Abstract
The development of resistance to tyrosine kinase inhibitors (TKIs) in metastatic non-small cell lung cancer (NSCLC) with oncogenic driver mutations highlights the challenge in improving the survival of these patients. The standard of care for ALK-rearranged advanced NSCLC refractory to various generations of ALK TKIs falls back to the use of chemotherapy and the prognosis remains poor. We report the case of a 41-year-old lady with an ALK-translocated metastatic lung adenocarcinoma, who demonstrated good response to an immune checkpoint inhibitor, atezolizumab in combination with bevacizumab and chemotherapy (pemetrexed and carboplatin), following disease progression on three generations of ALK TKIs. Six months into treatment, she continues to show improvement in her health-related quality of life and is tolerating treatment well. Our case suggests that this treatment regimen is a potential treatment option for TKI-refractory driver-mutated NSCLC.
Keywords: Lung cancer, Non-small cell, Adenocarcinoma, Atezolizumab, ALK, Immune checkpoint inhibitor
Abbreviations: TKI, tyrosine kinase inhibitor; NSCLC, non-small cell lung cancer; ICI, immune checkpoint inhibitor; ALK, anaplastic lymphoma kinase; PD-1, programmed death-1; PDL-1, programmed death ligand-1
Highlights
-
•
Treatment options for ALK-positive advanced lung cancer refractory to ALK tyrosine kinase inhibitors are limited.
-
•
Atezolizumab combination therapy is a promising therapeutic strategy for ALK-positive advanced lung cancer.
-
•
The potential of an atezolizumab combination therapy may be realized with tolerable treatment-related toxicities.
1. Introduction
Treating anaplastic lymphoma kinase (ALK)-translocated metastatic non-small cell lung cancer (NSCLC) with ALK tyrosine kinase inhibitors (TKIs) is a validated molecular-targeted strategy that is superior to chemotherapy in terms of progression-free survival (PFS) [1,2]. However, the durability of its clinical benefit remains limited by the emergence of resistance to ALK TKI. The standard of care for metastatic NSCLC with ALK translocation refractory to various generations of ALK TKIs falls back to chemotherapy and the prognosis remains poor.
Immune checkpoint inhibitors (ICIs), specifically programmed death-1 (PD-1) and programmed death-ligand 1 (PD-L1) inhibitors, have revolutionized the treatment landscape of metastatic NSCLC with their improved survival rates and controlled toxicity. Atezolizumab is the first PD-L1 inhibitor to have shown robust efficacy in metastatic NSCLC [3,4].
However, information on the efficacy of ICIs in advanced NSCLC with oncogenic driver alterations remains scarce and contradictory. Studies have shown low objective response rates and short median PFS of 0.6–1.9 months with the use of ICI monotherapy in advanced NSCLCs harboring ALK translocations regardless of PD-L1 expression status [[5], [6], [7]]. The IMPOWER150 trial demonstrated that the addition of atezolizumab to bevacizumab and chemotherapy significantly improved median PFS (9.7 months vs. 6.1 months; hazard ratio, 0.59; 95% CI, 0.37 to 0.94) in metastatic NSCLC with ALK or EGFR alterations, albeit the small number of ALK-positive patients in this study (n = 34, 4.3%) limits definitive conclusions from being drawn in this subgroup of patients [4]. Regardless, findings of this landmark trial suggest that driver-mutated NSCLC that has ceased to respond to TKIs may benefit from an atezolizumab combination therapy.
Herein, we report the case of a young patient with ALK-positive metastatic lung adenocarcinoma, with a remarkable response to a combinatorial regimen of atezolizumab, bevacizumab, pemetrexed, and carboplatin, following disease progression on three generations of ALK TKIs.
2. Case report
In December 2014, a 41-year-old lady with no smoking history and no symptoms was noted to have a nodule at the lingula of her left lung on chest X-ray. A computed tomography (CT)-guided percutaneous biopsy of the nodule was performed, and histopathological examination (HPE) confirmed adenocarcinoma. This patient underwent left thoracotomy and wedge excision of the left lingula, left lower lobe, and pleural nodules in January 2015. Multiple pleural nodules were noted intra-operatively. The pathological stage was pT2N0M1a (Stage IV). The tumor was found to be positive for ALK and negative for EGFR and ROS1.
From 2015 up until 2020, she had received three generations of ALK inhibitors; each switch in ALK inhibitor prompted by disease progression as per RECIST Criteria 1.1 [8]. She was first started on crizotinib, a first-generation ALK inhibitor from February 2015 up until September 2016. She was then switched to ceritinib, a second-generation ALK inhibitor from September 2016 to May 2017, and subsequently to lorlatinib, a third-generation ALK inhibitor up until July 2020.
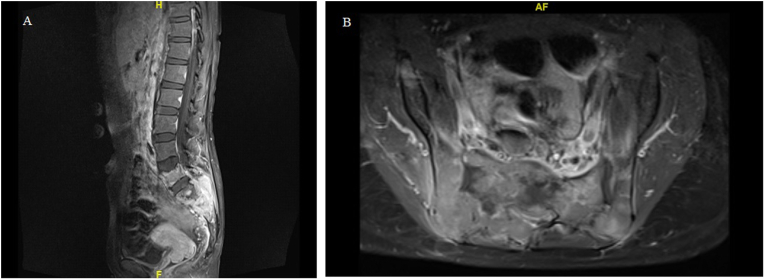
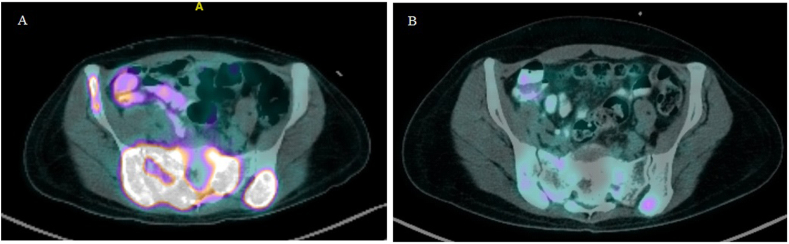
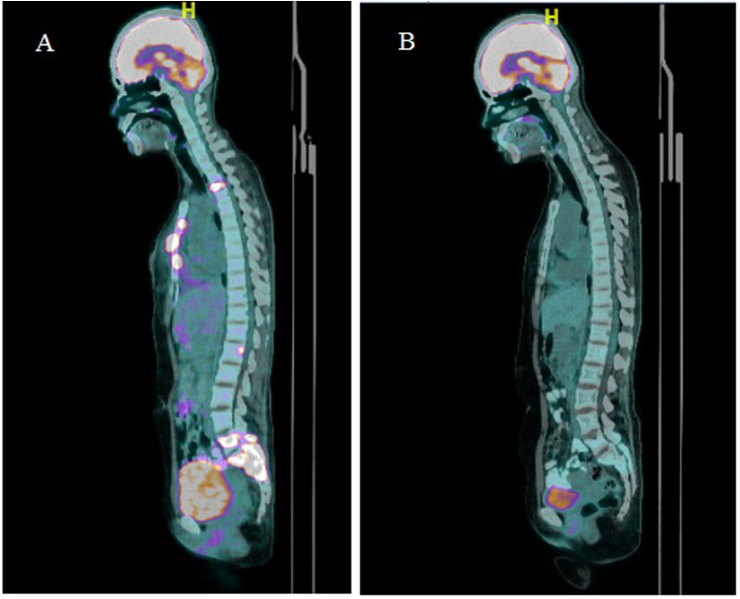
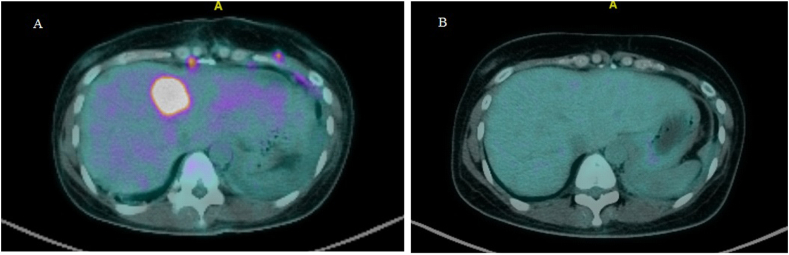
In July 2020, she presented with a two-day history of urinary retention, severe pain at the sacrum causing immobility and paresthesia at the S1 to S4 dermatome. On examination, a new left supraclavicular fossa node was palpable. A biopsy was performed, and HPE confirmed metastatic adenocarcinoma. Next-generation sequencing of the metastatic lesion revealed ALK positivity. PD-L1 IHC testing was done using SP142 and 22C3 assays. PD-L1 was found to be positive in the SP142 assay and PD-L1 expression was found to be high (tumor proportion score ≥ 50%) in the 22C3 assay. Magnetic resonance imaging (MRI) of the spine showed extensive bone metastases with S1 pathological fracture causing spinal canal stenosis at S1 and S2 (Fig. 1a and b). Positron emission tomography–computed tomography (PET-CT) demonstrated recurrence at the left lower lobe. There were extensive metastatic lesions at the bone and liver (Fig. 2, Fig. 3, Fig. 4A). Her disease was progressing. At this point, a spine surgeon was involved in her care. She was counseled for minimally invasive stabilization surgery of L3 to the pelvis to relieve her symptoms. This patient declined surgical intervention.
Fig. 1.
MRI of the whole spine demonstrating S1 pathological fracture with spinal canal stenosis at S1 and S2. (A) Sagittal view (B) Axial view.
Fig. 2.
PET-CT scans of the patient during treatment. (A) Baseline before treatment: Multiple avid pelvic bone lesions including right iliac bone (SUV 9.2), sacral bone (SUV 9.2), right acetabulum (SUV 6.4), left iliac bone (SUV 7.7). (B) After 4 cycles of treatment: Partial metabolic response in left iliac bone (SUV 2.0) and sacral bone (SUV 2.1), complete metabolic response in the remaining lesions.
Fig. 3.
PET-CT scans of the patient during treatment. (A) Baseline before treatment: Avid bony lesions at the sternum (SUV 7.1), T2 (SUV 6.0), L1 (SUV 6.2), L4 (SUV 7.3), and L5 vertebrae (SUV 7.8) and sacral bone (SUV 9.2). (B) After 4 cycles of treatment: Partial metabolic response at L1 (SUV 2.3) and L5/S1 (SUV 2.1), complete metabolic response in the remaining lesions.
Fig. 4.
PET-CT of the patient during treatment. (A) Baseline scan before treatment: Intra-hepatic avid lesions. (B) After 4 cycles of treatment: Complete metabolic response.
She was then started on atezolizumab (1200mg) combined with bevacizumab (7.5mg/kg), pemetrexed (500mg/m2), and carboplatin (AUC 5), every 3 weeks since the July 14, 2020.
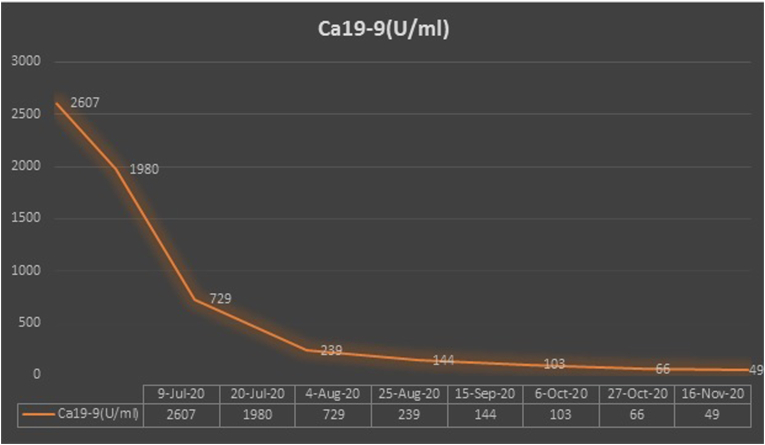
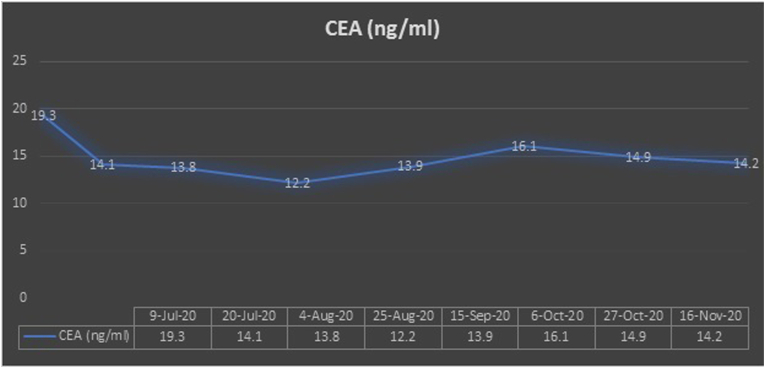
Following her first cycle, improvement in her clinical symptoms was observed. She had previously been bedbound due to her severe sacral pain and had a urinary catheter inserted for urinary retention. Her pain and paresthesia improved markedly following her first cycle of treatment. She was able to ambulate using a walking frame and her urinary retention resolved. There was also a significant decrease in her tumor markers – carcinoembryonic antigen (CEA) and carbohydrate antigen 19–9 (Ca19-9) (Fig. 5, Fig. 6).
Fig. 5.
Carbohydrate antigen 19–9 (Ca19-9) trend.
Fig. 6.
Carcinoembryonic antigen (CEA) trend.
A PET-CT following Cycle 4 was arranged to assess treatment response. There was a complete metabolic response of the liver lesions, and a partial metabolic response of the bony lesions (Fig. 2, Fig. 3, Fig. 4B). A decrease in size of the left lower lobe pleural mass was noted measuring 2.34 × 3.12cm (previously 2.47 × 3.63cm). Of note, this patient regained her ability to ambulate independently. Her sacral pain and paresthesia improved significantly.
Following Cycle 4, she was continued on maintenance atezolizumab, bevacizumab, and pemetrexed. At the point of writing, she has received up to Cycle 8 of this treatment. This lady tolerated treatment well. Treatment-related toxicities were mainly grade 1 nausea and grade 2 hypertension. She continues to show improvement in her health-related quality of life. Her Ca19-9 continues to decrease while her CEA plateaus.
3. Discussion
We describe a patient with ALK-translocated metastatic lung adenocarcinoma whose disease progressed on three generations of ALK TKIs within a span of five years and subsequently responded to an ICI combinatorial regimen comprised of atezolizumab, bevacizumab, carboplatin, and pemetrexed. Following the discontinuation of carboplatin after the fourth cycle of treatment, the patient continued demonstrating clinical and biochemical response, suggesting that the benefit of atezolizumab, bevacizumab, and pemetrexed remained.
The emergence of resistance mutations during or after sequential ALK TKIs narrows the subsequent therapeutic options available for this group of patients. Examples of mutations include C1156Y/I1171N after progression on sequential crizotinib, ceritinib, and alectinib, and L1198F contributing to lorlatinib resistance [9]. The development of such resistance is an expected consequence of tumor evolution.
There is a growing body of evidence that the strategy to target the tumor micro-environment is to induce an anti-tumoral immune response and inhibit angiogenesis. Vascular endothelial growth factor (VEGF), the critical driver of tumor angiogenesis, is a potent immunosuppressive factor in anti-tumor immunity [10]. The abnormal tumor vasculature induced by VEGF serves as a physical barrier for cytotoxic T-cells [10]. Furthermore, VEGF inhibits the maturation of dendritic cells, thereby interrupting T-cell priming against tumors [10]. There is pre-clinical evidence demonstrating that anti-angiogenic therapy with anti-vascular endothelial growth factor receptor 2 (VEGFR2) enhanced the efficacy of anti-PD-L1 immunotherapy in pancreatic and breast tumor mouse models [11]. VEGF inhibition reverses VEGF-mediated immunosuppression and limits blood supply to the tumor [10]. The reduction in tumor vasculature increases vascular permeability and may enhance intra-tumoral T-cell infiltration and delivery of chemotherapy into tumor cells [10,12]. Several mechanisms in which chemotherapy has been found to increase the efficacy of immunotherapy include enhancement of tumor antigen cross-presentation and elimination of immunosuppressive cells such as T-regulatory cells [[13], [14], [15], [16], [17]].
Atezolizumab is an immune checkpoint inhibitor that binds to PD-L1, an immune checkpoint protein and blocks its interaction with programmed death 1 (PD-1) and B7–1 receptors on T-cells (3). The interaction between PD-L1 and its receptors, PD-1 and B7-1 results in T-cell dysfunction, hence, reducing the tumor-killing ability of effector T-cells [18]. Hindering this interaction restores anti-tumor T-cell activity [18]. Bevacizumab is a humanized monoclonal antibody that selectively binds circulating vascular endothelial growth factor (VEGF), thereby inhibiting the binding of VEGF to its cell surface receptors [12].
Several phase 3 trials have demonstrated that ICI monotherapy is ineffective for patients with driver-mutated NSCLC [3, 19]. It is postulated that EML4-ALK fusion induces immune escape in NSCLC by upregulating PD-L1 via the activation of downstream oncogenic signaling pathways such as the PI3K-AKT pathway [20]. Hence, the notion of altering tumor microenvironment via the synergistic effects of immunotherapy, anti-angiogenesis, and chemotherapy is an appealing combination strategy to improve the survival of this subgroup of patients after TKI failure. Findings of the IMPOWER150 trial consolidates the above theories and pre-clinical evidence demonstrating significant PFS benefit and an acceptable safety profile when atezolizumab is used in combination with bevacizumab and platinum doublet chemotherapy in driver-mutated NSCLC [4].
The use of atezolizumab, bevacizumab, carboplatin, and pemetrexed in our case represents a novel combination that has yet to be explored in ALK-mutated metastatic NSCLC. The rationale of using pemetrexed in this case is in tandem with evidence demonstrating superior overall response rate and PFS in ALK-translocated patients with the use of pemetrexed compared with EGFR mutant or wild type NSCLC patients (46.7 versus 4.7 versus 16.2%, p = 0.001); 9.2 versus 1.4 versus 2.9 months, p = 0.001) [21]. In addition, Shaw et al. reported that among patients who received platinum and pemetrexed combination in any setting, ALK-positive patients showed a trend towards longer PFS compared with the ALK-negative patients [22]. The authors offered a possible explanation to this highlighting that thymidylate synthase (TS), one of the key folate enzymes targeted by pemetrexed, is observed to be lower in ALK-positive patients [22]. This is postulated to increase pemetrexed sensitivity [22]. This applies to our patient who has an ALK-translocated metastatic NSCLC.
The fact that our patient showed good response to this treatment regimen with tolerable side effects highlights that the potential of such a combination regimen may be realized without causing significant treatment-related toxicities. Our patient had a good baseline Eastern Cooperative Oncology Group (ECOG) performance status of 0. Upon disease progression, a drastic decline in performance status was observed due to the sacral pain and paresthesia secondary to her bone metastases. The decision to offer an ICI combination therapy was made given her age, good baseline performance status, and that her poor performance status was potentially reversible if her disease responded to treatment. Her performance status did improve significantly as her disease responded to treatment. Carboplatin and pemetrexed were the choices of platinum doublet chemotherapeutic agents in this case in view of their favorable toxicity profiles [23,24].
Findings of the phase 2 trial on the use of atezolizumab, bevacizumab, pemetrexed, and carboplatin combination for metastatic EGFR-mutated NSCLC after TKI failure demonstrated promising efficacy and an acceptable safety profile (25). At a median follow-up of 11 months, the objective response rate was 62.5% and median PFS was 9.43 months (95% CI: 7.62–12.1 months) [25]. Grade 3 and above treatment-related adverse events were reported in 37.5% of the patients [25].
At the point of writing, there is an ongoing phase 2 randomized trial investigating the use of carboplatin, pemetrexed, and bevacizumab, with or without atezolizumab in stage 4 EGFR-mutated NSCLC [26]. The subject on the optimal ICI combinatorial regimen in driver-mutated NSCLC after TKI failure is of great importance and relevance in the quest to prolong the survival of this subgroup of patients.
We believe that our case report is an essential contribution to the scarce literature on the use of ICI combination therapy in ALK-positive metastatic NSCLC. Further challenges moving forward include the need to better define patients who may benefit from this novel combination, hence, necessitating the identification of biomarkers predicting efficacy. The optimal timing and duration of treatment is an important factor that will need to be addressed for the formulation of an effective therapeutic model. We hope that our case report serves as an impetus for further research to be done on this subject.
4. Conclusion
NSCLC is a malignancy with high incidence and poor prognosis. The optimal approach in managing driver-mutated metastatic NSCLC that is refractory to TKIs is a pertinent question that needs to be addressed to improve the survival of these patients. The use of atezolizumab in combination with bevacizumab and chemotherapy represents a promising therapeutic strategy. The use of this treatment regimen should be researched more extensively to optimize its therapeutic benefits.
Ethical approval statement
Written informed consent was obtained from the patient. Ethics committee approval was not necessary for this case report because it does not constitute research where it was conducted.
Author contributions
Cheong E Von: Conceptualization, drafting the work, revising the work critically for important intellectual content. Ho Gwo Fuang: Final approval of the version to be published, agrees to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgment
We would like to thank our patient who consented to have her case discussed for educational purposes.
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Contributor Information
Cheong E Von, Email: evon@ummc.edu.my.
Ho Gwo Fuang, Email: gwoho@um.edu.my.
References
- 1.Shaw A.T., Kim D.W., Nakagawa K., Seto T., Crinó L., Ahn M.J. Crizotinib versus chemotherapy in advanced ALK-positive lung cancer. N. Engl. J. Med. 2013;368(25):2385–2394. doi: 10.1056/NEJMoa1214886. [DOI] [PubMed] [Google Scholar]
- 2.Shaw A.T., Kim T.M., Crinò L., Gridelli C., Kiura K., Liu G. Ceritinib versus chemotherapy in patients with ALK-rearranged non-small-cell lung cancer previously given chemotherapy and crizotinib (ASCEND-5): a randomised, controlled, open-label, phase 3 trial. Lancet Oncol. 2017;18(7):874–886. doi: 10.1016/S1470-2045(17)30339-X. [DOI] [PubMed] [Google Scholar]
- 3.Rittmeyer A., Barlesi F., Waterkamp D., Park K., Ciardiello F., von Pawel J. Atezolizumab versus docetaxel in patients with previously treated non-small-cell lung cancer (OAK): a phase 3, open-label, multicentre randomised controlled trial. Lancet. 2017;389(10066):255–265. doi: 10.1016/S0140-6736(16)32517-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Socinski M.A., Jotte R.M., Cappuzzo F., Orlandi F., Stroyakovskiy D., Nogami N. Atezolizumab for first-line treatment of metastatic nonsquamous NSCLC. N. Engl. J. Med. 2018;378(24):2288–2301. doi: 10.1056/NEJMoa1716948. [DOI] [PubMed] [Google Scholar]
- 5.Gainor J.F., Shaw A.T., Sequist L.V., Fu X., Azzoli C.G., Piotrowska Z. EGFR mutations and ALK rearrangements are associated with low response rates to PD-1 pathway blockade in non-small cell lung cancer: a retrospective analysis. Clin. Canc. Res. : an official journal of the American Association for Cancer Research. 2016;22(18):4585–4593. doi: 10.1158/1078-0432.CCR-15-3101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Garassino M.C., Cho B.C., Kim J.H., Mazières J., Vansteenkiste J., Lena H. Durvalumab as third-line or later treatment for advanced non-small-cell lung cancer (ATLANTIC): an open-label, single-arm, phase 2 study. Lancet Oncol. 2018;19(4):521–536. doi: 10.1016/S1470-2045(18)30144-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Oya Y., Kuroda H., Nakada T., Takahashi Y., Sakakura N., Hida T. Efficacy of immune checkpoint inhibitor monotherapy for advanced non-small-cell lung cancer with ALK rearrangement. Int. J. Mol. Sci. 2020;21(7):2623. doi: 10.3390/ijms21072623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Schwartz L.H., Litière S., de Vries E., Ford R., Gwyther S., Mandrekar S. vol. 62. European journal of cancer; 2016. pp. 132–137. (RECIST 1.1-Update and Clarification: from the RECIST Committee). (Oxford, England : 1990) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lin J.J., Riely G.J., Shaw A.T. Targeting ALK: precision medicine takes on drug resistance. Canc. Discov. 2017;7(2):137–155. doi: 10.1158/2159-8290.CD-16-1123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lee W.S., Yang H., Chon H.J., Kim C. Combination of anti-angiogenic therapy and immune checkpoint blockade normalizes vascular-immune crosstalk to potentiate cancer immunity. Exp. Mol. Med. 2020;52(9):1475–1485. doi: 10.1038/s12276-020-00500-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Allen E., Jabouille A., Rivera L.B., Lodewijckx I., Missiaen R., Steri V. Combined antiangiogenic and anti-PD-L1 therapy stimulates tumor immunity through HEV formation. Sci. Transl. Med. 2017;9(385) doi: 10.1126/scitranslmed.aak9679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kazazi-Hyseni F., Beijnen J.H., Schellens J.H.M. Bevacizumab. Oncologist. 2010;15(8):819–825. doi: 10.1634/theoncologist.2009-0317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Uemura T., Hida T. Additive effects of atezolizumab and bevacizumab plus chemotherapy for patients with non-small cell lung cancer regardless of presence of EGFR mutations, ALK rearrangements, or PD-L1 expression. Transl. Cancer Res. 2018:S796–S801. [Google Scholar]
- 14.Apetoh L., Ladoire S., Coukos G., Ghiringhelli F. Combining immunotherapy and anticancer agents: the right path to achieve cancer cure? Ann. Oncol. 2015;26(9):1813–1823. doi: 10.1093/annonc/mdv209. [DOI] [PubMed] [Google Scholar]
- 15.Zitvogel L., Galluzzi L., Smyth M.J., Kroemer G. Mechanism of action of conventional and targeted anticancer therapies: reinstating immunosurveillance. Immunity. 2013;39(1):74–88. doi: 10.1016/j.immuni.2013.06.014. [DOI] [PubMed] [Google Scholar]
- 16.Lesterhuis W.J., Punt C.J., Hato S.V., Eleveld-Trancikova D., Jansen B.J., Nierkens S. Platinum-based drugs disrupt STAT6-mediated suppression of immune responses against cancer in humans and mice. J. Clin. Invest. 2011;121(8):3100–3108. doi: 10.1172/JCI43656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhang P., Ma Y., Lv C., Huang M., Li M., Dong B. Upregulation of programmed cell death ligand 1 promotes resistance response in non-small-cell lung cancer patients treated with neo-adjuvant chemotherapy. Canc. Sci. 2016;107(11):1563–1571. doi: 10.1111/cas.13072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tang F., Zheng P. Tumor cells versus host immune cells: whose PD-L1 contributes to PD-1/PD-L1 blockade mediated cancer immunotherapy? Cell Biosci. 2018;8(1):34. doi: 10.1186/s13578-018-0232-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Borghaei H., Paz-Ares L., Horn L., Spigel D.R., Steins M., Ready N.E. Nivolumab versus docetaxel in advanced nonsquamous non–small-cell lung cancer. N. Engl. J. Med. 2015;373(17):1627–1639. doi: 10.1056/NEJMoa1507643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ota K., Azuma K., Kawahara A., Hattori S., Iwama E., Tanizaki J. Induction of PD-L1 expression by the EML4–ALK oncoprotein and downstream signaling pathways in non–small cell lung cancer. Clin. Canc. Res. 2015;21(17):4014–4021. doi: 10.1158/1078-0432.CCR-15-0016. [DOI] [PubMed] [Google Scholar]
- 21.Lee J.O., Kim T.M., Lee S.H., Kim D.W., Kim S., Jeon Y.K. Anaplastic lymphoma kinase translocation: a predictive biomarker of pemetrexed in patients with non-small cell lung cancer. J. Thorac. Oncol. : official publication of the International Association for the Study of Lung Cancer. 2011;6(9):1474–1480. doi: 10.1097/JTO.0b013e3182208fc2. [DOI] [PubMed] [Google Scholar]
- 22.Shaw A.T., Varghese A.M., Solomon B.J., Costa D.B., Novello S., Mino-Kenudson M. Pemetrexed-based chemotherapy in patients with advanced, ALK-positive non-small cell lung cancer. Ann. Oncol. 2013;24(1):59–66. doi: 10.1093/annonc/mds242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Santana-Davila R., Szabo A., Arce-Lara C., Williams C.D., Kelley M.J., Whittle J. Cisplatin versus carboplatin-based regimens for the treatment of patients with metastatic lung cancer. An analysis of Veterans Health Administration data. J. Thorac. Oncol. : official publication of the International Association for the Study of Lung Cancer. 2014;9(5):702–709. doi: 10.1097/JTO.0000000000000146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Scagliotti G.V., Parikh P., von Pawel J., Biesma B., Vansteenkiste J., Manegold C. Phase III study comparing cisplatin plus gemcitabine with cisplatin plus pemetrexed in chemotherapy-naive patients with advanced-stage non-small-cell lung cancer. J. Clin. Oncol. : official journal of the American Society of Clinical Oncology. 2008;26(21):3543–3551. doi: 10.1200/JCO.2007.15.0375. [DOI] [PubMed] [Google Scholar]
- 25.Lam T.C., Tsang K., Choi H., Lee V.H.F., Lam K.O., Chiang C.L. 380MO A phase II trial of atezolizumab, bevacizumab, pemetrexed and carboplatin combination for metastatic EGFR-mutated NSCLC after TKI failure. Ann. Oncol. 2020;31:S1389. doi: 10.1016/j.lungcan.2021.07.004. [DOI] [PubMed] [Google Scholar]
- 26.Bodor J.N., Patel J.D., Ross E.A., Litwin S., Clapper M., Levy B.P. Phase II randomized trial of carboplatin + pemetrexed + bevacizumab, +/- atezolizumab in stage IV non-squamous non-small lung cancer (NSCLC) patients who harbor a sensitizing EGFR mutation or have never smoked. J. Clin. Oncol. 2020;38(15_suppl) doi: 10.1016/j.cllc.2023.05.003. TPS9629-TPS. [DOI] [PMC free article] [PubMed] [Google Scholar]