Abstract
Necrotic enteritis (NE) is a significant enteric disease in commercial poultry with considerable economic effect on profitability manifested by an estimated $6 billion in annual losses to the global industry. NE presents a unique challenge, being a complex enteric disease that often leads to either clinical (acute) or subclinical (chronic) form. The latter typically results in poor performance (reduced feed intake, weight gain and eventually higher feed conversion ratio [FCR]) with low mortality rates, and represents the greatest economic impact on poultry production. The use of antibiotic growth promoters (AGPs) has been an effective tool in protecting birds from enteric diseases by maintaining enteric health and modifying gut microbiota, thus improving broilers’ production efficiency and overall health. The removal of AGPs presented the poultry industry with several challenges, including reduced bird health and immunity as well as questioning the safety of poultry products. Consequently, research on antibiotic alternatives that can support gut health was intensified. Probiotics, prebiotics, essential oils, and organic acids were among various additives that have been tested for their efficacy against NE with some being effective but not to the level of AGPs. The focus of this review is on the relationship between NE pathogenesis, microbiome, and host immune responses, along with references to recent reviews addressing production aspects of NE. With a comprehensive understanding of these dynamic changes, new and programmed strategies could be developed to make use of the current products more effectively or build a stepping stone toward the development of a new generation of supplements.
Key words: necrotic enteritis, pathology, tight junctions, immune response, gut microbiome
INTRODUCTION
Necrotic enteritis (NE) was first reported in chickens 60 yr ago (Parish, 1961) and despite years of research, the reemergence of NE continues to present a major challenge in commercial poultry production (Stanley et al., 2014). Caused by the Gram-positive bacteria Clostridium perfringens, NE is a significant enteric disease in poultry with considerable economic effect on profitability represented by estimated annual losses of ~$6 billion worldwide (Wade and Keyburn, 2015).
The use of antibiotic growth promoters (AGPs) has been an effective tool in maintaining gut health and modifying its resident microbiota, thus improving production efficiency and improving enteric health of broilers (Cervantes, 2015). Over the past few years, several countries across the world, including the European Union and United States, have eliminated the use of antibiotics as growth promoters in poultry feed due to grave concerns for antibiotic resistance (Dibner and Richards, 2005; Castanon, 2007; Martin et al., 2015). This policy has created an urgent need for nonantibiotic alternatives in order to prevent the spread of foodborne illnesses and preserve the poultry industry's ability to meet demand (Cox and Dalloul, 2010). The removal of AGPs faced the poultry industry with several challenges, including reduced bird health and immunity and the safety of poultry products (Cervantes, 2015). Consequently, research on antibiotic alternatives that can support gut health has recently intensified (Lee et al., 2011; Cox and Dalloul, 2015). Probiotics, prebiotics, essential oils and organic acids were among various additives that have been tested for their efficacy against NE with some being effective but not to the level of AGPs (M'Sadeq et al., 2015).
The lack of reliable alternative strategies to control NE is mainly due to limited insight into the relationship between NE pathogenesis, microbiome, and host responses (Wang et al., 2019). Therefore, key to overcoming NE is to define the cellular and molecular mechanisms that are involved in the development of the disease, especially with regard to mucosal immune responses and dynamics of the gut microbiome. Also, assessing the impact of these changes on intestinal cell metabolism and function is of great importance. This review aims to provide an update on the recent advances in NE research and the interactions of C. perfringens with the host. Collectively, this review could be a valuable guide to the poultry community regarding our knowledge of the disease to provide an insight into preventing the onset or alleviating the negative consequences of NE on the health and performance of poultry.
Etiology of Necrotic Enteritis
C. perfringens is the pathogen responsible for NE (Parish, 1961). This organism is a Gram-positive, rod-shaped, spore-forming, anaerobic bacterium (Shojadoost et al., 2012; To et al., 2017). C. perfringens strains are classified into 7 toxinogenic types (A, B, C, D, E, F, G), which differ based on their ability to produce various types of toxins: α, β, ε, ι, NetB, and enterotoxins (Table 1) (Rood et al., 2018). In broiler chickens, NE commonly occurs around 2 to 6 wk of age, but also affects 12 to 16 wk old cage-reared replacement pullets as well as layers at 3 to 6 mo of age (Broussard et al., 1986; Frame and Bickford, 1986; Dhillon et al., 2004; Cooper et al., 2013; To et al., 2017). NE is caused mostly by NetB-positive C. perfringens type G strains in broiler chickens; yet, some researchers were able to induce NE with NetB-negative strains or C. perfringens type A, indicative of the possibility of other toxins being involved (Keyburn et al., 2008, Rood et al., 2018; Goossens et al., 2020; Shini et al., 2020). In a healthy bird, C. perfringens population is ~102–104 CFU/g digesta; however, at the time of disease this number will rise to ~107–109 CFU/g digesta (Kondo, 1988). There is a greater prevalence of netB gene carriage in C. perfringens isolates from diseased poultry compared to healthy birds. NetB is known to be encoded exclusively on conjugative plasmids, indicating that horizontal gene transfer may play a role in the dissemination to NetB-negative strains (Rood et al., 2016). Therefore, it is now accepted that NE is a complex enteric disease with C. perfringens as the causal agent, but predisposing factors are requisite to make the gastrointestinal tract environment suitable for these bacteria to replicate and produce toxins (Van Immerseel et al., 2004).
Table 1.
C. perfringens typing.
| Toxin productiona |
||||||
|---|---|---|---|---|---|---|
| Type | α (plc or cpa) | β (cpb) | ε (etx) | ι (iap and ibp) | CPE (cpe) | NetB (netB) |
| A | + | – | – | – | – | – |
| B | + | + | + | – | – | – |
| C | + | + | – | – | – | |
| D | + | – | + | – | – | |
| E | + | – | – | + | – | |
| F | + | – | – | – | + | – |
| G | + | – | – | – | – | + |
“+” indicates production of that toxin, while “–” indicates lack of toxin production.
The names of toxin structural genes are shown in parentheses (Rood et al., 2018).
NE occurs in a clinical (acute) or subclinical (chronic) form (Wu et al., 2010; To et al., 2017). In severe clinical forms, mortality is high and associated symptoms include ruffled feathers, depression, diarrhea, huddling, anorexia, sternal recumbency, and a sudden rise in flock mortality (To et al., 2017). In certain cases, sudden rise in flock mortality is the first and only sign with no other premonitory symptoms. Pathologically, clinical forms show necrotic foci in the small intestine, which in the most severe cases appear as broad necrosis of the mucosal lining (Van Immerseel et al., 2009). In subclinical NE, chronic damage to the small intestinal mucosa leads to poor performance manifested by decreased feed intake and weight gain, and eventually increased FCR, without significant mortality. Interestingly, the subclinical forms of NE are responsible for the greatest economic impact on poultry production (Van Immerseel et al., 2009; Shojadoost et al., 2012).
C. perfringens Virulence Factors
Pathogenic strains of C. perfringens have developed several virulence factors that contribute to the progression of NE in chickens. These virulence factors are very important for bacterial attachment to the mucosa (adhesins) and for providing nutrients for their rapid proliferation (degradative enzymes), as well as for toxin production. A positive association of virulence genes including netB, pfoA, cpb2, tpeL, and cna variants is linked to NE-inducing C. perfringens isolates (Kiu et al., 2019).
Several C. perfringens proteins, including collagen adhesion protein (CNA), have been proposed to function as adhesins during disease (Martin and Smyth, 2010). Adherence of C. perfringens strains to extracellular matrix proteins is important in NE pathogenesis and is correlated strongly with their virulence (Prescott et al., 2016; Wade et al., 2016). Presence of adhesin-encoding gene cnaA, which is found in the VR-10B chromosomal locus, is critical for binding of the pathogen to collagen types IV and V and gelatin (Wade et al., 2015, 2016). C. perfringens also produces an adhesive pilus required for adhering to the intestine and is consisted of 3 subunits (CnaA, FimA, and FimB) encoded within the VR-10B chromosomal locus (Lepp et al., 2021). Fibronectin, an extracellular matrix protein, is the target for C. perfringens fibrinogen-binding proteins FbpA and FbpB to facilitate host cell contact and further colonization (Katayama et al., 2009; Mehdizadeh Gohari et al., 2021).
Sialic acid (N-acetyl neuraminic acid) is a component of the mucus glycoproteins and there is evidence of a linear relationship between alpha toxin antibody levels and sialic acid concentrations in the ileal digesta (Fernando et al., 2011). Some pathogens including influenza viruses, Vibrio cholerae, Streptococcus pneumoniae and C. perfringens produce sialidase enzymes, which by releasing sialic acid could provide nutrients for these microbes (Juge et al., 2016). C. perfringens produces various sialidases including NanH, NanI, and NanJ that increase its adherence to intestinal tissues and break the sialic acid linkages at different points to generate nutrients for its growth (Li et al., 2016; Mehdizadeh Gohari et al., 2021). At the time of C. perfringens intestinal infections, toxins are produced, absorbed to reach other organs such as the liver and brain, and eventually cause enterotoxaemia (Li et al., 2016). In addition, intestinal damage by bacterial toxins stimulates goblet cells to produce more mucus, which in turn may lead to higher proliferation of pathogenic organisms (Fernando et al., 2011). Other virulence factors are zinc metalloproteases (mucinases) encoded by the zmpA and zmpB genes. The zmpB gene is located on a chromosome while zmpA is located within a plasmid-encoded region called NELoc-1, which encodes NetB as well (Lepp et al., 2010; Wade et al., 2020). The proteins (proteases) are usually secreted and are generally produced by bacteria that reside on or in the mucosal surfaces and degrade mucin (the primary constituent glycoprotein of mucosa); thus, enhance pathogen colonization (Wade et al., 2016). It is hypothesized that both proteases are involved in the same virulence-enhancing catabolic process, and loss of either protease is sufficient to halt the process resulting in the reduction in virulence (Wade et al., 2020).
Predisposing Factors
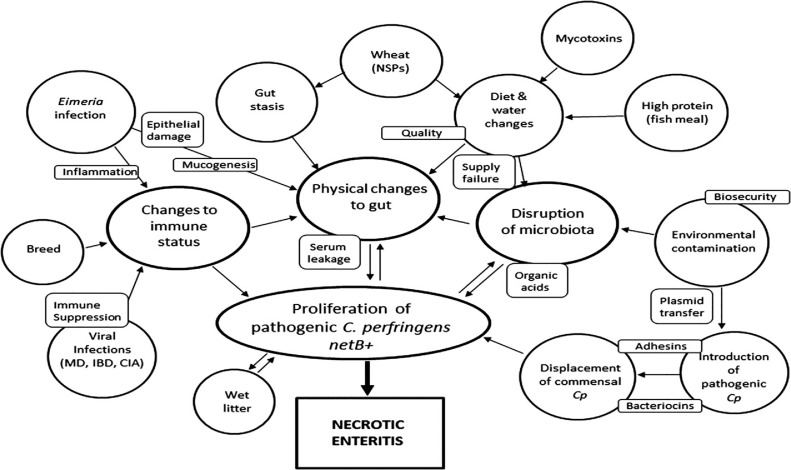
Major risk factors for NE include 1) Eimeria infection; 2) removal of coccidiostats or AGPs; 3) environmental and management conditions; 4) physiological stress and immunosuppression; and 5) nature and form of the diets (Dahiya et al., 2006). In recent years, several factors including Eimeria, fish meal, Fusarium mycotoxins and certain grains with high content of nonstarch polysaccharides have been identified as stress factors (Figure 1) that could stimulate the onset of NE (Annett et al., 2002; Wu et al., 2014; Antonissen et al., 2016; Moore, 2016). These factors predispose birds to NE by affecting the gastrointestinal tract in different ways such as altering the microbial profile in favor of C. perfringens proliferation (e.g., high levels of crude protein), changing the digesta viscosity thus increasing the production rate of mucins (e.g., mucinogenic effect of wheat), damaging the epithelial layer (e.g., biogenic amines in fish meal; mycotoxins), and acting as a source of C. perfringens contamination (e.g., fish meal) (Annett et al., 2002; Drew et al., 2004; Antonissen et al., 2014; Wu et al., 2014). Wheat and other grains with high amount of soluble nonstarch polysaccharides are mucogenic compounds and regarded as predisposing factors for NE (Moore, 2016). Furthermore, damage to the epithelium induced by Eimeria would release serum compounds and other nutrients, or causes mucogenesis that promotes the proliferation of Clostridium. Fish meal is a source of biogenic amines and clostridial contamination (Moore, 2016). The ceca are the site of fermentation, and protein products that bypass the ileum and reach this site will be used by putrefactive bacteria. Fermentation and putrefaction produce compounds such as amines, indoles, phenols, cresol and ammonia, which in high concentrations can adversely affect the gut and cell health and have a negative impact on performance of broilers (Apajalahti and Vienola, 2016). Mycotoxins (deoxynivalenol and fumonisins) inflict damage to intestinal epithelial cells resulting in decreased absorption of dietary proteins, which in turn could lead to proliferation of bacteria such as C. perfringens (Antonissen et al., 2014). Additionally, mycotoxins (fumonisins) predispose broiler chickens to NE by affecting the intestinal microbial homeostasis and reducing abundance of Candidatus Savagella and Lactobacillus spp. (Antonissen et al., 2015).
Figure 1.
Summary of predisposing factors for necrotic enteritis development in chickens. Predisposing factors and the major effects of these factors are shown in ovals. Important factors that may drive the influence of the predisposing factors are shown in the small rectangular boxes. Abbreviations: CIA, chicken infectious anemia; Cp, Clostridium perfringens; IBD, infectious bursal disease; MD, Marek's disease; NSPs, nonstarch polysaccharides (Moore, 2016).
The Role of Intestinal Microbiota
The cecum is the main site of C. perfringens replication; however, NE lesions are more prominent in the small intestine than the ceca (Van Immerseel et al., 2004; Stanley et al., 2012; Cooper et al., 2013). Shifts in microbial diversity and population could make the gut environment suitable for proliferation or pathogenesis of bacteria such as C. perfringens by providing a desirable ecological environment or nutrients (Shojadoost et al., 2012; Antonissen et al., 2016). Shifts in microbial diversity and population during NE have been vastly studied. However, contradicting results have been reported in certain cases possibly due to differences in challenge models, age of the birds at challenge, health status of the birds, breed, sex, disease status (clinical vs. subclinical), and site (ileum vs. cecum) and type (mucosal scrapings vs. digesta) of the samples collected. Therefore, in this section the goal is to summarize shifts in bacterial taxa with direct relevance to NE.
Prevotellaceae is among the bacterial taxa associated with NE (Latorre et al., 2018; Emami et al., 2020). Prevotellaceae can degrade mucus oligosaccharides resulting in the disruption of intestinal mucosal barrier, thus causing intestinal inflammation (Rho et al., 2005). Ileal microbial profiling showed increased abundance of Prevotella in NE-challenged compared to nonchallenged birds (Latorre et al., 2018). Supplementation of a probiotic (B. licheniformis) or a multicomponent feed additive (probiotics/prebiotics/essential oils) reduced relative abundance of Prevotellaceae in the ileal mucosa microbiome compared to control (no additive) during a naturally occurring subclinical NE. Greater relative abundance of Prevotellaceae in the control (basal broiler diet) group likely led to the disruption of the mucosal barrier rendering it prone to bacterial pathogens, which coincided with higher FCR and lesion scores in the control birds (Emami et al., 2020).
There is positive correlation between the relative abundance of Escherichia-Shigella with NE occurrence in broiler chickens (Du et al., 2015). C. perfringens infection increased the relative abundance of Escherichia-Shigella in the ileum of broiler chickens (Li et al., 2017). This genus pair is mainly composed of opportunistic pathogens and higher presence might be an indicator of disrupted gut integrity that allows the overgrowth of such pathogens. C. perfringens and its coinfection with Eimeria increased relative abundance of Escherichia-Shigella in the jejunum of broiler chickens (Yang et al., 2019). A L. acidophilus-based probiotic improved intestinal health by decreasing the relative abundance of Escherichia-Shigella populations in the ileum (Li et al., 2017). The probiotic bacteria B. subtilis DSM 32315 decreased the abundance Escherichia-Shigella and improved the growth performance and intestinal structure of broilers (Ma et al., 2018).
Studies mostly support decrease in the number of Lactobacillus and overgrowth of Clostridium sensu stricto 1 associated with NE (Antonissen et al., 2016; Fasina et al., 2016; Li et al., 2017). Mixed C. perfringens and Eimeria challenge significantly increased Clostridium sensu stricto 1 and reduced Lactobacillus abundance with the concurrent increase in NE lesions (Yang et al., 2019). Similarly, NE challenge decreased cecal Lactobacillus and Bifidobacterium spp. counts while increasing C. perfringens counts, and these changes were reversed by the dietary addition of a prebiotic to broiler feed (Ahiwe et al., 2019). L. acidophilus supplementation in the diet increased Lactobacillus abundance in the ileum and cecum, and decreased Escherichia abundance in the ileum of male Arbor Acres broilers at d 21, and these shifts were associated with better performance and improved gut morphology (Li et al., 2018). Supplementation of a probiotic (B. licheniformis) or a multicomponent feed additive (probiotics/prebiotics/essential oils) increased relative abundance of Lactobacillus in the ileal microbiota and improved intestinal health compared to control during NE (Emami et al., 2020). The abundance of Lactobacillaceae and Clostridiaceae families was significantly increased in the cecal digesta of probiotic-fed birds subjected to NE challenge (Whelan et al., 2019). However, there are reports on the higher abundance of Lactobacillus in the ileal digesta of NE-challenged broilers compared to nonchallenged birds (Latorre et al., 2018).
Reports on the shifts in the relative abundance of taxa such as Ruminococcaceae during NE are inconsistent. Relative frequency of Ruminococcus and Ruminococcaceae significantly decreased in the cecal content of broiler chickens subjected to NE compared to control (Bortoluzzi et al., 2019). On the contrary, ileal microbial profiling showed increased abundance of the genera Ruminococcus in NE-challenged compared to nonchallenged birds (Latorre et al., 2018). Supplementation of a probiotic or multicomponent feed additive to broiler diets subjected to a naturally occurring subclinical NE led to lower relative abundance of Ruminococcaceae UCG_014 and better performance compared to the control group (Emami et al., 2020). Relative abundance of Ruminococcaceae was decreased in the cecal digesta of NE-challenged broiler chickens fed a diet supplemented with Bacillus-based probiotic compared to the challenged control (Whelan et al., 2019). However, dietary supplementation of a Bacillus-based probiotic increased the relative frequency of Ruminococcus and unclassified members of the family Ruminococcaceae in the ileal digesta of NE-challenged birds, which were associated with lower severity of NE related lesions and better FCR (Bortoluzzi et al., 2019). These reports show that shifts in the microbial community of each segment of the gastrointestinal tract (e.g., ileum vs. cecum) during the NE challenge are specific to that section and might substantially differ from the shifts occurring in other sections. Meta-analysis of the data generated by various studies would be valuable in identifying the correlation between specific bacteria or bacterial communities with NE. Additionally, it is imperative to further explore how such changes influence the physiological processes in birds in order to find innovative practical solutions for NE control.
Besides changes in taxonomy, few studies delved into shifts in bacterial function (predicted) during NE. Supplementation of a multicomponent feed additive (probiotics/prebiotics/essential oils) enriched the predicted metabolism of propanoate in the ileal bacteria compared to control during a subclinical NE challenge (Emami et al., 2020). Furthermore, supplementation of B. licheniformis enriched the predicted metabolism of butanoate and propanoate in the ileal bacterial populations compared to control during a subclinical NE challenge (Emami et al., 2020). Similarly, supplementation of B. licheniformis enriched butanoate metabolism in the microbiota of broiler chickens challenged with NE compared to challenged nonsupplemented group (Lin et al., 2017).
Yeast cell wall extract increased formic acid concentration in cecal contents during NE challenge and increased butyric acid concentration in unchallenged birds (Xue et al., 2017). Further, dietary supplementation of a prebiotic (but not probiotic and symbiotic) to laying hens enriched cecal microbial genes involved in butanoate and propanoate metabolism (Pineda-Quiroga et al., 2019). Optimal butyrate production relies on the presence of butyrate-producing bacteria and various others including lactate-producing bacteria that cross-feed butyrate producers (De Maesschalck et al., 2015; Hwang et al., 2017). Butyrate could enhance epithelial regeneration by stimulating villus growth; however, it does not inhibit C. perfringens (Kien et al., 2007; Timbermont et al., 2010). Absorption of butyrate and propionate by chicken cecal mucosa could improve host energy metabolism and improve performance (Pineda-Quiroga et al., 2019).
In summary, factors such as Eimeria and fish meal alter the microbial balance in the gastrointestinal tract, and predispose birds to enteric diseases such as NE. Natural feed additives provide an opportunity for rebalancing the gastrointestinal microbial community to avoid the proliferation of opportunistic pathogenic bacteria. Accordingly, manipulation of the gut microbiota during NE challenge might prevent/alleviate its negative effects. The effect of each additive is signature-like, and indicates the tailoring potential of precision feeding of effective additives to alleviate and/or prevent a specific enteric disturbance.
Immune Responses
In order to induce NE under experimental conditions, Eimeria infection is widely used as a predisposing factor for the proliferation of C. perfringens (Williams, 2005). This is also the case in the naturally occurring NE model previously used by our group (Ҫalik et al., 2019a; Emami et al., 2019, 2020, 2021). Therefore, the immune responses to coccidia challenge and NE are discussed in this section. Eimeria spp. are intracellular enteric parasites that invade the intestinal mucosa, cause epithelial damage and induce inflammation (Dalloul and Lillehoj, 2005, 2006). A cascade of signaling pathways is triggered upon invasion and proliferation of Eimeria parasites, eventually inducing mRNA expression of cytokines such as IFN-γ, TGF-β, IL-1β, IL-10, and IL-17 (Cosmi et al., 2014; Fasina and Lillehoj, 2019). Higher abundance of IL-21 (a T-helper type 17 [Th17]-associated cytokine) was reported in the small intestines of E. maxima- or E. tenella-infected Ross 308 broilers, while its role was not identified (Bremner, 2018). Abundance of IFN-γ and IL-1β was greater in the ileum of coccidiosis-challenged Cobb 500 male broilers compared with controls (Ҫalik et al., 2019b). T-helper 1 (Th1) responses are necessary for dealing with Eimeria infections and helping to maximize clearance of pathogens, which may result in additional tissue damage (Couper et al., 2008; Bremner, 2018). During infection with protozoa and bacteria, IL-10 acts as an immune regulator to ameliorate excessive Th1 and CD8+ T cell responses (Couper et al., 2008; Lee et al., 2018). IL-10 was also shown to be essential for maintaining the integrity of the epithelial barrier as reduced production of IL-10 by macrophages compromised the recovery of the small intestine epithelial barrier in mice (Morhardt et al., 2019). Anti-inflammatory properties of IL-10 are typically necessary to prevent further inflammation that may be detrimental to host tissues (Arendt et al., 2016). However, intracellular parasites such as Eimeria induce IL-10 production to exploit its anti-inflammatory effects in order to elude host immune responses, allowing the parasite to complete its life cycle within intestinal epithelial cells (Hong et al., 2006; Arendt et al., 2016). The effect of Eimeria species on variability of cytokine responses should not be ruled out, as spatial regulation of IL-10 has been shown (increased IL-10 in the duodenum and reduced presence of IL-10 in the jejunum and cecum after challenging broiler chickens with high dose of a commercial vaccine containing various Eimeria species) (Arendt et al., 2019). Broiler chickens less susceptible to Eimeria had pronounced proinflammatory Th1-skewed response with increased IFN-y and reduced IL-10 suggesting involvement of IL-10 in susceptibility to Eimeria (Bremner, 2018). Oral IL-10 neutralizing antibodies were ineffective at preventing increased Eimeria-induced intestinal luminal IL-10; however, it improved body weight compared to the control (no antibodies) challenged birds (Arendt et al., 2016; Sand et al., 2016). Results for the concentration of IL-10 in the circulation after Eimeria challenge are not consistent. The level of circulating IL-10 was substantially increased around 5 d following E. tenella challenge compared to nonchallenged birds (Wu et al., 2016). Conversely, serum concentration of LPS-induced tumor necrosis factor (TNF)-alpha factor (LITAF) increased, while concentration of IL-10 decreased in broilers challenged with E. tenella or E. acervulina (Alcala-Canto et al., 2014). Discrepancies in the reported results might be due to one or several factors including Eimeria species inoculated, their pathogenicity, time of sampling post challenge, tissues collected and tested (e.g., blood, luminal digesta, intestine), as well as age of birds at challenge.
Several studies reported that dietary probiotics could regulate immune responses. Supplementation of probiotics to Cobb 500 broiler diets increased ileal abundance of TLR-4 and Muc-2 mRNA, and decreased abundance of IFN-γ, LITAF and IL-4 with no effect on IL-13 (Pender et al., 2017). Both IL-4 and IL-13 are Th2 cytokines that function via inhibiting the production of proinflammatory modulators. The authors concluded that supplementation of probiotics reduced colonization of pathogens, thus dampened the immune response by lowering the abundance of immune-related genes (Pender et al., 2017). In contrast, the use of a direct-fed microbial did not alleviate the impact of coccidia increased abundance of IL-1β and IFN-γ in the ileum of Cobb 500 male broiler chickens, while improved body weight gain (Ҫalik et al., 2019b). Three days post E. acervulina challenge, intestinal IFN-γ concentration was higher in birds fed a diet supplemented with Lactobacillus-based probiotic; however, there was no difference between treatments on d 6, 9, and 12 postchallenge (Dalloul et al., 2005).
High-throughput sequencing revealed differentially expressed cytokines and their receptors in the intestine and spleen of chicken lines differing in NE-susceptibility, which could provide insights in host-pathogen interaction and potential biomarkers of NE resistance (Truong et al., 2015a,b). However, identification of the most important and relevant genes and their correlation with performance parameters is yet to be defined and warrants further research. Therefore, our focus in this review is on the better identified and reported responses. To this end, upregulation of IL-1β, IL-10, TNF-α, and IFN-γ in the intestine and spleen of chickens during NE challenge is a consistent trend.
Proinflammatory cytokines are primarily produced by lamina propria macrophages upon encountering bacteria (such as C. perfringens) and could trigger the activation of T cells and neutrophils (Sartor, 2006). IL-1 and TNF-α represent the archetypal proinflammatory cytokines that are rapidly released upon tissue injury or infection (Lawrence, 2009). Coinfection with Eimeria and C. perfringens, induced a complex and dynamic expression of immune-related genes (Park et al., 2008). mRNA abundance of IFN-γ, IL-1β, IL-12, IL-13, and IL-17 were decreased, while abundance of IL-8, and IL-10 were increased by E. maxima/C. perfringens coinfection compared with single E. maxima or C. perfringens challenge (Park et al., 2008). Subclinical NE challenge decreased abundance of IL-8 and IL-2, increased abundance of IFN-γ, IL-10 and matrix metalloproteinase (MMP)-2, with no impact on IL-6, IL-17 and LITAF abundance in the ileum of NE-challenged Cobb 500 male broiler chickens compared to controls (Wang et al., 2017). Abundance of IL-1β, IFN-γ, and TNF-α was greater in the spleen of C. perfringens-challenged broiler chickens; while in the jejunum only IL-1β abundance increased (Li et al., 2018). NE-induced inflammatory IFN-γ and LITAF mRNA accumulation in chicken ileal tissues compared to nonchallenged controls (Wang et al., 2019). mRNA abundance of IL-1β, IL-10, and MMP-7 increased in NE challenged birds (Eimeria + C. perfringens) compared to nonchallenged group (Lorenzoni et al., 2019).
C. perfringens infection could induce an inflammatory response in the intestine of broiler chickens, and the mechanisms of inflammation are probably mediated via Th2 and Th17 cells (Fasina and Lillehoj, 2019). C. perfringens infection induced mRNA abundance of IL-17, which was significantly reduced following coinfection with E. maxima and C. perfringens (Park et al., 2008). During infection and inflammation (e.g., NE), cell proliferation in the intestine commonly occurs in order to replace damaged enterocytes (Kim et al., 2017). Nonavian studies evidenced the role of IL-17 and IL-22, tissue-signaling cytokines that favor protection and regeneration of cells in barrier organs such as the skin, lung, and gastrointestinal tract (Eyerich et al., 2017). In addition, IL-17A is important in inflammatory and antimicrobial defense against pathogens (extracellular bacteria and fungi) at mucosal surfaces and regulates mucosal immune defenses (Dann et al., 2015; Cai et al., 2016). Emami and colleagues (2019) showed that supplementation of a multistrain probiotic to broiler diets during a naturally occurring NE increased mRNA abundance of IL-10 and IL-17 in the jejunum of Cobb 500 male broilers compared to challenged control birds. These changes were associated with better performance and lower lesion scores in birds fed multistrain probiotic supplemented diet. Supplementation of a probiotic (L. johnsonii BS15) alleviated the negative impact of subclinical NE on Cobb 50 male broilers and increased the abundance of IL-8 and NRF-2 (nuclear factor erythroid 2–related factor 2), while decreased IFN-γ, IL-10 and MMP-2 in the ileum with no impact on IL-2 (Wang et al., 2017). L. acidophilus treatment significantly decreased the mRNA abundance of IL-1β, IL-8, and IFN-γ in the jejunum of broiler chickens challenged with NE (Li et al., 2018). Also, the inclusion of deoxycholic acid attenuated NE-induced inflammatory IFN-γ and LITAF mRNA abundance in the ileum of Cobb 500 broiler chickens (Wang et al., 2019). Greater mRNA abundance of IL-17 and lower abundance of IFN-γ were reported in antibiotic (BMD) fed Cobb 500 male broiler chickens on d 7 post NE challenge compared to a nonmedicated control (Fasina and Lillehoj, 2019). Yeast cell wall extract suppressed inflammatory response to NE challenge by reducing serum concentration of IL-1 in Ross 308 male broilers compared to challenged control; however, there was no effect on IL-10 levels (Xue et al., 2017). Supplementation of a probiotic (B. licheniformis) or a multicomponent feed additive consisted of probiotics, prebiotics, and essential oils reduced mRNA abundance of IFN-γ, while increased IL-10 abundance in the jejunum of broiler chickens subjected to a subclinical naturally occurring NE (Emami et al., 2020). Discrepancies in the results among several studies could be attributed to various factors including the use of different challenge models, severity of infection, type and dose of the probiotics/additives, time of sampling, sex, breed, and potentially the different sampling sites (Ҫalik et al., 2019a; Emami et al., 2019).
To recap, mucosal immune responses to Eimeria and C. perfringens are specific, and lead to inflammation and tissue injury that influence birds’ performance. Special attention should be paid to identifying the role of less studied cytokines and other immune-related genes involved in Eimeria and C. perfringens challenges to evaluate their function and potential as therapeutic targets (Broom and Kogut , 2019). Finally, natural feed additives such as probiotics and multicomponent additives are capable of modulating mucosal immune responses in favor of anti-inflammatory/regulatory phenotypes, thus alleviating/preventing the negative impact of NE on performance and health of poultry.
Gut Health for Disease Prevention and Productivity
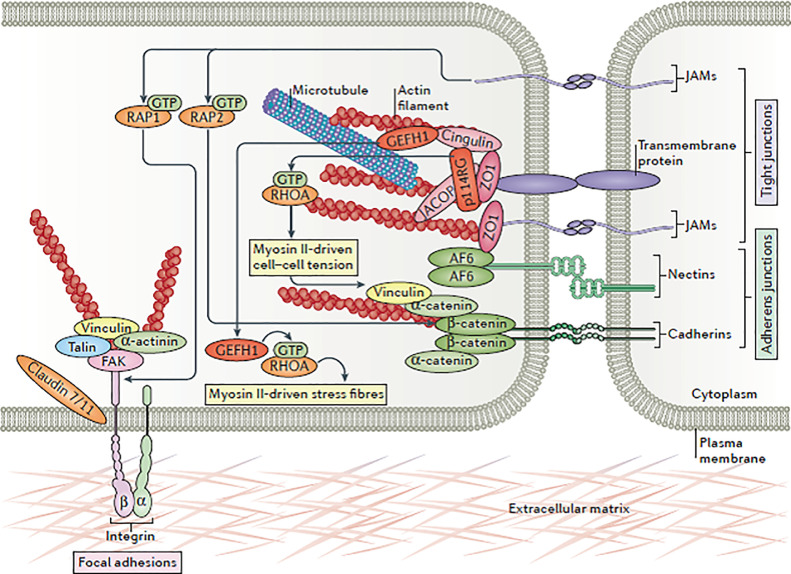
Gut integrity, robust immunity, and balanced microbial profiles are pivotal for intestinal health which if compromised would negatively affect digestion, absorption and metabolism of nutrients thus leading to poor performance and onset of enteric disease (Yegani and Korver, 2008; Ritzi et al., 2014). Tight junctions serve as regulated barriers that restrict the diffusion of solutes through the paracellular route in epithelial cell lining, as such, they maintain a barricade between the apical and basolateral sides of the cell (internal tissue compartments from external environments; Figure 2) (Karcher and Applegate, 2008; Saitoh et al., 2015). Tight junctions, which connect the adjacent epithelial cells at the apicolateral borders, are of great importance for the maintenance of gut integrity and are key contributors to epithelial cell polarization (Tang et al., 2010). Tight junctions are subject to change and remodel in response to external stimuli in the gut lumen such as nutrients, pathogens, and commensal bacteria. Therefore, these barriers are dynamic and subject to constant remodeling in response to various enteric stimuli (Ulluwishewa et al., 2011).
Figure 2.
Components of an individual tight junction. Tight junctions are protein complexes composed of 3 subcomponents: 1) transmembrane proteins (such as claudin family of proteins), 2) cytoskeletal elements (such as actin and myosin filaments), and 3) scaffolding proteins called zonula occludens (Zihni et al., 2016).
Natural feed additives appear to be effective in NE challenged birds by maintaining the integrity of the epithelial barrier and improving FCR (Emami et al., 2019; 2020; Shini et al., 2020). Nutrient absorption is facilitated through active or passive transport. Active transport is facilitated by nutrient transporters at the apical membrane of the small intestine that are important in moving nutrients into the enterocytes. Damage to the epithelial cells due to NE or higher viscosity of digesta due to addition of grains (with high content of soluble fiber) to the diet decreases the absorption capacity of nutrients thus negatively affects bird performance (Jha and Mishra, 2021). Sodium glucose cotransporter 1 (SGLT1) and peptide transporter 1 (PepT1) mediate absorption of carbohydrates (glucose and galactose) and di- and tri-peptides, respectively (Gilbert et al., 2007; Shimizu et al., 2018). Therefore, these transporters are critical for maintaining the energy and amino acid supplies. SGLT1 is a cotransporter of glucose and sodium; concurrent absorption of glucose and sodium establishes a gradient that facilitates the movement of sodium and water through the paracellular space (Nighot and Nighot, 2018). This might be helpful in reducing diarrhea, which is a common symptom during enteric diseases, subsequently alleviating its negative impact on the bird (Emami et al., 2019). Dietary supplementation of epidermal growth factor upregulates mRNA abundance of nutrient transporters in birds challenged with Eimeria compared to control challenged birds (Kim et al., 2017). Addition of xylanase to the diet of broiler chickens challenged with subclinical NE led to greater abundance of SGLT1 and PepT1 in the jejunum compared to the control challenged birds (Hosseini et al., 2017). A recent study showed that supplementation of a multistrain probiotic to the diet increased mRNA abundance of SGLT1 and AMP-activated protein kinase (AMPK)-α1 in the jejunum of broiler chickens challenged with NE compared to control challenged birds. In addition, multistrain probiotic increased mRNA abundance of PepT1 in the jejunum compared to birds fed virginiamycin-supplemented diet. Higher abundance of SGLT1 and PepT1 was accompanied by lower NE lesion scores in the small intestine of probiotic fed birds (Emami et al., 2019). These findings indicate that improving gut health during a disease challenge might be beneficial in increasing the absorptive capacity of the gastrointestinal tract, thus improving performance. Supplementation of toxin binders to the diet could decrease the effect of NE challenge in broiler chickens through alleviating the impact of mycotoxins on gut health, microbiota, and immune responses (Cravens et al., 2015; Liew and Mohd-Redzwan, 2018).
The mucosal barrier and epithelial lining play a pivotal role in separating the internal body environment from the intestinal lumen and are the first lines of defense against invading microbial pathogens (Oshima and Miwa, 2016; Pender et al., 2016). The immune system and an intact epithelial cell layer (gastrointestinal mucosal barrier) are the 2 defense mechanisms that act at the epithelial lining to prevent pathogens from gaining access to the host (Tang et al., 2010). However, selective paracellular diffusion is critical for the regulation of homeostasis in tissues (Zihni et al., 2016). A small portion of antigens that exist in the gastrointestinal tract may cross the mucosal barrier as intact proteins through microfold (M) cells or the paracellular pathway (Fasano, 2008). Interestingly, a close relationship exists between tight junctions and the gut-associated lymphoid tissues; thus, proper immune function is critical for the integrity of the tight junctions and vice versa.
In recent years, research on non-avian model species has outlined the role of chemokines and cytokines in the regulation and maintenance of the intestinal barrier. Treatment of Caco-2 cells and primary human intestinal epithelium with IL-22 increased paracellular permeability for ions through upregulating claudin-2, a cation–channel-forming tight junction protein. Upregulated claudin-2 protein was also shown in colonic epithelial cells of mice as a result of treatment with IL-22 (Tsai et al., 2017). Downregulation of proinflammatory cytokines alleviated intestinal mucosal disruption in pigs (Tan et al., 2014). In addition, intestinal barrier function is greatly under the influence of the intestinal microbiota and its interaction with the bird's immune status. Proinflammatory cytokines, such as IL-1, TNF-α and IFN-γ, are primarily produced by lamina propria macrophages upon encounters with bacteria and could trigger the activation of T cells and neutrophils (Sartor, 2006). Proinflammatory cytokines, including IFN-γ, could affect the structure of tight junctions through suppressing AMPK abundance, and are thus etiological factors in intestinal barrier dysfunction (Aznar et al., 2016; Sun and Zhu, 2017). IL-10 can also be involved in the restoration of the epithelial barrier as a lack of or reduced production of IL-10 by macrophages compromised the recovery of the small intestine epithelial barrier in mice (Morhardt et al., 2019). Therefore, changes in microbial composition could lead to hyper immune stimulation, epithelial dysfunction, and enhanced mucosal permeability. Conversely, manipulation of microbial composition in favor of beneficial bacteria could positively affect and modulate the host immune responses and eventually lead to the improvement of gut barrier function (Zou et al., 2016).
Tight junctions are key signaling platforms that transmit/receive signals to/from the cell interior, thus regulate the cytoskeleton, morphogenesis, gene abundance, cell polarity, proliferation and differentiation during various cellular processes (Schneeberger and Lynch, 2004; Zihni et al., 2016). Lymphocytes could accentuate the assembly of tight junctions in epithelial MDCK cells as mediated by AMPK (Tang et al., 2010). AMPK is the master regulator of energy metabolism homeostasis in eukaryotic cells and this kinase will be activated in response to high AMP/ATP ratio in the cell. AMPK activation shifts the metabolism from anabolism to catabolism by turning off ATP-consuming processes and activating ATP-producing pathways (Zhang et al., 2006). However, ATP-independent activation of AMPK by a proinflammatory cytokine such as TNF-α has been previously reported (Tang et al., 2010). Additionally, cytokines could affect the structure of tight junctions through AMPK (Aznar et al., 2016) highlighting the regulatory role of AMPK in their assembly and disassembly (Zhang et al., 2006; Zheng and Cantley, 2007; Zihni et al., 2016). During a pathologic response to bacteria, AMPK supports maintenance of cell-cell junctions in different tissues including the intestine (Seo-Mayer et al., 2011; Spruss et al., 2012). However, such effects as well as the impact of changes in tight junction proteins on cell energy metabolism have yet to be studied in birds. In a recent study, supplementation of a multistrain probiotic to the diet of broiler chickens during a naturally occurring NE increased mRNA abundance of AMPK-α1 and claudin-3 in the jejunum of broilers (Emami et al., 2019). In another study, supplementation of a multicomponent feed additive (probiotic/prebiotic/essential oils) increased mRNA abundance of claudin-3, PGC-1α and mTOR in the jejunum of broilers subjected to subclinical NE; however, it did not have any effect on AMPK-α1 abundance (Emami et al., 2020).
Tight junctions remodel during intestinal disorders; therefore, structure and localization of tight junction proteins have been identified as biomarkers of specific diseases (Oshima and Miwa, 2016). C. perfringens infection significantly decreased the mRNA abundance of occludin; however, the relative mRNA levels of claudin-1 and ZO-1 in the jejunum were not affected by C. perfringens infection or L. acidophilus treatment (Li et al., 2018). The inclusion of probiotics and multicomponent additives in poultry feed increased mRNA abundance of claudin-3 in the jejunum of broilers during a subclinical naturally occurring NE, while there was no change in the abundance of claudin-1, occludin, ZO-1, and ZO-2 (Emami et al., 2019, 2020). Higher abundance of claudin-3 coincided with lower NE lesion scores in the small intestine and lower relative abundance of Clostridium sensu stricto 1 in the jejunum of additive supplemented groups (Emami et al., 2019, 2020).
To re-emphasize, cytokines and energy status of the enterocytes could affect tight junctions and therefore gut integrity. Tight junctions are targets for many pathogens and their toxins, and growing evidence shows that beneficial bacteria affect gut integrity via modulating mucosal immune responses and cell metabolism. Furthermore, evidence shows the effect of feed additives in the remodeling of tight junction proteins, thus reducing the negative impact of pathogens on intestinal health and integrity. However, the detailed mode of action and mechanisms still are largely unknown, further validating the need for this type of research. This promising research area could open the door to the future of disease control by remodeling the tight junctions through targeted feeding and precision nutrition with more predictable host responses.
CONCLUDING REMARKS
Pathogen invasion and penetration of enterocytes triggers a cascade of signaling events in the intestine leading to secretion of various cytokines, which subsequently influence the integrity and function of the intestinal barrier, nutrient uptake, and epithelial cell energy metabolism. In a drug-free era, maintaining gut integrity and balancing the interaction between the gut microbiota and its host is critical for sustainable poultry production, and improving birds’ health and welfare through targeted feeding and precision nutrition.
Healthy birds have a specific microbial signature that is totally different from NE challenged birds. In other words, it is not just one species or genus of bacteria that is more abundant, but the whole microbial profile is different in these birds. Thus, considering the microbial community instead of focusing on one bacterial species (such as using single-strain probiotic) might be a more reliable approach when dealing with bacterial diseases in poultry production. As the findings of recent studies showed, multicomponent additives containing fructooligosaccharides or mannan oligosaccharides functioned better than probiotics alone, indicating that “feeding” the intestinal microbiome, along with the use of beneficial bacteria to promote “immune education” and to enhance gut integrity, might be more effective and the most “natural way” compared to using specific bacterial strains (Emami et al., 2020, 2021). Predicted functional analysis of microbiome revealed enrichment of functional genes involved in propanoate and butanoate metabolism in healthier birds (Emami et al., 2020, 2021). Thus, evaluating the synergistic effects of fructooligosaccharides, fiber and short chain fatty acids in the diet should be considered in future research.
Employing 16S rDNA sequencing highlighted the limitation of the current approach in facing disease challenges. As an example, using Virginiamycin (an AGP) prevented NE outbreak but increased the number of Escherichia-Shigella in the ileal mucosa (Emami et al., 2020, 2021). Escherichia-Shigella is among the pathogenic bacteria in poultry and the causal agents of certain diseases such as colibacillosis and shigellosis. As there are several pathogenic bacteria that negatively affect the birds’ performance in a commercial setting, especially when raising birds on built-up litter, having a more holistic approach to deal with bacterial challenges and to balance gut microbiome and health is necessary. Therefore, targeted feeding of the inoculated microbiome by adding fiber, fructooligosaccharides and short chain fatty acids to the diet should be given consideration in upcoming research investigating drug alternatives. This type of research will eventually lead us to defining a holistic approach in restraining enteric pathogens in commercial poultry settings regardless of the type of bacterial challenges.
DISCLOSURES
The authors declare no conflict of interest.
REFERENCES
- Ahiwe E.U., Chang'a E.P., Abdallh M.E., Al-Qahtani M., Kheravii S.K., Wu S., Graham H., Iji P.A. Dietary hydrolysed yeast cell wall extract is comparable to antibiotics in the control of subclinical necrotic enteritis in broiler chickens. Br. Poult. Sci. 2019;60:757–765. doi: 10.1080/00071668.2019.1664727. [DOI] [PubMed] [Google Scholar]
- Alcala-Canto Y., Ramos-Martinez E., Tapia-Perez G., Gutierrez L., Sumano H. Pharmacodynamic evaluation of a reference and a generic toltrazuril preparation in broilers experimentally infected with Eimeria tenella or E. acervulina. Br. Poult. Sci. 2014;55:44–53. doi: 10.1080/00071668.2013.872770. [DOI] [PubMed] [Google Scholar]
- Annett C.B., Viste J.R., Chirino-Trejo M., Classen H.L., Middleton D.M., Simko E. Necrotic enteritis: effect of barley, wheat and corn diets on proliferation of Clostridium perfringens type A. Avian Pathol. 2002;31:598–601. doi: 10.1080/0307945021000024544. [DOI] [PubMed] [Google Scholar]
- Antonissen G., Van Immerseel F., Pasmans F., Ducatelle R., Haesebrouck F., Timbermont L., Verlinden M., Janssens G.P.J., Eeckhaut V., Eeckhout M., De Saeger S., Hessenberger S., Martel A., Croubels S. The mycotoxin deoxynivalenol predisposes for the development of Clostridium perfringens-induced necrotic enteritis in broiler chickens. PLoS One. 2014;9 doi: 10.1371/journal.pone.0108775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antonissen G., Croubels S., Pasmans F., Ducatelle R., Eeckhaut V., Devreese M., Verlinden M., Haesebrouck F., Eeckhout M., De Saeger S., Antlinger B., Novak B., Martel A., Van Immerseel F. Fumonisins affect the intestinal microbial homeostasis in broiler chickens, predisposing to necrotic enteritis. Vet. Res. 2015;46:98. doi: 10.1186/s13567-015-0234-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antonissen G., Eeckhaut V., Van Driessche K., Onrust L., Haesebrouck F., Ducatelle R., Moore R.J., Van Immerseel F. Microbial shifts associated with necrotic enteritis. Avian Pathol. 2016;45:308–312. doi: 10.1080/03079457.2016.1152625. [DOI] [PubMed] [Google Scholar]
- Apajalahti J., Vienola K. Interaction between chicken intestinal microbiota and protein digestion. Anim. Feed Sci. Technol. 2016;221:323–330. [Google Scholar]
- Arendt M.K., Sand J.M., Marcone T.M., Cook M.E. Interleukin-10 neutralizing antibody for detection of intestinal luminal levels and as a dietary additive in Eimeria challenged broiler chicks. Poult. Sci. 2016;95:430–438. doi: 10.3382/ps/pev365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arendt M.K., Elissa J., Schmidt N., Micheal E., Potter N., Cook M., Knoll L.J. Investigating the role of interleukin 10 on Eimeria intestinal pathogenesis in broiler chickens. Vet. Immunol. Immunopathol. 2019;218 doi: 10.1016/j.vetimm.2019.109934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aznar N., Patel A., Rohena C.C., Dunkel Y., Joosen L.P., Taupin V., Kufareva I., Farquhar M.G., Ghosh P. AMP-activated protein kinase fortifies epithelial tight junctions during energetic stress via its effector GIV/Girdin. Elife. 2016;5:e20795. doi: 10.7554/eLife.20795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bortoluzzi C., Serpa Vieira B., de Paula Dorigam J.C., Menconi A., Sokale A., Doranalli K., Applegate T.J. Bacillus subtilis DSM 32315 supplementation attenuates the effects of Clostridium perfringens challenge on the growth performance and intestinal microbiota of broiler chickens. Microorganisms. 2019;7:71. doi: 10.3390/microorganisms7030071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bremner A. The University of Edinburgh; Edinburgh, UK: 2018. Innate Responses and Biomarkers of Resistance to Eimeria Infection in the Chicken. PhD thesis. [Google Scholar]
- Broussard C.T., Hofacre C.L., Page R.K., Fletcher O.J. Necrotic enteritis in cage-reared commercial layer pullets. Avian Dis. 1986;30:617–619. [PubMed] [Google Scholar]
- Broom L.J., Kogut. M.H. Deciphering desirable immune responses from disease models with resistant and susceptible chickens. Poult. Sci. 2019;98:1634–1642. doi: 10.3382/ps/pey535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai C.W., Blase J.R., Zhang X., Eickhoff C.S., Hoft D.F. Th17 cells are more protective than Th1 cells against the intracellular parasite Trypanosoma cruzi. PLoS Pathog. 2016;12 doi: 10.1371/journal.ppat.1005902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ҫalik A., Omara I.I., White M.B., Evans N.P., Karnezos T.P., Dalloul R.A. Dietary non-drug feed additive as an alternative for antibiotic growth promoters for broilers during a necrotic enteritis challenge. Microorganisms. 2019;7:257. doi: 10.3390/microorganisms7080257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ҫalik A., Omara I.I., White M.B., Li W., Dalloul R.A. Effects of dietary direct fed microbial supplementation on performance, intestinal morphology and immune response of broiler chickens challenged with coccidiosis. Front. Vet. Sci. 2019;6:463. doi: 10.3389/fvets.2019.00463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castanon J.I.R. History of the use of antibiotic as growth promoters in European poultry feeds. Poult. Sci. 2007;86:2466–2471. doi: 10.3382/ps.2007-00249. [DOI] [PubMed] [Google Scholar]
- Cervantes H.M. Antibiotic-free poultry production: is it sustainable? J. Appl. Poult. Res. 2015;24:91–97. [Google Scholar]
- Cooper K.K., Songer J.G., Uzal F.A. Diagnosing clostridial enteric disease in poultry. J. Vet. Diagn. Invest. 2013;25:314–327. doi: 10.1177/1040638713483468. [DOI] [PubMed] [Google Scholar]
- Cosmi L., Maggi L., Santarlasci V., Liotta F., Annunziato F. T helper cells plasticity in inflammation. Cytometry A. 2014;85:36–42. doi: 10.1002/cyto.a.22348. [DOI] [PubMed] [Google Scholar]
- Couper K.N., Blount D.G., Riley E.M. IL-10: the master regulator of immunity to infection. J. Immunol. 2008;180:5771–5777. doi: 10.4049/jimmunol.180.9.5771. [DOI] [PubMed] [Google Scholar]
- Cox C.M., Dalloul R.A. Beta-glucans as immunomodulators in poultry: use and potential applications. Avian Biol. Res. 2010;3:171–178. [Google Scholar]
- Cox C.M., Dalloul R.A. Immunomodulatory role of probiotics in poultry and potential in ovo application. Benef. Microbes. 2015;6:45–52. doi: 10.3920/BM2014.0062. [DOI] [PubMed] [Google Scholar]
- Cravens R.L., Goss G.R., Chi F., DeBoer E.D., Davis S.W., Hendrix S.M., Johnston S.L. Products to alleviate the effects of necrotic enteritis and aflatoxin on growth performance, lesion scores, and mortality in young broilers. J. Appl. Poult. Res. 2015;24:145–156. [Google Scholar]
- Dahiya J.P., Wilkie D.C., Van Kessel A.G., Drew M.D. Potential strategies for controlling necrotic enteritis in broiler chickens in post-antibiotic era. Anim. Feed Sci. Technol. 2006;129:60–88. [Google Scholar]
- Dalloul R.A., Lillehoj H.S., Tamim N.M., Shellem T.A., Doerr J.A. Induction of local protective immunity to Eimeria acervulina by a Lactobacillus-based probiotic. Comp. Immunol. Microbiol. Infect. Dis. 2005;28:351–361. doi: 10.1016/j.cimid.2005.09.001. [DOI] [PubMed] [Google Scholar]
- Dalloul R.A., Lillehoj H.S. Recent advances in immunomodulation and vaccination strategies against coccidiosis. Avian Dis. 2005;49:1–8. doi: 10.1637/7306-11150R. [DOI] [PubMed] [Google Scholar]
- Dalloul R.A., Lillehoj H.S. Poultry coccidiosis: recent advancements in control measures and vaccine development. Expert Rev. Vaccines. 2006;5:143–163. doi: 10.1586/14760584.5.1.143. [DOI] [PubMed] [Google Scholar]
- Dann S.M., Manthey C.F., Le C., Miyamoto Y., Gima L., Abrahim A., Cao A.T., Hanson E.M., Kolls J.K., Raz E., Cong Y., Eckmann L. IL-17A promotes protective IgA responses and expression of other potential effectors against the lumen-dwelling enteric parasite Giardia. Exp. Parasitol. 2015;156:68–78. doi: 10.1016/j.exppara.2015.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Maesschalck C., Eeckhaut V., Maertens L., De Lange L., Marchal L., Nezer C., De Baere S., Croubels S., Daube G., Dewulf J., Haesebrouck F., Ducatelle R., Taminau B., Van Immerseel F. Effects of xylo-oligosaccharides on broiler chicken performance and microbiota. Appl. Environ. Microbiol. 2015;81:5880–5888. doi: 10.1128/AEM.01616-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Dhillon A.S., Roy P., Lauerman L., Schaberg D., Weber S., Bandli D., Wier F. High mortality in egg layers as a result of necrotic enteritis. Avian Dis. 2004;48:675–680. doi: 10.1637/7113. [DOI] [PubMed] [Google Scholar]
- Dibner J.J., Richards J.D. Antibiotic growth promoters in agriculture: history and mode of action. Poult. Sci. 2005;84:634–643. doi: 10.1093/ps/84.4.634. [DOI] [PubMed] [Google Scholar]
- Drew M.D., Syed N.A., Goldade B.G., Laarveld B., Van Kessel A.G. Effects of dietary protein source and level on intestinal populations of Clostridium perfringens in broiler chickens. Poult. Sci. 2004;83:414–420. doi: 10.1093/ps/83.3.414. [DOI] [PubMed] [Google Scholar]
- Du E., Gan L., Li Z., Wang W., Liu D., Guo Y. In vitro antibacterial activity of thymol and carvacrol and their effects on broiler chickens challenged with Clostridium perfringens. J. Anim. Sci. Biotechnol. 2015;6:58. doi: 10.1186/s40104-015-0055-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emami N.K., Ҫalik A., White M.B., Young M., Dalloul R.A. Necrotic enteritis in broiler chickens: the role of tight Junctions and mucosal immune responses in alleviating the effect of the disease. Microorganisms. 2019;7:231. doi: 10.3390/microorganisms7080231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emami N.K., Ҫalik A., White M.B., Kimminau E.A., Dalloul R.A. Effect of probiotics and multi-component feed additives on microbiota, gut barrier and immune responses in broiler chickens during subclinical necrotic enteritis. Front. Vet. Sci. 2020;7 doi: 10.3389/fvets.2020.572142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emami N.K., White M.B., Ҫalik A., Kimminau E.A., Dalloul R.A. Managing broilers gut health with antibiotic-free diets during subclinical necrotic enteritis. Poult. Sci. 2021;100 doi: 10.1016/j.psj.2021.101055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eyerich K., Dimartino V., Cavani A. IL-17 and IL-22 in immunity: driving protection and pathology. Eur. J. Immunol. 2017;47:607–614. doi: 10.1002/eji.201646723. [DOI] [PubMed] [Google Scholar]
- Fasano A. Physiological, pathological, and therapeutic implications of zonulin-mediated intestinal barrier modulation: living life on the edge of the wall. Am. J. Pathol. 2008;173:1243–1252. doi: 10.2353/ajpath.2008.080192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fasina Y.O., Lillehoj H.S. Characterization of intestinal immune response to Clostridium perfringens infection in broiler chickens. Poult. Sci. 2019;98:188–198. doi: 10.3382/ps/pey390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fasina Y.O., Newman M.M., Stough J.M., Liles M.R. Effect of Clostridium perfringens infection and antibiotic administration on microbiota in the small intestine of broiler chickens. Poult. Sci. 2016;95:247–260. doi: 10.3382/ps/pev329. [DOI] [PubMed] [Google Scholar]
- Fernando P.S., Rose S.P., Mackenzie A.M., Silva S.S.P. Effect of diets containing potato protein or soya bean meal on the incidence of spontaneously-occurring subclinical necrotic enteritis and the physiological response in broiler chickens. Br. Poult. Sci. 2011;52:106–114. doi: 10.1080/00071668.2010.549105. [DOI] [PubMed] [Google Scholar]
- Frame D.D., Bickford A.A. An outbreak of coccidiosis and necrotic enteritis in 16-week-old cage-reared replacement pullets. Avian Dis. 1986;30:601–602. [PubMed] [Google Scholar]
- Gilbert E.R., Li H., Emmerson D.A., Webb K.E., Jr., Wong E.A. Developmental regulation of nutrient transporter and enzyme mRNA abundance in the small intestine of broilers. Poult. Sci. 2007;86:1739–1753. doi: 10.1093/ps/86.8.1739. [DOI] [PubMed] [Google Scholar]
- Goossens, E., E. Dierick, R. Ducatelle, and F. Van Immerseel. Spotlight on avian pathology: untangling contradictory disease descriptions of necrotic enteritis and necro-haemorrhagic enteritis in broilers. Avian Pathol. 49:423-427. [DOI] [PubMed]
- Hosseini S.M., Manafi M., Nazarizadeh H. Effects of xylanase supplementation and citric acid on performance, ileal nutrients digestibility, and gene expression of intestinal nutrient transporters in broilers challenged with Clostridium perfringens. J. Poult. Sci. 2017;25:149–156. doi: 10.2141/jpsa.0160099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hwang N., Eom T., Gupta K.S., Jeong S.-Y., Jeong D.-Y., Kim S.Y., Lee J.-H., Sadowsky M.J., Unno T. Genes and gut bacteria involved in luminal butyrate reduction caused by diet and loperamide. Genes. 2017;8:350. doi: 10.3390/genes8120350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong Y.H., Lillehoj H.S., Lee S.H., Dalloul R.A., Lillehoj E.P. Analysis of chicken cytokine and chemokine gene expression following Eimeria acervulina and Eimeria tenella infections. Vet. Immunol. Immunopathol. 2006;114:209–223. doi: 10.1016/j.vetimm.2006.07.007. [DOI] [PubMed] [Google Scholar]
- Jha R., Mishra P. Dietary fiber in poultry nutrition and their effects on nutrient utilization, performance, gut health, and on the environment: a review. J. Anim. Sci. Biotechnol. 2021;12:51. doi: 10.1186/s40104-021-00576-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Juge N., Tailford L., Owen C.D. Sialidases from gut bacteria: a mini-review. Biochim. Soc. Trans. 2016;44:166–175. doi: 10.1042/BST20150226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karcher D.M., Applegate T.J. Survey of enterocyte morphology and tight junction formation in the small intestine of avian embryos. Poult. Sci. 2008;87:339–350. doi: 10.3382/ps.2007-00342. [DOI] [PubMed] [Google Scholar]
- Katayama S., Nozu N., Okuda M., Hirota S., Yamasaki T., Hitsumoto Y. Characterization of two putative fibronectin-binding proteins of Clostridium perfringens. Anaerobe. 2009;15:155–159. doi: 10.1016/j.anaerobe.2009.03.001. [DOI] [PubMed] [Google Scholar]
- Keyburn A.L., Boyce J.D., Vaz P., Bannam T.L., Ford M.E., Parker D., Di Rubbo A., Rood J.I., Moore R.J. NetB, a new toxin that is associated with avian necrotic enteritis caused by Clostridium perfringens. PLoS Pathog. 2008;4:e26. doi: 10.1371/journal.ppat.0040026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kien C.L., Blauwiekel R., Bunn J.Y., Jetton T.L., Frankel W.L., Holst J.J. Cecal infusion of butyrate increases intestinal cell proliferation in piglets. J. Nutr. 2007;137:916–922. doi: 10.1093/jn/137.4.916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim E., Leung H., Akhtar N., Li J., Barta J.R., Wang Y., Yang C., Kiarie E. Growth performance and gastrointestinal responses of broiler chickens fed corn-soybean meal diet without or with exogenous epidermal growth factor upon challenge with Eimeria. Poult. Sci. 2017;96:3676–3686. doi: 10.3382/ps/pex192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiu R., Brown J., Bedwell H., Leclaire C., Caim S., Pickard D., Dougan G., Dixon R.A., Hall L.J. Genomic analysis on broiler-associated Clostridium perfringens strains and exploratory caecal microbiome investigation reveals key factors linked to poultry necrotic enteritis. Anim. Microbiome. 2019;1:12. doi: 10.1186/s42523-019-0015-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kondo F. In vitro lecithinase activity and sensitivity to 22 antimicrobial agents of Clostridium perfringens isolated from necrotic enteritis of broiler chickens. Res. Vet. Sci. 1988;45:337–340. [PubMed] [Google Scholar]
- Latorre J.D., Adhikari B., Park S.H., Teague K.D., Graham L.E., Mahaffey B.D., Baxter M.F.A., Hernandez-Velasco X., Kwon Y.M., Ricke S.C., Bielke L.R., Hargis B.M., Tellez G. Evaluation of the epithelial barrier function and ileal microbiome in an established necrotic enteritis challenge model in broiler chickens. Front. Vet. Sci. 2018;5:199. doi: 10.3389/fvets.2018.00199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawrence T. The nuclear factor NF-kappaB pathway in inflammation. Cold Spring Harb. Perspect. Biol. 2009;1 doi: 10.1101/cshperspect.a001651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee K.W., Lillehoj H.S., Jeong W., Jeoung H.Y., An D.J. Avian necrotic enteritis: experimental models, host immunity, pathogenesis, risk factors, and vaccine development. Poult. Sci. 2011;90:1381–1390. doi: 10.3382/ps.2010-01319. [DOI] [PubMed] [Google Scholar]
- Lee Y., Kim W.H., Lee S.-j., Lillehoj H.S. Detection of chicken interleukin-10 production in intestinal epithelial cells and necrotic enteritis induced by Clostridium perfringens using capture ELISA. Vet. Immunol. Immunopathol. 2018;204:52–58. doi: 10.1016/j.vetimm.2018.10.001. [DOI] [PubMed] [Google Scholar]
- Lepp D., Roxas B., Parreira V.R., Marri P.R., Rosey E.L., 5, Gong J., Songer J.G., Vedantam G., Prescott J.F. Identification of novel pathogenicity loci in Clostridium perfringens strains that cause avian necrotic enteritis. PLoS One. 2010;5:e10795. doi: 10.1371/journal.pone.0010795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lepp D., Zhou Y., Ojha S., Mehdizadeh Gohari I., Carere J., Yang C., Prescott J.F., Gong J. Clostridium perfringens produces an adhesive pilus required for the pathogenesis of necrotic enteritis in poultry. J. Bacteriol. 2021;203 doi: 10.1128/JB.00578-20. e00578-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J., Uzal F.A., McClane B.A. Clostridium perfringens sialidases: potential contributors to intestinal pathogenesis and therapeutic targets. Toxins (Basel). 2016;8:341. doi: 10.3390/toxins8110341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Z., Wang W., Liu D., Guo Y. Effects of Lactobacillus acidophilus on gut microbiota composition in broilers challenged with Clostridium perfringens. PLoS One. 2017;12 doi: 10.1371/journal.pone.0188634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Z., Wang W., Liu D., Guo Y. Effects of Lactobacillus acidophilus on the growth performance and intestinal health of broilers challenged with Clostridium perfringens. J. Anim. Sci. Biotechnol. 2018;9:25. doi: 10.1186/s40104-018-0243-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liew W.-P.-P., Mohd-Redzwan S. Mycotoxin: its impact on gut health and microbiota. Front. Cell. Infect. Microbiol. 2018;8:60. doi: 10.3389/fcimb.2018.00060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin Y., Xu S., Zeng D., Ni X., Zhou M., Zeng Y., Wang H., Zhou Y., Zhu H., Pan K., Li G. Disruption in the cecal microbiota of chickens challenged with Clostridium perfringens and other factors was alleviated by Bacillus licheniformis supplementation. PLoS One. 2017;12 doi: 10.1371/journal.pone.0182426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lorenzoni A.G., Rojas-Nunez I., Moore A.F. Effect of aspirin on the intestinal response to a necrotic enteritis challenge. Avian Dis. 2019;63:686–692. doi: 10.1637/aviandiseases-D-19-00093. [DOI] [PubMed] [Google Scholar]
- M'Sadeq S.A., Wu S., Swick R.A., Choct M. Towards the control of necrotic enteritis in broiler chickens with in-feed antibiotics phasing-out worldwide. Anim. Nutr. 2015;1:1–11. doi: 10.1016/j.aninu.2015.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma Y., Wang W., Zhang H., Wang J., Zhang W., Gao J., Wu S., Qi G. Supplemental Bacillus subtilis DSM 32315 manipulates intestinal structure and microbial composition in broiler chickens. Sci. Rep. 2018;8:15358. doi: 10.1038/s41598-018-33762-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin M.J., Thottathil S.E., Newman T.B. Antibiotics overuse in animal agriculture: a call to action for health care providers. Am. J. Public Health. 2015;105:2409–2410. doi: 10.2105/AJPH.2015.302870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin T.G., Smyth J.A. The ability of disease and non-disease producing strains of Clostridium perfringens from chickens to adhere to extracellular matrix molecules and Caco-2 cells. Anaerobe. 2010;16:533–539. doi: 10.1016/j.anaerobe.2010.07.003. [DOI] [PubMed] [Google Scholar]
- Mehdizadeh Gohari I., Navarro M.A., Li J., Shrestha A., Uzal F., McClane B.A. Pathogenicity and virulence of Clostridium perfringens. Virulence. 2021;12:723–753. doi: 10.1080/21505594.2021.1886777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore R.J. Necrotic enteritis predisposing factors in broiler chickens. Avian Pathol. 2016;45:275–281. doi: 10.1080/03079457.2016.1150587. [DOI] [PubMed] [Google Scholar]
- Morhardt T.L., Hayashi A., Ochi T., Quirós M., Kitamoto S., Nagao-Kitamoto H., Kuffa P., Atarashi K., Honda K., Kao J.Y., Nusrat A., Kamada N. IL-10 produced by macrophages regulates epithelial integrity in the small intestine. Sci. Rep. 2019;9 doi: 10.1038/s41598-018-38125-x. 1223-1223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nighot M., Nighot P. Pathophysiology of avian intestinal ion transport. World's Poult. Sci. J. 2018;74:347–360. doi: 10.1017/S004393391800003X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oshima T., Miwa H. Gastrointestinal mucosal barrier function and diseases. J. Gastroenterol. 2016;51:768–778. doi: 10.1007/s00535-016-1207-z. [DOI] [PubMed] [Google Scholar]
- Parish W.E. Necrotic enteritis in the fowl (Gallus gallus domesticus). I. Histopathology of the disease and isolation of a strain of Clostridium welchii. J. Comp. Pathol. 1961;71:377–393. [PubMed] [Google Scholar]
- Park S.S., Lillehoj H.S., Allen P.C., Park D.W., FitzCoy S., Bautista D.A., Lillehoj E.P. Immunopathology and cytokine responses in broiler chickens coinfected with Eimeria maxima and Clostridium perfringens with the use of an animal model of necrotic enteritis. Avian Dis. 2008;52:14–22. doi: 10.1637/7997-041707-Reg. [DOI] [PubMed] [Google Scholar]
- Pender C.M., Kim S., Potter T.D., Ritzi M.M., Young M., Dalloul R.A. Effects of in ovo supplementation of probiotics on performance and immunocompetence of broiler chicks to an Eimeria challenge. Benef. Microbes. 2016;7:699–705. doi: 10.3920/BM2016.0080. [DOI] [PubMed] [Google Scholar]
- Pender C.M., Kim S., Sumners L.H., Ritzi M.M., Young M., Dalloul R.A. In ovo and dietary supplementation of probiotics affects post-hatch expression of immune-related genes in broiler chicks. J. Immunobiol. 2017;2:126–134. [Google Scholar]
- Pineda-Quiroga C., Borda-Molina D., Chaves-Moreno D., Ruiz R., Atxaerandio R., Camarinha-Silva A., García-Rodríguez A. Microbial and functional profile of the ceca from laying hens affected by feeding prebiotics, probiotics, and synbiotics. Microorganisms. 2019;7:123. doi: 10.3390/microorganisms7050123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prescott J.F., Parreira V.R., Mehdizadeh Gohari I., Lepp D., Gong J. The pathogenesis of necrotic enteritis in chickens: what we know and what we need to know: a review. Avian Pathol. 2016;45:288–294. doi: 10.1080/03079457.2016.1139688. [DOI] [PubMed] [Google Scholar]
- Rho J.-h., Wright D.P., Christie D.L., Clinch K., Furneaux R.H., Roberton A.M. A novel mechanism for desulfation of mucin: identification and cloning of a mucin-desulfating glycosidase (sulfoglycosidase) from Prevotella strain RS2. J. Bacteriol. 2005;187:1543–1551. doi: 10.1128/JB.187.5.1543-1551.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ritzi M.M., Abdelrahman W., Mohnl M., Dalloul R.A. Effects of probiotics and application methods on performance and response of broiler chickens to an Eimeria challenge. Poult. Sci. 2014;93:2772–2778. doi: 10.3382/ps.2014-04207. [DOI] [PubMed] [Google Scholar]
- Rood J.I., Keyburn A.L., Moore R.J. NetB and necrotic enteritis: the hole movable story. Avian Pathol. 2016;45:295–301. doi: 10.1080/03079457.2016.1158781. [DOI] [PubMed] [Google Scholar]
- Rood J.I., Adams V., Lacey J., Lyras D., A.McClane B., B.Melville S., J.Moore R., R.Popoff M., R.Sarker M., Songer J.G., Uzal F.A., Van Immerseel F. Expansion of the Clostridium perfringens toxin-based typing scheme. Anaerobe. 2018;53:5–10. doi: 10.1016/j.anaerobe.2018.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saitoh Y., Suzuki H., Tani K., Nishikawa K., Irie K., Ogura Y., Tamura A., Tsukita S., Fujiyoshi Y. Structural insight into tight junction disassembly by Clostridium perfringens enterotoxin. Science. 2015;347:775. doi: 10.1126/science.1261833. [DOI] [PubMed] [Google Scholar]
- Sand J.M., Arendt M.K., Repasy A., Deniz G., Cook M.E. Oral antibody to interleukin-10 reduces growth rate depression due to Eimeria spp. infection in broiler chickens. Poult. Sci. 2016;95:439–446. doi: 10.3382/ps/pev352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sartor R.B. Mechanisms of disease: pathogenesis of Crohn's disease and ulcerative colitis. Nat. Clin. Prac. Gastroenterol. Hepatol. 2006;3:390–407. doi: 10.1038/ncpgasthep0528. [DOI] [PubMed] [Google Scholar]
- Schneeberger E.E., Lynch R.D. The tight junction: a multifunctional complex. Am. J. Physiol. Cell Physiol. 2004;286:C1213–C1228. doi: 10.1152/ajpcell.00558.2003. [DOI] [PubMed] [Google Scholar]
- Seo-Mayer P.W., Thulin G., Zhang L., Alves D.S., Ardito T., Kashgarian M., Caplan M.J. Preactivation of AMPK by metformin may ameliorate the epithelial cell damage caused by renal ischemia. Am. J. Physiol. Renal Physiol. 2011;301:F1346–F1357. doi: 10.1152/ajprenal.00420.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimizu K., Komaki Y., Fukano N., Bungo T. Transporter gene expression and transference of fructose in broiler chick intestine. J. Poult. Sci. 2018;55:137–141. doi: 10.2141/jpsa.0170095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shini S., Zhang D., Aland R.C., Li X., Dart P.J., Callaghan M.J., Speight R.E., Bryden W.L. Probiotic Bacillus amyloliquefaciens H57 ameliorates subclinical necrotic enteritis in broiler chicks by maintaining intestinal mucosal integrity and improving feed efficiency. Poult. Sci. 2020;99:4278–4293. doi: 10.1016/j.psj.2020.05.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shojadoost B., Vince A.R., Prescott J.F. The successful experimental induction of necrotic enteritis in chickens by Clostridium perfringens: a critical review. Vet. Res. 2012;43:74. doi: 10.1186/1297-9716-43-74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spruss A., Kanuri G., Stahl C., Bischoff S.C., Bergheim I. Metformin protects against the development of fructose-induced steatosis in mice: role of the intestinal barrier function. Lab. Invest. 2012;92:1020–1032. doi: 10.1038/labinvest.2012.75. [DOI] [PubMed] [Google Scholar]
- Stanley D., Keyburn A.L., Denman S.E., Moore R.J. Changes in the caecal microflora of chickens following Clostridium perfringens challenge to induce necrotic enteritis. Vet. Microbiol. 2012;159:155–162. doi: 10.1016/j.vetmic.2012.03.032. [DOI] [PubMed] [Google Scholar]
- Stanley D., Wu S.-B., Rodgers N., Swick R.A., Moore R.J. Differential responses of cecal microbiota to fishmeal, Eimeria and Clostridium perfringens in a necrotic enteritis challenge model in chickens. PLoS One. 2014;9 doi: 10.1371/journal.pone.0104739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun X., Zhu M.-J. AMP-activated protein kinase: a therapeutic target in intestinal diseases. Open Biol. 2017;7 doi: 10.1098/rsob.170104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan J., Applegate T.J., Liu S., Guo Y., Eicher S.D. Supplemental dietary L-arginine attenuates intestinal mucosal disruption during a coccidial vaccine challenge in broiler chickens. Br. J. Nutr. 2014;112:1098–1109. doi: 10.1017/S0007114514001846. [DOI] [PubMed] [Google Scholar]
- Tang X.X., Chen H., Yu S., Zhang L., Caplan M.J., Chan H.C. Lymphocytes accelerate epithelial tight junction assembly: Role of AMP-activated protein kinase (AMPK) PLoS One. 2010;5:e12343. doi: 10.1371/journal.pone.0012343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Timbermont L., Lanckriet A., Dewulf J., Nollet N., Schwarzer K., Haesebrouck F., Ducatelle R., Van Immerseel F. Control of Clostridium perfringens-induced necrotic enteritis in broilers by target-released butyric acid, fatty acids and essential oils. Avian Pathol. 2010;39:117–121. doi: 10.1080/03079451003610586. [DOI] [PubMed] [Google Scholar]
- To H., Suzuki T., Kawahara F., Uetsuka K., Nagai S., Nunoya T. Experimental induction of necrotic enteritis in chickens by a netB-positive Japanese isolate of Clostridium perfringens. J. Vet. Med. Sci. 2017;79:350–358. doi: 10.1292/jvms.16-0500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsai P.-Y., Zhang B., He W.-Q., Zha J.-M., Odenwald M.A., Singh G., Tamura A., Shen L., Sailer A., Yeruva S.l, Kuo W.-T., Fu Y.-X., Tsukita S., Turner J.R. IL-22 upregulates epithelial claudin-2 to drive diarrhea and enteric pathogen clearance. Cell Host Microbe. 2017;21:671–681. doi: 10.1016/j.chom.2017.05.009. e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Truong A.D., Hong Y.H., Lillehoj H.S. RNA-seq profiles of immune related genes in the spleen of necrotic enteritis-afflicted chicken lines. Asian Australas. J. Anim. Sci. 2015;28:1496–1511. doi: 10.5713/ajas.15.0143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Truong A.D., Hong Y.H., Lillehoj H.S. High-throughput sequencing reveals differing immune responses in the intestinal mucosa of two inbred lines afflicted with necrotic enteritis. Vet. Immunol. Immunopathol. 2015;166:116–124. doi: 10.1016/j.vetimm.2015.06.008. [DOI] [PubMed] [Google Scholar]
- Ulluwishewa D., Anderson R.C., McNabb W.C., Moughan P.J., Wells J.M., Roy N.C. Regulation of tight junction permeability by intestinal bacteria and dietary components. J. Nutr. 2011;141:769–776. doi: 10.3945/jn.110.135657. [DOI] [PubMed] [Google Scholar]
- Van Immerseel F., Buck J.D., Pasmans F., Huyghebaert G., Haesebrouck F., Ducatelle R. Clostridium perfringens in poultry: an emerging threat for animal and public health. Avian Pathol. 2004;33:537–549. doi: 10.1080/03079450400013162. [DOI] [PubMed] [Google Scholar]
- Van Immerseel F., Rood J.I., Moore R.J., Titball R.W. Rethinking our understanding of the pathogenesis of necrotic enteritis in chickens. Trends Microbiol. 2009;17:32–36. doi: 10.1016/j.tim.2008.09.005. [DOI] [PubMed] [Google Scholar]
- Wade, B., and A. L. Keyburn. 2015. The true cost of necrotic enteritis. Poultry World – Meat. Accessed March, 2021.
- Wade B., Keyburn A.L., Seemann T., Rood J.I., Moore R.J. Binding of Clostridium perfringens to collagen correlates with the ability to cause necrotic enteritis in chickens. Vet. Microbiol. 2015;180:299–303. doi: 10.1016/j.vetmic.2015.09.019. [DOI] [PubMed] [Google Scholar]
- Wade B., Keyburn A.L., Haring V., Ford M., Rood J.I., Moore R.J. The adherent abilities of Clostridium perfringens strains are critical for the pathogenesis of avian necrotic enteritis. Vet. Microbiol. 2016;197:53–61. doi: 10.1016/j.vetmic.2016.10.028. [DOI] [PubMed] [Google Scholar]
- Wade B., Keyburn A.L., Haring V., Ford M., Rood J.I., Moore R.J. Two putative zinc metalloproteases contribute to the virulence of Clostridium perfringens strains that cause avian necrotic enteritis. J. Vet. Diagn. Invest. 2020;32:259–267. doi: 10.1177/1040638719898689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H., Ni X., Qing X., Liu L., Lai J., Khalique A., Li G., Pan K., Jing B., Zeng D. Probiotic enhanced intestinal immunity in broilers against subclinical necrotic enteritis. Front. Immunol. 2017;8:1592. doi: 10.3389/fimmu.2017.01592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H., Latorre J.D., Bansal M., Abraha M., Al-Rubaye B., Tellez-Isaias G., Hargis B., Sun X. Microbial metabolite deoxycholic acid controls Clostridium perfringens-induced chicken necrotic enteritis through attenuating inflammatory cyclooxygenase signaling. Sci. Rep. 2019;9:14541. doi: 10.1038/s41598-019-51104-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whelan R.A., Doranalli K., Rinttilä T., Vienola K., Jurgens G., Apajalahti J. The impact of Bacillus subtilis DSM 32315 on the pathology, performance, and intestinal microbiome of broiler chickens in a necrotic enteritis challenge. Poult. Sci. 2019;98:3450–3463. doi: 10.3382/ps/pey500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams R.B. Intercurrent coccidiosis and necrotic enteritis of chickens: rational, integrated disease management by maintenance of gut integrity. Avian Pathol. 2005;34:159–180. doi: 10.1080/03079450500112195. [DOI] [PubMed] [Google Scholar]
- Wu S.B., Rodgers N., Choct M. Optimized necrotic enteritis model producing clinical and subclinical infection of Clostridium perfringens in broiler chickens. Avian Dis. 2010;54:1058–1065. doi: 10.1637/9338-032910-Reg.1. [DOI] [PubMed] [Google Scholar]
- Wu S.-B., Stanley D., Rodgers N., Swick R.A., Moore R.J. Two necrotic enteritis predisposing factors, dietary fishmeal and Eimeria infection, induce large changes in the caecal microbiota of broiler chickens. Vet. Microbiol. 2014;169:188–197. doi: 10.1016/j.vetmic.2014.01.007. [DOI] [PubMed] [Google Scholar]
- Wu Z., Hu T., Rothwell L., Vervelde L., Kaiser P., Boulton K., Nolan M.J., Tomley F.M., Blake D.P., Hume D.A. Analysis of the function of IL-10 in chickens using specific neutralising antibodies and a sensitive capture ELISA. Dev. Comp. Immunol. 2016;63:206–212. doi: 10.1016/j.dci.2016.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xue G.-D., Wu S.-B., Choct M., Swick R.A. Effects of yeast cell wall on growth performance, immune responses and intestinal short chain fatty acid concentrations of broilers in an experimental necrotic enteritis model. Anim. Nutr. 2017;3:399–405. doi: 10.1016/j.aninu.2017.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang W.-Y., Lee Y., Lu H., Chou C.-H., Wang C. Analysis of gut microbiota and the effect of lauric acid against necrotic enteritis in Clostridium perfringens and Eimeria side-by-side challenge model. PLoS One. 2019;14 doi: 10.1371/journal.pone.0205784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yegani M., Korver D.R. Factors affecting intestinal health in poultry. Poult. Sci. 2008;87:2052–2063. doi: 10.3382/ps.2008-00091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang L., Li J., Young L.H., Caplan M.J. AMP-activated protein kinase regulates the assembly of epithelial tight junctions. Proc. Natl. Acad. Sci. U.S.A. 2006;103:17272–17277. doi: 10.1073/pnas.0608531103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng B., Cantley L.C. Regulation of epithelial tight junction assembly and disassembly by AMP-activated protein kinase. Proc. Natl. Acad. Sci. U.S.A. 2007;104:819–822. doi: 10.1073/pnas.0610157104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zihni C., Mills C., Matter K., Balda M.S. Tight junctions: from simple barriers to multifunctional molecular gates. Nat. Rev. Mol. Cell Biol. 2016;17:564. doi: 10.1038/nrm.2016.80. [DOI] [PubMed] [Google Scholar]
- Zou Y., Xiang Q., Wang J., Peng J., Wei H. Oregano essential oil improves intestinal morphology and expression of tight junction proteins associated with modulation of selected intestinal bacteria and immune status in a pig model. Biomed. Res. Int. 2016;2016 doi: 10.1155/2016/5436738. [DOI] [PMC free article] [PubMed] [Google Scholar]