Abstract
Pleural empyema of extra pulmonary origin is uncommon and empyema secondary to a fistula between the urinary tract and thorax is extremely rare. We report a case of nephropleural fistula causing massive pleural empyema in a 64-year-old woman with a long history of urological problems, including nephrolitiasis and urinary tract infection. She was admitted with sepsis, fever, chills, tachypnea, productive cough and pyuria. At clinical examination, breath sounds were reduced over the left hemithorax. CT revealed a fistulous connection from the upper left calyceal group and the pleural space. Drainage of thoracic and perinephric collection was carried out, but nephrectomy and pleural decortication were required due to haemopurulent urine and decreased hemoglobin levels during the hospitalization. This case demonstrates the unusual and prolonged evolution of an obstructive hydroureteronephrosis complicated by pyonephrosis, culminating in retroperitoneal abscess that fistulized into the pleural space, leading to empyema.
Keywords: Nephropleural fistula, Pleural empyema, Thoracic empyema, Abscess, Pyonephrosis, Computed Tomography
Introduction
Empyema thoracis is an infectious process defined by the presence of pus in the pleural space and generally it is the result of an extension of infection from a contiguous source and it is commonly related to lung infections. Pulmonary manifestations of intra-abdominal pathologies are usually represented by non-complicated pleural effusions, and they are associated with a wide range of diseases of subdiaphragmatic origin, these can be transudative or exudative secondary to conditions such as pancreatitis, subphrenic collections or paraneoplastic syndromes [1]. The development of parapneumonic effusion and empyema occurs into three stages over a period of 3 to 6 weeks: the exudative stage, fibrinopurulent stage and the organizing/late stage [2]. Pleural effusions related to the renal system are defined as “urothorax” and characterized by the presence of extravasated urine crossing the diaphragm via lymphatic channels in the case of obstructive uropathy, and they are characterized by low pH and protein levels. Extra-pulmonary origin of pleural empyema are infrequent [3], and empyema that forms secondary to a fistula extending between the urinary tract and thorax represents a very rare clinical entity [4,5], although pulmonary complications may occur in up to 20% of renal infections [6].
We report a case of nephropleural fistula developing in 64-year-old woman with longstanding urological problems.
Case
A 64-year-old woman, with a long history of urological problems including recurrent nephrolithiasis and urinary tract infections, referred at our hospital with a diagnosis of pulmonary effusion. She presented high fever and chills, she was tachypneic and complained of a recent history of progressive dyspnea and productive cough. Urine was cloudy and foul smelling, indicating the presence of pyuria. Physical examination revealed diminished breath sounds over the left hemithorax. Blood tests showed leukocytosis (11.3 103/mm3, n.v. 4.2-10.5), thrombocytosis (PLT 525 103/mm3, n.v. 150-400), increased fibrinogen (>900 mg/dl, n.v. 160-380), and increased C reactive protein levels (16.3 mg/dl, n.v. 0.0-0.5)). Because of the diagnosis of recurrent nephrolitiasis and urinary tract infections, the patient underwent chest-abdomen-pelvis CT before and after contrast administration [5,7]. CT documented a left conspicuous multiloculated pleural effusion with pleural thickening and contrast enhancement of pleural layers. Pleural effusion was characterized by fluid/over fluid density with contextual air inclusions (Fig. 1A). The left kidney showed delayed parenchymographic enhancement and absent excretion of the contrast agent even in the late phases of acquisition. The left calycopelvic system was markedly dilated with parietal thickening. The upper calyceal group showed an anterio- posterior caliber of 25 mm and inhomogeneous high density content extending (Fig. 1B) into a semilunar fluid collection, with hyperemic walls, attached to the posterior renal fascia (Fig. 1C). The semilunar fluid collection was adherent to the lower surface of the left hemidiaphragm and continuous through a fistulous connection directly into the pleural empyema (Fig. 2 A,B). There was perirenal fat stranding, thickening of Gerota's fascia and lymph nodes (Fig. 1D). A CT diagnosis of left pleural empyema secondary to nephropleural fistula, determined by complicated pyonephrosis, was formulated. Prompt drainage, by placing a mono J ureteral stent and a percutaneous drainage, was performed in order to drain both the calycopelvic system and the perirenal abscess, with the emission of purulent fluid [8,9]. The need of urgent therapeutic drainage was established also for the pleural empyema. An endopleural drainage was positioned allowing the aspiration of about 1200 cc of fluid with a frankly purulent appearance [10,11]. A broad spectrum intravenous antibiotic was administered (Meropenem 1000 mg every 8 hours for 14 days). Microbiological cultures of urine and pleural fluid revealed the presence of two obligate anaerobic gram negative bacteria: Bacteroides fragilis and Parabacteroides distasonis. All microbiological cultures presented the same spectrum of antibiotic resistance. In the following days, the patient complained of a treatment-resistant severe left lumbar pain, and urine became haemopurulent from the bladder catheter and the perirenal drainage, hemoglobin levels dropped to 6 g/dL, requiring blood transfusions. Radical left nephrectomy was performed. A few weeks after nephrectomy, the persistence of pleural empyema, despite the endopleural drains, required the execution of a pleural cavity toilette and pulmonary decortication for the evacuation of the infected pleural collection, with the placement of suction drainages, performed under Video-Assisted-Thoracic-Surgery, [2,10,12]. After about two months of hospitalization, drainages were removed, and the patient was discharged with no CT evidence of pleural effusion or fluid collection in the renal lodge.
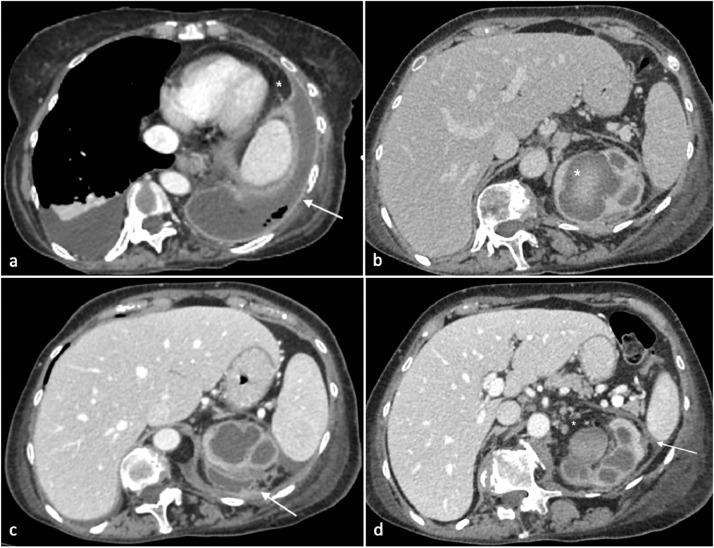
Fig. 1.
Axia CT image after intravenous contrast. (A) Pleural effusion on the right side. On the left hemithorax, a fluid density collection is detected within the pleural space with small air bubbles inside. The fluid collection forms an obtuse angle with the adjacent lung (*). The pleura is thickened, smooth and enhancing. At the margins of empyema, the pleura is dived into the parietal and the visceral layer (split pleural sign). (B) Dilated calicopelvic system of the left kidney with parietal thickening and inhomogeneous urine density. High density material is appreciable in the upper calyceal group. (C) On the upper pole of the left kidney adjacent to the dilated and inhomogeneous calicopelvic system, a semilunar fluid collection is appreciated adherent to Gerota's fascia. (D) Dilated left calicopelvic system with inhomogeneous high density content. Gerota's fascia is thickened (white arrow); perirenal fat is inhomogeneous. Lumboaortic nodes are detected (*).
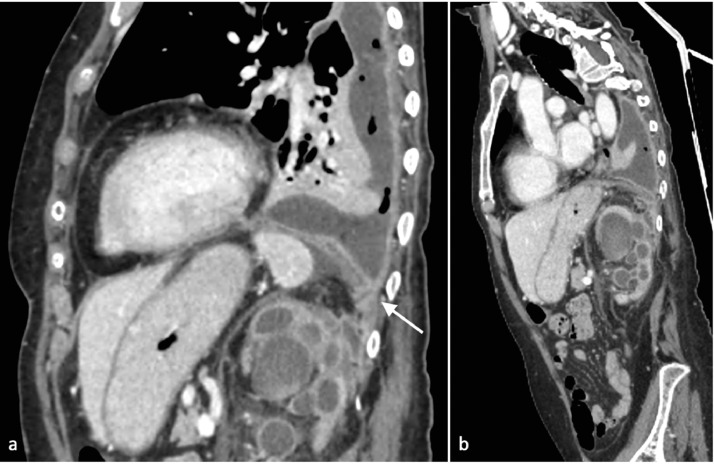
Fig. 2.
(A,B) MPR CT images after intravenous contrast. (A) Sagittal MPR image. The left kidney appeared enlarged with high grade of calicopelvic system dilatation, with parietal thickening and inhomogeneous high density urine. An abscess is appreciated posteriorly on the upper pole of the left kidney. The abscess continues with a fistulous connection that goes through the diaphragm into the pleural empyema. (B) Parasagittal MPR image. The fistulous connection between the perineal abscess and pleural empyema is clearly appreciable.
Discussion
Parapneumonic pleural effusion are classified into uncomplicated or exudative, complicated (resulting from bacterial introduction into the pleural space) and empyema thoracis. Empyema thoracis is characterized by the presence of frank pus in the pleural space or by the evidence of bacterial infection of the pleural fluid by Gram stain or a positive culture [10,11]. The terms pleural infection, complicated parapneumonic effusion (CPPE) and empyema are often used interchangeably, supported by the fact that their clinical management is identical [10,[13], [14], [15]]. The difference between effusion and empyema is based on the concentration of leucocytes; in case of empyema, leucocytes become macroscopically evident and pleural fluid appears as a thick and turbid fluid. Light's criteria used for the diagnosis of empyema are: exudate/pus with polymorphonuclear predominance; Gram stain showing organisms; low glucose; elevated lactate dehydrogenase >1000; and pH < 7.2 [13]. Accumulation of exudative pleural fluid associated with an ipsilateral pulmonary infection that does not look like pus but satisfies the above criteria is also called empyema-like fluid, since positive cultures are only obtained in 56% of cases. Nowadays the role of chest radiography is limited, because it cannot diagnose empyema, but it can only reveal the presence of parapneumonic fluid. Radiographically empyema appears as pleural fluid that is usually unilateral. Helpful differentiating sign at chest x-ray between pleural effusion and empyema, is that the first changes in position with the change in posture of the patient, but this characteristic is limited and can also be confusing in loculated, or very purulent fluid collections [16,17]. Moreover, on standard posterior-anterior and lateral chest radiograph, pleural fluid is detectable in the costophrenic recesses after 50mL has accumulated, and blunting of the costophrenic recesses and obliteration of the hemidiaphragm are seen only when >200mL and >500mL of pleural fluid have accumulated [18], [19], [20]. Ultrasound can rapidly differentiate conditions that demonstrate a non-specific, radiopaque appearance of lower lung fields on chest radiographs, including pleural effusions, pneumonia, atelectasis, elevated hemidiaphragm, and lung or pleural masses with direct contact with the pleural surface [20,21]. Multiple studies have demonstrated the superior diagnostic accuracy of ultrasound compared to chest radiography for the detection of pleural effusions [20]. Pleural ultrasound can detect physiologic amounts and it is 100% sensitive for pleural effusion >100 ml [22]. Based on their sonographic appearance, pleural effusions are categorized as simple or complex. Simple pleural effusions are anechoic, and are usually transudative. Complex pleural effusions are subcategorized as homogeneously or heterogeneously echogenic, with or without septations, and are more often exudative. Homogenously echogenic effusions are most often due to hemothorax or empyema. Empyemas develop from complex effusions that organize into collections of pus and usually have a homogeneously echogenic, speckled appearance [20]. Sonographic evidence of septations in the presence of empyema predicts the need for intrapleural fibrinolytic therapy, longer duration of drainage, and possible surgical intervention [23,24] . Moreover ultrasound-guided needle biopsy is a safe and convenient procedure with a high accuracy for the diagnosis of pleural effusions [25]. Multidetector CT scan allows high-resolution imaging of the pleura with multiplanar coronal and sagittal reconstruction that assist in the evaluation of complex pleural abnormalities adjacent to lung, mediastinal and chest wall lesions. Intravenous contrast administration allows the differentiation of pleural membranes from parenchymal process for patients with empyema and associated pulmonary infections or neoplasms [26]. The CT imaging appearance of empyema is represented by a unilateral pleural effusion associated with an area of consolidation. In advanced stage of the disease the pleural collection may appear loculated [26], although the diagnostic capability of ultrasound in defining the type of fluid and the presence of septations remains superior [5,20,27]. Contrast enhanced CT is helpful demonstrating the characteristic “split pleural sign”, which refers to the presence of thickened enhancing parietal and visceral pleural layers separated by pleural fluid [28]. Pleural thickening and enhancement are seen more frequently in empyemas compared with parapneumonic effusions and it is often associated with pleural thickness [26,27]. Septations are less clearly seen on CT, although they can be inferred by the presence of gas within separate locules [26]. The presence of intrapleural gas is often indicative of infection sustained by aerogenic bacteria, but that can also be a consequence of esophageal-pleural or bronchopleural fistulization. Tsujimoto et al [29] demonstrated a high diagnostic yield of both split pleura sign and large pleural effusion (≥30 mm) on thoracic CT for discriminating between complicated parapneumonic effusion/empyema and parapneumonic effusion. Other CT signs are represented by thickening of the extrapleural tissues and increased attenuation of the extrapleural fat, defined as an increase of greater than 50 HU when compared with the fat posterior to the spine [26,27]. Mediastinal lymph node enlargement is frequently seen in community- acquired parapneumonic effusions and empyemas, and it is usually ipsilateral. Pulmonary complications are not uncommonly associated with abdominal pathologies and simple (non infected) pleural effusions, in particular, are associated with a wide range of diseases of subdiaphragmatic origin. Pyonephrosis is defined as the collection of pus in an obstructed collecting system (pus under pressure); the obstruction may be due to stricture, stone, congenital anomaly, or tumor and may also be associated with catheterization or placement of other devices, endoscopic maneuvers, renal failure, kidney transplantation, acquired or congenital immune system impairment. Pyonephrosis represents a urological emergency that can rapidly progress to sepsis and septic shock. Pyonephrosis can determine generalized peritonitis as a result of a rupture of the pyonephrotic kidney or can develop fistulae [30,31]. Ultrasound and CT are the imaging methods of choice in the diagnosis and staging of pyonephrosis. Although ultrasound is highly accurate in the diagnosis, it may not be able to stage the pathology. CT stages pyonephrosis accurately, detecting extrarenal peritoneal and retroperitoneal complications. Locally, CT can clearly demonstrate renal and perinephric abscesses that not infrequently involve the retroperitoneal spaces and iliopsoas muscle and assess the presence of fistulae to pleura, colon and duodenum. A perinephric abscess affects the renal capsule and Gerota's fascia. Ascending urological infection towards the pleura are rare and only few cases are reported [5,32], pleural empyema secondary to a nephropleural fistula is a rare clinical entity [1,[33], [34], [35]]. In our case, pulmonary symptoms dominated the clinical picture, and it may be challenging if underlying urological pathology remains silent. In conclusion complicated or recurrent pleural effusions and empyemas in patients with underlying urological pathology should alert the clinician in order to exclude possible pulmonary extension.
Patient consent
Patient's consent not required as patient's identity is not disclosed or compromised.
Footnotes
Competing Interest: The authors declare no conflict of interest.
Contributor Information
Stefania Tamburrini, Email: tamburrinistefania@gmail.com.
Marina Lugarà, Email: marinalugara82@gmail.com.
Pietro Paolo Saturnino, Email: pietropsaturnino@libero.it.
Giovanni Ferrandino, Email: gianni.ferrandino90@gmail.com.
Pasquale Quassone, Email: pasquale.quassone@gmail.com.
Silvio Leboffe, Email: silvio.leboffe@gmail.com.
Giuseppe Sarti, Email: sartigiuseppe@gmail.com.
Concetta Rocco, Email: dottimmarocco@gmail.com.
Claudio Panico, Email: cl.panico@tiscali.it.
Francesco Raffaele, Email: raffaelefrancesco@gmail.com.
Teresa Cesarano, Email: cesaranot@yahoo.iy.
Michele Iannuzzi, Email: michele.iannuzzi74@gmail.com.
Lucio Cagini, Email: lucio.cagini@unipg.it.
Ines Marano, Email: ines.marano@tiscali.it.
Bibliography
- 1.Jones G.H., Kalaher H.R., Misra N., Curtis J., Parker R.J. Empyema and respiratory failure secondary to nephropleural fistula caused by chronic urinary tract infection: a case report. Case Rep Pulmonol. 2012;2012 doi: 10.1155/2012/595402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Reichert M., Hecker M., Witte B., Bodner J., Padberg W., Weigand M.A. Stage-directed therapy of pleural empyema. Langenbecks Arch Surg. 2017;402(1):15–26. doi: 10.1007/s00423-016-1498-9. [DOI] [PubMed] [Google Scholar]
- 3.Bobbio A., Bouam S., Frenkiel J., Zarca K., Fournel L., Canny E. Epidemiology and prognostic factors of pleural empyema. Thorax. 2021 doi: 10.1136/thoraxjnl-2020-215267. Epub ahead of print. PMID: 33785584. [DOI] [PubMed] [Google Scholar]
- 4.Irving A.D., Turner M.A. Pleural empyema in association with renal sepsis. Br J Surg. 1976;63(1):70–72. doi: 10.1002/bjs.1800630116. [DOI] [PubMed] [Google Scholar]
- 5.Tamburrini S., Lugara M., Iannuzzi M., Cesaro E., De Simone F., Del Biondo D. Pyonephrosis ultrasound and computed tomography features: a pictorial review. Diagnostics (Basel) 2021;11(2):331. doi: 10.3390/diagnostics11020331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Abd Karim S.H., Wan Zain W.Z., Mohd Hashim M.N., Zakaria A.D., Hayati F., Ng C.Y. Empyema thoracis presented as giant back abscess. Radiol Case Rep. 2021;16(5):1061–1064. doi: 10.1016/j.radcr.2021.02.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Demertzis J., Menias C.O. State of the art: imaging of renal infections. Emerg Radiol. 2007;14(1):13–22. doi: 10.1007/s10140-007-0591-3. [DOI] [PubMed] [Google Scholar]
- 8.Florido C., Herren J.L., Pandhi M.B., Niemeyer M.M. Emergent percutaneous nephrostomy for pyonephrosis: a primer for the on-call interventional radiologist. Semin Intervent Radiol. 2020;37(1):74–84. doi: 10.1055/s-0039-3401842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Thornton R.H., Covey A.M. Urinary drainage procedures in interventional radiology. Tech Vasc Interv Radiol. 2016;19(3):170–181. doi: 10.1053/j.tvir.2016.06.002. [DOI] [PubMed] [Google Scholar]
- 10.Sundaralingam A., Banka R., Rahman N.M. Management of pleural infection. Pulm Ther. 2021;7(1):59–74. doi: 10.1007/s41030-020-00140-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Corcoran J.P., Wrightson J.M., Belcher E., DeCamp M.M., Feller-Kopman D., Rahman N.M. Pleural infection: past, present, and future directions. Lancet Respir Med. 2015;3(7):563–577. doi: 10.1016/S2213-2600(15)00185-X. [DOI] [PubMed] [Google Scholar]
- 12.Coote N., Kay E.S. WITHDRAWN: Surgical versus non-surgical management of pleural empyema. Cochrane Database Syst Rev. 2009;(4) doi: 10.1002/14651858.CD001956.pub3. PMID: 19821285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Light R.W. Parapneumonic effusions and empyema. Proc Am Thorac Soc. 2006;3(1):75–80. doi: 10.1513/pats.200510-113JH. [DOI] [PubMed] [Google Scholar]
- 14.Porcel J.M., Light R.W. [Parapneumonic pleural effusions and empyema in adults:current practice] Rev Clin Esp. 2009;209(10):485–494. doi: 10.1016/s0014-2565(09)72634-7. [DOI] [PubMed] [Google Scholar]
- 15.Vaziri M., Abed O. Management of thoracic empyema: review of 112 cases. Acta Med Iran. 2012;50(3):203–207. [PubMed] [Google Scholar]
- 16.King S., Thomson A. Radiological perspectives in empyema. Br Med Bull. 2002;61:203–214. doi: 10.1093/bmb/61.1.203. [DOI] [PubMed] [Google Scholar]
- 17.Light R.W. Diseases of the pleura. Curr Opin Pulm Med. 1997;3(4):303–304. doi: 10.1097/00063198-199707000-00011. [DOI] [PubMed] [Google Scholar]
- 18.Blackmore C.C., Black W.C., Dallas R.V., Crow H.C. Pleural fluid volume estimation: a chest radiograph prediction rule. Acad Radiol. 1996;3(2):103–109. doi: 10.1016/s1076-6332(05)80373-3. [DOI] [PubMed] [Google Scholar]
- 19.Brixey A.G., Luo Y., Skouras V., Awdankiewicz A., Light R.W. The efficacy of chest radiographs in detecting parapneumonic effusions. Respirology. 2011;16(6):1000–1004. doi: 10.1111/j.1440-1843.2011.02006.x. [DOI] [PubMed] [Google Scholar]
- 20.Soni N.J., Franco R., Velez M.I., Schnobrich D., Dancel R., Restrepo M.I. Ultrasound in the diagnosis and management of pleural effusions. J Hosp Med. 2015;10(12):811–816. doi: 10.1002/jhm.2434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Heffner J.E., Klein J.S., Hampson C. Diagnostic utility and clinical application of imaging for pleural space infections. Chest. 2010;137(2):467–479. doi: 10.1378/chest.08-3002. [DOI] [PubMed] [Google Scholar]
- 22.Grimberg A., Shigueoka D.C., Atallah A.N., Ajzen S., Iared W. Diagnostic accuracy of sonography for pleural effusion: systematic review. Sao Paulo Med J. 2010;128(2):90–95. doi: 10.1590/S1516-31802010000200009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tu C.Y., Hsu W.H., Hsia T.C., Chen H.J., Tsai K.D., Hung C.W. Pleural effusions in febrile medical ICU patients: chest ultrasound study. Chest. 2004;126(4):1274–1280. doi: 10.1378/chest.126.4.1274. [DOI] [PubMed] [Google Scholar]
- 24.McLoud T.C., Flower C.D. Imaging the pleura: sonography, CT, and MR imaging. AJR Am J Roentgenol. 1991;156(6):1145–1153. doi: 10.2214/ajr.156.6.2028857. [DOI] [PubMed] [Google Scholar]
- 25.Lin Z., Wu D., Wang J., Wang C., Huang M. Diagnostic value of ultrasound-guided needle biopsy in undiagnosed pleural effusions: A systematic review and meta-analysis. Medicine (Baltimore) 2020;99(27):e21076. doi: 10.1097/MD.0000000000021076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Qureshi N.R., Gleeson F.V. Imaging of pleural disease. Clin Chest Med. 2006;27(2):193–213. doi: 10.1016/j.ccm.2006.02.001. [DOI] [PubMed] [Google Scholar]
- 27.Kearney S.E., Davies C.W., Davies R.J., Gleeson F.V. Computed tomography and ultrasound in parapneumonic effusions and empyema. Clin Radiol. 2000;55(7):542–547. doi: 10.1053/crad.1999.0480. [DOI] [PubMed] [Google Scholar]
- 28.Algin O., Gokalp G., Topal U. Signs in chest imaging. Diagn Interv Radiol. 2011;17(1):18–29. doi: 10.4261/1305-3825.DIR.2901-09.1. [DOI] [PubMed] [Google Scholar]
- 29.Tsujimoto N., Saraya T., Light R.W., Tsukahara Y., Koide T., Kurai D. A simple method for differentiating complicated parapneumonic effusion/empyema from parapneumonic effusion using the split pleura sign and the amount of pleural effusion on thoracic CT. PLoS One. 2015;10(6) doi: 10.1371/journal.pone.0130141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kim C.J., Kato K., Yoshiki T., Okada Y., Tani T. [Intractable duodenocutaneous fistula after nephrectomy for stone pyonephrosis: report of a case] Hinyokika Kiyo. 2003;49(9):547–550. [PubMed] [Google Scholar]
- 31.Kondapi D., Tambe V., Hegazy H. Pyeloduodenal fistula as a result of pyonephrosis. Urol Case Rep. 2018;21:36–37. doi: 10.1016/j.eucr.2018.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Quaresima S., Manzelli A., Ricciardi E., Petrou A., Brennan N., Mauriello A. Spontaneous intraperitoneal rupture of pyonephrosis in a patient with unknown kidney carcinosarcoma: a case report. World J Surg Oncol. 2011;9:39. doi: 10.1186/1477-7819-9-39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Evangelista I., Colombo A., Mazzone A., Mumoli N. Renal pyonephrosis with massive pleural empyema. Intensive Care Med. 2021 doi: 10.1007/s00134-021-06403-4. [DOI] [PubMed] [Google Scholar]
- 34.Cleto Marinho R., Pinheiro G., Almeida S. Empyema secondary to obstructive pyelonephritis. BMJ Case Rep. 2019;12(11):e231985. doi: 10.1136/bcr-2019-231985. PMID: 31690689; PMCID: PMC6855852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tufano A., Minelli R., Di Lascio G., Delicato G., Baffigo G., Signore S. Infected kidney stone progressing to perinephric abscess and thoracic empyema. Arch Ital Urol Androl. 2020;92(3) doi: 10.4081/aiua.2020.3.203. PMID: 33016044. [DOI] [PubMed] [Google Scholar]