Abstract
Phosphatidylinositol-specific phospholipase Cγ2 (PLCγ2) is a critical signaling molecule activated downstream from a variety of cell surface receptors that contain an intracellular immunoreceptor tyrosine-based activation motif. These receptors recruit kinases such as Syk, BTK, and BLNK to phosphorylate and activate PLCγ2, which then generates 1D-myo-inositol 1,4,5-trisphosphate and diacylglycerol. These well-known second messengers are required for diverse membrane functionality including cellular proliferation, endocytosis, and calcium flux. As a result, PLCγ2 dysfunction is associated with a variety of diseases including cancer, neurodegeneration, and immune disorders. The diverse pathologies associated with PLCγ2 are exemplified by distinct genetic variants. Inherited mutations at this locus cause PLCγ2-associated antibody deficiency and immune dysregulation, in some cases with autoinflammation. Acquired mutations at this locus, which often arise as a result of BTK inhibition to treat chronic lymphocytic leukemia, result in constitutive downstream signaling and lymphocyte proliferation. Finally, a third group of PLCγ2 variants actually has a protective effect in a variety of neurodegenerative disorders, presumably by increased uptake and degradation of deleterious neurological aggregates. Therefore, manipulating PLCγ2 activity either up or down could have therapeutic benefit; however, we require a better understanding of the signaling pathways propagated by these variants before such clinical utility can be realized. Here, we review the signaling roles of PLCγ2 in hematopoietic cells to help understand the effect of mutations driving immune disorders and cancer and extrapolate from this to roles which may relate to protection against neurodegeneration.
Keywords: phospholipase C, cancer, neurodegeneration, immunodeficiency, inflammation
Abbreviations: Aβ, amyloid β; AD, Alzheimer’s disease; APLAID, PLCγ2-associated antibody deficiency and immune dysregulation with autoinflammation; BCR, B cell receptor; BM, bone marrow; Cbl, c-Casitas B-lineage lymphoma; CLL, chronic lymphocytic leukemia; cSH2, C-terminal Src homology 2; CVID, common variable immunodeficiency; DAG, diacylglycerol; DCs, dendritic cells; DLBCL, diffuse large B cell lymphoma; EBL, endemic Burkitt lymphoma; EBV, Epstein–Barr virus; Ig, immunoglobulin; IP3, inositol 1,4,5-trisphosphate; PIP2, phosphatidylinositol-4,5-bisphosphate; PLAID, PLCγ2-associated antibody deficiency and immune dysregulation; PLCγ2, phosphatidylinositol-specific phospholipase Cγ2; RA, rheumatoid arthritis; RANKL, receptor activator of nuclear factor kappa-B ligand; ROS, reactive oxygen species; SLE, systemic lupus erythematosus; SLP-76, SH2 domain–containing leukocyte protein of 76 kDa; TB, tuberculosis; TCR, T cell receptor
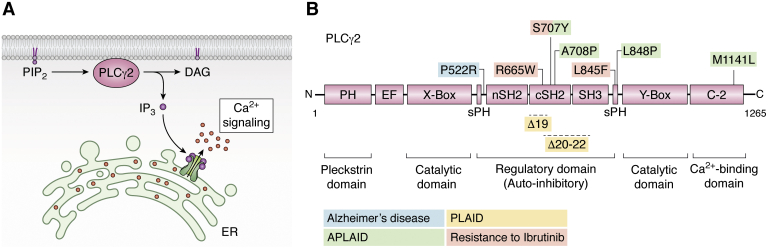
Phosphatidylinositol-specific phospholipase Cγ2 (PLCγ2) belongs to a group of intracellular enzymes that cleave the membrane phospholipid phosphatidylinositol-4,5-bisphosphate (PIP2) into diacylglycerol (DAG) and inositol 1,4,5-trisphosphate (IP3) (1) (Fig. 1A). IP3 and DAG then facilitate secondary signaling for membrane-bound immunological and growth factor receptors (1, 2), including activation of PKC and intracellular calcium release (2, 3). PLCγ2 was first cloned in a functional form, including the promoter region, in the hematopoietic Raji cell line (4). PLCγ2 has since been revealed to be involved in a plethora of normal cellular signaling functions in the majority of cells in the hematopoietic system, bone marrow (BM) niche, and bone remodeling as well as various other roles in tissue development and function.
Figure 1.
Activity, structural domains, and known mutations of PLCγ2. A, PLCγ2 belongs to a group of intracellular enzymes that cleave the membrane phospholipid phosphatidylinositol-4,5-bisphosphate (PIP2) to diacylglycerol (DAG) and inositol 1,4,5-trisphosphate (IP3), resulting in increased calcium signaling. B, PLAID-causing genomic deletions (Δ19 and Δ20–22) and APLAID-associated somatic mutations are located within the regulatory domain (S707Y, L848P, A708P) or in the C2 domain (M1141L) of PLCγ2. Acquired mutations as a result of BTK inhibition (S707Y, L845F) and the protective P522R variant in Alzheimer’s disease are found in the regulatory domain of PLCγ2. APLAID, PLCγ2-associated antibody deficiency and immune dysregulation with autoinflammation; C, carboxyl terminus; N, amino terminus; PLAID, PLCγ2-associated antibody deficiency and immune dysregulation; PLCγ2, phosphatidylinositol-specific phospholipase Cγ2.
Structurally, human PLCγ2 is characterized by a multidomain insert between the X box and Y box, which consists of a split pleckstrin homology domain, N-terminal SH2 domain, C-terminal Src homology 2 (cSH2) domain, and SH3 domain (Fig. 1B). Interaction between the cSH2 domain and residues surrounding the catalytic active site results in autoinhibition of PLCγ2. Upon Y759 phosphorylation, the cSH2 domain is removed from the catalytic active site and interacts with phosphorylated residue, allowing the substrate PIP2 access to the active site (4). Biochemically, PLCγ2 is downstream of many signaling molecules including the kinases Syk Btk, and Lyn, the guanine nucleotide exchange factors Vav, and the GTPase Rac2 that will be discussed in this review. The split pleckstrin homology domain of PLCγ2 (Fig. 1B) is essential and sufficient for activation by Rac2, with the crucial amino acids at the PLCγ2–Rac2 interface determined by NMR spectroscopy (5) and protein crystallization (6). Rac2-mediated PLCγ2 activation requires stable translocation to the plasma membrane, and it appears that this proximity to the membrane releases PLCγ2 from autoinhibition (7).
PLCγ2 is also a known risk factor and an important driver in a multitude of diverse disease circumstances including those with an immunological basis such as inflammation, autoimmunity, immunodeficiency, and allergy, as well as in hematological malignancies (Fig. 2). Moreover, research has also suggested PLCγ2 may have a role in Alzheimer’s disease (AD) and some solid cancers. However, the links between PLCγ2’s critical biochemical functions and its diverse biological impacts are poorly understood. Gaining insight into relevant pathways will be important in considering causes of and treatments for a diverse range of diseases. In this review, we describe the current understanding of PLCγ2’s roles in the adaptive and innate immune systems and explore open questions in the field as to how these roles relate to disease pathology. We hope that this is a timely and useful review for researchers interested in learning about how their pathway of interest may be a key avenue involved in unexpected pathological situations.
Figure 2.
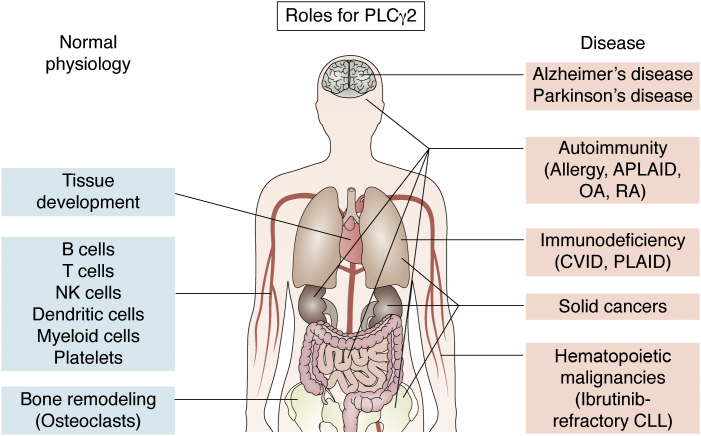
Organs and tissues influenced by PLCγ2 in health and disease. An overview of the involvement of PLCγ2 at various anatomical sites in both normal physiology (left) and disease settings (right) in humans. PLCγ2, phosphatidylinositol-specific phospholipase Cγ2.
PLCγ2 in the adaptive immune system
PLCγ2 plays a fundamental role in development of mature B cells as a component of the signal transduction cascade that occurs when a B cell receptor (BCR) is stimulated by its cognate antigen. Early studies of PLCγ2 function, typically conducted in the chicken B cell line DT40, revealed that BCR signaling through PLCγ2 required its association with other signal transduction molecules including Syk and Btk in a complex located in a lipid raft, or signalosome. PLCγ2 would then be phosphorylated at four tyrosines (Y753, Y759, Y1197, and Y1217 (8, 9, 10, 11)). Moreover, when maximally stimulated, Y1217 was capable of a 3-fold greater level of phosphorylation than residues Y753 and Y759 in murine splenic B cells potentially demonstrating a potent and unique function of a specific critical tyrosine residue within PLCγ2 (10). In addition to the production of IP3, DAG, and calcium flux, PLCγ2 activation results in activation of phospholipase D and cell spreading (12, 13, 14, 15, 16, 17), bringing on other vital cell signaling processes such as Erk (18). Studies using DT40 cells showed that Rac2 could also stimulate PLCγ2 to enhance the BCR-induced calcium flux (19). The BCR coreceptor CD19 is also a vital component of the BCR/PLCγ2 signaling complex requiring phosphorylation of its Y391 residue, which is costimulated with the BCR in response to antigen engagement (20). Upon BCR signaling, PLCγ2 also interacts with B cell adaptor protein with ankyrin repeats (21). PLCγ2 immunoprecipitates with CD19, in a signaling complex with Lyn, Vav, Grb2, and p85 of PI3K, potentially via their SH2 domains, suggesting a tight multimeric association of these molecules and with the cell membrane (22).
In addition to this crucial role in BCR signaling, PLCγ2 is also required for the signaling of CD72, a regulatory molecule important in B cell development, although it is thought this process relies on Btk instead of Syk (23). The use of Btk- or Blnk-deficient mice showed their activation was necessary for PLCγ2 signaling (24). BLNK was also noted as being specifically required in human B cells in vitro for PLCγ2 phosphorylation and resultant calcium flux after BCR stimulation (25).
The functional requirement of PLCγ2-mediated signaling in B cells was first addressed in mice engineered to lack PLCγ2 enzymatic activity, through the replacement of the second exon (encoding the enzymatic function) with a neomycin cassette (24). Unlike the germline deletion of PLCγ2 that results in embryonic lethality, mice lacking PLCγ2 enzymatic activity were viable, but with profound defects in B cell development and function. These mice exhibited reduced mature B cells, disrupted differentiation of pro-B cells, and a lack of B1 B cells (24). As expected, BCR stimulation failed to induce calcium flux or mitogenic stimulation of proliferation in B cells and serum immunoglobulin (Ig) M, G2a, and G3 and T cell–independent antibody production was reduced in the absence of PLCγ2 function (24). Mice were developed where the exon of PLCγ2, bearing the catalytic region containing the PIP2-binding site, was floxed and inducibly deleted in adult mice (26). Indeed, PLCγ2-deficient and inducible PLCγ2−/− mice have been widely shown to lack the ability to respond to antigens (24, 26, 27, 28).
The importance of BLNK to PLCγ2 function during B cell development was revealed through the use of BLNK−/−PLCγ2−/− mice where floxed enzymatic PIP2 domains of PLCγ2 were inducibly deleted (26, 29). BLNK/PLCγ2 double-deficient mice showed a more pronounced defect in early B cell progenitors from around the Pro-B cell stage than in the single KO of BLNK or PLCγ2 (29). This study also showed that PLCγ2 dosage was important as PLCγ2 biochemical function and B cell development were reduced in the PLCγ2 heterozygous mice on a BLNK-deficient background, compared with BLNK−/−PLCγ2+/+ mice (29). It is worth noting that PLCγ2 concentration is not constant through B cell ontogeny, as immature B cells express 3-fold more PLCγ2, as well as BLNK and Btk, leading to enhanced BCR-mediated phosphorylation of PLCγ2 (30). Significantly higher levels of PLCγ2 were also observed in human IgM memory and switched B cells than naïve B cells (31).
The first PLCγ2−/− mouse studies showed a block in B cell maturation from transitional 2 to follicular B cells, with these cells also exhibiting increased levels of BCR-induced apoptosis (28, 32). The importance of PLCγ2 was additionally shown in terms of light-chain loci activation, required for recombination and receptor editing of autoreactive B cells (28). B cells in these PLCγ2−/− mice showed a downregulation of the potent prosurvival protein Bcl-2 and impaired BCR-induced expression of A1, another prosurvival Bcl2 family member (32). Bcl-2 overexpression however did stop BCR-induced apoptosis in all splenic B cells and partially restored numbers of follicular B cells (32). The incomplete nature of the rescue of PLCγ2 deficiency by Bcl2 overexpression was due to Bcl2 not being able to compensate for an additional function of PLCγ2 in mediating B cell–activating factor receptor signaling, which is also necessary for B cell survival and maturation (33).
Broader regulation of PLCγ2 function may involve several transcription factors, such as Ikaros, which was shown to be essential in generating B cells, but not T cells, in mice (34, 35, 36). Deletion of Ikaros in the DT40 cell line also showed its absence impaired BCR signaling via PLCγ2 and associated calcium flux, suggesting a role of Ikaros in functional control of PLCγ2 (37). In addition, transcription factors, NFAT and AP-1, are also induced after BCR stimulation in B cells mediated by PLCγ2 (38). Enzymatic deficiency of PLCγ2 also downregulated BCR-induced upregulation of IRF4 and IRF8, both vital transcription factors for rearrangement process of λ and κ light chains (28). Collectively, these studies reveal that PLCγ2 is an important component in several immunological pathways resulting in the induction of key transcription factors necessary for subsequent immunological functions.
Several biochemical pathways and processes were shown to negatively regulate PLCγ2 activity and the resulting calcium flux after BCR engagement. These include RasGRP3 (39, 40), Bam32 (41), hematopoietic adaptor protein Dok-3 (downstream of kinase-3) with Grb2 (42), Themis2, an adaptor protein with no known enzymatic activity, via constitutive binding to Lyn, Grb2, and PLCγ2 to mediate its function (43), BLNK via impairing its association with the C2-phosphotyrosine domain of PLCγ2 (44), and SFRP2 (secreted frizzled-related protein 2), a potential Wnt inhibitor (45). BCR signaling, via Syk, BLNK, PLCγ2, and NF-κB, is also inhibited via BTLA (B and T lymphocyte attenuator) associating with the BCR and via binding its known ligand, herpes virus entry mediator and activating SHP-1 (46). Activation of PLCγ2 and subsequent calcium flux is also negatively regulated by the E3-ubiquitin protein ligase isoforms c-Casitas B-lineage lymphoma (Cbl) and Cbl-b (47, 48, 49, 50). B cell double c-Cbl and Cbl-b KO studies in mice resulted in a systemic lupus erythematosus (SLE)-like autoimmune disease (50). Engagement of the BCR in these mice resulted in enhanced PLCγ2 signaling, including Syk and calcium flux, but reduced BLNK and no detectable PLCγ2 ubiquitination, a known function of Cbl (50, 51). Examining the pre-B cells of mice lacking both c-Cbl and BLNK, compared with those with one allele of c-Cbl, but still in the absence of BLNK, revealed elevated levels of Btk and PLCγ2 (51). The plethora of negative regulators suggested to help control BCR/PLCγ2/calcium flux signaling within B cells underscores the importance of tight regulation of this cellular process in mediating immune responses, without which would very likely further contribute to development of inflammatory disease.
Aside from BCR engagement, there are alternative signaling pathways initiated through extracellular immunological receptors that also utilize PLCγ2 to function, which include CD38 (16), CD40 (52), IL-4R (53, 54, 55) and the LPS/TLR4/MyD88 pathway, the latter of which induces ERK and NK-κB phosphorylation in MEKs in a PI3K-independent fashion (56). CD86 engagement was also observed to signal via PLCγ2 inducing Oct-2 expression (57, 58). TGF-βR stimulation has also been shown to inhibit PLCγ2 function in B cells (59). These alternative extracellular receptor signaling pathways reveal the utility of PLCγ2 in delivering important cellular messages for B cells to mediate a range of immunological functions.
The role of PLCγ2 in the immunological functions of T cells is comparatively more limited than that of B cells, but it still serves important functions in certain cellular contexts. Inducible KO mice showed T cell development and T cell receptor (TCR)-mediated proliferation were normal in the absence of PLCγ2 (24, 26), with development instead depending on the closely related family member PLCγ1 (60). However, PLCγ2 is required in primary T cells, in association with a linker for activated T cells and SH2 domain–containing leukocyte protein of 76 kDa (SLP-76), when signaling upon TCR stimulation, with PLCγ1/2 double-mutant cells showing impaired TCR signal transduction with regard to calcium flux and subsequent Erk activation compared with the PLCγ1 KO alone (61). Double deficiency for PLCγ1/2 also has a more severe T cell developmental phenotype, particularly in the transition of CD4/CD8 double-positive thymocytes to the CD4 or CD8 lineage (61). The calpain-calpastatin system is a fundamental part of membrane fusion events mediated by the proteolysis of amyloid precursor proteins which is an important process for effective responses to antigenic challenges. In resting human T cells, the calpain-calpastatin system, specifically calpain inhibition, also played a role for PLCγ2 phosphorylation and the subsequent calcium flux, along with the phosphorylation of p56Lck and NF-κB (62).
In summary, PLCγ2 plays a fundamental role in B cell development affecting the survival of mature B cells and antibody production, as well as being an absolutely necessary and immediately proximal component of the BCR signaling cascade. While PLCγ1 plays a greater role in T cells, PLCγ2 is still important in terms of TCR signaling and the calpain-calpastatin system and when combined with PLCγ1 deletion. Considering these vital roles for PLCγ2 in the adaptive immune system, it is of little surprise its dysregulation or malfunction is also then of crucial importance in a number of disease settings of an immunological and hematological nature.
PLCγ2 and immune deficiency
Given its prominent role in the adaptive immune system, PLCγ2 has been implicated in manifestations of immunodeficiency, including both the immunodeficiency syndrome, PLCγ2-associated antibody deficiency and immune dysregulation (PLAID) and common variable immunodeficiency (CVID). PLAID is specifically driven by a gain-of-function amino acid mutation in the PLCγ2 gene (63, 64), whereas CVID is much more genetically diverse with 2% of published cases of CVID estimated to be due to mutations in the PLCG2 gene (65). Patients with PLAID typically exhibit symptoms of cold urticaria, a form of cold temperature-induced hives, beginning in infancy, but otherwise shares many of the common clinical manifestations of CVID such as granulomatous disease, allergy, autoimmune disease, and an abnormally low concentration of serum IgG with recurrent infections (63, 66). The cellular infiltrate of the skin granulomas observed in patients with PLAID is neutrophilic and monocytic in nature, as a result of leukocyte hyperactivation, making it histopathologically very similar to that seen in CVID (67). Chemotactic ability of neutrophils from patients with PLAID was also noted to be defective in vitro (67). Patients with PLAID also typically have suboptimal numbers of NK cells, low serum IgM and IgA, weak vaccination responses, and low frequency of circulating switched memory B cells (68).
The biochemical mechanism behind the PLAID phenotype may be at least partially explained by examining the role of the cSH2 domain of PLCγ2, which is affected in patients with PLAID (69). In vitro studies revealed that after BCR signaling, the cSH2 domain of PLCγ2, coupled with poor phosphorylation of Syk, Btk, and BLNK, stabilized the early signaling complex (69). This in turn resulted in reduced clustering with the BCR and impaired downstream signaling (69). The authors of this study concluded that the impaired cellular movement of the antigen-engaged BCR may be responsible, at least in part, for the PLAID syndrome through reduction of specific antigen engagement by T and B cells that are required to activate and differentiate B cells allowing antibody secretion and formation of B cell memory (69). A common symptom of patients with PLAID is cold-induced urticaria, which may also be linked to mutations in the SH2 region of PLCγ2 as the lack of that region resulted in over a 100-fold rapid increase in activation status after only a few degrees Celsius of cooling but was distinct from a lack of autoinhibition (70).
Overall, these studies of CVID and PLAID lay bare the crucial role of PLCγ2 in the etiology of these diseases because of PLCγ2’s underlying role in the vital immunological signaling of lymphoid cells. Further research into these immunological diseases involving PLCγ2 will help reveal much needed therapeutic inventions.
PLCγ2 in the innate immune system
PLCγ2 is also very important in the context of myeloid cells including monocytes, macrophages, NK cells, dendritic cells (DCs), and mast cells often because of its utility in facilitating downstream signaling after FcR engagement. Moreover, PLCγ2 is reported to be involved in essential signaling processes associated with integrin activation and signaling downstream of critical myeloid receptors including cytokine receptors and TLRs. This signaling drives subsequent antibacterial functions including degranulation and production of reactive oxygen species (ROS) and inflammatory responses.
In monocytes, macrophages, and mast cells, PLCγ2 is activated through crosslinking of the FcεR and FcγR (71). While PLCγ2 KO mice have normal numbers of myeloid cells, and mast cells exhibit normal MAPK activation and cytokine mRNA levels, they show impaired FcεR-stimulated calcium flux, IP3 production, degranulation, and inflammatory cytokine release (71). FcγR meanwhile is normal in macrophages and monocytes, but PLCγ2-deficient mice are resistant to IgE-mediated cutaneous inflammatory skin reaction (71). Macrophages signaling through FcγRI and FcγRIII express both SLP-76 and BLNK, which can phosphorylate PLCγ2 (72). PLCγ2 is also essential in neutrophils for β-2 integrin and FcγR-mediated effects including production of ROS, degranulation, and cell spreading (73). PLCγ2 was also shown to be necessary for ROS production in the neutrophil response to inflammatory microcrystals (74). Research exploring the CD16b (FcγRIII) inhibitor LFM-A13 in human neutrophils also revealed that CD16b signals through Btk/PLCγ2 and associated calcium flux (75), although PLCγ2-deficient mouse neutrophils respond normally to agonists including chemokines, bacterial formyl peptides, TLR ligands, and proinflammatory cytokines (73).
The Fms (M-CSF) receptor, essential for macrophage development, also associates with PLCγ2, leading to its activation, resulting in PI3K activation, thus showing a potential role in myeloid cell differentiation (76, 77). Blocking of either Src family kinases (78) or PLCγ2 directly showed it was required downstream of M-CSF signaling to facilitate human monocyte differentiation (79).
In addition to FcR signaling requiring PLCγ2 for efficient ROS production in murine neutrophils, PLCγ2 is also essential for postintegrin signaling induction of ROS, as well as being critical for the neutrophil-dependent host defense against bacterial infections in vivo (80). Murine macrophages lacking PLCγ2 are also defective in the pattern recognition receptor for fungal cell walls, dectin-2 signaling in response to fungal infections as highlighted by reduced inflammatory cytokine responses, NF-κB and MAPK signaling, ROS, and clearance of fungal infections in vivo (81). PLCγ2 can also be exploited by some intracellular bacteria, such as Ehrlichia chaffeensis in the monocytic THP-1 cell line, where the recruitment and activation of PLCγ2 is required to maintain stable calcium levels to facilitate intracellular infections (82). PLCγ2 was also found to be phosphorylated (at Y1217) by Mycobacterium tuberculosis (the causative agent of tuberculosis (TB)) in the macrophage cell lines (83). Knockdown of PLCγ2 enhanced TB uptake and killing via NO and blocked the TB-mediated inhibition of proinflammatory cytokine production including TNF-α and RANTES (83). These studies underscore the importance of PLCγ2 activation for the survival of intracellular bacteria.
The use of BM-derived macrophages and DCs from PLCγ2 KO mice showed that PLCγ2 was required after peptidoglycan/TLR2 and LPS/TLR4 stimulation to induce normal levels of TNF-α and IL-6 (84). siRNA and inhibitor studies in a macrophage cell line further highlighted the requirement of PLCγ2 in LPS-induced TLR4 signaling in terms of IP3 production calcium flux and endocytosis, as well as downregulation of IRF3, but with the increase of NF-κB signaling (85). The activity of both PLCγ2 and PLCγ1 is triggered in primary macrophages in response to titanium wear particles (generated as a consequence of the degradation of titanium arthritic joint replacements) in vitro, suggesting an advantage to inhibiting PLCγ2 in the context of joint replacement (86). PLCγ2 also has a role in neutrophil recruitment via Btk in regulating E-selectin–mediated integrin activation, which facilitates leukocyte rolling and infiltration into inflamed tissues, and subsequent PI3Kγ pathways (87). Indeed, PLCγ2 phosphorylation, along with Syk, Atk, and p38 MAPK, is required for successful E-selectin–mediated slowing of leukocyte rolling and subsequent transmigration (88). DCs also use PLCγ2 for signaling of major histocompatibility complex (89), integrin receptors, TLRs, and the dectin-1 pathways in a manner similar to macrophages regulating DC migration (90, 91) and antifungal immunity (92).
NK cells also use PLCγ2 during their development and to elicit various cellular functions including CD69 signaling, secretion of cytotoxic granules, response to infection, and tumor immunosurveillance. CD69-mediated cytotoxicity of human NK cells has been shown to signal through Syk/Src family tyrosine kinase and PLCγ2/Vav1 (93). Indeed, PLCγ2 is essential for murine NK cell cytotoxic function as it is required for cytotoxic granule secretion and calcium flux (94, 95). This lack of cytotoxicity in the absence of PLCγ2 in NK cells resulted in the failure of NK cell–mediated rejection of major histocompatibility complex I–negative lymphoma cells and NK cell–mediated control CMV infection in vivo (94, 95). The PLCγ2-deficient mouse also revealed a defect in lineage-committed NK precursor cell expression of Ly49 receptors, which impaired the terminal maturation of NK cells (96).
A number of studies also noted that PLCγ2 is required for osteoclastogenesis and within the BM niche. Using colony assays and a PLCγ2 inhibitor in both mouse and human hematopoietic stem and progenitor cells, it was found that PLCγ2 is responsible for IL-3 and GM-CSF-induced signaling through the resultant calcium flux, IP3 production, and subsequent MEK/ERK activation (97). In megakaryocytes, PLCγ2 is also a crucial downstream signaling molecule, along with Syk and SLP-76, for the surface protein CLEC-2 (98). CLEC-2 in turn regulates the production of thrombopoietin that is essentially required for the BM niche and the proper maintenance of hematopoietic stem cells (98).
Receptor activator of nuclear factor kappa-B ligand (RANKL) is a fundamental cytokine governing bone formation and destruction via the RANK receptor expressed on osteocytes. RANKL-induced osteoclastogenesis requires PLCγ2, which controls downstream functions including NFATc1, AP1, and NF-κB (99). In contrast, another study reported that PLCγ2-deficient mice showed impaired RANKL-induced activation of MAPK, p38, and JNK but not ERK and NF-κB, AP-1, and NFATc1 (100). They also noted a failure of PLCγ2-deficient BM macrophage precursors to differentiate into osteoclasts after RANKL stimulation, which could still be rescued with PLCγ2 and not PLCγ1 (100). Moreover, PLCγ2-deficient mouse studies showed that blocking PLCγ2 impedes the early development and function of osteoclasts, thereby preventing bone loss in arthritis (99, 100). This effect was independent of PLCγ1 (99). Another group also observed that PLCγ2 was required in the regulation of integrin-mediated osteoclast cell adhesion migration and resorption of the bone (101). Ephrin A1 reverse signaling, a process responsible for bone resorption, may also be mediated by PLCγ2 (102). These numerous reports give great scope to the incredibly important innate immune functions that require PLCγ2 in their execution and exemplify why the corruption of these processes is of great relevance for inflammatory disease, as now discussed below.
PLCγ2 and allergy
Mast cells are one of the primary mediators in allergic responses. They are activated via engagement of their antigen-specific IgE receptors that in turn release their proinflammatory payload. PLCγ2 is implicated in allergic responses, specifically with regard to the function of mast cells and their crosslinking of FcεRI (IgE-binding receptor) and FcγR and subsequent degranulation (71). Studies of PLCγ2-deficient mice revealed they had normal numbers of mast cells but impaired FcεR-stimulated calcium flux, IP3 generation, degranulation, and cytokine responses despite normal MAPK activation and cytokine mRNA levels (71). The PLCγ2-deficient mice were also resistant to IgE-mediated cutaneous inflammatory skin reactions (71). By way of a potential therapy, dexamethasone was found to inhibit mast cell inflammatory mediators by blocking FcεRI-mediated PI3K activation via downstream signaling molecules Grb2 and PLCγ2, and associated calcium flux, by blocking degranulation and the production of inflammatory cytokines (103). Targeting the transcription factor Zeb2 using siRNA in mast cells revealed it may regulate Btk/PLCγ2, as evident by their decreased expression, which impacted signal transduction via the FcεRI∖Grb2∖PLCγ2 pathway, which was in turn responsible for the production of inflammatory mediators (104). And another study in mast cells, in which the signaling adaptor protein T cell ubiquitin ligand-2 was knocked down, showed it, like SHIP-1, functions as a negative regulator of FcεRI signaling via Syk/PLCγ2 phosphorylation and the resultant degranulation (105). These studies collectively show that targeting PLCγ2 may have benefits for the treatment of diseases associated with an overreactive immune response.
PLCγ2 and autoinflammatory disease
Autoinflammatory diseases are characterized by recurrent episodes of systemic inflammation, skin lesions, and arthritic joints. Understanding the mechanisms behind the role of PLCγ2 in autoinflammatory diseases was enabled by the identification of the Ali5 mice that bear a gain-of-function point mutation resulting in a single amino acid change (D993G) in PLCγ2 resulting in spontaneous inflammation (106). These mice are characterized by an expansion of innate immune cells, T/B cell–independent inflammation, hyperactive calcium flux in their B cells, and formation of autoantibody complexes (106). Further work utilizing N-ethyl-N-nitrosourea alkylating mutagenesis resulted in the creation of the Ali14 mutant mice, in which gene mapping revealed a Y to C missense mutation in PLCγ2 at amino acid position 495 within the SH2 domain (107). These mice exhibited a dominantly inherited disease characterized by spontaneous hind paw swelling/inflammation (107). Hyperactive calcium flux was observed in their B cells, with an abnormally high T:B cell ratio, especially in the peripheral blood, as well as increased serum Ig, cholesterol, and triglycerides (107). Male mice presented with increased IgG1 and IgG2b, whereas serum IgM was elevated in both sexes (107). Ali14 mutant mice also exhibited changes to their body composition with reduced fat mass and a lesser bone mineral content, the latter suggesting an impact on osteoblastic functions (107).
In humans, seven cases of autoinflammation caused by PLCγ2 gain-of-function point mutations, summarized under the acronym APLAID, have been reported (69). These patients exhibit both phenotypic differences and similarities with PLAID, including involvement of the humoral immune (Fig. 3A) and innate system (Fig. 3B) as well as various forms of inflammation (64). Common clinical features in APLAID include early-onset, recurrent blistering skin lesions, pulmonary disease, arthralgia, inflammatory eye and bowel disease, and mild immunodeficiency (64). Laboratory analysis of patients with APLAID showed decreased IgM, IgG, and IgA levels, typically lacking class-switched memory B cells, with normal NK and T cell counts. There are no standard treatment guidelines for APLAID’s management. Patients were refractory to treatment with TNF-α inhibitors and responded partially to the IL-1 receptor antagonist Anakinra (108).
Figure 3.
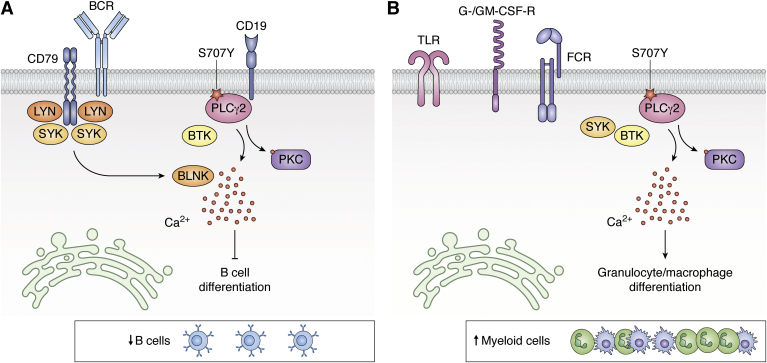
Opposing cellular effects of PLCγ2 mutations in APLAID. In APLAID, PLCγ2 mutations result in (A) stronger BCR signaling including Syk, Btk, and BLNK and elevated calcium responses and therefore negatively impact B cell development and (B) increased production of granulocytes and macrophages, which possibly depends on an increased GM-CSF receptor signaling. APLAID, PLCγ2-associated antibody deficiency and immune dysregulation with autoinflammation; PLCγ2, phosphatidylinositol-specific phospholipase Cγ2.
Mechanistically, the pathogenesis in patients with APLAID is not yet well understood. The inflammation found in APLAID has partially been attributed to the activation of the NLRP3 inflammasome as patient’s blood mononuclear cells secreted increased levels of IL-1β (109). In contrast, whole-blood assays revealed a reduced production of IL-10 and IL-1β after LPS stimulation (110).
Most mutations found in APLAID lie within the autoinhibitory domain (Fig. 1), causing failure of autoinhibition and resulting in constitutive phospholipase activity resulting ultimately in an increased production of both intracellular IP3 and calcium. The C2 domain of PLCγ2, in which the APLAID mutation M1141L resides, has previously been shown to be critical for calcium-dependent translocation of PLCγ2 to the plasma membrane for the sustained influx of extracellular calcium. It has been proposed that as this mutation is proximal to the calcium-binding site, it may result in higher affinity binding, leading to increased IP3 and DAG production. As a consequence, binding of the SH2 domain to BLNK is also stabilized by the C2 domain in a calcium-regulated manner (44).
PLCγ2 and autoimmune disease
PLCγ2 also appears to play a role in the chronic inflammatory disorder, rheumatoid arthritis (RA), as evident by an upregulation of the PLCγ2 gene signature of patient peripheral blood mononuclear cells (111). Indeed, PLCγ2 was revealed to be involved in RA-related gene pathways such as inflammasome activation and both platelet aggregation and activation, which makes it a promising drug target to stem inflammation in RA (111). In a K/BxN serum-transfer murine model of RA, Vav/PLCγ2-deficient mice were protected from inflammation and bone erosion (73, 112). This involvement of PLCγ2 was downstream of integrin stimulation and neutrophil activation, including their spreading and degranulation (112). B cell adaptor protein with ankyrin repeats is a known risk factor in autoimmunity including SLE and systemic sclerosis (113, 114). Indeed, patients with SLE saw clinical benefit through treatment with an anti-CD22 mAb therapy, Epratuzumab (115). The potential mode of action in human B cells by Epratuzumab was observed to be elicited by a downregulation of Syk/PLCγ2 signaling, including calcium flux, after BCR stimulation (116). Thymocyte-expressed, positive selection–associated 1 is critical in thymic T cell development and activation of mast cells via FcεRI, as well as being of importance to B cell functions including proliferation, activation, germinal center formation, and serum antibody production (117). Pathogenic B cells which form in the thymocyte-expressed, positive selection–associated 1 KO mice in a collagen-induced arthritis model were impaired in PLCγ2 function, and associated calcium flux, thereby reducing the severity of their disease in comparison with control mice (117).
Lyn tyrosine kinase autoactivation (gain-of-function) mice, after BCR stimulation, showed constitutive phosphorylation of Syk/PLCγ2, with a resultant increased calcium flux, observed in their resting B cells (118). These circumstances of PLCγ2 hyperactivation led to an increased level of autoantibodies in these mice and ultimately a lethal autoimmune glomerulonephritis (118). Common genetic variants in PLCG2 also link the enzyme to type 1 diabetes (119) and venous thromboembolism (120), which could have a shared autoimmune etiology.
Overall, PLCγ2 clearly exhibits an important role in the manifestation of autoinflammatory disease with hypermorphic PLCγ2 signaling resulting in spontaneous autoinflammatory disease. These PLCγ2 signaling-induced autoimmune diseases produce a range of inflammatory phenotypes in both patients and disease models, typically driven by autoantibodies (with the exception of APLAID) and broad autoimmune cell expansion. These disease situations highlight the potential to develop PLCγ2-modulating therapies that treat immune disease but must be tailored so as to avoid effects on other disease relevant processes such as cancer and neurodegeneration. Next, we explore recent insights and advances in these fields.
PLCγ2 and cancer
The crucial nature of PLCγ2 in both the innate and adaptive immune system is further underscored by its vital roles in hematological malignancy. This is perhaps most evident in chronic lymphocytic leukemia (CLL), but PLCγ2 is also observed to play a role in diffuse large B cell lymphoma (DLBCL), the most common form of non-Hodgkin’s lymphoma, Hodgkin’s lymphoma, myelodysplastic syndrome, endemic Burkitt lymphoma (EBL), MALT-associated gastric lymphoma and multiple myeloma. Finally, PLCγ2 has also been shown to be a relevant part of Epstein–Barr virus (EBV) transformation of B cells.
A powerful tool for validating the roles of oncogenes and tumor suppressors is the Eμ-Myc mice lymphoma model where all mice will succumb to lymphoma. Using this Eμ-Myc lymphomagenesis mouse model in the absence of PLCγ2 demonstrated that PLCγ2 was indeed vital to early B cell development, resulting in an accumulation of large pre-B cells (121). PLCγ2 deficiency affects the functionality of pre-BCR, resulting in increased IL-7 signaling and upregulation of the RAG recombinase in selected large pre-B cells, thus leaving them vulnerable to transformation and accelerating c-myc-mediated lymphomagenesis (121).
While the absence of PLCγ2 can further enable lymphogenesis in certain murine lymphomas, in humans, PLCγ2 signaling can drive leukemogenesis in some cases. Ibrutinib, which inhibits Btk and thus blocks PLCγ2 signaling, has become an important and effective treatment for CLL. However, a subset of patients become refractory to this treatment over time and relapse, with PLCγ2 as a primary driver of the recurrent disease (122, 123). Mutations in PLCγ2 and/or Btk have been reported in 11 to 90% cases of Ibrutinib-refractory CLL, further underscoring the importance of better understanding the process by which the partnership of Btk and PLCγ2 drives CLL disease progression (122, 123, 124, 125, 126). Interestingly, two separate studies observed that there were no PLCγ2 or BTK mutations detected in patients with CLL before Ibrutinib treatment (127, 128). However, after Ibrutinib treatment, it was observed that CLL progression favored the selection of rare subclones bearing PLCγ2 mutations (129, 130). Collectively, these studies speak to the significant merit of monitoring patients with CLL for development of PLCγ2 mutations after their Ibrutinib treatment. This knowledge may be beneficial to clinicians by serving as an early clinical indicator of likely relapse and potentially help inform a more prudent treatment course for the patient.
Hypermorphic gain-of-function genetic changes in the putative calcium-regulated domain (C2) of PLCG2 were noted to be correlated with CLL resistance to Ibrutinib (131). It was also demonstrated in vitro SYK and LYN were essential in the mutated hypermorphic PLCγ2 (R665W) signaling, a common relapse patient PLCγ2 mutation, suggesting SYK and LYN as alternative targets in Ibrutinib resistance (132). SYK/PLCγ2 activity is also suggested as a potential biomarker for responsiveness in the treatment of CLL and DLBCL, as their signaling activity correlated with cell death induced by treatment with the Src tyrosine kinase inhibitor, Dasatinib (133, 134). Furthermore, PLCγ2 mutations R665W and L845F in Ibrutinib-resistant CLL were shown to be hypersensitive to the Rho GTPase, Rac2 protein in vitro, which may suggest Rac2 as a potential target of refractory disease (135).
PLCγ2 has also been implicated in 63% of DLBCL cases (54 of 86 patients in the study) (136). Treatment of these patient cells with an inhibitor of PLC (U73122) in vitro suppressed their proliferation and induced apoptosis and cell cycle arrest (136). However, unusually, high PLCγ2 expression was actually associated with better overall survival, which the authors speculated that potentially the stronger PLCγ2 signaling enhancing cellular proliferation and thus making the cells more susceptible to cytotoxic drugs (136). Downregulation of PLCγ2 gene pathways, and other BCR-related genes, along with EBV infection, is also associated with Hodgkin’s lymphoma (137). The use of the Ali5 heterozygous point mutation, gain-of-function PLCγ2 mice (106) in a model of Helicobacter felis infection showed PLCγ2 protects mice from developing gastric MALT lymphoma (138). These mice also had decreased levels of inflammatory cytokines and antibody responses to H. felis, suggesting a weakened immune response after infection that the authors ventured was due to the increased presence of regulatory T cells (138). Mutations in the PLCγ2 (Q548R) gene, along with PIK3CD (D133E) and AKT3 (D280G), were implicated in myelodysplastic syndrome progression after the chemotherapeutic combination treatment of Azacitidine/Lenalidomide in the cohort of patients who were either first to become refractory to therapy or failed entirely to respond to therapy (139). These mutations also were noted to correlate with shorter overall survival and duration of response to treatment (139).
In addition to the transformative influence of PLCγ2, in certain the hematological malignancies, PLCγ2 also serves a role in EBV transformation of activated B cells. It was noted that PLCγ2 and its BCR-associated signaling are blocked via the virally encoded integral latent membrane protein 2A in combination with BLNK (140). While PLCγ2 signaling is apparently blocked during EBV infection, it was also conversely shown to be upregulated in EBV-transformed lymphoblasts (141). This potentially indicates that after initial EBV infection, PLCγ2 function may be restored in the transformed lymphoblasts. However, it remains to be confirmed beyond this initial observation as to whether this is indeed the process that occurs during EBV transformation and why. In addition, an RNA-seq study of patient tumors of EBL, a common childhood cancer from equatorial Africa where malaria is endemic, showed that the vast majority of cases which harbor EBV contain PLCγ2 mutations, along with mutations in other genes including MYC (142). Interestingly, while MYC translocation and hyperexpression is a hallmark of EBL, it in itself is not sufficient to induce lymphogenesis (143), which may at least in part explain why mutations in PLCγ2 are relevant to the disease initiation (142).
PLCγ2 is also potentially involved in a small number of solid cancers such as Wilms' tumor (kidney), osteosarcoma, esophageal squamous cell carcinoma, esophageal adenocarcinoma, cervical adenocarcinoma, Birt-Hogg-Dube tumors, non–small-cell lung cancer, and breast cancer. PLCγ2 was implicated in the Wnt signaling pathway necessary for kidney development and development of Wilms' tumor (144). In addition, PLCG2 was implicated as a potential tumor suppressor gene in Wilms' tumor with a focal deletion of chromosome 16q22.1q24.3, which resulted in a copy number alteration and downregulation of PLCG2 (145). PLCγ2 was also found to potentially contribute to other tumorogenic situations such as the formation of esophageal adenocarcinoma (146). This is thought to be through PLCγ2’s necessary role in contributing to excessive ROS production and cell proliferation, induced by taurodeoxycholic acid, causing the activation of PI-PLCγ2, ERK-2, and MAPK and production of NADPH oxidase NOX5-S. The resulting mutagenic stress may also be a factor in the development of esophageal adenocarcinoma (146). PLCγ2 was also implicated in cervical adenocarcinoma, where in vitro siRNA knockdown of PLCγ2, and calmodulin 1, in human cervical adenocarcinoma cells led to an increased sensitivity to doxorubicin and paclitaxel, but not Cisplatin (147). However, PLCγ2 was noted to be involved in Cisplatin resistance in another study of seven cancer cell lines, suggesting that the influence of PLCγ2 on tumorogenesis may be dependent on the cellular context (148). Population studies and microarray analyses also revealed PLCγ2 associated with the EGFR pathway was a risk factor in esophageal squamous cell carcinoma and gastric cancer (149), in non–small-cell lung cancer associated with HMGB1 expression (150), and as upregulated and potentially involved in triple-negative breast cancer–associated BRAC1 in a Chinese patient cohort (151). Indeed, PLCγ2 SNPs were identified as breast cancer risk factors in patients on menopausal hormone replacement therapy (152, 153).
Using patient material and mouse/human xenograft models, PLCγ2 was revealed to be an overexpressed upstream mediator, among other factors resulting in ERK signaling, that induced increased FGF-mediated bevacizumab resistance in head and neck squamous cell carcinoma (154). siRNA targeting Ezrin, a cytoplasmic peripheral protein involved in metastatic spreading of osteosarcoma and dependent on PIP2, also resulted in PLCγ2 upregulation in human osteosarcoma cell lines (155, 156), while another study of osteosarcoma cell lines found PLCγ2 to be a differentially regulated gene (157). PLCγ2 as was also noted as a potential biomarker of radiation exposure through its upregulation after ionizing radiation (158). The detection PLCγ2 in such wide array of solid cancers speak to its great potential as a prognostic indicator and drug target.
Overall, these studies demonstrate that PLCγ2 is a driver or risk factor in a number of human cancers, both solid and hematological. However, in mice, the absence of PLCγ2 can help drive lymphomagenesis. Thus, more research is needed to understand the distinct roles of PLCγ2. Nevertheless, PLCγ2 clearly plays a crucial role in CLL that has become refractory to Ibrutinib treatment. In this context, PLCγ2 serves as a vital biomarker for CLL progression during Ibrutinib treatment in the clinical setting. It is also fascinating that the same mutation can drive both resistance to Ibrutinib and the inherited immune disease APLAID (Fig. 1). Therefore, efforts to therapeutically target the effect of these mutations could be broadly applicable across a range of pathologies.
PLCγ2 and neurological diseases
Late-onset AD is the most common form of dementia, characterized by accumulation of amyloid β (Aβ) and neurofibrillary tangles. A rare variant in the PLCG2 gene (Pro522Arg) has been associated with decreased risk of AD by genome-wide association study (159). The initial genetic results were replicated by various independent cohorts (160, 161, 162). Recently, the reduced risk of the P522R mutation for AD has been extended to other dementia subtypes including Lewy bodies and frontotemporal dementia (163) (Fig. 4). This variant was also associated with an increased chance of reaching at least 90 years of age and then becoming a cognitively healthy centenarian, that is, people in whom the absence of dementia and extreme longevity is combined (163). In contrast, the PLCγ2 variant had no protective effect on the risk of progressive supranuclear palsy, amyotrophic lateral sclerosis, Parkinson's disease, or multiple sclerosis (163). On a functional level, the P522R mutation shows an increased enzymatic activity in cell lines compared with WT enzyme, acting as a functional hypermorph (164).
Figure 4.
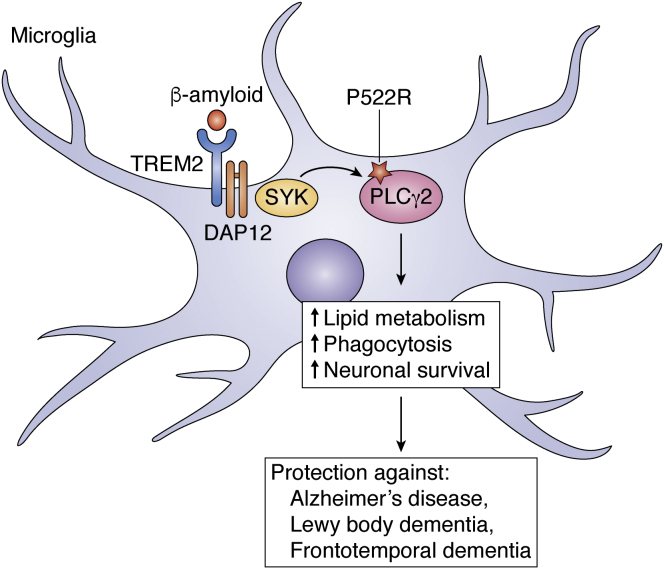
The PLCγ2 variant P522R is protective against neurodegeneration. In the brain, the γ2 isoform is predominantly expressed by microglia (right). When triggered by TREM2, the PLCγ2 variant P522R supports lipid metabolism, phagocytosis, and survival (left), thus protecting against Alzheimer’s disease (AD), Lewy body dementia, and frontotemporal dementia (FTD). PLAID, PLCγ2-associated antibody deficiency and immune dysregulation.
Human genetic data were further corroborated by using well-studied animal models of AD (165, 166). Comparative gene expression profiling of known genetic risk factors in AD revealed an increase of PLCG2 gene expression in the cortex of AppNL-G-F/NL-G-F mice (165). Single microglia sequencing of two different mouse models of AD, one displaying either Aβ or tau pathology, further confirmed the expression of AD risk genes, including PLCG2 gene, and also confirmed that more microglia adopt an activated phenotype when facing Aβ than tau pathology (166).
Although PLCγ2 is active in various cell types and tissues as highlighted in this article, within the brain, PLCγ2 is, interestingly, primarily expressed in microglia, in both humans and mice (164) (Fig. 4). A recent study using genetically engineered human induced pluripotent stem cell–derived microglia-like cells found that PLCγ2 activity in microglia regulates multiple processes downstream of TREM2 including survival, myelin phagocytosis, processing of neuronal debris, and metabolism of lipids (Fig. 4), as well as inflammation downstream of TLRs (167). Interestingly, in the absence of TREM2, TLR-dependent PLCγ2 signaling was intensified as a result of aberrant lipid metabolism, resulting in a hyperinflammatory state (167).
Taken together, these results provide further evidence that PLCγ2 may play an important role in AD pathophysiology. Future studies investigating how these variants in PLCγ2 modulate its function and microglia phenotypes in AD could lead to the identification of novel therapeutic strategies.
Conclusion
In summary, PLCγ2 is a fascinating enzyme regulating diverse biological functions through critical immunological molecules used by a variety of extracellular receptor signaling pathways. Conclusive genetic evidence demonstrates how important normal PLCγ2 function is to immune health, the overaction of which can lead to immunodeficiency, autoimmunity, or autoinflammation. Some of these same mutations can also drive hematopoietic cell proliferation, for example, in Ibrutinib-refractory CLL, and so inhibition of PLCγ2 could be considered as a therapeutic modality in some malignancies and immune disorders. However, such intervention would need to be approached with caution, as the constitutive roles of PLCγ2 are important, as are other gain-of-function variants that provide protection against neurodegenerative disease. Therefore, improved understanding of PLCγ2 functions could inform cellular and temporal targeting of this pathway for therapeutic benefit in the future.
Conflict of interest
S. L. M. is a scientific advisor for IFM Therapeutics. J. T. J., E. M., and S. L. N. declare no conflicts of interest.
Acknowledgments
Author contributions
J. T. J., E. M., and S. L. M. conceptualization; J. T. J., E. M., and S. L. M. writing–original draft; J. T. J., E. M., S. L. N., and S. L. M. writing–review and editing.
Funding and additional information
E. M. is funded by the German Research Foundation (DFG, reference no. SCHU 3126/3-1). S. L. M. acknowledges funding from National Health and Medical Research Council Grant (2003159), The Sylvia and Charles Viertel Foundation, and Howard Hughes Medical Institute-Wellcome International Research Scholarship. S. L. M. is a scientific advisor for IFM therapeutics. S. L. N. acknowledges funding from National Health and Medical Research Council Grant (1155342).
Edited by Alex Toker
References
- 1.Berridge M.J. Inositol trisphosphate and calcium signalling. Nature. 1993;361:315–325. doi: 10.1038/361315a0. [DOI] [PubMed] [Google Scholar]
- 2.Nishizuka Y. Protein kinase C and lipid signaling for sustained cellular responses. FASEB J. 1995;9:484–496. [PubMed] [Google Scholar]
- 3.Hernandez D., Egan S.E., Yulug I.G., Fisher E.M. Mapping the gene that encodes phosphatidylinositol-specific phospholipase C-gamma 2 in the human and the mouse. Genomics. 1994;23:504–507. doi: 10.1006/geno.1994.1533. [DOI] [PubMed] [Google Scholar]
- 4.Koss H., Bunney T.D., Behjati S., Katan M. Dysfunction of phospholipase Cγ in immune disorders and cancer. Trends Biochem. Sci. 2014;39:603–611. doi: 10.1016/j.tibs.2014.09.004. [DOI] [PubMed] [Google Scholar]
- 5.Walliser C., Retlich M., Harris R., Everett K.L., Josephs M.B., Vatter P., Esposito D., Driscoll P.C., Katan M., Gierschik P., Bunney T.D. Rac regulates its effector phospholipase Cgamma2 through interaction with a split pleckstrin homology domain. J. Biol. Chem. 2008;283:30351–30362. doi: 10.1074/jbc.M803316200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bunney T.D., Opaleye O., Roe S.M., Vatter P., Baxendale R.W., Walliser C., Everett K.L., Josephs M.B., Christow C., Rodrigues-Lima F., Gierschik P., Pearl L.H., Katan M. Structural insights into formation of an active signaling complex between Rac and phospholipase C gamma 2. Mol. Cell. 2009;34:223–233. doi: 10.1016/j.molcel.2009.02.023. [DOI] [PubMed] [Google Scholar]
- 7.Everett K.L., Buehler A., Bunney T.D., Margineanu A., Baxendale R.W., Vatter P., Retlich M., Walliser C., Manning H.B., Neil M.A., Dunsby C., French P.M., Gierschik P., Katan M. Membrane environment exerts an important influence on Rac-mediated activation of phospholipase Cγ2. Mol. Cell. Biol. 2011;31:1240–1251. doi: 10.1128/MCB.01408-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ozdener F., Dangelmaier C., Ashby B., Kunapuli S.P., Daniel J.L. Activation of phospholipase Cgamma2 by tyrosine phosphorylation. Mol. Pharmacol. 2002;62:672–679. doi: 10.1124/mol.62.3.672. [DOI] [PubMed] [Google Scholar]
- 9.Rodriguez R., Matsuda M., Storey A., Katan M. Requirements for distinct steps of phospholipase Cgamma2 regulation, membrane-raft-dependent targeting and subsequent enzyme activation in B-cell signalling. Biochem. J. 2003;374:269–280. doi: 10.1042/BJ20021778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kim Y.J., Sekiya F., Poulin B., Bae Y.S., Rhee S.G. Mechanism of B-cell receptor-induced phosphorylation and activation of phospholipase C-gamma2. Mol. Cell. Biol. 2004;24:9986–9999. doi: 10.1128/MCB.24.22.9986-9999.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Watanabe D., Hashimoto S., Ishiai M., Matsushita M., Baba Y., Kishimoto T., Kurosaki T., Tsukada S. Four tyrosine residues in phospholipase C-gamma 2, identified as Btk-dependent phosphorylation sites, are required for B cell antigen receptor-coupled calcium signaling. J. Biol. Chem. 2001;276:38595–38601. doi: 10.1074/jbc.M103675200. [DOI] [PubMed] [Google Scholar]
- 12.Hitomi T., Yanagi S., Inatome R., Yamamura H. Cross-linking of the B cell receptor induces activation of phospholipase D through Syk, Btk and phospholipase C-gamma2. FEBS Lett. 1999;445:371–374. doi: 10.1016/s0014-5793(99)00153-2. [DOI] [PubMed] [Google Scholar]
- 13.Aman M.J., Ravichandran K.S. A requirement for lipid rafts in B cell receptor induced Ca(2+) flux. Curr. Biol. 2000;10:393–396. doi: 10.1016/s0960-9822(00)00415-2. [DOI] [PubMed] [Google Scholar]
- 14.Lang M.L., Chen Y.W., Shen L., Gao H., Lang G.A., Wade T.K., Wade W.F. IgA Fc receptor (FcalphaR) cross-linking recruits tyrosine kinases, phosphoinositide kinases and serine/threonine kinases to glycolipid rafts. Biochem. J. 2002;364(Pt 2):517–525. doi: 10.1042/BJ20011696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yokozeki T., Adler K., Lankar D., Bonnerot C. B cell receptor-mediated Syk-independent activation of phosphatidylinositol 3-kinase, Ras, and mitogen-activated protein kinase pathways. J. Immunol. 2003;171:1328–1335. doi: 10.4049/jimmunol.171.3.1328. [DOI] [PubMed] [Google Scholar]
- 16.Moreno-García M.E., López-Bojórques L.N., Zentella A., Humphries L.A., Rawlings D.J., Santos-Argumedo L. CD38 signaling regulates B lymphocyte activation via a phospholipase C (PLC)-gamma 2-independent, protein kinase C, phosphatidylcholine-PLC, and phospholipase D-dependent signaling cascade. J. Immunol. 2005;174:2687–2695. doi: 10.4049/jimmunol.174.5.2687. [DOI] [PubMed] [Google Scholar]
- 17.Weber M., Treanor B., Depoil D., Shinohara H., Harwood N.E., Hikida M., Kurosaki T., Batista F.D. Phospholipase C-gamma2 and Vav cooperate within signaling microclusters to propagate B cell spreading in response to membrane-bound antigen. J. Exp. Med. 2008;205:853–868. doi: 10.1084/jem.20072619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hashimoto A., Okada H., Jiang A., Kurosaki M., Greenberg S., Clark E.A., Kurosaki T. Involvement of guanosine triphosphatases and phospholipase C-gamma2 in extracellular signal-regulated kinase, c-Jun NH2-terminal kinase, and p38 mitogen-activated protein kinase activation by the B cell antigen receptor. J. Exp. Med. 1998;188:1287–1295. doi: 10.1084/jem.188.7.1287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Walliser C., Tron K., Clauss K., Gutman O., Kobitski A.Y., Retlich M., Schade A., Röcker C., Henis Y.I., Nienhaus G.U., Gierschik P. Rac-mediated stimulation of phospholipase Cγ2 amplifies B cell receptor-induced calcium signaling. J. Biol. Chem. 2015;290:17056–17072. doi: 10.1074/jbc.M115.645739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Brooks S.R., Li X., Volanakis E.J., Carter R.H. Systematic analysis of the role of CD19 cytoplasmic tyrosines in enhancement of activation in daudi human B cells: Clustering of phospholipase C and Vav and of Grb2 and sos with different CD19 tyrosines. J. Immunol. 2000;164:3123–3131. doi: 10.4049/jimmunol.164.6.3123. [DOI] [PubMed] [Google Scholar]
- 21.Bernal-Quiros M., Wu Y.Y., Alarcón-Riquelme M.E., Castillejo-López C. BANK1 and BLK act through phospholipase C gamma 2 in B-cell signaling. PLoS One. 2013;8 doi: 10.1371/journal.pone.0059842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Brooks S.R., Kirkham P.M., Freeberg L., Carter R.H. Binding of cytoplasmic proteins to the CD19 intracellular domain is high affinity, competitive, and multimeric. J. Immunol. 2004;172:7556–7564. doi: 10.4049/jimmunol.172.12.7556. [DOI] [PubMed] [Google Scholar]
- 23.Venkataraman C., Muthusamy N., Muthukkumar S., Bondada S. Activation of lyn, blk, and btk but not syk in CD72-stimulated B lymphocytes. J. Immunol. 1998;160:3322–3329. [PubMed] [Google Scholar]
- 24.Wang D., Feng J., Wen R., Marine J.C., Sangster M.Y., Parganas E., Hoffmeyer A., Jackson C.W., Cleveland J.L., Murray P.J., Ihle J.N. Phospholipase Cgamma2 is essential in the functions of B cell and several Fc receptors. Immunity. 2000;13:25–35. doi: 10.1016/s1074-7613(00)00005-4. [DOI] [PubMed] [Google Scholar]
- 25.Taguchi T., Kiyokawa N., Takenouch H., Matsui J., Tang W.R., Nakajima H., Suzuki K., Shiozawa Y., Saito M., Katagiri Y.U., Takahashi T., Karasuyama H., Matsuo Y., Okita H., Fujimoto J. Deficiency of BLNK hampers PLC-gamma2 phosphorylation and Ca2+ influx induced by the pre-B-cell receptor in human pre-B cells. Immunology. 2004;112:575–582. doi: 10.1111/j.1365-2567.2004.01918.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hashimoto A., Takeda K., Inaba M., Sekimata M., Kaisho T., Ikehara S., Homma Y., Akira S., Kurosaki T. Cutting edge: Essential role of phospholipase C-gamma 2 in B cell development and function. J. Immunol. 2000;165:1738–1742. doi: 10.4049/jimmunol.165.4.1738. [DOI] [PubMed] [Google Scholar]
- 27.Dai X., Chen Y., Schuman J., Hua Z., Adamson J.W., Wen R., Wang D. Distinct roles of phosphoinositide-3 kinase and phospholipase Cgamma2 in B-cell receptor-mediated signal transduction. Mol. Cell. Biol. 2006;26:88–99. doi: 10.1128/MCB.26.1.88-99.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bai L., Chen Y., He Y., Dai X., Lin X., Wen R., Wang D. Phospholipase Cgamma2 contributes to light-chain gene activation and receptor editing. Mol. Cell. Biol. 2007;27:5957–5967. doi: 10.1128/MCB.02273-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Xu S., Huo J., Chew W.K., Hikida M., Kurosaki T., Lam K.P. Phospholipase Cgamma2 dosage is critical for B cell development in the absence of adaptor protein BLNK. J. Immunol. 2006;176:4690–4698. doi: 10.4049/jimmunol.176.8.4690. [DOI] [PubMed] [Google Scholar]
- 30.Benschop R.J., Brandl E., Chan A.C., Cambier J.C. Unique signaling properties of B cell antigen receptor in mature and immature B cells: Implications for tolerance and activation. J. Immunol. 2001;167:4172–4179. doi: 10.4049/jimmunol.167.8.4172. [DOI] [PubMed] [Google Scholar]
- 31.Moens L., Kane A., Tangye S.G. Naïve and memory B cells exhibit distinct biochemical responses following BCR engagement. Immunol. Cell Biol. 2016;94:774–786. doi: 10.1038/icb.2016.41. [DOI] [PubMed] [Google Scholar]
- 32.Wen R., Chen Y., Xue L., Schuman J., Yang S., Morris S.W., Wang D. Phospholipase Cgamma2 provides survival signals via Bcl2 and A1 in different subpopulations of B cells. J. Biol. Chem. 2003;278:43654–43662. doi: 10.1074/jbc.M307318200. [DOI] [PubMed] [Google Scholar]
- 33.Hikida M., Johmura S., Hashimoto A., Takezaki M., Kurosaki T. Coupling between B cell receptor and phospholipase C-gamma2 is essential for mature B cell development. J. Exp. Med. 2003;198:581–589. doi: 10.1084/jem.20030280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wang J.H., Nichogiannopoulou A., Wu L., Sun L., Sharpe A.H., Bigby M., Georgopoulos K. Selective defects in the development of the fetal and adult lymphoid system in mice with an Ikaros null mutation. Immunity. 1996;5:537–549. doi: 10.1016/s1074-7613(00)80269-1. [DOI] [PubMed] [Google Scholar]
- 35.Georgopoulos K., Bigby M., Wang J.H., Molnar A., Wu P., Winandy S., Sharpe A. The Ikaros gene is required for the development of all lymphoid lineages. Cell. 1994;79:143–156. doi: 10.1016/0092-8674(94)90407-3. [DOI] [PubMed] [Google Scholar]
- 36.Papathanasiou P., Perkins A.C., Cobb B.S., Ferrini R., Sridharan R., Hoyne G.F., Nelms K.A., Smale S.T., Goodnow C.C. Widespread failure of hematolymphoid differentiation caused by a recessive niche-filling allele of the Ikaros transcription factor. Immunity. 2003;19:131–144. doi: 10.1016/s1074-7613(03)00168-7. [DOI] [PubMed] [Google Scholar]
- 37.Nera K.P., Alinikula J., Terho P., Narvi E., Törnquist K., Kurosaki T., Buerstedde J.M., Lassila O. Ikaros has a crucial role in regulation of B cell receptor signaling. Eur. J. Immunol. 2006;36:516–525. doi: 10.1002/eji.200535418. [DOI] [PubMed] [Google Scholar]
- 38.de Gorter D.J., Vos J.C., Pals S.T., Spaargaren M. The B cell antigen receptor controls AP-1 and NFAT activity through Ras-mediated activation of Ral. J. Immunol. 2007;178:1405–1414. doi: 10.4049/jimmunol.178.3.1405. [DOI] [PubMed] [Google Scholar]
- 39.Oh-hora M., Johmura S., Hashimoto A., Hikida M., Kurosaki T. Requirement for Ras guanine nucleotide releasing protein 3 in coupling phospholipase C-gamma2 to Ras in B cell receptor signaling. J. Exp. Med. 2003;198:1841–1851. doi: 10.1084/jem.20031547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Aiba Y., Oh-hora M., Kiyonaka S., Kimura Y., Hijikata A., Mori Y., Kurosaki T. Activation of RasGRP3 by phosphorylation of Thr-133 is required for B cell receptor-mediated Ras activation. Proc. Natl. Acad. Sci. U. S. A. 2004;101:16612–16617. doi: 10.1073/pnas.0407468101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Marshall A.J., Niiro H., Yun T.J., Clark E.A. Regulation of B-cell activation and differentiation by the phosphatidylinositol 3-kinase and phospholipase Cgamma pathway. Immunol. Rev. 2000;176:30–46. doi: 10.1034/j.1600-065x.2000.00611.x. [DOI] [PubMed] [Google Scholar]
- 42.Stork B., Neumann K., Goldbeck I., Alers S., Kähne T., Naumann M., Engelke M., Wienands J. Subcellular localization of Grb2 by the adaptor protein Dok-3 restricts the intensity of Ca2+ signaling in B cells. EMBO J. 2007;26:1140–1149. doi: 10.1038/sj.emboj.7601557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Cheng D., Deobagkar-Lele M., Zvezdova E., Choi S., Uehara S., Baup D., Bennett S.C., Bull K.R., Crockford T.L., Ferry H., Warzecha C., Marcellin M., de Peredo A.G., Lesourne R., Anzilotti C. Themis2 lowers the threshold for B cell activation during positive selection. Nat. Immunol. 2017;18:205–213. doi: 10.1038/ni.3642. [DOI] [PubMed] [Google Scholar]
- 44.Engelke M., Oellerich T., Dittmann K., Hsiao H.H., Urlaub H., Serve H., Griesinger C., Wienands J. Cutting edge: Feed-forward activation of phospholipase Cγ2 via C2 domain-mediated binding to SLP65. J. Immunol. 2013;191:5354–5358. doi: 10.4049/jimmunol.1301326. [DOI] [PubMed] [Google Scholar]
- 45.Tokuda Y., Tanaka M., Yagi T., Tashiro K. The defect of SFRP2 modulates an influx of extracellular calcium in B lymphocytes. BMC Res. Notes. 2014;7:780. doi: 10.1186/1756-0500-7-780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Vendel A.C., Calemine-Fenaux J., Izrael-Tomasevic A., Chauhan V., Arnott D., Eaton D.L. B and T lymphocyte attenuator regulates B cell receptor signaling by targeting Syk and BLNK. J. Immunol. 2009;182:1509–1517. doi: 10.4049/jimmunol.182.3.1509. [DOI] [PubMed] [Google Scholar]
- 47.Yasuda T., Maeda A., Kurosaki M., Tezuka T., Hironaka K., Yamamoto T., Kurosaki T. Cbl suppresses B cell receptor-mediated phospholipase C (PLC)-gamma2 activation by regulating B cell linker protein-PLC-gamma2 binding. J. Exp. Med. 2000;191:641–650. doi: 10.1084/jem.191.4.641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Yasuda T., Tezuka T., Maeda A., Inazu T., Yamanashi Y., Gu H., Kurosaki T., Yamamoto T. Cbl-b positively regulates Btk-mediated activation of phospholipase C-γ2 in B cells. J. Exp. Med. 2002;196:51–63. doi: 10.1084/jem.20020068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sohn H.W., Gu H., Pierce S.K. Cbl-b negatively regulates B cell antigen receptor signaling in mature B cells through ubiquitination of the tyrosine kinase Syk. J. Exp. Med. 2003;197:1511–1524. doi: 10.1084/jem.20021686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kitaura Y., Jang I.K., Wang Y., Han Y.C., Inazu T., Cadera E.J., Schlissel M., Hardy R.R., Gu H. Control of the B cell-intrinsic tolerance programs by ubiquitin ligases Cbl and Cbl-b. Immunity. 2007;26:567–578. doi: 10.1016/j.immuni.2007.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Song H., Zhang J., Chiang Y.J., Siraganian R.P., Hodes R.J. Redundancy in B cell developmental pathways: c-Cbl inactivation rescues early B cell development through a B cell linker protein-independent pathway. J. Immunol. 2007;178:926–935. doi: 10.4049/jimmunol.178.2.926. [DOI] [PubMed] [Google Scholar]
- 52.Ying H., Li Z., Yang L., Zhang J. Syk mediates BCR- and CD40-signaling integration during B cell activation. Immunobiology. 2011;216:566–570. doi: 10.1016/j.imbio.2010.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Mizuno K., Tagawa Y., Mitomo K., Watanabe N., Katagiri T., Ogimoto M., Yakura H. Src homology region 2 domain-containing phosphatase 1 positively regulates B cell receptor-induced apoptosis by modulating association of B cell linker protein with Nck and activation of c-Jun NH2-terminal kinase. J. Immunol. 2002;169:778–786. doi: 10.4049/jimmunol.169.2.778. [DOI] [PubMed] [Google Scholar]
- 54.Mizuno K., Tagawa Y., Watanabe N., Ogimoto M., Yakura H. SLP-76 is recruited to CD22 and dephosphorylated by SHP-1, thereby regulating B cell receptor-induced c-Jun N-terminal kinase activation. Eur. J. Immunol. 2005;35:644–654. doi: 10.1002/eji.200425465. [DOI] [PubMed] [Google Scholar]
- 55.Guo B., Rothstein T.L. B cell receptor (BCR) cross-talk: IL-4 creates an alternate pathway for BCR-induced ERK activation that is phosphatidylinositol 3-kinase independent. J. Immunol. 2005;174:5375–5381. doi: 10.4049/jimmunol.174.9.5375. [DOI] [PubMed] [Google Scholar]
- 56.Dye J.R., Palvanov A., Guo B., Rothstein T.L. B cell receptor cross-talk: Exposure to lipopolysaccharide induces an alternate pathway for B cell receptor-induced ERK phosphorylation and NF-kappa B activation. J. Immunol. 2007;179:229–235. doi: 10.4049/jimmunol.179.1.229. [DOI] [PubMed] [Google Scholar]
- 57.Kin N.W., Sanders V.M. CD86 stimulation on a B cell activates the phosphatidylinositol 3-kinase/Akt and phospholipase C gamma 2/protein kinase C alpha beta signaling pathways. J. Immunol. 2006;176:6727–6735. doi: 10.4049/jimmunol.176.11.6727. [DOI] [PubMed] [Google Scholar]
- 58.Lucas C.R., Cordero-Nieves H.M., Erbe R.S., McAlees J.W., Bhatia S., Hodes R.J., Campbell K.S., Sanders V.M. Prohibitins and the cytoplasmic domain of CD86 cooperate to mediate CD86 signaling in B lymphocytes. J. Immunol. 2013;190:723–736. doi: 10.4049/jimmunol.1201646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Roes J., Choi B.K., Cazac B.B. Redirection of B cell responsiveness by transforming growth factor beta receptor. Proc. Natl. Acad. Sci. U. S. A. 2003;100:7241–7246. doi: 10.1073/pnas.0731875100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Fu G., Chen Y., Yu M., Podd A., Schuman J., He Y., Di L., Yassai M., Haribhai D., North P.E., Gorski J., Williams C.B., Wang D., Wen R. Phospholipase C{gamma}1 is essential for T cell development, activation, and tolerance. J. Exp. Med. 2010;207:309–318. doi: 10.1084/jem.20090880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Fu G., Chen Y., Schuman J., Wang D., Wen R. Phospholipase Cγ2 plays a role in TCR signal transduction and T cell selection. J. Immunol. 2012;189:2326–2332. doi: 10.4049/jimmunol.1103458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Mikosik A., Jasiulewicz A., Daca A., Henc I., Frąckowiak J.E., Ruckemann-Dziurdzińska K., Foerster J., Le Page A., Bryl E., Fulop T., Witkowski J.M. Roles of calpain-calpastatin system (CCS) in human T cell activation. Oncotarget. 2016;7:76479–76495. doi: 10.18632/oncotarget.13259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Ombrello M.J., Remmers E.F., Sun G., Freeman A.F., Datta S., Torabi-Parizi P., Subramanian N., Bunney T.D., Baxendale R.W., Martins M.S., Romberg N., Komarow H., Aksentijevich I., Kim H.S., Ho J. Cold urticaria, immunodeficiency, and autoimmunity related to PLCG2 deletions. N. Engl. J. Med. 2012;366:330–338. doi: 10.1056/NEJMoa1102140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Zhou Q., Lee G.S., Brady J., Datta S., Katan M., Sheikh A., Martins M.S., Bunney T.D., Santich B.H., Moir S., Kuhns D.B., Long Priel D.A., Ombrello A., Stone D., Ombrello M.J. A hypermorphic missense mutation in PLCG2, encoding phospholipase Cγ2, causes a dominantly inherited autoinflammatory disease with immunodeficiency. Am. J. Hum. Genet. 2012;91:713–720. doi: 10.1016/j.ajhg.2012.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Bogaert D.J., Dullaers M., Lambrecht B.N., Vermaelen K.Y., De Baere E., Haerynck F. Genes associated with common variable immunodeficiency: One diagnosis to rule them all? J. Med. Genet. 2016;53:575–590. doi: 10.1136/jmedgenet-2015-103690. [DOI] [PubMed] [Google Scholar]
- 66.Giannelou A., Zhou Q., Kastner D.L. When less is more: Primary immunodeficiency with an autoinflammatory kick. Curr. Opin. Allergy Clin. Immunol. 2014;14:491–500. doi: 10.1097/ACI.0000000000000117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Aderibigbe O.M., Priel D.L., Lee C.C., Ombrello M.J., Prajapati V.H., Liang M.G., Lyons J.J., Kuhns D.B., Cowen E.W., Milner J.D. Distinct cutaneous manifestations and cold-induced leukocyte activation associated with PLCG2 mutations. JAMA Dermatol. 2015;151:627–634. doi: 10.1001/jamadermatol.2014.5641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Milner J.D. PLAID: A syndrome of complex patterns of disease and unique phenotypes. J. Clin. Immunol. 2015;35:527–530. doi: 10.1007/s10875-015-0177-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Wang J., Sohn H., Sun G., Milner J.D., Pierce S.K. The autoinhibitory C-terminal SH2 domain of phospholipase C-gamma2 stabilizes B cell receptor signalosome assembly. Sci. Signal. 2014;7 doi: 10.1126/scisignal.2005392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Schade A., Walliser C., Wist M., Haas J., Vatter P., Kraus J.M., Filingeri D., Havenith G., Kestler H.A., Milner J.D., Gierschik P. Cool-temperature-mediated activation of phospholipase C-γ2 in the human hereditary disease PLAID. Cell. Signal. 2016;28:1237–1251. doi: 10.1016/j.cellsig.2016.05.010. [DOI] [PubMed] [Google Scholar]
- 71.Wen R., Jou S.T., Chen Y., Hoffmeyer A., Wang D. Phospholipase C gamma 2 is essential for specific functions of Fc epsilon R and Fc gamma R. J. Immunol. 2002;169:6743–6752. doi: 10.4049/jimmunol.169.12.6743. [DOI] [PubMed] [Google Scholar]
- 72.Bonilla F.A., Fujita R.M., Pivniouk V.I., Chan A.C., Geha R.S. Adapter proteins SLP-76 and BLNK both are expressed by murine macrophages and are linked to signaling via Fcgamma receptors I and II/III. Proc. Natl. Acad. Sci. U. S. A. 2000;97:1725–1730. doi: 10.1073/pnas.040543597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Jakus Z., Simon E., Frommhold D., Sperandio M., Mócsai A. Critical role of phospholipase Cgamma2 in integrin and Fc receptor-mediated neutrophil functions and the effector phase of autoimmune arthritis. J. Exp. Med. 2009;206:577–593. doi: 10.1084/jem.20081859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Jackon J.K., Tudan C., Burt H.M. The involvement of phospholipase C in crystal induced human neutrophil activation. J. Rheumatol. 2000;27:2877–2885. [PubMed] [Google Scholar]
- 75.Fernandes M.J., Lachance G., Paré G., Rollet-Labelle E., Naccache P.H. Signaling through CD16b in human neutrophils involves the Tec family of tyrosine kinases. J. Leukoc. Biol. 2005;78:524–532. doi: 10.1189/jlb.0804479. [DOI] [PubMed] [Google Scholar]
- 76.Bourette R.P., Myles G.M., Choi J.L., Rohrschneider L.R. Sequential activation of phoshatidylinositol 3-kinase and phospholipase C-gamma2 by the M-CSF receptor is necessary for differentiation signaling. EMBO J. 1997;16:5880–5893. doi: 10.1093/emboj/16.19.5880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Jack G.D., Zhang L., Friedman A.D. M-CSF elevates c-Fos and phospho-C/EBPalpha(S21) via ERK whereas G-CSF stimulates SHP2 phosphorylation in marrow progenitors to contribute to myeloid lineage specification. Blood. 2009;114:2172–2180. doi: 10.1182/blood-2008-11-191536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Bourgin-Hierle C., Gobert-Gosse S., Thérier J., Grasset M.F., Mouchiroud G. Src-family kinases play an essential role in differentiation signaling downstream of macrophage colony-stimulating factor receptors mediating persistent phosphorylation of phospholipase C-gamma2 and MAP kinases ERK1 and ERK2. Leukemia. 2008;22:161–169. doi: 10.1038/sj.leu.2404986. [DOI] [PubMed] [Google Scholar]
- 79.Obba S., Hizir Z., Boyer L., Selimoglu-Buet D., Pfeifer A., Michel G., Hamouda M.A., Gonçalvès D., Cerezo M., Marchetti S., Rocchi S., Droin N., Cluzeau T., Robert G., Luciano F. The PRKAA1/AMPKα1 pathway triggers autophagy during CSF1-induced human monocyte differentiation and is a potential target in CMML. Autophagy. 2015;11:1114–1129. doi: 10.1080/15548627.2015.1034406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Graham D.B., Robertson C.M., Bautista J., Mascarenhas F., Diacovo M.J., Montgrain V., Lam S.K., Cremasco V., Dunne W.M., Faccio R., Coopersmith C.M., Swat W. Neutrophil-mediated oxidative burst and host defense are controlled by a Vav-PLCgamma2 signaling axis in mice. J. Clin. Invest. 2007;117:3445–3452. doi: 10.1172/JCI32729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Gorjestani S., Yu M., Tang B., Zhang D., Wang D., Lin X. Phospholipase Cγ2 (PLCγ2) is key component in Dectin-2 signaling pathway, mediating anti-fungal innate immune responses. J. Biol. Chem. 2011;286:43651–43659. doi: 10.1074/jbc.M111.307389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Lin M., Zhu M.X., Rikihisa Y. Rapid activation of protein tyrosine kinase and phospholipase C-gamma2 and increase in cytosolic free calcium are required by Ehrlichia chaffeensis for internalization and growth in THP-1 cells. Infect. Immun. 2002;70:889–898. doi: 10.1128/IAI.70.2.889-898.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Paroha R., Chaurasiya S.K., Chourasia R. Phospholipase C-γ2 promotes intracellular survival of mycobacteria. J. Cell Biochem. 2019;120:5062–5071. doi: 10.1002/jcb.27783. [DOI] [PubMed] [Google Scholar]
- 84.Aki D., Minoda Y., Yoshida H., Watanabe S., Yoshida R., Takaesu G., Chinen T., Inaba T., Hikida M., Kurosaki T., Saeki K., Yoshimura A. Peptidoglycan and lipopolysaccharide activate PLCgamma2, leading to enhanced cytokine production in macrophages and dendritic cells. Genes Cells. 2008;13:199–208. doi: 10.1111/j.1365-2443.2007.01159.x. [DOI] [PubMed] [Google Scholar]
- 85.Chiang C.Y., Veckman V., Limmer K., David M. Phospholipase Cγ-2 and intracellular calcium are required for lipopolysaccharide-induced Toll-like receptor 4 (TLR4) endocytosis and interferon regulatory factor 3 (IRF3) activation. J. Biol. Chem. 2012;287:3704–3709. doi: 10.1074/jbc.C111.328559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Palmbos P.L., Sytsma M.J., DeHeer D.H., Bonnema J.D. Macrophage exposure to particulate titanium induces phosphorylation of the protein tyrosine kinase lyn and the phospholipases Cgamma-1 and Cgamma-2. J. Orthop. Res. 2002;20:483–489. doi: 10.1016/S0736-0266(01)00147-4. [DOI] [PubMed] [Google Scholar]
- 87.Mueller H., Stadtmann A., Van Aken H., Hirsch E., Wang D., Ley K., Zarbock A. Tyrosine kinase Btk regulates E-selectin-mediated integrin activation and neutrophil recruitment by controlling phospholipase C (PLC) gamma2 and PI3Kgamma pathways. Blood. 2010;115:3118–3127. doi: 10.1182/blood-2009-11-254185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Rossaint J., Spelten O., Kässens N., Mueller H., Van Aken H.K., Singbartl K., Zarbock A. Acute loss of renal function attenuates slow leukocyte rolling and transmigration by interfering with intracellular signaling. Kidney Int. 2011;80:493–503. doi: 10.1038/ki.2011.125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Andreae S., Buisson S., Triebel F. MHC class II signal transduction in human dendritic cells induced by a natural ligand, the LAG-3 protein (CD223) Blood. 2003;102:2130–2137. doi: 10.1182/blood-2003-01-0273. [DOI] [PubMed] [Google Scholar]
- 90.Spurrell D.R., Luckashenak N.A., Minney D.C., Chaplin A., Penninger J.M., Liwski R.S., Clements J.L., West K.A. Vav1 regulates the migration and adhesion of dendritic cells. J. Immunol. 2009;183:310–318. doi: 10.4049/jimmunol.0802096. [DOI] [PubMed] [Google Scholar]
- 91.Zanoni I., Ostuni R., Capuano G., Collini M., Caccia M., Ronchi A.E., Rocchetti M., Mingozzi F., Foti M., Chirico G., Costa B., Zaza A., Ricciardi-Castagnoli P., Granucci F. CD14 regulates the dendritic cell life cycle after LPS exposure through NFAT activation. Nature. 2009;460:264–268. doi: 10.1038/nature08118. [DOI] [PubMed] [Google Scholar]
- 92.Xu S., Huo J., Lee K.G., Kurosaki T., Lam K.P. Phospholipase Cgamma2 is critical for Dectin-1-mediated Ca2+ flux and cytokine production in dendritic cells. J. Biol. Chem. 2009;284:7038–7046. doi: 10.1074/jbc.M806650200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Pisegna S., Zingoni A., Pirozzi G., Cinque B., Cifone M.G., Morrone S., Piccoli M., Frati L., Palmieri G., Santoni A. Src-dependent Syk activation controls CD69-mediated signaling and function on human NK cells. J. Immunol. 2002;169:68–74. doi: 10.4049/jimmunol.169.1.68. [DOI] [PubMed] [Google Scholar]
- 94.Tassi I., Presti R., Kim S., Yokoyama W.M., Gilfillan S., Colonna M. Phospholipase C-gamma 2 is a critical signaling mediator for murine NK cell activating receptors. J. Immunol. 2005;175:749–754. doi: 10.4049/jimmunol.175.2.749. [DOI] [PubMed] [Google Scholar]
- 95.Caraux A., Kim N., Bell S.E., Zompi S., Ranson T., Lesjean-Pottier S., Garcia-Ojeda M.E., Turner M., Colucci F. Phospholipase C-gamma2 is essential for NK cell cytotoxicity and innate immunity to malignant and virally infected cells. Blood. 2006;107:994–1002. doi: 10.1182/blood-2005-06-2428. [DOI] [PubMed] [Google Scholar]
- 96.Regunathan J., Chen Y., Kutlesa S., Dai X., Bai L., Wen R., Wang D., Malarkannan S. Differential and nonredundant roles of phospholipase Cgamma2 and phospholipase Cgamma1 in the terminal maturation of NK cells. J. Immunol. 2006;177:5365–5376. doi: 10.4049/jimmunol.177.8.5365. [DOI] [PubMed] [Google Scholar]
- 97.Leon C.M., Barbosa C.M., Justo G.Z., Borelli P., Resende J.D., de Oliveira J.S., Ferreira A.T., Paredes-Gamero E.J. Requirement for PLCγ2 in IL-3 and GM-CSF-stimulated MEK/ERK phosphorylation in murine and human hematopoietic stem/progenitor cells. J. Cell Physiol. 2011;226:1780–1792. doi: 10.1002/jcp.22507. [DOI] [PubMed] [Google Scholar]
- 98.Nakamura-Ishizu A., Takubo K., Kobayashi H., Suzuki-Inoue K., Suda T. CLEC-2 in megakaryocytes is critical for maintenance of hematopoietic stem cells in the bone marrow. J. Exp. Med. 2015;212:2133–2146. doi: 10.1084/jem.20150057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Mao D., Epple H., Uthgenannt B., Novack D.V., Faccio R. PLCgamma2 regulates osteoclastogenesis via its interaction with ITAM proteins and GAB2. J. Clin. Invest. 2006;116:2869–2879. doi: 10.1172/JCI28775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Chen Y., Wang X., Di L., Fu G., Chen Y., Bai L., Liu J., Feng X., McDonald J.M., Michalek S., He Y., Yu M., Fu Y.X., Wen R., Wu H. Phospholipase Cgamma2 mediates RANKL-stimulated lymph node organogenesis and osteoclastogenesis. J. Biol. Chem. 2008;283:29593–29601. doi: 10.1074/jbc.M802493200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Epple H., Cremasco V., Zhang K., Mao D., Longmore G.D., Faccio R. Phospholipase Cgamma2 modulates integrin signaling in the osteoclast by affecting the localization and activation of Src kinase. Mol. Cell. Biol. 2008;28:3610–3622. doi: 10.1128/MCB.00259-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Irie N., Takada Y., Watanabe Y., Matsuzaki Y., Naruse C., Asano M., Iwakura Y., Suda T., Matsuo K. Bidirectional signaling through ephrinA2-EphA2 enhances osteoclastogenesis and suppresses osteoblastogenesis. J. Biol. Chem. 2009;284:14637–14644. doi: 10.1074/jbc.M807598200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Andrade M.V., Hiragun T., Beaven M.A. Dexamethasone suppresses antigen-induced activation of phosphatidylinositol 3-kinase and downstream responses in mast cells. J. Immunol. 2004;172:7254–7262. doi: 10.4049/jimmunol.172.12.7254. [DOI] [PubMed] [Google Scholar]
- 104.Barbu E.A., Zhang J., Berenstein E.H., Groves J.R., Parks L.M., Siraganian R.P. The transcription factor Zeb2 regulates signaling in mast cells. J. Immunol. 2012;188:6278–6286. doi: 10.4049/jimmunol.1102660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.de Castro R.O., Zhang J., Groves J.R., Barbu E.A., Siraganian R.P. Once phosphorylated, tyrosines in carboxyl terminus of protein-tyrosine kinase Syk interact with signaling proteins, including TULA-2, a negative regulator of mast cell degranulation. J. Biol. Chem. 2012;287:8194–8204. doi: 10.1074/jbc.M111.326850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Yu P., Constien R., Dear N., Katan M., Hanke P., Bunney T.D., Kunder S., Quintanilla-Martinez L., Huffstadt U., Schröder A., Jones N.P., Peters T., Fuchs H., de Angelis M.H., Nehls M. Autoimmunity and inflammation due to a gain-of-function mutation in phospholipase C gamma 2 that specifically increases external Ca2+ entry. Immunity. 2005;22:451–465. doi: 10.1016/j.immuni.2005.01.018. [DOI] [PubMed] [Google Scholar]
- 107.Abe K., Fuchs H., Boersma A., Hans W., Yu P., Kalaydjiev S., Klaften M., Adler T., Calzada-Wack J., Mossbrugger I., Rathkolb B., Rozman J., Prehn C., Maraslioglu M., Kametani Y. A novel N-ethyl-N-nitrosourea-induced mutation in phospholipase Cγ2 causes inflammatory arthritis, metabolic defects, and male infertility in vitro in a murine model. Arthritis Rheum. 2011;63:1301–1311. doi: 10.1002/art.30280. [DOI] [PubMed] [Google Scholar]
- 108.Martín-Nalda A., Fortuny C., Rey L., Bunney T.D., Alsina L., Esteve-Solé A., Bull D., Anton M.C., Basagaña M., Casals F., Deyá A., García-Prat M., Gimeno R., Juan M., Martinez-Banaclocha H Severe autoinflammatory manifestations and antibody deficiency due to novel hypermorphic PLCG2 mutations. J. Clin. Immunol. 2020;40:987–1000. doi: 10.1007/s10875-020-00794-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Chae J.J., Park Y.H., Park C., Hwang I.Y., Hoffmann P., Kehrl J.H., Aksentijevich I., Kastner D.L. Connecting two pathways through Ca 2+ signaling: NLRP3 inflammasome activation induced by a hypermorphic PLCG2 mutation. Arthritis Rheumatol. 2015;67:563–567. doi: 10.1002/art.38961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Neves J.F., Doffinger R., Barcena-Morales G., Martins C., Papapietro O., Plagnol V., Curtis J., Martins M., Kumararatne D., Cordeiro A.I., Neves C., Borrego L.M., Katan M., Nejentsev S. Novel PLCG2 mutation in a patient with APLAID and Cutis Laxa. Front. Immunol. 2018;9:2863. doi: 10.3389/fimmu.2018.02863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Afroz S., Giddaluru J., Vishwakarma S., Naz S., Khan A.A., Khan N. A comprehensive gene expression meta-analysis identifies novel immune signatures in rheumatoid arthritis patients. Front. Immunol. 2017;8:74. doi: 10.3389/fimmu.2017.00074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Cremasco V., Graham D.B., Novack D.V., Swat W., Faccio R. Vav/Phospholipase Cgamma2-mediated control of a neutrophil-dependent murine model of rheumatoid arthritis. Arthritis Rheum. 2008;58:2712–2722. doi: 10.1002/art.23757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Kozyrev S.V., Abelson A.K., Wojcik J., Zaghlool A., Linga Reddy M.P., Sanchez E., Gunnarsson I., Svenungsson E., Sturfelt G., Jönsen A., Truedsson L., Pons-Estel B.A., Witte T., D'Alfonso S., Barrizzone N. Corrigendum: Functional variants in the B-cell gene BANK1 are associated with systemic lupus erythematosus. Nat. Genet. 2008;40:484. doi: 10.1038/ng0408-484. [DOI] [PubMed] [Google Scholar]
- 114.Rueda B., Gourh P., Broen J., Agarwal S.K., Simeon C., Ortego-Centeno N., Vonk M.C., Coenen M., Riemekasten G., Hunzelmann N., Hesselstrand R., Tan F.K., Reveille J.D., Assassi S., Garcia-Hernandez F.J. BANK1 functional variants are associated with susceptibility to diffuse systemic sclerosis in Caucasians. Ann. Rheum. Dis. 2010;69:700–705. doi: 10.1136/ard.2009.118174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Jacobi A.M., Goldenberg D.M., Hiepe F., Radbruch A., Burmester G.R., Dörner T. Differential effects of epratuzumab on peripheral blood B cells of patients with systemic lupus erythematosus versus normal controls. Ann. Rheum. Dis. 2008;67:450–457. doi: 10.1136/ard.2007.075762. [DOI] [PubMed] [Google Scholar]
- 116.Sieger N., Fleischer S.J., Mei H.E., Reiter K., Shock A., Burmester G.R., Daridon C., Dörner T. CD22 ligation inhibits downstream B cell receptor signaling and Ca(2+) flux upon activation. Arthritis Rheum. 2013;65:770–779. doi: 10.1002/art.37818. [DOI] [PubMed] [Google Scholar]
- 117.Yao Y., Huang W., Li X., Li X., Qian J., Han H., Sun H., An X., Lu L., Zhao H. Tespa1 deficiency dampens thymus-dependent B-cell activation and attenuates collagen-induced arthritis in mice. Front. Immunol. 2018;9:965. doi: 10.3389/fimmu.2018.00965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Hibbs M.L., Harder K.W., Armes J., Kountouri N., Quilici C., Casagranda F., Dunn A.R., Tarlinton D.M. Sustained activation of Lyn tyrosine kinase in vivo leads to autoimmunity. J. Exp. Med. 2002;196:1593–1604. doi: 10.1084/jem.20020515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Bergholdt R., Brorsson C., Palleja A., Berchtold L.A., Fløyel T., Bang-Berthelsen C.H., Frederiksen K.S., Jensen L.J., Størling J., Pociot F. Identification of novel type 1 diabetes candidate genes by integrating genome-wide association data, protein-protein interactions, and human pancreatic islet gene expression. Diabetes. 2012;61:954–962. doi: 10.2337/db11-1263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Lindström S., Wang L., Smith E.N., Gordon W., van Hylckama Vlieg A., de Andrade M., Brody J.A., Pattee J.W., Haessler J., Brumpton B.M., Chasman D.I., Suchon P., Chen M.H., Turman C., Germain M. Genomic and transcriptomic association studies identify 16 novel susceptibility loci for venous thromboembolism. Blood. 2019;134:1645–1657. doi: 10.1182/blood.2019000435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Wen R., Chen Y., Bai L., Fu G., Schuman J., Dai X., Zeng H., Yang C., Stephan R.P., Cleveland J.L., Wang D. Essential role of phospholipase C gamma 2 in early B-cell development and Myc-mediated lymphomagenesis. Mol. Cell. Biol. 2006;26:9364–9376. doi: 10.1128/MCB.00839-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Ahn I.E., Underbayev C., Albitar A., Herman S.E., Tian X., Maric I., Arthur D.C., Wake L., Pittaluga S., Yuan C.M., Stetler-Stevenson M., Soto S., Valdez J., Nierman P., Lotter J. Clonal evolution leading to ibrutinib resistance in chronic lymphocytic leukemia. Blood. 2017;129:1469–1479. doi: 10.1182/blood-2016-06-719294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Gango A., Alpár D., Galik B., Marosvári D., Kiss R., Fésüs V., Aczél D., Eyüpoglu E., Nagy N., Nagy Á., Krizsán S., Reiniger L., Farkas P., Kozma A., Ádám E. Dissection of subclonal evolution by temporal mutation profiling in chronic lymphocytic leukemia patients treated with ibrutinib. Int. J. Cancer. 2020;146:85–93. doi: 10.1002/ijc.32502. [DOI] [PubMed] [Google Scholar]
- 124.Kadri S., Lee J., Fitzpatrick C., Galanina N., Sukhanova M., Venkataraman G., Sharma S., Long B., Petras K., Theissen M., Ming M., Kobzev Y., Kang W., Guo A., Wang W. Clonal evolution underlying leukemia progression and Richter transformation in patients with ibrutinib-relapsed CLL. Blood Adv. 2017;1:715–727. doi: 10.1182/bloodadvances.2016003632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Lampson B.L., Brown J.R. Are BTK and PLCG2 mutations necessary and sufficient for ibrutinib resistance in chronic lymphocytic leukemia? Expert Rev. Hematol. 2018;11:185–194. doi: 10.1080/17474086.2018.1435268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Quinquenel A., Fornecker L.M., Letestu R., Ysebaert L., Fleury C., Lazarian G., Dilhuydy M.S., Nollet D., Guieze R., Feugier P., Roos-Weil D., Willems L., Michallet A.S., Delmer A., Hormigos K. Prevalence of BTK and PLCG2 mutations in a real-life CLL cohort still on ibrutinib after 3 years: A FILO group study. Blood. 2019;134:641–644. doi: 10.1182/blood.2019000854. [DOI] [PubMed] [Google Scholar]
- 127.Kanagal-Shamanna R., Jain P., Patel K.P., Routbort M., Bueso-Ramos C., Alhalouli T., Khoury J.D., Luthra R., Ferrajoli A., Keating M., Jain N., Burger J., Estrov Z., Wierda W., Kantarjian H.M. Targeted multigene deep sequencing of Bruton tyrosine kinase inhibitor-resistant chronic lymphocytic leukemia with disease progression and Richter transformation. Cancer. 2019;125:559–574. doi: 10.1002/cncr.31831. [DOI] [PubMed] [Google Scholar]
- 128.Maddocks K.J., Ruppert A.S., Lozanski G., Heerema N.A., Zhao W., Abruzzo L., Lozanski A., Davis M., Gordon A., Smith L.L., Mantel R., Jones J.A., Flynn J.M., Jaglowski S.M., Andritsos L.A. Etiology of ibrutinib therapy discontinuation and outcomes in patients with chronic lymphocytic leukemia. JAMA Oncol. 2015;1:80–87. doi: 10.1001/jamaoncol.2014.218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Landau D.A., Sun C., Rosebrock D., Herman S.E.M., Fein J., Sivina M., Underbayev C., Liu D., Hoellenriegel J., Ravichandran S., Farooqui M.Z.H., Zhang W., Cibulskis C., Zviran A., Neuberg D.S. The evolutionary landscape of chronic lymphocytic leukemia treated with ibrutinib targeted therapy. Nat. Commun. 2017;8:2185. doi: 10.1038/s41467-017-02329-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Burger J.A., Landau D.A., Taylor-Weiner A., Bozic I., Zhang H., Sarosiek K., Wang L., Stewart C., Fan J., Hoellenriegel J., Sivina M., Dubuc A.M., Fraser C., Han Y., Li S. Clonal evolution in patients with chronic lymphocytic leukaemia developing resistance to BTK inhibition. Nat. Commun. 2016;7:11589. doi: 10.1038/ncomms11589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Jones D., Woyach J.A., Zhao W., Caruthers S., Tu H., Coleman J., Byrd J.C., Johnson A.J., Lozanski G. PLCG2 C2 domain mutations co-occur with BTK and PLCG2 resistance mutations in chronic lymphocytic leukemia undergoing ibrutinib treatment. Leukemia. 2017;31:1645–1647. doi: 10.1038/leu.2017.110. [DOI] [PubMed] [Google Scholar]
- 132.Liu T.M., Woyach J.A., Zhong Y., Lozanski A., Lozanski G., Dong S., Strattan E., Lehman A., Zhang X., Jones J.A., Flynn J., Andritsos L.A., Maddocks K., Jaglowski S.M., Blum K.A. Hypermorphic mutation of phospholipase C, γ2 acquired in ibrutinib-resistant CLL confers BTK independency upon B-cell receptor activation. Blood. 2015;126:61–68. doi: 10.1182/blood-2015-02-626846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Yang C., Lu P., Lee F.Y., Chadburn A., Barrientos J.C., Leonard J.P., Ye F., Zhang D., Knowles D.M., Wang Y.L. Tyrosine kinase inhibition in diffuse large B-cell lymphoma: Molecular basis for antitumor activity and drug resistance of dasatinib. Leukemia. 2008;22:1755–1766. doi: 10.1038/leu.2008.163. [DOI] [PubMed] [Google Scholar]
- 134.Song Z., Lu P., Furman R.R., Leonard J.P., Martin P., Tyrell L., Lee F.Y., Knowles D.M., Coleman M., Wang Y.L. Activities of SYK and PLCgamma2 predict apoptotic response of CLL cells to SRC tyrosine kinase inhibitor dasatinib. Clin. Cancer Res. 2010;16:587–599. doi: 10.1158/1078-0432.CCR-09-1519. [DOI] [PubMed] [Google Scholar]
- 135.Walliser C., Hermkes E., Schade A., Wiese S., Deinzer J., Zapatka M., Désiré L., Mertens D., Stilgenbauer S., Gierschik P. The phospholipase Cγ2 mutants R665W and L845F identified in ibrutinib-resistant chronic lymphocytic leukemia patients are hypersensitive to the Rho GTPase Rac2 protein. J. Biol. Chem. 2016;291:22136–22148. doi: 10.1074/jbc.M116.746842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Huynh M.Q., Goßmann J., Gattenlöehner S., Klapper W., Wacker H.H., Ramaswamy A., Bittner A., Kaiser U., Neubauer A. Expression and pro-survival function of phospholipase Cγ2 in diffuse large B-cell lymphoma. Leuk. Lymphoma. 2015;56:1088–1095. doi: 10.3109/10428194.2014.941832. [DOI] [PubMed] [Google Scholar]
- 137.Kuang Z., Guo L., Li X. Identification of key genes and pathways associated with classical Hodgkin lymphoma by bioinformatics analysis. Mol. Med. Rep. 2017;16:4685–4693. doi: 10.3892/mmr.2017.7158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Gossmann J., Stolte M., Lohoff M., Yu P., Moll R., Finkernagel F., Garn H., Brendel C., Bittner A., Neubauer A., Huynh M.Q. A gain-of-function mutation in the Plcg2 gene protects mice from Helicobacter felis-induced gastric MALT lymphoma. PLoS One. 2016;11 doi: 10.1371/journal.pone.0150411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Follo M.Y., Pellagatti A., Armstrong R.N., Ratti S., Mongiorgi S., De Fanti S., Bochicchio M.T., Russo D., Gobbi M., Miglino M., Parisi S., Martinelli G., Cavo M., Luiselli D., McCubrey J.A. Response of high-risk MDS to azacitidine and lenalidomide is impacted by baseline and acquired mutations in a cluster of three inositide-specific genes. Leukemia. 2019;33:2276–2290. doi: 10.1038/s41375-019-0416-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Engels N., Merchant M., Pappu R., Chan A.C., Longnecker R., Wienands J. Epstein-Barr virus latent membrane protein 2A (LMP2A) employs the SLP-65 signaling module. J. Exp. Med. 2001;194:255–264. doi: 10.1084/jem.194.3.255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Dai Y., Tang Y., He F., Zhang Y., Cheng A., Gan R., Wu Y. Screening and functional analysis of differentially expressed genes in EBV-transformed lymphoblasts. Virol. J. 2012;9:77. doi: 10.1186/1743-422X-9-77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Kaymaz Y., Oduor C.I., Yu H., Otieno J.A., Ong'echa J.M., Moormann A.M., Bailey J.A. Comprehensive transcriptome and mutational profiling of endemic Burkitt lymphoma reveals EBV type–specific differences. Mol. Cancer Res. 2017;15:563–576. doi: 10.1158/1541-7786.MCR-16-0305-T. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Janz S., Potter M., Rabkin C.S. Lymphoma- and leukemia-associated chromosomal translocations in healthy individuals. Genes Chromosomes Cancer. 2003;36:211–223. doi: 10.1002/gcc.10178. [DOI] [PubMed] [Google Scholar]
- 144.Maschietto M., de Camargo B., Brentani H., Grundy P., Sredni S.T., Torres C., Mota L.D., Cunha I.W., Patrão D.F., Costa C.M., Soares F.A., Brentani R.R., Carraro D.M. Molecular profiling of isolated histological components of wilms tumor implicates a common role for the Wnt signaling pathway in kidney and tumor development. Oncology. 2008;75:81–91. doi: 10.1159/000155210. [DOI] [PubMed] [Google Scholar]
- 145.Krepischi A.C.V., Maschietto M., Ferreira E.N., Silva A.G., Costa S.S., da Cunha I.W., Barros B.D.F., Grundy P.E., Rosenberg C., Carraro D.M. Genomic imbalances pinpoint potential oncogenes and tumor suppressors in Wilms tumors. Mol. Cytogenet. 2016;9:20. doi: 10.1186/s13039-016-0227-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Hong J., Behar J., Wands J., Resnick M., Wang L.J., Delellis R.A., Lambeth D., Cao W. Bile acid reflux contributes to development of esophageal adenocarcinoma via activation of phosphatidylinositol-specific phospholipase Cgamma2 and NADPH oxidase NOX5-S. Cancer Res. 2010;70:1247–1255. doi: 10.1158/0008-5472.CAN-09-2774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Stanislaus A., Bakhtiar A., Salleh D., Tiash S., Fatemian T., Hossain S., Akaike T., Chowdhury E.H. Knockdown of PLC-gamma-2 and calmodulin 1 genes sensitizes human cervical adenocarcinoma cells to doxorubicin and paclitaxel. Cancer Cell Int. 2012;12:30. doi: 10.1186/1475-2867-12-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Yang Y., Li H., Hou S., Hu B., Liu J., Wang J. Differences in gene expression profiles and carcinogenesis pathways involved in cisplatin resistance of four types of cancer. Oncol. Rep. 2013;30:596–614. doi: 10.3892/or.2013.2514. [DOI] [PubMed] [Google Scholar]
- 149.Li W.Q., Hu N., Wang Z., Yu K., Su H., Wang L., Wang C., Chanock S.J., Burdett L., Ding T., Qiao Y.L., Fan J.H., Wang Y., Xu Y., Giffen C. Genetic variants in epidermal growth factor receptor pathway genes and risk of esophageal squamous cell carcinoma and gastric cancer in a Chinese population. PLoS One. 2013;8 doi: 10.1371/journal.pone.0068999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Feng A., Tu Z., Yin B. The effect of HMGB1 on the clinicopathological and prognostic features of non-small cell lung cancer. Oncotarget. 2016;7:20507–20519. doi: 10.18632/oncotarget.7050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Qi F., Qin W.X., Zang Y.S. Molecular mechanism of triple-negative breast cancer-associated BRCA1 and the identification of signaling pathways. Oncol. Lett. 2019;17:2905–2914. doi: 10.3892/ol.2019.9884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Rudolph A., Fasching P.A., Behrens S., Eilber U., Bolla M.K., Wang Q., Thompson D., Czene K., Brand J.S., Li J., Scott C., Pankratz V.S., Brandt K., Hallberg E., Olson J.E. A comprehensive evaluation of interaction between genetic variants and use of menopausal hormone therapy on mammographic density. Breast Cancer Res. 2015;17:110. doi: 10.1186/s13058-015-0625-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Rudolph A., Hein R., Lindström S., Beckmann L., Behrens S., Liu J., Aschard H., Bolla M.K., Wang J., Truong T., Cordina-Duverger E., Menegaux F., Brüning T., Harth V., GENICA Network Genetic modifiers of menopausal hormone replacement therapy and breast cancer risk: A genome-wide interaction study. Endocr. Relat. Cancer. 2013;20:875–887. doi: 10.1530/ERC-13-0349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Gyanchandani R., Ortega Alves M.V., Myers J.N., Kim S. A proangiogenic signature is revealed in FGF-mediated bevacizumab-resistant head and neck squamous cell carcinoma. Mol. Cancer Res. 2013;11:1585–1596. doi: 10.1158/1541-7786.MCR-13-0358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Lo Vasco V.R., Leopizzi M., Puggioni C., Della Rocca C. Ezrin silencing remodulates the expression of Phosphoinositide-specific phospholipase C enzymes in human osteosarcoma cell lines. J. Cell. Commun. Signal. 2014;8:219–229. doi: 10.1007/s12079-014-0235-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Lo Vasco V.R., Leopizzi M., Stoppoloni D., Della Rocca C. Silencing of phosphoinositide-specific phospholipase C ε remodulates the expression of the phosphoinositide signal transduction pathway in human osteosarcoma cell lines. Anticancer Res. 2014;34:4069–4075. [PubMed] [Google Scholar]
- 157.Sun L., Li J., Yan B. Gene expression profiling analysis of osteosarcoma cell lines. Mol. Med. Rep. 2015;12:4266–4272. doi: 10.3892/mmr.2015.3958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Park W.Y., Hwang C.I., Im C.N., Kang M.J., Woo J.H., Kim J.H., Kim Y.S., Kim J.H., Kim H., Kim K.A., Yu H.J., Lee S.J., Lee Y.S., Seo J.S. Identification of radiation-specific responses from gene expression profile. Oncogene. 2002;21:8521–8528. doi: 10.1038/sj.onc.1205977. [DOI] [PubMed] [Google Scholar]
- 159.Sims R., van der Lee S.J., Naj A.C., Bellenguez C., Badarinarayan N., Jakobsdottir J., Kunkle B.W., Boland A., Raybould R., Bis J.C., Martin E.R., Grenier-Boley B., Heilmann-Heimbach S., Chouraki V., Kuzma A.B. Rare coding variants in PLCG2, ABI3, and TREM2 implicate microglial-mediated innate immunity in Alzheimer's disease. Nat. Genet. 2017;49:1373–1384. doi: 10.1038/ng.3916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Conway O.J., Carrasquillo M.M., Wang X., Bredenberg J.M., Reddy J.S., Strickland S.L., Younkin C.S., Burgess J.D., Allen M., Lincoln S.J., Nguyen T., Malphrus K.G., Soto A.I., Walton R.L., Boeve B.F. ABI3 and PLCG2 missense variants as risk factors for neurodegenerative diseases in Caucasians and African Americans. Mol. Neurodegener. 2018;13:53. doi: 10.1186/s13024-018-0289-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Tesi N., van der Lee S.J., Hulsman M., Jansen I.E., Stringa N., van Schoor N., Meijers-Heijboer H., Huisman M., Scheltens P., Reinders M.J.T., van der Flier W.M., Holstege H. Centenarian controls increase variant effect sizes by an average twofold in an extreme case-extreme control analysis of Alzheimer's disease. Eur. J. Hum. Genet. 2019;27:244–253. doi: 10.1038/s41431-018-0273-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Dalmasso M.C., Brusco L.I., Olivar N., Muchnik C., Hanses C., Milz E., Becker J., Heilmann-Heimbach S., Hoffmann P., Prestia F.A., Galeano P., Avalos M.S.S., Martinez L.E., Carulla M.E., Azurmendi P.J. Transethnic meta-analysis of rare coding variants in PLCG2, ABI3, and TREM2 supports their general contribution to Alzheimer's disease. Transl. Psychiatry. 2019;9:55. doi: 10.1038/s41398-019-0394-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.van der Lee S.J., Conway O.J., Jansen I., Carrasquillo M.M., Kleineidam L., van den Akker E., Hernández I., van Eijk K.R., Stringa N., Chen J.A., Zettergren A., Andlauer T.F.M., Diez-Fairen M., Simon-Sanchez J., Lleó A. A nonsynonymous mutation in PLCG2 reduces the risk of Alzheimer's disease, dementia with Lewy bodies and frontotemporal dementia, and increases the likelihood of longevity. Acta Neuropathol. 2019;138:237–250. doi: 10.1007/s00401-019-02026-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Magno L., Lessard C.B., Martins M., Lang V., Cruz P., Asi Y., Katan M., Bilsland J., Lashley T., Chakrabarty P., Golde T.E., Whiting P.J. Alzheimer's disease phospholipase C-gamma-2 (PLCG2) protective variant is a functional hypermorph. Alzheimers Res. Ther. 2019;11:16. doi: 10.1186/s13195-019-0469-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Castillo E., Leon J., Mazzei G., Abolhassani N., Haruyama N., Saito T., Saido T., Hokama M., Iwaki T., Ohara T., Ninomiya T., Kiyohara Y., Sakumi K., LaFerla F.M., Nakabeppu Y. Comparative profiling of cortical gene expression in Alzheimer's disease patients and mouse models demonstrates a link between amyloidosis and neuroinflammation. Sci. Rep. 2017;7:17762. doi: 10.1038/s41598-017-17999-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Sierksma A., Lu A., Mancuso R., Fattorelli N., Thrupp N., Salta E., Zoco J., Blum D., Buée L., De Strooper B., Fiers M. Novel Alzheimer risk genes determine the microglia response to amyloid-β but not to TAU pathology. EMBO Mol. Med. 2020;12 doi: 10.15252/emmm.201910606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Andreone B.J., Przybyla L., Llapashtica C., Rana A., Davis S.S., van Lengerich B., Lin K., Shi J., Mei Y., Astarita G., Di Paolo G., Sandmann T., Monroe K.M., Lewcock J.W. Alzheimer's-associated PLCγ2 is a signaling node required for both TREM2 function and the inflammatory response in human microglia. Nat. Neurosci. 2020;23:927–938. doi: 10.1038/s41593-020-0650-6. [DOI] [PubMed] [Google Scholar]