Abstract
Introduction and importance
Immunocompromised patients are at high risk of unexpectedly serious infections caused by uncommon bacteria or fungi. We experienced a case of Cryptococcus neoformans-induced necrotizing fasciitis (NF) of the lower extremities. The progress so far has been reported by the urology department [1].
Moreover, after the NF had been treated, the patient developed immune reconstitution inflammatory syndrome (IRIS). We report from surgeon's view point.
Case presentation
A 51-year-old male renal transplant patient complained of pain in both lower extremities (LE). After the initial debridement, periodic acid-Schiff after diastase digestion (D-PAS) staining confirmed the diagnosis. No symptoms were seen in the lungs or cerebrospinal system. The patient was reluctant to undergo surgical treatment but several debridement improved patient's condition. After the LE wound healed, prednisolone was discontinued, then painful nodules appeared on both LE. Based on the negative culture results and the fact that the patient had been treated with flucytosine and fluconazole, we suspected that the nodules had been caused by IRIS.
Clinical discussion
It was difficult to diagnose Cryptococcus-induced NF and paradoxical IRIS. Cooperation from other specialists was essential.
Conclusion
We think this patient needed earlier and more definitive debridement. Fortunately, we were able to save the patient's life and maintain his LE function. In immunocompromised patients, cryptococcus can be a pathogen. In addition, IRIS can occur during treatment. Management of IRIS is the capital point of sepsis management, careful anti-inflammatory drug control by specialists is required.
Keywords: Cryptococcus, Immune reconstitution inflammatory syndrome (IRIS), Necrotizing fasciitis
Highlights
-
•
A case of necrotizing fasciitis due to cryptococcosis in both lower extremity of a kidney transplant patient
-
•
Debridement and systemic drug treatment saved the patient's life.
-
•
Extensive necrosis of the gastrocnemius tendon was noted. However, we could maintain the patient's lower limb function.
-
•
The patient also developed immune reconstitution syndrome.
1. Introduction
Necrotizing fasciitis (NF) is a destructive soft-tissue infection, which is typically caused by group A streptococci or a combination of facultative and anaerobic bacteria [2]. Immunocompromised patients are at high risk of NF [3] and we sometimes encounter unexpectedly serious infections caused by uncommon bacteria or fungi. We experienced a rare case of Cryptococcus neoformans (C. neoformans)-induced NF of the lower extremities (LE) in a renal transplant patient [1]. Moreover, after the NF had been successfully treated the patient developed immune reconstitution inflammatory syndrome (IRIS) [4], [5]. We report this rare case from surgeon's view point. This work has been reported in line with the SCARE criteria [6].
2. Case report
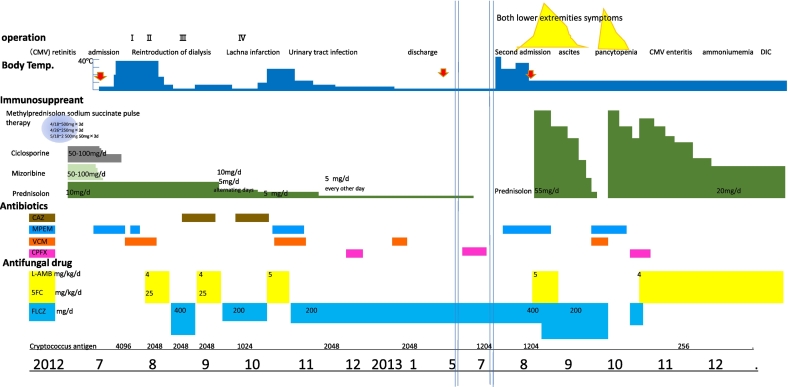
A 51-year-old male had received a living-donor kidney transplant from his wife due to renal failure secondary to polycystic kidney disease in August 2011. He was administered prednisolone (PSL), cyclosporine A, and mizoribine.
However, rejection occurred 2 months later, and methylprednisolone pulse therapy was administered.
Ten months after transplantation, the patient complained of pain in the right LE and the same symptoms appeared on the left. He was hospitalized on suspicion of cellulitis. Hemodialysis was resumed due to decreased renal function (Table 1).
Table 1.
Patient course, symptoms, drugs.
Some courses of antibiotics did not bring any improvement, and fungal infections such as candida were suspected.
On July 12th 2012, the patient was referred to our department by the urology department for the indication of surgical debridement. The patient was complaining of severe pain and a high fever. Edema, erythema, ecchymosis, vesicle formation, and erosion were also seen on both LE, indicating NF.
However, the patient refused to undergo emergency surgical debridement. A vesicle-derived bacterial culture revealed yeast, and treatment with lipid amphotericin B and flucytosine was started immediately.
2.1. July 24th, operation I (debridement)
The necrotic region had extended, and the patient agreed to undergo minimal debridement. Periodic acid-Schiff after diastase digestion (D-PAS) staining showed many yeast cells in the debrided skin. Cryptococcus neoformans (C. neoformans) was detected in tissue cultures. Conversely, cerebrospinal fluid and blood cultures were negative. Chest radiography and cranial computed tomography showed normal findings.
Around this time, methicillin-resistant Staphylococcus aureus (MRSA) and Pseudomonas aeruginosa (P. aeruginosa) were detected in wound cultures. A prostate abscess was caused by P. aeruginosa.
2.2. July 27th,operation II (second debridement)
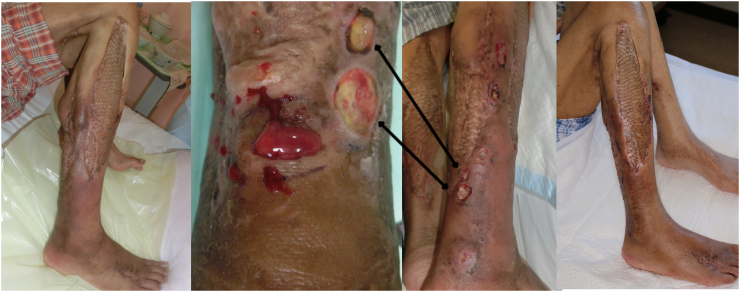
The patient's fever persisted. After the second debridement, patient's condition stabilized. Some necrotic tissue remained on the transitional surface of the gastrocnemius tendon, but the patient wanted to preserve as much LE function as possible. Therefore, we waited for granulation tissue to form; however, little had formed one month later (Fig. 1).
Fig. 1.
Necrotic tissue on gastrocnemius tendon transitional surface remained, the patient wanted to preserve the lower leg function as possible. Therefore, we waited for granulation formation without further debridement. One month later, granulation scarcely formed.
2.3. August 23th, operation III (third debridement)
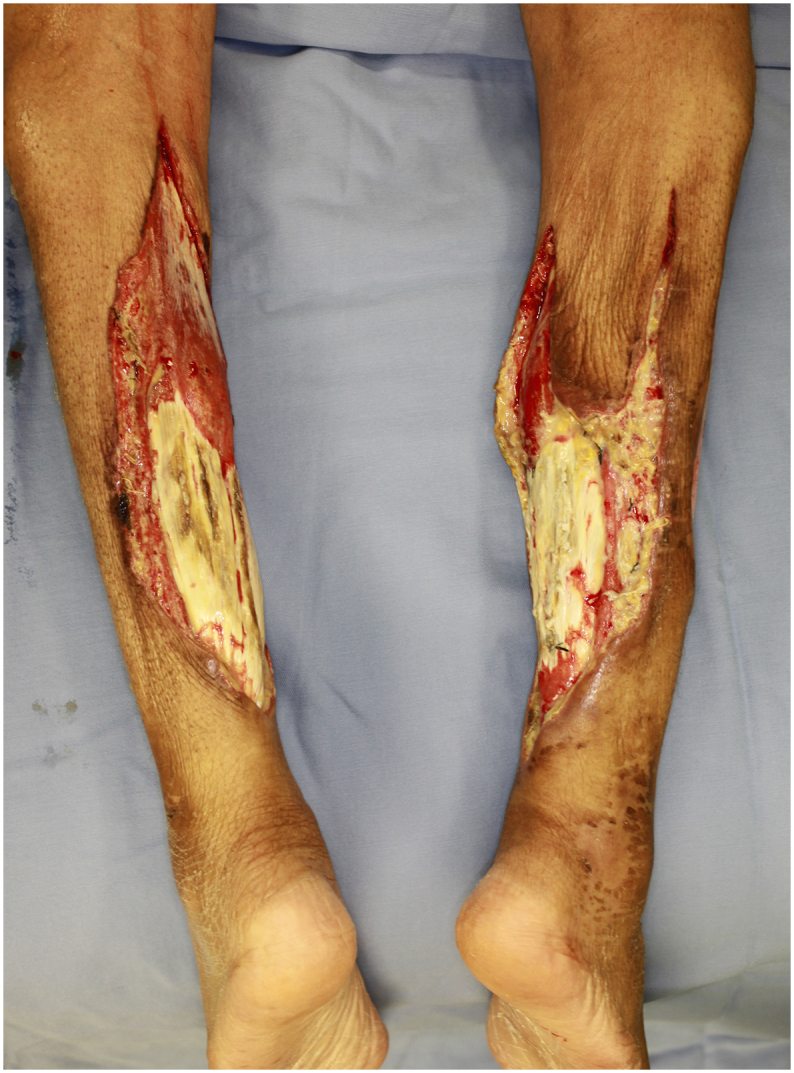
We made multiple small incisions over the gastrocnemius tendon to expose the underlying muscle body (Fig. 2).
Fig. 2.
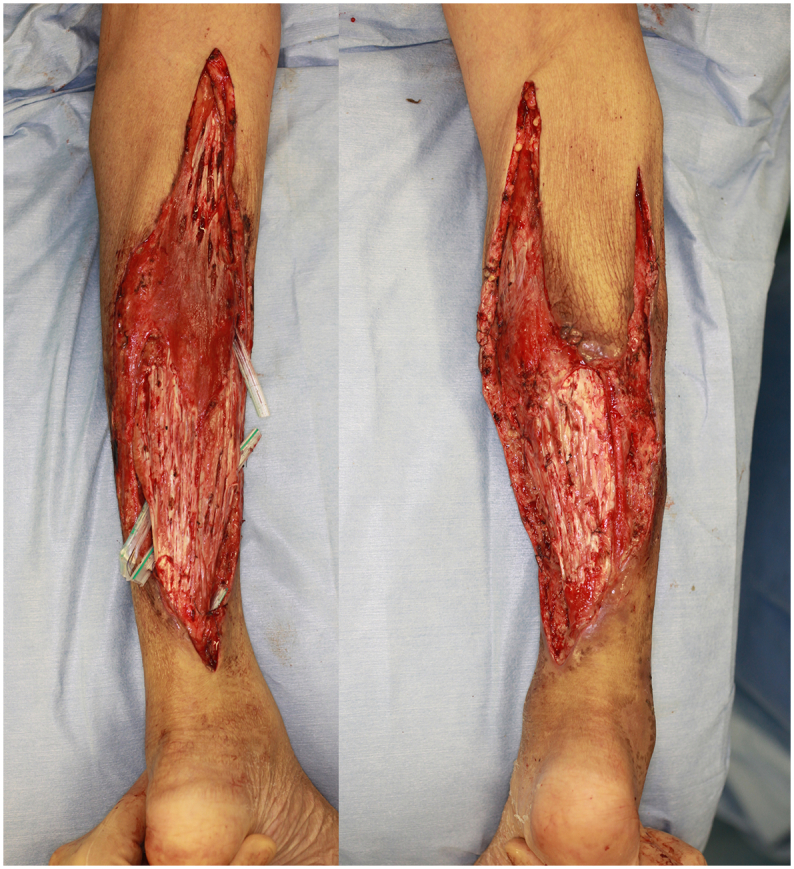
August 23th, operation III.
Debridement was performed as less as possible. We decided to make a small incision on the tendon transition area to expose the underlying muscular body and wait for granulation formation from here.
2.4. October 2nd, operation IV (skin graft)
As good granulation tissue formed on the tendon, meshed skin grafting was performed. The graft exhibited good survival (Fig. 3). The fluconazole treatment was continued. In early June 2013, the patient was discharged. PSL was discontinued in June (Table 1).
Fig. 3.
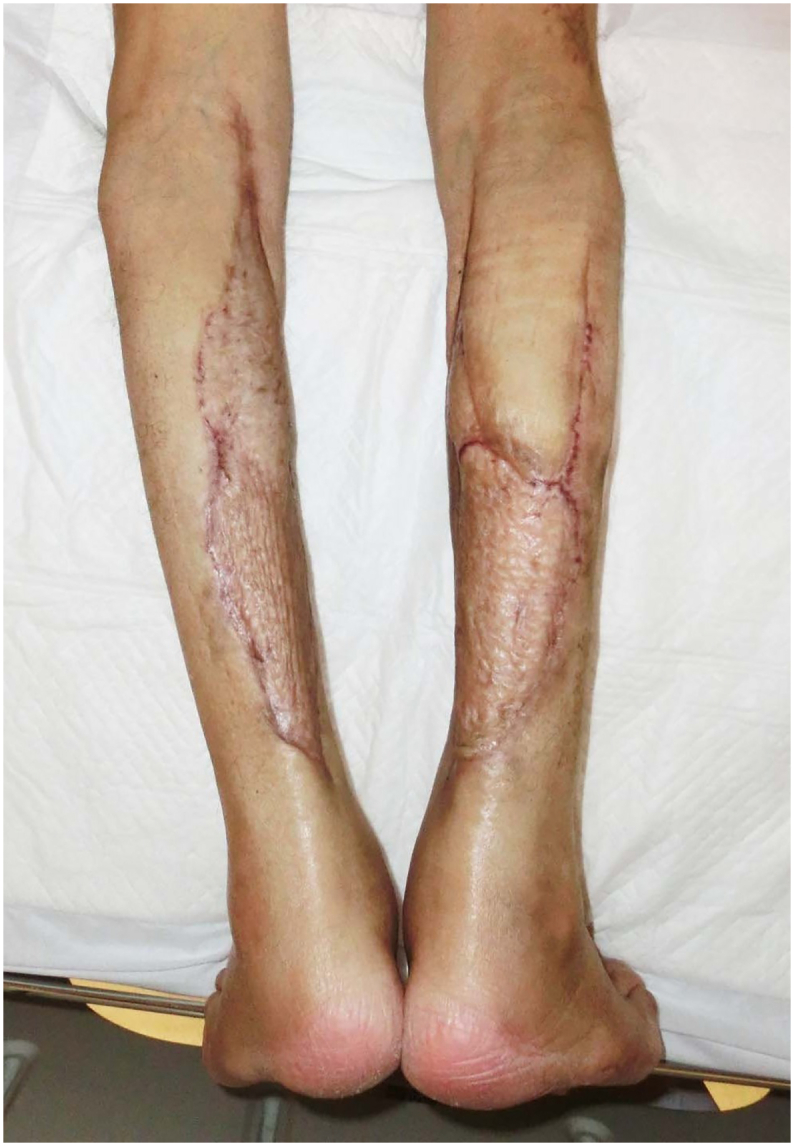
The skin graft survival was good, and rehabilitation was started 3 weeks after surgery. There was no restriction on the range of motion of the ankle joints, and it is possible to walk by itself.
July 27th and July 29th, painful nodules with abscesses appeared on the left calf and right sole on respectively, and were drained.
On July 30th, the patient was readmitted to our hospital, and lipid amphotericin B therapy was started. But new abscesses appeared on both LE, on August (Fig. 4).
Fig. 4.
Left. Immune reconstructive syndrome (IRIS) was suspected.
August 12th, a new abscess appeared on the dorsal side of the right lower leg and an incision was added.
Center. Abscess drainage and granulation formation.
Right. After resumed PSL, his symptoms improved.
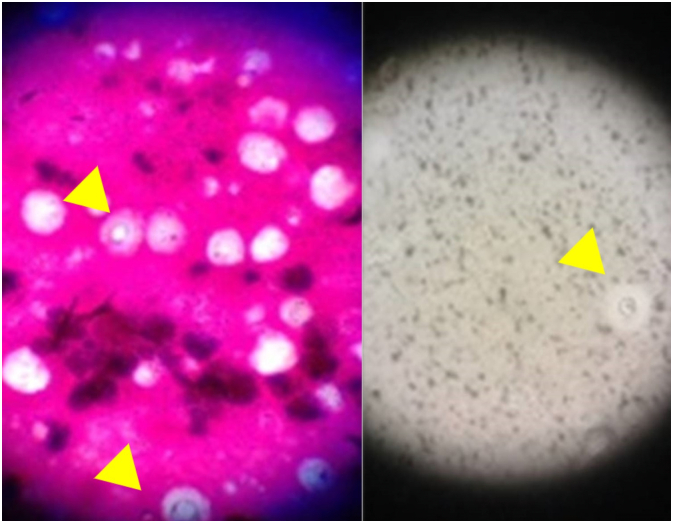
Microscopy revealed cryptococcal spores (Fig. 5). However, neither bacteria nor cryptococcal organisms were detected in the abscess and tissue culture.
Fig. 5.
Cryptococcus was detected in the abscess by microscopic examination.
Gram stain and India ink stain.
We suspected that the patient was suffering from immune reconstruction inflammatory syndrome (IRIS) based on the negative culture results and the fact that he had been treated with flucytosine and fluconazole.
Thus, we resumed the PSL, and the patient's symptoms began to improve (Fig. 4). The drained site gradually contracted and healed. The PSL dose was slowly reduced and was discontinued on September 4th.
However, on September 7th swelling of both LE re-appeared, and it was considered that the IRIS had recurred.
After the PSL treatment was resumed, the patient's symptoms improved rapidly. The PSL dose was gradually decreased to a maintenance dose of 20 mg/day.
After the patient's first admission, a prostate abscess and cytomegalovirus (CMV)-induced retinitis of the left eye occurred repeatedly. During the patient's second admission, severe pancytopenia, most likely due to vancomycin, occurred repeatedly. From September 2013, the CMV-induced enteritis and prostate abscess caused ammonemia, and intractable ascites occurred. The patient subsequently developed disseminated intravascular coagulation and died on January 10th, 2014.
3. Discussion
Cryptococcosis is an infection caused by C. neoformans, in which the primary lesions usually form in the lungs through the respiratory tract and cause secondary lesions in the central nervous system (CNS) and skin, mainly through hematogenous spread [5]. Therefore, the appearance of cutaneous lesions, which are observed in 10–15% of cases, is indicative of a disseminated infection [5], [7]. Primary cutaneous cryptococcosis is rare and is usually associated with skin injuries [7], [8].
In our case CNS, and chest radiographs did not suggest disseminated cryptococcosis. However, there was no clear preceding skin damage. Moreover, the patient's infection was severe, causing NF, rather than being limited to a small area. Therefore, disseminated cryptococcosis was diagnosed.
Cryptococcal cellulitis is uncommon and is indistinguishable from acute bacterial cellulitis [9], [10]. And NF is often misdiagnosed as cellulitis [11]. The LE are the most common sites of such infections, and in contrast to bacterial NF, bilateral disease is common in cryptococcosis [12].
There have been 14 reported cases of cryptococcal NF [9], [10], [13], and its mortality rate in immunocompromised patients is very high (57%) [13]. Therefore, early diagnosis and treatment, including debridement, are recommended [13].
A definitive diagnosis of cryptococcosis can be made by isolating Cryptococcus from a clinical specimen or directly detecting the fungus by staining bodily fluids with India ink [5]. In our case was diagnosed based on D-PAS staining of debrided tissue.
This patient was reluctant to undergo surgical treatment and earlier and more definitive debridement must have improved the patient's conditions faster. Therefore, we think earlier and more definitive debridement was appropriate from retrospective view.
Amphotericin B deoxycholate (AmBd) is the main drug treatment, Liposomal amphotericin B is an alternative and causes less nephrotoxicity. Flucytosine or fluconazole can be used in combination with AmBd as a first-line treatment. Various treatment protocols have been proposed, depending on the patient's main symptoms and immunosuppressive status [14].
Maintaining an immunosuppressed state to protect the transplanted kidney makes susceptible to other bacterial infections. Indeed, MRSA and P. aeruginosa infections repeatedly occurred in the wound and urinary tract. After the third operation, the steroid dose was gradually reduced.
The patient's wound healing occurred very slowly. In addition to immunosuppressed state, this was attributed to various factors, including renal failure-induced anemia; malnutrition; and severe arteriosclerosis, which caused a lacunar infarction.
Over the gastrocnemius tendon, we could obtain granulation tissue from the deeper muscles (Figs. 5). This resulted in almost no sacrifice of LE function.
IRIS involves exaggerated inflammatory responses to persistent foreign antigens [15]. Cryptococcal IRIS is associated with significant morbidity and mortality. IRIS is estimated to occur in 5–11% of organ transplant recipients with cryptococcal infections and is associated with an increased risk of allograft failure [4], [5], [16]. In up to a third of patients, so-called paradoxical cryptococcal IRIS; i.e., a worsening of disease or recurrent disease at the same or new sites, despite microbiological evidence of effective antifungal treatment, occurs during treatment [5], [16]. Cryptococcal IRIS is considered to be caused by the reactions of T cells to Cryptococcus antigens [17]. On skin and soft tissue, IRIS can manifest as subcutaneous abscesses, lymphadenitis [18], or nodular and plaque-like erythematous lesions [19]. In a previously reported cases [19] and present case, IRIS arose during steroid withdrawal.
The following diagnostic criteria for IRIS have been suggested: Histopathologically, a granulomatous lesion should be present. Symptoms that occur during appropriate antifungal therapy and cannot be explained by a newly acquired infection or another process should also be present. Finally, cultures should produce negative results. All 3 criteria were met in our case [5], [20].
The management of cryptococcal IRIS is based on expert opinion. Confirming the effectiveness of antifungal therapy is essential, and steroid treatment can reduce the need for hospitalization and improve short-term quality of life [5].
In conclusion, we encountered cryptococcosis in immunocompromised patients. It manifested as severe NF and even gave rise to IRIS. We think this patient needed earlier and more definitive debridement. Fortunately, we were able to save the patient's life and maintain his LE function.
In immunocompromised patients, cryptococcus can be a pathogen and caution should be exercised in the search for causative organisms. In addition, IRIS can occur during treatment. Management of IRIS is the capital point of sepsis management, careful anti-inflammatory drug control by infection control specialists is required.
Provenance and peer review
Not commissioned, externally peer-reviewed.
Consent
Written informed consent was obtained from the patient for publication of this case report and accompanying images. A copy of the written consent is available for review by the Editor-in-Chief of this journal on request.
Ethical approval
Japanese Ethical Guidelines for Medical Research for Humans https://www.mhlw.go.jp/file/06-Seisakujouhou-10600000-Daijinkanboukouseikagakuka/0000153339.pdf.
Treatment of this patient complies with the Japanese insurance system and does not include clinical trials, experimental interventional treatments or drug use.
This is not a medical procedure that the ethics committee or IRB should discuss.
Funding
There were no sources of funding support for this study except for English check fee that was paid by dermatology department in our university.
None of the authors have conflicting financial interests.
Author contribution
Masamitsu Kawahara; wrote manuscript.
Satoshi Yurugi; surgeon 1.
Kumi Mashiba; surgeon 2.
Junji Ando; assistant surgeon.
Mika Takeuchi; doctor; patient care at ward.
Riyo Miyata; doctor; patient care at ward.
Masayuki Harada; doctor; patient care at ward.
Yasumitsu Masuda; doctor; patient care at ward.
Saori Kanagawa; doctor; patient care at ward.
Tatsuo Yoneda; Physician who performed kidney transplantation.
and adjustment of immunosuppressant.
Tatsuya Fukumori; Infectious disease control doctor; patient care at ward.
Taku Ogawa; Infectious disease control doctor; patient care at ward.
Fukumi Nakamura-Uchiyama; Infectious disease control doctor; patient care at ward.
Kei Kasahara; Infectious disease control department physician who gives instructions to ward doctors.
There are more than six authors, but these doctors were essential for treatment of this patient.
Guarantor
Masamitsu Kuwahara.
Registration of research studies
Not applicable.
Declaration of competing interest
There are no conflicts of interest.
Contributor Information
Masamitsu Kuwahara, Email: makuwa@naramed-u.ac.jp, plastic-surg@naramed-u.ac.jp.
Satoshi Yurugi, Email: plastic-surg@naramed-u.ac.jp.
Junji Ando, Email: plastic-surg@naramed-u.ac.jp.
Mika Takeuchi, Email: plastic-surg@naramed-u.ac.jp.
Riyo Miyata, Email: plastic-surg@naramed-u.ac.jp.
Masayuki Harada, Email: plastic-surg@naramed-u.ac.jp.
Yasumitsu Masuda, Email: plastic-surg@naramed-u.ac.jp.
Saori Kanagawa, Email: plastic-surg@naramed-u.ac.jp.
Tatsuo Yoneda, Email: urology@naramed-u.ac.jp.
Tatsuya Fukumori, Email: cid@naramed-u.ac.jp.
Taku Ogawa, Email: cid@naramed-u.ac.jp.
Fukumi Nakamura-Uchiyama, Email: cid@naramed-u.ac.jp, S8000403@section.metro.tokyo.jp.
Kei Kasahara, Email: cid@naramed-u.ac.jp.
References
- 1.Yoneda T., Itami Y., Hirayama A. Cryptococcal necrotizing fasciitis in a patient after renal transplantation–a case report. Transplant. Proc. 2014;46(2):620–622. doi: 10.1016/j.transproceed.2013.11.034. [DOI] [PubMed] [Google Scholar]
- 2.McHenry C.R., Piotrowski J.J., Petrinic D., Malangoni M.A. Determinants of mortality for necrotizing soft-tissue infections. Ann. Surg. 1995;221:558–563. doi: 10.1097/00000658-199505000-00013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gupta R.K. Opportunistic infections in renal allograft recipients. Transplant. Proc. 2007;39:731–733. doi: 10.1016/j.transproceed.2007.01.063. [DOI] [PubMed] [Google Scholar]
- 4.Müller M., Wandel S., Colebunders R. Immune reconstitution inflammatory syndrome in patients starting antiretroviral therapy for HIV infection: a systematic review and meta-analysis. Lancet Infect. Dis. 2010;10:251–261. doi: 10.1016/S1473-3099(10)70026-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Maziarz E.K., Perfect J.R. Cryptococcosis. Infect. Dis. Clin. N. Am. 2016;30:179–206. doi: 10.1016/j.idc.2015.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.for the SCARE Group. Agha R.A., Franchi T., Sohrabi C., Mathew G. The SCARE 2020 guideline: updating consensus Surgical CAse REport (SCARE) guidelines. Int. J. Surg. 2020;84:226–230. doi: 10.1016/j.ijsu.2020.10.034. [DOI] [PubMed] [Google Scholar]
- 7.Christianson J.C., Engber W., Andes D. Primary cutaneous cryptococcosis in immunocompetent and immunocompromised hosts. Med. Mycol. 2003;41:177–188. doi: 10.1080/1369378031000137224. [DOI] [PubMed] [Google Scholar]
- 8.Noguchi H., Matsumoto T., Kimura U., Hiruma M., Kusuhara M., Ihn H. Cutaneous cyptococcosis. Med. Mycol. J. 2019;60:101–107. doi: 10.3314/mmj.19.008. [DOI] [PubMed] [Google Scholar]
- 9.Shrader S.K., Watts J.C., Dancik J.A., Band J.D. Disseminated cryptococcosis presenting as cellulitis with necrotizing vasculitis. J. Clin. Microbiol. 1986;24:860–862. doi: 10.1128/JCM.24.5.860-862.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kimura M., Kadota E., Satou T., Yoneda E., Furuta T. Case report Cryptococcal cellulitis showing necrotizing vasculitis. Mycoses. 2001;44:115–158. [PubMed] [Google Scholar]
- 11.Goh T., Goh L.G., Ang C.H., Wong C.H. Early diagnosis of necrotizing fasciitis. Br. J. Surg. 2014;101(1):119e–125e. doi: 10.1002/bjs.9371. [DOI] [PubMed] [Google Scholar]
- 12.Baer S., Baddley J.W., Gnann J.W., Pappas P.G. Cryptococcal disease presenting as necrotizing cellulitis in transplant recipients. Transpl. Infect. Dis. 2009;11(4):353–358. doi: 10.1111/j.1399-3062.2009.00399.x. [DOI] [PubMed] [Google Scholar]
- 13.Hoshino T., Omura K., Kimura S., Takahashi H., Kamei K., Ohkusu M. A case of disseminated cryptococcosis with necrotizing fasciitis in a non-HIV patient. Acute Med Surg. 2017;4:454–457. doi: 10.1002/ams2.298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Perfect J.R., Dismukes W.E., Dromer F., Goldman D.L., Graybill J.R., Hamill R.J. Clinical practice guidelines for the management of cryptococcal disease: 2010 update by the infectious diseases society of america. Clin. Infect. Dis. 2010;50:291–322. doi: 10.1086/649858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wiesner D.L., Boulware D.R. Cryptococcus-related immune reconstitution inflammatory Syndrome(IRIS): pathogenesis and its clinical implications. Curr. Fungal Infect Rep. 2011;1(5):252–261. doi: 10.1007/s12281-011-0064-8. Dec. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jenny-Avital E.R., Abadi M. Immune reconstitution cryptococcosis after initiation of successful highly active antiretroviral therapy. Clin. Infect. Dis. 2002;35:128e–133e. doi: 10.1086/344467. 15. [DOI] [PubMed] [Google Scholar]
- 17.Breton G., Seilhean D., Chérin P., Herson S., Benveniste O. Paradoxical intracranial cryptococcoma in a human immunodeficiency virus-infected man being treated with combination antiretroviral therapy. Am. J. Med. 2002;113:155–157. doi: 10.1016/S0002-9343(02)01130-0. [DOI] [PubMed] [Google Scholar]
- 18.Burton R., Gogela N., Rebe K., McNally M., Meintjes G. Cryptococcal immune reconstitution inflammatory syndrome presenting with erosive bone lesions, arthritis and subcutaneous abscesses. AIDS. 2009;13(23):2371–2373. doi: 10.1097/QAD.0b013e328330975f. [DOI] [PubMed] [Google Scholar]
- 19.Narayanan S., Banerjee C., Holt P.A. Cryptococcal immune reconstitution syndrome during steroid withdrawal treated with hydroxychloroquine. Int. J. Infect. Dis. 2011;15:70e–73e. doi: 10.1016/j.ijid.2010.09.006. [DOI] [PubMed] [Google Scholar]
- 20.Ft. Sun Hsin-Yun, Barbara D.A., Shirish H., Graeme N., Didier Bruno G., Marshall L. Predictors of immune reconstitution syndrome in organ transplant recipients with cryptococcosis: implications for the management of immunosuppression. Clin. Infect. Dis. 2015;60:36–44. doi: 10.1093/cid/ciu711. [DOI] [PMC free article] [PubMed] [Google Scholar]