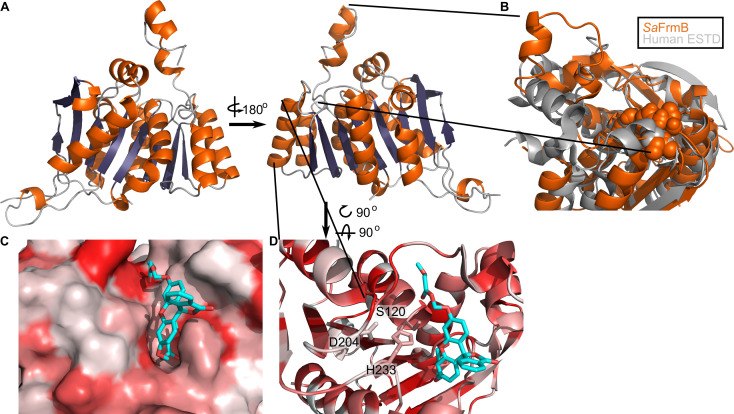
Figure 4. Three-dimensional structure of FrmB.
(A) Overall fold, a-helices colored in orange and β-strands colored in purple. (B) Comparison between SaFrmB (orange) and its closest human ortholog, ESTD (gray). Active site residues denoted in orange spheres. (C, D) Docking of substrate 1O (sticks) in the active site of FrmB. surface view, red indicates highly hydrophobic and white hydrophilic residues. Surface view (C) or stick view with catalytic triad (D).