Graphical abstract

Keywords: RCAN1.4, Calcineurin, NFAT, Osteoclast, Osteosarcoma
Highlights
-
•
Protein level of RCAN1.4 in osteosarcoma specimens was lower than that of chondroma.
-
•
RCAN1.4 loss promoted osteosarcoma growth, migration and invasion.
-
•
RCAN1.4-calcineurin/NFAT pathway regulated the osteosarcoma growth and metastasis.
Abstract
Calcipressin-1, also known as a regulator of calcineurin 1 (RCAN1), is one of the families of endogenous regulators of calcineurin activation and can specifically constrain the activity of calcineurin, but its function in osteosarcoma is still unknown. Firstly, we examined the protein level of RCAN1 in osteosarcoma specimens was lower than that of chondroma specimens. RCAN1.4 rather than RCAN1.1 had a higher endogenous protein level in six osteosarcoma cell lines by western blot. Further, we created stable RCAN1.4-deficient 143B and Hos cells using CRISPR-Cas9. RCAN1.4 loss promoted tumor growth in subcutaneous xenograft models. RCAN1.4 knockdown promoted tumor metastases to the lungs using intravenous metastasis models. Furthermore, we found that higher activity of calcineurin in RCAN1.4-deficient cells enhanced the nuclear translocation of NFATc1 to induce the cyclin D1 and MMPs expression. In addition, RCAN1.4 overexpression restrained osteosarcoma cell growth and invasion and inhibited the activity of calcineurin. Finally, we discovered that conditioned medium (20%) derived from RCAN1.4-deficient cells significantly promoted osteoclastogenesis, indicating Receptor Activator of Nuclear factor κB (RANK) signaling activation during osteosarcoma metastasis. In conclusion, RCAN1.4 may be a potential therapeutic target for osteosarcoma.
1. Introduction
Osteosarcoma (OS) originating from mesenchymal stem cells is the most common malignant tumor of bone in children and adolescents younger than 20 years old [1]. This disease presents a huge challenge to improve the overall survival, especially in metastatic patients [2]. Historically, <20% of patients with metastatic osteosarcoma survived without recurrence of their cancer [3]. Though the situation got better with new effective chemotherapy, the mechanism of osteosarcoma development and metastasis, which remain largely unclear, can be crucial to improve the survival rate.
Calcipressin-1, also known as a regulator of calcineurin 1 (RCAN1) [4], is one of the families of negative endogenous regulators of calcineurin activation and can specifically bind to the catalytic domain of calcineurin A and downregulate the activity of calcineurin [5], [6]. Calcineurin, composed of calcineurin A (a catalytic domain, CnA) and calcineurin B (a binding domain, CnB), is a protein phosphatase enzyme that primarily promotes nuclear translocation of NFAT. RCAN1 localizes on the q22.12 region of chromosome 21 [7]. It includes seven exons, and two main isoforms: RCAN1.1 (252 amino acids, 39 KDa) and RCAN1.4, (192 amino acids; 29 KDa) are generated by alternative splicing. Recent researches showed that RCAN1.4 played a critical role in cancer growth [8], [9], [10], [11], [12], endothelial cells migration [13], [14] and neuronal apoptosis [15]. However, further exploration is indeed needed to elucidate the mechanism of RCAN1.4 in osteosarcoma.
Researches showed that RCAN1 interacts with calcineurin (CaN) to inhibit the nuclear translocation of nuclear factor of activated T-cells (NFAT) [9], [16]. While NFAT isoforms have been proved to motivate multiple events during cancer progression, including cellular phenotype like proliferation, survival, migration, and invasion, as well as tumor angiogenesis [17]. Furthermore, downregulation of specific RCAN1 isoform has been observed in numerous types of solid tumor involving various organs. The RCAN1.4 expression of hepatocellular carcinoma (HCC) cells was obviously down-regulated and RCAN1.4 overexpression of HCC cells prevented tumor proliferation, migration and invasion in vitro by constraining calcineurin/NFAT1 signaling pathway, as well as prevented tumor metastases in the intravenous metastasis models [18]. As for thyroid cancer, small cell lung cancer and renal cell carcinoma, similar results have been reported, indicating that RCAN1.4 acts as a suppressor during cancer progression [19], [8], [20]. Above all, RCAN1.4 may be regarded as a potential therapeutic target for cancer in many individuals [21].
Nevertheless, the association of RCAN1 with osteosarcoma remains to be elucidated. In this study, we verified that the protein expression of RCAN1.1 was pretty low in osteosarcoma cell lines. We, therefore, set out to explore the role of RCAN1.4 in the tumorigenesis and metastasis of osteosarcoma. Using certain types of osteosarcoma cell lines (Hos and 143B) with stable deficiency or overexpression of RCAN1.4, we accessed the effect of RCAN1.4 on osteosarcoma cells, the subcutaneous xenograft models and intravenous metastasis models. Moreover, we explored the role of the calcineurin/NFAT pathway in osteosarcoma development and metastasis. Finally, we analyzed the association between osteoclastogenesis and osteosarcoma metastasis.
2. Materials and methods
2.1. Clinical samples
OS or chondroma specimens from 6 osteosarcoma patients or 6 chondroma patients were collected from the surgical specimen archives of Sir Run Run Shaw Hospital, respectively. All the procedures were conducted in accordance with the Declaration of Helsinki and approved by the Institutional Review Board of Sir Run Run Shaw Hospital. Informed consent was acquired from each patient before the beginning of this study. All the tissues were stored in liquid nitrogen.
2.2. Cell culture
Human OS cells were purchased from the American Type Culture Collection (ATCC, Manassas, VA, USA), including the 143B (CRL-8303), Hos (CRL-1543), MG-63 (CRL-1427), U2OS (HTB96), SJSA-1 (CRL-2098) and Saos-2 (HTB-85) cell lines. These cell lines were cultured in Dulbecco’s modified Eagle’s medium (DMEM, Gibco) with 10% fetal bovine serum (Gibco), 1% penicillin/streptomycin (Invitrogen, Carlsbad, CA, USA) in an incubator at 37 °C with 5% CO2.
2.3. Generation of stable cell lines
The 143B and Hos cells were infected with the viral supernatants of non-targeting scrambled vector or CRISPR-Cas9-RCAN1.4 for 8 h. After 48 h, they were selected with 1 μg/ml and 2 μg/ml puromycin (Invitrogen) for at least 2 weeks, respectively. The target sequences are TGTCTGGATTTGGCGGAGCC. RCAN1.4 knockdown clones were screened using western blot.
2.4. Wound-healing assay
Osteosarcoma cells were seeded in 6-well plates until they reached at least 95% confluence. Subsequently, cell monolayer was scratched using a 200 μl pipette tip. Representative images of cell migration were captured at 0 h, 6 h, and 18 h after injury. Diminishing distance across the scratched area was measured, normalized to the 0 h cells.
2.5. RNA extraction and quantitative PCR
The cells were cultured in 12-well plates (1 × 104 cells/well). All the RNAs of cells, stimulated with the indicated agents, were extracted using Ultrapure RNA Kit (CW0581, CWBIO, China). Quantity of RNAs was measured by Nanodrop 2000. Reverse transcription was performed using the HiFiScript cDNA Synthesis kit (CW2569, CWBIO, China). RT-qPCR used the UltraSYBR Mixture (CW0957, CWBIO) to quantify transcriptional gene levels. The experimental reaction was performed at preincubation (95 °C for 10 min), amplification (40 cycles) (95 °C for 15 s, 60 °C for 60 s, and 72 °C for 20 s) and melting curves (95 °C for 15 s, 60 °C for 60 s). The specificity of amplification was verified by analyzing the melting curves. The cyclin D1, Acp5, c-Fos, NFATc1, CTSK, DC-STAMP and β-actin primer sequences were presented in supplementary Table 1.
2.6. Western blot analysis
The cells were cultured (1 × 105 cells/well) in six-well plates and then pretreated with the indicated agents. All the proteins were harvested using RIPA (Solarbio, Beijing, China) with 100 mM phenylmethanesulfonyl fluoride (Beyotime, Zhengzhou, China), 100 × protease inhibitor cocktail (Millipore, USA). Supernatants were extracted followed by the 15 min centrifugation at 12,000 rpm. Supernatants were resolved by 10% SDS-PAGE and transferred by electroblotting to PVDF membranes (Millipore, USA). Blocked in 5% (w/v) nonfat dry milk in TBS with Tween 20 (TBST) at RT for 45 min, membranes incubated with indicated antibodies (1:1000 dilution) at 4 °C overnight. Washed 5 × 7 min with TBST, bands were incubated with the HRP-conjugated goat anti-mouse/rabbit IgG (1:5000 dilution; Abcam) for 1 h at RT. Bands were detected by Image Lab software (Bio-Rad, Hercules, CA). The images were quantified by ImageJ (National Institutes of Health, Bethesda, MD, USA).
2.7. Immunofluorescence staining
The stable 143B and Hos cell lines were placed on slides in 12-well plates (1.5 × 104 cells/well). Slides were then fixed in 4% paraformaldehyde for 30 min, and then permeabilizated with 0.5% Triton-X100 for 30 min at RT. After, slides were incubated with 5% bovine serum albumin (BSA) for 1 h, followed by anti-NFATc1 (ET1704-45, Huabio, Hangzhou, China), anti-MMP2 (EM1706-99, Huabio) and anti-MMP9 (ER1706-40, Huabio) (1:100–200 dilution) antibodies at 4 °C overnight. Nuclei were stained with 0.1 μg/mL DAPI (Sigma-Aldrich) for 30 min at RT. Cells were imaged by fluorescence microscope model BX51TRF (Olympus Corporation, Tokyo, Japan). Images were analyzed by ImageJ software (National Institutes of Health).
2.8. Immunohistochemistry (IHC)
Human tumor specimens and subcutaneous xenograft tumors were dehydrated with a graded series of ethanol, and then embedded in paraffin. 4 μm slices were cut using a microtome (Leica, Germany). To examine the immunoreactivity of Ki67, MMP2 and MMP9, immunohistochemical analysis of slices was conducted using an SP Rabbit & Mouse HRP Kit (CW2069, CWBIO). Rabbit anti-RCAN1 (ab140131, Abcam), anti-Ki67 (ER1802-31, Huabio), anti-MMP2 and anti-MMP9 polyclonal antibodies were used (1:100–1:200). Three pathologists who were blind to the tissue data were responsible for counting numbers of total cells, Ki67, RCAN1, MMP2 or MMP9-positive cells under high-power fields (magnification 200x) for three sections in each specimen. If the intraclass correlation coefficient was below 0.8, the sections were recounted.
2.9. BMMs preparation and osteoclast differentiation in vitro
Primary BMMs were isolated from the whole bone marrow of male 6-week-old C57BL/6 mice, as described previously [22]. Briefly, cells that were isolated from the femurs and tibias were cultured for 6 days with 10% FBS (Gibco), alpha-MEM, 1% penicillin/streptomycin, and 25 ng/ml MCSF (Novoprotein, Shanghai, China) under 5% CO2, 37 °C. BMMs were cultured in 24-well plates at a density of 5 × 104 cells with or without 10 ng/ml RANKL (R&D, Minneapolis, MN, USA), conditioned medium (20%) derived from knockdown RCAN1.4 cells and vector cells. After being induced for 6 days, multinucleated osteoclasts with more than three nuclei were immediately fixed in 4% paraformaldehyde for 20 min (RT) and then stained using the TRAP solution (Sigma).
2.10. Subcutaneous xenograft tumor models
A total of 2 × 106 143B or Hos stable cells were injected subcutaneously using the athymic nude mice (male, 8-week-old) (n = 4). Animal experiments were approved and conducted in compliance with the institutional guidelines set by the Institutional Review Board of Sir Run Run Shaw Hospital. Tumor volume was calculated using the following formula: tumor volume (mm3) = ½ × (length × width × width) [8]. Thirty days later, the xenograft tumors were harvested after the animals were sacrificed, followed by in 4% paraformaldehyde. Tumor weight was shown as the mean ± SEM of each group.
2.11. Tail vein metastasis model
143B and Hos cells were stably transfected with vector or CRISPR-Cas9-RCAN1.4. The stable 143B and Hos cell lines were suspended in sterile PBS (1 × 106 cells/100 μl) and injected into the nude mice tail vein (male, 8-week-old) using 29-gauge insulin syringes (Terumo). Mice (n = 4) were sacrificed 4 weeks later for the stable 143B cell lines and 5 weeks later for the stable Hos cell lines. Lung tissues were obtained to perform the histology staining and examination.
2.12. Statistical analysis
Data analyses were performed by SPSS 19.0 (SPSS, Chicago, IL, USA)). The data were presented as the mean and standard deviation. Statistical differences were analyzed by one-way ANOVA or Student’s t-test, followed by Tukey’s post hoc analyses where appropriate. P values < 0.05 were considered statistically significant.
3. Results
3.1. RCAN1 was downregulated in human osteosarcoma samples and stable RCAN1.4 knockdown cell lines were established
To investigate whether RCAN1 downregulation was related to human osteosarcoma, we detected RCAN1 expression in osteosarcoma or chondroma specimens from 6 osteosarcoma patients or 6 chondroma patients. The protein level of RCAN1 in the osteosarcoma specimens was lower than that of chondroma specimens (Fig. 1A and B). In general, RCAN1 expression was higher in chondroma tissues and lower expression in the invasive osteosarcoma, indicating the RCAN1 played a vital role in osteosarcoma. To confirm the levels of endogenous RCAN1 isoforms in osteosarcoma cells, we observed a series of confirmed human osteosarcoma cell lines by western blot. All the cell lines had little protein expression of RCAN1.1, and 143B and Hos cells had higher endogenous RCAN1.4 protein levels versus the other cell lines (Fig. 1C). Therefore, we created stable RCAN1.4-deficient 143B and Hos cells using CRISPR-Cas9 specifically targeting RCAN1.4. Western blot showed that both 143B and Hos clones had an appropriately 100% knockdown of RCAN1.4, compared with non-targeting scrambled vector (Fig. 1D). RCAN1.1 expression was almost not altered (Fig. 1D).
Fig. 1.
RCAN1 was downregulated in human osteosarcoma samples and stable RCAN1.4 knockdown cell lines were established. (A) Protein level of RCAN1 in the osteosarcoma specimens was lower than that of chondroma specimens. (B) Quantification of RCAN1-positive cells in osteosarcoma and chondroma specimens. (C) Endogenous RCAN1.1 and RCAN1.4 protein levels of six osteosarcoma cell lines. (D) Both 143B and Hos clones with knockdown RCAN1.4 were constructed, compared with non-targeting scrambled vector. All experiments were performed at least three times. Scale bar: 100 μm. *P < 0.05, **P < 0.01, ***P < 0.005.
3.2. RCAN1.4 modulated tumor proliferation in vitro and in vivo
In both 143B and Hos cells, RCAN1.4-deficient cells had a significant difference in cell proliferation by CCK-8 assay (Fig. 2A and B). Also, knockdown RCAN1.4 promoted colony formation in both 143B and Hos cells (Fig. 2C and D). Then, the vector and knockdown RCAN1.4 cells were subcutaneously injected in the lower back side of athymic nude mice. Tumor growth was monitored using caliper measurements. For both 143B and Hos cells, a difference in tumor weight demonstrated that knockdown RCAN1.4 xenografts had faster growth compared with vector xenografts (Fig. 2E). The final average tumor weight was 0.2825 g and 1.0925 g for 143B vector and knockdown RCAN1.4 cells, and 0.2450 g and 0.9875 g for Hos vector and knockdown RCAN1.4 cells (Fig. 2F). The average tumor volumes were 0.2375 cm3 and 1.1180 cm3 for 143B vector and knockdown RCAN1.4 cells, and 0.2275 cm3 and 1.0300 cm3 for Hos vector and knockdown RCAN1.4 cells (Fig. 2G). Further, RT-qPCR demonstrated that knockdown RCAN1.4 induced the gene expression of cyclin D1, which positively promoted cell proliferation (Fig. 2H). In addition, in both 143B and Hos cells, knockdown RCAN1.4 cells had significantly more Ki67–positive cells compared with vector tumors (Fig. 2I and J). In conclusion, knockdown RCAN1.4 promoted subcutaneous xenograft growth, which was strongly associated with enhanced cell proliferation in vitro.
Fig. 2.
RCAN1.4 modulated tumor growth in vitro and in vivo. (A) Knockdown RCAN1.4 promoted the 143B and (B) Hos cells growth by CCK-8 assay. (C) Knockdown RCAN1.4 also promoted colony formation in both 143B and Hos cells. (D) Number of formatted colony in 143B and Hos cells with knockdown RCAN1.4. (E) For both 143B and Hos cells, knockdown RCAN1.4 xenografts had faster growth compared with vector xenografts (n = 4). (F) The final average tumor weight was 0.2825 g and 1.0925 g for 143B vector and knockdown RCAN1.4 cells, and 0.2450 g and 0.9875 g for Hos vector and knockdown RCAN1.4 cells. (G) The average tumor volumes were 0.2375 cm3 and 1.1180 cm3 for 143B vector and knockdown RCAN1.4 cells, and 0.2275 cm3 and 1.0300 cm3 for Hos vector and knockdown RCAN1.4 cells. (H) RT-qPCR demonstrated that knockdown RCAN1.4 induced the gene expression of cyclin D1. (H) In both 143B and Hos cells, knockdown RCAN1.4 cells had significantly more Ki67–positive cells compared with vector tumors. (I) Quantification of Ki67-positive cells in two groups. All experiments were performed at least three times. Scale bar: 100 μm. *P < 0.05, **P < 0.01, ***P < 0.005, ****P < 0.001.
3.3. Knockdown RCAN1.4 promoted tumor metastasis in vitro and in vivo.
We next evaluated the effects of knockdown RCAN1.4 on migration and invasion using trans-well filters with or without matrigel. The trans-well migration and invasion assay revealed that the migration and invasion capacities of both 143B and Hos cells were enhanced by knockdown RCAN1.4. The number of migrated or invaded cells per field was quantized. Representative images were shown in Fig. 3A. The mean number of migrated or invaded cells was enhanced more than 2–3-fold in 143B or Hos cells by knockdown RCAN1.4 versus control cells (Fig. 3B and C). Besides, knockdown RCAN1.4 raised the healing of scratches in the wound-healing assay, indicating that osteosarcoma cell migration was increased (Fig. 3D). Furthermore, subsequent metastasis in a tail vein model was monitored weekly. After the indicated time, the mice were sacrificed. The lungs were harvested and stained with H&E. There was a visible difference in lung tumor numbers between the two groups (Fig. 3E and F). We also observed the higher frequency of lung metastases in knockdown RCAN1.4 cells versus vector cells by histology examination (Fig. 3G). Taken together, knockdown RCAN1.4 promoted osteosarcoma cells metastasis in vitro and in vivo.
Fig. 3.
Knockdown RCAN1.4 promoted tumor metastasis in vitro and in vivo. (A) The trans-well migration and invasion assay revealed that the migration and invasion capacities of both 143B and Hos cells were enhanced by knockdown RCAN1.4. (B) Number of migrated or (C) invaded cells per field was counted for quantitation. (D) Knockdown RCAN1.4 raised the healing of scratches at 6 h and 18 h in the wound-healing assay. (E) There was a visible difference of lung tumor number between vector and knockdown RCAN1.4 cells. (F) Quantification of lung tumor number in two groups. (G) Histology examination confirmed the higher frequency of lung metastases in knockdown RCAN1.4 cells versus vector cells. All experiments were performed at least three times. *P < 0.05, **P < 0.01, ***P < 0.005, ****P < 0.001.
3.4. The calcineurin/NFAT signaling pathway was activated in knockdown RCAN1.4 cells
RCAN1.4 could interfere with the calcineurin/NFAT signaling pathway, which was thought to facilitate tumor growth and metastasis. Thus, we detected the calcineurin activity in 143B or Hos cells. As shown in Fig. 4A, the activity of calcineurin in 143B and Hos cells with knockdown RCAN1.4 was upregulated. Because the mRNA level of NFATc1 among the NFAT1-5 in 143B and Hos cells was relatively higher (Fig. 4B), we detected its nuclear translocation. The IF assay demonstrated the nuclear translocation of NFATc1 was obviously increased in knockdown RCAN1.4 cells, compared with the vector cells (Fig. 4C), indicating the calcineurin/NFAT signaling pathway was activated by downregulation of RCAN1.4. We also found the increasing expression of NFATc1 in OS specimens compared to chondroma (Fig. 4D) and OS cell lines with high expression of RCAN1.4 were almost the lower expression of NFATc1 (Fig. 4E). Further, in order to clear enhanced growth and metastasis, we demonstrated that MMP2 and MMP9, which assisted tumor metastasis, had a higher expression in knockdown RCAN1.4 cells by and IF assay (Fig. 4F and G) and western blot (Fig. 4H). In addition, there were significantly more MMP2 or MMP9–positive cells in knockdown RCAN1.4 tumor slices, compared with vector tumors (Fig. 4I). Together, the calcineurin/NFAT signaling pathway was activated in knockdown RCAN1.4 cells, motivating the tumor growth and metastasis.
Fig. 4.
The calcineurin/NFAT signaling pathway was activated in knockdown RCAN1.4 cells. (A) The activity of calcineurin in 143B and Hos cells with knockdown RCAN1.4 was upregulated. (B) mRNA level of NFATc1 among the NFAT1-5 in 143B and Hos cells was relatively higher. (C) The nuclear translocation of NFATc1 by immunofluorescence staining was obviously increased in knockdown RCAN1.4 cells, compared with the vector cells. (D) The increasing expression of NFATc1 in OS specimens compared to chondroma. (E) OS cell lines with high expression of RCAN1.4 were almost the lower expression of NFATc1. (F) The IF assay also showed MMP2 and (G) MMP9 were higher expression in knockdown RCAN1.4 cells compared with the vector cells. (H) MMP2 and MMP9 were higher expression in knockdown RCAN1.4 cells compared with the vector cells by western blot. (I) There were significantly more MMP2 or MMP9–positive cells in knockdown RCAN1.4 tumor slices, compared with vector tumors. All experiments were performed at least three times. Scale bar: 100 μm. *P < 0.05, **P < 0.01, ***P < 0.005, ****P < 0.001.
3.5. RCAN1.4 overexpression suppressed the growth, migration and invasion in osteosarcoma cells
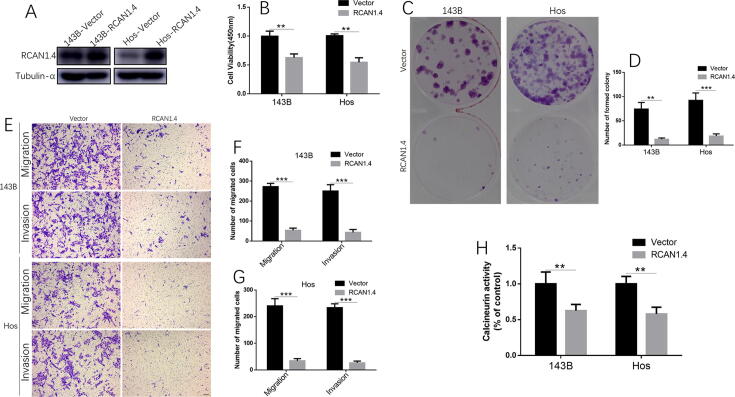
To investigate if RCAN1.4 overexpression can reduce osteosarcoma cell growth and invasion, RCAN1.4-overexpressed cell lines were created with vector or RCAN1.4 plasmids. We confirmed that the induction of RCAN1.4 was achieved in 143B and Hos cells by western blot (Fig. 5A). RCAN1.4 overexpression inhibited 143B and Hos cell proliferation at 48 h by CCK-8 assay (Fig. 5B). Also, RCAN1.4 overexpression inhibited colony formation in both 143B and Hos cells (Fig. 5C and D). Induction of RCAN1.4 in 143B and Hos cells indeed reduced migration and matrigel invasion (Fig. 5E–G). In addition, there was a lower activity of calcineurin in 143B and Hos cells with RCAN1.4 overexpression (Fig. 5H). Therefore, we concluded that RCAN1.4 acted as a suppressor of human osteosarcoma progression.
Fig. 5.
RCAN1.4 overexpression suppressed the growth, migration and invasion in osteosarcoma cells. (A) Induction of RCAN1.4 was achieved in 143B and Hos cells by western blot. (B) RCAN1.4 overexpression inhibited 143B and Hos cell proliferation at 48 h by CCK-8 assay. (C) RCAN1.4 overexpression inhibited colony formation in both 143B and Hos cells. (D) Quantification of formatted colony. (E) In both cell lines, induction of RCAN1.4 reduced migration and matrigel invasion. (F-G) Number of migrated or invaded cells per field was counted for quantitation in (F) 143B cells and (G) Hos cells. (H) Lower activity of calcineurin in 143B and Hos cells with RCAN1.4 overexpression, compared with vector cells. All experiments were performed at least three times. *P < 0.05, **P < 0.01, ***P < 0.005, ****P < 0.001.
3.6. Knockdown RCAN1.4 cells accelerated the osteoclastogenesis
The RANK expression had been reported in osteosarcoma cells, multiple myeloma and breast cancer-associated osteolysis, indicating that RANKL-RANK signaling inhibition could be as an adjuvant for the treatment of osteosarcoma [23], [24], [25]. As shown in the representative images in Fig. 6A and B, conditioned medium (20%) derived from knockdown RCAN1.4 cells significantly promoted osteoclast differentiation with 10 ng/ml RANKL, compared with vector cells. The number and area of TRAP-positive multinucleated osteoclasts (percentage of a well) also demonstrated that the conditioned medium accelerated the osteoclast differentiation (Fig. 6C and D). We then detected the RANKL expression, which strongly promoted the osteoclastogenesis. There is no significant difference in the expression of RANKL in the cells, but more secretion of RANKL in the RCAN1.4-deficient cell supernatant, compared to the control (Fig. 6E). Further, protein and mRNA levels of representative osteoclast genes were strongly facilitated, stimulated with conditioned medium (20%) derived from knockdown RCAN1.4 cells, compared with vector cells (Fig. 6F–K). Finally, the schematic representation that RCAN1.4 interfered with the calcineurin/NFAT signaling pathway to suppress the osteosarcoma growth and metastasis was presented (Fig. 7). Taken together, knockdown RCAN1.4 cells accelerated the osteoclastogenesis, perhaps facilitating the osteosarcoma metastasis.
Fig. 6.
Knockdown RCAN1.4 cells accelerated the osteoclastogenesis. (A) Conditioned medium (20%) derived from knockdown RCAN1.4 cells significantly promoted osteoclast differentiation with 10 ng/ml RANKL, compared with vector cells. (B) Representative images of osteoclasts were shown. (C-D) Quantification of TRAP-positive multinuclear cells, (C) osteoclast area and (D) number (percentage of well). (E) There is no significant difference in the expression of RANKL in the cells, but more secretion of RANKL in the RCAN1.4-deficient cell supernatant, compared to the control. (F) Protein levels of representative osteoclast genes c-Fos and NFATc1 were strongly induced, stimulated with conditioned medium (20%) derived from knockdown RCAN1.4 cells, compared with vector cells. (G–K) mRNA levels of (G) c-Fos, (H) NFATc1, (I) Acp5, (J) DC-STEMP and (K) CTSK in osteoclasts stimulated with conditioned medium from knockdown RCAN1.4 cells were strongly induced, compared with vector cells. All experiments were performed at least three times. SN: supernatant. Scale bar: 100 μm. *P < 0.05, **P < 0.01, ***P < 0.005, ****P < 0.001.
Fig. 7.
The schematic representation of this experiment. RCAN1.4 interfered with the calcineurin/NFAT signaling pathway to suppress the osteosarcoma growth and metastasis. Arrows indicated activation of the signaling pathways, while T bars indicated inhibition of the signaling pathways.
4. Discussion
The underlying mechanisms that suppress osteosarcoma are essential for future treatments. This study characterized the decreased changes in expression of RCAN1.4 in human OS, which promoted OS cell proliferation, migration and metastasis mainly via interfering with the calcineurin/NFAT signaling pathway. Activation of calcineurin through RCAN1.4 downregulation could promote the nuclear translocation of NFATc1 further to increase the expression levels of cyclin D1, MMP2 and MMP9. We also demonstrated that knockdown RCAN1.4 cells accelerated the osteoclastogenesis, which facilitated the osteosarcoma associated bone destruction and metastasis. Additional experiments in the subcutaneous xenograft tumor models and tail vein metastasis model further confirmed that RCAN1.4 could play an important role in osteosarcoma development and progression.
Numerous epidemiological studies showed that reduced solid tumors in Down syndrome, indicating the potential tumor-suppressive genes on chromosome 21 [26]. The in vitro and in vivo results in the several studies indicated that RCAN1.4 localizing on the q22.12 region of chromosome 21 might be an endogenous tumor suppressor [8], [9], [10], [11]. The human RCAN1 gene included seven exons and six introns, which were predicted to generate different isoforms by alternative splicing [27]. Isoform RCAN1.4 (appropriately 28KDa) consisted of the 197 amino acids [28], [29]. In view of cell of origin, content of stroma and immune cells in OS or chondromas, it is a limitation that RCAN1.4 gene expression may differ between two tumor types. OS tumors with any different grades rapidly progresses with the high malignancy while chondroma is a kind of the benign tumors. Here, our main purpose is to show that the lower expression of RCAN1.4 was strongly correlated with the malignancy of the tumor. We constructed the RCAN1 knockout plasmid and verified the efficiency of knockout RCAN1.4 without affecting RCAN1.1 expression by western blot. In view that RCAN1.4 protein level was much higher than RCAN1.1 in human OS cell lines, we just focused on the function of RCAN1.4. Because the primary calcineurin-binding portion of RCAN1.4 included two independent motifs, ELHA and PxIxxT, that interacted with calcineurin to suppress nuclear translocation of NFATs by inhibiting its dephosphorylation [30], we detected the increasing activity of calcineurin and nuclear translocation of NFATc1 in knockdown RCAN1.4 cells. In addition, we demonstrated that RCAN1.4 overexpression could inhibit the activity of calcineurin and tumor growth and metastasis in human osteosarcoma cells. Together, these data indicated that RCAN1.4 was an endogenous suppressor in osteosarcoma, though we did not detect the intracellular calcium level in RCAN1.4-deficient cells. A recent study showed 143B cells with a high metastatic potential [31], but we detected the RCAN1.4 at high levels in 143B cells. We did not find the 143B with the high expression of RCAN1.4 had a high metastatic potential. We did not detect its lung metastases in subcutaneous xenograft tumor models. However, using the OS/chondromas specimens, we indeed found the negative relationship between the RCAN1.4 expression and malignancy of the tumor.
It was reported that blockade of store-operated Ca2+ channels involved down-regulation of NFATc1 in osteosarcoma cells [32]. Activated NFATc1 was strongly associated with osteosarcoma cell growth [33]. The calcineurin/NFAT signaling pathway is critical for many physiological functions, such as lung morphogenesis, cardiac morphogenesis, immune responses, epithelial stem cell maintenance, neural crest diversification, and axon outgrowth [34]. NFATc1 and its upstream activator calcineurin are essential to the tumorigenic and metastatic properties of glioblastoma [35]. The targets of NFAT signaling are largely cytokines, growth factors and their receptors, proteins involved in cell–cell interactions, and microRNAs [35], [36]. Here, we found there were significantly increasing nuclear translocation of NFATc1 and higher expression of cyclin D1 and more MMP2 or MMP9–positive cells in knockdown RCAN1.4 cells, indicating cyclin D1 and MMPs might be the target genes of NFATc1. In addition, the ectopic activation of NFATc1 in tumor cells upregulates the expression of oncogenes c-MYC and STAT3, which are a vital transcription factor for tumor cell growth and metastasis [37].
RANK from osteosarcoma cells increased the expression of the anti-apoptotic oncogene c-Flip and the pro-proliferation oncogene c-Myc [24], [38]. And a positive effect of the RANK-RANKL system in cancer development had previously been reported in diverse organs.[39] There was a vicious cycle between tumor cells and RANKL-dependent osteoclasts [40], [41]. To a great degree, all the cells that express elements of the RANKL/RANK/OPG triad in the tumor microenvironment might participate in the vicious cycle and affect tumor manners. So a potential therapeutic strategy was urgent to disrupt this cycle by targeting osteoclast differentiation as well as tumor cell proliferation [42]. Here, we showed that conditioned medium (20%) derived from RCAN1.4-deficient cells significantly promoted osteoclast differentiation, compared with vector cells. And we further discovered the increased RANKL secretion in the RCAN1.4-deficient cells inducing osteoclastogenesis. Enhanced osteoclast differentiation indeed raised the capacity of osteosarcoma metastasis [39]. Because RCAN1.4 was an endogenous inhibitor of calcineurin/NFAT pathway promoting the osteoclastogenesis [43], we could regard RCAN1.4 as a target to inhibit both osteoclastogenesis and tumor metastasis. The exact mechanism was still needed to further clarify RCAN1.4 function in tumor metastasis.
In conclusion, we found out that RCAN1.4 loss promoted cancer cell proliferation, invasion and migration in vitro, as well as enhanced tumorigenesis and metastasis in the subcutaneous xenograft models and intravenous metastasis models. Further, we confirmed the positive role of the calcineurin/NFAT pathway in osteosarcoma development and metastasis. Moreover, we also uncovered the effect of RCAN1.4-deficient osteosarcoma cells on the RANKL-dependent osteoclastogenesis. These results indicated that RCAN1.4 acted as a potential therapeutic target for osteosarcoma.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
This work was supported by the Zhejiang Provincial Natural Science Foundation of China (LQ19H060005) and the Natural Science Foundation of China (81871796).
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.jbo.2021.100383.
Contributor Information
Bao Huang, Email: huangbao@zju.edu.cn.
Zenghui Jiang, Email: jiangzenghui@zju.edu.cn.
Saishuang Wu, Email: 3150103259@zju.edu.cn.
Hao Wu, Email: harwu@ucdavis.edu.
Xuyang Zhang, Email: jiurizhang@zju.edu.cn.
Jian Chen, Email: chenjian-bio@zju.edu.cn.
Fengdong Zhao, Email: zhaofengdong@zju.edu.cn.
Junhui Liu, Email: ljhzju@zju.edu.cn.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
Supplementary figure 1.
References
- 1.Program S. National Cancer Institute; 1999. Cancer incidence and survival among children and adolescents: United States SEER Program, 1975-1995. [Google Scholar]
- 2.Gianferante D.M., Mirabello L., Savage S.A. Germline and somatic genetics of osteosarcoma – connecting aetiology, biology and therapy. Nat. Rev. Endocrinol. 2017;13(8):480–491. doi: 10.1038/nrendo.2017.16. [DOI] [PubMed] [Google Scholar]
- 3.Daw N.C., Chou A.J., Jaffe N. Recurrent osteosarcoma with a single pulmonary metastasis: a multi-institutional review. Br. J. Cancer. 2015;112(2):278–282. doi: 10.1038/bjc.2014.585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Davies K.J., Ermak G., Rothermel B.A. Renaming the DSCR1/Adapt78 gene family as RCAN: regulators of calcineurin. FASEB J. 2007;21(12):3023–3028. doi: 10.1096/fj.06-7246com. [DOI] [PubMed] [Google Scholar]
- 5.Fuentes J.J., Genescà L., Kingsbury T.J. DSCR1, overexpressed in Down syndrome, is an inhibitor of calcineurin-mediated signaling pathways. Hum. Mol. Genet. 2000;9(11):1681–1690. doi: 10.1093/hmg/9.11.1681. [DOI] [PubMed] [Google Scholar]
- 6.Rothermel B., Vega R.B., Yang J., Wu H., Bassel-Duby R., Williams R.S. A protein encoded within the Down syndrome critical region is enriched in striated muscles and inhibits calcineurin signaling. J. Biol. Chem. 2000;275(12):8719–8725. doi: 10.1074/jbc.275.12.8719. [DOI] [PubMed] [Google Scholar]
- 7.Fuentes J.-J., Pritchard M.A., Planas A.M., Bosch A., Ferrer I., Estivill X. A new human gene from the Down syndrome critical region encodes a proline-rich protein highly expressed in fetal brain and heart. Hum. Mol. Genet. 1995;4(10):1935–1944. doi: 10.1093/hmg/4.10.1935. [DOI] [PubMed] [Google Scholar]
- 8.Wang C., Saji M., Justiniano S.E. RCAN1-4 is a thyroid cancer growth and metastasis suppressor. JCI Insight. 2017;2(5) doi: 10.1172/jci.insight.90651. e90651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jin H., Wang C., Jin G. Regulator of calcineurin 1 gene isoform 4, downregulated in hepatocellular carcinoma, prevents proliferation, migration, and invasive activity of cancer cells and growth of orthotopic tumors by inhibiting nuclear translocation of NFAT1. Gastroenterology. 2017 doi: 10.1053/j.gastro.2017.05.045. [DOI] [PubMed] [Google Scholar]
- 10.Kim D.H., Park S., Kim H. Tumor-derived exosomal miR-619-5p promotes tumor angiogenesis and metastasis through the inhibition of RCAN1.4. Cancer Lett. 2020;475:2–13. doi: 10.1016/j.canlet.2020.01.023. [DOI] [PubMed] [Google Scholar]
- 11.Song Z., Cao Q., Ruan H. RCAN1.4 acts as a suppressor of cancer progression and sunitinib resistance in clear cell renal cell carcinoma. Exp. Cell Res. 2018;372(2):118–128. doi: 10.1016/j.yexcr.2018.09.017. [DOI] [PubMed] [Google Scholar]
- 12.Xy P., Hm Y., Wang L. Methylation of RCAN1.4 mediated by DNMT1 and DNMT3b enhances hepatic stellate cell activation and liver fibrogenesis through Calcineurin/NFAT3 signaling. Theranostics. 2019;9(15):4308–4323. doi: 10.7150/thno.32710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Alghanem A.F., Wilkinson E.L., Emmett M.S. RCAN1.4 regulates VEGFR-2 internalisation, cell polarity and migration in human microvascular endothelial cells. Angiogenesis. 2017;20(3):341–358. doi: 10.1007/s10456-017-9542-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Villahoz S., Yunes-Leites P.S., Mendez-Barbero N. Conditional deletion of Rcan1 predisposes to hypertension-mediated intramural hematoma and subsequent aneurysm and aortic rupture. Nat. Commun. 2018;9(1):4795. doi: 10.1038/s41467-018-07071-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yun Y., Zhang Y., Zhang C. Regulator of calcineurin 1 is a novel RNA-binding protein to regulate neuronal apoptosis. Mol. Psychiatry. 2021;26(4):1361–1375. doi: 10.1038/s41380-019-0487-0. [DOI] [PubMed] [Google Scholar]
- 16.Minami T., Jiang S., Schadler K. The calcineurin-NFAT-angiopoietin-2 signaling axis in lung endothelium is critical for the establishment of lung metastases. Cell Rep. 2013;4(4):709–723. doi: 10.1016/j.celrep.2013.07.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mancini Maria, Toker Alex. NFAT proteins: emerging roles in cancer progression. Nat. Rev. Cancer. 2009;9(11):810–820. doi: 10.1038/nrc2735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jin H., Wang C., Jin G. Regulator of calcineurin 1 gene isoform 4, down-regulated in hepatocellular carcinoma, prevents proliferation, migration, and invasive activity of cancer cells and metastasis of orthotopic tumors by inhibiting nuclear translocation of NFAT1. Gastroenterology. 2017;153(3):799–811.e33. doi: 10.1053/j.gastro.2017.05.045. [DOI] [PubMed] [Google Scholar]
- 19.Song Z., Cao Q., Ruan H. 4 acts as a suppressor of cancer progression and sunitinib resistance in clear cell renal cell carcinoma. Exp. Cell Res. 2018;372(2):118–128. doi: 10.1016/j.yexcr.2018.09.017. [DOI] [PubMed] [Google Scholar]
- 20.Ma N., Shen W., Pang H. The effect of RCAN1 on the biological behaviors of small cell lung cancer. Tumor Biol. 2017;39(6) doi: 10.1177/1010428317700405. 1010428317700405. [DOI] [PubMed] [Google Scholar]
- 21.Baek K.-H., Zaslavsky A., Lynch R.C. Down's syndrome suppression of tumour growth and the role of the calcineurin inhibitor DSCR1. Nature. 2009;459(7250):1126–1130. doi: 10.1038/nature08062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Huang B., Wang J., Zhang X. Administration of SB239063 Ameliorates Ovariectomy-Induced Bone Loss via Suppressing Osteoclastogenesis in Mice. Front. Pharmacol. 2019;10:900. doi: 10.3389/fphar.2019.00900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jie Z., Xie Z., Xu W. SREBP-2 aggravates breast cancer associated osteolysis by promoting osteoclastogenesis and breast cancer metastasis. Biochim. Biophys. Acta, Mol. Basis Dis. 2019;1865(1):115–125. doi: 10.1016/j.bbadis.2018.10.026. [DOI] [PubMed] [Google Scholar]
- 24.Navet B., Ando K., Vargas-Franco J.W. The Intrinsic and Extrinsic Implications of RANKL/RANK Signaling in Osteosarcoma: from Tumor Initiation to Lung Metastases. Cancers. 2018;10(11):398. doi: 10.3390/cancers10110398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Marino S., Carrasco G., Li B. JZL184, A Monoacylglycerol Lipase Inhibitor, Induces Bone Loss in a Multiple Myeloma Model of Immunocompetent Mice. Calcif. Tissue Int. 2020 doi: 10.1007/s00223-020-00689-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Satge D., Seidel M.G. The Pattern of Malignancies in Down Syndrome and Its Potential Context With the Immune System. Front. Immunol. 2018;9:3058. doi: 10.3389/fimmu.2018.03058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jj F., Ma P., Genomics E.X.J. Genomic organization, alternative splicing, and expression patterns of the DSCR1 (Down syndrome candidate region 1) Gene. 1997;44(3):358–361. doi: 10.1006/geno.1997.4866. [DOI] [PubMed] [Google Scholar]
- 28.Chan B., Greenan G., McKeon F., Ellenberger T. Identification of a peptide fragment of DSCR1 that competitively inhibits calcineurin activity in vitro and in vivo. Proc. Natl. Acad. Sci. U.S.A. 2005;102(37):13075–13080. doi: 10.1073/pnas.0503846102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Davies K.J., Ermak G., Rothermel B.A. Renaming the DSCR1/Adapt78 gene family as RCAN: regulators of calcineurin. FASEB J. 2007;21(12):3023–3028. doi: 10.1096/fj.06-7246com. [DOI] [PubMed] [Google Scholar]
- 30.Rao A., Luo C., Hogan P.G. Transcription factors of the NFAT family regulation and function. Annu. Rev. Immunol. 1997;15(1):707–747. doi: 10.1146/annurev.immunol.15.1.707. [DOI] [PubMed] [Google Scholar]
- 31.Mohseny A.B., Machado I., Cai Y. Functional characterization of osteosarcoma cell lines provides representative models to study the human disease . Lab. Investig. 2011;91(8):1195–1205. doi: 10.1038/labinvest.2011.72. [DOI] [PubMed] [Google Scholar]
- 32.Zang Jie, Zuo Dongqing, Shogren Kristen L. STIM1 expression is associated with osteosarcoma cell survival. Chin. J. Cancer Res. 2019;31(1):203–211. doi: 10.21147/j.issn.1000-9604.2019.01.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Giuliani A.L., Colognesi D., Ricco T. Trophic activity of human P2X7 receptor isoforms A and B in osteosarcoma. PLoS ONE. 2014;9(9) doi: 10.1371/journal.pone.0107224. e107224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Crabtree G.R., Schreiber S.L. SnapShot Ca2+-calcineurin-NFAT signaling. Cell. 2009;138(1) doi: 10.1016/j.cell.2009.06.026. 210, 210 e211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Song Yifu, Jiang Yang, Tao Dongxia. NFAT2-HDAC1 signaling contributes to the malignant phenotype of glioblastoma. Neuro Oncol. 2020;22(1):46–57. doi: 10.1093/neuonc/noz136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mognol G.P., González-Avalos E., Ghosh S. Targeting the NFAT:AP-1 transcriptional complex on DNA with a small-molecule inhibitor. PNAS. 2019;116(20):9959–9968. doi: 10.1073/pnas.1820604116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Manda K.R., Tripathi P., Hsi A.C. NFATc1 promotes prostate tumorigenesis and overcomes PTEN loss-induced senescence. Oncogene. 2016;35(25):3282–3292. doi: 10.1038/onc.2015.389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Stine Z.E., Walton Z.E., Altman B.J., Hsieh A.L., Dang C.V. MYC, Metabolism, and Cancer. Cancer Discovery. 2015;5(10):1024–1039. doi: 10.1158/2159-8290.CD-15-0507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Renema N., Navet B., Heymann M.F., Lezot F., Heymann D. RANK-RANKL signalling in cancer. Biosci. Rep. 2016;36(4) doi: 10.1042/BSR20160150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lamoureux F., Baud'huin M., Rodriguez Calleja L. Selective inhibition of BET bromodomain epigenetic signalling interferes with the bone-associated tumour vicious cycle. Nat. Commun. 2014;5(1):3511. doi: 10.1038/ncomms4511. [DOI] [PubMed] [Google Scholar]
- 41.Lamoureux F., Richard P., Wittrant Y. Therapeutic relevance of osteoprotegerin gene therapy in osteosarcoma: blockade of the vicious cycle between tumor cell proliferation and bone resorption. Cancer Res. 2007;67(15):7308–7318. doi: 10.1158/0008-5472.CAN-06-4130. [DOI] [PubMed] [Google Scholar]
- 42.Walsh M.C., Choi Y. Biology of the RANKL-RANK-OPG System in Immunity, Bone, and Beyond. Front. Immunol. 2014;5:511. doi: 10.3389/fimmu.2014.00511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bai H., Zhu H., Yan Q. TRPV2-induced Ca(2+)-calcineurin-NFAT signaling regulates differentiation of osteoclast in multiple myeloma. Cell Commun Signal. 2018;16(1):68. doi: 10.1186/s12964-018-0280-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.