SUMMARY
We recently introduced CUT&Tag, an epigenomic profiling strategy in which antibodies are bound to chromatin proteins in situ in permeabilized nuclei, and then used to tether the cut-and-paste transposase Tn5. Activation of the transposase simultaneously cleaves DNA and adds adapters (“tagmentation”) for paired-end DNA sequencing. Here, we introduce a streamlined CUT&Tag protocol that suppresses DNA accessibility artifacts to ensure high-fidelity mapping of the antibody-targeted protein and improves signal-to-noise over current chromatin profiling methods. Streamlined CUT&Tag can be performed in a single PCR tube from cells to amplified libraries, providing low-cost genome-wide chromatin maps. By simplifying library preparation, CUT&Tag requires less than a day at the bench from live cells to sequencing-ready barcoded libraries. Because of low background levels, barcoded and pooled CUT&Tag libraries can be sequenced for as little as $25 per sample, enabling routine genome-wide profiling of chromatin proteins and modifications that requires no special skills or equipment.
Keywords: transcription factors, histone modifications, epigenomic profiling, DNA sequencing
INTRODUCTION
DEVELOPMENT OF THE PROTOCOL
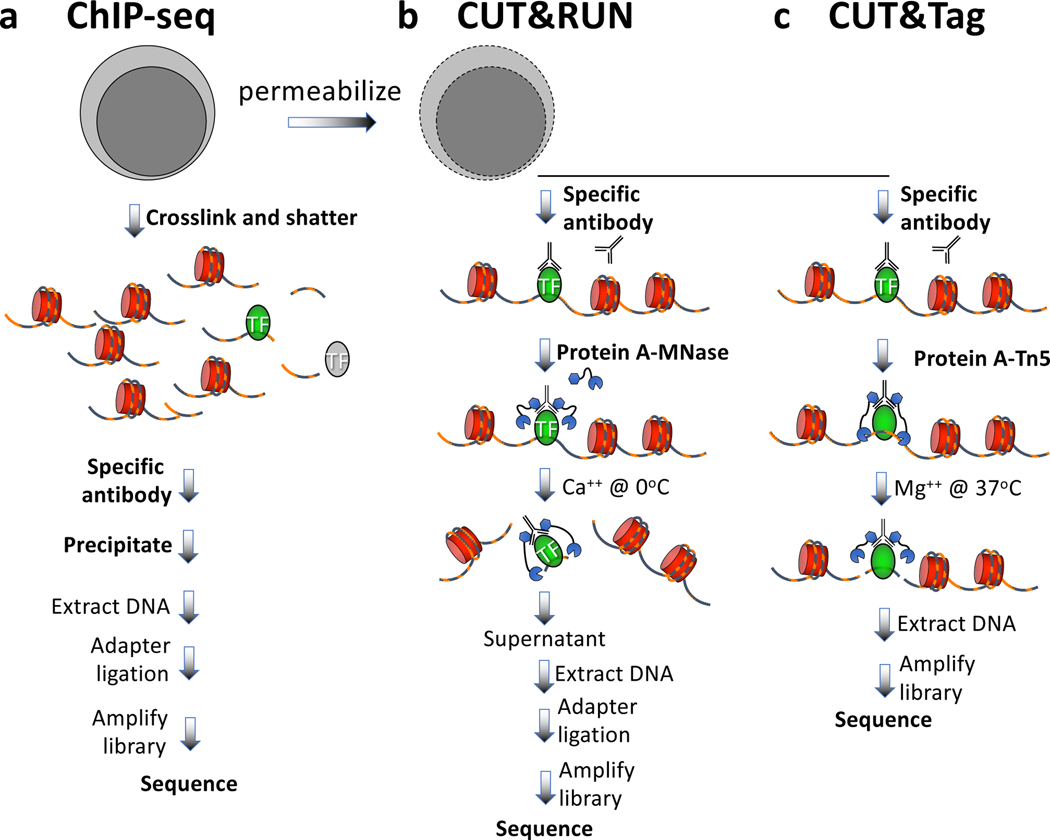
All dynamic processes that take place on DNA in the nucleus occur in the context of a chromatin landscape that comprises nucleosomes and their modifications, transcription factors and chromatin-associated complexes. A variety of chromatin features mark sites of transcriptional regulatory elements and regions of activation and silencing that differ between cell types and change during development and disease progression. The mapping of chromatin features genome-wide has traditionally been performed using chromatin immunoprecipitation (ChIP), in which chromatin is cross-linked and solubilized and an antibody to a protein or modification of interest is used to immunoprecipitate the bound DNA1 (Fig. 1a). This basic protocol has not changed since ChIP was first described 35 years ago2. Rather, the enormous progress since then using ChIP is attributable to improvements in readout technologies, progressing from southern blotting to quantitative PCR, to microarrays, and over the past decade to high-throughput sequencing (ChIP-seq). However, ChIP-seq remains challenging with small samples and is fraught with signal-to-noise issues and artifacts, although recent modifications of the basic strategy have greatly increased resolution3–6 and efficiency7–9.
Figure 1 |. Steps in antibody-targeted chromatin profiling strategies.
a) ChIP-seq, b) CUT&RUN; c) CUT&Tag. Cells are indicated in grey, chromatin as red nucleosomes, and a specific chromatin protein in green. See text for details of each procedure.
An alternative chromatin profiling strategy that is becoming increasingly popular is enzyme tethering in situ whereby the chromatin protein or modification of interest is targeted by an antibody or fusion protein, and the underlying DNA is marked or released. A succession of enzyme-tethering methods have been introduced over the past two decades, including DamID10, ChEC (Chromatin Endogenous Cleavage)11 and ChIC (Chromatin ImmunoCleavage)11. In DamID, expression of a fusion between a chromatin protein of interest and E. coli Dam methyltransferase results in targeted DNA methylation of GATC motifs near sites of binding, and a GATC-specific restriction enzyme is used to cleave fragments for mapping. In ChEC and ChIC Micrococcal Nuclease (MNase) is tethered to a target protein either directly as a fusion protein (ChEC) or indirectly to an antibody via a protein A-MNase fusion protein (ChIC). Addition of calcium ions activates MNase to cleave and release the targeted DNA fragments for DNA sequencing. Both strategies have been adapted for a sequencing readout (ChEC-seq and CUT&RUN/ChIC-seq)12,13 (Fig. 1b), and CUT&RUN has been fully automated for high-throughput application14. The improved signal-to-noise of CUT&RUN relative to ChIP-seq translates to an order-of-magnitude reduction in the amount of sequencing required to map chromatin features. Unlike ChIP, which requires cross-linking, CUT&RUN is performed on intact unfixed cells or nuclei, and so is free of epitope masking and other artifacts attributable to the harsh conditions required for ChIP. Importantly, the high efficiency of CUT&RUN makes it suitable for much lower cell numbers than is practical with ChIP-seq15. These and other advantages of CUT&RUN have resulted in a surge in popularity of the method since its introduction in 2017, replacing ChIP-seq for many chromatin profiling applications16–19, including for single-cell analysis17. During this time, novel computational tools have been introduced to take advantage of the near base-pair resolution and low background levels of CUT&RUN, including CUT&RUNTools20, SEACR21 and EChO22.
A very recent development in enzyme-tethering chromatin profiling technologies has been the substitution of the Tn5 transposase for MNase in CUT&RUN, what we refer to as Cleavage Under Targets & Tagmentation (CUT&Tag)23 (Fig. 1c). The protein A-Tn5 (pA-Tn5) transposome, when loaded with mosaic end adapters and activated by magnesium ions, integrates the adapters into nearby DNA to create fragments that are amplifiable to generate sequencing libraries. Antibody-tethered Tn5-based methods, not only CUT&Tag23, but also ChIL-seq24, ACT-seq25 and CoBATCH26, achieve high sensitivity owing to the high efficiency of tethered Tn5 integration. Although these methods are based on the same principle and all have been used for single-cell profiling, there are important differences in the protocols that can result in different outcomes. Like CUT&Tag, ACT-seq and CoBATCH use a Protein A-Tn5 fusion protein, whereas ChIL-seq uses a secondary antibody conjugated to a double-stranded template for Tn5 transposome binding and linear amplification by T7 RNA polymerase. As Tn5 remains bound following the cut-and-paste tagmentation reaction, fragments are retained within the cell, making these methods suitable for single-cell profiling. Indeed, these methods were introduced with proof-of-concept single-cell profiling data, suggesting that this basic strategy represents an important future direction for single-cell chromatin profiling in studies of development and disease.
CUT&Tag builds on the CUT&RUN protocol, in which cells are permeabilized, incubated with primary antibody, then a secondary antibody and pA-Tn5 are successively tethered to antibody-bound sites. Stringent washing with 300 mM NaCl is critical to limit the affinity of Tn5 for exposed DNA. We describe here the need for controlling background Tn5 affinity for accessible DNA and describe how our CUT&Tag protocol effectively suppresses this artifact for unambiguous mapping of chromatin epitopes. We present a protocol that can process either native or fixed nuclei, and includes alternative methods for DNA isolation. To illustrate the method, we describe a typical experiment, including evaluation of the results. Further, we validate a single-tube format for CUT&Tag that requires no DNA isolation but instead uses tagmented material directly for library amplification. We document critical steps for the CUT&Tag protocol, informed by our experiences helping users establish this method in their research.
Applications of the method
In this protocol we describe CUT&Tag for bench-top application, suitable for profiling a dozen or so samples in a day. The major practical advantage of CUT&Tag over other methods is that it eliminates the time and expense of preparing sequencing libraries, but CUT&Tag also has other important attributes that makes it the protocol of choice for most chromatin profiling applications. CUT&Tag has improved signal-to-noise for histone marks, at least in part because an antibody-tethered Tn5 integrates its mosaic-end adapters and remains bound during the incubation. We also find that CUT&Tag is more efficient than traditional chromatin profiling methods, likely because integration by targeted Tn5 is more efficient than enzymatic end-polishing and ligation in traditional library preparation steps. The higher efficiency of tagmentation and the retention of targeted Tn5-bound particles makes CUT&Tag preferable for single-cell profiling, and we have previously described this application using the Takara ICELL8 robotic nanowell chip system23.
Although we are excited by the prospects for routine single-cell CUT&Tag chromatin profiling, all current single-cell profiling methods produce sparse data, and this translates into orders-of-magnitude lower information content per dollar spent than the simple bench-top protocol described here. CUT&Tag profiling identifies on the order of 10,000 high-quality chromatin features such as promoter marks for as little as $25 per sample, which places the method in the realm of clinical sample testing. We previously described a fully automated CUT&RUN protocol14, which is currently in operation as a core facility at the Fred Hutch serving researchers and clinicians, and adaptation of this CUT&Tag protocol for automation is an attractive prospect.
Comparison with other methods
Compared to ChIP-seq, both CUT&RUN15 and CUT&Tag23 require less input material and fewer reads to map features, have a higher signal-to-noise ratio, have little or no fragmentation bias, and are amenable to calibration to equalize samples in a series for comparison. By eliminating library preparation, CUT&Tag, like ATAC-seq, requires less time and effort than the other methods to produce libraries that are ready for sequencing. Although ATAC-seq can be performed in just a few hours, CUT&Tag requires additional incubation and wash steps that require a day. However, because fewer reads are required for peak detection, sequencing of pooled CUT&Tag active chromatin libraries is less expensive than performing ChIP-seq or ATAC-seq on the same samples, which becomes especially critical in low-cell-number applications.
Experimental Design
Overview.
The CUT&Tag method for in situ tagmentation of chromatin complexes can be performed on the bench-top and completed in a day using standard lab equipment. Our detailed protocol applies to any chromatin feature for which an antibody is available and should be adaptable to any cell line, primary cell or tissue for which there is a standard isolation protocol. In brief, native or cross-linked nuclei are prepared and immobilized on magnetic beads (Steps 1–16). Beads are incubated with a primary antibody followed by incubation with a secondary antibody to increase the number of IgG molecules at each epitope bound by the primary antibody (Steps 17–28). Beads are washed and incubated with protein A-Tn5 loaded with mosaic-end adapters (Steps 29–34) and washed under stringent conditions. Tn5 is activated by addition of Mg2+, whereupon integration of adapters effectively inactivates the pA-Tn5 transposome, so that tagmentation will reach completion during the incubation period. Therefore, timing is not as critical for pA-Tn5 as for pA-MNase used in CUT&RUN, in which the nuclease continues to cleave DNA in the presence of Ca2+. A small amount of residual E. coli DNA that is carried over from pA-Tn5 purification becomes tagmented and serves as a calibration standard in lieu of a spike-in for comparing samples in an experimental series23,27. DNA is extracted with phenol-chloroform (Step 35A) or released in a small volume of SDS and then mixed with Triton-X100 to neutralize the SDS (Step 35B). Samples are enriched by PCR amplification (Steps 36–37) and a single Solid Phase Reversible Immobilization (SPRI) magnetic bead cleanup step (Steps 38–45). Up to 96 barcoded libraries from multiple experiments may be pooled for a 2-lane flow cell, as 3 million mapped paired-end reads are usually sufficient for a genome-wide profile of a histone modification in human cells.
Controls.
For CUT&Tag we recommend using a positive control antibody that targets an abundant epitope and therefore the library DNA can be easily detected following amplification. For histone modifications, a nucleosomal ladder is expected by TapeStation or other capillary electrophoretic analysis method. Once the expected library DNA pattern is observed by capillary electrophoresis for a positive control such as the H3K27me3 histone modification, it is not necessary to sequence this sample. As a negative control, we recommend simply omitting the primary antibody so that the secondary antibody will randomly coat the chromatin at low efficiency without sequence bias. Although the negative control is useful for ascertaining the success of an experiment by capillary gel analysis, sequencing it is optional because for large genomes the reads are too sparsely distributed to be useful for data analysis. An exception is peak-calling such as using SEACR (https://seacr.fredhutch.org), where comparing each experimental sample versus a negative control is the preferred option.
Limitations.
As is the case with ChIP-seq and CUT&RUN, CUT&Tag relies on the affinity of the primary antibody for its target and its specificity under the conditions used for binding. Although low background levels reduce read-depth requirements and thus lower costs, antibody-specific problems such as low affinity and epitope masking will require deeper sequencing to identify features. Unlike ChIP-seq, in which antibodies bind their epitopes in solution, CUT&RUN and CUT&Tag bind chromatin targets in situ. Therefore, we expect that antibodies successfully tested for specificity by immunofluorescence (IF) are likely to work.
Whereas CUT&RUN provides base-pair resolution that can be especially valuable for mapping transcription factors22, the pA-Tn5 complex is bulkier than MNase28 and steric effects evidently reduce resolution. Furthermore, the requirement for more stringent washes to avoid binding to and tagmentation of accessible DNA also reduces occupancy of transcription factors (TFs) in unfixed cells, making CUT&RUN preferable for both resolution and signal-to-noise for profiling many TFs. In addition, CUT&RUN has been used to distinguish direct DNA binding from nucleosome binding in characterizing pioneer transcription factors22, and for detecting 3D contact sites13, but these applications have not been demonstrated for CUT&Tag.
Because CUT&Tag is a new method, there are no bioinformatics tools expressly designed for it. However, widely used data processing tools, including bowtie229 for alignment, bedtools30 and Picard tools (https://github.com/broadinstitute/picard) for analysis, and Deeptools31 for heatmaps, are recommended for these tasks. Also, we expect that tools designed for CUT&RUN, including SEACR21 and CUT&RUNTools20 will work for CUT&Tag.
MATERIALS
Biological Materials
Cell suspension. We have used human K562 (RRID:CVCL_0004) and other mammalian cell lines, Drosophila S2 cells (RRID:CVCL_IZ08) and dissected Drosophila tissues such as brains and imaginal disks.
CAUTION:
The cell lines used in your research should be regularly checked to ensure they are authentic and are not infected with mycoplasma.
REAGENTS
Distilled, deionized or RNAse-free H2O (dH2O e.g., Promega, cat. no. P1197)
1 M Hydroxyethyl piperazineethanesulfonic acid pH 7.9 (HEPES (K+); Sigma-Aldrich, cat. no. H3375)
1 M Potassium Chloride (KCl; Sigma-Aldrich, cat. no. P3911)
10% (vol/vol) Triton X-100 (Sigma-Aldrich, cat. no. X100)
2 M Spermidine (Sigma-Aldrich, cat. no. S2501)
Glycerol (Sigma-Aldrich, cat. no. G5516)
Roche Complete Protease Inhibitor EDTA-Free tablets (Sigma-Aldrich, cat. no. 5056489001)
1 M Hydroxyethyl piperazineethanesulfonic acid pH 7.5 (HEPES (Na+); Sigma-Aldrich, cat. no. H3375)
0.5 M Ethylenediaminetetraacetic acid (EDTA; Research Organics, cat. no. 3002E)
5 M Sodium chloride (NaCl; Sigma-Aldrich, cat. no. S5150–1L)
30% Bovine Serum Albumin (BSA, Sigma-Aldrich, cal. no. A8577)
Dimethyl sulfoxide (DMSO; Sigma-Aldrich cat. no. D4540)
Concanavalin-coated magnetic beads (Bangs Laboratories, ca. no. BP531)
1 M Manganese Chloride (MnCl2; Sigma-Aldrich, cat. no. 203734)
1 M Calcium Chloride (CaCl2; Fisher, cat. no. BP510)
1 M Potassium Chloride (KCl; Sigma-Aldrich, cat. no. P3911)
Antibody to a chromatin epitope of interest, e.g., α-H3K4me3 rabbit polyclonal antibody (Active Motif cat. no. 39159, RRID:AB_2616029)
Positive control antibody to an abundant epitope, e.g. anti-H3K27me3 rabbit monoclonal antibody (Cell Signaling Technology, cat. no. 9733, RRID:AB_2615077)
Secondary antibody to increase the number of IgG molecules per targeted epitope, e.g. guinea pig anti-rabbit (Antibodies-Online ABIN101961, RRID:AB_10775589) or rabbit anti-mouse (Abcam ab46540, RRID:AB_2614925)
CRITICAL:
The immunoglobin-binding moiety of pA-Tn5 binds tightly only to certain IgG isotypes of some host species. Thus, the secondary amplifying antibody must be chosen to provide high affinity for protein-A.
Protein A–Tn5 (pA-Tn5) fusion protein (loaded enzyme provided in 50% (vol/vol) glycerol) aliquots are available from the authors at cutnrun@fredhutch.org. until commercially available.
CRITICAL:
Store at −20 oC. pA-Tn5 is loaded with oligonucleotide adapters with 19mer Tn5 mosaic ends. Adapters are generated by annealing Mosaic end_Adapter A (TCGTCGGCAGCGTCAGATGTGTATAAGAGACAG) and Mosaic end_Adapter B (GTCTCGTGGGCTCGGAGATGTGTATAAGAGACAG) with Mosaic end_reverse ([PHO]CTGTCTCTTATACACATCT)32.
3XFlag-pA-Tn5-Fl (pA-Tn5) plasmid (AddGene cat. no. 124601)
100 mM Magnesium Chloride (MgCl2; Sigma-Aldrich, cat. no. M8266–100G) 3002E)
10% Sodium dodecyl sulfate (SDS; Sigma-Aldrich, cat. no. L4509)
NEBNext High-Fidelity 2X PCR Master Mix (cat. no. M0541L)
Proteinase K (Thermo Fisher Scientific, cat. no. EO0492) (for Option A)
Phenol-chloroform-isoamyl alcohol 25:24:1 (PCI; Invitrogen, cat. no. 15593049) (for Option A)
CAUTION Phenol and chloroform are toxic, so they should be handled in a hood while wearing disposable gloves.
Chloroform (Sigma, cat. no. 366919–1L) (for Option A)
1 M TAPS (to pH 8.5 with NaOH) (for Option B)
0.33% Triton-X100 (Sigma-Aldrich, cat. no. X100) (for Option B)
Solid Phase Reversible Immobilization (SPRI) magnetic beads (e.g. Agencourt AMPure XP, Beckman Coulter, cat. no. A63880)
1 M Tris-HCl pH 8.0 (Fisher cat no. BP1521)
Ethanol (Decon Labs, cat. no. 2716)
PCR primers: 10 μM stock solutions of a universal i5 primer and 16 i7 primers with unique barcodes by Buenrostro et al33. Sequences are listed in Supplementary Table 1. We order oligonucleotides from IDT in salt-free format, Oligonucleotides are dissolved in water and stored as 100 mM and 10 mM stock solutions at 4 oC; higher purification formats are optional. Our PCR conditions are optimized for these custom primers.
CAUTION:
Do not use Nextera primers, which will not work efficiently.
Qubit dsDNA HS kit (Life Technologies, cat. no. Q32851)
EQUIPMENT
Centrifuge Eppendorf 5810, swing bucket
Centrifuge Eppendorf 5424, fixed angle rotor
Centrifuge Eppendorf 5415R, refrigerated fixed angle rotor
Macsimag magnetic separator (Miltenyi, cat. no. 130–092-168), which allows clean withdrawal of the liquid from the bottom of 0.5, 1.5 and 2 mL microfuge tubes.
Vortex mixer (e.g., VWR Vortex Genie)
Micro-centrifuge (e.g., VWR Model V)
Conical centrifuge tubes (15 mL or 50 mL)
Cryogenic screw-cap vials (Corning cat. no. 430658)
Mr. Frosty containers (Thermo cat. no. 5100–0001)
0.5 mL thin-wall PCR tubes (Axygen, Fisher 14–222-292)
1.5-mL microcentrifuge tubes (Genesee, cat. no. 22–282)
2-mL microcentrifuge tubes (Axygen, cat. no. MCT-200-C)
Tube rotator (Labquake, Thermo Fisher)
Heater block with wells for 1.5-mL microcentrifuge tubes
Water baths (set to 37 °C and 70 °C)
MaXtract phase-lock microcentrifuge tubes (Qiagen, cat. no. 139046)
Capillary electrophoresis instrument (e.g. Agilent Tapestation 4200)
Qubit Fluorometer (Life Technologies, cat. no. Q33216)
Software
Bowtie2 version 2.2.5 (http://bowtie-bio.sourceforge.net/bowtie2/index.shtmL)
Picard version 2.15 (https://broadinstitute.github.io/picard/index.htmL)
SEACR peak-caller is available as a public web server (https://seacr.fredhutch.org) or as scripts from https://github.com/FredHutch/SEACR/
REAGENT SETUP
NE1 buffer
Mix 1 mL 1M HEPES-KOH pH 7.9, 500 μL 1M KCl, 12.5 μL 2 M spermidine, 500 μL 10% Triton X-100, and 10 mL glycerol in 38 mL dH2O, and add 1 Roche Complete Protease Inhibitor EDTA-Free tablet. Chill on ice before use. Store the buffer at 4 °C for 6 months.
Wash buffer
Mix 1 mL 1 M HEPES pH 7.5, 1.5 mL 5 M NaCl, 12.5 μL 2 M spermidine, bring the final volume to 50 mL with dH2O, and add 1 Roche Complete Protease Inhibitor EDTA-Free tablet. Store the buffer at 4 °C for up to 1 month.
Binding buffer
Mix 200 μL 1M HEPES-KOH pH 7.5, 100 μL 1M KCl, 10 μL 1M CaCl2 and 10 μL 1M MnCl2, and bring the final volume to 10 mL with dH2O. Store the buffer at 4 °C for 6 months.
Antibody buffer
Mix 8 μL 0.5 M EDTA and 6.7 μL 30% BSA with 2 mL Wash buffer and chill on ice. Make fresh.
300-wash buffer
Mix 1 mL 1 M HEPES pH 7.5, 3 mL 5 M NaCl and 12.5 μL 2 M spermidine, bring the final volume to 50 mL with dH2O and add 1 Roche Complete Protease Inhibitor EDTA-Free tablet. Store at 4 °C for up to a month.
Tagmentation buffer
Mix 1 mL 300-wash buffer and 10 μL 1 M MgCl2 (to 10 mM). Make fresh.
Post-tagmentation wash buffer
Mix 10 μL 1 M TAPS pH 8.5 in 1 mL dH2O (to 10 mM). Make fresh.
SDS Release buffer
Mix 10 μL 10% SDS and 10 μL 1 M TAPS pH 8.5 in 1 mL dH2O. Make fresh.
Tn5-adapter complex formation:
Anneal each of Mosaic end - adapter A (ME-A) and Mosaic end - adapter B (ME-B) oligonucleotides with Mosaic end – reverse oligonucleotides25.
To anneal, dilute oligonucleotides to 200 μM in annealing buffer (10mM Tris pH8, 50mM NaCl, 1 mM EDTA). Each pair of oligos, ME-A+ME-Reverse and ME-B+ME-Reverse, is mixed separately resulting in 100 μM annealed product.
Place the tubes in a 90–95 °C hot block and leave for 3–5 minutes, then remove the hot block from the heat source allowing for slow cooling to room temperature (~45 minutes).
Mix 16 μL of 100 μM equimolar mixtures of preannealed ME-A and ME-B oligonucleotides with 100 μL of 5.5 μM protein A - Tn5 fusion protein.
- Incubate the mixture on a rotating platform for 1 hour at room temperature and then store at −20 °C for up to 1 year.
- CRITICAL STEP: pA-Tn5 aliquots received from the CUT&RUN team are pre-loaded with adapters suitable for single- or dual-indexing on a paired-end Illumina flow-cell platform.
PROCEDURE
CRITICAL STEP:
The following procedure is for 16 samples and can be scaled up or down by increasing volumes at all steps proportionally.
CRITICAL:
Step 35 Option A requires tube transfers for DNA purification prior to PCR, including handling toxic materials. Option B is performed in single PCR tubes and requires no toxic materials. We have obtained high quality data for low cell numbers using either option.
Prepare Concanavalin A beads
TIMING 15 min
1) Resuspend and withdraw enough of the ConA bead slurry such that there will be 10 μL (Option A) or 3 μL (Option B) for each final sample of up to ~500,000 mammalian cells (Option A) or up to ~100,000 cells (Option B).
2) Transfer 160 μL (Option A) or 55 μL (Option B) ConA bead slurry into 1.5 mL Binding buffer in a 2 mL tube, mix by pipetting, and place the tube on a magnet stand to clear (30 s to 2 min).
3) Withdraw the liquid completely, and remove from the magnet stand. Add 2 mL Binding buffer and mix by pipetting.
4) Place on magnet stand to clear, withdraw liquid, resuspend in 160 μL (Option A) or 55 μL (Option B) Binding buffer, and hold on ice until nuclei are ready (freshly cross-linked or thawed).
Prepare nuclei, optionally fix and cryopreserve
TIMING 1 hr
- 5) Transfer a fresh culture of up to 20 million cells to a conical centrifuge tube (15 mL or 50 mL) at room temperature and count cells. This protocol can be used for up to ~500,000 mammalian (e.g., human K562) cells per sample to be sequenced.
- CRITICAL STEP: The single-tube protocol (Option B) should only be used with nuclei, with an upper limit of ~100,000 starting cells per sample.
- CRITICAL STEP: Cross-linking is not required for CUT&Tag and can cause epitope masking. However for this protocol light cross-linking to fix nuclei may be beneficial, because it helps keep nuclei intact and reduces clumping throughout the procedure.
6) Centrifuge 3 min 600 x g in a swing-bucket rotor at room temperature and drain liquid.
7) Resuspend in 1 volume PBS at room temperature while pipetting.
8) Centrifuge 3 min 600 x g in a swing-bucket rotor at room temperature and drain liquid.
9) Resuspend in ½ volume (relative to starting culture) ice-cold NE1 with gentle vortexing. Let sit on ice 10 min.
10) Centrifuge 4 min 1300 x g at 4 oC in a swing-bucket rotor and drain liquid by pouring off, then inverting onto a paper towel for a few seconds.
11) Resuspend in 1/2 volume of PBS (relative to starting culture). For unfixed nuclei, skip to Step 14.
12) While gently vortexing add 16% formaldehyde to 0.1% (e.g., 62 μL to 10 mL) and incubate at room temperature for 2 min.
13) Stop cross-linking by addition of 2.5 M glycine to twice the molar concentration of formaldehyde (e.g., 300 μL to 10 mL).
14) Centrifuge 4 min 1300 x g at 4 oC and drain the liquid by pouring off, then inverting onto a paper towel for a few seconds.
15) Resuspend in Wash buffer to a concentration of ~1 million cells per mL. Count nuclei using ViCell, cell counter slide or equivalent.
16) OPTIONAL: Nuclei may be slow-frozen by aliquoting 900 μL into cryogenic vials containing 100 μL DMSO, mixed, then placed in a Mr. Frosty container filled to the line with isopropanol and placed in a −80 oC freezer overnight for storage at −80 oC indefinitely.
Bind nuclei to ConA beads
TIMING 15 min
17) Use fresh nuclei on ice or thaw frozen nuclei aliquot at room temperature, for example by placing in a 20 mL beaker of water.
18) Mix 50–200 μL of cell suspension with 10 μL (Option A) or 3 μL (Option B) beads (from Step 4) in thin-wall 0.5 mL PCR tubes and let sit at room temperature for 10 min.
- 19) Place the tubes on a magnet stand to clear and withdraw and discard the liquid.
- CRITICAL STEP: Surface tension will cause bead-bound cells to slide down to the bottom of the tube, so to avoid losses here and below, set the pipettor at 45 μL for a 50 μL volume.
- CRITICAL STEP: If loss of bead-bound cells is occurring during liquid withdrawal for single-tube CUT&Tag (Option B), then up to 5 μL of ConA beads may be used without causing PCR inhibition.
- CRITICAL STEP: If desired, the supernatants may be counted on a ViCell, cell counter slide or equivalent to check efficiency of nuclei binding to beads, which is usually >90%.
Bind primary antibody
TIMING 1 hr 15 min
- CRITICAL: To evaluate success of the procedure by capillary electrophoresis (e.g. TapeStation) analysis, include in parallel a positive control antibody (e.g. anti-H3K27me3) and a no-primary antibody negative control.
- 20) Resuspend cells in 50 μL Antibody buffer pre-mixed with antibody (1:100) with gentle vortexing.
CRITICAL STEP: For bulk processing of up to 16 samples, resuspend in Antibody buffer and antibody (1:100) with gentle vortexing. We use 1:100 by default or the manufacturer’s recommended concentration for immunofluorescence.
? TROUBLESHOOTING
- 21) Place on a Rotator at room temperature and incubate at least 1 hr or at 4 oC overnight.
- PAUSE POINT Antibody incubation may proceed on a Rotator overnight at 4 oC. We have not noticed any difference between the efficiency of a 1–2 hr room temperature incubation and an overnight 4 °C incubation.
Bind secondary antibody
TIMING 45 min
- CRITICAL: The secondary antibody step is required for CUT&Tag to increase the number of Protein A binding sites for each chromatin target. We have found that without the secondary antibody the efficiency of tagmentation is very low.
- 22) After a quick spin, place each tube on the magnet stand to clear and withdraw and discard the liquid.
- CRITICAL STEP: A quick spin on a micro-centrifuge (~100 x g) will minimize carry-over of reagents.
- 23) Mix secondary antibody 1:100 in Wash buffer and squirt in 50 μL per sample while gently vortexing to allow the solution to dislodge the beads from the sides.
- 24) Place the tubes on a Rotator at room temperature for 30 min.
- PAUSE POINT Secondary antibody incubation may proceed overnight at 4 oC.
- 25) After a quick spin, place the tubes on a magnet stand to clear and withdraw and discard the liquid.
- 26) With tubes still on the magnet stand, carefully add 500 μL Wash buffer. The surface tension will cause the beads to slide up along the side of the tube closest to the magnet.
- 27) Slowly withdraw the liquid with a 1 mL pipette tip and discard.
- CRITICAL STEP: To withdraw the wash liquid without losing beads, set the pipettor to 600 μL, and keep the plunger depressed while lowering the tip to the bottom. The liquid level will rise to near the top completing the wash. Then ease off on the plunger until all the liquid is withdrawn, and remove the pipettor. When using low-retention PCR tubes, a strong magnet stand such as the Macsimag is recommended to minimize loss of beads during this and other washing steps.
- 28) After a quick spin, place the tubes on a magnet stand to remove the last drop with a 20 μL pipettor.
Bind pA-Tn5 adapter complex
TIMING 1 hr 15 min
29) Mix pA-Tn5 adapter complex in 300-wash buffer to a final concentration of 1:200.
30) Squirt in 50 μL per sample of the pA-Tn5 mix while gently vortexing to allow the solution to dislodge most or all of the beads.
31) Place the tubes on a Rotator at room temperature for 1 hr.
32) After a quick spin, place the tubes on a magnet stand to clear and pull off the liquid, setting the pipettor to 45 μL to avoid losses.
33) With the tubes still on the magnet stand, carefully add 500 μL 300-wash buffer.
34) Remove the liquid, and after a quick spin, place the tubes on a magnet stand to remove the last drop with a 20 μL pipettor, and proceed immediately to the next step.
Tagmentation and DNA purification
35) To tagment and extract the DNA, follow Option A. For single-tube CUT&Tag, follow Option B. Both options provide similar results.
Tagmentation with DNA extraction
TIMING 3 hr
i) Add 200 μL 300-wash buffer. Invert 10x to allow the solution to dislodge most or all of the beads.
ii) After a quick spin, place the tubes on a magnet stand to clear and pull off the liquid.
iii) Add 300 μL Tagmentation buffer while gently vortexing.
- iv) Incubate at 37 ºC for 1 hr.
- CRITICAL STEP: It is typical for the beads to form a large clump during incubation owing to the viscoelasticity of DNA. However, for abundant chromatin epitopes, extensive fragmentation of the genome will normally result in reduced clumping and release of beads into suspension, turning the liquid brownish relative to samples profiling less abundant epitopes and to negative controls.
v) Add 10 μL 0.5M EDTA, 3 μL 10% SDS and 2.5 μL 20 mg/mL Proteinase K to each sample. Mix by inversion and incubate 10 min at 70 oC.
vi) Add 300 μL PCI and mix by full-speed vortexing ~2 s.
CAUTION: Phenol and chloroform are toxic, so they should be handled wearing disposable gloves in a hood.
vii) Transfer the solution to a phase-lock tube, and centrifuge for 5 min at room temperature at 16,000 x g.
viii) Add 300 μL chloroform, invert ~10x to mix, and centrifuge for 5 min at room temperature at 16,000 x g.
ix) Remove the aqueous phase by pipetting to a fresh 1.5 mL Eppendorf tube containing 1 mL 100% ethanol and mix by vortexing or tube inversion.
x) Chill on ice for 5 min and centrifuge for at least 10 min at 4 °C at 16,000 x g.
xi) Pour off the liquid and drain on a paper towel.
xii) Rinse the pellet by adding 1 mL 100% ethanol, invert ~10x to mix, and centrifuge 1 min at 4 °C at 16,000 x g.
xiii) Carefully pour off the liquid and drain on a paper towel. Air dry until the ethanol evaporates (~5 min).
xiv) When the tube is dry, dissolve the pellet in 21 μL 1 mM Tris-HCl pH8 0.1 mM EDTA.
- xv) Vortex hard and after a quick spin transfer the solution to a 0.5 mL thin-wall PCR tube.
- CRITICAL STEP: Do not add glycogen, which will inhibit the PCR.
- CRITICAL STEP: Do not perform TapeStation analysis at this stage as the tagmented DNA is not yet released.
Option B: Single-tube tagmentation and pA-Tn5 release
TIMING 2 hr 30 min
i) Add 50 μL 300-wash buffer to resuspend the bead/nuclei pellet by inversions or vortexing.
ii) After a quick spin, place the tubes on a magnet stand to clear and pull off the liquid, setting the pipettor to 45 μL to avoid losses.
iii) After a second quick spin, place the tubes on a magnet stand to remove the last drop with a 20 μL pipette tip.
iv) Resuspend the bead/nuclei pellet in 50 μL tagmentation buffer with gentle vortexing.
v) Incubate at 37 ºC for 1 hr in a PCR cycler with heated lid.
vi) Place the tubes on a magnet stand and remove the liquid.
vii) After a quick spin replace the tubes on a magnet stand and remove any remaining liquid using a 20 μL pipette tip.
viii) Resuspend the beads in 50 μL 10 mM TAPS buffer and mix.
ix) After a quick spin place the tubes on a magnet stand and remove the liquid.
x) After a second quick spin replace the tubes on a magnet stand and remove any remaining liquid using a 20 μL pipette tip.
- xi) Resuspend the beads in 5 μL (0.1%) SDS Release buffer.
- CRITICAL STEP: Use a 20 μL pipette tip to dispense while wetting the sides of the tubes to recover the fraction of beads sticking to the sides (which can be large for unfixed nuclei). During and after dispensing the liquid “twirl” the tube between thumb and forefinger with the tip rubbing along the side. Do not withdraw liquid with the pipettor.
xii) After a quick spin, incubate at 58 ºC for 1 hr in a PCR cycler with heated lid to release the tagmented particles into solution.
- xiii) Add 15 μL 0.67% Triton-X and briefly vortex on full speed.
- CRITICAL STEP: SDS will inhibit PCR even after dilution down to 0.01%, but addition of excess non-ionic detergent such as Triton-X100 neutralizes the SDS.
PCR Amplification
TIMING 40 min
36) Add 2 μL of 10 μM Universal or barcoded i5 primer + 2 μL of 10 μM uniquely barcoded i7 primers, using a different barcode for each sample. Indexed primers designed by Buenrostro et al.33 are listed in Supplementary Table 1.
37) Move tubes to an aluminum block on ice. Add 25 μL NEBNext HiFi 2x PCR Master mix and perform PCR with the conditions below:
| Cycle number | Denature | Anneal | Extend | Final |
|---|---|---|---|---|
| 1 | 58 °C, 5 min | |||
| 72 °C, 5 min | ||||
| 2 | 98 °C, 45 s | |||
| 3–14 | 98 °C, 15 s | 63° C, 10 s | ||
| 15 | 72 °C, 1 min | |||
| 16 | 8 °C, hold |
CRITICAL STEP: To minimize the contribution of large DNA fragments, PCR cycles should be 12 cycles, with a 10 s 60–63 oC combined annealing/extension step.
CRITICAL STEP: Tagmentation leaves a gap between each 3’ end of the insert and the reverse mosaic end oligonucleotide. Step 1 of the PCR is required to displace the oligonucleotide while extending the 3’ ends, producing blunt-ended adapter insert fragments. Hot-start polymerases are not active during this critical extension step and should not be used. We combined annealing/extension to minimize the amplification of large fragments.
CRITICAL STEP: The cycle times are based on using conventional Peltier cycler (e.g., BioRad/MJ PTC-200), and the 3 °C/sec ramp rate of this machine allows sufficient time for annealing as the sample cools from 98 °C to 60 °C. Use of a rapid cycler with faster ramp rates will require adjustment to assure extension.
Post-PCR Clean-up
TIMING 30 min
38) Remove tubes from the cycler and add 1.3 volume (65 μL) Ampure XP beads, mixing by pipetting up and down ~10x.
39) Quick spin and let sit at room temperature 5–10 min.
40) Place on magnet and allow to clear before carefully withdrawing liquid. While still on the magnet add 200 μL 80% ethanol.
41) While still on the magnet, withdraw liquid and add 200 μL 80% ethanol.
- 42) Withdraw the liquid, and after a quick spin remove the remaining liquid with a 20 μL pipette.
- CRITICAL STEP: Overdrying can reduce recovery of DNA, so continue to the elution step within 5 min.
43) Remove from the magnet stand, add 22 μL 10 mM Tris-HCl pH 8 and vortex on full speed.
44) After 5 min place on magnet stand and allow to clear.
45) Remove liquid to a fresh 0.5 mL tube with a pipette.
Library pooling and DNA sequencing
TIMING: 1 d
46) Determine the size distribution of libraries by capillary electrophoresis (e.g. Agilent 4200 TapeStation), using 2 μL following the manufacturer’s instructions. For mixing single-tube samples with load buffer use a low-volume (e.g. 2 μL) pipettor to avoid injecting bubbles.
? TROUBLESHOOTING
47) OPTIONAL: Quantify the library yield for a dsDNA-specific assay, such as Qubit following the manufacturer’s instructions. CRITICAL STEP: Molarity estimates are based on the capillary electrophoresis profile by choosing the region between 160 bp and 1000 bp and using the value reported as “Region Molarity [pmol/L]”.
CRITICAL STEP:
Because of the very low background with CUT&Tag, typically 3 million mapped paired-end reads per sample suffices, even for the human genome. For maximum economy, we mix up to 96 barcoded samples per lane on a 2-lane flow cell, and perform paired-end 25×25 bp sequencing, which is sufficient for high-confidence mapping with high-affinity histone modification antibodies. Single-end sequencing is not recommended for CUT&Tag, as it sacrifices resolution and fragment size information.
48) Pool libraries with compatible barcodes in equimolar amounts to achieve similar read counts.
OPTIONAL: If necessary reduce the volume by Speedvac to obtain the molar concentration and volume required by the sequencing facility (e.g. 2 nM)
OPTIONAL: If there is not enough of a very low-yield samples to achieve an equimolar amount in a pool, use the whole sample.
49) Repeat Steps 38–45.
50) Recheck libraries by capillary electrophoresis to make sure that primers have been sufficiently depleted and to obtain a final concentration estimate.
CRITICAL STEP: This also provides a length distribution for the pool, which is used to estimate the molar concentration based on a Qubit measurement. The two molar concentration estimates should be similar.
51) Perform paired-end Illumina sequencing on the barcoded libraries using an Illumina HiSeq 2500 or other massively parallel DNA sequencer following the manufacturer’s instructions.
Data processing and analysis
TIMING 1 d (variable)
52) Align paired-end reads using Bowtie2 version 2.2.5 (http://bowtie-bio.sourceforge.net/bowtie2/index.shtmL) with options: --end-to-end --very-sensitive --no-unal --no-mixed --no-discordant --phred33 -I 10 -X 700. For mapping E. coli carry-over fragments for calibration27, we also use the --no-overlap --no-dovetail options to avoid cross-mapping of the experimental genome to that of the carry-over DNA.
? TROUBLESHOOTING
53) A Unix-compatible script for processing CUT&RUN and Tag spike-in and E. coli carry-over data is available from GitHub (https://github.com/Henikoff/Cut-and-Run). We use the Picard version 2.15 “MarkDuplicates” command (https://broadinstitute.github.io/picard/index.htmL) to mark presumed PCR duplicates and to estimate library size.
? TROUBLESHOOTING
54) Optional: Call peaks using SEACR21, MACS234 or other peak-calling program. The Sparse Enrichment Analysis for CUT&RUN (SEACR) peak-caller was designed for low-read-count, low-background paired-end sequencing data of the type obtained using CUT&RUN and CUT&Tag and is available as a public web server: https://seacr.fredhutch.org or as scripts from https://github.com/FredHutch/SEACR/. Calculate the fraction of reads in peaks (FRiPs)35 to evaluate signal-to-noise.
? TROUBLESHOOTING
TIMING
Day 1 Cells to library
Steps 1–4, Prepare Concanavalin A beads: 15 min
Steps 5–16, Prepare nuclei, optionally fix and cryopreserve: 1 hr
Steps 17–19 Bind nuclei to ConA beads: 15 min
Steps 20–21, bind primary antibody: 1 hr 15 min
Steps 22–28, bind secondary antibody: 45 min
Steps 29–34, bind pA-Tn5 adapter complex: 1 hr 15 min
Step 35A, Tagmentation with DNA extraction: 3 hr
Step 35B, Tagmentation and particle release: 2 hr 30 min
Step 36–37 PCR amplification: 40 min
Steps 38–45, Post-PCR clean-up: 30 min
Day 2 Library pooling and DNA sequencing
Steps 46–51, library preparation and sequencing: 2 days
Day 3 (variable) Data processing and analysis
Steps 52–54, ≥1 day
TROUBLESHOOTING
Troubleshooting advice can be found in Table 1.
Table 1:
Troubleshooting table.
| Steps | Problem | Possible reasons | Solutions |
|---|---|---|---|
| 20 | Beads clump and cannot be disaggregated | Cells lyse | Small clumps (2–5 cells) do not cause problems; but for large clumps, reduce the number of cells or treat nuclei more gently. |
| 20 | Beads stick to the walls or cap of the tube and cannot be resuspended | Beads get out of suspension during incubation and dry | Use low-bind tubes. Make sure that beads are immersed in the buffer during incubations. |
| 46 | No insert for positive control is detected by capillary electrophoresis | May indicate PCR failure | Make sure that sample DNA is free from PCR inhibitors. Make sure that PCR reaction works using a positive control. |
| 46 | Little or no yield of insert | Expected for low cell number samples or rare epitopes, but may indicate antibody failure | Replace antibody. Antibody binding may be tested by immunofluorescence. |
| 46 | A smear covering a large region, but no nucleosomal ladder is detected by capillary electrophoresis | E. coli DNA tagmentation due to high concentration of pA-Tn5 or very low cell number or rare epitope | Decrease pA-Tn5 concentration. If possible start with more material. Replace antibody. |
| 52 | The majority of reads map to E. coli genome | E. coli reads are expected to increase with decreasing cell numbers or rare epitopes, but may indicate antibody failure | Replace antibody. Antibody binding may be tested by immunofluorescence. |
| 53 | >50% Duplicates | Too few cells or rare epitope | Increase cell number |
| 54 | Signals are weak and FRiPs are low | High background caused by a weak antibody or unstable binding of target protein | Sequence more deeply |
ANTICIPATED RESULTS
High CUT&Tag robustness with low DNA sequencing requirements
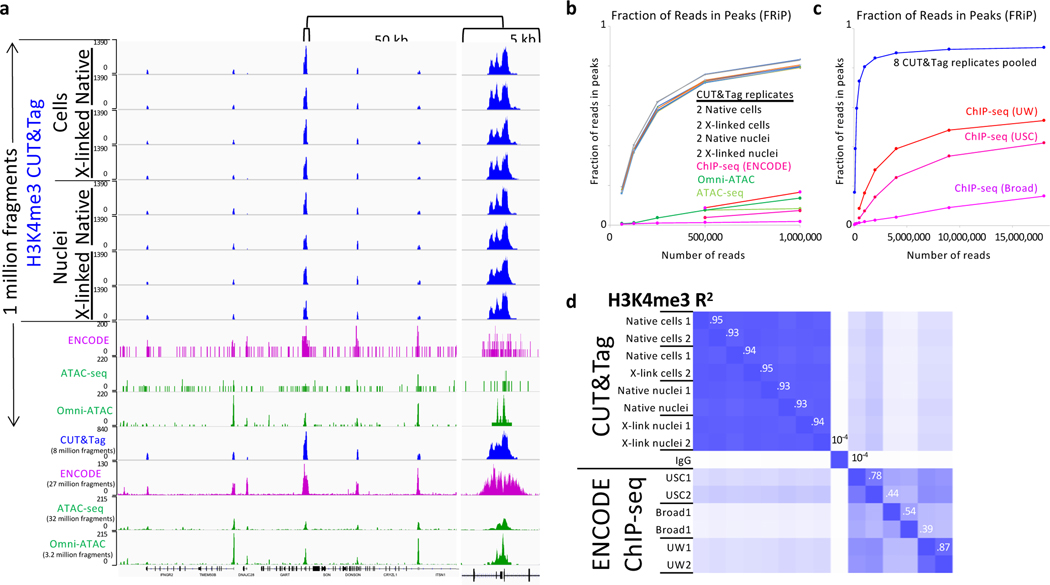
Improvements in signal-to-noise can lower the cost of an experiment both by reducing the amount of sequencing needed to identify features and by reducing the size of files that must be transferred, analyzed and stored. For ChIP-seq of histone modifications such as H3K4me3, H3K36me3 and H3K27me3, 40–50 million is the recommended minimum number of reads for human cells36. We previously showed that CUT&Tag for permeabilized cells improves signal-to-noise and thus reduces sequencing costs relative to ChIP-seq by an order of magnitude23. To confirm that our protocol for nuclei also provides improved signal-to-noise relative to popular chromatin profiling methods, we performed CUT&Tag using both our original protocol for cells23 and our current protocol for nuclei with an antibody to histone H3K4me3, a mark of active promoters. Human K562 cells were washed in phosphate-buffered saline (PBS) and half of the culture was used to prepare nuclei. The cells and nuclei were each split such that half of each suspension was lightly fixed (0.1% formaldehyde 2 minutes at room temperature)37. H3K4me3 CUT&Tag was performed on cells permeabilized by digitonin followed by extraction using our previous protocol23 (Option A), then barcoded during PCR amplification. The same experiment was also done using 0.2% formaldehyde. All 8 samples were pooled with 70 other barcoded samples and subjected to paired-end 25×25 sequencing on a single flow cell. Examination of a representative 300-kb region shows clean promoter peaks essentially identical for all 8 samples when only 1 million fragments were mapped (Fig. 2a). This demonstrates that CUT&Tag provides highly reproducible data for native or lightly fixed cells or nuclei.
Figure 2 |. CUT&Tag provides high signal-to-noise and reproducibility for native and lightly cross-linked cells and nuclei.
H3K4me3 CUT&Tag was performed on native and cross-linked cells and nuclei from the same batch on two different occasions (biological replicates 1 and 2 using the DNA extraction option for nuclei). Barcoded libraries were mixed with 70 other barcoded libraries and sequenced, yielding at least 1 million fragments. a) H3K4me3 CUT&Tag (blue), ENCODE H3K4me3 ChIP-seq (magenta GSM733680), ATAC-seq (GSM2695560) and Omni-ATAC (SRX2894091–2) tracks (green) for K562 cells. b) MACS2 was used to call peak counts for datasets shown in (a) and additional datasets from ENCODE (H3K4me3) and GEO (Omni-ATAC) to calculate the Fraction of Reads in Peaks (FRiP). The multicolored curves without marker dots represent the 8 CUT&Tag replicates. c) The 8 CUT&Tag datasets were pooled for FRiP comparisons to ChIP-seq for up to 18 million fragments. d) A correlation matrix of MACS2 narrow peak calls for biological replicates representing the 8 CUT&Tag datasets and pooled IgG datasets and the 6 ENCODE datasets from three laboratories. Peaks were called using MACS2 on the pooled CUT&Tag H3K4me3 resulting in 12,224 peaks, from which 223 were removed because of high IgG density. Pairwise correlations of mean normalized counts were calculated over a span of ±150 bp around each summit. Numbers along the diagonal are R2 values between successive rows; for example between IgG and USC1 R2 = 10−4, between USC1 and USC2 R2 = 0.74, and between USC2 and Broad1 R2 = 0.44.
For comparison, we sampled 1 million fragments from 6 ENCODE H3K4me3 ChIP-seq datasets from 3 different laboratories and ATAC-seq and Omni-ATAC datasets obtained from GEO, which revealed weak barely detectable peaks over a sparse background for ChIP-seq and ATAC-seq. When ~30 million ChIP-seq and ATAC-seq fragments were mapped, the same promoter peaks that were seen in each of the 1 million fragment CUT&Tag tracks were confirmed by the other methods. To extend this comparison genome-wide, we called peaks using MACS2 and calculated the fraction of reads in peaks (FRiPs), a measure of signal-to-noise adopted by the ENCODE project35. Based on FRiP, CUT&Tag shows nearly identical performance for all 8 samples (two biological replicates from native and lightly cross-linked cells and nuclei), confirming the high robustness of CUT&Tag regardless of which protocol is used (Fig. 2c). In contrast, ChIP-seq and Omni-ATAC datasets were much less suitable for peak-calling when downsampled to 1 million reads, with both ChIP-seq and Omni-ATAC showing an order-of-magnitude lower FRiP relative to CUT&Tag. We also pooled the fragments from the 8 CUT&Tag datasets and downsampled from the pool to compare FRiP curves at high read depths, and observed a FRiP of 0.90 for CUT&Tag versus 0.15–0.53 For ChIP-seq and Omni-ATAC (Fig. 2c). Reproducibility of H3K4me3 CUT&Tag biological replicates was also much better than that for ChIP-seq, with R2 values for narrow peaks called by MACS2 ranging from 0.93–0.95 compared to 0.39–0.87 for H3K4me3 ChIP-seq peaks from three ENCODE laboratories (Fig. 2d).
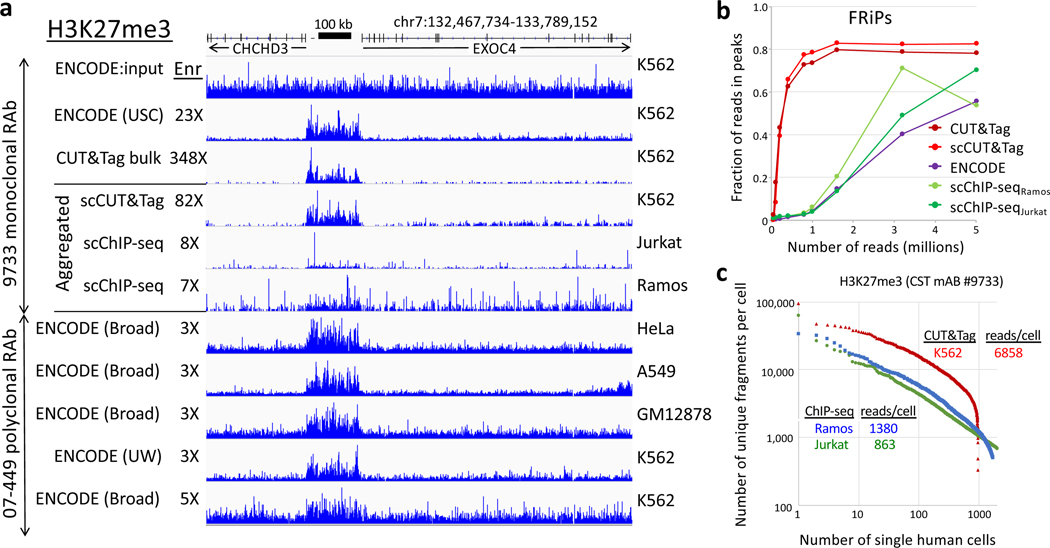
CUT&Tag has also been implemented for single-cell chromatin profiling, and we previously reported a dataset consisting of 807 H3K27me3 single-cell profiles, which we find outperforms a recent single-cell ChIP-seq method8 (Box 1).
Box 1: Single-cell CUT&Tag compared to single-cell ChIP-seq.
We previously showed that CUT&Tag is suitable for high-throughput single-cell profiling using the Takara ICELL8 nanowell platform23. Meanwhile, another group introduced a high-throughput single-cell version of native ChIP-seq (scChIP-seq) using a microfluidic device8. That study included scChIP-seq experiments on Jurkat and Ramos hematopoietic cancer cell lines using an H3K27me3 rabbit monoclonal antibody, the same antibody as we had used for single-cell CUT&Tag of K562 cells, also a cancer cell line of hematopoetic origin. Direct comparison of a representative H3K27me3 domain shows that signal-to-noise is similar between bulk and single-cell CUT&Tag, but is ~10-fold lower using scChIP-seq for both Jurkat and Ramos cells (Fig. 3a). The efficiency of scCUT&Tag was also better than that for scChIP-seq, with a ~6-fold higher median fragment-per-cell ratio over the distribution of cells reported in both studies. When we used MACS2 to call broad peaks on these domains genome-wide, we found close correspondence between bulk and single-cell CUT&Tag, with FRiPs leveling off at 1 million fragments, ~30-fold higher than that for scChIP-seq at 1 million fragments (Fig. 3b). The median level of reads per cell was ~6-fold higher for single-cell CUT&Tag than for scChIP-seq (Fig. 3c). We conclude that scCUT&Tag on the ICELL8 is very suitable for efficient single-cell profiling, and anticipate that CUT&Tag will be soon adopted for other high-throughput single-cell platforms.
From nuclei to sequencing-ready library in a single tube
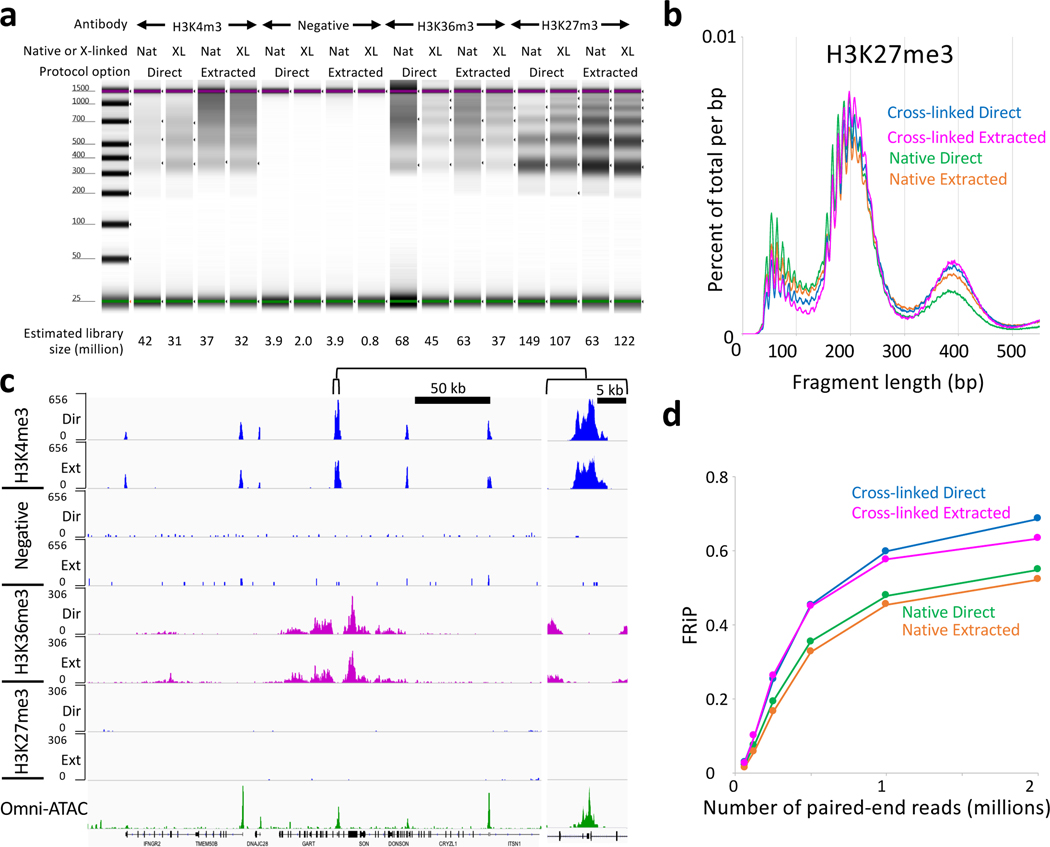
Our original protocol uses organic extraction to prepare tagmented samples for PCR. We have developed a protocol that proceeds from tagmentation directly to PCR, releasing pA-Tn5 from the DNA using a low concentration of SDS in a small volume and incubating for 1 hr at 58oC, which also reverses the cross-links (Option B). Triton-X100 is added to the beads to neutralize the SDS, which would otherwise inhibit Taq polymerase, followed by addition of PCR primers and master mix. Post-PCR clean-up is performed by addition of SPRI beads, such that the sequencing-ready library is withdrawn from the same tube used for all previous steps. Results using this method are comparable to results using organic DNA extraction (Fig. 4).
Figure 4 |. Similar results are obtained using DNA extraction and single-tube CUT&Tag options.
a) Image of a capillary electrophoretic gel for a typical experiment. Native and cross-linked nuclei were prepared and frozen in advance. Samples were thawed, mixed with beads and ~120,000 nuclei were aliquoted into each PCR tube. All steps through TapeStation analysis were performed in one day in parallel for both the extraction and single-tube (direct) options. The 16 samples were mixed in equimolar amounts with 56 other samples and sequenced (paired-end 25×25), yielding a median of 2.7 million mapped reads per sample. Estimated library size is indicated below. b) Length distribution for sequenced fragments from H3K27me3 CUT&Tag. The striking 10-bp sawtooth pattern that diminishes with length suggests a tagmentation preference for one surface of the DNA double-helix at a fixed distance on and around the bound particle. c) The same 300-kb GART-SON region displayed in Figure 2 is shown for the Direct-to-PCR single-tube samples. The negative control had been incubated with a validated H3K27ac mouse monoclonal antibody followed by the anti-rabbit secondary antibody, which suppressed the signal. d) Mapped fragments were sampled and narrow peaks were called using MACS2 with p-value = 10−5 and FRiP values were calculated.
CUT&Tag stringent salt conditions suppress accessible DNA detection
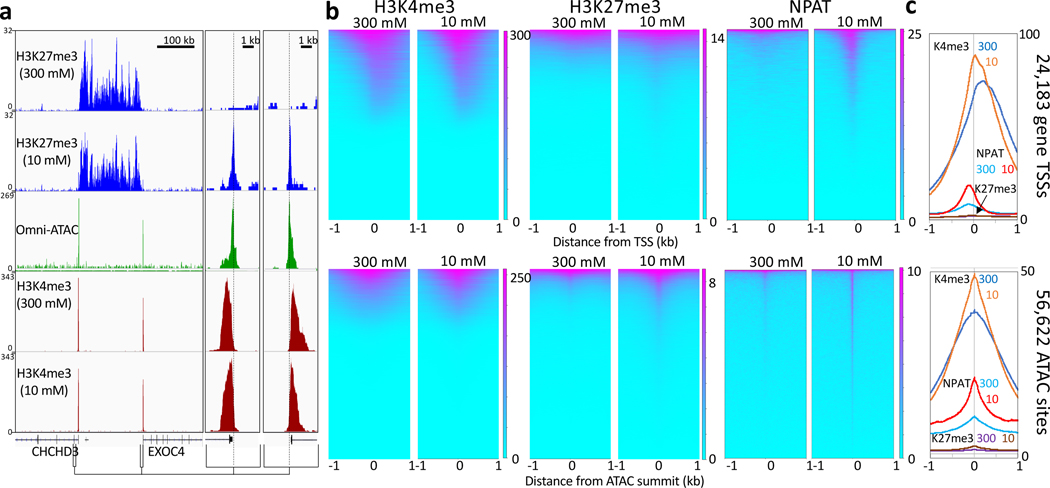
CoBATCH and ACT-seq are alternative versions of the CUT&Tag strategy, but there are key differences that have implications for suitability in different applications. We have found that stringent binding conditions during pA-Tn5 tethering and in subsequent washes and during tagmentation are critical to limit the affinity of Tn5 for exposed DNA. Such affinity, if not properly controlled, leads to tagmentation of exposed DNA in an ATAC-like profile that can be mis-interpreted as a genomic profile of a chromatin epitope. We find that performing all steps from pA-Tn5 binding through tagmentation in the presence of 300 mM NaCl simply and effectively suppresses this artifact (Fig. 5a). Heatmaps aligned to TSSs and ATAC-seq sites show close correspondence for H3K4me3, a positive control that marks nucleosomes just downstream of active promoters when tagmentation is done using either 300 mM or 10 mM monovalent ionic conditions (Fig. 5b). However, tracks and heatmaps for H3K27me3, an abundant histone modification that marks transcriptionally silent domains, show a weak signal directly over regulatory sites for 10 mM monovalent ionic conditions. For NPAT, a transcription factor that marks <100 annotated sites mostly within the histone gene clusters on human Chromosomes 1 and 6, the artifact is very conspicuous when tagmentation is performed in 10 mM monovalent ionic conditions, and is strongly suppressed by 300 mM NaCl (Fig. 5c). Taken together, these results imply that rare epitopes bound at regulatory elements are most susceptible to the artifact, which is suppressed by 300 mM NaCl, although this high salt concentration may also reduce the signal of loosely-bound factors by displacing them from chromatin.
Figure 5|. Suppression of accessible DNA tagmentation.
a) A representative region spanning a large H3K27me3 domain flanked by oppositely oriented promoters marked by H3K4me3 nucleosomes just downstream of the TSSs in K562 cells. CUT&Tag with DNA extraction was performed on ~70,000 lightly cross-linked frozen and thawed nuclei per sample using the DNA extraction option except that the third wash and tagmentation steps were done in 10 mM TAPS pH8.5. This resulted in peaks over the TSSs for H3K27me3 that are nearly absent in the presence of 300 mM NaCl. For clarity 5 kb regions around both promoters are expanded on the right, revealing that the low-salt peaks align with the Omni-ATAC peaks and the promoters, but are offset from the H3K4me3 nucleosomes downstream (dashed lines). b) Heatmap representations and c) average plots of 24,183 annotated promoters (top) and 56,622 ATAC-seq MACS2 peak summits (bottom) for CUT&Tag histone modifications and the NPAT chromatin protein, showing genome-wide suppression of accessible DNA peaks when tagmentation is performed in the presence of 300 mM NaCl. Heatmaps were separately ordered by signal over the displayed region using Deeptools.
Suppression of tagmentation of accessible DNA is essential for accurate antibody-dependent CUT&Tag chromatin profiling. We find that variations in the basic CUT&Tag strategy, such as that used in CoBATCH and ACT-seq (Box 2) detect accessible DNA with high efficiency. The improved signal at accessible sites for ACT-seq and CoBATCH methods relative to ATAC-seq from many laboratories is not fully understood, but may be due to the wash steps following pA-Tn5 tethering that limit background tagmentation of the genome.
Box 2: Accessible DNA detection by CoBATCH and ACT-seq.
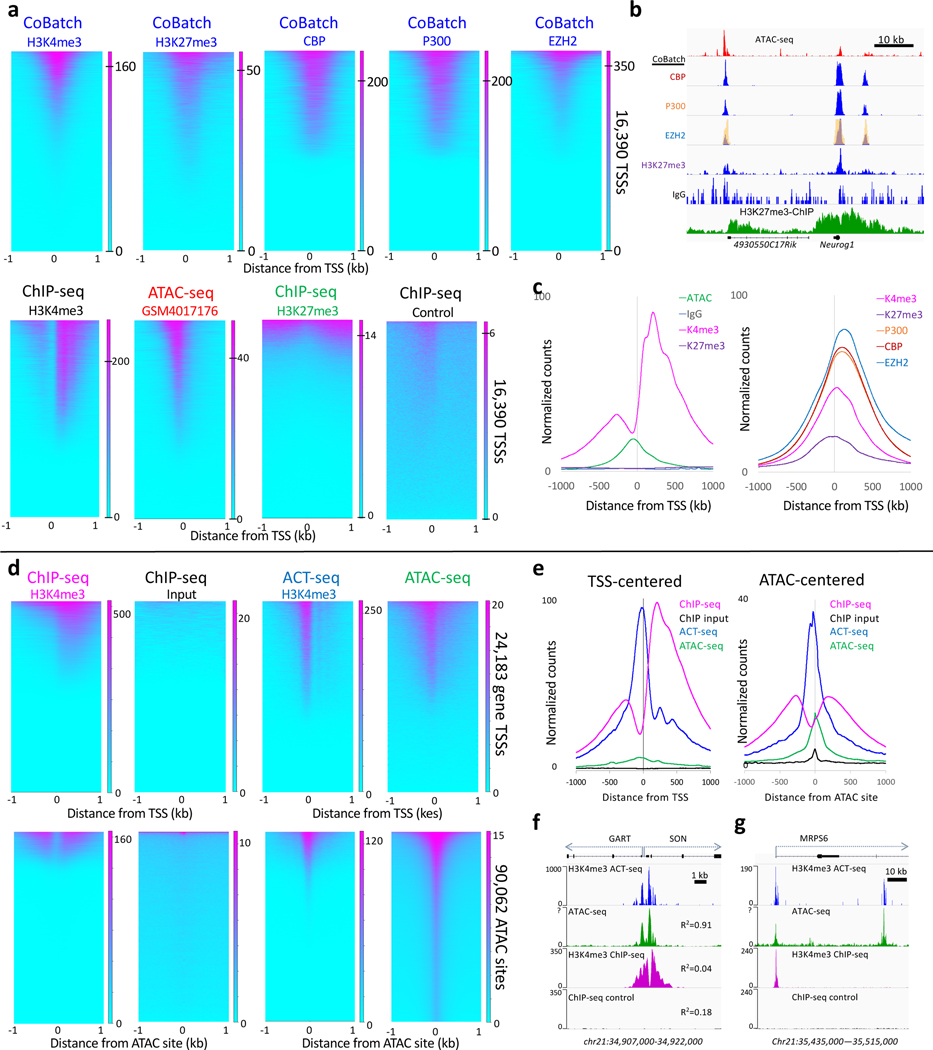
Analysis of published data from two groups demonstrates the possibility of accessibility artifacts in Tn5-based profiling, previously described by Harada et al.24 One recent publication applied CUT&Tag in a single-cell-compatible format called CoBATCH26. This study mapped multiple activating and repressing chromatin proteins and histone modifications, but with strikingly similar results for all proteins (Fig. 6a-b). CoBATCH mapping of H3K27me3 is particularly telling, as this is discordant for mapping of the same modification by ChIP-seq. Instead, each peak in the CoBATCH tracks corresponds to an ATAC-seq profiling peak regardless of which antibody was used. This is likely attributable to the low salt concentrations this study used before and during tagmentation, as the ratio between antibody-tethered and accessibility tagmentation decreases at reduced salt concentration (Fig. 5). It is also apparent that CoBATCH conditions improve the detection of accessible DNA in mouse embryonic stem cells (mESCs) (Fig. 6c), as these sites are better represented with much lower backgrounds than 10 ATAC-seq profiles for mESCs from different laboratories with 5–25X more reads (Supplementary Fig. 1).
ACT-seq is a strategy similar to CUT&Tag, except that the antibody is mixed together with pA-Tn5 and applied to permeabilized cells with a single in situ binding reaction in a buffer containing 150 mM NaCl25. Analysis of the ACT-seq dataset from GEO for aggregate H3K4me3 single-cell data from human HEK293 cells reveals that the ACT-seq signal differs from the H3K4me3 ENCODE ChIP-seq signal for HEK293 cells in that it is not centered over the H3K4me3 nucleosome, but rather in the adjacent accessible DNA site (Fig. 6d). As for CoBATCH, the signal-to-noise of the accessible DNA signal is much better than that of ATAC-seq for the same HEK293 cell type (Fig. 6e). When mapped with base-pair resolution, the offset of ACT-seq signal correlates strongly with ATAC-seq signal but shows essentially no similarity with H3K4me3 ChIP-seq (Fig. 6f). ACT-seq peaks are seen not only at promoters marked by H3K4me3 using ChIP-seq, but also at non-promoter sites that lack a ChIP-seq signal (Fig. 6g).
Figure 6 |. CoBATCH and ACT-seq peaks correspond to ATAC-seq peak summits genome-wide.
a) Heatmaps are similar between ATAC-seq summits and CoBATCH H3K27me3 signals but correspond to depleted H3K27me3 ChIP-seq signals in mESCs. LIkewise, CoBATCH CBP, P300 and EZH2 summits correspond to one another genome-wide with an ~10-fold larger dynamic range than is seen for ATAC-seq (0 to 200–350 for CoBATCH, 0–20 for ATAC-seq). For each dataset, heatmaps are ordered by decreasing normalized count density. The ATAC-seq track shown is better than average based on comparing mESC ATAC-seq tracks from 8 different laboratories (Supplementary Fig. 1). b) Representative examples from tracks shown Figure 2C of Ref. 19. The EZH2 track from the figure image (yellow) is superimposed over the track of the same region reproduced from the data in GEO, confirming correspondence between the published CoBATCH image and the source data used here. Profiles for CBP, P300 and EZH2 closely correspond to one another and to ATAC-seq peaks, although with much lower background, and lack the broad domains seen for H3K27me3 ChIP-seq profiles. c) Average plots of the data shown in panel a. d) Comparison of H3K4me3 ChIP-seq to ACT-seq and ATAC-seq heatmaps. ACT-seq shows a chromatin accessibility profile with a ~10-fold larger dynamic range than is seen for ATAC-seq (0 to 250 for ACT-seq, 0–20 for ATAC-seq). e) Average plot of the dataset shown in top panels of (d). f) A representative region showing bidirectional housekeeping promoters and R2 Pearson correlation coefficients over the region between ACT-seq, ATAC-seq and ChIP-seq from human K562 cells. g) A nearby representative gene region showing the promoter marked by ACT-seq, ATAC-seq and H3K4me3 ChIP and an intronic region marked by ACT-seq and ATAC-seq, but not by ChIP-seq. Heatmaps were separately ordered by signal over the displayed region using Deeptools.
In conclusion, the ability to easily obtain high quality chromatin profiles at low cost by using our CUT&Tag protocol enables studies that might otherwise be cost prohibitive. For example, chromatin profiling of small numbers of selected cells provides an attractive alternative to single-cell profiling for developmental studies, where applying CUT&Tag to cells purified by fluorescence-activated cell-sorting38, laser-assisted microdissection39, INTACT40 and other cell-isolation methods promises to provide highly robust cell function information that can be used to directly characterize cell types.
Supplementary Material
Figure 3 |. Comparison of single-cell CUT&Tag (scCUT&Tag) to single-cell ChIP-seq.
The same monoclonal antibody was used for H3K27me3 scCUT&Tag and scChIP-seq allowing a direct comparison of aggregated single-cell datasets. a) Tracks from a representative region of the human genome. Although different hematopoetic cancer cell lines were used, this region shows similar profiles for diverse cancer and normal lines (indicated on right). To quantify approximate signal-to-noise differences, we calculated the fold enrichment over the H3K27me3-enriched domain (132,768,919–132,935,822) relative to that for the flanking regions (132,467,734–132,768,918 and 132,935,823–133,789,152) for each track relative to the fold enrichment for the ENCODE USC K562 input track (“Enr” numbers for each track). b) Noisiness of scChIP-seq data relative to CUT&Tag data is confirmed by FRiP analysis. c) Knee plot showing the read/cell distribution estimated from aggregate data using the Picard Mark Duplicates program. Medians are indicated, where for scCUT&Tag all cells were scored and for scChIP-seq cells below a threshold were not scored.
ACKNOWLEDGEMENTS
We thank Christine Codomo for pooling Illumina sequencing libraries and members of our laboratory and colleagues at the Fred Hutch for providing input. We are especially grateful to the many Protocols.io subscribers around the world who have tried CUT&Tag and provided helpful comments and feedback that have enriched this protocol. This work was supported by the Howard Hughes Medical Institute (H.S.K. and S.H.), grants R01 HG010492 (S.H.) and R01 GM108699 (K.A.) from the National Institutes of Health and an HCA Seed Network grant from the Chan-Zuckerberg Initiative (S.H.).
Footnotes
DATA AVAILABILITY
Publically available datasets analyzed in this work are available in Supplementary Note 1. All sequencing data generated in this study have been deposited in GEO under accession GSE145187.
COMPETING FINANCIAL INTERESTS
The authors declare that they have no competing financial interests.
REFERENCES
- 1.Rodriguez-Ubreva J & Ballestar E Chromatin immunoprecipitation. Methods Mol Biol 1094, 309–18 (2014). [DOI] [PubMed] [Google Scholar]
- 2.Solomon MJ & Varshavsky A Formaldehyde-mediated DNA-protein crosslinking: a probe for in vivo chromatin structures. Proc Natl Acad Sci U S A 82, 6470–4 (1985). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rossi MJ, Lai WKM & Pugh BF Simplified ChIP-exo assays. Nat Commun 9, 2842 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.He Q, Johnston J & Zeitlinger J ChIP-nexus enables improved detection of in vivo transcription factor binding footprints. Nat Biotechnol 33, 395–401 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Skene PJ & Henikoff S A simple method for generating high-resolution maps of genome wide protein binding. eLife 4, e09225 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kasinathan S, Orsi GA, Zentner GE, Ahmad K & Henikoff S High-resolution mapping of transcription factor binding sites on native chromatin. Nature Methods 11, 203–9 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ai S et al. Profiling chromatin states using single-cell itChIP-seq. Nat Cell Biol 21, 1164–1172 (2019). [DOI] [PubMed] [Google Scholar]
- 8.Grosselin K et al. High-throughput single-cell ChIP-seq identifies heterogeneity of chromatin states in breast cancer. Nat Genet 51, 1060–1066 (2019). [DOI] [PubMed] [Google Scholar]
- 9.Schmidl C, Rendeiro AF, Sheffield NC & Bock C ChIPmentation: fast, robust, low-input ChIP-seq for histones and transcription factors. Nat Methods 12, 963–965 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.van Steensel B & Henikoff S Identification of in vivo DNA targets of chromatin proteins using tethered Dam methyltransferase. Nature Biotechnology 18, 424–428 (2000). [DOI] [PubMed] [Google Scholar]
- 11.Schmid M, Durussel T & Laemmli UK ChIC and ChEC; genomic mapping of chromatin proteins. Mol Cell 16, 147–57 (2004). [DOI] [PubMed] [Google Scholar]
- 12.Zentner GE, Kasinathan S, Xin B, Rohs R & Henikoff S ChEC-seq kinetics discriminate transcription factor binding sites by DNA sequence and shape in vivo. Nature Communications 6, 8733 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Skene PJ & Henikoff S An efficient targeted nuclease strategy for high-resolution mapping of DNA binding sites. Elife 6, e21856 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Janssens DH et al. Automated in situ chromatin profiling efficiently resolves cell types and gene regulatory programs. Epigenetics Chromatin 11, 74 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Skene PJ, Henikoff JG & Henikoff S Targeted in situ genome-wide profiling with high efficiency for low cell numbers. Nat Protoc 13, 1006–1019 (2018). [DOI] [PubMed] [Google Scholar]
- 16.Liu N et al. Direct Promoter Repression by BCL11A Controls the Fetal to Adult Hemoglobin Switch. Cell 173, 430–442 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hainer SJ, Boškovic A, McCannell KN, Rando OJ & Fazzio TG Profiling of pluripotency factors in individual stem cells and early embryos. Cell 177, 1319–1329 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Roth TL et al. Reprogramming human T cell function and specificity with non-viral genome targeting. Nature 559, 405–409 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Oomen ME, Hansen AS, Liu Y, Darzacq X & Dekker J CTCF sites display cell cycle-dependent dynamics in factor binding and nucleosome positioning. Genome Res 29, 236–249 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhu Q, Liu N, Orkin SH & Yuan GC CUT&RUNTools: a flexible pipeline for CUT&RUN processing and footprint analysis. Genome Biol 20, 192 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Meers MP, Tenenbaum D & Henikoff S Peak calling by Sparse Enrichment Analysis for CUT&RUN chromatin profiling. Epigenetics Chromatin 12, 42 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Meers MP, Janssens DH & Henikoff S Pioneer Factor-Nucleosome Binding Events during Differentiation Are Motif Encoded. Mol Cell (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kaya-Okur HS et al. CUT&Tag for efficient epigenomic profiling of small samples and single cells. Nature communications 10, 1930 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Harada A et al. A chromatin integration labelling method enables epigenomic profiling with lower input. Nat Cell Biol 21, 287–296 (2019). [DOI] [PubMed] [Google Scholar]
- 25.Carter B et al. Mapping histone modifications in low cell number and single cells using antibody-guided chromatin tagmentation (ACT-seq). Nat Commun 10, 3747 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wang Q et al. CoBATCH for High-Throughput Single-Cell Epigenomic Profiling. Mol Cell 76, 206–216 e7 (2019). [DOI] [PubMed] [Google Scholar]
- 27.Meers MP, Bryson TD, Henikoff JG & Henikoff S Improved CUT&RUN chromatin profiling tools. Elife 8, e46314 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Buenrostro JD, Giresi PG, Zaba LC, Chang HY & Greenleaf WJ Transposition of native chromatin for fast and sensitive epigenomic profiling of open chromatin, DNA-binding proteins and nucleosome position. Nat Methods 10, 1213–8 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Langmead B & Salzberg SL Fast gapped-read alignment with Bowtie 2. Nat Methods 9, 357–9 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Quinlan AR & Hall IM BEDTools: a flexible suite of utilities for comparing genomic features. Bioinformatics 26, 841–2 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ramirez F et al. deepTools2: a next generation web server for deep-sequencing data analysis. Nucleic Acids Res 44, W160–5 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Picelli S et al. Tn5 transposase and tagmentation procedures for massively scaled sequencing projects. Genome Res 24, 2033–40 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Buenrostro JD et al. Single-cell chromatin accessibility reveals principles of regulatory variation. Nature 523, 486–90 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Liu T Use model-based Analysis of ChIP-Seq (MACS) to analyze short reads generated by sequencing protein-DNA interactions in embryonic stem cells. Methods Mol Biol 1150, 81–95 (2014). [DOI] [PubMed] [Google Scholar]
- 35.Landt SG et al. ChIP-seq guidelines and practices of the ENCODE and modENCODE consortia. Genome Res 22, 1813–31 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Jung YL et al. Impact of sequencing depth in ChIP-seq experiments. Nucleic Acids Res 42, e74 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Oh KS, Ha J, Baek S & Sung MH XL-DNase-seq: improved footprinting of dynamic transcription factors. Epigenetics Chromatin 12, 30 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ernst C, Eling N, Martinez-Jimenez CP, Marioni JC & Odom DT Staged developmental mapping and X chromosome transcriptional dynamics during mouse spermatogenesis. Nat Commun 10, 1251 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Org T et al. Genome-wide histone modification profiling of inner cell mass and trophectoderm of bovine blastocysts by RAT-ChIP. PLoS One 14, e0225801 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mo A et al. Epigenomic signatures of neuronal diversity in the mammalian brain. Neuron 86, 1369–84 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.