Abstract
Background
Canine adipose-derived stem cells (cADSCs) exhibit various differentiation properties and are isolated from the canine subcutaneous fat. Although cADSCs are valuable as tools for research on adipogenic differentiation, studies focusing on adipogenic differentiation methods and the underlying mechanisms are still lacking.
Objectives
In this study, we aimed to establish an optimal method for adipogenic differentiation conditions of cADSCs and evaluate the role of peroxisome proliferator-activated receptor gamma (PPARγ) and estrogen receptor (ER) signaling in the adipogenic differentiation.
Methods
To induce adipogenic differentiation of cADSCs, 3 different adipogenic medium conditions, MDI, DRI, and MDRI, using 3-isobutyl-1-methylxanthine (M), dexamethasone (D), insulin (I), and rosiglitazone (R) were tested.
Results
MDRI, addition of PPARγ agonist rosiglitazone to MDI, was the most significantly facilitated cADSC into adipocyte. GW9662, an antagonist of PPARγ, significantly reduced adipogenic differentiation induced by rosiglitazone. Adipogenic differentiation was also stimulated when 17β-estradiol was added to MDI and DRI, and this stimulation was inhibited by the ER antagonist ICI182,780.
Conclusions
Taken together, our results suggest that PPARγ and ER signaling are related to the adipogenic differentiation of cADSCs. This study could provide basic information for future research on obesity or anti-obesity mechanisms in dogs.
Keywords: Dogs, canine adipose-derived stem cells, adipogenesis, PPAR gamma, estrogen receptor
INTRODUCTION
Adipose-derived stem cells (ADSCs) are adult stem cells (ASCs) that have self-regenerative ability, long-term viability, and multipotency [1]. ADSCs are easy to access and plentiful, and therefore can be obtained in large quantities [2]. Bone marrow stem cells (BMSCs), also a type of ASCs, are difficult to obtain and available in small amount [3]. Therefore, ADSCs are considered as a potential alternative for BMSCs and serve as an attractive model [4]. ADSCs undergo differentiation, including adipogenic, osteogenic, chondrogenic, myogenic, and neurogenic differentiation, into various cell types and have potential therapeutic applications in various fields [5,6]. In particular, studies on the control of the adipogenic differentiation of ADSCs suggest their therapeutic application for the management of obesity and aging diseases [4,7].
Adipogenesis refers to the complex process of differentiation from pre-adipocytes into adipocytes [7]. Various hormones and nutritional signals, including glucocorticoids and insulin, influence adipogenesis in vivo and in vitro [8]. In vitro, 3T3-L1 pre-adipocyte is the representative cell line for adipogenic differentiation [9]. For adipogenesis studies using 3T3-L1 cells, 3-isobutyl-1-methylxanthine (IBMX), dexamethasone, and insulin are commonly used as differentiation inducers [10]. In the present study, we used rosiglitazone and 17β-estradiol (E2) as adipogenic differentiation inducers. Rosiglitazone is a thiazolidinediones (TZD) that acts as a peroxisome proliferator-activated receptor gamma (PPARγ) agonist [11]. Rosiglitazone increases insulin sensitivity by binding to PPARγ ligand, resulting in the stimulation of adipogenic differentiation through upregulating of the expression of adipogenesis-related genes, including CCAAT/enhancer-binding protein alpha (C/EBPα) and PPARγ [11]. E2, an active form of estrogen, functions by binding to estrogen receptor (ER) α and ERβ [12]. Estrogen affects cell proliferation, differentiation, and metabolism in various tissues such as the adipose, cartilage, bone, and muscle tissues [13]. Whether estrogen stimulates adipogenic differentiation during adipogenesis is, however, unknown [13,14,15,16].
Obesity refers to the excessive accumulation of fat in the body and may be caused by multiple factors such as environmental factors, genetics, imbalance energy expenditure and intake, and endocrine system change [17]. As observed in obese humans, obese dogs have a high risk of disease including orthopedic and respiratory diseases, endocrine and metabolic disorders, and dyslipidemia, which may degrade the quality of life [18].
In recent years, there is an increase in the awareness about obesity in companion dogs. However, only a few reports have focused on the obesity mechanism in dogs using canine cells. The present study aimed to examine the optimal adipogenic differentiation conditions for cADSC and evaluate the effect of rosiglitazone and estrogen on the adipogenic differentiation of cADSCs.
MATERIALS AND METHODS
Materials
Canine adipose-derived stem cells (cADSCs) were kindly provided by Prof. Oh-kyeng Kweon, Seoul National University [19,20]. Dulbecco's modified Eagle's medium (DMEM) and Dulbecco's phosphate-buffered saline (PBS) were purchased from CORNING (USA). Fetal bovine serum (FBS), antibiotic-antimycotic (Anti-Anti), 0.5% trypsin-ethylenediaminetetraacetic acid (EDTA), and insulin were obtained from Gibco BRL (USA). The compound IBMX, dexamethasone, rosiglitazone, E2, and GW9662 (PPARγ antagonist) were provided by Sigma (USA), while ICI182,780 (ER antagonist) was supplied by Tocris (USA).
Cell culture and adipogenic differentiation
Cells were maintained in the growth medium, 10% FBS-DMEM with 1% Anti-Anti at 37°C in a 5% CO2 incubator. When cells were approximately 70% confluent, the medium was removed and cells were washed with PBS. The cells were trypsinized with 0.25% trypsin-EDTA and subcultured. cADSCs were used until passage 7.
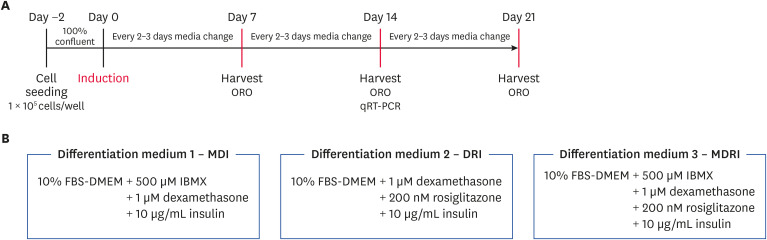
For adipogenic differentiation, 1 × 105 cells/well were seeded into six-well plates. After 2 days, cADSCs were induced with three different adipogenic medium conditions (day 0), 3-isobutyl-1-methylxanthine + dexamethasone + insulin (MDI), dexamethasone + rosiglitazone + insulin (DRI), and 3-isobutyl-1-methylxanthine + dexamethasone + rosiglitazone + insulin (MDRI) (Fig. 1). Adipogenic medium was changed every 2 or 3 days for 7, 14, and 21 days. Cultures were maintained at 37°C in a 5% CO2 incubator.
Fig. 1. Schematic diagram of the treatment schedule and the medium to induce adipogenic differentiation of cADSCs.
cADSC, canine adipose-derived stem cell; qRT-PCR, quantitative real-time reverse-transcription polymerase chain reaction; FBS, fetal bovine serum; DMEM, Dulbecco's modified Eagle's medium; IBMX, 3-siobutyl-1-methylxanthine; MDI, 3-isobutyl-1-methylxanthine + dexamethasone + insulin; DRI, dexamethasone + rosiglitazone + insulin; MDRI, and 3-isobutyl-1-methylxanthine + dexamethasone + rosiglitazone + insulin.
PPARγ inhibition
For PPARγ antagonist studies, cADSCs were seeded at 1 × 105 cells/well into 6-well plates. After 2 days (day 0), adipogenic differentiation was induced with MDRI in the absence or presence of 10 µM GW9662, a known PPARγ antagonist. The medium was replaced every 2 or 3 days for 14 and 21 days, and the cultures were maintained at 37°C in a 5% CO2 incubator.
ER inhibition
For ER antagonist studies, cADSCs were plated in six-well plates at a density of 1 × 105 cells/well. At 100% confluence (day 0), adipogenic differentiation was induced with MDIE (500 µM IBMX, 1 µM dexamethasone, 10 µg/mL insulin, and 10 nM E2) and DRIE (1 µM dexamethasone, 200 nM rosiglitazone, 10 µg/mL insulin, and 10 nM E2) in the absence or presence of 10 µM ER antagonist ICI182,780. The medium was replaced every 2 or 3 days for 14 and 21 days. Cultures were maintained at 37 °C in a 5% CO2 incubator.
Oil Red-O staining
In brief, 1 × 105 cells/well were plated into six-well plates and differentiation was induced as per the protocols described above. After 7, 14, and 21 days, cADSCs were stained with an Oil Red-O working solution for 30 min. After washing 4 times with distilled water, the stained cells were photographed using an ECLIPSE Ts2 microscope (Nikon, Japan). To quantify staining, the stained lipid droplets were eluted with 100% isopropanol and the absorbance was measured at 500 nm wavelength with an Epoch Microplate Spectrophotometer (BioTek Instruments, Inc., USA).
RNA extraction and cDNA synthesis
On day 14 of adipogenic differentiation, cADSCs were harvested and the total RNA was extracted with the easy-spin total RNA extraction kit (iNtRON Biotechnology, Korea). The concentration of extracted RNA was quantified 50 ng/µL using a Take3 Micro-Volume plate (BioTek Instruments, Inc.). cDNA was synthesized with a thermal cycler (Takara Bio Inc., Japan) using the GoScript Reverse Transcriptase (Promega, Madison, WI, USA).
Quantitative real-time reverse-transcription polymerase chain reaction (qRT-PCR)
qRT-PCR was performed using a QuantStudio 3 Real-Time PCR Instrument (Thermo Fisher Scientific, USA). All reactions were performed in total volume of 20 uL containing 5.5 µL cDNA template, 10 µL TB Green® Premix Ex Taq II (Takara Bio Inc.), 1 µL of 10 pmol forward primer, 1 µL of 10 pmol reverse primer, and 2.5 µL diethylpyrocarbonate-treated water. Primers were purchased from Macrogen (Korea) and their sequences are shown in Table 1.
Table 1. Primer sequences used for qRT-PCR.
| Gene | Forward (5′-3′) | Reverse (5′-3′) | Gene bank No. |
|---|---|---|---|
| PPARγ | ACACGATGCTGGCGTCCTTGATG | TGGCTCCATGAAGTCACCAAAGG | NM_001024632.2 |
| C/EBPα | AGTCAAGAAGTCGGTGGACAAG | GCGGTCATTGTCACTGGTGAG | XM_022407214.1 |
| FABP4 | ATCAGTGTAAACGGGGATGTG | GACTTTTCTGTCATCCGCAGTA | XM_845069.5 |
| LEPTIN | CTATCTGTCCTGTGTTGAAGCTG | GTGTGTGAAATGTCATTGATCCTG | NM_001003070 |
| LPL | ACACATTCACAAGAGGGTCACC | CTCTGCAATCACACGGATGGC | XM_005635734.3 |
| β-actin | GCCAACCGTGAGAAGATGACT | CCCAGAGTCCATGACAATACCAG | AF021873 |
qRT-PCR, quantitative real-time reverse-transcription polymerase chain reaction; PPARγ, peroxisome proliferator-activated receptor gamma; C/EBPα, CCAAT/enhancer-binding protein alpha; FABP4, fatty acid-binding protein 4; LPL, lipoprotein lipase.
Statistical analysis
All results are presented as the mean ± standard error of mean (SEM) of at least three separate experiments. The data were analyzed by one-way analysis of variance (ANOVA), followed by Tukey's post-hoc tests using GraphPad Prism 5 software (Graph Pad Software Inc., USA). The p values less than 0.05 were considered statistically significant.
RESULTS
MDRI induced the highest lipid accumulation for 21 days in cADSCs
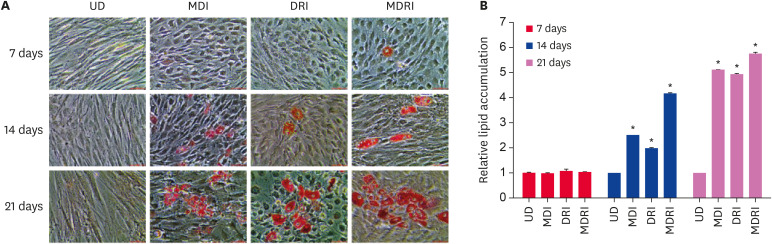
We first investigated the lipid accumulation of ADSCs cultured in the three different adipogenic media, MDI, DRI, and MDRI, for 7, 14, and 21 days by Oil Red-O staining (Fig. 2). Almost no staining was observed in cells from MDI, DRI, and MDRI conditions at day 7 of differentiation, similar to undifferentiated (UD) cells. In contrast, the staining of cells significantly increased under MDI, DRI, and MDRI conditions than UD condition on day 14 and 21 of differentiation. More lipid droplets were observed at day 21 than at day 14. In particular, treatment with MDRI significantly increased the lipid droplet staining by 5.8-fold as compared with UD condition, and this effect was higher than that observed under MDI and DRI conditions. Although the level of lipid accumulation was similar in MDI and DRI conditions, the lipid droplet size was larger under DRI condition.
Fig. 2. Differentiation of cADSCs into adipocytes according to the differentiation medium. cADSCs were induced to adipogenic differentiation with MDI, DRI, and MDRI conditions for 7, 14, and 21 days. (A) Oil Red-O staining images. Scale bar indicated 50 µm. (B) Quantification of the stained lipid droplets. The results are presented as mean ± SEM.
cADSC, canine adipose-derived stem cell; MDI, 3-isobutyl-1-methylxanthine + dexamethasone + insulin; DRI, dexamethasone + rosiglitazone + insulin; MDRI, and 3-isobutyl-1-methylxanthine + dexamethasone + rosiglitazone + insulin; UD, undifferentiated; SEM, standard error of mean.
*p < 0.001 vs. UD.
Rosiglitazone induced adipogenesis via PPARγ signaling in cADSCs
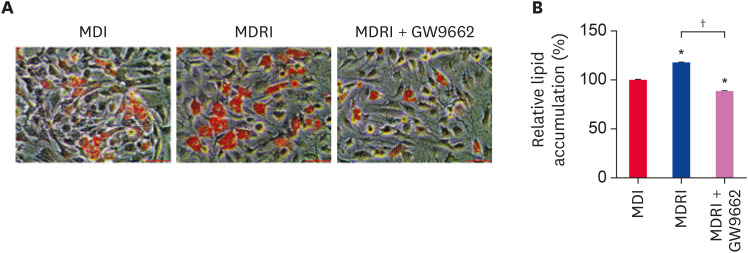
To confirm whether rosiglitazone, a PPARγ agonist, induces adipogenic differentiation via PPARγ, we induced adipogenic differentiation of cADSCs with MDI and MDRI in the absence or presence of GW9662. At 21 days of adipogenic differentiation, we assessed lipid accumulation by Oil Red-O staining (Fig. 3A). Addition of rosiglitazone to MDI increased the number and size of stained lipid droplets as compared with MDI condition. In contrast, addition of GW9662 to MDRI visibly reduced the number and size of stained lipid droplets. The quantification value was in line with these results (Fig. 3B).
Fig. 3. Effect of rosiglitazone on the adipogenic differentiation of cADSCs. cADSCs were induced to adipogenic differentiation under MDI, MDRI, and MDRI + GW9662 conditions for 21 days. (A) Oil Red-O staining images. Scale bar indicates 50 µm. (B) Quantification of the stained lipid droplets. The results are presented as mean ± SEM.
cADSC, canine adipose-derived stem cell; MDI, 3-isobutyl-1-methylxanthine + dexamethasone + insulin; MDRI, and 3-isobutyl-1-methylxanthine + dexamethasone + rosiglitazone + insulin; SEM, standard error of mean.
*p < 0.001 vs. MDI, †p < 0.001 vs. MDRI.
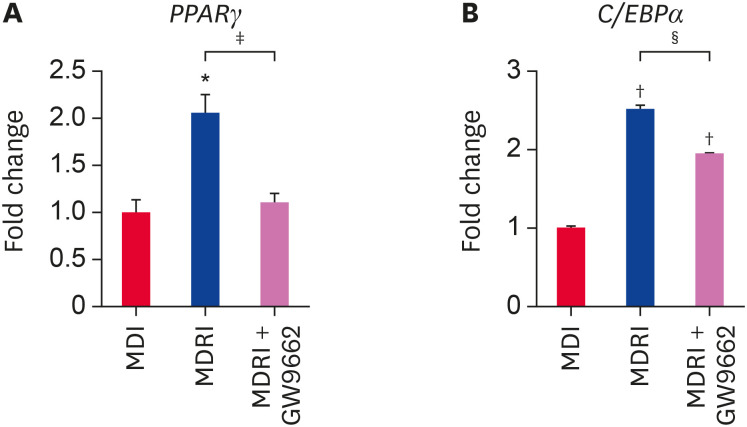
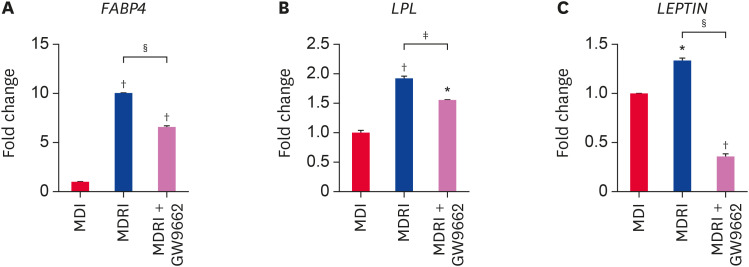
In addition, we assessed the mRNA expression of adipogenic factors by qRT-PCR at 14 days of adipogenic differentiation. PPARγ and C/EBPα are essential adipogenic transcription factors that regulate terminal differentiation process and stimulate adipogenesis-related gene expression. Addition of rosiglitazone to MDI significantly enhanced PPARγ mRNA expression by 2.1-fold as compared to MDI condition (Fig. 4A). GW9662 significantly suppressed PPARγ mRNA expression under MDRI condition to the level observed under MDI condition (Fig. 4A). C/EBPα mRNA expression showed a trend similar to that of PPARγ mRNA expression (Fig. 4B). MDRI treatment significantly upregulated FABP4, LPL, and LEPTIN mRNA expression as compared with MDI condition (Fig. 5A-C). FABP4, LPL, and LEPTIN are regulated by PPARγ and C/EBPα, and their expression was inhibited by GW9662 (Fig. 5A-C). Taken together, these results show that rosiglitazone induces adipogenic differentiation in cADSCs via PPARγ.
Fig. 4. Effect of rosiglitazone on the mRNA gene expression of transcription factors related to adipogenic differentiation of cADSCs. cADSCs were induced to adipogenic differentiation in MDI, MDRI, and MDRI + GW9662 conditions for 14 days. Expression of (A) PPARγ and (B) C/EBPα mRNAs was quantified by qRT-PCR. The results are presented as mean ± SEM.
cADSC, canine adipose-derived stem cell; MDI, 3-isobutyl-1-methylxanthine + dexamethasone + insulin; MDRI, and 3-isobutyl-1-methylxanthine + dexamethasone + rosiglitazone + insulin; PPARγ, peroxisome proliferator-activated receptor gamma; C/EBPα, CCAAT/enhancer-binding protein alpha; qRT-PCR, quantitative real-time reverse-transcription polymerase chain reaction; SEM, standard error of mean.
*p < 0.05, †p < 0.001 vs. MDI, ‡p < 0.05, §p < 0.001 vs. MDRI.
Fig. 5. Effect of rosiglitazone on the mRNA expression of gene expressions related to adipogenic differentiation of cADSCs. cADSCs were induced to adipogenic differentiation under MDI, MDRI, and MDRI + GW9662 for 14 days. Expression of (A) FABP4, (B) LPL, and (C) LEPTIN mRNAs was quantified by qRT-PCR. The results are presented as mean ± SEM.
cADSC, canine adipose-derived stem cell; MDI, 3-isobutyl-1-methylxanthine + dexamethasone + insulin; MDRI, and 3-isobutyl-1-methylxanthine + dexamethasone + rosiglitazone + insulin; FABP4, fatty acid-binding protein 4; LPL, lipoprotein lipase; qRT-PCR, quantitative real-time reverse-transcription polymerase chain reaction; SEM, standard error of mean.
*p < 0.01, †p < 0.001 vs. MDI, ‡p < 0.05, §p < 0.001 vs. MDRI.
Estrogen induced adipogenesis via ER in cADSCs
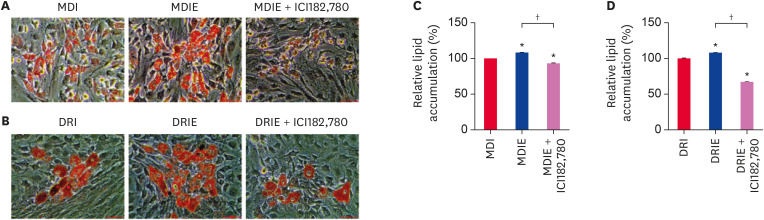
To investigate whether estrogen induces adipogenic differentiation via ER, cADSCs were induced to adipogenic differentiation in MDIE and DRIE conditions in the absence or presence the ER antagonist ICI182,780. The molecule E2 is a potent estrogen and functions by binding to the ER. Lipid accumulation was measured using Oil Red-O staining after 21 days of adipogenic differentiation. We observed that the addition of E2 to MDI produced sufficient lipid droplets, while the number of stained lipid droplets visibly reduced when ICI182,780 was added to MDIE condition (Fig. 6A). The quantification value was also consistent with these results (Fig. 6C). The adipogenic differentiation of cADSCs was induced by DRIE and inhibited by ICI182,780 (Fig. 6B and D).
Fig. 6. Effect of estrogen on the adipogenic differentiation of cADSCs. cADSCs were induced to adipogenic differentiation in MDIE and DRIE condition for 21 days. Oil Red-O staining images of (A) MDIE condition and (B) DRIE condition. Scale bar indicates 50 µm. Quantification of the stained lipid droplets of (C) MDIE condition and (D) DRIE condition. The results are presented as mean ± SEM. cADSC, canine adipose-derived stem cell; MDI, 3-isobutyl-1-methylxanthine + dexamethasone + insulin; DRI, dexamethasone + rosiglitazone + insulin; SEM, standard error of mean; MDIE condition, MDI, MDIE, and MDIE+ICI182,780; DRIE condition, DRI, DRIE, and DRIE+ICI182,780.
*p < 0.001 vs. MDI or DRI, †p < 0.001 vs. MDIE or DRIE.
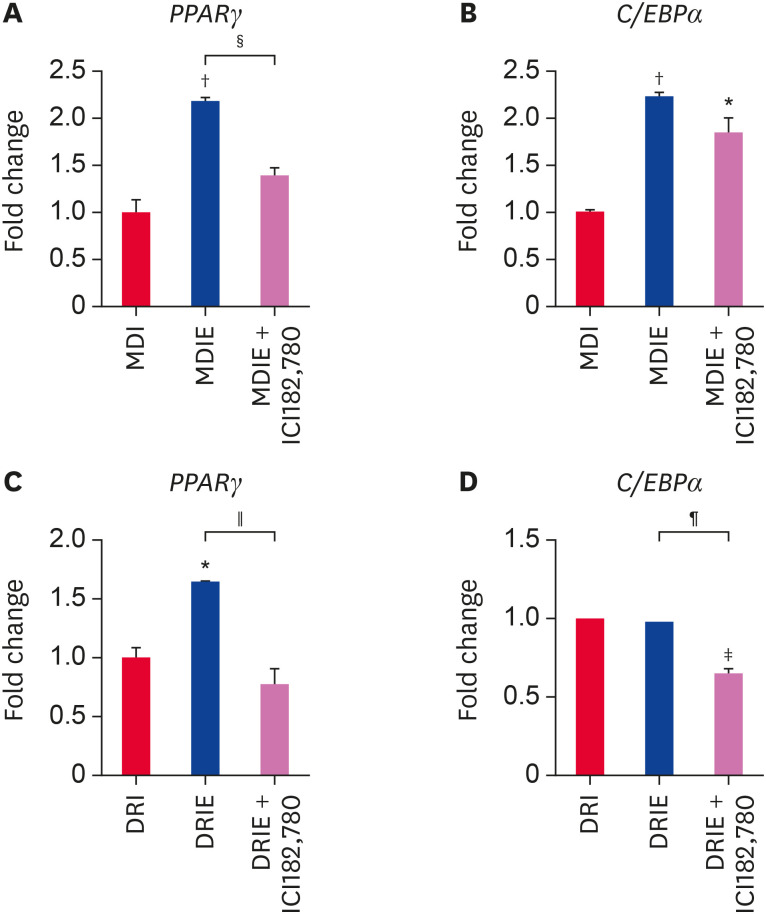
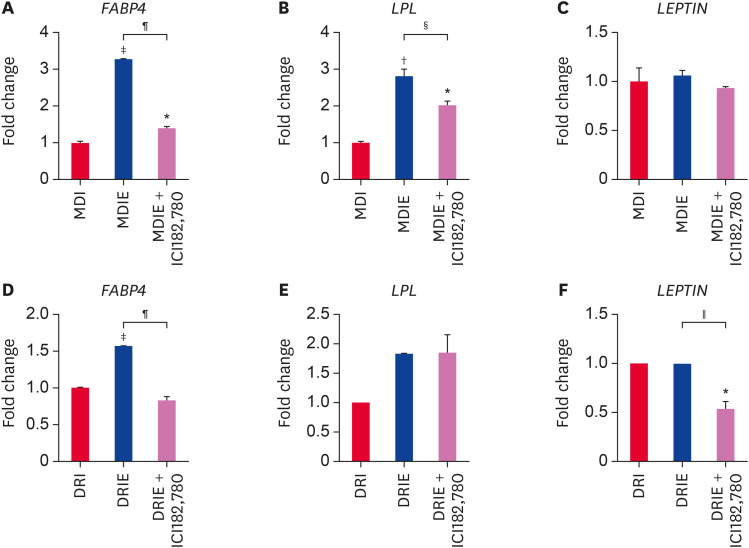
At 14 days of adipogenic differentiation, we examined the mRNA expression of adipogenic transcription factors by qRT-PCR. Addition of E2 to MDI condition significantly upregulated PPARγ and C/EBPα mRNA expression as compared with MDI condition and this effect was attenuated in the presence of ICI182,780 (Fig. 7A and B). In contrast, while addition of E2 to DRI significantly stimulated PPARγ mRNA expression as compared with DRI conditon (Fig. 7C), no difference was observed in C/EBPα mRNA expression (Fig. 7D). However, PPARγ and C/EBPα mRNA expression was significantly suppressed by ICI182,780 (Fig. 7C and D). Furthermore, we assessed the mRNA expression of adipogenesis-related genes. In the MDIE condition, FABP4 and LPL mRNA expression were effectively stimulated by E2 and inhibited by ICI182,780 (Fig. 8A and B), whereas LEPTIN mRNA expression was not significantly different (Fig. 8C). In the DRIE condition, only FABP4 mRNA expression was significantly upregulated by E2 (Fig. 8D). ICI182,780 significantly suppressed expression of adipogenesis-related genes excepted for LPL (Fig. 8D-F). These results suggest that estrogen stimulates adipogenic differentiation via ER in cADSCs.
Fig. 7. Effect of estrogen on the mRNA expression of transcription factors related to adipogenic differentiation of cADSCs. cADSCs were induced to adipogenic differentiation under MDIE and DRIE condition for 21 days. (A) PPARγ and (B) C/EBPα mRNA expression in MDIE condition and (C) PPARγ and (D) C/EBPα mRNA expression in DRIE condition were quantified by qRT-PCR. The results are presented as mean ± SEM.
cADSC, canine adipose-derived stem cell; PPARγ, peroxisome proliferator-activated receptor gamma; C/EBPα, CCAAT/enhancer-binding protein alpha; qRT-PCR, quantitative real-time reverse-transcription polymerase chain reaction; SEM, standard error of mean; MDI, 3-isobutyl-1-methylxanthine + dexamethasone + insulin; DRI, dexamethasone + rosiglitazone + insulin.
*p < 0.05, †p < 0.01, ‡p < 0.001 vs. MDI or DRI, §p < 0.05, ∥p < 0.01, ¶p < 0.001 vs. MDIE or DRIE.
Fig. 8. Effect of estrogen on the mRNA gene expressions related to adipogenic differentiation of cADSCs. cADSCs were induced to adipogenic differentiation in MDIE and DRIE condition for 21 days. (A) FABP4, (B) LPL, and (C) LEPTIN mRNA expression in MDIE condition and (D) FABP4, (E) LPL, and (F) LEPTIN mRNA expression in DRIE condition were quantified by qRT-PCR.
cADSC, canine adipose-derived stem cell; FABP4, fatty acid-binding protein 4; LPL, lipoprotein lipase; qRT-PCR, quantitative real-time reverse-transcription polymerase chain reaction; MDI, 3-isobutyl-1-methylxanthine + dexamethasone + insulin; DRI, dexamethasone + rosiglitazone + insulin.
*p < 0.05, †p < 0.01, ‡p < 0.001 vs. MDI or DRI, §p < 0.05, ∥p < 0.01, ¶p < 0.001 vs. MDIE or DRIE.
DISCUSSION
ADSCs are considered as effective treatment models because they can be differentiated into adipocytes, osteoblasts, myocytes, and chondrocytes [1]. Basic studies with ADSCs isolated from donors such as human, murine, and canine to understand their characterization and potential have been widely conducted for many years [2,3,4]. Some in vitro studies that investigated the control of several differentiation processes of ADSCs suggested therapeutic applications such as tissue, bone, cartilage, and cardiovascular regeneration and improved our understanding of the underlying mechanisms [21].
Obesity refers to the excessive accumulation of fat. There has been a growing interest health problems related to companion dogs, especially obesity [22]. Similar to obese humans, obese dogs have a high risk of secondary diseases. However, cADSCs have been rarely employed to study the mechanism underlying obesity in dogs.
In the present study, we performed adipogenic differentiation by developing optimal differentiation conditions using cADSCs to understand the obesity mechanism in dogs. cADSCs were induced to adipogenic differentiation in DMEM containing 10% FBS or 5% rabbit serum supplemented with MDI, DRI, MDRI, and MDI-indomethacin (M, IBMX; D, dexamethasone; R, rosiglitazone; I, insulin). While indomethacin is known to drive PPARγ expression and induce adipogenesis [23], we found it to be cytotoxicity under MDI-indomethacin-induced adipogenesis condition (not shown). Although some studies have reported rabbit serum to enhance adipogenesis in vitro [24,25], we observed no significant difference in the results using rabbit serum instead of FBS (not shown).
We examined cADSCs that were induced with three adipogenic differentiation medium conditions, namely MDI, DRI, and MDRI, to investigate the optimal method for adipogenic differentiation. Among these conditions, MDRI-induced adipogenesis was the most effective for 21 days, and the effects of MDI and DRI were similar.
Addition of IBMX to DRI led to effective differentiation ability as compared with DRI condition. IBMX is important during the early stage of adipogenic differentiation [26]. IBMX is commonly used as differentiation inducers and increases intracellulatr cyclic adenosine monophosphate (cAMP), stimulating expression of C/EBPβ [27]. C/EBPβ and C/EBPδ induce expression of C/EBPα and PPARγ, known as regulating terminal differentiation procedure [28]. These previous studies support our results that MDRI induced better adipogenesis than DRI.
Unlike previous studies [27,28], we observe that DRI treatment induced adipogenic differentiation of cADSCs without IBMX. A previous study reported dexamethasone and rosiglitazone (D&R) to induce sufficient adipogenic differentiation. D&R increased the expression of PPARγ and C/EBPα, and this effect was attenuated by the glucocorticoid receptor (GR) antagonist RU486 and the PPARγ antagonist GW9662 [29]. These previous study demonstrated the validity of our results and confirm that DRI can induce adipogenesis without IBMX.
In the present study, we investigated whether rosiglitazone stimulates adipogenesis via PPARγ signaling using a PPARγ antagonist. Rosiglitazone functions as a PPARγ agonist by binding to PPARγ ligand [30]. PPARγ is a major transcription factor during adipogenic differentiation and, cross-regulates with C/EBPα [31]. Moreover, PPARγ and C/EBPα regulate adipogenesis-related gene expression [32]. Our results show that MDRI medium with rosiglitazone significantly stimulated expression of PPARγ and C/EBPα as compared with MDI. The increased expression of PPARγ and C/EBPα subsequently stimulated the expression of fatty acid-binding protein 4 (FABP4), lipoprotein lipase (LPL), and LEPTIN, which are adipogenesis-regulated factors. These results are in accordance with previous studies [23,24]. Furthermore, the reduction in PPARγ expression by GW9662 led to the inhibition of MDRI-induced adipogenic differentiation of cADSCs, thereby decreasing the expression of other adipogenesis-related genes. Previous study demonstrated that PPARγ is activated by binding to its ligand, and GW9662 is a selective ligand for PPARγ [33]. In addition, rosiglitazone effect was inhibited by GW9662 [34]. Therefore, we may conclude that rosiglitazone-induced adipogenesis was stimulated via PPARγ signaling.
We next investigated whether estrogen stimulates adipogenesis via ER, using the ER antagonist ICI182,780. Estrogen is a sex steroid hormone that mainly acts by binding to ERα and ERβ expressed on the adipose tissue [35]. In fact, the clear role of estrogen in adipogenesis remains controversial. Some studies have reported that estrogen inhibits adipogenesis in vitro and in vivo. Estrogen downregulated the expression of ERα, ERβ, C/EBPβ, and PPARγ in pre-adipocytes and mesenchymal stem cells (MSCs) [13,16,36] and increases the adipose tissue in an ERα knockout mouse model [37]. In contrast, some studies have reported the ability of estrogen to increase adipocyte number and lipid accumulation [14,38]. Moreover, in vitro studies have reported the estrogen-mediated upregulation in PPARγ, LPL, ERα, and ERβ mRNA expression in adipocytes [15,36].
In this study, the addition of estrogen to MDI and DRI differentiation conditions led to a lipid accumulation and upregulated adipogenesis-related gene expressions in cADSCs. This differentiation ability was suppressed by ICI182,780. These results are in line with those previously published showing that estrogen contributes to increased adipocyte number and lipid accumulation [39].
Several studies have reported the interaction between ER signaling and PPARγ [40,41]. These mechanisms support our results that PPARγ and FABP4 mRNA expression were stimulated by the addition of estrogen to MDIE and DRIE media.
ICI182,780 inhibited the expression of adipogenic-related genes, except LEPTIN, in MDIE condition but not LPL in DRIE condition. This indicates that ICI182,780 may be gene-specific, as reported in a previous study [42]. Furthermore, the levels of C/EBPα, FABP4, and LEPTIN under DRIE condition were lower than those under DRI condition. These results are sufficient to suggest that estrogen stimulates adipogenic differentiation via ER.
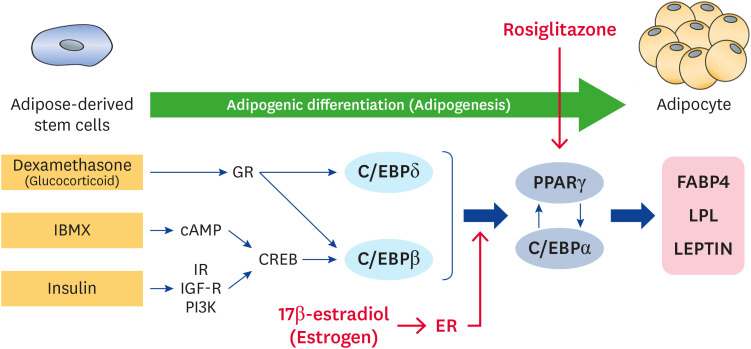
In conclusion, MDRI condition showed the most effective adipogenic differentiation ability as compared to MDI and DRI conditions in cADSCs for 21 days. Rosiglitazone and estrogen could sufficiently stimulate adipogenic differentiation via PPARγ and ER signaling, and these effects were inhibited by the PPARγ antagonist GW9662 and ER antagonist ICI182,780, respectively. Our results suggest that PPARγ and ER are adipogenic differentiation-related signaling, and their agonists and antagonists can to be used as important molecules to study the mechanism of adipogenic differentiation in dogs (Fig. 9). This study provides the basis of obesity or anti-obesity mechanisms in dogs, however, further studies are warranted to demonstrate the ER-dependent mechanism of adipogenesis in cADSCs.
Figure 9. An overview of the adipogenic differentiation process in cADSCs.
cADSC, canine adipose-derived stem cell; IBMX, 3-isobutyl-1-methylxantine; GR, glucocorticoid receptor; cAMP, cyclic adenosine monophosphate; IR, insulin receptor; IGF-R, insulin-like growth factor receptor; CREB, cAMP response element-binding protein; C/EBP, CCAAT/enhancer-binding protein; PPAR, peroxisome proliferator activated receptor; FABP, fatty acid binding protein; LPL, lipoprotein lipase; ER, estrogen receptor.
ACKNOWLEDGEMENTS
We are grateful to Prof. Oh-kyeong Kweon (Seoul National University) for providing the cADSCs.
Footnotes
Funding: This work was supported by “Cooperative Research Program of Center for Companion Animal Research (project No. PJ01398403)” Rural Development Administration, Republic of Korea and partly supported by Gachon University research fund of 2018 (GCU-2018-0704).
Conflict of Interest: The authors declare no conflicts of interest.
- Conceptualization: Lee HJ.
- Formal analysis: Kim JY, Park EJ, Kim SM.
- Investigation: Kim JY, Kim SM.
- Methodology: Lee HJ, Kim JY, Park EJ.
- Project administration: Park EJ.
- Supervision: Lee HJ.
- Visualization: Kim JY, Park EJ.
- Writing - original draft: Kim JY, Park EJ.
- Writing - review & editing: Lee HJ.
References
- 1.Bunnell BA, Flaat M, Gagliardi C, Patel B, Ripoll C. Adipose-derived stem cells: isolation, expansion and differentiation. Methods. 2008;45(2):115–120. doi: 10.1016/j.ymeth.2008.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Schwarz C, Leicht U, Rothe C, Drosse I, Luibl V, Röcken M, et al. Effects of different media on proliferation and differentiation capacity of canine, equine and porcine adipose derived stem cells. Res Vet Sci. 2012;93(1):457–462. doi: 10.1016/j.rvsc.2011.08.010. [DOI] [PubMed] [Google Scholar]
- 3.Zuk PA, Zhu M, Mizuno H, Huang J, Futrell JW, Katz AJ, et al. Multilineage cells from human adipose tissue: implications for cell-based therapies. Tissue Eng. 2001;7(2):211–228. doi: 10.1089/107632701300062859. [DOI] [PubMed] [Google Scholar]
- 4.Jankowski M, Dompe C, Sibiak R, Wąsiatycz G, Mozdziak P, Jaśkowski JM, et al. In vitro cultures of adipose-derived stem cells: an overview of methods, molecular analyses, and clinical applications. Cells. 2020;9(8):1783. doi: 10.3390/cells9081783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kingham PJ, Kolar MK, Novikova LN, Novikov LN, Wiberg M. Stimulating the neurotrophic and angiogenic properties of human adipose-derived stem cells enhances nerve repair. Stem Cells Dev. 2014;23(7):741–754. doi: 10.1089/scd.2013.0396. [DOI] [PubMed] [Google Scholar]
- 6.Ma T, Sun J, Zhao Z, Lei W, Chen Y, Wang X, et al. A brief review: adipose-derived stem cells and their therapeutic potential in cardiovascular diseases. Stem Cell Res Ther. 2017;8(1):124. doi: 10.1186/s13287-017-0585-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hong W, Park J, Yun W, Kang PJ, Son D, Jang J, et al. Inhibitory effect of celastrol on adipogenic differentiation of human adipose-derived stem cells. Biochem Biophys Res Commun. 2018;507(1-4):236–241. doi: 10.1016/j.bbrc.2018.11.014. [DOI] [PubMed] [Google Scholar]
- 8.Dieudonne MN, Pecquery R, Leneveu MC, Giudicelli Y. Opposite effects of androgens and estrogens on adipogenesis in rat preadipocytes: evidence for sex and site-related specificities and possible involvement of insulin-like growth factor 1 receptor and peroxisome proliferator-activated receptor γ2. Endocrinology. 2000;141(2):649–656. doi: 10.1210/endo.141.2.7293. [DOI] [PubMed] [Google Scholar]
- 9.Farmer SR. Transcriptional control of adipocyte formation. Cell Metab. 2006;4(4):263–273. doi: 10.1016/j.cmet.2006.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Scott MA, Nguyen VT, Levi B, James AW. Current methods of adipogenic differentiation of mesenchymal stem cells. Stem Cells Dev. 2011;20(10):1793–1804. doi: 10.1089/scd.2011.0040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fayyad AM, Khan AA, Abdallah SH, Alomran SS, Bajou K, Khattak MNK. Rosiglitazone enhances browning adipocytes in association with MAPK and PI3-K pathways during the differentiation of telomerase-transformed mesenchymal stromal cells into adipocytes. Int J Mol Sci. 2019;20(7):1618. doi: 10.3390/ijms20071618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Foryst-Ludwig A, Kintscher U. Metabolic impact of estrogen signalling through ERalpha and ERbeta. J Steroid Biochem Mol Biol. 2010;122(1-3):74–81. doi: 10.1016/j.jsbmb.2010.06.012. [DOI] [PubMed] [Google Scholar]
- 13.Jeong S, Yoon M. 17β-Estradiol inhibition of PPARγ-induced adipogenesis and adipocyte-specific gene expression. Acta Pharmacol Sin. 2011;32(2):230–238. doi: 10.1038/aps.2010.198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dieudonné MN, Leneveu MC, Giudicelli Y, Pecquery R. Evidence for functional estrogen receptors alpha and beta in human adipose cells: regional specificities and regulation by estrogens. Am J Physiol Cell Physiol. 2004;286(3):C655–C661. doi: 10.1152/ajpcell.00321.2003. [DOI] [PubMed] [Google Scholar]
- 15.Hong L, Colpan A, Peptan IA, Daw J, George A, Evans CA. 17-β estradiol enhances osteogenic and adipogenic differentiation of human adipose-derived stromal cells. Tissue Eng. 2007;13(6):1197–1203. doi: 10.1089/ten.2006.0317. [DOI] [PubMed] [Google Scholar]
- 16.Stubbins RE, Holcomb VB, Hong J, Núñez NP. Estrogen modulates abdominal adiposity and protects female mice from obesity and impaired glucose tolerance. Eur J Nutr. 2012;51(7):861–870. doi: 10.1007/s00394-011-0266-4. [DOI] [PubMed] [Google Scholar]
- 17.Kopelman PG. Obesity as a medical problem. Nature. 2000;404(6778):635–643. doi: 10.1038/35007508. [DOI] [PubMed] [Google Scholar]
- 18.German AJ. The growing problem of obesity in dogs and cats. J Nutr. 2006;136(7) Suppl:1940S–1946S. doi: 10.1093/jn/136.7.1940S. [DOI] [PubMed] [Google Scholar]
- 19.Ryu HH, Kang BJ, Park SS, Kim Y, Sung GJ, Woo HM, et al. Comparison of mesenchymal stem cells derived from fat, bone marrow, Wharton's jelly, and umbilical cord blood for treating spinal cord injuries in dogs. J Vet Med Sci. 2012;74(12):1617–1630. doi: 10.1292/jvms.12-0065. [DOI] [PubMed] [Google Scholar]
- 20.Ryu HH, Lim JH, Byeon YE, Park JR, Seo MS, Lee YW, et al. Functional recovery and neural differentiation after transplantation of allogenic adipose-derived stem cells in a canine model of acute spinal cord injury. J Vet Sci. 2009;10(4):273–284. doi: 10.4142/jvs.2009.10.4.273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tobita M, Orbay H, Mizuno H. Adipose-derived stem cells: current findings and future perspectives. Discov Med. 2011;11(57):160–170. [PubMed] [Google Scholar]
- 22.Animal and Plant Quarantine Agency. 2017 Animal Protection and Welfare Survey. Gimcheon: Animal and Plant Quarantine Agency; 2017. [Google Scholar]
- 23.Saben J, Thakali KM, Lindsey FE, Zhong Y, Badger TM, Andres A, et al. Distinct adipogenic differentiation phenotypes of human umbilical cord mesenchymal cells dependent on adipogenic conditions. Exp Biol Med (Maywood) 2014;239(10):1340–1351. doi: 10.1177/1535370214539225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lu YH, Dallner OS, Birsoy K, Fayzikhodjaeva G, Friedman JM. Nuclear Factor-Y is an adipogenic factor that regulates leptin gene expression. Mol Metab. 2015;4(5):392–405. doi: 10.1016/j.molmet.2015.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yu G, Floyd ZE, Wu X, Hebert T, Halvorsen YD, Buehrer BM, et al. Adipogenic differentiation of adipose-derived stem cells. Methods Mol Biol. 2011;702:193–200. doi: 10.1007/978-1-61737-960-4_14. [DOI] [PubMed] [Google Scholar]
- 26.Lala-Tabbert N, Fu D, Wiper-Bergeron N. Induction of CCAAT/enhancer-binding protein β expression with the phosphodiesterase inhibitor isobutylmethylxanthine improves myoblast engraftment into dystrophic muscle. Stem Cells Transl Med. 2016;5(4):500–510. doi: 10.5966/sctm.2015-0169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kim SP, Ha JM, Yun SJ, Kim EK, Chung SW, Hong KW, et al. Transcriptional activation of peroxisome proliferator-activated receptor-γ requires activation of both protein kinase A and Akt during adipocyte differentiation. Biochem Biophys Res Commun. 2010;399(1):55–59. doi: 10.1016/j.bbrc.2010.07.038. [DOI] [PubMed] [Google Scholar]
- 28.Madsen MS, Siersbæk R, Boergesen M, Nielsen R, Mandrup S. Peroxisome proliferator-activated receptor γ and C/EBPα synergistically activate key metabolic adipocyte genes by assisted loading. Mol Cell Biol. 2014;34(6):939–954. doi: 10.1128/MCB.01344-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Contador D, Ezquer F, Espinosa M, Arango-Rodriguez M, Puebla C, Sobrevia L, et al. Dexamethasone and rosiglitazone are sufficient and necessary for producing functional adipocytes from mesenchymal stem cells. Exp Biol Med (Maywood) 2015;240(9):1235–1246. doi: 10.1177/1535370214566565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rosen ED, Spiegelman BM. PPARγ: a nuclear regulator of metabolism, differentiation, and cell growth. J Biol Chem. 2001;276(41):37731–37734. doi: 10.1074/jbc.R100034200. [DOI] [PubMed] [Google Scholar]
- 31.Berger JP, Akiyama TE, Meinke PT. PPARs: therapeutic targets for metabolic disease. Trends Pharmacol Sci. 2005;26(5):244–251. doi: 10.1016/j.tips.2005.03.003. [DOI] [PubMed] [Google Scholar]
- 32.Farmer SR. Regulation of PPARγ activity during adipogenesis. Int J Obes. 2005;29(Suppl 1):S13–S16. doi: 10.1038/sj.ijo.0802907. [DOI] [PubMed] [Google Scholar]
- 33.Leesnitzer LM, Parks DJ, Bledsoe RK, Cobb JE, Collins JL, Consler TG, et al. Functional consequences of cysteine modification in the ligand binding sites of peroxisome proliferator activated receptors by GW9662. Biochemistry. 2002;41(21):6640–6650. doi: 10.1021/bi0159581. [DOI] [PubMed] [Google Scholar]
- 34.Seargent JM, Yates EA, Gill JH. GW9662, a potent antagonist of PPARγ, inhibits growth of breast tumour cells and promotes the anticancer effects of the PPARγ agonist rosiglitazone, independently of PPARγ activation. Br J Pharmacol. 2004;143(8):933–937. doi: 10.1038/sj.bjp.0705973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Heine PA, Taylor JA, Iwamoto GA, Lubahn DB, Cooke PS. Increased adipose tissue in male and female estrogen receptor-α knockout mice. Proc Natl Acad Sci U S A. 2000;97(23):12729–12734. doi: 10.1073/pnas.97.23.12729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tao Z, Zheng LD, Smith C, Luo J, Robinson A, Almeida FA, et al. Estradiol signaling mediates gender difference in visceral adiposity via autophagy. Cell Death Dis. 2018;9(3):309. doi: 10.1038/s41419-018-0372-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wada T, Ihunnah CA, Gao J, Chai X, Zeng S, Philips BJ, et al. Estrogen sulfotransferase inhibits adipocyte differentiation. Mol Endocrinol. 2011;25(9):1612–1623. doi: 10.1210/me.2011-1089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hong L, Colpan A, Peptan IA. Modulations of 17-β estradiol on osteogenic and adipogenic differentiations of human mesenchymal stem cells. Tissue Eng. 2006;12(10):2747–2753. doi: 10.1089/ten.2006.12.2747. [DOI] [PubMed] [Google Scholar]
- 39.Pedersen SB, Kristensen K, Hermann PA, Katzenellenbogen JA, Richelsen B. Estrogen controls lipolysis by up-regulating α2A-adrenergic receptors directly in human adipose tissue through the estrogen receptor α. Implications for the female fat distribution. J Clin Endocrinol Metab. 2004;89(4):1869–1878. doi: 10.1210/jc.2003-031327. [DOI] [PubMed] [Google Scholar]
- 40.Wang X, Kilgore MW. Signal cross-talk between estrogen receptor alpha and beta and the peroxisome proliferator-activated receptor gamma1 in MDA-MB-231 and MCF-7 breast cancer cells. Mol Cell Endocrinol. 2002;194(1-2):123–133. doi: 10.1016/s0303-7207(02)00154-5. [DOI] [PubMed] [Google Scholar]
- 41.Surazynski A, Jarzabek K, Miltyk W, Wolczynski S, Palka J. Estrogen-dependent regulation of PPAR-gamma signaling on collagen biosynthesis in adenocarcinoma endometrial cells. Neoplasma. 2009;56(5):448–454. doi: 10.4149/neo_2009_05_448. [DOI] [PubMed] [Google Scholar]
- 42.Boucher JG, Ahmed S, Atlas E. Bisphenol S induces adipogenesis in primary human preadipocytes from female donors. Endocrinology. 2016;157(4):1397–1407. doi: 10.1210/en.2015-1872. [DOI] [PubMed] [Google Scholar]