Abstract
Stress is a frequent precipitant of relapse to drug use. Pharmacotherapies targeting a diverse array of neural systems have been assayed for efficacy in attenuating stress-induced drug-seeking in both rodents and in humans, but none have shown enough evidence of utility to warrant routine use in the clinic. We posit that a critical barrier in effective translation is inattention to sex as a biological variable at all phases of the research process. In this review, we detail the neurobiological systems implicated in stress-induced relapse to cocaine, opioids, methamphetamine, and cannabis, as well as the pharmacotherapies that have been used to target these systems in rodent models, the human laboratory, and in clinical trials. In each of these areas we additionally describe the potential influences of biological sex on outcomes, and how inattention to fundamental sex differences can lead to biases during drug development that contribute to the limited success of large clinical trials. Based on these observations, we determine that of the pharmacotherapies discussed only α2-adrenergic receptor agonists and oxytocin have a body of research with sufficient consideration of biological sex to warrant further clinical evaluation. Pharmacotherapies that target β-adrenergic receptors, other neuroactive peptides, the hypothalamic-pituitary-adrenal axis, neuroactive steroids, and the endogenous opioid and cannabinoid systems require further assessment in females at the preclinical and human laboratory levels before progression to clinical trials can be recommended.
Keywords: Stress, Addiction, Translation, Sex, Treatment
1. Introduction
Drug addiction is often characterized by cycles of abstinence and relapse, and it is this relapsing nature of addiction that makes clinical intervention particularly challenging (McLellan et al., 2000). Stress has been identified as an important contributor to relapse (Brownwell et al., 1986; Sinha, 2001), and accordingly represents a valuable therapeutic target. In this vein, several techniques have been developed to attempt to simulate stress-induced relapse in both animal and human experimental models. These stress induction paradigms have then been used as screening tools for a vast array of pharmacological compounds, with the ultimate goal of discerning which agents might be efficacious in attenuating stress-induced relapse on a broader, clinical scale. However, despite these efforts, no such treatment has yet emerged. Positive pharmacotherapeutic outcomes observed in rodents rarely translate to humans, and even those promising findings from the human laboratory often fail to replicate in the clinic.
We posit that a significant contributor to this failure to translate is insufficient consideration for sex as a biological variable at each phase of the research process. Substantial evidence demonstrates important sex and gender differences in the behavioral, biological, and clinical correlates of substance use disorders. Across addictive substances, men tend to initiate use earlier than women, while women demonstrate an accelerated progression from initiation to use disorder and treatment entry (Diehl et al., 2007; Greenfield et al., 2010; Haas and Peters, 2000; Hernandez-Avila et al., 2004; Khan et al., 2013; Lewis and Nixon, 2014; Randall et al., 1999). Women experience greater withdrawal symptom severity in stimulant, nicotine, benzodiazepine, or cannabis dependence (Becker and Koob, 2016; Herrmann et al., 2015); experience greater withdrawal-related negative affect (Hogle and Curtin, 2006), and functional impairment (Sherman et al., 2017b); and women more often use substances to reduce negative affect (Lehavot et al., 2014; McHugh et al., 2013; Sinha et al., 2007a). In addition, women report greater subjective effects of delta-9-tetrahydrocannabinol (THC), the psychoactive component of cannabis (Cooper and Haney, 2014; Sholler et al., 2020), and oxycodone (Lofwall et al., 2012), metabolize nicotine more rapidly than men (Benowitz et al., 2006), and show higher peak plasma levels of cocaine (Lukas et al., 1996), suggesting greater abuse liability. These patterns are supported by preclinical studies, where female rodents consistently acquire self-administration more rapidly, demonstrate an accelerated shift from voluntary to compulsive drug intake, and exhibit greater withdrawal (except alcohol and opioids) and greater drug reinstatement (see Becker and Koob, 2016 for review). As such, consideration of sex and gender is critical in interpretation of both clinical and preclinical investigations of addiction pharmacotherapy.
The purpose of this review is to synthesize the available scientific literature evaluating pharmacotherapeutic strategies for the prevention of stress-induced relapse to cocaine, opioids, methamphetamine, and cannabis in both animals and humans. Of note, studies primarily concerned with treatment options for alcohol and nicotine addiction have been intentionally omitted from this work as these have been reviewed extensively elsewhere (Bruijnzeel, 2012; Greenwald, 2018; Mantsch et al., 2016; Sinha et al., 2011). We highlight the critical importance of sex and gender differences when gauging pharmacotherapeutic efficacy by illustrating how their lack of consideration may contribute to the present dearth of available addiction treatments. By doing so, we provide further support to the 2015 initiative by the National Institutes of Health requiring consideration of sex as a biological variable in both human and animal research.
To this end, we first provide a background in stress physiology, the sex differences therein, and its role in relapse. We then discuss the circuitry of several neurobiological signaling systems that have been pharmacologically targeted for their role in stress-induced relapse, including the noradrenergic, hypothalamic-pituitary-adrenal (HPA) axis, neuroactive steroidal, opioid, neuroactive peptide, and endocannabinoid systems; results of pharmacotherapeutic research targeting these signaling systems in animal, human laboratory, and clinical models; and the role of sex and gender in the generation of these outcomes. We close with recommendations for future research, including the importance of highlighting differential outcomes on the basis of biological sex and gender and promising pharmacotherapeutic targets that have yet to be fully explored.
2. Role of stress in relapse—and significance of biological sex
Stress is defined as a state of challenged homeostasis that can be alleviated through a coordinated behavioral and neuroendocrine response. While moderate and short-term activation of the stress response is healthy and adaptive, more severe and protracted stress can promote disease states (Selye, 1950), including depression, anxiety, and substance use disorders (Chrousos and Gold, 1992). The manner in which stress precipitates relapse has been shown to depend on neuroadaptations resulting from a variety of factors, including history of drug intake (Graf et al., 2013; Mantsch et al., 2008), history of stress exposure at the time of initial drug use (Mantsch and Katz, 2007), and biological sex (Doncheck et al., 2020). Herein we focus on the latter of these, as females appear not only more vulnerable to relapse than their male counterparts, but this disparity is magnified during times of stress. Females reportedly progress through the stages of addiction in a more rapid, “telescoping” manner compared to their male counterparts (Brady and Randall, 1999; Kosten et al., 1993), a sex difference which can be further exaggerated by stress. Stress can also more readily predispose females to drug dependence (Bertholomey et al., 2016; Hyman et al., 2008; Thomas and Becker, 2019) and can elicit heightened responses in females following the development of drug dependence (Back et al., 2005). Taken together, these effects may manifest as stress-induced escalation of drug intake (Holly et al., 2012).
Several key sex differences have been observed in signaling processes that involve dysregulation of the HPA axis and hyperarousal (see Fig. 1 for an overview of the HPA axis). A classic example is the higher level of corticosterone (CORT) observed in female rodents compared to males both at baseline (Bertholomey et al., 2016; Critchlow et al., 1963; Doncheck et al., 2020; Garcia-Keller et al., in press) and upon stress exposure (Critchlow et al., 1963; Doncheck et al., 2020; Lu et al., 2015). In humans, while baseline cortisol levels are typically comparable between men and women, sex differences in cortisol levels have been observed following stress exposure. These findings have been inconsistent, however, with some studies reporting higher levels in women and others reporting higher levels in men following exposure to a stressor (Kirschbaum et al., 1999; Kudielka et al., 1998; Kudielka and Kirschbaum, 2005; Seeman et al., 2001; Uhart et al., 2006). Conflicting outcomes across studies may be attributable to the use of different stressors or to participant characteristics, including hormonal status. Further supporting a human sexual dimorphism is that sex differences in cortisol levels have been reported in stress-associated neuropsychiatric disorders which have high comorbidity with substance use disorder, such as posttraumatic stress disorder or major depressive disorder (Freidenberg et al., 2010; Meewisse et al., 2007; Young, 1995; Young and Altemus, 2004). However, inconsistent outcomes overall make it difficult to determine if there are sex differences in human cortisol response to stress that are comparable to those observed for CORT in rodents.
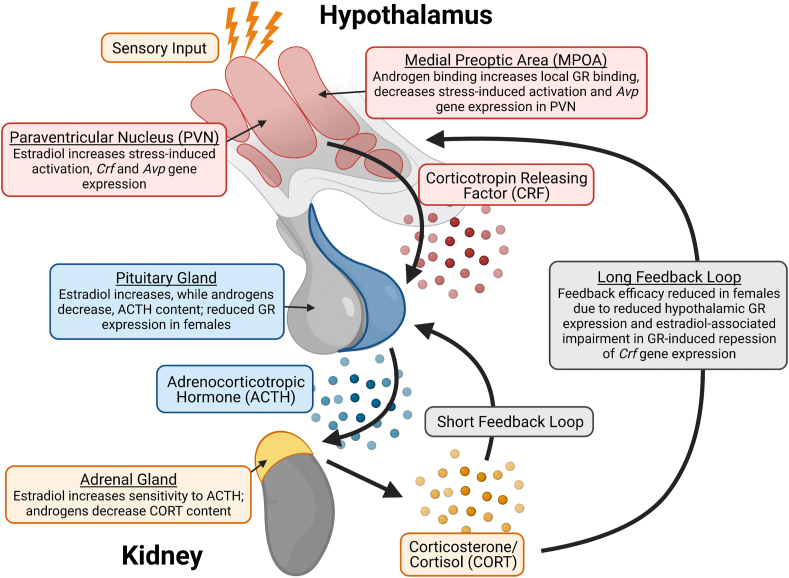
Fig. 1.
The physiological stress response is composed of signalling processes in the hypothalamic-pituitary-adrenal (HPA) axis which are modulated by sex hormones. HPA axis activation is initiated by corticotropin-releasing factor (CRF), a neuropeptide released from parvocellular neurons in the hypothalamic paraventricular nucleus (PVN). CRF targets its Gs protein-coupled CRFR1 receptor in the anterior pituitary, which stimulates adrenocorticotropic hormone (ACTH) release. ACTH is then released into circulation and targets the adrenal cortex to induce glucocorticoid production and secretion. Glucocorticoids (predominantly cortisol in humans and corticosterone in rodents) provide both rapid and delayed negative feedback to the HPA axis. Each component of the HPA axis response is modulated by sex hormones, with estrogen-mediated effects being generally stress-promoting and androgen-mediated effects being generally stress-inhibiting. GR = glucocorticoid receptor, Avp = arginine vasopressin.
Sex differences are also evident in corticotropin releasing factor (CRF) and noradrenergic (NA) signaling. These sex differences are most clearly apparent in the locus coeruleus (LC), a key brain area involved in stress responses (Fig. 2). This is a sexually dimorphic structure in which females manifest both greater volume and neuron count than do males (Pinos et al., 2001), and in which women show greater functional activation relative to men in response to emotional stimuli (Filkowski et al., 2017). On a molecular level, stress elicits strikingly divergent electrophysiological responses in LC noradrenergic neurons on the basis of sex (Bangasser et al., 2016). During stress, female LC neurons fire faster and exhibit enhanced CRF sensitivity relative to males (Curtis et al., 2006). This effect is mediated by CRF activation of its own receptor which, in females, results in greater activation of the stimulatory molecular (i.e., cAMP/PKA) pathway (Bangasser et al., 2010). In addition to this enhanced stimulatory mechanism, the sex differences in LC-mediated arousal are also linked to sex differences in CRF receptor internalization (Bangasser et al., 2010). Stress only induces LC CRF receptor internalization in males; in fact, stress may induce CRF receptor trafficking in the opposite direction in females. These internalization differences may be related to sex differences in CRF receptor association with β-arrestin2, which has increased co-localization in male, but not female, brains (Bangasser et al., 2010). Together, this body of literature suggests significant sex differences in CRF receptor activity, which may enhance local activity as can be observed in LC noradrenergic neurons. The differences in LC neuronal firing may lead to increased arousal in females following stress, which, if dysregulated, could promote maladaptive behaviors (Bangasser and Valentino, 2014).
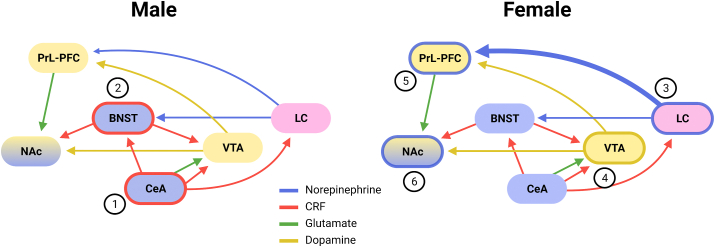
Fig. 2.
Sex differences in overlapping stress and reward circuitry. The extended amygdala (in blue) is a central system involved in stress and addiction pathology. The central nucleus of the amygdala (CeA) receives direct innervation from the paraventricular nucleus of the hypothalamus (PVN, not shown). (1) Within this nucleus, neurons are more responsive to corticotrophin releasing factor (CRF) in males relative to females. (2) The extended amygdala also includes the bed nucleus of the striatal terminals (BNST), an area in which stress effects are increased in males relative to females, perhaps due to differences in brain-derived neurotrophic factor (BDNF). (3) The locus coeruleus (LC) is one of the most well-documented sexually dimorphic brain areas (in pink) and is a source of noradrenergic and CRF signaling. LC neurons fire faster and have an increased responsiveness to CRF in females. Sex differences in CRF receptor trafficking also increases LC signaling to the prelimbic prefrontal cortex (PrL-PFC). The ventral tegmental (VTA), nucleus accumbens (NAc), and PrL-PFC are canonically considered part of the reward system (in yellow) and interface with the extended amygdala. (4) In the VTA, stress reduces the number of spontaneously firing dopamine neurons, whereas estradial enhances dopamine firing in females. Increased motivation in females is attributed to sex differences in dopamine regulation. (5) Both the LC and the VTA innervate the PrL-PFC. Estrogens are thought to amplify stress responses in females in this area. (6) In the NAc, estradiol promotes drug reward and modulates synaptic physiology. Bolded outlines indicate areas with documented sexual dimorphisms. Bolded arrows indicate greater signaling of projections to targeted brain area. (For interpretation of the references to colour in this figure legend, the reader is referred to the Web version of this article.)
There are also indications that gonadal hormones may produce differences in stress signaling and reactivity that contribute to drug seeking (Goldstein et al., 2010; Handa and Weiser, 2014). In particular, it appears that circulating levels of the primary estrogen 17β-estradiol typically co-vary with stress reactivity in females, such that higher levels of 17β-estradiol coincide with greater stress reactivity (Doncheck et al., 2020; Feltenstein et al., 2011; Shansky et al., 2003). Higher levels of 17β-estradiol are also associated with enhanced propensity for relapse (Doncheck et al., 2018; Feltenstein et al., 2011; Larson et al., 2007). Conversely, higher levels of progestins coincide with diminished stress reactivity and relapse propensity in females (Feltenstein et al., 2011; Sinha et al., 2007a). While these interactions have been demonstrated in females, the influence of these hormones has not been thoroughly contrasted between the sexes. Moreover, while some studies indicate an interaction with stress reactivity (Knight et al., 2017; Lund et al., 2006), the effects of “male” sex hormones such as testosterone and dihydroxytestosterone in stress-associated relapse are wholly understudied. This lack of research may be attributable to the historical perspective that only “female” sex hormones underlie observed sex differences—a perspective which has recently come under fire as sexist (Shansky, 2019). While females do show enhanced relapse vulnerability relative to males (for review, see Becker and Hu, 2008), fluctuations in sex hormones represent only one of several potential explanations for why females differ from “norms” set by largely male-centric research, as we have already highlighted in this section. That said, sex hormones certainly influence stress signaling and stress-associated relapse through a wide variety of signaling mechanisms. While therefore an important area of research for both sexes, complete synthesis of such information is beyond the scope of this review.
3. Sex differences in stress and motivation neural circuitry
While interactions between circuits governing stress and motivated behaviors may have initially developed to promote adaptive coping responses to stressors, in substance use disorders stress can hijack these circuit interactions to promote drug seeking. Several different pathways have been shown to contribute to stress-triggered relapse (see Mantsch et al., 2016), but key stress signaling processes such as NA and CRF signaling generally integrate within the extended amygdala, a region composed of the bed nucleus of the stria terminalis (BNST), central amygdala (CeA), and nucleus accumbens (NAc)-shell. Inactivation studies show each of these subregions are required for stress-triggered drug seeking (McFarland et al., 2004), and efferents from each subregion are active during stress-triggered drug seeking (Briand et al., 2010). These activated efferents interface with the ventral tegmental area (VTA) (Briand et al., 2010), a major node within the motivation circuitry and first component of the mesocorticolimbic pathway. In this pathway, the VTA provides the critical dopaminergic input to the prelimbic prefrontal cortex (PrL-PFC)—a region homologous with the human dorsal anterior cingulate cortex (van Heukelum et al., 2020)—that is necessary for stress-induced reinstatement in rodents (Vranjkovic et al., 2018).
Further characterization of sex differences in the neural circuits governing stress and motivated behavior is still required. Much of the research conducted with females relies upon circuits defined in males, yet some key sex differences have still been observed within this circuitry. As mentioned in the previous section, the most well-characterized is the interaction between CRF and NA signaling in the LC, which facilitates NA output to the PrL-PFC in female but not male rodents (Bangasser et al., 2010; Bangasser and Valentino, 2014; Curtis et al., 2006). Interestingly, CRF conversely increases activation of CeA neurons to a greater extent in males relative to females, highlighting that sex differences in signaling are not generalizable across brain regions (Agoglia et al., 2020). Activity in the BNST may also enhance the effect of stress exposure in males but not females (Bangasser et al, 2005, 2016; Bangasser and Wicks, 2017), and females show greater PrL-PFC sensitivity (Shansky et al., 2003) and reactivity (Bland et al., 2005) to stressors. Finally, sex differences in how the VTA regulates dopamine dynamics may drive enhanced motivation in females relative to males (Calipari et al., 2017). These differences in signaling and circuit activation across sexes provide a framework upon which stress-targeting pharmacotherapeutics may produce differential outcomes by sex, which are elaborated upon further in the following sections.
4. Noradrenergic targets
Noradrenergic signaling plays a critical role in stress-induced drug seeking, particularly in the BNST and the extended amygdala. Both of these structures receive the majority of their noradrenergic input from the lateral tegmentum via the α1-and α2-adrenergic neurons that comprise the ventral noradrenergic bundle (Aston-Jones et al., 1999), and the BNST additionally receives glutamatergic input from the parabrachial nucleus that is regulated by noradrenergic signaling (Fetterly et al., 2019). This noradrenergic signaling at alpha receptor subtypes has been shown to be integral in stress-induced reinstatement behavior independent of LC involvement (Shaham et al., 2000; Wang et al., 2001). Beta-adrenergic signaling has also been implicated in stress-induced reinstatement: a β1/β2 antagonist combination infused into either the BNST or the CeA was sufficient to block stress-induced reinstatement in rats (Leri et al., 2002), and this observation has since been further narrowed to be a consequence of β2-adrenergic receptor (AR) antagonism in the vBNST (Vranjkovic et al., 2014). These β2-ARs regulate vBNST CRF projections to the VTA, and are critical for stress-induced reinstatement of drug-seeking behavior (McReynolds et al., 2014; Vranjkovic et al., 2014). Given this clearly delineated circuitry, it logically follows that pharmacologic manipulations of noradrenergic signaling have been mostly restricted to α2-AR agonists or β2-AR antagonists.
4.1. Animal
Alpha2-AR agonists such as clonidine, lofexidine, and guanabenz, which functionally inhibit noradrenergic signaling, all attenuate footshock-induced reinstatement of cocaine seeking in rodents (see Table 1). These results are not generalizable to cocaine-induced reinstatement (Erb et al., 2000), nor do they generalize to treatments that act primarily at α1-ARs (Mantsch et al., 2010). Comparably, inhibition of noradrenaline synthesis using the dopamine beta-hydroxylase inhibitor, nepicastat, suppresses both footshock stress- and yohimbine-induced reinstatement (Schroeder et al., 2013). Similar results are yielded when testing for effects of α2-AR agonists on heroin-cocaine mixtures (lofexidine, clonidine) (Highfield et al., 2001) or heroin alone (clonidine) (Shaham et al., 2000), indicating that the noradrenergic neurochemistry implicated in stress-induced drug-seeking may be similar across these drug classes. Anxiety associated with withdrawal, which predicts subsequent reinstatement of cocaine-seeking, is also attenuated by α2-AR activation by guanfacine (Buffalari et al., 2012b).
Table 1.
Pharmacotherapy for stress-induced reinstatement across translational level and in consideration of biological sex.
| Intervention Class |
Drug of Abuse |
Preclinical |
Human Laboratory |
Clinical Trial |
Citations |
||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Efficacy | % Female (Pooled) | Sex Effect | Efficacy | % Female (Pooled) | Sex Effect | Efficacy | % Female (Pooled) | Sex Effect | |||
| Noradrenergic Targets | |||||||||||
| α-2 Adrenergic Receptor Agonist | Cocaine | Yes | 0% | – | Mixed | 19% | Yes ♀ | (Buffalari et al., 2012a, Buffalari et al., 2012b; Erb et al., 2000; Fox et al., 2012, Fox et al., 2014, a ; Jobes et al., 2011; Mantsch et al., 2010; Moran-Santa Maria et al., 2015) | |||
| Opioid | Yes | 0% | – | No | 19% | – | (Krupitsky et al., 2013; Shaham et al., 2000) | ||||
| Cocaine/Opioid | Yes | 0% | – | Highfield et al. (2001) | |||||||
| Cannabis | No | 20% | – | (Haney et al, 2008, 2019; Holst et al., 2019) | |||||||
| + Naltrexone | Opioid | Yes | 17% | – | Mixed | 16% | – | (Krupitsky et al., 2013; Sinha et al., 2007b) | |||
| + Dronabinol | Cannabis | Yes | 0% | – | No | 31% | – | (Haney et al., 2008; Levin et al., 2016) | |||
| + Buprenorphine | Opioid | Yes | 22% | – | Kowalczyk et al. (2015) | ||||||
| α-1 Adrenergic Receptor Agonist | Cocaine | No | 0% | – | Mantsch et al. (2010) | ||||||
| β-1 Adrenergic Antagonist | Cocaine | No | 0% | – | (Mantsch et al., 2010; Vranjkovic et al., 2012) | ||||||
| β-2 Adrenergic Antagonist | Cocaine | Yes | 0% | – | (Mantsch et al., 2010; McReynolds et al., 2014; Vranjkovic et al., 2012) | ||||||
| β-1/β-2 Adrenergic Antagonist | Cocaine | Yes | 0% | – | (Kampman et al, 2001, 2006; Mantsch et al., 2010) | ||||||
| Opioid | Yes | 0% | – | Zhao et al. (2010) | |||||||
| + Amantadine | Cocaine | No | 31% | – | Kampman et al. (2006) | ||||||
| Dopamine β-Hydroxylase Inhibitor | Cocaine | Yes | 0% | – | Schroeder et al. (2013) | ||||||
| Norepinephrine Reuptake Inhibitor | Cannabis | No | 23% | – | Tirado et al. (2008) | ||||||
| + Buprenorphine | Cocaine/Opioid | Yes | 34% | – | Kosten et al. (2003) | ||||||
| HPA Axis Targets | |||||||||||
| CRFR1/CRFR2 Antagonist | Cocaine | Yes | 0% | – | (Brown et al., 2009; Erb et al., 1998; Lu et al., 2001) | ||||||
| CRFR1/CRFR2 Antagonist | Opioid | Yes | 0% | – | (Shaham et al., 1997; Shalev et al., 2006) | ||||||
| Methamphetamine | Yes | 0% | – | Nawata et al. (2012) | |||||||
| CRFR1 Antagonist | Cocaine | Yes | 0% | – | (Lu et al., 2001; McReynolds et al., 2014; Shaham et al., 1998; Twining et al., 2015; Vranjkovic et al, 2014, 2018) | ||||||
| Opioid | Yes | 0% | – | Shaham et al. (1998) | |||||||
| CRFR2 Antagonist | Cocaine | No | 0% | – | Lu et al. (2001) | ||||||
| Cortisol Synthesis Inhibitor | Opioid | No | 0% | – | Shaham et al. (1997) | ||||||
| + Oxazepam | Cocaine | Yes | 35% | – | Kablinger et al. (2012) | ||||||
| Neuroactive Steroidal Targets | |||||||||||
| Progesterone Receptor Agonist | Cocaine | Mixed | 43% | Yes ♀ | Yes | 100% | – | (Fox et al., 2013; Yonkers et al., 2014) | |||
| Cannabis | Yes | 100% | – | Sherman et al. (2019) | |||||||
| + Methadone | Cocaine | No | 0% | – | Sofuoglu et al. (2007) | ||||||
| Progesterone Receptor Antagonist | Cocaine | No | 0% | – | Graf et al. (2013) | ||||||
| Opioid | Yes | 0% | – | Karimi et al. (2014) | |||||||
| Allopregnanolone | Cocaine | Mixed | 91% | Yes ♀ | Yes | 37% | No | (Anker and Carroll, 2010; Milivojevic et al., 2016; Regier et al., 2014) | |||
| Opioidergic Targets | |||||||||||
| κ Opioid Receptor Antagonist | Cocaine | Yes | 0% | – | (Aldrich et al, 2009, 2013; Beardsley et al, 2005, 2010; Carey et al., 2007; Graziane et al., 2013; Grimwood et al., 2011; McLaughlin et al., 2003; Redila and Chavkin, 2008) | ||||||
| κ Opioid Receptor Antagonist | Opioid | Yes | 0% | – | Sedki et al. (2015) | ||||||
| μ Opioid Receptor Agonist | Cocaine/Opioid | No | 0% | – | Leri et al. (2004) | ||||||
| μ Opioid Receptor Antagonist | Opioid | No | 0% | – | No | 21% | – | (Hyman et al., 2007; Sedki et al., 2015; Shaham and Stewart, 1996) | |||
| Cannabis | Yes | 42% | – | Notzon et al. (2018) | |||||||
| + Buprenorphine/Naloxone | Cocaine/Opioid | Mixed | 22% | – | Ling et al. (2016) | ||||||
| κ/μ Opioid Receptor Antagonist | Cocaine/Opioid | No | 0% | – | Sorge et al. (2005) | ||||||
| Neuropeptidergic Targets | |||||||||||
| Oxytocin Receptor Agonist | Cocaine | Yes | Not reported | – | Mixed | 37% | Yes ♀ | (Ferrer-Pérez et al., 2019; Flanagan et al., 2015; Sherman et al., 2020b) | |||
| Opioid | Yes | 0% | – | Zanos et al. (2014) | |||||||
| Cannabis | Mixed | 46% | Yes ♂ | Yes | 38% | – | (McRae-Clark et al., 2013; Reed et al., 2019; Sherman et al., 2017a) | ||||
| Methamphetamine | Yes | 28% | Yes ♀ | No | 0% | – | (Cox et al., 2013; Everett et al., 2020; Han et al., 2014; Qi et al., 2009b; Stauffer et al., 2020) | ||||
| + Methadone | Cocaine/Opioid | Yes | 50% | – | Stauffer et al. (2016) | ||||||
| AVP V1b Receptor Antagonist | Opioid | Yes | 0% | – | Zhou et al. (2008) | ||||||
| NPY5 Receptor Antagonist | Opioid | Yes | 0% | – | Maric et al. (2011) | ||||||
| NPY1 Receptor Antagonist | Opioid | No | 0% | – | Maric et al. (2011) | ||||||
| NPS Receptor Antagonist | Cocaine | Yes | 0% | – | Schmoutz et al. (2012) | ||||||
| NK-1 Receptor Antagonist | Cocaine | Yes | 0% | – | Schank et al. (2014) | ||||||
| PAC1 Receptor Antagonist | Cocaine | Yes | 0% | – | Miles et al. (2018) | ||||||
| Cannabinoid Targets | |||||||||||
| CB1 Receptor Inverse Agonist | Cocaine | Yes | 6% | No | (De Vries et al., 2001; Doncheck et al., 2020; Kupferschmidt et al., 2012; McReynolds et al., 2018, 2016; Vaughn et al., 2012) | ||||||
| DAGL Inhibitor | Cocaine | Yes | 0% | – | McReynolds et al. (2018) | ||||||
| FAAH Inhibitor | Cocaine | Yes | 0% | – | Chauvet et al. (2014) | ||||||
| Cannabis | Yes | 0% | – | D'Souza et al. (2019) | |||||||
| Methamphetamine | No | 0% | – | Nawata et al. (2019) | |||||||
| MAGL Inhibitor | Methamphetamine | Yes | 0% | – | Nawata et al. (2019) | ||||||
| Cannabidiol | Cocaine | Yes | 0% | – | (Calpe-López et al., 2021; Gonzalez-Cuevas et al., 2018) | ||||||
| Cannabis | Mixed | 26% | No | (Freeman et al., 2020; Solowij et al., 2018) | |||||||
Notes. Efficacy: “yes” if more than 60% of primary outcomes for that intervention/drug of abuse combination indicated efficacy across included studies, “mixed” if between 40 and 60% of primary outcomes indicated efficacy across included studies, “no” if less than 40% of primary outcomes indicated efficacy across included studies.
Sex effect: “yes” if reported in any of the studies for that intervention/drug of abuse combination, “no” if sex effect assessed but determined absent, "-" if not examined in any included studies. “♂” indicates superior efficacy in males, “♀” indicates superior efficacy in females.
Overlapping participants in both studies; larger cohort was used to calculate % female.
Study did not disclose how many animals were used, though they were all male; study was not included in % female calculation.
Stress-induced reinstatement of cocaine-seeking can also be inhibited by antagonism of beta-adrenergic receptors. This is evidenced following systemic administration of the dual β1/β2-AR antagonist propanolol (Mantsch et al., 2010) and infusion of the β2-AR antagonist ICI-118,551 (Mantsch et al., 2010; McReynolds et al., 2014; Vranjkovic et al., 2012), but not the β1-AR antagonist betaxolol (Mantsch et al., 2010; Vranjkovic et al., 2012). Yet, despite consistent findings that inhibition of noradrenergic signaling via both alpha and beta receptor subtypes can block stress-induced reinstatement or attenuate withdrawal-induced anxiety in male rodents, these positive effects have yet to be extended to females.
4.2. Human
Research examining the impact of noradrenergic interventions on stress-induced drug craving in both sexes has been conducted in the human laboratory, most notably in the context of cocaine use disorder. The α2-AR agonist clonidine effectively attenuates cocaine craving in response to stressful imagery (Jobes et al., 2011). Guanfacine, in contrast, has generated more mixed results: in an initial study incorporating stress and drug imagery, guanfacine reduced cue-induced, but not stress-induced, cocaine craving and anxiety, with no effect on negative affect or cardiovascular outcomes (Fox et al., 2012). This net negative outcome was a product of conflicting sex-specific effects of guanfacine; women that received guanfacine treatment reported attenuated cocaine craving, anxiety, and negative affect following all imagery scripts versus placebo, while men that received guanfacine conversely reported relatively greater cocaine craving (Fox et al., 2014). Unfortunately, guanfacine had no apparent effect on social stress reactivity regardless of sex in a larger study of men and women with cocaine dependence (Moran-Santa Maria et al., 2015). These disparate outcomes may be a product of methodological differences, including medication dosing regimen prior to test and method of stress induction (i.e. imagery versus a social stressor). Notably, different methods of stress induction are also uniquely sensitive to sex differences (Fig. 3): women typically demonstrate an enhanced response to social stressors relative to men, while the opposite is true for stressful imagery.
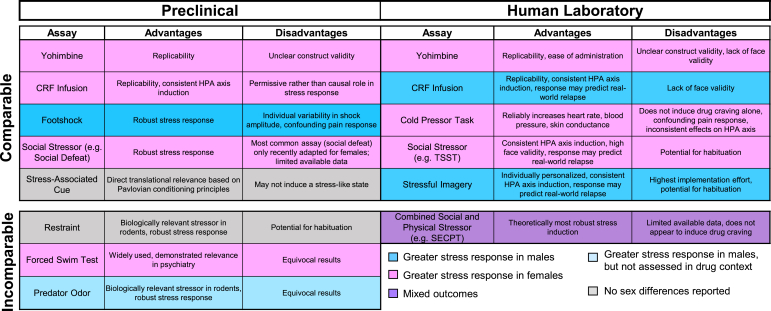
Fig. 3.
Methods of stress induction. Multiple methods can be used to promote stress-induced reinstatement of drug seeking in human and animal models. Methods vary in translational relevance, elements of the stress response being probed, and in which sex a greater stress response is often produced. These factors should be considered in both the design and interpretation of research into stress-induced relapse.
Noradrenergic approaches have also been assessed for their impact on stress-induced cannabis and opioid use in the human laboratory. One week of lofexidine treatment was found to reduce the likelihood of men engaging in a laboratory model of cannabis relapse (Haney et al., 2008). Also, while lofexidine alone did not decrease cannabis craving, this effect was achieved when combined with 60 mg THC. Later research in people of both sexes that use cannabis, however, found no effect of guanfacine on craving, withdrawal-precipitated anxiety, or relapse-like behavior (Haney et al., 2019; Holst et al., 2019). In people with opioid dependence, combined lofexidine and naltrexone treatment attenuated imagery stress-induced craving (Sinha et al., 2007b). This effect was likely driven by lofexidine, as naltrexone has been shown to have little efficacy for stress-induced relapse on its own (Hyman et al., 2007; Sinha et al., 2007b). Finally, Zhao et al. (2010) conducted three studies in abstinent men with heroin dependence. These studies demonstrated that social stress exposure or exogenous cortisol administration enhanced recall of heroin-related words, and that this effect was attenuated by 40 mg propranolol, but not placebo (Zhao et al., 2010). The effects of β-AR antagonists on laboratory stress-induced craving or relapse behavior have not been assessed in women of any substance-using population.
Noradrenergic treatment efficacy in clinical trials has been dependent on the addictive substance of concern. In the clinical trial segment of the aforementioned lofexidine/naltrexone study, the intervention group had higher rates of urine-verified opioid abstinence (Sinha et al., 2007b). A much larger trial found no effect of guanfacine with or without adjunct naltrexone on abstinence, but these participants reported reduced stress and opioid craving relative to those receiving placebo at later points in the study (Krupitsky et al., 2013). Likewise, a study of people with opioid dependence maintained on buprenorphine found that while clonidine had no effect on overall relapse rate, it prolonged the time until first opioid-positive urine sample and partially dissociated stress from drug craving (Kowalczyk et al., 2015). In buprenorphine-maintained people that also used cocaine, highest rates of urine-verified abstinence from cocaine and opioids were observed in the group randomized to combined desipramine (a norepinephrine reuptake inhibitor) and contingency management, though curiously this difference was not replicated in measures of self-reported abstinence (Kosten et al., 2003).
In non-opioid-using populations, noradrenergic drugs have appeared less effective at attenuating relapse. Propranolol reduced relapse rates in people with severe cocaine withdrawal symptoms, but there was no difference from placebo in those with mild or moderate symptoms (Kampman et al., 2001). Propranolol also had no effect on anxiety or cocaine craving, even when stratifying by withdrawal symptom severity (Kampman et al., 2001). The effect of propranolol on abstinence was then lost in a larger trial of only people with severe cocaine withdrawal, and was not rescued by additional amantadine treatment (Kampman et al., 2006). Sex differences were not assessed in any of these cocaine treatment trials, including those that involved comorbid opioid use. This may contribute to the lack of positive findings, particularly for β-AR antagonists, as controlled human laboratory and preclinical studies have thus far only demonstrated efficacy of these drugs in attenuating stress-induced relapse in men and male rodents. In people that use cannabis, atomoxetine, an inhibitor of the norepinephrine transporter, increased self-reported abstinence, but side effects were so frequent and unpleasant that the drug was not assessed further (Tirado et al., 2008). Levin et al. (2016) also attempted to expand on the laboratory findings reported in Haney et al. (2008), comparing the combined effects of lofexidine and dronabinol with a placebo in promoting abstinence from cannabis. This treatment had no effect on abstinence rates or withdrawal symptoms in the larger sample that included women.
4.3. Conclusions
Alpha2-AR agonists consistently attenuate stress-induced relapse in animal models across addictive substances. This efficacy translates to the human laboratory in individuals that use cocaine or opioids, where it appears to be modulated by biological sex; namely, women appear to receive more benefit from α2-AR agonist treatment than men. This may be opposite in people that use cannabis, where the only laboratory study with positive outcomes enrolled exclusively male participants (Haney et al., 2008). Clinical trials demonstrate limited to mixed efficacy across substances, but conclusions were drawn in the absence of attention to sex differences shown to be critical in the human laboratory. Inclusion of female participants in these clinical trials was relatively low and individuals that use predominantly cocaine, rather than co-use of cocaine and opioids, have not been directly assessed. With the current strength of preclinical and human laboratory evidence, we believe further clinical trials examining the efficacy α2-AR agonists, particularly as a function of sex, are warranted.
Beta-AR antagonists, particularly those that target β2, show limited preliminary evidence of efficacy. However, given the interesting albeit exclusively male preclinical and human laboratory work, these should be examined more specifically in rodent models of stress-induced reinstatement and in women in the human laboratory before conducting additional clinical trials.
5. HPA axis targets
Given its role in precipitating HPA axis signaling, CRF has been a subject of extensive study in the context of stress-induced drug seeking. CRF receptor activation appears to occur downstream of noradrenergic stimulation (Brown et al., 2009), and activation of these CRF receptors has been shown to be integral in stress-induced drug seeking (Blacktop et al., 2011; Chen et al., 2014; Vranjkovic et al., 2014; Wang et al., 2005 but see Wang, You, Rice and Wise, 2007). The CeA has CRF projections to both the BNST (Erb et al., 2001) and the VTA (Rodaros et al., 2007), and upregulated CRF receptor expression and CRF release have been observed within the amygdala during cocaine withdrawal (Richter and Weiss, 1999; Zorrilla et al., 2001). The BNST, in turn, contains both CRF interneurons (Erb et al., 2001) and makes CRF projections to the VTA (Rodaros et al., 2007) that have been implicated in stress-induced drug seeking. The paraventricular nucleus of the hypothalamus (PVN) also makes CRF-containing projections to the VTA (Rodaros et al., 2007). VTA dopaminergic release is regulated by CRF (Wanat et al., 2013; Wang et al., 2005), and CRF receptor-regulated dopaminergic projections to the prelimbic cortex are necessary for stress-induced reinstatement of cocaine seeking in rodents. CRF receptor expression is also upregulated in these neurons following extended access to cocaine (Vranjkovic et al., 2018). Taken together, these mechanistic insights suggest that CRF receptor antagonism may present a promising strategy for reducing stress-induced relapse.
5.1. Animal
Acute administration of CRF reinstates cocaine seeking in male and female rats with a high degree of individual variability, though females overall show greater sensitivity (Buffalari et al., 2012a). Not surprisingly, administration of nonspecific CRF receptor antagonists (Astressin, α-helical CRF, D-Phe CRF12–41) consistently block stress-induced reinstatement of cocaine (Brown et al., 2009; Erb et al., 1998; Lu et al., 2001), methamphetamine (Nawata et al., 2012), and heroin seeking (Shaham et al., 1997; Shalev et al., 2006). This is likely through CRF receptor 1 (CRFR1) activity: specific CRFR1 antagonists block stress-induced reinstatement of cocaine-conditioned place preference (Lu et al., 2001; McReynolds et al., 2014; Shaham et al., 1998; Vranjkovic et al, 2014, 2018) while a specific CRFR2 antagonist (AS-30) fails to do so (Lu et al., 2001). Finally, CRF-mediated activation of VTA dopaminergic projections to the NAc provide a mechanism for quinine stress-induced reinstatement of cocaine seeking, and this is abolished following infusion of the CRFR1 antagonist CP-376395 into the VTA (Twining et al., 2015). It thus appears from the animal literature that CRFR1, but not CRFR2, antagonists present an ideal candidate for therapeutic development. Conversely, neither acute nor chronic administration of the CORT synthesis inhibitor metyrapone impacted footshock-induced reinstatement of heroin seeking in male rats (Shaham et al., 1997).
As with noradrenergic interventions discussed above, none of the treatments listed herein have been assessed in females. This is despite the fact that females purportedly have greater HPA axis response to stress than males and may thus serve to benefit the most from treatments that target this system.
5.2. Human
Research endeavors targeting CRF or the HPA axis have largely been halted following the failed translation of CRFR1 antagonists to attenuate stress-induced craving in individuals with alcohol dependence (Kwako et al., 2015; Schwandt et al., 2016), in spite of the overwhelmingly positive findings in the preclinical literature. It is therefore presently unclear if this lack of efficacy generalizes to other addictive substances in humans. In contrast, combined administration of oxazepam, a benzodiazepine, and metyrapone shows preliminary indication of clinical efficacy. Results from a small pilot study indicated that oxazepam/metyrapone treatment may effectively reduce cocaine craving, use, and anxiety in a cocaine-dependent population (Kablinger et al., 2012). The effect of this combination on stress-induced drug craving has not yet been directly assessed in humans, however, and outcomes may instead be associated with attenuation of cue-associated relapse, as has been observed in male rodents (Keller et al., 2013). Human laboratory research examining the impact of oxazepam/metyrapone on stress response may provide mechanistic insight into the positive findings observed thus far.
5.3. Conclusions
The failure of CRFR1 antagonists to translate from rodents to humans thus far has proven disappointing. There remains the possibility that the severe anxiety present in individuals enrolled in these studies negatively impacted outcomes (Kwako et al., 2015; Schwandt et al., 2016), as Schwandt and colleagues successfully demonstrated blockade of HPA axis response via the CRFR1 antagonist, vecuferont, in women. Notably, CRF increases activation of CeA neurons in male, but not female, rodents (Agoglia et al., 2020), suggesting a potential role of sex differences in outcomes. Efficacy may also differ by participant drug of choice given only people with alcohol dependence were assessed, though outcomes in animals were positive across drug classes. Of note, oxazepam/metyrapone showed promise in humans with cocaine dependence (Kablinger et al., 2012) despite unimpressive outcomes in heroin-dependent animals (Shaham et al., 1997). This may be due to the different substances assessed or because these studies focused on different outcomes: Kablinger and colleagues (Kablinger et al., 2012) only assessed general outcomes related to cocaine use and withdrawal in an outpatient setting, whereas Shaham and colleagues (Shaham et al., 1997) sought an effect on stress-induced reinstatement specifically. Human researchers should look to clarify if the improved abstinence rate associated with oxazepam/metyrapone is a result of an effect on stress or on other factors, such as cue-induced craving.
6. Neuroactive steroidal targets
Neuroactive steroids are synthesized in the brain and peripheral tissues from cholesterol or steroidal precursors. Stress and drug seeking can regulate their concentrations and, in turn, neuroactive steroids can increase concentrations of neuromodulators (e.g., dopamine and serotonin) (Maayan et al., 2006) and modulate receptors within the reward circuitry (e.g., GABAA, NMDA, and sigma-1) (for review, see Yadid Gal et al., 2010). Allopregnanolone (ALLO) and allotetrahydrodeoxycorticosterone (THDOC), neuroactive steroid metabolites of progesterone and deoxycorticosterone respectively, act as GABAA-receptor allosteric modulators (Crawley et al., 1986; Majewska et al., 1986), and have been directly implicated in the stress response (Barbaccia et al., 1996, Barbaccia et al., 1997; Herrera et al., 2016; Purdy et al., 1991; Reddy and Rogawski, 2002). Progesterone, likely via ALLO, may also interact with CRF signaling (Torres et al., 2001; Toufexis et al., 2004).
6.1. Animal
Relapse vulnerability in female rodents covaries with the estrous cycle, such that peak circulating levels of progesterone appear protective against relapse (Feltenstein and See, 2007; Larson et al., 2007), especially within the context of stress (Doncheck et al., 2020). Conversely, low progesterone has been associated with increased cocaine seeking (Doncheck et al., 2020; Feltenstein and See, 2007; Kippin et al., 2005). These effects of progesterone may be mediated by its 5α-reductase metabolite ALLO (Anker et al., 2009; Anker and Carroll, 2010; Frye et al., 2006; Regier et al., 2014). There is also some evidence of sex-specificity for the effects of progesterone (i.e. superior efficacy in females) (Anker and Carroll, 2010; Evans and Foltin, 2006; Fox et al., 2013; Russo et al., 2010; Sinha et al., 2007a; Sofuoglu et al., 2007), but research in males has been severely limited to this point. This lack of interest in progestin-mediated relapse protection in males may be predicated on the biased assumption that progestins are only relevant to females, even though these hormones are present in both sexes. In support of the notion that progesterone is critical for stress-induced drug-seeking in males as well as females, the dual glucocorticoid/progesterone receptor antagonist, mifepristone (RU38486), has been assessed for its impact on drug-seeking behavior. Mifepristone administration was shown to block forced swim stress-induced reinstatement of morphine seeking (Karimi et al., 2014), but not footshock stress-induced reinstatement of cocaine seeking (Graf et al., 2013), in male rats. It is unclear if these divergent outcomes are attributable to the addictive substance or to the method of stress induction, and if effects of mifepristone on drug-seeking are attributable to progesterone or glucocorticoid antagonism; further research is necessary to clarify these points.
More specific approaches targeting enzymatic conversion may also serve to elucidate the role of glucocorticoids or progestins, as glucocorticoids are metabolites of progesterone. Related to this, the androgen testosterone—which is also derived from progesterone—has been shown to reduce cocaine seeking in female rhesus monkeys (Mello et al., 2011). However, as testosterone can be converted by aromatase into the primary estrogen 17β-estradiol which promotes drug seeking (Doncheck et al., 2020; 2018; Feltenstein et al., 2011; Feltenstein and See, 2007; Kippin et al., 2005; Larson et al., 2005; Terner and de Wit, 2006), approaches inhibiting progesterone-derived synthesis (i.e., 17β-hydroxysteroid dehydrogenase) and estradiol-yielding aromatization may be necessary to isolate effects of testosterone on drug seeking.
6.2. Human
Cocaine-dependent women with higher levels of endogenous progesterone consistently report reduced stress-induced drug craving compared to those with lower progesterone (Sherman et al., 2020a; Sinha et al., 2007a), but inconsistencies exist in the effects of menstrual cycle phase on opioid and cannabis use (Moran-Santa Maria et al., 2014; Sherman et al., 2020a). Importantly, these studies in opioid- or cannabis-using participants did not examine the role of stress in craving for or relapse to these addictive substances. It is possible that in these populations, as in women that use tobacco, attenuation of HPA axis response to a stressor by progesterone is uncoupled from an effect on craving (Nakajima et al., 2019). It remains unknown if this uncoupling from HPA axis response extends further to an effect on relapse, and to which substances this may be specific.
However, given the demonstrated efficacy of endogenous progesterone to attenuate stress-induced craving for cocaine, it follows that the majority of the research to date involving administration of exogenous progesterone has been conducted with individuals that use cocaine. In the absence of a stressor, exogenous progesterone does not reduce desire for or self-administration of cocaine following an acute cocaine challenge (Evans and Foltin, 2006; Reed et al., 2011; Sofuoglu et al., 2004), but progesterone may reduce the positive subjective effects of cocaine, particularly in women (Evans and Foltin, 2006; Sofuoglu et al., 2004). In abstinent people with cocaine dependence, one week of progesterone treatment attenuated increases in drug craving and cortisol following cue exposure (Fox et al., 2013). Progesterone also curbed increases in negative emotion and systolic blood pressure in response to stressful imagery, though only in women (Fox et al., 2013). Curiously, a later study with exogenous progesterone, which analyzed participant outcomes as a function of plasma allopregnanolone, found reduced cocaine craving following both stress and cocaine cue imagery, but also observed a greater increase in plasma cortisol following stress imagery in the high ALLO group relative to the low ALLO group (Milivojevic et al., 2016). This may be attributed in part to the lower baseline cortisol found in the high ALLO group, but it is unclear to what extent this would account for the observed effect (Milivojevic et al., 2016). In women that use cannabis, a small study found that progesterone could attenuate cannabis craving during a brief abstinence period (Sherman et al., 2019). Further research is necessary to determine the reliability of this finding and if this effect extends to men.
Clinical trials incorporating exogenous progesterone are similarly limited to examining its efficacy in treating cocaine dependence. Unfortunately, this efficacy has yet to be demonstrated: progesterone treatment failed to improve rates of abstinence in methadone-maintained men that also use cocaine (Sofuoglu et al., 2007), or in postpartum cocaine-using women (Yonkers et al., 2014). The latter of these studies found lower self-reported cocaine use in women randomized to progesterone, however. Additional research with this neuroactive steroid may further elucidate its effects on stress-induced drug seeking and confirm these early observations that beneficial effects of progesterone may be amplified in women.
6.3. Conclusions
Research in rodents and in humans consistently indicate a role for endogenous progesterone in reduction of stress-induced relapse, and the efficacy of exogenous progesterone has been demonstrated in the human laboratory for multiple addictive substances. Notably, the lack of translation from the human laboratory to the clinic thus far may be related to study design (Anker and Carroll, 2010; Evans and Foltin, 2006; Fox et al., 2013; Russo et al., 2010; Sinha et al., 2007a), as only men (Sofuoglu et al., 2007) and postpartum women (Yonkers et al., 2014) were assessed. Progesterone may have superior efficacy in women due to its ability to alleviate stress-associated negative affect (Fox et al., 2013), as negative affect has been shown to be a key contributor to stress-induced relapse in women in particular (Sinha et al., 2007a). However, the dramatic hormonal fluctuation that occurs postpartum (Hendrick et al., 1998) may overpower many effects of exogenous progesterone, although some positive effects with respect to cocaine use were still observed (Yonkers et al., 2014). The ability of exogenous progesterone to attenuate stress-induced drug seeking in freely-cycling women should be examined at the clinical trial level for individuals that use cocaine, and at the human laboratory level for other addictive drugs, such as cannabis and opioids.
There may also be a role for DHEA and its sulfate ester DHEAS in regulation of stress-associated drug seeking. Promisingly, DHEA administration has been shown to suppress relapse to heroin seeking in humans (Maayan et al., 2008) and cocaine reinstatement in rodents (Doron et al., 2006) in the absence of a stressor. As DHEA has been shown to promote adaptive responding to stressful stimuli (Shields et al., 2016), it presents a promising target for pharmacotherapeutic investigation in stress-induced drug-seeking.
7. Opioid targets
There are three classic opioid receptors: mu, kappa, and delta. These receptors are all G-protein coupled inhibitory receptors, which when ligand-bound lead to an overall reduction in transmission of nerve impulses and inhibition of neurotransmitter release (McDonald and Lambert, 2005). The most common opioids used in human and animal research are naloxone and methadone, which clinically are used to prevent opioid overdose and to help maintain abstinence from other opioidergic drugs, respectively. These drugs, however, both act at the mu opioid receptor, which has not generally been implicated in mechanistic studies of stress-related drug seeking. In contrast, kappa opioid receptor (KOR) signaling induced by binding with its endogenous ligand, dynorphin, is prevalent in many of the major neurobiological structures implicated in stress-induced drug seeking, and has been shown to play a clear role in maintaining stress-induced relapse-like behavior (Redila and Chavkin, 2008). Noradrenergic neurons in the LC receive KOR-mediated dynorphin afferents (Kreibich et al., 2008; Reyes et al., 2007), and this signaling alone is sufficient for partial recovery of stress-induced reinstatement of cocaine seeking in an otherwise KOR-knockout mouse model (Al-Hasani et al., 2013). Dynorphin/KOR signaling in the BNST has been implicated in stress-induced alcohol seeking, though it has not yet been assessed for other addictive substances (Lê et al., 2018), and dynorphin/KOR has been shown to mediate stress-induced changes to GABAergic signaling in the VTA that are integral for stress-induced reinstatement of cocaine seeking (Graziane et al., 2013). Additionally, dynorphin/KOR inputs onto serotonergic neurons in the dorsal raphe nucleus are necessary for stress-induced reinstatement of cocaine conditioned place preference, potentially by producing a hyposerotonergic state in the NAc (Land et al., 2009; Schindler et al., 2012).
Taken together, these findings suggest the most optimal treatment strategy to reduce stress-induced drug seeking via the dynorphin/KOR system would be KOR antagonism. Other therapeutic options that target primarily mu opioid receptors (MOR), such as buprenorphine, methadone, or naltrexone, should also be considered for their documented efficacy in reducing rates of opioid relapse in a clinical setting. However, the systemic role of MOR in stress-induced drug seeking is less clear than that of KOR, and it is possible that the therapeutic efficacy of MOR-active drugs is achieved via a different mechanism, as suggested by Graziane et al. (2013).
7.1. Animal
KOR antagonists are consistently effective at reducing stress-precipitated relapse across behavioral models, species, and addictive substance. For example, the KOR antagonist nor-BNI has been shown to block stress-potentiated reinstatement of cocaine CPP, and mice lacking the prodynorphin gene did not show stress-induced reinstatement of cocaine CPP (McLaughlin et al., 2003; Redila and Chavkin, 2008). Both PF-04455242 and CJ-15,208 also prevented forced swim stress-induced reinstatement of cocaine CPP in mice (Aldrich et al., 2013; Grimwood et al., 2011), JDTic and its analogue RTI 194 blocked footshock-induced reinstatement of cocaine seeking in rats (Beardsley et al, 2005, 2010), and nor-BNI blocked food deprivation-induced reinstatement of heroin seeking (Sedki et al., 2015). Additionally, site-specific antagonism of KORs in the VTA also blocks forced swim stress-induced reinstatement (Graziane et al., 2013). All antagonists listed above are long-lasting, but even the shorter-acting antagonist Zyklophin blocked stress-induced reinstatement of cocaine CPP (Aldrich et al., 2009). These relapse-preventive effects of KOR antagonists may be specific to stress-induced reinstatement, as these drugs have no apparent impact on cocaine-primed reinstatement (Beardsley et al., 2005; Carey et al., 2007). Importantly, however, none of these resoundingly positive studies included any female animals. Given the well-documented presence of sex differences in KOR function (Chartoff and Mavrikaki, 2015), one must be skeptical that such outcomes would hold were both sexes included in assessment. In support of this notion, nor-BNI has been shown to reduce forced swim stress-induced immobility in male, but not female, mice (Laman-Maharg et al., 2018), and this difference is likely attributable to a moderating effect of estrogens (Reichard et al., 2020). Thus, it is difficult to generalize the apparent efficacy of KOR antagonists to both sexes.
In contrast to KORs, MORs have not been implicated in stress-primed reinstatement of drug seeking, though maintenance on the MOR agonist methadone reduces reinstatement to cocaine- and heroin-primed reinstatement (Leri et al., 2004). Likewise, the MOR antagonist, naltrexone, is without an effect on stress-induced reinstatement (Sedki et al., 2015; Shaham and Stewart, 1996). Surprisingly, combined MOR/KOR antagonism via buprenorphine reduced heroin- and cocaine-primed reinstatement, but was without an effect on stress-induced reinstatement (Sorge et al., 2005). This is of particular interest given the contrast with the aforementioned KOR antagonist data and the frequency of buprenorphine-prescribing in the treatment of opioid dependence in humans.
7.2. Human
Though findings from the preclinical literature generally suggest efficacy of KOR antagonists to treat stress-induced drug seeking at least in males, these findings have yet to be replicated in humans. Initial excitement surrounding the use of this drug class has been tempered by observations of significant side effects in the promising therapeutic candidate, JDTic (Buda et al., 2015). However, PET imaging in humans further supports the notion that KOR antagonists may be a suitable drug target at least in individuals that use cocaine (Martinez et al., 2019), and a tolerability study with a different candidate medication, Opra Kappa, did not replicate the adverse effects of JDTic (Reed et al., 2018). Notably, the cocaine users in these two trials skewed heavily male (39M/5F) (Martinez et al., 2019; Reed et al., 2018), Opra Kappa did not attenuate baseline craving for cocaine, and increased levels of ACTH and cortisol were observed at time points expected to correspond with peak KOR antagonism (Reed et al., 2018). While additional research with these compounds is warranted from the strength of the preclinical literature, expectations should be adjusted in a manner according to these preliminary findings. The stark lack of research in females affords a further layer of skepticism, particularly given the abundance of sex differences in KOR signaling referenced in the previous section. PET imaging findings also indicate that women have lower KOR density relative to men (Vijay et al., 2016).
More frequently assessed in human models is the MOR antagonist, naltrexone. As in animal models, it does not appear to attenuate stress-induced drug craving or anxiety in opioid-dependent individuals in a laboratory setting (Hyman et al., 2007). Further, naltrexone appears to increase positive subjective effects of and desire for THC in individuals that use cannabis (Haney et al., 2003), though it was paradoxically associated with reduced self-report cannabis use in a small, open-label pilot study (Notzon et al., 2018). In surprising contrast to preclinical data, naltrexone combined with buprenorphine/naloxone was associated with an increase in cocaine-negative urine samples relative to placebo in a large clinical trial (Ling et al., 2016). Yet, consistent with the animal literature and with human laboratory findings, this drug combination had no effect on opioid use or craving (Ling et al., 2016). Taken together, these outcomes suggest that even though naltrexone shows clear efficacy in relapse prevention, it is likely ineffective at attenuating specifically stress-induced craving for or relapse to opioids in humans. Further assessment is necessary to more clearly elucidate naltrexone's effects on stress-induced relapse to cannabis or cocaine.
7.3. Conclusions
Both MOR agonists and antagonists show limited efficacy in the treatment of stress-induced relapse in both human and animal models despite their prevalence in the clinic, suggesting their ability to reduce drug use is derived via a different mechanism. In contrast, KOR antagonists appear to be on the cusp of translation from rodent to human models. Yet, it seems unlikely that the same degree of positive effects observed in males thus far will be seen across both sexes, given the abundance of sex differences present in KOR function more generally. It is therefore critical that the effects of KOR antagonists on stress-induced drug seeking be fully evaluated in preclinical models of both sexes prior to advancing to the human laboratory stage or resource-intensive clinical trials.
8. Neuroactive peptide targets
Although most neuroactive peptides remain in the early phases of exploration, the role of oxytocin in stress-induced drug seeking behavior has developed a substantial body of research, followed by vasopressin. Oxytocin neurons residing in the PVN and supraoptic nucleus (SON) regulate both the stress response and reward circuitry through local release mechanisms and projections to the forebrain, posterior pituitary, and other regions (Knobloch et al., 2012; Landgraf and Neumann, 2004; Neumann, 2007), making oxytocin a clear candidate pharmacotherapy. Similarly, stress exposure is associated with arginine vasopressin (AVP) release in the PVN and SON that appears to regulate HPA axis activity (Landgraf and Neumann, 2004; Tanoue et al., 2004), and stress exposure increases AVP mRNA expression in the amygdala (Zhou et al, 2008, 2015). Despite structural similarities and comparable release patterns between these neuroactive peptides, however, oxytocin and AVP differ in their downstream activity and sex-dependent effects (Bisagno and Cadet, 2014). For example, a study in humans found that increased plasma oxytocin following stress exposure in women, but not men, was associated with increased positive affect, but increased plasma vasopressin in men, but not women, was associated with increased post-stress anger (Moons et al., 2014). Oxytocin receptor agonism may therefore be an effective treatment for stress-induced relapse in women and vasopressin receptor antagonism may be an effective treatment in men, but it is unclear how therapeutic efficacy may translate across sexes.
Other peptides, such as neuropeptide Y (NPY), neuropeptide S (NPS), substance P, and pituitary adenylate cyclase-activating peptide (PACAP) have a less comprehensive supportive body of work, but hold promise given their regulation of both stress-associated brain regions and behaviors. For example, NPY signaling in the amygdala and septum reduces anxiety (Heilig, 2004), while NPS promotes drug seeking through projections to the BNST and PVN (Kallupi et al., 2010; Ubaldi et al., 2016). Acute stress coincides with elevation in substance P levels in regions regulating the stress response (Ebner et al., 2008a, Ebner et al., 2008b), while chronic stress exposure results in adaptations mediated by activation of PACAP in the BNST (Miles et al., 2019). Further, increased CRF transmission is observed in response to PACAP in hypothalamic cells and ICV PACAP administration increases CRF mRNA in the PVN (Hashimoto et al., 2011). Circulating PACAP levels and a single nucleotide polymorphism in the PAC1 receptor gene have both been associated with PTSD and other stress-related disorders in women to further implicate PACAP/PAC1R function in stress- and anxiety-related states such as addiction (Hashimoto et al, 2011, 2016; Ressler et al., 2011). While these latter neuroactive peptides have produced some exciting, albeit very preliminary, results in the context of stress-induced reinstatement (Maric et al., 2011; Miles et al., 2018; Schank et al., 2014; Schmoutz et al., 2012), none of these have been assessed in humans nor have their effects been examined in female animals. Thus, further discussion of these compounds has been excluded from the following sections.
8.1. Animal
Exogenous oxytocin treatment appears to attenuate stress-associated drug seeking and has been most explored in the context of methamphetamine addiction in rodents. Acute oxytocin treatment inhibits stress-induced reinstatement of methamphetamine seeking (Han et al., 2014; Qi et al., 2009), and this is prevented by the oxytocin receptor antagonist, atosiban (Qi et al., 2009). Acute oxytocin exposure has also been shown to decrease yohimbine stress-induced reinstatement of methamphetamine and sucrose seeking in both male and female rats (Cox et al., 2013). In contrast to studies of acute exposure, chronic oxytocin treatment suppressed both incubation of methamphetamine craving and methamphetamine-induced reinstatement in both sexes, but only attenuated yohimbine-induced reinstatement in females (Everett et al., 2020). This illustrates that even if sex differences are not immediately apparent following acute drug treatment, differences may arise as a function of time—an important consideration when considering eventual translation to a clinical setting, where most medications are administered chronically. Beyond methamphetamine, oxytocin has also been shown to curb social defeat stress-induced cocaine seeking (Ferrer-Pérez et al., 2019) and the oxytocin analogue carbetocin attenuates forced-swim stress-induced opioid seeking (Zanos et al., 2014). It has not yet been examined if the sex-specific effects of long-term oxytocin exposure carry over to stress-induced reinstatement of other addictive substances beyond methamphetamine.
One study in male rats has also shown potential for the AVP V1b receptor antagonist SSR149415 to attenuate footshock stress-induced reinstatement of heroin seeking during a withdrawal period (Zhou et al., 2008). Dose-dependent reductions in plasma ACTH following footshock in SSR149415-treated animals suggest treatment efficacy may be specific to stress-induced reinstatement. It is unclear, however, if these positive outcomes would generalize to females given the sexual dimorphism of AVP, particularly in a stress context (Bisagno and Cadet, 2014).
8.2. Human
Assessment of oxytocin's effect on stress-induced drug seeking has only recently shifted to human models, and results have been mixed and sex-dependent. A specific variant of the OXTR gene in humans has been associated with reduced stress reactivity (Rodrigues et al., 2009). In the laboratory, a small study of people with cannabis dependence found that oxytocin effectively reduced drug craving and anxiety, but not subjective stress, following the Trier Social Stress Test (TSST) (McRae-Clark et al., 2013). However, another trial involving a larger sample size and proportionally more women did not find the same effect on craving (Reed et al., 2019). This latter study additionally found that while oxytocin attenuated subjective stress in men following the TSST, it actually led to increased stress in women relative to placebo. Oxytocin also had no effect on cannabis self-administration, though neither did stress. In people with cocaine dependence, acute oxytocin administration was found to increase desire to use cocaine prior to any sort of stimulus exposure and had no effect on anxiety (Lee et al., 2014). In people with both cocaine dependence and exposure to early childhood adversity, oxytocin reduced cortisol response to the TSST, though craving and subjective stress data were not reported (Flanagan et al., 2015). Interestingly, Sherman et al. (2020a) also observed this reduced cortisol response, but this time only in women; no effect was found as a function of childhood trauma. This study also reported no effect of oxytocin on subjective stress or cocaine craving in either sex (Sherman et al., 2020a).
Clinical efficacy of oxytocin has only briefly been touched upon at the time of this writing. A pilot trial in methadone-maintained people with concurrent opioid/cocaine use disorders found some ability of oxytocin to reduce craving for cocaine and heroin (Stauffer et al., 2016). This study also found reduced self-report cocaine use in the oxytocin group, but there was no impact of this treatment on urine-verified abstinence. Other studies have evaluated oxytocin as an adjunct to motivational therapy in people that use methamphetamine or cannabis (Sherman et al., 2017a; Stauffer et al., 2020). While combined oxytocin and therapy had no effect on anxiety, craving, or drug use in individuals that use methamphetamine (Stauffer et al., 2020), positive, albeit small, effects on use behavior were observed in the trial for cannabis dependence (Sherman et al., 2017a). Results from these pilot trials are promising despite conflict in the laboratory research, and warrant further investigation.
Replicating findings in rodents, AVP V1b receptor antagonism by ABT-436 reduced levels of resting plasma ACTH and serum cortisol in a sample of healthy volunteers that was 95% male (Katz et al., 2016). ABT-436 was then evaluated in a larger clinical trial for the treatment of alcohol dependence with moderately positive outcomes (Ryan et al., 2017). Results in this study did not differ significantly by sex, but were surprisingly more favorable in women relative to men. It is unclear how these findings generalize to addictive substances besides alcohol, but results warrant further investigation, particularly in people with opioid dependence. Mechanistic investigations into gender differences in outcomes are also warranted; increased efficacy in women may be associated with a reduction in negative affect produced by ABT-436, but this remains to be seen.
8.3. Conclusions
While a handful of neuroactive peptides show some evidence of efficacy for attenuation of stress-induced reinstatement, many of these are still in the early preclinical stages of research. Oxytocin has shown some evidence of successful translation, though outcomes are mediated by sex. In light of this, the observation that chronic oxytocin treatment only effectively attenuated stress-induced reinstatement in female rodents (Everett et al., 2020) should be of substantial consideration when designing upcoming clinical trials. AVP V1b receptor antagonism also shows preliminary evidence of promise, but further research both preclinically and in the human laboratory should be conducted to assess the contributions of sex and stress exposure in outcomes, respectively. Researchers exploring other neuroactive peptides discussed in this section should ascertain if any positive outcomes observed extend to females before progressing with trials in humans.
9. Cannabinoid targets
The endocannabinoid system plays an integral role in regulation of the HPA axis, stress, and drug seeking. First discovered as the targets for THC, the cannabinoid type-1 and -2 receptors (CB1R and CB2R, respectively) have both been implicated in cocaine seeking (De Vries et al., 2001; Li et al., 2018; Peterson et al., 2016; Xi et al, 2006, 2011) and the promotion thereof by stress (Doncheck et al., 2020; McReynolds et al., 2018; 2016; Vaughn et al., 2012; but see De Vries et al., 2001). A high density of CB1Rs can be observed in brain regions associated with stress reactivity—namely the PFC, amygdala, and hippocampus—as well as those regions implicated in addiction including the NAc and VTA (Hill et al., 2011; Kucera et al., 2018; Mackie, 2005). Additionally, recent evidence suggests that CB2Rs are also expressed in regions critical for stress-promoted drug seeking, including the VTA (Chen et al., 2017; Zhang et al., 2017). Mobilization of the endogenous ligands for CB1/2R, 2-arachidonoylglycerol (2-AG) and anandamide (AEA), has been implicated in regulating both HPA axis activity and drug seeking in both sexes (Di et al., 2003; Doncheck et al., 2020; Hill et al., 2011; McReynolds et al., 2018). Clinical data also indicate the genetic variant for reduced expression of fatty acid amide hydrolase (FAAH), the primary hydrolytic enzyme for AEA, is associated with reduced stress reactivity and anxiety (Dincheva et al., 2015; Gunduz-Cinar et al., 2013). Conversely, activity of the synthetic enzyme for 2-AG, diacylglycerol lipase (DAGL), is necessary for stress-potentiated drug seeking (McReynolds et al., 2018). Given the apparent role of the endocannabinoid system in stress and drug seeking, it presents a promising area of research for the identification of therapeutic targets for relapse prevention. Furthermore, the phytocannabinoid cannabidiol (CBD) presents an emerging area of study for a range of stress-related neurological disorders, including substance use disorders, though its pharmacological activity appears to fundamentally differ from both THC and the endogenous cannabinoids (Compton et al., 1993; De Petrocellis et al., 2011; Tham et al., 2019).
9.1. Animal
Exogenous CB1R agonists promote reinstatement of drug-seeking (De Vries et al., 2001; McReynolds et al., 2016, 2018; Vaughn et al., 2012). In contrast, systemic administration of the CB1R inverse agonist AM251 blocked forced swim stress- (Vaughn et al., 2012) and CRF-induced reinstatement of drug seeking (Kupferschmidt et al., 2012). AM251 has additionally been shown to block footshock-, restraint-, and CORT-induced reinstatement of cocaine-seeking in male rodents, and restraint- and CORT-induced reinstatement in females (Doncheck et al., 2020; McReynolds et al., 2016; but see Kupferschmidt et al., 2012), and this effect of AM251 is dependent on 2-AG synthesis in the PrL-PFC (McReynolds et al., 2018). This is in surprising contrast to the finding that CB1R antagonists also increase circulating ACTH and CORT (Manzanares et al., 1999; Patel et al., 2004). To further complicate interpretation, chronic administration of the FAAH inhibitor URB597 during withdrawal suppressed pharmacological stressor-induced reinstatement of cocaine-seeking (Chauvet et al., 2014), while acute URB597 administration did not (Nawata et al., 2019). Nawata et al. (2019) also report that systemic inhibition of monoacylglycerol lipase, the primary hydrolytic enzyme for 2-AG, using JZL184 reduced both anxiety and methamphetamine-seeking during footshock in a CB1R-dependent manner. This seemingly contrasts with the findings by McReynolds et al. (2018), which showed that 2-AG synthesis in PrL-PFC was necessary for potentiated cocaine seeking following footshock. However, these disparities may be due to the time at which drug seeking was assessed relative to footshock exposure, as footshock stress-induced analgesia involves mobilization of both 2-AG and AEA in the periaqueductal grey and inhibition of their hydrolysis therein enhances analgesia in a CB1R-dependent manner (Hohmann et al., 2005). This highlights the complex relationship between endocannabinoid signaling and stress-precipitated drug seeking, as the specific endocannabinoid, brain region, temporal relationship to and subtype of the stressor, as well as the specific drug of abuse may strongly influence the outcome of endocannabinoid manipulations.
In addition to synthetic pharmacotherapeutic approaches, the phytocannabinoid CBD has recently emerged as a potential treatment for stress-induced drug-seeking. Gonzalez-Cuevas et al. (2018) demonstrated that sub-chronic transdermal CBD treatment could attenuate stress-induced cocaine seeking without tolerance, sedative effects, or interference with natural reward seeking behavior. Similar outcomes have been produced using intraperitoneal CBD (Calpe-López et al., 2021). As mentioned earlier, CB1R agonists like THC do not provide protection against drug seeking in line with CBD. THC may, rather, produce changes within the endogenous opioid system that make animals more sensitive and vulnerable to opioid use (Rubio et al., 1998; Vela et al., 1998), and this plasticity may vary by sex (Corchero et al., 2002). Despite these data, potential effects of THC on drug-seeking and the role of stress therein need to be more extensively studied in males and females, given its pharmacological similarity to the endogenous cannabinoids.
Taken together, current data highlight manipulation of CB1R activity as a potentially efficacious method for reducing stress-induced reinstatement. The directionality of this manipulation that produces best results—whether agonism or antagonism—remains to be seen, and may be related to individual differences. No sex differences were observed in the efficacy of intra-PrL-PFC AM251 in attenuating CORT or restraint stress-induced reinstatement to cocaine (Doncheck et al., 2020), which is surprising when considering the contribution of estradiol to sexual dimorphisms in the endocannabinoid system (Huang and Woolley, 2012; López, 2010). As the PrL-PFC only represents one node in the stress-associated reinstatement neurocircuitry, however, it is possible that sex differences emerge when examining pharmacological effects of AM251 when administered systemically or intracranially into other brain regions.
9.2. Human
The potential for cannabinoid interventions in stress-induced drug seeking in humans is an area that has yet to be explored despite the promise shown in the preclinical literature. Several laboratory studies have assessed the effects of oral THC during periods of cannabis abstinence, however, with mixed outcomes with respect to cannabis craving, withdrawal-associated anxiety, and relapse-like behavior (Budney et al., 2007; Haney et al, 2004, 2008, 2013; Schlienz et al., 2018). Most commonly reported are that oral THC attenuates cannabis craving (Budney et al., 2007; Haney et al, 2004, 2013; Schlienz et al., 2018) with no effect on anxiety (Budney et al., 2007; Haney et al, 2008, 2013; Schlienz et al., 2018) and reduces cannabis self-administration in the laboratory (Haney et al., 2013; Schlienz et al., 2018). Another study in individuals with cannabis dependence found no effect of Sativex®, a compound with a 1:1 ratio of THC:CBD, on craving or anxiety associated with withdrawal (Trigo et al., 2016). CBD similarly had no impact on cannabis self-administration in the human laboratory (Haney et al., 2016). Finally, a trial in abstinent individuals with heroin use disorder found that cannabidiol significantly attenuated ratings of cue-induced craving and anxiety relative to placebo, as well as cue-induced increases in salivary cortisol, but effects on stress were not directly assessed (Hurd et al., 2019). No sex differences were reported in any of these trials, but samples were overwhelmingly male, and it is likely that CBD-associated alleviation of anxiety will confer additional efficacy in women with respect to relapse prevention.
Clinical trials incorporating cannabinoid approaches are also presently limited. CB1R agonists appear to have limited efficacy in promoting abstinence from cannabis (Allsop et al., 2014; Levin et al., 2011), though nabiximols may reduce cannabis craving and anxiety associated with withdrawal. Two brief trials examining the efficacy of CBD in attenuating cannabis use also had modest results (Freeman et al., 2020; Solowij et al., 2018). Lastly, and by far the most promising, was a trial examining the efficacy of the FAAH inhibitor PF-04457845 in the treatment of cannabis dependence (D'Souza et al., 2019). Authors reported that PF-04457845 both reduced anxiety and improved rates of urine-verified abstinence, though only male participants were assessed (D'Souza et al., 2019). Given the lack of gender diversity present in both other clinical and laboratory trials, it is difficult to hypothesize how these results will translate to a larger sample that includes women. However, FAAH inhibitors may present an effective therapeutic option to reduce stress-induced relapse.
9.3. Conclusions
Endocannabinoid signaling plays a clear and critical role in the stress response, but research on this system in relation to stress-induced reinstatement is still in its infancy. Preclinical models suggest efficacy of CB1R antagonists, endogenous agonists, and CBD, but no method has emerged as clearly superior. Work in female animals is also limited at this point to only one study of intra-PrL-PFC CB1R antagonism in which no sex differences were observed (Doncheck et al., 2020). Given negative psychiatric effects associated with CB1R antagonism in humans (Christensen et al., 2007), research efforts should likely remain focused on the potential of enhanced endocannabinoid signaling and CBD to attenuate stress-induced relapse. In particular, effects on stress-induced drug craving, especially for stimulant drugs, should be assessed in the human laboratory. Clinical trials of FAAH inhibitors should be extended to include women.
10. Conclusions
In this review, we discuss neural systems associated with stress-induced drug-seeking behavior and the pharmacological interventions used to target them in both human and animal models. In this context, we highlight the crucial importance of biological sex in both research design and in interpreting outcomes; many of the models used to assess stress-induced reinstatement produce differential outcomes by sex, and of the small handful of studies that adequately integrated assessment of sex-specific findings, nearly all reported differential outcomes on the basis of sex. Given the above-listed evidence, we firmly believe that inattention to sex as a biological variable has contributed significantly to failed translation across species and between the human laboratory and the clinic.
In this vein, we acknowledge that differences between male and female physiology—particularly at the molecular level—may complicate research into basic neural systems. It is also well-known that overall more men than women use addictive substances (SAMHSA, 2020), potentially making women less likely to participate in human subjects research, though women do progress more quickly from use to dependence (Haas and Peters, 2000; Kosten et al., 1993). However, continuous attempts to side-step these hurdles, rather than modification of study design, have led to the heavily male-centric research program we detail in this review, where pharmacological interventions are not designed for female brains and are not adequately tested in female subjects prior to late-stage clinical trials. There are evident drawbacks to this approach, such as limiting discovery and development of potential effective treatment options for women as well as investment of significant time and resources into research endeavors that ultimately fail due to inattention to sex differences.
That being said, there remains an opportunity to assess many of the interventions described herein in females prior to this point. At the preclinical level, CRF antagonists, DHEA/DHEAS, KOR antagonists, AVP V1b antagonists, NPS, NPY, substance P, and PACAP show at least some evidence of efficacy in males, but remain unexplored in females in the context of stress-induced reinstatement. Not only should these signaling systems be assessed pharmacologically for potential treatment efficacy, but their function should also be examined and reported simply in the context of the female brain. In the human laboratory, β-AR antagonists, oxazepam/metyrapone, progesterone, and multiple cannabinoids (THC, CBD, enhancers of endocannabinoid signaling) should be similarly assessed, bearing in mind sex differences inherent to different laboratory stress-induction methods. Finally, evidence supporting the efficacy of α2-AR agonists and oxytocin appears sufficient at this stage to recommend progression to clinical trials. In conducting these trials, however, investigators must continually bear in mind how sex has been shown in preclinical and human laboratory studies to influence outcomes for these therapeutics. Only by remaining conscientious in this regard can we as researchers in stress-induced relapse hope to reliably produce clinically meaningful results.
Funding
This work was supported by the National Institute on Drug Abuse (NIDA) grants R25-DA020537 (Martin), T32-DA007288 (Martin, Doncheck), U54-DA016511 (McRae-Clark), and R01-DA033049 (Reichel).
Author contributions
Erin Martin: Conceptualization, Investigation, Writing – Original draft, Writing – Review and Editing, Visualization. Elizabeth Doncheck: Conceptualization, Investigation, Writing – Original draft, Writing – Review and Editing, Carmela Reichel: Conceptualization, Writing – Original draft, Writing – Review and Editing, Supervision, Aimee McRae-Clark: Conceptualization, Writing – Original draft, Writing – Review and Editing, Supervision, Project Administration.
Declaration of interests
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Declaration of competing interest
None.
Contributor Information
Erin L. Martin, Email: marterin@musc.edu.
Elizabeth M. Doncheck, Email: doncheck@musc.edu.
Carmela M. Reichel, Email: reichel@musc.edu.
Aimee L. McRae-Clark, Email: mcraeal@musc.edu.
References
- Agoglia A.E., Tella J., Herman M.A. Sex differences in corticotropin releasing factor peptide regulation of inhibitory control and excitability in central amygdala corticotropin releasing factor receptor 1-neurons. Neuropharmacology. 2020;180:108296. doi: 10.1016/j.neuropharm.2020.108296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al-Hasani R., McCall J.G., Foshage A.M., Bruchas M.R. Locus coeruleus kappa-opioid receptors modulate reinstatement of cocaine place preference through a noradrenergic mechanism. Neuropsychopharmacology. 2013;38:2484–2497. doi: 10.1038/npp.2013.151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aldrich J.V., Patkar K.A., McLaughlin J.P. Zyklophin, a systemically active selective kappa opioid receptor peptide antagonist with short duration of action. Proc. Natl. Acad. Sci. U. S. A. 2009;106:18396–18401. doi: 10.1073/pnas.0910180106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aldrich J.V., Senadheera S.N., Ross N.C., Ganno M.L., Eans S.O., McLaughlin J.P. The macrocyclic peptide natural product CJ-15,208 is orally active and prevents reinstatement of extinguished cocaine-seeking behavior. J. Nat. Prod. 2013;76:433–438. doi: 10.1021/np300697k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allsop D.J., Copeland J., Lintzeris N., Dunlop A.J., Montebello M., Sadler C., Rivas G.R., Holland R.M., Muhleisen P., Norberg M.M., Booth J., McGregor I.S. Nabiximols as an agonist replacement therapy during cannabis withdrawal: a randomized clinical trial. JAMA Psychiatry. 2014;71:281–291. doi: 10.1001/jamapsychiatry.2013.3947. [DOI] [PubMed] [Google Scholar]
- Anker J.J., Carroll M.E. Sex differences in the effects of allopregnanolone on yohimbine-induced reinstatement of cocaine seeking in rats. Drug Alcohol Depend. 2010;107:264–267. doi: 10.1016/j.drugalcdep.2009.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anker J.J., Holtz N.A., Zlebnik N., Carroll M.E. Effects of allopregnanolone on the reinstatement of cocaine-seeking behavior in male and female rats. Psychopharmacology (Berl) 2009;203:63–72. doi: 10.1007/s00213-008-1371-9. [DOI] [PubMed] [Google Scholar]
- Aston-Jones G., Delfs J.M., Druhan J., Zhu Y. The bed nucleus of the stria terminalis. A target site for noradrenergic actions in opiate withdrawal. Ann. N. Y. Acad. Sci. 1999;877:486–498. doi: 10.1111/j.1749-6632.1999.tb09284.x. [DOI] [PubMed] [Google Scholar]
- Back S.E., Brady K.T., Jackson J.L., Salstrom S., Zinzow H. Gender differences in stress reactivity among cocaine-dependent individuals. Psychopharmacology (Berl) 2005;180:169–176. doi: 10.1007/s00213-004-2129-7. [DOI] [PubMed] [Google Scholar]
- Bangasser D.A., Curtis A.L., Reyes B.A.S., Bethea T.T., Parastatidis I., Ischiropoulos H., Van Bockstaele E.J., Valentino R.J. Sex differences in corticotropin-releasing factor receptor signaling and trafficking: potential role in female vulnerability to stress-related psychopathology. Mol. Psychiatr. 2010;15:896–904. doi: 10.1038/mp.2010.66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bangasser D.A., Santollo J., Shors T.J. The bed nucleus of the stria terminalis is critically involved in enhancing associative learning after stressful experience. Behav. Neurosci. 2005;119:1459–1466. doi: 10.1037/0735-7044.119.6.1459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bangasser D.A., Valentino R.J. Sex differences in stress-related psychiatric disorders: neurobiological perspectives. Front. Neuroendocrinol. 2014;35:303–319. doi: 10.1016/j.yfrne.2014.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bangasser D.A., Wicks B. Sex-specific mechanisms for responding to stress. J. Neurosci. Res. 2017;95:75–82. doi: 10.1002/jnr.23812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bangasser D.A., Wiersielis K.R., Khantsis S. Sex differences in the locus coeruleus-norepinephrine system and its regulation by stress. Brain Res. 2016;1641:177–188. doi: 10.1016/j.brainres.2015.11.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barbaccia M.L., Roscetti G., Trabucchi M., Mostallino M.C., Concas A., Purdy R.H., Biggio G. Time-dependent changes in rat brain neuroactive steroid concentrations and GABAA receptor function after acute stress. Neuroendocrinology. 1996;63:166–172. doi: 10.16309/j.cnki.issn.1007-1776.2003.03.004. [DOI] [PubMed] [Google Scholar]
- Barbaccia M.L., Roscetti G., Trabucchi M., Purdy R.H., Mostallino M.C., Concas A., Biggio G. The effects of inhibitors of GABAergic transmission and stress on brain and plasma allopregnanolone concentrations. Br. J. Pharmacol. 1997;120:1582–1588. doi: 10.1038/sj.bjp.0701046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beardsley P.M., Howard J.L., Shelton K.L., Carroll F.I. Differential effects of the novel kappa opioid receptor antagonist, JDTic, on reinstatement of cocaine-seeking induced by footshock stressors vs cocaine primes and its antidepressant-like effects in rats. Psychopharmacology (Berl) 2005;183:118–126. doi: 10.1007/s00213-005-0167-4. [DOI] [PubMed] [Google Scholar]
- Beardsley P.M., Pollard G.T., Howard J.L., Carroll F.I. Effectiveness of analogs of the kappa opioid receptor antagonist (3R)-7-Hydroxy-N-((1S)-1-{[(3R,4R)-4-(3-hydroxyphenyl)-3, 4-dimethyl-1- piperidinyl] methyl} -2-methylpropyl)-1,2,3, 4-tetrahydro-3- isoquinolinecarboxamide (JDTic) to reduce U50, 488-induce. Psychopharmacology (Berl) 2010;210:189–198. doi: 10.1007/s00213-010-1846-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becker J.B., Hu M. Sex differences in drug abuse. Front. Neuroendocrinol. 2008;29:36–47. doi: 10.1016/j.yfrne.2007.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becker J.B., Koob G.F. Sex differences in animal models: focus on addiction. Pharmacol. Rev. 2016;68:242–263. doi: 10.1124/pr.115.011163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benowitz N.L., Lessov-Schlaggar C.N., Swan G.E., Jacob P. Female sex and oral contraceptive use accelerate nicotine metabolism. Clin. Pharmacol. Ther. 2006;79:480–488. doi: 10.1016/j.clpt.2006.01.008. [DOI] [PubMed] [Google Scholar]
- Bertholomey M.L., Nagarajan V., Torregrossa M.M. Sex differences in reinstatement of alcohol seeking in response to cues and yohimbine in rats with and without a history of adolescent corticosterone exposure. Psychopharmacology (Berl) 2016;233:2277–2287. doi: 10.1007/s00213-016-4278-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bisagno V., Cadet J.L. Stress, sex, and addiction: potential roles of corticotropin-releasing factor, oxytocin, and arginine-vasopressin. Behav. Pharmacol. 2014;25:445–457. doi: 10.1097/FBP.0000000000000049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blacktop J.M., Seubert C., Baker D.A., Ferda N., Lee G., Graf E.N., Mantsch J.R. Augmented cocaine seeking in response to stress or CRF delivered into the ventral tegmental area following long-access self-administration is mediated by CRF receptor type 1 but not CRF receptor type 2. J. Neurosci. 2011;31:11396–11403. doi: 10.1523/JNEUROSCI.1393-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bland S.T., Schmid M.J., Der-Avakian A., Watkins L.R., Spencer R.L., Maier S.F. Expression of c-fos and BDNF mRNA in subregions of the prefrontal cortex of male and female rats after acute uncontrollable stress. Brain Res. 2005;1051:90–99. doi: 10.1016/j.brainres.2005.05.065. [DOI] [PubMed] [Google Scholar]
- Brady K.T., Randall C.L. Gender differences in substance use disorders. Addict. Dis. 1999;22:241–252. doi: 10.1016/s0193-953x(05)70074-5. [DOI] [PubMed] [Google Scholar]
- Briand L.A., Vassoler F.M., Pierce R.C., Valentino R.J., Blendy J.A. Ventral tegmental afferents in stress-induced reinstatement: the role of cAMP response element-binding protein. J. Neurosci. 2010;30:16149–16159. doi: 10.1523/JNEUROSCI.2827-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown Z.J., Tribe E., D'Souza N.A., Erb S. Interaction between noradrenaline and corticotrophin-releasing factor in the reinstatement of cocaine seeking in the rat. Psychopharmacology (Berl) 2009;203:121–130. doi: 10.1007/s00213-008-1376-4. [DOI] [PubMed] [Google Scholar]
- Brownwell K.D., Marlatt G.A., Lichtenstein E., Wilson G.T. Understanding and preventing relapse. Am. Psychol. 1986;41:765–782. doi: 10.1037//0003-066x.41.7.765. [DOI] [PubMed] [Google Scholar]
- Bruijnzeel A.W. Tobacco addiction and the dysregulation of brain stress systems. Neurosci. Biobehav. Rev. 2012;36:1418–1441. doi: 10.1016/j.neubiorev.2012.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buda J.J., Carroll F.I., Kosten T.R., Swearingen D., Walters B.B. A double-blind, placebo-controlled trial to evaluate the safety, tolerability, and pharmacokinetics of single, escalating oral doses of JDTic. Neuropsychopharmacology. 2015;40:2059–2065. doi: 10.1038/npp.2015.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Budney A.J., Vandrey R., Hughes J.R., Moore B.A., Bahrenburg B. Oral delta-9-tetrahydrocannabinol suppresses cannabis withdrawal symptoms. Drug Alcohol Depend. 2007;86:22–29. doi: 10.1016/j.drugalcdep.2006.04.014. [DOI] [PubMed] [Google Scholar]
- Buffalari D.M., Baldwin C.K., Feltenstein M.W., See R.E. Corticotrophin releasing factor (CRF) induced reinstatement of cocaine seeking in male and female rats. Physiol. Behav. 2012;105:209–214. doi: 10.1016/j.physbeh.2011.08.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buffalari D.M., Baldwin C.K., See R.E. Treatment of cocaine withdrawal anxiety with guanfacine: relationships to cocaine intake and reinstatement of cocaine seeking in rats. Psychopharmacology (Berl) 2012;223:179–190. doi: 10.1007/s00213-012-2705-1. [DOI] [PubMed] [Google Scholar]
- Calipari E.S., Juarez B., Morel C., Walker D.M., Cahill M.E., Ribeiro E., Roman-Ortiz C., Ramakrishnan C., Deisseroth K., Han M.H., Nestler E.J. Dopaminergic dynamics underlying sex-specific cocaine reward. Nat. Commun. 2017;8:13877. doi: 10.1038/ncomms13877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calpe-López C., Gasparyan A., Navarrete F., Manzanares J., Miñarro J., Aguilar M.A. Cannabidiol prevents priming- and stress-induced reinstatement of the conditioned place preference induced by cocaine in mice. J. Psychopharmacol. 2021 doi: 10.1177/0269881120965952. [DOI] [PubMed] [Google Scholar]
- Carey A.N., Borozny K., Aldrich J.V., McLaughlin J.P. Reinstatement of cocaine place-conditioning prevented by the peptide kappa-opioid receptor antagonist arodyn. Eur. J. Pharmacol. 2007;569:84–89. doi: 10.1016/j.ejphar.2007.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chartoff E.H., Mavrikaki M. Sex differences in kappa opioid receptor function and their potential impact on addiction. Front. Neurosci. 2015;9:466. doi: 10.3389/fnins.2015.00466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chauvet C., Nicolas C., Thiriet N., Lardeux V., Duranti A., Solinas M. Chronic stimulation of the tone of endogenous anandamide reduces cue-and stress-induced relapse in rats. Int. J. Neuropsychopharmacol. 2014;18:1–5. doi: 10.1093/ijnp/pyu025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen D.J., Gao M., Gao F.F., Su Q.X., Wu J. Brain cannabinoid receptor 2: expression, function and modulation. Acta Pharmacol. Sin. 2017;38:312–316. doi: 10.1038/aps.2016.149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen N.A., Jupp B., Sztainberg Y., Lebow M., Brown R.M., Kim J.H., Chen A., Lawrence A.J. Knockdown of CRF1 receptors in the ventral tegmental area attenuates cue- and acute food deprivation stress-induced cocaine seeking in mice. J. Neurosci. 2014;34:11560–11570. doi: 10.1523/JNEUROSCI.4763-12.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christensen R., Kristensen P.K., Bartels E.M., Bliddal H., Astrup A. Efficacy and safety of the weight-loss drug rimonabant: a meta-analysis of randomised trials. Lancet. 2007;370:1706–1713. doi: 10.1016/S0140-6736(07)61721-8. [DOI] [PubMed] [Google Scholar]
- Chrousos G.P., Gold P.W. The concepts of stress and stress system disorders: overview of physical and behavioral homeostasis. J. Am. Med. Assoc. 1992;267:1244–1252. doi: 10.1001/jama.1992.03480090092034. [DOI] [PubMed] [Google Scholar]
- Compton D.R., Rice K.C., De Costa B.R., Razdan R.K., Melvin L.S., Johnson M.R., Martin B.R. Cannabinoid structure-activity relationships: correlation of receptor binding and in vivo activities. J. Pharmacol. Exp. Therapeut. 1993;265:218–226. [PubMed] [Google Scholar]
- Cooper Z.D., Haney M. Investigation of sex-dependent effects of cannabis in daily cannabis smokers. Drug Alcohol Depend. 2014;136:85–91. doi: 10.1016/j.drugalcdep.2013.12.013.INVESTIGATION. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corchero J., Fuentes J.A., Manzanares J. Gender differences in proenkephalin gene expression response to δ9-tetrahydrocannabinol in the hypothalamus of the rat. J. Psychopharmacol. 2002;16:283–289. doi: 10.1177/026988110201600401. [DOI] [PubMed] [Google Scholar]
- Cox B.M., Young A.B., See R.E., Reichel C.M. Sex differences in methamphetamine seeking in rats: impact of oxytocin. Psychoneuroendocrinology. 2013;38:2343–2353. doi: 10.1016/j.psyneuen.2013.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crawley J.N., Glowa J.R., Majewska M.D., Paul S.M. Anxiolytic activity of an endogenous adrenal steroid. Brain Res. 1986;398:382–385. doi: 10.1016/0006-8993(86)91500-3. [DOI] [PubMed] [Google Scholar]
- Critchlow V., Liebelt R.A., Bar-Sela M., Mountcastle W., Lipscomb H.S. Sex difference in resting pituitary-adrenal function in the rat. Am. J. Physiol. 1963;205:807–815. doi: 10.1152/ajplegacy.1963.205.5.807. [DOI] [PubMed] [Google Scholar]
- Curtis A.L., Bethea T., Valentino R.J. Sexually dimorphic responses of the brain norepinephrine system to stress and corticotropin-releasing factor. Neuropsychopharmacology. 2006;31:544–554. doi: 10.1038/sj.npp.1300875. [DOI] [PubMed] [Google Scholar]
- D'Souza D.C., Cortes-Briones J., Creatura G., Bluez G., Thurnauer H., Deaso E., Bielen K., Surti T., Radhakrishnan R., Gupta A., Gupta S., Cahill J., Sherif M.A., Makriyannis A., Morgan P.T., Ranganathan M., Skosnik P.D. Efficacy and safety of a fatty acid amide hydrolase inhibitor (PF-04457845) in the treatment of cannabis withdrawal and dependence in men: a double-blind, placebo-controlled, parallel group, phase 2a single-site randomised controlled trial. The Lancet Psychiatry. 2019;6:35–45. doi: 10.1016/S2215-0366(18)30427-9. [DOI] [PubMed] [Google Scholar]
- De Petrocellis L., Ligresti A., Moriello A.S., Allarà M., Bisogno T., Petrosino S., Stott C.G., Di Marzo V. Effects of cannabinoids and cannabinoid-enriched Cannabis extracts on TRP channels and endocannabinoid metabolic enzymes. Br. J. Pharmacol. 2011;163:1479–1494. doi: 10.1111/j.1476-5381.2010.01166.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Vries T.J., Shaham Y., Homberg J.R., Crombag H., Schuurman K., Dieben J., Vanderschuren L.J.M.J., Schoffelmeer A.N.M. A cannabinoid mechanism in relapse to cocaine seeking. Nat. Med. 2001;7:1151–1154. doi: 10.1038/nm1001-1151. [DOI] [PubMed] [Google Scholar]
- Di S., Malcher-Lopes R., Halmos K.C., Tasker J.G. Nongenomic glucocorticoid inhibition via endocannabinoid release in the hypothalamus: a fast feedback mechanism. J. Neurosci. 2003;23:4850–4857. doi: 10.1523/jneurosci.23-12-04850.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diehl A., Croissant B., Batra A., Mundle G., Nakovics H., Mann K. Alcoholism in women: is it different in onset and outcome compared to men? Eur. Arch. Psychiatr. Clin. Neurosci. 2007;257:344–351. doi: 10.1007/s00406-007-0737-z. [DOI] [PubMed] [Google Scholar]
- Dincheva I., Drysdale A.T., Hartley C.A., Johnson D.C., Jing D., King E.C., Ra S., Gray J.M., Yang R., DeGruccio A.M., Huang C., Cravatt B.F., Glatt C.E., Hill M.N., Casey B.J., Lee F.S. FAAH genetic variation enhances fronto-amygdala function in mouse and human. Nat. Commun. 2015;6:6395. doi: 10.1038/ncomms7395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doncheck E.M., Liddiard G.T., Konrath C.D., Liu X., Yu L., Urbanik L.A., Herbst M.R., DeBaker M.C., Raddatz N., Van Newenhizen E.C., Mathy J.C., Gilmartin M.R., Liu Q. song, Hillard C.J., Mantsch J.R. Sex, stress, and prefrontal cortex: influence of biological sex on stress-promoted cocaine seeking. Neuropsychopharmacology. 2020;45:1974–1985. doi: 10.1038/s41386-020-0674-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doncheck E.M., Urbanik L.A., Debaker M.C., Barron L.M., Liddiard G.T., Tuscher J.J., Frick K.M., Hillard C.J., Mantsch J.R. 17β-estradiol potentiates the reinstatement of cocaine seeking in female rats: role of the prelimbic prefrontal cortex and cannabinoid type-1 receptors. Neuropsychopharmacology. 2018;43:781–790. doi: 10.1038/npp.2017.170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doron R., Fridman L., Gispan-Herman I., Maayan R., Weizman A., Yadid G. DHEA, a neurosteroid, decreases cocaine self-administration and reinstatement of cocaine-seeking behavior in rats. Neuropsychopharmacology. 2006;31:2231–2236. doi: 10.1038/sj.npp.1301013. [DOI] [PubMed] [Google Scholar]
- Ebner K., Muigg P., Singewald G.M., Singewald N. Substance P in stress and anxiety: NK-1 receptor antagonism interacts with key brain areas of the stress circuitry. Ann. N. Y. Acad. Sci. 2008;1144:61–73. doi: 10.1196/annals.1418.018. [DOI] [PubMed] [Google Scholar]
- Ebner K., Rupniak N.M., Saria A., Singewald N. Substance P in the medial amygdala: emotional stress-sensitive release and modulation of anxiety-related behavior in rats. Proc. Natl. Acad. Sci. U. S. A. 2004;101:4280–4285. doi: 10.1073/pnas.0400794101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ebner K., Singewald G.M., Whittle N., Ferraguti F., Singewald N. Neurokinin 1 receptor antagonism promotes active stress coping via enhanced septal 5-HT transmission. Neuropsychopharmacology. 2008;33:1929–1941. doi: 10.1038/sj.npp.1301594. [DOI] [PubMed] [Google Scholar]
- Erb S., Hitchcott P.K., Rajabi H., Mueller D., Shaham Y., Stewart J. Alpha-2 adrenergic receptor agonists block stress-induced reinstatement of cocaine seeking. Neuropsychopharmacology. 2000;23:138–150. doi: 10.1016/S0893-133X(99)00158-X. [DOI] [PubMed] [Google Scholar]
- Erb S., Salmaso N., Rodaros D., Stewart J. A role for the CRF-containing pathway from central nucleus of the amygdala to bed nucleus of the stria terminalis in the stress-induced reinstatement of cocaine seeking in rats. Psychopharmacology (Berl) 2001;158:360–365. doi: 10.1007/s002130000642. [DOI] [PubMed] [Google Scholar]
- Erb S., Shaham Y., Stewart J. The role of corticotropin-releasing factor and corticosterone in stress- and cocaine-induced relapse to cocaine seeking in rats. J. Neurosci. 1998;18:5529–5536. doi: 10.1523/jneurosci.18-14-05529.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans S.M., Foltin R.W. Exogenous progesterone attenuates the subjective effects of smoked cocaine in women, but not in men. Neuropsychopharmacology. 2006;31:659–674. doi: 10.1038/sj.npp.1300887. [DOI] [PubMed] [Google Scholar]
- Everett N.A., Baracz S.J., Cornish J.L. The effect of chronic oxytocin treatment during abstinence from methamphetamine self-administration on incubation of craving, reinstatement, and anxiety. Neuropsychopharmacology. 2020;45:597–605. doi: 10.1038/s41386-019-0566-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feltenstein M.W., Henderson A.R., See R.E. Enhancement of cue-induced reinstatement of cocaine-seeking in rats by yohimbine: sex differences and the role of the estrous cycle. Psychopharmacology (Berl) 2011;216:53–62. doi: 10.1007/s00213-011-2187-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feltenstein M.W., See R.E. Plasma progesterone levels and cocaine-seeking in freely cycling female rats across the estrous cycle. Drug Alcohol Depend. 2007;89:183–189. doi: 10.1016/j.drugalcdep.2006.12.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrer-Pérez C., Castro-Zavala A., Luján M.Á., Filarowska J., Ballestín R., Miñarro J., Valverde O., Rodríguez-Arias M. Oxytocin prevents the increase of cocaine-related responses produced by social defeat. Neuropharmacology. 2019;146:50–64. doi: 10.1016/j.neuropharm.2018.11.011. [DOI] [PubMed] [Google Scholar]
- Fetterly T.L., Basu A., Nabit B.P., Awad E., Williford K.M., Centanni S.W., Matthews R.T., Silberman Y., Winder D.G. α2A-adrenergic receptor activation decreases parabrachial nucleus excitatory drive onto BNST CRF neurons and reduces their activity in vivo. J. Neurosci. 2019;39:472–484. doi: 10.1523/JNEUROSCI.1035-18.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filkowski M.M., Olsen R.M., Duda B., Wanger T.J., Sabatinelli D. Sex differences in emotional perception: meta analysis of divergent activation. Neuroimage. 2017;147:925–933. doi: 10.1016/j.neuroimage.2016.12.016. [DOI] [PubMed] [Google Scholar]
- Flanagan J.C., Baker N.L., McRae-Clark A.L., Brady K.T., Moran-Santa Maria M.M. Effects of adverse childhood experiences on the association between intranasal oxytocin and social stress reactivity among individuals with cocaine dependence. Psychiatr. Res. 2015;229:94–100. doi: 10.1016/j.psychres.2015.07.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox H.C., Morgan P.T., Sinha R. Sex differences in guanfacine effects on drug craving and stress arousal in cocaine-dependent individuals. Neuropsychopharmacology. 2014;39:1527–1537. doi: 10.1038/npp.2014.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox H.C., Seo D., Tuit K., Hansen J., Kimmerling A., Morgan P.T., Sinha R. Guanfacine effects on stress, drug craving and prefrontal activation in cocaine dependent individuals: preliminary findings. J. Psychopharmacol. 2012;26:958–972. doi: 10.1177/0269881111430746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox H.C., Sofuoglu M., Morgan P.T., Tuit K.L., Sinha R. The effects of exogenous progesterone on drug craving and stress arousal in cocaine dependence: impact of gender and cue type. Psychoneuroendocrinology. 2013;38:1532–1544. doi: 10.1016/j.psyneuen.2012.12.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freeman T.P., Craft S., Wilson J., Stylianou S., Elsohly M., Forti M. Di, Lynskey M.T. Changes in delta‐9‐tetrahydrocannabinol (THC) and cannabidiol (CBD) concentrations in cannabis over time: systematic review and meta‐analysis. Addiction. 2020;116(5):1000–1010. doi: 10.1111/add.15253. [DOI] [PubMed] [Google Scholar]
- Freidenberg B.M., Gusmano R., Hickling E.J., Blanchard E.B., Bremner J.D., Frye C. Women with PTSD have lower basal salivary cortisol levels later in the day than do men with PTSD: a preliminary study. Physiol. Behav. 2010;99:234–236. doi: 10.1016/j.physbeh.2009.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frye C.A., Rhodes M.E., Petralia S.M., Walf A.A., Sumida K., Edinger K.L. 3alpha-hydroxy-5alpha-pregnan-20-one in the midbrain ventral tegmental area mediates social, sexual, and affective behaviors. Neuroscience. 2006;138:1007–1014. doi: 10.1016/j.neuroscience.2005.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia-Keller, C., Carter, J.S., Kruyer, A., Kearns, A.M, Hopkins, J.L., Hodebourg, R., Kalivas, P.W., Reichel, C.M. BehAvioral and accumbens synaptic plasticity induced by cues associated with restraint stress. Neuropsychopharmacology, (in press). [DOI] [PMC free article] [PubMed]
- Goldstein J.M., Jerram M., Abbs B., Whitfield-Gabrieli S., Makris N. Sex differences in stress response circuitry activation dependent on female hormonal cycle. J. Neurosci. 2010;30:431–438. doi: 10.1523/JNEUROSCI.3021-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez-Cuevas G., Martin-Fardon R., Kerr T.M., Stouffer D.G., Parsons L.H., Hammell D.C., Banks S.L., Stinchcomb A.L., Weiss F. Unique treatment potential of cannabidiol for the prevention of relapse to drug use: preclinical proof of principle. Neuropsychopharmacology. 2018;43:2036–2045. doi: 10.1038/s41386-018-0050-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graf E.N., Wheeler R.A., Baker D.A., Ebben A.L., Hill J.E., Mcreynolds J.R., Robble M.A., Vranjkovic O., Wheeler D.S., Mantsch J.R., Gasser P.J. Corticosterone acts in the nucleus accumbens to enhance dopamine signaling and potentiate reinstatement of cocaine seeking. J. Neurosci. 2013;33:11800–11810. doi: 10.1523/JNEUROSCI.1969-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graziane N.M., Polter A.M., Briand L.A., Pierce R.C., Kauer J.A. Kappa opioid receptors regulate stress-induced cocaine seeking and synaptic plasticity. Neuron. 2013;77:942–954. doi: 10.1016/j.neuron.2012.12.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenfield S.F., Back S.E., Lawson K., Brady K.T. Substance abuse in women. Psychiatr. Clin. 2010;33:339–355. doi: 10.1016/j.psc.2010.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenwald M.K. Anti-stress neuropharmacological mechanisms and targets for addiction treatment: a translational framework. Neurobiol. Stress. 2018;9:84–104. doi: 10.1016/j.ynstr.2018.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grimwood S., Lu Y., Schmidt A.W., Vanase-Frawley M.A., Sawant-Basak A., Miller E., McLean S., Freeman J., Wong S., McLaughlin J.P., Verhoest P.R. Pharmacological characterization of 2-methyl-N-((2′-(pyrrolidin-1- ylsulfonyl)biphenyl-4-yl)methyl)propan-1-amine (pf-04455242), a high-affinity antagonist selective for κ-opioid receptors. J. Pharmacol. Exp. Therapeut. 2011;339:555–566. doi: 10.1124/jpet.111.185108. [DOI] [PubMed] [Google Scholar]
- Gunduz-Cinar O., MacPherson K.P., Cinar R., Gamble-George J., Sugden K., Williams B., Godlewski G., Ramikie T.S., Gorka A.X., Alapafuja S.O., Nikas S.P., Makriyannis A., Poulton R., Patel S., Hariri A.R., Caspi A., Moffitt T.E., Kunos G., Holmes A. Convergent translational evidence of a role for anandamide in amygdala-mediated fear extinction, threat processing and stress-reactivity. Mol. Psychiatr. 2013;18:813–823. doi: 10.1038/mp.2012.72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haas A.L., Peters R.H. Development of substance abuse problems among drug-involved offenders: evidence for the telescoping effect. J. Subst. Abuse. 2000;12:241–253. doi: 10.1016/S0899-3289(00)00053-5. [DOI] [PubMed] [Google Scholar]
- Han W.Y., Du P., Fu S.Y., Wang F., Song M., Wu C.F., Yang J.Y. Oxytocin via its receptor affects restraint stress-induced methamphetamine CPP reinstatement in mice: involvement of the medial prefrontal cortex and dorsal hippocampus glutamatergic system. Pharmacol. Biochem. Behav. 2014;119:80–87. doi: 10.1016/j.pbb.2013.11.014. [DOI] [PubMed] [Google Scholar]
- Handa R.J., Weiser M.J. Gonadal steroid hormones and the hypothalamo-pituitary-adrenal axis. Front. Neuroendocrinol. 2014;35:197–220. doi: 10.1016/j.yfrne.2013.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haney M., Bisaga A., Foltin R.W. Interaction between naltrexone and oral THC in heavy marijuana smokers. Psychopharmacology (Berl) 2003;166:77–85. doi: 10.1007/s00213-002-1279-8. [DOI] [PubMed] [Google Scholar]
- Haney M., Cooper Z.D., Bedi G., Herrmann E.S., Comer S.D., Reed S.C., Foltin R.W., Levin F.R. Guanfacine decreases symptoms of cannabis withdrawal in daily cannabis smokers. Addict. Biol. 2019;24:707–716. doi: 10.1111/adb.12621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haney M., Cooper Z.D., Bedi G., Vosburg S.K., Comer S.D., Foltin R.W. Nabilone decreases marijuana withdrawal and a laboratory measure of marijuana relapse. Neuropsychopharmacology. 2013;38:1557–1565. doi: 10.1038/npp.2013.54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haney M., Hart C.L., Vosburg S.K., Comer S.D., Reed S.C., Foltin R.W. Effects of THC and lofexidine in a human laboratory model of marijuana withdrawal and relapse. Psychopharmacology (Berl) 2008;197:157–168. doi: 10.1007/s00213-007-1020-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haney M., Hart C.L., Vosburg S.K., Nasser J., Bennett A., Zubaran C., Foltin R.W. Marijuana withdrawal in humans: effects of oral THC or divalproex. Neuropsychopharmacology. 2004;29:158–170. doi: 10.1038/sj.npp.1300310. [DOI] [PubMed] [Google Scholar]
- Haney M., Malcolm R.J., Babalonis S., Nuzzo P.A., Cooper Z.D., Bedi G., Gray K.M., McRae-Clark A.L., Lofwall M.R., Sparenborg S., Walsh S.L. Oral cannabidiol does not alter the subjective, reinforcing or cardiovascular effects of smoked cannabis. Neuropsychopharmacology. 2016;41:1974–1982. doi: 10.1038/npp.2015.367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hashimoto H., Shintani N., Ago Y., Hayata-Takano A., Nakazawa T., Hashimoto R., Matsuzaki S., Katayama T., Tohyama M., Matsuda T., Baba A. Pituitary Adenylate Cyclase Activating Polypeptide—PACAP. 2016. Implications of PACAP signaling in psychiatric disorders; pp. 757–766. [DOI] [Google Scholar]
- Hashimoto H., Shintani N., Tanida M., Hayata A., Hashimoto R., Baba A. PACAP is implicated in the stress axes. Curr. Pharmaceut. Des. 2011;17:985–989. doi: 10.2174/138161211795589382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heilig M. The NPY system in stress, anxiety and depression. Neuropeptides. 2004;38:213–224. doi: 10.1016/j.npep.2004.05.002. [DOI] [PubMed] [Google Scholar]
- Hendrick V., Altshuler L.L., Suri R. Hormonal changes in the postpartum and implications for postpartum depression. Psychosomatics. 1998;39:93–101. doi: 10.1016/S0033-3182(98)71355-6. [DOI] [PubMed] [Google Scholar]
- Hernandez-Avila C.A., Rounsaville B.J., Kranzler H.R. Opioid-, cannabis- and alcohol-dependent women show more rapid progression to substance abuse treatment. Drug Alcohol Depend. 2004;74:265–272. doi: 10.1016/j.drugalcdep.2004.02.001. [DOI] [PubMed] [Google Scholar]
- Herrera A.Y., Nielsen S.E., Mather M. Stress-induced increases in progesterone and cortisol in naturally cycling women. Neurobiol. Stress. 2016;3:96–104. doi: 10.1016/j.ynstr.2016.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herrmann E.S., Weerts E.M., Vandrey R. Sex differences in cannabis withdrawal symptoms among treatment-seeking cannabis users. Exp. Clin. Psychopharmacol. 2015;23:415–421. doi: 10.1016/j.physbeh.2017.03.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Highfield D., Yap J., Grimm J.W., Shalev U., Shaham Y. Repeated lofexidine treatment attenuates stress-induced, but not drug cues-induced reinstatement of a heroin-cocaine mixture (speedball) seeking in rats. Neuropsychopharmacology. 2001;25:320–331. doi: 10.1016/S0893-133X(01)00227-5. [DOI] [PubMed] [Google Scholar]
- Hill M.N., McLaughlin R.J., Pan B., Fitzgerald M.L., Roberts C.J., Lee T.T.Y., Karatsoreos I.N., Mackie K., Viau V., Pickel V.M., McEwen B.S., Liu Q. song, Gorzalka B.B., Hillard C.J. Recruitment of prefrontal cortical endocannabinoid signaling by glucocorticoids contributes to termination of the stress response. J. Neurosci. 2011;31:10506–10515. doi: 10.1523/JNEUROSCI.0496-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hogle J.M., Curtin J.J. Sex differences in negative affective response during nicotine withdrawal. Psychophysiology. 2006;43:344–356. doi: 10.1111/j.1469-8986.2006.00406.x. [DOI] [PubMed] [Google Scholar]
- Hohmann A.G., Suplita R.L., Bolton N.M., Neely M.H., Fegley D., Mangieri R., Krey J.F., Walker J.M., Holmes P.V., Crystal J.D., Duranti A., Tontini A., Mor M., Tarzia G., Piomelli D. An endocannabinoid mechanism for stress-induced analgesia. Nature. 2005;435:1108–1112. doi: 10.1038/nature03658. [DOI] [PubMed] [Google Scholar]
- Holly E.N., Shimamoto A., Debold J.F., Miczek K.A. Sex differences in behavioral and neural cross-sensitization and escalated cocaine taking as a result of episodic social defeat stress in rats. Psychopharmacology (Berl) 2012;224:179–188. doi: 10.1007/s00213-012-2846-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holst M., Mathai D.S., Patel M.M., Rodgman C., Keller J., Hussain M.Z., De La Garza R., Kosten T.R., Verrico C.D. Use of guanfacine for cannabis use disorder and related symptomology. Am. J. Addict. 2019;28:455–464. doi: 10.1111/ajad.12959. [DOI] [PubMed] [Google Scholar]
- Huang G.Z., Woolley C.S. Estradiol acutely suppresses inhibition in the hippocampus through a sex-specific endocannabinoid and mGluR-dependent mechanism. Neuron. 2012;74:801–808. doi: 10.1016/j.neuron.2012.03.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hurd Y.L., Spriggs S., Alishayev J., Winkel G., Gurgov K., Kudrich C., Oprescu A.M., Salsitz E. Cannabidiol for the reduction of cue-induced craving and anxiety in drug-abstinent individuals with heroin use disorder: a double-blind randomized placebo-controlled trial. Am. J. Psychiatr. 2019;176:911–922. doi: 10.1176/appi.ajp.2019.18101191. [DOI] [PubMed] [Google Scholar]
- Hyman S.M., Fox H.C., Hong K.I.A., Doebrick C., Sinha R. Stress and drug-cue-induced craving in opioid-dependent individuals in naltrexone treatment. Exp. Clin. Psychopharmacol. 2007;15:134–143. doi: 10.1037/1064-1297.15.2.134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hyman S.M., Paliwal P., Chaplin T.M., Mazure C.M., Rounsaville B.J., Sinha R. Severity of childhood trauma is predictive of cocaine relapse outcomes in women but not men. Drug Alcohol Depend. 2008;92:208–216. doi: 10.1016/j.drugalcdep.2007.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jobes M.L., Ghitza U.E., Epstein D.H., Phillips K.A., Heishman S.J., Preston K.L. Clonidine blocks stress-induced craving in cocaine users. Psychopharmacology (Berl) 2011;218:83–88. doi: 10.1007/s00213-011-2230-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kablinger A.S., Lindner M.A., Casso S., Hefti F., Demuth G., Fox B.S., McNair L.A., McCarthy B.G., Goeders N.E. Effects of the combination of metyrapone and oxazepam on cocaine craving and cocaine taking: a double-blind, randomized, placebo-controlled pilot study. J. Psychopharmacol. 2012;26:973–981. doi: 10.1177/0269881111430745. [DOI] [PubMed] [Google Scholar]
- Kallupi M., Cannella N., Economidou D., Ubaldi M., Ruggeri B., Weiss F., Massi M., Marugan J., Heilig M., Bonnavion P., De Lecea L., Ciccocioppo R. Neuropeptide S facilitates cue-induced relapse to cocaine seeking through activation of the hypothalamic hypocretin system. Proc. Natl. Acad. Sci. U. S. A. 2010;107:19567–19572. doi: 10.1073/pnas.1004100107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kampman K.M., Dackis C.A., Lynch K.G., Pettinati H., Tirado C., Gariti P., Sparkman T., Atzram M., O'Brien C.P. A double-blind, placebo-controlled trial of amantadine, propranolol, and their combination for the treatment of cocaine dependence in patients with severe cocaine withdrawal symptoms. Drug Alcohol Depend. 2006;85:129–137. doi: 10.1016/j.drugalcdep.2006.04.002. [DOI] [PubMed] [Google Scholar]
- Kampman K.M., Volpicelli J.R., Mulvaney F., Alterman A.I., Cornish J., Gariti P., Cnaan A., Poole S., Muller E., Acosta T., Luce D., O'Brien C. Effectiveness of propranolol for cocaine dependence treatment may depend on cocaine withdrawal symptom severity. Drug Alcohol Depend. 2001;63:69–78. doi: 10.1016/S0376-8716(00)00193-9. [DOI] [PubMed] [Google Scholar]
- Karimi S., Attarzadeh-Yazdi G., Yazdi-Ravandi S., Hesam S., Azizi P., Razavi Y., Haghparast A. Forced swim stress but not exogenous corticosterone could induce the reinstatement of extinguished morphine conditioned place preference in rats: involvement of glucocorticoid receptors in the basolateral amygdala. Behav. Brain Res. 2014;264:43–50. doi: 10.1016/j.bbr.2014.01.045. [DOI] [PubMed] [Google Scholar]
- Katz D.A., Liu W., Locke C., Dutta S., Tracy K.A. Clinical safety and hypothalamic-pituitary-adrenal axis effects of the arginine vasopressin type 1B receptor antagonist ABT-436. Psychopharmacology (Berl) 2016;233:71–81. doi: 10.1007/s00213-015-4089-5. [DOI] [PubMed] [Google Scholar]
- Keller C.M., Cornett E.M., Guerin G.F., Goeders N.E. Combinations of oxazepam and metyrapone attenuate cocaine and methamphetamine cue reactivity. Drug Alcohol Depend. 2013;133:405–412. doi: 10.1016/j.drugalcdep.2013.06.025. [DOI] [PubMed] [Google Scholar]
- Khan S.S., Secades-Villa R., Okuda M., Wang S., Pérez-Fuentes G., Kerridge B.T., Blanco C. Gender differences in cannabis use disorders: results from the national epidemiologic survey of alcohol and related conditions. Drug Alcohol Depend. 2013;130:101–108. doi: 10.1016/j.drugalcdep.2012.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kippin T.E., Fuchs R.A., Mehta R.H., Case J.M., Parker M.P., Bimonte-Nelson H.A., See R.E. Potentiation of cocaine-primed reinstatement of drug seeking in female rats during estrus. Psychopharmacology (Berl) 2005;182:245–252. doi: 10.1007/s00213-005-0071-y. [DOI] [PubMed] [Google Scholar]
- Kirschbaum C., Kudielka B.M., Gaab J., Schommer N.C., Hellhammer D.H. Impact of gender, menstrual cycle phase, and oral contraceptives on the activity of the hypothalamus-pituitary-adrenal axis. Psychosom. Med. 1999;61:154–162. doi: 10.1097/00006842-199903000-00006. [DOI] [PubMed] [Google Scholar]
- Knight E.L., Christian C.B., Morales P.J., Harbaugh W.T., Mayr U., Mehta P. Exogenous testosterone enhances cortisol and affective responses to social-evaluative stress in dominant men. Psychoneuroendocrinology. 2017;85:151–157. doi: 10.1016/j.psyneuen.2017.08.014.Exogenous. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knobloch H.S., Charlet A., Hoffmann L.C., Eliava M., Khrulev S., Cetin A.H., Osten P., Schwarz M.K., Seeburg P.H., Stoop R., Grinevich V. Evoked axonal oxytocin release in the central amygdala attenuates fear response. Neuron. 2012;73:553–566. doi: 10.1016/j.neuron.2011.11.030. [DOI] [PubMed] [Google Scholar]
- Kosten T., Oliveto A., Feingold A., Poling J., Sevarino K., McCance-Katz E., Stine S., Gonzalez G., Gonsai K. Desipramine and contingency management for cocaine and opiate dependence in buprenorphine maintained patients. Drug Alcohol Depend. 2003;70:315–325. doi: 10.1016/S0376-8716(03)00032-2. [DOI] [PubMed] [Google Scholar]
- Kosten T.A., Gawin F.H., Kosten T.R., Rounsaville B.J. Gender differences in cocaine use and treatment response. J. Subst. Abuse Treat. 1993;10:63–66. doi: 10.1016/0740-5472(93)90100-G. [DOI] [PubMed] [Google Scholar]
- Kowalczyk W.J., Phillips K.A., Jobes M.L., Kennedy A.P., Ghitza U.E., Agage D.A., Schmittner J.P., Epstein D.H., Preston K.L. Clonidine maintenance prolongs opioid abstinence and decouples stress from craving in daily life: a randomized controlled trial with ecological momentary assessment. Am. J. Psychiatr. 2015;172:760–767. doi: 10.1176/appi.ajp.2014.14081014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kreibich A.S., Reyes B.A.S., Curtis A.L., Ecke L., Chavkin C., Van Bockstaele E.J., Valentino R.J. Presynaptic inhibition of diverse afferents to the locus ceruleus by κ-opiate receptors: a novel mechanism for regulating the central norepinephrine system. J. Neurosci. 2008;28:6516–6525. doi: 10.1523/JNEUROSCI.0390-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krupitsky E., Zvartau E., Blokhina E., Verbitskaya E., Tsoy M., Wahlgren V., Burakov A., Masalov D., Romanova T.N., Palatkin V., Tyurina A., Yaroslavtseva T., Sinha R., Kosten T.R. Naltrexone with or without guanfacine for preventing relapse to opiate addiction in St.-Petersburg, Russia. Drug Alcohol Depend. 2013;132:674–680. doi: 10.1016/j.drugalcdep.2013.04.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kucera R., Bouskila J., Elkrief L., Fink-Jensen A., Palmour R., Bouchard J.F., Ptito M. Expression and localization of CB1R, NAPE-PLD, and FAAH in the vervet monkey nucleus accumbens. Sci. Rep. 2018;8:8689. doi: 10.1038/s41598-018-26826-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kudielka B.M., Hellhammer J., Hellhammer D.H., Wolf O.T., Pirke K.M., Varadi E., Pilz J., Kirschbaum C. Sex differences in endocrine and psychological responses to psychosocial stress in healthy elderly subjects and the impact of a 2-week dehydroepiandrosterone treatment. J. Clin. Endocrinol. Metab. 1998;83:1756–1761. doi: 10.1210/jc.83.5.1756. [DOI] [PubMed] [Google Scholar]
- Kudielka B.M., Kirschbaum C. Sex differences in HPA axis responses to stress: a review. Biol. Psychol. 2005;69:113–132. doi: 10.1016/j.biopsycho.2004.11.009. [DOI] [PubMed] [Google Scholar]
- Kupferschmidt D.A., Klas P.G., Erb S. Cannabinoid CB 1 receptors mediate the effects of corticotropin-releasing factor on the reinstatement of cocaine seeking and expression of cocaine-induced behavioural sensitization. Br. J. Pharmacol. 2012;167:196–206. doi: 10.1111/j.1476-5381.2012.01983.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwako L.E., Spagnolo P.A., Schwandt M.L., Thorsell A., George D.T., Momenan R., Rio D.E., Huestis M., Anizan S., Concheiro M., Sinha R., Heilig M. The corticotropin releasing hormone-1 (CRH1) receptor antagonist Pexacerfont in alcohol dependence: a randomized controlled experimental medicine study. Neuropsychopharmacology. 2015;40:1053–1063. doi: 10.1038/npp.2014.306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laman-Maharg A., Williams A.V., Zufelt M.D., Minie V.A., Ramos-Maciel S., Hao R., Sanchez E.O., Copeland T., Silverman J.L., Leigh A., Snyder R., Ivy Carroll F., Fennell T.R., Trainor B.C. Sex differences in the effects of a kappa opioid receptor antagonist in the forced swim test. Front. Pharmacol. 2018;9:93. doi: 10.3389/fphar.2018.00093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Land B.B., Bruchas M.R., Schattauer S., Giardino W.J., Aita M., Messinger D.I., Hnasko T.S., Palmiter R.D., Chavkin C. Activation of the kappa opioid receptor in the dorsal raphe nucleus mediates the aversive effects of stress and reinstates drug seeking. Proc. Natl. Acad. Sci. U. S. A. 2009;106:19168–19173. doi: 10.1073/pnas.0910705106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landgraf R., Neumann I.D. Vasopressin and oxytocin release within the brain: a dynamic concept of multiple and variable modes of neuropeptide communication. Front. Neuroendocrinol. 2004;25:150–176. doi: 10.1016/j.yfrne.2004.05.001. [DOI] [PubMed] [Google Scholar]
- Larson E.B., Anker J.J., Gliddon L.A., Fons K.S., Carroll M.E. Effects of estrogen and progesterone on the escalation of cocaine self-administration in female rats during extended access. Exp. Clin. Psychopharmacol. 2007;15:461–471. doi: 10.1037/1064-1297.15.5.461. [DOI] [PubMed] [Google Scholar]
- Larson E.B., Roth M.E., Anker J.J., Carroll M.E. Effect of short- vs. long-term estrogen on reinstatement of cocaine-seeking behavior in female rats. Pharmacol. Biochem. Behav. 2005;82:98–108. doi: 10.1016/j.pbb.2005.07.015. [DOI] [PubMed] [Google Scholar]
- Lê A.D., Funk D., Coen K., Tamadon S., Shaham Y. Role of κ-opioid receptors in the bed nucleus of stria terminalis in reinstatement of alcohol seeking. Neuropsychopharmacology. 2018;43:838–850. doi: 10.1038/npp.2017.120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee M.R., Glassman M., King-Casas B., Kelly D.L., Stein E.A., Schroeder J., Salmeron B.J. Complexity of oxytocin's effects in a chronic cocaine dependent population. Eur. Neuropsychopharmacol. 2014;24:1483–1491. doi: 10.1016/j.euroneuro.2014.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehavot K., Stappenbeck C.A., Luterek J.A., Kaysen D., Simpson T.L. Gender differences in relationships among PTSD severity, drinking motives, and alcohol use in a comorbid alcohol dependence and PTSD sample. Psychol. Addict. Behav. 2014;28:42–52. doi: 10.1037/a0032266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leri F., Flores J., Rodaros D., Stewart J. Blockade of stress-induced but not cocaine-induced reinstatement by infusion of noradrenergic antagonists into the bed nucleus of the stria terminalis or the central nucleus of the amygdala. J. Neurosci. 2002;22:5713–5718. doi: 10.1523/jneurosci.22-13-05713.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leri F., Tremblay A., Sorge R.E., Stewart J. Methadone maintenance reduces heroin- and cocaine-induced relapse without affecting stress-induced relapse in a rodent model of poly-drug use. Neuropsychopharmacology. 2004;29:1312–1320. doi: 10.1038/sj.npp.1300435. [DOI] [PubMed] [Google Scholar]
- Levin F.R., Mariani J.J., Brooks D.J., Pavlicova M., Cheng W., Nunes E.V. Dronabinol for the treatment of cannabis dependence: a randomized, double-blind, placebo-controlled trial. Drug Alcohol Depend. 2011;116:142–150. doi: 10.1016/j.drugalcdep.2010.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levin F.R., Mariani J.J., Pavlicova M., Brooks D.J., Glass A., Mahony A., Nunes E.V., Bisaga A., Dakwar E., Carpenter K.M., Sullivan M.A., Choi J.C. Dronabinol and lofexidine for cannabis use disorder: a randomized, double-blind, placebo-controlled trial. Drug Alcohol Depend. 2016;159:53–60. doi: 10.1016/j.drugalcdep.2015.11.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis B., Nixon S.J. Characterizing gender differences in treatment seekers. Alcohol Clin. Exp. Res. 2014;38:275–284. doi: 10.1111/acer.12228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X., Peng X.Q., Jordan C.J., Li J., Bi G.H., He Y., Yang H.J., Zhang H.Y., Gardner E.L., Xi Z.X. mGluR 5 antagonism inhibits cocaine reinforcement and relapse by elevation of extracellular glutamate in the nucleus accumbens via a CB1 receptor mechanism. Sci. Rep. 2018;8:3686. doi: 10.1038/s41598-018-22087-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ling W., Hillhouse M.P., Saxon A.J., Mooney L.J., Thomas C.M., Ang A., Matthews A.G., Hasson A., Annon J., Sparenborg S., Liu D.S., McCormack J., Church S., Swafford W., Drexler K., Schuman C., Ross S., Wiest K., Korthuis P.T., Lawson W., Brigham G.S., Knox P.C., Dawes M., Rotrosen J. Buprenorphine + naloxone plus naltrexone for the treatment of cocaine dependence: the Cocaine Use Reduction with Buprenorphine (CURB) study. Addiction. 2016;111:1416–1427. doi: 10.1111/add.13375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lofwall M.R., Nuzzo P.A., Walsh S.L. Effects of cold pressor pain on the abuse liability of intranasal oxycodone in male and female prescription opioid abusers. Drug Alcohol Depend. 2012;123:229–238. doi: 10.1016/j.drugalcdep.2011.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- López H.H. Cannabinoid-hormone interactions in the regulation of motivational processes. Horm. Behav. 2010;58:100–110. doi: 10.1016/j.yhbeh.2009.10.005. [DOI] [PubMed] [Google Scholar]
- Lu J., Wu X.Y., Zhu Q. Bin, Li J., Shi L.G., Wu J.L., Zhang Q.J., Huang M.L., Bao A.M. Sex differences in the stress response in SD rats. Behav. Brain Res. 2015;284:231–237. doi: 10.1016/j.bbr.2015.02.009. [DOI] [PubMed] [Google Scholar]
- Lu L., Liu D., Ceng X. Corticotropin-releasing factor receptor type 1 mediates stress-induced relapse to cocaine-conditioned place preference in rats. Eur. J. Pharmacol. 2001;415:203–208. doi: 10.1016/S0014-2999(01)00840-8. [DOI] [PubMed] [Google Scholar]
- Lukas S.E., Sholar M., Lundahl L.H., Lamas X., Kouri K., Wines J.D., Kragie L., Mendelson J.H. Sex differences in plasma cocaine levels and subjective effects after acute cocaine administration in human volunteers. Psychopharmacology (Berl) 1996;125:346–354. doi: 10.1007/BF02246017. [DOI] [PubMed] [Google Scholar]
- Lund T.D., Hinds L.R., Handa R.J. The androgen 5α-dihydrotestosterone and its metabolite 5α-androstan-3β,17β-diol inhibit the hypothalamo-pituitary- adrenal response to stress by acting through estrogen receptor β-expressing neurons in the hypothalamus. J. Neurosci. 2006;26:1448–1456. doi: 10.1523/JNEUROSCI.3777-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maayan R., Lotan S., Doron R., Shabat-Simon M., Gispan-Herman I., Weizman A., Yadid G. Dehydroepiandrosterone (DHEA) attenuates cocaine-seeking behavior in the self-administration model in rats. Eur. Neuropsychopharmacol. 2006;16:329–339. doi: 10.1016/j.euroneuro.2005.10.002. [DOI] [PubMed] [Google Scholar]
- Maayan R., Touati-Werner D., Shamir D., Yadid G., Friedman A., Eisner D., Weizman A., Herman I. The effect of DHEA complementary treatment on heroin addicts participating in a rehabilitation program: a preliminary study. Eur. Neuropsychopharmacol. 2008;18:406–413. doi: 10.1016/j.euroneuro.2007.12.003. [DOI] [PubMed] [Google Scholar]
- Mackie K. In: Pertwee R., editor. 2005. Distribution of cannabinoid receptors in the central and peripheral nervous system; pp. 299–325. Cannabinoids. [DOI] [PubMed] [Google Scholar]
- Majewska M.D., Harrison N.L., Schwartz R.D., Barker J.L., Paul S.M. Steroid hormone metabolites are barbiturate-like modulators of the GABA receptor. Science. 1986;232(80):1004–1007. doi: 10.1126/science.2422758. [DOI] [PubMed] [Google Scholar]
- Mantsch J.R., Baker D.A., Funk D., Lê A.D., Shaham Y. Stress-induced reinstatement of drug seeking: 20 years of progress. Neuropsychopharmacology. 2016;41:335–356. doi: 10.1038/npp.2015.142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mantsch J.R., Baker D.A., Serge J.P., Hoks M.A., Francis D.M., Katz E.S. Surgical adrenalectomy with diurnal corticosterone replacement slows escalation and prevents the augmentation of cocaine-induced reinstatement in rats self-administering cocaine under long-access conditions. Neuropsychopharmacology. 2008;33:814–826. doi: 10.1038/sj.npp.1301464. [DOI] [PubMed] [Google Scholar]
- Mantsch J.R., Katz E.S. Elevation of glucocorticoids is necessary but not sufficient for the escalation of cocaine self-administration by chronic electric footshock stress in rats. Neuropsychopharmacology. 2007;32:367–376. doi: 10.1038/sj.npp.1301077. [DOI] [PubMed] [Google Scholar]
- Mantsch J.R., Weyer A., Vranjkovic O., Beyer C.E., Baker D.A., Caretta H. Involvement of noradrenergic neurotransmission in the stress- but not cocaine-induced reinstatement of extinguished cocaine-induced conditioned place preference in mice: role for β-2 adrenergic receptors. Neuropsychopharmacology. 2010;35:2165–2178. doi: 10.1038/npp.2010.86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manzanares J., Corchero J., Fuentes J.A. Opioid and cannabinoid receptor-mediated regulation of the increase in adrenocorticotropin hormone and corticosterone plasma concentrations induced by central administration of Δ9-tetrahydrocannabinol in rats. Brain Res. 1999;839:173–179. doi: 10.1016/S0006-8993(99)01756-4. [DOI] [PubMed] [Google Scholar]
- Maric T., Sedki F., Chafetz D., Schoela N., Shalev U. A role for neuropeptide y Y5 but not the Y1-receptor subtype in food deprivation-induced reinstatement of heroin seeking in the rat. Psychopharmacology (Berl) 2011;218:693–701. doi: 10.1007/s00213-011-2362-9. [DOI] [PubMed] [Google Scholar]
- Martinez D., Slifstein M., Matuskey D., Nabulsi N., Zheng M.Q., Lin S. fei, Ropchan J., Urban N., Grassetti A., Chang D., Salling M., Foltin R.W., Carson R.E., Huang Y. Kappa-opioid receptors, dynorphin, and cocaine addiction: a positron emission tomography study. Neuropsychopharmacology. 2019;44:1720–1727. doi: 10.1038/s41386-019-0398-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDonald J., Lambert D.G. Opioid receptors. Cont. Educ. Anaesth. Crit. Care Pain. 2005;5:22–25. doi: 10.1093/bjaceaccp/mki004. [DOI] [Google Scholar]
- McFarland K., Davidge S.B., Lapish C.C., Kalivas P.W. Limbic and motor circuitry underlying footshock-induced reinstatement of cocaine-seeking behavior. J. Neurosci. 2004;24:1551–1560. doi: 10.1523/JNEUROSCI.4177-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McHugh R.K., DeVito E.E., Dodd D., Carroll K.M., Potter J.S., Greenfield S.F., Connery H.S., Weiss R.D. Gender differences in a clinical trial for prescription opioid dependence. J. Subst. Abuse Treat. 2013;45:38–43. doi: 10.1016/j.jsat.2012.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLaughlin J.P., Marton-Popovici M., Chavkin C. Κ opioid receptor antagonism and prodynorphin gene disruption block stress-induced behavioral responses. J. Neurosci. 2003;23:5674–5683. doi: 10.1523/jneurosci.23-13-05674.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLellan A.T., Lewis D.C., O'Brien C.P., Kleber H.D. Drug dependence, a chronic medical illness: implications for treatment, insurance, and outcomes evaluation. J. Am. Med. Assoc. 2000;284:1689–1695. doi: 10.1001/jama.285.4.409. [DOI] [PubMed] [Google Scholar]
- McRae-Clark A.L., Baker N.L., Maria M.M.S., Brady K.T. Effect of oxytocin on craving and stress response in marijuana-dependent individuals: a pilot study. Psychopharmacology (Berl) 2013;228:623–631. doi: 10.1007/s00213-013-3062-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McReynolds J.R., Doncheck E.M., Li Y., Vranjkovic O., Graf E.N., Ogasawara D., Cravatt B.F., Baker D.A., Liu Q.S., Hillard C.J., Mantsch J.R. Stress promotes drug seeking through glucocorticoid-dependent endocannabinoid mobilization in the prelimbic cortex. Biol. Psychiatr. 2018;84:85–94. doi: 10.1016/j.biopsych.2017.09.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McReynolds J.R., Doncheck E.M., Vranjkovic O., Ganzman G.S., Baker D.A., Hillard C.J., Mantsch J.R. CB1 receptor antagonism blocks stress-potentiated reinstatement of cocaine seeking in rats. Psychopharmacology (Berl) 2016;233:99–109. doi: 10.1007/s00213-015-4092-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McReynolds J.R., Vranjkovic O., Thao M., Baker D.A., Makky K., Lim Y., Mantsch J.R. Beta-2 adrenergic receptors mediate stress-evoked reinstatement of cocaine-induced conditioned place preference and increases in CRF mRNA in the bed nucleus of the stria terminalis in mice. Psychopharmacology (Berl) 2014;231:3953–3963. doi: 10.1007/s00213-014-3535-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meewisse M.L., Reitsma J.B., De Vries G.J., Gersons B.P.R., Olff M. Cortisol and post-traumatic stress disorder in adults: systematic review and meta-analysis. Br. J. Psychiatry. 2007;191:387–392. doi: 10.1192/bjp.bp.106.024877. [DOI] [PubMed] [Google Scholar]
- Mello N.K., Knudson I.M., Kelly M., Fivel P.A., Mendelson J.H. Effects of progesterone and testosterone on cocaine self-administration and cocaine discrimination by female rhesus monkeys. Neuropsychopharmacology. 2011;36:2187–2199. doi: 10.1038/npp.2011.130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miles O.W., May V., Hammack S.E. Pituitary adenylate cyclase-activating peptide (PACAP) signaling and the dark side of addiction. J. Mol. Neurosci. 2019;68:453–464. doi: 10.1007/s12031-018-1147-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miles O.W., Thrailkill E.A., Linden A.K., May V., Bouton M.E., Hammack S.E. Pituitary adenylate cyclase-activating peptide in the bed nucleus of the stria terminalis mediates stress-induced reinstatement of cocaine seeking in rats. Neuropsychopharmacology. 2018;43:978–986. doi: 10.1038/npp.2017.135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milivojevic V., Fox H.C., Sofuoglu M., Covault J., Sinha R. Effects of progesterone stimulated allopregnanolone on craving and stress response in cocaine dependent men and women. Psychoneuroendocrinology. 2016;65:44–53. doi: 10.1016/j.psyneuen.2015.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moons W.G., Way B.M., Taylor S.E. Oxytocin and vasopressin receptor polymorphisms interact with circulating neuropeptides to predict human emotional reactions to stress. Emotion. 2014;14:562–572. doi: 10.1037/a0035503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moran-Santa Maria M.M., Baker N.L., Ramakrishnan V., Brady K.T., McRae-Clark A.L. Impact of acute guanfacine administration on stress and cue reactivity in cocaine-dependent individuals. Am. J. Drug Alcohol Abuse. 2015;41:146–152. doi: 10.3109/00952990.2014.945590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moran-Santa Maria M.M., Flanagan J.C., Brady K.T. Ovarian hormones and drug abuse. Curr. Psychiatr. Rep. 2014;16:511. doi: 10.1007/s11920-014-0511-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakajima M., Allen S., Al'Absi M. Influences of the menstrual phase on cortisol response to stress in nicotine dependent women: a preliminary examination. Nicotine Tob. Res. 2019;21:617–622. doi: 10.1093/ntr/nty071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nawata Y., Kitaichi K., Yamamoto T. Increases of CRF in the amygdala are responsible for reinstatement of methamphetamine-seeking behavior induced by footshock. Pharmacol. Biochem. Behav. 2012;101:297–302. doi: 10.1016/j.pbb.2012.01.003. [DOI] [PubMed] [Google Scholar]
- Nawata Y., Yamaguchi T., Fukumori R., Yamamoto T. Inhibition of monoacylglycerol lipase reduces the reinstatement of methamphetamine-seeking and anxiety-like behaviors in methamphetamine self-administered rats. Int. J. Neuropsychopharmacol. 2019;22:165–172. doi: 10.1093/ijnp/pyy086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neumann I.D. Stimuli and consequences of dendritic release of oxytocin within the brain. Biochem. Soc. Trans. 2007;35:1252–1257. doi: 10.1042/BST0351252. [DOI] [PubMed] [Google Scholar]
- Notzon D.P., Kelly M.A., Choi C.J., Pavlicova M., Mahony A.L., Brooks D.J., Mariani J.J., Levin F.R. Open-label pilot study of injectable naltrexone for cannabis dependence. Am. J. Drug Alcohol Abuse. 2018;44:619–627. doi: 10.1080/00952990.2017.1423321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel S., Roelke C.T., Rademacher D.J., Cullinan W.E., Hillard C.J. Endocannabinoid signaling negatively modulates stress-induced activation of the hypothalamic-pituitary-adrenal axis. Endocrinology. 2004;145:5431–5438. doi: 10.1210/en.2004-0638. [DOI] [PubMed] [Google Scholar]
- Peterson B.M., Martinez L.A., Meisel R.L., Mermelstein P.G. Estradiol impacts the endocannabinoid system in female rats to influence behavioral and structural responses to cocaine. Neuropharmacology. 2016;110:118–124. doi: 10.1016/j.neuropharm.2016.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinos H., Collado P., Rodríguez-Zafra M., Rodríguez C., Segovia S., Guillamón A. The development of sex differences in the locus coeruleus of the rat. Brain Res. Bull. 2001;56:73–78. doi: 10.1016/S0361-9230(01)00540-8. [DOI] [PubMed] [Google Scholar]
- Purdy R.H., Morrow A.L., Moore P.H., Paul S.M. Stress-induced elevations of γ-aminobutyric acid type a receptor-active steroids in the rat brain. Proc. Natl. Acad. Sci. U. S. A. 1991;88:4553–4557. doi: 10.1073/pnas.88.10.4553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qi J., Yang J.Y., Wang F., Zhao Y.N., Song M., Wu C.F. Effects of oxytocin on methamphetamine-induced conditioned place preference and the possible role of glutamatergic neurotransmission in the medial prefrontal cortex of mice in reinstatement. Neuropharmacology. 2009;56:856–865. doi: 10.1016/j.neuropharm.2009.01.010. [DOI] [PubMed] [Google Scholar]
- Randall C.L., Roberts J.S., Del Boca F.K., Carroll K.M., Connors G.J., Mattson M.E. Telescoping of landmark events associated with drinking: a gender comparison. J. Stud. Alcohol. 1999;60:252–260. doi: 10.15288/jsa.1999.60.252. [DOI] [PubMed] [Google Scholar]
- Reddy D.S., Rogawski M.A. Stress-induced deoxycorticosterone-derived neurosteroids modulate GABA a receptor function and seizure susceptibility. J. Neurosci. 2002;22:3795–3805. doi: 10.1523/jneurosci.22-09-03795.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Redila V.A., Chavkin C. Stress-induced reinstatement of cocaine seeking is mediated by the kappa opioid system. Psychopharmacology (Berl) 2008;200:59–70. doi: 10.1007/s00213-008-1122-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reed B., Butelman E.R., Fry R.S., Kimani R., Kreek M.J. Repeated administration of opra kappa (LY2456302), a novel, short-acting, selective KOP-r antagonist, in persons with and without cocaine dependence. Neuropsychopharmacology. 2018;43:739–750. doi: 10.1038/npp.2017.205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reed S.C., Evans S.M., Bedi G., Rubin E., Foltin R.W. The effects of oral micronized progesterone on smoked cocaine self-administration in women. Horm. Behav. 2011;59:227–235. doi: 10.1016/j.yhbeh.2010.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reed S.C., Haney M., Manubay J., Campagna B.R., Reed B., Foltin R.W., Evans S.M. Sex differences in stress reactivity after intranasal oxytocin in recreational cannabis users. Pharmacol. Biochem. Behav. 2019;176:72–82. doi: 10.1016/j.pbb.2018.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Regier P.S., Claxton A.B., Zlebnik N.E., Carroll M.E. Cocaine-, caffeine-, and stress-evoked cocaine reinstatement in high vs. low impulsive rats: treatment with allopregnanolone. Drug Alcohol Depend. 2014;143:58–64. doi: 10.1016/j.drugalcdep.2014.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reichard K.L., Newton K.A., Rivera Z.M.G., De Menezes P.M.S., Schattauer S.S., Land B.B., Chavkin C. Regulation of kappa opioid receptor inactivation depends on sex and cellular site of antagonist action. Mol. Pharmacol. 2020;98:548–558. doi: 10.1124/MOLPHARM.120.000124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ressler K.J., Mercer K.B., Bradley B., Jovanovic T., Mahan A., Kerley K., Norrholm S.D., Kilaru V., Smith A.K., Myers A.J., Ramirez M., Engel A., Hammack S.E., Toufexis D., Braas K.M., Binder E.B., May V. Post-traumatic stress disorder is associated with PACAP and the PAC1 receptor. Nature. 2011;470:492–497. doi: 10.1038/nature09856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reyes B.A.S., Johnson A.D., Glaser J.D., Commons K.G., Van Bockstaele E.J. Dynorphin-containing axons directly innervate noradrenergic neurons in the rat nucleus locus coeruleus. Neuroscience. 2007;145:1077–1086. doi: 10.1016/j.neuroscience.2006.12.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richter R.M., Weiss F. In vivo CRF release in rat amygdala is increased during cocaine withdrawal in self-administering rats. Synapse. 1999;32:254–261. doi: 10.1002/(SICI)1098-2396(19990615)32:4<254::AID-SYN2>3.0.CO;2-H. [DOI] [PubMed] [Google Scholar]
- Rodaros D., Caruana D.A., Amir S., Stewart J. Corticotropin-releasing factor projections from limbic forebrain and paraventricular nucleus of the hypothalamus to the region of the ventral tegmental area. Neuroscience. 2007;150:8–13. doi: 10.1016/j.neuroscience.2007.09.043. [DOI] [PubMed] [Google Scholar]
- Rodrigues S.M., Saslow L.R., Garcia N., John O.P., Keltner D. Oxytocin receptor genetic variation relates to empathy and stress reactivity in humans. Proc. Natl. Acad. Sci. U. S. A. 2009;106:21437–21441. doi: 10.1073/pnas.0909579106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubio P., De Fonseca F.R., Martín-Calderón J.L., Del Arco I., Bartolomé S., Villanúa M.A., Navarro M. Maternal exposure to low doses of Δ9-tetrahydrocannabinol facilitates morphine-induced place conditioning in adult male offspring. Pharmacol. Biochem. Behav. 1998;61:229–238. doi: 10.1016/S0091-3057(98)00099-9. [DOI] [PubMed] [Google Scholar]
- Russo S.J., Sun W.L., Minerley A.C.E., Weierstall K., Nazarian A., Festa E.D., Niyomchai T., Akhavan A., Jenab S., Quiñones-Jenab V. Progesterone does not affect cocaine-induced conditioned place preference or locomotor activity in male rats. Ethn. Dis. 2010;20(S1):73–77. [PubMed] [Google Scholar]
- Ryan M.L., Falk D.E., Fertig J.B., Rendenbach-Mueller B., Katz D.A., Tracy K.A., Strain E.C., Dunn K.E., Kampman K., Mahoney E., Ciraulo D.A., Sickles-Colaneri L., Ait-Daoud N., Johnson B.A., Ransom J., Scott C., Koob G.F., Litten R.Z. A phase 2, double-blind, placebo-controlled randomized trial assessing the efficacy of ABT-436, a novel V1b receptor antagonist, for alcohol dependence. Neuropsychopharmacology. 2017;42:1012–1023. doi: 10.1038/npp.2016.214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SAMHSA . 2020. Results from the 2018 National Survey on Drug Use and Health: Detailed Tables. [Google Scholar]
- Schank J.R., King C.E., Sun H., Cheng K., Rice K.C., Heilig M., Weinshenker D., Schroeder J.P. The role of the neurokinin-1 receptor in stress-induced reinstatement of alcohol and cocaine seeking. Neuropsychopharmacology. 2014;39:1093–1101. doi: 10.1038/npp.2013.309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schindler A.G., Messinger D.I., Smith J.S., Shankar H., Gustin R.M., Schattauer S.S., Lemos J.C., Chavkin N.W., Hagan C.E., Neumaier J.F., Chavkin C. Stress produces aversion and potentiates cocaine reward by releasing endogenous dynorphins in the ventral striatum to locally stimulate serotonin reuptake. J. Neurosci. 2012;32:17582–17596. doi: 10.1523/JNEUROSCI.3220-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlienz N.J., Lee D.C., Stitzer M.L., Vandrey R. The effect of high-dose dronabinol (oral THC) maintenance on cannabis self-administration. Drug Alcohol Depend. 2018;187:254–260. doi: 10.1016/j.drugalcdep.2018.02.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmoutz C.D., Zhang Y., Runyon S.P., Goeders N.E. Antagonism of the neuropeptide S receptor with RTI-118 decreases cocaine self-administration and cocaine-seeking behavior in rats. Pharmacol. Biochem. Behav. 2012;103:332–337. doi: 10.1016/j.pbb.2012.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schroeder J.P., Alisha Epps S., Grice T.W., Weinshenker D. The selective dopamine β-hydroxylase inhibitor nepicastat attenuates multiple aspects of cocaine-seeking behavior. Neuropsychopharmacology. 2013;38:1032–1038. doi: 10.1038/npp.2012.267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwandt M.L., Cortes C.R., Kwako L.E., George D.T., Momenan R., Sinha R., Grigoriadis D.E., Pich E.M., Leggio L., Heilig M. The CRF1 antagonist verucerfont in anxious alcohol-dependent women: translation of neuroendocrine, but not of anti-craving effects. Neuropsychopharmacology. 2016;41:2818–2829. doi: 10.1038/npp.2016.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sedki F., Eigenmann K., Gelinas J., Schouela N., Courchesne S., Shalev U. A role for kappa-, but not mu-opioid, receptor activation in acute food deprivation-induced reinstatement of heroin seeking in rats. Addiction Biol. 2015;20:423–432. doi: 10.1111/adb.12133. [DOI] [PubMed] [Google Scholar]
- Seeman T.E., Singer B., Wilkinson C.W., McEwen B.S. Gender differences in age-related changes in HPA axis reactivity. Psychoneuroendocrinology. 2001;26:225–240. doi: 10.1016/S0306-4530(00)00043-3. [DOI] [PubMed] [Google Scholar]
- Selye H. Stress and the general adaptation syndrome. Br. Med. J. 1950;1:1383–1392. doi: 10.1159/000227975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaham Y., Erb S., Leung S., Buczek Y., Stewart J. CP-154,526, a selective, non-peptide antagonist of the corticotropin-releasing factor 1 receptor attenuates stress-induced relapse to drug seeking in cocaine- and heroin-trained rats. Psychopharmacology (Berl) 1998;137:184–190. doi: 10.1007/s002130050608. [DOI] [PubMed] [Google Scholar]
- Shaham Y., Funk D., Erb S., Brown T.J., Walker C.D., Stewart J. Corticotropin-releasing factor, but not corticosterone, is involved in stress-induced relapse to heroin-seeking in rats. J. Neurosci. 1997;17:2605–2614. doi: 10.1523/jneurosci.17-07-02605.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaham Y., Highfield D., Delfs J.M., Leung S., Stewart J. Clonidine blocks stress-induced reinstatement of heroin seeking in rats: an effect independent of locus coeruleus noradrenergic neurons. Eur. J. Neurosci. 2000;12:292–302. doi: 10.1046/j.1460-9568.2000.00899.x. [DOI] [PubMed] [Google Scholar]
- Shaham Y., Stewart J. Effects of opioid and dopamine receptor antagonists on relapse induced by stress and re-exposure to heroin in rats. Psychopharmacology (Berl) 1996;125:385–391. doi: 10.1007/BF02246022. [DOI] [PubMed] [Google Scholar]
- Shalev U., Finnie P.S., Quinn T., Tobin S., Wahi P. A role for corticotropin-releasing factor, but not corticosterone, in acute food-deprivation-induced reinstatement of heroin seeking in rats. Psychopharmacology (Berl) 2006;187:376–384. doi: 10.1007/s00213-006-0427-y. [DOI] [PubMed] [Google Scholar]
- Shansky R.M. Are hormones a “female problem” for animal research? Science. 2019;364(80):825–826. doi: 10.1126/science.aaw7570. [DOI] [PubMed] [Google Scholar]
- Shansky R.M., Glavis-Bloom C., Lerman D., McRae P., Benson C., Miller K., Cosand L., Horvath T.L., Arnsten A.F.T. Estrogen mediates sex differences in stress-induced prefrontal cortex dysfunction. Mol. Psychiatr. 2003;9:531–538. doi: 10.1038/sj.mp.4001435. [DOI] [PubMed] [Google Scholar]
- Sherman B.J., Baker N.L., Brady K.T., Joseph J.E., Nunn L.M., McRae-Clark A.L. The effect of oxytocin, gender, and ovarian hormones on stress reactivity in individuals with cocaine use disorder. Psychopharmacology (Berl) 2020;237:2031–2042. doi: 10.1007/s00213-020-05516-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherman B.J., Baker N.L., McRae-Clark A.L. Effect of oxytocin pretreatment on cannabis outcomes in a brief motivational intervention. Psychiatr. Res. 2017;249:318–320. doi: 10.1016/j.psychres.2017.01.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherman B.J., Baker N.L., Schmarder K., McRae-Clark A.L., Gray K.M. Latency to cannabis dependence mediates the relationship between age at cannabis use initiation and cannabis use outcomes during treatment in men but not women. Drug Alcohol Depend. 2020:108383. doi: 10.1016/j.drugalcdep.2020.108383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherman B.J., Caruso M.A., McRae-Clark A.L. Exogenous progesterone for cannabis withdrawal in women: feasibility trial of a novel multimodal methodology. Pharmacol. Biochem. Behav. 2019;179:22–26. doi: 10.1016/j.pbb.2019.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherman B.J., McRae-Clark A.L., Baker N.L., Sonne S.C., Killeen T.K., Cloud K., Gray K.M. Gender differences among treatment-seeking adults with cannabis use disorder: clinical profiles of women and men enrolled in the achieving cannabis cessation—evaluating N-acetylcysteine treatment (ACCENT) study. Am. J. Addict. 2017;26:136–144. doi: 10.1111/ajad.12503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shields G.S., Lam J.C.W., Trainor B.C., Yonelinas A.P. Exposure to acute stress enhances decision-making competence: evidence for the role of DHEA. Psychoneuroendocrinology. 2016;67:51–60. doi: 10.1016/j.psyneuen.2016.01.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sholler D.J., Strickland J.C., Spindle T.R., Weerts E.M., Vandrey R. Sex differences in the acute effects of oral and vaporized cannabis among healthy adults. Addiction Biol. 2020 doi: 10.1111/adb.12968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinha R. How does stress increase risk of drug abuse and relapse? Psychopharmacology (Berl) 2001;158:343–359. doi: 10.1007/s002130100917. [DOI] [PubMed] [Google Scholar]
- Sinha R., Fox H.C., Hong K.I.A., Sofuoglu M., Morgan P.T., Bergquist K.T. Sex steroid hormones, stress response, and drug craving in cocaine-dependent women: implications for relapse susceptibility. Exp. Clin. Psychopharmacol. 2007;15:445–452. doi: 10.1037/1064-1297.15.5.445. [DOI] [PubMed] [Google Scholar]
- Sinha R., Kimmerling A., Doebrick C., Kosten T.R. Effects of lofexidine on stress-induced and cue-induced opioid craving and opioid abstinence rates: preliminary findings. Psychopharmacology (Berl) 2007;190:569–574. doi: 10.1007/s00213-006-0640-8. [DOI] [PubMed] [Google Scholar]
- Sinha R., Shaham Y., Heilig M. Translational and reverse translational research on the role of stress in drug craving and relapse. Psychopharmacology (Berl) 2011;218:69–82. doi: 10.1007/s00213-011-2263-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sofuoglu M., Mitchell E., Kosten T.R. Effects of progesterone treatment on cocaine responses in male and female cocaine users. Pharmacol. Biochem. Behav. 2004;78:699–705. doi: 10.1016/j.pbb.2004.05.004. [DOI] [PubMed] [Google Scholar]
- Sofuoglu M., Poling J., Gonzalez G., Gonsai K., Oliveto A., Kosten T.R. Progesterone effects on cocaine use in male cocaine users maintained on methadone: a randomized, double-blind, pilot study. Exp. Clin. Psychopharmacol. 2007;15:453–460. doi: 10.1037/1064-1297.15.5.453. [DOI] [PubMed] [Google Scholar]
- Solowij N., Broyd S.J., Beale C., Prick J.A., Greenwood L.M., Van Hell H., Suo C., Galettis P., Pai N., Fu S., Croft R.J., Martin J.H., Yücel M. Therapeutic effects of prolonged cannabidiol treatment on psychological symptoms and cognitive function in regular cannabis users: a pragmatic open-label clinical trial. Cannabis Cannabinoid Res. 2018;3:21–34. doi: 10.1089/can.2017.0043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sorge R.E., Rajabi H., Stewart J. Rats maintained chronically on buprenorphine show reduced heroin and cocaine seeking in tests of extinction and drug-induced reinstatement. Neuropsychopharmacology. 2005;30:1681–1692. doi: 10.1038/sj.npp.1300712. [DOI] [PubMed] [Google Scholar]
- Stauffer C.S., Moschetto J.M., McKernan S., Meinzer N., Chiang C., Rapier R., Hsiang E., Norona J., Borsari B., Woolley J.D. Oxytocin-enhanced group therapy for methamphetamine use disorder: randomized controlled trial. J. Subst. Abuse Treat. 2020;116:108059. doi: 10.1016/j.jsat.2020.108059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stauffer C.S., Musinipally V., Suen A., Lynch K.L., Shapiro B., Woolley J.D. A two-week pilot study of intranasal oxytocin for cocaine-dependent individuals receiving methadone maintenance treatment for opioid use disorder. Addiction Res. Theor. 2016;24:490–498. doi: 10.3109/16066359.2016.1173682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanoue A., Ito S., Honda K., Oshikawa S., Kitagawa Y., Koshimizu T.A., Mori T., Tsujimoto G. The vasopressin V1b receptor critically regulates hypothalamic-pituitary-adrenal axis activity under both stress and resting conditions. J. Clin. Invest. 2004;113:302–309. doi: 10.1172/JCI200419656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terner J.M., de Wit H. Menstrual cycle phase and responses to drugs of abuse in humans. Drug Alcohol Depend. 2006;84:1–13. doi: 10.1016/j.drugalcdep.2005.12.007. [DOI] [PubMed] [Google Scholar]
- Tham M., Yilmaz O., Alaverdashvili M., Kelly M.E.M., Denovan-Wright E.M., Laprairie R.B. Allosteric and orthosteric pharmacology of cannabidiol and cannabidiol-dimethylheptyl at the type 1 and type 2 cannabinoid receptors. Br. J. Pharmacol. 2019;176:1455–1469. doi: 10.1111/bph.14440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas M.B., Becker J.B. Sex differences in prenatal stress effects on cocaine pursuit in rats. Physiol. Behav. 2019;203:3–9. doi: 10.1016/j.physbeh.2017.10.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tirado C.F., Goldman M., Lynch K., Kampman K.M., Obrien C.P. Atomoxetine for treatment of marijuana dependence: a report on the efficacy and high incidence of gastrointestinal adverse events in a pilot study. Drug Alcohol Depend. 2008;94:254–257. doi: 10.1016/j.drugalcdep.2007.10.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torres J.M., Ruiz E., Ortega E. Effects of CRH and ACTH administration on plasma and brain neurosteroid levels. Neurochem. Res. 2001;26:555–558. doi: 10.1023/A:1010925331768. [DOI] [PubMed] [Google Scholar]
- Toufexis D.J., Davis C., Hammond A., Davis M. Progesterone attenuates corticotropin-releasing factor-enhanced but not fear-potentiated startle via the activity of its neuroactive metabolite, allopregnanolone. J. Neurosci. 2004;24:10280–10287. doi: 10.1523/JNEUROSCI.1386-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trigo J.M., Lagzdins D., Rehm J., Selby P., Gamaleddin I., Fischer B., Barnes A.J., Huestis M.A., Le Foll B. Effects of fixed or self-titrated dosages of Sativex on cannabis withdrawal and cravings. Drug Alcohol Depend. 2016;161:298–306. doi: 10.1016/j.drugalcdep.2016.02.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Twining R.C., Wheeler D.S., Ebben A.L., Jacobsen A.J., Robble M.A., Mantsch J.R., Wheeler R.A. Aversive stimuli drive drug seeking in a state of low dopamine tone. Biol. Psychiatr. 2015;77:895–902. doi: 10.1016/j.biopsych.2014.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ubaldi M., Giordano A., Severi I., Li H., Kallupi M., De Guglielmo G., Ruggeri B., Stopponi S., Ciccocioppo R., Cannella N. Activation of hypocretin-1/orexin-a neurons projecting to the bed nucleus of the stria terminalis and paraventricular nucleus is critical for reinstatement of alcohol seeking by neuropeptide S. Biol. Psychiatr. 2016;79:452–462. doi: 10.1016/j.biopsych.2015.04.021. [DOI] [PubMed] [Google Scholar]
- Uhart M., Chong R.Y., Oswald L., Lin P.I., Wand G.S. Gender differences in hypothalamic-pituitary-adrenal (HPA) axis reactivity. Psychoneuroendocrinology. 2006;31:642–652. doi: 10.1016/j.psyneuen.2006.02.003. [DOI] [PubMed] [Google Scholar]
- van Heukelum S., Mars R.B., Guthrie M., Buitelaar J.K., Beckmann C.F., Tiesinga P.H.E., Vogt B.A., Glennon J.C., Havenith M.N. Where is cingulate cortex? A cross-species view. Trends Neurosci. 2020;43:285–299. doi: 10.1016/j.tins.2020.03.007. [DOI] [PubMed] [Google Scholar]
- Vaughn L.K., Mantsch J.R., Vranjkovic O., Stroh G., LaCourt M., Kreutter M., Hillard C.J. Cannabinoid receptor involvement in stress-induced cocaine reinstatement: potential interaction with noradrenergic pathways. Neuroscience. 2012;204:117–124. doi: 10.1016/j.neuroscience.2011.08.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vela G., Martín S., García-Gil L., Crespo J.A., Ruiz-Gayo M., Javier Fernández-Ruiz J., García-Lecumberri C., Pélaprat D., Fuentes J.A., Ramos J.A., Ambrosio E. Maternal exposure to δ9-tetrahydrocannabinol facilitates morphine self-administration behavior and changes regional binding to central μ opioid receptors in adult offspring female rats. Brain Res. 1998;807:101–109. doi: 10.1016/S0006-8993(98)00766-5. [DOI] [PubMed] [Google Scholar]
- Vijay A., Wang S., Worhunsky P., Zheng M.-Q., Nabulsi N., Ropchan J., Krishnan-Sarin S., Huang Y., Morris E.D. PET imaging reveals sex differences in kappa opioid receptor availability in humans, in vivo. Am. J. Nucl. Med. Mol. Imaging. 2016;6:205–214. [PMC free article] [PubMed] [Google Scholar]
- Vranjkovic O., Gasser P.J., Gerndt C.H., Baker D.A., Mantsch J.R. Stress-induced cocaine seeking requires a beta-2 adrenergic receptor-regulated pathway from the ventral bed nucleus of the stria terminalis that regulates CRF actions in the ventral tegmental area. J. Neurosci. 2014;34:12504–12514. doi: 10.1523/JNEUROSCI.0680-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vranjkovic O., Hang S., Baker D.A., Mantsch J.R. β-adrenergic receptor mediation of stress-induced reinstatement of extinguished cocaine-induced conditioned place preference in mice: roles for β1 and β2 adrenergic receptors. J. Pharmacol. Exp. Therapeut. 2012;342:541–551. doi: 10.1124/jpet.112.193615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vranjkovic O., Van Newenhizen E.C., Nordness M.E., Blacktop J.M., Urbanik L.A., Mathy J.C., McReynolds J.R., Miller A.M., Doncheck E.M., Kloehn T.M., Stinnett G.S., Gerndt C.H., Ketchesin K.D., Baker D.A., Seasholtz A.F., Mantsch J.R. Enhanced CRFR1-dependent regulation of a ventral tegmental area to prelimbic cortex projection establishes susceptibility to stress-induced cocaine seeking. J. Neurosci. 2018;38:10657–10671. doi: 10.1523/JNEUROSCI.2080-18.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wanat M.J., Bonci A., Phillips P.E.M. CRF acts in the midbrain to attenuate accumbens dopamine release to rewards but not their predictors. Nat. Neurosci. 2013;16:383–385. doi: 10.1038/nn.3335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang B., Shaham Y., Zitzman D., Azari S., Wise R.A., You Z.B. Cocaine experience establishes control of midbrain glutamate and dopamine by corticotropin-releasing factor: a role in stress-induced relapse to drug seeking. J. Neurosci. 2005;25:5389–5396. doi: 10.1523/JNEUROSCI.0955-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang B., You Z.B., Rice K.C., Wise R.A. Stress-induced relapse to cocaine seeking: roles for the CRF2 receptor and CRF-binding protein in the ventral tegmental area of the rat. Psychopharmacology (Berl) 2007;193:283–294. doi: 10.1007/s00213-007-0782-3. [DOI] [PubMed] [Google Scholar]
- Wang X., Cen X., Lu L. Noradrenaline in the bed nucleus of the stria terminalis is critical for stress-induced reactivation of morphine-conditioned place preference in rats. Eur. J. Pharmacol. 2001;432:153–161. doi: 10.1016/S0014-2999(01)01487-X. [DOI] [PubMed] [Google Scholar]
- Xi Z.X., Gilbert J.G., Peng X.Q., Pak A.C., Li X., Gardner E.L. Cannabinoid CB1 receptor antagonist AM251 inhibits cocaine-primed relapse in rats: role of glutamate in the nucleus accumbens. J. Neurosci. 2006;26:8531–8536. doi: 10.1523/JNEUROSCI.0726-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xi Z.X., Peng X.Q., Li X., Song R., Zhang H.Y., Liu Q.R., Yang H.J., Bi G.H., Li J., Gardner E.L. Brain cannabinoid CB2 receptors modulate cocaine's actions in mice. Nat. Neurosci. 2011;14:1160–1168. doi: 10.1038/nn.2874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yadid Gal G., Sudai E., Maayan R., Gispan I., Weizman A. The role of dehydroepiandrosterone (DHEA) in drug-seeking behavior. Neurosci. Biobehav. Rev. 2010;35:303–314. doi: 10.1016/j.neubiorev.2010.03.003. [DOI] [PubMed] [Google Scholar]
- Yonkers K.A., Forray A., Nich C., Carroll K.M., Hine C., Merry B.C., Shaw H., Shaw J., Sofuoglu M. Progesterone for the reduction of cocaine use in post-partum women with a cocaine use disorder: a randomised, double-blind, placebo-controlled, pilot study. The Lancet Psychiatry. 2014;1:360–367. doi: 10.1016/S2215-0366(14)70333-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young E.A. Glucocorticoid cascade hypothesis revisited: role of gonadal steroids. Depression. 1995;3:20–27. doi: 10.1002/depr.3050030105. [DOI] [Google Scholar]
- Young E.A., Altemus M. Puberty, ovarian steroids, and stress. Ann. N. Y. Acad. Sci. 2004;1021:124–133. doi: 10.1196/annals.1308.013. [DOI] [PubMed] [Google Scholar]
- Zanos P., Georgiou P., Wright S.R., Hourani S.M., Kitchen I., Winsky-Sommerer R., Bailey A. The oxytocin analogue carbetocin prevents emotional impairment and stress-induced reinstatement of opioid-seeking in morphine-abstinent mice. Neuropsychopharmacology. 2014;39:855–865. doi: 10.1038/npp.2013.285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang H.Y., Gao M., Shen H., Bi G.H., Yang H.J., Liu Q.R., Wu J., Gardner E.L., Bonci A., Xi Z.X. Expression of functional cannabinoid CB2 receptor in VTA dopamine neurons in rats. Addiction Biol. 2017;22:752–765. doi: 10.1111/adb.12367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao L.Y., Shi J., Zhang X.L., Epstein D.H., Zhang X.Y., Liu Y., Kosten T.R., Lu L. Stress enhances retrieval of drug-related memories in abstinent heroin addicts. Neuropsychopharmacology. 2010;35:720–726. doi: 10.1038/npp.2009.179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Y., Leri F., Cummins E., Hoeschele M., Kreek M.J. Involvement of arginine vasopressin and V1b receptor in heroin withdrawal and heroin seeking precipitated by stress and by heroin. Neuropsychopharmacology. 2008;33:226–236. doi: 10.1038/sj.npp.1301419. [DOI] [PubMed] [Google Scholar]
- Zhou Y., Leri F., Cummins E., Kreek M.J. Individual differences in gene expression of vasopressin, D2 receptor, POMC and orexin: vulnerability to relapse to heroin-seeking in rats. Physiol. Behav. 2015;139:127–135. doi: 10.1016/j.physbeh.2014.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zorrilla E.P., Valdez G.R., Weiss F. Changes in levels of regional CRF-like-immunoreactivity and plasma corticosterone during protracted drug withdrawal in dependent rats. Psychopharmacology (Berl) 2001;158:374–381. doi: 10.1007/s002130100773. [DOI] [PubMed] [Google Scholar]