Abstract
Background
Circadian rhythm disturbance is common postoperatively in older patients with hip fractures, which may contribute to the development of postoperative delirium (POD). As a reliable biomarker of endogenous circadian rhythms, melatonin regulates the sleep-wake cycle and environmental adaptation, and its secretory rhythm may be modified by anaesthesia and surgery. This study compared the impact of subarachnoid anaesthesia (SA) and general anaesthesia (GA), on the peak of melatonin secretion (primary outcome), the circadian rhythm of melatonin, cortisol and sleep, and the POD incidence (secondary outcome).
Methods
In this prospective cohort observational study, hip fracture surgery patients were enrolled and assigned to receive either SA or GA. Postoperative plasma melatonin and cortisol levels were dynamically measured every six hours on seven time-points, and the circadian rhythm parameters including mesor, amplitude, and acrophase were calculated. Subjective and objective sleep assessments were performed by sleep diaries and sleep trackers, respectively. The Confusion Assessment Method was used twice daily by a specific geriatrician to screen for POD occurrence.
Findings
In a cohort of 138 patients who underwent hip fracture surgery, the circadian rhythm disruption of the patients in the GA group (n=69) was greater than the SA group (n=69). Compared with SA, GA provided the lower peak concentration, mesor, and amplitude of melatonin secretion on postoperative day 1 (p < 0.05). Patients in the GA group experienced higher awakenings, more sleep deprivation, and poor sleep quality on surgery day (p < 0.05). A proportion of 12 patients in the SA group (17.4%) and 24 patients in the GA group (34.8%) experienced POD (p = 0.020).
Interpretation
These results suggest that SA may be superior to GA in elderly patients undergoing hip fracture surgery as SA is associated with less impairment of the melatonin rhythm and sleep patterns, and fewer POD occurrences.
Keywords: Circadian rhythm, melatonin, general anaesthesia, subarachnoid anaesthesia, postoperative delirium
Research in context.
Evidence before this study
Anaesthesia contributes to circadian rhythm disruption in elderly patients, resulting in disturbed sleep and postoperative delirium (POD). We searched PubMed, MEDLINE, Embase and Cochrane Library for relevant articles with the terms “circadian rhythm” in combination with “melatonin”, “anaesthesia methods”, “postoperative delirium”, or “hip fractures”. Few studies have evaluated the effects of different anaesthesia methods (subarachnoid and general anaesthesia) on the circadian rhythm of melatonin, and explored their associations with POD occurrence in elderly patients. In this study, we explore the effects of subarachnoid and general anaesthesia on the circadian rhythm of melatonin in elderly patients undergoing hip surgery.
Added value of this study
We observed less disruption of melatonin secretion and sleep, and a lower POD incidence in elderly patients undergoing hip fracture when subarachnoid rather than general anaesthesia was used.
Implications of all the available evidence
Anaesthesia may modify the melatonin circadian secretion and disturb postoperative sleep, which may relate to POD, especially in elderly patients. Subarachnoid anaesthesia may be superior to general anaesthesia in elderly patients after hip fracture surgery as it was associated with less melatonin and sleep disturbances and POD occurrence. This hypothesis should be tested in randomised controlled trials. Our findings support to explore the hypothesis that melatonin administration could be beneficial in the postoperative period in the elderly patients.
Alt-text: Unlabelled box
1. Introduction
Hip fractures are a serious injury among the elderly [1]. The number of hip fractures increased approximately 4 folds from 2012 to 2016 with a worldwide hip fracture rate being expected to 4.5 million by 2050 [1,2]. Hip fractures are associated with an increased risk of morbidity, mortality, as well as postoperative delirium (POD), with a prevalence rate up to 53% [3,4]. Elderly patients undergoing hip fracture surgery usually coexist with multiple conditions, such as pain, inflammation, sleep disturbance, which serve as precipitating factors to accelerate the development of POD [4], [5], [6].
A possible mechanism underlying POD is circadian rhythm disruption [6]. The sleep-wake cycle, hormones (melatonin and cortisol) secretion, core body temperature, and cardiometabolic function represent the apparent circadian rhythms, that are generated by the internal clock at the suprachiasmatic nucleus (SCN) [7]. Among all indicators, plasma melatonin is reliable biomarker of endogenous circadian rhythms and is governed by the light and dark cycle [7], [8], [9], [10]. It produces a hypnotic effect by accelerating sleep initiation and improving sleep maintenance and efficiency [11]. The circadian melatonin rhythm has been shown to be altered after surgery, and the lowest concentration of night melatonin was observed on the first night postoperatively, which may lead to an abnormal sleeping pattern [9,12,13]. In addition, previous studies revealed a potential linkage of abnormal plasma melatonin secretion pattern to POD [14], [15], [16]. The occurrence of perioperative circadian rhythm disorder is likely to be multifactorial, including anaesthetic drugs, surgery, and environmental factors [5,17]. The disruption of the circadian rhythm caused by anaesthesia is poorly understood [17,18].
A clinical trial found that patients undergoing subarachnoid anaesthesia (SA) experienced less sleep disturbance after surgery than those receiving general anaesthesia (GA) [19]. This may be because patients receiving SA remain awake during the operation, while GA induces a state of depressed consciousness through anaesthetics. Besides, patients may remember the strange state of disorientation for a long time after waking up from GA. Patients often feel that time stands still and experience an anaesthetic-induced jet-lag, probably because loosing endogenous time perception cues [18,20]. Meanwhile, many studies showed that GA modifies the internal clock and disrupts circadian rhythms [17,18]. Few studies have been conducted to explore the exact impact of different anaesthesia methods on the circadian rhythm and the choice of anaesthesia methods (SA versus GA) remains ambiguous for elderly patients undergoing hip fracture surgery [21]. We hypothesised that SA is superior to GA as SA may have a higher peak melatonin level measured at 04:00, and thus SA causing less disruption of the melatonin circadian rhythm, sleep, and POD occurrence than the GA group.
2. Methods
A prospective single-center, cohort observational clinical study was conducted at Beijing Jishuitan Hospital of geriatric orthopaedics unit from November 2019. The primary aim of this study was to compare the impact of different anaesthesia methods (SA or GA) on the peak of melatonin (the melatonin level measured at 04:00). A secondary objective was to compare the circadian rhythm of melatonin, cortisol, sleep, and the POD incidence, and determine the potential relationship between circadian rhythm disturbance and the POD incidence in elderly patients undergoing hip fracture surgery.
2.1. Ethics statement
This trial was in agreement with the Declaration of Helsinki (2013), and approved by the Medical Science Research Ethics Committees of Beijing Jishuitan Hospital (JLKS201901-04), and registered at the Chinese Clinical Trial Registry (ChiCTR1900027393). Written consent was obtained prior to the study from each participant.
2.2. Patients
The inclusion criteria were: age of ≥65 years, hospital admission for surgical treatment of hip fracture, and American Society of Anesthesiologists (ASA) physical status classification of I to III. The exclusion criteria were: preoperative delirium, Parkinson's disease, dementia, a stroke within a period of prior 6 months, multiple traumas, communication difficulties, severe hearing or vision impairment, taking medications related to melatonin, night shift duty, and unwillingness to participate in the study or unexpected discharge.
2.3. Preoperative interview
The research protocol has been published elsewhere [22].
All participants were interviewed the day before surgery and the baseline data were collected, including demographic information, laboratory results, ASA physical status, age-adjusted Charlson comorbidity index (ACCI) [23], Mini-Mental State Exam (MMSE) [24], Activities of Daily Living (ADL) [25] and Pittsburgh Sleep Quality Index (PSQI) [26]. The sleep quality and pain intensity were assessed by a numerical rating scale (NRS) [27]. Other information including comorbidities, past medical history, and fracture classification was collected according to the medical records of patients. History collection, physical evaluation, and cognitive assessment were conducted by trained investigators.
2.4. Perioperative management
Each participant was placed in a standard room (e.g., light, temperature), and free from noise, television, mobile phone, and computer. The nurses kept quiet during the blood drawing to avoid disturbing the patients' sleep. Their diet was refrained from alcohol, nicotine, and beverages or foods containing caffeine, and their activities were restricted throughout the observation period. The lights-on (06:00) and lights-off (20:00) times were determined following the hospital discharge policy. Light exposure (controlled to be < 10 lux) was measured during a night-time sleep period [28]. To avoid light exposure and minimize disruption of participant sleep, a penlight was used for taking blood sample and nursing interventions at night instead of turning on the light. A digital light meter was used for light intensity readings to ensure that light levels were maintained at 10 lux or less at the patient's eye level during sleep period [7,28].
2.5. Anaesthesia and analgesia
Patients were assigned to the GA group or SA group based on their wishes, individual situations, as well as the experience of anaesthesiologists and surgeons. Participants in the SA group received a single-dose subarachnoid spinal anaesthesia, and no epidural catheter was placed. Ropivacaine was used at the L2-3 or L3-4 levels, and no additional anaesthetic agents were administered. In the GA group, anaesthesia was induced and maintained with a combination of intravenous propofol, sevoflurane, etomidate, sufentanil. Muscle relaxation was maintained with cisatracurium.
All patients received standard perioperative pain management [29]. To reduce acute postoperative pain, each participant received a standardized ultrasound-guided fascia iliac block (a single injection of 30 mL of 0.33% ropivacaine) before anaesthesia [29]. All patients received intravenous patient-controlled analgesia (100 µg sufentanil and 8 mg ondansetron in 100 ml saline). Furthermore, intramuscular injection of pethidine (50 mg) or oral use of oxycodone (5 mg)/acetaminophen (325 mg) was made as remedy analgesia as needed.
2.6. Postoperative assessment
Each participant was followed up twice daily by a trained geriatrician (XPL). Delirium was diagnosed with the Confusion Assessment Method (CAM) as previously described [29]. NRS and sleep diaries were used to assess subjective sleep (total time of night sleep, time of sleep onset, number of awakenings during the night, and fatigue). Objective sleep [total sleep time, rapid-eye-movement (REM) sleep, and “light sleep” and “deep sleep” time] was assessed with the sleep tracker (Fitbit Charge; Fitbit, Inc., San Francisco, CA, USA) [30]. NRS was used to assess the intensity of postoperative pain.
2.7. Blood specimen processing
Blood samples (2 ml) were sampled every 6 hours from 22:00 on the surgery day to 10:00 on postoperative day 2 for assays of melatonin and cortisol levels. Plasma melatonin and cortisol concentrations were measured by an enzyme immunoassay method (IBL International GmbH, Germany) with a limit of detection of 1.6 pg/mL, and an electrochemiluminescence immunoassay method (Roche Diagnostics GmbH, Germany), respectively [31,32]. We measured plasma C-reactive protein (CRP) and interleukin-6 (IL-6) before anaesthesia and 24 h after surgery using an immunoturbidimetry (Beckman Coulter, Inc., USA) and electrochemiluminescence immunoassay method (Roche, Germany), respectively.
2.8. Circadian rhythm parameters
To minimize disruption of participant sleep by multiple blood drawings, plasma melatonin and cortisol levels were dynamically measured every six hours during the postoperative follow-up period for seven times (22:00 on the surgery day, 4:00, 10:00, 16:00, and 22:00 on postoperative day 1, and 4:00 and 10:00 on postoperative day 2, respectively) [9,15]. Circadian rhythm parameters were analyzed using Origin (OriginLab Corp, USA), and subsequently smoothed with GraphPad Prism 8 (GraphPad Software, Inc) based on cosinor regression y = a + b × cos(x × π/12-c × π/12), in which a, b, and c represent mesor, amplitude, and acrophase, respectively [33,34]. The regression was considered significant at p < 0.05, and R2 indicated the goodness of regression [34]. Mesor is the average level of values among 24 h [33]. Amplitude corresponds to the distance between the mesor and the peak of the wave [33]. Acrophase is the phase of the maximal value assumed by the curve [33]. To observe the effect of different postoperative periods on the circadian rhythm, postoperative rhythm markers were calculated four times with cosinor method based on four different starting time points which successively were at 22:00 (on the surgery day), 4:00, 10:00, and 16:00 (on the postoperative day 1).
2.9. Sample-size estimation
The melatonin shows a clear circadian rhythm, presenting a low level during the daytime, rising between 20:00 and 22:00 and reaching a peak between 02:00 and 04:00 [8,35]. The nocturnal levels of melatonin were significantly lower on the first night postoperatively than on the second or third nights [9]. The finding implies that the peak of melatonin is significantly suppressed on the first night postoperatively, which may contribute to a melatonin rhythm disturbance. As such, in this study, we defined the sample size based on the melatonin level measured at 04:00, which reflects the peak value observed on the first postoperative day between two groups. In our preliminary study, the plasma melatonin levels probed at 04:00 on postoperative day 1 were 13.40 ± 6.85 and 10.48 ± 4.04 pg/mL in the SA and GA groups, respectively (n = 15 each). It was estimated that group sample sizes of 60 and 60 would achieve 80.386% power to reject the null hypothesis of equal means with a significance level (alpha) of 0.050 using a two-sided two-sample unequal-variance t-test. Assuming a dropout rate of 15%, we set a target of 69 participants per group for a total of 138 participants.
2.10. Statistics
All normality variables were tested with the Kolmogorov-Smirnov method. The between-group differences were compared with a chi-square test for categorical variables, an independent-samples t-test for continuous variables, or a Mann-Whitney U-test for the non-normal variables. The association between melatonin parameters and sleep quality was validated by Pearson or Spearman analysis. The superior effect of anaesthesia method (GA vs. SA) over time was analyzed using a repeated-measures analysis of variance (RM-ANOVA), taking into consideration time, the effect of the anaesthesia method, and their interaction. We used the Benjamini-Hochberg procedure for multiple testing. In this method, the p-values are ranked in ascending order, with the smallest p-value getting the rank of 1. Each p-value is then multiplied by the number of tests and divided by its rank [36,37]. Although the Benjamini-Hochberg procedure may provide less stringent control of Type I errors compared to familywise error rate controlling procedures, it was applied to the multiple analysis of cortisol data in another study, in which the sampling protocol was similar to that used in the present study, and the cortisol levels were dynamically measured at five equally spaced time points [38]. All analyses were performed using SPSS software, version 25.0 (SPSS Inc., USA) or Prism 8 software (GraphPad, USA). p values < 0.05 were considered significant.
2.11. Blinding
Investigators responsible for the preoperative interview including history collection, physical evaluation, and cognitive assessment were blinded to anaesthesia allocation. And the trained geriatrician responsible for postoperative delirium evaluation was blinded to the study group assignment.
2.12. Role of the funding source
The study was supported by the National Natural Science Foundation of China, Key Clinical Projects of Peking University Third Hospital, and Peking University “Clinical Medicine plus X” Youth Project. All funders had no role in study design, data collection, data analysis, result interpretation, or writing of the report. All the data and the content in the study are the responsibility of the authors.
3. Results
3.1. Baseline Characteristics
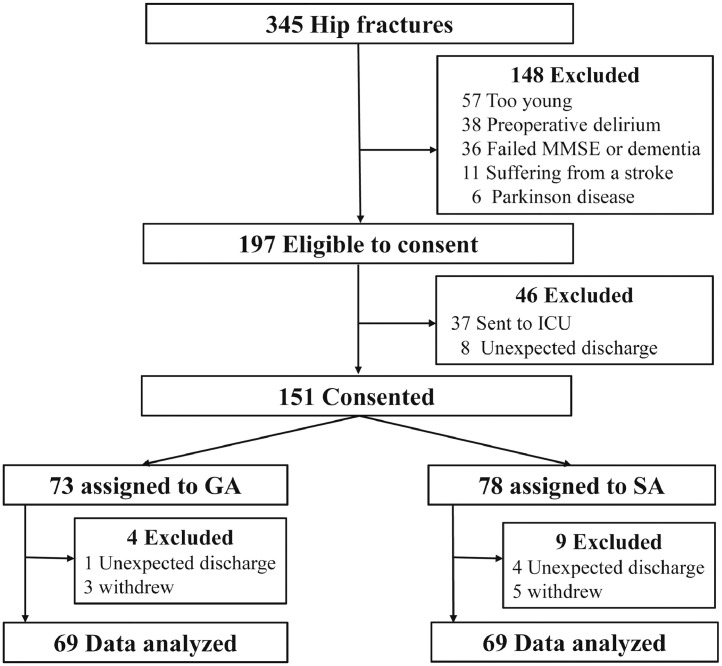
345 patients that had hip fractures were screened from November 2019 to July 2020, 138 participants received either GA or SA were eligible for inclusion (Fig. 1). The mean age was 79.0 (7.1) years and 77.7 (6.8) years respectively for group SA and GA. There were 46 male patients (66.7%) in the SA group and 56 male patients (81.2%) in the GA group (p > 0.05). 52.2% (36/69) of patients in the SA group and 47.8% (33/69) of patients in the GA group were diagnosed with a femoral neck fracture. Patients in the GA group had higher MMSE scores (p = 0.020). There was no significant difference in baseline demographics between the two groups except MMSE (Table 1).
Fig. 1.
Study flow chart.
MMSE, Mini-Mental State Examination; ICU, intensive care unit; GA, general anaesthesia; SA, subarachnoid anaesthesia.
Table 1.
Patients' demographics and baseline characteristics.
| Variable | SA (n=69) | GA (n=69) | p-value |
| Baseline demographics | |||
| Age, yr | 79.0 ± 7.1 | 77.7 ± 6.8 | 0.260 |
| Sex, female | 46 (66.7%) | 56 (81.2%) | 0.081 |
| BMI, kg/m2 | 23.4 ± 3.5 | 23.4 ± 3.4 | 0.987 |
| Medical history | |||
| ASA physical status class | 0.239 | ||
| I-II | 45 (65.2%) | 52 (75.4%) | |
| III | 24 (34.8%) | 17 (24.6%) | |
| ACCI | 4.3 ± 1.4 | 4.0 ± 1.5 | 0.334 |
| Ischemic heart disease | 15 (21.7%) | 13 (18.8%) | 0.672 |
| Chronic obstructive pulmonary disease | 9 (13.0%) | 7 (10.1%) | 0.595 |
| Hypertension | 40 (58.0%) | 45 (65.2%) | 0.382 |
| Diabetes | 20 (29.0%) | 26 (37.7%) | 0.279 |
| Stroke | 16 (23.2%) | 9 (13.0%) | 0.122 |
| Smoking status, yes | 18 (26.1%) | 10 (14.5%) | 0.138 |
| ADL, points | 14.0 (14.0, 14.0) | 14.0 (14.0, 14.0) | 0.613 |
| Education, yr | 9.0 (4.0, 13.0) | 9.0 (6.0, 12.0) | 0.953 |
| MMSE, points | 25.3 ± 3.6 | 26.7 ± 2.8 | 0.020 |
| Preoperative medication use | |||
| β-blockers | 8 (11.6%) | 9 (13.0%) | 0.796 |
| Calcium channel blockers | 23 (33.3%) | 23 (33.3%) | 1.000 |
| ACEI or ARB | 10 (14.5%) | 12 (17.4%) | 0.642 |
| Insulin or oral hypoglycemic drugs | 17 (24.6%) | 18 (26.1%) | 0.845 |
| Baseline laboratory | |||
| Leukocyte, × 10^9 /L | 10.0 ± 2.9 | 9.8 ± 2.7 | 0.670 |
| Platelet, × 10^9 /L | 204.5 ± 61.4 | 201.1 ± 76.2 | 0.774 |
| Hemoglobin, g/L | 120.8 ± 16.7 | 117.1 ± 20.1 | 0.247 |
| ALT, IU/L | 15.5 ± 8.3 | 15.8 ± 5.6 | 0.857 |
| AST, IU/L | 20.6 ± 7.4 | 20.3 ± 4.9 | 0.751 |
| Creatinine, μmol/L | 65.0 ± 26.0 | 59.1 ± 17.6 | 0.123 |
| Albumin, g/L | 41.1 ± 2.4 | 40.3 ± 3.2 | 0.098 |
| TSH, mIU/L | 1.6 (1.0, 2.8) | 1.6 (1.0, 2.9) | 0.968 |
| Blood glucose, mmol/L | 7.8 ± 1.7 | 8.8 ± 2.5 | 0.753 |
| PaO2, mmHg | 75.0 ± 10.6 | 75.0 ±11.4 | 0.307 |
| SaO2, % | 94.9 ± 2.3 | 95.5 ± 1.8 | 0.137 |
| Surgical fracture | |||
| Time from injury to hospital, hours | 18.0 (4.6, 47.7) | 9.0 (3.0, 29.8) | 0.069 |
| Time from injury to operation, hours | 91.0 (71.0, 118.0) | 73.5 (51.7, 126.3) | 0.057 |
| Type of fracture | 0.703 | ||
| Femoral neck | 36 (52.2%) | 33 (47.8%) | |
| Inter/subtrochanteric | 33 (47.8%) | 36 (52.2%) | |
| Intraoperative | |||
| Duration of anaesthesia, min | 86.0 (64.5, 106.5) | 93.0 (68.0, 110.5) | 0.247 |
| Duration of surgery, min | 62.0 (44.5, 79.5) | 71.0 (48.0, 92.0) | 0.157 |
| Start time of surgery (morning/afternoon) | 20/49 | 26/43 | 0.279 |
| Blood loss, mL | 200.0 (100.0, 200.0) | 150.0 (150.0, 250.0) | 0.138 |
| Postoperative | |||
| POD | 12 (17.4%) | 24 (34.8%) | 0.020 |
| Pneumonia | 3 (4.3%) | 4 (5.8%) | 1.000 |
| Deep venous thrombosis | 8 (11.6%) | 5 (7.2%) | 0.562 |
| Hospital stay, days | 4.4 ± 1.6 | 4.2 ± 1.3 | 0.056 |
The categorical variables are expressed as n (%). Normal data are given as mean ± SD, whereas non-normaldata are expressed as median (25th percentile, 75th percentile). ADL, activities of daily living; ACCI, age-adjusted Charlson comorbidity index; ACEI, angiotensin-converting enzyme inhibitor; ALT, alanine aminotransferase; ARB, Angiotensin receptor blocker; ASA, American Society of Anesthesiologists; AST, aspartate aminotransferase; BMI, body mass index; GA, general anaesthesia; MMSE, Mini-Mental State Examination; PaO2, partial arterial oxygen concentration; SA, subarachnoid anaesthesia; SaO2, arterial blood saturation values; TSH, thyroid stimulating hormone.
3.2. Postoperative plasma melatonin concentration and circadian rhythm parameters
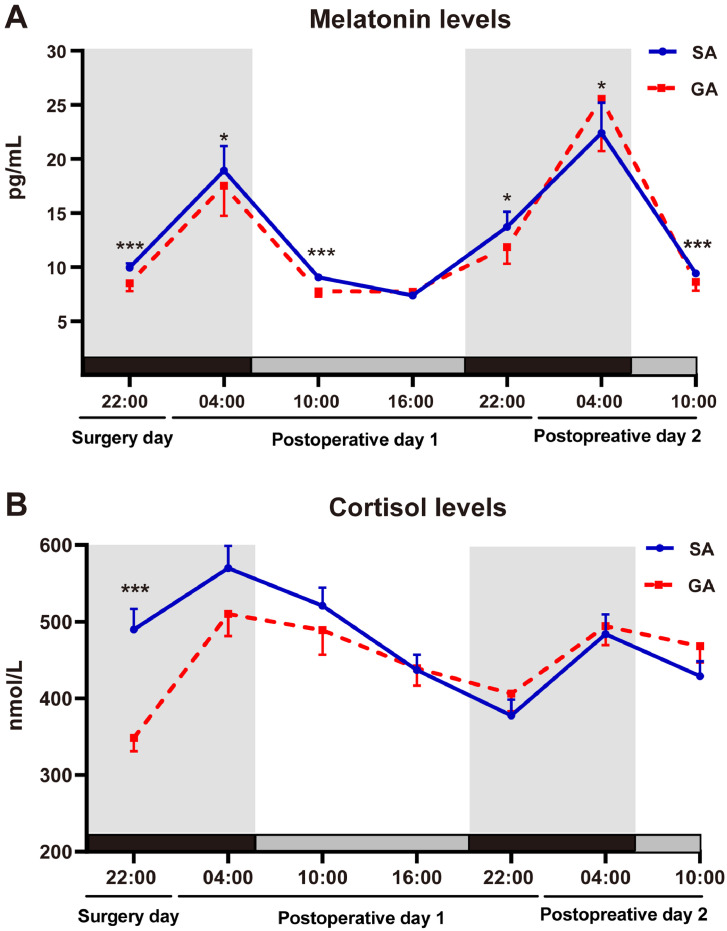
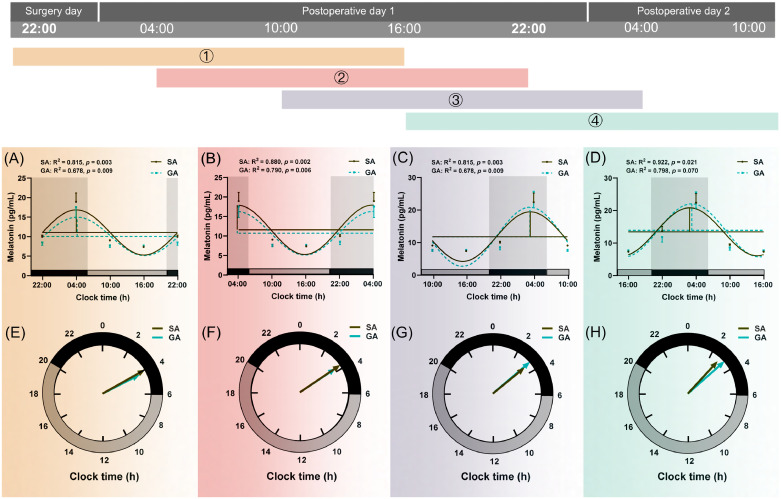
Patients in the GA group displayed lower concentrations of plasma melatonin than the SA group as measured at 22:00 on surgery day (p < 0.001), 04:00 on postoperative day 1 (p = 0.030), 10:00 on postoperative day 1 (p < 0.001), 22:00 on postoperative day 1 (p = 0.018), and 10:00 on postoperative day 2 (p < 0.001) (Fig. 2A, Supplementary Table 1). The melatonin level at 04:00 on postoperative day 2 was significantly higher in GA patients than SA patients (p = 0.014, Fig. 2A, Supplementary Table 1). We performed linear regression models to assess the effect of anaesthesia method on melatonin level at 4:00 on postoperative day 1. In the adjusted models, analyses were adjusted for a range of confounder including gender, age, MMSE, ASA, ACCI, hypertension, diabetes, ischemic heart disease, chronic obstructive pulmonary disease, PaO2, SaO2, albumin, time from injury to hospital, time from injury to operation and the start time of surgery. The results indicated that SA tended to have higher melatonin secretion after adjusting for confounders (p = 0.044, Supplementary Table 2). Circadian variations of melatonin secretion are presented in Fig. 3 and Table 2. Compared with the SA group, four mesor values and the amplitude (4) were all diminished significantly in the GA group (all p < 0.05).
Fig. 2.
Postoperative plasma concentrations of melatonin and cortisol.
Melatonin (A) and cortisol (B) concentration profiles from 69 SA patients (solid curve) and 69 GA patients (broken curve). Data were shown as the mean and SEM. Patients in the GA group displayed significantly lower concentrations of plasma melatonin than the SA group as measured at 22:00 (surgery day), 04:00 (postoperative day 1), 10:00 (postoperative day 1), 22:00 (postoperative day 1), and 10:00 (postoperative day 2) (A). The melatonin level at 04:00 (postoperative day 2) was significantly higher in GA patients than SA patients (A). The plasma cortisol concentration in the GA group was significantly lower at 22:00 on the surgery day (B). Compared to SA group, *p < 0.05, **p < 0.01 and *** p < 0.001 (Benjamini-Hochberg correction). GA, general anaesthesia; SA, subarachnoid anaesthesia.
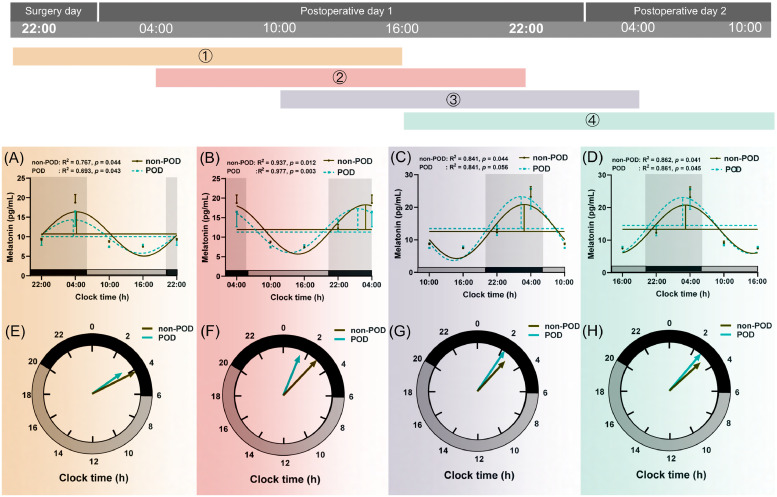
Fig. 3.
Circadian rhythm of plasma melatonin secretion in SA (n=69) and GA (n=69) group.
Data were shown as mean and SEM. Different colours (①,②,③,④) represented different rhythm curves based on four starting time points which successively were at 22:00 (on the surgery day), 4:00, 10:00, and 16:00 (on postoperative day 1). The upper part (Fig. A, B, C, and D) was a cosine curve, and the lower part (Fig. E, F, G, and H) was a continuous clock face from 0:00 to 24:00, as computed by the cosinor method. The last point in each clock was a duplicate of the start point, which was for visualizing periodically. And the data was subsequently smoothed with the cosinor method curve-fitting procedure (GraphPad Prism 8; GraphPad Software, Inc). The acrophase was the phase of the maximal value assumed by the curve, and horizontal and vertical lines represented mesor and amplitude, respectively in figures (A, B, C, and D). The goodness of rhythmicity (R2 and p-value) was shown on the top, and the black bar indicated the night or light-off period (20:00-06:00), and grey bars represented the day or light-on period (06:00-20:00) at the bottom of the lower figures (A, B, C, and D). The amplitude and acrophase of a rhythm were plotted on a continuous clock face from 0:00 to 24:00, and the acrophase indicated by the angle of a vector whose length corresponds to the amplitude (E, F, G, and H). Compared to SA group, *p < 0.05, **p < 0.01 and *** p < 0.001. GA, general anaesthesia; SA, subarachnoid anaesthesia.
Table 2.
Postoperative rhythm markers of melatonin and cortisol based on different starting points.
| Variable | SA (n=69) | GA (n=69) | p-value | |
| Melatonin | ||||
| Mesor (pg/mL) | 1 | 9.5 (8.9, 10.8) | 8.7 (6.9, 10.4) | 0.004 |
| 2 | 9.7 (8.9, 13.8) | 9.0 (7.4, 11.4) | 0.009 | |
| 3 | 10.5 (9.1, 14.7) | 9.0 (7.7, 13.3) | 0.012 | |
| 4 | 11.0 (9.0, 15.3) | 9.0 (7.8, 14.6) | 0.008 | |
| Amplitude (pg/mL) | 1 | 2.5 (1.2, 6.0) | 1.5 (0.9, 5.8) | 0.230 |
| 2 | 3.2 (1.4, 9.8) | 2.4 (1.6, 6.9) | 0.536 | |
| 3 | 4.3 (1.8, 13.1) | 2.4 (1.3, 11.6) | 0.245 | |
| 4 | 3.9 (2.0, 12.1) | 2.3 (1.2, 10.0) | 0.024 | |
| Acrophase (h) | 1 | 4.1 (1.8, 4.9) | 4.2 (0.1, 5.8) | 0.646 |
| 2 | 3.2 (0.1, 4.3) | 2.8 (23.0, 4.0) | 0.201 | |
| 3 | 3.4 (2.0, 4.0) | 2.7 (23.3, 3.5) | 0.176 | |
| 4 | 3.6 (1.6, 4.1) | 2.5 (23.1, 4.2) | 0.208 | |
| Cortisol | ||||
| Mesor (nmol/L) | 1 | 490.0 (401.1, 595.0) | 437.8 (352.6, 514.1) | 0.008 |
| 2 | 459.3 (376.4, 569.6) | 455.3 (359.8, 536.7) | 0.840 | |
| 3 | 448.8 (340.4, 534.9) | 452.9 (369.5, 523.9) | 0.882 | |
| 4 | 443.2 (335.9, 489.7) | 451.3 (357.4, 525.0) | 1.000 | |
| Amplitude (nmol/L) | 1 | 120.9 (89.2, 190.7) | 121.5 (78.2, 194.4) | 0.983 |
| 2 | 128.6 (85.9, 183.0) | 131.3 (76.7, 164.9) | 0.512 | |
| 3 | 117.6 (76.0, 172.1) | 105.7 (70.5, 143.3) | 0.904 | |
| 4 | 94.2 (65.0, 132.4) | 87.3 (65.8, 133.4) | 0.603 | |
| Acrophase (h) | 1 | 5.1 (1.8, 9.5) | 8.1 (4.0, 10.7) | 0.104 |
| 2 | 7.1 (5.1, 9.9) | 6.8 (4.01, 10.2) | 0.748 | |
| 3 | 7.6 (4.1, 11.7) | 7.2 (2.9, 10.1) | 0.254 | |
| 4 | 7.5 (3.9, 14.2) | 8.0 (4.9, 12.2) | 0.967 | |
Normal data are given as mean ± SD, whereas non-normal data are expressed as median (25th percentile,
75th percentile). GA, general anaesthesia; SA, subarachnoid anaesthesia. Postoperative rhythm markers were calculated four times with cosinor method based on four different starting time points which successively were at 22:00 (on the surgery day), 4:00, 10:00, and 16:00 (on the postoperative day 1). To be specific, the term “mesor 1 and 2” refers to the early postoperative period rhythm mesor marker which analyzed starting form 22:00 on the surgery day and 4:00 on the postoperative day 1. And the term “mesor 3 and 4” refers to the late postoperative period rhythm mesor marker which analyzed starting form 10:00 and 16:00 on the postoperative day 1.
RM-ANOVA demonstrated that there was a significant interaction between group and time in melatonin levels (F = 2.422, p = 0.030), and acrophase of melatonin (F = 3.000, p = 0.033). Additionally, RM-ANOVA showed a significant effect for time in melatonin levels (F = 13.699, p < 0.001), mesor of melatonin (F = 10.289, p < 0.001), amplitude of melatonin (F = 9.783, p < 0.001), and acrophase of melatonin (F = 1100.149, p < 0.001). RM-ANOVA showed a significant effect for time in melatonin concentrations and circadian rhythm parameters. Additionally, there was a significant interaction between group and time in melatonin levels and acrophase of melatonin (Supplementary Table 3).
3.3. Postoperative plasma cortisol concentration and circadian rhythm parameters
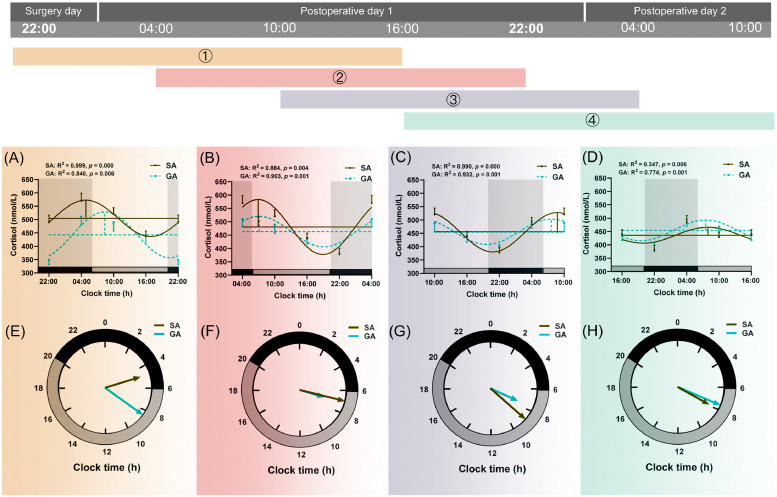
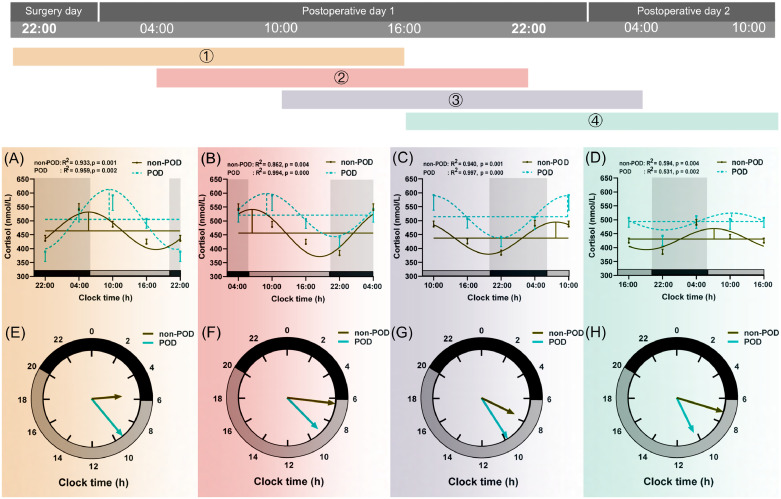
The plasma cortisol concentration in the GA group was significantly lower at 22:00 on the surgery day (p < 0.001, Fig. 2B, Supplementary Table 1). The difference gradually dismissed thereafter. At 16:00 on postoperative day 1, the cortisol concentrations in both groups were not different from each other. The cortisol concentration in the GA group was gradually elevated afterward to a level higher than that in the SA group (Fig. 2B, Supplementary Table 1). Circadian variations of cortisol levels were depicted in Fig. 4 and Table 2. Compared with SA, the mesor (1) value was decreased significantly (p = 0.008, Table 2) in the GA group.
Fig. 4.
Circadian rhythm of plasma cortisol secretion in SA (n=69) and GA (n=69) group.
See above legend shown in Fig. 3.
RM-ANOVA demonstrated that there was a significant interaction between group and time in cortisol levels (F = 3.934, p < 0.001), mesor of cortisol (F = 6.081, p < 0.001). Additionally, RM-ANOVA showed a significant effect for time in cortisol (F = 17.808, p < 0.001), mesor of cortisol (F = 4.492, p < 0.001), amplitude of cortisol (F = 6.489, p < 0.001), and acrophase of cortisol (F = 371.753, p < 0.001, Supplementary Table 3). RM-ANOVA showed a significant effect for time in all variables of cortisol concentrations and circadian rhythm parameters. Additionally, there was a significant interaction between group and time in cortisol levels, mesor of cortisol (Supplementary Table 3).
3.4. Perioperative subjective and objective sleep data
An insignificant difference in the preoperative PSQI and sleep quality was found between the two groups (Table 3). There was a higher number of patients in the GA group that suffered from sleep deprivation (p = 0.040). The sleep quality of patients in the GA group was inferior to that in the SA group as evaluated by the number of awakenings, sleep quality score, and feeling after waking up (all p < 0.05) at the night on the surgery day. At the night on postoperative day 1, there was no significant difference between the two groups in all measurements of subjective sleep and objective sleep.
Table 3.
Perioperative sleep data.
| Variable | SA (n=69) | GA (n=69) | p-value |
| Preoperative | |||
| PSQI score | 5.0 (3.0, 11.0) | 5.0 (2.0, 8.5) | 0.322 |
| Sleep quality | 5.0 (3.0, 6.0) | 4.0 (3.0, 6.0) | 0.409 |
| Postoperative | |||
| Surgery day | |||
| Sleep deprivation | 6 (8.6%) | 16 (22.95%) | 0.040 |
| Subjective sleep | |||
| Night sleep time, min | 253.0 ±135.2 | 212.2 ±160.5 | 0.090 |
| Sleep onset time, h | 22.4 ± 2.2 | 21.7 ± 2.4 | 0.242 |
| Sleep offset time, h | 5.5 ± 1.0 | 5.4 ± 1.2 | 1.000 |
| Number of awakenings | 2.9 ± 1.1 | 6.4 ± 1.2 | <0.001 |
| Sleep quality | 5.0 (3.0, 6.0) | 3.0 (1.0, 6.0) | 0.032 |
| Feeling after waking up | 0.032 | ||
| Refreshed | 8 (11.6%) | 1 (1.4%) | |
| Somewhat refreshed | 33 (47.8%) | 28 (40.6%) | |
| Fatigued | 28 (40.6%) | 40 (58.0%) | |
| Objective sleep | |||
| Total sleep time, min | 309.0 (184, 410.0) | 250.0 (3.0, 376.0) | 0.220 |
| REM time, min | 36.0 (15.0, 57.0) | 25.5 (9.0, 54.0) | 0.300 |
| Light sleep time, min | 231.0 (139.0, 281.0) | 178.0 (114.0, 297.0) | 0.250 |
| Deep sleep time, min | 39.0 (8.0, 62.0) | 14.0 (6.0, 45.0) | 0.072 |
| Day 1 | |||
| Sleep deprivation, % | 2 (2.8%) | 6 (8.5%) | 0.275 |
| Subjective sleep | |||
| Night sleep time, min | 303.2 ±130.1 | 269.5 ±116.5 | 0.122 |
| Sleep onset time, h | 22.2 ± 2.0 | 21.7 ± 1.6 | 0.154 |
| Sleep offset time, h | 5.3 ± 0.9 | 5.0 ± 1.1 | 0.209 |
| Number of awakenings | 3.0 ± 0.6 | 2.7 ± 0.9 | 0.068 |
| Sleep quality | 6.0 (4.0, 7.0) | 5.0 (3.0, 7.0) | 0.568 |
| Feeling after waking up | 0.357 | ||
| Refreshed | 20 (29.0%) | 28 (40.6%) | |
| Somewhat refreshed | 33 (47.8%) | 27 (39.1%) | |
| Fatigued | 16 (23.2%) | 14 (20.3%) | |
| Objective sleep | |||
| Total sleep time, min | 337.0 (280.0, 439.0) | 306.0 (181.0, 420.0) | 0.229 |
| REM time, min | 44.0 (30.0, 70.0) | 43.0 (13.0, 75.0) | 0.652 |
| Light sleep time, min | 250.0 (194.0, 316.0) | 235.0 (128.0, 342.0) | 0.369 |
| Deep sleep time, min | 41.0 (23.0, 63.0) | 31.0 (11.0, 51.0) | 0.153 |
Sleep quality was assessed with numeric rating scale (NRS, 0 = worst and 10 = best); Sleep diaries were used to assess other subjective sleep data. Objective sleep data was calculated with sleep tracker (Fitbit Charge). Stage N1 and N2 sleep (polysomnography) were categorized as “light sleep” and stage N3 (polysomnography) as “deep sleep” (according to Fitbit Inc.). GA, general anaesthesia; PSQI, Pittsburgh Sleep Quality Index; REM, rapid-eye-movement; SA, subarachnoid anaesthesia; TST, total sleep time.
3.5. Correlation analysis of melatonin with sleep quality
We validated the association between the melatonin concentration at 4:00 and the sleep quality by Pearson or Spearman analysis in the SA group, GA group and all patients, respectively. On postoperative day 1 and 2, the melatonin concentration at 4:00 showed a positive correlation with the sleep quality in SA group (r = 0.360, p = 0.002; r = 0.288, p = 0.016; respectively), in GA group (r = 0.274, p = 0.023; r = 0.269, p = 0.025; respectively), and in all patients (r = 0.454, p = 0.029; r = 0.593, p = 0.003; respectively).
3.6. POD and circadian rhythm parameters
36 patients (26.1%) developed POD, with 12 patients (17.4%) in the SA group and 24 (34.8%) in the GA group (p = 0.020). There was no significant difference in baseline demographics and perioperative characteristics between POD and non-POD groups (Supplementary Table 4). The hypertension incidence (77.8%) and the pneumonia incidence (13.9%) were higher in the POD group (all p < 0.05). For secondary outcome (POD occurrence), we used binary logistic regression analysis to adjust for confounding (hypertension, platelet, ALT, Albumin, TSH, ACEI or ARB and MMSE). The results showed that anaesthesia method was an independent influencing factor of POD occurrence (OR = 0.287, 95% CI: 0.115 - 0.718, p = 0.008, Supplementary Table 5).
Comparisons of circadian variations of melatonin and cortisol secretion between POD and non-POD groups are presented in Fig. 5, Fig. 6 and Supplementary Table 6. Mesor, amplitude, and acrophase (1, 2, 3, and 4) of melatonin were similar between the two groups (Fig. 5 and Supplementary Table 6). The POD group tended to have a higher cortisol mesor (1, 2, 3, and 4), as well as a more delayed acrophase (1) than the non-POD group (all p < 0.05, Fig. 6 and Supplementary Table 6).
Fig. 5.
Circadian rhythm of plasma melatonin secretion in non-POD (n=102) and POD (n=36) group.
See above legend shown in Fig. 3.
Fig. 6.
Circadian rhythm of plasma cortisol secretion in non-POD (n=102) and POD (n=36) group.
See above legend shown in Fig. 3.
3.7. Perioperative pain data
There was no difference in the preoperative pain score between the two groups (Table 4). Patients in the GA group required more rescue analgesia than those in the SA group on surgery day (p = 0.038). Besides, a higher pain score on activity in the GA group was observed on postoperative day 1 (p < 0.001).
Table 4.
Perioperative pain data.
| Variable | SA (n=69) | GA (n=69) | p- value |
| Preoperative | |||
| Pain at rest score | 3.0 (2.0, 5.0) | 3.0 (3.0, 4.0) | 0.795 |
| Pain on activity score | 8.0 (8.0, 9.0) | 8.0 (7.0, 9.0) | 0.140 |
| Postoperative | |||
| Surgery day | |||
| Remedy analgesia, % | 11 (15.9%) | 24 (34.8%) | 0.038 |
| Day 1 | |||
| Remedy analgesia, % | 15 (21.7%) | 17 (24.6%) | 1.000 |
| Pain at rest score | 2.0 (2.0, 3.0) | 2.0 (2.0, 3.0) | 0.120 |
| Pain on activity score | 6.0 (5.0, 7.0) | 7.0 (6.0, 8.0) | < 0.001 |
| Day 2 | |||
| Remedy analgesia, % | 13 (18.8%) | 13 (18.8%) | 1.000 |
| Pain at rest score | 2.0 (1.0, 2.0) | 2.0 (1.0, 2.0) | 0.502 |
| Pain on activity score | 5.0 (3.0, 6.0) | 5.0 (5.0, 6.0) | 0.454 |
Pain intensity was measured using the numeric rating scale. The categorical variables are expressed as n (%). Normal data are given as mean ± SD, whereas non-normal data are expressed as median (25th percentile, 75th percentile). GA, general anaesthesia; SA, subarachnoid anaesthesia.
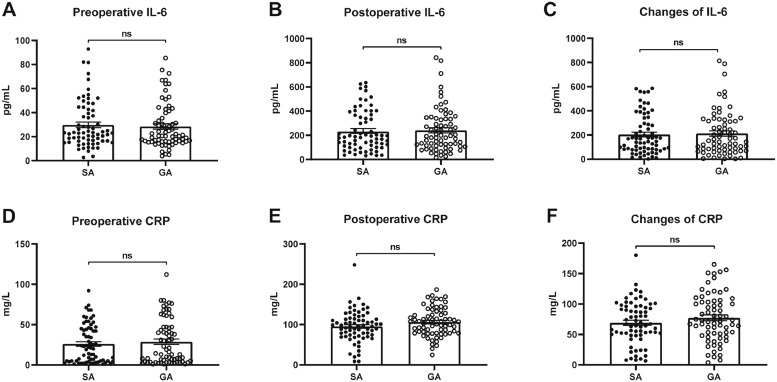
3.8. Perioperative plasma inflammatory cytokines concentration
There was no significant difference in plasma IL-6 and CRP concentrations at preoperative and 24h after surgery (Fig. 7). Besides, the changes in CRP and IL-6 between preoperatively and postoperatively were comparable between the two groups (Fig. 7).
Fig. 7.
Perioperative plasma levels of inflammatory cytokines.
Data were presented as mean and SEM. There was no significant difference between the two groups (n=69, each) in CRP and IL-6 concentration at baseline and on postoperative day 1, and changes of CRP and IL-6 (A–F).
4. Discussion
The results of this study show that both GA and SA could affect postoperative circadian melatonin secretion and sleep, but SA was associated with less impairment of the melatonin rhythm and sleep, and fewer POD.
In GA patients, we observed a significant decrease in the peak level of melatonin at 04:00, as well as a considerable decrease in the mesor and amplitude of melatonin was observed on postoperative day 1. This was followed by an increase in the melatonin peak level at 04:00 and an advanced phase on postoperative day 2, indicating a fluctuating trend in the circadian melatonin secretion. It is worth mentioning that the difference in melatonin level at 04:00 on postoperative day 1 (the primary aim) between the two groups [10.1 (7.3, 15.4) vs. 11.6 (9.4, 18.0) pg/mL], seemed small. Melatonin, an important neuroendocrine hormone, like other hormones, has the characteristics of tiny amounts and high efficiency, and acts on the biological clock and regulates the sleep-wake cycle [7,8,39]. It may be this slight melatonin fluctuation that causes clinical outcomes. Gögenur et al. found that the difference of melatonin concentration [36 (5-110) vs. 36 (8-196) pg/mL] led to the change of melatonin rhythm, as well as the change of cortisol and body temperature [40]. Shenshen et al. also reported a small difference between preoperative and postoperative melatonin concentrations (3.79 ± 0.68 vs. 5.51 ± 0.82 pg/mL) in aging cataract surgery patients, with the differences in PSQI and Epworth Sleepiness Scale scores [41]. Another study demonstrated that Δ melatonin concentration at 1 hour after the operation was significantly lower in patients with delirium than in those without delirium (- 1.1 vs. 0 pg/mL, p = 0.036) [42]. The magnitude of the difference between the groups in the current study was in accordance with the above-mentioned findings [40], [41], [42].
In this study, we controlled external factors (light, meal, activity, temperature, the start time of surgery, and preoperative medication use) known to influence melatonin secretion [17,43,44]. Our results support that GA influences postoperative melatonin secretion as reported by others [17,18]. A study by Karkela et al. [13] reported that there was no significant difference in the melatonin level between SA and GA groups, they however studied a younger group of patients (20 orthopaedic patients, 29 ± 11 years of age), and they measured melatonin at only five time points (21:00, 22:00, 23:00, and 24:00 and 08:00) and might have missed the highest level of melatonin [13]. Our findings are supported by observations in an animal study that showed a decrease in the melatonin level immediately after propofol anaesthesia (without surgery), followed by an increase 20h after anaesthesia and a phase advance of the circadian melatonin rhythm [45].
It is thought that both surgery and anaesthesia may inhibit melatonin secretion after an operation. Many clinical studies have demonstrated that the surgical procedure causes trauma and thus increased inflammatory cytokine that affects the circadian clock [17]. As shown in a large number of reports, GA anaesthetics strongly affects the circadian clock [18]. In our report, the surgical trauma and inflammatory cytokine levels in the two groups of patients were similar, except for the pain score. We therefore suggest that the difference in melatonin secretion between the two groups may be attributed to the anaesthetic method and associated pain management.
Sevoflurane or propofol anaesthesia may decrease the expression of clock genes in the SCN, resulting in a decrease in melatonin secretion and a phase advance of the circadian rhythms [46], [47], [48], [49]. The reason for an increased melatonin peak on postoperative day 2 in the GA group is unclear. It is possible that, on the second day after surgery, GA anaesthetics are metabolized and a rebound is observed after the anaesthetic-induced inhibition of melatonin secretion stops.
Another finding in this study was that the plasma cortisol level was significantly decreased at 22:00 on the surgery day in the GA group compared to the SA group. This decrease had recovered at 16:00 on postoperative day 1. Afterwards, the plasma cortisol was higher in the GA than the SA group. A prospective study conducted by Jansen et al. [50] found that a group of patients receiving local anaesthesia exhibited a higher level of cortisol at the beginning of surgery and 20 min after incision compared to the GA group.
Many clinical studies have shown that the cortisol was disturbed by surgical aggression, stress, inflammatory biomarkers, and general anaesthetics [51], [52], [53]. One possible reason for the higher cortisol level in the SA group is that patients are awake, and the perioperative anxiety may lead to a significant increase in cortisol levels [50]. Besides, GA anaesthetics i.e. opioids and etomidate that are known to abolish cortisol secretion [53]. The anaesthetic etomidate interfered with the production of cortisol in the adrenal cortex, and a single induction dose of etomidate suppressed cortisol production for 6 -12 h [53,54].
Our study shows that the peak concentration of melatonin (04:00) was positively correlated with sleep quality. Patients in the GA group experienced significant sleep disturbance in the first postoperative. These findings are consistent with what has been previously reported by Kjolhede et al. [19] and Su et al. [5]. This might be explained by more intense postoperative pain in the GA group. Reciprocally, poor sleep quality might lead to increased sensitivity to pain and more analgesics [5,55]. A lower melatonin concentration (especially the peak concentration of melatonin at 4:00) serves as a major element in mechanisms underlying the sleep disturbance in the GA group.
Circadian rhythm disturbance may account for POD [56]. Several previous studies have demonstrated the link between a disturbed circadian pattern of melatonin secretion and delirium [12,57,58]. In this study, the POD incidence in the GA group was significantly higher than that in the SA group, which is consistent with previous studies [59]. As perioperative inflammatory markers were not different between the two groups, we suggest that the different incidence of POD mainly derives from pain and rhythm disturbance.
To further explore the relationship between circadian rhythm disturbance and POD, we divided the patients into POD versus non-POD groups for comparison (this should be considered as exploratory as the sample size calculation was not based on POD incidence). The results indicated that the POD group experienced more severe circadian rhythm disturbance. Future studies will need to explore whether exogenous treatment with melatonin to re-establish the rhythms could be beneficial for the mitigation of POD [60].
The major strength of our study is that we shed light on the effect of GA and SA on the circadian rhythm and explore their associations with POD in elderly patients. However, the current study has multiple limitations. First, the results may only show a general trend of changes in postoperative melatonin secretion and might have missed the highest level due to a limited number of sampling time points. In general, sampling at higher frequencies may contribute to the accurate analysis of rhythm parameters. Nevertheless, too dense measurements are redundant and do not bring additional information. It also has been recommended to choose Δt≤6h in the case of equidistant data obtained at Δt intervals [33,61]. In order to protect the welfare of our participants and ensure the sleep was not disrupted by multiple blood drawings, we, therefore, took blood samples every 6 hours. Second, we only collected data for two days according to the hospital discharge policy, and both the melatonin and sleep disturbance might have lasted for a longer period. Third, considering geriatric hip fracture patients with at least one significant comorbidity and often experience frailty, the method of anaesthesia (GA or SA) depends on patient wishes, individual situations, as well as the experience of anaesthesiologists and surgeons [62]. Therefore, this study was a non-randomized exploratory and observational cohort study. We cannot therefore exclude the possibility of unmeasured confounders between groups. Fourth, we herein excluded patients with dementia who were at a higher risk for delirium development [63,64]. Lastly, we conduced explorative analysis that require validation in prospective patient cohorts.
5. Conclusion
In summary, the current study provides evidence supporting that SA is superior to GA in elderly patients after hip fracture surgery as SA was associated with less impairment of the melatonin rhythm and sleep, and fewer POD. Our results suggests that melatonin administration provides potential therapeutic benefits for the elderly hip fracture population, by improving sleep and reducing the POD incidence.
Contributors
YNS and YJL contributed equally.
ZQL and XYG contributed equally.
ZQL, XYG, YJL, JQW, and AV contributed to the study design; XYG and ZQL obtained funding; WCZ, GW, YY, and NC and performed the anaesthesia; YYJ was responsible for blood sample; YNS and XPL contributed to data collection; XNM, CL, and YL are laboratory technicians; XXJ, WTL, and LL contributed to circadian rhythm analysis and figures; XXW, YZ, YZH, and CMS are responsible for data statistics; ZQL, XYG, and YNS verified the underlying data; YNS, YY, and ZQL drafted the manuscript; XYG, JQW, and AV reviewed the manuscript; All authors read and approved the final version of the manuscript.
Data sharing
The data used to support the findings of this study are given in the main text.
Funding
The study was supported by the National Natural Science Foundation of China (81971012, 81873726, 81901095, 81701052, and 81801070), Key Clinical Projects of Peking University Third Hospital (BYSYZD2019027), and Peking University “Clinical Medicine plus X” Youth Project (PKU2020LCXQ016).
Footnotes
The research protocol published in the journal of BMJ Open can be found in the online version at doi: 10.1136/bmjopen-2020-043720.
Declaration of Competing Interest
The authors declare no competing interests.
Acknowledgments
All authors thank all participants for their huge contribution to this study. This study was supported by the National Natural Science Foundation of China, Key Clinical Projects of Peking University Third Hospital, and Peking University “Clinical Medicine plus X” Youth Project.
Footnotes
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.ebiom.2021.103490.
Contributor Information
Xiangyang Guo, Email: puthmzk@bjmu.edu.cn.
Zhengqian Li, Email: zhengqianli@bjmu.edu.cn.
Appendix. Supplementary materials
References
- 1.Cauley JA, Chalhoub D, Kassem AM, Fuleihan GH. Geographic and ethnic disparities in osteoporotic fractures. Nat Rev Endocrinol. 2014;10(6):338–351. doi: 10.1038/nrendo.2014.51. [DOI] [PubMed] [Google Scholar]
- 2.Zhang CG, Feng JN, Wang SF, Gao P, Xu L, Zhu JX. Incidence of and trends in hip fracture among adults in urban China: A nationwide retrospective cohort study. Plos Med. 2020;17(8) doi: 10.1371/journal.pmed.1003180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ravi B, Pincus D, Choi S, Jenkinson R, Wasserstein DN, Redelmeier DA. Association of Duration of Surgery With Postoperative Delirium Among Patients Receiving Hip Fracture Repair. Jama Netw Open. 2019;2(2) doi: 10.1001/jamanetworkopen.2019.0111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jin Z, Hu J, Ma D. Postoperative delirium: perioperative assessment, risk reduction, and management. Br J Anaesth. 2020;125(4):492–504. doi: 10.1016/j.bja.2020.06.063. [DOI] [PubMed] [Google Scholar]
- 5.Su X, Wang DX. Improve postoperative sleep: what can we do? Curr Opin Anesthesio. 2018;31(1):83–88. doi: 10.1097/ACO.0000000000000538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fitzgerald JM, Adamis D, Trzepacz PT, O'regan N, Timmons S, Dunne C. Delirium: a disturbance of circadian integrity? Med Hypotheses. 2013;81(4):568–576. doi: 10.1016/j.mehy.2013.06.032. [DOI] [PubMed] [Google Scholar]
- 7.Videnovic A, Noble C, Reid KJ, Peng J, Turek FW, Marconi A. Circadian Melatonin Rhythm and Excessive Daytime Sleepiness in Parkinson Disease. Jama Neurol. 2014;71(4):463–469. doi: 10.1001/jamaneurol.2013.6239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Brzezinski A. Melatonin in humans. N Engl J Med. 1997;336(3):186–195. doi: 10.1056/NEJM199701163360306. [DOI] [PubMed] [Google Scholar]
- 9.Cronin AJ, Keifer JC, Davies MF, King TS, Bixler EO. Melatonin secretion after surgery. Lancet. 2000;356(9237):1244–1245. doi: 10.1016/S0140-6736(00)02795-1. [DOI] [PubMed] [Google Scholar]
- 10.Lewy AJ. Melatonin as a marker and phase-resetter of circadian rhythms in humans. Adv Exp Med Biol. 1999;460:425–434. doi: 10.1007/0-306-46814-x_51. [DOI] [PubMed] [Google Scholar]
- 11.Akerstedt T, Ingre M, Broman JE, Kecklund G. Disturbed sleep in shift workers, day workers, and insomniacs. Chronobiol Int. 2008;25(2-3):333–348. doi: 10.1080/07420520802113922. [DOI] [PubMed] [Google Scholar]
- 12.Miyazaki T, Kuwano H, Kato H, Ando H, Kimura H, Inose T. Correlation between serum melatonin circadian rhythm and intensive care-unit psychosis after thoracic esophagectomy. Surgery. 2003;133(6):662–668. doi: 10.1067/msy.2003.149. [DOI] [PubMed] [Google Scholar]
- 13.Karkela J, Vakkuri O, Kaukinen S, Huang WQ, Pasanen M. The influence of anaesthesia and surgery on the circadian rhythm of melatonin. Acta Anaesth Scand. 2002;46(1):30–36. doi: 10.1034/j.1399-6576.2002.460106.x. [DOI] [PubMed] [Google Scholar]
- 14.Scholtens RM, Van Munster BC, Van Faassen M, Van Kempen MF, Kema IP, De Rooij SE. Plasma melatonin levels in hip fracture patients with and without delirium: A confirmation study. Mech Ageing Dev. 2017;167:1–4. doi: 10.1016/j.mad.2017.08.016. [DOI] [PubMed] [Google Scholar]
- 15.Piotrowicz K, Klich-Raczka A, Pac A, Zdzienicka A, Grodzicki T. The diurnal profile of melatonin during delirium in elderly patients–preliminary results. Exp Gerontol. 2015;72:45–49. doi: 10.1016/j.exger.2015.09.007. [DOI] [PubMed] [Google Scholar]
- 16.Yousaf F, Seet E, Venkatraghavan L, Katznelson R, Chung F. Melatonin and postoperative delirium: a possible link? Can J Anaesth. 2010;57(8):794–795. doi: 10.1007/s12630-010-9340-2. [DOI] [PubMed] [Google Scholar]
- 17.Coppola S, Caccioppola A, Chiumello D. Internal clock and the surgical ICU patient. Curr Opin Anesthesio. 2020;33(2):177–184. doi: 10.1097/ACO.0000000000000816. [DOI] [PubMed] [Google Scholar]
- 18.Poulsen RC, Warman GR, Sleigh J, Ludin NM, Cheeseman JF. How does general anaesthesia affect the circadian clock? Sleep Med Rev. 2018;37:35–44. doi: 10.1016/j.smrv.2016.12.002. [DOI] [PubMed] [Google Scholar]
- 19.Kjolhede P, Langstrom P, Nilsson P, Wodlin NB, Nilsson L. The Impact of Quality of Sleep on Recovery from Fast-Track Abdominal Hysterectomy. J Clin Sleep Med. 2012;8(4):395–402. doi: 10.5664/jcsm.2032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wirz-Justice A, Frank M. Does anaesthesia stop the clock? Sleep Med Rev. 2018;37:3. doi: 10.1016/j.smrv.2017.06.004. [DOI] [PubMed] [Google Scholar]
- 21.Tzimas P, Samara E, Petrou A, Korompilias A, Chalkias A, Papadopoulos G. The influence of anesthetic techniques on postoperative cognitive function in elderly patients undergoing hip fracture surgery: General vs spinal anesthesia. Injury. 2018;49(12):2221–2226. doi: 10.1016/j.injury.2018.09.023. [DOI] [PubMed] [Google Scholar]
- 22.Yuan Y, Song Y, Wang G, Jia Y, Zhou Y, Mi X. Effects of general versus regional anaesthesia on circadian melatonin rhythm and its association with postoperative delirium in elderly patients undergoing hip fracture surgery: study protocol for a prospective cohort clinical trial. BMJ Open. 2021;11(2) doi: 10.1136/bmjopen-2020-043720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Charlson M, Szatrowski TP, Peterson J, Gold J. Validation of a combined comorbidity index. J Clin Epidemiol. 1994;47(11):1245–1251. doi: 10.1016/0895-4356(94)90129-5. [DOI] [PubMed] [Google Scholar]
- 24.Girard TD, Thompson JL, Pandharipande PP, Brummel NE, Jackson JC, Patel MB. Clinical phenotypes of delirium during critical illness and severity of subsequent long-term cognitive impairment: a prospective cohort study. Lancet Respir Med. 2018;6(3):213–222. doi: 10.1016/S2213-2600(18)30062-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Katz S, Ford AB, Moskowitz RW, Jackson BA, Jaffe MW. Studies of Illness in the Aged. The Index of Adl: A Standardized Measure of Biological and Psychosocial Function. JAMA. 1963;185:914–919. doi: 10.1001/jama.1963.03060120024016. [DOI] [PubMed] [Google Scholar]
- 26.Buysse DJ, Reynolds CF, Monk TH, Berman SR, Kupfer DJ. The Pittsburgh Sleep Quality Index - a New Instrument for Psychiatric Practice and Research. Psychiat Res. 1989;28(2):193–213. doi: 10.1016/0165-1781(89)90047-4. [DOI] [PubMed] [Google Scholar]
- 27.Bahreini M, Jalili M, Moradi-Lakeh M. A comparison of three self-report pain scales in adults with acute pain. J Emerg Med. 2015;48(1):10–18. doi: 10.1016/j.jemermed.2014.07.039. [DOI] [PubMed] [Google Scholar]
- 28.Lockley SW. Journal of Pineal Research guideline for authors: Measuring melatonin in humans. J Pineal Res. 2020;69(2):e12664. doi: 10.1111/jpi.12664. [DOI] [PubMed] [Google Scholar]
- 29.Yuan Y, Li Z, Yang N, Han Y, Ji X, Han D. Exosome α-Synuclein Release in Plasma May be Associated With Postoperative Delirium in Hip Fracture Patients. Front Aging Neurosci. 2020;12(13):67. doi: 10.3389/fnagi.2020.00067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.De Zambotti M, Goldstone A, Claudatos S, Colrain IM, Baker FC. A validation study of Fitbit Charge 2™ compared with polysomnography in adults. Chronobiol Int. 2018;35(4):465–476. doi: 10.1080/07420528.2017.1413578. [DOI] [PubMed] [Google Scholar]
- 31.Terzieva DD, Mateva ND, Vladimirova-Kitova LG. Melatonin Reference Limits at 3:00 AM and 8:00 AM in Healthy Adults. Clin Lab. 2009;55(9-10):359–361. [PubMed] [Google Scholar]
- 32.Kennaway DJ. A critical review of melatonin assays: Past and present. J Pineal Res. 2019;67(1):e12572. doi: 10.1111/jpi.12572. [DOI] [PubMed] [Google Scholar]
- 33.Refinetti R, Lissen GC, Halberg F. Procedures for numerical analysis of circadian rhythms. Biol Rhythm Res. 2007;38(4):275–325. doi: 10.1080/09291010600903692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Liu L, Wang Z, Cao J, Dong Y, Chen Y. Effect of melatonin on monochromatic light-induced changes in clock gene circadian expression in the chick liver. J Photochem Photobiol B. 2019;197 doi: 10.1016/j.jphotobiol.2019.111537. [DOI] [PubMed] [Google Scholar]
- 35.Karasek M, Winczyk K. Melatonin in humans. J Physiol Pharmacol. 2006;57(5):19–39. Official Journal of the Polish Physiological SocietySuppl. [PubMed] [Google Scholar]
- 36.Chung KF, Poon YPY, Ng TK, Kan CK. Subjective-Objective Sleep Discrepancy in Schizophrenia. Behav Sleep Med. 2020;18(5):653–667. doi: 10.1080/15402002.2019.1656077. [DOI] [PubMed] [Google Scholar]
- 37.Benjamini Y, Hochberg Y. Controlling the False Discovery Rate - a Practical and Powerful Approach to Multiple Testing. J R Stat Soc B. 1995;57(1):289–300. [Google Scholar]
- 38.Dornbierer DA, Boxler M, Voegel CD, Stucky B, Steuer AE, Binz TM. Nocturnal Gamma-Hydroxybutyrate Reduces Cortisol-Awakening Response and Morning Kynurenine Pathway Metabolites in Healthy Volunteers. Int J Neuropsychopharmacol. 2019;22(10):631–639. doi: 10.1093/ijnp/pyz047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ray S, Valekunja UK, Stangherlin A, Howell SA, Snijders AP, Damodaran G. Circadian rhythms in the absence of the clock gene Bmal1. Science. 2020;367(6479):800–806. doi: 10.1126/science.aaw7365. [DOI] [PubMed] [Google Scholar]
- 40.Gögenur I, Ocak U, Altunpinar O, Middleton B, Skene DJ, Rosenberg J. Disturbances in melatonin, cortisol and core body temperature rhythms after major surgery. World J Surg. 2007;31(2):290–298. doi: 10.1007/s00268-006-0256-5. [DOI] [PubMed] [Google Scholar]
- 41.Shenshen Y, Minshu W, Qing Y, Yang L, Suodi Z, Wei W. The effect of cataract surgery on salivary melatonin and sleep quality in aging people. Chronobiol Int. 2016;33(8):1064–1072. doi: 10.1080/07420528.2016.1197234. [DOI] [PubMed] [Google Scholar]
- 42.Yoshitaka S, Egi M, Morimatsu H, Kanazawa T, Toda Y, Morita K. Perioperative plasma melatonin concentration in postoperative critically ill patients: Its association with delirium. J Crit Care. 2013;28(3):236–242. doi: 10.1016/j.jcrc.2012.11.004. [DOI] [PubMed] [Google Scholar]
- 43.Farsi H, Harti D, Achaâban MR, Piro M, Raverot V, Bothorel B. Melatonin rhythm and other outputs of the master circadian clock in the desert goat (Capra hircus) are entrained by daily cycles of ambient temperature. J Pineal Res. 2020;68(3):e12634. doi: 10.1111/jpi.12634. [DOI] [PubMed] [Google Scholar]
- 44.Brown TM. Melanopic illuminance defines the magnitude of human circadian light responses under a wide range of conditions. J Pineal Res. 2020;69(1):e12655. doi: 10.1111/jpi.12655. [DOI] [PubMed] [Google Scholar]
- 45.Dispersyn G, Pain L, Touitou Y. Propofol Anesthesia Significantly Alters Plasma Blood Levels of Melatonin in Rats. Anesthesiology. 2010;112(2):333–337. doi: 10.1097/ALN.0b013e3181c920e2. [DOI] [PubMed] [Google Scholar]
- 46.Yoshida Y, Nakazato K, Takemori K, Kobayashi K, Sakamoto A. The influences of propofol and dexmedetomidine on circadian gene expression in rat brain. Brain Res Bull. 2009;79(6):441–444. doi: 10.1016/j.brainresbull.2009.04.015. [DOI] [PubMed] [Google Scholar]
- 47.Ben-Hamouda N, Poirel VJ, Dispersyn G, Pevet P, Challet E, Pain L. Short-term propofol anaesthesia down-regulates clock genes expression in the master clock. Chronobiol Int. 2018;35(12):1735–1741. doi: 10.1080/07420528.2018.1499107. [DOI] [PubMed] [Google Scholar]
- 48.Sakamoto A, Imai J, Nishikawa A, Honma R, Ito E, Yanagisawa Y. Influence of inhalation anesthesia assessed by comprehensive gene expression profiling. Gene. 2005;356:39–48. doi: 10.1016/j.gene.2005.03.022. [DOI] [PubMed] [Google Scholar]
- 49.Reppert SM, Weaver DR. Coordination of circadian timing in mammals. Nature. 2002;418(6901):935–941. doi: 10.1038/nature00965. [DOI] [PubMed] [Google Scholar]
- 50.Jansen P, Stoffels I, Museler AC, Petri M, Brinker TJ, Schedlowski M. Salivary cortisol levels and anxiety in melanoma patients undergoing sentinel lymph node excision under local anesthesia versus general anesthesia: a prospective study. World J Surg Oncol. 2020;18(1):53. doi: 10.1186/s12957-020-01823-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Dispersyn G, Pain L, Challet E, Touitou Y. General anesthetics effects on circadian temporal structure: an update. Chronobiol Int. 2008;25(6):835–850. doi: 10.1080/07420520802551386. [DOI] [PubMed] [Google Scholar]
- 52.Khoo B, Boshier PR, Freethy A, Tharakan G, Saeed S, Hill N. Redefining the stress cortisol response to surgery. Clin Endocrinol. 2017;87(5):451–458. doi: 10.1111/cen.13439. [DOI] [PubMed] [Google Scholar]
- 53.Desborough JP. The stress response to trauma and surgery. Br J Anaesth. 2000;85(1):109–117. doi: 10.1093/bja/85.1.109. [DOI] [PubMed] [Google Scholar]
- 54.Wagner RL, White PF. Etomidate inhibits adrenocortical function in surgical patients. Anesthesiology. 1984;61(6):647–651. doi: 10.1097/00000542-198412000-00003. [DOI] [PubMed] [Google Scholar]
- 55.Wang JP, Lu SF, Guo LN, Ren CG, Zhang ZW. Poor preoperative sleep quality is a risk factor for severe postoperative pain after breast cancer surgery A prospective cohort study. Medicine. 2019;98(44) doi: 10.1097/MD.0000000000017708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Scott BK. Disruption of Circadian Rhythms and Sleep in Critical Illness and its Impact on the Development of Delirium. Curr Pharm Des. 2015;21(24):3443–3452. doi: 10.2174/1381612821666150706110656. [DOI] [PubMed] [Google Scholar]
- 57.Maldonado JR. Neuropathogenesis of Delirium: Review of Current Etiologic Theories and Common Pathways. Am J Geriat Psychiat. 2013;21(12):1190–1222. doi: 10.1016/j.jagp.2013.09.005. [DOI] [PubMed] [Google Scholar]
- 58.Olofsson K, Alling C, Lundberg D, Malmros C. Abolished circadian rhythm of melatonin secretion in sedated and artificially ventilated intensive care patients. Acta Anaesth Scand. 2004;48(6):679–684. doi: 10.1111/j.0001-5172.2004.00401.x. [DOI] [PubMed] [Google Scholar]
- 59.Ehsani R, Djalali Motlagh S, Zaman B, Sehat Kashani S, Ghodraty MR. Effect of General Versus Spinal Anesthesia on Postoperative Delirium and Early Cognitive Dysfunction in Elderly Patients. Anesthesiol Pain Medicine. 2020;10(4) doi: 10.5812/aapm.101815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Han Y, Wu J, Qin Z, Fu W, Zhao B, Li X. Melatonin and its analogues for the prevention of postoperative delirium: A systematic review and meta-analysis. J Pineal Res. 2020:e12644. doi: 10.1111/jpi.12644. [DOI] [PubMed] [Google Scholar]
- 61.Cornelissen G. Cosinor-based rhythmometry. Theor Biol Med Model. 2014;11:16. doi: 10.1186/1742-4682-11-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Wenk M, Frey S. Elderly hip fracture patients: surgical timing and factors to consider. Curr Opin Anaesthesiol. 2021;34(1):33–39. doi: 10.1097/ACO.0000000000000941. [DOI] [PubMed] [Google Scholar]
- 63.Wang P, Velagapudi R, Kong C, Rodriguiz RM, Wetsel WC, Yang T. Neurovascular and immune mechanisms that regulate postoperative delirium superimposed on dementia. Alzheimers Dement. 2020;16(5):734–749. doi: 10.1002/alz.12064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Cortese GP, Burger C. Neuroinflammatory challenges compromise neuronal function in the aging brain: Postoperative cognitive delirium and Alzheimer's disease. Behav Brain Res. 2017;322(Pt B) doi: 10.1016/j.bbr.2016.08.027. 269-279. Pt. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.