Abstract
Background & aims
Overweight and obesity have been consistently reported to carry an increased risk for poorer outcomes in coronavirus disease 2019 (COVID-19) in adults. Existing reports mainly focus on in-hospital and intensive care unit mortality in patient cohorts usually not representative of the population with the highest mortality, i.e. the very old and frail patients. Accordingly, little is known about the risk patterns related to body mass and nutrition in very old patients. Our aim was to assess the relationship between body mass index (BMI), nutritional status and in-geriatric hospital mortality among geriatric patients treated for COVID-19. As a reference, the analyses were performed also in patients treated for other diagnoses than COVID-19.
Methods
We analyzed up to 10,031 geriatric patients with a median age of 83 years of which 1409 (14%) were hospitalized for COVID-19 and 8622 (86%) for other diagnoses in seven geriatric hospitals in the Stockholm region, Sweden during March 2020–January 2021. Data were available in electronic hospital records. The associations between 1) BMI and 2) nutritional status, assessed using the Mini-Nutritional Assessment - Short Form (MNA-SF) scale, and short-term in-geriatric hospital mortality were analyzed using logistic regression.
Results
After adjusting for age, sex, comorbidity, polypharmacy, frailty and the wave of the pandemic (first vs. second), underweight defined as BMI<18.5 increased the risk of in-hospital mortality in COVID-19 patients (odds ratio [OR] = 2.30; confidence interval [CI] = 1.17–4.31). Overweight and obesity were not associated with in-hospital mortality. Malnutrition; i.e. MNA-SF 0–7 points, increased the risk of in-hospital mortality in patients treated for COVID-19 (OR = 2.03; CI = 1.16–3.68) and other causes (OR = 6.01; CI = 2.73–15.91).
Conclusions
Our results indicate that obesity is not a risk factor for very old patients with COVID-19, but emphasize the role of underweight and malnutrition for in-hospital mortality in geriatric patients with COVID-19.
Keywords: COVID-19, Mortality, BMI, MNA-SF, Obesity, Malnutrition
1. Introduction
Globally, in April 2021, 141 million individuals have been confirmed positive of Corona virus disease-2019 (COVID-19) [1], and of these, 3 million have died [2]. Age is the most significant risk factor for severe COVID-19 infection. For example, in Sweden, through March 2021, 91% of the ~13,000 COVID-19-related deaths have occurred in individuals aged >70 years [3]. Other risk factors for adverse COVID-19 outcomes include male sex, lower socioeconomic position, obesity, hypertension, chronic kidney disease, and diabetes [[4], [5], [6], [7], [8], [9], [10], [11], [12], [13], [14], [15]], In addition, a recent meta-analysis based on 76 studies suggests that in addition to age and sex, severe obesity and active cancer are the most decisive risk factors for severe outcomes of COVID-19 [6]. However, there is evidence to suggest that the risk factors for COVID-19 outcomes are not uniform with age [9,13,14], although information on age-group specific risk patterns is currently lacking. A patient group underrepresented in the literature is especially the very old i.e. aged>80 years hospitalized adults as it is likely that the care home residents have been treated at their residence rather than at hospital. Statistics from 21 countries show that 41% of all deaths have been reported in care home residents (in February 2021) [16].
During the first COVID-19 wave in the spring 2020 in Sweden, we reported that in-hospital mortality was 24% among older hospitalized geriatric patients [7]. The risk for death was almost double for patients classified as frail according to the Clinical Frailty Scale (CFS) as compared to robust older patients. In these patients, acute kidney injury was also a strong risk factor for in-hospital mortality [17]. In addition, we [7,10] and others [11] have reported that multi-morbidity is associated with COVID-19 mortality in older adults.
While obesity is considered as risk factor for worse COVID-19 outcomes in community samples, the role of body composition in COVID-19 pathology has not been well characterized in hospitalized older adults (>65 years). Current knowledge indicates that overweight/obesity is a risk factor for adverse outcomes in midlife [18], but often related to reduced mortality in advanced age [19]. Due to this obesity or body mass index (BMI) paradox, it is conceivable that risk factors other than obesity may play a pivotal role for serious outcomes among older hospitalized adults. For example, underweight and malnutrition are related to a decline of immune functions [[20], [21], [22]]. Reduction in the capacity to mount leukocyte responses, leukocyte chemotaxis, B-lymphocyte antibody production, cytokine production is only the few of the immune aberrations taking place with aging and malnutrition [23]. Aging per se is also associated with compromised immune functions and increased susceptibility to various infections, aggravating the infection trajectories in older adults [24]. Furthermore, malnutrition is linked to muscle wasting, including respiratory and cardiac muscles, further jeopardizing crucial organ functions [[25], [26], [27], [28], [29]]. Thus, underweight and malnutrition are potential mortality risk factors in older patients with COVID-19, but this hypothesis is lacking empirical evidence.
The objective of this study is to assess whether 1) deviations from normal BMI and 2) nutritional status characterized by Mini-Nutritional Assessment-Short Form (MNA-SF) associate with the risk of COVID-19 mortality in hospitalized geriatric patients. For a reference, we also perform an analogous analysis in geriatric patients hospitalized for other causes than COVID-19 at the same geriatric hospitals during the same time period.
2. Material & methods
2.1. Study samples
The data were sourced from electronic medical records for 14,014 patients older than 65 years who were admitted to one of the seven geriatric hospitals in the Stockholm region, Sweden between March 2, 2020 and January 5, 2021. Patients without date of death or discharge were excluded from the analysis. In patients hospitalized for COVID-19, 2741 (20%) were diagnosed for COVID-19 based on ICD-10-codes U07.1 or U07.2, and 11,273 patients were hospitalized for other causes. 94% of the COVID-19 patients were admitted to hospital once, 5.8% twice and 0.2% three times. Three out of four of the non-COVID patients were admitted to hospital once, 16% two times and 8% from three to 13 times. Study samples in the main analysis comprised electronic records only from the first hospital admission. A sensitivity analysis was performed using data only from the last admission to a hospital (see Statistical analysis). The median (interquartile range) duration of a hospital visit was 9 (8) days in COVID-19 patients, and 7 (5) days in non-COVID patients.
Of the 14,014 patients, BMI was available for 1409 COVID-19 patients and 8622 non-COVID patients. MNA-SF score was available for 1297 COVID-19 patients and 6942 non-COVID patients (Supporting information Table S1). A flowchart showing the selection of the samples for the analysis is shown in Supporting information Figure S1. Proportion of patients with both BMI and MNA-SF information available was 34% (n = 934) for the 2741 COVID-19, and 52% (n = 5823) for the 11,273 non-COVID-19 patients (Supporting information, Figure S2). Because the number of patients with both BMI and MNA-SF information available was limited, the analyses were performed in four separate samples: COVID-19 patients and non-COVID-19 patients with BMI available and COVID-19 patients and non-COVID-19 patients with MNA-SF available.
2.2. Assessment of COVID-19 diagnosis
Clinical COVID-19 diagnosis was determined as described previously [7]. Briefly, diagnosis was determined in extracts from nasopharyngeal swabs using reverse transcriptase polymerase chain reaction (RT-PCR). If the patient had a negative RT-PCR but typical symptoms and findings on a computed tomography scan with no other explanation for the symptoms, a clinical COVID-19 diagnosis was made in collaboration with infection disease specialists.
2.3. Body mass index and Mini Nutritional Assessment-Short Form
BMI was calculated as weight (kg)/height (m)2. Weight and height and the MNA-SF were assessed by nurses and assistant nurses after admission to the hospital. Outlier values of BMI (<10, n = 22 and > 70, n = 154) were excluded from the analysis. The MNA-SF score was the sum of the scores across six items in the MNA-SF [30,31]. These items indicate decrease in the food intake, weight loss, psychological stress or acute disease during the past three months, and current mobility, and current BMI.
2.4. Other study variables
All information on the study variables was retrieved from the medical records, including age, sex and date of death or discharge. Number of diseases was defined as the sum of individual ICD-codes. Charlson comorbidity index (CCI) [32] was calculated using ICD-codes with ICD-9 scoring system. Hospital frailty risk score (HFRS) [33] was used as an indicator of frailty and it was calculated using ICD-codes that are over-represented in the more frail individuals. Polypharmacy was defined as the sum of individual ATC-codes after one day of admission. The pandemic waves (1st vs. 2nd) were considered in the analysis so that the 1st wave was defined as the period from March 2, 2020 until August 31, 2020, and the second wave starting from September 1 2020.
2.5. Statistical analysis
The associations between BMI, MNA-SF and the outcome, in-hospital-mortality were assessed using logistic regressions, fitting separate models for BMI and MNA-SF. The multivariate models adjusting for age, sex, number of diseases, CCI, HFRS, number of drugs, and the pandemic wave in a multivariate model were termed as the fully adjusted models. Age, number of diseases, CCI, HFRS, and number of drugs were used as continuous variables. For sex, women was used as the reference, and for the pandemic wave, the 1st wave was set as the reference category.
In the main analysis (Analysis strategy in Supporting information, Figure S1), BMI and the MNA-SF score were analyzed as categorized variables. BMI was categorized as follows according to World Health Organization standards: ‘Underweight’ = BMI < 18.5, ‘Normal weight’ = 18.5 ≤ BMI<25, ‘Overweight’ 25 ≤BMI<30 and ‘Obesity’ = BMI ≥ 30. MNA-SF was categorized according the guidelines of the assessment as follows: ‘Malnutrition’ = MNA-SF score 0–7 points, ‘At risk of malnutrition’ = MNA-SF score 8–11 points, ‘Normal nutrition’ = 12–14 points. For both variables, the category ‘Normal’ was set as the reference category.
We performed several sensitivity analyses. The first sensitivity analysis was performed on the COVID-19 patients stratifying the fully adjusted models by the pandemic wave. The second sensitivity analysis was performed using the last hospital admission for all patients. MNA-SF and BMI score were additionally analyzed as continuous variables in the fully adjusted models. To assess how the risk of death varies at different levels of nutritional status, MNA-SF was analyzed as a continuous variable in the model and the relationship was visualized as a scatterplot and loess-fitted splines. BMI was also analyzed using age-specific BMI categories [34]: ‘Underweight’ = BMI < 22 if age≥70 years and BMI<20 if age <70 years, ‘Normal weight’ = 22≤BMI<30 if age≥70 years and 20≤BMI < 25 if age<70 years, ‘Overweight’ = 30≤BMI<35 if age≥70 years, and 25≤BMI<30 if age<70 years, ‘Obese’ = BMI≥35 if age≥70 years, BMI≥30 if age<70 years.
Analyses and visualizations were performed using R software version 4.0.3. Results from the logistic models were visualized using R-package forestmodel and loess-fitted splines were produced using DescTools.
3. Results
3.1. BMI and mortality in geriatric hospitals
Sample characteristics of the patients with BMI available (1409 COVID-19 and 8622 non-COVID-19 patients) are shown in Table 1 . Mortality rates in these samples were 7.9% and 0.8%, and the age range was 65–104 and 65–105 years, respectively. Eighty percent of patients in the samples were at least 75 years old. Median length of hospital stay was 9 and 7 days in COVID-19 and non-COVID-19 patients, respectively.
Table 1.
Sample characteristics of geriatric patients hospitalized for COVID-19 (A) and other causes (B) in the BMI analysis.
| Sample in BMI analysis |
||||||
|---|---|---|---|---|---|---|
| A. COVID-19 |
B. Non-COVID-19 |
|||||
| All | Died | Survived | All | Died | Survived | |
| n (%) | 1409 (100) | 112 (8) | 1297 (92) | 8622 (100) | 67 (0.8) | 8555 (99.2) |
| Age, median (IQR) | 83 (12) | 88 (10.3) | 83 (12) | 84 (12) | 91 (9) | 84 (12) |
| Men, n (%) | 644 (45.7) | 64 (57.1) | 580 (44.7) | 3375 (39.1) | 34 (50.8) | 3341 (39.1) |
| BMI, median (IQR) | 24.2 (6.2) | 23.3 (6.0) | 24.2 (6.3) | 23.7 (6.2) | 22.7 (4.9) | 23.7 (6.24) |
| BMI, categorised | ||||||
| <18.5, n (%) | 112 (8.0) | 16 (14.3) | 96 (7.4) | 847 (9.8) | 6 (9.0) | 841 (9.8) |
| 18.5–25, n (%) | 709 (50.3) | 56 (50) | 653 (50.4) | 4408 (51.1) | 43 (64.2) | 4365 (51.0) |
| 25–30, n (%) | 394 (28.0) | 29 (25.9) | 365 (28.1) | 2228 (25.8) | 12 (17.9) | 2216 (25.9) |
| >30, n (%) | 194 (13.8) | 11 (9.8) | 183 (14.1) | 1139 (13.2) | 6 (9.0) | 1133 (13.2) |
| Number of diseases, median (IQR, Min, Max) | 6 (2,1,24) | 6.5 (3,2,17) | 6 (2,1,24) | 5 (2,1,17) | 7 (2.5,1,12) | 5 (2,1,17) |
| CCI, median (IQR, Min, Max) | 1 (2,0,9) | 2 (2,0,7) | 1 (2,0,9) | 1 (2,0,9) | 2 (2,0,8) | 1 (2,0,9) |
| HFRS, median (IQR, Min, Max) | 1 (1,0,8) | 2 (2,0,8) | 1 (1,0,8) | 2 (2,0,12) | 2 (2,0,7) | 2 (2,0,12) |
| Number of drugs, median (IQR, Min, Max) | 10 (7,1,29) | 11 (7,2,29) | 10 (6,1,29) | 9 (6,1,40) | 12 (7.5,1,27) | 9 (6,1,40) |
| 1st wave: prior to September 1st 2020, n (% within the 1st wave) | 846 (100) | 69 (8.2) | 777 (91.8) | 5484 (100) | 50 (0.9) | 5434 (99.1) |
| 2nd wave: after September 1st 2020, n (% within the 2nd wave) | 563 (100) | 43 (7.6) | 520 (92.4) | 3138 (100) | 17 (0.5) | 3121 (99.5) |
Abbreviations: BMI = body mass index (m/kg2), CCI = Charlson comorbidity index, HFRS = Hospital Frailty Risk Score, IQR = interquartile range.
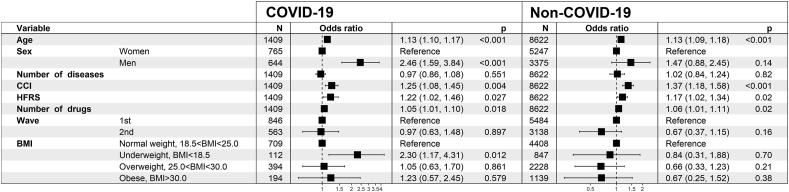
Results from the fully adjusted model for the BMI categories are shown in Fig. 1 . Underweight (BMI<18.5) was associated with a higher mortality in COVID-19 patients (OR = 2.30; CI = 1.17–4.31). The cut-offs for underweight at BMI <20 or <22 for patients <70 and ≥70 years of age, respectively, did not discriminate the mortality risk (results from the logistic regression shown in Supporting information, Figure S3) as the associations were non-significant. BMI as a continuous (linear) variable was likewise not associated with mortality in patients hospitalized for COVID-19 (OR = 0.97; CI = 0.93–1.02) or other causes (OR = 0.95; CI = 0.89–1.00) in the fully adjusted models.
Fig. 1.
The fully adjusted mortality risk model for the BMI analysis in geriatric patients hospitalized for COVID-19 (n = 1409, 112 died) and other causes (n = 8622, 67 died). Abbreviations: BMI = body mass index, CCI = Charlson comorbidity index, HFRS = Hospital Frailty Risk Score.
In the sensitivity analysis stratified by the pandemic wave (Supporting information, Figure S4), in the fully adjusted model, in patients hospitalized for COVID-19, underweight was associated with higher mortality in the 2nd pandemic wave data (OR = 3.19; CI = 1.13–8.42), but not in the 1st wave (OR = 1.83; CI = 0.72–4.27). In the sensitivity analysis using the records from the last hospital admission (in Supporting information; sample characteristics presented in Table S2 and results from the logistic regression in Figure S5), the association of underweight with mortality was statistically significant (OR = 2.26; CI = 1.15–4.26) in COVID-19 patients, but not in the non-COVID-19 patients.
3.2. MNA-SF and and mortality in geriatric hospitals
Sample characteristics of the patients in the MNA-SF analysis (1297 COVID-19 and 6942 non-COVID-19 patients) are shown in Table 2 . Mortality rates were in these samples, 11.2% and 1.2%, respectively, and the age range was 65–104 and 65–105 years, respectively. Eighty percent of patients in the samples were at least 75 years old. Median length of hospital stay was 9 and 7 days in COVID-19 and non-COVID-19 patients, respectively.
Table 2.
Descriptive statistics of geriatric patients hospitalized for COVID-19 (A) and for other causes (B) in the MNA-SF analysis.
| Sample in MNA-SF analysis |
||||||
|---|---|---|---|---|---|---|
| A. COVID-19 |
B. Non-COVID-19 |
|||||
| All | Died | Survived | All | Died | Survived | |
| n (%) | 1297 (100) | 145 (11.2) | 1152 (88.8) | 6942 (100) | 85 (1.2) | 6857 (98.8) |
| Age, median (IQR) | 83 (12) | 88 (11) | 83 (12) | 84 (12) | 90 (10) | 84 (11) |
| Men, n (%) | 592 (45.6) | 79 (54.5) | 513 (44.5) | 2746 (39.6) | 41 (48.2) | 2705 (39.5) |
| MNA-SF score, median (IQR) | 9 (4) | 8 (4) | 9 (4) | 9 (3) | 7 (4) | 9 (3) |
| MNA-SF score, categorised | ||||||
| Normal: 12–14, n (%) | 227 (17.5) | 19 (13.1) | 208 (18.1) | 1612 (23.2) | 6 (7.06) | 1606 (23.4) |
| At risk: 8–11, n (%) | 690 (53.2) | 59 (40.7) | 631 (54.8) | 3634 (52.4) | 29 (34.1) | 3605 (52.6) |
| Malnourished: 0–7, n (%) | 380 (29.3) | 67 (46.2) | 313 (27.2) | 1696 (24.4) | 50 (58.8) | 1646 (24.0) |
| Number of diseases, median (IQR, Min, Max) | 6 (3,1,24) | 7 (2,3,14) | 6 (2,1,24) | 5 (2,1,17) | 6 (2,1,14) | 5 (2,1,17) |
| CCI, median (IQR, Min, Max) | 1 (2,0,9) | 2 (2,0,7) | 1 (2,0,9) | 1 (2,0,9) | 2 (2,0,8) | 1 (2,0,9) |
| HFRS, median (IQR, Min, Max) | 1 (1,0,8) | 2 (1,0,8) | 1 (1,0,8) | 2 (1,0,12) | 2 (2,0,6) | 2 (1,0,12) |
| Number of drugs, median (IQR, Min, Max) | 10 (7,1,33) | 11 (7,1,33) | 10 (7,1,29) | 9 (6,1,40) | 11 (7,1,24) | 9 (6,1,40) |
| 1st wave: prior to September 1st 2020, n (% within the 1st wave) | 818 (100) | 104 (12.7) | 714 (87.3) | 4501 (100) | 61 (1.4) | 4440 (98.6) |
| 2nd wave: after September 1st 2020, n (% within the 2nd wave) | 479 (100) | 41 (8.6) | 438 (91.4) | 2441 (100) | 24 (1.0) | 2417 (99.0) |
Abbreviations: CCI = Charlson comorbidity index, HFRS = Hospital Frailty Risk Score, IQR = interquartile range, MNA-SF = Mini Nutritional Assessment Short Form.
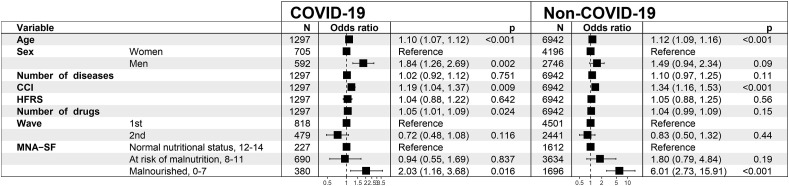
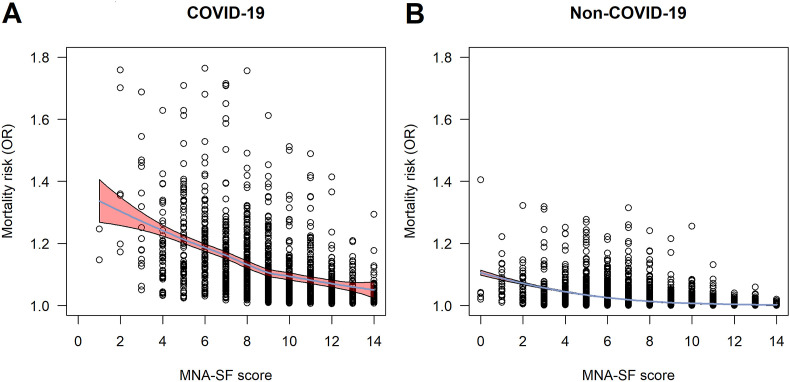
Results from the fully adjusted mortality risk model in the MNA-SF analysis are shown in Fig. 2 . Malnutrition, i.e. MNA-SF 0–7 points, was associated with higher mortality in COVID-19 patients (OR = 2.03; CI = 1.16–3.68), and in patients hospitalized for other causes (OR = 6.01; CI = 2.73–15.91). MNA-SF score as a continuous variable was significantly associated with mortality in COVID-19 (OR = 0.85; CI = 0.79–0.92), and non-COVID-19 patients (OR = 0.76; CI = 0.70–0.82) in the fully adjusted models. The relationship between continuous MNA-SF score and mortality risk is presented in Fig. 3 .
Fig. 2.
The fully adjusted mortality risk model in the MNA-SF analysis in geriatric patients hospitalized for COVID-19 (n = 1297, 145 died) and patients hospitalized for other causes (n = 6942, 85 died). Abbreviations: CCI = Charlson comorbidity index, HFRS = Hospital Frailty Risk Score, MNA-SF = Mini Nutritional Assessment Short Form.
Fig. 3.
The risk of all-cause in-hospital mortality and the MNA-SF scores. The odds ratios (ORs) for the risk of death in the fully adjusted mortality model are shown in the y-axis and MNA-SF score in x-axis in patients hospitalized for COVID-19 (A) and for other diagnoses (B). The blue line represents a loess fitted and the red-shaded area represents 95% confidence intervals. Abbreviations: MNA-SF = Mini Nutritional Assessment Short Form, OR = odds ratio. (For interpretation of the references to colour in this figure legend, the reader is referred to the Web version of this article.)
When compared to normal nutritional status, the OR of malnutrition with the risk of mortality in the 1st pandemic wave 1 was 1.93 (CI = 0.97–4.04) and in the 2nd wave it was 2.43 (CI = 0.94–7.19) (Supporting information, Figure S6). In the sensitivity analysis where the hospital records from the last admission were used instead of the 1st admission (Supporting information; sample characteristics in Table S3 and results from the logistic regression in Figure S7), the OR of malnutrition in patients hospitalized for COVID-19 was 1.88 (CI = 1.08–3.37) and in patients hospitalized for other causes the OR was 5.34 (CI = 2.64–12.33).
4. Discussion
In this study, we assessed the associations of 1) BMI and 2) nutritional status according to the MNA-SF with in-hospital mortality in geriatric patients with a median age of 83 years in seven geriatric hospitals in the region of Stockholm, Sweden. The electronic hospital records were obtained between March 2020 and January 2021. In COVID-19 patients, very low BMI, but not obesity or overweight, was associated with an increased risk of in-geriatric hospital mortality after adjusting for other risk factors; i.e. age, sex, multimorbidity, frailty, and polypharmacy. The associations were similar when using either the 1st or last hospital admission data. In contrast, in patients hospitalized for other causes than COVID-19, BMI was not associated with in-hospital mortality in any of the analyses. MNA-SF scores indicating malnutrition were strongly associated with a higher risk of in-hospital mortality in both COVID-19 patients and patients hospitalized for other causes after adjusting for age, sex, multimorbidity, frailty, polypharmacy, and when using either the 1st or last hospital admission data.
4.1. Body mass index and mortality
Considerable overweight is a risk factor for poor health outcomes [18] including COVID-19 disease [6]. In older adults, the association between body composition and adverse health events is complex [35]. After the age 65, obesity is less common than in midlife [36], possibly due to high mortality rates in late midlife (i.e., selection) because of cardio-metabolic risk and subsequent risk of death among obese and overweight individuals. Furthermore, obese and overweight older survivors may display metabolically healthier fat tissue [37], and obesity or overweight in older people may rather be related to a reduced risk than being a risk factor in itself. Accordingly, low body weight becomes a risk factor for mortality at older age [38]. We have also reported that underweight is a significant risk factor for frailty, whereas overweight might confer some protection against the progression of frailty in very old ages [39]. Our current findings and previous findings by others [40] on the association between very low body weight and in-hospital mortality in COVID-19 patients is thus expected. Nevertheless, these findings are important as reports on body weight and the risk for serious outcomes in very old patients with COVID-19 are scarce. The results indicate that, perhaps contrary to a general perception, obesity is not a risk factor for serious outcomes in COVID-19 across the whole life span. Noticeably, we found no association between underweight and in-hospital mortality in patients hospitalized for other causes, or association between age group specific BMI categories in either of the patient groups. Potential reasons for the inconsistency with the findings by others [41] are that we lacked statistical power due to low number of deaths across the short observation period in our data. Moreover, a potential selection bias cannot be overruled due to missing BMI data.
4.2. Mini Nutritional Assessment Short Form and mortality
We show that malnutrition is strongly associated with an increased risk of in-hospital mortality in COVID-19 patients, independent of other risk factors. To the best of our knowledge, only few previous studies have analyzed malnutrition in relation to COVID-19 disease pathology and outcomes using smaller groups of older individuals (n < 300) in the first wave of the pandemic. In addition to MNA-SF, also other assessments, including Nutrition Risk Screening 2002 and Nutritional Risk Index (NRI) are used as parallel tools to identify COVID-19 patients at nutritional risk [42]. In studies performed in Spain and China, malnutrition indicated by the MNA [43] and by Nutritional risk in critically ill score [40], was associated with higher in-hospital-mortality. In studies performed in China, more than 50% of the patients were categorized as malnourished by MNA [44], and by other measures [40], and in France, assessment by the NRI indicated that 40% of the patients with COVID-19 were malnourished [45]. In addition, the hospitalization period appears to be longer in malnourished COVID-19 patients [42,46]. The observations by us and others thus underline that malnutrition, a common problem in very old age, is a risk factor for severe outcomes in old COVID-19 patients in different societies.
4.3. Potential mechanisms for the excess COVID-19 mortality related to undernutrition
The major finding of this study is that indicators of undernutrition; i.e., underweight and low MNA-SF scores, are strongly related to short-term mortality. The observation periods were on average less than 10 days. It could be speculated that in-hospital mortality would be more strongly related to the severity of the infection, rather than to other mechanisms. However, as already indicated, undernutrition is linked to impairments of the immune system [23]. In addition, muscle wasting deranges respiratory muscle function [[26], [27], [28], [29]]. As the MNA-SF partially indexes frailty through weight loss and physical functioning (mobility), it is possible that a certain degree of the risk conferred by worse MNA-SF is explained by frailty. This hypothesis is supported by the finding that the effect of the HFRS attenuated in the MNA-SF model, whereas in the BMI model the HFRS was significant. Thus, the combined effect of old age and undernutrition likely undermine the immune system capacity to protect from infections in general and specifically in COVID-19.
In our analysis, MNA-SF predicted mortality also in patients hospitalized for other causes. This is in line with previous studies [47], as for example MNA is associated with in-hospital mortality in geriatric patients [48], and in patients aged 27–56 years [49], and also with 10-years-mortality in community-living older women [50]. Particularly notable in this study is that MNA-SF was able to predict mortality in a setting where the mortality rate was very low and the observation period very short.
4.4. Strengths and limitations
The strengths of the study were a large collection of data over a long period of time during the pandemic and inclusion of several geriatric hospitals and a study population of very old hospitalized individuals that are underpresented in the current literature. This study has also limitations that need to be acknowledged. One is that information on many mortality risk factors e.g. smoking, and socioeconomic position was missing. In the electronic medical records, a substantially high proportion of patients had missing information on BMI and MNA-SF. The overlap between those who had data on both BMI and MNA-SF was moderate. Thus, to increase the statistical power, the analyses were performed separately for BMI and MNA. The proportion of missing data on BMI and MNA-SF was higher in COVID-19 patients and patients who died. This is probably an effect of e.g. difficulties to examine and communicate with patients with a more severe COVID-19 condition. The implication of this is unclear, but our results may indicate that the relationship between underweight/malnutrition and mortality might have been even stronger if data would have been more complete. We also acknowledge that our follow-up time was relatively short and analyses on long-term mortality after hospitalization are thus needed.
4.5. Conclusion
In geriatric patients hospitalized for COVID-19, the MNA-SF appears as a powerful tool to assess short-term prognosis. In COVID-19 patients, very low BMI is associated with higher in-geriatric hospital mortality. No evidence was found that high BMI would confer an increased risk of death due to COVID-19 in very old patients admitted and treated in geriatric settings.
Ethical statement
The study was approved by the Swedish Ethical Review Authority in Stockholm Dnr 2020-02146, 2020-03345 and 2021-00595.
Sources of funding
This work was supported financially by the Swedish Research Council grants (2016-02317, 2018–02077, 2020-06101) and the regional agreement on medical training and clinical research between the Stockholm county council and the Karolinska Institutet (ALF), The Strategic Research Area in Epidemiology and Biostatistics grant, and the Academy of Finland through its funding to the Centre of Excellence in Research of Ageing and Care (CoEAgeCare, grant numbers 335870, 326567 and 336670).
The funders had no role in study design, data collection and interpretation, or the decision to submit the work for publication.
Author contribution
L.K. and J.J. and T.C. conceived and designed the analysis. L.K. processed and analysed the data. L.K. and J.J and T.C. wrote the manuscript. All authors gave analysis suggestions, participated in writing and reviewing of the manuscript.
Conflict of interest
The authors declare no conflicts of interest.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.clnu.2021.07.025.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.Shanmugam C., Mohammed A.R., Ravuri S., Luthra V., Rajagopal N., Karre S. COVID-2019 - a comprehensive pathology insight. Pathol Res Pract. 2020 Oct;216(10):153222. doi: 10.1016/j.prp.2020.153222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.World Health Organization . 2021. WHO coronavirus (COVID-19) dashboard.https://covid19.who.int/ Available at: [Google Scholar]
- 3.Stewart C., Statista Number of coronavirus (COVID-19) deaths in Sweden, by age groups. 2021. https://www.statista.com/statistics/1107913/number-of-coronavirus-deaths-in-sweden-by-age-groups/ Available at:
- 4.Strang P., Fürst P., Schultz T. Excess deaths from COVID-19 correlate with age and socio-economic status. A database study in the Stockholm region. ujms. 2020 10/14;125(4):297–304. doi: 10.1080/03009734.2020.1828513. 2021/03. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Figliozzi S., Masci P.G., Ahmadi N., Tondi L., Koutli E., Aimo A., et al. Predictors of adverse prognosis in COVID-19: a systematic review and meta-analysis. Eur J Clin Invest. 2020 10/01;50(10) doi: 10.1111/eci.13362. 2021/03. [DOI] [PubMed] [Google Scholar]
- 6.Booth A., Reed A.B., Ponzo S., Yassaee A., Aral M., Plans D., et al. Population risk factors for severe disease and mortality in COVID-19: a global systematic review and meta-analysis. PloS One. 2021 03/04;16(3) doi: 10.1371/journal.pone.0247461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hägg S., Jylhävä J., Wang Y., Xu H., Metzner C., Annetorp M., et al. Age, frailty, and comorbidity as prognostic factors for short-term outcomes in patients with coronavirus disease 2019 in geriatric care. J Am Med Dir Assoc. 2020 Nov;21(11):1555–1559. doi: 10.1016/j.jamda.2020.08.014. e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bergman J., Ballin M., Nordström A., Nordström P. Risk factors for COVID-19 diagnosis, hospitalization, and subsequent all-cause mortality in Sweden: a nationwide study. Eur J Epidemiol. 2021 Mar;36(3):287–298. doi: 10.1007/s10654-021-00732-w. Epub 2021 Mar 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Svensson P., Hofmann R., Häbel H., Jernberg T., Nordberg P. Association between cardiometabolic disease and severe COVID-19: a nationwide case–control study of patients requiring invasive mechanical ventilation. BMJ Open. 2021 02/01;11(2) doi: 10.1136/bmjopen-2020-044486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mak J.K.L., Kuja-Halkola R., Wang Y., Hägg S., Jylhävä J. Frailty and comorbidity in predicting community COVID-19 mortality in the U.K. Biobank: the effect of sampling. J Am Geriatr Soc. 2021 May;69(5):1128–1139. doi: 10.1111/jgs.17089. Epub 2021 Mar 5. PMID: 33619733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.D’ascanio M., Innammorato M., Pasquariello L., Pizzirusso D., Guerrieri G., Castelli S., et al. Age is not the only risk factor in COVID-19: the role of comorbidities and of long staying in residential care homes. BMC Geriatr. 2021 01/15;21(1):63. doi: 10.1186/s12877-021-02013-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Goyal P., Ringel J.B., Rajan M., Choi J.J., Pinheiro L.C., Li H.A., et al. Obesity and COVID-19 in New York City: a retrospective cohort study. Ann Intern Med. 2020 11/17;173(10):855–858. doi: 10.7326/M20-2730. 2021/03. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hendren Nicholas S., de Lemos James A., Colby A., Das Sandeep R., Anjali R., Spencer C., et al. Association of body mass index and age with morbidity and mortality in patients hospitalized with COVID-19. Circulation. 2021 01/12;143(2):135–144. doi: 10.1161/CIRCULATIONAHA.120.051936. 2021/03. [DOI] [PubMed] [Google Scholar]
- 14.Kompaniyets L., Goodman A.B., Belay B., Freedman D.S., Sucosky M.S., Lange S.J., et al. Body mass index and risk for COVID-19-related hospitalization, intensive care unit admission, invasive mechanical ventilation, and death - United States, March-December 2020. MMWR Morb Mortal Wkly Rep. 2021 Mar 12;70(10):355–361. doi: 10.15585/mmwr.mm7010e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Singh S., Bilal M., Pakhchanian H., Raiker R., Kochhar G.S., Thompson C.C. Impact of obesity on outcomes of patients with coronavirus disease 2019 in the United States: a multicenter electronic health records network study. Gastroenterology. 2020 Dec;159(6):2221–2225. doi: 10.1053/j.gastro.2020.08.028. e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Comas-Herrera A., Zalakaín J., Lemmon E., Henderson D., Litwin C., Hsu A., et al. Mortality associated with COVID-19 in care homes: international evidence. Article LTCcovid. org, Int Long-term Care Polic Network, CPEC-LSE. 2021 February 1st;2021 [Google Scholar]
- 17.Xu H., Garcia-Ptacek S., Annetorp M., Bruchfeld A., Cederholm T., Johnson P., et al. Acute kidney injury and mortality risk in older adults with COVID-19. J Nephrol. 2021 Mar 22:1–10. doi: 10.1007/s40620-021-01022-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Aune D., Sen A., Prasad M., Norat T., Janszky I., Tonstad S., et al. BMI and all cause mortality: systematic review and non-linear dose-response meta-analysis of 230 cohort studies with 3.74 million deaths among 30.3 million participants. BMJ. 2016 May 4;353:i2156. doi: 10.1136/bmj.i2156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Javed A.A., Aljied R., Allison D.J., Anderson L.N., Ma J., Raina P. Body mass index and all-cause mortality in older adults: a scoping review of observational studies. Obes Rev. 2020 08/01;21(8) doi: 10.1111/obr.13035. 2021/03. [DOI] [PubMed] [Google Scholar]
- 20.Alam I., Almajwal A.M., Alam W., Alam I., Ullah N., Abulmeaaty M., et al. The immune-nutrition interplay in aging – facts and controversies. Nutr Healthy Aging. 2019;5:73–95. [Google Scholar]
- 21.Cederholm T., Gyllenhammar H. Impaired granulocyte formylpeptide-induced superoxide generation in chronically ill, malnourished, elderly patients. J Intern Med. 1999 May;245(5):475–482. doi: 10.1046/j.1365-2796.1999.00481.x. [DOI] [PubMed] [Google Scholar]
- 22.Lesourd B. Nutritional factors and immunological ageing. Proc Nutr Soc. 2006 Aug;65(3):319–325. doi: 10.1079/pns2006507. [DOI] [PubMed] [Google Scholar]
- 23.Bourke C.D., Berkley J.A., Prendergast A.J. Immune dysfunction as a cause and consequence of malnutrition. Trends Immunol. 2016 Jun;37(6):386–398. doi: 10.1016/j.it.2016.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Franceschi C., Garagnani P., Parini P., Giuliani C., Santoro A. Inflammaging: a new immune–metabolic viewpoint for age-related diseases. Nat Rev Endocrinol. 2018 10/01;14(10):576–590. doi: 10.1038/s41574-018-0059-4. [DOI] [PubMed] [Google Scholar]
- 25.Arora N.S., Rochester D.F. Effect of body weight and muscularity on human diaphragm muscle mass, thickness, and area. J Appl Physiol Respir Environ Exerc Physiol. 1982 Jan;52(1):64–70. doi: 10.1152/jappl.1982.52.1.64. [DOI] [PubMed] [Google Scholar]
- 26.Arora N.S., Rochester D.F. Respiratory muscle strength and maximal voluntary ventilation in undernourished patients. Am Rev Respir Dis. 1982 Jul;126(1):5–8. doi: 10.1164/arrd.1982.126.1.5. [DOI] [PubMed] [Google Scholar]
- 27.Thurlbeck W.M. Diaphragm and body weight in emphysema. Thorax. 1978 Aug;33(4):483–487. doi: 10.1136/thx.33.4.483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Keys A., Henschel A., Taylor H.L. The size and function of the human heart at rest in semi-starvation and in subsequent rehabilitation. Am J Physiol. 1947 Jul 1;150(1):153–169. doi: 10.1152/ajplegacy.1947.150.1.153. [DOI] [PubMed] [Google Scholar]
- 29.Heymsfield S.B., Bethel R.A., Ansley J.D., Gibbs D.M., Felner J.M., Nutter D.O. Cardiac abnormalities in cachectic patients before and during nutritional repletion. Am Heart J. 1978 May;95(5):584–594. doi: 10.1016/0002-8703(78)90300-9. [DOI] [PubMed] [Google Scholar]
- 30.Rubenstein L.Z., Harker J.O., Salvà A., Guigoz Y., Vellas B. Screening for undernutrition in geriatric practice: developing the short-form mini-nutritional assessment (MNA-SF) J Gerontol A Biol Sci Med Sci. 2001 06/01;56(6):M366–M372. doi: 10.1093/gerona/56.6.m366. 3/17. [DOI] [PubMed] [Google Scholar]
- 31.Vellas B., Guigoz Y., Garry P.J., Nourhashemi F., Bennahum D., Lauque S., et al. The Mini Nutritional Assessment (MNA) and its use in grading the nutritional state of elderly patients. Nutrition. 1999 Feb;15(2):116–122. doi: 10.1016/s0899-9007(98)00171-3. [DOI] [PubMed] [Google Scholar]
- 32.Charlson M.E., Pompei P., Ales K.L., MacKenzie C.R. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chron Dis. 1987;40(5):373–383. doi: 10.1016/0021-9681(87)90171-8. [DOI] [PubMed] [Google Scholar]
- 33.Gilbert T., Neuburger J., Kraindler J., Keeble E., Smith P., Ariti C., et al. Development and validation of a Hospital Frailty Risk Score focusing on older people in acute care settings using electronic hospital records: an observational study. Lancet. 2018 May 5;391(10132):1775–1782. doi: 10.1016/S0140-6736(18)30668-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cederholm T., Bosaeus I., Barazzoni R., Bauer J., Van Gossum A., Klek S., et al. Diagnostic criteria for malnutrition - an ESPEN consensus statement. Clin Nutr. 2015 Jun;34(3):335–340. doi: 10.1016/j.clnu.2015.03.001. [DOI] [PubMed] [Google Scholar]
- 35.Bosello O., Vanzo A. Obesity paradox and aging. Eating and weight disorders - studies on anorexia. Bulim Obes. 2021 02/01;26(1):27–35. doi: 10.1007/s40519-019-00815-4. [DOI] [PubMed] [Google Scholar]
- 36.GBD 2015 Obesity Collaborators Health Effects of Overweight and obesity in 195 countries over 25 Years. N Engl J Med. 2017 07/06;377(1):13–27. doi: 10.1056/NEJMoa1614362. 2021/03. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Blüher M. Metabolically healthy obesity. Endocr Rev. 2020 May 1;41(3):405–420. doi: 10.1210/endrev/bnaa004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Cederholm T., Jensen G.L., Correia M.I.T.D., Gonzalez M.C., Fukushima R., Higashiguchi T., et al. GLIM criteria for the diagnosis of malnutrition - a consensus report from the global clinical nutrition community. Clin Nutr. 2019 Feb;38(1):1–9. doi: 10.1016/j.clnu.2018.08.002. [DOI] [PubMed] [Google Scholar]
- 39.Raymond E., Reynolds C.A., Dahl Aslan A.K., Finkel D., Ericsson M., Hägg S., et al. Drivers of frailty from adulthood into old age: results from a 27-year longitudinal population-based study in Sweden. J Gerontol A Biol Sci Med Sci. 2020 09/25;75(10):1943–1950. doi: 10.1093/gerona/glaa106. 3/18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Li G., Zhou C.L., Ba Y.M., Wang Y.M., Song B., Cheng X.B., et al. Nutritional risk and therapy for severe and critical COVID-19 patients: a multicenter retrospective observational study. Clin Nutr. 2021 Apr;40(4):2154–2161. doi: 10.1016/j.clnu.2020.09.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Cereda E., Klersy C., Hiesmayr M., Schindler K., Singer P., Laviano A., et al. Body mass index, age and in-hospital mortality: the NutritionDay multinational survey. Clin Nutr. 2017 06/01;36(3):839–847. doi: 10.1016/j.clnu.2016.05.001. 2021/04. [DOI] [PubMed] [Google Scholar]
- 42.Liu G., Zhang S., Mao Z., Wang W., Hu H. Clinical significance of nutritional risk screening for older adult patients with COVID-19. Eur J Clin Nutr. 2020 Jun;74(6):876–883. doi: 10.1038/s41430-020-0659-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Abadía Otero J., Briongos Figuero L.S., Gabella Mattín M., Usategui Martín I., Cubero Morais P., Cuellar Olmedo L., et al. The nutritional status of the elderly patient infected with COVID-19: the forgotten risk factor? Curr Med Res Opin. 2021 01/29:1–9. doi: 10.1080/03007995.2021.1882414. [DOI] [PubMed] [Google Scholar]
- 44.Li T., Zhang Y., Gong C., Wang J., Liu B., Shi L., et al. Prevalence of malnutrition and analysis of related factors in elderly patients with COVID-19 in Wuhan, China. Eur J Clin Nutr. 2020 Jun;74(6):871–875. doi: 10.1038/s41430-020-0642-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Allard L., Ouedraogo E., Molleville J., Bihan H., Giroux-Leprieur B., Sutton A., et al. Malnutrition: percentage and association with prognosis in patients hospitalized for coronavirus disease 2019. Nutrients. 2020 Nov 28;12(12):3679. doi: 10.3390/nu12123679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Yu Y., Ye J., Chen M., Jiang C., Lin W., Lu Y., et al. Malnutrition prolongs the hospitalization of patients with COVID-19 infection: a clinical epidemiological analysis. J Nutr Health Aging. 2021 03/01;25(3):369–373. doi: 10.1007/s12603-020-1541-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Guigoz Y., Vellas B. Nutritional assessment in older adults: MNA® 25 Years of a screening tool & a reference standard for care and research; what next? J Nutr Health Aging. 2021;25:528–583. doi: 10.1007/s12603-021-1601-y. [DOI] [PubMed] [Google Scholar]
- 48.Asiimwe S.B., Muzoora C., Wilson L.A., Moore C.C. Bedside measures of malnutrition and association with mortality in hospitalized adults. Clin Nutr. 2015 Apr;34(2):252–256. doi: 10.1016/j.clnu.2014.03.013. [DOI] [PubMed] [Google Scholar]
- 49.Asiimwe S.B. Simplifications of the mini nutritional assessment short-form are predictive of mortality among hospitalized young and middle-aged adults. Nutrition. 2016 Jan;32(1):95–100. doi: 10.1016/j.nut.2015.07.017. [DOI] [PubMed] [Google Scholar]
- 50.Lundin H., Sääf M., Strender L., Mollasaraie H.A., Salminen H. Mini nutritional assessment and 10-year mortality in free-living elderly women: a prospective cohort study with 10-year follow-up. Eur J Clin Nutr. 2012 09/01;66(9):1050–1053. doi: 10.1038/ejcn.2012.100. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.