Abstract
The aim of this study was to analyze the relationship between the kinetic behavior, carcass characteristics, oxidative status (blood and meat), and meat fatty acid profiles of 6 organically reared slower growing chicken genotypes (SrG). One hundred male chickens of 6 SrG were used: Ranger Classic (RC), Ranger Gold (RG), Rowan Ranger (RR), RedJA (RJ), CY Gen 5 JA87 (CY), and M22 × JA87 (M). Twenty chickens/genotype were selected to analyze behavior, while, 15 individuals were slaughtered and different traits were analyzed in the blood and drumstick meat. The variables were grouped into different principal components: kinetic activity (PC1, with explorative attitude as the highest score), productive performance (PC2, carcass and head/feet yields), blood (PC3, carbonyls, and TBARS) and meat (PC6, thiols, and TBARS) markers, technological traits (PC4, pH, and color), proximate meat composition (PCA5, moisture, lipids, protein, and ash), fatty acid profile, and nutritional indexes (PC7, IP, and PUFAn-3). Uni- and bivariate analyses showed a strong positive association between kinetic behaviors and blood and meat oxidation and a medium positive association with fatty acid profile and nutritional indexes, whereas a negative association was found between productive performance loads and the technological traits of meat. Generalized linear models showed that all PCs were influenced by genotype. In particular, CY and M resulted as less active genotypes; conversely, RR showed more kinetic activity, whereas RJ, RG, and RC exhibited intermediate levels of activity. Cluster analysis of kinetic behavior and blood or meat oxidative status highlighted 2 groups: nonwalking (NW: CY and M) and walking (W: RC, RG, RR, and RJ) animals. However, in the W group, another was visualized, constituted by genotypes with high kinetic activity resulting in the worst oxidative balance (Walking not trained-genotypes, Wnt: RR and RJ). The present results confirmed that the kinetic behavior of SrG genotypes is negatively correlated with productive performance. Furthermore, a significant association between kinetic behavior and blood (positively correlated) or meat (negatively correlated) oxidative status was noted. Such differences are mainly due to the intrinsic response of the genotypes used (i.e., training-walking capacity).
Key words: organic, poultry genotypes, kinetic behaviors, meat quality, oxidation
INTRODUCTION
Over the past 50 yr, the human perception of animal production systems has drastically changed: humans have evolved from animal housing and management strategies in industrialized systems that have only focused on productive traits to the “one welfare” concept (Tarazona et al., 2020). The welfare concept emphasizes the link between human welfare and animal welfare, and poor animal welfare that makes production systems inefficient is likely to have negative effects on human welfare too. Both stress and pain require energy for compensation, so part of the energy consumed by the animal is used to manage welfare problems, instead of productive efficiency and qualitative traits (Rauw, 2008).
Poultry production is very sensitive to welfare issues, considering the large increase of alternative commercial rearing systems (i.e., organic, free-range; Castellini et al., 2002a; Castellini et al., 2012; dal Bosco et al., 2010, 2016; Hofmann et al., 2020). In this context, many efforts have been made to study the adaptability of different chickens strains reared in less-controlled environments, such as organic rearing systems, with the aim of improving the welfare and health of animals and furnishing products that better meet consumer preferences (Avilés-Esquivel et al., 2018; Mancinelli et al., 2020a,b).
Until a few years ago, due to the lack of appropriate legislation in many EU countries, poultry strains reared in extensive systems were mainly fast-growing (FG), characterized by a rapid growth rate (>40 g/d) and higher breast yield, although they were not adapted to less-controlled environments (Gálvez et al., 2020). These strains are less resistant to environmental stress (e.g., heat stress), disease, and only marginally utilize pasture (dal Bosco et al., 2010, 2012). In addition, the higher kinetic behavior of these strains worsens the health status of the animals (Mattioli et al., 2017; Mancinelli et al., 2020a), as well as the quality of meat (Castellini et al., 2002b; dal Bosco et al., 2012).
On this basis, slow growing chicken genotypes consists of a heterogeneous group of animals represented by pure breeds and commercial genotypes, defined by the most breed companies as slower growing (SrG) strains.
For all these reasons, many companies have started using SrG instead of FG, characterized as having an intermediate growth rate (<38–40 g/d; Mancinelli et al., 2020a) and to be more suitable for free-range systems, since they represent a good compromise between adaptability and product quality (Castellini et al., 2002b, 2016).
It is well documented that pure breeds showed higher foraging behavior than FG chickens, and that this behavior increases the quality of products (Lindqvist, 2008; dal Bosco et al., 2016). dal Bosco et al. (2016) demonstrated that the intake of pasture positively affects antioxidant compounds (vitamins and carotenes) and other bioactive molecules (polyunsaturated fatty acids) in the blood and meat (dal Bosco et al., 2011; dal Bosco et al., 2012; Castellini and Dal Bosco, 2017).
However, some studies have demonstrated that the kinetic activity of animals, if not well balanced (with good management practices, that is, stocking density, dietary administration, suitable genotypes, and appropriate slaughtering age), triggers an oxidative thrust, which negatively influences the health status of animals and, in turn, the quality of the meat (Castellini and Dal Bosco, 2017).
In this context, after analyzing the behavioral response of the genotypes (Cartoni Mancinelli et al., 2020a), the present study investigated the relationships between kinetic behaviors (as a set of different parameters), carcass and meat characteristics, oxidative status (blood and meat), and fatty acid profiles of 6 organically reared SrG.
MATERIAL AND METHODS
Animals and Farming System
The experiment was carried out at the experimental farm of the University of Perugia (Italy). Chickens were reared according to EU Regulation 834/07, EU Regulation 889/2008, and the Italian directives (European Parliament and Council of the European Union, 2013) on animal welfare for experimental and other scientific purposes. The experimental protocol was positively evaluated and approved by the Ethical Committee of the University of Perugia (ID number: 112606).
A total of 100 male chickens of 6 SrGs were used: Ranger Classic (RC), Ranger Gold (RG), Rowan Ranger (RR), RedJA (RJ), CY Gen 5 × JA87 (CY), and M22 × JA87 (M).
The birds were provided by 2 commercial poultry farms: RC, RG, and RR by Aviagen (Cocconato, AT, Italy) and RJ, CY, and M from Hubbard (Le Foeil-Quintin, France). Both breeder companies define the provided genotypes as SrG broilers (growing rate ≤40 g/d).
The rearing system management was reported in detailed in the companion paper of Mancinelli et al. (2020a).
Behavior Observations
Behavioral observations were performed using a computerized system (Noldus Technology, Wageningen, the Netherlands) comprising of 2 different software programs, Media Recorder and Observer XT, to record and analyze the videos. The behavior observations consisted of the evaluation of Explorative Attitude (EA) and the Behavior Patterns (BP). The EA involved the count of the animals that left the shelter for the first time within 5 min. The EAs were recorded for each genotype at 21 d, the age corresponding to the first access to the pasture. A 5-min long video was recorded for each shelter opening. Three videos were taken for each genotype. The videos were analyzed with Observer XT by counting the number of animals that left the shelter at the pre-established time (5 min), as reported in detail by Mancinelli et al. (2020a). Furthermore, the EA scores were calculated as reported by Mancinelli et al. (2020a). The longer is the EA time, the lesser is the ability of the genotype to use the outdoor space (Castellini et al., 2006).
The BP included all behaviors expressed by the animals in the outdoor area. The BP was evaluated 1 wk before slaughtering, and for each genotype, 3 videos of 20 min length were recorded. All videos were analyzed with Observer XT software by observing the behaviors for 1 min as described by Mancinelli et al. (2020a). The BP expressed from the 20 identified animals/genotype was analyzed using the focal sampling method.
The BP was divided into 3 main macro groups of behaviors: static (rest: body in line with the ground, with erect head and open eyes, or roost: standing stationary, no body movement, head erect, or relaxed with open eyes), kinetic (running, walking), and eating (feeding, grass, and drink consumed), as reported in Table 1.
Table 1.
Main behaviors expressed by the chickens in outdoor area.
| Behavior category | Behaviors | Description |
|---|---|---|
| Kinetic | Walking | Bird that moves more than three steps |
| Run | Bird that rapidly walking | |
| Static | Rest | Bird that presents the body in line with the ground with an erect head and open eyes |
| Roost | Bird in lying position with the ventral body region in contact with the floor | |
| Eating | Feed | Bird that pecks inside the feeder |
| Grass | Bird that presents its head down and beak in contact with the grass | |
| Drink | Bird that pecks the drinker |
Productive Performances and Carcass Traits
At 81 days of age, 15 chickens/genotype were randomly selected and slaughtered in a commercial slaughterhouse 12 h after feed withdrawal. The animals were electrically stunned (110 V; 350 Hz) before killed. After bleeding, the carcasses were placed in hot water (56.5°C for 1 min) and then plucked, eviscerated (nonedible viscera: intestines, proventriculus, gall bladder, spleen, esophagus, and full crop), and the carcasses were stored for 24 h at 4°C to obtain the cold carcass. The head and feet yield (% cold carcass weight including head, neck, and feet to the live weight), bust weight, and yield (% cold carcass without head, neck, and feet to the live weight), as well as the breast and drumstick weights, were registered and calculated. The drumstick meat was excised from the carcasses and entirely removed from the bone.
Blood Collection and In Vivo Oxidative Status Evaluations
Blood samples were collected at slaughtering for 90 chickens (15 chicken/genotype) and collected in heparinized vacutainers to obtain the plasma for measuring the in vivo oxidative status, or in empty tubes to recover the serum for fatty acid determination and immunity traits. After collection, the blood samples were immediately sent to the laboratory of the Department of Agricultural, Food and Environmental Science, where the plasma tubes were centrifuged at 1,500 × g for 10 min at + 4°C, and the serum tubes were left to separate for 2 h at room temperature. Finally, the plasma and serum samples were frozen at −80°C until analysis (at least 2 wk later).
The extent of plasma lipid peroxidation was evaluated using a spectrophotometer (set at 532 nm, Shimadzu Corporation UV-2550, Kyoto, Japan), which measured the absorbance of thiobarbituric acid reactive substances (TBARS), and a tetraethoxypropane calibration curve in sodium acetate buffer (pH = 3.5; Mattioli et al., 2019). The results were expressed as nmol of malondialdehyde (MDA)/mL of plasma.
The detection of protein carbonyl groups followed the method of Dalle-Donne et al. (2003), using 2,4-dinitrofenilhidrazina (DNPH) as the reactive. The serum was diluted to 1:40 with phosphate-buffered saline (PBS) before analysis. Carbonyl content was determined from the absorbance at 366 nm using a molar absorption coefficient of 22,000 M 1/cm. The results were expressed as nmol/mg of protein.
The tocols (α-tocopherol and its isoforms γ and δ, and α and γ-tocotrienol) and retinol levels were measured according to Schüep and Rettenmaier (1994). Briefly, 0.2 mL of plasma was mixed with 1 mL of water and 4 mL of an ethanol solution of 0.06 % butylated hydroxytoluene (BHT). The mixture was saponified with water/potassium hydroxide (KOH) (60%) at 70°C for 30 min and extracted with hexane/ethyl acetate (9/1, v/v). Following centrifugation, 2 mL of the supernatant was transferred into a glass tube, dried under N2, and resuspended in 200 μL of acetonitrile. The pellet was re-extracted twice. A 50 μL volume of filtrate was then injected into the HPLC/FD (pump model Perkin Elmer series 200, equipped with an autosampler system, model AS 950-10, Jasco, Tokyo, Japan) on a Sinergy Hydro-RP column (4 µm, 4.6 × 100 mm; Phenomenex, Bologna, Italy). The flow rate was 2 mL/min. All tocopherols and tocotrienols were identified using an FD detector (model Jasco, FP-1525 - excitation and emission wavelengths of 295 and 328 nm, respectively) and quantified using external calibration curves prepared with increasing amounts of pure standard solutions (Sigma-Aldrich, Bornem, Belgium) in ethanol. The tocols sum was used for statistical analysis. Retinol was analyzed with the same HPLC system using a UV-VIS spectrophotometer detector (Jasco UV2075 Plus) set at λ 325 nm. Retinol was identified and quantified by comparing the sample with a pure commercial standard in chloroform (Sigma-Aldrich, Steinheim, Germany; Extrasynthese, Genay, France).
Reactive oxygen molecules (ROMs) of the plasma were evaluated with a commercial kit (Diacron, GROMsseto, Italy) and expressed as mmol H2O2.
The antioxidant power of plasma (PAO) was measured with a commercial kit (Diacron, GROMsseto, Italy), which evaluated the ability of the plasma to oppose the massive oxidative action of a hypochlorous acid (HClO) solution. The PAO levels of each sample were expressed as μmol of neutralized HClO.
Proximate Composition and Technological Traits
Moisture, ash, and total nitrogen were assessed using the AOAC methods (AOAC, 1995–N. 950.46B, 920.153, and 928.08, respectively). Total protein was calculated by Kjeldahl using a 6.25 conversion factor. Total lipids were extracted in duplicate from 5 g of each homogenized sample and calculated gravimetrically (Folch et al., 1957).
The ultimate pH (24 h) was measured with a Knick digital pH meter (Broadly Corp., Santa Ana, CA) after homogenization of 1 g of raw muscle for 30 s in 10 mL of 5 M iodoacetate (Korkeala et al., 1986).
The water-holding capacity (WHC) was estimated by placing 1 g of whole muscle on tissue paper inside a tube and centrifuging for 4 min at 1,500 × g. The water remaining after centrifugation was quantified by drying the samples at 70°C overnight. WHC was calculated as follows: (weight after centrifugation − weight after drying)/initial weight × 100.
At 24 h postmortem, L* value (degree of lightness) was measured on the cut surface of each fillet using a tristimulus analyzer (Minolta Chroma meter CR-200, Osaka, Japan), following the CIELab color system (Robertson, 1977).
Oxidative Status of Meat and Fatty Acid Profiles
All oxidative parameters and fatty acid profiles were analyzed in duplicate. The α, γ and δ-tocopherol, α and γ-tocotrienol, and retinol contents of the meat were quantified using the HPLC system described above, according to Hewavitharana et al. (2004). Five milliliters of distilled water and 4 mL of ethanol were added to 2 g of sample and vortexed for 10 s. After mixing, 4 mL of hexane containing BHT (200 mg/L) was added and the mixture was carefully shaken and centrifuged at 8000 × g for 10 min. An aliquot of the supernatant (3 mL) was dried under a stream of nitrogen and dissolved in 200 μL of acetonitrile; 50 μL was then injected into the same HPLC.
Lipid oxidation was evaluated using a spectrophotometer set at 532 nm (Shimadzu Corporation UV- 2550, Kyoto, Japan) that measured the absorbance of TBARS and a 1,1,3,3-tetraethoxypropane calibration curve (Ke et al., 1977). Oxidation products were quantified as malondialdehyde equivalents (μg MDA/g).
Carbonyl derivatives of proteins were detected according to the method of Mattioli et al. (2018). Briefly, the pellets from trichloroacetic acid (TCA) extracts were mixed with 1 mL of 10 mM DNPH in 2 M HCl. Samples were incubated for 1 h at RT and then centrifuged at 13,000 × g for 5 min. Supernatants were discarded and the pellets were washed 3 times with 1 mL of ethanol–ethylacetate (1:1, v/v) in order to remove unreacted DNPH. The pellets were then dissolved in 1.5 mL of 6 M guanidine-HCl and centrifuged as above to pellet insoluble particles. The carbonyl content of the resulting supernatants was evaluated spectrophotometrically at 370 nm using a molar extinction coefficient of 22,000 1/M*cm; values were expressed as nmol of carbonyl/mg of protein in the guanidine chloride solution. Protein concentrations were measured using the Bradford method with Coomassie Brilliant Blue G-250 (Bradford, 1976), using bovine serum albumin as the standard. The same trichloroacetic acid extract was also used to evaluate thiol groups based on 5,5’ dithio-bis-2-dinitrobenzoic acid assay, with an extinction coefficient of 13,600 1/M*cm and expressed as µmoL SH – group per g.
The lipid fraction for fatty acid evaluation was extracted from the meat following the method reported by Folch et al. (1957). To obtain the fatty acid methyl esters, the lipid extract was dried with a rotavapor and 1 mL of n-hexane was added. Finally, the transmetilation procedure was performed with 0.5 mL of 2 M KOH methanol solution at 60°C for 15 min. To calculate the amount of each fatty acid, heneicosanoic acid was used as the internal standard (C21:0, Sigma-Aldrich analytical standard). The average amount of each fatty acid was used to calculate the sum of the total saturated (SFA), total monounsaturated (MUFA), and total polyunsaturated (PUFA) acids from the n-3 and n-6 series. The n-6/n-3 fatty acid ratio was also calculated.
In the meat samples, some nutritional indexes of lipids were evaluated, as reported below.
The peroxidability index (IP) was calculated according to the method of Arakawa and Sagai (1986):
IP = (% monoenoic × 0.025) + (% dienoic × 1) + (% trienoic × 2) + (% tetraenoic × 4) + (% pentaenoic × 6) + (% hexaenoic × 8).
Indexes of atherogenicity (IA) was calculated according to Ulbricht and Southgate (1991). In particular:
Statistical Evaluation
First, a multivariate approach was used to reduce the number of variables using principal component analysis (PCA). The variables that could measure aspects of the same underlying dimensions were stratified and included in several PCAs. Thus, 7 different models were used to analyze the kinetic behavior variables (PCA1), productive performance traits (PCA2), markers of blood oxidative stress (PCA3), technological traits (PCA4), and proximate composition (PCA5) of meat, markers of meat oxidative stress (PCA6), and fatty acid profile and nutritional indices (PCA7). The variables were included in each PCA after inspection of the correlation matrix and communalities (Righi et al., 2019; Menchetti et al., 2020a). However, some metabolically or qualitatively important variables (e.g., tocols) were retained despite their low commonalities. One component (PC) for each PCA was retained to evaluate its performance through the total variance explained, the Kaiser criterion, and the Bartlett's test (Garson, 2008;Menchetti et al., 2020a,b). The sign of the loadings (and of the scores) of PC6 was reversed to facilitate its interpretation.
The seven PCs describing behavior, productive, chemical, metabolic, and/or physiological aspects were analyzed using bi- and univariate approaches (Cardinali et al., 2017; Menchetti et al., 2018). A matrix of Pearson product-moment correlations was built to detect associations among the PCs. Correlation was defined as high when the Pearson coefficient (r) > |0.5|, medium when r ranged from 0.3 to 0.5, and low when r <|0.3| (Cardinali et al., 2017). Furthermore, generalized linear models (GLMs) were used to assess whether the genotype influenced the profiles defined by each PC setting identity link function and normal distribution (Menchetti et al., 2018). Graphics tests were used to verify the assumptions, while the least significant difference (LSD) method was used for pairwise comparisons. The PCs were included in the GLMs as dependent variables and the genotype was included as the independent variable.
Finally, a two-step cluster analysis (TCA) was used to identify clusters of animals with similar characteristics in regards to the relationship between kinetic behavior (PC1) and meat (PC6) or blood (PC3) oxidative stress. Log likelihood was used as a distance measure while Bayesian information criteria (BIC) were used as clustering criteria (Garson, 2008). The Silhouette coefficient was used as a measure of goodness-of-fit (Rousseeuw, 1987). Chi-square (χ2) or Fisher's tests were conducted to determine the associations between clusters and genotype.
Statistical analyses and visualizations were performed using SPSS Statistics version 25 (IBM, SPSS Inc., Chicago, IL) and GraphPad Prism, version 7.0 (GraphPad Software, San Diego, CA). The level of statistical significance was set at P <0.05.
RESULTS
Multivariate Analysis
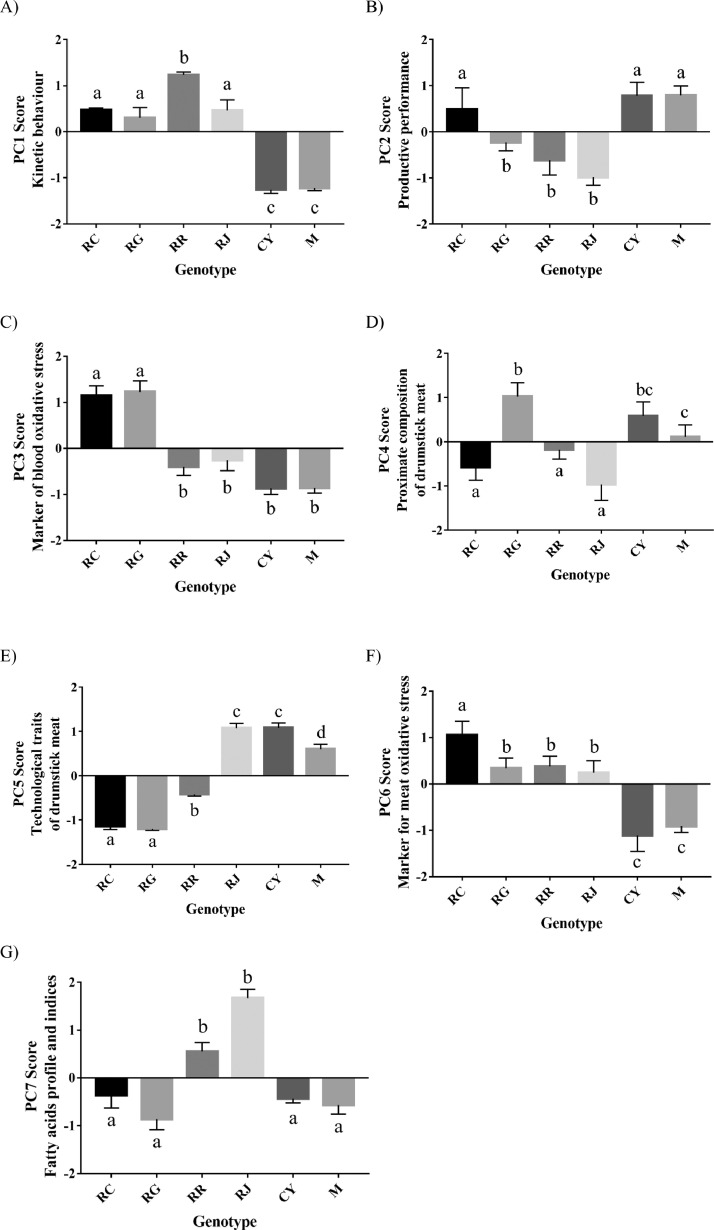
The variables included in each PC and the respective loadings are detailed in Table 2. Moreover, mean values and scores were reported in Supplemental Tables S1 and S2. PC labels were chosen according to the highest positive loadings. In particular, indicators of kinetic activity emerged in PC1, while carcass and head/feet yields were the heaviest indicators of performance (PC2). The highest loadings in the PC of marker oxidative stress were carbonyls and TBARS in the blood (PCA3), and thiols and TBARS in the meat (PCA6). Negative scores were obtained from tocols and carbonyls. The PCs of the technological traits and proximate composition of meat (PCA4 and PCA5, respectively) were also strongly bipolar: the positive loading of pH was opposed to the negative loading of a*, while protein and ash were opposed to moisture.
Table 2.
Labels of Principal Components (PCs) extracted with the Principal Component Analyses (PCA), loadings of variables, and total variance explained by each PC.
| N° PCA | Label of PC | Item | Loading | Variance explained |
|---|---|---|---|---|
| PCA1 | Kinetic behavior | EA_reversed (*) | 0.892 | 67.2% |
| Eating, % | 0.777 | |||
| Kinetic, % | 0.607 | |||
| Static, % | -0.960 | |||
| PCA2 | Productive performance | Carcass yield, % | 0.878 | 56.9% |
| Head and feet yield, % | 0.750 | |||
| DWG, g | 0.684 | |||
| Drumstick, g | 0.642 | |||
| PCA3 | Marker of blood oxidative stress | Carbonyls, nmol/mg proteins | 0.816 | 40.1% |
| TBARS, nmol MDA/mL | 0.728 | |||
| ROMs, mmol H2O2 | 0.634 | |||
| Retinol, nmol/mL | 0.619 | |||
| Σ Tocols, nmol/mL | −0.161 | |||
| PCA4 | Technological traits of drumstick meat | pH | 0.678 | 40.4% |
| L * | 0.497 | |||
| a * | −0.710 | |||
| PCA5 | Proximate composition of drumstick meat | Protein, % | 0.957 | 71.6% |
| Ash, % | 0.936 | |||
| Lipids, % | 0.744 | |||
| Moisture, % | −0.721 | |||
| PCA6 | Marker for meat oxidative stress | Thiols, µmol SH-group/g | 0.529 | 41.5% |
| TBARS, µg MDA/g | 0.524 | |||
| Carbonyls, nmol/mg proteins | −0.721 | |||
| Σ Tocols, µg/g | −0.764 | |||
| PCA7 | Fatty acids profile and indices | IP | 0.780 | 56.6% |
| n-3 PUFA, % of total fatty acids | 0.620 | |||
| IA | 0.556 | |||
| PUFA, % of total fatty acids | −0.794 | |||
| n-6 PUFA, % of total fatty acids | −0.948 |
(*)The signs of this traits were reversed.
Abbreviations: DWG, daily weight gain; EA, explorative attitude; IA, index of atherogenicity; IP, index of peroxidability; MDA, malondialdehyde; PUFA, polyunsaturated fatty acids; ROMs, reactive oxygen molecules; TBARS, thiobarbituric reactive substances.
Similarly, in PCA7, the IP index and n-3 PUFA exhibited opposite signs with respect to PUFA and total n-6. The total variance explained by the analyses ranged from 40.1% for PCA3 to 71.6% for PCA5. All eigenvalues were above 1.0, meeting the Kaiser criterion, and Bartlett's tests were significant, confirming adequate correlations among the variables.
Uni- and Bivariate Analyses
Table 3 shows the correlations among the PCs. Strong negative associations were found between PC1 and PC2 or PC5 (P < 0.01), whereas PC1 was positively correlated with PC3, PC6 (P < 0.01), and PC7 (P < 0.05). Positive association was also found between PC3 and PC6 (P < 0.01), and between PC5 and PC7 (P < 0.05). Negative associations were found between PC2 and PC6 or PC7 (P > 0.01), PC3 and PC5 (P < 0.01), and PC4 and PC7 (P < 0.01).
Table 3.
Matrix of Pearson product-moment correlations.
| Label of PC | PC2 Productive performance | PC3 Marker of blood oxidative stress | PC4 Technological traits of drumstick | PC5 Proximate composition of drumstick | PC6 Marker for meat oxidative stress | PC7 Fatty acids profile and nutritional indices |
|---|---|---|---|---|---|---|
| PC1 Kinetic behavior | −0.487⁎⁎ | 0.400⁎⁎ | −0.257 | −0.512** | 0.609** | 0.382* |
| PC2 Productive performance | −0.078 | 0.030 | 0.036 | −0.472** | −0.523** | |
| PC3 Marker of blood oxidative stress | 0.110 | −0.734⁎⁎ | 0.568⁎⁎ | −0.203 | ||
| PC4 Technological traits of drumstick | −0.098 | −0.112 | −0.411⁎⁎ | |||
| PC5 Proximate composition of drumstick | −0.564⁎⁎ | 0.373* | ||||
| PC6 Marker for meat oxidative stress | −0.194 |
**Correlation is significant for 0.01 level (2-tailed).
*Correlation is significant for 0.05 level (2-tailed).
Boldface indicates the significant correlations.
Abbreviation: PC, principal component.
GLMs showed that all PCs were influenced by the genotype (P < 0.001; Tables S1 and S2). In particular, CY and M resulted in less active genotypes (PC1); conversely, RR showed more kinetic activity, whereas RJ, RG, and RC showed intermediate levels. Productive performance scores also showed higher values in the same genotypes, followed by that of RC (PC2). Considering the markers of blood and meat oxidative stress (Figure 1C - PC3 and 1F - PC6, respectively), CY, M, RC, and RG showed a similar trend regarding in vivo markers, whereas RC showed a different trend in PC6 respect to RG, RR, and RG. RR and RJ had lower loadings for in vivo oxidative marker and higher in drumstick meat. Considering the TBARS and tocols scores, the RR and RG showed lower and higher values for in vivo oxidative markers and the contrary in meat. However, protein oxidation (carbonyls) showed an opposite trend in the blood and meat, independent of genotype.
Figure 1.
PC scores of different poultry genotypes. (A) Kinetic behaviors (PC1); (B) productive performance (PC2); (C) marker of blood oxidative stress (PC3); (D) proximate composition of drumstick meat (PC4); (E) technological traits of drumstick meat (PC5); (F) marker for meat oxidative stress (PC6); (G) fatty acid profile and nutritional indices (PC7). Bars not sharing any superscript are significantly different at P < 0.05.
The technological traits and proximate composition PC (Figures 1D- PC4 and 1E- PC4 and 5) showed a unique trend depending on the genotype: CY and M presented higher pH, lower redness (a*), and higher protein and lipid respect to RC and RR. Conversely, RJ showed lower pH and L* values, and higher redness, but displayed a lower percentage of all main nutrients compared to that of the other genotypes.
Figure 1G shows the PC scores of the fatty acid profile and indices. The most kinetic birds (RJ and RR) showed a higher n-3 PUFA amount than others associated with a higher IP and IA. Conversely, RC, RG, CY, and M showed a higher n-6 PUFA presence in the drumstick meat.
Cluster Analysis
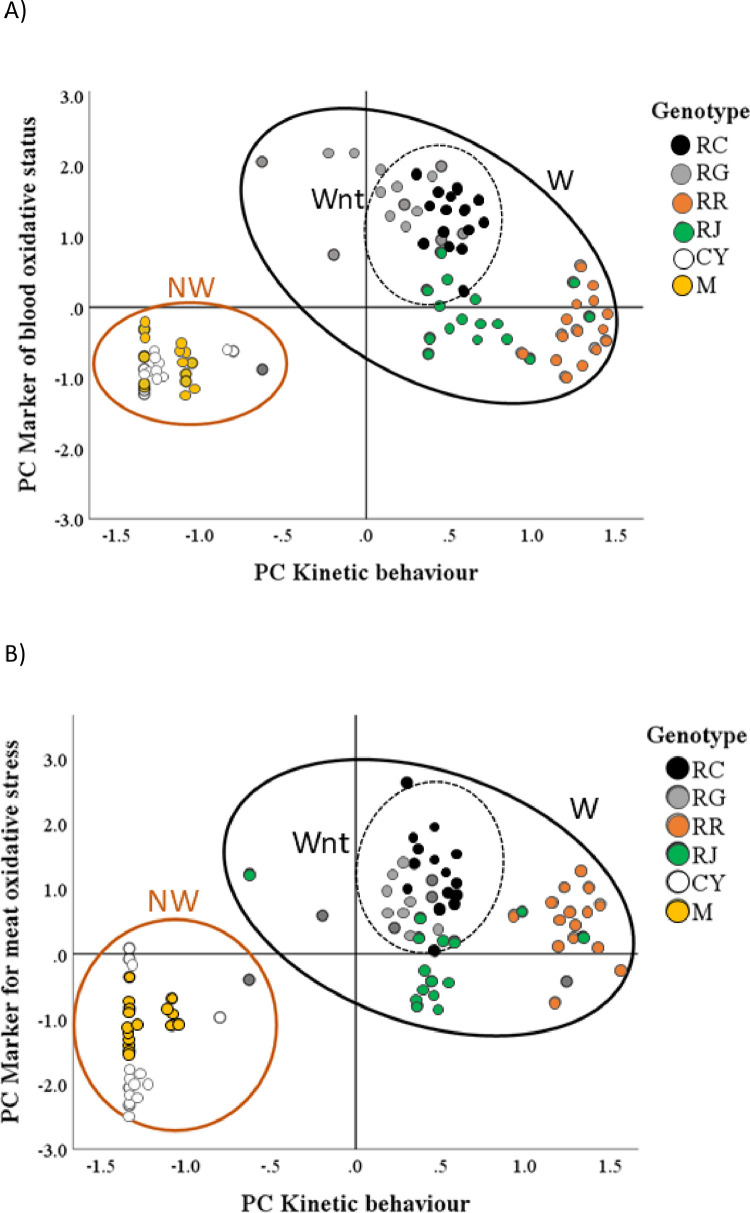
Figures 2A and 2B show the two-step cluster analyses based on kinetic behavior and blood (TCA1, Panel A) or meat (TCA2, Panel B) oxidative status across the different genotypes. From the analysis, two clusters emerged with a good level of cohesion and separation (Silhouette coefficient >0.5). The most important predictor was kinetic behavior (PC1).
Figure 2.
Two step cluster analysis (TCA) of kinetic behaviour (PC1) and marker of blood (PC3, Panel A) and meat (PC6, Panel B) oxidative stress. Red circle contains the samples included in the cluster 1 (Not walking genotypes, NW) showing negative mean scores for both PC1 and PC3 or PC6; Solid Black circle contains the samples included in the cluster 2 (Walking genotypes, W) showing positive mean scores for both PC1 and PC3 or PC6; Dotted black line: walking not-trained-genotypes (Wnt).
Cluster 1 showed a negative mean score for both the PCs and included all of the samples of the CY and M genotypes. Cluster 2 showed positive mean scores for both PCs and included almost all samples of the RC, RG, RR, and RJ genotypes (χ2 = 40.0 and χ2 = 38.3 for TCA1 and TCA2, respectively; P < 0.001). Thus, the clusters denoted clearly that the genotypes with higher kinetic activity (Walking – W: RR and RJ, Figures 2A and 2B) also showed a higher blood/meat oxidation than did the nonwalking genotypes (NW: CY and M - Figures 2A and 2B) that were grouped separately. However, in the walking group, a third sub-cluster, composed mainly of RC and RG genotypes (Figure 2, dotted black line), is possible to visualize. In this sub-group, a higher kinetic score determined a particularly high blood and meat oxidation (may be defined as walking not-trained-genotypes, Wnt) compared to typical Walking genotype. Furthermore, the RC genotype showed a tendency oriented toward a lower kinetic activity respect to RG which are mainly located in the right square of the TCA graph.
DISCUSSION
PCA Correlations
Literature has reported that extensive poultry rearing systems improve meat quality as the kinetic activity and the scavenger aptitude of the birds (Castellini et al., 2006, 2016) increase the ingestion of bioactive compounds (tocopherols, carotenoids, polyphenols, and PUFAs, especially α-linolenic) contained in grass, insects, and earthworms (dal Bosco et al., 2016).
However, kinetic activity may be also associated with a worsening of productive performances, antioxidant status, and shelf-life of meat due to the interaction of other factors, including energy balance, genetic features, dietary intake, or metabolic stress (Celi et al., 2017; He et al., 2018).
This study confirms that kinetic behavior (PC1) and productive performance (PC2) are negatively correlated. The daily weight gain (DWG), carcass and head/feet yield, and drumstick weight were lower in more walking birds, due to a great expenditure of energy compared to that of the more static individuals. Probably, on one hand, the kinetic behavior triggered regulatory changes (an increased mobilization of energy and a shift in metabolism) further reducing growth performance (Bureau et al., 2009), on the other hand, it is also true that the less weight of animals favored the kinetic activity of them.
There is a strict relationship between genotype and DWG. However, it is important to note that genotypes with similar DWG may exhibit different behavioral patterns, predominantly in kinetic activity (Mancinelli et al., 2020a). On the same time, this study confirms that kinetic (PC1) and blood (PC3) or meat (PC6) oxidative status was highly correlated (0.400 and 0.609 correlation values, respectively). Chickens with high EA or kinetic activity increased their production of ROMs with consequent in vivo oxidation of proteins (carbonyls) and lipids (TBARS); meanwhile, retinol enhanced and tocols decreased in the plasma (Table 2; Figures 1C and 1F).
The oxidative markers of the drumstick meat (PC6) showed a positive correlation with kinetic behavior: reduced protein oxidation and tocols content, and increased TBARS and thiols were associated with higher kinetic behavior. At the same time, the IP of the meat also increased, most likely due to the higher n-3 PUFA content (PCA7).
Other authors (Lewis et al., 1997; Castellini et al., 2002b) confirmed that free-range chickens could exhibit lower meat oxidative stability due to higher motor activity that increases oxidative metabolism and free radical production. Furthermore, high ROMs production can be stimulated by stressful conditions, which can cause lipid peroxidation and oxidative damage to proteins (Dröge, 2002).
Mancinelli et al. (2020b) evaluated the effect of the cooking procedure on breast meat in different chicken genotypes and defined the kinetic chickens as “more vulnerable” toward lipid oxidation compared to that of the static chickens. Genotypes with higher kinetic activity consume more antioxidants than static animals to counteract the production of ROM by movement; hence, they have less antioxidant availability during the cooking process.
However, it should be underlined that not always, higher kinetic activity means higher foraging, and then, pasture intake. The present data clearly indicated that the overproduction of free radicals compromised in vivo antioxidant defenses and oxidative stress, but it was neutralized by antioxidant intake (foraging behaviors) resulting in the not oxidatively damaged meat (i.e., walking genotypes). Fresh grass has approximately 6 times more α-tocopherol and 2 to 8 times more carotenoids than that of standard poultry feed (Smet et al., 2008). Ponte et al. (2008) demonstrated that the synergistic cooperation of the terpene content of grass (i.e., α-tocopherol, γ-tocotrienol, and β-carotene) acts to prevent radical formation in extensively reared chickens. Dietary intake of pasture increases the antioxidant content of the plasma (Castellini et al., 2002a, b; Castellini et al., 2002a, Mugnai et al., 2014), and, if necessary, these antioxidants are utilized to balance the oxidative thrust of the body. It is possible to hypothesize a turnover/loop mechanism of in vivo antioxidants: higher kinetic activity increases ROMs production and, thus, lipid and protein oxidation (higher ROMs = higher TBARS). Contemporary, the foraging behavior enhances the antioxidants recovered by grass intake (higher intake = higher antioxidants); consequently, the ingestion of enough antioxidants through grass, counteracts the oxidative thrust, resulting in a lower oxidation (higher antioxidants = lower TBARS); finally, a reduction of available antioxidants may occur in the different tissues if not followed by a progressive ingestion (lower intake = lower antioxidants = higher TBARS).
The apparent discrepancy between the pro- and antioxidant molecules in the blood has to be related to the intrinsic balance of the body; it is most likely associated with specific response of genotypes (§ next paragraph). A similar finding was observed for some chemical components of the meat, which was negatively correlated with kinetic behavior. Indeed, such correlation was mainly due to the different proximate compositions, attributable to the genotypes.
Michiels et al. (2014) observed a higher TBARS values associated with lower α-tocopherol content in the plasma of outdoor chickens compared to that of indoor chickens. However, such outcomes are referred to as fast-growing chickens, which have low kinetic activity and poor foraging abilities (dal Bosco et al., 2010), confirming that foraging ability and pasture availability are crucial for organic chickens as an antioxidant source.
In the drumstick tissue, the antioxidants showed a different trend. The pro-oxidative environment created in postmortem muscles upon animal slaughter led to the occurrence of oxidative reactions in the poultry muscle, mainly in the drumstick, which is the muscle that supports walking. Branciari et al. (2009) suggested that a shift of muscle fibers from α-white, which are more glycolytic, to α-red fibers, which are more oxidative, could be a kinetic-adaptation mechanism (primarily the Ileo-Tibialis lateralis). This shift renders the muscle more efficient and resistant to fatigue; in addition, other muscle modifications, such as the expression of contractile proteins (e.g., myosin) and an increase in muscle mitochondria (Kikusato and Toyomizu, 2013), increased cellular respiration rate and oxidative capacity. Both of these changes resulted in higher oxidative metabolism and, thus, in ROMs production, which leads to the oxidative status of tissues. In this study, lipid oxidation (TBARS) increased, but not proteins (carbonyls, PCA6). This trend was likely due to the higher susceptibility of lipid oxidation, mainly because the tissues were constituted by PUFAs (Mancinelli et al., 2020b). In connection with PUFA reduction, vitamin E decreased, whereas no significant correlation was found in PC6 for retinol.
The different trends observed for the antioxidant molecules were most likely due to their specific biological effects. Tocols are the combination of tocopherols and tocotrienols, which constitute vitamin E. Vitamin E, like fat-soluble antioxidants, are more active against lipid oxidation, and work as chain-breakers in the hydroperoxide formation that begins with PUFA (Galli et al., 2017). Retinol derivatives from the metabolism of carotenes are more abundant in grazing birds and stored in animal tissues, and its amount is predominantly related to specific intake due to its late action in the antioxidant chain. Thiol content, consisting of indirect measures of the enzyme glutathione, increases with movement as a response to exogenous stressors (Śliwa-Jóźwik et al., 2002) for restoring optimal oxidative conditions (Fellenberg and Speisky, 2006).
Among animal-source foods, poultry meat has been recognized as a very sensitive source of oxidative processes owing to the high degree of unsaturation in the muscle lipids (Min and Ahn, 2005). Even the fatty acid profile PC (PCA7) showed that the kinetic behaviors (as foraging one) positively affected n-3 content due to grass ingestion, whereas n-6 was negatively correlated. Furthermore, PUFA content decreased due to oxidation, as has already been demonstrated by Mancinelli et al. (2020b) during cooking.
Comparison of Genotypes
We found that chickens with higher DWG (RC, CY, and M; Supplemental Table S1) showed different responses compared to that of less productive birds.
It is known that the selection for higher growth rate decreases all functions not directly connected with muscle growth (e.g., locomotive activity, immune response, thermoregulation; Zampiga et al., 2021; Siegel and Honaker, 2009; Zhou et al., 2014); thus, high-performance chickens do not take advantage of outdoor runs, however, they produce a limited amount of oxidative mediators. Equilibrium between a “sufficient” movement for taking advantage of the pasture and the antioxidant power of the body should be determined by comparing different genotypes and/or managing the dietary intake of antioxidants (solid feed supplement/favor intake of more antioxidants in the pasture; Benbrook, 2005).
Indeed, the adaptation of chicken strains to outdoor environments affects animal equilibrium by modulating the intake of grass (Castellini et al., 2002b; Mugnai et al., 2014) and body metabolism (Branciari et al., 2009; Dransfield and Sosnicki, 1999), with implications on the health status and the oxidative stability of the meat (Castellini et al., 2006; Mancinelli et al., 2020b). Such differences seem not only related to the growing performances of animals but also to the genotype itself.
In a recent paper, Mancinelli et al. (2020a), demonstrated that the adaptability of chickens to organic system mainly depends on the genotype defined as an “independent prediction factor of adaptability,” whereas the DWG has less predictive power. Based on such evidence, DWG should be considered as a prerequisite but a “true” adaptability can be assessed only characterizing the single poultry genotype.
In the present study, the 6 SrG poultry genotypes showed important differences: first, the kinetic score was lower in the genotypes with higher growth rates (CY and M; Figures 1A and 1B), while the opposite was true for RJ, RR, and RG. Only RC showed a positive trend between kinetic scores and productive performance, indeed it showed higher live weight (Mancinelli et al., 2020a), although the kinetic behaviors resulted better than CY and M.
Such trend was probably due to the explication of kinetic actions different to the movements (walking, running), such as eating. Indeed, the positive items included in the PC1 are EA, kinetic, and eating (Table 2). In agreement, Mancinelli et al. (2020a) demonstrated that RC genotypes spent more time in the feeding action respect to other kinetic actions as walking or running.
Moreover, Wallenbeck et al. (2016) found more kinetic behavior in RR than in Ross 308. In addition, Pulcini et al. (2021), demonstrated that the same genotypes here tested, classified as walking strains (RG, RR, and RedJ), showed a less curved tibia that not-walking ones; on the same time, altered tibia shape was positively correlated with carcass and growth rate.
Comparing the oxidative scores, this research confirmed that heavier chicken strains are more sedentary than the lighter one and have better in vivo antioxidant status (respectively, about 0 vs. 20% kinetic activity; 4.50 vs. 5.80 mmol H2O2 for ROMs, Supplemental Table S1), whereas the others had a strain-specific trend (Figures 1C and 1F).
Accordingly, the cluster plots (Figures 2A and 2B) clearly showed the in vivo and postmortem trends: the heavier animals (CY and M) were grouped in the less oxidative and less kinetic shape, separately from the others. These genotypes may be classified as nonwalking chickens (NW). However, in the “Walking” (W) chickens group, not all the birds showed a better oxidative profile; thus, physical exercise in this group enhanced muscle training, reducing, as a consequence, ROMs production.
However, within W chickens RC and RG clustered separately from the other “more kinetic animals” (RJ and RR), because they showed a higher oxidative profile correlated with the movement, whereas RR and RJ demonstrated a lower ROMs production. It should be underlined that RC showed also a lower (although not significantly different) kinetic activity than RG. To explain this trend, it is possible to formalize 2 different hypotheses:
1) Walking activity is different from scavenging (often, kinetic behavior, is only related to the walking activity of birds, without including pasture intake; Mancinelli et al., 2020a);
2) Different muscular adaptation to the movement occurred: for RG and mainly RC, the walking has not training effect (ntW) whereas in RJ and RR (W) the activity resulted in training.
Mattioli et al. (2017) found that FG chickens (Ross 308) subjected to “forced” and moderate kinetic activity (approximately 4 km/day) increased the oxidative thrust of the plasma and skeletal muscles ( Dal Bosco et al., 2011) due to the high production of free radicals (Dröge, 2002). In contrast, pure breed chickens (i.e., Leghorn), which show high exploratory attitude and marked rusticity, have a better response to exercise.
Furthermore, even the technological traits and proximate composition of meat (PC 4, 5; Figures 1D and 1E) showed an unusual trend, primarily related to kinetic thrust. The main differences were recorded in the technological parameters between M and CY vs. RC, RR, and RJ, and between RJ and CY and RC and RG for the proximate composition. Considering the animals were housed under the same conditions and with the same dietary plan, the main differences in the technological parameters could be related to the genotype (intrinsic item) and movement (extrinsic item). Accordingly, the kinetic birds (RR, RJ, and RC) showed higher values of PC4 with higher redness and lower pH or L*, justified by a shift in muscle fibers from white/glycolytic to red/oxidative (Petracci et al., 2015). Similarly, proximate composition showed differing trends, predominantly in the less kinetic animals, associated with higher moisture and less protein and lipid content, probably linked to the higher live weights of the animals. The reduction in protein content coupled with moisture increase may also be an indirect index of fiber degeneration and atrophy (Petracci and Cavani, 2012).
CONCLUSIONS
The present results confirmed that the kinetic behavior, a prerequisite for the adaptation of SrG genotypes to outdoor systems, is negatively correlated with productive performance. Furthermore, our data demonstrated, for the first time, that there were opposing trends between kinetic behavior and blood (positively correlated) or meat (negatively correlated) oxidative status. Such differences are mainly due to the intrinsic response of the genotypes used. Indeed, every genotype showed a specific response to movement that may be identified as training-walking capacity.
Nevertheless, the genotypes investigated in the present study were classified as SrG, many differences regarding kinetic behavior, performance, and oxidative status were recorded. Such differences allowed to identify the least suitable genotypes (not walking); whereas, in order to define the best one, is necessary manage various aspects: that is, rearing system, pasture and/or environmental enrichments availability, diet, stocking density, slaughtering age, etc., which influence all herein evaluated parameters.
ACKNOWLEDGMENTS
Authors wish to thank Mr Giovanni Migni, for his contribute in animal handling and ungraduated students of Perugia University for their interest in following the present experimentation. Authors wish to thank breeders’ companies (Aviagen, Hubbard and Amadori to provide animals for experimentation). This research was partly funded by GESCO2020 (private fund) and TIPIBIO Projects (MIPAAF fund).
DISCLOSURES
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Footnotes
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.psj.2021.101297.
Appendix. Supplementary materials
REFERENCES
- AOAC . Association of Official Analytical Chemists. 16th ed. Sci. Educ.; Washington, DC: 1995. Official methods of analysis. [Google Scholar]
- Arakawa K., Sagai M. Species differences in lipid peroxide levels in lung tissue and investigation of their determining factors. Lipids. 1986;21:769–775. doi: 10.1007/BF02535410. [DOI] [PubMed] [Google Scholar]
- Avilés-Esquivel, D. F., M. A. Montero, H. Zurita-Vásquez, and M. Barros-Rodríguez. 2018. Animal welfare and poultry productivity: a short review. Tropical and Subtropical Agroecosystems. 21:114–123.
- Benbrook C. Org. Cent. Press; Washington, DC: 2005. Elevating Antioxidant Levels in Food through Organic Farming and Food Processing. [Google Scholar]
- Bradford M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Branciari R., Mugnai C., Mammoli R., Miraglia D., Ranucci D., Dal Bosco A., Castellini C. Effect of genotype and rearing system on chicken behavior and muscle fiber characteristics. J. Anim. Sci. 2009;87:4109–4117. doi: 10.2527/jas.2009-2090. [DOI] [PubMed] [Google Scholar]
- Bureau C., Hennequet-Antier C., Couty M., Guémené D. Gene array analysis of adrenal glands in broiler chickens following ACTH treatment. BMC Genomics. 2009;10:1–8. doi: 10.1186/1471-2164-10-430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cardinali L., Troisi A., Verstegen J.P., Menchetti L., Ngonput A.Elad, Boiti C., Canello S., Zelli R., Polisca A. Serum concentration dynamic of energy homeostasis hormones, leptin, insulin, thyroid hormones, and cortisol throughout canine pregnancy and lactation. Theriogenology. 2017;97:154–158. doi: 10.1016/j.theriogenology.2017.04.040. [DOI] [PubMed] [Google Scholar]
- Castellini C., Boggia A., Cortina C., Dal Bosco A., Paolotti L., Novelli E., Mugnai C. A multicriteria approach for measuring the sustainability of different poultry production systems. J. Clean. Prod. 2012;37:192–201. [Google Scholar]
- Castellini C., Dal Bosco A., Mugnai C., Bernardini M. Performance and behaviour of chickens with different growing rate reared according to the organic system. Ital. J. Anim. Sci. 2002;1:290–300. [Google Scholar]
- Castellini C., Dal Bosco A., Mugnai C., Pedrazzoli M. Comparison of two chicken genotypes organically reared: oxidative stability and other qualitative traits of the meat. Ital. J. Anim. Sci. 2006;5:29–42. [Google Scholar]
- Castellini C., Mugnai C., Dal Bosco A. Effect of organic production system on broiler carcass and meat quality. Meat Sci. 2002;60:219–225. doi: 10.1016/s0309-1740(01)00124-3. [DOI] [PubMed] [Google Scholar]
- Castellini C., Mugnai C., Moscati L., Mattioli S., Amato M.G., Mancinelli A.C., Dal Bosco A. Adaptation to organic rearing system of eight different chicken genotypes: behaviour, welfare and performance. Ital. J. Anim. Sci. 2016;15:37–46. [Google Scholar]
- Castellini, C., and A. Dal Bosco. 2017. Animal welfare and poultry meat in alternative production systems (and ethics of poultry meat production). Pages 335-357 in Poultry Quality Evaluation: Quality Attributes and Consumer Values. Woodhead publishing, Chennai, India.
- Celi P., Cowieson A.J., Fru-Nji F., Steinert R.E., Kluenter A.M., Verlhac V. Gastrointestinal functionality in animal nutrition and health: new opportunities for sustainable animal production. Anim. Feed Sci. Technol. 2017;234:88–100. [Google Scholar]
- Dal Bosco A., Mugnai C., Castellini C. Performance and meat quality of pure Ancona and Cornish × Ancona chickens organically reared. Arch. Geflugelkd. 2011;75:7–12. [Google Scholar]
- Dal Bosco A., Mugnai C., Mattioli S., Rosati A., Ruggeri S., Ranucci D., Castellini C. Transfer of bioactive compounds from pasture to meat in organic free-range chickens. Poult. Sci. 2016;95:2464–2471. doi: 10.3382/ps/pev383. [DOI] [PubMed] [Google Scholar]
- Dal Bosco A., Mugnai C., Ruggeri S., Mattioli S., Castellini C. Fatty acid composition of meat and estimated indices of lipid metabolism in different poultry genotypes reared under organic system. Poult. Sci. 2012;91:2039–2045. doi: 10.3382/ps.2012-02228. [DOI] [PubMed] [Google Scholar]
- Dal Bosco A., Mugnai C., Sirri F., Zamparini C., Castellini C. Assessment of a global positioning system to evaluate activities of organic chickens at pasture. J. Appl. Poult. Res. 2010;19:213–218. [Google Scholar]
- Dalle-Donne I., Rossi R., Giustarini D., Milzani A., Colombo R. Protein carbonyl groups as biomarkers of oxidative stress. Clin. Chim. Acta. 2003;329:23–38. doi: 10.1016/s0009-8981(03)00003-2. [DOI] [PubMed] [Google Scholar]
- Dransfield E., Sosnicki A.A. Relationship between muscle growth and poultry meat quality. Poult. Sci. 1999;78:743–746. doi: 10.1093/ps/78.5.743. [DOI] [PubMed] [Google Scholar]
- Dröge W. Free radicals in the physiological control of cell function. Physiol. Rev. 2002;82:47–95. doi: 10.1152/physrev.00018.2001. [DOI] [PubMed] [Google Scholar]
- European Parliament and Council of the European Union. 2013. Directive 2010/63/EU of the European Parliament and of the Council of 22 September 2010 on the protection of animals used for scientific purposes. Accessed Jan. 2020. https://ec.europa.eu/environment/archives/lab_animals/works_en.htm
- Fellenberg M.A., Speisky H. Antioxidants: their effects on broiler oxidative stress and its meat oxidative stability. Worlds. Poult. Sci. J. 2006;62:53–70. [Google Scholar]
- Folch J., Lees M., Sloane Stanley G.H. A simple method for the isolation and purification of total lipides from animal tissues. J. Biol. Chem. 1957;226:497–509. [PubMed] [Google Scholar]
- Galli F., Azzi A., Birringer M., Cook-Mills J.M., Eggersdorfer M., Frank J., Cruciani G., Lorkowski S., Özer N.K. Vitamin E: emerging aspects and new directions. Free Radic. Biol. Med. 2017;102:16–36. doi: 10.1016/j.freeradbiomed.2016.09.017. [DOI] [PubMed] [Google Scholar]
- Gálvez F., Domínguez R., Maggiolino A., Pateiro M., Carballo J., De Palo P., Barba F.J., Lorenzo J.M. Meat quality of commercial chickens reared in different production systems: industrial, range and organic. Ann. Anim. Sci. 2020;20:263–285. [Google Scholar]
- Garson, D. G. 2008. Factor analysis: Statnotes.in from North Carolina State University Public Administration Program. Accessed June 2021.http://www.stat.fsu.edu~jfrade/HOMEWORKS/STA5707/STA5707fall07/files/project/PA%20765_%20Factor%20Analysis.pdf
- He S.P., Arowolo M.A., Medrano R.F., Li S., Yu Q.F., Chen J.Y., He J.H. Impact of heat stress and nutritional interventions on poultry production. Worlds. Poult. Sci. J. 2018;74:647–664. [Google Scholar]
- Hewavitharana A.K., Lanari M.C., Becu C. Simultaneous determination of Vitamin E homologs in chicken meat by liquid chromatography with fluorescence detection. J. Chromatogr. A. 2004;1025:313–317. doi: 10.1016/j.chroma.2003.10.052. [DOI] [PubMed] [Google Scholar]
- Hofmann T., Schmucker S.S., Bessei W., Grashorn M., Stefanski V. Impact of housing environment on the immune system in chickens: a review. Animals. 2020;10:1138. doi: 10.3390/ani10071138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ke P.J., Ackman R.G., Linke B.A., Nash D.M. Differential lipid oxidation in various parts of frozen mackerel. Int. J. Food Sci. Technol. 1977;12:37–47. [Google Scholar]
- Kikusato M., Toyomizu M. Crucial role of membrane potential in heat stress-induced overproduction of reactive oxygen species in avian skeletal muscle mitochondria. PLoS One. 2013;8:e64412. doi: 10.1371/journal.pone.0064412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korkeala H., Mäki-Petäys O., Alanko T., Sorvettula O. Determination of pH in meat. Meat Sci. 1986;18:121–132. doi: 10.1016/0309-1740(86)90088-4. [DOI] [PubMed] [Google Scholar]
- Lewis P.D., Perry G.C., Farmer L.J., Patterson R.L.S. Responses of two genotypes of chicken to the diets and stocking densities typical of UK and “Label Rouge” production systems: I. Performance, behaviour and carcass composition. Meat Sci. 1997;45:501–516. doi: 10.1016/s0309-1740(96)00084-8. [DOI] [PubMed] [Google Scholar]
- Lindqvist C. PhD Thesis, IFM Biology. Linkoping University; Sweden: 2008. Domestication effects on foraging behaviour: consequences for adaptability in chickens. [Google Scholar]
- Mancinelli A.C., Mattioli S., Bosco A.D., Aliberti A., Amato M.G., Castellini C. Performance, behavior, and welfare status of six different organically reared poultry genotypes. Animals. 2020;10:550. doi: 10.3390/ani10040550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mancinelli A.C., Silletti E., Mattioli S., Bosco A.D., Sebastiani B., Menchetti L., Koot A., van Ruth S., Castellini C. Fatty acid profile, oxidative status, and content of volatile organic compounds in raw and cooked meat of different chicken strains. Poult. Sci. 2020;100:1273–1282. doi: 10.1016/j.psj.2020.10.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattioli S., Dal Bosco A., Duarte J.M.M., D'Amato R., Castellini C., Beone G.M., Fontanella M.C., Beghelli D., Regni L., Businelli D., Trabalza-Marinucci M., Proietti P. Use of Selenium-enriched olive leaves in the feed of growing rabbits: effect on oxidative status, mineral profile and Selenium speciation of Longissimus dorsi meat. J. Trace Elem. Med. Biol. 2019;51:98–105. doi: 10.1016/j.jtemb.2018.10.004. [DOI] [PubMed] [Google Scholar]
- Mattioli S., Dal Bosco A., Ruggeri S., Martino M., Moscati L., Pesca C., Castellini C. Adaptive response to exercise of fast-growing and slow-growing chicken strains: blood oxidative status and non-enzymatic antioxidant defense. Poult. Sci. 2017;96:4096–4102. doi: 10.3382/ps/pex203. [DOI] [PubMed] [Google Scholar]
- Mattioli S., Machado Duarte J.M., Castellini C., D'Amato R., Regni L., Proietti P., Businelli D., Cotozzolo E., Rodrigues M., Dal Bosco A. Use of olive leaves (whether or not fortified with sodium selenate) in rabbit feeding: effect on performance, carcass and meat characteristics, and estimated indexes of fatty acid metabolism. Meat Sci. 2018;143:230–236. doi: 10.1016/j.meatsci.2018.05.010. [DOI] [PubMed] [Google Scholar]
- Menchetti L., Barbato O., Filipescu I.E., Traina G., Leonardi L., Polisca A., Troisi A., Guelfi G., Piro F., Brecchia G. Effects of local lipopolysaccharide administration on the expression of Toll-like receptor 4 and pro-inflammatory cytokines in uterus and oviduct of rabbit does. Theriogenology. 2018;107:162–174. doi: 10.1016/j.theriogenology.2017.10.046. [DOI] [PubMed] [Google Scholar]
- Menchetti L., Curone G., Andoni E., Barbato O., Troisi A., Fioretti B., Polisca A., Codini M., Canali C., Vigo D., Brecchia G. Impact of goji berries (Lycium barbarum) supplementation on the energy homeostasis of rabbit does: uni- and multivariate approach. Animals. 2020;10:2000. doi: 10.3390/ani10112000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menchetti L., Padalino B., Fernandes F.Brasileiro, Nanni Costa L. Comparison of nonlinear growth models and factors affecting body weight at different ages in Toy Poodles. Ital. J. Anim. Sci. 2020;19:792–802. [Google Scholar]
- Michiels J., Tagliabue M.M., Akbarian A., Ovyn A., De Smet S. Oxidative status, meat quality and fatty acid profile of broiler chickens reared under free-range and severely feed-restricted conditions compared with conventional indoor rearing. Avian Biol. Res. 2014;7:74–82. [Google Scholar]
- Min B., Ahn D.U. Mechanism of lipid peroxidation in meat and meat products - a review. Food Sci. Biotechnol. 2005;14:52–163. [Google Scholar]
- Mugnai C., Sossidou E.N., Dal Bosco A., Ruggeri S., Mattioli S., Castellini C. The effects of husbandry system on the grass intake and egg nutritive characteristics of laying hens. J. Sci. Food Agric. 2014;94:459–467. doi: 10.1002/jsfa.6269. [DOI] [PubMed] [Google Scholar]
- Petracci M., Cavani C. Muscle growth and poultry meat quality issues. Nutrients. 2012;4:1–12. doi: 10.3390/nu4010001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petracci M., Mudalal S., Soglia F., Cavani C. Meat quality in fast-growing broiler chickens. Worlds. Poult. Sci. J. 2015;71:363–374. [Google Scholar]
- Pulcini, D., D. M. Zilio, F. Cenci, and C. Castellini. 2021. Differences in Tibia shape in organically reared chicken lines measured by means of geometric morphometrics. 11:101. [DOI] [PMC free article] [PubMed]
- Rauw, W. M. 2008. Pages 1–21 in Resource Allocation Theory Applied to Farm Animal Production. CABI, Wallingford, Oxfordshire, UK.
- Ponte P.I.P., Rosado C.M.C., Crespo J.P., Crespo D.G., Mourão J.L., Chaveiro-Soares M.A., Brás J.L.A., Mendes I., Gama L.T., Prates J.A.M., Ferreira L.M.A. Pasture intake improves the performance and meat sensory attributes of free-range broilers. Poult. Sci. 2008;87:71–79. doi: 10.3382/ps.2007-00147. [DOI] [PubMed] [Google Scholar]
- Righi C., Menchetti L., Orlandi R., Moscati L., Mancini S., Diverio S. Welfare assessment in shelter dogs by using physiological and immunological parameters. Animals. 2019;9:340. doi: 10.3390/ani9060340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robertson A.R. The CIE 1976 Color-Difference Formulae. Color Res. Appl. 1977;2:7–11. [Google Scholar]
- Rousseeuw P.J. Silhouettes: a graphical aid to the interpretation and validation of cluster analysis. J. Comput. Appl. Math. 1987;20:53–65. [Google Scholar]
- Schüep W., Rettenmaier R. Analysis of vitamin E homologs in plasma and tissue: high-performance liquid chromatography. Methods Enzymol. 1994;234:294–302. doi: 10.1016/0076-6879(94)34096-x. [DOI] [PubMed] [Google Scholar]
- Siegel P.B., Honaker C.F. Impact of genetic selection for growth and immunity on resource allocations. J. Appl. Poult. Res. 2009;18:125–130. [Google Scholar]
- Śliwa-Jóźwik A., Jóźwik A., KoŁataj A. Influence of exogenous glutathione (GSH), as stressfactor, on the activity of lysosome enzymes in some organs of mice. Arch. Anim. Breed. 2002;45:307–314. [Google Scholar]
- Smet K., Raes K., Huyghebaert G., Haak L., Arnouts S., De Smet S. Lipid and protein oxidation of broiler meat as influenced by dietary natural antioxidant supplementation. Poult. Sci. 2008;87:1682–1688. doi: 10.3382/ps.2007-00384. [DOI] [PubMed] [Google Scholar]
- Tarazona A.M., Ceballos M.C., Broom D.M. Human relationships with domestic and other animals: one health, one welfare, one biology. Animals. 2020;10:43. doi: 10.3390/ani10010043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ulbricht T.L.V., Southgate D.A.T. Coronary heart disease: seven dietary factors. Lancet. 1991;338:985–992. doi: 10.1016/0140-6736(91)91846-m. [DOI] [PubMed] [Google Scholar]
- Wallenbeck A., Wilhelmsson S., Jönsson L., Gunnarsson S., Yngvesson J. Behaviour in one fast-growing and one slower-growing broiler (Gallus gallus domesticus) hybrid fed a high- or low-protein diet during a 10-week rearing period. Acta Agric. Scand. A Anim. Sci. 2016;66:168–176. [Google Scholar]
- Zampiga M., Laghi L., Zhu C., Mancinelli A.C., Mattioli S., Sirri F. Breast muscle and plasma metabolomics profile of broiler chickens exposed to chronic heat stress conditions. Animal. 2021;15:100275. doi: 10.1016/j.animal.2021.100275. [DOI] [PubMed] [Google Scholar]
- Zhou, S., W. Sun, Z. Zhang, and Y. Zheng. 2014. The role of Nrf2-mediated pathway in cardiac remodeling and heart failure. Oxid. Med. Cell. Longev. 2014:260429. [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.