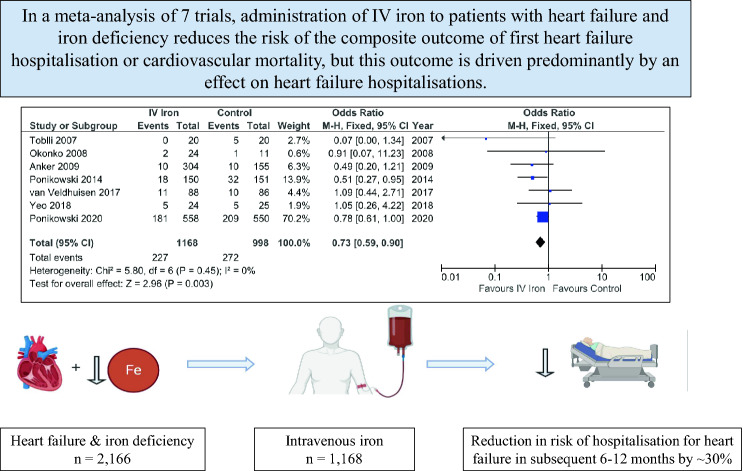
Abstract
Background
The recent AFFIRM-AHF trial assessing the effect of intravenous (IV) iron on outcomes in patients hospitalised with worsening heart failure who had iron deficiency (ID) narrowly missed its primary efficacy endpoint of recurrent hospitalisations for heart failure (HHF) or cardiovascular (CV) death. We conducted a meta-analysis to determine whether these results were consistent with previous trials.
Methods
We searched for randomised trials of patients with heart failure investigating the effect of IV iron vs placebo/control groups that reported HHF and CV mortality from 1st January 2000 to 5th December 2020. Seven trials were identified and included in this analysis. A fixed effect model was applied to assess the effects of IV iron on the composite of first HHF or CV mortality and individual components of these.
Results
Altogether, 2,166 patients were included (n = 1168 assigned to IV iron; n = 998 assigned to control). IV iron reduced the composite of HHF or CV mortality substantially [OR 0.73; (95% confidence interval 0.59–0.90); p = 0.003]. Outcomes were consistent for the pooled trials prior to AFFIRM-AHF. Whereas first HHF were reduced substantially [OR 0.67; (0.54–0.85); p = 0.0007], the effect on CV mortality was uncertain but appeared smaller [OR 0.89; (0.66–1.21); p = 0.47].
Conclusion
Administration of IV iron to patients with heart failure and ID reduces the risk of the composite outcome of first heart failure hospitalisation or cardiovascular mortality, but this outcome may be driven predominantly by an effect on HHF. At least three more substantial trials of intravenous iron are underway.
Graphic abstract
Supplementary Information
The online version contains supplementary material available at 10.1007/s00392-021-01837-8.
Keywords: Iron deficiency, Heart failure, Intravenous iron, Meta-analysis
Introduction
Patients with heart failure often have evidence of iron deficiency (ID), with or without anaemia, which is associated with more severe symptoms, lower exercise capacity and higher rates of hospitalisations for heart failure (HHF) and mortality [1, 2]. In an individual patient meta-analysis of four trials including 839 patients with heart failure with reduced ejection fraction (HFrEF) and serum markers of ID, Anker and colleagues suggested that administration of intravenous iron (IV) reduced the risk of first and recurrent HHF when compared to placebo [3]. Recently, the AFFIRM-AHF trial narrowly missed its primary efficacy endpoint of recurrent HHF or cardiovascular (CV) death [4]. Therefore, we produced an updated the meta-analysis to investigate whether the effects of IV iron were consistent amongst the randomised trials reported so far and whether sufficient evidence had accumulated to indicate a conclusive effect on HHF and CV mortality.
Methods
We searched for English language trials from 1st January 2000 to 5th December 2020 in PubMed using pre-specified search terms (see Supplements), and from additional sources including a recent systematic review [5]. Only published randomised trials investigating the effects of IV iron compared to a control group that did not receive IV iron in patients with heart failure, regardless of participants’ left ventricular ejection fraction, the formulation of IV iron, concomitant therapy, or definition of ID, that reported either HHF or CV mortality were included in the main report. If mortality was not explicitly reported but HHF was, it was assumed that no deaths had occurred. An additional analysis was done including two unpublished trials, with data derived from the meta-analysis reported by Anker et al. [3].
Data were extracted by two independent reviewers (FG and PP). Deaths not clearly declared as CV or non-CV were adjudicated independently by two authors, both of whom are experienced in clinical end-point adjudication. Adjudication was based on the clinical information provided by authors in the text. Disparities were resolved by discussion or by checking with a third author (JGFC). Outcomes assessed were the composite of HHF or CV mortality as first events, and HHF as a first event and CV mortality separately. Data analysed were the numbers of first events and numbers of participants in each treatment arm for each trial. Odds ratios and 95% confidence intervals for the effect of treatment with IV iron relative to control were calculated for each trial. The data were analysed using both fixed effects (primary analysis) and random effects models. Forest plots with odds ratios and corresponding (95% confidence intervals) were produced and reported. A level of significance of 5% was considered statistically significant.
To assess the impact of results from the largest trial to date, additional analysis comparing odds ratios for studies excluding AFFIRM-AHF to the AFFIRM-AHF trial alone were carried out.
All analyses were conducted with Review Manager (RevMan) Version 5.4 (The Cochrane Collaboration, 2020).
Results
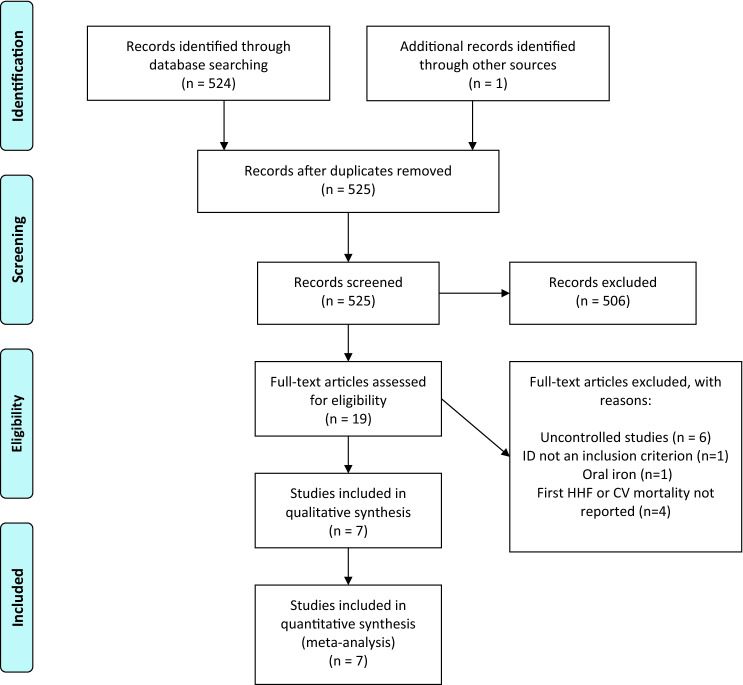
We identified 19 reports assessing the effect of iron therapy in patients with heart failure (Fig. 1). After excluding 12 reports [6–17], mainly because they were not randomised-controlled trials or did not report relevant outcomes (Supplementary Table S1), seven trials (Table 1) that enrolled 2,166 patients (n = 1168 assigned to IV iron; n = 998 assigned to the control/placebo) were included in the primary analysis [4, 18–23]. The most common definition of ID was a ferritin < 100 µg/L and/or, if ferritin was 100–300 µg/L, a TSAT of < 20%. Most trials excluded patients with a very low haemoglobin (less than 8–10 g/dL) or with values greater than 15 g/dL [4, 19]. Only two trials followed patients for > 6 months [4, 19]. Five trials used ferric carboxymaltose and two used iron sucrose [21, 22].
Fig. 1.
PRISMA diagram detailing the number of records identified, screened, included, and excluded, with a summary of the reasons for exclusion. Modified from Moher D, Liberati A, Tetzlaff J, Altman DG, The PRISMA Group (2009). Preferred Reporting Items for Systematic Reviews and Meta-Analyses: the PRISMA statement. PLoS Med 6(7): e1000097. 10.1371/journal.pmed1000097. ID iron deficiency, HHF hospitalisation for heart failure, CV cardiovascular
Table 1.
Characteristics of included trials
| Toblli et al | FERRIC-HF | FAIR-HF | CONFIRM-HF | EFFECT-HF | PRACTICE- ASIA-HF |
AFFIRM-AHF | ||
|---|---|---|---|---|---|---|---|---|
| Year of publication | 2007 | 2008 | 2009 | 2014 | 2017 | 2018 | 2020 | |
| Country | Argentina | UK and Poland | Europe and Argentina | Europe | Europe and Australia | Singapore | 15 countries (International) | |
| Number of patients (IV iron: control) | 40 (1:1) | 35 (2:1) | 459 (2:1) | 301 (1:1) | 174 (1:1) | 49 (1:1) | 1108 (1:1) | |
| Double-blind | Yes | No | Yes | Yes | No | No | Yes | |
| Definition of ID | F < 100 and/or T ≤ 20% | F < 100 or T < 20% + F100–300 | F < 100 or T < 20% + F100–299 | F < 100 or T < 20% + F100–300 | F < 100 or T < 20% + F100–300 |
T < 20% and F < 300 |
F < 100 or T < 20% + F 100–299 | |
| Main inclusion criteria (Hb: g/dL) |
• LVEF ≤ 35% • NYHA II-IV • Anaemia |
• LVEF ≤ 45% • NYHA II-III • Hb ≤ 14.5 |
• LVEF ≤ 45% • NYHA II-III • Hb 9.5–13.5 |
• LVEF ≤ 45% • NYHA II/III • Hb < 15 |
• LVEF ≤ 45% • NYHA II or III • Hb < 15 |
• HF Hosp • Hb < 14 |
• LVEF < 50% • HF Hosp • NT-proBNP↑ • Hb 8–15 |
|
| Age (years) | 75 | 63 | 68 | 70 | 64 | 63 | 71 | |
| Women (%) | – | 29 | 54 | 47 | 25 | 22 | 45 | |
| Ischaemic aetiology (%) | 63 | 74 | 80 | 83 | – | – | 47 | |
| LVEF (%) | 31 ± 4 | 30 ± 7 | 32 ± 6 | 37 ± 8 | 33 ± 9 | 39 ± 18 | 33 (10) | |
| NT-proBNP (pg/ml) | 256 ± 125 | – | – | 2511 ± 5006 | 1576* | – | 4743 (2781–8128)* | |
| eGFR (ml/min/1.73m2) | – | – | 64 | 66 | 52 | – | – | |
| Haemoglobin (g/dL) | 10.3 ± 0.6 | 12.6 ± 1.2 | 11.9 ± 1.3 | 12.3 ± 1.4 | 12.9 ± 1.3 | 11.6 ± 1.9 | 12.3 ± 1.6 | |
| Ferritin (µg/L) | 73 ± 30 | 62 ± 37 | 53 ± 55 | 57 ± 48 | 48* | 91 ± 80 | 84 ± 62 | |
| TSAT (%) | 20 ± 1 | 20 ± 8 | 18 ± 13 | 20 ± 18 | 17* | 16 ± 10 | 15 ± 8 | |
| Form of iron therapy (mean dose) | Iron sucrose; 1000 mg | Iron sucrose; 1433 mg | FCM; n/a | FCM; 1500 mg | FCM; 1204 mg | FCM; 1000 mg | FCM; 1352 mg | |
| Follow-up | 24 weeks | 18 weeks | 24 weeks | 52 weeks | 24 weeks | 12 weeks | 52 weeks | |
| Outcomes reported | HHF | + | + | + | + | + | + | + |
| CVM | − | + | + | + a | + | − | + | |
Data shown are for the active group only, but this is also representative of the control group. Data presented as mean ± SD or count and (%) unless otherwise stated
If data not available/reported, cell filled (–). *Median and (Q1–Q3) reported
aNot specifically reported but derived from reported outcomes in the paper and from the individual-patient-data meta-analysis by Anker et al.—see reference [3]
ID iron deficiency, F ferritin (µg/L), T transferrin saturation (%), LVEF left ventricular ejection fraction, NYHA New York Heart Association, Hb haemoglobin, NT-proBNP N-terminal pro-brain natriuretic peptide, IV intravenous, eGFR estimated glomerular filtration rate, FCM ferric carboxymaltose, HHF hospitalisation for heart failure, CVM cardiovascular mortality
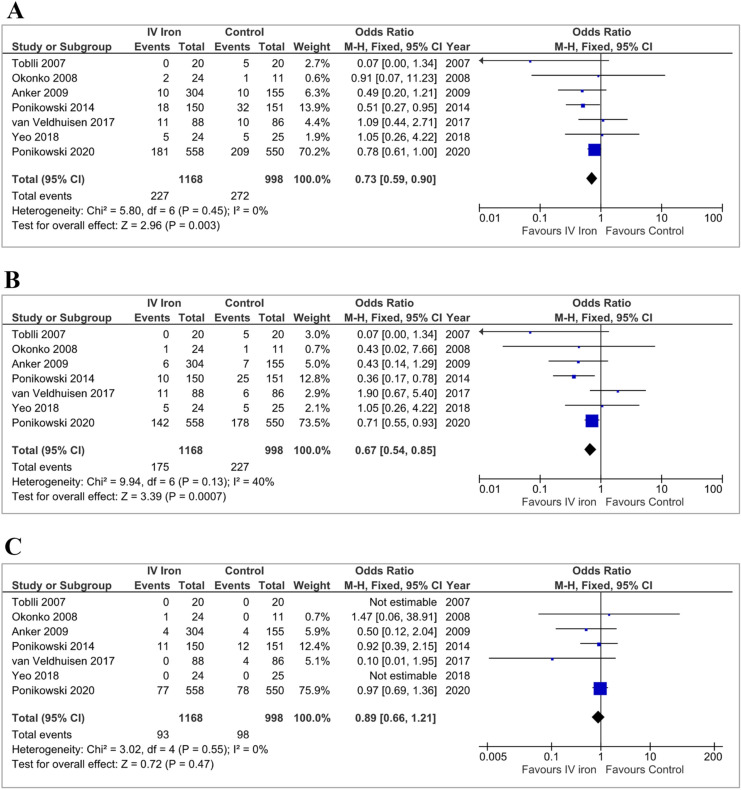
In the primary analysis, IV iron reduced the composite outcome of HHF or CV death: OR 0.73 [0.59–0.90]; p = 0.003 (Fig. 2a). HHF occurred in 175 (15%) patients administered IV iron and 227 (23%) assigned to control: OR 0.67 [0.54–0.85]; p = 0.0007 (Fig. 2b). CV deaths occurred in 93 (8%) patients administered IV iron and in 98 (10%) assigned to control: OR 0.89 [0.66–1.21]; p = 0.47 (Fig. 2c). Adding data from the two unpublished trials to the main analysis did not substantially alter these results (Supplementary Figure S1).
Fig. 2.
Fixed-effects meta-analysis model of all included trials detailing the pooled effect of intravenous iron on the composite of first hospitalisations for heart failure or cardiovascular mortality (a), and first hospitalisation for heart failure (b) and cardiovascular mortality (c) alone. IV intravenous, CI confidence interval
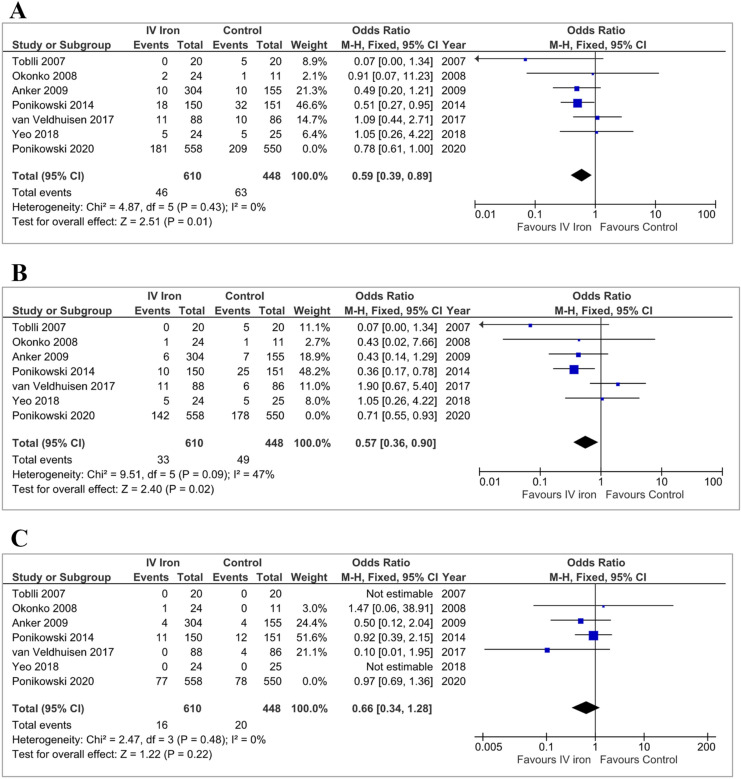
When AFFIRM-AHF was excluded from the model, the point estimates for the effect of IV iron were OR 0.59 [0.39–0.89]; p = 0.01 for the composite outcome, OR 0.57 [0.36–0.90]; p = 0.02 for HHF and OR 0.66 [0.34–1.28]; p = 0.22 for CV mortality (Table 2 and Fig. 3). The odds ratios for all outcomes were not significantly different for the pooled data excluding AFFIRM-AHF compared to AFFIRM-AHF alone (Table 3).
Table 2.
Summary of results from meta-analysis models, with and without AFFIRM-AHF, and AFFIRM-AHF alone, assessing the effect of IV iron on outcomes
| Outcome | IV iron | Controls | Fixed effect | Random effect | ||||
|---|---|---|---|---|---|---|---|---|
| Events | Patients | Events | Patients | OR (95% CI) | p | OR (95% CI) | p | |
| AFFIRM-AHF excluded | ||||||||
| CVM or HHF | 46 | 610 | 63 | 448 | 0.59 (0.39, 0.89) | 0.01 | 0.62 (0.41, 0.93) | 0.02 |
| HHF | 33 | 610 | 49 | 448 | 0.57 (0.36, 0.90) | 0.02 | 0.60 (0.28, 1.28) | 0.19 |
| CVM | 16 | 610 | 20 | 448 | 0.66 (0.34, 1.28) | 0.22 | 0.72 (0.36, 1.43) | 0.35 |
| AFFIRM-AHF | ||||||||
| CVM or HHF | 181 | 558 | 209 | 550 | 0.78 (0.61, 1.00) | – | 0.78 (0.61, 1.00) | – |
| HHF | 142 | 558 | 178 | 550 | 0.71 (0.55, 0.93) | – | 0.71 (0.55, 0.93) | – |
| CVM | 77 | 558 | 78 | 550 | 0.97 (0.69, 1.36) | – | 0.97 (0.69, 1.36) | – |
| All Trials | ||||||||
| CVM or HHF | 227 | 1168 | 272 | 998 | 0.73 (0.59, 0.90) | 0.003 | 0.74 (0.60–0.91) | 0.005 |
| HHF | 175 | 1168 | 227 | 998 | 0.67 (0.54, 0.85) | 0.0007 | 0.64 (0.40, 1.04) | 0.07 |
| CVM | 93 | 1168 | 98 | 998 | 0.89 (0.66, 1.21) | 0.47 | 0.91 (0.67, 1.24) | 0.56 |
IV intravenous, OR odds ratio, CI confidence interval, CVM cardiovascular mortality, HHF hospitalisation for heart failure
Fig. 3.
Fixed-effects meta-analysis model of all trials, excluding AFFIRM-AHF, detailing the pooled effect of intravenous iron on the composite of first hospitalisations for heart failure or cardiovascular mortality (a), and first hospitalisation for heart failure (b) and cardiovascular mortality (c) alone. Although not included in the pooled analysis, odds ratios and 95% confidence intervals are presented for AFFIRM-AHF for comparison. IV intravenous, CI confidence interval
Table 3.
Comparison of fixed-effects odds ratios (ORs) between pooled trials prior to AFFIRM-AHF and AFFIRM-AHF
| Odds ratio (95% confidence interval) | P for comparison | OR (95% confidence interval) of comparison | |
|---|---|---|---|
| Composite endpoint | |||
| All trials except AFFIRM-AHF | 0.59 (0.39, 0.89) | 0.26 | 0.76 (0.47, 1.22) |
| AFFIRM-AHF | 0.78 (0.61, 1.00) | ||
| Hospitalisation for heart failure | |||
| All trials except AFFIRM-AHF | 0.57 (0.36, 0.90) | 0.41 | 0.80 (0.47, 1.36) |
| AFFIRM-AHF | 0.71 (0.55, 0.93) | ||
| Cardiovascular mortality | |||
| All trials except AFFIRM-AHF | 0.66 (0.34, 1.28) | 0.31 | 0.68 (0.32, 1.43) |
| AFFIRM-AHF | 0.97 (0.69, 1.36) | ||
In random effects models, IV iron reduced the composite outcome [OR 0.74 (0.60–0.91); p = 0.005] but neither HHF [OR 0.64 (0.40–1.04); p = 0.07] nor CV mortality [OR 0.91 (0.67–1.24); p = 0.56] (Supplementary Figure S2). Results were similar when AFFIRM-AHF was excluded (Table 2 and Supplementary Figure S3).
Discussion
This meta-analysis suggests that IV iron reduces the risk of the composite outcome of first HHF or CV death for patients with serum markers of ID and heart failure. This result was driven predominantly by an effect on HHF with no convincing evidence of a reduction in CV mortality. Because HHF is associated with a higher risk of CV mortality, the effect of IV iron for each outcome might be expected to be rather similar. The relatively small number of deaths, the short duration of follow-up and the play of chance might explain this possible anomaly. A longer duration of follow-up might show a greater effect on CV mortality, but the trial with the longest follow-up to date, albeit only one year, showed little effect on this outcome [4]. The AFFIRM-AHF trial suggests that the reduction in hospitalisations for heart failure is not observed until 8–12 weeks after administration of IV iron, consistent with its benefits being mediated through the synthesis of new red blood cells, myoglobin and other metalloproteins. Accordingly, large effects observed in some small trials lasting 3 months or less may reflect chance effects.
The effects of IV iron appeared somewhat greater in a pooled analysis of trials excluding AFFIRM-AHF, although differences were not statistically significant. IV iron might be more effective in clinically stable populations. Differences in study design, inclusion criteria, iron dosing and length of follow-up may affect the outcome. The small size of some trials confounded statistical assessment of heterogeneity. Instead, we produced both fixed and random effects models which, for the composite outcome, yielded similar results, although less secure for effects on HHF in the random effects model.
Whether the definition of ID used in these trials is optimal is uncertain. Using a TSAT < 20% alone might be a better guide to ID than one based on ferritin [24–26]. This is important because giving IV iron to patients who are not iron-deplete is unlikely to be beneficial. Fortunately, ID appears common in patients with heart failure and therefore an effect might be detected even if the diagnostic accuracy of the test for ID is poor. Perhaps most patients with heart failure have ID and the key question is how severe it is, rather than whether it is present; ID should not be a binary, all-or-nothing classification.
We did not conduct subgroup analyses, which are best left to an individual-patient-data (IPD) meta-analysis that can adjust for confounding variables. In an IPD meta-analysis [3], lower TSAT but not lower serum ferritin predicted greater benefit from IV iron. In AFFIRM-AHF it appeared that lower serum ferritin or a lower TSAT were associated with greater benefit from IV iron, but > 80% of participants had a TSAT < 20%. Further analyses are required. Haemoglobin concentration has not predicted benefit but, because women have lower concentrations than men, such analyses may be confounded by participants’ sex. The AFFIRM-AHF trial enrolled patients with new-onset heart failure, which is unusual for trials of heart failure; these patients may have had somewhat less benefit from IV iron possibly because they were less likely to have true ID or because the determinants of outcome in such patients are different. In AFFIRM-AHF patients with ischaemic cardiomyopathy appeared to have greater benefit; the reasons for this are unclear. The reduction in events with IV iron, compared to control, might have been underestimated because treatments for heart failure might have been more likely to be intensified in the control group who did not receive the symptomatic benefits of iron therapy. This possibility should be explored in the future analysis of substantial long-term trials.
Results from three other large ongoing trials should clarify the effects of IV iron on morbidity and mortality in patients with HFrEF and ID and provide further insights into the possible predictors of response [27]. Trials in heart failure with preserved ejection fraction are also underway but limited data currently exist [23].
Limitations
We did not investigate the effect of IV iron on all-cause mortality as this is not yet reported for AFFIRM-AHF. The composite outcome reported for CONFIRM-HF [19] was HHF and all-cause mortality, which included one non-CV death amongst patients assigned to iron and two to placebo. This would not materially alter our overall results. An analysis of recurrent HHF rather than just the first event would make the result more robust but requires access to IPD. An IPD meta-analysis has many advantages when exploring the interaction amongst variables [28–30]. In particular, an IPD would have allowed analysis of the potential interaction between sex and the effects of IV iron. However, aggregate data have the advantage that it includes all the published data rather than the proportion where IPD is available to the authors. Each type of meta-analysis has advantages, and they are complimentary. All meta-analyses should be interpreted cautiously, particularly for analyses involving a number of small studies where there will be little power to detect heterogeneity. Fixed effects meta-analysis provides an estimate of an average treatment effect in the studies conducted but uncertainty about heterogeneity may make it difficult to extrapolate that effect to a particular clinical context. Random effects analyses assume that studies have underlying treatment effects arising from a random distribution and provide estimates of the average of, and variation in, the treatment effect in that distribution. However, if the variation is systematic and not random then the random effects analysis may not be helpful in extrapolating a treatment effect to a new situation. In the context of this analysis, length of follow-up, clinical status of patients at recruitment and IV iron dosing strategy are systematically different amongst the studies. Whether these factors systematically impact the treatment effect is difficult to determine with the data available.
Conclusion
In a meta-analysis of seven trials, administration of IV iron to patients with heart failure and ID reduced the risk of the composite outcome of heart failure hospitalisation or cardiovascular mortality in the following 12 months. To date, this outcome is driven predominantly by an effect on HHF. Longer term effects of repeated administration of IV iron are unknown. More evidence is desirable.
Supplementary Information
Below is the link to the electronic supplementary material.
Funding
No funding was received for conducting this study.
Data availability
Not applicable.
Code availability
Not applicable.
Declarations
Conflict of interest
FG reports receipt of sponsorship from Vifor to attend an international meeting. JGFC reports receipt of personal honoraria for lectures and advisory boards from Pharmacosmos and Vifor, and from AstraZeneca, Amgen, Bayer, Novartis and Servier. IF reports receipt of research grants from Vifor and Pharmacosmos. MCP reports receipts for Consultancy and/or Endpoint Committees for Boehringer Ingleheim, Novartis, AstraZeneca, Novo Nordisk, Abbvie, Bayer, Takeda, Cardiorentis and Pharmacosmos. MCP and JGFC are supported by the British Heart Foundation (BHF) Centre of Research Excellence Award (RE/13/5/30177 and RE/18/6/34217 +). PRK reports receipt of personal honoraria for lectures, advisory boards and research/quality improvement grants from Pharmacosmos and Vifor. PRK reports receipt of personal honoraria for lectures and advisory boards from Ackea, Amgen, AstraZeneca, Bayer and Servier.
Ethics approval
Not applicable.
Informed consent
Not applicable.
Consent for publication
All authors consent to the publication of this manuscript in its current form.
References
- 1.Klip IT, Comin-Colet J, Voors AA, Ponikowski P, Enjuanes C, Banasiak W, Lok DJ, Rosentryt P, Torrens A, Polonski L, van Veldhuisen DJ, van der Meer P, Jankowska EA. Iron deficiency in chronic heart failure: an international pooled analysis. Am Heart J. 2013;165(4):575–582. doi: 10.1016/j.ahj.2013.01.017. [DOI] [PubMed] [Google Scholar]
- 2.Jankowska EA, Rozentryt P, Witkowska A, Nowak J, Hartmann O, Ponikowska B, Borodulin-Nadzieja L, Banasiak W, Polonski L, Filippatos MJJV, Anker SD, Ponikowski P. Iron deficiency: an ominous sign in patients with systolic chronic heart failure. Eur Heart J. 2010;31(15):1872–1880. doi: 10.1093/eurheartj/ehq158. [DOI] [PubMed] [Google Scholar]
- 3.Anker SD, Kirwan BA, van Veldhuisen DJ, Filippatos G, Comin-Colet J, Ruschitzka F, Lüscher TF, Arutyunov GP, Motro M, Mori C, Roubert B, Pocock SJ, Ponikowski P. Effects of ferric carboxymaltose on hospitalisations and mortality rates in iron-deficient heart failure patients: an individual patient data meta-analysis. Eur J Heart Fail. 2018;20(1):125–133. doi: 10.1002/ejhf.823. [DOI] [PubMed] [Google Scholar]
- 4.Ponikowski P, Kirwan B-A, Anker SD, McDonagh T, Dorobantu M, Drozdz J, Fabien V, Filippatos G, Göhring UM, Keren A, Khintibidze I, Kragten H, Martinez FA, Metra M, Milicic D, Nicolau JC, Ohlsson M, Parkhomenko A, Pascual-Figal DA, Ruschitzka F, Sim D, Skouri H, van der Meer P, Lewis BS, Comin-Colet J, von Haehling S, Cohen-Solal A, Danchin N, Doehner W, Dargie HJ, Morto M, Butler J, Friede T, Jensen KH, Pocock S, Jankowska EA. Ferric carboxymaltose for iron deficiency at discharge after acute heart failure: a multicentre, double-blind, randomised, controlled trial. Lancet. 2020;396(10266):1895–1904. doi: 10.1016/S0140-6736(20)32339-4. [DOI] [PubMed] [Google Scholar]
- 5.Anand IS, Gupta P. Anemia and iron deficiency in heart failure. Circulation. 2018;138(1):80–98. doi: 10.1161/CIRCULATIONAHA.118.030099. [DOI] [PubMed] [Google Scholar]
- 6.Silverberg DS, Wexler D, Blum M, Tchebiner JZ, Sheps D, Keren G, Schwartz D, Baruch R, Yachnin T, Shaked M, Schwartz I, Steinbruch S, Iaina A. The effect of correction of anaemia in diabetics and non-diabetics with severe resistant congestive heart failure and chronic renal failure by subcutaneous erythropoietin and intravenous iron. Nephrol Dial Transplant. 2003;18(1):141–146. doi: 10.1093/ndt/18.1.141. [DOI] [PubMed] [Google Scholar]
- 7.Silverberg DS, Wexler D, Blum M, Schwartz D, Keren G, Iaina SD, A, Effect of correction of anemia with erythropoietin and intravenous iron in resistant heart failure in octogenarians. Isr Med Assoc J. 2003;5(5):337–339. [PubMed] [Google Scholar]
- 8.Charles-Edwards G, Amaral N, Sleigh A, Ayis S, Catibog N, McDonagh T, Monaghan M, Amin-Youssef G, Kemp GJ, Shah AM, Okonko DO. Effect of iron isomaltoside on skeletal muscle energetics in patients with chronic heart failure and iron deficiency: FERRIC-HF II randomized mechanistic trial. Circulation. 2019;139(21):2386–2398. doi: 10.1161/CIRCULATIONAHA.118.038516. [DOI] [PubMed] [Google Scholar]
- 9.Núñez J, Miñana G, Cardells I, Palau P, Llàcer P, Fácila L, Almenar L, López-Lereu MP, Monmeneu JV, Amiguet M, González J, Serrano A, Montagud V, López-Vilella R, Valero E, García-Blas S, Bodi V, de la Espriella-Juan R, Lupón J, Navarro J, Górriz JL, Sanchis J, Chorro FJ, Comín-Colet J. Noninvasive imaging estimation of myocardial iron repletion following administration of intravenous iron: the myocardial-IRON trial. J Am Heart Assoc. 2020;9(4):e014254. doi: 10.1161/JAHA.119.014254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bolger AP, Bartlett FR, Penston HS, O’Leary J, Pollock N, Kaprielian R, Chapman CM. Intravenous iron alone for the treatment of anemia in patients with chronic heart failure. J Am Coll Cardiol. 2006;48(6):1225–1227. doi: 10.1016/j.jacc.2006.07.015. [DOI] [PubMed] [Google Scholar]
- 11.Usmanov RI, Zueva EB, Silverberg DS, Shaked M. Intravenous iron without erythropoietin for the treatment of iron deficiency anemia in patients with moderate to severe congestive heart failure and chronic kidney insufficiency. J Nephrol. 2008;21(2):236–242. [PubMed] [Google Scholar]
- 12.Reed BN, Blair EA, Thudium EM, Waters SB, Sueta CA, Jensen BC, Rodgers JE. Effects of an accelerated intravenous iron regimen in hospitalized patients with advanced heart failure and iron deficiency. Pharmacother J Hum Pharmacol Drug Ther. 2015;35(1):64–71. doi: 10.1002/phar.1525. [DOI] [PubMed] [Google Scholar]
- 13.Mirdamadi A, Arefeh A, Garakyaraghi M, Pourmoghadas A. Beneficial effects of the treatment of iron deficiency on clinical condition, left ventricular function, and quality of life in patients with chronic heart failure. Acta Biomed. 2018;89(2):214–219. doi: 10.23750/abm.v89i2.5020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Silverberg DS, Wexler D, Sheps D, Blum M, Keren G, Baruch R, Schwartz D, Yachni T, Steinbruch S, Shapira I, Laniado S, Iaina A. The effect of correction of mild anemia in severe, resistant congestive heart failure using subcutaneous erythropoietin and intravenous iron: a randomized controlled study. J Am Coll Cardiol. 2001;37(7):1775–1780. doi: 10.1016/S0735-1097(01)01248-7. [DOI] [PubMed] [Google Scholar]
- 15.Lewis GD, Malhotra R, Hernandez AF, McNulty SE, Smith A, Michael Felker G, Tang WHW, LaRue SJ, Redfield MM, Semigran MJ, Givertz MM, van Buren P, Whellan D, Anstrom KJ, Shah MR, Desvigne-Nickens P, Butler J, Braunwald E. Effect of oral iron repletion on exercise capacity in patients with heart failure with reduced ejection fraction and iron deficiency the IRON OUT HF randomized clinical trial. JAMA. 2017;317(19):1958–1966. doi: 10.1001/jama.2017.5427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Beck-Da-Silva L, Piardi D, Soder S, Rohde LE, Pereira-Barretto AC, De Albuquerque D, Bocchi E, Vilas-Boas F, Moura LZ, Montera MW, Rassi S, Clausell NL. IRON-HF study: a randomized trial to assess the effects of iron in heart failure patients with anemia. Int J Cardiol. 2013;168(4):3439–3442. doi: 10.1016/j.ijcard.2013.04.181. [DOI] [PubMed] [Google Scholar]
- 17.Toblli JE, Di Gennaro F, Rivas C. Changes in echocardiographic parameters in iron deficiency patients with heart failure and chronic kidney disease treated with intravenous iron. Hear Lung Circ. 2015;24(7):686–695. doi: 10.1016/j.hlc.2014.12.161. [DOI] [PubMed] [Google Scholar]
- 18.Anker SD, Colet JC, Filippatos G, Willenheimer R, Dickstein K, Drexler H, Lüscher TF, Bart B, Banasiak W, Niegowska J, Kirwan B, Mori C, von Eisenhart RB, Pocock SJ, Poole-Wilson PA, Ponikowski P. FAIR-HF trial investigators. Ferric carboxymaltose in patients with heart failure and iron deficiency. N Engl J Med. 2009;361(25):2436–2448. doi: 10.1056/NEJMoa0908355. [DOI] [PubMed] [Google Scholar]
- 19.Ponikowski P, Van Veldhuisen DJ, Comin-Colet J, Ertl G, Komajda M, Mareev V, McDonagh T, Parkhomenko A, Taazzi L, Levesque V, Mori C, Roubert B, Filippatos G, Ruschitzka F, Anker SD. CONFIRM-HF Investigators. Beneficial effects of long-term intravenous iron therapy with ferric carboxymaltose in patients with symptomatic heart failure and iron deficiency. Eur Heart J. 2015;36(11):657–668. doi: 10.1093/eurheartj/ehu385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.van Veldhuisen DJ, Ponikowski P, Van Der Meer P, Metra M, Böhm M, Doletsky A, Voors AA, Macdougall IC, Anker SD, Roubert B, Zakin L, Cohen-Solal A. EFFECT-HF investigators. Effect of ferric carboxymaltose on exercise capacity in patients with chronic heart failure and iron deficiency. Circulation. 2017;136(15):1374–1383. doi: 10.1161/CIRCULATIONAHA.117.027497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Toblli JE, Lombraña A, Duarte P, Di Gennaro F. Intravenous iron reduces NT-pro-brain natriuretic peptide in anemic patients with chronic heart failure and renal insufficiency. J Am Coll Cardiol. 2007;50(17):1657–1665. doi: 10.1016/j.jacc.2007.07.029. [DOI] [PubMed] [Google Scholar]
- 22.Okonko DO, Grzeslo A, Witkowski T, Mandal AKJ, Slater RM, Roughton M, Foldes G, Thum T, Majda J, Banasiak W, Missouris CG, Poole-Wilson PA, Anker SD, Ponikowski P. Effect of intravenous iron sucrose on exercise tolerance in anemic and nonanemic patients with symptomatic chronic heart failure and iron deficiency. FERRIC-HF: a randomized, controlled observer-blinded trial. J Am Coll Cardiol. 2008;51(2):103–112. doi: 10.1016/j.jacc.2007.09.036. [DOI] [PubMed] [Google Scholar]
- 23.Yeo TJ, Yeo PSD, Hadi FA, Cushway T, Lee KY, Yin FF, Ching A, Li R, Loh SL, Lim SL, Wong RC, Tai BC, Richards AM, Lam CSP. Single-dose intravenous iron in Southeast Asian heart failure patients: a pilot randomized placebo-controlled study (PRACTICE-ASIA-HF) ESC Hear Fail. 2018;5(2):344–353. doi: 10.1002/ehf2.12250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Beverborg NG, Klip IjT, Meijers WC, Voors AA, Vegter EL, Van Der Wal HH, Swinkels DW, van Pelt J, Mulder AB, Bulstra SK, Vellenga E, Mariani MA, de Boer RA, van Veldhuisen DJ, van der Meer P. Definition of iron deficiency based on the gold standard of bone marrow iron staining in heart failure patients. Circ Heart Fail. 2018;11(2):e004519. doi: 10.1161/CIRCHEARTFAILURE.117.004519. [DOI] [PubMed] [Google Scholar]
- 25.Cleland JGF, Zhang J, Pellicori P, Dicken B, Dierckx R, Shoaib A, Wong K, Rigby A, Goode K, Clark AL. Prevalence and outcomes of anemia and hematinic deficiencies in patients with chronic heart failure. JAMA Cardiol. 2016;1(5):539–547. doi: 10.1001/jamacardio.2016.1161. [DOI] [PubMed] [Google Scholar]
- 26.Moliner P, Jankowska EA, van Veldhuisen DJ, Farre N, Rozentryt P, Enjuanes C, Polonski L, Moroño O, Voors AA, Ponikowski P, van der Meer P, Comin-Colet J. Clinical correlates and prognostic impact of impaired iron storage versus impaired iron transport in an international cohort of 1821 patients with chronic heart failure. Int J Cardiol. 2017;243:360–366. doi: 10.1016/j.ijcard.2017.04.110. [DOI] [PubMed] [Google Scholar]
- 27.Pellicori P, Khan MJI, Graham FJ, Cleland JGF. New perspectives and future directions in the treatment of heart failure. Heart Fail Rev. 2020;25(1):147–159. doi: 10.1007/s10741-019-09829-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cleland JG, Abraham WT, Linde C, Gold MR, Young JB, Claude Daubert J, Sherfesee L, Wells GA, Tang ASL. An individual patient meta-analysis of five randomized trials assessing the effects of cardiac resynchronization therapy on morbidity and mortality in patients with symptomatic heart failure. Eur Heart J. 2013;34(46):3547–3556. doi: 10.1093/eurheartj/eht290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cleland JGF, Bunting KV, Flather MD, Altman DG, Holmes J, Coats AJS, Manzano L, McMurray JJV, Rutschitzka F, van Veldhuisen DJ, von Lueder TG, Böhm M, Andersson B, Kjekshus J, Packer M, Rigby AS, Rosano G, Wedel H, Hjalmarson A, Wikstrand J, Koetcha D. Beta-blockers in Heart Failure Collaborative Group. Beta-blockers for heart failure with reduced, mid-range, and preserved ejection fraction: an individual patient-level analysis of double-blind randomized trials. Eur Heart J. 2018;39(1):26–35. doi: 10.1093/eurheartj/ehx564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Linde C, Cleland JGF, Gold MR, Claude Daubert J, Tang ASL, Young JB, Sherfesee L, Abraham WT. The interaction of sex, height, and QRS duration on the effects of cardiac resynchronization therapy on morbidity and mortality: an individual-patient data meta-analysis. Eur J Heart Fail. 2018;20(4):780–791. doi: 10.1002/ejhf.1133. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Not applicable.
Not applicable.