Abstract
The zinc element is an essential nutrient for human health. Zinc is involved in the glucose, lipid, and protein metabolism and antioxidant processes in biological pathways. Zinc deficiency can lead to several chronic liver diseases. Nonalcoholic fatty liver disease (NAFLD) is one of the most common liver diseases where zinc deficiency plays a critical role in pathogenesis. Human and animal studies showed that both NAFLD risk factors (i.e., insulin resistance, diabetes mellitus, dyslipidemia, obesity, hypertension) and NAFLD itself are associated with decreased blood levels of zinc. Additionally, endoplasmic reticulum stress and inflammation due to unfolded protein response, inadequate dietary zinc intake, and decreased zinc absorption from the gastrointestinal tract can result in zinc deficiency leading to NAFLD. Herein, we reviewed the mechanistic links between zinc deficiency and NAFLD development and the role of zinc in the prevention and treatment of NAFLD.
Keywords: Zinc, Nonalcoholic fatty liver disease, Nonalcoholic steatohepatitis, Fatty liver, Hepatic steatosis, Metabolic syndrome, Oxidative stress, Inflammation, NAFLD, NASH, Obesity, Diabetes, Dyslipidemia, Hypertension
1. Introduction
In 2016, the World Health Organization estimated that there were more than 1.9 billion adult overweight people, of whom more than 650 million were obese [1]. The healthcare and economic burdens related to chronic metabolic diseases have been increasing in the US such that one-third of the adult population in the US is now obese [2]. Due to the rising obesity epidemic, one-fourth of the adult population worldwide is affected by nonalcoholic fatty liver disease (NAFLD) [3]. Additionally, cirrhosis secondary to NAFLD is on the verge of becoming the most common indication for liver transplantation, not only in the US but worldwide [4,5]. As such, there is an urgent need for effective interventions to treat NAFLD. These effective interventions should not only be limited to weight reduction, exercise, and control of risk factors (e.g., obesity, insulin resistance, dyslipidemia), but also repletion of essential micronutrients. Among several micronutrient deficiencies in NAFLD [6], zinc deficiency appears to play the most pivotal role in NAFLD development. This review aims to identify the mechanistic links between NAFLD and zinc deficiency, determine the role of zinc in the prevention and treatment of NAFLD, and underline the key points in supplementation and laboratory evaluation of zinc element.
2. Conditions associated with zinc deficiency in NAFLD
NAFLD is a significant cause of cirrhosis and liver cancer due to the rising global obesity epidemic [7]. Patients with NAFLD were shown to have significantly reduced serum zinc concentrations compared with controls [8]. Additionally, lower zinc concentrations were reported to be associated with higher stages of hepatic fibrosis in subjects with biopsy-proven NAFLD [9]. A cross-sectional study conducted among 300 subjects with NAFLD defined by NAFLD liver fat score [10] showed that significant hepatic fibrosis estimated by the fibrosis-4 index [11] was associated with reduced zinc levels [12]. In contrast to the results of these studies, a study that evaluated subjects with NAFLD who underwent bariatric surgery showed no significant correlation between serum zinc levels and the severity of hepatic steatosis, steatohepatitis, and fibrosis [13]. Regarding the animal studies, zinc supplementation in rats with diet-induced NAFLD reduced the severity of hepatic steatosis in the periportal areas, reduced lipid deposition in hepatocytes, improved glucose metabolism and insulin signaling, and reduced liver injury [[14], [15], [16]].
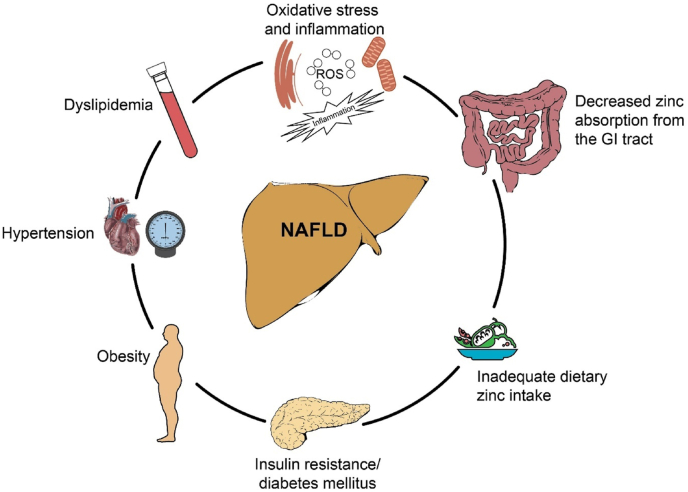
Several conditions in patients with NAFLD can be associated with zinc deficiency, including oxidative stress and inflammation increasing the demand for zinc supplementation, insulin resistance, diabetes mellitus, obesity, hypertension, dyslipidemia, decreased zinc absorption from the gastrointestinal tract, and inadequate dietary zinc intake (Fig. 1).
Fig. 1.
Several conditions can be associated with zinc deficiency, including oxidative stress and inflammation increasing the demand for zinc supplementation, insulin resistance, diabetes mellitus, obesity, hypertension, dyslipidemia, decreased zinc absorption from the gastrointestinal tract, and inadequate dietary zinc intake. NAFLD=Nonalcoholic fatty liver disease; ROS = Reactive oxygen species, GI = Gastrointestinal (with permission from Baylor College of Medicine).
2.1. Zinc deficiency associated with oxidative stress and inflammation
Endoplasmic reticulum stress that leads to unfolded protein response, and thereby oxidative stress and inflammation play a central role in the development of NAFLD [17]. The endoplasmic reticulum stress occurs when the demand for protein folding exceeds the capacity of the endoplasmic reticulum resulting in the accumulation of unfolded and/or misfolded proteins [18,19]. Several factors can stimulate endoplasmic reticulum stress (e.g., obesity, hyperlipidemia, hyperglycemia), which is expected to occur specifically in the cells that produce proteins in large amounts (e.g., pancreatic beta cells and adipocytes) [[17], [18], [19]]. The endoplasmic reticulum stress induces an unfolded protein response, an adaptive response to reduce protein folding demand, inducing endoplasmic reticulum--associated degradation for cell survival [[17], [18], [19]]. When endoplasmic reticulum stress is unresolved, chronic unfolded protein response can lead to inflammation via the activation of the endoplasmic reticulum sensors, including protein kinase R (PKR)-like endoplasmic reticulum kinase (PERK), inositol-requiring enzyme 1α (IRE1α), and activating transcription factor-6 (ATF6) that in turn trigger the formation of intracellular reactive oxygen species (ROS), and activation of nuclear factor-κB and JUN N-terminal kinase [18,19]. While hepatic steatosis was suggested to result from chronic unfolded protein response in the hepatocytes, steatohepatitis and hepatocellular carcinoma were suggested to result from terminal and resistant unfolded protein response, respectively [17]. An adequate zinc supply plays a central role in the adaption of the endoplasmic reticulum to oxidative stress and attenuation of inflammation. Indeed, zinc supplementation was shown to reduce oxidative stress and downregulate inflammatory cytokines (e.g., tumor necrosis factor-alpha) [[20], [21], [22], [23]]. As mentioned above, zinc deficiency originates from increased demand in endoplasmic reticulum stress, but also zinc deficiency itself can induce endoplasmic reticulum stress [24,25]. Homma et al. [25] showed that zinc deficiency exposed the Derlin-1 binding region of the copper-zinc superoxide dismutase (SOD1) and led to SOD1-Derlin-1 interaction, and this, in turn, triggered the endoplasmic reticulum stress response and upregulated expression of ZIP14, which is the zinc transporter abundant in the hepatocytes. In addition to the critical role of zinc, two major zinc transporter groups, including zinc transporter (ZnT) that regulates efflux of zinc from the cytoplasm and Zrt-, Irt-like protein (ZIP) that regulates the influx of zinc into the cytoplasm, were shown to be involved in the attenuation of oxidative stress and inflammation [26,27]. Among the zinc transporters, hepatic ZIP14-mediated zinc transport was reported to play a critical role in the adaptation of the cells to the endoplasmic reticulum stress [26].
2.2. Zinc deficiency associated with diabetes mellitus
Patients with NAFLD can have zinc deficiency associated with diabetes mellitus. According to a cross-sectional study conducted among 2839 patients with type 2 diabetes mellitus, nearly 70% of patients had NAFLD diagnosed by ultrasound [28]. A more recent study showed that the prevalence of NAFLD diagnosed by magnetic resonance imaging was 76% in patients with insulin-naive type 2 diabetes mellitus and only 8.8% in patients with type 1 diabetes mellitus [29]. Zinc plays a key role in insulin synthesis, storage, secretion, and signaling [30]. Pancreatic beta cells store insulin in the form of zinc-insulin crystals [30], and therefore pancreas has the highest zinc concentration per tissue wet weight compared with other organs [30,31]. The zinc concentration was found to be 50% lower in the pancreas of diabetic patients compared with the pancreas of non-diabetic patients [32]. Additionally, several studies showed that serum zinc concentration was lower in patients with type 1 or type 2 diabetes mellitus than patients without diabetes [[33], [34], [35]].
Zinc deficiency in diabetes mellitus is multifactorial. Zinc deficiency in type 2 diabetes mellitus could be secondary to increased oxidative stress and hyperinsulinemia, all of which increase the demand for zinc and result in intracellular depletion of zinc [18,36,37]. Specifically, hyperinsulinemia can deplete pancreatic zinc stores, inducing more insulin secretion, further reducing pancreatic zinc levels [36]. This vicious cycle in the form of exacerbation of hyperinsulinemia in diabetic patients can be overcome by zinc supplementation and reducing insulin production by the pancreatic beta cells [36,37]. A randomized, double-blind, placebo-controlled trial conducted in 200 adult subjects with prediabetes showed that the subjects who received 20 mg of zinc daily had decreased blood glucose levels, improved insulin resistance, and beta-cell function, and reduced progression to diabetes compared with the subjects who received placebo [38]. A meta-analysis that included placebo-controlled trials, showed that zinc supplement alone without co-supplements significantly lowered the fasting and 2-h postprandial glucose levels, homeostatic model assessment for insulin resistance (HOMA-IR), and hemoglobin A1c [39].
Zinc deficiency in diabetes mellitus also can occur secondary to dysfunction of zinc transporters [30,40]. The gene SLC30A8 encodes for the zinc efflux transporter ZnT8 that plays a key role in zinc storage and insulin secretion in pancreatic beta cells [30,40]. The polymorphic variants of the gene SLC30A8, including rs13266634 and rs11558471, were shown to be associated with type 2 diabetes mellitus and glucose-increasing effect, respectively [[41], [42], [43]]. According to the results of a large meta-analysis that included 14 cohort studies, a higher daily zinc intake was shown to be associated with lower fasting glucose levels in subjects who had polymorphic variant rs11558471 of the gene SLC30A8 [42].
Zinc deficiency in diabetic patients also can result from hyperzincuria [44] that appears to be secondary to glycosuria, hyperglycemia and/or decreased zinc absorption [45], [46], [47]. McNair et al. [45] showed a positive correlation between urinary zinc excretion and the degree of glycosuria in diabetic patients. Kinlaw et al. [46] reported a positive correlation between urinary zinc excretion and serum glucose levels in patients with type 2 diabetes mellitus. Additionally, they showed that patients with type 2 diabetes mellitus and proteinuria had a higher urinary zinc excretion than those without proteinuria [46]. Escobar et al. [47] showed that the levels of metallothionein were higher in the liver and small intestine of the rats with streptozotocin-induced diabetes compared with the control rats and attributed the decreased zinc absorption and hyperzincuria to the increased zinc intake secondary to hyperphagia in diabetic rats rather than diabetes-induced defect in zinc transport [47].
2.3. Zinc deficiency associated with obesity
Zinc appears to play an essential role in appetite regulation and obesity prevention through its interaction with the leptin hormone [48]. Leptin, a hormone that is secreted by adipocytes, inhibits hunger, controls energy balance, and thereby regulates body weight [49]. Zinc supplementation in rats that had high-fat/high-fructose diet-induced obesity significantly reduced leptin and insulin levels along with body weight and abdominal fat pad [50]. Liu et al. [51] reported a similar negative association between leptin and zinc levels showing that zinc deficiency enhanced leptin production and macrophage infiltration in the adipocytes of the high-fat diet-induced obese mice. Maxel et al. [52] showed that obese subjects had a downregulated zinc influx transporter ZIP14 expression compared with non-obese subjects and an upregulated ZIP14 expression after losing weight. Additionally, ZIP14 gene expression had a negative correlation with leptin gene expression and blood leptin levels [52].
A cross-sectional study conducted using US National Health and Nutrition Examination Survey 2011–2014 (NHANES) database showed a negative correlation between serum zinc levels and body mass index in children and adolescents [53]. A randomized, double-blind, placebo-controlled trial showed that adult obese subjects who had 30 mg of zinc daily for 15 weeks and a restricted-calorie diet had significantly reduced body weight, body mass index, waist circumference, and appetite score compared with obese subjects who had placebo and a restricted-calorie diet [54]. Another randomized, double-blind, placebo-controlled trial showed that adult obese or overweight subjects who had 30 mg of zinc daily and a restricted-calorie diet for 12 weeks had significantly reduced waist circumference, alanine aminotransferase, and gamma-glutamyl transpeptidase levels compared with obese subjects who had placebo and a restricted-calorie diet [55].
2.4. Zinc deficiency associated with hypertension
Although multiple hypotheses were proposed for zinc deficiency-induced hypertension, a murine study shed light on its mechanism. Williams et al. [56] showed that zinc deficiencyinduced hypertension by upregulating the Na+- Cl- cotransporter and increasing sodium reabsorption, which was reversed by zinc repletion and administration of hydrochlorothiazide, an inhibitor of Na +- Cl- cotransporter. According to a meta-analysis that included data from nine randomized clinical trials, zinc supplementation significantly reduced systolic blood pressure without any significant effect on diastolic blood pressure [57].
2.5. Zinc deficiency associated with dyslipidemia
According to the results of a randomized, double-blind, placebo-controlled trial, obese subjects who received zinc gluconate 30 mg daily had reduced triglyceride levels compared with obese subjects who received placebo; however, they had no significant reduction in other lipid profile biomarker levels compared with the placebo group [58]. A meta-analysis that included nine randomized controlled trials showed that patients with type 2 diabetes mellitus who had zinc supplementation had a significantly reduced triglyceride and total cholesterol levels compared with patients with type 2 diabetes mellitus who had placebo; however there was no significant improvement on high-density lipoprotein and low-density lipoprotein levels with zinc supplementation [59]. In contrast to the findings of these studies conducted in subjects with obesity and type 2 diabetes mellitus, a study conducted in healthy subjects showed that zinc supplementation lowered HDL levels, potentially increasing the risk of cardiovascular disease [60].
2.6. Zinc deficiency associated with decreased absorption from the gastrointestinal tract
In humans, zinc is absorbed from the small intestine [61]. The absorption of zinc was shown to be concentration-dependent, occurring at the highest rate in the jejunum and at the lowest rate in the ileum [61]. In rats, the highest zinc absorption was reported in the ileum [62]. While the resection of the distal small intestine resulted in a compensatory increase of zinc absorption in the proximal small intestine, the resection of the proximal small intestine did not increase zinc absorption in the distal small intestine [62]. Given zinc absorption in the small intestine, zinc deficiency can occur in patients with impaired small bowel absorption (e.g., short bowel syndrome, Crohn's disease, severe diarrhea) [62,63]. Zinc deficiency also occurs in patients with exocrine pancreatic insufficiency [64]. Taken altogether, zinc deficiency secondary to impaired small bowel absorption and pancreatic exocrine insufficiency may be playing a role in the development of NAFLD after pancreatectomy and pancreatoduodenectomy. In fact, several studies showed that patients undergoing pancreatectomy or pancreaticoduodenectomy developed post-op NAFLD [[65], [66], [67]].
Besides the impaired small bowel absorption, pancreatic exocrine insufficiency, and pancreatoduodenectomy that can result in impaired zinc absorption, several other factors (e.g., low protein diet, toxic levels of cadmium, phytic acid in fiber-rich diet) also can reduce the zinc absorption from the gastrointestinal tract [68]. Specifically, patients with type 2 diabetes mellitus often have an increased fiber intake in their diet to achieve a better control on their blood glucose and lipid levels [69]. The phytic acid in a fiber-rich diet (e.g., beans, nuts, grains, and seeds) can reduce zinc absorption [68]. A cross-sectional study conducted in healthy and diabetic patients showed a positive association between dietary fiber and (calcium)(phytic acid)/zinc molar ratio [70]. It is unknown whether phytic acid-induced zinc deficiency results in NAFLD, and the results of the animal studies are conflicting. Omoruyi et al. [71] observed that there was no significant change in the lipid profile of the phytic acid-treated diabetic rats compared with the lipid profile of the normal control rats and untreated diabetic rats. Also, they showed that phytic acid-fed diabetic rats had significantly increased alanine aminotransferase, alkaline phosphatase, and inflammatory biomarker levels, including interleukin-1 beta and interleukin-6 [71]. In contrast, Sekita et al. [72] showed that phytic acid improved high sucrose-induced hepatic steatosis in rats by altering the gut microbiota and downregulating the genes regulating lipogenesis.
2.7. Zinc deficiency associated with inadequate dietary zinc intake
Several reports link the development of obesity to empty calorie intake (e.g., solid fat, added sugars) [[73], [74], [75]] which can result in micronutrient deficiency. According to a cross-sectional study using the NHANES database, almost 40% of total caloric intake in children between the ages of 2 and 18 was from solid fats (e.g., pizza, whole milk, regular cheese, fatty meats, grain desserts) and added sugars (e.g., soda, fruit drinks, candy, grain and dairy desserts) [75]. A survey conducted among 645 children between ages 1 and 5 revealed a negative relationship between milk intake and sweetened beverages (i.e., juice drinks, soda, and added sugar beverages), leading to multiple nutrient deficiencies [76]. A 26-week randomized, double-blind, placebo-controlled trial conducted among 87 obese women showed that multivitamin and mineral supplementation significantly decreased body weight and fat mass, increased resting energy expenditure, and improved lipid profile compared to placebo [77]. Taken together, subjects with NAFLD should be evaluated for adequate zinc and other micronutrients in their diet.
3. Interactions of zinc with other micronutrients: key points to know in zinc supplementation
Human studies demonstrated benefits of zinc supplementation in subjects with NAFLD risk factors. Table 1 shows several clinical trials and meta-analysis studies where zinc supplementation outcomes were assessed without co-supplements. Zinc has a good safety profile; however, excessive zinc intake can be toxic. A total of daily 150 mg to 450 mg zinc intake can result in copper deficiency and several other complications (e.g., anemia, altered iron metabolism, decreased HDL level, neurotoxicity) [78]. Excess zinc intake can upregulate intestinal metallothionein, a metal-binding protein, displace zinc and bind dietary copper with high affinity, and reduce copper absorption leading to copper deficiency [78,79]. Therefore, blood copper levels should be monitored during zinc supplementation to prevent zinc-induced copper deficiency [79].
Table 1.
Summary of human studies that used zinc supplementation in subjects with nonalcoholic fatty liver disease (NAFLD) risk factors and conditions associated with NAFLD.
| NAFLD risk factor/condition associated with NAFLD | Study | Study population | Study design | Zinc supplementation (intervention) | Study duration | Summary of study outcome |
|---|---|---|---|---|---|---|
| Oxidative stress and inflammation | Prasad et al. (2004) [20] | Healthy subjects, age range: 19–50 years | Randomized, placebo-controlled | Zinc gluconate 45 mg daily | 8 weeks | Reduction in lipid peroxidation products and downregulation of inflammatory cytokines in the intervention group |
| Prasad et al. (2007) [23] | Healthy subjects, age range: 55–87 years | Randomized, double-blinded, placebo-controlled | Zinc gluconate capsule (15 mg elemental zinc), one capsule before breakfast, and two capsules at bedtime | 12 months | Reduction in the incidence of infections, tumor necrosis factor-alpha, and oxidative stress markers in the intervention group | |
| Bao et al. (2008) [21] | Subjects with sickle cell disease, mean age (SD): 32.9 (9.7) years | Randomized, placebo-controlled | Zinc acetate 25 mg three times a day | 3 months | Reduction in oxidative stress, and inflammatory cytokines, increase in red blood cell, and hemoglobin/hematocrit in the intervention group | |
| Diabetes mellitus/insulin resistance | Ranasinghe et al. (2018) [38] | Subjects with prediabetes, mean age (SD): 51.8 (7.3) years | Randomized, double-blinded, placebo-controlled | Zinc 20 mg daily | 12 months | Reduction in blood glucose level, insulin resistance, total cholesterol, low-density lipoprotein, and progression to diabetes, and improvement in beta-cell function in the intervention group |
| Wang et al. (2019) [39] | Varies by study | Meta-analysis of 20 randomized, placebo-controlled trials | Varies by study | Varies by study | Reduction in fasting and 2-h postprandial glucose levels, homeostatic model assessment for insulin resistance (HOMA-IR), and hemoglobin A1c in the intervention group | |
| Kanoni et al. (2011) [42] | Subjects without type 2 diabetes mellitus, age range as mean (SD): 11.2 (0.7)-74.8 (2.9) | Meta-analysis of 14 cohort studies | Average total zinc intake from food sources and supplements: 13.8 mg daily, range: 8.7–17.3 mg daily | Varies by study | Inverse correlation between fasting glucose levels and total zinc intake in subjects with SLC30A8 rs11558471 variant | |
| Obesity | Khorsandi et al. (2019) [54] | Subjects with obesity, mean age (SD) in zinc group: 35.63 (3.2), in placebo group: 32.95 (1.7) years | Randomized, double-blind, placebo-controlled | Zinc 30 mg daily | 15 weeks | Reduction in body weight, body mass index, waist circumference, and appetite score in the intervention group |
| Fathi et al. (2020) [55] | Overweight and obese subjects, age range: 18–65 years | Randomized, double-blind, placebo-controlled | Zinc 30 mg daily | 12 weeks | Reduction in waist circumference, alanine aminotransferase, and gamma-glutamyl transpeptidase levels in the intervention group | |
| Hypertension | Mousavi et al. (2020) [57] | Subjects with type 2 diabetes mellitus, prediabetes, diabetic retinopathy, polycystic ovary syndrome, hypertension, obesity, and subjects on hemodialysis, age range as mean: 25–57 years | Meta-analysis of 9 randomized, controlled trials | Varies by study (elemental zinc 5–150 mg daily) | Varies by study (1–12 months) | Reduction in systolic blood pressure in the intervention group |
| Dyslipidemia | Hooper et al. (1980) [60] | Healthy male subjects, age range: 23–35 years | Zinc sulfate 440 mg daily | Zinc sulfate 440 mg daily | 5 weeks | Reduction in high-density lipoprotein levels |
| Payahoo et al. (2013) [58] | Subjects with obesity, age range: 18–45 years | Randomized, double-blind, placebo-controlled | Zinc gluconate 30 mg daily | 1 month | Reduction in triglyceride levels in the intervention group | |
| Asbaghi et al. (2020) [59] | Subjects with type 2 diabetes mellitus | Meta-analysis of 9 randomized, controlled trials, age range as means: 48.2–65.9 | Varies by study (zinc sulfate 30–660 mg daily, zinc gluconate 50–240 mg daily) | Varies by study (6–52 weeks) | Reduction in triglyceride and total cholesterol levels in the intervention group |
4. Laboratory evaluation of zinc levels: key points to know in the assessment of zinc levels
According to a systematic review, zinc levels in healthy subjects can be reliably evaluated in the plasma, urine, or hair [80]. A study that assessed plasma zinc kinetics showed that after 5-week of zinc depletion in humans, plasma and urine zinc concentrations decreased by 65% and 96%, respectively [81], demonstrating the response of plasma zinc level to zinc depletion. Although blood zinc concentration is reliable to assess zinc status [80], caution is needed in the interpretation of zinc levels as several factors can interfere with blood zinc levels (e.g., inflammation, hypoalbuminemia, fasting, starvation, pregnancy, intravascular hemolysis, myocardial infarction, contamination of biological samples with zinc) [79,82,83]. Hypoalbuminemia can spuriously lower blood zinc levels as albumin is the primary transporter for zinc [79,84]. The systemic inflammatory response also was reported to cause low blood zinc levels, and checking C-reactive protein level was recommended for reliable zinc level in patients with a systemic inflammatory response [79,85]. Although the utility of assessing zinc levels in every patient with NAFLD remains to be further defined, plasma zinc levels should be assessed in subjects with NAFLD who have advanced fibrosis/cirrhosis for identification and treatment of zinc deficiency and interpreted by taking potential interfering factors into account, specifically inflammation and hypoalbuminemia that are common in these patients.
5. Conclusions
Zinc deficiency in patients with NAFLD is associated with multiple factors, including endoplasmic reticulum stress and inflammation, insulin resistance, diabetes, dyslipidemia, obesity, hypertension, decreased absorption from the gastrointestinal tract, and inadequate dietary intake. Several studies have suggested that zinc supplementation has beneficial metabolic effects in patients with the NAFLD risk factors. Plasma zinc levels should be evaluated in patients with NAFLD and NAFLD risk factors, specifically in those with advanced hepatic fibrosis/cirrhosis. Randomized placebo-controlled trials are required to verify the beneficial effects of zinc supplementation in patients with NAFLD, specifically to evaluate the histological improvement of hepatic steatosis and fibrosis.
Author contributions
Mary Barbara, MD contributed to the drafting and writing the manuscript and performed a critical review of the manuscript for important intellectual content. Ayse L. Mindikoglu, MD, MPH contributed to the drafting and writing the manuscript and performed a critical review of the manuscript for important intellectual content.
Declaration of competing interest
None of the authors has a conflict of interest.
Acknowledgements
Authors thank Scott C Holmes, C.M.I., a member of the Michael E. DeBakey Department of Surgery Research Core at Baylor College of Medicine, for his assistance during the preparation of Fig. 1.
References
- 1.Obesity and overweight. http://www.who.int/mediacentre/factsheets/fs311/en/ Available at. Accessed on May 4, 2019.
- 2.Flegal K.M., Kruszon-Moran D., Carroll M.D., Fryar C.D., Ogden C.L. Trends in obesity among adults in the United States, 2005 to 2014. J Am Med Assoc. Jun 7 2016;315:2284–2291. doi: 10.1001/jama.2016.6458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Younossi Z.M., Koenig A.B., Abdelatif D., Fazel Y., Henry L., Wymer M. Global epidemiology of nonalcoholic fatty liver disease-Meta-analytic assessment of prevalence, incidence, and outcomes. Hepatology. Jul 2016;64:73–84. doi: 10.1002/hep.28431. [DOI] [PubMed] [Google Scholar]
- 4.Calzadilla-Bertot L., Jeffrey G.P., Jacques B., McCaughan G., Crawford M., Angus P. Increasing incidence of nonalcoholic steatohepatitis as an indication for liver transplantation in Australia and New Zealand. Liver Transplant. 2019;25:25–34. doi: 10.1002/lt.25361. [DOI] [PubMed] [Google Scholar]
- 5.Sayiner M., Younossi Z.M. Nonalcoholic steatohepatitis is becoming a top indication for liver transplantation worldwide. Liver Transplant. 2019;25:10–11. doi: 10.1002/lt.25387. [DOI] [PubMed] [Google Scholar]
- 6.Pickett-Blakely O., Young K., Carr R.M. Micronutrients in nonalcoholic fatty liver disease pathogenesis. Cell Mol Gastroenterol Hepatol. 2018;6:451–462. doi: 10.1016/j.jcmgh.2018.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Baffy G., Brunt E.M., Caldwell S.H. Hepatocellular carcinoma in non-alcoholic fatty liver disease: an emerging menace. J Hepatol. Jun 2012;56:1384–1391. doi: 10.1016/j.jhep.2011.10.027. [DOI] [PubMed] [Google Scholar]
- 8.Akdas S., Yazihan N. Serum zinc level and dietary zinc intake status in non-alcoholic fatty liver disease: a meta-analysis and systematic review. Hepatology Forum. 2020;2(1):59–67. doi: 10.14744/hf.2020.2020.0006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ito T., Ishigami M., Ishizu Y., Kuzuya T., Honda T., Ishikawa T. Correlation of serum zinc levels with pathological and laboratory findings in patients with nonalcoholic fatty liver disease. Eur J Gastroenterol Hepatol. 2020;32:748–753. doi: 10.1097/MEG.0000000000001587. [DOI] [PubMed] [Google Scholar]
- 10.Kotronen A., Peltonen M., Hakkarainen A., Sevastianova K., Bergholm R., Johansson L.M. Prediction of non-alcoholic fatty liver disease and liver fat using metabolic and genetic factors. Gastroenterology. Sep 2009;137:865–872. doi: 10.1053/j.gastro.2009.06.005. [DOI] [PubMed] [Google Scholar]
- 11.Sterling R.K., Lissen E., Clumeck N., Sola R., Correa M.C., Montaner J. Development of a simple noninvasive index to predict significant fibrosis in patients with HIV/HCV coinfection. Hepatology. Jun 2006;43:1317–1325. doi: 10.1002/hep.21178. [DOI] [PubMed] [Google Scholar]
- 12.Kim M.C., Lee J.I., Kim J.H., Kim H.J., Cho Y.K., Jeon W.K. Serum zinc level and hepatic fibrosis in patients with nonalcoholic fatty liver disease. PloS One. 2020;15 doi: 10.1371/journal.pone.0240195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bertol F.S., Araujo B., Jorge B.B., Rinaldi N., De Carli L.A., Tovo C.V. Role of micronutrients in staging of nonalcoholic fatty liver disease: a retrospective cross-sectional study. World J Gastrointest Surg. Jun 27 2020;12:269–276. doi: 10.4240/wjgs.v12.i6.269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gatiatulina E.R., Sheina E.A., Nemereshina O.N., Popova E.V., Polyakova V.S., Agletdinov E.F. Effect of Zn supplementation on trace element status in rats with diet-induced non-alcoholic fatty liver disease. Biol Trace Elem Res. Sep 2020;197:202–212. doi: 10.1007/s12011-019-01985-z. [DOI] [PubMed] [Google Scholar]
- 15.Qi Y., Zhang Z., Liu S., Aluo Z., Zhang L., Yu L. Zinc supplementation alleviates lipid and glucose metabolic disorders induced by a high-fat diet. J Agric Food Chem. May 6 2020;68:5189–5200. doi: 10.1021/acs.jafc.0c01103. [DOI] [PubMed] [Google Scholar]
- 16.Vivero A., Ruz M., Rivera M., Miranda K., Sacristán C., Espinosa A. Zinc supplementation and strength exercise in rats with type 2 diabetes: akt and PTP1B phosphorylation in nonalcoholic fatty liver. Biol Trace Elem Res. Jun 2021;199:2215–2224. doi: 10.1007/s12011-020-02324-3. [DOI] [PubMed] [Google Scholar]
- 17.Lebeaupin C., Vallee D., Hazari Y., Hetz C., Chevet E., Bailly-Maitre B. Endoplasmic reticulum stress signalling and the pathogenesis of non-alcoholic fatty liver disease. J Hepatol. Oct 2018;69:927–947. doi: 10.1016/j.jhep.2018.06.008. [DOI] [PubMed] [Google Scholar]
- 18.Zhang K., Kaufman R.J. From endoplasmic-reticulum stress to the inflammatory response. Nature. Jul 24 2008;454:455–462. doi: 10.1038/nature07203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hotamisligil G.S. Endoplasmic reticulum stress and the inflammatory basis of metabolic disease. Cell. 2010;140:900–917. doi: 10.1016/j.cell.2010.02.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Prasad A.S., Bao B., Beck F.W., Kucuk O., Sarkar F.H. Antioxidant effect of zinc in humans. Free Radic Biol Med. Oct 15 2004;37:1182–1190. doi: 10.1016/j.freeradbiomed.2004.07.007. [DOI] [PubMed] [Google Scholar]
- 21.Bao B., Prasad A.S., Beck F.W., Snell D., Suneja A., Sarkar F.H. Zinc supplementation decreases oxidative stress, incidence of infection, and generation of inflammatory cytokines in sickle cell disease patients. Transl Res. Aug 2008;152:67–80. doi: 10.1016/j.trsl.2008.06.001. [DOI] [PubMed] [Google Scholar]
- 22.Prasad A.S. Zinc: role in immunity, oxidative stress and chronic inflammation. Curr Opin Clin Nutr Metab Care. Nov 2009;12:646–652. doi: 10.1097/MCO.0b013e3283312956. [DOI] [PubMed] [Google Scholar]
- 23.Prasad A.S., Beck F.W., Bao B., Fitzgerald J.T., Snell D.C., Steinberg J.D. Zinc supplementation decreases incidence of infections in the elderly: effect of zinc on generation of cytokines and oxidative stress. Am J Clin Nutr. Mar 2007;85:837–844. doi: 10.1093/ajcn/85.3.837. [DOI] [PubMed] [Google Scholar]
- 24.Ellis C.D., Wang F., MacDiarmid C.W., Clark S., Lyons T., Eide D.J. Zinc and the Msc2 zinc transporter protein are required for endoplasmic reticulum function. J Cell Biol. Aug 2 2004;166:325–335. doi: 10.1083/jcb.200401157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Homma K., Fujisawa T., Tsuburaya N., Yamaguchi N., Kadowaki H., Takeda K. SOD1 as a molecular switch for initiating the homeostatic ER stress response under zinc deficiency. Mol Cell. Oct 10 2013;52:75–86. doi: 10.1016/j.molcel.2013.08.038. [DOI] [PubMed] [Google Scholar]
- 26.Kim M.H., Aydemir T.B., Kim J., Cousins R.J. Hepatic ZIP14-mediated zinc transport is required for adaptation to endoplasmic reticulum stress. Proc Natl Acad Sci U S A. Jul 18 2017;114:E5805–e5814. doi: 10.1073/pnas.1704012114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hara T., Takeda T.-a., Takagishi T., Fukue K., Kambe T., Fukada T. Physiological roles of zinc transporters: molecular and genetic importance in zinc homeostasis. J Physiol Sci. 2017/03/01 2017;67:283–301. doi: 10.1007/s12576-017-0521-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Targher G., Bertolini L., Padovani R., Rodella S., Tessari R., Zenari L. Prevalence of nonalcoholic fatty liver disease and its association with cardiovascular disease among type 2 diabetic patients. Diabetes Care. 2007;30:1212–1218. doi: 10.2337/dc06-2247. [DOI] [PubMed] [Google Scholar]
- 29.Cusi K., Sanyal A.J., Zhang S., Hartman M.L., Bue-Valleskey J.M., Hoogwerf B.J. Non-alcoholic fatty liver disease (NAFLD) prevalence and its metabolic associations in patients with type 1 diabetes and type 2 diabetes. Diabetes Obes Metabol. Nov 2017;19:1630–1634. doi: 10.1111/dom.12973. [DOI] [PubMed] [Google Scholar]
- 30.Chimienti F. Zinc, pancreatic islet cell function and diabetes: new insights into an old story. Nutr Res Rev. Jun 2013;26:1–11. doi: 10.1017/S0954422412000212. [DOI] [PubMed] [Google Scholar]
- 31.Rutter G.A., Chabosseau P., Bellomo E.A., Maret W., Mitchell R.K., Hodson D.J. Intracellular zinc in insulin secretion and action: a determinant of diabetes risk? Proc Nutr Soc. Feb 2016;75:61–72. doi: 10.1017/S0029665115003237. [DOI] [PubMed] [Google Scholar]
- 32.Scott D.A., Fisher A.M. The insulin and the zinc content OF normal and diabetic pancreas. J Clin Invest. Nov 1938;17:725–728. doi: 10.1172/JCI101000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Basaki M., Saeb M., Nazifi S., Shamsaei H.A. Zinc, copper, iron, and chromium concentrations in young patients with type 2 diabetes mellitus. Biol Trace Elem Res. Aug 2012;148:161–164. doi: 10.1007/s12011-012-9360-6. [DOI] [PubMed] [Google Scholar]
- 34.Jansen J., Rosenkranz E., Overbeck S., Warmuth S., Mocchegiani E., Giacconi R. Disturbed zinc homeostasis in diabetic patients by in vitro and in vivo analysis of insulinomimetic activity of zinc. J Nutr Biochem. Nov 2012;23:1458–1466. doi: 10.1016/j.jnutbio.2011.09.008. [DOI] [PubMed] [Google Scholar]
- 35.Isbir T., Tamer L., Taylor A., Isbir M. Zinc, copper and magnesium status in insulin-dependent diabetes. Diabetes Res. 1994;26:41–45. [PubMed] [Google Scholar]
- 36.Sprietsma J.E., Schuitemaker G.E. Diabetes can be prevented by reducing insulin production. Med Hypotheses. Jan 1994;42:15–23. doi: 10.1016/0306-9877(94)90029-9. [DOI] [PubMed] [Google Scholar]
- 37.Chausmer A.B. Zinc, insulin and diabetes. J Am Coll Nutr. Apr 1998;17:109–115. doi: 10.1080/07315724.1998.10718735. [DOI] [PubMed] [Google Scholar]
- 38.Ranasinghe P., Wathurapatha W.S., Galappatthy P., Katulanda P., Jayawardena R., Constantine G.R. Zinc supplementation in prediabetes: a randomized double-blind placebo-controlled clinical trial. J Diabetes. May 2018;10:386–397. doi: 10.1111/1753-0407.12621. [DOI] [PubMed] [Google Scholar]
- 39.Wang X., Wu W., Zheng W., Fang X., Chen L., Rink L. Zinc supplementation improves glycemic control for diabetes prevention and management: a systematic review and meta-analysis of randomized controlled trials. Am J Clin Nutr. Jul 1 2019;110:76–90. doi: 10.1093/ajcn/nqz041. [DOI] [PubMed] [Google Scholar]
- 40.Chimienti F., Devergnas S., Pattou F., Schuit F., Garcia-Cuenca R., Vandewalle B. In vivo expression and functional characterization of the zinc transporter ZnT8 in glucose-induced insulin secretion. J Cell Sci. Oct 15 2006;119:4199–4206. doi: 10.1242/jcs.03164. [DOI] [PubMed] [Google Scholar]
- 41.Cauchi S., Guerra S.D., Choquet H., D'Aleo V., Groves C.J., Lupi R. Meta-analysis and functional effects of the SLC30A8 rs13266634 polymorphism on isolated human pancreatic islets. Mol Genet Metabol. 2010/05/01/2010;100:77–82. doi: 10.1016/j.ymgme.2010.01.001. [DOI] [PubMed] [Google Scholar]
- 42.Kanoni S., Nettleton J.A., Hivert M.F., Ye Z., van Rooij F.J., Shungin D. Total zinc intake may modify the glucose-raising effect of a zinc transporter (SLC30A8) variant: a 14-cohort meta-analysis. Diabetes. Sep 2011;60:2407–2416. doi: 10.2337/db11-0176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sladek R., Rocheleau G., Rung J., Dina C., Shen L., Serre D. A genome-wide association study identifies novel risk loci for type 2 diabetes. Nature. Feb 22 2007;445:881–885. doi: 10.1038/nature05616. [DOI] [PubMed] [Google Scholar]
- 44.el-Yazigi A., Hannan N., Raines D.A. Effect of diabetic state and related disorders on the urinary excretion of magnesium and zinc in patients. Diabetes Res. 1993;22:67–75. [PubMed] [Google Scholar]
- 45.McNair P., Kiilerich S., Christiansen C., Christensen M.S., Madsbad S., Transbol I. Hyperzincuria in insulin treated diabetes mellitus—its relation to glucose homeostasis and insulin administration. Clin Chim Acta. 1981/05/28/1981;112:343–348. doi: 10.1016/0009-8981(81)90457-5. [DOI] [PubMed] [Google Scholar]
- 46.Kinlaw W.B., Levine A.S., Morley J.E., Silvis S.E., McClain C.J. Abnormal zinc metabolism in type II diabetes mellitus. Am J Med. Aug 1983;75:273–277. doi: 10.1016/0002-9343(83)91205-6. [DOI] [PubMed] [Google Scholar]
- 47.Escobar O., Sandoval M., Vargas A., Hempe J.M. Role of metallothionein and cysteine-rich intestinal protein in the regulation of zinc absorption by diabetic rats. Pediatr Res. 1995/03/01 1995;37:321–327. doi: 10.1203/00006450-199503000-00012. [DOI] [PubMed] [Google Scholar]
- 48.Miao X., Sun W., Fu Y., Miao L., Cai L. Zinc homeostasis in the metabolic syndrome and diabetes. Front Med. Mar 2013;7:31–52. doi: 10.1007/s11684-013-0251-9. [DOI] [PubMed] [Google Scholar]
- 49.Izquierdo A.G., Crujeiras A.B., Casanueva F.F., Carreira M.C. Leptin, obesity, and leptin resistance: where are we 25 Years later? Nutrients. 2019;11:2704. doi: 10.3390/nu11112704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Thoen R.U., Barther N.N., Schemitt E., Bona S., Fernandes S., Coral G. Zinc supplementation reduces diet-induced obesity and improves insulin sensitivity in rats. Appl Physiol Nutr Metabol. Jun 2019;44:580–586. doi: 10.1139/apnm-2018-0519. [DOI] [PubMed] [Google Scholar]
- 51.Liu M.J., Bao S., Bolin E.R., Burris D.L., Xu X., Sun Q. Zinc deficiency augments leptin production and exacerbates macrophage infiltration into adipose tissue in mice fed a high-fat diet. J Nutr. Jul 2013;143:1036–1045. doi: 10.3945/jn.113.175158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Maxel T., Smidt K., Larsen A., Bennetzen M., Cullberg K., Fjeldborg K. Gene expression of the zinc transporter ZIP14 (SLC39a14) is affected by weight loss and metabolic status and associates with PPARγ in human adipose tissue and 3T3-L1 pre-adipocytes. BMC Obesity. 2015;2(46) doi: 10.1186/s40608-015-0076-y. 2015/11/24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Fan Y., Zhang C., Bu J. Relationship between selected serum metallic elements and obesity in children and adolescent in the U.S. Nutrients. Feb 3 2017;9 doi: 10.3390/nu9020104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Khorsandi H., Nikpayam O., Yousefi R., Parandoosh M., Hosseinzadeh N., Saidpour A. Zinc supplementation improves body weight management, inflammatory biomarkers and insulin resistance in individuals with obesity: a randomized, placebo-controlled, double-blind trial. Diabetol Metab Syndrome. 2019;11:101. doi: 10.1186/s13098-019-0497-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Fathi M., Alavinejad P., Haidari Z., Amani R. The effect of zinc supplementation on steatosis severity and liver function enzymes in overweight/obese patients with mild to moderate non-alcoholic fatty liver following calorie-restricted diet: a double-blind, randomized placebo-controlled trial. Biol Trace Elem Res. Oct 2020;197:394–404. doi: 10.1007/s12011-019-02015-8. [DOI] [PubMed] [Google Scholar]
- 56.Williams C.R., Mistry M., Cheriyan A.M., Williams J.M., Naraine M.K., Ellis C.L. Zinc deficiency induces hypertension by promoting renal Na(+) reabsorption. Am J Physiol Ren Physiol. Apr 1 2019;316:F646–F653. doi: 10.1152/ajprenal.00487.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Mousavi S.M., Mofrad M.D., do Nascimento I.J.B., Milajerdi A., Mokhtari T., Esmaillzadeh A. The effect of zinc supplementation on blood pressure: a systematic review and dose-response meta-analysis of randomized-controlled trials. Eur J Nutr. Aug 2020;59:1815–1827. doi: 10.1007/s00394-020-02204-5. [DOI] [PubMed] [Google Scholar]
- 58.Payahoo L., Ostadrahimi A., Mobasseri M., Khaje Bishak Y., Farrin N., Asghari Jafarabadi M. Effects of zinc supplementation on the anthropometric measurements, lipid profiles and fasting blood glucose in the healthy obese adults. Adv Pharmaceut Bull. 2013;3:161–165. doi: 10.5681/apb.2013.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Asbaghi O., Sadeghian M., Fouladvand F., Panahande B., Nasiri M., Khodadost M. Effects of zinc supplementation on lipid profile in patients with type 2 diabetes mellitus: a systematic review and meta-analysis of randomized controlled trials. Nutr Metabol Cardiovasc Dis. 2020;30:1260–1271. doi: 10.1016/j.numecd.2020.03.021. 2020/07/24. [DOI] [PubMed] [Google Scholar]
- 60.Hooper P.L., Visconti L., Garry P.J., Johnson G.E. Zinc lowers high-density lipoprotein-cholesterol levels. J Am Med Assoc. 1980;244:1960–1961. [PubMed] [Google Scholar]
- 61.Lee H.H., Prasad A.S., Brewer G.J., Owyang C. Zinc absorption in human small intestine. Am J Physiol. Jan 1989;256:G87–G91. doi: 10.1152/ajpgi.1989.256.1.G87. [DOI] [PubMed] [Google Scholar]
- 62.Antonson D.L., Vanderhoof J.A. Zinc absorption following massive small-bowel resection in the rat. Dig Dis Sci. 1982;27:789–793. doi: 10.1007/BF01391371. 1982/09/01. [DOI] [PubMed] [Google Scholar]
- 63.Jeejeebhoy K.N. Short bowel syndrome: a nutritional and medical approach. CMAJ (Can Med Assoc J) : Canadian Medical Association journal = journal de l'Association medicale canadienne. 2002;166:1297–1302. [PMC free article] [PubMed] [Google Scholar]
- 64.Dutta S.K., Procaccino F., Aamodt R. Zinc metabolism in patients with exocrine pancreatic insufficiency. J Am Coll Nutr. Dec 1998;17:556–563. doi: 10.1080/07315724.1998.10718803. [DOI] [PubMed] [Google Scholar]
- 65.Murata Y., Mizuno S., Kato H., Kishiwada M., Ohsawa I., Hamada T. Nonalcoholic steatohepatitis (NASH) after pancreaticoduodenectomy: association of pancreatic exocrine deficiency and infection. Clin J Gastroenterol. Aug 2011;4:242–248. doi: 10.1007/s12328-011-0226-9. [DOI] [PubMed] [Google Scholar]
- 66.Nomura R., Ishizaki Y., Suzuki K., Kawasaki S. Development of hepatic steatosis after pancreatoduodenectomy. AJR Am J Roentgenol. Dec 2007;189:1484–1488. doi: 10.2214/AJR.07.2809. [DOI] [PubMed] [Google Scholar]
- 67.Takemura N., Saiura A., Koga R., Yamamoto J., Yamaguchi T. Risk factors for and management of postpancreatectomy hepatic steatosis. Scand J Surg. Sep 2017;106:224–229. doi: 10.1177/1457496916669630. [DOI] [PubMed] [Google Scholar]
- 68.Lönnerdal B. Dietary factors influencing zinc absorption. J Nutr. 2000;130:1378S–1383S. doi: 10.1093/jn/130.5.1378S. [DOI] [PubMed] [Google Scholar]
- 69.Chandalia M., Garg A., Lutjohann D., von Bergmann K., Grundy S.M., Brinkley L.J. Beneficial effects of high dietary fiber intake in patients with type 2 diabetes mellitus. N Engl J Med. 2000;342:1392–1398. doi: 10.1056/NEJM200005113421903. [DOI] [PubMed] [Google Scholar]
- 70.Foster M., Karra M., Picone T., Chu A., Hancock D.P., Petocz P. Dietary fiber intake increases the risk of zinc deficiency in healthy and diabetic women. Biol Trace Elem Res. Nov 2012;149:135–142. doi: 10.1007/s12011-012-9408-7. [DOI] [PubMed] [Google Scholar]
- 71.Omoruyi F.O., Budiaman A., Eng Y., Olumese F.E., Hoesel J.L., Ejilemele A. The potential benefits and adverse effects of phytic acid supplement in streptozotocin-induced diabetic rats. Advances in Pharmacological Sciences. 2013:172494. doi: 10.1155/2013/172494. 2013/12/22 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Sekita A., Okazaki Y., Katayama T. Dietary phytic acid prevents fatty liver by reducing expression of hepatic lipogenic enzymes and modulates gut microflora in rats fed a high-sucrose diet. Nutrition. Jun 2016;32:720–722. doi: 10.1016/j.nut.2016.01.003. [DOI] [PubMed] [Google Scholar]
- 73.Vartanian L.R., Schwartz M.B., Brownell K.D. Effects of soft drink consumption on nutrition and health: a systematic review and meta-analysis. Am J Publ Health. Apr 2007;97:667–675. doi: 10.2105/AJPH.2005.083782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Astrup A., Bügel S. Micronutrient deficiency in the aetiology of obesity. Int J Obes. 2010;34:947–948. doi: 10.1038/ijo.2010.81. 2010/06/01. [DOI] [PubMed] [Google Scholar]
- 75.Reedy J., Krebs-Smith S.M. Dietary sources of energy, solid fats, and added sugars among children and adolescents in the United States. J Am Diet Assoc. Oct 2010;110:1477–1484. doi: 10.1016/j.jada.2010.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Marshall T.A., Eichenberger Gilmore J.M., Broffitt B., Stumbo P.J., Levy S.M. Diet quality in young children is influenced by beverage consumption. J Am Coll Nutr. Feb 2005;24:65–75. doi: 10.1080/07315724.2005.10719445. [DOI] [PubMed] [Google Scholar]
- 77.Li Y., Wang C., Zhu K., Feng R.N., Sun C.H. Effects of multivitamin and mineral supplementation on adiposity, energy expenditure and lipid profiles in obese Chinese women. Int J Obes. Jun 2010;34:1070–1077. doi: 10.1038/ijo.2010.14. [DOI] [PubMed] [Google Scholar]
- 78.Cai L., Li X.K., Song Y., Cherian M.G. Essentiality, toxicology and chelation therapy of zinc and copper. Curr Med Chem. 2005;12:2753–2763. doi: 10.2174/092986705774462950. [DOI] [PubMed] [Google Scholar]
- 79.Duncan A., Yacoubian C., Watson N., Morrison I. The risk of copper deficiency in patients prescribed zinc supplements. J Clin Pathol. Sep 2015;68:723–725. doi: 10.1136/jclinpath-2014-202837. [DOI] [PubMed] [Google Scholar]
- 80.Lowe N.M., Fekete K., Decsi T. Methods of assessment of zinc status in humans: a systematic review. Am J Clin Nutr. Jun 2009;89:2040s–2051s. doi: 10.3945/ajcn.2009.27230G. [DOI] [PubMed] [Google Scholar]
- 81.King J.C., Shames D.M., Lowe N.M., Woodhouse L.R., Sutherland B., Abrams S.A. Effect of acute zinc depletion on zinc homeostasis and plasma zinc kinetics in men. Am J Clin Nutr. Jul 2001;74:116–124. doi: 10.1093/ajcn/74.1.116. [DOI] [PubMed] [Google Scholar]
- 82.Prasad A.S. Laboratory diagnosis of zinc deficiency. J Am Coll Nutr. 1985;4:591–598. doi: 10.1080/07315724.1985.10720101. 1985/01/01. [DOI] [PubMed] [Google Scholar]
- 83.Wieringa F.T., Dijkhuizen M.A., Fiorentino M., Laillou A., Berger J. Determination of zinc status in humans: which indicator should we use? Nutrients. 2015;7:3252–3263. doi: 10.3390/nu7053252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Chesters J.K., Will M. Zinc transport proteins in plasma. Br J Nutr. Jul 1981;46:111–118. doi: 10.1079/bjn19810014. [DOI] [PubMed] [Google Scholar]
- 85.Duncan A., Talwar D., McMillan D.C., Stefanowicz F., O'Reilly D.S. Quantitative data on the magnitude of the systemic inflammatory response and its effect on micronutrient status based on plasma measurements. Am J Clin Nutr. Jan 2012;95:64–71. doi: 10.3945/ajcn.111.023812. [DOI] [PubMed] [Google Scholar]