Abstract
Objectives
On 21 December 2020 the European Commission granted conditional marketing authorisation in the European Union for the anti-COVID-19 mRNA vaccine Bnt162b2 (Comirnaty, Pfizer/BioNTech). The main endpoint of this epidemiological, observational, prospective and monocentric study was to identify the number, types, and severity of adverse events following immunisation that occurred in subjects who had been previously infected with COVID-19, and in those who had not, after vaccination with Comirnaty, and to compare the two groups of subjects looking at events that occurred within a month after the first and the second dose.
Methods
Data were gathered by a questionnaire. The results included the responses of all healthcare workers (2030) of the IRCCS Sacro Cuore Don Calabria Hospital (Italy) vaccinated between 1st January and 28th February 2021. Adverse effects of the vaccine were reported after the first and the second doses.
Results
There was a statistically significant increase (p<0.001, χ2=35.60) in participants who experienced some side-effects after receiving the first dose of the vaccine and who had previously been infected with the coronavirus, compared with participants who had not previously been infected. 46.76% (136) of the participants who had previously been infected experienced some side-effects after the first dose of vaccine, and 63.23% (184) experienced some side-effects after the second dose, compared with 29.15% (507) after the first dose and 70.79% (1231) after the second dose in those who had not been previously infected. The number of participants who experienced side-effects after the second dose and had previously been infected was significantly lower compared with participants who had not previously been infected (p=0.0094, χ2=6.743).
Conclusions
Most of the side-effects identified in this trial were also reported by the manufacturer and the US Food and Drug Administration. Active surveillance should always continue to constantly check the vaccine’s risk/benefit ratio over time.
Keywords: COVID-19, drug monitoring, drug-related side effects and adverse reactions, evidence-based medicine, immunization, pharmacy service, hospital
Introduction
On 30th January 2020, WHO declared that the outbreak of a new beta-coronavirus, severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) was an international public health concern. By 12th March 2020, it was declared a pandemic and resulted in a substantial increase in hospitalisations for pneumonia with multiorgan disease called coronavirus disease 2019 (COVID-19). Up to 31th March 2021, there have been 128 540 982 confirmed cases of COVID-19, including 2 808 308 deaths, reported to WHO.1–4 SARS-CoV-2 infection may be asymptomatic or it can cause a wide spectrum of signs, also life-threatening sepsis. Approximately 17–35% of hospitalised patients are treated in intensive care units.4 Moreover, genetic variants of SARS-CoV-2 are emerging, such as VOC 202012/01 identified in the UK, 501Y.V2 identified in South Africa, and P.1 identified in Brazil and Japan.5
A key priority is to identify the combination of measures that minimise social and economic disruption while adequately controlling infection.4
Vaccination has become increasingly relevant as the SARS-CoV-2 pandemic continues to worsen around the world.6 On 21th December 2020, the European Commission granted the conditional marketing authorisation (CMA) to Pfizer-BioNTech COVID-19 mRNA vaccine (nucleoside-modified) BNT162b2 (Comirnaty), for active immunisation of individuals aged 16 years and older to prevent COVID-19.1 7 8 During pre-authorisation studies, BNT162b2 showed a vaccine efficacy of 95% (95% CI 90.3% to 97.6%) after a two-dose regimen (30 µg per dose administered 21 days apart).1
Given the importance of vaccines in fighting this public health crisis, it is crucial to understand their efficacy and also their safety profile.9
An adverse event following immunisation (AEFI) is any episode of a medical nature that occurs after the administration of a vaccine (temporal relationship) but not necessarily caused by vaccination (causal relationship). Each AEFI must be reported to the pharmacovigilance system to allow the assessment of any changes in the safety profile of the vaccine.10
The most frequent adverse reactions during the Comirnaty registration trial were injection site pain, fatigue, headache, myalgia and chills, arthralgia, fever and swelling at the injection site. They were usually mild or moderate in intensity and resolved within a few days after vaccination. Currently, the only major risk identified is anaphylaxis.1 7 8
The mRNA vaccines developed by Pfizer/BioNtech use a lipid-based nanoparticle carrier system that is further stabilised by a polyethylene glycol (PEG) 2000 lipid conjugate that provides a hydrophilic layer, prolonging half-life. It is possible that some populations are at higher risk for non-IgE-mediated mast cell activation or complement activation related to either the lipid or the PEG-lipid component of the vaccine.6 9 11
This study was undertaken to highlight the role of the hospital pharmacist in vaccine surveillance. Therefore, we considered it appropriate to describe accurately the detection of adverse events (from the least to the most serious) manifested by all employees of the hospital, vaccinated for COVID-19 during the period 1st January 2021 to 28th February 2021.
Methods
Study design
The main endpoint of this epidemiological, observational, prospective and monocentric study was to identify the number, types and severity of adverse events that occurred in two groups of subjects—those who had been previously infected with COVID-19, and those who had not been previously infected— and to compare these two groups by looking at events that occurred within a month after the first and second dose of the vaccine.
All hospital healthcare workers, male and female, over 18 years old, who received vaccinations in the period 1st January to 28th February 2021, at the hospital and agreed to participate in the trial, were included. All subjects were asked to complete independently a nine-item self-administered questionnaire, used in printed form (see online supplemental material 1) after both the first and second doses of the vaccine.
ejhpharm-2021-002933supp001.pdf (86.7KB, pdf)
Follow-up for each participant lasted 30 days from the second vaccination until any outcome occurred or the end of the study period (31st March 2021), whichever came first.
We assessed the percentage of adverse events which occurred in the whole observed population, their onset and duration, distinguishing between serious and non-serious adverse drug reactions (ADRs), as defined by the European Medicines Agency.12 Subjects were stratified by previous COVID-19 infection versus no previous COVID-19 infection. We compared the incidence of events following both the first and second doses of the vaccine, according to the subject stratification.
Subjects were asked to return all questionnaires to the pharmacy by 31st March 2021 if no events occurred after vaccine administration. Otherwise, if post-administration events occurred, they were asked to contact the hospital pharmacovigilance representatives immediately and return the completed questionnaire within 24 to 72 hours.
The hospital pharmacy was responsible for the design and management of the study (data collection and analysis).
Statistical analysis
All statistical tests were performed using Prism (version 8.4.0, GraphPad Software Inc, La Jolla, CA, USA). The level of significance was set at p<0.05.
Primarily, descriptive statistics were carried out for demographic variables (gender and age), and COVID-19-related anamnesis (previous infection, hospitalisation, duration of infection); vaccine side effects (local and general side effects, fatigue, headache, muscle and joint pain, chills, skin rashes, tachycardia and fever) were represented by frequencies, percentages, means and standard deviations. Inferential statistics were then performed to assess the association between side-effects and COVID-19-related anamnesis, and the association of various side-effects and each other using the χ2 test and Student’s t-test, with a confidence level of 95% and significance value of p<0.05.
Results
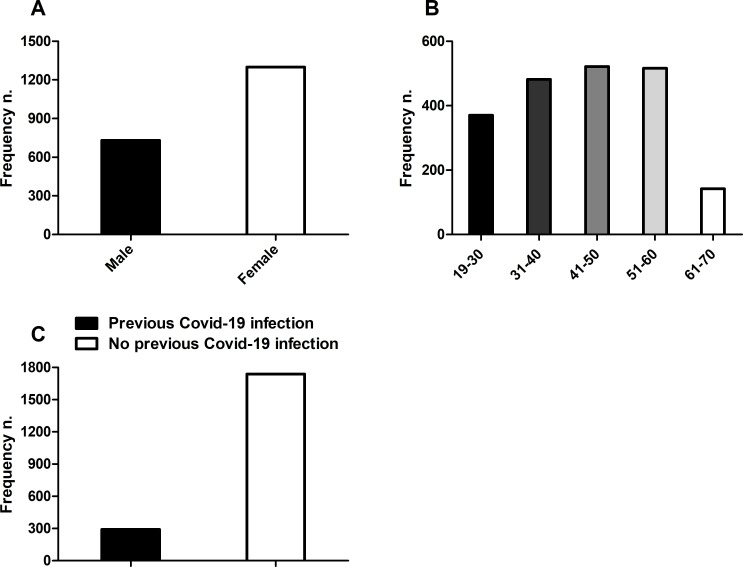
Between 1st January and 28th February 2021, a total of 2148 people were screened and 2030 people aged 19 years and over were included in the observational study. A total of 2030 participants received injections of BNT162b2. By 31st March, safety data from at least 1 month of follow-up were available for a total of 2030 participants after the second vaccine dose and were included in the final analyses. Of 2030 participants, 63.99% (1299) were female and 36.01% (731) were male. The median age was 44 years (33;53 years, first;third quartile) and 64.88% of participants were over 50 years old. Past medical history of the population revealed that 291 subjects (14.33%) had previously been infected with the SARS-CoV-2 virus and that most of the participants (1739, 85.67%) had never been infected. All subjects (2030, 100%) received both doses of the Pfizer/BioNTech COVID-19 vaccine. The characteristics of the subjects are shown in figure 1.
Figure 1.
Characteristics of study participants based on gender (male and female) (panel A), age (divided into five decades) (panel B), and previous COVID-19 infection (panel C). Data are expressed as frequency (number (n)).
In the population analysed, only one serious adverse event (0.05%) was reported in an approximately 30-year-old female subject, who was immediately hospitalised after the second dose of vaccine due to symptoms similar to anaphylaxis (skin rash and glottal oedema). This serious adverse reaction was completely resolved within 24 hours.
A total of 99.95% of reported reactions were related to non-serious events, such as injection site pain, fever, fatigue, headache, muscle and joint pain, chills and rash. Paresthesia, dizziness, nausea and abdominal pain were also observed, although in negligible percentages.
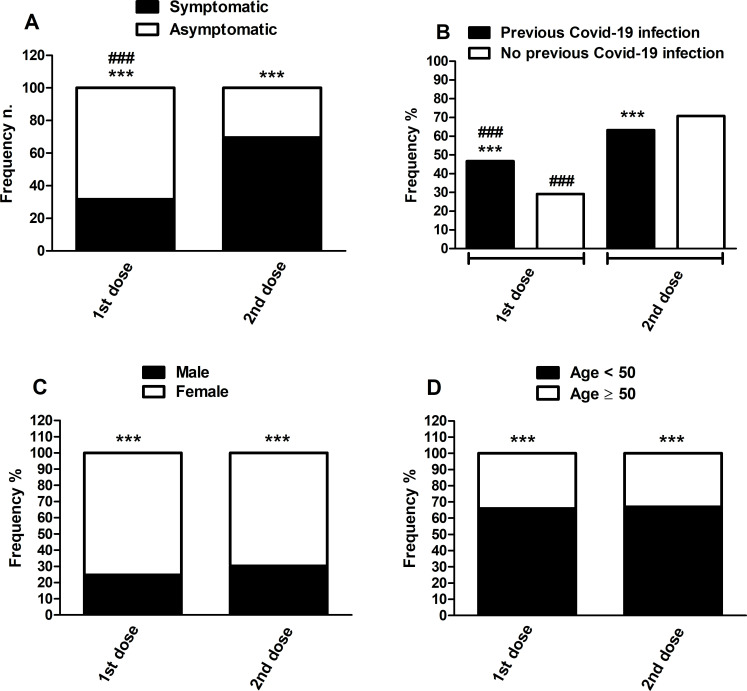
A χ2 test revealed a significant increase (p<0.001; χ2=587.3) in the distribution of subjects who experienced side effects after receiving the second dose of the vaccine (1415, 69.70%) compared with those who reported side effects after the first dose (643, 31.67%) (figure 2, panel A). On the other hand, there were more asymptomatic subjects after the first vaccine dose (1387, 68.33%) than after the second one (615, 33.30%) (figure 2, panel A).
Figure 2.
Pfizer/BioNTech COVID-19 vaccine side effects distribution following first and second doses (symptomatic and asymptomatic subjects) (panel A), stratified also for gender (panel B), age under and over 50 years (panel C), and previous and non-previous COVID-19 infection (panel D). Frequency data are expressed as a percentage of the total population analysed (2030 subjects; panel A), as a percentage of symptomatic subjects after the first and second doses (643 and 1415, respectively; panels B and C), and as a percentage of previous and non-previous COVID-19 infected subjects (291 and 1739, respectively; panel D). A statistical analysis of the comparison between the different frequencies was performed with a χ2 test. **p<0.01 versus non-previous COVID-19 infection after the second dose; ***p<0.001 versus asymptomatic, female and over 50 years in the same dose; ###p<0.001 versus second dose.
The number of female participants who reported different side effects after receiving both doses of the vaccine was significantly higher when compared with males (p<0.001, respectively, χ2=51.98 for the first dose and χ2=65.67 for the second dose). Among the population that suffered from some side-effects of the COVID-19 vaccine after the first dose, 24.73% (159) were male and 75.27% (484) were female; after the second dose, 30.32% (429) were male and 69.68% (986) were female (figure 2, panel C).
643 (31.67%) participants after the first dose and 1415 (69.70%) participants after the second dose reported at least one non-serious adverse reaction on completion of the questionnaire, with a statistically significant difference between the <50-year-old group and the ≥50-year-old group for both the injections: 424 (65.94%) vs 219 (34.06%) and 947 (66.93%) vs 468 (33.07%), respectively (p<0.001) (figure 2, panel D).
Results showed a statistically significant increase (p<0.001, χ2=35.60) in the number of participants who experienced some side-effects after receiving the first dose of the vaccine who had previously been infected with the coronavirus, compared with participants who had not previously been infected (figure 2, panel B). In particular, 46.76% (136) of the participants who had previously been infected with the coronavirus manifested some side-effects after the first dose of COVID-19 vaccine, and 63.23% (184) after the second dose, compared with those who had not previously had COVID-19 infection (29.15% (507) after the first dose and 70.79% (1231) after the second dose) (figure 2, panel B). On the other hand, the number of participants who experienced some side-effects after the second dose, and had previously been infected with the coronavirus, was significantly lower, compared with participants who had not previously been infected (p=0.0094, χ2=6.743) (figure 2, panel B).
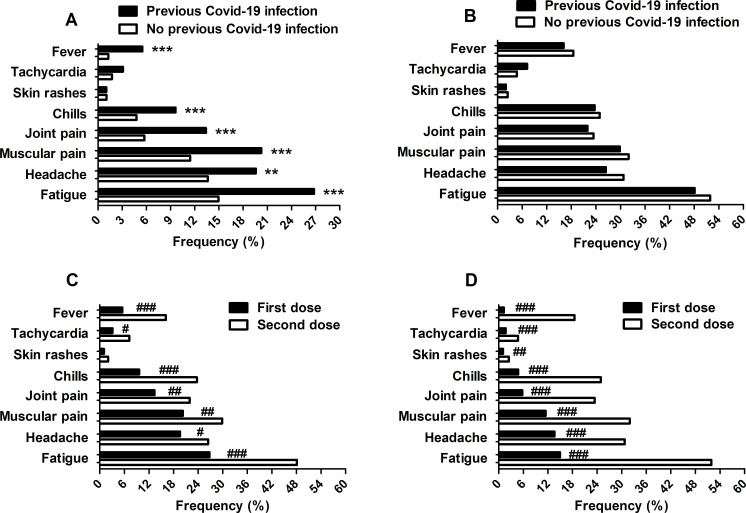
Injection site pain (even if mild) was the most common local reaction reported by all subjects (100%) analysed after both the first and second vaccine doses. Among systemic reactions, fatigue, headache, muscle pain, joint pain, chills, tachycardia and fever were the most common events, and the frequency was significantly higher after the second dose compared with the first in both the group of subjects previously infected with coronavirus and the group of subjects not previously infected (previous COVID-19 infection group (48.11% vs 26.80%; p<0.001), (26.56% vs 19.59%; p=0.0489), (29.90% vs 20.27%; p=0.0074), (21.99% vs 13.40%; p=0.0066), (23.71% vs 9.62%; p<0.001), (7.22% vs 3.09%; p=0.0245), (16.15% vs 5.50%; p<0.001); no previous COVID-19 infection group (51.93% vs 14.95%; p<0.001), (30.76% vs 13.63%; p<0.001), (31.97% vs 11.44%; p<0.001), (23.46% vs 5.75%; p<0.001), (24.96% vs 4.77%; p<0.001), (7.72% vs 1.73%; p<0.001), (18.52% vs 1.27%; p<0.001)) (figure 3, panel C, D). Moreover, the frequency of fatigue, headache, muscle pain, joint pain, chills and fever immediately after the first dose was significantly higher in the previously infected group compared with the not previously infected group ((26.80% vs 14.95%; p<0.001), (19.59% vs 13.63%; p=0.0075), (20.27% vs 11.44%; p<0.001), (13.40% vs 5.75%; p<0.001), (9.62% vs 4.77%; p=0.0008), (5.50% vs 1.27%; p<0.001), respectively) (figure 3, panel A, B).
Figure 3.
Adverse reactions reported after the first and second doses of vaccine (panels A and B, respectively) and in previously COVID-19 infected subjects and not previously infected subjects (panels C and D, respectively), stratified by previous and no previous COVID-19 infection, and for first and second dose. Frequency data are expressed as a percentage of previous and non-previous COVID-19 infected subjects (respectively 291 and 1739; panels A–D). A statistical analysis of the comparison between the different frequencies among variables was performed using the χ2 test. **p<0.01 and ***p<0.001 versus non-previous COVID-19 infection after the first dose. #p≤0.05, ##p<0.01 and ###p<0.001 versus the second dose.
Fever was higher after the second dose compared to the first one (median 38°C (37.5°C;38.5°C, first;third quartile) and median 37.6°C (37.4°C; 38°C, first;third quartile), respectively).
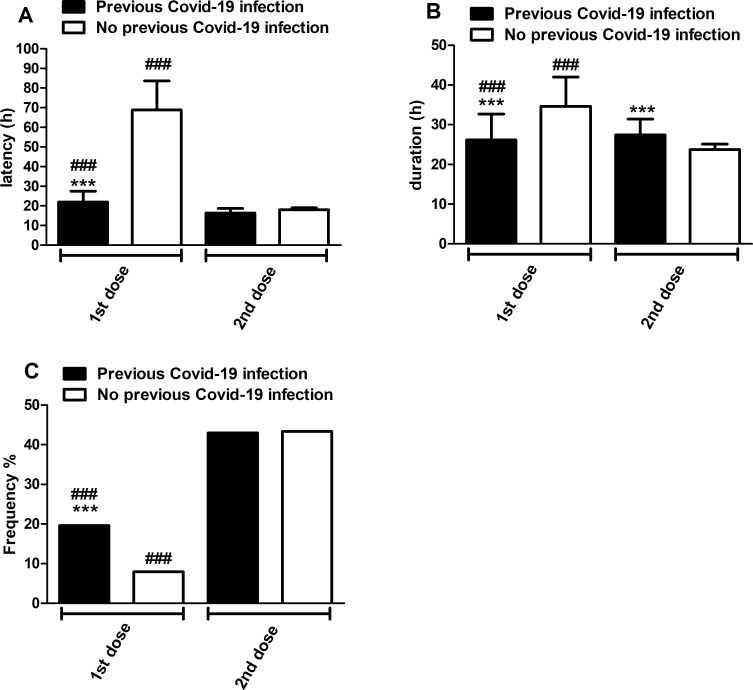
Overall, local and systemic reactions were transient, had a rapid onset (48.6±7.9 hours after the first dose and 17.8±0.9 hours after the second one), were of short duration (31±5 hours after the first dose and 24.2±1.3 hours after the second one), and resolved within a few days after vaccination; mostt of them had mild to moderate intensity. The results showed a significant reduction (p<0.001) in symptom latencies in the previous COVID-19 infection group compared with the non-previous COVID-19 infection group, both after the first and second vaccine doses (mainly after the first dose). There was also a significant reduction (p<0.001) in symptom latencies after the booster dose of the vaccine compared with the first dose, for both groups: previous COVID-19 infection and no previous COVID-19 infection, but mainly in the latter one (figure 4, panel A). On the other hand, the duration of symptoms after the first dose was significantly reduced (p<0.001) in the previous COVID-19 infection group compared with the no previous COVID-19 infection group, while the duration of symptoms for this latter group was significantly reduced (p<0.001) after the second dose compared with the first dose (figure 4, panel B).
Figure 4.
Onset (panel A) and duration (panel B) of adverse effects and frequency of symptomatic drug intake (panel C) after the first and second doses of Pfizer/BioNTech COVID-19 vaccine stratified by previous and non-previous COVID-19 infection. Latency and duration are expressed as mean±SD in hours, while the frequency of symptomatic drug intake after vaccination is expressed as a percentage of previous and non-previous COVID-19 infected subjects (respectively 291 and 1739; panel C). Statistical analyses of latency and duration were performed with the Student t-test, while a comparison of frequencies was performed with the χ2 test. ***p<0.001 versus non-previous COVID-19 infection; ###p<0.001 versus second dose.
Gastrointestinal discomfort (nausea, abdominal pain, diarrhoea) (40 (2%) after the first dose vs 136 (6.7%) after the second dose, respectively) and rarely syncope and dizziness (5 (0.25%) after first dose vs 23 (1.13%) after the second), paresthesia (4 (0.2%) after the first dose vs 15 (0.74%) after the second) and dyspnoea (5 cases (0.25%) only after the second dose) occurred.
Bizarre reactions such as herpes zoster reactivation and lymphadenopathy (both supraclavicular and axillary) were also observed in 5 (0.25%) and 19 (0.94%) subjects, respectively, after the first dose and in 10 (0.5%) and 72 (3.5%) subjects after the vaccine booster. Data showed a statistically significant increase only in the incidence of lymphadenopathy after the second dose of vaccine (p<0.001; χ2=31.52).
Use of antipyretic/pain medications (ie, acetaminophen, ibuprofen, ketoprofen, naproxen, acetylsalicylic acid, nimesulide and ketorolac) was more common and statistically significantly greater after the booster dose compared with the first dose in both previous and no previous COVID-19 infection groups (p<0.001; respectively χ2=36.97 and χ2=572.1). Moreover, after the first vaccine dose the use of antipyretic/pain medication drugs was statistically greater in the group of subjects with previous COVID-19 infection (p<0.001; χ2=38.98). In particular after the first dose, 19.59% (57 subjects) of the previous COVID-19 infection group and 7.94% (138 subjects) of the no previous COVID-19 infection group took at least one drug. After the vaccine booster, 42.96% (125 subjects) of the previous COVID-19 infection group and 43.36% (754 subjects) of the no previous COVID-19 infection group used drugs (figure 4, panel C).
Discussion
The efficacy and safety of COVID-19 vaccines are not easy to establish, given their rapid development, thus actions should be implemented to identify possible AEFIs. Constant monitoring and data collection should be implemented to improve the efficacy and safety profile of COVID-19 vaccines. To contribute to this, we carried out an epidemiological, observational, prospective and monocentric study, analysing short and medium term (immediately after and within 1 month later) adverse events following administration of the Pfizer/BioNTech COVID-19 vaccine, the first mRNA vaccine ever developed.13
Although the present study was conducted in a limited population (2030 subjects), it reported one severe AEFI with an incidence rate of 0.05%, potentially due to an anaphylactic syndrome. However, the real aetiology remains unknown.
Usually, for most vaccines, anaphylaxis is uncommon (occurring at a rate of less than one per million doses).14 15 On the other hand, anaphylaxis following Pfizer/BioNTech COVID-19 vaccine administration has been reported more frequently with a rate closer to 1:125 000 doses, probably due to the presence of pegylated nanoparticles (PEG2000) as excipients.9
A small number of people previously exposed to PEG may have high levels of antibodies against it, which puts them at risk of an anaphylactic reaction to the vaccine.16
Our results, according to the literature, showed that the main AEFIs reported are non-serious, described as local injection site or systemic reactions, which mainly lasted only a few days after vaccination.1 17–19
Systemic adverse reactions were generally reported more frequently after the second dose than after the first one. Furthermore, AEFIs were generally more frequent and severe in females compared with males, and in people under the age of 50 compared with those over 50.
These findings are in agreement with El-Shitany et al 17 and Polack et al 1, stating that the vaccine-associated systemic side effects are more common among younger people and after the second dose of the vaccine, but disagree with them in reporting that injection site pain was more frequent among people younger than 55 years compared with older people (in fact, all people vaccinated reported injection site pain).
This observation could be justified by the stimulation of the immune response induced by the vaccine, as well as by the stronger and more efficient immune systems of younger people compared with older ones.1 17 20 This could explain both the higher prevalence of side effects in people under 50 compared with older people, and the higher frequency of AEFIs in people previously infected with COVID-19 compared with people not previously infected.
These data are in agreement with emerging real-world evidence suggesting that the antibody responses to the first vaccine dose in individuals with prior SARS-CoV-2 infection is equal to or exceeds the antibody titres found in naïve individuals after the second dose. Indeed, recent findings report high antibody titres and neutralisation activity after the first dose of the Comirnaty vaccine in individuals who already had SARS-CoV-2 infection.21 Indeed, the immune system may produce cytokines that could have an inflammatory effect on blood vessels, muscles, and other tissues. It may also produce flu-like symptoms that last several days after vaccination.20
We assume that since side effects are greater after the second dose of the vaccine in patients who have not previously been infected, similarly the first dose of the vaccine may represent a booster dose in previous COVID-19 infected patients and therefore triggers an ADR more frequently (figure 2 panel B).
Our study confirms recently published evidences suggesting that antibody responses after a single dose of the vaccine were significantly higher in previously infected vaccinated people. Furthermore, it seems that vaccination of individuals with evidence of a previous infection leads to a booster response, reaching IgG titres of approximately one order of magnitude higher than naïve individuals.16–18 22 23
Furthermore, a recent study highlights that the simultaneous production of neutralising antibodies, activation of virus-specific CD4+ and CD8+ T cells, and robust release of immunomodulatory cytokines such as interferon γ (key cytokines along with type I interferons for several antiviral responses), represent a coordinated immune response to counter viral intrusion, supporting a favourable TH1 profile to the detriment of a potentially deleterious TH2 immune response.20
Finally, the most frequent symptoms, justifying the use of non-steroidal anti-inflammatory drugs, analgesic drugs and antipyretic drugs, were injection site pain, fatigue, headache and muscle and joint pain.
These findings are similar to those from clinical trials, in which injection site pain, fatigue, headache and myalgia were most frequently reported after the second dose.22
These results are in accordance with the previously reported side-effects of the vaccine and the information of the summary of the product characteristics of the Pfizer/BioNTech COVID-19 vaccine. Moreover, systemic adverse reactions had a median onset of 1–2 days after vaccine receipt and resolved completely within a few days.
Nevertheless, paresthesia, gastrointestinal discomfort (nausea, abdominal pain, diarrhoea), dizziness, dyspnoea, reactivation of herpes zoster and lymphadenopathy (both supraclavicular and axillary) were also reported.
Conclusion
The side-effects that have been identified after administration of the Pfizer/BioNTech COVID-19 vaccine are typical symptoms of most vaccines, and most of them are tolerated.12 24 Most of the symptoms have been reported by the manufacturer and FDA fact sheets.25 However, we must continue to monitor the Pfizer/BioNTech COVID-19 vaccine over a long period of time through active surveillance to confirm the efficacy of immunisation and also to detect novel and serious AEFIs.
Key messages.
What is already known on this subject
Current scientific knowledge on adverse reactions from the Comirnaty (Pfizer/BioNTech) vaccine confirms what is reported in the summary of product characteristics. However, there is no clear information on the different responses to the vaccine, especially on the number, types and severity of adverse events following immunisation (AEFIs) that occurred in subjects either previously infected with COVID-19 or not previously infected, both after the first and second dose.
Active surveillance is important to confirm the efficacy of immunisation and also to detect novel and serious AEFIs.
What this study adds
This study represents real-world evidence, involving a significant number of subjects monitored for 1 month after both doses and highlighting the safety of the Comirnaty vaccine.
This study, for the first time, clearly shows that the number and severity of AEFIs that occurred in subjects previously infected with COVID-19 after the first dose of Comirnaty are similar to AEFIs that occurred after the second dose in subjects not previously infected with COVID-19.
These data also support the emerging real-world evidence suggesting that the antibody response to the first vaccine dose in individuals with prior SARS-CoV-2 infection is equal to or exceeds the antibody titres found in naïve individuals after the second dose.
Footnotes
AO and RT contributed equally.
Contributors: Contributorship statement: AO: conceptualisation, writing - review and editing. RT: conceptualisation, writing and editing. CT: writing - review and editing. TZ: methodology, review and editing. NR: supervision, data curation, formal analysis. FM: conceptualisation, writing - review and editing, supervision.
Funding: The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests: None declared.
Provenance and peer review: Not commissioned; internally peer reviewed.
Supplemental material: This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.
Data availability statement
No data are available. Not applicable.
Ethics statements
Patient consent for publication
Not required.
Ethics approval
The study was examined and approved by the referral Ethics Committee for the hospital “Comitato etico per la Sperimentazione Clinica (CESC) delle Province di Verona e Rovigo” on 20 December 2020 with ID number: 3112CESC. Printed informed consent was obtained from each participant before participation. All participants were allowed to withdraw from the study at any moment without justification.
References
- 1. Polack FP, Thomas SJ, Kitchin N, et al. Safety and efficacy of the BNT162b2 mRNA Covid-19 vaccine. N Engl J Med 2020;383:2603–15. 10.1056/NEJMoa2034577 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. World Health Organization (WHO) . Weekly epidemiological update - 22 December 2020. Geneva: WHO, 2020. https://www.who.int/publications/m/item/weekly-epidemiological-update---22-december-2020 [Google Scholar]
- 3. World Health Organization (WHO) . Coronavirus disease 2019 (COVID-19): dashboard with vaccination data. Geneva: WHO, 2021. https://covid19.who.int/ [Google Scholar]
- 4. Wiersinga WJ, Rhodes A, Cheng AC, et al. Pathophysiology, transmission, diagnosis, and treatment of coronavirus disease 2019 (COVID-19): a review. JAMA 2020;324:782–93. 10.1001/jama.2020.12839 [DOI] [PubMed] [Google Scholar]
- 5. European Centre for Disease Control and Prevention (ECDC) . Risk assessment: risk related to the spread of new SARS-CoV-2 variants of concern in the EU/EEA – first update. Stockholm: ECDC, 2021. https://www.ecdc.europa.eu/en/publications-data/covid-19-risk-assessment-spread-new-variants-concern-eueea-first-update [Google Scholar]
- 6. Banerji A, Wickner PG, Saff R, et al. mRNA vaccines to prevent COVID-19 disease and reported allergic reactions: current evidence and suggested approach. J Allergy Clin Immunol Pract 2021;9:1423–37. 10.1016/j.jaip.2020.12.047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. European Medicines Agency (EMA) . Committee for Medicinal Products for Human Use (CHMP). procedure No. EMEA/H/C/005735/0000. assessment report Comirnaty, COVID-19 mRNA vaccine (nucleoside-modified), 2021. Available: https://www.ema.europa.eu/en/documents/assessment-report/comirnaty-epar-public-assessment-report_en.pdf
- 8. Cavaleri M, Enzmann H, Straus S, et al. The European Medicines Agency's EU conditional marketing authorisations for COVID-19 vaccines. Lancet 2021;397:355–7. 10.1016/S0140-6736(21)00085-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Turner PJ, Ansotegui IJ, Campbell DE, et al. COVID-19 vaccine-associated anaphylaxis: a statement of the World Allergy Organization Anaphylaxis Committee. World Allergy Organ J 2021;14:100517. 10.1016/j.waojou.2021.100517 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Council for International Organizations of Medical Sciences (CIOMS), World Health Organization (WHO) . Definition and application of terms for vaccine pharmacovigilance. Report of CIOMS/WHO working group on vaccine pharmacovigilance. CIOMS/WHO 2012, 2021. Available: https://www.who.int/vaccine_safety/initiative/tools/CIOMS_report_WG_vaccine.pdf
- 11. Castells MC, Phillips EJ. Maintaining safety with SARS-CoV-2 vaccines. N Engl J Med 2021;384:643–9. 10.1056/NEJMra2035343 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. European Medicines Agency (EMA) . ICH topic E 2 a clinical safety data management: definitions and standards for expedited reporting, 2021. Available: https://www.ema.europa.eu/en/documents/scientific-guideline/international-conference-harmonisation-technical-requirements-registration-pharmaceuticals-human-use_en-15.pdf
- 13. World Health Organization (WHO) . Weekly epidemiological update on COVID-19 - 27 April 2021. Geneva: WHO, 2021. https://apps.who.int/iris/handle/10665/341070 [Google Scholar]
- 14. McNeil MM, Weintraub ES, Duffy J, et al. Risk of anaphylaxis after vaccination in children and adults. J Allergy Clin Immunol 2016;137:868–78. 10.1016/j.jaci.2015.07.048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Su JR, Moro PL, Ng CS, et al. Anaphylaxis after vaccination reported to the vaccine adverse event reporting system, 1990-2016. J Allergy Clin Immunol 2019;143:1465–73. 10.1016/j.jaci.2018.12.1003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. de Vrieze J. Pfizer's vaccine raises allergy concerns. Science 2021;371:10–11. 10.1126/science.371.6524.10 [DOI] [PubMed] [Google Scholar]
- 17. El-Shitany NA, Harakeh S, Badr-Eldin SM, et al. Minor to moderate side effects of Pfizer-BioNTech COVID-19 vaccine among Saudi residents: a retrospective cross-sectional study. Int J Gen Med 2021;14:1389–401. 10.2147/IJGM.S310497 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Subbarao S, Warrener LA, Hoschler K, et al. Robust antibody responses in 70-80-year-olds 3 weeks after the first or second doses of Pfizer/BioNTech COVID-19 vaccine, United Kingdom, January to February 2021. Euro Surveill 2021;26. 10.2807/1560-7917.ES.2021.26.12.2100329 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Menni C, Klaser K, May A, et al. Vaccine side-effects and SARS-CoV-2 infection after vaccination in users of the COVID symptom study APP in the UK: a prospective observational study. Lancet Infect Dis 2021;21:939–49. 10.1016/S1473-3099(21)00224-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Sahin U, Muik A, Derhovanessian E, et al. COVID-19 vaccine BNT162b1 elicits human antibody and TH1 T cell responses. Nature 2020;586:594–9. 10.1038/s41586-020-2814-7 [DOI] [PubMed] [Google Scholar]
- 21. Frieman M, Harris AD, Herati RS, et al. SARS-CoV-2 vaccines for all but a single dose for COVID-19 survivors. EBioMedicine 2021;68:103401. 10.1016/j.ebiom.2021.103401 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Gee J, Marquez P, Su J, et al. First month of COVID-19 vaccine safety monitoring - United States, December 14, 2020-January 13, 2021. MMWR Morb Mortal Wkly Rep 2021;70:283–8. 10.15585/mmwr.mm7008e3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Abu Jabal K, Ben-Amram H, Beiruti K, et al. Impact of age, ethnicity, sex and prior infection status on immunogenicity following a single dose of the BNT162b2 mRNA COVID-19 vaccine: real-world evidence from healthcare workers, Israel, December 2020 to January 2021. Euro Surveill 2021;26. 10.2807/1560-7917.ES.2021.26.6.2100096 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Aps LRdeMM, Piantola MAF, Pereira SA, et al. Adverse events of vaccines and the consequences of non-vaccination: a critical review. Rev Saude Publica 2018;52:40. 10.11606/s1518-8787.2018052000384 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Dreskin SC, Halsey NA, Kelso JM, et al. International consensus (ICON): allergic reactions to vaccines. World Allergy Organ J 2016;9:32. 10.1186/s40413-016-0120-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
ejhpharm-2021-002933supp001.pdf (86.7KB, pdf)
Data Availability Statement
No data are available. Not applicable.