarcinoma reveals antigen-presenting cancer-associated fibroblasts. Cancer Discov. 9, 1102–1123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Öhlund D. et al. (2017) Distinct populations of inflammatory fibroblasts and myofibroblasts in pancreatic cancer. J. Exp. Med. 214, 579–596 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Brechbuhl HM et al. (2017) Fibroblast subtypes regulate responsiveness of luminal breast cancer to estrogen. Clin. Cancer Res. 23, 1710–1721 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Su S. et al. (2018) CD10+GPR77+ cancer-associated fibroblasts promote cancer formation and chemoresistance by sustaining cancer stemness. Cell 172, 841–856.e16 [DOI] [PubMed] [Google Scholar]
- 51.Cogle CR et al. (2016) Acute myeloid leukemia in the vascular niche. Cancer Lett. 380, 552–560 [DOI] [PubMed] [Google Scholar]
- 52.Bajaj J. et al. (2016) CD98-mediated adhesive signaling enables the establishment and propagation of acute myelogenous leukemia. Cancer Cell 30, 792–805 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Zhang B. et al. (2018) Bone marrow niche trafficking of miR-126 controls the self-renewal of leukemia stem cells in chronic myelogenous leukemia. Nat. Med. 24, 450–462 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Chaffer CL and Weinberg RA (2011) A perspective on cancer cell metastasis. Science 331, 1559–1564 [DOI] [PubMed] [Google Scholar]
- 55.Suarez-Carmona M. et al. (2017) EMT and inflammation: inseparable actors of cancer progression. Mol. Oncol. 11, 805–823 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Linde N. et al. (2018) Macrophages orchestrate breast cancer early dissemination and metastasis. Nat. Commun. 9, 21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Talmadge JE and Fidler IJ (2010) AACR centennial series: the biology of cancer metastasis: historical perspective. Cancer Res. 70, 5649–5669 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Lee CC et al. (2018) Macrophage-secreted interleukin-35 regulates cancer cell plasticity to facilitate metastatic colonization. Nat. Commun. 9, 3763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Wang D. et al. (2017) CXCL1 is critical for premetastatic niche formation and metastasis in colorectal cancer. Cancer Res. 77, 3655–3665 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Wculek SK and Malanchi I (2015) Neutrophils support lung colonization of metastasis-initiating breast cancer cells. Nature 528, 413–417 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.De Cock JM et al. (2016) Inflammation triggers Zeb1-dependent escape from tumor latency. Cancer Res. 76, 6778–6784 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Albrengues J. et al. (2018) Neutrophil extracellular traps produced during inflammation awaken dormant cancer cells in mice. Science 361, 6409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Agudo J. et al. (2018) Quiescent tissue stem cells evade immune surveillance. Immunity 48, 271–285.e5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Turdo A. et al. (2019) Meeting the challenge of targeting cancer stem cells. Front. Cell Dev. Biol. 7, 16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Burr ML et al. (2019) An evolutionarily conserved function of polycomb silences the MHC class I antigen presentation pathway and enables immune evasion in cancer. Cancer Cell 36, 385–401 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Casey SC et al. (2016) MYC regulates the antitumor immune response through CD47 and PD-L1. Science 352, 227–231 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Paczulla AM et al. (2019) Absence of NKG2D ligands defines leukaemia stem cells and mediates their immune evasion. Nature 572, 254–259 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Mohme M. et al. (2017) Circulating and disseminated tumour cells – mechanisms of immune surveillance and escape. Nat. Rev. Clin. Oncol. 14, 155. [DOI] [PubMed] [Google Scholar]
- 69.Wang B. et al. (2014) Metastatic consequences of immune escape from NK cell cytotoxicity by human breast cancer stem cells. Cancer Res. 74, 5746–5757 [DOI] [PubMed] [Google Scholar]
- 70.Malladi S. et al. (2016) Metastatic latency and immune evasion through autocrine inhibition of WNT. Cell 165, 45–60 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Kai FB et al. (2019) The extracellular matrix modulates the metastatic journey. Dev. Cell 49, 332–346 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Labernadie A. et al. (2017) A mechanically active heterotypic E-cadherin/N-cadherin adhesion enables fibroblasts to drive cancer cell invasion. Nat. Cell Biol. 19, 224–237 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Dongre A and Weinberg RA (2019) New insights into the mechanisms of epithelial–mesenchymal transition and implications for cancer. Nat. Rev. Mol. Cell Biol. 20, 69–84 [DOI] [PubMed] [Google Scholar]
- 74.Calon A. et al. (2014) TGF-beta in CAF-mediated tumor growth and metastasis. Semin. Cancer Biol. 25, 15–22 [DOI] [PubMed] [Google Scholar]
- 75.Yu Y. et al. (2014) Cancer-associated fibroblasts induce epithelial-mesenchymal transition of breast cancer cells through paracrine TGF-β signalling. Br. J. Cancer 110, 724–732 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Zhuang J. et al. (2015) TGFβ1 secreted by cancer-associated fibroblasts induces epithelial-mesenchymal transition of bladder cancer cells through lncRNA-ZEB2NAT. Sci. Rep. 5, 11924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Ebbing EA et al. (2019) Stromal-derived interleukin 6 drives epithelial-to-mesenchymal transition and therapy resistance in esophageal adenocarcinoma. Proc. Natl. Acad. Sci. U. S. A. 116, 2237–2242 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Waghray M. et al. (2016) GM-CSF mediates mesenchymal– epithelial cross-talk in pancreatic cancer. Cancer Discov. 6, 886–899 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Ao Z. et al. (2015) Identification of cancer-associated fibroblasts in circulating blood from patients with metastatic breast cancer. Cancer Res. 75, 4681–4687 [DOI] [PubMed] [Google Scholar]
- 80.Massagué J and Obenauf AC (2016) Metastatic colonization by circulating tumour cells. Nature 529, 298–306 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Celià-Terrassa T and Kang Y (2018) Metastatic niche functions and therapeutic opportunities. Nat. Cell Biol. 20, 868–877 [DOI] [PubMed] [Google Scholar]
- 82.Esposito M. et al. (2019) Bone vascular niche E-selectin induces mesenchymal–epithelial transition and Wnt activation in cancer cells to promote bone metastasis. Nat. Cell Biol. 21, 627–639 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Goddard ET et al. (2018) Dormant tumour cells, their niches and the influence of immunity. Nat. Cell Biol. 20, 1240–1249 [DOI] [PubMed] [Google Scholar]
- 84.Gao H. et al. (2016) Multi-organ site metastatic reactivation mediated by non-canonical discoidin domain receptor 1 signaling. Cell 166, 47–62 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Sansone P. et al. (2017) Packaging and transfer of mitochondrial DNA via exosomes regulate escape from dormancy in hormonal therapy-resistant breast cancer. Proc. Natl. Acad. Sci. U. S. A. 114, E9066–E9075 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Fabian A. et al. (2019) Metastasis of pancreatic cancer: an uninflamed liver micromilieu controls cell growth and cancer stem cell properties by oxidative phosphorylation in pancreatic ductal epithelial cells. Cancer Lett. 453, 95–106 [DOI] [PubMed] [Google Scholar]
- 87.Carlson P. et al. (2019) Targeting the perivascular niche sensitizes disseminated tumour cells to chemotherapy. Nat. Cell Biol. 21, 238–250 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Drost J and Clevers H (2018) Organoids in cancer research. Nat. Rev. Cancer 18, 407–418 [DOI] [PubMed] [Google Scholar]
- 89.Aboulkheyr Es H. et al. (2018) Personalized cancer medicine: an organoid approach. Trends Biotechnol. 36, 358–371 [DOI] [PubMed] [Google Scholar]
- 90.Brancato V. et al. (2020) Could 3D models of cancer enhance drug screening? Biomaterials 232, 119744 [DOI] [PubMed] [Google Scholar]
- 91.Rodrigues T. et al. (2018) Emerging tumor spheroids technologies for 3D in vitro cancer modeling. Pharmacol. Ther. 184, 201–211 [DOI] [PubMed] [Google Scholar]
- 92.Spinler K. et al. (2020) A stem cell reporter based platform to identify and target drug resistant stem cells in myeloid leukemia. Nat. Commun. 11, 5998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Fox RG et al. (2016) Image-based detection and targeting of therapy resistance in pancreatic adenocarcinoma. Nature 534.7607, 407–411 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Ellenbroek SIJ and Van Rheenen J (2014) Imaging hallmarks of cancer in living mice. Nat. Rev. Cancer 14, 406–418 [DOI] [PubMed] [Google Scholar]
- 95.Dierks C. et al. (2008) Expansion of Bcr-Abl-positive leukemic stem cells Is dependent on Hedgehog pathway activation. Cancer Cell 14, 238–249 [DOI] [PubMed] [Google Scholar]
- 96.Zhao C. et al. (2009) Hedgehog signalling is essential for maintenance of cancer stem cells in myeloid leukaemia. Nature 458, 776–779 [DOI] [PMC free article] [PubMed] [Google Scholar]