Abstract
Objective:
Nearly half of individuals living with HIV in the USA are now 50 or older. This rapidly aging populace may be at an increasingly greater risk of Alzheimer’s disease (AD). However, the potential interaction between HIV-disease and AD pathogenesis (i.e., AD genetic risk factors) on brain function remains an open question. The present study aimed to investigate the impact of APOE ε4 on brain function in middle-aged to older PWH, as well as the putative interaction between ε4 and HIV disease severity.
Methods:
Ninety-nine PWH participated in a cross-sectional study (56.3±6.5yrs, range 41–70yrs, 27 females, 26 ε4 carriers and 73 noncarriers). Structural MRI and resting-state functional MRI were collected to assess alterations in brain structure and functional connectivity (FC), respectively.
Results:
APOE ε4 was associated with worse memory performance and reduced FC in the memory network. The FC reduction was centered at the caudate nucleus rather than hippocampus and correlated with worse memory performance. In ε4 carriers, low CD4 nadir was associated with reduced FC in the memory network, but this association was absent in noncarriers. Furthermore, there was an indirect detrimental impact of ε4 on memory performance through memory network FC. However, this indirect effect was contingent on CD4 nadir – that is, the indirect effect of ε4 on memory was only significant when CD4 nadir was low.
Interpretation:
APOE ε4 is associated with reduced memory and reduced FC within the memory network, and low CD4 nadir—indicating a history of severe immunosuppression—may exacerbate the effects of ε4.
Keywords: APOE, HIV, memory, CD4 nadir, resting state fMRI
INTRODUCTION
APOE ε4 is a known genetic risk factor for late-onset sporadic Alzheimer’s disease (AD), atherosclerosis, and worse clinical outcomes after a traumatic brain injury [1]. In people with HIV (PWH), ε4 is associated with increased amyloid pathology [2–4], but the association between ε4 and neurocognitive impairment is unclear: while some studies found that ε4 was associated with a higher risk of neurocognitive impairment or dementia [5–8], others found no association [9–14], supporting a need for additional research, especially as ε4 may predispose to damage caused by agents like HIV.
Resting state functional MRI (fMRI) is a useful technique to study brain function [15]. In APOE ε4, studies have shown that functional connectivity (FC) is altered in ε4 carriers compared to noncarriers [16], even prior to the onset of detectable amyloid deposition [17]. A recent study found reduced FCs between hippocampus and caudate, and between hippocampus and other key regions of the Papez circuit in cognitively normal middle-aged ε4 carriers compared to noncarriers (despite a lack of significant difference in memory performance), and across subjects, FC correlated with memory performance [18]. This finding is of particular interest for several reasons: first, the Papez circuit is a vital pathway in episodic memory formation and consolidation [19], and is involved in AD [20]; second, the caudate nucleus [21,22] and several regions in the Papez circuit are preferentially affected in PWH, including thalamus, hippocampus, and cingulate cortex [22,23], especially the caudate, which has been shown to play a critical a role in HIV-associated neurocognitive disorders (HAND) [21,22]; third, while the caudate and the hippocampus belong to separate and competing memory systems, the caudate-hippocampus FC correlates with memory performance (e.g., [18,24]). Therefore, investigating the impact of ε4 on FCs between these regions (the Papez circuit plus the caudate) in PWH may provide important insight into the potential interactions between HIV-disease and APOE ε4 on brain function.
The present study was conducted to understand whether HIV-disease and APOE ε4 may concomitantly and/or interactively affect brain function (with a focus on the memory network). We first examined whether ε4 was associated with worse memory performance in PWH; then using resting-state FC technique, we investigated the impact of ε4 on memory-related brain regions (focusing on the Papez circuit, plus the caudate), and the potential interaction between ε4 status and CD4 nadir.
METHODS
See supplemental materials for additional details on methods.
Participants
One hundred and four PWH were recruited from the greater Washington DC metropolitan area between 11/01/2015 and 06/28/2019. Blood specimens were collected to measure viral load and current CD4 counts. Saliva samples were collected for genotyping. Self-reported CD4 nadirs and estimated duration of HIV infection were documented. In addition, we applied bootstrapping techniques to data analyses to assess the robustness of the results. Five subjects were excluded due to the lack of genotype information (n=3) or MRI anomalies (n=2). All procedures were performed in accordance with the guidelines and regulations from the Institutional Review Board. Written informed consent from every participant was obtained prior to enrollment.
Neuropsychological testing
A comprehensive neuropsychological battery was administered (including Hopkins Verbal Learning Test-Revised (HVLT-R), see Table S1) to assess performance of cognitive domains that are affected in PWH [25]. Neuropsychological test scores were used to calculate global deficit score (GDS) [26] and to diagnose HAND (together with the Lawton and Brody Activities of Daily Living (ADL) index) following the standard Frascati guideline [27].
MRI acquisition and pre-processing
High-resolution (1×1×1mm3) T1-weighted images and one run of resting state fMRI images (n=264; resolution 3.2×3.2×4mm2) were acquired from each participant at the local institute.
The software package SPM12 (https://www.fil.ion.ucl.ac.uk/spm/), the Computational Anatomy Toolbox (CAT, version 12.5) (www.neuro.uni-jena.de/cat/), and the CONN functional connectivity toolbox (https://www.nitrc.org/projects/conn/) [28] were used for pre-processing and analyzing MRI data, following default processing pipeline settings with default parameters.
Statistical analyses
The statistical analyses were conducted in SPSS 25.0 (Chicago, IL), and MATLAB 2018b (Math Works, Natick, MA). We divided the PWH into two groups based on their genotypes: carriers, PWH with at least one copy of ε4 allele; and noncarriers, PWH with zero copy of ε4 allele. All statistical analyses (including MRI) were two-tailed, and controlled for age, education years, sex, and race. Additional corresponding MRI covariates were included in MRI analyses.
Contingency χ2 tests, and two-sample t-tests were used to examine group differences in demographics, HIV disease (current CD4 counts, CD4 nadir, and disease duration), HAND diagnoses between carriers and noncarriers, and history of illicit drug use (see Table 1). As our sample of PWH was predominantly African American (AA), race was defined as a dichotomous variable: AA (1), non-AA (0).
Table 1.
Demographics and HIV disease information of APOE ε4 carriers and noncarriers.
| Carriers a (n=26) | Noncarriers b (n=73) | p-value | |
|---|---|---|---|
| Age (years) | 55.1 (5.9) c | 56.8 (6.7) | n.s. d |
| Education (years) | 13.62 (3.1) | 14.5 (2.9) | n.s. |
| Sex (Female%) | 26.9% | 21.9% | n.s. |
| Race (AA%) e | 76.9% | 57.5% | n.s. |
| Current CD4 (cells/μl) | 684.5 (561.0) | 612.0(450.3) | n.s. |
| CD4 nadir (cells/μl) | 152 (330) | 200 (285) f | n.s. |
| Disease duration (years) | 26.0 (9.8) | 26.0 (9.3) | n.s. |
| GDS g | 0.34 (0.29) | 0.34 (0.44) | n.s. |
| HAND diagnosis h | 26.9% | 26.0% | n.s. |
| On stable cART i | 100% | 97.3% | n.s. |
| Undetectable VL j | 84.6% | 82.2% | n.s. |
| History of illicit drug use k | 53.8% | 45.2% | n.s. |
| Taking medications for | |||
| - Hypertension | 42.3% | 45.2% | n.s. |
| - Diabetes | 19.2% | 11.0% | n.s. |
| - Cholesterol level l | 46.2% | 41.1% | n.s. |
Note
ε2/ε4 (n=2), ε3/ε4 (n=21), ε4/ε4 (n=3);
ε2/ε2 (n=4), ε2/ε3 (n=13), ε3/ε3 (n=56);
Age, education, disease duration, and GDS were presented as mean (standard deviation), versus current CD4 and CD4 nadir were resented as median (IQR);
n.s., not significant;
AA, African-Americans, similar results were observed in the AA subgroup (n=62) (see Table S3 and Fig. S7 to S10);
one noncarrier did not provide CD4 nadir (treated as a missing value);
GDS, global deficits score, which was calculated from the seven neurocognitive domains [26];
HAND, HIV-associated neurocognitive disorders, 7 carriers (6 with asymptomatic neurocognitive impairment (ANI), and 1 with mild neurocognitive disorder (MND)), and 19 noncarriers (18 with ANI, and 1 with MND) met the HAND criteria [27];
cART, combination antiretroviral therapy;
Subjects with undetectable plasma viral load (VL) (<20 copies/ml), including 22 carriers and 60 noncarriers (similar results were observed in this subgroup (n=82), see Table S2 and Fig. S3 to S6), and only six PWH (2 carriers, 4 noncarriers) had a VL higher than 200 copies/ml in their blood specimens.
Subjects who have at least one drug abuse/dependent diagnoses based on Composite International Diagnostic Interview. Note that subjects with current illicit use is not qualified to participate the current study. In additional analyses, we included the history of illicit drug use and diabetes as covariates and obtained equivalent results.
11 APOE ε4 carriers and 26 noncarriers are taking medications to dyslipidemia, and 1 carrier and 4 noncarriers are taking medications for the purpose of general heart health.
The CAT software package was used to test the effect of ε4 status on cortical thickness and GMv, using a non-parametric permutation-based approach [29] at a threshold of p<0.001 (uncorrected, at least 50 contiguous voxels).
Three different types of FC analyses were conducted using the CONN software package: region of interest (ROI) based (ROI-to-ROI), seed-to-voxel, and multivariate seed-to-voxel. The Papez circuit and bilateral caudate ROIs were identified, including thalamus (THA), caudate (CAU), mammillary body (MB), anterior and posterior hippocampus (aHIP, pHIP), entorhinal cortex (EC), parahippocampal cortex (PHC), anterior and posterior cingulate cortex (ACC, PCC) ROIs. Based on the results of ROI-to-ROI analyses, the right caudate (CAUr) and the right anterior hippocampus (aHIPr) were chosen as the seed ROIs for the seed-to-voxel and the multivariate seed-to-voxel analyses. The multivariate seed-to-voxel analyses were conducted to compare the roles of hippocampus and caudate in the functional disruptions in ε4 carriers: when the CAUr was chosen as the seed region, the time series in the aHIPr were controlled; when the aHIPr was chosen as the seed region, the time series in the CAUr were controlled. A threshold of p<0.05 (false discovery rate (FDR) corrected) was applied in ROI-to-ROI FC analysis. Seed-to-voxel FC analyses and multivariate seed-to-voxel FC analyses used a threshold of voxel-wise p<0.001 (uncorrected), cluster-wise p<0.05 (FDR corrected).
A moderated mediation analysis was conducted in SPSS toolbox PROCESS V3.4 to investigate the indirect (via caudate-hippocampal FC, FCCAUr-aHIPr) effect of ε4 status on memory performance, with the indirect effect contingent on CD4 nadir.
RESULTS
There was no significant difference in demographics, HIV disease, HAND diagnosis, and GDS between carriers and noncarriers (Table 1). Among the study sample, 22 carriers and 60 noncarriers had undetectable viral load and were on cART. The results in the virologically suppressed subgroup did not differ from those in the entire study sample (see Table S2 and Fig. S3–S6). Similar results were found in the AA-subgroup (see Table S3 and Fig. S7–S10).
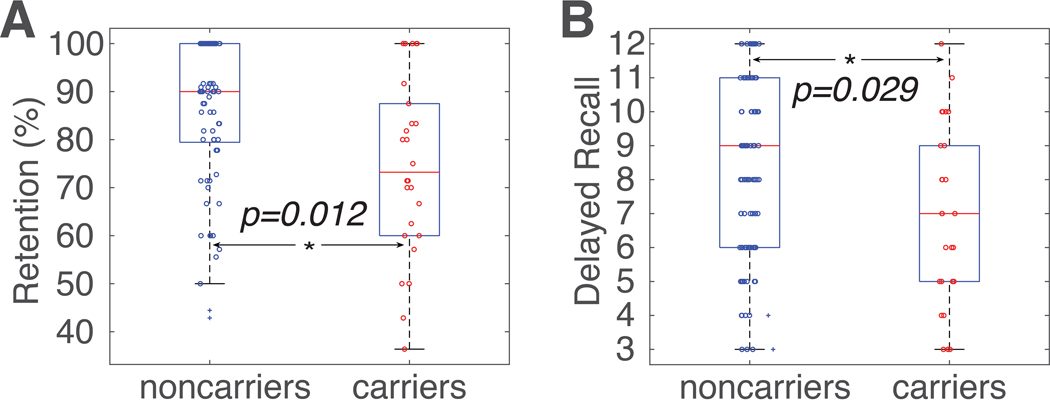
ANCOVA analysis revealed significant differences between carriers and noncarriers in two HVLT-R scores that are related to memory. After controlling for age, education, sex, and race, we found that ε4 carriers had worse HVLT-R retention rate (F(1,93)=6.42, p=0.012, Fig. 1A) and delayed recall (F(1,93)=4.92, p=0.029, Fig. 1B). As expected, no significant group differences were found in any other neurocognitive domains (Table S1), supporting that memory was the primary neurocognitive domain affected in these ε4 carriers. Additional analyses revealed no significant interaction between age and ε4 status on any memory scores.
Figure 1. Group differences in HVLT-R retention and delayed recall.
(A) APOE ε4 carriers (red circles) had significantly lower HVLT-R retention rate than noncarriers (blue circles; blue crosses denote outliers that were more than three scaled median absolute deviations away from the median). (B) APOE ε4 carriers (red circles) had significantly lower HVLT-R delayed recall scores than noncarriers (blue circles). On each box, the central mark (red line) indicates the median, the bottom and top edges of the box are the 25th and 75th percentiles of the samples, respectively, and the whiskers extend to the most extreme data points not considered outliers. The two outlier subjects (depicted as blue +) in Figure 1A were identified using the isoutlier function in MATLAB. Similar results were obtained when the two outlier subjects were excluded (retention rate, F(1,91)=9.77, p=0.002; delayed recall, F(1,91)=7.08, p=0.008). HVLT-R, the Hopkins Verbal Learning Test–Revised. *, p<0.05.
For both cortical thickness and GMv, there was no significant difference between the carriers and noncarriers at a threshold of p<0.001 uncorrected. Additionally, we extracted the GMv of the medial temporal lobe (MTL) subregions, and there were no significant differences between carriers and noncarriers (Table S1).
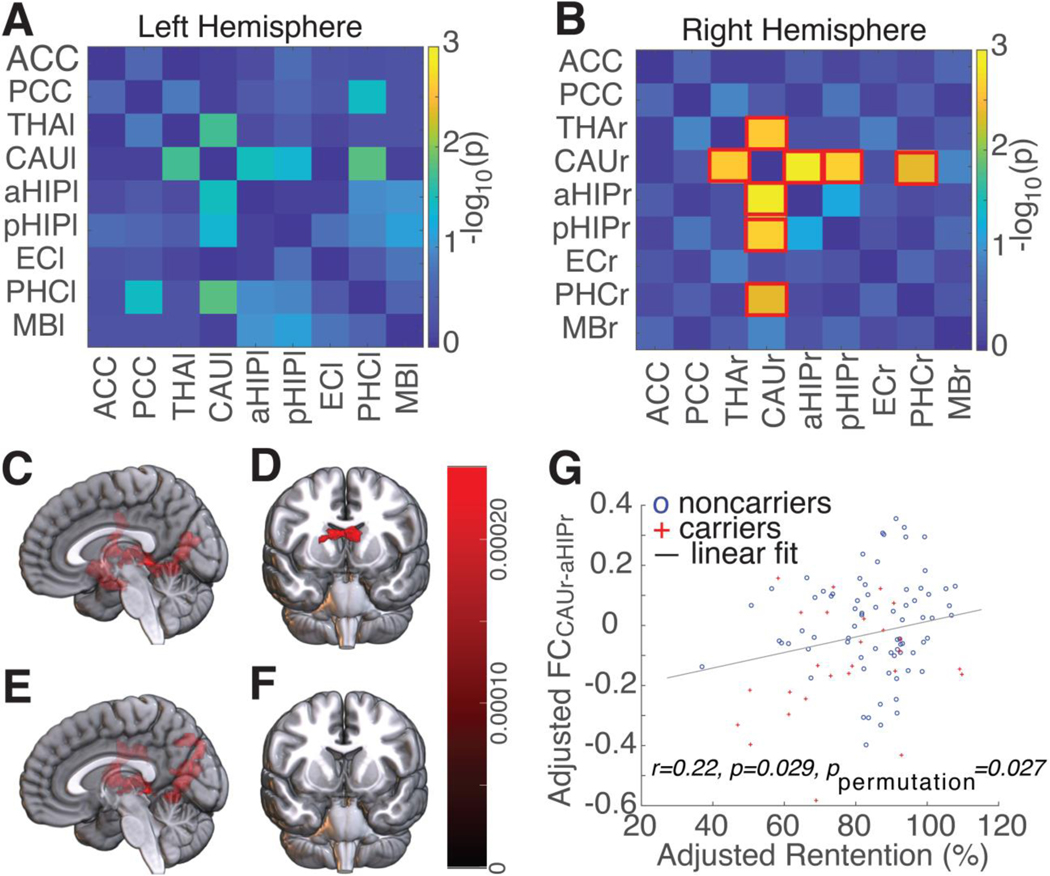
ROI-to-ROI FC analysis revealed that, compared to noncarriers, carriers had significantly lower FCs between the CAU and several key regions (aHIP, pHIP, PHC, and THA) in the Papez circuit (Fig. 2). After correction for multiple comparisons, the effect was still significant in the right (Fig. 2B) but not in the left hemisphere (Fig. 2A). The strongest group difference in FC was between the CAUr and the aHIPr (FCCAUR-aHIPr, F(1,93)=12.42, p=0.0007). Additional seed-to-voxel analyses and multivariate seed-to-voxel analyses confirmed the central role of CAUr. In seed-to-voxel analyses, CAUr as the seed region revealed reduced FCs between CAUr and a large cluster in the right limbic system, including hippocampus, thalamus, parahippocampus, putamen, and occipital cortex, in carriers compared to noncarriers (Fig. 2C); whereas aHIPr as the seed region revealed a group difference largely limited to bilateral caudate nuclei (Fig. 2D). Similar results were found in multivariate seed-to-voxel analyses. After controlling for BOLD timeseries in the aHIPr, CAUr as the seed region revealed reduced FCs between CAUr and putamen, thalamus, posterior hippocampus, posterior cingulate cortex, and occipital cortex, in carriers compared to noncarriers (Fig. 2E). In contrast, when aHIPr as the seed region and controlling for BOLD timeseries in the CAUr, there was no significant group difference (Fig. 2F). There were no increased FCs in carriers compared to noncarriers with either seed region.
Figure 2. Reduced functional connectivity (FC) in carriers compared to noncarriers, and the correlation between FC and memory performance.
The group comparisons (carriers versus noncarriers) of the ROI-to-ROI functional connectivity (FC) in the (A) left and (B) right hemisphere, respectively (each with nine ROIs). Bilateral ACC and bilateral PCC were each treated as one single ROI and were included in FC analyses in the left and right hemisphere. The pairwise ROI-to-ROI FC comparisons that reached significant difference (with FDR correction) were highlighted with a red square box. The colormap represented negative log p-values of group comparisons. ε4 carriers had significant lower FCs between CAUr and aHIPr, pHIPr, THAr, and PHCr than noncarriers. (C) Using the right caudate (CAUr) as the seed region, the seed-to-voxel analysis revealed that carriers had reduced FC between CAUr and a large cluster of brain regions in the right limbic system and the right occipital cortex. (D) By contrast, using the right anterior hippocampus (aHIPr) as the seed region, the seed-to-voxel analysis revealed that the reduced FC in carriers was largely limited to bilateral caudate nuclei. (E) After controlling for the BOLD timeseries in the aHIPr ROI, the seed-to-voxel analysis with CAUr as the seed region revealed that carriers had reduced FC between CAUr and a large cluster of brain regions in the right limbic system and the right occipital cortex. (F) By contrast, after controlling for the BOLD timeseries in the CAUr ROI, the seed-to-voxel analysis with aHIPr as the seed region did not found any significant cluster. All results showing here were controlled for age, for age, education, sex, and race. Seed-to-voxel analysis (C and D) were thresholded at voxel-wise p<0.001 (uncorrected) and cluster-wise p<0.05 (FDR corrected). Multivariate seed-to-voxel analysis (E and F) were thresholded at voxel-wise p<0.005 (uncorrected) and cluster-wise p<0.05 (FDR corrected). (G) Pearson correlation revealed a significant correlation (r=0.220, p=0.029, ppermutation=0.027 with 10000 permutations) between the adjusted FCCAUr-aHIPr and adjusted HVLT-R retention (adjusted for age, education, sex, and race). Red crosses, carriers; blue circles, noncarriers. Abbreviations: ACC/PCC, anterior/posterior cingulate cortex; aHIP/pHIP, anterior/posterior hippocampus; CAU, caudate; FC, functional connectivity; FDR, false discovery rate; MB, mammillary body; OC, occipital cortex; ROI, region-of-interest; PUT, putamen. THA, thalamus; -l/-r: left/right (e.g., CAUl/CAUr, left and right caudate, respectively).
In addition, the ROI-to-ROI FC between CAUr and aHIPr (FCCAUr-aHIPr) was significantly correlated with HVLT-R retention rate (r=0.220, p=0.029, ppermutation=0.027 with 10000 permutations, Fig. 2G). There was no significant correlation between FCCAUr-aHIPr and HVLT-R delayed recall (r=0.105, p=0.301).
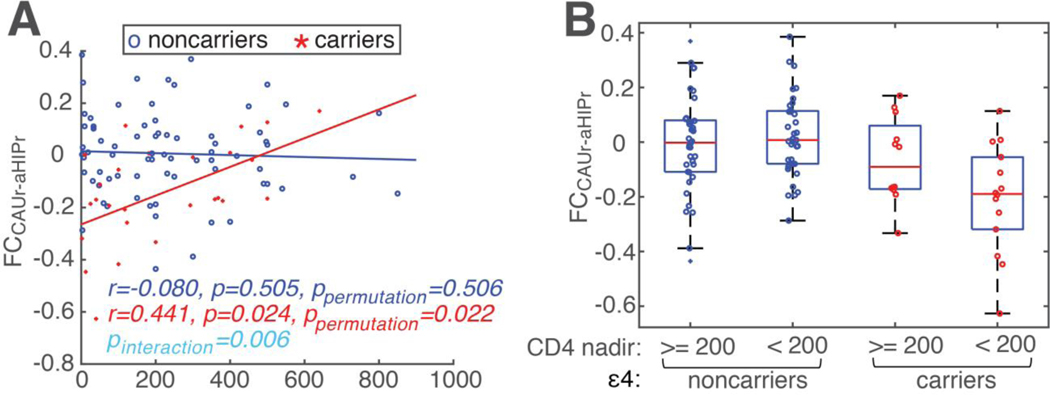
A general linear model (GLM) analysis revealed a significant interaction between CD4 nadir and ε4 status on FCCAUr-aHIPr (F(1,90)=7.68, p=0.006, Fig. 3A). Post-hoc correlation analyses revealed a significant correlation between CD4 nadir and FCCAUr-aHIPr in carriers (r=0.441, p=0.024, ppermutation=0.022 with 10000 permutations), but not in noncarriers (p=0.505), suggesting low CD4 nadir has a negative impact on the FCCAUr-aHIPr, but only in carriers. We further divided the PWH into four groups based on ε4 status (carriers versus noncarriers) and CD4 nadir counts (<200 cells/μl versus ≥200 cells/μl) (Fig. 3B). ANCOVA analysis on ε4 status (carriers vs noncarriers) and CD4 nadir counts (<200 cells/μl versus ≥200 cells/μl) revealed a main effect of ε4 status (p=0.003) and a significant interaction between ε4 and CD4 nadir counts (p=0.048), further supporting that low CD4 nadir (i.e., 200 cells/μl or lower) might exacerbate the detrimental effects of ε4, which was also supported by the moderated mediation analysis below. By contrast, there were no interactions between FCCAUr-aHIPr and disease duration nor current CD4 (at least p>0.1).
Figure 3. The interaction of ε4 status and CD4 nadir on FC between CAUr and aHIPr (FCCAUr-aHIPr).
(A) A general linear model (GLM) analysis revealed significant interaction of ε4 and CD4 nadir on FC between CAUr and aHIPr (FCCAUr-aHIPr) (F(1,90)=7.67, p=0.006, the cyan text in the figure), after controlling for age, education, sex, and race. For carriers: red crosses, data of each individual subject; red line, fitted regression line; red text, correlation coefficient between FCCAUr-aHIPr and CD4 nadir in carriers. Noncarriers were shown in blue color (markers (circles), line, and text). (B) The subjects were further divided into four groups, ε4 status (carriers versus noncarriers) x CD4 nadir (<200 cells/μl versus ≥200 cells/μl). A significant interaction between ε4 status and CD4 nadir was observed.
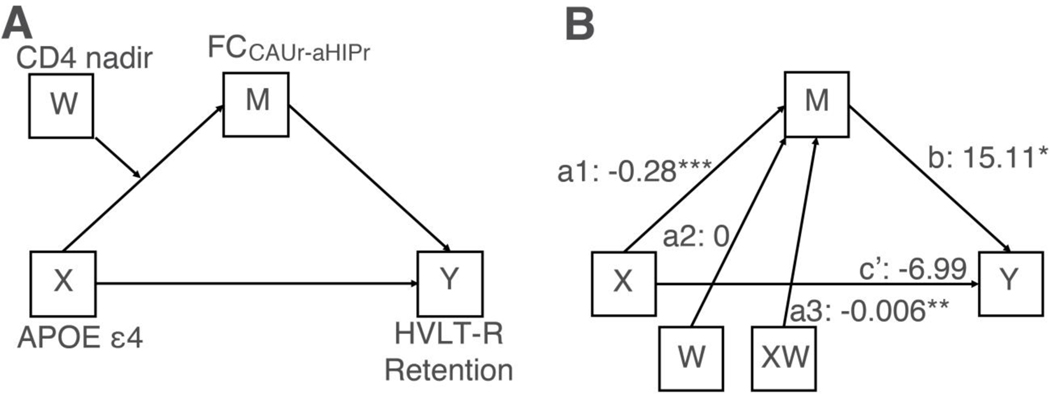
As shown in Fig. S11, the moderated mediation analysis (Fig. 4A) was motivated by findings from two previous studies [24,30] and the results in Fig. 2 and 3. This analysis revealed a significant moderated mediation effect (index = 0.009) with 95% confidence interval (CI) ranging from 0.0002 to 0.02213, which did not encompass zero, suggesting a significant model (Fig. 4B). In short, the moderated mediation analysis revealed two key findings: i) ε4 was associated with reduced FCCAUr-aHIPr, but the association depended on nadir CD4 (in line with Fig. 3); and ii) ε4 had an indirect detrimental effect on memory performance (HVLT-R retention rate) through FCCAUr-aHIPr, but the indirect effect was significant only when CD4 nadir was low (i.e., 199.5 cells/μl or lower) and not when CD4 nadir was high (i.e., 462.4 cells/μl or higher).
Figure 4. The moderated mediation analysis.
(A) The conceptual diagram of the moderated mediation model. X, APOE ε4 status; Y, HVLT-R retention rate; M, FCCAUr-aHIPr; W, CD4 nadir. (A) The statistical diagram of the moderated mediation model. a1, the effect of ε4 (X) on FCCAUr-aHIPr (M); a2, the effect of CD4 nadir (W) on FCCAUr-aHIPr (M); a3, the interaction effect between ε4 (X) and CD4 nadir (W) on FCCAUr-aHIPr (M); b, the effect of mediator FCCAUr-aHIPr (M) on HVLT-R retention rate (Y); c’, the direct effect of ε4 (X) on HVLT-R retention rate (Y). Note: * denotes p<0.05; ** denotes p<0.01; *** denotes p<0.001.
DISCUSSION
In this sample of PWH, ε4 was associated with reduced verbal memory performance and reduced FC between the caudate and regions in the Papez circuit, especially the hippocampus. The caudate (but not the hippocampus) assumed the predominant role in this functional disruption. There was a significant correlation between the FC between right caudate and right anterior hippocampus (FCCAUr-aHIPr) and memory performance. Low CD4 nadir was associated with reduced FCCAUr-aHIPr in ε4 carriers, but not in noncarriers; this interaction was further supported by the moderated mediation analysis. In addition, the moderated mediation analysis revealed an indirect detrimental effect of ε4 status on memory performance through FCCAUr-aHIPr, but the indirect effect was contingent on CD4 nadir counts.
Impaired episodic memory is the cognitive hallmark of Alzheimer’s disease, and ε4 is associated with reduced episodic memory in HIV-uninfected [31] and HIV-infected [32–34] “cognitively normal” adults – suggesting the presence of early neural injury to the memory network in some of the “cognitively normal” ε4 carriers. Reduced executive function is also highly prevalent [8,31,33,35]. In the present study, we did not find a significant impact of ε4 on executive function, nor global cognition, but rather the effect of ε4 was limited to episodic memory. Thus, memory may be the most affected cognitive domain in these HIV+ ε4 carriers, similar to HIV-uninfected ε4 carriers [31]. The lack of interaction between age and ε4 on memory performance might be due to a relatively young sample of PWH (with an average age of 56 years), along with a relatively narrow age range (41–70 years). The narrow age range was intentional by study design to investigate a critical transitional period (from middle-age to old age) and to produce a relatively homogeneous group of PWH (to improve sensitivity).
The lack of a significant effect of ε4 on HAND diagnosis in the present study is in line with many previous studies [9–14,32–35], but in contrast to several other studies [5–8]. The inconsistency may be partially due to differences in study samples: the PWH in these studies [5–8] either have poor immune restoration [8] or low education (5.5 years) (which in turn implicates low cognitive reserve) [7], or are at more advanced stages of HIV brain disease (i.e., 25–26% of the study sample [5] or the older subgroup [6] are demented); in contrast, the cohort of PWH in the present study are relatively healthy (Table 1). Taken together, this and previous studies suggest that ε4 may be associated with increased risk of neurocognitive decline, especially memory, implicating an early and mild neural injury that may be largely confined to brain regions/networks involving memory (or plus executive function). The mild neural injury may make HIV-infected ε4 carriers more susceptible to neurocognitive impairment or even dementia, especially when combining with additional comorbidities [5–8].
Using resting state FC technique, we investigated the neural mechanisms underlying the impact of ε4 on memory. The FC analyses revealed that ε4 in PWH was associated with reduced FCs between the caudate and several key regions in the Papez circuit (especially the hippocampus), with a stronger effect in the right than the left hemisphere (Fig. 2A and 2B). Future studies are needed to investigate potential hemispheric difference. In line with a previous study with HIV-uninfected middle-aged adults [18], across ε4 carriers and noncarriers, there was a significant correlation between FCCAUr-aHIPr and memory performance, suggesting altered FC between caudate and hippocampus might contribute to reduced memory in both HIV-infected and uninfected ε4 carriers. However, a key and important difference between the previous study [18] and the present study is that the ε4-associated network disruption is centered at the hippocampus in the previous study with HIV-uninfected adults [18], versus at the caudate in the present study with PWH (Fig. 2C–F, especially Fig. 2E & 2F). This difference is interesting: while the hippocampus (along with other MTL subregions) is at the center of AD pathology, the caudate (along with other subcortical regions) has been proposed to be at the center of HAND pathology [21,22]. The inconsistency suggests that, in addition to common ε4 pathology shared with HIV-uninfected carriers, unique ε4 pathology may exist in HIV-infected carriers, i.e., injury to the caudate and other subcortical regions, probably due to interactions between ε4 and HIV-disease severity. Amyloid PET scans may help to examine whether amyloid deposition is more prominent in caudate (or other subcortical regions) than MTL in HIV-infected ε4 carriers, similar to individuals with Down syndrome or autosomal dominant AD [36,37].
Low CD4 nadir, which indicates a history of severe immunosuppression, is a strong predicator of neurocognitive impairment in PWH [25,38–40]. This suggests that the depth of immunosuppression (represented by a low CD4 nadir count) may have caused irreversible neural injury persisting years later, or it may have triggered certain neuropathology “cascades” in some patients (e.g., due to interaction with host genes) that evolve over time. Both mechanisms may contribute to the high prevalence of HAND in the cART era. However, it remains largely unknown whether and how CD4 nadir and host genes interactively impacts brain health/function. In the present study, we observed a significant interaction between ε4 and CD4 nadir on the FCCAUr-aHIPr, suggesting that the memory network is more vulnerable to low CD4 nadir in ε4 carriers. Interestingly, two previous studies have found an interaction of ε4 and current immunosuppression on HAND status [5,8]. The PWH in the present study had successful immune restoration (Table 1), thus we could not assess the potential interaction of ε4 and severity of current immunosuppression. Nevertheless, the results suggest that in PWH, the co-existence of ε4 allele and low CD4 nadir may result in an increased risk of neurocognitive impairment, especially in the memory domain (along with disruption to the memory network). The underlying neural mechanisms might be due to an interaction of AD pathology (through ε4) and HIV-disease pathology (i.e., immunosuppression).
Multiple factors may have contributed to the impact of CD4 nadir on the FCCAUr-aHIPr in HIV+ ε4 carriers. The association of ε4 with alterations in brain structure and function in PWH is consistent with a model where ε4 predisposes to damage caused by other agents, such as acute injuries or aging. This predisposition could be related to inflammation or lipid homeostasis [41], conditions that could be present in the brains of PWH and might correlate with HIV disease severity. For instance, both ε4 [1] and HIV-disease (including low CD4 nadir) [42] are risk factors for atherosclerosis. Therefore, the findings of the interactive impact of low CD4 nadir and ε4 on the memory network in the present study may be due to a double-hit – low CD4 nadir and APOE ε4 – perhaps mediated by atherosclerosis. Another potential contributing factor is dopamine deficit. In older adults, the availability of D2 dopamine receptors (D2DR) in caudate correlates with FC between the caudate and the hippocampus, as well as episodic memory performance (the latter two also correlated with each other) [24]. In this earlier study [24], a mediation analysis further revealed an indirect of D2DR in the caudate on episodic memory through the caudate-hippocampus FC, suggesting that dopamine deficits in PWH might contribute to reduced caudate-hippocampus FC and worse memory performance in ε4 carriers with low CD4 nadir. However, it is not clear whether there is an interaction of ε4 and immune suppression (current or history) on dopamine deficits in the caudate of PWH. Future studies are necessary to understand the biological mechanisms underlying the interaction between APOE ε4 and immunosuppression.
There are several limitations of this study. First, the participants in the present study were relatively young, with only six of them older than 65 and none of them older than 70, limiting our capability to detect the potential age X ε4 interaction. The young age might also contribute to the relatively weak group difference in memory, similar to other studies [18]. Second, the ε4 allele has a higher prevalence and probably a reduced strength in people with African ancestry than people of other races [43–45], but the impact of race (i.e., with African ancestry) on ε4 in PWH is unknown. In the present study, nearly two-thirds of participants were African American (AA), and the ε4 allele was more prevalent in AA participants (32.3%) than non-AA participants (16.2%) (Table 1). We did find similar results in the AA-subgroup (see Table S4 and Fig. S7–S10), but due to limited sample size, we could not directly compare AA vs. non-AA subgroups. Third, female sex is a risk factor for Alzheimer’s disease in APOE ε4 carriers [44] (but also see [46]). In the present study, sex was always included as a covariate in data analyses, and additional post-hoc data analyses revealed no significant effect of sex (p>0.5). However, the lack of significance may be due to a small number of female participants, and thus lack of statistical power. Fourth, due to a lack of medical records more than 10 years old, CD4 nadirs were based on self-report. Although self-reported CD4 nadir is largely accurate [38,47], future large cohort studies with evidence from medical records is needed to further investigate the impact of CD4 nadir, current CD4, and disease duration. Fifth, previous studies suggest a stronger effect of ε4 in PWH at more advanced stages of HAND [5,6], but it is unclear whether and how more advanced stages of HAND would interfere with the interaction between ε4 and CD4 nadir, as only two PWH met the MND criteria in the current study. Sixth, a combination of multimodality imaging and other techniques (such as CSF specimens) is necessary for a better understanding of how ε4 impacts brain health/function in PWH, by acting alone as well as interactively with HIV disease severity. For example, amyloid PET scans can help to assess and compare amyloid deposition at different regions (i.e., caudate versus hippocampus), as well as the relationship between FCs and amyloid deposition at different regions.
In summary, we provide evidence that ε4 is associated with reduced memory and reduced FC within the memory network. In this functional disruption, the caudate (but not the hippocampus) assumed the predominant role. In addition, low CD4 nadir has a negative impact on memory network FC, but only in ε4 carriers and not in noncarriers, suggesting that HIV disease severity may exacerbate the effects of ε4 on brain in middle-aged and older PWH.
Supplementary Material
Acknowledgements
We wish to thank all participants for their time and participation, Harvey R. Fernandez for assistance with APOE genotyping, and the assistance for patient care from the Georgetown University Clinical Research Unit (GU-CRU), which has been supported by Grant # UL1TR000101 (previously UL1RR031975) through the Clinical and Translational Science Awards Program (CTSA).
Funding
The study is supported by award 1R01MH108466 (X.J.) from the National Institutes of Health.
Footnotes
Potential Conflicts of Interest
None.
REFERENCES
- 1.Mahley RW, Rall SC. Apolipoprotein E: far more than a lipid transport protein. Annu Rev Genomics Hum Genet 2000; 1:507–537. [DOI] [PubMed] [Google Scholar]
- 2.Cysique LA, Hewitt T, Croitoru-Lamoury J, Taddei K, Martins RN, Chew CSN, et al. APOE ε4 moderates abnormal CSF-abeta-42 levels, while neurocognitive impairment is associated with abnormal CSF tau levels in HIV+ individuals - a cross-sectional observational study. BMC Neurol 2015; 15:51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Soontornniyomkij V, Moore DJ, Gouaux B, Soontornniyomkij B, Tatro ET, Umlauf A, et al. Cerebral β-amyloid deposition predicts HIV-associated neurocognitive disorders in APOE ε4 carriers. AIDS 2012; 26:2327–2335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Levine AJ, Soontornniyomkij V, Achim CL, Masliah E, Gelman BB, Sinsheimer JS, et al. Multilevel analysis of neuropathogenesis of neurocognitive impairment in HIV. J Neurovirol 2016; 22:431–441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Corder EH, Robertson K, Lannfelt L, Bogdanovic N, Eggertsen G, Wilkins J, et al. HIV-infected subjects with the E4 allele for APOE have excess dementia and peripheral neuropathy. Nature Medicine 1998; 4:1182–1184. [DOI] [PubMed] [Google Scholar]
- 6.Valcour V, Shikuma C, Shiramizu B, Watters M, Poff P, Selnes O, et al. Higher frequency of dementia in older HIV-1 individuals: the Hawaii Aging with HIV-1 Cohort. Neurology 2004; 63:822–827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Spector SA, Singh KK, Gupta S, Cystique LA, Jin H, Letendre S, et al. APOE epsilon4 and MBL-2 O/O genotypes are associated with neurocognitive impairment in HIV-infected plasma donors. AIDS 2010; 24:1471–1479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Panos SE, Hinkin CH, Singer EJ, Thames AD, Patel SM, Sinsheimer JS, et al. Apolipoprotein-E genotype and human immunodeficiency virus-associated neurocognitive disorder: the modulating effects of older age and disease severity. Neurobehav HIV Med 2013; 5:11–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Burt TD, Agan BK, Marconi VC, He W, Kulkarni H, Mold JE, et al. Apolipoprotein (apo) E4 enhances HIV-1 cell entry in vitro, and the APOE epsilon4/epsilon4 genotype accelerates HIV disease progression. Proc Natl Acad Sci USA 2008; 105:8718–8723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pemberton LA, Stone E, Price P, van Bockxmeer F, Brew BJ. The relationship between ApoE, TNFA, IL1a, IL1b and IL12b genes and HIV-1-associated dementia. HIV Med 2008; 9:677–680. [DOI] [PubMed] [Google Scholar]
- 11.Sun B, Abadjian L, Rempel H, Calosing C, Rothlind J, Pulliam L. Peripheral biomarkers do not correlate with cognitive impairment in highly active antiretroviral therapy–treated subjects with human immunodeficiency virus type 1 infection. Journal of Neurovirology 2010; 16:115–124. [DOI] [PubMed] [Google Scholar]
- 12.Joska JA, Combrinck M, Valcour VG, Hoare J, Leisegang F, Mahne AC, et al. Association between apolipoprotein E4 genotype and human immunodeficiency virus-associated dementia in younger adults starting antiretroviral therapy in South Africa. J Neurovirol 2010; 16:377–383. [DOI] [PubMed] [Google Scholar]
- 13.Morgan EE, Woods SP, Letendre SL, Franklin DR, Bloss C, Goate A, et al. Apolipoprotein E4 genotype does not increase risk of HIV-associated neurocognitive disorders. J Neurovirol 2013; 19:150–156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Becker JT, Martinson JJ, Penugonda S, Kingsley L, Molsberry S, Reynolds S, et al. No association between Apoε4 alleles, HIV infection, age, neuropsychological outcome, or death. J Neurovirol 2015; 21:24–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.van den Heuvel MP, Hulshoff Pol HE. Exploring the brain network: a review on resting-state fMRI functional connectivity. Eur Neuropsychopharmacol 2010; 20:519–534. [DOI] [PubMed] [Google Scholar]
- 16.Pietzuch M, King AE, Ward DD, Vickers JC. The Influence of Genetic Factors and Cognitive Reserve on Structural and Functional Resting-State Brain Networks in Aging and Alzheimer’s Disease. Frontiers in Aging Neuroscience 2019; 11. doi: 10.3389/fnagi.2019.00030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sheline YI, Morris JC, Snyder AZ, Price JL, Yan Z, D’Angelo G, et al. APOE4 allele disrupts resting state fMRI connectivity in the absence of amyloid plaques or decreased CSF Aβ42. J Neurosci 2010; 30:17035–17040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Li W, Antuono PG, Xie C, Chen G, Jones JL, Ward BD, et al. Aberrant functional connectivity in Papez circuit correlates with memory performance in cognitively intact middle-aged APOE4 carriers. Cortex 2014; 57:167–176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Aggleton JP, Brown MW. Interleaving brain systems for episodic and recognition memory. Trends Cogn Sci (Regul Ed) 2006; 10:455–463. [DOI] [PubMed] [Google Scholar]
- 20.Jicha GA, Carr SA. Conceptual evolution in Alzheimer’s disease: Implications for understanding the clinical phenotype of progressive neurodegenerative disease. J Alzheimers Dis 2010; 19:253–272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Paul R, Cohen R, Navia B, Tashima K. Relationships between cognition and structural neuroimaging findings in adults with human immunodeficiency virus type-1. Neurosci Biobehav Rev 2002; 26:353–359. [DOI] [PubMed] [Google Scholar]
- 22.Israel SM, Hassanzadeh-Behbahani S, Turkeltaub PE, Moore DJ, Ellis RJ, Jiang X. Different roles of frontal versus striatal atrophy in HIV-associated neurocognitive disorders. Hum Brain Mapp Published Online First: 28 March 2019. doi: 10.1002/hbm.24577 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Moore DJ, Masliah E, Rippeth JD, Gonzalez R, Carey CL, Cherner M, et al. Cortical and subcortical neurodegeneration is associated with HIV neurocognitive impairment. AIDS 2006; 20:879–887. [DOI] [PubMed] [Google Scholar]
- 24.Nyberg L, Karalija N, Salami A, Andersson M, Wåhlin A, Kaboovand N, et al. Dopamine D2 receptor availability is linked to hippocampal-caudate functional connectivity and episodic memory. Proc Natl Acad Sci USA 2016; 113:7918–7923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Heaton RK, Clifford DB, Franklin DR, Woods SP, Ake C, Vaida F, et al. HIV-associated neurocognitive disorders persist in the era of potent antiretroviral therapy. Neurology 2010; 75:2087–2096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Blackstone K, Moore DJ, Franklin DR, Clifford DB, Collier AC, Marra CM, et al. Defining neurocognitive impairment in HIV: deficit scores versus clinical ratings. Clin Neuropsychol 2012; 26:894–908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Antinori A, Arendt G, Becker JT, Brew BJ, Byrd DA, Cherner M, et al. Updated research nosology for HIV-associated neurocognitive disorders. Neurology 2007; 69:1789–1799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Whitfield-Gabrieli S, Nieto-Castanon A. Conn: a functional connectivity toolbox for correlated and anticorrelated brain networks. Brain connectivity 2012; 2:125–41. [DOI] [PubMed] [Google Scholar]
- 29.Smith SM, Nichols TE. Threshold-free cluster enhancement: Addressing problems of smoothing, threshold dependence and localisation in cluster inference. NeuroImage 2009; 44:83–98. [DOI] [PubMed] [Google Scholar]
- 30.Li W, Antuono PG, Xie C, Chen G, Jones JL, Ward BD, et al. Aberrant functional connectivity in Papez circuit correlates with memory performance in cognitively intact middle-aged APOE4 carriers. Cortex 2014; 57:167–176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Small BJ, Rosnick CB, Fratiglioni L, Bäckman L. Apolipoprotein E and cognitive performance: a meta-analysis. Psychol Aging 2004; 19:592–600. [DOI] [PubMed] [Google Scholar]
- 32.Morales D, Acevedo SF, Skolasky RL, Hechavarria R, Santiago S, De La Torre T, et al. Translational spatial task and its relationship to HIV-associated neurocognitive disorders and apolipoprotein E in HIV-seropositive women. J Neurovirol 2012; 18:488–502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chang L, Jiang C, Cunningham E, Buchthal S, Douet V, Andres M, et al. Effects of APOE ε4, age, and HIV on glial metabolites and cognitive deficits. Neurology 2014; 82:2213–2222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hoare J, Westgarth-Taylor J, Fouche J-P, Combrinck M, Spottiswoode B, Stein DJ, et al. Relationship between apolipoprotein E4 genotype and white matter integrity in HIV-positive young adults in South Africa. Eur Arch Psychiatry Clin Neurosci 2013; 263:189–195. [DOI] [PubMed] [Google Scholar]
- 35.Wendelken LA, Jahanshad N, Rosen HJ, Busovaca E, Allen I, Coppola G, et al. ApoE ε4 Is Associated With Cognition, Brain Integrity, and Atrophy in HIV Over Age 60. J Acquir Immune Defic Syndr 2016; 73:426–432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tentolouris-Piperas V, Ryan NS, Thomas DL, Kinnunen KM. Brain imaging evidence of early involvement of subcortical regions in familial and sporadic Alzheimer’s disease. Brain Res 2017; 1655:23–32. [DOI] [PubMed] [Google Scholar]
- 37.Cohen AD, McDade E, Christian B, Price J, Mathis C, Klunk W, et al. Early striatal amyloid deposition distinguishes Down syndrome and autosomal dominant Alzheimer’s disease from late-onset amyloid deposition. Alzheimers Dement 2018; 14:743–750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ellis RJ, Badiee J, Vaida F, Letendre S, Heaton RK, Clifford D, et al. CD4 nadir is a predictor of HIV neurocognitive impairment in the era of combination antiretroviral therapy. AIDS 2011; 25:1747–1751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Robertson KR, Smurzynski M, Parsons TD, Wu K, Bosch RJ, Wu J, et al. The prevalence and incidence of neurocognitive impairment in the HAART era. AIDS 2007; 21:1915–1921. [DOI] [PubMed] [Google Scholar]
- 40.Valcour V, Yee P, Williams AE, Shiramizu B, Watters M, Selnes O, et al. Lowest ever CD4 lymphocyte count (CD4 nadir) as a predictor of current cognitive and neurological status in human immunodeficiency virus type 1 infection--The Hawaii Aging with HIV Cohort. J Neurovirol 2006; 12:387–391. [DOI] [PubMed] [Google Scholar]
- 41.Rebeck GW. The role of APOE on lipid homeostasis and inflammation in normal brains. J Lipid Res 2017; 58:1493–1499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Post WS, Budoff M, Kingsley L, Palella FJ, Witt MD, Li X, et al. Associations between HIV infection and subclinical coronary atherosclerosis. Ann Intern Med 2014; 160:458–467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Tang MX, Stern Y, Marder K, Bell K, Gurland B, Lantigua R, et al. The APOE-epsilon4 allele and the risk of Alzheimer disease among African Americans, whites, and Hispanics. JAMA 1998; 279:751–755. [DOI] [PubMed] [Google Scholar]
- 44.Farrer LA, Cupples LA, Haines JL, Hyman B, Kukull WA, Mayeux R, et al. Effects of age, sex, and ethnicity on the association between apolipoprotein E genotype and Alzheimer disease. A meta-analysis. APOE and Alzheimer Disease Meta Analysis Consortium. JAMA 1997; 278:1349–56. [PubMed] [Google Scholar]
- 45.Logue MW, Schu M, Vardarajan BN, Buros J, Green RC, Go RCP, et al. A comprehensive genetic association study of Alzheimer disease in African Americans. Arch Neurol 2011; 68:1569–1579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Neu SC, Pa J, Kukull W, Beekly D, Kuzma A, Gangadharan P, et al. Apolipoprotein E Genotype and Sex Risk Factors for Alzheimer Disease: A Meta-analysis. JAMA Neurol 2017; 74:1178–1189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Buisker TR, Dufour M-SK, Myers JJ. Recall of Nadir CD4 Cell Count and Most Recent HIV Viral Load Among HIV-Infected, Socially Marginalized Adults. AIDS Behav 2015; 19:2108–2116. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.