Abstract
Antiphospholipid syndrome (APS) is a systemic autoimmune disease defined by thrombotic or obstetrical events and persistent antiphospholipid antibodies (aPLs). Chemokine-like factor-like MARVEL transmembrane domain-containing family (CMTM) is widely expressed in the immune system and may closely related to APS. This review aimed to systematically summarize the possible effects of CMTM on APS. Publications were collected from PubMed and Web of Science databases up to August 2020. CKLF, CKLFSF, CMTM, antiphospholipid syndrome, immune cells, and immune molecules were used as search criteria. Immune cells, including neutrophil, dendritic cells (DCs), T-cells, B-cells, and inflammatory cytokines, play an important role in the development of APS. Chemokine-like factor 1 (CKLF1) has a chemotactic effect on many cells and can affect the expression of inflammatory cytokines and adhesion molecules through the nuclear factor-kB (NF-kB) pathway or mitogen-activated protein kinase (MARK) pathway. CKLF1 can participate in the maturation of DCs, T lymphocyte activation, and the activation of neutrophils through the MAPK pathway. CMTM1 may act on Annexin A2 by regulating Ca2+ signaling. CMTM2 and CMTM6 are up-regulated in neutrophils of APS patients. Some CMTM family members influence the activation and accumulation of platelets. CMTM3 and CMTM7 are binding partners of B-cell linker protein (BLNK), thereby linking B cell receptor (BCR) and activating BLNK-mediated signal transduction in B cells. Moreover, CMTM3 and CMTM7 can act on DCs and B-1a cell development, respectively. CMTM may have potential effects on the development of APS by acting on immune cells and immune molecules. Thus, CMTM may act as a novel prognostic factor or immunomodulatory treatment option of APS.
Keywords: Antiphospholipid syndrome, CMTM, Pathogenesis
Introduction
Antiphospholipid syndrome (APS) is a systemic autoimmune disease defined by thrombotic or obstetrical events and persistent antiphospholipid antibodies (aPLs), namely lupus anticoagulant (LA), anticardiolipin antibodies (aCL), or anti-β2 glycoprotein-I (β2GPI) antibodies. APS can occur as an isolated diagnosis (primary APS) or can be associated with systemic lupus erythematosus (SLE) or another rheumatic disease.[1] The presence of aPLs plays a critical role in the pathogenesis of APS but is not sufficient for the clinical manifestations of APS.[2] Further insight is needed to identify the pathogenically relevant underlying mechanisms of APS.
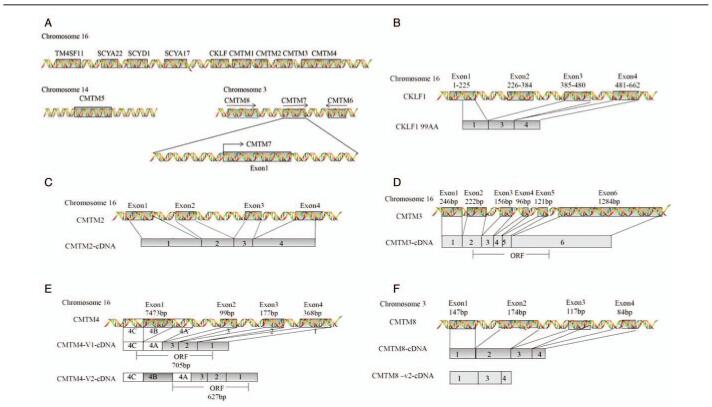
Chemokine-like factor superfamily members (CKLFSF) were first cloned and described by the Peking University Human Disease Gene Research Center in 2001.[3,4] Chemokine-like factor 1 (CKLF1) was isolated from a leukemia cell line U937 after the use of phytohemagglutinin (PHA), and cloned and validated CKLF-like MARVEL transmembrane domain-containing members (CMTM) by reverse transcription PCR.[3,5,6] In 2005, according to the molecular structures, the International Human Genetics Nomenclature Committee renamed CKLFSF1-8 to CMTM1-8.[7] CMTM comprises nine genes, CKLFs and CKLFSF1-8, which are located on different chromosomes. CKLF and CMTM1-4 are co-located on chromosome 16q22.1, CMTM5 is independently located on 14q11.2, and CMTM6-8 are co-located on chromosome 3p23[8–11] [Figure 1A]. Their gene products include chemokines and the transmembrane 4 superfamily (TM4SF). CMTM1 is most similar to chemokines in particular, whereas CMTM8 resembles TM4SF, and the biological characteristics of CMTM2-7 are somewhere in between.[12]
Figure 1.
Chromosomal location of CMTM members and related genes. (A) CMTM consists of nine genes, CKLF, and CMTM1-8. CKLF and CMTM1-4 form a gene cluster on chromosome 16, CMTM5 is mapped to chromosome 14q11, and CMTM6-8 constitute another cluster on chromosome 3p23. The gene density of the first cluster is much higher compared to that of the second cluster. The cluster consisting of SCYA22, SCYD1, and SCYA17 is not far from TM4SF11. (B–F) Chromosome localizations and genomic structures of CKLF1, CMTM2-4, CMTM8. BLNK: B-cell linker protein; CKLF: Chemokine-like factor; CMTM: Chemokine-like factor-like MARVEL transmembrane domain-containing family.
Various studies have shown that CMTM family members are widely expressed throughout the immune system, exhibit critical functions in the immune system, and are closely related to autoimmune diseases, such as APS.[6,13] This review aimed to systematically summarize the possible effects of CMTM on APS. CMTM members may be promising targets for the diagnosis and treatment of APS.
Pathogenesis of APS
APS is a systemic autoimmune disease characterized by the persistent presence of aPL, which is defined as LAC and/or significant titers of IgG and/or IgM class aCL and/or IgG and/or IgM class anti-β2GPI in the classification criteria, as a serologic hallmark, and obstetric complications or thrombosis as clinical criteria. The obstetric complications include recurrent early abortions, fetal loss, and premature birth due to (pre-)eclampsia or recognized features of placental insufficiency.[1]
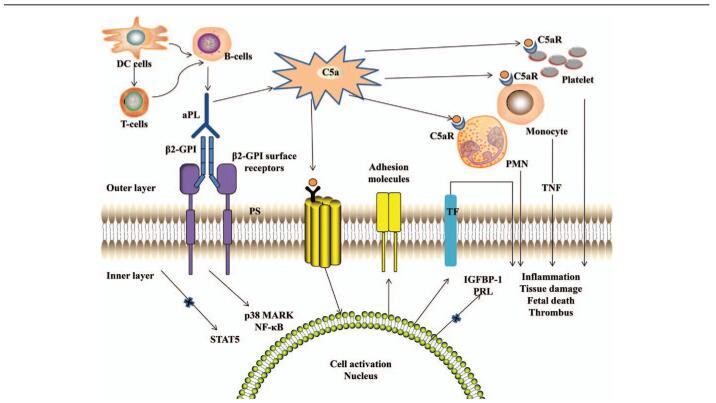
The pathogenesis of APS has been reviewed elsewhere.[14] Potential pathogenic pathways are illustrated in Figure 2.[15] The aPL induces thrombosis and placental injury of APS using multiple mechanisms.[2] Phosphatidylserine (PS), a negatively charged phospholipid, migrates from the inner to the outer cell membrane during activation or apoptosis of platelets and endothelial cells.[16] Subsequently, dimeric β2-GPI binds to PS, probably via β2-GPI surface receptors such as apoER2′, Annexin A2, or a Toll-like receptor, and aPL binds to β2-GPI, thereby activating the classic complement pathway, leading to the generation of C5a.[17–19] C5a can induce the expression of adhesion molecules (eg, intracellular adhesion molecule-1 [ICAM-1] and tissue factor [TF]), and activation of monocytes, polymorphonuclear neutrophils (PMN), and platelets, resulting in the release of pro-inflammatory mediators (eg, tumor necrosis factor-α (TNF-α) and vascular endothelial growth factor receptor-1), and initiation of the proadhesive and prothrombotic state.[20–22] Both nuclear factor-κB (NF-κB) and p38 mitogen-activated protein kinase (p38 MAPK) play a role in the intracellular signaling cascade.[23,24] The aPL can also downregulate the expression of trophoblast signal transducer and activator of transcription 5 (STAT5) to reduce the endometrial stromal cell production of prolactin (PRL) and insulin growth factor binding protein-1 (IGFBP-1), and adversely affect the formation of a trophoblast syncytium, trophoblast migration, invasion, and placental apoptosis, which are required for normal establishment of placental development.[25]
Figure 2.
Proposed mechanism of aPL-related thrombosis and placental injury. aPL: Antiphospholipid antibody; β2GPI: β2 glycoprotein-I; β2-GPI surface receptors: Referring to apoER2′, Annexin A2, or a Toll-like receptor; C5aR: C5a receptor; DCs: Dendritic cells; IGFBP-1: Insulin growth factor binding protein-1; NF-κB: Nuclear factor-κB; p38 MAPK: p38 mitogen-activated protein kinase; PMN: Polymorphonuclear neutrophils; PRL: Prolactin; STAT5: Signal transducer and activator of transcription 5; TF: Tissue factor; TNF: Tumor necrosis factor α.[14]
The presence of aPLs is necessary, but not sufficient for the clinical manifestations of APS.[14] In recent years, further insight has been provided into relevant mechanisms of pathogenesis of APS. Growing evidence has suggested a role of innate immune cells, in particular neutrophils and dendritic cells (DCs), and adaptive immune cells in APS. Neutrophil activation, including the expression of TF and the release of neutrophil extracellular traps (NETs), and interleukin-8 (IL-8), may be an important factor of aPL-associated thrombosis.[26] DCs play an important role in the sustained production of aPLs triggered by endothelial damage in APS.[27] B-cell activating factor (BAFF), which is crucial for B-cell survival, may play a role in the prevention of thrombosis associated with APS.[28] Furthermore, T-cell plays an important role in the activation of endothelial cells, thrombocytes, and placental tissue by anti-β2GPI antibodies related to clinical manifestations of APS.[26]
Features of CMTM Family Members
CKLF1
CKLF1 (GenBank accession No. AF096895) maps to chromosome 16q 22.1 and composed of four exons and three introns, with a calculated molecular mass of 10.9 KD [Figure 1B]. The full-length cDNA of CKLF1 is 530 bp long, with a single open reading frame encoding 99 amino acid residues, forming a highly hydrophobic alkaline protein.[6] CKLF1 has the remarkable characteristics of the CC chemokines family and bears two successive cysteine residues in the sequence but does not have an obvious homology as a classical CC subfamily members. Mature CKLF1 protein only contains a single conserved CC motif and lacks the additional C-terminus cysteine.[6]
The CKLF1 motif shares similar amino acids with the thymus, and activation-regulated chemokine (TARC)/C-C class chemokine (CCL) 17 and macrophage-derived chemokine (MDC)/CCL22, which are specific ligands for C–C chemokine receptor 4 (CCR4).[29–31] Several studies have shown that CKLF1 is a functional ligand for CCR4 and shows a high affinity for CCR4.[32] C19 and C27 are the main secreted forms of CKLF1 at the C-terminus, both of which can interact with CCR4. C27 acts as an agonist of CCR4, whereas C19 acts as an antagonist.[33]
CMTM1-4
CKLF and CMTM1-4 are grouped on chromosome 16q22.1 to form a gene cluster.[12] CMTM1 comprises seven exons, six introns, and 23 isoforms. CMTM2 is tightly linked to CMTM1 [Figure 1C].[7] CMTM3 [Figure 1D] is highly expressed in multiple immune cells, such as resting B lymphocytes, CD4+T lymphocytes, and monocytes.[7]. CMTM3 can enhance Rab5 activity, which plays important roles in T cell receptor (TCR), B cell receptor (BCR), and Toll-like receptors (TLRs).[34,35] It is suggested that CMTM3 may play a role in the development of autoimmune diseases by promoting Rab5 activity.[36]
CMTM4, with a MARVEL domain and a four-time transmembrane structure, has three transcript variants, such as CMTM4-v1, CMTM4-v2, and CMTM4-v3, of which CMTM4-v2 is the full-length cDNA product and has been highly conserved during evolution. CMTM4 contains four exons and three introns and the last exon can be divided into three parts; that is, A, B, and C. CMTM4-v2 contains all exons, whereas CMTM4-v1 contains exons 1, 2, 3 and part A and part C of exon 4 [Figure 1E].[37,38]
CMTM5
CMTM5 is independently located on 14q11.2 and closely linked to the interleukin 25 gene. CMTM5 comprises at least six mRNA splicing bodies, CMTM5-v1-v6, of which CMTM5-v1 is the most conserved.[3]
CMTM6-8
CMTM6-8 is located in a gene cluster on chromosome 3p22. CMTM6, adjacent to CMTM7, is 21,600 nucleotides long and encodes three transcripts, CMTM6-001 to CMTM6-003, but only CMTM6-001 bearing four introns can be successfully translated. CMTM6 is a 183 amino acid protein with a typical MARVEL domain.[3] CMTM7 is located between CMTM6 and CMTM8, within the same cluster, and is 63858 bp in length [Figure 1A]. CMTM7 is highly expressed in leukocytes and has six splicing isoforms: CMTM7-001 to CMTM7-006. CMTM7-001 is the main splicing isoform and can be detected by Northern blot analysis, and its cDNA is 1369 bp long, including four introns, five exons, a classical promoter sequence, and a poly (A) tail.[6] The cDNA of CMTM8 has a full length of 1185bp, of which nucleotides 295–816 encode CMTM8. The expression product is a four-time transmembrane protein, which consists of 173 amino acids and MARVEL domains for vesicular transport and membrane ligation [Figure 1F].[1]
Possible Effects of the CMTM Family on APS
Endothelial cells
The aPL can bind to the immunogenic β2GPI, thereby resulting in endothelial-cell activation, and causing some proinflammatory and prothrombotic changes.[20–22] The presence of aPL may up-regulate cell-surface adhesive molecules (such as ICAM-1) and stimulate the release of TNF-α.[39] CKLF1 has a broad spectrum of chemotactic activity and can affect the expression of inflammatory cytokines and adhesion molecules.[40] Kong et al[41] reported that an anti-CKLF1 antibody could decrease the production of inflammatory factors TNF-a, IL-1b, macrophage inflammatory protein-2, and IL-8 as well as that of adhesion molecules, ICAM-1, and vascular cell adhesion molecule 1 (VCAM-1). Furthermore, CMTM3 possesses the capability of mediating intercellular adhesion at endothelial adherens junctions, which play a key role in maintaining endothelial barrier function, through participating in VE-cadherin turnover and regulating the cell surface pool of VE-cadherin.[42]
NF-κB plays an important role in the intracellular signaling cascade of the classic complement activation pathway in APS.[23,24] Targeting NF-κB is a therapeutic option.[43] It has been reported that CKLF1 can activate the NF-κB signaling pathway, which can regulate the expression of pro-inflammatory mediators. Keith et al[44] showed that WAY-169916, a selective NF-kB transcriptional inhibitor, caused a marked decrease in CKLF1 expression in the rat spleen. Thus, CKLF1 may act on inflammation through the NF-kB pathway.
Calcium (Ca2+) plays an important role in the pathogenesis of autoimmune diseases.[13] In the presence of Ca2+, Annexin A2 is associated with anionic phospholipid and participates in the thrombosis of APS.[45] Liu et al[46] demonstrated that the expression of CMTM1 was down-regulated in rheumatoid arthritis synovial fibroblasts (RASFs) from rheumatoid arthritis (RA) patients treated with celastrol, which can induce Ca2+ signaling and mobilize cytosolic Ca2+ in RASFs. In addition, Wong et al[47] showed that CMTM1 may be suppressed by calmodulin. Moreover, CMTM1-v5 can interact with calcium-modulating cyclophilin ligand (CAML), which can negatively participate in the intracellular calcium signaling to negatively regulate the Ca2+ response in the endoplasmic reticulum (ER), thereby causing an increase in calcium influx and in turn activating the calcineurin, leading to the activation of NF-kB.[48] Therefore, CMTM1 can play a role in the regulation of Ca2+ signaling and accordingly act on Annexin A2.
Platelets
In vitro, aPLs can act on platelets from healthy donors and increase the expression of glycoprotein IIb/IIIa (the receptor for fibrinogen).[49,50] Platelets may play a key role in the prothrombotic interactions between aPLs and endothelial cells in APS.[22]
The CMTM family may influence the activation and accumulation of platelets and play a role in the process of hemostasis and thrombosis. Through paired-end next-generation RNA sequencing to identify functional differences in platelets of human and mouse, it was suggested that CMTM5 can be expressed in human platelets, but not in mouse platelets.[51] Platelets possess palmitoylation machinery that is required for both platelet activation and platelet accumulation into thrombi.[52] Dowal et al[53] showed that CMTM3, CMTM5, and CMTM7 were significantly enriched in the hydroxylamine+ (HA+) sample, which suggested that they were palmitoyl proteins. CMTM3, CMTM5, and CMTM7 may play a specific role in platelet function and be potential targets for the modulation of hemostasis and thrombosis. Moreover, the expression of CKLF, CMTM1-3, and CMTM5-7 is up-regulated in platelets of SLE patients when compared to those of healthy individuals, implying that they may affect platelet activation and contribute to the development of vascular disease in SLE.[54]
Innate immunity cells
DCs
The presence of DCs, the most potent antigen-presenting cells that link innate and adaptive immunity, is necessary for generating and maintaining the production of aPLs triggered by exposed intracellular phospholipids on the outer surface of apoptotic cells in APS.[27]
In previous studies, Shao et al[55] showed that CKLF1 was highly expressed in monocytes. During differentiation from monocytes to immature DCs, CKLF1 was significantly increased on day 2, then decreased from day 3 to 5. CKLF1 was down-regulated upon the maturation of DCs activated by different stimuli. Hence, CKLF1 plays a key role in the maturation of DCs.[55] Two peptides of CKLF1, C19, and C27 can promote the effect of immature DCs (imDCs) on T-cell proliferation and IFN-γ production. In addition, they up-regulate the secretion of HLA-DR and IL-12, without obvious effects on CD80, CD83, or CD86 in immature DCs. Thus, CKLF1-C19 and -C27 stimulate the antigen-presenting capability of imDCs.[55] B-cell linker protein (BLNK) has distinct functions in endocytosis and signaling through a cell-surface receptor in DCs. It has been reported that CMTM3, as a binding partner of BLNK, is highly expressed in DCs.[56] CMTM3 can also bind to SLP76 in DC2.4 cells. Consequently, CMTM3 may have an important role in DCs via BLNK.[57]
Neutrophils
Neutrophils are involved in the pathogenesis of APS. Neutrophil activation, including the expression of TF and the release of NETs and IL-8, may be an important factor of aPL-associated thrombosis.[58]
Previous studies have shown that CKLF1 exhibits a broad spectrum of chemotactic activity on neutrophils and can activate neutrophils through the MAPK pathway.[40] Additional studies showed that when administrated an anti-CKLF1 antibody, numbers of myeloperoxidase (MPO)-positive neutrophils and the activity of MPO, a marker enzyme for measuring neutrophils accumulation, decreased. An anti-CKLF1 antibody can also inhibit the phosphorylation level of p38, extracellular signal-regulated kinase (ERK), and c-Jun-N-terminal kinase (JNK) of the MAPK signal transduction pathway, which are the most important signaling molecules that are thought to mediate inflammatory responses.[41,59–61] Therefore, anti-CKLF1 antibodies can inhibit neutrophil infiltration via acting on MAPK signaling pathways. Recently, Knight et al[62] showed that CMTM2 and CMTM6 were up-regulated in neutrophils from APS patients.
Adaptive Immune Cells
T-cells
The protein β2GPI is regarded as the most important autoantigen in APS. By activating endothelial cells, thrombocytes, and placental tissue, T-cell-dependent anti-β2GPI autoantibodies are associated with the development of autoimmune coagulation and obstetric complications in APS.[26]
As mentioned above, CKLF1 is a novel functional ligand of CCR4.[26] CCR4 can facilitate the recruitment, homing, and education of activated leukocytes (mainly CD4+ Th2 lymphocytes).[30,63] In addition, CKLF1 itself has chemotactic effects on leukocytes.[40] Therefore, the interaction of CKLF1 with CCR4 might play a role in T-cells.
CKLF1 may be involved in the activation of T lymphocytes. When studying the expression profile of CKLF1 in activated T lymphocytes, Li et al demonstrated that CKLF1 was up-regulated in activated CD4+ and CD8+ cells, with no obvious changes in CD19+ cells. They further performed kinetic analyses of CKLF1 expression in PHA-stimulated human peripheral blood lymphocytes (PBL) at both mRNA and protein levels. They found that the expression of CKLF1 in lymphocytes was remarkably up-regulated by PHA, appearing at 8 h after PHA-stimulation and persisting up to 72 h, which showed that it could be up-regulated by PHA-activation in a time-dependent manner.[64]
Furthermore, the expression of CKLF1, as well as that of C–X–C motif chemokine ligand (CXCL)13 and inducible co-stimulator (ICOS), is significantly up-regulated in germinal center T helper cells (GC-Th cells), which are mostly nonpolarized (lacking IL-4 and interferon γ [IFN-γ] production) but are efficient in inducing B-cell production of immunoglobulin.[65] It has been suggested that CKLF1 may participate in the humoral immune response and germinal center formation via acting on GC-Th cells.
B-cells
B-cells serve a central role in the pathophysiology of an autoantibody-mediated disease, such as APS.[2] Increased percentages and absolute counts of naive B cells were observed in APS women.[66] Moreover, B-cell activating factor (BAFF), which is crucial for B-cell survival, may play a role in the prevention of thrombosis associated with APS.[28]
BLNK is a pivotal adaptor protein in the signal transduction pathway from the IgM class BCR.[67–69] In previous studies, it was identified that CMTM3 was a binding partner of BLNK that could bind the N-terminal part of BLNK.[57] In the chicken B cell line DT40, CMTM3 may act as a scaffold for signaling proteins and enhance ERK activation by BCR signaling. CMTM3 can enhance Rab5 activity, which is a key check-point in the endocytic pathways of BCR trafficking.[36] CMTM7 is also a binding partner of BLNK.[57] CMTM7 can link sIgM and BLNK in the plasma membrane to recruit BLNK to the environment of Syk and to initiate BLNK-mediated signaling transduction. In general, CMTM7 can link BCR and activate BLNK-mediated signal transduction in B cells, specifically involved in BCR expression.[57]
Innate-like B-1a cells (also termed CD5-positive B-cells) are an important cell population for the secretion of natural IgM and IL-1, and they act as the first line against pathogens.[70,71] Increased percentages of B-1a cells in primary APS patients correlated with levels of IgM aPLs.[72] CMTM7 is essential for B-1a cells development. CMTM7 is specifically involved in the survival of B-1a cells and the plasma cell generation of B-1a and B-1b cells, while having little effect on the development and function of B-2 cells.[73] Further investigations demonstrated that CMTM7 specifically acted on the B-1a cell development at the transitional B-1a (TrB-1a) stage. Loss of CMTM7 resulted in B-1a cell developmental arrest at TrB-1a, resulting in reduced numbers of mature B-1a cells in spleen and PerC, followed by the marked decrease of B-1a cell numbers in all investigated tissues, which results from B-cell-intrinsic defects. Because of B-1a cells loss, CMTM7-deficient mice produced less IgM and IL-10 and were more susceptible to microbial sepsis.[74]
Summary and Prospect
APS has a broad spectrum of thrombotic and nonthrombotic clinical manifestations.[1] The presence of aPLs plays a critical role in the pathogenesis of APS but is not sufficient for the clinical manifestations of APS.[2] Further insight on the pathogenesis of APS is needed.
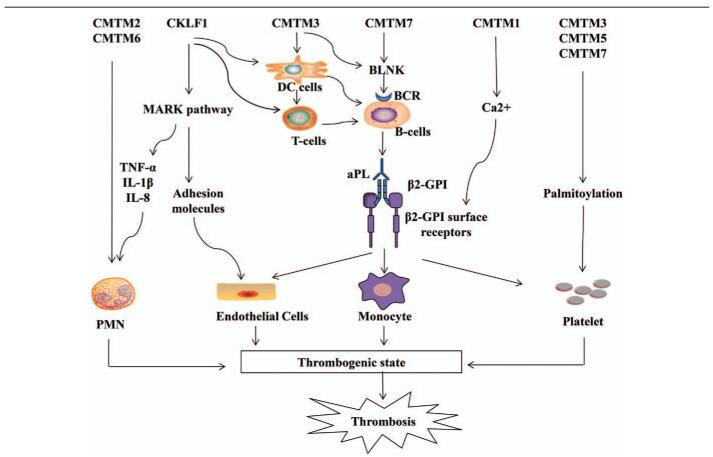
CMTM family members are widely expressed in the immune system, participate in T cell and B cell activation, and are closely related to autoimmune diseases, such as APS.[6,13] In a large number of studies, it was suggested that CMTM may have potential effects on the development of APS through acting on immune cells and immune molecules [Figure 3]. CKLF1 has a broad spectrum of chemotactic effects on many cells, including lymphocytes, macrophages, and neutrophils.[39] CKLF1s can affect the expression of inflammatory cytokines and adhesion molecules in terms of NF-kB or MAPK pathways.[43,56] CKLF1 plays a key role in the maturation of DCs, as well as on the activation of T lymphocytes, and participates in the humoral immune response and germinal center formation via acting on GC-Th cells.[53,62,63] Furthermore, CKLF1 can activate neutrophils through the MAPK pathway.[56] CMTM1 may act on Annexin A2 by regulating Ca2+ signaling.[13,45,46] CMTM2 and CMTM6 are up-regulated in neutrophils of APS patients.[59] Some CMTM family members may have an effect on the activation and accumulation of platelets and play a role in processes, such as hemostasis and thrombosis.[49–52] CMTM3 and CMTM7 are binding partners of BLNK, linking BCR and activating BLNK-mediated signal transduction in B cells.[55] Furthermore, CMTM3 may play an important role in DCs.[54] CMTM7 is essential for B-1a cells development and specifically acts on the transitional B-1a (TrB-1a) stage.[70,71]
Figure 3.
Potential effects of CMTM on APS. CKLF1 has a chemotactic effect on many cells and can affect the expression of inflammatory cytokines and adhesion molecules through the MARK pathway. CKLF1 can participate in the maturation of DCs, T lymphocyte activation, and the activation of neutrophils through the MAPK pathway. CMTM1 may act on Annexin A2 by regulating Ca2+ signaling. CMTM2 and CMTM6 are up-regulated in the neutrophils of APS patients. CMTM3, CMTM5, CMTM7 influence the activation and accumulation of platelets. CMTM3 and CMTM7 are binding partners of BLNK, thereby linking BCR and activating BLNK-mediated signal transduction in B cells. CMTM3 and CMTM7 can act on DCs and B-1a cell development, respectively. aPL: Antiphospholipid antibody; β2GPI: β2 glycoprotein-I; β2-GPI surface receptors: Referring to apoER2′, annexin A2, or a Toll-like receptor; BCR: B cell receptor; BLNK: B-cell linker protein; CKLF1: chemokine-like factor 1; CMTM: Chemokine-like factor-like MARVEL transmembrane domain-containing family; DCs: Dendritic cells; IL: Interleukin; MAPK: Mitogen-activated protein kinase; PMN: Polymorphonuclear neutrophils; TNF-α: Tumor necrosis factor-α.
However, relatively a few in-depth studies on CMTM have been performed in APS. Advances in our understanding of how CMTM participates in the pathogenesis of APS are needed. Thus, CMTM may act as a novel prognostic factor or immunomodulatory treatment option of APS in the future.
Funding
This work was supported by a grant from the National Natural Science Foundation (No. 81501390).
Footnotes
How to cite this article: Ge YY, Duan HJ, Deng XL. Possible effects of chemokine-like factor-like MARVEL transmembrane domain-containing family on antiphospholipid syndrome. Chin Med J 2021;134:1661–1668. doi: 10.1097/CM9.0000000000001449
References
- 1.Miyakis S, Lockshin MD, Atsumi T, Branch DW, Brey RL, Cervera R, et al. International consensus statement on an update of the classification criteria for definite antiphospholipid syndrome (APS). J Thromb Haemost 2006; 4:295–306. doi: 10.1111/j.1538-7836.2006.01753.x. [DOI] [PubMed] [Google Scholar]
- 2.van den Hoogen LL, van Roon JAG, Radstake TRDJ, Fritsch-Stork RDE, Derksen RHWM. Delineating the deranged immune system in the antiphospholipid syndrome. Autoimmun Rev 2016; 15:50–60. doi: 10.1016/j.autrev.2015.08.011. [DOI] [PubMed] [Google Scholar]
- 3.Han W, Ding P, Xu M, Wang L, Rui M, Shi S, et al. Identification of eight genes encoding chemokine-like factor superfamily members 1-8 (CKLFSF1-8) by in silico cloning and experimental validation. Genomics 2003; 81:609–617. doi: 10.1016/s0888-7543(03)00095-8. [DOI] [PubMed] [Google Scholar]
- 4.Lu J, Wu QQ, Zhou YB, Zhang KH, Pang BX, Li L, et al. Cancer research advance in CKLF-like MARVEL transmembrane domain containing member family (Review). Asian Pac J Cancer Prev 2016; 17:2741–2744. [PubMed] [Google Scholar]
- 5.Yang GY, Chen X, Sun YC, Ma CL, Qian G. Chemokine-like factor 1 (CLFK1) is over-expressed in patients with atopic dermatitis. Int J Biol Sci 2013; 9:759–765. doi: 10.7150/ijbs.6291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Han W, Lou Y, Tang J, Zhang Y, Chen Y, Li Y, et al. Molecular cloning and characterization of chemokine-like factor 1 (CKLF1), a novel human cytokine with unique structure and potential chemotactic activity. Biochem J 2001; 357:127–135. doi: 10.1042/0264-6021:3570127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhong J, Wang Y, Qiu X, Mo X, Liu Y, Li T, et al. Characterization and expression profile of CMTM3/CKLFSF3. J Biochem Mol Biol 2006; 39:537–545. doi: 10.5483/bmbrep.2006.39.5.537. [DOI] [PubMed] [Google Scholar]
- 8.Wang Y, Li J, Cui Y, Li T, Ng KM, Geng H, et al. CMTM3, located at the critical tumor suppressor locus 16q22.1, is silenced by CpG methylation in carcinomas and inhibits tumor cell growth through inducing apoptosis. Cancer Res 2009; 69:5194–5201. doi: 10.1158/0008-5472.CAN-08-3694. [DOI] [PubMed] [Google Scholar]
- 9.Li H, Guo X, Shao L, Plate M, Mo X, Wang Y, et al. CMTM5-v1, a four-transmembrane protein, presents a secreted form released via a vesicle-mediated secretory pathway. BMB Rep 2010; 43:182–187. doi: 10.5483/bmbrep.2010.43.3.182. [DOI] [PubMed] [Google Scholar]
- 10.Wang L, Wu C, Zheng Y, Qiu X, Wang L, Fan H, et al. Molecular cloning and characterization of chemokine-like factor super family member 1 (CKLFSF1), a novel human gene with at least 23 alternative splicing isoforms in testis tissue. Int J Biochem Cell Biol 2004; 36:1492–1501. doi: 10.1016/j.biocel.2003.11.017. [DOI] [PubMed] [Google Scholar]
- 11.Gao DH, Hu H, Fang ZW, Huo F, Wang HR, Xu KX, et al. Research advances in chemokine-like factor super family member 8 (In Chinese). Acta Acad Med Sin 2016; 38:746–749. doi: 10.3881/j.issn.1000-503X.2016.06.021. [DOI] [PubMed] [Google Scholar]
- 12.Wu K, Li X, Gu H, Yang Q, Liu Y, Wang L. Research advances in CKLF-like MARVEL transmembrane domain-containing family in non-small cell lung cancer. Int J Biol Sci 2019; 15:2576–2583. doi: 10.7150/ijbs.33733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Duan HJ, Li XY, Liu C, Deng XL. Chemokine-like factor-like MARVEL transmembrane domain-containing family in autoimmune diseases. Chin Med J 2020; 133:951–958. doi: 10.1097/CM9.0000000000000747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Giannakopoulos B, Krilis SA. The pathogenesis of the antiphospholipid syndrome. N Engl J Med 2013; 368:1033–1044. doi: 10.1056/NEJMra1112830. [DOI] [PubMed] [Google Scholar]
- 15.Firestein GS, Gabriel SE, McInnes IB, O’Dell JR. Kelley and Firestein's textbook of rheumatology, 10th edn. Philadelphia, PA: Elsevier; 2017. [Google Scholar]
- 16.Lutters BCH, Derksen RHWM, Tekelenburg WL, Lenting PJ, Arnout J, de Groot PG. Dimers of beta 2-glycoprotein I increase platelet deposition to collagen via interaction with phospholipids and the apolipoprotein E receptor 2’. J Biol Chem 2003; 278:33831–33838. doi: 10.1074/jbc.M212655200. [DOI] [PubMed] [Google Scholar]
- 17.Romay-Penabad Z, Montiel-Manzano MG, Shilagard T, Papalardo E, Vargas G, Deora AB, et al. Annexin A2 is involved in antiphospholipid antibody-mediated pathogenic effects in vitro and in vivo. Blood 2009; 114:3074–3083. doi: 10.1182/blood-2008-11-188698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.van Lummel M, Pennings MTT, Derksen RH, Urbanus RT, Lutters BC, Kaldenhoven N, et al. The binding site in {beta}2-glycoprotein I for ApoER2’ on platelets is located in domain V. J Biol Chem 2005; 280:36729–36736. doi: 10.1074/jbc.M504172200. [DOI] [PubMed] [Google Scholar]
- 19.Erkan D, Lockshin MD. What is antiphospholipid syndrome? Curr Rheumatol Rep 2004; 6:451–457. doi: 10.1007/s11926-004-0024-1. [DOI] [PubMed] [Google Scholar]
- 20.Zuo Y, Shi H, Li C, Knight JS. Antiphospholipid syndrome: a clinical perspective. Chin Med J 2020; 133:929–940. doi: 10.1097/CM9.0000000000000705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bordron A, Dueymes M, Levy Y, Jamin C, Ziporen L, Piette JC, et al. Anti-endothelial cell antibody binding makes negatively charged phospholipids accessible to antiphospholipid antibodies. Arthritis Rheum 1998; 41:1738–1747. doi: 10.1002/1529-0131(199810)41:10<1738::AID-ART6>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- 22.Font J, Espinosa G, Tassies D, Pino M, Khamashta MA, Gallart T, et al. Effects of beta2-glycoprotein I and monoclonal anticardiolipin antibodies in platelet interaction with subendothelium under flow conditions. Arthritis Rheum 2002; 46:3283–3289. doi: 10.1002/art.10634. [DOI] [PubMed] [Google Scholar]
- 23.Pierangeli SS, Vega-Ostertag M, Harris EN. Intracellular signaling triggered by antiphospholipid antibodies in platelets and endothelial cells: A pathway to targeted therapies. Thromb Res 2004; 114:467–476. doi: 10.1016/j.thromres.2004.06.031. [DOI] [PubMed] [Google Scholar]
- 24.Dunoyer-Geindre S, de Moerloose P, Galve-de Rochemonteix B, Reber G, Kruithof EKO. NFkappaB is an essential intermediate in the activation of endothelial cells by anti-beta(2)-glycoprotein 1 antibodies. Thromb Haemost 2002; 88:851–857. [PubMed] [Google Scholar]
- 25.Mak IYH, Brosens JJ, Christian M, Hills FA, Chamley L, Regan L, et al. Regulated expression of signal transducer and activator of transcription, Stat5, and its enhancement of PRL expression in human endometrial stromal cells in vitro. J Clin Endocrinol Metab 2002; 87:2581–2588. doi: 10.1210/jcem.87.6.8576. [DOI] [PubMed] [Google Scholar]
- 26.Garcia D, Erkan D. Diagnosis and management of the Antiphospholipid syndrome. N Engl J Med 2018; 378:2010–2021. doi: 10.1056/NEJMra1705454. [DOI] [PubMed] [Google Scholar]
- 27.Broder A, Chan JJ, Putterman C. Dendritic cells: An important link between antiphospholipid antibodies, endothelial dysfunction, and atherosclerosis in autoimmune and non-autoimmune diseases. Clin Immunol 2013; 146:197–206. doi: 10.1016/j.clim.2012.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kahn P, Ramanujam M, Bethunaickan R, Huang W, Tao H, Madaio MP, et al. Prevention of murine antiphospholipid syndrome by BAFF blockade. Arthritis Rheum 2008; 58:2824–2834. doi: 10.1002/art.23764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Scheu S, Ali S, Ruland C, Arolt V, Alferink J. The C-C chemokines CCL17 and CCL22 and their receptor CCR4 in CNS autoimmunity. Int J Mol Sci 2017; 18:2306.doi: 10.3390/ijms18112306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Imai T, Chantry D, Raport CJ, Wood CL, Nishimura M, Godiska R, et al. Macrophage-derived chemokine is a functional ligand for the CC chemokine receptor 4. J Biol Chem 1998; 273:1764–1768. doi: 10.1074/jbc.273.3.1764. [DOI] [PubMed] [Google Scholar]
- 31.Imai T, Baba M, Nishimura M, Kakizaki M, Takagi S, Yoshie O. The T cell-directed CC chemokine TARC is a highly specific biological ligand for CC chemokine receptor 4. J Biol Chem 1997; 272:15036–15042. doi: 10.1074/jbc.272.23.15036. [DOI] [PubMed] [Google Scholar]
- 32.Wang Y, Zhang Y, Yang X, Han W, Liu Y, Xu Q, et al. Chemokine-like factor 1 is a functional ligand for CC chemokine receptor 4 (CCR4). Life Sci 2006; 78:614–621. doi: 10.1016/j.lfs.2005.05.070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wang Y, Zhang Y, Han W, Li D, Tian L, Yin C, et al. Two C-terminal peptides of human CKLF1 interact with the chemokine receptor CCR4. Int J Biochem Cell Biol 2008; 40:909–919. doi: 10.1016/j.biocel.2007.10.028. [DOI] [PubMed] [Google Scholar]
- 34.Andre P, Boretto J, Hueber AO, Regnier-Vigouroux A, Gorvel JP, Ferrier P, et al. A dominant-negative mutant of the Rab5 GTPase enhances T cell signaling by interfering with TCR down-modulation in transgenic mice. J Immunol 1997; 159:5253–5263. [PubMed] [Google Scholar]
- 35.Hoogeboom R, Tolar P. Molecular mechanisms of B cell antigen gathering and endocytosis. Curr Top Microbiol Immunol 2016; 393:45–63. doi: 10.1007/82_2015_476. [DOI] [PubMed] [Google Scholar]
- 36.Yuan W, Liu B, Wang X, Li T, Xue H, Mo X, et al. CMTM3 decreases EGFR expression and EGF-mediated tumorigenicity by promoting Rab5 activity in gastric cancer. Cancer Lett 2017; 386:77–86. doi: 10.1016/j.canlet.2016.11.015. [DOI] [PubMed] [Google Scholar]
- 37.Plate M, Li T, Wang Y, Mo X, Zhang Y, Ma D, et al. Identification and characterization of CMTM4, a novel gene with inhibitory effects on HeLa cell growth through Inducing G2/M phase accumulation. Mol Cells 2010; 29:355–361. doi: 10.1007/s10059-010-0038-7. [DOI] [PubMed] [Google Scholar]
- 38.Li T, Guo XH, Wang Y, Markus P, Shao LN, Song QS, et al. Preparation, purification and characterization of the polyclonal antibody against human CMTM4 (in Chinese). Chin J Cell Mol Immunol 2008; 24:41–44. [PubMed] [Google Scholar]
- 39.Vega-Ostertag M, Casper K, Swerlick R, Ferrara D, Harris EN, Pierangeli SS. Involvement of p38 MAPK in the up-regulation of tissue factor on endothelial cells by antiphospholipid antibodies. Arthritis Rheum 2005; 52:1545–1554. doi: 10.1002/art.21009. [DOI] [PubMed] [Google Scholar]
- 40.Ke X, Jia L, Jing H, Liu Y, Zhang Y, Di C. Effects of novel human chemokine-like factor 1 (CKLF1) on bone marrow hematopoietic stem cell/progenitor cell in vitro (in Chinese). Chin J Hematol 2002; 23:301–303. [PubMed] [Google Scholar]
- 41.Kong LL, Wang ZY, Han N, Zhuang XM, Wang ZZ, Li H, et al. Neutralization of chemokine-like factor 1, a novel C-C chemokine, protects against focal cerebral ischemia by inhibiting neutrophil infiltration via MAPK pathways in rats. J Neuroinflammation 2014; 11:112.doi: 10.1186/1742-2094-11-112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Chrifi I, Louzao-Martinez L, Brandt M, van Dijk C, Burgisser P, Zhu C, et al. CMTM3 (CKLF-like marvel transmembrane domain 3) mediates angiogenesis by regulating cell surface availability of VE-cadherin in endothelial adherens junctions. Arterioscler Thromb Vasc Biol 2017; 37:1098–1114. doi: 10.1161/ATVBAHA.116.308792. [DOI] [PubMed] [Google Scholar]
- 43.Montiel-Manzano G, Romay-Penabad Z, Papalardo DME, Meillon-Garcia LA, Garcia-Latorre E, Reyes-Maldonado E, et al. In vivo effects of an inhibitor of nuclear factor-kappa B on thrombogenic properties of antiphospholipid antibodies. Ann N Y Acad Sci 2007; 1108:540–553. doi: 10.1196/annals.1422.057. [DOI] [PubMed] [Google Scholar]
- 44.Keith JC, Jr, Albert LM, Leathurby Y, Follettie M, Wang L, Borges-Marcucci L, et al. The utility of pathway selective estrogen receptor ligands that inhibit nuclear factor-kappa B transcriptional activity in models of rheumatoid arthritis. Arthritis Res Ther 2005; 7:R427–R438.doi: 10.1186/ar1692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Gerke V, Moss SE. Annexins: from structure to function. Physiol Rev 2002; 82:331–371. doi: 10.1152/physrev.00030.2001. [DOI] [PubMed] [Google Scholar]
- 46.Dias IDSR, Mok SWF, Gordillo-Martinez F, Khan I, Hsiao WWL, Law BYK, et al. The calcium-induced regulation in the molecular and transcriptional circuitry of human inflammatory response and autoimmunity. Front Pharmacol 2017; 8:962.doi: 10.3389/fphar.2017.00962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wong VKW, Qiu C, Xu SW, Law BYK, Zeng W, Wang H, et al. Ca2+ signalling plays a role in celastrol-mediated suppression of synovial fibroblasts of rheumatoid arthritis patients and experimental arthritis in rats. Br J Pharmacol 2019; 176:2922–2944. doi: 10.1111/bph.14718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Cao L, Yang C, Zhu B, Zhang G, Zhao N, Liu X, et al. A novel protein CMTM1-v5 specifically induced human lymphoma cells apoptosis in vitro and in vivo. Exp Cell Res 2019; 385:111623.doi: 10.1016/j.yexcr.2019.111623. [DOI] [PubMed] [Google Scholar]
- 49.Espinola RG, Pierangeli SS, Gharavi AE, Harris EN. Hydroxychloroquine reverses platelet activation induced by human IgG antiphospholipid antibodies. Thromb Haemost 2002; 87:518–522. doi: 10.1055/s-0037-1613033. [PubMed] [Google Scholar]
- 50.Proulle V, Furie RA, Merrill-Skoloff G, Furie BC, Furie B. Platelets are required for enhanced activation of the endothelium and fibrinogen in a mouse thrombosis model of APS. Blood 2014; 124:611–622. doi: 10.1182/blood-2014-02-554980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Rowley JW, Oler AJ, Tolley ND, Hunter BN, Low EN, Nix DA, et al. Genome-wide RNA-seq analysis of human and mouse platelet transcriptomes. Blood 2011; 118:e101–e111. doi: 10.1182/blood-2011-03-339705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Sim DS, Dilks JR, Flaumenhaft R. Platelets possess and require an active protein palmitoylation pathway for agonist-mediated activation and in vivo thrombus formation. Arterioscler Thromb Vasc Biol 2007; 27:1478–1485. doi: 10.1161/ATVBAHA.106.139287. [DOI] [PubMed] [Google Scholar]
- 53.Dowal L, Yang W, Freeman MR, Steen H, Flaumenhaft R. Proteomic analysis of palmitoylated platelet proteins. Blood 2011; 118:e62–e73. doi: 10.1182/blood-2011-05-353078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Lood C, Amisten S, Gullstrand B, Jönsen A, Allhorn M, Truedsson L, et al. Platelet transcriptional profile and protein expression in patients with systemic lupus erythematosus: Up-regulation of the type I interferon system is strongly associated with vascular disease. Blood 2010; 116:1951–1957. doi: 10.1182/blood-2010-03-274605. [DOI] [PubMed] [Google Scholar]
- 55.Shao L, Li T, Mo X, Majdic O, Zhang Y, Seyerl M, et al. Expressional and functional studies of CKLF1 during dendritic cell maturation. Cell Immunol 2010; 263:188–195. doi: 10.1016/j.cellimm.2010.03.015. [DOI] [PubMed] [Google Scholar]
- 56.Imamura Y, Katahira T, Kitamura D. Identification and characterization of a novel BASH N terminus-associated protein, BNAS2. J Biol Chem 2004; 279:26425–26432. doi: 10.1074/jbc.M403685200. [DOI] [PubMed] [Google Scholar]
- 57.Miyazaki A, Yogosawa S, Murakami A, Kitamura D. Identification of CMTM7 as a transmembrane linker of BLNK and the B-cell receptor. PLoS One 2012; 7:e31829.doi: 10.1371/journal.pone.0031829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Meng H, Yalavarthi S, Kanthi Y, Mazza LF, Elfline MA, Luke CE, et al. In vivo role of neutrophil extracellular traps in antiphospholipid antibody-mediated venous thrombosis. Arthritis Rheumatol 2017; 69:655–667. doi: 10.1002/art.39938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Maddahi A, Ansar S, Chen Q, Edvinsson L. Blockade of the MEK/ERK pathway with a raf inhibitor prevents activation of pro-inflammatory mediators in cerebral arteries and reduction in cerebral blood flow after subarachnoid hemorrhage in a rat model. J Cereb Blood Flow Metab 2011; 31:144–154. doi: 10.1038/jcbfm.2010.62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Benakis C, Bonny C, Hirt L. JNK inhibition and inflammation after cerebral ischemia. Brain Behav Immun 2010; 24:800–811. doi: 10.1016/j.bbi.2009.11.001. [DOI] [PubMed] [Google Scholar]
- 61.Kaminska B. MAPK signalling pathways as molecular targets for anti-inflammatory therapy - from molecular mechanisms to therapeutic benefits. Biochim Biophys Acta 2005; 1754:253–262. doi: 10.1016/j.bbapap.2005.08.017. [DOI] [PubMed] [Google Scholar]
- 62.Knight JS, Meng H, Coit P, Yalavarthi S, Sule G, Gandhi AA, et al. Activated signature of antiphospholipid syndrome neutrophils reveals potential therapeutic target. JCI Insight 2017; 2:e93897.doi: 10.1172/jci.insight.93897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Clark-Lewis I, Kim KS, Rajarathnam K, Gong JH, Dewald B, Moser B, et al. Structure-activity relationships of chemokines. J Leukoc Biol 1995; 57:703–711. doi: 10.1002/jlb.57.5.703. [DOI] [PubMed] [Google Scholar]
- 64.Li T, Zhong J, Chen Y, Qiu X, Zhang T, Ma D, et al. Expression of chemokine-like factor 1 is upregulated during T lymphocyte activation. Life Sci 2006; 79:519–524. doi: 10.1016/j.lfs.2006.01.042. [DOI] [PubMed] [Google Scholar]
- 65.Kim CH, Lim HW, Kim JR, Rott L, Hillsamer P, Butcher EC. Unique gene expression program of human germinal center T helper cells. Blood 2004; 104:1952–1960. doi: 10.1182/blood-2004-03-1206. [DOI] [PubMed] [Google Scholar]
- 66.Carbone J, Gallego A, Lanio N, Navarro J, Orera M, Aguaron A, et al. Quantitative abnormalities of peripheral blood distinct T, B, and natural killer cell subsets and clinical findings in obstetric antiphospholipid syndrome. J Rheumatol 2009; 36:1217–1225. doi: 10.3899/jrheum.081079. [DOI] [PubMed] [Google Scholar]
- 67.Xu S, Tan JE, Wong EP, Manickam A, Ponniah S, Lam KP. B cell development and activation defects resulting in xid-like immunodeficiency in BLNK/SLP-65-deficient mice. Int Immunol 2000; 12:397–404. doi: 10.1093/intimm/12.3.397. [DOI] [PubMed] [Google Scholar]
- 68.Tan JE, Wong SC, Gan SK, Xu S, Lam KP. The adaptor protein BLNK is required for b cell antigen receptor-induced activation of nuclear factor-kappa B and cell cycle entry and survival of B lymphocytes. J Biol Chem 2001; 276:20055–20063. doi: 10.1074/jbc.M010800200. [DOI] [PubMed] [Google Scholar]
- 69.Hayashi K, Nittono R, Okamoto N, Tsuji S, Hara Y, Goitsuka R, et al. The B cell-restricted adaptor BASH is required for normal development and antigen receptor-mediated activation of B cells. Proc Natl Acad Sci U S A 2000; 97:2755–2760. doi: 10.1073/pnas.040575697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Haas KM, Poe JC, Steeber DA, Tedder TF. B-1a and B-1b cells exhibit distinct developmental requirements and have unique functional roles in innate and adaptive immunity to S. pneumoniae. Immunity 2005; 23:7–18. doi: 10.1016/j.immuni.2005.04.011. [DOI] [PubMed] [Google Scholar]
- 71.Martin F, Kearney JF. B-cell subsets and the mature preimmune repertoire. Marginal zone and B1 B cells as part of a “natural immune memory”. Immunol Rev 2000; 175:70–79. [PubMed] [Google Scholar]
- 72.Velasquillo MC, Alcocer-Varela J, Alarcon-Segovia D, Cabiedes J, Sanchez-Guerrero J. Some patients with primary antiphospholipid syndrome have increased circulating CD5+ B cells that correlate with levels of IgM antiphospholipid antibodies. Clin Exp Rheumatol 1991; 9:501–505. [PubMed] [Google Scholar]
- 73.Zhang Y, Wang J, Han W. A role for CMTM7 in BCR expression and survival in B-1a but not B-2 cells. Int Immunol 2014; 26:47–57. doi: 10.1093/intimm/dxt042. [DOI] [PubMed] [Google Scholar]
- 74.Liu Z, Liu Y, Li T, Wang P, Mo X, Lv P, et al. Cmtm7 knockout inhibits B-1a cell development at the transitional (TrB-1a) stage. Int Immunol 2019; 31:715–728. doi: 10.1093/intimm/dxz041. [DOI] [PubMed] [Google Scholar]