Abstract
Background and Objective:
The Food and Drug Administration has cleared a probe-based NIRAF detection system called PTeye™ as an adjunct tool for label-free intraoperative parathyroid gland identification. Since PTeye™ has been investigated only in a ‘blinded’ manner to date, this study describes the preliminary impressions of PTeye™ when used by surgeons without being blinded to the device output.
Methods:
Patients undergoing thyroid and parathyroid procedures were prospectively recruited. Target tissues were intraoperatively assessed with PTeye™. The surgeon’s confidence in PG identification was recorded concomitantly with NIRAF parameters that were output in real-time from PTeye™.
Results:
A retrospective review of prospectively collected data on 83 patients was performed. PTeye™ was used for interrogating 336 target tissues in 46 parathyroid and 37 thyroid procedures. PTeye™ yielded an overall accuracy of 94.3% with a positive predictive value of 93.0% and a negative predictive value of 100%. An increase in confidence for intraoperative PG identification with PTeye™ was observed by all three participating high-volume surgeons, irrespective of their level of accrued surgical experience.
Conclusions:
Probe-based NIRAF detection with PTeye™ can be a valuable adjunct device to intraoperatively identify PGs for surgeons of varied training and experience.
Keywords: Parathyroid gland, surgical guidance, thyroidectomy, parathyroidectomy, near-infrared, autofluorescence
Introduction
Approximately 150,000 thyroid and at least 100,000 parathyroid operative procedures occur annually in the US 1-3. For thyroidectomies, post-operative hypocalcemia is an undesirable and unwarranted sequela that may follow due to inadvertent trauma/excision of healthy parathyroid glands (PGs) or unintentional damage to parathyroid vasculature. While postoperative hypocalcemia can be transient as seen in 5-35% of cases or permanent as observed in 2-7% of the patients, this condition can be debilitating for most patients 4-6. In comparison, a surgeon may fail to localize all diseased/hyperfunctioning PGs in 5-10% of parathyroidectomies 7,8, leading to persistent hyperparathyroidism and unnecessary re-operative procedures thereafter. The root of the aforementioned complications is mainly due to surgeons having difficulty in accurately identifying or localizing PGs during the operations, as PGs may often be mistaken for thyroid nodules, fat, or lymph nodes.
Ultrasound imaging (US), 99mtechnetium-sestamibi scintigraphy, and computed tomography (CT) are valuable preoperative modalities to localize enlarged PGs 9-11 and the gamma probe can be utilized to locate diseased PGs intraoperatively with varied success 12,13. However, these techniques are not useful to localize and preserve healthy PGs. More importantly, preoperative localization does not always match with what is observed by the surgeon intraoperatively. Thus, surgeons still tend to visually identify both healthy or diseased PGs by relying on their own accrued surgical experience, which may be highly subjective and variable. Frozen section analyses (FSA) or tissue aspirate parathyroid hormone (PTH) analysis serves as the current gold standard to intraoperatively identify if a tissue is a PG or not, whenever a surgeon is unsure. However, FSA or tissue aspirate PTH analysis is possibly injurious to healthy PGs and requires a waiting period of 20 to 30 minutes per sample 14. Therefore, surgeons could highly benefit from non-invasive intraoperative tools that can help preserve healthy PGs as well as detect/identify diseased PGs.
About a decade ago, strong near-infrared autofluorescence (NIRAF) was discovered in PGs compared to other soft tissues in the neck 15. Since the instrumentation required for NIRAF detection was relatively simple 15-17, several studies implemented this technique for PG identification with high success 18-23. Thus, NIRAF detection proved to be a rapid, non-invasive, and label-free approach that could effectively identify both healthy and diseased PGs, which had not been feasible with conventional intraoperative modalities available to date.
The implemented approaches for NIRAF detection so far can be broadly categorized as (a) imaging-based (Fluobeam) and (b) probe-based (PTeye™). While several studies have been able to evaluate the impact and outcome of the imaging-based approach due to easily accessible commercial near infrared (NIR) cameras 24-30, the scope of probe-based NIRAF detection has only been investigated with surgeons always remaining blinded to the device output 15,17,18,31,32. Without true feedback from the modality, the actual benefits from a probe-based system for surgical guidance remain only theoretical at best. Given that the probe-based approach can provide real-time quantitative information and was observed to be more sensitive in NIRAF detection from PGs than an imaging-based approach 33, the utility of this approach needs to be further explored in a non-blinded manner. In this study, we present our early clinical impressions upon utilizing PTeye™ as an adjunct device across a range of thyroid and parathyroid procedures, while providing an overview of its merits and demerits. Based on our initial experiences with this device, we sought to elaborate regarding our learning curve to effectively use PTeye™ in the operating room, while highlighting the clinical scenarios where PTeye™ was the most beneficial during neck endocrine procedures.
Methods
Description of PTeye™
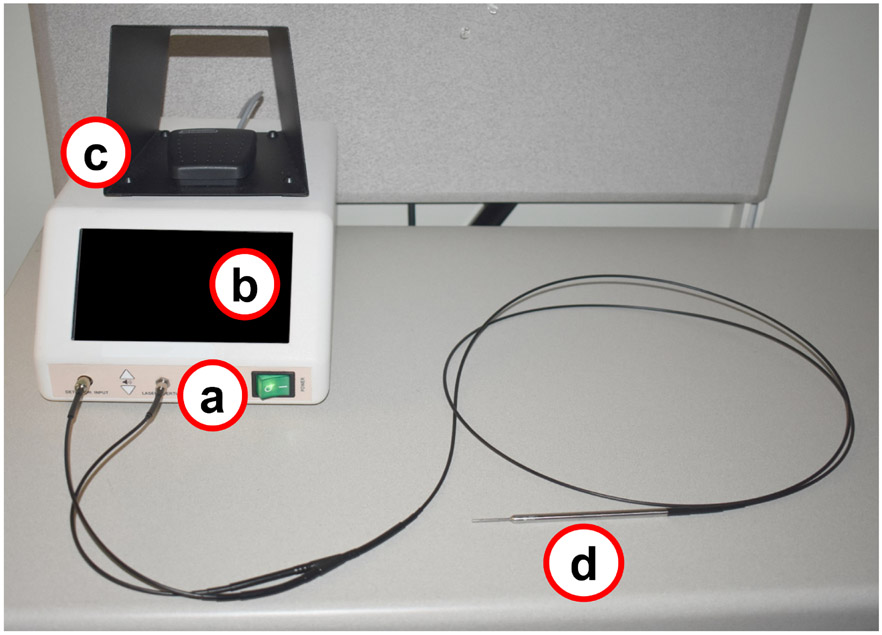
PTeye™ (see Figure 1), mainly consists of (i) a display console that also encloses a 785 nm laser source, a photo-diode detector, and relevant internal circuitry, (ii) a foot-pedal to activate the laser source, and (iii) a detachable sterile fiber-optic probe that is placed in contact with the tissue for NIRAF measurements. The distinct internal circuitry of PTeye™ enables NIRAF detection from tissues without interference from ambient operation room lights. Tissue NIRAF measured with PTeye™ is conveyed to the surgeon via display console as (i) Detection level – absolute NIRAF intensity measured from tissue and (ii) Detection ratio – absolute NIRAF intensity of tissue normalized to the baseline NIRAF intensity established in the patient (see equation below).
Figure 1:
Probe-based near-infrared autofluorescence (NIRAF) detection system – PTeye™. The device consists of (a) a console that houses a near-infrared (NIR) laser and a detector, (b) a display that informs the surgeon whether tissue is a parathyroid or not, (c) a foot-pedal for activating the NIR laser for tissue measurements and (d) a sterile detachable fiber probe that is placed in contact with the target tissue. (Figure adapted from Solórzano et al.34)
Based on earlier data, the device threshold was set such that only tissues with a detection ratio exceeding 1.2 were classified as ‘parathyroid’ 18,32. To obtain a NIRAF measurement, the surgeon places the sterile fiber-optic probe on the tissue and presses the foot-pedal. For tissue measurements with PTeye™, the surgeon first establishes a baseline NIRAF for each patient by obtaining NIRAF measurements on five random sites on the patient’s thyroid (or neck muscle if the thyroid is absent). After establishing the baseline, subsequent NIRAF measurements would indicate detection levels and ratios on the display console as NIRAF measurements are recorded from tissues of interest.
Study Design
A retrospective review was performed of the initial experiences of three high-volume surgeons utilizing NIRAF detection with the probe-based system PTeye™ as part of routine practice. Inclusion criteria for patients that were reviewed and considered for analysis were adult patients (≥18 years of age) who underwent thyroid or parathyroid procedures between February and October 2020 during which PTeye™ was utilized. Patient demographics, body mass index (BMI), operative indication, and procedure type were collected. Procedure types consisted of (i) parathyroidectomy – including focused or bilateral neck explorations (BNE) and (ii) thyroidectomy – including thyroid lobectomy (TL), total thyroidectomy (TT), TT with central lymph node dissection (CND), and TT with CND and modified lateral neck dissection (MLND). It was also noted if the surgery was a primary or re-operative procedure. The number of PGs preoperatively localized with US, 99mTechnetium-sestamibi scintigraphy, or CT for parathyroid procedures was also noted.
During the first 19 cases, PTeye™ was arbitrarily used as intended and described in its instruction manual, without following a specific protocol per se. The surgeon relied on the audio-visual feedback from the device simply to determine if the tissue was PG or not. Based on the learning curve obtained with PTeye™ after enrolling 19 patients, a study protocol was designed to standardize the use of PTeye™ across the participating surgeons for subsequent patients to be recruited. The study protocol ensured that the device setup, intraoperative utilization, and interpretation of PTeye™ output, were unanimously adopted and followed by all three surgeons for the remaining 64 patients (Table 1). Detection level, ratios, baseline values, and sources of device error were additionally noted for the subsequent patient dataset.
Table 1:
Protocol designed for PTeye™ after surgeons’ learning curve with initial 19 patients.
| Step of PTeye™ Use |
Description of Protocol |
|---|---|
| Step 1: Set-up | Turn on and set up the device as instructed |
| Step 2: Thyroid exposure | Expose as much of the thyroid lobe and isthmus as possible. This may not be possible in cases with a substernal component and/or retropharyngeal thyroid extension. Ligation of the middle thyroid vein is recommended. |
| Step 3: Obtain the baseline | The goal is to assess the highest areas of autofluorescence on the thyroid so that baseline reflects its NIRAF heterogeneity. Place the sterile probe in contact with the thyroid, press the foot pedal to activate the NIR light to detect thyroid NIRAF. Repeat 5 times on the thyroid. After the fifth measurement, the device will automatically set the baseline. * If there is no thyroid the baseline can be set using neck muscle. |
| Step 4: Double-check the baseline | After the device baseline is set the surgeon should always scan/survey the thyroid/muscle with the probe to ensure accuracy of baseline NIRAF. To double-check the baseline- place the probe on the thyroid lobe and press the foot-pedal to scan the thyroid lobe in as many places as possible. If any areas show “high” detection ratios (ratios > 1.2) after the initial baseline, then the surgeon should aim to re-adjust the baseline to include those thyroid areas with high detection ratio. *Beware of subcapsular parathyroids when obtaining/checking baseline. |
| Step 5: Readjust the baseline if necessary | Turn the device off and then back on. Repeat Steps 3 and 4 above. The goal is to scan the thyroid with the probe and not detect any areas that give high detection ratios on the thyroid. |
| Step 6: Expose suspected PG tissue | Properly expose the tissue of interest before obtaining the NIRAF measurement. Obtain measurements at various locations on the possible PG tissue. Parathyroid adenomas tend to have heterogenous NIRAF and could have areas of low detection ratio and areas of very high ratios. Always ensure that the probe tip is clean and free from tissue/blood residue before and after interrogation. |
| Step 7: Interpreting the PTeye™ display | When the probe touches PG tissue, the device should typically display a detection ratio >1.2 and generate high-frequency auditory beep. The surgeon should however question low detection ratios that range from 1.2-2.0 particularly when he/she has low confidence that the tissue is a PG. Or when he/she has high confidence it is a parathyroid adenoma. |
| (a) During thyroidectomy | The probe can be used to confirm PGs when the surgeon has high confidence. When the surgeon has lower confidence and the PG has not been visualized clearly, the surgeon can use the probe to interrogate or map suspicious PG, fat, thyroid, thymus, lymph nodes. |
| (b) During parathyroidectomy | The probe can be used to confirm PGs when the surgeon has high confidence. When the surgeon has lower confidence and/or the PG has not been localized or visualized clearly, the surgeon can use the probe to interrogate or map suspicious PG, fat, thyroid, thymus, and lymph nodes. |
| (c) Excised specimen(s) | Any removed specimen can be interrogated/scanned with the probe to look for possible PGs. The excised thyroid should be scanned with the probe to look for incidentally excised PGs. Parathyroid adenomas tend to have heterogenous NIRAF and could have areas of low detection ratio and areas of very high ratios. |
To quantify the utility of PTeye™, surgeon confidence was recorded for each target PG. The surgeons first recorded their confidence level on whether the tissue is PG as high (>75%), medium (50-75%), or low (<50%) based on their visual examination. The surgeon's confidence was then recorded again after interrogating the tissue with PTeye™ and the detection ratio on the presumed PG was known. Confidence levels of the surgical trainee, if present during the procedure, were also collected. To eliminate the attending’s influence on the surgical resident, they were asked to first identify PGs independently by placing the PTeye™ probe on the tissue that was assumed to be the parathyroid. The resident would then interrogate the target tissue with PTeye™ and interpret the device output again stating their confidence prior to the surgical attending making their judgement. If the resident failed to see the gland or incorrectly placed the probe on a non-parathyroid tissue, their degree of confidence was marked as ‘low’.
Histopathology (FSA or permanent histology) served as the gold standard validation for excised specimens, while the surgeon’s expert opinion was used for the corroborating identity of in situ tissues investigated with PTeye™. It must be noted that while a surgeon’s expert opinion could be subjective and error-prone, it is the only option available to verify the identity of in-situ tissues that are typically left intact and not biopsied. After each case, surgeons quantified their interpretation of the results as true positive (TP), false positive (FP), true negative (TN), or false negative (FN). Definition and determination of TP, FP, TN, and FN are provided in Table 2. The number of PGs identified with high confidence by the surgeons and the residents, before and after PTeye™, were compared and analyzed using a paired 2-tailed student t-test, with a p-value < 0.05 being considered significant.
Table 2:
Definition and description of true positive (TP), false positive (FP), true negative (TN), and false-negative (FN) as determined for PTeye™ in this study. (FSA, Frozen section analyses)
| In situ tissues | Excised tissues | |||
|---|---|---|---|---|
| PTeye™ output |
Gold standard | PTeye™ output |
Gold standard | |
| True Positive (TP) | Detection ratio > 1.2 | The expert surgeon has HIGH/MEDIUM confidence that tissue is parathyroid | Detection ratio > 1.2 | FSA/Permanent histology is POSITIVE for parathyroid tissue |
| False Positive (FP) | Detection ratio > 1.2 | The expert surgeon has LOW confidence that tissue is parathyroid | Detection ratio > 1.2 | FSA/Permanent histology is NEGATIVE for parathyroid tissue |
| True Negative (TN) | Detection ratio < 1.2 | The expert surgeon has LOW confidence that tissue is parathyroid | Detection ratio < 1.2 | FSA/Permanent histology is NEGATIVE for parathyroid tissue |
| False Negative (FN) | Detection ratio < 1.2 | The expert surgeon has HIGH/MEDIUM confidence that tissue is parathyroid | Detection ratio < 1.2 | FSA/Permanent histology is POSITIVE for parathyroid tissue |
PTeye™ has been designed such that when detection ratio > 1.2, the device indicates a high probability of the tissue being parathyroid. Similarly, a detection ratio < 1.2 would suggest a low probability of the tissue being parathyroid.
After testing the device in this preliminary cohort, all three surgeons met to share their initial impressions and examine the perceived advantages and pitfalls during use with PTeye™. Specific scenarios in which the PTeye™ was found to be most helpful and the skills acquired while using the probe-based system in practice were discussed extensively. The quantitative and qualitative data from the three surgeons were pooled together, analyzed, jointly interpreted, and subsequently described below.
Results
A total of 83 cases, 46 (55.5%) parathyroidectomy, and 37 (44.6%) thyroidectomy met the inclusion criteria. Patient demographics and clinical information are included in Table 3. The median age of the cohort was 54 years with the majority of patients being female (n=60, 72.3%). Of the parathyroidectomy cases, 19.6% were focused and 80.4% were BNE. While the majority of parathyroidectomies were performed for primary hyperparathyroidism (95.7%), 13% of the parathyroid cases were performed in a re-operative setting. Of the thyroidectomy cases, 21.6% were lobectomy, 59.5% total thyroidectomy, 5.4% total thyroidectomy with CND, 8.1% total thyroidectomy with CND and MLND, 2.7% completion thyroidectomy, and 2.7% prophylactic thyroidectomy. Indications for thyroidectomy included multinodular goiter/thyroid nodule (37.8%), hyperthyroidism (40.6%), well-differentiated thyroid cancer (16.2%), recurrent thyroid disease (2.7%), and Multiple Endocrine Neoplasia Type IIA syndrome (2.7%)
Table 3:
Patient demographics and operative procedure information (CND, central neck dissection; MLND, modified lateral neck dissection).
| Total cases using PTeye™ | 19 (initial set) | 64 (2nd set) | 83 (overall) |
| Age (years, range) | 57 (20 – 75) | 52 (22 – 83) | 54 (20 – 83) |
| Gender | |||
| Male | 5 (26.3%) | 18 (28.1%) | 23 (27.7%) |
| Female | 14 (73.7%) | 46 (71.9%) | 60 (72.3%) |
| Body Mass Index (kg/m2, range) | 28 (22 – 39) | 30 (17 – 54) | 29 (17 – 54) |
| Race | |||
| Caucasian | 16 (84.2%) | 55 (85.5%) | 71 (85.5%) |
| Non-Caucasian | 2 (10.5%) | 9 (13.3%) | 11 (13.3%) |
| Unknown | 1 (5.3%) | 0 (1.2%) | 1 (1.2%) |
| Ethnicity | |||
| Hispanic | 0 (0%) | 2 (3.1%) | 2 (2.4%) |
| Non-Hispanic | 17 (89.5%) | 62 (96.9%) | 79 (95.2%) |
| Unknown | 2 (10.5%) | 0 (0%) | 2 (2.4%) |
| Operative procedure | |||
| Thyroidectomy | 9 (47.4%) | 28 (43.8%) | 37 (45.6%) |
| Parathyroidectomy | 10 (52.6%) | 36 (56.2%) | 46 (55.4%) |
| Parathyroid specific data | |||
| Procedure | |||
| Focused | 1 (10%) | 8 (22.2%) | 9 (19.6%) |
| Bilateral neck exploration | 9 (90%) | 28 (77.8%) | 37 (80.4%) |
| Re-operative surgery | |||
| Yes | 4 (40%) | 3 (8.3%) | 7 (15.2%) |
| No | 6 (60%) | 33 (91.7%) | 39 (84.8%) |
| Diagnosis | |||
| Primary hyperparathyroidism | 10 (100%) | 34 (94.4%) | 44 (95.7%) |
| Tertiary hyperparathyroidism | 0 (0%) | 2 (5.6%) | 2 (4.3%) |
| Thyroid specific data | |||
| Procedure | |||
| Thyroid lobectomy | 4 (44.5%) | 4 (14.2%) | 8 (21.6%) |
| Total thyroidectomy | 3 (33.3%) | 19 (67.9%) | 22 (59.5%) |
| Total thyroidectomy with CND | 1 (11.1%) | 1 (3.6%) | 2 (5.4%) |
| Total thyroidectomy with CND and MLND | 1 (11.1%) | 2 (7.1%) | 3 (8.1%) |
| Completion thyroidectomy | 0 (0%) | 1 (3.6%) | 1 (2.7%) |
| Prophylactic thyroidectomy | 0 (0%) | 1 (3.6%) | 1 (2.7%) |
| Diagnosis | |||
| Multinodular goiter/thyroid nodule | 1 (11.1%) | 13 (46.4%) | 14 (37.8%) |
| Hyperthyroidism (Graves/toxic nodule) | 5 (55.6%) | 10 (35.7%) | 15 (40.6%) |
| Well-differentiated thyroid cancer | 3 (33.3%) | 3 (10.7%) | 6 (16.2%) |
| Recurrent/residual thyroid disease | 0 (0%) | 1 (3.6%) | 1 (2.7) |
| Multiple Endocrine Neoplasia, Type IIA* | 0 (0%) | 1 (3.6%) | 1 (2.7) |
Asymptomatic patient underwent prophylactic thyroidectomy
Age, body mass index reported as median, all others n (%)
In this cohort, a total of 336 target PGs were interrogated using the PTeye™. The overall accuracy was 94.3% with a positive predictive value of 93.0% and a negative predictive value of 100%. Table 4 demonstrates the performance of the PTeye™ versus the surgeon’s confidence level. Without the PTeye™, the surgeons themselves exhibited high confidence in 67.2% (226/336), medium confidence in 8.3% (27/336), and low confidence in 22.2% (83/336) on whether the target tissue was PG. When the surgeon reported high confidence in tissue being PG (n = 226), the PTeye™ agreed with the surgeon 100% (226/226) of the time. However, there were 5 (2.2%) FP results in the high confidence group, where 4 were confirmed to be negative for PG by histology. The remaining FP was judged to be a non-PG tissue by the surgeon when the actual PG was found later with an even higher detection ratio in a similar location. Taking this into account, the TP rate in the high confidence group was 97.8% (221/226). In the group where surgeons reported medium confidence (n = 27), PTeye™ indicated PG for 92.6% of those tissues (25/27). Of these 25, PTeye™ exhibited a TP rate of 96% (24/25), with just one FP being found in the medium confidence group. The tissue was deemed as FP when it was later opined to be thyroid tissue based on the surgeon’s judgment after two confirmed PGs were later identified on the ipsilateral side.
Table 4:
Concurrence between the surgeons’ confidence in identifying parathyroid and PTeye™ output. (PG, parathyroid gland).
| Surgeon’s confidence in tissue being PG |
In-situ (Final surgeon’s opinion for validation) |
Excised (FSA/ Permanent histology for validation) |
Target tissues tested (n) |
Device Positive: Detection ratio >1.2 (n) |
True Positive: Detection ratio >1.2 (n, %) |
False Positive: Detection ratio >1.2 (n, %) |
Device Negative: Detection ratio <1.2 (n) |
True Negative: Detection ratio <1.2 (n, %) |
False Negative: Detection ratio <1.2 (n, %) |
|---|---|---|---|---|---|---|---|---|---|
| 19 patients – initial set | |||||||||
| High (>75%) | 33 | 21 | 54 | 54 | 52 (96.3) | 2 (3.7) | 0 | 0 (0) | 0 (0) |
| Medium (50-75%) | 1 | 1 | 2 | 2 | 2 (100) | 0 (0) | 0 | 0 (0) | 0 (0) |
| Low (<50%) | 8 | 6 | 14 | 3 | 2 (66.7) | 1 (33.3) | 11 | 11 (100) | 0 (0) |
| Total | 42 | 28 | 70 | 59 | 56 (94.9) | 3 (5.1%) | 11 | 11 (100) | 0 (0) |
| 64 patients – second set | |||||||||
| High (>75%) | 108 | 64 | 172 | 172 | 169 (98.3) | 3 (1.7) | 0 | 0 (0) | 0 (0) |
| Medium (50-75%) | 25 | 0 | 25 | 23 | 22 (95.7) | 1 (4.3) | 2 | 2 (100) | 0 (0) |
| Low (<50%) | 62 | 7 | 69 | 18 | 6 (33.3) | 12 (66.7) | 51 | 51 (100) | 0 (0) |
| Total | 195 | 71 | 266 | 213 | 197 (92.5) | 16 (7.5) | 53 | 53 (100) | 0 (0) |
| 83 patients – overall | |||||||||
| High (>75%) | 141 | 85 | 226 | 226 | 221 (97.8) | 5 (2.2) | 0 | 0 (0) | 0 (0) |
| Medium (50-75%) | 26 | 1 | 27 | 25 | 24 (96) | 1 (4) | 2 | 2 (100) | 0 (0) |
| Low (<50%) | 70 | 13 | 83 | 21 | 8 (38.1) | 13 (61.9) | 62 | 62 (100) | 0 (0) |
| Total | 237 | 99 | 336 | 272 | 253 (93.0) | 19 (7.0) | 64 | 64 (100) | 0 (0) |
Number of true positive, false positive, true negative and false negative (see Table 2) was determined by surgeon’s judgement (for in-situ tissues) or histology (for excised tissues).
When the surgeons gave low confidence in tissue being PG (n=83), the PTeye™ concurred with the surgeons at 74.7% (62/83) that the tissue was not PG. In the remaining 21 cases (25.3%) when the surgeon had low confidence in tissue being PG, the PTeye™ detection ratio was >1.2 indicative that parathyroid tissue may in fact be present. Eight of the 21 were validated to be TP by histology or prompted the surgeon to interrogate further and ultimately confirm the parathyroid. In these 8 cases it was particularly valuable, as the PTeye™ indicated PG tissue even when the surgeon did not think it was likely to be PG. The remaining 13 cases were deemed to be FP by surgeon judgement and/or histology. The overall data set yielded 19 FPs obtained from thyroid nodules, lymph nodes, brown/regular fat, thymus, and paratracheal tissues as indicated in Table 5. 50% of FPs exhibited a ratio from 1.2-2.0, while the remaining FPs gave detection ratios >2.0. There was however a 100% negative predictive value as there were no instances of FN observed with PTeye™, regardless of the surgeon’s confidence level. Although it must be mentioned that some adenomatous glands had NIRAF heterogeneity, leading to low detection ratios (or FNs) on the most diseased regions of these glands. These glands were in turn detected due to the high ratios in the healthy-appearing ‘cap’ of the diseased gland.
Table 5:
Distribution of non-parathyroid tissues in the dataset that was misclassified by PTeye™ (FP – false positives)
| Tissue type | Number of FP readings (n, %) |
Mean Detection Ratio | Range of Detection Ratios |
|---|---|---|---|
| Thyroid nodules | 10 (52.5%) | 3.1 | 1.4 – 12.3 |
| Lymph node | 4 (21.1%) | 4.5 | 1.8 – 9.0 |
| Brown fat | 2 (10.5%) | 8.4 | 5.7 – 11.0 |
| Yellow fat | 1 (5.3%) | 1.5 | 1.5 |
| Thymus | 1 (5.3%) | 1.4 | 1.4 |
| Paratracheal tissue | 1 (5.3%) | 2.0 | 2.0 |
| Total | 19 (100%) | 3.8 | 1.4 – 12.3 |
Overall, the number of PGs identified with high confidence by the surgeons increased significantly from (i) 2.5 PGs/case to 3.1 PGs/case during thyroid procedures (p=0.00013) and (ii) 3.2 PGs/case to 3.5 PGs/case during BNE parathyroid procedures (p=0.005). While PTeye™ increased the number of PGs identified with high confidence from 1.1 to 1.2 PGs/case during focused parathyroid procedures, the difference was not significant (p=0.35). With respect to resident trainees, a total of 10, were included in the study, the level of surgical training ranged from 1st to 5th year of residency. The level of experience with parathyroid/thyroid surgery and with PTeye™ was variable. In comparison, PTeye™ aided the resident trainee considerably in identifying more PGs with high confidence during thyroid (1.3 PGs/case vs 3.1 PGs/case with PTeye™, p=1.9×10−6), BNE parathyroid (1.2 PGs/case vs 3.4 PGs/case with PTeye™, p=4.8×10−7) and focused parathyroid (0.2 PGs/case vs 1.2 PGs/case with PTeye™, p=0.01).
For focused parathyroid procedures, preoperative imaging (CT, US, and/or 99mTechnetium-sestamibi) had visualized 100% of the diseased glands (9/9 PGs) that were also confirmed intraoperatively by surgeon and PTeye™. However, for BNE parathyroid procedures, preoperative imaging found only 42.8% of the diseased PGs (30/70), while PTeye™ detected all these 70 diseased PGs intraoperatively along with the ipsilateral/contralateral additional 58 healthy PGs visualized.
Discussion
With the recent emergence of NIRAF detection as a promising technology for label-free intraoperative PG identification, PTeye™ is currently the only FDA-cleared probe-based NIRAF detection device 23,34. As a relatively new technology, little has been published about the surgeon’s experiences on utilizing PTeye™ in a high-volume endocrine surgery practice (>150 endocrine cases per surgeon per year). This study describes our initial experiences upon using PTeye™ in our thyroid and parathyroid procedures while providing insights into the potential advantages as well as some of the pitfalls encountered while using PTeye™.
In this initial cohort, the overall concurrence of PTeye™ with the surgeons’ judgment was 94.1%. When validated with the respective gold standards (surgeon opinion and/or histology), the accuracy for the device was calculated to be 94.3%, which is comparable to 92-98% reported with the prototype version of PTeye™ used in earlier studies 31-33. It must, however, be noted that the surgeons were always blinded to PTeye™ output in prior studies, while the current study is the first where the surgeon(s) received audio-visual feedback for PG discrimination. The agreement of PTeye™ with the surgeons was highest (97.8%) when the surgeons were highly confident that they had already identified a PG. While it can be argued that in these cases PTeye™ did not provide any added benefit, all surgeons report feeling reassured by PTeye™ as it confirmed their judgment. For target tissues identified with medium confidence, PTeye™ was highly beneficial for further improving the surgeon’s confidence and confirming PG tissue in 88.9% of these cases (24/27) and prevented surgeon error in 7.4% (2/27) of times where the surgeon misidentified non-parathyroid tissue as PG. On the contrary, very few PGs (8 out of 21) were identified as TP when the surgeon had low confidence. Importantly a detection ratio with PTeye™>1.2 was more likely to be an FP than a TP in this group. As seen in the Table 6 (for the second dataset of 64 patients), it is clear that if surgeons were to consider a higher Detection Ratio for tissue discrimination when they have low confidence, the number of false positives decreases. One must note that while false positives drop considerably as the Detection Ratio threshold is raised to 2.0 from 1.2, false negatives with PTeye™ on parathyroid glands that the surgeon had high-medium confidence increased from 0 to 2. Therefore, it might be more appropriate to consider a threshold of higher Detection Ratio only when the surgeon has low confidence on the tissue. This also further reiterates that surgeon’s judgement should always be considered while interpreting Detection Ratios. Therefore, we conclude that when the surgeon has low confidence about a tissue that gives a detection ratio >1.2 with PTeye™, it should be interpreted with caution and the surgeon should beware of the potential for FP. In these cases when the surgeon has low confidence, consideration of the value of detection ratios may be more helpful. Higher detection ratios > 2.0 are likely to be more indicative of PGs (normal PGs in particular vs. adenomas) than lower ratios i.e., 1.2-2.0. Alternatively, intraoperative FSA or tissue aspirate PTH assay can be considered if accurate identification of the target gland is critical to conclude the case. Another aspect to be considered is that brown fat is a strong source of FP with exceptionally high detection ratios (Table 5). Since brown fat tends to occur in younger and leaner adults (as also observed in our study), the surgeon should be discerning of this finding while operating on these patients.
Table 6:
False positives and false negative levels with different thresholds set for Detection Ratios when using PTeye™. A Detection Ratio of 1.2 is the threshold set by the manufacturer of PTeye™ for parathyroid identification.
| Detection Ratio Threshold set in PTeye™ - 1.2 |
High confidence |
Medium confidence |
Low confidence |
Total | |
|---|---|---|---|---|---|
| 83 patients (overall) | 226 | 27 | 83 | 336 | |
| False Positive at 1.2 | 5 | 1 | 13 | 19 | |
| False Negative at 1.2 | 0 | 0 | 0 | 0 | |
| 19 patients (Initial set) | Detection Ratio not recorded | 54 | 2 | 14 | 70 |
| False Positive at 1.2 | 2 | 0 | 1 | 3 | |
| False Negative at 1.2 | 0 | 0 | 0 | 0 | |
| 64 patients (2nd set) | Detection Ratio recorded | 172 | 25 | 69 | 266 |
| False Positive at 1.2 | 3 | 1 | 12 | 16 | |
| False Positive at 1.5 | 3 | 0 | 9 | 12 | |
| False Positive at 1.8 | 3 | 0 | 6 | 9 | |
| False Positive at 2.0 | 2 | 0 | 6 | 8 | |
| False Negative at 1.2 | 0 | 0 | 0 | 0 | |
| False Negative at 1.5 | 0 | 0 | 0 | 0 | |
| False Negative at 1.8 | 1 | 1 | 0 | 2 | |
| False Negative at 2.0 | 1 | 1 | 0 | 2 |
After using the probe-based approach in practice, we have identified several advantages of PTeye™ utilization during thyroid and parathyroid surgery, which are detailed in Table 7. Overall, we believe there is an advantage in the real-time feedback provided to the surgeon regarding a suspect parathyroid tissue. Whether this advantage offered by PTeye™ could reduce operative time, minimize the number of FSAs, or simply improve a surgeon’s confidence in PG identification is yet to be determined. We, however, hypothesize that it may indeed impact all of the aforementioned. Interestingly in our group, all surgeons subjectively reported improved confidence in identifying PGs using the PTeye™, regardless of their level of experience (18, 6, and 2 years of independent surgical practice), particularly for thyroid and BNE parathyroid procedures. While this improved confidence may never translate into a measurable benefit, the ability of such modalities to provide increased certainty in often uncertain surgical environments should not be undervalued, particularly for early-career surgeons and trainees. In addition to improving the operating surgeons’ confidence, we have found PTeye™ useful as a teaching tool for our surgical trainees. Trainees gain real-time feedback on their interpretation of the anatomy by placing the probe on a target gland. Based on their feedback, the trainees also reported higher confidence in identifying parathyroid tissue when using PTeye™. From a cost-benefit perspective, it is extremely unrealistic and time-consuming to perform FSAs on every tissue a trainee would want to interrogate. Yet PTeye™ can be used to interrogate tissues rapidly with very little time wasted. However, we have learned that surgical experience matters while interpreting results from new technology such as PTeye™. Trainees seem more likely to accept FP results from PTeye™, particularly when they have low confidence on whether the tissue is parathyroid or not. As a result, trainees would likely trust the device more than their own surgical experience. Thus, it would be important for early-career and trainees to consider using FSA/tissue aspirate PTH analysis when they have low confidence and encounter a detection ratio above the threshold, particularly when they are just learning to use this device. Another aspect to be noted regarding this study was that it was not feasible to track the confidence and learning curve of the surgical trainees over a longer duration since they rotate frequently on the endocrine surgery service. The scope of PTeye™ and similar technologies being able to shorten the learning curve for residents or early career surgeons requires further investigations in a more comprehensive manner.
Table 7:
Advantages and pitfalls when utilizing probe-based NIRAF detection system – PTeye™ in thyroid and parathyroid operative procedures (PG, parathyroid gland; FSA, frozen section analyses; PTH, parathyroid hormone).
| Advantages | Possible pitfalls | |
|---|---|---|
| PTeye™ utilization in neck endocrine operative procedures |
|
|
Although our team has been investigating the feasibility of the probe-based lab-built system since 2009 15,17,18,35 and the original PTeye™ prototype since 2017 31,32 to identify PGs, the surgeons involved have always been blinded to the device output in these prior studies. While using the FDA-cleared PTeye™ as intended – without being blinded, we encountered several pitfalls in our initial cohort experience that allowed us to troubleshoot along the way and improve the learning curve to reliably utilize PTeye™ (Table 1). We learned that setting an appropriate baseline is critical to obtaining reliable results. Inappropriately high or low baseline levels can result in misleading FN and FP respectively, arising from the resultant incorrect detection ratios in PTeye™. Low baseline levels are particularly challenging as the rate of FP will increase and can lead to intraoperative confusion. After the device baseline is set, the surgeon should always scan/survey the thyroid (or muscle in absence of thyroid) with the probe to reduce the likelihood of an inaccurate low baseline. If any areas show detection ratios >1.2 after the initial baseline, the surgeon should then re-adjust the baseline to include the thyroid areas with the high ratios. While we did not encounter any FN in this cohort, there is certainly also the risk of an inappropriately high baseline from either unidentified intrathyroidal PGs, a toxic thyroid nodule, or thyroid cancer with very high NIRAF getting included in baseline measurements. Knowledge about the potential sources of high NIRAF within the thyroid can help surgeons have a higher suspicion for an FN, e.g., if the baseline NIRAF is high and a target PG has a detection ratio <1.2 despite high surgeon confidence. PTeye™ is not recommended for use in patients with secondary hyperparathyroidism or parathyroid cysts due to high FN rates observed earlier in these cases 18,23,32.
An additional lesson learned from this cohort study is that there is great intraglandular NIRAF heterogeneity within diseased PGs, as also reported in other studies. 36,37. This has been encountered when a PG gland has both a normal-appearing portion and an adenomatous portion with higher detection ratios encountered in the normal-appearing ‘cap’ portion of the gland. If only the most abnormal portion of the gland is interrogated with PTeye™, the surgeon may fail to see a detection ratio >1.2 with PTeye™. Therefore, we recommend interrogating the entirety of the target diseased PG with the probe before drawing a conclusion. This can be encountered particularly with large parathyroid adenomas or when the PG has cystic areas. The majority of pitfalls with this technology can be overcome as surgeons learn how to optimally use the modality or as they gain further surgical experience over time with more thyroid and/or parathyroid surgeries (akin to nerve monitoring device). Thus, it is imperative to state that surgeon discretion is essential in the use of this device 23,34.
There are several clinical scenarios in which PTeye™ has proven most beneficial. (Table 8). We anticipate this list will grow and/or change within increased utilization of this device in different clinical situations. In general, the PTeye™ has been most useful in (i) re-operative or non-localized parathyroid procedures, (ii) patients with Hashimoto’s thyroiditis with associated reactive lymphadenopathy during thyroid or parathyroid procedures, and (iii) identification/confirmation of at least one residual PG after total thyroidectomy. Furthermore, PTeye™ can assist surgeons to find residual diseased PGs that were not preoperatively localized or when intraoperative PTH levels fail to normalize, while helping preserve the remaining healthy PGs, which can be extremely valuable during BNE parathyroid procedures. Other studies utilizing image-based NIRAF detection cameras have already suggested that the use of this technology to visualize PGs and help avoid postoperative hypocalcemia after total thyroidectomy 24,38. We are currently investigating the impact of NIRAF detection through two clinical trials to systematically assess the benefits of PTeye™ during thyroidectomy or parathyroidectomy 39,40.
Table 8:
Clinical scenarios in which PTeye™ was found to be most helpful for endocrine surgeons (PG, parathyroid gland; FSA, frozen section analyses; PTH, parathyroid hormone)
| Type of operative procedure | The clinical scenario in which PTeye™ was most beneficial |
|---|---|
| Thyroid procedures |
|
| Parathyroid procedures |
|
In conclusion, we have found that in practice PTeye™ is highly accurate and overall, greatly improves the surgeon’s confidence in PG identification thus allowing the operator to move forward more assuredly. In this manner, the surgeons will need to worry less about whether there was tissue misidentification during a parathyroidectomy or if there were sufficient PGs left behind after thyroidectomy to ensure a euparathyroid state for the patient. This increase in surgeon confidence for confirming PGs was described by all three participating surgeons and felt to occur irrespective of surgeon experience level. As with the implementation of any new technology, there is bound to be a learning curve with PTeye™ as its utility becomes more widespread in the surgical community. Overall, PTeye™ is a user-friendly and easy to interpret platform that can aid surgeons in identifying PGs. More extensive and large-scale studies with feedback from surgeons at other surgical centers will be needed to corroborate our current findings.
Synopsis.
Near infrared autofluorescence (NIRAF) could be useful for label-free intraoperative parathyroid gland identification during thyroid and parathyroid procedures. This study describes the early clinical impressions of 3 high-volume surgeons who tested the probe-based NIRAF detection system - PTeye™ - for identifying parathyroid glands in 83 prospectively enrolled patients.
Acknowledgments
We would like to thank the OR staff and surgical trainees for their assistance in data collection. Dr. G. Thomas and Dr. C. Solórzano were supported by the National Institute of Health under Grant No. R01CA212147.
Footnotes
Disclosures
Dr. Giju Thomas is affiliated with Vanderbilt University who has a licensing agreement for PTeye™ with AiBiomed (now officially acquired by Medtronic).
Data Availability Statement
Data that support the findings of this study will be made available from the corresponding author upon reasonable request.
References
- 1.Grogan RH, Suh I, Chomsky-Higgins K, et al. Patient Eligibility for Transoral Endocrine Surgery Procedures in the United States. JAMA Network Open. 2019;2(5):e194829–e194829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Al-Qurayshi Z, Robins R, Hauch A, Randolph GW, Kandil E. Association of Surgeon Volume With Outcomes and Cost Savings Following Thyroidectomy: A National Forecast. JAMA Otolaryngology–Head & Neck Surgery. 2016;142(1):32–39. [DOI] [PubMed] [Google Scholar]
- 3.Kim SM, Shu AD, Long J, et al. Declining rates of inpatient parathyroidectomy for primary hyperparathyroidism in the US. PloS one. 2016;11(8):e0161192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bergenfelz A, Nordenström E, Almquist M. Morbidity in patients with permanent hypoparathyroidism after total thyroidectomy. Surgery. 2020;167(1):124–128. [DOI] [PubMed] [Google Scholar]
- 5.Edafe O, Antakia R, Laskar N, Uttley L, Balasubramanian SP. Systematic review and meta-analysis of predictors of post-thyroidectomy hypocalcaemia. The British journal of surgery. 2014;101(4):307–320. [DOI] [PubMed] [Google Scholar]
- 6.Antakia R, Edafe O, Uttley L, Balasubramanian SP. Effectiveness of Preventative and Other Surgical Measures on Hypocalcemia Following Bilateral Thyroid Surgery: A Systematic Review and Meta-Analysis. Thyroid. 2014;25:95–106. [DOI] [PubMed] [Google Scholar]
- 7.Cron DC, Kapeles SR, Andraska EA, et al. Predictors of operative failure in parathyroidectomy for primary hyperparathyroidism. The American Journal of Surgery. 2017;214:509–514. [DOI] [PubMed] [Google Scholar]
- 8.Simental A, Ferris RL. Reoperative Parathyroidectomy. Otolaryngologic Clinics of North America. 2008;41(6):1269–1274. [DOI] [PubMed] [Google Scholar]
- 9.Patel CN, Salahudeen HM, Lansdown M, Scarsbrook AF. Clinical utility of ultrasound and 99mTc sestamibi SPECT/CT for preoperative localization of parathyroid adenoma in patients with primary hyperparathyroidism. Clinical Radiology. 2010;65(4):278–287. [DOI] [PubMed] [Google Scholar]
- 10.Sukan A, Reyhan M, Aydin M, et al. Preoperative evaluation of hyperparathyroidism: the role of dual-phase parathyroid scintigraphy and ultrasound imaging. Annals of Nuclear Medicine. 2008;22(2):123–131. [DOI] [PubMed] [Google Scholar]
- 11.Mohebati A, Shaha AR. Imaging techniques in parathyroid surgery for primary hyperparathyroidism. American Journal of Otolaryngology. 2012;33(4):457–468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Koc ZP, Ozcan Kara P, Dag A, Berkesoglu M. Feasibility of portable gamma camera imaging in intraoperative radioguided parathyroid adenoma identification. Iranian Journal of Nuclear Medicine. 2018;26:62–65. [Google Scholar]
- 13.Hall NC, Plews RL, Agrawal A, et al. Intraoperative scintigraphy using a large field-of-view portable gamma camera for primary hyperparathyroidism: initial experience. BioMed research international. 2015;2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Novis DA, Zarbo RJ. Interinstitutional comparison of frozen section turnaround time. Archives of pathology & laboratory medicine. 1997;121(6):559. [PubMed] [Google Scholar]
- 15.Paras C, Keller M, White L, Phay J, Mahadevan-Jansen A. Near-infrared autofluorescence for the detection of parathyroid glands. Journal of biomedical optics. 2011;16(6):067012-067012-067014. [DOI] [PubMed] [Google Scholar]
- 16.Paras C, Pence I, Mahadevan-Jansen A. A novel optical approach to intraoperative detection of parathyroid glands. Nashville, TN, USA: Biomedical Engineering, Vanderbilt University; 2012. [Google Scholar]
- 17.McWade MA, Paras C, White LM, Phay JE, Mahadevan-Jansen A, Broome JT. A novel optical approach to intraoperative detection of parathyroid glands. Surgery. 2013;154(6):1371–1377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.McWade MA, Sanders ME, Broome JT, Solórzano CC, Mahadevan-Jansen A. Establishing the clinical utility of autofluorescence spectroscopy for parathyroid detection. Surgery. 2016;159(1):193–203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ladurner R, Sommerey S, Arabi NA, Hallfeldt KKJ, Stepp H, Gallwas JKS. Intraoperative near-infrared autofluorescence imaging of parathyroid glands. Surgical Endoscopy. 2016:1–6. [DOI] [PubMed] [Google Scholar]
- 20.Kim SW, Song SH, Lee HS, et al. Intraoperative Real-Time Localization of Normal Parathyroid Glands with Autofluorescence Imaging. The Journal of Clinical Endocrinology & Metabolism. 2016;0(0):jc.2016–2558. [DOI] [PubMed] [Google Scholar]
- 21.Shinden Y, Nakajo A, Arima H, et al. Intraoperative Identification of the Parathyroid Gland with a Fluorescence Detection System. World Journal of Surgery. 2017;41(6):1506–1512. [DOI] [PubMed] [Google Scholar]
- 22.Falco J, Dip F, Quadri P, de la Fuente M, Prunello M, Rosenthal RJ. Increased identification of parathyroid glands using near infrared light during thyroid and parathyroid surgery. Surgical Endoscopy. 2017;31(9):3737–3742. [DOI] [PubMed] [Google Scholar]
- 23.Solórzano CC, Thomas G, Baregamian N, Mahadevan-Jansen A. Detecting the Near Infrared Autofluorescence of the Human Parathyroid: Hype or Opportunity? Annals of Surgery. 2020;272(6):973–985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Benmiloud F, Godiris-Petit G, Gras R, et al. Association of Autofluorescence-Based Detection of the Parathyroid Glands During Total Thyroidectomy With Postoperative Hypocalcemia Risk: Results of the PARAFLUO Multicenter Randomized Clinical Trial. JAMA Surgery. 2020;155(2):106–112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Squires MH, Jarvis R, Shirley LA, Phay JE. Intraoperative Parathyroid Autofluorescence Detection in Patients with Primary Hyperparathyroidism. Annals of Surgical Oncology. 2019;26:1142–1148. [DOI] [PubMed] [Google Scholar]
- 26.Dip F, Falco J, Verna S, et al. Randomized Controlled Trial Comparing White Light with Near-Infrared Autofluorescence for Parathyroid Gland Identification During Total Thyroidectomy. Journal of the American College of Surgeons. 2019;228:744–751. [DOI] [PubMed] [Google Scholar]
- 27.DiMarco A, Chotalia R, Bloxham R, McIntyre C, Tolley N, Palazzo FF. Does fluoroscopy prevent inadvertent parathyroidectomy in thyroid surgery? Ann R Coll Surg Engl. 2019;101(7):508–513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.DiMarco A, Chotalia R, Bloxham R, McIntyre C, Tolley N, Palazzo FF. Autofluorescence in Parathyroidectomy: Signal Intensity Correlates with Serum Calcium and Parathyroid Hormone but Routine Clinical Use is Not Justified. World Journal of Surgery. 2019;43:1532–1537. [DOI] [PubMed] [Google Scholar]
- 29.Kahramangil B, Dip F, Benmiloud F, et al. Detection of Parathyroid Autofluorescence Using Near-Infrared Imaging: A Multicenter Analysis of Concordance Between Different Surgeons. Annals of Surgical Oncology. 2018;25:957–962. [DOI] [PubMed] [Google Scholar]
- 30.Kim YS, Erten O, Kahramangil B, Aydin H, Donmez M, Berber E. The impact of near infrared fluorescence imaging on parathyroid function after total thyroidectomy. Journal of Surgical Oncology. 2020;122(5):973–979. [DOI] [PubMed] [Google Scholar]
- 31.Thomas G, McWade MA, Nguyen JQ, et al. Innovative surgical guidance for label-free real-time parathyroid identification. Surgery. 2019;165(1):114–123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Thomas G, McWade MA, Paras C, et al. Developing a clinical prototype to guide surgeons for intraoperative label-free identification of parathyroid glands in real time. Thyroid. 2018;28(11):1517–1531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Thomas G, Squires MH, Metcalf T, Mahadevan-Jansen A, Phay JE. Imaging or Fiber Probe-Based Approach? Assessing Different Methods to Detect Near Infrared Autofluorescence for Intraoperative Parathyroid Identification. Journal of the American College of Surgeons. 2019;229(6):596–608.e593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Solórzano CC, Thomas G, Berber E, et al. Current state of intraoperative use of near infrared fluorescence for parathyroid identification and preservation. Surgery. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.McWade MA, Paras C, White LM, et al. Label-free Intraoperative Parathyroid Localization With Near-Infrared Autofluorescence Imaging. The Journal of Clinical Endocrinology & Metabolism. 2014;99(12):4574–4580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kose E, Kahramangil B, Aydin H, Donmez M, Berber E. Heterogeneous and low-intensity parathyroid autofluorescence: Patterns suggesting hyperfunction at parathyroid exploration. Surgery. 2019;165:431–437. [DOI] [PubMed] [Google Scholar]
- 37.Demarchi MS, Karenovics W, Bédat B, Triponez F. Intraoperative autofluorescence and indocyanine green angiography for the detection and preservation of parathyroid glands. Journal of Clinical Medicine. 2020;9(3):830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Benmiloud F, Rebaudet S, Varoquaux A, Penaranda G, Bannier M, Denizot A. Impact of autofluorescence-based identification of parathyroids during total thyroidectomy on postoperative hypocalcemia: a before and after controlled study. Surgery. 2018;163(1):23–30. [DOI] [PubMed] [Google Scholar]
- 39.ClinicalTrials.gov. Evaluating impact of NIRAF detection for identifying parathyroid glands during parathyroidectomy. 2020; https://clinicaltrials.gov/ct2/show/NCT04299425, 2020.
- 40.ClinicalTrials.gov. Assessing benefits of NIRAF detection for identifying parathyroid glands during total thyroidectomy. 2020; https://clinicaltrials.gov/ct2/show/NCT04281875, 2020.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data that support the findings of this study will be made available from the corresponding author upon reasonable request.