Abstract
Introduction
Ceriops decandra (CD) and Ceriops tagal (CT) are two traditionally used mangrove plants widely distributed along the coastal areas of South Asia, Africa, South Pacific. In this study, we evaluated the diuretic potential of aerial roots of CD, CT and assessed the effectiveness of the plants’ terpenoids enriched bioactive constituents against human carbonic anhydrase (hCA) enzyme through molecular docking.
Materials and methods
Firstly, the acute toxicity of CD and CT was evaluated in mice. In vivo diuretic activity was then studied in mice and the volume of excreted urine was measured. The urine was further examined for pH, density and Na+, K+, Cl− concentrations. From this, the saluretic, natriuretic, kaliuretic and CAI (carbonic anhydrase inhibitory) activities were calculated. Finally, total terpenoid contents (TTC) of the plant extracts were quantified and the terpenoids previously reported from both CD and CT were docked against four hCA isoforms - hCAII, hCAIV, hCAXII and hCAXIV.
Results
In the acute toxicity assessment, no sign of toxicity was found. In diuretic activity evaluation, both extracts displayed substantial increase in urine volume, with CD being at top. Concentrations of Na+, K+ and Cl− were also upsurged at a high dose of treatment (500 mg/kg). Both extracts at 500 mg/kg dose demonstrated potent saluretic, natriuretic and CAI activity. The TTC of CD was significantly higher than CT. In molecular docking analysis, greater binding affinity against hCA isoforms was demonstrated by the terpenoids reported from CD.
Conclusion
Aerial roots of both CD and CT possess substantial diuretic activity with an inhibitory effect on CA. Here, diuretic potential as well as the total terpenoid content of CD were much greater between the two.
Keywords: Ceriops decandra, Ceriops tagal, Diuretic, Carbonic anhydrase, Molecular docking
Ceriops decandra; Ceriops tagal; Diuretic; Carbonic anhydrase; Molecular docking.
1. Introduction
Since time immemorial, metabolites of plants have been a substantial source of novel drug leads and the mangrove plant species, offering a plethora of secondary metabolites due to their endurance of harsh environmental conditions like low oxygen, high salinity, low temperature etc., have been evidenced to have magnitude of bioactive compounds [1, 2, 3]. Many of these plants are of great importance to the people of tropical shoreline for their medicinal worth and Ceriops decandra (Griff.) (CD) and Ceriops tagal (Perr.) (CT) of family Rhizophoraceae are two of the most common mangrove plants that have tremendous traditional usage [4].
CD, also known as ‘Goran’ in Bengali, is medium sized, evergreen perennial shrub with brownish to grey colored bark, simple and spirally arranged leaves, small white flours, ovoid-conical fruits and stilt roots [5]. CT, also called ‘Mot Goran’, is a small tree with grey smooth bark, obovate-elliptic glossy-green leaves, pendulous white flower, stilt roots and fruits containing recurved and persistent sepals [6]. CD and CT are widely distributed along the coastal areas of South Asia, Africa, South Pacific islands and Madagascar [7]. The whole plant of CD is traditionally used for treating wounds, hemorrhage, angina and diabetes, the barks are used for viral infection, the leaves and fruits for ulcer, hepatitis and the roots for pain [8]. On the other hand, the ethno-medicinal uses of the barks, leaves and roots of CT include the treatment of hemorrhage, diabetes, sores, malaria, ulcers [9].
Magnitude of bioactive phytoconstituents, mostly terpenoids, have already been reported for both plants. Major di- and triterpenoids reported from the roots, leaves and barks of CD are ceriopsins A-G; decandrins A-K; tagalsin X; pinoresinol; lupeol; lupenone; betulin; betulinaldehyde; betulinic and epibetulinic acid; lupan-3β, 20-diol [8, 10, 11]. In considering CT, the bioactive terpenoids reported from the roots, stems and aerial parts of the plant include tagalsins A-H, P, X; ent-5α,2-oxodolabr-3-ene-3,15,16-triol; betulin; betulinic acid; ent-8(14)-pimarane-16,18-dihydroxy-15-one; (5S∗,8S∗,9S∗,10R∗)-13S∗-hydroxy-4S∗,18-epoxy-15,16-dinordolabr-1-en-3-one; lupeol [7, 12].
The versatile medicinal properties, diverse traditional utilization and vast amount of phytoconstituents steered us to evaluate its effectiveness against cardiac ailments, specifically hypertension. We investigated the potential diuretic effect of the plant extracts because the major compounds reported from the plants are largely terpenoids and many terpenoids have previously been reported for their diuretic potential [13, 14]. Here, the diuretic activity of aerial roots of both plants was first assayed in vivo using mice model and a comparison was drawn between the two. The concentrations of Na+, K+ and Cl− ions as well as the pH and density of the urine of extracts treated mice were also determined in order to measure the saluretic, natriuretic, kaliuretic and carbonic anhydrase inhibition (CAI) index. To identify the bioactive phytoconstituents that are responsible for the diuretic potential of the plant extracts, the previously reported terpenoids from both plants were evaluated in silico by site specific molecular docking. Here, human carbonic anhydrase (hCA) enzyme was the target protein as the catalytically active isoforms of hCA - CAII, CAIV, CAXII and CAXIV are highly expressed in the epithelial cells of renal tubule and are involved in the acid-base homeostasis, bicarbonate reabsorption and renal NH4+ output [15, 16, 17]. Hence, inhibition of the enzyme entirely abolishes the reabsorption of NaHCO3 and stimulates the excretion of Na+ and Cl− ions [18, 19].
2. Materials and methods
2.1. Chemicals
Analytical grade NaCl, KCl, AgNO3, K2CrO4, NaHCO3, Bi(NO3)3, tartaric acid, CuSO4, H2SO4, NaOH, sodium potassium tartrate, sodium citrate, Na2CO3, FeCl3, HCl, HNO3 and α-naphthol were purchased from Sigma-Aldrich Co., USA. Standard drug frusemide was purchased from Square Pharmaceuticals Ltd., Bangladesh.
2.2. Experimental animal
Swiss albino mice of either sex, 22–28 g of weight and 6–7 weeks aged were purchased from the animal house of Department of Pharmacy, Jahangirnagar University, Dhaka for this study. The mice were kept in a controlled environment of 27±2 °C temperature and 60–65% relative humidity for 1–2 weeks before the experiment. The animals were allowed to free access of water and were fed with standard laboratory animal feed. All the experiments conducted on animals were approved by the Animal Ethics Committee of Khulna University, Khulna, Bangladesh (Reference: KUAEC-2020/08/12).
2.3. Collection and extraction of plants
Aerial roots of CD and CT for this study were collected from the Sundarbans in October, 2017. Both species were identified by experts at Bangladesh National Herbarium, Mirpur, Dhaka where two voucher specimens were submitted for future reference (DACB 43819 for CD and DACB 43823 for CT). The collected aerial roots were first cleaned, shed dried to prevent any decomposition of active constituents, ground into fine powder and then macerated in 96% ethanol. After 14 days, the macerated samples were filtered, the solvent was evaporated with a LabTech Rotary Evaporator and traces amount of solvent was desiccated with Borosilicate Glass Laboratory Vacuum Desiccator. The yield for CD and CT was 6.65% and 8.05%, respectively.
2.4. Phytochemical screening
Qualitative assessment of phytoconstituents like reducing sugar, combined reducing sugar, phenolic compounds, flavonoids, tannins, saponins, gums, steroids, terpenoids, alkaloids, glycosides, acidic compounds, amino acids and proteins were conducted with freshly prepared CD and CT extract solutions following the methods described by Golder et al. [20].
2.5. Acute toxicity evaluation
Acute toxicity of plant extracts was evaluated using mice according to the method of Sarkar et al. [21]. The mice were orally administrated with different concentrations of extracts (250, 500, 1000, 2000 mg/kg) and then kept under observations for 14 days to identify effects of extracts on behavioral patterns like alertness, locomotion, salivation, convulsions and potential changes in grip strength, pain response, even mortality. On the 14th day, the mice were weighed and compared to control.
2.6. Evaluation of diuretic activity
First, the mice were divided into six groups with 5 mice in each group (n = 5). Each mouse was then placed in metabolic cage 24 h prior to the experiment. The animals were fasted overnight and pretreated with physiological saline (0.9% NaCl) for uniform water and salt load. Negative control group was orally administered with reconstitution vehicles (Tween 80 and distilled water). The standard group was given frusemide at 10 mg/kg body weight (b.w.) doses. The other four groups were treated with CD and CT extracts at 250 and 500 mg/kg b.w. doses. Urine of the mice were collected and volume was measured each hour for 6 h after administration of samples. After 6 h, the urine was stored under refrigeration (-20 °C) for future analysis. The effect of samples on the urinary excretion rate was analyzed using Eq. (1). Diuretic action of frusemide and plant extracts were then calculated by dividing the urinary excretion rate of test groups by the urinary excretion rate of control groups [22].
| (1) |
2.7. Analysis of urine
The freshly collected urine samples were assayed for pH using the D-50 Series Handheld Water Quality Meters by Horiba Scientific. Density of mice urine was estimated by measuring the volume and weight of urine. Concentrations of Na+ and K+ ions were measured in mEQ/L with a Jenway PFP7 Flame Photometer using a 100 times diluted solution. Concentration of Cl− ion was estimated by direct titration with AgNO3 solution (1%) using the potassium chromate (5%) indicator. From the concentrations of the ions, the saluretic (Na+ + Cl−), natriuretic (Na+/K+), kaliuretic (K+/Na+) and carbonic anhydrase inhibitory (CAI) activity [Cl−/(Na++K+)] were calculated [23, 24]. Finally, the Na+, K+, Cl−, saluretic, natriuretic, kaliuretic and CAI index were also measured by dividing the values in test group with the values in control [24].
2.8. Determination of total terpenoid content (TTC)
200 μL of extract solutions in methanol (0.1 mg/mL) was first mixed with 1 mL of perchloric acid and 300 μL vanillin/glacial acetic acid (5% w/v) solution. 5 mL of glacial acetic acid was then added to it and the absorbance was measured at 548 nm with a Shimadzu UV-Visible spectrophotometer. Ursolic acid at concentrations 0.0625–1 mg/mL was used to generate the standard calibration curve [25].
2.9. Preparation of bioactive ligands and hCA isozymes for molecular docking
To construct an exclusive library of bioactive compounds isolated from CD and CT, we gathered published research articles from PubMed, Scopus, Google scholar and 49 bioactive compounds were selected [7, 8, 10, 11, 12]. The compounds were further checked with databases like Pubchem, Drugbank and Chemspider. Compounds that are not available at those databases were drawn by BIOVIA Draw 2018. Finally, by using the B3LYP correlation function of the DFT method implemented at GaussView software package (version 5.0.8), energy of each compound was minimized [26, 27]. The three-dimensional X-ray crystallographic structures of hCAⅡ (PDBID 1Z9Y), hCAⅣ (PDBID 5JNC), hCAⅫ (PDBID 5MSB) and hCAⅩⅠⅤ (PDBID 5CJF) were collected from Protein Data Bank (www.rcsb.org). Then energy minimization process of the four targets were carried in vacuo with GROMOS 96 43B1 parameters, without reaction field, using the Swiss-PDB Viewer (version 4.1.0) [28]. Finally, water molecules, HETATM and unnecessary residues were removed from the crystal structures of hCA isoforms.
2.10. Site specific molecular docking study of reported compounds
AutoDock software package (version 4.2.6) was used to dock the reported compounds at the active site of hCAⅡ, hCAⅣ, hCAⅫ and hCAⅩⅠⅤ [29]. By using administered Universal Force Field (UFF) and steepest descent optimization algorithm, free energy of each compound was further minimized. 49 bioactive compounds were docked at the inhibitory sites of enzymes to identify potential candidates. Dimensions of Vina search space were kept at 26.98, 29.18 and 26.52Å along the XYZ directions. Binding affinity was shown in kcal/mol (as a negative value). Complex of the best pose at the protein pocket was made using PyMOL and visualized with Discovery studio (version 4.5) [30,31].
2.11. Statistical analysis
One-way ANOVA analysis of test results was conducted with Dunnet's t test (p < 0.05, versus control). Pair-wise comparisons were done with Post-hoc Tukey test (p < 0.05, versus standard/extract). For analyzing the data, SPSS software of IBM Corporation, New York, USA (version 16.0) was used [32].
3. Results
3.1. Phytochemical screening
Phytochemical investigation of both plant extracts demonstrated presence of reducing sugar, combined reducing sugar, polyphenols, tannins, flavonoids, saponins, steroids, terpenoids, alkaloids, glycosides, protein and acidic compounds in both CD and CT. Gums were present only in CT.
3.2. Acute toxicity evaluation
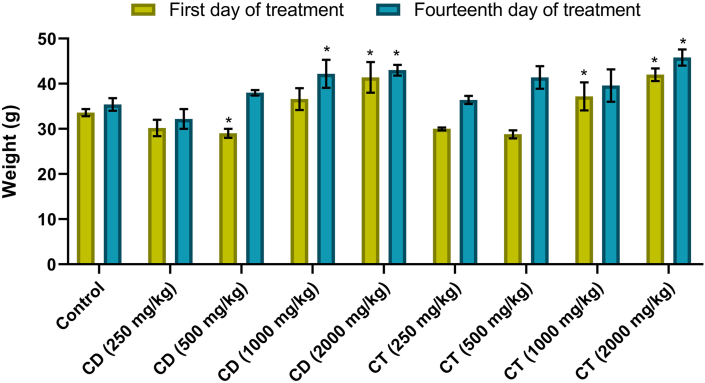
In evaluating the acute toxicity of plant extracts, no mortality or behavioral changes were observed in mice administrated with CD and CT. The weight of mice after 14 days was also measured and slight increase in weight was detected (Figure 1).
Figure 1.
Variation in the body weight of extracts administered mice in the acute toxicity evaluation. Data are means of 5 replicates with SD (standard deviation); ∗p < 0.05 vs. control (Dunnett's t test).
3.3. Evaluation of diuretic activity
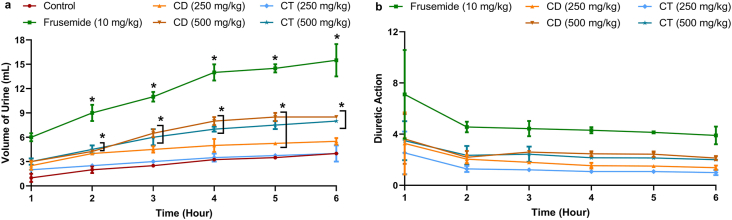
In the diuretic activity test, frusemide standard at 10 mg/kg b.w. and both CD and CT at 500 mg/kg b.w. demonstrated significant (p < 0.05) upsurge in urinary excretion rate in comparison with control. CD at 250 mg/kg b.w. also showed substantial increase in urinary excretion rate, however, for CT at 250 mg/kg, the variation in excretion was insignificant. The diuretic action was highest for frusemide (Figure 2).
Figure 2.
Presentation of urinary output results for 6 h following treatment with standard drug and extracts in the diuretic activity test. Here, ‘a’ presents the volume of urine at different time points and ‘b’ presents the diuretic action of treatment groups at the time points. Data are means of 5 replicates with SD (standard deviation); ∗p < 0.001 vs. control (Dunnett's t test).
3.4. Analysis of urine
The pH and density of urine of extract treated mice was found fairly comparable to that of control (pH ranging from 7.3 to 7.82 and density ranging from 0.93 to 0.99 g/mL). The urine of tested mice was also analyzed for the excretion of electrolytes like Na+, K+, Cl−. The most (p < 0.05) upsurge in the excretion of Na+ was found in case of frusemide, followed by CD at 500 mg/kg, CT at 500 mg/kg, CD at 250 mg/kg and finally CT at 250 mg/kg. However, excretion of K+ was fairly comparable to that of control with slight increase in case of mice treated with CD and CT at 500 mg/kg. In addition, substantial increase in the excretion of Cl− was demonstrated by frusemide and extracts at 500 mg/kg.
3.5. Saluretic, natriuretic, kaliuretic and CAI activity
The saluretic, natriuretic, kaliuretic and CAI activity as well as the saluretic, natriuretic, kaliuretic and CAI indexes after oral administrations of samples were presented in the tables (Tables 1 and 2). Significant (p < 0.05) saluretic effects were displayed by the standard frusemide and both extracts at 500 mg/kg. Natriuretic and kaliuretic effects represent the ratio of Na+ and K+ excretion. Here, frusemide and both extracts at 500 mg/kg showed the most natriuretic ratio. The kaliuretic ratio was comparable to that of control. Substantial fall in the CAI in sample treated mice were also observed. The frusemide standard and extracts at 500 mg/kg dose revealed the most CAI activity. The index values represent the ratio of the effectiveness of samples in relation to the effects in control. Hence, higher saluretic, natriuretic as well as the Na+, K+, Cl− indexes in frusemide and in CD and CT (at 500 mg/kg) represents a higher ratio of effectiveness.
Table 1.
Effect of plant extracts on urinary volume, pH, density, conductivity and electrolyte output of mice after 6 h of treatment in the diuretic activity test.
| Treatment Group | pH | Density (g/mL) | Na+ (mEq/L) | K+ (mEq/L) | Cl− (mEq/L) | Saluretic (Na+ + Cl−) | Natriuretic (Na+/K+) | Kaliuretic (K+/Na+) | CAI [Cl−/(Na++K+)] |
|---|---|---|---|---|---|---|---|---|---|
| Control | 7.3 ± 0.06 | 0.99 ± 0.01 | 50.61 ± 3.58 | 33.34 ± 2.5 | 65.83 ± 13.76 | 116.44 ± 10.62 | 1.52 ± 0.21 | 0.66 ± 0.09 | 0.79 ± 0.18 |
| Frusemide (10 mg/kg) | 7.71 ± 0.12∗|| | 0.99 ± 0.01∗‡§||¶ | 144.95 ± 10.95∗‡||¶ | 53.59 ± 4.24∗¶ | 113.33 ± 11.23∗ | 258.28 ± 20.56∗‡|| | 2.72 ± 0.42∗‡||¶ | 0.37 ± 0.06∗‡|| | 0.57 ± 0.07 |
| CD (250 mg/kg) | 7.39 ± 0.04†§¶ | 0.95 ± 0.03∗†|| | 90.02 ± 3.59∗†§¶ | 57.92 ± 4.15∗ | 100.83 ± 7.63∗ | 190.85 ± 4.17∗†§¶ | 1.56 ± 0.18† | 0.65 ± 0.07† | 0.68 ± 0.05 |
| CD (500 mg/kg) | 7.76 ± 0.02∗‡|| | 0.99 ± 0.01∗†|| | 128.23 ± 7.46∗‡|| | 62.26 ± 4.34∗ | 118.52 ± 6.29∗ | 246.56 ± 2.93∗‡|| | 2.06 ± 0.05 | 0.49 ± 0.01 | 0.62 ± 0.07 |
| CT (250 mg/kg) | 7.43 ± 0.15†§ | 0.93 ± 0.01∗†‡§¶ | 73.29 ± 5.41∗†§¶ | 47.8 ± 6.63∗||§¶ | 88.33 ± 11.27 | 161.63 ± 13.77∗†§¶ | 1.56 ± 0.34† | 0.65 ± 0.13† | 0.72 ± 0.07 |
| CT (500 mg/kg) | 7.82 ± 0.05∗‡|| | 0.99 ± 0.01∗†|| | 119.87 ± 5.47∗†‡|| | 66.6 ± 3.43∗†|| | 120.0 ± 15.61∗ | 239.87 ± 19.29∗‡|| | 1.81 ± 0.16† | 0.56 ± 0.05 | 0.64 ± 0.09 |
Data are means of 5 replicates ±SD (Standard deviation); ∗p < 0.05 vs. Control (Dunnett's t test); †p < 0.05 vs. Frusemide (10 mg/kg); ‡p < 0.05 vs CD (250 mg/kg); §p < 0.05 vs CD (500 mg/kg); ||p < 0.05 vs. CT (250 mg/kg); ¶p < 0.05 vs. CT (550 mg/kg); pair-wise comparison by Post-hoc Tukey test.
Table 2.
Effect of plant extracts on urine output and electrolytic excretion index after collecting 6 h of urine as well as index for the saluretic, natriuretic, kaliuretic and CAI activity.
| Treatment Group | Diuretic Index | Na+ Index | K+ Index | Cl− Index | Saluretic Index |
Natriuretic Index | Kaliuretic Index | CAI Index |
|---|---|---|---|---|---|---|---|---|
| Frusemide (10 mg/kg) | 3.88 | 2.86 | 1.61 | 1.72 | 2.22 | 1.79 | 0.56 | 0.73 |
| CD (250 mg/kg) | 1.38 | 1.78 | 1.74 | 1.53 | 1.64 | 1.02 | 0.97 | 0.87 |
| CD (500 mg/kg) | 2.13 | 2.53 | 1.87 | 1.80 | 2.12 | 1.35 | 0.73 | 0.79 |
| CT (250 mg/kg) | 1.13 | 1.45 | 1.43 | 1.34 | 1.39 | 1.02 | 0.99 | 0.93 |
| CT (500 mg/kg) | 2 | 2.37 | 1.99 | 1.82 | 2.06 | 1.18 | 0.84 | 0.82 |
Diuretic index, volume of urine in test group/volume of urine in control group; Na+ index, sodium excreted in test group/sodium excreted in control group; K+ index, potassium excreted in test group/potassium excreted in control group; Cl− index, chloride excreted in test group/chloride excreted in control group; saluretic index, saluretic activity in test group/saluretic activity in control group; natriuretic index, natriuretic activity in test group/natriuretic activity in control group; kaliuretic index, kaliuretic activity in test group/kaliuretic activity in control group; CAI index, CAI activity in test group/CAI activity in control group.
3.6. Determination of total terpenoid content (TTC)
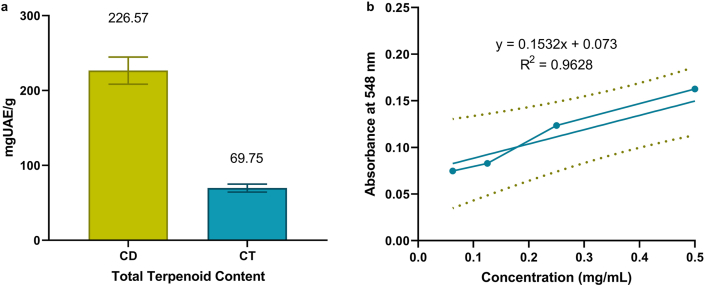
In determining TTC, ursolic acid was used to generate the standard calibration curve (calibration equation y = 0.1532x + 0.073; R2 = 0.9628). The TTC of CD and CT was calculated in terms of mg ursolic acid equivalent (mgUAE)/g of dried plant extract. Here, the TTC of CD and CT was found to be 226.57 and 69.75 mgUAE/g (Figure 3).
Figure 3.
Determination of total terpenoid content of CD and CT plant extract using ursolic acid standard calibration curve. Here, ‘a’ represents the total terpenoid contents of CD and CT; ‘b’ represents the calibration curve for the determination of total terpenoid contents.
3.7. Site specific molecular docking study of reported compounds
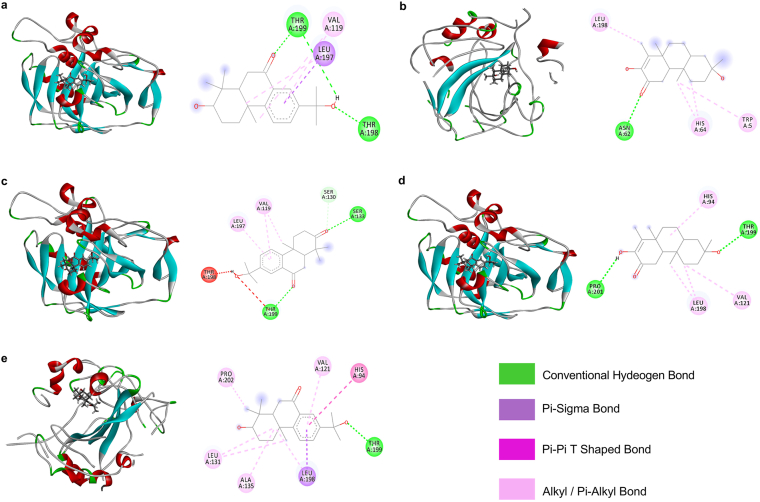
The binding affinity of the compounds obtained in the in-silico study as well as the interacting amino acids of components having high binding affinity have been presented in tables (Tables 3 and 4). Here, phytoconstituents of CD demonstrated greater binding affinity against hCA isoforms. Decandrin G of CD demonstrated the maximum binding affinity against hCAII (- 9.1 kcal/mol). The ligand formed multiple hydrogen bond with THR198 and THR199 at C15 OH and C7 O where, the bond distances were 1.93492 and 1.97098, respectively (Figures 4 and 5). Decandrin C was found to be the most potent inhibitor of hCAXII (- 10.0 kcal/mol), which formed hydrogen bonds with SER133 and THR199 at C3 and C7 O with bond distance of 2.47229 and 1.98688, respectively (Figures 4 and 5). For hCAIV, tagalsin P had the maximum binding affinity (- 7.4 kcal/mol) with a conventional hydrogen bond between ASN62 and C2 O (bond distance 2.56418, Figures 4 and 5). Finally, for hCAXIV, both tagalsin P and decandrin G demonstrated the highest binding affinity (- 8.8 kcal/mol). Here, C3 and C16 OH of tagalsin P formed hydrogen bonds with PRO201 and THR199 (bond distance 2.14257 and 2.43257, respectively). On the other hand, a hydrogen bond was formed between C15 OH of decandrin G and THR199 of hCAXIV with a bond distance of 2.03058 (Figures 4 and 5).
Table 3.
Binding affinity (kcal/mol) of the bioactive compounds previously reported in CD and CT with isomers of CA (CAII, CAIV, CAXII and CAXIV).
| Source |
Compound Name |
hCA II |
hCA IV |
hCA XII |
hCA XIV |
|---|---|---|---|---|---|
| Standard | Frusemide | - 8.3 | - 6.5 | - 7.7 | - 7.5 |
| Compounds found in both CD and CT | Lupeol | - 7.4 | - 6.8 | - 7 | - 8.1 |
| Betulin | - 7.5 | - 6.4 | - 6.5 | - 7.4 | |
| Betulinic acid | - 7.4 | - 6.5 | - 7.4 | - 7.7 | |
| Epibetulinic acid | - 7.7 | - 6.4 | - 7.1 | - 7.7 | |
| Tagalsin X | - 7.6 | - 5.9 | - 6.8 | - 8.6 | |
| Tagalsin P | - 8 | - 7.4 | - 8.6 | - 8.8 | |
| Beta-Sitosterol | - 7.7 | - 6.1 | - 8 | - 7.9 | |
| Compounds found in CD | Ceriopsin A | - 6.9 | - 6.7 | - 7.4 | - 7.9 |
| Ceriopsin B | - 6.8 | - 6.3 | - 6.8 | - 7.9 | |
| Ceriopsin C | - 7.8 | - 5.9 | - 7.3 | - 7.3 | |
| Ceriopsin D | - 8.1 | - 6.1 | - 7 | - 7.2 | |
| Ceriopsin E | - 6.4 | - 5.8 | - 7.3 | - 7.5 | |
| Ceriopsin F | - 6.8 | - 6.5 | - 7 | - 8.2 | |
| Ceriopsin G | - 7 | - 6.2 | - 7.4 | - 7.5 | |
| Decandrin A | - 9 | - 6.6 | - 9.1 | - 8 | |
| Decandrin B | - 8.1 | - 6.2 | - 7.6 | - 8 | |
| Decandrin C | - 9 | - 7.3 | - 10 | - 8.5 | |
| Decandrin D | - 8.8 | - 6.5 | - 8.9 | - 7.8 | |
| Decandrin E | - 8.5 | - 6.5 | - 8.8 | - 8.3 | |
| Decandrin F | - 6.6 | - 5.6 | - 6.5 | - 7.4 | |
| Decandrin G | - 9.1 | - 7.3 | - 9.1 | - 8.8 | |
| Decandrin H | - 8.8 | - 6.3 | - 8.8 | - 8.5 | |
| Decandrin I | - 8.1 | - 6.2 | - 8.6 | - 8.6 | |
| Decandrin J | - 8 | - 6 | - 7.2 | - 7.2 | |
| Decandrin K | - 7.9 | - 5.9 | - 7.4 | - 7.2 | |
| Ent-5α,2-oxodolabr-3-ene-3,15,16-triol | - 6.5 | - 6.1 | - 6.7 | - 7.1 | |
| Pinoresinol | - 7.8 | - 6.6 | - 7.9 | - 7.3 | |
| Betulinaldehyde | - 7.5 | - 6.7 | - 7.4 | - 7.8 | |
| Lupan-3β,20-diol | - 7.2 | - 6.9 | - 7.2 | - 8.1 | |
| Lupenone | - 7.5 | - 7.1 | - 7.1 | - 8.4 | |
| Compounds found in CT | Tagalsin A | - 7.7 | - 6.5 | - 6.9 | - 8 |
| Tagalsin B | - 8 | - 6.4 | - 7 | - 8 | |
| Tagalsin C | - 7.6 | - 6.2 | - 6.8 | - 7.3 | |
| Tagalsin D | - 7.3 | - 6.1 | - 7.3 | - 7.4 | |
| Tagalsin E | - 7.4 | - 6.1 | - 6.6 | - 7.6 | |
| Tagalsin F | - 7.6 | - 6.3 | - 6.9 | - 7.3 | |
| Tagalsin G | - 7.6 | - 6.1 | - 6.6 | - 7.8 | |
| Tagalsin H | - 6.6 | - 5.4 | - 6.2 | - 6.6 | |
| Squalene | - 7.3 | - 5.2 | - 7.2 | - 6.8 | |
| (5S∗, 8S∗,9S∗,10R∗,13S∗) -2,16-dihydroxydolabr-4R∗, 18 –epoxy 3, 15-dione | - 6.8 | - 5.8 | - 7.1 | - 6.8 | |
| Ent-5α,2-oxodolabr-3-ene-3,15,16-triol | - 6.6 | - 6.1 | - 6.4 | - 7.2 | |
| (5S∗,8S∗,9S∗,10R∗)-3,13S∗-dihydroxy-15,16-dinorlabr-3-en-2-one | - 6.8 | - 5.5 | - 6.4 | - 7.6 | |
| (5S∗,8S∗,9S∗,10R∗,13S∗)-3-hydroxy-16-nor-2-oxodolar-3-ene-15-oic acid | - 6.7 | - 6 | - 6.8 | - 7.2 | |
| (5S∗,8S∗,9S∗,10R∗)-13S∗,18-dihydroxy-15,16-dinordolabr-4(18)-ene-3-one | - 6.7 | - 5.5 | - 7.2 | - 7.2 | |
| (5S∗,8S∗,9S∗,10R∗,13S∗)-18-hydroxy-16-nor-3-oxodolabr-4(18)-en-15-oic acid | - 6.3 | - 5.8 | - 6.8 | - 7.2 | |
| (5S∗,8S∗,9S∗,10R∗)-13S∗-hydroxy-4S∗,18-epoxy-15,16-dinordolabr-1-en-3-one | - 6.5 | - 6.9 | - 8.4 | - 8.7 | |
| Ent-8(14)-pimarane-16,18-dihydroxy-15-one | - 7.5 | - 6.1 | - 6.9 | - 7.6 | |
| Ent-8(14)-pimarane-15,18-diol | - 6.6 | - 5.1 | - 6.6 | - 7.2 |
Table 4.
Interaction of selected components having high binding affinity with CAII, CAIV, CAXII and CAXIV.
| Protein | Compound Name | Binding affinity (kcal/mol) | Interacting Amino Acids |
|---|---|---|---|
| hCA II | Tagalsin P | - 8 | THR199 (conventional hydrogen bond), VAL119, LEU139, LEU197, PRO201 (alkyl bond) |
| Decandrin A | - 9 | THR199 (conventional hydrogen bond), LEU197 (pi-sigma bond), HIS91, HIS117, VAL119, VAL141, LEU197, VAL206, TRP208 (alkyl/pi-alkyl bond) | |
| Decandrin C | - 9 | SER133, THR199 (conventional hydrogen bond), LEU197 (pi-sigma bond), VAL119 (alkyl/pi-alkyl bond) | |
| Decandrin D | - 8.8 | THR199 (conventional hydrogen bond), LEU197 (pi-sigma bond), HIS91, HIS117, VAL119, VAL141, LEU197, VAL206, TRP208 (alkyl/pi-alkyl bond) | |
| Decandrin E | - 8.5 | THR199 (pi-donor hydrogen bond), HIS91 (pi-sigma bond), HIS91 (pi-pi T shaped bond), HIS66, HIS93, VAL119, ALA129, LEU139, LEU197 (alkyl/pi-alkyl bond) | |
| Decandrin G | - 9.1 | THR198, THR199 (conventional hydrogen bond), LEU197 (pi-sigma bond), VAL119 (alkyl/pi-alkyl bond) | |
| Decandrin H | - 8.8 | THR199 (conventional hydrogen bond), HIS91, HIS117, VAL119, LEU139, VAL141, LEU197, VAL206, TRP208 (alkyl/pi-alkyl bond) | |
| hCA IV | Tagalsin P | - 7.4 | ASN62 (conventional hydrogen bond), TRP5, HIS64, LEU198 (alkyl/pi-alkyl bond) |
| Decandrin C | - 7.3 | THR199, THR200 (conventional hydrogen bond), HIS94, LEU198 (pi-sigma bond), VAL121, LEU198 (alkyl/pi-alkyl bond) | |
| Decandrin G | - 7.3 | THR199, THR200 (conventional hydrogen bond), LEU198 (pi-sigma bond), VAL121, LEU198 (alkyl/pi-alkyl bond) | |
| (5S∗,8S∗,9S∗,10R∗)-13S∗-hydroxy-4S∗,18-epoxy-15,16-dinordolabr-1-en-3-one | - 6.9 | GLN92 (conventional hydrogen bond), HIS94 (pi-donor hydrogen bond), HIS94 (pi-sigma bond), HIS64, LEU198 (alkyl/pi-alkyl bond) | |
| hCA XII | Tagalsin P | - 8.6 | SER133, THR199 (conventional hydrogen bond), SER133 (carbon hydrogen bond), HIS91, ALA129, LEU197 (alkyl/pi-alkyl bond) |
| Decandrin A | - 9.1 | THR199 (conventional hydrogen bond), LEU197 (pi-sigma bond), HIS91, HIS117, VAL119, VAL141, VAL206, LEU197, TRP208 (alkyl/pi-alkyl bond) | |
| Decandrin C | - 10 | SER133, THR199 (conventional hydrogen bond), LEU197 (pi-sigma bond), VAL119 (alkyl/pi-alkyl bond) | |
| Decandrin D | - 8.9 | THR199 (conventional hydrogen bond), LEU197 (pi-sigma bond), HIS91, HIS117, VAL119, VAL141, LEU197, VAL206, TRP208 (alkyl/pi-alkyl bond) | |
| Decandrin E | - 8.8 | THR199 (pi-donor hydrogen bond), HIS91, VAL119, LEU139, LEU197 (alkyl/pi-alkyl bond) | |
| Decandrin G | - 9.1 | THR198, THR199 (conventional hydrogen bond), LEU197 (pi-sigma bond), VAL119, LEU197 (alkyl/pi-alkyl bond) | |
| Decandrin H | - 8.8 | THR199 (conventional hydrogen bond), HIS91, HIS117, VAL119, ALA129, LEU139, VAL141, LEU197, VAL206, TRP208 (alkyl/pi-alkyl bond) | |
| hCA XIV | Tagalsin P | - 8.8 | THR199, PRO201 (conventional hydrogen bond), HIS94, VAL121, LEU198 (alkyl/pi-alkyl bond) |
| Decandrin G | - 8.8 | THR199 (conventional hydrogen bond), HIS94 (pi-pi T shaped bond), LEU198 (pi-sigma bond), VAL121, LEU131, ALA135, LEU198, PRO202 (alkyl/pi-alkyl bond) | |
| Decandrin I | - 8.6 | HIS64, HIS119, THR199 (conventional hydrogen bond), LEU198 (pi-alkyl bond) | |
| (5S∗,8S∗,9S∗,10R∗)-13S∗-hydroxy-4S∗,18-epoxy-15,16-dinordolabr-1-en-3-one | - 8.7 | THR199 (conventional hydrogen bond), HIS94, VAL121, LEU198 (alkyl/pi-alkyl bond) |
Figure 4.
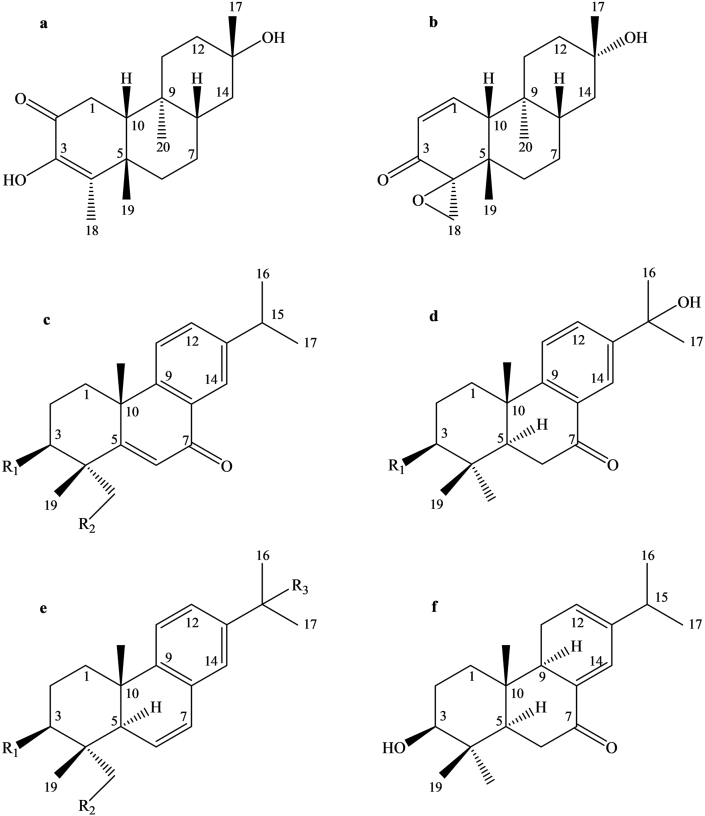
Bioactive compounds reported from CD and CT with substantial carbonic anhydrase (CA) enzyme inhibitory activity. Here, ‘a’ is tagalsin P; ‘b’ is (5S∗,8S∗,9S∗,10R∗)-13S∗-hydroxy-4S∗,18-epoxy-15,16-dinordolabr-1-en-3-one; ‘c’ is decandrin A (R1 OH; R2 H) and decandrin D (R1 H; R2 OH), ‘d’ is decandrin C (R1 O) and decandrin G (R1 OH); ‘e’ is decandrin E (R1 OH; R2 H; R3 H) and decandrin I (R1 H; R2 OH; R3 OH); ‘f’ is decandrin H.
Figure 5.
Molecular docking analysis of major bioactive compounds from CD and CT with the isoforms of CA. Here, ‘a’ is presenting the docking of decandrin G with hCA II; ‘b’ is presenting the docking of tagalsin P with hCA IV; ‘c’ is presenting the docking of decandrin C with hCA XII; ‘d’ is presenting the docking of tagalsin P with hCA XIV and ‘e’ is presenting the docking of decandrin G with hCA XIV.
4. Discussion
Diuretics are useful modulators of body fluids that are therapeutically effective in magnitude of clinical conditions like hypertension, nephritic cirrhosis, cardiovascular failures [24]. Plant oriented terpenoids have previously been reported to have substantial diuretic activity and both CD and CT have already been demonstrated to possess abundance of terpenoids [7, 13]. In addition, total terpenoid contents of both plant extracts were also found to be substantially high. Hence, this study focuses on scientific authentication of the presence of terpenoidal components in CD and CT that stand for their diuretic effectiveness.
Phytochemical screening of the ethanolic plant extracts exhibited a number of compounds which may partly or effusively responsible for multiple bioactivities. In the acute toxicity evaluation, a high dose of 2000 mg/kg in mice didn't demonstrated any signs of adverse reactions. Therefore, for the diuretic activity evaluation, the plant extracts were tested at 250 and 500 mg/kg b.w. doses and then compared with frusemide (10 mg/kg) standard.
Controlling the levels of Na+, K+ and Cl− in plasma is essential in regulating blood volume, blood pressure, acid-base balance as well as maintaining the functionality of cardiac and skeletal muscles [33]. Here, a dose related upsurge in the urinary output as well as a higher excretion of Na+ and Cl− ions were observed during the diuretic activity test along with a slight alkalization of the excreted urine. The upsurge in the level of K+ was marginal. When measuring the saluretic effect, CD and CT at 500 mg/kg b.w. doses showed a greater effectiveness compared to the lower doses, with CD being at top between the two. The ratio of Na+/K+ was the indicator for natriuretic activity and a value greater than 10.0 would indicate a potassium sparing effect. However, in our study, a value greater than 2.0 for CD and CT at 500 mg/kg b.w. dose represented a good natriuretic effect without the potassium sparing activity [34]. CAI activity [Cl−/(Na++K+)] was also calculated and the extracts exhibited noticeable CAI effect (at 500 mg/kg for both CD and CT with CD being at top), demonstrated by a reduction in CAI ratio with values lower than 0.8 (values between 1.0 and 0.8 do not represent CA inhibition) [35]. Hence, diuretic activity of the plant extracts may be attributed, but not limited to the inhibition of carbonic anhydrase enzyme. Inhibition of CA reduce the availability of proton for Na+ - H+ antiporter of proximal tubule. This maintains a low concentration of protein in cell and subsequently transports sodium bicarbonate to the interstitial space from tubular lumen. The overall effect of the process is the augmentation of water in renal tubule, causing diuresis [18].
In conducting the literature review of the plants, terpenoids were found to be the most abundant phytoconstituents [7]. Moreover, in our estimation of total terpenoid content, substantial amount of terpenoids were found in both extracts, with CD having the highest. So, for further in silico assessment, the previously reported terpenoids of CD and CT were docked against four isoforms of h CA - hCAII, hCAIV, hCAXII and hCAXIV as these isoenzymes have been reported to be present in the epithelial cells of renal tubulin [18]. The active site of the isoenzymes consists of a metal binding site as well as amino acid residues amongst which, Thr199, a catalytic residue, has been reported to form hydrogen bonds with the classical hCA inhibitors [36, 37, 38, 39]. Typical inhibitors of hCA bind to the metal at the active site, however, multitude of recently reported inhibitors bind to alternative parts of active site, without interacting with the catalytic metal [37]. In our work, reported terpenoids of CD and CT were docked at the active site of hCAⅡ, hCAⅣ, hCAⅫ and hCAⅩⅠⅤ without considering the catalytic metal. Decandrins A, C, D, E, G, H, I of CD as well as tagalsin P and (5S∗,8S∗,9S∗,10R∗)-13S∗-hydroxy-4S∗,18-epoxy-15,16-dinordolabr-1-en-3-one of CT demonstrated substantial binding affinity to the inhibitory site of hCA isoforms. In addition, all of the stated components formed strong conventional hydrogen bond with the catalytic residue Thr199.
5. Conclusion
To sum up, comparative diuretic activity assessment of the aerial roots of CD and CT demonstrated substantial diuretic effectiveness of both extracts at a high dose with CD being at top of the two. This is possibly attributed to the higher terpenoid content of CD as the molecular docking study demonstrated a greater binding affinity of terpenoidal constituents from CD against hCA isoforms. Further in vitro and in vivo assessments are required in future to establish the drug-ability as well as to assess the pharmacodynamic and pharmacokinetic parameters of the reported components.
Declarations
Author contribution statement
Biswajit Biswas; Mimi Golder: Performed the experiments; Analyzed and interpreted the data; Wrote the paper.
Md. Ahsan Abid: Performed the experiments.
Kishor Mazumder; Samir Kumar Sadhu: Conceived and designed the experiments; Contributed reagents, materials, analysis tools or data.
Funding statement
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Data availability statement
Data will be made available on request.
Declaration of interests statement
The authors declare no conflict of interest.
Additional information
No additional information is available for this paper.
Acknowledgements
The authors would like to acknowledge the contribution of Pharmacy Discipline, Khulna University for providing experimental instruments and laboratory facilities. The authors would also like to express their gratitude towards Department of Pharmacy, Jahangirnagar University for providing the laboratory animals and Bangladesh National Herbarium, Dhaka for identifying the plants.
References
- 1.Biswas B., Golder M., Islam T., Sadhu S.K. Comparative antioxidative and antihyperglycemic profiles of pneumatophores of two mangrove species Avicennia alba and Sonneratia apetala. Dhaka Univ. J. Pharm. Sci. 2018;17(2):205–211. [Google Scholar]
- 2.Gülcin I. Antioxidant activity of food constituents: an overview. Arch. Toxicol. 2012;86(3):345–391. doi: 10.1007/s00204-011-0774-2. [DOI] [PubMed] [Google Scholar]
- 3.Taslimi P., Gülçin İ. Antioxidant and anticholinergic properties of olivetol. J. Food Biochem. 2018;42(3) [Google Scholar]
- 4.Bandaranayake W. Traditional and medicinal uses of mangroves. Mangroves Salt Marshes. 1998;2(3):133–148. [Google Scholar]
- 5.Mahmood H. (SDBC - Sundarbans); Bangladesh: 2015. Handbook of Selected Plant Species of the Sundarbans and the Embankment Ecosystem. Sustainable Development and Biodiversity Conservation in Coastal protection Forests; pp. 10–11. [Google Scholar]
- 6.Tomlinson P.B. second ed. Cambridge University Press; London, UK: 2016. The Botany of Mangroves; pp. 315–355. [Google Scholar]
- 7.Wang H., Li M.Y., Wu J. Chemical constituents and some biological activities of plants from the genus Ceriops. Chem. Biodivers. 2012;9(1):1–11. doi: 10.1002/cbdv.201000299. [DOI] [PubMed] [Google Scholar]
- 8.Mahmud I., Shahria N., Yeasmin S., Iqbal A., Mukul E.H., Gain S. Ethnomedicinal, phytochemical and pharmacological profile of a mangrove plant Ceriops decandra GriffDin Hou. J. Compl. Integr. Med. 2018;16(1):20170129. doi: 10.1515/jcim-2017-0129. [DOI] [PubMed] [Google Scholar]
- 9.Tiwari P., Tamrakar A.K., Ahmad R., Srivastava M.N., Kumar R., Lakshmi V. Antihyperglycaemic activity of Ceriops tagal in normoglycaemic and streptozotocin-induced diabetic rats. Med. Chem. Res. 2008;17(2-7):74–84. [Google Scholar]
- 10.Wang H., Li M.-Y., Satyanandamurty T., Wu J. New diterpenes from a Godavari mangrove, Ceriops decandra. Planta Med. 2013;79(8):666–672. doi: 10.1055/s-0032-1328459. [DOI] [PubMed] [Google Scholar]
- 11.Linh K.T.P., Van Chien N., Trung N.Q., Thong V.H., Van Tuyen N., Thao N.P. Dolabrane-type diterpenoid and lignan constituents from the stem barks of Ceriops decandra (Griff.) W. Theob. Vietnam J. Sci. Technol. 2020;58(4):419–425. [Google Scholar]
- 12.Yuan P., Min-yi L. Diterpenoids from Hainan mangrove, Ceriops tagal. Nat. Prod. Res. 2016;28(12):1870–1874. [Google Scholar]
- 13.Wu J., Li J., Zhang J., Hu X., Yao D., Ma L. In Silico identification and experimental validation of diuresis compounds from Euphorbia lathyris for potential UT-B inhibitors. J. Taiwan Inst. Chem. Eng. 2016;60:124–137. [Google Scholar]
- 14.Kolak U., Ari S., Birman H., Hasancebi S., Ulubelen A. Cardioactive diterpenoids from the roots of Salvia amplexicaulis. Planta Med. 2001;67(8):761–763. doi: 10.1055/s-2001-18359. [DOI] [PubMed] [Google Scholar]
- 15.Topal M., Gülçin İ. Rosmarinic acid: a potent carbonic anhydrase isoenzymes inhibitor. Turkish J. Chem. 2014;38(5):894–902. [Google Scholar]
- 16.Arabaci B., Gülçin I., Alwasel S. Capsaicin: a potent inhibitor of carbonic anhydrase isoenzymes. Molecules. 2014;19(7):10103–10114. doi: 10.3390/molecules190710103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gülçin İ., Trofimov B., Kaya R., Taslimi P., Sobenina L., Schmidt E. Synthesis of nitrogen, phosphorus, selenium and sulfur-containing heterocyclic compounds –determination of their carbonic anhydrase, acetylcholinesterase, butyrylcholinesterase and α-glycosidase inhibition properties. Bioorg. Chem. 2020;103:104171. doi: 10.1016/j.bioorg.2020.104171. [DOI] [PubMed] [Google Scholar]
- 18.Carta F., Supuran C.T. Diuretics with carbonic anhydrase inhibitory action: a patent and literature review (2005–2013) Expert Opin. Ther. Pat. 2013;23(6):681–691. doi: 10.1517/13543776.2013.780598. [DOI] [PubMed] [Google Scholar]
- 19.Brunton L.L., Parker K.L., Blumenthal D.K., Buxton I.L.O. eleventh ed. McGraw-Hill Companies, Inc.; New York: 2008. Goodman and Gilman’s Manual of Pharmacology and Therapeutics; pp. 477–479. [Google Scholar]
- 20.Golder M., Sadhu S.K., Biswas B., Islam T. Comparative pharmacologic profiles of leaves and hypocotyls of a mangrove plant: Bruguiera gymnorrhiza. Adv. Tradit. Med. 2020;20(3):395–405. [Google Scholar]
- 21.Sarkar K.K., Rahman M.M., Shahriar A.A.E., Mitra T., Golder M., Zilani M.N.H. Comparative neuropharmacological and cytotoxic profiles of Alstonia scholaris (L.) and Mimusops elengi (L.) leaves. Adv. Tradit. Med. 2020 [Google Scholar]
- 22.Mekonnen T., Urga K., Engidawork E. Evaluation of the diuretic and analgesic activities of the rhizomes of Rumex abyssinicus Jacq in mice. J. Ethnopharmacol. 2010;127(2):433–439. doi: 10.1016/j.jep.2009.10.020. [DOI] [PubMed] [Google Scholar]
- 23.Abdala S., Martin-Herrera D., Benjumea D., Perez-Paz P. Diuretic activity of Smilax canariensis, an endemic Canary Island species. J. Ethnopharmacol. 2008;119(1):12–16. doi: 10.1016/j.jep.2008.05.025. [DOI] [PubMed] [Google Scholar]
- 24.Amuthan A., Chogtu B., Bairy K., Prakash M. Evaluation of diuretic activity of Amaranthus spinosus Linn. aqueous extract in Wistar rats. J. Ethnopharmacol. 2012;140(2):424–427. doi: 10.1016/j.jep.2012.01.049. [DOI] [PubMed] [Google Scholar]
- 25.Chang C.L., Lin C.S., Lai G.H. Phytochemical characteristics, free radical scavenging activities, and neuroprotection of five medicinal plant extracts. Evid Based Compl. Altern. Med. 2012;2012:984295. doi: 10.1155/2012/984295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lee C., Yang W., Parr R.G. Development of the Colle-Salvetti correlation-energy formula into a functional of the electron density. Phys. Rev. B. 1988;37(2):785. doi: 10.1103/physrevb.37.785. [DOI] [PubMed] [Google Scholar]
- 27.Becke A.D. Density-functional exchange-energy approximation with correct asymptotic behavior. Phys. Rev. A. 1988;38(6):3098. doi: 10.1103/physreva.38.3098. [DOI] [PubMed] [Google Scholar]
- 28.Johansson M.U., Zoete V., Michielin O., Guex N. Defining and searching for structural motifs using DeepView/Swiss-PdbViewer. BMC Bioinformat. 2012;13(1):173. doi: 10.1186/1471-2105-13-173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jaghoori M.M., Bleijlevens B., Olabarriaga S.D. 1001 Ways to run AutoDock Vina for virtual screening. J. Comput. Aided Mol. Des. 2016;30(3):237–249. doi: 10.1007/s10822-016-9900-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.DeLano W.L. Pymol: an open-source molecular graphics tool. CCP4 Newslett. Prot. Cryst. 2002;40(1):82–92. [Google Scholar]
- 31.Seeliger D., de Groot B.L. Ligand docking and binding site analysis with PyMOL and Autodock/Vina. J. Comput. Aided Mol. Des. 2010;24(5):417–422. doi: 10.1007/s10822-010-9352-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Debnath S.L., Kundu P., Golder M., Biswas B., Sadhu S.K. Phytochemical characterization and evaluation of pharmacological activities of leaves of a mangrove plant species - Aegiceras corniculatum (L.) Trop. J. Nat. Prod. Res. 2020;4(9):516–522. [Google Scholar]
- 33.Palmer B.F. Regulation of potassium homeostasis. Clin. J. Am. Soc. Nephrol. 2015;10(6):1050–1060. doi: 10.2215/CJN.08580813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Shanmuganathan P., Kumarappan M. Evaluation of diuretic, saluretic and natriuretic activity of hydrochlorothiazide in combination with misoprostol in Wistar rats. Natl. J. Phys. Pharm Pharmacol. 2018;8(8):1226–1229. [Google Scholar]
- 35.Tirumalasetty J., Chandrasekhar N., Naveen A. Evaluation of diuretic activity of ethanol extract of Benincasa hispida stem in Swiss albino rats. J. Chem. Pharm Res. 2013;5(3):91–97. [Google Scholar]
- 36.Mickevičiūtė A., Timm D.D., Gedgaudas M., Linkuvienė V., Chen Z., Waheed A. Intrinsic thermodynamics of high affinity inhibitor binding to recombinant human carbonic anhydrase IV. Eur. Biophys. J. 2018;47(3):271–290. doi: 10.1007/s00249-017-1256-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Eldehna W.M., Al-Ansary G.H., Bua S., Nocentini A., Gratteri P., Altoukhy A. Novel indolin-2-one-based sulfonamides as carbonic anhydrase inhibitors: synthesis, in vitro biological evaluation against carbonic anhydrases isoforms I, II, IV and VII and molecular docking studies. Eur. J. Med. Chem. 2017;127(15):521–530. doi: 10.1016/j.ejmech.2017.01.017. [DOI] [PubMed] [Google Scholar]
- 38.Čapkauskaitė E., Zubrienė A., Smirnov A., Torresan J., Kišonaitė M., Kazokaitė J. Benzenesulfonamides with pyrimidine moiety as inhibitors of human carbonic anhydrases I, II, VI, VII, XII, and XIII. Bioorg. Med. Chem. 2013;21(22):6937–6947. doi: 10.1016/j.bmc.2013.09.029. [DOI] [PubMed] [Google Scholar]
- 39.La Regina G., Coluccia A., Famiglini V., Pelliccia S., Monti L., Vullo D. Discovery of 1, 1′-biphenyl-4-sulfonamides as a new class of potent and selective carbonic anhydrase XIV inhibitors. J. Med. Chem. 2015;58(21):8564–8572. doi: 10.1021/acs.jmedchem.5b01144. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data will be made available on request.