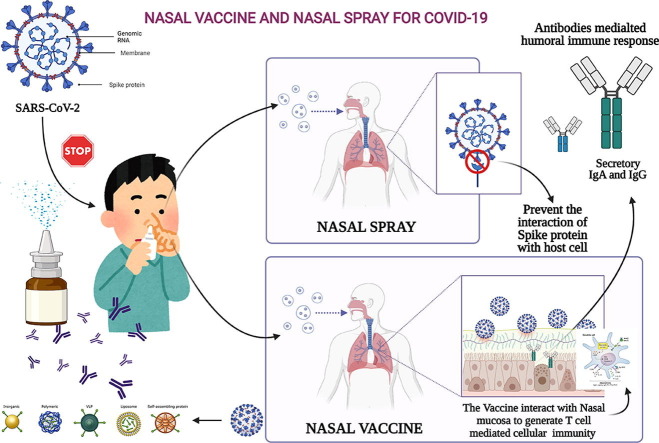
Graphical abstract
Keywords: COVID-19, SARS-CoV-2, Nasal vaccine, Nasal spray, Antigen-presenting cells (APCs), Dendritic cells
Abstract
Unlike conventional Coronavirus 2019 (COVID-19) vaccines, intranasal vaccines display a superior advantage because the nasal mucosa is often the initial site of infection. Preclinical and clinical studies concerning intranasal immunization elicit high neutralizing antibody generation and mucosal IgA and T cell responses that avoid severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection in both; the upper and lower respiratory tract. A nasal formulation is non-invasive with high appeal to patients. Intranasal vaccines enable self-administration and can be designed to survive at ambient temperatures, thereby simplifying logistical aspects of transport and storage. In this review, we provide an overview of nasal vaccines with a focus on formulation development as well as ongoing preclinical and clinical studies for SARS-CoV-2 intranasal vaccine products.
Introduction
Vaccines take advantage of the remarkable capacity of the human immune system to respond to, and recall, pathogenic material it encounters. An ideal vaccine should provide rapid, multifaceted, long-term protection by preventing the disease from causing severe disease, hospitalization, and death. Post vaccination, the adaptive immune response is mediated by B cells that produce antibodies and by T cells.1 More than 4.31 billion doses of COVID-19 vaccine have been administered across 180 countries at a rate of 42.5 million doses a day.2 As of May 2, 2021, more than 189 candidate vaccines against SARS-CoV-2 were under different stages of early-stage (75 vaccine candidates under active investigation in animals) and clinical development (99 vaccines in clinical trials on humans).3, 4 Intramuscular administration (Table 1 ) induces a strong serum IgG reflex that is believed to defend the lower respiratory tract but will not trigger the epithelial cell IgA responses (in both serum and respiratory fluids) necessary to protect the upper respiratory tract.5 IgA can reach to upper respiratory tact through mucociliary process but only when the serum IgG concentration is high.
Table 1.
Emergency use-approved COVID-19 vaccine candidates.
| Vaccine | Company | Country of origin | Type of vaccine | Dose | Clinical trials | Approval |
|---|---|---|---|---|---|---|
| Comirnaty (BNT162b2) | Pfizer, BioNtech, Fosun Pharma | Multinational | mRNA vaccine (nucleoside modified) | 2 | 18 trials in 12 countries | Approved in 85 countries with WHO approval |
| Moderna COVID-19(mRNA-1273) | Moderna, BARDA, NIAID | USA | LNP-encapsulated mRNA vaccine encoding S-protein | 2 | 16 trials in three countries | Approved in 45 countries |
| COVAXIN | Bharat Biotech, ICMR | India | Whole-virion inactivated Vero cells | 2 with a booster dose | Five trials in two countries | Approved in nine countries |
| JNJ-78436735 or Ad26.COV2.S | Johnson & Johnson | The Netherlands and USA | Adv serotype 26(Ad26)vector-based DNA vaccine (nonreplicating viral vector) | 1 | Eight trials in 17 countries | Approved in 41 countries with WHO approval |
| CoronaVac | Sinovac Biotech | China | Inactivated vaccine | 2 | 14 trials in seven countries | Approved in 24 countries |
| Sputnik V | Gamaleya Research Institute, Acellena Contract Drug Research, and Development | Russia | Adenovirus vector vaccine | 2 | 19 trials in six countries | Approved in 65 countries |
| COVID-19 Vaccine AstraZeneca (AZD1222); also known as Vaxzevria and Covishield | Jenner Institute (University of Oxford), Cobra Biologics, Oxford Biomedica, Merck KGaA, Halix BV, Pall Corporation, SGS, India’s Serum Institute, AstraZeneca, Catalent Biologics, CSL Limited | UK | Nonreplicating viral vector (genetically modified virus) | 2 | 25 trials in 15 countries | Approved in 93 countries, with WHO approval |
Consequently, most vaccines only protect against lower respiratory tract infections but do not induce sterilizing immunity in the upper airway. Nasal vaccine delivery could not only provide defense against symptomatic diseases, but also prevent virus dissemination by infected individuals. A vaccination that causes sterilizing immunity in the upper airway will be preferable for preventing virus transmission.6
An intranasal vaccine is a promising option because it closely matches the normal route of infection, self-administration is easy, and it could gain a fair market share in future years.7 Intranasal immunization elicited high neutralizing antibody responses and mucosal IgA and T cell responses that almost completely vanishes the SARS-CoV-2 infections in both the upper and lower respiratory tract.8 A nasal spray, unlike injection, is painless and appeals to those who are afraid of needles.
SARS-CoV-2 life cycle
Since the first report of a coronavirus-related pneumonia outbreak in December 2019, the virus SARS-CoV-2 that triggers the COVID-19 has become a pandemic, with > 200 million people in over 220 countries reported to be infected and around three million people dying from COVID-19, as of May 2021.9 A coronavirus comprises a lipid bilayer wrapping with spike-like projections on a surface formed of glycoprotein.10
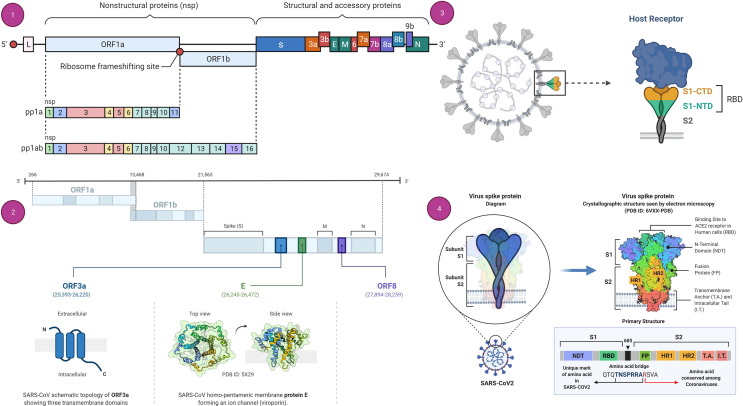
Its structural component mainly comprises five kinds of proteins: Spike protein (S), Membrane protein (M), Envelope protein (E), Nucleocapsid (N), and RNA Genome (R, 26–32 kb in length).11 The S-protein has two domains or subunits in addition to the receptor-binding motif; that is, an S1 subunit (bilobular receptor-binding domain) and an S2 subunit (stalk fusion domain). The viral structure and genomic organization of SARS-CoV-2 are shown in Fig. 1 . Genetically, it contains nonsegmented positive-sense single-stranded RNA (ssRNA), which is encapsulated in a capsid formed of protein; hence, it is an enveloped virus.12, 13 The S-protein attaches to the Angiotensin-converting enzyme 2 (ACE-2) receptor, which is found in the lung epithelia of the host.14, 15 Viral entry into the host cell is facilitated by endocytosis, for that virus uses human cell machinery.16, 17
Figure 1.
Genomic organization of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) and Spike (S)-protein structure. (a) Genome organization of SARS-CoV-2. (b) The genomic organization of SARS-CoV-2, highlighting the viroporins ORF3a (blue), E (green), and ORF8 (purple), as well as their propozed topology/3D structures. (c) Schematic of the S-protein–receptor binding mechanism of SARS-CoV-2. (d) The S-protein of SARS-CoV2 comprises two subunits, S1 and S2, and is commonly represented as a sword-like spike. The Protein Data Bank (PDB) model of this glycoprotein reveals how the subunits are formed of different regions that are fundamental to the infection process. S1 and S2 are linked by a polybasic amino acid bridge, which might be important for viral targeting.
The virus must access the host cell cytosol following receptor binding by endosomal cysteine protease cathepsins.18, 19 Transmembrane protease serine 2 (TMPRRS2) or TMPRSS11D implement S1/S2 cleavage to invoke S-protein followed by viral and cellular membrane fusion. S-protein cleavage operates at two locations inside the S2 protein element, with the first cleavage dividing the receptor-binding domain from the fusion domain of the S-protein while second cleavage facilitate membrane fusion.20 The coronavirus life cycle then proceeds to translate the replicase gene from the virion genomic RNA once the nucleocapsid has escaped and become uncoated.21, 22, 23
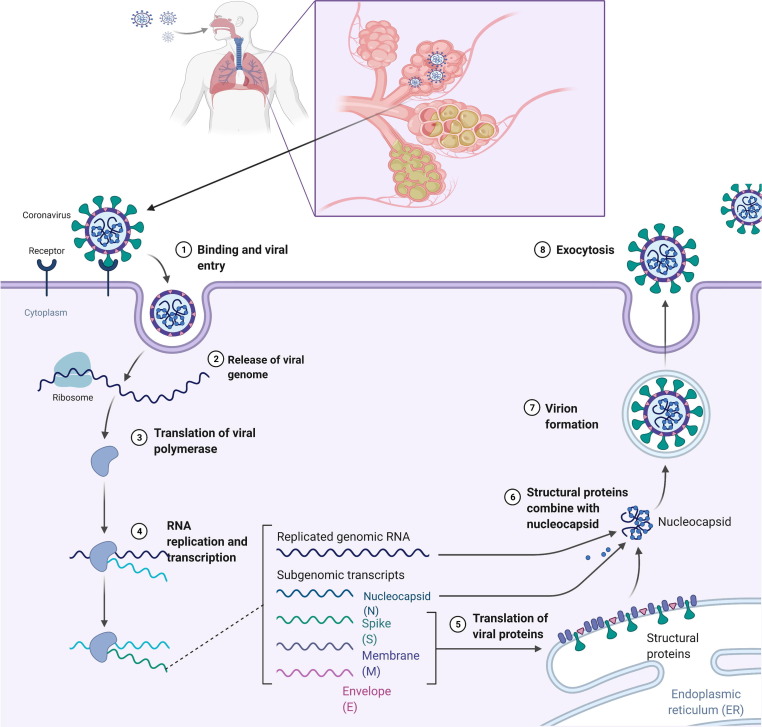
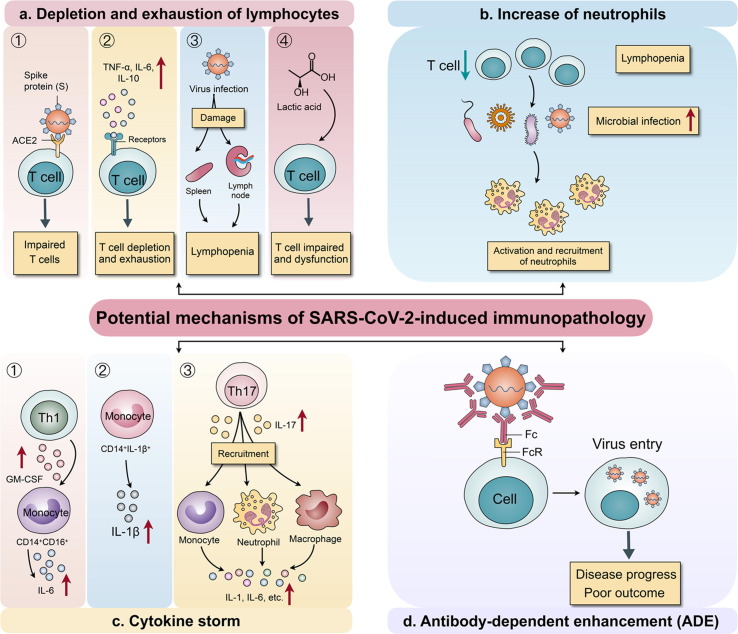
The transmission and life cycle of SARS-CoV-2 is summarized in Fig. 2 . Various proteins are generated once viral RNA fuses with the vesicle and is translated, assembled into new virus particles, and released by the damaged host cells.24 The host immune system starts acting against invading SARS-CoV-2 via a humoral immune response (i.e., antibodies). Levels of immunoglobulins (IgA, IgM, and IgG) increase, which alert the immune system to the infection.25 As a consequence, the host can experience respiratory symptoms that further lead to a cytokine storm, which causes vasodilation followed by hypotension.26 As a result, a reduced amount of blood reaches the organs, leading to organ damage. IL-1, IL-6, and TNF-α (cytokines) also act on the hypothalamus, leading to fever (Fig. 3 ).27
Figure 2.
Replication cycle of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) in the host cell.
Figure 3.
Immunological and pathological consequences of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection in the host. (a) Depletion and exhaustion of lymphocytes because of impaired T cell function as viruses invade T cells through angiotensin-converting enzyme 2 (ACE2) receptors, aided by proinflammatory mediators. Lymphopenia occurs as a result of the damaging effect of SARS-CoV-2 on the spleen and lymph; the increase in lactic acid also impacts T cell proliferation. (b) Neutrophils increase in response to infection caused by lymphopenia. (c) A cytokine storm results from increased monocyte production, which promotes IL-1β production, whereby Th17 cells impart synergism and CD4 + T cells mediate the increase in IL-6. (d) A neutralising antibody attacking the virus will increase virus entry into cells through the Fc region of the antibody attached to the Fc receptor (FcR) on cells; this is linked to disease progression and adverse outcomes in patients with coronavirus 2019 (COVID-19). Adapted under Creative Commons Attribution 4.0 from28.
Given the ability of SARS-CoV-2 to mutate and evolve new variants, it might be a battle to develop a fully competent vaccine.29 ‘Triple mutant’ mutants were discovered in India and California, classified as delta variant by the WHO.30 At the time of writing, there were five variants of concern as classified by the US Food and Drug Administration (FDA), whereas eight variants of interest have been identified and classified.31 The fear is that these new strains will evade natural infection-induced immunity as well as existing vaccination attempts.
Therapeutic potential of the nasal route against COVID-19
mRNA vaccines developed by Pfizer/BioNTech and Moderna are available in the USA, UK, and elsewhere. Oxford/AstraZeneca AZD1222 (Covishield in India), JNJ-78436735 (Janssen Pharmaceutical, USA) and Gamaleya Sputnik V (Russia) are three adenoviral vector vaccines that have also been approved. Inactivated coronavirus spore-based vaccines, produced by Sinopharm and Sinovac in China, constitute the third form of vaccine.32 They are usually administered in one or two doses, intramuscularly, in the upper arm. Intramuscular injections sometimes cause a local reaction, such as discomfort or swelling at the injection site.33
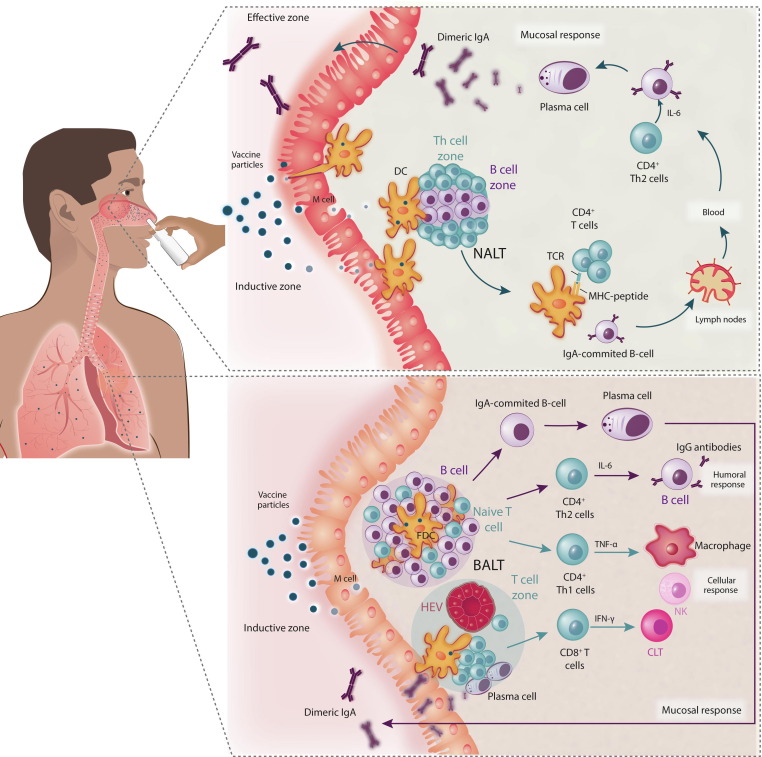
Intramuscular injections elicit a systemic humoral reaction to a vaccine, mediated by B cells, resulting in the generation of IgM antibodies first, followed by IgG.34 Humoral immune systems are generally characterised by primary IgM antibody levels, accompanied by secondary IgG, IgA, and IgE antibody responses consistent with acquired immunity.35 This mechanism functions complementary to the T cell immune response. However, in respiratory viruses, such as SARS-CoV-2, the mucosal immune system is the first line of protective immune defense in the nasopharynx-associated lymphoid tissue (NALT), with the pathogen-mediated reaction occurring predominantly from IgA antibodies generated by mucosal epithelial cells (Fig. 4 ).36 The palatine tonsils and other lymph epithelial complexes in Waldeyer’s pharyngeal ring, such as the adenoids in mammals, are examples of these structures in the upper airways. IgA and IgG responses have a negative association in COVID-19 infection; that is, a robust systemic IgG response to a vaccine cannot have an adequate mucosal IgA response.37 This means that systemically vaccinated individuals are susceptible to SARS-CoV-2 infection through the upper respiratory tract if they are asymptomatic because of a lack of mucosal immunity.32
Figure 4.
Effect of nasal vaccines on the upper and lower respiratory tract for the generation of mucosal and systemic immunity. (a) Protective immune responses in the nasopharynx-associated lymphoid tissue (NALT), with the pathogen-mediated reaction resulting mainly from by secretory IgA antibodies generated by mucosal epithelial cells. (b) Humoral immune response in the lower respiratory tract with bronchus-associated lymphoid tissue (BALT) having humoral as well as mucosal/local immune responses. Abbreviations: CTL, cytotoxic T lymphocyte; DC, dendritic cell; NK, natural killer; TCR, T cell receptor.
Mucosal immune activities are linked to lymphoid tissues, specifically mucosa-associated lymphoid tissue (MALT), found in mucosal tissue of the nose, lungs, gastrointestinal tract, and vaginal/rectal surfaces.38 It is again bifurcated as NALT in the nasal cavity and bronchus-associated lymphoid tissue (BALT) in the lower respiratory tract.39 In reaction to invasion by pathogenic organisms or immunogenic agents, epithelial cells, lymphocytes, and underlying antigen-presenting cells [APCs; e.g., dendritic cells (DCs) and macrophages], cytokines, and chemokines mount an endogenous, nonspecific, and adaptive immune response.40 By nonspecific endocytosis or communicating with pattern recognition receptors (PRRs), such as Toll-like receptors (TLRs), epithelial cells can recognize and take up pathogenic species and/or antigenic components.41 NALT comprizes lymphoid tissue, B cells, T cells, and APCs, and is covered by an epithelial layer containing memory (M) cells (antigen transporter cells).42 Activation of antigen-specific secretory IgA (sIgA) antibodies prevents pathogens and toxins from adhering to or infecting epithelial cells and disrupting the mucosal barrier.43 Pathogens or immunogenic agents might be able to cross the nasal epithelium and communicate with APCs, including macrophages and DCs. APCs filter the antigen before migrating to the lymph node, where the immunogenic component is presented to T cells to activate the immune cascade. APCs can recognize soluble antigens, whereas particulate antigen is normally taken up by M cells and transported to NALT. NALT drains to the lymph node, where further antigen synthesis occurs.44 As an antibody reaction, interaction with pathogens or antigens can result in IgA secretion. Intracellular antigens are usually stored in host cells before being bound to a major histocompatibility complex I (MHC-I), a cell surface molecule, and transferred to the cell surface. The presence of MHC-I on the cell surface causes CD8 + T cells to become cytotoxic T lymphocytes (CTLs). Endocytosed extracellular antigens are presented on MHC-II molecules for activation.45 A nasal vaccine might also stimulate Th-17 CD4 + cells. Th-17 cells are responsible for developing proinflammatory interleukins, such as IL-17A, IL-22, IL-17F, and IL-21.46 As a result, effective vaccines that protect at these sites are desperately needed. The memory T cells associated with CD8+ T cell have longevity of systemic immunity. Apart from the ease of administration, the mucosal IgA reaction to an intranasal vaccine should provide successful systemic defense.47
Following mucosal immunization, antigen-activated B and T cells exit the draining lymph nodes, travel across the lymph, penetrate the bloodstream, and ‘seed’ the mucosal tissues. Intranasally administered adjuvants improve immune responses primarily by augmenting the innate immune response through the upregulation of co-stimulatory molecule, chemokine, and cytokine expression.48 Intranasal immunization has been shown to activate cross-reactive antibodies, which might be suggestive of cross-protection. Given that cross-protective vaccines can develop cross-reactive antibodies that recognize more than one antigen, this effect might render vaccines more effective by reducing the number of vaccinations needed.49
Challenges to nasal vaccine development
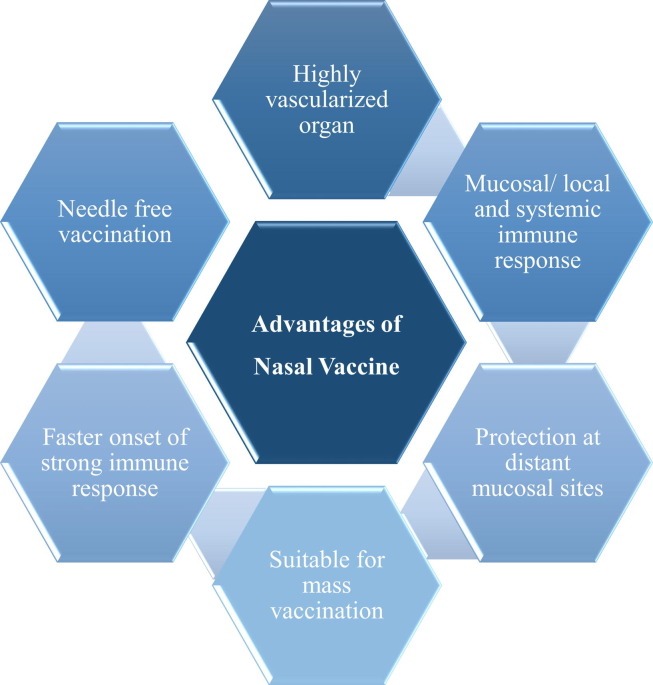
SARS-CoV-2 infection starts at the mucosal surface of the upper respiratory system; hence, mucosal immunity would provide effective and long-lasting defense against coronavirus invasion (Fig. 5 ). However, all commercial approved emergency-use vaccines are administered systemically, resulting in only humoral immune responses and no CoV-specific mucosal immunity. In general, several considerations, including antigen, adjuvant, formulation, and animal models for efficacy and safety evaluation, should be considered for effective and safe nasal vaccine production.50 Successful nasal vaccine development usually includes: (1) antigens for eliciting a particular adaptive immune response; (2) immunostimulants to activate the innate immune system; and (3) device for vaccine delivery. One of the most prominent difficulties in developing a nasal vaccine is nasal clearing. Mucus serves as a sticky solvent in the nasal mucosa. Cilia acts as a protective barrier, preventing infectious chemicals, bacteria, and debris from being transported into the lungs by inhaled breath. The absorption of antigen is influenced by the residence time in the mucosa. If mucociliary clearance increases, antigen absorption decreases. When administered intranasally, a vaccine is predisposed to elicit Th17 immune responses, which can be detrimental to the removal of SARS-CoV-2 from the lungs.51 Another constraint to a nasal or pulmonary COVID-19 vaccine is the need for a specialised delivery system, which can impose additional costs on the vaccine formulation. The unique biopharmaceutical obstacles that intranasally administered protein vaccines must overcome before reaching the target site, as well as the comparatively poor immunogenicity and low stability of the protein antigens, necessitate thoughtful and fine-tuned nasal vaccine formulations, including the use of immunostimulants and the determination of the appropriate vaccine delivery method.52 A successful vaccine composition for intranasal delivery keeps the antigen steady, guarantees its retention in the nasopharyngeal area long enough to communicate with the lymphatic system, and activates the immune system, with or without additional adjuvants, to provide long-term protection.53
Figure 5.
Advantages of nasal vaccines over conventional coronavirus 2019 (COVID-19) vaccines.
Formulation adjuvants/delivery systems for intranasal vaccination
To achieve effective mucosal immunization through the nasal route, the vaccine formulation has a crucial role in terms of maintenance of antigen stability, prolonged residence time in the nasal mucosa, and compatibility with other vaccine components, including adjuvants. Vaccine formulations along with antigenic components contain a variety of agents, including nanocarriers (in recent vaccines), stabilizers, adjuvants, cryoprotectants, buffers, antibiotics, and preservatives. These complement the antigenic component in maintaining immunogenicity before and after vaccine administration. The primary factors governing nasal vaccine stability are storage temperature and the formulation pH. However, vaccines comprizing biomolecules such as mRNA, peptides, or even carbohydrates can also face a problem pertaining to protein aggregation and hydrolysis, thereby necessitating the use of stabilizers.54 Stabilizers also improve the thermostability of vaccines. Commonly used intranasal viral vaccine stabilizers include monosodium glutamate, lactose, sorbitol, porcine gelatin, arginine, tricine, and sucrose.55 The role of adjuvants in vaccine formulations is to enhance the immunogenicity, and they can be classified on the basis of their origin, mechanism of action, and physicochemical properties.56 Adjuvants can also be hybrid in nature, a common example being a combination of monophosphoryl lipid A (MPL) and aluminium salt (AS04 used in the Cervarix vaccine) or MPL and QS-21, an extract from Chilean soapbark tree (AS01B used in the Shingrix vaccine).57 Chitosan, heat-labile enterotoxin (LT), and cholera toxin (CT) are some of the most commonly used mucosal adjuvants for the purpose of mucosal immunization, primarily via the intranasal route.56 Another interesting approach is the use of virus-like particles (VLP), which mimic the structural properties of a virus and can also be enveloped. Examples of virosome-based intranasal influenza vaccines include Inflexal® V, Nasalflu®, Invivac®, and Fluad®.58, 59 Adjuvants have to go through a series of evaluation studies to determine their safety and efficacy and are also continuously monitored by the Centers for Disease Control and Prevention (CDC) and FDA post approval.57 Apart from these major components, vaccine formulations might contain trace amounts of antibiotics (e.g., neomycin or gentamycin) to prevent bacterial contamination of viral tissue culture cells during manufacturing, and preservatives (e.g., thiomersal) in multidose vaccines for prevention of bacterial and fungal growth during storage. Implementation of aerosolized vaccines for inhalation is another formulation strategy wherein the use of an inhalable dry powder dosage form can enhance vaccine stability and shelf-life.60 WHO has enlisted three basic strategies for the development of intranasal vaccines: whole microbe approach, subunit approach, and genetic approach.61
Whole-microbe approach
Live attenuated vaccine is the most widely used and traditional approach to vaccine development, wherein the live viral strains are used in their weakened form. A range of influenza vaccines have been developed using this strategy, and FluMist® Quadrivalent nasal spray vaccine is one latest example. Manufactured by MedImmune LLC, this quadrivalent influenza vaccine is made using four flu viruses: influenza A(H1N1), A(H3N2), and two influenza B viruses.58 The FluMist® influenza vaccine is a prefilled refrigerated unit dose spray and, in addition to the weakened viruses, it contains several other ingredients: monosodium glutamate (stabiliser), hydrolysed porcine gelatin (stabiliser), arginine (immunostimulant), sucrose (cryoprotectant), dibasic potassium phosphate (buffer), monobasic potassium phosphate (buffer), and gentamicin sulfate (antibiotic), and is claimed to be free of preservatives.62
A single-dose intranasal live-attenuated vaccine against SARS-CoV-2, named COVI-VAC (Codagenix, Inc. in partnership with Serum Institute of India), has been developed using a proprietary Synthetic Attenuated Virus Engineering (SAVE) platform that uses synthetic biology to recode viral genes to make them safe and stable. The vaccine has minimal logistical challenges with respect to administration, cold storage, and large-scale manufacturing.63
Multiple inactivated vaccines are also under different stages of clinical development and mainly involve vaccine adjuvants. Vaccine adjuvants can target different arms of the immune system to activate innate immunity and, in turn, elicit antigen-specific T cell responses.53 Studies of vaccines against COVID-19 report the use of adjuvants such as aluminium salts, emulsions, and toll-like receptors (TLRs). TLR agonists, including like CpG, Poly I:C, glucopyranosyl lipid A, and resiquimod, combined with carriers, such as PLGA nanoparticles (NPs), and liposomes, have been evaluated for their intranasal activity against SARS-CoV and Middle East respiratory syndrome (MERS)-CoV, and their further examination is warranted to assess their effectiveness against SARS-CoV-2.64
Viral vector-based vaccines are another strong contender in this category of vaccines and involve using a safe virus to deliver components such as viral proteins of interest. Human adenovirus (Ads) vectors have been the most sought in this fight against coronavirus because they have been in vaccine development for more than seven decades. Ads vector-based vaccines are simple to prepare, easy to purify to high titre, genetically stable, and relatively cheap, and have the potential to be delivered via numerous routes, including intranasal, oral, intradermal, and intramuscular.65 They have the innate ability to induce both B and T cell responses. Johnson & Johnson’s approved vaccine (IM) is based on Janssen’s AdVac® platform, and comprises genetically modified recombinant Ad26 vector encoding a stabilized variant of SARS-CoV-2 S-protein.66 Excipients in this vaccine include polysorbate-80 (surfactant), 2-hydroxypropyl-β-cyclodextrin (adjuvant, solubiliser, and stabiliser), salts, and ethanol.67 ChAdOx1 nCoV-19 vaccine (IM) (Oxford-AstraZeneca) is based on the modified chimpanzee Ads containing a genetic code for SARS-CoV-2 S-protein. Other ingredients include polysorbate 80 (emulsifier), magnesium, and alcohol.68 The Sputnik V vaccine (Gamaleya Research Institute of Epidemiology and Microbiology, Russia) also has a modified replication-defective Ads, and is a two-component vaccine comprising recombinant Ads with serotypes 5 (rAd5) and 26 (rAd26). The vaccine also contains polysorbate 80 (stabilizer), salts, and ethanol.69 ChAd-SARS-CoV-2-S vaccine and Ad5-nCoV + ZF2001 vaccines are also based on the use of an Ads-based vector.
Subunit approach
Subunit vaccines are inactivated whole-cell vaccines that are devoid of the live components of the pathogen. They comprize the antigenic parts of the organism, which are vital for production of an immune response upon administration. The subunits are generally proteins or sugars. Subunit vaccines are also often developed in combination with vaccine adjuvants or with mucoadhesives as auxiliary agents to enhance vaccine efficacy. SARS-CoV-2 has been extensively studied for the S-protein component and its two subunits (S1 and S2). S-protein-mediated binding to the hACE2 receptor in the host cell allows entry of the virus to host cells. Thus, S-protein and the associated antigenic fragments are potential targets for designing subunit vaccines against SARS-CoV-2 infection.70
Genetic approach
Vaccines developed using a genetic approach include the use of nucleic acids. The most widely used vaccine approaches in this category include the delivery of DNA and mRNA, which direct the host cells toward the production of viral antibodies. Although not popular initially, they have gained attention recently because of positive results obtained for mRNA COVID-19 vaccine candidates. Moderna’s mRNA-based vaccine (mRNA-1273) and the Pfizer/BioNTech mRNA vaccine (Comirnaty®) are just two lipid NP-based vaccines receiving fast-track approval during the ongoing pandemic. The Moderna vaccine comprises a mixture of lipids, including PEG 2000 dimyristoyl glycerol, 1,2-distearoyl-sn-glycero-3-phosphocholine, cholesterol, and a proprietary lipid SM-102 with salts and sucrose.71 The lipids used in Comirnaty® include (4-hydroxybutyl)azanediyl)bis(hexane-6,1-diyl)bis or ALC-3015, (2-hexyldecanoate),2-[(polyethylene glycol)-2000]-N,N-ditetradecylacetamide (ALC-0159), 1,2-distearoyl-snglycero-3-phosphocholine (DPSC), and cholesterol along with salts and sucrose.72 The mRNA from the vaccine enters the host cell and induces translation into desired protein antigens that can be recognized by the host, thereby initiating antibody generation. The Chalmers University of Technology (Sweden) and AstraZeneca have also initiated mRNA-based nasal spray development, comprising biomimetic NPs, for nasal immunization against SARS-CoV-2.73
Some companies with IM vaccine candidates are experimenting with them for intranasal application. Such formulations could become viable for intranasal delivery by tweaking factors including pH, mucoadhesion, and nasal mucosal toxicity.
Nasal vaccination for COVID-19
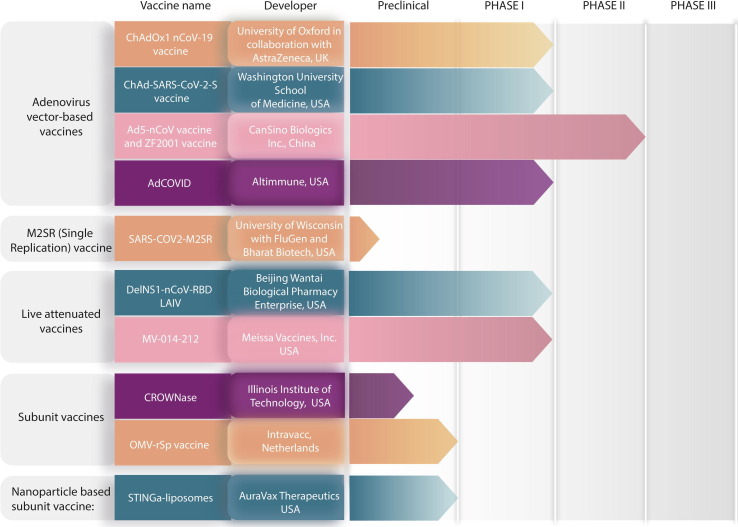
Nasal vaccines hold a superior position in infections such as SARS-CoV-2, where major viral invasion occurs via nasal mucosa. Here, we provide examples of intranasal vaccines that are being developed against SARS-CoV-2, and their clinical developmental status is summarized in Fig. 6 .
Figure 6.
Infographics of nasal vaccine development for severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2).
Adenovirus vector-based vaccines
Ads are regarded as excellent vectors for delivering target antigens to mammalian hosts, because of their potential to induce both adaptive and innate immune responses. In addition, their ability to generate such responses at the mucosal sites of pathogen entry makes them a preferred choice for intranasal vaccination.74 Furthermore, Ads can be produced safely and cheaply.75 Currently, Ads-based vaccines are used against a variety of pathogens. Nonetheless, only limited SARS-CoV-2 vaccine candidates are administered by mucosal routes, and mainly comprise a different nonreplicating type of Ads vector carrying genetic material of SARS-CoV-2, which is administered intranasally. Extensively studied Ads include human Ads serotype 5(AdHu5); however, because of the high frequency of AdHu5-neutralizing antibodies (NAs) across the human population, gene transfer efficiency by the vector is affected, leading to diminishing potency of the vaccine. To overcome this issue, Ads procured from numerous different species are used as potential immunization vectors. Ads derived from chimpanzees (AdCs) were found to be advantageous because they can be produced easily in a human cell line. Moreover, compared with Ad human serotypes (AdHus), their seroprevalence in the human population is low because they rarely circulate in humans.76, 77
With advances in the molecular tools and developmental strategies available to generating nonhuman Ads, AdCs have emerged as attractive platforms for vaccine design. The growing popularity of AdCs as vaccine vectors is associated with their ability to bypass the negative impact of pre-existing antihuman Ads immunity while inducing immune responses that are similar to, or more potent than, those elicited by their human Ads counterparts74. Here, we discuss various Ads based vaccines that are under preclinical and clinical development.
ChAdOx1 nCoV-19 vaccine
The UK-based ChAdOx1 nCoV-19 vaccine is being developed by the University of Oxford in collaboration with AstraZeneca. The same vaccine that is being delivered currently via the intramuscular route is being studied for its intranasal potential in a Phase I trial using an intranasal spray.78 Intranasal vaccination in rhesus macaques showed the presence of IgG and IgA in their serum specific to SARS-CoV-2, indicating activation of systemic immunity. A study in the sentinel hamster transmission model exhibited reduced viral shedding, indicating 100% transmission control.79 The preliminary data showed a possibility of SARS-CoV-2-specific mucosal immunity with a transmission control post-intranasal vaccination, demanding a clinical examination.
ChAd-SARS-CoV-2-S vaccine
The intranasal ChAd-SARS-CoV-2-S vaccine developed by Washington University School of Medicine in St Louis, USA, comprises an AdC vaccine encoding a stabilized S-protein that can prevent SARS-CoV-2 infection and pneumonia in mice. Single intranasal administration of the vaccine was studied in a stringent SARS-CoV-2 challenge model in C57BL/6 transgenic mice containing hACE2 with a K18 cytokeratin epithelial cell promoter (K18-hACE2 mice). A single dose prevented upper and lower respiratory tract infections, thereby providing potential protection against SARS-CoV-2 infection and transmission. Reports indicated the generation of systemic and mucosal immunity, suggesting this as a promising intranasal vaccine platform with a need for further clinical evidence.80 The vaccine is undergoing Phase I clinical trials in India under the name BBV154 and is being studies by Bharat Biotech.81 In the USA and Europe, Precision Virologics, Inc. is the licensee for this vaccine.82
Ad5-nCoV and ZF2001 vaccines
Chinese researchers from CanSino Biologics Inc. and a unit of Chongqing Zhifei Biological Products are developing an intranasal vaccine using their vaccine candidates (i.e., Ad5-nCoV vaccine and ZF2001 vaccine, respectively). A trial is planned of Ad5-nCoV vaccine followed by a dose of ZF 2001 vaccine at an interval of 28 and 56 days in 120 participants. The Ad5-nCoV inhalation vaccine (CanSino Biologics Inc. with Beijing Institute of Biotechnology and Jiangsu Province Centers for Disease Control and Prevention) is also undergoing independent Phase I/II clinical trials in a randomized double-blind, placebo-controlled study to evaluate its safety and immunogenicity in adults over 18 years of age (NCT04840992).83 By contrast, the intramuscular version of the ZF2001 vaccine is in Phase III clinical trials to determine its safety and efficacy (NCT04646590).84
AdCOVID
USA-based Altimmune has designed an intranasal adenovirus type 5 (Ad5)-vectored vaccine encoding the receptor-binding domain (RBD) of the SARS-CoV-2 S-protein. Single intranasal doses in a mouse model demonstrated activation of the immune system. The intranasal shot was able to stimulate a three-way immune response, namely serum neutralising activity (IgG), T cell based immunity, and mucosal immunity (IgA), which stimulated concomitant local mucosal and systemic immunity. The preclinical study data represents a promising COVID-19 vaccine candidate. Phase I trials were started in February 2021 with 180 participants aged 18–55 years. The vaccine also has added advantage of being stable under refrigerated conditions and retaining that stability on exposure to room temperature. Altimmune has also tested NasoVAX in Phase II clinical trials (NCT04442230). NasoVAX is an intranasally administered recombinant monovalent influenza vaccine with adenovector-mediated expression of the influenza antigen in the target cell. This leads to a broader and more rapid immune response compared with traditional influenza vaccines.85 A study of the highest NasoVAX dose and the approved Fluzone® influenza vaccine showed comparable hemagglutination inhibition (HAI) geometric mean titres (152.8 versus 293.4) and microneutralization (MN) geometric mean titres (142.5 versus 162.8), with NasoVAX HAI titres being maintained for more than 1 year on an average following a single dose. Hemagglutininspecific T cell responses were also documented in peripheral mononuclear cell (PBMC) preparations. Post-intranasal administration, NasoVAX elicited antigen-specific mucosal IgA responses in the nasopharyngeal cavity with a twofold increase in baseline GMT at mid–high doses. NasoVAX was deemed to be safe and was able to induce humoral, cellular, and mucosal immune responses.86, 87
M2SR (single replication) vaccines
SARS-COV2-M2sr
The University of Wisconsin-Madison, along with FluGen and Bharat Biotech, is developing a M2SR vaccine that contains M2SR (single replication) vaccine with a deleted M2 gene from M2SR. The deletion of the M2 gene leads to activation of immune responses in the host, inducing innate and cellular immunity.88, 89 The uniqueness of M2SR is that the vaccine virus offers multiple antigen targets to the immune system, similar to a wild-type virus, and activates the immune responses without the production of progeny virus. The M2SR vaccine provides broad-spectrum, long-lasting cross-protection against multiple influenza subtypes, including H5N1 in mice and ferrets.90 This concept of cross-protective immunity can help curb a pandemic of this magnitude to reduce host mortality and viral transmission. Preclinical trials for this vaccine are to be initiated in the near future.88, 90
Live-attenuated vaccines
DelNS1-nCoV-RBD LAIV
Another intranasal spray vaccine, DelNS1-nCoV-RBD LAIV, is being co-created by Beijing Wantai Biological Pharmacy Enterprise with researchers from Xiamen University and Hong Kong University, and received first clinical trial authorization in November 2020 from the China National Medical Products Administration with 100 participants. The Phase I randomized double-blinded placebo-controlled trial is currently recruiting participants (NCT04809389).91 The intranasal vaccine comprises weakened flu viruses, such as H1N1, H3N2, and B, with genetic segments of the S-protein of COVID-19, mimics infection by respiratory viruses, and stimulates the immune response.92 A second early-stage clinical trial is approved to begin in Hong Kong.93
Mv-014-212
Meissa Vaccines, Inc. has developed an intranasal live-attenuated chimeric vaccine candidate (MV-014-212) against SARS-CoV-2. Preliminary preclinical results in nonhuman primates demonstrated induced neutralizing antibodies against S-expressing pseudovirus and a SARS-CoV-2 S-specific mucosal IgA response, and was highly protective against SARS-CoV-2 challenge in the upper and lower respiratory tract. These outstanding preclinical outcomes led to clearance from the FDA for a Phase I clinical study of MV-014-212 (NCT04798001) in 130 healthy adult volunteers between the ages of 18 and 69 years, and is currently enrolling participants at two sites in the USA. This easy-to-manufacture single adjuvant-free vaccine dose could induce mucosal and systemic immunity to change the paradigm of vaccine rollout.94
Subunit vaccines
CROWNase
A new COVID-19 vaccine in experimental stages at the Illinois Institute of Technology, Chicago, an inhaled therapy called CROWNase, shows the potential to limit COVID-19 infection. S-protein gives the SARS-CoV-2 virus its crown-like appearance, enabling the virus to attach to hACE2 and cause infection. The S-protein has a coating of human-derived molecules, which help the virus evade the immune system and infect host cells. CROWNase works by removing the coating from the S-protein, exposing the protein component of the S-protein to the immune system. This further stops the virus from infecting human cells. CROWNase also includes the ACE2 receptor, which helps improve its viral-binding efficacy.95
CovOMV
Intravacc’s nasal SARS-CoV-2 vaccine embeds viral S antigen in bacterial outer membrane vesicles (OMVs). Animal studies for this intranasal vaccine have been initiated in mouse and hamster models. Animals in preclinical studies received vaccines based on OMVs mixed with recombinant S-protein (rSp) (CovOMV) and another based on OMVs coupled to rSp based on Intravacc’s proprietary OMV click technology (CovOMVclick). The success of preclinical trials was recently announced. The intramuscular counterpart of the same vaccine has already been demonstrated to be a safe platform, with cheaper manufacturing and stability at 4 °C.96
Nanoparticle-based subunit vaccines
STINGa-liposomes
AuraVax Therapeutics has a liposomal stimulator of interferon genes or STING agonist technology licensed from Massachusetts General Hospital (MGH) for use as an adjuvant in vaccines against COVID-19 and other respiratory diseases. Research evidenced that triggering a mucosal immune response can evoke rapid and durable protection against pandemic viruses, such as SARS-CoV-2. Researchers identified the cyclic GMP-AMP Synthase (cGAS) and developed STING agonists (STINGa),97 encapsulating STING and 2′3′-cGAMP or cGAMP in liposomes. These were used as the adjuvant for intranasal vaccination with the trimeric or monomeric versions of the S-protein. The NP-based colloidal nasal vaccine was manufactured by gently mixing the trimeric S proteins with the STINGa-liposomes at room temperature to allow adsorption of the protein on the surface of liposomes. The adsorbed trimer-STINGa-liposomes showed a mean particle diameter of 105 nm and a mean zeta potential of −29 mV. Preclinical results in mice showed that this vaccine is safe and provokes systemic immunity, mucosal immunity (IgA in the nasal compartment and lung, and IgA-secreting cells in the spleen) and cellular immunity (spleen and lung). In July 2020, the first COVID-19 vaccine candidate to elicit mucosal immunity was reported, to support further translational studies as an intranasal nonviral candidate that can induce systemic immunity and confer immunity at the primary site of viral entry.98
Nasal spray for nasal disinfection and COVID-19 treatment
High levels of SARS-CoV-2 are expelled from the nasal cavity of infected patients both before and after symptoms appear, including those who are asymptomatic. Disinfecting the nasal cavity of those who have or do not have COVID-19 could help to reduce contagiousness or serve as a preventative measure, respectively.99
Since undertaking initial clinical trials in Canada and, most recently, in the UK, a self-administered nitric oxide nasal spray (NONS) developed by Vancouver-based biotech company SaNOtize was found to significantly minimize COVID-19 viral load in infected patients.100, 101, 102, 103 The desired result of inhaled NO, pulmonary vasodilation, is induced in part by increased cellular cGMP. cGMP phosphorylates the calcium channel and increases the uptake of Ca2+, that leads to vasodilation and increases oxygen uptake by the lung.104 NO inhibits SARS-CoV replication through two distinct mechanisms. Primarily, NO or its derivatives reduce palmitoylation of nascently expressed S-protein, which affects the fusion of the S-protein with its cognate receptor, ACE2. Second, NO or its derivatives reduce viral RNA generation in the body.105 In a randomized, double-blind, placebo-controlled Phase II trial of 79 confirmed COVID-19 cases, SaNOtize’s early therapy significantly reduced the level of SARS-CoV-2, particularly in patients with high viral loads contaminated by the Alpha variant of COVID-19.106 It was reported that the viral load decreased by more than 99% within 72 h of nasal spray application.107 There are also two platforms, Beyond Air LungFit™ platform108 and VERO’s GeNOsyl® iNO system,109 that are based on inhaled NO delivery system for the treatment of COVID-19.
In recent joint research with the Israeli Ministry of Health and the Sheba Medical Center at Tel Hashomer Hospital, Nasus Pharma reported that its patented Taffix™ spray ‘obstructed 100 percent’ of the two new SARS-CoV-2 virus strains, the Alpha variant, and beta variant.110 The key cause of SARS-CoV-2 infection is thought to be viral penetration into the nasal mucosa. Taffix’s key component, hydroxypropyl methyl cellulose (hypromellose or HPMC), is a cellulose derivative that forms a mucoadhesive gel. As HPMC reaches the nasal mucosa, it is understood to absorb fluids and form a micron-sized gel. The gel coats the nasal cells, preventing viruses from interacting with the receptors required for viral penetration into the cells. Taffix also produces a local acidic microenvironment with a pH of 3.5 on mucosal surfaces that is stable for up to 5 h, which is detrimental for viral survival.110, 111 There were no recorded adverse events in this open-label user survey. Regulated clinical trials can be useful in identifying more accurately target groups and methods of promoting adherence.
Researchers at the University of Birmingham have created a nasal spray that can provide adequate protection against COVID-19.112 A spray containing polysaccharides known for their mucoadhesive properties was developed and tested for mechanical, spray pattern, and antiviral properties. A comprehensive understanding of a hybrid mixture comprizing two polymers, gellan and carrageenan, resulted in the ability to engineer core behaviors, such as yielding.113 The spray functions in two ways. It first captures and coats the virus inside the nose, from where it is then removed via normal routes (either by blowing the nose or swallowing). Second, because the virus is encapsulated in the viscous layer of the spray, it is not absorbed by the body. Thus, it will reduce the viral load in the body if virus particles are present.112
Neurimmune, a Swiss antibody company, and Ethris, a German RNA biotech, are working to produce inhaled mRNA antibodies that can treat the respiratory effects of CoV disease.114 Neurimmune G contributes to this work by studying the Ig sequences of patients recovered from COVID-19, whereas Ethris focuses on using a novel pulmonary therapeutics tool to do the same. Ethris’ stabilized non-immunogenic mRNA (SNIM®RNA) technologies will be used in the planned antibody therapy. The pulmonary SNIM®RNA technology developed by Ethris will aid in the administration of mRNA-encoded, neutralizing anti-SARS-CoV-2 antibodies directly into patients’ lungs, allowing for the rapid attainment of successful pulmonary antibody concentrations. Thus, this cutting-edge pulmonary research holds the potential to cure viral lung disease, which is the leading cause of morbidity and mortality.115
Marinomed Biotech’s nasal spray engineered to block novel coronavirus infection has been shown to inactivate new, quickly spreading variants. According to the Korneuburg-based company, in vitro testing revealed that the compound was successful against the SARS-CoV-2 wild-type as well as three variants known as the Alpha, Beta, and Gamma. Carragelose, a sulfated polymer obtained from red seaweed, is the company’s most recent invention, and acts by creating a membrane on the mucosa that wraps invading viruses, inactivating them.116
The PH94B neuroactive nasal spray was developed by VistaGen Therapeutics, Inc. in the USA. PH94B is a novel, odourless, and fast-acting nasal drug delivery mechanism that binds directly to nasal chemosensory receptors, activating synaptic pathways in the brain that inhibit anxiety and apprehension associated with daily social environments and other repetitive situations.117 VistaGen Therapeutics, Inc. has successfully completed Phase II (NCT01217788) and Phase III (NCT02622958) clinical trials of PH94B.118 They also demonstrated the effectiveness of the PH94B nasal spray (8 g spray) in the emergency treatment of social anxiety disorders. Following these clinical trials, VistaGen Therapeutics, Inc. initiated a Phase IIa review of PH94B nasal spray for the treatment of COVID-19-related anxiety.119
Ciclesonide, a glucocorticoid commonly used to treat asthma, is marketed under the brand name Alvesco (Covis Pharma, Luxembourg). In the USA, the Alvesco pressurized metered-dose inhaler (80–320 g ciclesonide/actuation) is used for the management of asthma as prophylactic therapy in adult and juvenile patients aged 12 and over. Ciclesonide is expected to minimize COVID-19 signs and restrain viral replication.130 The Randomised Ciclesonid COVID-19 (RACCO) trial provided conclusive comparative efficacy results as well as significant clinical effects data comparing ciclesonide and symptomatic treatment classes.131 The 400 patients in the multicenter, randomized, double-blind, placebo-controlled trial (NCT04377711) had symptomatic COVID-19 infection. In addition to routine supportive treatment, patients were randomly selected to receive either ciclesonide 320 mcg inhaled twice daily or placebo.123 In a subgroup study, ciclesonide therapy was associated with a reduction in the period to cough relief by day 6 in 75% of the population. Furthermore, the ciclesonide arm had a 70% decrease in subsequent emergency room visits or hospital admissions because of COVID-19, relative to a 30% reduction in the placebo arm (P = 0.0301); a total of ten incidents occurred.132
With recent developments in science and technology, collaborative and team projects around the world demonstrate the speed with which COVID-19 therapy can be conceived and developed. There are many other nasal products that are under different stages of clinical development, as summarized in Table 2 .
Table 2.
Nasal sprays for COVID-19 treatment.
| Product | Developer | Delivery platform | Mechanism against COVID-19 | Development status | Country of origin | Refs |
|---|---|---|---|---|---|---|
| NONS | SaNOtize | NORS™ as an upper airway ‘disinfectant’ | Reduces viral RNA synthesis and fusion of S-protein | Phase III completed in UK and Canada; received investigational drug exception (IDE) to allow clinical trials in USA | Canada | 120 |
| Beyond Air | LungFit™ | Reduces viral RNA synthesis and fusion of S-protein | Applied to FDA for IDE | USA | 108 | |
| Vero Biotech LLC | GeNOsyl® Chronic D.S. | Reduces viral RNA synthesis and fusion of S-protein | NA | Georgia | 109 | |
| Powder for nebuliser suspension | APEPTICO | Water-soluble synthetic peptide-Solnatide | Restores injured endothelial–epithelial barrier of pulmonary alveoli | Phase II | Austria | 121 |
| HPMC nasal spray | Nasus Pharma | Taffix spray | Prevents viral interaction with host receptor | Open-label user survey | Israel | 110 |
| Nasal spray containing polysaccharides | University of Birmingham | Mucoadhesive nasal spray | Coats viral particles | N/A | UK | 113 |
| Inhaled mRNA antibodies | Neurimmune, and Ethris | SNIM®RNA technology | mRNA-encoded, neutralising anti-SARS-CoV-2 antibodies | Phase I imminent | Germany | 114 |
| Sinapultide; lyophilised synthetic peptide KL4 surfactant | Windtree Therapeutics Inc. | AEROSURF® delivery technology | Reduces frequency of nasal continuous positive airway pressure (nCPAP) failure | Phase IIa | USA | 122 |
| Alvesco | Covis Pharma | Ciclesonide | Restrains viral replication | Phase III | Luxembourg | 123 |
| Nasal spray | Marinomed Biotech | Carragelose Spray | Coats viral particles | Preclinical | Austria | 116 |
| Nebulisation of peptide | NeuroRx, Inc. | Aviptadil (a synthetic form of vasoactive intestinal polypeptide) | Blocks replication of SARS-CoV-2 and prevents synthesis of cytokines | FDA emergency use IND authorisation | USA | 124 |
| Leukine sargramostim nebuliser | Partner Therapeutics | iLeukPulm | For acute hypoxemia in COVID-19 | Phase II | USA | 125 |
| Neuroactive nasal spray | VistaGen Therapeutics, Inc. | PH94B | Activates synaptic pathways to reduce anxiety | Phase III | USA | 117 |
| Anti-IL-6 receptor monoclonal antibody by nebulisation | Tiziana Life Sciences, UK and STC Biologics, US and Sciarra Laboratories, US | TZLS-501 | Depletes circulating levels of IL-6 in blood | Phase I | USA and UK | 126 |
| Aspartyl-alanyl diketopiperazine for nebulisation | Ampio Pharmaceuticals | Ampion™ | Interrupts hyperinflammatory response and respiratory disease associated with COVID-19 | Phase I | USA | 127 |
| Interferon beta for nebulisation | Synairgen | SNG001 | Upregulates pulmonary antiviral defenses | Phase II EudraCT | UK | 128 |
| Nasal spray | Biohaven Pharmaceuticals, Inc | Zavegepant (BHV-3500) | CGRP receptor antagonist | Phase II/III | USA | 129 |
Safety and efficacy
The ever-growing number of clinical trials signify the accepted need for intranasal vaccines that are easy to self-administer and offer superior advantages over other vaccine routes in terms of cost of formulation. The apparent gains of directly stimulating the mucosal immune response intranasally are clear, although have not yet been entirely realized other than those for influenza, which demonstrate the effectiveness of this route. However, the sudden requirement to immunize large populations quickly against COVID-19 has shown the clear need to have strategies in place.38
There are several safety-related events that, because of rarity or pathogenesis, might be detected only during long-term surveillance for adverse events after immunization.133, 134 Vigorous follow-up systems for safety, both during emergency use and after licensure, will be crucial to uncovering any possible rare events. Given that, at the time of writing, pregnant women and children had not yet been included in clinical trials of SARS-CoV-2 vaccines, these investigations must be done for both efficacy and safety. RNA-based vaccines are a new platform, so there are existing data on safety in children or during pregnancy. Advector based vaccines have been authorized for Ebola (Ad26- and Ad5-based), but significant data on safety during pregnancy are not available.135, 136
Most of the intranasal vaccines for SARS-CoV-2 are in early-stage clinical trials and, therefore, their safety and efficacy profiles are yet to be established in humans. Thus, it is easier to rely on the safety and efficacy data available from intranasal influenza vaccines. Licensed intranasal influenza vaccines, FluMist/Fluenz™, and the Nasovac™ live-attenuated influenza nasal spray, have no serious side effects reported, indicating their safety. However, sufficient Nasovac efficacy data are not available yet, whereas FluMist is considered one of the most well-tolerated, effective intranasal vaccines.48
Vaccine effectiveness, particularly intranasal spray, can differ from year to year, among different age and risk groups, by vaccine type, and even by virus type and subtype. Therefore, clinically established effective vaccines need to be re-evaluated from time to time in different population data sets.58
Consideration of nasal vaccine delivery devices
Among the numerous routes of mucosal immunization, the intranasal route is one the most explored alternatives because of the pre-established commercial setup. The nose is a convenient and easy site for self-administration, and delivery devices to be used to enable the vaccine delivery are already available on the market. An intranasal vaccine device that enables optimum and reproducible dosing, ease of handling, convenience of packaging, ability to refill, adaptability for mass vaccinations, and offers resistance to changing environmental conditions can be considered ideal. Given that the vaccine formulations can either be liquids or solid powders, the device should also be made suitable for the delivery of all dosage forms. For powder formulations, a special emphasis on moisture-resistant packaging is essential. For example, Activ-Vial™ is one such platform packaging technology developed by AptarGroup, Inc., which protects powder formulations, whereas their Activ-Polymer™ technology was approved by FDA in 2019 for intranasal unidose powder administration and has also been approved by European regulatory authorities.137 OptiNose US, Inc. has developed exhalation delivery systems (EDS) that are suitable for intranasal delivery of both liquids and powders with a unique option of bi-directional delivery to the mouth as well as nose.138 A single-dose (0.2 ml suspension) prefilled device is being used for delivery of the first FDA-approved influenza vaccine, FluMist®, wherein the liquid dose is administered via the spray tip fitted for nasal delivery.62 The prefilled device, known as the BD Accuspray™ nasal spray system, has been developed by Becton, Dickinson and Company, USA.139
Considerations for device selection include vaccine type, the volume of administration, single or multiple dose strategies, possibility of automated filling, protection during transport and storage, and, most importantly, the cost:benefit ratio, especially when mass immunisation for a pandemic such as COVID-19 is to be planned.7 Thus, the pathophysiological rationale of the delivery site, type of dosage form, suitability of the delivery device, and right regulatory pathway form the key attributes in devizing a rapid and strong intranasal vaccine delivery strategy. In these pandemic times, companies generally prefer using delivery devices that already have regulatory approval to hasten the process of product development. However, if companies develop a delivery device of their own, they need to comply with the specific regulatory guidelines and ensure that the same device is used throughout the clinical trials to ensure the safety and efficacy of the vaccine–device combination.140, 141
Concluding remarks and prospects
The COVID-19 global pandemic has hastened the momentum of the global vaccine market like never before. The global statistics (as of 30 April 2021) pertaining to vaccine administration drives state that China was leading the vaccination drive (>250 million), followed by USA (>200 million), India (>150 million), and UK (>50 million), with other countries slowly catching up.142 Looking at the mass scale of vaccination drives that would be needed to combat the pandemic, it is imperative for formulation scientists to explore alternative routes of vaccine delivery for both prophylaxis as well as therapy. The nasal vaccine approach has been at the forefront in this regard because major viral invasion occurs via the nasal mucosa. It adds a second line of defense immediately at the infection site, paving the way for an effective reduction in viral transmission and shedding.
However, intranasal vaccine development comes with its own set of challenges and opportunities. Strategies concerning the selection of vaccine type, functional excipients, such as vaccine adjuvants and mucoadhesive agents, vaccine carriers, including NPs, surface-modified NPs, and virus-like particles, all determine the immunogenic efficacy of the formulation. Challenges to manufacturing include large-scale production, in-process quality control, meeting regulatory requirements, while also taking care of market needs and demands. Nevertheless, we consider that it will not be too long before an intranasal COVID-19 vaccine enters the market. From antibody cocktails to nasal sprays targeting the inhalation airways and powders for inhalation targeting the lungs, these second-generation COVID-19 vaccines are being developed with the aim of overcoming current logistical drawbacks of multiple doses, use of cold storage, and mandated trained vaccine administration staff. The nasal route is especially preferred for easy vaccination of the pediatric population and Altimmune is in talks with the FDA to launch a paediatric trial of their nasal vaccine candidate AdCOVID.143 Another mucosal vaccine based on viral protein-based NP-coated liposomes (virus-like particles) is being developed by Avalon GloboCare.144 The vaccine, comprizing an artificial SARS-CoV-2 envelope, will help preventing viral invasion. As per current data, 13 companies are working on intranasal vaccines, with five having reached the early clinical stages.145 Similar to the first-generation intramuscular vaccines helping gain control over virus spread, it is expected that the second-generation intranasal vaccines would assist in slowing the transmission rate, strengthening our ability to eradicate this virus.
A major takeaway from this global pandemic is that the current state of regulatory mechanisms in the healthcare industry is not capable of fast-tracking products such as vaccines unless otherwise forced to do so. Vaccine development has not been a focus of research, and it has taken a global pandemic to garner the attention of global researchers and governments, working together to develop vaccines and other treatments for COVID-19. There also needs to be a separate financial and regulatory system in place to cater for healthcare emergencies of this stature to assure minimum loss of life. In short, the IM Vaccine delivery elucidate a long-lasting systemic IgG response along with generation of memory B and T cells; a follow up booster dose by intranasal route recruit memory B and T cells in the upper respiratory tract for providing mucosal immunity to avoid viral transmission and infection. Most innovators of COVID-19 vaccines are also trialling nasal-based vaccine platforms as a part of a booster dose strategy. We are hopeful that the numerous ongoing efforts will pave the way for new-generation vaccines as well as regulatory mechanisms in the near future and will be able to come us through the current crisis.
CRediT authorship contribution statement
VPC and LKV have prepared the plot of the manuscript and written the first draft of the manuscript along with AP. VPC, LKV, and VP revised the manuscript. VPC and LKV have prepared the figures of the manuscript. All authors have read the final version of the manuscript and approved the same.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
Figure 1, Figure 2 were prepared using templates from BioRender.com. A.K.P. would like to thank the Department of Science & Technology (DST) INSPIRE program of the Ministry of Science & Technology, Government of India, for providing her fellowship. V.B.P. would like to acknowledge DBT BIRAC COVID-19 Research Consortium, Government of India, for her grant to work toward the development of an intranasal mucosal vaccine for COVID-19 infection (BT/PR40394/COV/140/1/2020).
Biographies

Vivek Chavda is an assistant professor in the Department of Pharmaceutics and Pharmaceutical Technology, L.M. College of Pharmacy, Ahmedabad, Gujarat, India. He was awarded a B Pharm and M Pharm by Gujarat Technological University. Before joining academia, he worked in industry in the research and development of biologics with two successful regulatory filings. His research interests include the development of biologics processing and formulations, medical device development, nanodiagnostics and nanocarrier formulations, long-acting parenteral formulations, and nanovaccines.

Lalitkumar Vora is a postdoctoral research fellow at the School of Pharmacy, Queen’s University, Belfast. UK He was awarded a PhD in pharmaceutics in 2017 by the Institute of Chemical Technology, Mumbai, India. His research interests include polymeric drug delivery, microneedle-assisted non-invasive drug delivery, and long-acting drug delivery, specifically for infectious diseases.

Anjali Pandya is a recipient of a doctoral fellowship at the Institute of Chemical Technology, Mumbai, India, awarded by the Department of Science and Technology (DST) - Innovation in Science Pursuit for Inspired Research (INSPIRE), Government of India, for research on oral protein and peptide delivery,.

Vandana Patravale is a professor of pharmaceutics at the Institute of Chemical Technology, Mumbai, India. Her research interests include development of nanocarriers with a major emphasis on malaria, cancer, and neurodegenerative disorders; medical device development, nanodiagnostics, and nanovaccines. Professor Patravale has transferred many technologies to various industries including technology on drug eluting stents, which is being marketed in more than 60 countries.
References
- 1.Pollard A.J., Bijker E.M. A guide to vaccinology: from basic principles to new developments. Nat Rev Immunol. 2021;21(2):83–100. doi: 10.1038/s41577-020-00479-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bloomberg. Vaccine Tracker. www.bloomberg.com/graphics/covid-vaccine-tracker-global-distribution/ [accessed August 6, 2021].
- 3.Zimmer C, Corum J, Wee S-L. Coronavirus vaccine tracker. NYTimes. www.nytimes.com/interactive/2020/science/coronavirus-vaccine-tracker.html [accessed August 6, 2021].
- 4.Machhi J., Shahjin F., Das S., Patel M., Abdelmoaty M.M., Cohen J.D., et al. Nanocarrier vaccines for SARS-CoV-2. Adv Drug Deliv Rev. 2021;171:215–239. doi: 10.1016/j.addr.2021.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kashte S., Gulbake A., El-Amin Iii S.F., Gupta A. COVID-19 vaccines: rapid development, implications, challenges and future prospects. Hum Cell. 2021;34:711–733. doi: 10.1007/s13577-021-00512-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Krammer F. SARS-CoV-2 vaccines in development. Nature. 2020;586(7830):516–527. doi: 10.1038/s41586-020-2798-3. [DOI] [PubMed] [Google Scholar]
- 7.Birkhoff M., Leitz M., Marx D. Advantages of intranasal vaccination and considerations on device selection. Indian J Pharm Sci. 2009;71(6):729–731. [Google Scholar]
- 8.Lundstrom K. Viral vectors for COVID-19 vaccine development. Viruses. 2021;13(2):317. doi: 10.3390/v13020317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wang C., Wang Z., Wang G., Lau J.-Y.-N., Zhang K., Li W. COVID-19 in early 2021: current status and looking forward. Signal Transduction Targeted Therapy. 2021;6(1):114. doi: 10.1038/s41392-021-00527-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mittal A., Manjunath K.R.R., Kaushik S., Kumar S.V.V. COVID-19 pandemic: insights into structure, function, and hACE2 receptor recognition by SARS-CoV-2. PLoS Pathogens. 2020;16(8) doi: 10.1371/journal.ppat.1008762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lan J., Ge J., Yu J., Shan S.Z.H. Structure of the SARS-CoV-2 spike receptor-binding domain bound to the ACE2 receptor. Nature. 2020;581:215–220. doi: 10.1038/s41586-020-2180-5. [DOI] [PubMed] [Google Scholar]
- 12.Wang C., Liu Z., Chen Z., Huang X.X.M. The establishment of reference sequence for SARSCoV-2 and variation analysis. J Med Virol. 2020;92(6):667–674. doi: 10.1002/jmv.25762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Monpara J.D., Sodha S.J., Gupta P.K. COVID-19 associated complications and potential therapeutic targets. Eur J Pharmacol. 2020;886 doi: 10.1016/j.ejphar.2020.173548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wang Q., Wu J., Wang H., Gao Y.L.Q. Structural basis for RNA replication by the SARS-CoV-2 polymerase. Cell. 2020;182(2):417–428. doi: 10.1016/j.cell.2020.05.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Machhi J., et al. A Role for Extracellular Vesicles in SARS-CoV-2 Therapeutics and Prevention. Journal of Neuroimmune Pharmacology. 2021;16:270–288. doi: 10.1007/s11481-020-09981-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rothan H.A., Byrareddy S.N. The epidemiology and pathogenesis of coronavirus disease (COVID-19) outbreak. J Autoimmun. 2020;109 doi: 10.1016/j.jaut.2020.102433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhou P., Yang X.L., Wang X.G., Hu B., Zhang L.Z.W. A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature. 2020;579:270–273. doi: 10.1038/s41586-020-2012-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Qian Z., Dominguez S.R., Holmes K.V. Role of the Spike glycoprotein of human Middle East respiratory syndrome coronavirus (MERS-CoV) in virus entry and syncytia formation. PLoS ONE. 2013;8(10) doi: 10.1371/journal.pone.0076469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bosch B.J., Bartelink W., Rottier P.J.M. Cathepsin L Functionally cleaves the severe acute respiratory syndrome coronavirus Class I fusion protein upstream of rather than adjacent to the fusion peptide. J Virol. 2008;82(17):8887–8890. doi: 10.1128/JVI.00415-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.He Y., Zhou Y., Liu S., et al. Receptor-binding domain of SARS-CoV spike protein induces highly potent neutralizing antibodies: Implication for developing subunit vaccine. Biochem Biophys Res Commun. 2004;324(2):773–781. doi: 10.1016/j.bbrc.2004.09.106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.de Wilde A.H., Snijder E.J., Kikkert M., van Hemert M.J. Host factors in coronavirus replication. Curr Top Microbiol Immunol. 2018;419:1–42. doi: 10.1007/82_2017_25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lim Y., Ng Y., Tam J., Liu D. Human coronaviruses: a review of virus–host interactions. Diseases. 2016;4(4):26. doi: 10.3390/diseases4030026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fehr A.R., Coronaviruses P.S. an overview of their replication and pathogenesis. Coronaviruses: Methods Protocols. 2015;1282:1–23. doi: 10.1007/978-1-4939-2438-7_1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zheng M., Gao Y., Wang G., Song G., Liu S.S.D. Functional exhaustion of antiviral lymphocytes in COVID-19 patients. CellMol Immunol. 2020;17:533–535. doi: 10.1038/s41423-020-0402-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kim S.J., Nguyen V.G., Park Y.H., Park B.K.C.H. A novel synonymous mutation of SARS-CoV-2: is this possible to affect their antigenicity and immunogenicity? Vaccines. 2020;8:E220. doi: 10.3390/vaccines8020220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Moore B.J., June C.J. Cytokine release syndrome in severe COVID-19. Science. 2020;368:473–474. doi: 10.1126/science.abb8925. [DOI] [PubMed] [Google Scholar]
- 27.Acharya D., Liu G.G.M.U. Dysregulation of type I interferon responses in COVID-19. Nat Rev Immunol. 2020;20:397–398. doi: 10.1038/s41577-020-0346-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yang L., Liu S., Liu J., Zhang Z., Wan X., Huang B., et al. COVID-19: immunopathogenesis and immunotherapeutics. Signal Transduction Targeted Therapy. 2020;5(1):128. doi: 10.1038/s41392-020-00243-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Verardi P. How worried should you be about coronavirus variants? A virologist explains his concerns. www.downtoearth.org.in/blog/health/how-worried-should-you-be-about-coronavirus-variants-a-virologist-explains-his-concerns-76384 [accessed July 2, 2021].
- 30.Bentley M. Known unknowns: Covid-19 and biological warfare. E-International Relations. www.e-ir.info/2020/08/08/known-unknowns-covid-19-and-biological-warfare/ [accessed July 2, 2021].
- 31.CDC. Cases, data, and surveillance. www.cdc.gov/coronavirus/2019-ncov/cases-updates/variant-surveillance/variant-info.html [accessed July 2, 2021].
- 32.Bleier B.S., Ramanathan M.J., Lane A.P. COVID-19 vaccines may not prevent nasal SARS-CoV-2 infection and asymptomatic transmission. Otolaryngol Head Neck Surgery. 2021;164(2):305–307. doi: 10.1177/0194599820982633. [DOI] [PubMed] [Google Scholar]
- 33.McLenon J., Rogers M.A.M. The fear of needles: a systematic review and meta-analysis. J Adv Nurs. 2019;75(1):30–42. doi: 10.1111/jan.13818. [DOI] [PubMed] [Google Scholar]
- 34.Olive C., Sun H.K., Ho M.F., Dyer J., Horváth A., Toth I., et al. Intranasal administration is an effective mucosal vaccine delivery route for self-adjuvanting lipid core peptides targeting the Group A streptococcal M protein. J Infect Dis. 2006;194(3):316–324. doi: 10.1086/505580. [DOI] [PubMed] [Google Scholar]
- 35.Sterlin D., Mathian A., Miyara M., Mohr A., Anna F., Claër L., et al. IgA dominates the early neutralizing antibody response to SARS-CoV-2. Sci Transl Med. 2021;13(577) doi: 10.1126/scitranslmed.abd2223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Peek L.J., Middaugh C.R., Berkland C. Nanotechnology in vaccine delivery. Adv Drug Deliv Rev. 2008;60(8):915–928. doi: 10.1016/j.addr.2007.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Butler S.E., Crowley A.R., Natarajan H., et al. Features and functions of systemic and mucosal humoral immunity among SARS-CoV-2 convalescent individuals. medRxiv. 2020 doi: 10.3389/fimmu.2020.618685. 2020.08.05.20168971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yusuf H., Kett V. Current prospects and future challenges for nasal vaccine delivery. Human Vacc Immunotherap. 2017;13(1):34–45. doi: 10.1080/21645515.2016.1239668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.van de Pavert S.A., Mebius R.E. New insights into the development of lymphoid tissues. Nat Rev Immunol. 2010;10(9):664–674. doi: 10.1038/nri2832. [DOI] [PubMed] [Google Scholar]
- 40.Kagnoff M.F., Eckmann L. Epithelial cells as sensors for microbial infection. J Clin Invest. 1997;100(1):6–10. doi: 10.1172/JCI119522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hargreaves D.C., Medzhitov R. Innate sensors of microbial infection. J Clin Immunol. 2005;25(6):503–510. doi: 10.1007/s10875-005-8065-4. [DOI] [PubMed] [Google Scholar]
- 42.Kiyono H., Fukuyama S. NALT- versus Peyer’s-patch-mediated mucosal immunity. Nat Rev Immunol. 2004;4(9):699–710. doi: 10.1038/nri1439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Brandtzaeg P. Function of mucosa-associated lymphoid tissue in antibody formation. Immunol Invest. 2010;39(4–5):303–355. doi: 10.3109/08820131003680369. [DOI] [PubMed] [Google Scholar]
- 44.Illum L. Nanoparticulate systems for nasal delivery of drugs: a real improvement over simple systems? J Pharm Sci. 2007;96(3):473–483. doi: 10.1002/jps.20718. [DOI] [PubMed] [Google Scholar]
- 45.Burgdorf S., Kautz A., Böhnert V., Knolle P.A., Kurts C. Distinct pathways of antigen uptake and intracellular routing in CD4 and CD8 T cell activation. Science. 2007;316(5824):612–616. doi: 10.1126/science.1137971. [DOI] [PubMed] [Google Scholar]
- 46.Sansonetti P.J., Di Santo J.P. Debugging how bacteria manipulate the immune response. Immunity. 2007;26(2):149–161. doi: 10.1016/j.immuni.2007.02.004. [DOI] [PubMed] [Google Scholar]
- 47.Skwarczynski M., Toth I. Non-invasive mucosal vaccine delivery: advantages, challenges and the future. Expert Opin Drug Deliv. 2020;17(4):435–437. doi: 10.1080/17425247.2020.1731468. [DOI] [PubMed] [Google Scholar]
- 48.Lycke N. Recent progress in mucosal vaccine development: potential and limitations. Nat Rev Immunol. 2012;12(8):592–605. doi: 10.1038/nri3251. [DOI] [PubMed] [Google Scholar]
- 49.Lijek R.S., Luque S.L., Liu Q., Parker D., Bae T., Weiser J.N. Protection from the acquisition of Staphylococcus aureus nasal carriage by cross-reactive antibody to a pneumococcal dehydrogenase. PNAS. 2012;109(34):13823–13828. doi: 10.1073/pnas.1208075109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Pashine A., Valiante N.M., Ulmer J.B. Targeting the innate immune response with improved vaccine adjuvants. Nat Med. 2005;11(4 Suppl):S63–S68. doi: 10.1038/nm1210. [DOI] [PubMed] [Google Scholar]
- 51.Linehan J.L., Dileepan T., Kashem S.W., Kaplan D.H., Cleary P., Jenkins M.K. Generation of Th17 cells in response to intranasal infection requires TGF-β1 from dendritic cells and IL-6 from CD301b+ dendritic cells. PNAS. 2015;112(41):12782–12787. doi: 10.1073/pnas.1513532112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wang S., Liu H., Zhang X., Qian F. Intranasal and oral vaccination with protein-based antigens: advantages, challenges and formulation strategies. Protein & Cell. 2015;6(7):480–503. doi: 10.1007/s13238-015-0164-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Jabbal-Gill I. Nasal vaccine innovation. J Drug Target. 2010;18(10):771–786. doi: 10.3109/1061186X.2010.523790. [DOI] [PubMed] [Google Scholar]
- 54.Vora L., et al. Self-assembled nanocomplexes of anionic pullulan and polyallylamine for DNA and pH-sensitive intracellular drug delivery. Journal of Nanoparticle Research volume. 2014;16 [Google Scholar]
- 55.Cardoso F.M.C., Petrovajová D., Horňáková T. Viral vaccine stabilizers: status and trends. Acta Virol. 2017;61(3):231–239. doi: 10.4149/av_2017_301. [DOI] [PubMed] [Google Scholar]
- 56.Apostólico J.de.S., Lunardelli V.A.S., Coirada F.C., Boscardin S.B., Rosa D.S. Adjuvants: classification, modus operandi, and licensing. J Immunol Res. 2016:e1459394. doi: 10.1155/2016/1459394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.CDC. Adjuvants and Vaccines | Vaccine Safety. www.cdc.gov/vaccinesafety/concerns/adjuvants.html (accessed July 2, 2021).
- 58.CDC. Live attenuated influenza vaccine (the nasal spray flu vaccine). www.cdc.gov/flu/prevent/nasalspray.html (accessed July 2, 2021).
- 59.de Bruijn I.A., Nauta J., Gerez L., Palache A.M. The virosomal influenza vaccine Invivac®: Immunogenicity and tolerability compared to an adjuvanted influenza vaccine (Fluad®) in elderly subjects. Vaccine. 2006;24(44):6629–6631. doi: 10.1016/j.vaccine.2006.05.035. [DOI] [PubMed] [Google Scholar]
- 60.Heida R, Hinrichs WL, Frijlink HW. Inhaled vaccine delivery in the combat against respiratory viruses: a 2021 overview of recent developments and implications for COVID-19. Expert Rev Vacc [published online March 22, 2021]. http://dx.doi.org/10.1080/14760584.2021.1903878. [DOI] [PubMed]
- 61.WHO. The different types of COVID-19 vaccines. www.who.int/news-room/feature-stories/detail/the-race-for-a-covid-19-vaccine-explained. [accessed July 2, 2021].
- 62.FluMist – FDA prescribing information, side effects and uses. www.drugs.com/pro/flumist.html [accessed July 2, 2021].
- 63.Balfour H. First patient dosed with intranasal COVID-19 vaccine candidate. European Pharmaceutical Review. www.europeanpharmaceuticalreview.com/news/139089/first-patient-dosed-with-covi-vac-an-intranasal-covid-19-vaccine-candidate/ [accessed July 2, 2021].
- 64.Liang Z., Zhu H., Wang X., Jing B., Li Z., Xia X., et al. Adjuvants for coronavirus vaccines. Front Immunol. 2020;11 doi: 10.3389/fimmu.2020.589833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Kremer E.J. Pros and cons of adenovirus-based SARS-CoV-2 vaccines. Mol Ther. 2020;28(11):2303–2304. doi: 10.1016/j.ymthe.2020.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Janssen. Vaccine technology. www.janssen.com/infectious-diseases-and-vaccines/vaccine-technology [accessed July 2, 2021].
- 67.CDC. Janssen COVID-19 Vaccine (Johnson & Johnson): Vaccine preparation and administration summary. www.cdc.gov/vaccines/covid-19/info-by-product/janssen/downloads/Janssen-Prep-and-Admin-Summary.pd. [accessed July 2, 2021].
- 68.Vaccine Knowledge Project. COVID-19 vaccines. https://vk.ovg.ox.ac.uk/vk/covid-19-vaccines [accessed July 2, 2021].
- 69.Precision Vaccinations. Sputnik V Vaccine. www.precisionvaccinations.com/vaccines/sputnik-v-vaccine [accessed July 2, 2021].
- 70.Wang N., Shang J., Jiang S., Du L. Subunit vaccines against emerging pathogenic human coronaviruses. Front Microbiol. 2020;11:298. doi: 10.3389/fmicb.2020.00298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.FDA. Moderna COVID-19 vaccine. www.fda.gov/emergency-preparedness-and-response/coronavirus-disease-2019-covid-19/moderna-covid-19-vaccine [accessed July 2, 2021].
- 72.WHO. WHO recommendation Tozinameran – COVID-19 mRNA vaccine (nucleoside modified) – COMIRNATY®. https://extranet.who.int/pqweb/vaccines/who-recommendation-covid-19-mrna-vaccine-nucleoside-modified-comirnaty [accessed July 2, 2021].
- 73.Chalmers. Nasal spray could deliver vaccine against COVID-19. www.chalmers.se/en/departments/physics/news/Pages/Nasal_spray_could_deliver_a_future_mRNA_vaccine_against_COVID_19.aspx [accessed July 2, 2021].
- 74.Afkhami S., Yao Y., Xing Z. Methods and clinical development of adenovirus-vectored vaccines against mucosal pathogens. Mol Therapy Methods Clin Develop. 2016;3:16030. doi: 10.1038/mtm.2016.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Choi Y., Chang J. Viral vectors for vaccine applications. Clin Exp Vaccine Res. 2013;2(2):97. doi: 10.7774/cevr.2013.2.2.97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Malik J.A., Mulla A.H., Farooqi T., Pottoo F.H., Anwar S., Rengasamy K.R.R. Targets and strategies for vaccine development against SARS-CoV-2. Biomed Pharmacother. 2021;137 doi: 10.1016/j.biopha.2021.111254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Guo J., Mondal M., Zhou D. Development of novel vaccine vectors: chimpanzee adenoviral vectors. Human Vacc Immunotherap. 2018;14(7):1679–1685. doi: 10.1080/21645515.2017.1419108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.University of Oxford. University of Oxford to study nasal administration of COVID-19 vaccine. www.ox.ac.uk/news/2021-03-25-university-oxford-study-nasal-administration-covid-19-vaccine [accessed July 2, 2021].
- 79.Van Doremalen N., Purushotham J.N., Schulz J.E., et al. Intranasal ChAdOx1 nCoV-19/AZD1222 vaccination reduces shedding of SARS-CoV-2 D614G in rhesus macaques. IoRxiv. 2021 doi: 10.1126/scitranslmed.abh0755. 2021.01.09.426058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Hassan A.O., Kafai N.M., Dmitriev I.P., Fox J.M., Smith B.K., Harvey I.B., et al. A single-dose intranasal ChAd vaccine protects upper and lower respiratory tracts against SARS-CoV-2. Cell. 2020;183(1):169–184. doi: 10.1016/j.cell.2020.08.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Bharat Biotech. Intranasal vaccine For Covid-19. www.bharatbiotech.com/intranasal-vaccine.html [accessed July 2, 2021].
- 82.St. Louis-based Precision Virologics and India’s Biotech leader Bharat Biotech obtain rights to intranasal COVID-19 vaccine technology. www.biostl.org/news-and-media/home/st-louis-based-precision-virologics-and-indias-biotech-leader-bharat-biotech-obtain-rights-to-intranasal-covid-19-vaccine-technology [accessed July 2, 2021].
- 83.CanSino Biologics Inc. A randomized, double-blind, placebo-controlled Phase I/II clinical trial to evaluate the safety and immunogenicity of Ad5-NCoV for inhalation in adults 18 years of age and older. https://clinicaltrials.gov/ct2/show/NCT04840992 [accessed July 2, 2021].
- 84.Anhui Zhifei Longcom Biologic Pharmacy Co., Ltd. A Phase III randomized, double-blind, placebo-controlled clinical trial in 18 years of age and above to determine the safety and efficacy of ZF2001, a recombinant novel coronavirus vaccine (CHO Cell) for prevention of COVID-19. https://clinicaltrials.gov/ct2/show/NCT04646590 [accessed July 2, 2021].
- 85.Altimmune, Inc. Phase 2, double-blind, randomized, placebo-controlled study of NasoVAX in the prevention of clinical worsening in patients with early Coronavirus infectious disease 2019 (COVID-19). https://clinicaltrials.gov/ct2/show/NCT04442230 [accessed July 2, 2021].
- 86.King R.G., Silva-Sanchez A., Peel J.N., Botta D., Meza-Perez S., Allie R., et al. Single-dose intranasal administration of AdCOVID elicits systemic and mucosal immunity against SARS-CoV-2 in mice. bioRxiv. 2020 doi: 10.3390/vaccines9080881. 2020.10.10.331348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.NasoVAX Influenza Vaccine. www.precisionvaccinations.com/vaccines/nasovax-influenza–vaccine [accessed July 2, 2021].
- 88.FluGen Inc. Focused on efficacy – M2SR: the vaccine. http://flugen.com/m2sr/ [accessed July 2, 2021].
- 89.Clinical Research and Development Company – Bharat Biotech. www.bharatbiotech.com/r&d_pipeline.html [accessed July 2, 2021].
- 90.Bilsel P. M2SR, a single replication universal flu vaccine. Virol-Mycol. 2015;4:2. [Google Scholar]
- 91.The University of Hong Kong. A Phase 1, randomized, double-blinded, placebo-controlled, dose-escalation and dose-expansion study to evaluate the safety and immunogenicity of DelNS1-NCoV-RBD LAIV for COVID-19 in Healthy Adults. https://clinicaltrials.gov/ct2/show/NCT04809389 [accessed July 2, 2021]. [DOI] [PMC free article] [PubMed]
- 92.Clinical Trials Arena. Nasal spray vaccine for Covid-19. www.clinicaltrialsarena.com/comment/nasal-spray-vaccine-covid-19/ [accessed July 2, 2021].
- 93.The Scientist Magazine. Track COVID-19 vaccines advancing through clinical trials. www.the-scientist.com/news-opinion/track-covid-19-vaccines-advancing-through-clinical-trials-67382 [accessed July 2, 2021].
- 94.Meissa Vaccines, Inc. Phase 1, open-label, dose-escalation study to evaluate tolerability, safety, and immunogenicity of an intranasal live attenuated respiratory syncytial virus vaccine expressing Spike protein of SARS-CoV-2 in healthy adults ages 18–69 Years. https://clinicaltrials.gov/ct2/show/NCT04798001 [accessed July 2, 2021].
- 95.Illinois Institute of Technology. Promising new COVID-19 treatment in development at Illinois Tech. www.iit.edu/news/promising-new-covid-19-treatment-development-illinois-tech. [Accessed July 2, 2021].
- 96.Intravacc. Intravacc announces positive pre-clinical data for its SARS-CoV-2 nose spray vaccine. www.intravacc.nl/news/intravacc-announces-positive-pre-clinical-data-intranasal-sars-cov-2-candidate-vaccine/ [accessed July 2, 2021].
- 97.Koshy S.T., Cheung A.S., Gu L., Graveline A.R., Mooney D.J. Liposomal delivery enhances immune activation by STING agonists for cancer immunotherapy. Adv Biosyst. 2017;1(1–2):1600013. doi: 10.1002/adbi.201600013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.AuraVax Therapeutics licences intranasal vaccine adjuvant technology from Massachusetts General Hospital. www.oindpnews.com/2021/01/auravax-therapeutics-licences-intranasal-vaccine-adjuvant-technology-from-massachusetts-general-hospital/ [accessed July 2, 2021].
- 99.Mitchell J.P., Berlinski A., Canisius S., Cipolla D., Dolovich M.B., Gonda I., et al. Urgent appeal from International Society for Aerosols in Medicine (ISAM) during COVID-19: clinical decision makers and governmental agencies should consider the inhaled route of administration: a statement from the ISAM Regulatory and Standardization Issues Networking Group. J Aerosol Med Pulmonary Drug Deliv. 2020;33(4):235–238. doi: 10.1089/jamp.2020.1622. [DOI] [PubMed] [Google Scholar]
- 100.Parkins K. SaNOtize’s ‘revolutionary’ Covid-19 nasal spray bolstered by Phase II trial data. www.clinicaltrialsarena.com/news/sanotize-nasal-spray-reduces-covid-19-viral-load-uk-clinical-trail/ [accessed July 2, 2021].
- 101.Regev-Shoshani G., Vimalanathan S., McMullin B., Road J., Av-Gay Y., Miller C. Gaseous nitric oxide reduces influenza infectivity in vitro. Nitric Oxide. 2013;31:48–53. doi: 10.1016/j.niox.2013.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Regev-Shoshani G., McMullin B., Nation N., Church J.S., Dorin C., Miller C. Non-inferiority of nitric oxide releasing intranasal spray compared to sub-therapeutic antibiotics to reduce incidence of undifferentiated fever and bovine respiratory disease complex in low to moderate risk beef cattle arriving at a commercial feedlot. Preventive Veterinary Med. 2017;138:162–169. doi: 10.1016/j.prevetmed.2015.04.008. [DOI] [PubMed] [Google Scholar]
- 103.Miller C., Miller M., McMullin B., Regev G., Serghides L., Kain K., et al. A phase I clinical study of inhaled nitric oxide in healthy adults. J Cyst Fibros. 2012;11(4):324–331. doi: 10.1016/j.jcf.2012.01.003. [DOI] [PubMed] [Google Scholar]
- 104.Weinberger B., Laskin D.L., Heck D.E., Laskin J.D. The toxicology of inhaled nitric oxide. Toxicol Sci. 2001;59(1):5–16. doi: 10.1093/toxsci/59.1.5. [DOI] [PubMed] [Google Scholar]
- 105.Akerström S., Gunalan V., Keng C.T., Tan Y.-J., Mirazimi A. Dual effect of nitric oxide on SARS-CoV replication: viral RNA production and palmitoylation of the S protein are affected. Virology. 2009;395(1):1–9. doi: 10.1016/j.virol.2009.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Business Wire. UK clinical trial confirms SaNOtize’s breakthrough treatment for COVID-19. www.businesswire.com/news/home/20210315005197/en/UK-Clinical-Trial-Confirms-SaNOtize’s-Breakthrough-Treatment-for-COVID-19 [accessed July 2, 2021].
- 107.SaNOtize Research and Development Corp. Nitric oxide releasing solutions to prevent and treat mild/moderate COVID-19 infection (NOCOVID). https://clinicaltrials.gov/ct2/show/NCT04337918 [accessed July 2, 2021].
- 108.Beyond Air. Revolutionizing nitric oxide therapy. www.beyondair.net/ [accessed July 2, 2021].
- 109.VERO Biotech. Technology. www.vero-biotech.com/technology/. Published 2020 [accessed July 2, 2021].
- 110.Shmuel K, Dalia M, Tair L, Yaakov N. Low pH Hypromellose (Taffix) nasal powder spray could reduce SARS-CoV-2 infection rate post mass-gathering event at a highly endemic community: an observational prospective open label user survey. Expert Rev Anti-infective Therapy Published online April 24, 2021. http://dx.doi.org/10.1080/14787210.2021.1908127. [DOI] [PMC free article] [PubMed]
- 111.Hull D., Rennie P., Noronha A., Poore C., Harrington N., Fearnley V., et al. Effects of creating a non-specific, virus-hostile environment in the nasopharynx on symptoms and duration of common cold. Acta Otorhinolaryngol Italica. 2007;27(2):73–77. [PMC free article] [PubMed] [Google Scholar]
- 112.Healthcare Technologies Institute. Anti-COVID-19 nasal spray ‘ready for use in humans’. www.birmingham.ac.uk/news/latest/2020/11/anti-covid-19-nasal-spray-ready-for-use-in-humans.aspx [accessed July 2, 2021].
- 113.Moakes R.J.A., Davies S.P., Stamataki Z., Grover L.M. Formulation of a composite nasal spray enabling enhanced surface coverage and prophylaxis of SARS-COV-2. bioRxiv. 2020 doi: 10.1002/adma.202008304. 2020.11.18.388645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Buller F. Neurimmune and Ethris sign collaboration agreement to rapidly develop inhaled mRNA-based antibody therapy for the treatment of Covid-19. Neurimmune AG. www.neurimmune.com/news/neurimmune-and-ethris-sign-collaboration-agreement-to-rapidly-develop-inhaled-mrna-based-antibody-therapy-for-the-treatment-of-covid-19 [accessed July 2, 2021].
- 115.Chakraverty A. Swiss and German team to develop inhaled mRNA coronavirus treatment. labiotech. www.labiotech.eu/trends-news/ethris-neurimmune-mrna-coronavirus/ [accessed July 2, 2021].
- 116.Prieschl-Grassauer E. Nasal spray works against COVID-19 variants. www.thepharmaletter.com/article/nasal-spray-works-against-covid-19-variants [accessed July 2, 2021].
- 117.Liebowitz Michael R., Salman Ester, Humberto Nicolini N.R. Effect of an acute intranasal aerosol dose of PH94B on social and performance anxiety in women with social anxiety disorder. Am J Psychiatry. 2014;171(6):675–682. doi: 10.1176/appi.ajp.2014.12101342. [DOI] [PubMed] [Google Scholar]
- 118.Zehr L. VistaGen readies Phase 3 trial of novel, fast-acting neuroactive nasal spray in social anxiety disorder. https://biotuesdays.com/2021/02/09/vistagen-readies-phase-3-trial-of-novel-fast-acting-neuroactive-nasal-spray-in-social-anxiety-disorder/ [accessed July 2, 2021].
- 119.VistaGen Therapeutics Inc. PH94B in the treatment of adjustment disorder with anxiety. https://clinicaltrials.gov/ct2/show/NCT04404192 [accessed July 2, 2021].
- 120.SaNOtize. NORSTMas an upper airway ‘disinfectant.’ https://sanotize.com/covid-19/ [accessed July 2, 2021].
- 121.APEPTICO Forschung und Entwicklung GmbH. APEPTICO’s global survey on incidence of ARDS and outcomes in hospitalized patients with COVID-19 has been published by Critical Care. www.apeptico.com/index-news [accessed July 2, 2021].
- 122.ClinicalTrials.gov. NCT02074059. Trial to assess the safety and tolerability of lucinactant for inhalation in premature neonates. https://clinicaltrials.gov/ct2/show/NCT02074059 [accessed July 2, 2021].
- 123.ClinicalTrials.gov. NCT04330586. A Trial of ciclesonide in adults with mild-to-moderate COVID-19. https://clinicaltrials.gov/ct2/show/NCT04330586 [accessed July 2, 2021].
- 124.NeuroRx IRTHA. FDA grants inhaled use IND for RLF-100 (aviptadil) to treat patients with moderate and severe COVID-19 aiming to prevent progression to respiratory failure. www.prnewswire.com/news-releases/fda-grants-inhaled-use-ind-for-rlf-100-aviptadil-to-treat-patients-with-moderate-and-severe-covid-19-aiming-to-prevent-progression-to-respiratory-failure-301107288.html [accessed July 2, 2021].
- 125.Partner Therapeutics. Partner Therapeutics initiates patient enrollment in U.S. clinical trial evaluating Leukine® (rhuGM-CSF, sargramostim) in COVID-19 patients. www.partnertx.com/partner-therapeutics-initiates-patient-enrollment-in-u-s-clinical-trial-evaluating-leukine-rhugm-csf-sargramostim-in-covid-19-patients/ [accessed July 2, 2021].
- 126.Lacroix M., Rousseau F., Guilhot F., Malinge P., Magistrelli G., Herren S., et al. Novel insights into interleukin 6 (IL-6) cis- and trans-signaling pathways by differentially manipulating the assembly of the IL-6 signaling complex. J Biol Chem. 2015;290(45):26943–26953. doi: 10.1074/jbc.M115.682138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.ClinicalTrials.gov. NCT04456452. Study of Ampion for the treatment of Adult COVID-19 patients requiring oxygen supplementation. ClinicalTrials.gov. NCT04456452. https://clinicaltrials.gov/ct2/show/NCT04456452 [accessed July 2, 2021].
- 128.EudraCT: 2020-001023-14. A randomized double-blind placebo-controlled trial to determine the safety and efficacy of inhaled SNG001 (IFNβ-1a for nebulization) for the treatment of patients with confirmed SARS-CoV-2 infection (COVID-19). www.clinicaltrialsregister.eu/ctr-search/trial/2020-001023-14/GB [accessed July 2, 2021].
- 129.ClinicalTrials.gov. NCT04408794. Long-term safety study of BHV-3500 (Zavegepant*) for the acute treatment of migraine. https://clinicaltrials.gov/ct2/show/NCT04408794. Published 2021 [accessed July 2, 2021].
- 130.Covis Pharma. Covis Pharma B.V. Initiates Phase 3 Clinical Trial of Alvesco (Ciclesonide) Inhaler for the Treatment of COVID-19. prnewswire. www.prnewswire.com/news-releases/covis-pharma-bv-initiates-phase-3-clinical-trial-of-alvesco-ciclesonide-inhaler-for-the-treatment-of-covid-19-301061105.html [accessed July 2, 2021].
- 131.Terada-Hirashima J., Suzuki M., Uemura Y., et al. Efficacy and safety of inhaled ciclesonide in treating patients with asymptomatic or mild COVID-19 in the RACCO Trial: protocol for a multicenter, open-label, randomized controlled trial. JMIR Res Protocols. 2020;9(12) doi: 10.2196/23830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Covis Pharma. Covis Pharma Group announces top-line safety and efficacy data from a phase 3 placebo-controlled COVID-19 study using inhaled corticosteroid (ciclesonide). www.globenewswire.com/news-release/2021/04/15/2210630/11011/en/COVIS-PHARMA-GROUP-Announces-Top-line-Safety-and-Efficacy-Data-from-a-Phase-3-Placebo-Controlled-COVID-19-Study-Using-Inhaled-Corticosteroid-ciclesonide.html [accessed July 2, 2021].
- 133.Lambert P.H., Ambrosino D.M., Andersen S.R., Baric R.S., Black S.B., Chen R.T., et al. Consensus summary report for CEPI/BC March 12–13, 2020 meeting: assessment of risk of disease enhancement with COVID-19 vaccines. Vaccine. 2020;38(31):4783–4791. doi: 10.1016/j.vaccine.2020.05.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.CIOMS/WHO Working Group on Vaccine Pharmacovigilance. Definition and application of terms for vaccine pharmacovigilance: report of CIOMS/WHO Working Group on Vaccine Pharmacovigilance. Geneva; WHO; 2012.
- 135.Jacob S.T., Crozier I., Fischer W.A., 2nd, Hewlett A., Kraft C.S., Vega M.A., et al. Ebola virus disease. Nat Rev Dis Primers. 2020;6(1):1–31. doi: 10.1038/s41572-020-0147-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.WHO. Ebola vaccine candidates. www.who.int/blueprint/priority-diseases/key-action/ebola-vaccine-candidates/en/ [accessed July 2, 2021].
- 137.Aptar. Nasal vaccines. www.aptar.com/products/pharmaceutical/nasal-vaccines/ [accessed July 2, 2021].
- 138.Optinose. Exhalation delivery systems. www.optinose.com/exhalation-delivery-systems/technical-overview [accessed July 2, 2021].
- 139.BD. BD Accuspray™ nasal spray system. https://drugdeliverysystems.bd.com/products-and-services/products/prefillable-syringe-systems/vaccine-syringes/accuspray-nasal-spray-system [accessed July 2, 2021].
- 140.ICDRA. Vaccines. https://cdsco.gov.in/opencms/opencms/en/biologicals/Vaccines/index.html [accessed July 2, 2021].
- 141.FDA Nasal spray and inhalation solution, suspension, and spray drug products--chemistry, manufacturing, and controls documentation. www.fda.gov/regulatory-information/search-fda-guidance-documents/nasal-spray-and-inhalation-solution-suspension-and-spray-drug-products-chemistry-manufacturing-and [accessed July 2, 2021].
- 142.Our World in Data. Coronavirus (COVID-19) vaccinations - statistics and research. https://ourworldindata.org/covid-vaccinations [accessed July 2, 2021].
- 143.Business Insider. A future COVID-19 vaccine could be squirted up the nose. The nasal spray could stop transmission, especially in kids. www.businessinsider.in/science/news/a-future-covid-19-vaccine-could-be-squirted-up-the-nose-the-nasal-spray-could-stop-transmission-especially-in-kids-/articleshow/81355447.cms [accessed July 2, 2021].
- 144.Avalon GloboCare. Clinical trials aim to tackle COVID-19, with nasal spray vaccine and ‘cytokine storm’ blood filtering system. www.nature.com/articles/d43747-020-01139-4 [accessed July 2, 2021].
- 145.The San Diego Union-Tribune. Future COVID-19 vaccines could come in a capsule or spray -. www.sandiegouniontribune.com/news/health/story/2021-04-19/covid-19-vaccines-minus-the-needle-researchers-working-on-capsules-nasal-sprays [accessed July 2, 2021].