Abstract
Though electrophysiological measures (EEG and ERP) offer complementary information to MRI and a variety of advantages for studying infants and young children, these measures have not yet been included in large cohort studies of neurodevelopment. This review summarizes the types of EEG and ERP measures that could be used in the HEALthy Brain and Cognitive Development (HBCD) study, and the promises and challenges in doing so. First, we provide brief overview of the use of EEG/ERP for studying the developing brain and discuss exemplar findings, using resting or baseline EEG measures as well as the ERP mismatch negativity (MMN) as exemplars. We then discuss the promises of EEG/ERP such as feasibility, while balancing challenges such as ensuring good signal quality in diverse children with different hair types. We then describe an ongoing multi-site EEG data harmonization from our groups. We discuss the process of alignment and provide preliminary usability data for both resting state EEG data and auditory ERP MMN in diverse samples including over 300 infants and toddlers. Finally, we provide recommendations and considerations for the HBCD study and other studies of neurodevelopment.
Keywords: EEG, ERP, Mismatch negativity, Neurodevelopment, HBCD
1. EEG and ERP methods and their contribution to neurodevelopmental research
There is a longstanding precedent for using electroencephalography (EEG) as an indicator of brain function in developmental science, but its potential has not been fully realized as big data approaches have become drivers of neurodevelopmental research. EEG is a unique indicator of neural activity, is developmentally sensitive for use with infants from birth, and is more pragmatic (e.g., less expensive and portable) than MRI methods. In this paper, we provide a brief primer about EEG methods, their utility for large scale consortia, and challenges and opportunities for this next generation of neurodevelopmental research.
1.1. What are EEG/ERPs?
EEG is a noninvasive measurement tool designed to assess electrical activity in the brain (Pizzagalli, 2007). Neural activity and oscillations are recorded as EEG signals from electrodes that are placed on the scalp. Event-related potentials (ERPs) are EEG signals that are time-locked to a stimulus, such as the presentation of a sound or an image of a face. EEG and ERP measures can reflect several key types of neural activity that underlie cognitive and emotional processes that occur rapidly and transiently, within milliseconds (whereas temporal resolution for fMRI is several seconds). Because EEG is a direct measure of neuronal activity, provides millisecond time precision, is cost effective and feasible, and because it is well-suited to studying infants and children (as we describe in detail in Section 4.1, below), it is a powerful tool for developmental science.
Both EEG and ERPs offer crucial insights into the functioning of the typical and atypical developing brain. For over 70 years, clinicians and researchers have examined the utility of EEG measures for improving understanding of neurodevelopment and disorders (Jasper et al., 1938; Jasper, 1949). Multiple researchers have previously suggested that EEG may be of particular interest and applicability to assessing traits related to cognitive and emotional development and psychopathology in children, as well as biomarkers of relevant disorders (Banaschewski and Brandeis, 2007; Brooker et al., 2020; Loo et al., 2016). Studies examining risk for autism spectrum disorder have indicated that EEG markers early in life can provide good sensitivity and specificity for later diagnosis (Bosl et al., 2018).
1.2. Salience for the HEALthy Brain and Child Development (HBCD) study and other neurodevelopmental consortia
Because EEG/ERP measures can presage later cognitive and language skills (Bishop, 2007; Norton et al., 2019) and can be measured early in life, these are ideal for studying large samples of infants and children. However, traditional large national neurodevelopmental consortia focusing on broad brain development have had a sole focus on magnetic resonance imaging (MRI) and have not included EEG modalities. While MRI offers unique and important information that cannot be obtained from EEG/ERP, the higher cost and lower feasibility make it more challenging. Fortunately, the planned HEALthy Brain and Child Development (HBCD) Study requires EEG as a neuroimaging method. HBCD will be a longitudinal, nation-wide study, funded by the National Institutes of Health (NIH; Volkow et al., 2020).
Beginning prenatally, HBCD will characterize normative infant brain and behavioral development, as well as early life risk and resilience factors that influence typical and atypical development from birth to middle childhood (Morris et al., 2020). The study will include a representative cohort to examine normative brain development, oversampling for exposure to opioids and other substances, and adverse perinatal exposures that capture the developmental origins of health disparities. This multi-site study will include ˜7,500 pregnant women with planned follow-up of children for a decade. Importantly, HBCD will be the first and largest longitudinal consortium explicitly designed to prospectively examine the effects of early exposures on development and the first large-scale study of broad neurodevelopment to implement EEG and ERP measures.
1.3. Types of EEG measures and illustrative scientific utility
1.3.1. Baseline EEG procedure
EEG can be measured during developmentally appropriate tasks as well as during a neutral baseline or “rest” state. Baseline EEG features may reflect potentially meaningful differences in affective and cognitive tendencies that underlie behavioral response patterns and are widely studied on their own. Baseline EEG data collection protocols typically include a short period of quiet wakefulness, usually lasting less than 5 min. Infants and young children may watch a video of abstract images moving on a screen, a minimally arousing video clip, colorful balls spinning in a bingo wheel, or a person blowing bubbles, as just some examples. Researchers may mimic the “eyes-open” and “eyes-closed” segments of the standard adult and older children protocols by alternating turning lights on and off throughout the session. A baseline recording can also serve as an important point of comparison for EEG measured during a task. In infancy and toddlerhood, baseline EEG assessment is perhaps an ideal measure of neural activity because it is task-free; directing or engaging young children in specific tasks (e.g., attending to repetitive stimuli on a screen or pressing a button to indicate recognition given stimulus, etc.) is difficult (Bell and Cuevas, 2012). There are various ways to quantify EEG data; some widely used approaches are described below.
1.3.2. Baseline EEG power
Power is conceptualized as the signal produced from a neuronal population at the same time, and therefore represents an index of neural activity in a particular location (Klimesch, 1999). Power values are typically calculated for specific frequency bands, which are associated with different emotional and cognitive processes in children and adults. These values are derived by taking EEG data that have been preprocessed to remove artifacts and applying a Fourier transform that produces spectral power at the different frequencies, expressed in mean square microvolts. Measuring power within certain frequency bands that correspond to different established cognitive processes can provide important information about child development (Saby and Marshall, 2012).
The 6–9 Hz alpha band is the dominant frequency band in infancy and early childhood; this corresponds to the characteristics of the 8–13 Hz alpha band that is most prominent in awake children and adults (Cuevas and Bell, 2012; Marshall et al., 2002; Stroganova et al., 1999; Stroganova and Orekhova, 2007). Changes in power in this frequency band have been associated with several domains of development such as cognition (e.g., working memory) and emotional regulation (Bell, 2002; Fox et al., 2001). Other oscillations that have been studied in young children include high-frequency oscillations that typically increase across development, such as beta, and low-frequency oscillations that are shown to decrease, such as theta and delta (Matoušek and Petersen, 1973). Beta (approximately 10–18 Hz in infants, 13−30 Hz in adults) has been associated with cognitive processing, including attentional control and regulation (Bell, 1998; Ray and Cole, 1985). Activity in the theta band (3–6 Hz in infants and 4–7 Hz in older children and adults) has been related to social stimulation and affective state (Orekhova et al., 2006), as well as attention and readiness to learn (Begus and Bonawitz, 2020). Greater relative power in the low-frequency delta band (typically <2 Hz in infants/toddlers, up to 3 Hz in adults) as compared with higher bands such as alpha and beta, has been linked to a variety of mood, mental health, and learning disorders in children (Chabot et al., 2005).
1.3.3. Frontal EEG asymmetry
Frontal EEG asymmetry is the relative response or power in frontal electrodes in one hemisphere compared to the other. Asymmetry scores are typically calculated as a subtraction or ratio of power between electrodes in the two hemispheres. Alpha asymmetry is a widely-studied such metric, typically comparing power from a left frontal electrode (e.g., F3) with one on the right (e.g., F4). Frontal asymmetry measured at rest has been conceptualized as a trait-like biological marker of approach/avoidance tendencies, biasing how an individual processes affective information in their environment that may be a marker of risk for maladjustment (Coan et al., 2006; Davidson, 2000; Gatzke-Kopp et al., 2014). Individual differences in resting frontal EEG asymmetry emerge within the child’s first year and remain relatively stable over development (Henderson et al., 2001). Researchers can also measure changes in EEG asymmetry from baseline to task to examine how an individual currently responds to an emotion-eliciting situation (e.g., Atzaba-Poria et al., 2017; Bell and Diaz, 2012; Diaz and Bell, 2012). How well an individual is able to regulate their emotional distress may be related to these differences in the activation of the left and right frontal cortices (Fox, 1994).
1.3.4. Baseline EEG coherence
Coherence is a metric of similarity between electrode sites, typically calculated as the squared correlation coefficient of signals between a pair of electrodes in a specific frequency band. Coherence values range from 0 to 1, such that a value closer to 1, or a high coherence between sites, is thought to indicate a stronger level of synchronization or connectivity between the two sites (Nunez, 1981; Thatcher, 1992; Thatcher et al., 1986). Measured across age, increases and decreases in coherence depend on the relative distance between electrodes and may indicate brain maturation (Thatcher, 1994). Changes in coherence from baseline to task may index crosstalk of regions during specific cognitive processes elicited by the task. For instance, increases in coherence from baseline to task have been associated with executive function ability in preschoolers (Swingler et al., 2011).
1.3.5. Cross-frequency coupling
Correlations of oscillations during continuous recordings of EEG may provide new insights into trait-level relations between an individual’s cortical and subcortical circuitry. Most notable is delta-beta coupling, which is thought to reflect connections between the subcortical limbic system and the cortical regulatory system, both of which are implicated in emotional behaviors (Knyazev, 2007). Emerging research with infants and young children finds associations between delta-beta coupling and fearful or reactive behaviors. In a study of 6-month-old infants, researchers found key differences in delta-beta coupling based on levels of cortisol reactivity. Compared to those infants who were low cortisol-reactive, infants who were high cortisol-reactive showed greater delta-beta coupling in frontal, central, and parietal regions at baseline (Brooker et al., 2016). The temperamental profile of behavioral inhibition was also associated with higher delta-beta coupling in central and parietal regions for early school-age children (Pool et al., 2018).
1.4. Types of event-related EEG oscillations and spectral perturbations and illustrative scientific utility
Following some input or event, changes in EEG oscillations can be recorded from different frequency bands, which are termed event-related oscillations (EROs) or event-related spectral perturbations (ERSPs). In contrast to ERPs which measure event-related voltage changes, EROs reflect changes in oscillatory activity, typically within a given frequency band (Basar et al., 1998); such differences may reflect individual differences in how the brain responds to stimuli, such as sensory, emotional, or cognitive stimulation. In development, event-related differences in ranges studies with continuous EEG, such as theta, alpha, and mu oscillations, have been studied widely (Begus and Bonawitz, 2020; Marshall and Meltzoff, 2011; Yordanova and Kolev, 2009).
Studies indicate that EROs/ERSPs reflect crucial processes of early neural tuning for language. Three-month-old infants display heightened gamma power when listening to utterances in their native language and rhythmically similar languages (Pena et al., 2010). By 6 months of age, that preference appears to be honed, as gamma power increases exclusively when listening to sentences in their native language only (Ortiz-Mantilla et al., 2013). Similarly, event-related alpha power changes in response to both human speech and lemur calls (but not backward human speech) at 3–4 months of age; however, at 6 months, only human speech elicits the alpha oscillatory changes (Woodruff Carr et al., 2021).
Atypical oscillatory modulations may be biomarkers for psychopathology; for example, children with ADHD have demonstrated differential theta oscillations during a visual attention task compared to typically developing peers (Guo et al., 2020). Altered patterns of theta and gamma oscillations were observed when children on the Autism spectrum who were minimally verbal were visually processing semantically relevant information (Ortiz-Mantilla et al., 2019). Alterations in evoked theta power have been linked to attention and emotion as early as infancy, as well as processes such as error or change detection (Begus and Bonawitz, 2020), which are impaired in multiple mental health disorders.
1.5. Types of ERP measures and illustrative scientific utility
ERPs are time-locked segments of electrical activity when processing a particular stimulus, averaged across trials (Luck, 2005). ERPs offer a method to address an extremely wide variety of sensory, perceptual, cognitive, and affective processes, from pre-attentive processing within milliseconds of stimulus onset to conscious stimulus processing, to post-stimulus emotional appraisal. For a review of components related to child psychopathology, see Banaschewski and Brandeis (2007) and related to child language, see McWeeny and Norton (2020). ERPs are typically measured in terms of the amplitude or latency of a given component within a certain time window. Each component is typically defined by a spatial topography on the scalp, though spatial location of the peak or asymmetry of the amplitude are also sometimes measured (Norton et al., 2021). The goal of these measurements is typically to then compare across groups, ages, or to relate to behavioral measures.
ERPs are useful for capturing how the brain responds to, and differentiates between, discrete stimuli. For instance, infants at 7 months of age have demonstrated amplitude differences in the Nc component, associated with attention allocation to visual information, when shown happy versus threatening faces (de Haan et al., 2004). Depending on the stimulus, multiple ERP components can be measured that reflect different cognitive processes that occur on the order of milliseconds and would otherwise be challenging to capture via a behavioral response. An example of this is attention processes that unfold over the presentation of emotional faces. Researchers measure attention processes in multiple ways, but behavioral measures that require button presses are prevalent in the literature (e.g., the dot-probe task; Bradley et al., 1999; Mogg et al., 1997). However, multiple emotional, perceptual, and cognitive processes can occur between the stimulus presentation and one’s motor response, suggesting behavioral attention paradigms may not be reliable indicators of attention when shifts can occur under 100 milliseconds (Kappenman et al., 2014; Muller & Rabbitt, 1989). Further, rapid responses via button responses cannot be reliably assessed with young children.
ERPs can capture both bottom-up and top-down attention processes that occur at the millisecond-level without requiring overt attention or behavioral responses, making it an ideal measure to employ with young children. For instance, N2 and P2 ERP components, indexing different levels of attention at approximately 200 milliseconds after presentation of an emotional face, may help disentangle the links between threat avoidance and threat vigilance in anxious children (Thai et al., 2016). In infants as young as 7 months old, researchers have found differences in informational processing of emotional expressions as indexed by ERPs (Leppanen et al., 2007).
2. EEG/ERPs as an index of brain maturation
One interest of the HBCD study is indexing brain maturation, as maturation may vary based on the variety of exposures and environmental factors that will be studied. Charting intra-individual change or development for a given construct can be challenging in early development, given that a measure that is developmentally appropriate at one age may not be appropriate even mere months earlier or later. In order to truly track the same constructs over time, the way that EEG/ERPs are measured may need to change with the age of the child, including factors like the task length, task demand or need for child compliance, hardware factors like tolerance for electrodes placed on the face, or factors related to processing pipelines such as how eye blinks are detected and corrected or rejected). However, some EEG tasks are scalable across early development to capture individual differences in behavior while indexing brain maturation. These tasks are often relatively passive (do not require overt behavioral responses), such as EEG measured at baseline. ERPs such as the mismatch negativity (MMN) may also be a useful tool, as they are elicited via passive auditory paradigms. Here, we discuss these two paradigms as exemplars, given their widespread use and potential for inclusion in HBCD and other longitudinal studies.
2.1. Baseline EEG as an index of brain maturation
Repeated measures of baseline EEG across early development can provide multiple indexes of brain maturation. Developmental cognitive neuroscientists consider higher EEG power in certain bands to be indicative of broad brain development, as previous work has found linear increases in power across infancy and that higher power values are related to increased cognitive performance (Bell and Fox, 1992; Cuevas and Bell, 2011). Baseline power in the 6- to 9-Hz frequency band (the child alpha and mu frequency range) increases linearly from 6 to 12 months of age across frontal, parietal, and occipital sites (Bell and Fox, 1994; MacNeill et al., 2018). This band shows age-related developments through age 4 (Marshall et al., 2002). Such increases in baseline EEG power in particular regions over time are thought to index neuronal maturation and excitability in those groups of neurons (Nunez, 1981). Particularly in infancy, the brain undergoes rapid maturation that underlies the “bursts” in behavioral change that are evident in the developing infant (Fischer and Van Geert, 2014). The month-to-month changes in EEG power across the first year provide evidence to suggest whole brain maturation, further supported by research finding that power over time is linked to gains in cognitive performance, such as the A-not-B task (Bell and Fox, 1992; MacNeill et al., 2018). EEG power changes in various frequency bands continue in a protracted fashion through childhood and into early adulthood (e.g., Tierney et al., 2013.)
EEG coherence between electrode pairs over time may be markers of integrating neural networks that underlie maturation of the central nervous system (Thatcher, 1994). From birth to age 3, Thatcher and colleagues (Thatcher et al., 1987) found that some electrode pairs showed decreases in coherence while others showed increases over time. However, patterns of coherence were relatively phase-locked and stable beyond three years of age. These developmental shifts in coherence stability were posited to parallel bursts of cognitive change across childhood. Increases and decreases in coherence as indexes of maturation may also depend on electrode proximity and the hemisphere in question. In the left hemisphere, coherence that decreases across age for short-distance sites, but increases across age for long-distance sites, may indicate greater maturation. The right hemisphere follows an opposite pattern indicative of maturation. Such differential patterns of coherence across early development have been linked to critical skill gains in domains such as language and motor development (Bell and Fox, 1996; Mundy et al., 2003).
2.2. ERP MMN as an index of brain maturation
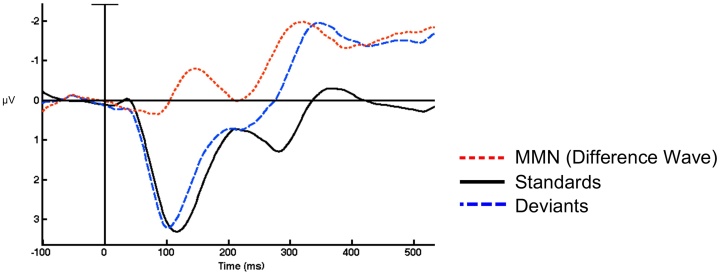
The mismatch negativity (MMN) ERP component is an indicator of auditory processing and reflects automatic discrimination of auditory input (Naatanen, 2001; Naatanen et al., 2012). It is elicited by an oddball paradigm, in which a repeated standard sound is occasionally replaced by a deviant sound (often a different syllable or tone of a different frequency than the standard sound). The MMN component is the difference between the response to the standards and deviants (see Fig. 1), as the brain discriminates the sound that is different from the one that the individual had stored in memory.
Fig. 1.
Example of MMN group grand average waveform. Measurements of the MMN typically examine the amplitude or peak of the difference wave (in red, the difference between the response to standard vs. deviant stimuli). (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article).
The auditory oddball MMN paradigm is a passive task and thus feasible in infancy, making the MMN a prime candidate for characterizing brain maturation across early development (Chen et al., 2017; Guttorm et al., 2010). This paradigm is also ideal for studying longitudinal, within-person change because task demands do not change. Further, passive auditory tasks have prominently lower attrition rates than visual ERP studies (Stets et al., 2012) because the child doesn’t have to attend to stimuli on a screen, allowing researchers to retain more quality ERP data, increasing signal-to-noise ratio and decreasing subject attrition.
The MMN changes markedly with age. Infants typically show a positive voltage wave for the MMN, sometimes called a mismatch response (MMR). Infants born preterm or with very low birthweight show atypical MMRs (Bisiacchi et al., 2009; Fellman et al., 2004). Earlier studies examining the MMN in developmental populations indicated that an adult-like MMN (to approx. 1000 vs. 1200 Hz tones) was present by about 4 years of age (Morr et al., 2002; Shafer et al., 2000), and that the peak latency decreased at a rate of approximately 1 ms per month (Morr et al., 2002). However, later studies indicate that these findings may be due to the use of peak measurements that are influenced by noise, and that the MMN to tones and syllables continues to mature through adolescence (Bishop et al., 2011). Other studies indicate that children with language impairment show immature or delayed MMN responses, similar to those seen earlier in development among typically developing children (Bishop and McArthur, 2004).
3. Exemplar early EEG/ERP predictors of later abilities and outcomes
In this section, we describe three areas of research in which EEG/ERP measures have been shown across multiple studies to be relevant indicators of important constructs known to predict developmental psychopathology (e.g., early life adversity) or predictors of later functioning such as language ability and mental health. Importantly, the results regarding these indicators are robust, seen across labs and ages. Of course, these are merely exemplars, as there are myriad ways to employ EEG/ERP to characterize neurodevelopmental mechanisms. Importantly, the ERP mismatch negativity (MMN) and baseline EEG techniques that form the basis of these studies are ideal for large consortia because they are highly feasible and cost effective for work with infants and children across ages.
3.1. ERP MMN and later language and psychopathology outcomes
The MMN is an ERP that represents the difference in response between a standard stimulus that is presented frequently versus a deviant or oddball stimulus that is presented intermittently among the standards. In neurodevelopmental research, the stimuli are almost always auditory, such as tones that differ in pitch or different speech syllables or phonemes. In studies of infants and children, the MMN often has two peaks, often called the early MMN and late MMN (or late discriminative negativity, LDN). It is not yet clear whether the MMN is an index of the brain’s ability to build a memory trace/template, detect deviation from that trace, or some combination (Garrido et al., 2009).
The MMN is one of the most widely studied ERPs in infancy and childhood because it can be completed passively, even during sleep. The bulk of studies in this area relate the MMN to language impairment, reading ability/dyslexia (Norton et al., 2019), and autism spectrum disorder (Schwartz et al., 2018). Though there are substantial differences in the results across studies, the general findings are that children who are at risk for or who have poor language or reading abilities have reduced amplitude of the MMN. Meta-analyses reveal that children and adults with dyslexia have reduced amplitude MMN to speech stimuli (Gu and Bi, 2020), but that groups with autism spectrum disorder (ASD) do not show significant MMN differences (Schwartz et al., 2018) relative to peers. Importantly, various aspects of the MMN also relate to key pre-literacy abilities including rapid automatized naming and phonological awareness (Norton et al., 2021).
Multiple studies suggest that the MMN measured very early in life (even within days after birth) relates to risk for or later diagnosis of language and reading disorders. Reading disorders such as dyslexia are among the most common learning disabilities, affecting approximately 7% of the population, and they are difficult to identify early in reading development, the timepoint when intervention is most effective (Peterson and Pennington, 2012). Numerous studies find that infants with family history of dyslexia (FHD) which indicates genetic risk (as dyslexia is approximately 50 % heritable) show altered or absent mismatch responses as compared to peers (e.g., Leppanen et al., 2002; Pihko et al., 1999; van Leeuwen et al., 2006). MMN responses in infancy also relate to later reading ability. Studies indicate that 2-month-old Dutch infants who were later diagnosed with dyslexia had absent MMN responses, whereas peers showed MMNs (van Zuijen et al., 2013). Another study of Finnish infants found that those with FHD had atypical MMNs in the first week of life, regardless of whether they later became typical readers or poor readers (Leppanen et al., 2010). One study in English-speaking infants observed that ERP responses to syllables, though not in a typical MMN oddball paradigm, combined in a discriminant function could classify 81 % of children as typical, dyslexic, or poor readers (defined as low reading ability and lower IQ) 8 years later (Molfese, 2000). These longitudinal studies from infancy to reading age have had relatively small groups (typically 24 or fewer children per group) and so more work is needed in large samples to assess how the MMN relates to later reading ability.
Studies have also linked the MMN to numerous factors related to psychopathology and altered auditory or conscious processing (Naatanen et al., 2012), though fewer longitudinal studies exist in these areas. In specific diagnoses, as heterogeneous as ADHD and schizophrenia, many studies have been conducted and meta-analyses show significant overall effects of reduced MMN for those with the disorder (for ADHD, Cheng et al., 2016; schizophrenia, Umbricht and Krljes, 2005). In addition, the MMN has been linked with underlying dimensional risk phenotypes, including social withdrawal (Bar-Haim et al., 2003) and impulsivity (Franken et al., 2005); in the case of young adults with high impulsivity, the MMN amplitude was greater than for controls. A similar auditory change detection paradigm also showed differences in children at 9 months with high levels of behavioral inhibition (Marshall et al., 2009), which is a risk factor for later anxiety disorder. To our knowledge, no studies have examined these mechanistic pathways to mental health in the early phase of the clinical sequence in young children.
3.2. Resting frontal alpha asymmetry and later psychopathology and socio-emotional outcomes
An individual’s relative frontal alpha asymmetry during a baseline session can reveal important insight into their approach-withdrawal motivational tendencies and their risk for developing internalizing and externalizing problems. Relative right frontal alpha asymmetry, a biomarker of avoidance, has typically been linked to higher levels of negative affect, difficulties regulating emotions, and internalizing problems. Relative left frontal alpha asymmetry, a biomarker of approach, has been related to positive affect and exploratory behaviors, but in some cases, difficulty controlling approach behaviors and greater externalizing problems (Davidson, 1994, 1998; Davidson, 2004; Davidson and Fox, 1989; Smith and Bell, 2010). Relative frontal alpha asymmetry has also been extensively studied as a risk or protective factor for developing internalizing and externalizing problems as a function of child temperament. Infants with higher negative reactivity show more social wariness only when they have right frontal alpha asymmetry (Henderson et al., 2001). Probability of belonging to an exuberant profile (defined as positivity, approach, and sociability) over time was related to higher levels of externalizing problems at age 5 only for children who exhibited left frontal alpha asymmetry at age 3 (Degnan et al., 2011).
Although relative frontal asymmetry has been conceptualized as an individual-level trait, there is evidence to suggest that salient aspects of the environment can impact frontal asymmetry development. Young children of depressed mothers have shown decreases in left frontal asymmetry from ages 3–6 years, and children of non-depressed mothers have shown stability in asymmetry at these ages (Goldstein et al., 2016). Positive associations have been found between frontal alpha asymmetry in parents and their 12-month-old infants, and that mothers’ depression predicts their infants’ frontal asymmetry. These findings suggest intergenerational transmission of frontal asymmetry, with potential consequences for offspring depression risk (Hill et al., 2019).
3.3. Early adverse experiences assessed via EEG/ERP and later broad outcomes
A key focus of HBCD is charting pathways from early life adverse exposures and brain and behavioral development. Children who experience early life stress, such as socioeconomic disadvantage, institutionalization, and maltreatment, are at greater risk for maladaptive socioemotional and cognitive outcomes. Disruptions in typical brain development likely underlie such trajectories, particularly during the first few years of life when brain plasticity is heightened (Blair and Raver, 2016). Children growing up in families with lower SES have shown different patterns of brain activity than children from higher SES backgrounds. For example, ERPs in selective attention tasks have been found to differ for young children from low and high SES groups (D’Angiulli et al., 2008, 2012). Young children from low SES backgrounds show disparities in EEG power, with differences in frontal theta and occipital/left temporal alpha band continuing through early school age (Otero et al., 2003). However, other work finds no association between SES and brain activity measured at birth (Brito et al., 2016). The complexity of the concept of SES and the variety of ways it is measured may explain some of these differences across studies.
Normative development may also be disrupted when a child lacks a responsive caregiver to scaffold self-regulation and underlying physiology, which is often the case with children growing up in institutional care. Children with a history of institutional care are more likely to have mental health problems than children without this history. ERP studies have demonstrated that institutionalized children and previously institutionalized children in foster care show reduced P300 magnitude during an inhibitory control task than control children, potentially reflecting diminished attention processing in children with a history of institutionalization (McDermott et al., 2012). However, some studies have found that placement into foster care at early ages may dampen the effects of institutionalization on psychophysiology and behavioral outcomes, potentially indicating a recovery process. Indeed, early institutionalized children who were randomized to more nurturing foster care settings were less likely to have an internalizing disorder than children who remained institutionalized (Zeanah et al., 2009). Age of foster care placement is related to both alpha power and short-distanced EEG coherence, providing further support for the importance of early interventions in optimizing healthy brain development (Marshall et al., 2008).
ERPs have also been a particularly useful tool for revealing underlying neural differences in emotion processing for maltreated children. Physically abused children have been shown to respond more quickly to targets following angry faces than happy faces compared to control children and have shown an increased P3b, an ERP component involved in attention allocation, to angry emotion faces (Pollak and Tolley-Schell, 2003). This group also showed larger P1s, an ERP component involved in early perceptual processing, in occipital regions for angry faces, compared to controls. However, no ERP differences for happy faces were found. Other work has demonstrated that maltreated children show greater P260 and Nc amplitudes compared to non-maltreated children when viewing angry faces. However, there were no differences between groups in the N150, an early perceptual processing component (Cicchetti and Curtis, 2005). These findings suggest that maltreated children do not show global deficits in emotion processing, and using ERPs is a highly informative way of disentangling specific attention processes that may be more susceptible to extreme forms of early adversity such as childhood maltreatment.
These findings from the early adversity literature speak to the complex associations between adversity and brain activity, and they underscore the need for more research on the specific mechanisms that shape these associations over time. In their model of early deprivation and threat, Sheridan and McLaughlin (2014) posit that early stressful experiences (e.g., poverty, institutionalization, maltreatment) influence neurodevelopment via the absence of important social and cognitive input and increased exposure to threat. However, many of the above studies represent early extreme adversity, making it unclear at which thresholds of adversity do we see differences in psychophysiological activity. Further, there is little understanding in the EEG/ERP literature regarding when EEG/ERP becomes a biomarker of clinically concerning behavior. For example, although some survey measures have clinical cutoff scores, no such score exists for right asymmetry or P300 amplitude. Large neurodevelopmental studies, such as HBCD, with comprehensive characterization of early life exposures spanning more commonly occurring adversities and stressful rearing environments, are essential for ameliorating the identification of biomarkers and atypical brain-behavior trajectories in development.
4. Promises and challenges of using EEG/ERPs in HBCD and studies of neurodevelopment
Major large-scale studies of children’s neurodevelopment such as the Pediatric Imaging, Neurocognition, and Genetics study (PING, e.g., Fjell et al., 2012; LeWinn et al., 2017), the Adolescent Brain and Cognitive Development study (ABCD, e.g., Casey et al., 2018; Jernigan et al., 2018; Volkow et al., 2018), and the Baby Connectome Project (BCP, Howell et al., 2019) have focused on MRI as their shared neuroscience modality. One large study consortium has specifically focused on EEG, the Autism Biomarkers Consortium for Clinical Trials, suggesting that it is feasible to align across sites and collect data that can be pooled for larger analyses (McPartland et al., 2020).
In addition to the specific advantages of EEG/ERP measures discussed below, there are reasons for collecting and analyzing different types of information in parallel. (Assessing which levels or types of neural data add incremental utility to clinical prediction is a goal of our multi-site MHESC study, see Section 5, below.) As an example, one recent study found a small but significant advantage for adding genetic information to behavioral data in terms of predicting language and literacy outcomes (Dale et al., 2020). Multiple modalities of measurement from MRI, including structural anatomical characteristics and diffusion-weighted measures, provided complementary (that is, non-redundant) information in accounting for cognitive control abilities that are central to mental health and academic success (Fjell et al., 2012). Here, we discuss the advantages of including EEG/ERP measures in the HBCD study, as well some challenges that are specific to engaging the diverse sample that will need to be considered.
4.1. Advantages for assessing brain activity with EEG/ERPs
As noted, MRI has been the primary method for large longitudinal and cross-sectional studies in the past. EEG/ERP has several scientific and practical advantages in a large cohort study of infants and young children such as HBCD.
4.1.1. Complementary information to other assessment types
EEG provides complementary information about neurodevelopment both to behavioral indicators (Coch, 2021) and other brain measures such as structural and functional MRI. MRI provides key information that EEG cannot, including about the structure of the brain (e.g., cortical thickness, structural connectivity) and provides much more precise information about the location of functional processes and functional connectivity. Thus, EEG/ERP and MRI are ideal to use in tandem. In some cases, structural neuroanatomical changes may relate to functional changes observed in EEG (Whitford et al., 2007). Thus, the combination of EEG, MRI, and behavioral assessment should provide a level of detail about how functional, structural, and phenotypic changes interact and unfold over time.
4.1.2. Functional information with temporal precision
Psychophysiological measures such as EEG (as well as magnetoencephalography, MEG) surpass all other neuroimaging methods in their temporal resolution, such that researchers can capture shifts in brain functioning that may parallel the rapid emotional and cognitive processes that unfold at the level of milliseconds, both at rest and in real-world paradigms (Bell, 1998; Bell and Cuevas, 2012). Multiple researchers make the case that this level of information can be particularly useful for understanding development and its disorders (Banaschewski and Brandeis, 2007; Cavanagh, 2019).
4.1.3. Feasibility and practicality
EEG hardware is relatively low cost to purchase and to use per session compared to other neuroscience methods, which makes it an accessible tool for capturing neural signals in large samples (McLoughlin et al., 2014). EEG/ERP activity may be a particularly useful measurement of early-emerging individual differences in brain activity and maturation, given its relative ease of application with awake infants. Baseline EEG and auditory MMN ERP paradigms are also highly feasible because the demand on the child is quite low, and relatively informative data can be collected in as little as two minutes of baseline and approximately 10 min of MMN.
EEG is also often portable and can be brought to participants’ homes or schools, which eases participant burden and allows researchers to reach families who are typically underrepresented in neuroscience research (See Section 4.2, below for discussion of using EEG in diverse groups). It is also more inclusive in that individuals with metal in their body can participate safely, which is typically a contraindication for MRI. Functional near-infrared spectroscopy (fNIRS) shares some of these advantages of cost and potential portability, but EEG has been so widely used and established as a developmental science tool that it far outpaces fNIRS in terms of existing literature with which to compare and availability of toolboxes and software for data processing.
4.1.4. Naturalistic assessment during wake states in early life
Increasingly there is a call to action for examining brain activity in more naturalistic settings to increase the ecological validity of our measurements (Melnik et al., 2017). Unlike other non-invasive neuroscience measures, such as MRI and MEG, EEG can tolerate constrained participant movements and can be employed in relatively naturalistic settings. MRI and functional near-infrared spectroscopy (fNIRS) afford better spatial resolution, and like EEG, fNIRS allows the participant to be mobile. Although EEG is sensitive to motion, it can be used for a myriad of relatively stationary behavioral tasks, which capture changes in power, coherence, or asymmetry from baseline to task. EEG also offers the opportunity for face-to-face interaction with another person; child EEG features and parent-child EEG synchrony have been examined during shared eye gaze and structured tasks (e.g., Leong et al., 2017). Our lab is pursuing these measures during baseline and naturalistic parent-child interaction (Norton et al., under review).
4.2. Engaging diverse children and families in EEG/ERP research
EEG may provide both advantages and challenges in addressing the lack of diversity in large-scale developmental studies’ samples, which is well documented (Rowley and Camacho, 2015; Sugden and Moulson, 2015). Because the developing brain is shaped by early experiences rooted in culture and other features of the environment (Greenough et al., 2002), it is unlikely that basic neural processes are truly invariant across sociodemographic and sociocultural groups. The field, therefore, can make little claim that our developmental cognitive neuroscience findings are generalizable when failing to collect data from underrepresented groups.
The field of developmental cognitive neuroscience needs models that (a) draw from economically and ethno-racially diverse samples, and (b) assess brain maturation early in development, to more comprehensively understand how experiences shape brain development. Further, large studies have the responsibility to thoughtfully examine how race, ethnicity, education, and poverty can foster unique experiences and physiological changes that shape brain development, rather than simply inputting these variables as controls. Thus, tailored approaches critical to effectively engaging diverse populations are essential to characterization of normative and atypical brain:behavior patterns and their links to developmental outcomes in a manner broadly representative of the varied contexts in which children learn and grow (Dotson and Duarte, 2019; Gross et al., 2001).
The benefits of EEG discussed here – that it is portable, inexpensive, and noninvasive – make it one of the best tools for collection of neural data from large and typically underrepresented samples. Thus far, research has examined relations among EEG/ERP, socioeconomic status (SES), and cognition in infancy and early childhood, finding some support for differences in brain depending on SES (e.g., Neville et al., 2013; Stevens et al., 2009; Tomalski et al., 2013), while other studies find no differences (e.g., Brito et al., 2016). These studies, while informative for understanding brain activity in the context of SES, have relied on largely White and/or well-educated samples. Creating a pooled cohort from independent, diverse samples, such as in the MHESC study that we currently have underway, may be an ideal solution for examining cross-cultural differences in brain activity. Collaborative studies can pool resources and share costs often associated with increasing diversity in samples (Dotson and Duarte, 2019).
This lack of diversity in large-scale developmental studies’ samples raises a particular consideration for EEG/ERP studies. EEG research often both overtly and implicitly excludes participants with thick, curly, and/or coarse hair, a feature that makes collection of EEG/ERP data more difficult. As a consequence, many research datasets tend to under-represent non-white participants. In some cases, regardless of the amount of gel used or the fit of the EEG cap or net, impedances often remain too high and a good cap-scalp connection cannot be established. While there is a nascent literature about this challenge (Etienne et al., 2020), social media and other platforms have helped to amplify this concern and begin to raise awareness. A similar issue is present for children who wear their hair in braids or dreadlocks (most often, children of color), as they would not be easily able to wash out the EEG gel or saline. In the past, our groups have worked with participants to schedule an EEG visit at a time just before they are about to have their hair re-braided so that the gel can easily be rinsed out, or to have a staff member or outside person with braiding expertise on site to help re-braid their hair.
4.3. Challenges and best practices for data acquisition in young children
Although young children tolerate EEG well relative to other non-invasive neuroscience measures, data loss is common due to gross participant motion, large muscle and eye movement, cap refusal, and fussing out during data collection. Successful electrode application is therefore necessary for acquiring a high-quality EEG signal and can be accomplished through comprehensive training and practice (Bell and Cuevas, 2012). Further, newer processing pipelines have been designed specifically for child EEG data in order to retain the most usable data possible (Debnath et al., 2020; Gabard-Durnam et al., 2018).
Perhaps the largest consideration for quality of EEG data is motion-related artifacts. Researchers can ask adults and older children to keep still, but younger populations are not amenable to such instructions, which often results in high levels of artifactual data, and missing data across time points. Because a data attrition rate of ˜50 % has been suggested as typical in infancy, recruiting twice the numbers of infants needed to detect meaningful developmental changes may be needed (Noreika et al., 2020). However, studies pointing to stability in data loss over time importantly advise that removing children from the analysis due to data attrition, even if enough power is achieved, can bias the results. In a study examining the impacts of child-related, measurement-related, and longitudinal-related factors on EEG data attrition, infants who had high data loss at one session were more likely to have high data loss at the second (van der Velde and Judge, 2020). The authors posited that stability in data loss over time may be in part due to infants’ temperament, and therefore data loss is not considered missing completely at random, posing challenges for data analysis and generalizability of the findings. This is problematic for pinpointing the early phase of the clinical sequence, as those infants most at risk may be excluded. For example, irritability is a highly robust transdiagnostic indicator of mental health risk (Beauchaine and Tackett, 2020; Damme et al., 2021; Wakschlag et al., 2015; Wakschlag et al., 2018), which is measurable from the first year of life, but attrition may be more likely in irritable children. Capturing neural mechanisms of dysregulation is essential, and these children cannot simply be dropped from the study of brain-behavior relations.
There are a number of published reviews of best practices for engaging children in neuroimaging studies mostly focusing on MRI (Nordahl et al., 2008; Perlman, 2012; Raschle et al., 2012), but more recently expanding into EEG/ERP (Brooker et al., 2019). Some of these principles apply across imaging modalities. For example, communicating with parents/caregivers before each visit to find the best time to schedule the visit (e.g., after the child has napped and been fed) is necessary to increase the likelihood of collecting high-quality EEG data across timepoints. Not only is a less fussy visit important for quality data, it is also key for retaining families over time. For parents whose infants fussed out during an EEG session, they may be more hesitant to return for follow-up appointments in the longitudinal study. The experimenters must assure parents that their child did well, as fussing during the EEG is a normal occurrence. Letting parents know that the time spent in the lab was valuable will help to retain them in the study.
4.4. Multi-site alignment: training, ongoing monitoring, data processing and analysis
All multi-site studies face challenges related to alignment at different levels. The specific procedures for data collection including creating a welcoming environment and setting up the child participant vary substantially even among labs that focus on pediatric EEG. Further, EEG recordings can be sensitive to the environment in the lab, such as the presence of electrical noise, and different types of system hardware can lead to different results. Small differences or variability in stimulus presentation and time-locking stimuli to the EEG recording can also have major effects on final results. Thus, alignment of sites for EEG work can be challenging. Webb and colleagues in the Autism Biomarkers Consortium for Clinical Trials (Webb et al., 2020) provide helpful recommendations about setup and ongoing, active quality control measures. Large-scale studies of adults indicate that data from across sites can be combined when basic data curation and processing steps are aligned (Bigdely-Shamlo et al., 2020).
Once data are collected, harmonization of multiple, diverse samples with neuroimaging data has the potential to enhance generalizability and risk enrichment. Data harmonization is a systematic strategy of combining study-specific variables in a pooled dataset for analysis. Along with this greater representativeness comes the population heterogeneity introduced via combining samples with different demographic, sampling, regional, and site characteristics. Accounting for these in risk prediction models is possible with a synthetic cohort approach, in which observed and imputed behavioral data at all timepoints are combined to reduce the loss of information (Allen et al., 2017; Siddique et al., 2015). The synthetic approach does not ignore sampling differences, but rather explicitly adjusts for this heterogeneity and tests its empirical salience. Thus, rather than reduce generalizability, the use of diverse pooled samples with methods that rigorously account for these differences enhances generalizability. The ability to harmonize and leverage multiple diverse samples enhances generalizability and enables risk enrichment.
5. MHESC study collaboration as an exemplar
The Mental Health Earlier Synthetic Cohort study (MHESC), an NIMH-funded collaboration between researchers at Northwestern University (NU) and researchers at Washington University in St. Louis School of Medicine (WUSM), provides an example of an aligned multi-site, longitudinal study using multiple cognitive neuroscience measures: both MRI (structural, resting state, and diffusion-weighted scans during natural sleep) and EEG/ERP. This overarching study combines and harmonizes data from multiple ongoing behavioral and cognitive neuroscience studies with children from infancy through preschool at both sites, with the overall goals of generating and validating an infant mental health risk calculator that specifies those brain, behavioral and environmental risk and resilience factors that determine an individual child’s probabilistic risk of developing preschool psychopathology. This study is explicitly designed to employ cutting-edge synthetic cohort harmonization methods and epidemiologic risk modeling methods to specify which types of brain and behavioral indicators (and what timing of their assessment) have clinically significant added value for prediction above and beyond less burdensome and costly measures (Luby et al., 2019; Wakschlag et al., under review).
One important question for studies like HBCD is whether adding additional modalities of neural assessment such as EEG, meaningfully improves detection of early life exposure effects, and prediction of later abilities or outcomes. This is a central question of the MHESC study, which examines multiple potential indicators that vary in their feasibility, cost, and potential burden for participants and uses rigorous epidemiologic risk methods to generate the most parsimonious, least burdensome and most precise risk prediction algorithm, a method highly consistent with state-of-the-science pragmatic methods (Glasgow, 2013). The study is designed to directly test whether less-intensive measures can meaningfully provide accurate prediction of later mental health risk. For example, we are testing sequential models, such that a parent survey measure may be the least burdensome, followed by a direct lab-based observation, followed by EEG, and then MRI. The study will also test hypotheses that neural markers enable earlier risk detection, and whether inclusion of repeated measures enhances predictive precision, as has been shown in detection of cardiovascular risk
As participant samples in imaging consortia are typically at low risk, the MHESC’s clinically enriched cohort with deep phenotyping is a unique strength. The increased prevalence of low base rate phenomena when samples are pooled is of particularly high significance to understand dimensionally defined psychopathology. Creating a synthetic cohort is necessary to provide power, methods, timepoints and growth patterns for generation of the risk calculator not feasible within a single study.
The studies involved have different patterns of enrollment. The team at NU is conducting the When to Worry (W2W) studies enrolling 12 month-olds enriched for irritable behavior and 24 month-olds with language delay, followed longitudinally through age 54 months. The Promoting Healthy Brains Project (PHBP) enrolls families during pregnancy and follows children through 24 months (data collection has just begun, thus no data are yet reported). The team at WUSM is conducting the Early Life Adversity, Biological Embedding, and Risk for Developmental Precursors of Mental Disorders (ELABE) study, which enrolls children with early adversity beginning at about 12 months.
5.1. Process of alignment between sites
Although data collection and processing span two sites, protocols and procedures are aligned to allow for analyses across sites. The process of aligning data collection and analysis procedures has been multi-tiered. First, the EEG investigator from NU (Norton) traveled to the WUSM site to give an overview of procedures for EEG and provide advice on lab setup. Then, once WUSM’s EEG equipment, with identical hardware to NU, was installed and calibrated by the vendor, initial pilot data (that were de-identified) were reviewed by Norton.
To ensure alignment of data collection procedures, two researchers from WUSM spent two days at NU in Chicago observing and learning about the NU study team’s best practices for ERP data collection and processing. The first day focused on ERP data collection; guided by the NU study team, they were given a tour and explanation of the EEG/ERP data collection facilities and equipment and watched a recording of an actual participant family’s visit (with consent from the family and in alignment with IRB protocols). The two teams discussed and compared best practices for equipment maintenance, participant capping, and general data collection. The second day focused on ERP data processing. The NU study team showed the WUSM team examples of usable and unusable data collected as part of the W2W study, and outlined the basic ERP processing pipeline steps in relation to those data. The WUSM study team also showed the NU team their data, and the NU study team offered suggestions for data collection improvement based on the presented data. To continually ensure fidelity and reliability since October 2019, the WUSM team have met with Dr. Norton, and used scripts and training procedures developed at NU for data processing. This ongoing process of alignment will enable analyses of the data together from both sites.
5.2. Rates of ERP MMN and baseline EEG data usability for MHESC studies
Here, we provide data on rates of usable data across sites as exemplars. Note that at both sites, EEG is acquired at the end of a visit lasting multiple hours. Both sites use a BioSemi ActiveTwo EEG system that requires gelling each of 32 electrode sites on the cap.
The WUSM sample at age 1 (n = 193) includes 123 Black/African-American participants (63.7 %), 66 white participants (34.2 %) and 4 participants who indicated “other” or more than one race (2.1 %). The mean age and ranges in the WUSM sample are 12.5 months (range 11.0–19.4 months) at Year 1, 25.6 months (range 23.1–31.7 months) at Year 2, and 38.1 months (range 36.4–39.2 months) at Year 3. The NU sample in Year 2 (n = 190) includes 29 Black/African-American participants (15.3 %), 120 white participants (63.2 %) and 31 participants who indicated “other” or more than one race (16.3 %). 10 participants did not report their race/ethnicity (5.3 %). The average ages in the NU sample are 27.0 months (range 24.2–32.2 months) at Year 2 and 40.4 months (range 35.8–45.6 months) at Year 3.
Table 1 lists the number of ERP MMN sessions that were attempted (researchers tried to cap the child), and then each session is categorized as either successful (completed and session notes indicate data are clean enough to be analyzed), partial (some good data was collected and can be analyzed, but session was not complete), unsuccessful (not able to be analyzed, data were too artifactual, child removed the cap, or other reason), or were not usable because of a technology issue. RAs judged if approximately 50 % or more of the ERP MMN data were clean (no large artifacts) and whether approx. 1 min of clean data was collected during baseline.
Table 1.
Usability of MMN Data Across Sites and Waves (for data collected to date).
| Site | Year/ Age | Attempted | Successful | Partial | Unsuccessful | Tech Issue | % with successful or partial |
|---|---|---|---|---|---|---|---|
| WUSM | Age 1 | 193 | 115 | 24 | 46 | 8 | 72.0 % |
| WUSM | Age 2 | 59 | 45 | 3 | 11 | 0 | 81.4 % |
| WUSM | Age 3 | 6 | 6 | 0 | 0 | 0 | 100 % |
| NU | Age 2 | 120 | 101 | 6 | 11 | 3 | 89.2 % |
| NU | Age 3 | 30 | 30 | 0 | 0 | 0 | 100 % |
Later waves provided more usable data, 100 % to date for the most recent longitudinal wave at both sites, though the sample sizes were smaller because the studies are in progress. The lowest rate was at WUSM in Year 1, with 72.0 % of sessions providing usable data. This is both when the researchers were newest to EEG data collection and when the children were youngest, yielding the most challenging conditions. Greater RA experience running EEG sessions has previously been shown to relate to reduced data loss (van der Velde and Junge, 2020). Even still, this rate of 28 % attrition is perhaps better than expected, as previous studies have suggested planning on at least 50 % attrition and a previous meta-analysis of infant EEG/ERP data found an overall average attrition of 49.2 % in infants through age 12 months (Stets et al., 2012). Table 2 gives the rates of usable data at the NU site for baseline EEG, conducted while the child watched a neutral movie. These rates are quite high, suggesting that baseline EEG may be one of the measures that is least prone to attrition.
Table 2.
Rates of usable baseline EEG data for the NU cohort across waves (for data collected to date).
| Year/ Age | Attempted | Successful | Partial | Unsuccessful | Tech Issue | % with successful or partial |
|---|---|---|---|---|---|---|
| Age 2 | 182 | 153 | 6 | 21 | 2 | 87.4 % |
| Age 3 | 31 | 31 | 0 | 0 | 0 | 100 % |
6. Conclusion: considerations and directions for HBCD
Though collecting EEG data in infants and toddlers, especially data that is highly aligned across multiple sites, is a challenge, it presents unique opportunities for HBCD to achieve its goals of understanding the myriad factors that shape early neural development and its implications for development and health. Importantly, the potential to track longitudinal change and assess not just static timepoints but trajectories of brain maturation and development will provide important insights.
Some opportunities that future studies may consider include collecting data from a child during more naturalistic tasks/interactions or from a child and parent together (“dual brain” or “hyperscanning” approaches, e.g., Norton et al., under review). These may provide a new level of information about how the child interacts with their caregiver and without constraints such as needing to attend to repetitive stimuli. Another possibility is to use a portable EEG approach where data collection could happen in families’ homes, community centers, pediatricians’ offices, or childcare centers. This and other more approaches that are community-engaged will enhance reach and engagement of diverse populations who may be reluctant to travel to research laboratories due to history of institutional racism and/or mistrust. Similarly, ensuring that EEG/ERP studies are able to collect data from people with various hair types will be crucial to engaging and representing diverse populations (Choy et al., 2021).
We recognize that it will not only take large and diverse samples to elucidate the complex relations from brain structure and function to neurodevelopment. Emerging evidence suggests that children who share many similarities in local brain structure and function can present with highly varied neurodevelopmental profiles, and that factors such as network organization within and between hubs may be important for distinguishing which profiles are associated with risk or disorder (Siugzdaite et al., 2020). Nevertheless, the HBCD study will be a key first step in understanding the normative and varied profiles of neurodevelopment.
Data statement
This is a review and descriptive paper, and thus there are no data to make available.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
We thank Roshaye Poleon and Shannon O’Hara for assistance with manuscript and data preparation. We thank the study team members who assisted with study coordination, EEG acquisition, and data processing, including at Northwestern: Amy Biel, Jessica Page, Kaitlyn Fredian, Kiera Cook, Ewa Gut, Hannah Stroup, Julia Nikolaeva, and Jinnie Choi, and at WUSM: Mary Haman, Samantha Cohen, Katherine Lane, Michayla Ruscitti, Jacob Bjork, Lourdes Bernardez, Shannon O'Hara, Annie Lee, Toni DeNap, and Sloane Wolter. ESN thanks the members of the HBCD Phase I EEG Workgroup for discussions about directions of EEG in HBCD Phase II.
This work was funded by the National Institutes of Health grants under award numbers R01MH121877, R34DA050266, R01DC016273, R01MH107652, and R01MH113883. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
References
- Allen N.B., Ning H., Lloyd Jones D., Zhao L., Siddique J. Cardiovascular health across the lifespan: the development and validation of a synthetic cardiovascular cohort. Circulation. 2017;135(suppl_1) AP196-AP196. [Google Scholar]
- Atzaba-Poria N., Deater-Deckard K., Bell M.A. Mother-child interaction: links between mother and child frontal electroencephalograph asymmetry and negative behavior. Child Dev. 2017;88(2):544–554. doi: 10.1111/cdev.12583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banaschewski T., Brandeis D. Annotation: what electrical brain activity tells us about brain function that other techniques cannot tell us - a child psychiatric perspective. J. Child Psychol. Psychiatry. 2007;48(5):415–435. doi: 10.1111/j.1469-7610.2006.01681.x. [DOI] [PubMed] [Google Scholar]
- Bar-Haim Y., Marshall P.J., Fox N.A., Schorr E.A., Gordon-Salant S. Mismatch negativity in socially withdrawn children. Biol. Psychiatry. 2003;54(1):17–24. doi: 10.1016/s0006-3223(03)00175-6. [DOI] [PubMed] [Google Scholar]
- Başar E., Rahn E., Demiralp T., Schürmann M. Spontaneous EEG theta activity controls frontal visual evoked potential amplitudes. Electroencephalogr. Clin. Neurophysiol. 1998;108(2):101–109. doi: 10.1016/s0168-5597(97)00039-7. [DOI] [PubMed] [Google Scholar]
- Beauchaine T.P., Tackett J.L. Irritability as a transdiagnostic vulnerability trait:current issues and future directions. Behav. Ther. 2020;51:350–364. doi: 10.1016/j.beth.2019.10.009. [DOI] [PubMed] [Google Scholar]
- Begus K., Bonawitz E. The rhythm of learning: Theta oscillations as an index of active learning in infancy. Dev. Cogn. Neurosci. 2020:100810. doi: 10.1016/j.dcn.2020.100810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bell M.A. Cognitive Neuroscience of Attention: a Developmental Perspective. Lawrence Erlbaum Associates Publishers; 1998. Frontal lobe function during infancy: implications for the development of cognition and attention; pp. 287–316. [Google Scholar]
- Bell M.A. Power changes in infant EEG frequency bands during a spatial working memory task. Psychophysiology. 2002;39(4):450–458. doi: 10.1017.S0048577201393174. https://doi.org/10.1017.S0048577201393174. [DOI] [PubMed] [Google Scholar]
- Bell M.A., Cuevas K. Using EEG to study cognitive development: issues and practices. J. Cogn. Dev. 2012;13(3):281–294. doi: 10.1080/15248372.2012.691143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bell M.A., Diaz A. EEG/ERP measures of emotion-cognition integration during development. Monogr. Soc. Res. Child Dev. 2012;77(2):8–16. doi: 10.1111/j.1540-5834.2011.00656.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bell M.A., Fox N.A. The relations between frontal brain electrical activity and cognitive development during infancy. Child Dev. 1992;63(5):1142–1163. doi: 10.2307/1131523. [DOI] [PubMed] [Google Scholar]
- Bell M.A., Fox N.A. Human Behavior and the Developing Brain. Guilford Press; 1994. Brain development over the first year of life: relations between electroencephalographic frequency and coherence and cognitive and affective behaviors; pp. 314–345. [Google Scholar]
- Bell M.A., Fox N.A. Crawling experience is related to changes in cortical organization during infancy: evidence from EEG coherence. Dev. Psychobiol. 1996;29(7):551–561. doi: 10.1002/(SICI)1098-2302(199611)29:7<551::AID-DEV1>3.0.CO;2-T. [DOI] [PubMed] [Google Scholar]
- Bigdely-Shamlo N., Touryan J., Ojeda A., Kothe C., Mullen T., Robbins K. Automated EEG mega-analysis I: Spectral and amplitude characteristics across studies. NeuroImage. 2020;207(116361) doi: 10.1016/j.neuroimage.2019.116361. [DOI] [PubMed] [Google Scholar]
- Bishop D.V. Using mismatch negativity to study central auditory processing in developmental language and literacy impairments: where are we, and where should we be going? Psychol. Bull. 2007;133(4):651–672. doi: 10.1037/0033-2909.133.4.651. [DOI] [PubMed] [Google Scholar]
- Bishop D.V., McArthur G.M. Immature cortical responses to auditory stimuli in specific language impairment: evidence from ERPs to rapid tone sequences. Dev. Sci. 2004;7(4):F11–18. doi: 10.1111/j.1467-7687.2004.00356.x. [DOI] [PubMed] [Google Scholar]
- Bishop D.V., Hardiman M.J., Barry J.G. Is auditory discrimination mature by middle childhood? A study using time-frequency analysis of mismatch responses from 7 years to adulthood. Dev. Sci. 2011;14(2):402–416. doi: 10.1111/j.1467-7687.2010.00990.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bisiacchi P.S., Mento G., Suppiej A. Cortical auditory processing in preterm newborns: an ERP study. Biol. Psychol. 2009;82(2):176–185. doi: 10.1016/j.biopsycho.2009.07.005. [DOI] [PubMed] [Google Scholar]
- Blair C., Raver C.C. Poverty, stress, and brain development: new directions for prevention and intervention. Acad. Pediatr. 2016;16(3 Suppl):S30–36. doi: 10.1016/j.acap.2016.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bosl W.J., Tager-Flusberg H., Nelson C.A. EEG analytics for early detection of autism spectrum disorder: a data-driven approach. Sci. Rep. 2018;8(1):6828. doi: 10.1038/s41598-018-24318-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradley B.P., Mogg K., White J., Groom C., de Bono J. Attentional bias for emotional faces in generalized anxiety disorder. Br. J. Clin. Psychol. 1999;38(3):267–278. doi: 10.1348/014466599162845. [DOI] [PubMed] [Google Scholar]
- Brito N.H., Fifer W.P., Myers M.M., Elliott A.J., Noble K.G. Associations among family socioeconomic status, EEG power at birth, and cognitive skills during infancy. Dev. Cogn. Neurosci. 2016;19:144–151. doi: 10.1016/j.dcn.2016.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brooker R.J., Phelps R.A., Davidson R.J., Goldsmith H.H. Context differences in delta beta coupling are associated with neuroendocrine reactivity in infants. Dev. Psychobiol. 2016;58(3):406–418. doi: 10.1002/dev.21381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brooker R.J., Bates J.E., Buss K.A., Canen M.J., Dennis-Tiwary T.A., Gatzke-Kopp L.M. Conducting event-related potential (ERP) research with young children. J. Psychophysiol. 2019;34(3):137–158. doi: 10.1027/0269-8803/a000243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casey B.J., Cannonier T., Conley M.I., Cohen A.O., Barch D.M., Heitzeg M.M., Soules M.E., Teslovich T., Dellarco D.V., Garavan H., Orr C.A., Wager T.D., Banich M.T., Speer N.K., Sutherland M.T., Riedel M.C., Dick A.S., Bjork J.M., Thomas K.M., Chaarani B., Mejia M.H., Hagler D.J., Cornejo D.M., Sicat C.S., Harms M.P., Dosenbach N.U.F., Rosenberg M., Earl E., Bartsch H., Watts R., Polimeni J.R., Kuperman J.M., Fair D.A., Dale A.M. The adolescent brain cognitive development (ABCD) study: imaging acquisition across 21 sites. Dev. Cogn. Neurosci. 2018;32:43–54. doi: 10.1016/j.dcn.2018.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavanagh J.F. Electrophysiology as a theoretical and methodological hub for the neural sciences. Psychophysiology. 2019;56(2) doi: 10.1111/psyp.13314. e13314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chabot R.J., di Michele F., Prichep L. The role of quantitative electroencephalography in child and adolescent psychiatric disorders. Child Adolesc. Psychiatr. Clin. N. Am. 2005;14(1):21–53. doi: 10.1016/j.chc.2004.07.005. [DOI] [PubMed] [Google Scholar]
- Chen C., Hu C.H., Cheng Y. Mismatch negativity (MMN) stands at the crossroads between explicit and implicit emotional processing. Hum. Brain Mapp. 2017;38(1):140–150. doi: 10.1002/hbm.23349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng C.H., Chan P.S., Hsieh Y.W., Chen K.F. A meta-analysis of mismatch negativity in children with attention deficit-hyperactivity disorders. Neurosci. Lett. 2016;612:132–137. doi: 10.1016/j.neulet.2015.11.033. [DOI] [PubMed] [Google Scholar]
- Choy T., Baker E., Stavropoulos K. Systemic racism in EEG Research: considerations and potential solutions. Affect. Sci. 2021 doi: 10.1007/s42761-021-00050-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cicchetti D., Curtis W.J. An event-related potential study of the processing of affective facial expressions in young children who experienced maltreatment during the first year of life. Dev. Psychopathol. 2005;17(3):641–677. doi: 10.1017/S0954579405050315. [DOI] [PubMed] [Google Scholar]
- Coan J.A., Allen J.J., McKnight P.E. A capability model of individual differences in frontal EEG asymmetry. Biol. Psychol. 2006;72(2):198–207. doi: 10.1016/j.biopsycho.2005.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coch D. Uncoupled brain and behavior changes in lexical, phonological, and memory processing in struggling readers. Dev. Psychol. 2021;46(1):33–53. doi: 10.1080/87565641.2020.1871481. [DOI] [PubMed] [Google Scholar]
- Cuevas K., Bell M.A. EEG and ECG from 5 to 10 months of age: developmental changes in baseline activation and cognitive processing during a working memory task. Int. J. Psychophysiol. 2011;80(2):119–128. doi: 10.1016/j.ijpsycho.2011.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Damme K.S.F., Norton E.S., Briggs-Gowan M., Wakschlag L.S., Mittal V. Developmental patterning of irritability enhances prediction of psychopathology in pre-adolescence: Improving RDoC with developmental science. Journal of Abnormal Psychology. 2021 doi: 10.1101/2020.04.30.070714. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D’Angiulli A., Herdman A., Stapells D., Hertzman C. Children’s event-related potentials of auditory selective attention vary with their socioeconomic status. Neuropsychology. 2008;22(3):293–300. doi: 10.1037/0894-4105.22.3.293. [DOI] [PubMed] [Google Scholar]
- D’Angiulli A., Van Roon P.M., Weinberg J., Oberlander T.F., Grunau R.E., Hertzman C., Maggi S. Frontal EEG/ERP correlates of attentional processes, cortisol and motivational states in adolescents from lower and higher socioeconomic status. Front. Hum. Neurosci. 2012;6:306. doi: 10.3389/fnhum.2012.00306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dale P.S., von Stumm S., Selzam S., Hayiou-Thomas M.E. Does the inclusion of a genome-wide polygenic score improve early risk prediction for later language and literacy delay? J. Speech Lang. Hear. Res. 2020;63(5):1467–1478. doi: 10.1044/2020_JSLHR-19-00161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davidson R.J. Asymmetric brain function, affective style, and psychopathology: The role of early experience and plasticity. Dev. Psychopathol. 1994;6(4):741–758. doi: 10.1017/S0954579400004764. [DOI] [Google Scholar]
- Davidson R.J. Anterior electrophysiological asymmetries, emotion, and depression: conceptual and methodological conundrums. Psychophysiology. 1998;35(5):607–614. doi: 10.1017/s0048577298000134. [DOI] [PubMed] [Google Scholar]
- Davidson R.J. Affective style, psychopathology, and resilience: brain mechanisms and plasticity. Am. Psychol. 2000;55(11):1196–1214. doi: 10.1037//0003-066x.55.11.1196. [DOI] [PubMed] [Google Scholar]
- Davidson R.J. What does the prefrontal cortex “do” in affect: perspectives on frontal EEG asymmetry research. Biol. Psychol. 2004;67(1–2):219–233. doi: 10.1016/j.biopsycho.2004.03.008. [DOI] [PubMed] [Google Scholar]
- Davidson R.J., Fox N.A. Frontal brain asymmetry predicts infants’ response to maternal separation. J. Abnorm. Psychol. 1989;98(2):127–131. doi: 10.1037//0021-843x.98.2.127. [DOI] [PubMed] [Google Scholar]
- de Haan M., Belsky J., Reid V., Volein A., Johnson M.H. Maternal personality and infants’ neural and visual responsivity to facial expressions of emotion. J. Child Psychol. Psychiatry. 2004;45(7):1209–1218. doi: 10.1111/j.1469-7610.2004.00320.x. [DOI] [PubMed] [Google Scholar]
- Debnath R., Buzzell G.A., Morales S., Bowers M.E., Leach S.C., Fox N.A. The Maryland analysis of developmental EEG (MADE) pipeline. Psychophysiology. 2020;57(6) doi: 10.1111/psyp.13580. e13580. [DOI] [PubMed] [Google Scholar]
- Degnan K.A., Hane A.A., Henderson H.A., Moas O.L., Reeb-Sutherland B.C., Fox N.A. Longitudinal stability of temperamental exuberance and social-emotional outcomes in early childhood. Dev. Psychol. 2011;47(3):765–780. doi: 10.1037/a0021316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diaz A., Bell M.A. Frontal EEG asymmetry and fear reactivity in different contexts at 10 months. Dev. Psychobiol. 2012;54(5):536–545. doi: 10.1002/dev.20612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dotson V.M., Duarte A. The importance of diversity in cognitive neuroscience. Ann. N. Y. Acad. Sci. 2019;1464(1):181–191. doi: 10.1111/nyas.14268. [DOI] [PubMed] [Google Scholar]
- Etienne A., Laroia T., Weigle H., Afelin A., Kelly S.K., Krishnan A., Grover P. 2020. Novel Electrodes for Reliable EEG Recordings on Coarse and Curly Hair. bioRxiv 2020.02.26.965202. [DOI] [PubMed] [Google Scholar]
- Fellman V., Kushnerenko E., Mikkola K., Ceponiene R., Leipälä J., Näätänen R. Atypical auditory event-related potentials in preterm infants – a possible sign of cognitive dysfunction? Pediatr. Res. 2004;56:291–297. doi: 10.1203/01.PDR.0000132750.97066.B9. [DOI] [PubMed] [Google Scholar]
- Fischer K.W., van Geert P. Handbook of Developmental Systems Theory and Methodology. Guilford Press; 2014. Dynamic development of brain and behavior; pp. 287–315. [Google Scholar]
- Fjell A.M., Walhovd K.B., Brown T.T., Kuperman J.M., Chung Y., Hagler D.J., Jr., Venkatraman V., Roddey J.C., Erhart M., McCabe C., Akshoomoff N., Amaral D.G., Bloss C.S., Libiger O., Darst B.F., Schork N.J., Casey B.J., Chang L., Ernst T.M., Gruen J.R., Kaufmann W.E., Kenet T., Frazier J., Murray S.S., Sowell E.R., van Zijl P., Mostofsky S., Jernigan T.L., Dale A.M., Pediatric Imaging, N, Genetics, S Multimodal imaging of the self-regulating developing brain. Proc. Natl. Acad. Sci. U. S.A. 2012;109(48):19620–19625. doi: 10.1073/pnas.1208243109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox N.A. Dynamic cerebral processes underlying emotion regulation. Monogr. Soc. Res. Child Dev. 1994;59(2–3):250–283. doi: 10.2307/1166143. 152-166. [DOI] [PubMed] [Google Scholar]
- Fox N.A., Henderson H.A., Rubin K.H., Calkins S.D., Schmidt L.A. Continuity and discontinuity of behavioral inhibition and exuberance: psychophysiological and behavioral influences across the first four years of life. Child Dev. 2001;72(1):1–21. doi: 10.1111/1467-8624.00262. [DOI] [PubMed] [Google Scholar]
- Franken I.H., Nijs I., Van Strien J.W. Impulsivity affects mismatch negativity (MMN) measures of preattentive auditory processing. Biol. Psychol. 2005;70(3):161–167. doi: 10.1016/j.biopsycho.2005.01.007. [DOI] [PubMed] [Google Scholar]
- Gabard-Durnam L.J., Mendez Leal A.S., Wilkinson C.L., Levin A.R. The Harvard automated processing pipeline for electroencephalography (HAPPE): standardized processing software for developmental and high-artifact data. Front. Neurosci. 2018;12:97. doi: 10.3389/fnins.2018.00097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garrido M.I., Kilner J.M., Stephan K.E., Friston K.J. The mismatch negativity: a review of underlying mechanisms. Clin. Neurophysiol. 2009;120(3):453–463. doi: 10.1016/j.clinph.2008.11.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gatzke-Kopp L.M., Jetha M.K., Segalowitz S.J. The role of resting frontal EEG asymmetry in psychopathology: afferent or efferent filter? Dev. Psychobiol. 2014;56(1):73–85. doi: 10.1002/dev.21092. [DOI] [PubMed] [Google Scholar]
- Glasgow R.E. What does it mean to be pragmatic? Pragmatic methods, measures, and models to facilitate research translation. Health Educ. Behav. 2013;40(3):257–265. doi: 10.1177/1090198113486805. [DOI] [PubMed] [Google Scholar]
- Goldstein B.L., Shankman S.A., Kujawa A., Torpey-Newman D.C., Olino T.M., Klein D.N. Developmental changes in electroencephalographic frontal asymmetry in young children at risk for depression. J. Child Psychol. Psychiatry. 2016;57(9):1075–1082. doi: 10.1111/jcpp.12567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenough W.T., Black J.E., Wallace C.S. 2nd ed. Blackwell Publishing; 2002. Experience and Brain Development. [Google Scholar]
- Gross D., Julion W., Fogg L. What motivates participation and dropout among low‐income urban families of color in a prevention intervention? Fam. Relat. 2001;50(3):246–254. doi: 10.1111/j.1741-3729.2001.00246.x. [DOI] [Google Scholar]
- Gu C., Bi H.-Y. Auditory processing deficit in individuals with dyslexia: a meta-analysis of mismatch negativity. Neurosci. Biobehav. Rev. 2020;116:396–405. doi: 10.1016/j.neubiorev.2020.06.032. [DOI] [PubMed] [Google Scholar]
- Guo J., Luo X., Li B., Chang Q., Sun L., Song Y. Abnormal modulation of theta oscillations in children with attention-deficit/hyperactivity disorder. Neuroimage Clin. 2020;27:102314. doi: 10.1016/j.nicl.2020.102314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guttorm T.K., Leppanen P.H., Hamalainen J.A., Eklund K.M., Lyytinen H.J. Newborn event-related potentials predict poorer pre-reading skills in children at risk for dyslexia. J. Learn. Disabil. 2010;43(5):391–401. doi: 10.1177/0022219409345005. [DOI] [PubMed] [Google Scholar]
- Henderson H.A., Fox N.A., Rubin K.H. Temperamental contributions to social behavior: the moderating roles of frontal EEG asymmetry and gender. J. Am. Acad. Child Adolesc. Psychiatry. 2001;40(1):68–74. doi: 10.1097/00004583-200101000-00018. [DOI] [PubMed] [Google Scholar]
- Hill K.E., Neo W.S., Hernandez A., Hamrick L.R., Kelleher B.L., Foti D. Intergenerational transmission of frontal alpha asymmetry among mother-infant dyads. Biol. Psychiatry Cogn. Neurosci. Neuroimaging. 2019;5(4):420–428. doi: 10.1016/j.bpsc.2019.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howell B.R., Styner M.A., Gao W., Yap P.T., Wang L., Baluyot K., Yacoub E., Chen G., Potts T., Salzwedel A., Li G., Gilmore J.H., Piven J., Smith J.K., Shen D., Ugurbil K., Zhu H., Lin W., Elison J.T. The UNC/UMN Baby Connectome Project (BCP): an overview of the study design and protocol development. Neuroimage. 2019;185:891–905. doi: 10.1016/j.neuroimage.2018.03.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jasper H.H. Electroencephalography in child neurology and psychiatry. Pediatrics. 1949;3(6):783–800. https://www.ncbi.nlm.nih.gov/pubmed/18146164 [PubMed] [Google Scholar]
- Jasper H.H., Solomon P., Bradley C. Electroencephalographic analyses of behavior problem children. Am. J. Psychiatry. 1938;95(3):641–658. doi: 10.1176/ajp.95.3.641. [DOI] [Google Scholar]
- Jernigan T.L., Brown S.A. Introduction (Open Access) Dev. Cogn. Neurosci. 2018;32:1–3. doi: 10.1016/j.dcn.2018.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kappenman E.S., Farrens J.L., Luck S.J., Proudfit G.H. Behavioral and ERP measures of attentional bias to threat in the dot-probe task: poor reliability and lack of correlation with anxiety. Front. Psychol. 2014;5:1368. doi: 10.3389/fpsyg.2014.01368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klimesch W. EEG alpha and theta oscillations reflect cognitive and memory performance: a review and analysis. Brain Res. Rev. 1999;29(2–3):169–195. doi: 10.1016/s0165-0173(98)00056-3. [DOI] [PubMed] [Google Scholar]
- Knyazev G.G. Motivation, emotion, and their inhibitory control mirrored in brain oscillations. Neurosci. Biobehav. Rev. 2007;31(3):377–395. doi: 10.1016/j.neubiorev.2006.10.004. [DOI] [PubMed] [Google Scholar]
- Leong V., Byrne E., Clackson K., Georgieva S., Lam S., Wass S. Speaker gaze increases information coupling between infant and adult brains. Proc. Natl. Acad. Sci. U. S. A. 2017;114(50):13290–13295. doi: 10.1073/pnas.1702493114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leppanen P.H., Richardson U., Pihko E., Eklund K.M., Guttorm T.K., Aro M., Lyytinen H. Brain responses to changes in speech sound durations differ between infants with and without familial risk for dyslexia. Dev. Neuropsychol. 2002;22(1):407–422. doi: 10.1207/S15326942dn2201_4. [DOI] [PubMed] [Google Scholar]
- Leppanen J.M., Moulson M.C., Vogel-Farley V.K., Nelson C.A. An ERP study of emotional face processing in the adult and infant brain. Child Dev. 2007;78(1):232–245. doi: 10.1111/j.1467-8624.2007.00994.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leppanen P.H., Hamalainen J.A., Salminen H.K., Eklund K.M., Guttorm T.K., Lohvansuu K., Puolakanaho A., Lyytinen H. Newborn brain event-related potentials revealing atypical processing of sound frequency and the subsequent association with later literacy skills in children with familial dyslexia. Cortex. 2010;46(10):1362–1376. doi: 10.1016/j.cortex.2010.06.003. [DOI] [PubMed] [Google Scholar]
- LeWinn K.Z., Sheridan M.A., Keyes K.M., Hamilton A., McLaughlin K.A. Sample composition alters associations between age and brain structure. Nat. Commun. 2017;8(1):874. doi: 10.1038/s41467-017-00908-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loo S.K., Lenartowicz A., Makeig S. Research review: use of EEG biomarkers in child psychiatry research - current state and future directions. J. Child Psychol. Psychiatry. 2016;57(1):4–17. doi: 10.1111/jcpp.12435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luby J., Allen N., Estabrook R., Pine D.S., Rogers C., Krogh-Jespersen S., Norton E.S., Wakschlag L. Mapping infant neurodevelopmental precursors of mental disorders: How synthetic cohorts & computational approaches can be used to enhance prediction of early childhood psychopathology. Behav. Res. Ther. 2019;123:103–484. doi: 10.1016/j.brat.2019.103484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luck S.J. MIT Press; 2005. Introduction to the Event Related Potential Technique. [Google Scholar]
- MacNeill L.A., Ram N., Bell M.A., Fox N.A., Perez-Edgar K. Trajectories of infants’ biobehavioral development: timing and rate of A-Not-B performance gains and EEG maturation. Child Dev. 2018;89(3):711–724. doi: 10.1111/cdev.13022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marshall P.J., Meltzoff A.N. Neural mirroring systems: exploring the EEG mu rhythm in human infancy. Dev. Cogn. Neurosci. 2011;1(2):110–123. doi: 10.1016/j.dcn.2010.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marshall P.J., Bar-Haim Y., Fox N.A. Development of the EEG from 5 months to 4 years of age. Clin. Neurophysiol. 2002;113(8):1199–1208. doi: 10.1016/s1388-2457(02)00163-3. [DOI] [PubMed] [Google Scholar]
- Marshall P.J., Reeb B.C., Fox N.A., Nelson C.A., 3rd, Zeanah C.H. Effects of early intervention on EEG power and coherence in previously institutionalized children in Romania. Dev. Psychopathol. 2008;20(3):861–880. doi: 10.1017/S0954579408000412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marshall P.J., Reeb B.C., Fox N.A. Electrophysiological responses to auditory novelty in temperamentally different 9‐month‐old infants. Dev. Sci. 2009;12(4):568–582. doi: 10.1111/j.1467-7687.2008.00808.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matoušek M., Petersen I. Automation of Clinical Electroencephalography. Raven Press; New York: 1973. Frequency analysis of the EEG in normal children and in normal adolescents; pp. 75–102. [Google Scholar]
- McDermott J.M., Westerlund A., Zeanah C.H., Nelson C.A., Fox N.A. Early adversity and neural correlates of executive function: implications for academic adjustment. Dev. Cogn. Neurosci. 2012;2(Suppl 1):S59–66. doi: 10.1016/j.dcn.2011.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLoughlin G., Makeig S., Tsuang M.T. In search of biomarkers in psychiatry: EEG-based measures of brain function. J. Neuropsychiatric Genet. Part B Am. J. Med. Genetics. 2014;165B(2):111–121. doi: 10.1002/ajmg.b.32208. [DOI] [PubMed] [Google Scholar]
- McPartland J.C., Bernier R.A., Jeste S.S., Dawson G., Nelson C.A., Chawarska K., Earl R., Faja S., Johnson S.P., Sikich L., Brandt C.A., Dziura J.D., Rozenblit L., Hellemann G., Levin A.R., Murias M., Naples A.J., Platt M.L., Sabatos-DeVito M., Shic F., Senturk D., Sugar C.A., Webb S.J., Autism Biomarkers Consortium for Clinical, T The autism biomarkers consortium for clinical trials (ABC-CT): scientific context, study design, and progress toward biomarker qualification. Front. Integr. Neurosci. 2020;14:16. doi: 10.3389/fnint.2020.00016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McWeeny S., Norton E.S. Understanding event related potentials (ERPs) in clinical and basic and language and communication disorders research: a tutorial. Int. J. Lang. Commun. Disord. 2020;55(4):445–457. doi: 10.1111/1460-6984.12535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melnik A., Legkov P., Izdebski K., Karcher S.M., Hairston W.D., Ferris D.P., Konig P. Systems, subjects, sessions: to what extent do these factors influence EEG data? Front. Hum. Neurosci. 2017;11:150. doi: 10.3389/fnhum.2017.00150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mogg K., Bradley B.P., de Bono J., Painter M. Time course of attentional bias for threat information in non-clinical anxiety. Behav. Res. Ther. 1997;35(4):297–303. doi: 10.1016/s0005-7967(96)00109-x. [DOI] [PubMed] [Google Scholar]
- Molfese D.L. Predicting dyslexia at 8 years of age using neonatal brain responses. Brain Lang. 2000;72(3):238–245. doi: 10.1006/brln.2000.2287. [DOI] [PubMed] [Google Scholar]
- Morr M.L., Shafer V.L., Kreuzer J.A., Kurtzberg D. Maturation of mismatch negativity in typically developing infants and preschool children. Ear Hear. 2002;23(2):118–136. doi: 10.1097/00003446-200204000-00005. [DOI] [PubMed] [Google Scholar]
- Morris A.S., Wakschlag L., Krogh-Jespersen S., Fox N.A., Planalp B., Perlman S.B., Shuffrey L.C., Smith B., Lorenzo N.E., Amso D., Coles C.D., Johnson S.P. Principles for guiding the selection of early childhood neurodevelopmental risk and resilience measures: HEALthy brain and child development study as an exemplar. Adversity and Resilience Science. 2020;1(21):247–267. doi: 10.1007/s42844-020-00025-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muller H.J., Rabbitt P.M. Reflexive and voluntary orienting of visual attention: time course of activation and resistance to interruption. J. Exp. Psychol. Hum. Percept. Perform. 1989;15(2):315–330. doi: 10.1037//0096-1523.15.2.315. [DOI] [PubMed] [Google Scholar]
- Mundy P., Fox N.A., Card J. EEG coherence, joint attention and language development in the second year. Dev. Sci. 2003;6(1):48–54. doi: 10.1111/1467-7687.00253. [DOI] [Google Scholar]
- Naatanen R. The perception of speech sounds by the human brain as reflected by the mismatch negativity (MMN) and its magnetic equivalent (MMNm) Psychophysiology. 2001;38(1):1–21. doi: 10.1017/s0048577201000208. [DOI] [PubMed] [Google Scholar]
- Naatanen R., Kujala T., Escera C., Baldeweg T., Kreegipuu K., Carlson S., Ponton C. The mismatch negativity (MMN)--a unique window to disturbed central auditory processing in ageing and different clinical conditions. Clin. Neurophysiol. 2012;123(3):424–458. doi: 10.1016/j.clinph.2011.09.020. [DOI] [PubMed] [Google Scholar]
- Neville H.J., Stevens C., Pakulak E., Bell T.A., Fanning J., Klein S., Isbell E. Family-based training program improves brain function, cognition, and behavior in lower socioeconomic status preschoolers. Proc. Natl. Acad. Sci. U. S. A. 2013;110(29):12138–12143. doi: 10.1073/pnas.1304437110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nordahl C.W., Simon T.J., Zierhut C., Solomon M., Rogers S.J., Amaral D.G. Brief report: methods for acquiring structural MRI data in very young children with autism without the use of sedation. J. Autism Dev. Disord. 2008;38(8):1581–1590. doi: 10.1007/s10803-007-0514-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noreika V., Georgieva S., Wass S., Leong V. 14 challenges and their solutions for conducting social neuroscience and longitudinal EEG research with infants. Infant Behav. Dev. 2020;58:101393. doi: 10.1016/j.infbeh.2019.101393. [DOI] [PubMed] [Google Scholar]
- Norton E.S., Gabrieli J.D., Gaab N. Developmental Dyslexia Across Languages and Writing Systems. Cambridge University Press; 2019. Neural predictors of developmental dyslexia; pp. 253–276. [Google Scholar]
- Norton, E. S., Manning, B. L., L., M., E., H., Nikolaeva, J., Krogh-Jespersen, S., & Wakschlag, L. (under review). Social EEG: A novel approach to studying brain-behavior links and brain-to-brain synchrony during naturalistic toddler-parent interactions. [DOI] [PMC free article] [PubMed]
- Norton E.S., Beach S.D., Eddy M.D., McWeeny S., Ozernov-Palchik O., Gaab N., Gabrieli J.D.E. ERP mismatch negativity amplitude and asymmetry reflect phonological and rapid automatized naming skills in English-speaking kindergartners. Front. Hum. Neurosci. 2021 doi: 10.3389/fnhum.2021.624617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nunez P.L. Oxford University Press; 1981. Electric Fields of the Brain: the Neurophysics of EEG. [Google Scholar]
- Orekhova E.V., Stroganova T.A., Posikera I.N., Elam M. EEG theta rhythm in infants and preschool children. Clin. Neurophysiol. 2006;117(5):1047–1062. doi: 10.1016/j.clinph.2005.12.027. [DOI] [PubMed] [Google Scholar]
- Ortiz-Mantilla S., Hämäläinen J.A., Musacchia G., Benasich A.A. Enhancement of gamma oscillations indicates preferential processing of native over foreign phonemic contrasts in infants. J. Neurosci. 2013;33(48):18746–18754. doi: 10.1523/JNEUROSCI.3260-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ortiz-Mantilla S., Cantiani C., Shafer V.L., Benasich A.A. Minimally-verbal children with autism show deficits in theta and gamma oscillations during processing of semantically-related visual information. Sci. Rep. 2019;9(1):1–12. doi: 10.1038/s41598-019-41511-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otero G.A., Pliego-Rivero F.B., Fernandez T., Ricardo J. EEG development in children with sociocultural disadvantages: a follow-up study. Clin. Neurophysiol. 2003;114(10):1918–1925. doi: 10.1016/s1388-2457(03)00173-1. [DOI] [PubMed] [Google Scholar]
- Pena M., Pittaluga E., Mehler J. Language acquisition in premature and full-term infants. Proc. Nati. Acad. Sci. U. S. A. 2010;107(8):3823–3828. doi: 10.1073/pnas.0914326107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perlman S.B. Neuroimaging in child clinical populations: considerations for a successful research program. J. Am. Acad. Child Adolesc. Psychiatry. 2012;51(12):1232–1235. doi: 10.1016/j.jaac.2012.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peterson R.L., Pennington B.F. Developmental dyslexia. Lancet. 2012;379(9830):1997–2007. doi: 10.1016/s0140-6736(12)60198-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pihko E., Leppänen P.H., Eklund K.M., Cheour M., Guttorm T.K., Lyytinen H. Cortical responses of infants with and without a genetic risk for dyslexia: I. Age effects. Neuroreport. 1999;10(5):901–905. doi: 10.1097/00001756-199904060-00002. [DOI] [PubMed] [Google Scholar]
- Pizzagalli D.A. Handbook of Psychophysiology. 3rd ed. Cambridge University Press; 2007. Electroencephalography and high-density electrophysiological source localization; pp. 56–84. [DOI] [Google Scholar]
- Pollak S.D., Tolley-Schell S.A. Selective attention to facial emotion in physically abused children. J. Abnorm. Psychol. 2003;112(3):323–338. doi: 10.1037/0021-843x.112.3.323. [DOI] [PubMed] [Google Scholar]
- Pool L.R., Ning H., Wilkins J., Lloyd-Jones D.M., Allen N.B. Use of long-term cumulative blood pressure in cardiovascular risk prediction models. JAMA Cardiol. 2018;3(11):1096–1100. doi: 10.1001/jamacardio.2018.2763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raschle N., Zuk J., Ortiz-Mantilla S., Sliva D.D., Franceschi A., Grant P.E., Benasich A.A., Gaab N. Pediatric neuroimaging in early childhood and infancy: challenges and practical guidelines. Ann. N. Y. Acad. Sci. 2012;1252:43–50. doi: 10.1111/j.1749-6632.2012.06457.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ray W.J., Cole H.W. EEG alpha activity reflects attentional demands, and beta activity reflects emotional and cognitive processes. Science. 1985;228(4700):750–752. doi: 10.1126/science.3992243. [DOI] [PubMed] [Google Scholar]
- Rowley S.J., Camacho T.C. Increasing diversity in cognitive developmental research: Issues and solutions. J. Cogn. Dev. 2015;16(5):683–692. doi: 10.1080/15248372.2014.976224. [DOI] [Google Scholar]
- Saby J.N., Marshall P.J. The utility of EEG band power analysis in the study of infancy and early childhood. Dev. Neuropsychol. 2012;37(3):253–273. doi: 10.1080/87565641.2011.614663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz S., Shinn-Cunningham B., Tager-Flusberg H. Meta-analysis and systematic review of the literature characterizing auditory mismatch negativity in individuals with autism. Neurosci. Biobehav. Rev. 2018;87:106–117. doi: 10.1016/j.neubiorev.2018.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shafer V.L., Morr M.L., Kreuzer J.A., Kurtzberg D. Maturation of mismatch negativity in school-age children. Ear Hear. 2000;21(3):242–251. doi: 10.1097/00003446-200006000-00008. [DOI] [PubMed] [Google Scholar]
- Sheridan M.A., McLaughlin K.A. Dimensions of early experience and neural development: deprivation and threat. Trends Cogn. Sci. 2014;18(11):580–585. doi: 10.1016/j.tics.2014.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siddique J., Reiter J.P., Brincks A., Gibbons R.D., Crespi C.M., Brown C.H. Multiple imputation for harmonizing longitudinal non‐commensurate measures in individual participant data meta‐analysis. Stat. Med. 2015;34(26):3399–3414. doi: 10.1002/sim.6562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siugzdaite R., Bathelt J., Holmes J., Astle D.E. Transdiagnostic brain mapping in developmental disorders. Curr. Biol. 2020;30(7):1245–1257. doi: 10.1016/j.cub.2020.01.078. e1244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith C.L., Bell M.A. Stability in infant frontal asymmetry as a predictor of toddlerhood internalizing and externalizing behaviors. Dev. Psychobiol. 2010;52(2):158–167. doi: 10.1002/dev.20427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stets M., Stahl D., Reid V.M. A meta-analysis investigating factors underlying attrition rates in infant ERP studies. Dev. Neuropsychol. 2012;37(3):226–252. doi: 10.1080/87565641.2012.654867. [DOI] [PubMed] [Google Scholar]
- Stevens C., Lauinger B., Neville H. Differences in the neural mechanisms of selective attention in children from different socioeconomic backgrounds: An event-related brain potential study. Dev. Sci. 2009;12(4):634–646. doi: 10.1111/j.1467-7687.2009.00807.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stroganova T.A., Orekhova E.V. Infant EEG and Event-related Potentials. 2007. EEG and infant states; pp. 251–288. [Google Scholar]
- Stroganova T.A., Orekhova E.V., Posikera I.N. EEG alpha rhythm in infants. Clin. Neurophysiol. 1999;110(6):997–1012. doi: 10.1016/s1388-2457(98)00009-1. [DOI] [PubMed] [Google Scholar]
- Sugden N.A., Moulson M.C. Recruitment strategies should not be randomly selected: empirically improving recruitment success and diversity in developmental psychology research. Front. Psychol. 2015;6:523. doi: 10.3389/fpsyg.2015.00523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swingler M.M., Willoughby M.T., Calkins S.D. EEG power and coherence during preschoolers’ performance of an executive function battery. Dev. Psychobiol. 2011;53(8):771–784. doi: 10.1002/dev.20588. [DOI] [PubMed] [Google Scholar]
- Thai N., Taber-Thomas B.C., Perez-Edgar K.E. Neural correlates of attention biases, behavioral inhibition, and social anxiety in children: an ERP study. Dev. Cogn. Neurosci. 2016;19:200–210. doi: 10.1016/j.dcn.2016.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thatcher R.W. Cyclic cortical reorganization during early childhood. Brain Cogn. 1992;20(1):24–50. doi: 10.1016/0278-2626(92)90060-y. [DOI] [PubMed] [Google Scholar]
- Thatcher R.W. Cyclic cortical reorganization: origins of human cognitive development. In: Dawson G., Fischer K.W., editors. Human Behavior and the Developing Brain. Guilford Press; 1994. pp. 232–266. [Google Scholar]
- Thatcher R.W., Krause P.J., Hrybyk M. Cortico-cortical associations and EEG coherence: a two-compartmental model. Electroencephalogr. Clin. Neurophysiol. 1986;64(2):123–143. doi: 10.1016/0013-4694(86)90107-0. [DOI] [PubMed] [Google Scholar]
- Thatcher R.W., Walker R.A., Giudice S. Human cerebral hemispheres develop at different rates and ages. Science. 1987;236(4805):1110–1113. doi: 10.1126/science.3576224. [DOI] [PubMed] [Google Scholar]
- Tierney A., Strait D.L., O’Connell S., Kraus N. Developmental changes in resting gamma power from age three to adulthood. Clin. Neurophysiol. 2013;124(5):1040–1042. doi: 10.1016/j.clinph.2012.09.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomalski P., Moore D.G., Ribeiro H., Axelsson E.L., Murphy E., Karmiloff-Smith A., Johnson M.H., Kushnerenko E. Socioeconomic status and functional brain development - associations in early infancy. Dev. Sci. 2013;16(5):676–687. doi: 10.1111/desc.12079. [DOI] [PubMed] [Google Scholar]
- Umbricht D., Krljes S. Mismatch negativity in schizophrenia: a meta-analysis. Schizophr. Res. 2005;76(1):1–23. doi: 10.1016/j.schres.2004.12.002. [DOI] [PubMed] [Google Scholar]
- van der Velde B., Junge C. Limiting data loss in infant EEG: putting hunches to the test. Dev. Cogn. Neurosci. 2020;45:100809. doi: 10.1016/j.dcn.2020.100809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Leeuwen T., Been P., Kuijpers C., Zwarts F., Maassen B., van der Leij A. Mismatch response is absent in 2-month-old infants at risk for dyslexia. Neuroreport. 2006;17(4):351–355. doi: 10.1097/01.wnr.0000203624.02082.2d. [DOI] [PubMed] [Google Scholar]
- van Zuijen T.L., Plakas A., Maassen B.A., Maurits N.M., van der Leij A. Infant ERPs separate children at risk of dyslexia who become good readers from those who become poor readers. Dev. Sci. 2013;16(4):554–563. doi: 10.1111/desc.12049. [DOI] [PubMed] [Google Scholar]
- Volkow N.D., Koob G.F., Croyle R.T., Bianchi D.W., Gordon J.A., Koroshetz W.J., Pérez-Stable E.J., Riley W.T., Bloch M.H., Conway K., Deeds B.G., Dowling G.J., Grant S., Howlett K.D., Matochik J.A., Morgan G.D., Murray M.M., Noronha A., Spong C.Y., Wargo E.M., Warren K.R., Weiss S.R.B. The conception of the ABCD study: from substance use to a broad NIH collaboration. Dev. Cogn. Neurosci. 2018;32:4–7. doi: 10.1016/j.dcn.2017.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volkow N.D., Gordon J.A., Freund M.P. The healthy brain and child development study-shedding light on opioid exposure, COVID-19, and health disparities. JAMA Psychiatry. 2020 doi: 10.1001/jamapsychiatry.2020.3803. [DOI] [PubMed] [Google Scholar]
- Wakschlag L.S., Estabrook R., Petitclerc A., Henry D., Burns J.L., Perlman S.B., Voss J.L., Pine D.S., Leibenluft E., Briggs-Gowan M.L. Clinical implications of a dimensional approach: the normal:abnormal spectrum of early irritability. J. Am. Acad. Child Adolesc. Psychiatry. 2015;54:626–634. doi: 10.1016/j.jaac.2015.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wakschlag L.S., Perlman S.B., Blair R.J., Leibenluft E., Briggs-Gowan M.J., Pine D.S. The neurodevelopmental basis of early childhood disruptive behavior: irritable and callous phenotypes as exemplars. Am. J. Psychiatry. 2018;175(2):114–130. doi: 10.1176/appi.ajp.2017.17010045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wakschlag L., Luby J., Pool L., MacNeill L.A., Adam H., Barch D., Norton E.S., Krogh-Jespersen S., Rogers C., Allen N. 2020. Accelerating Neurodevelopmental Discovery to Clinical Translation: Proof-of-concept for a Personalized Early Childhood Mental Health Risk Calculator. submitted manuscript. [Google Scholar]
- Autism Biomarkers Consortium for Clinical Trials. Webb S.J., Shic F., Murias M., Sugar C.A., Naples A.J., Barney E., Borland H., Hellemann G., Johnson S., Kim M., Levin A.R., Sabatos-DeVito M., Santhosh M., Senturk D., Dziura J., Bernier R.A., Chawarska K., Dawson G., Faja S., Jeste S., McPartland J. Biomarker acquisition and quality control for multi-site studies: the autism biomarkers consortium for clinical trials. Front. Integr. Neurosci. 2020;13:71. doi: 10.3389/fnint.2019.00071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitford T.J., Rennie C.J., Grieve S.M., Clark C.R., Gordon E., Williams L.M. Brain maturation in adolescence: concurrent changes in neuroanatomy and neurophysiology. Hum. Brain Mapp. 2007;28(3):228–237. doi: 10.1002/hbm.20273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodruff Carr K.L., Perszyk D.R., Norton E.S., Voss J.L., Poeppel D., Waxman S.R. Developmental changes in auditory-evoked alpha activity underlie the increasing precision with which infants link language and cognition. Dev. Sci. 2021 doi: 10.1111/desc.13121. epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yordanova J., Kolev V. Event-related brain oscillations: developmental effects on power and synchronization. J. Psychophysiol. 2009;23(4):174–182. doi: 10.1027/0269-8803.23.4.174. [DOI] [Google Scholar]
- Zeanah C.H., Egger H.L., Smyke A.T., Nelson C.A., Fox N.A., Marshall P.J., Guthrie D. Institutional rearing and psychiatric disorders in Romanian preschool children. Am. J. Psychiatry. 2009;166(7):777–785. doi: 10.1176/appi.ajp.2009.08091438. [DOI] [PubMed] [Google Scholar]