Abstract
Objective:
This review synthesizes literature on changes in binge eating (BE) and loss of control eating (LOC) following weight loss, and the association between BE/LOC and weight loss in children and adolescents.
Methods:
A systematic literature search was conducted in PubMed, Scopus, and PsycInfo. Eligible studies included all peer-reviewed journal articles of primary research that assessed BE/LOC and weight change following a weight loss intervention in individuals under 18 years of age.
Results:
The 29 articles included studies on behavioral therapy, pharmacotherapy, and surgical interventions. Four of 14 studies showed that higher baseline BE/LOC was associated with less weight loss, while 10 showed no significant association. BE/LOC behaviors significantly decreased following weight loss interventions in 20 of 21 studies. A greater decrease in BE/LOC was associated with improved weight loss in four of nine studies that assessed this change.
Conclusions:
Weight loss interventions are associated with improved BE/LOC in youth with obesity. Persistence of BE/LOC symptoms may be associated with less weight loss. These results can aid in guiding future treatment for youth with BE/LOC seeking weight loss treatment.
Keywords: Binge eating disorder, loss of control eating, obesity, weight loss
Introduction
Pediatric obesity is a global health concern. Rates of obesity in pediatric populations remain high in developed countries such as the United States and Canada with prevalence rates of 18.5% and 13.1%, respectively (1). This is of particular importance due to the association of pediatric obesity with metabolic syndrome, inflammation, and a variety of other comorbidities (2). Current weight management treatments for pediatric patients include behavioral therapy, pharmacotherapy, and bariatric surgery (3). These treatments can help patients lose weight. However, there has been concern that, in children and adolescents, they may promote or worsen disordered eating behaviors, such as binge eating (BE) and loss of control eating (LOC) (4). Several mechanisms have been proposed that may underlie this relationship (5). High levels of rigid dietary restraint have been identified as a central risk and maintaining factor of BE. Dieting may result in hunger and associated physiological responses, which may contribute to BE. In some individuals, externally or self-imposed dietary rules may be strict and increasingly difficult to sustain over the long term. The inevitable breaking of these rules may induce an all-or-none reaction, resulting in binge eating (i.e., the abstinence violation effect).
BE is reported by 20–35% of youth seeking weight loss (6). BE is defined as “the subjective experience of loss of control while eating a reportedly objectively large amount of food” (7). In 2013, the Diagnostic and Statistical Manual of Mental Disorders, 5th edition (DSM 5) added binge eating disorder (BED) as an independent diagnosis for those who have recurrent binge eating episodes while experiencing associated features (Table 1). Though it is a new diagnosis, it is the most prevalent eating disorder among adolescents (8, 9). Of note, one of the first descriptions of BE among adults was in 1959 (10). It was not until 1993 that more research was published regarding binge eating in adolescents with obesity, with studies demonstrating that BE was associated with depression (11).
Table 1:
Defining Terms Around Binge and Loss of Control Eating
| Loss of Control Eating (LOC) | “Subjective experience of loss of control while eating, irrespective of reported amount of food consumed” (7) |
| Subjective Binge Episode (SBE) | “Experience LOC but do not consume an objectively large amount of food” (16) |
| Binge Eating (BE) | “Subjective experience of loss of control while eating a reportedly objectively large amount of food” (7) |
| Objective Binge Episode (OBE) | “Experience LOC and consume an objectively large amount of food” (16) |
| Objective Overeating Episode (OOE) | “An unambiguously large amount of food is consumed in the absence of loss of control overeating.” (73) |
| Subthreshold BED | “Recurrent binge-eating episodes below the threshold of diagnostic criteria” (7) |
| Binge-Eating Disorder (BED) | “A: Recurrent episodes of binge eating B: Binge-eating episodes are associated with three (or more) of five specified features C: Marked distress regarding binge eating D: Binge eating occurs at least once a week for 3 months E: Binge eating is not associated with the regular use of inappropriate compensatory behaviors” and not exclusively during anorexia nervosa, bulimia nervosa, or avoidant-restrictive food intake disorder. (74) |
In pediatric patients, there are challenges in defining “a large amount of food” when considering the possibilities such as increases in calories related to growth spurts. Studies have found high levels of psychopathology regardless of whether the amount of food is objectively large, thus questioning the salience of this attribute (12–15). Consequently, several investigators have examined loss of control eating (LOC) in substitution for, or in addition to, BE, as it does not include the requirement of an objectively large amount of food consumed during such an episode (7). Instead, LOC can be broken down into subjective binge episodes (SBEs), in which the participant experiences a loss of control and does not consume an objectively large amount of food, and objective binge episodes (OBEs), in which they experience both a loss of control and consume an objectively large amount of food (Table 1) (16). Rates of LOC are 31.2% in youth with overweight (17). BE and LOC have been shown to be significantly correlated to weight and weight loss in adults (7). Adults tend to have decreases in BE/LOC with weight loss interventions, and persistent BE/LOC is also associated with less weight loss (18, 19). Previous literature has indicated that BE and LOC may have a different impact in children and adolescents than in adults (14). Therefore, identification of the effects of weight loss treatment on these behaviors in youth is critical.
The first-line treatment for BED is cognitive behavioral therapy (CBT), which is associated with declines in BE but typically does not produce weight loss (20, 21). Cognitive and behavioral models of BED hypothesize that increased dietary restraint is a mechanism linked to BE (22–24). Thus, there is controversy about whether those with binge eating should seek weight loss treatment or whether they should seek CBT first (25). In addition, it has been hypothesized that BE may impair weight loss outcomes and may be a factor that helps to explain the heterogeneity in weight loss seen with different weight management treatments (26, 27). Research is needed on factors that may help to identify individuals who need additional treatments.
A systematic review and meta-analysis in 2019 examined eating disorder risk in youth receiving weight management treatment, but focused solely on treatment-seeking patients who were overweight and received either health education or a diet regimen (28). The review excluded all studies of patients who received bariatric surgery, pharmacotherapy, or online programs, the last of which is commonly used by youth. The review analyzed overall eating disorder risk and had limited data on BE/LOC. The authors reported on five studies that examined BE, but did not include LOC. The results of this review did indicate a reduction in BE, along with other eating disorder symptoms, following weight loss interventions, when compared to pre-intervention. The present review expands on the topic by analyzing all forms of weight loss interventions. Additionally, this review focuses specifically on BE/LOC, as opposed to general eating disorder behaviors or psychopathology. As there is currently minimal evidence from clinical trials to guide treatment of BE in youth, a systematic review of the relationship of weight loss interventions, weight loss outcomes, and BE/LOC can aid researchers and clinicians in future treatment models (29).
The aim of this systematic review was to synthesize research on the association between BE/LOC and weight loss outcomes in children and adolescents. This review encapsulates studies that involved weight loss interventions and compared the study population before and after the intervention, studying outcomes of weight change and, when available, BE/LOC change. The two specific aims were to: a) determine if BE/LOC increased during weight loss treatment; and b) determine if BE/LOC impaired weight loss outcomes.
Methods
We conducted a systematic search for relevant articles published before June 13th, 2020, in consultation with a science literature librarian at the University of Pennsylvania. Data extraction and synthesis was completed in accordance with Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) 2020 guidelines for systematic reviews (30). Manual searches were conducted by reviewing references from relevant articles and previous systematic reviews on similar topics.
Eligibility Criteria
This review sought to include all published, peer-reviewed journal articles, written in English, that contained primary data on BE and/or LOC and weight loss outcomes in patients under 18 years of age with overweight or obesity who received any method of weight loss intervention. Unpublished data, conference reports, review articles, abstracts, protocols, and editorials were excluded.
Search Strategy
The search strategy for this review was created in consultation with an experienced librarian. Three databases were used: Scopus, PsycInfo, and PubMed. In accordance with the PICO strategy of the PRISMA 2020 guidelines, three search strings were utilized for each search, related to population (children/adolescents), intervention (any weight loss intervention) and outcomes (BE/LOC and weight loss). Detailed information on the search strategy is included in Table S1.
Study Selection
This search was completed by one author (AFM) and yielded 923 results from Scopus, 761 from PsycInfo, and 677 from PubMed (Figure 1). An additional 5 records were identified by AFM from reference lists of other articles and reviews that resulted from this search. Once duplicates (n=654) were removed, 1712 articles remained. AFM screened these articles’ titles and abstracts, and 1512 articles were excluded. Reasons for exclusion were: a) no data on weight loss outcomes (n=623); b) no data on patients under 18 years old (n=474); c) no data on BE/LOC (n=406); or d) subjects were not human (n=9). 200 full-text articles remained.
Figure 1.
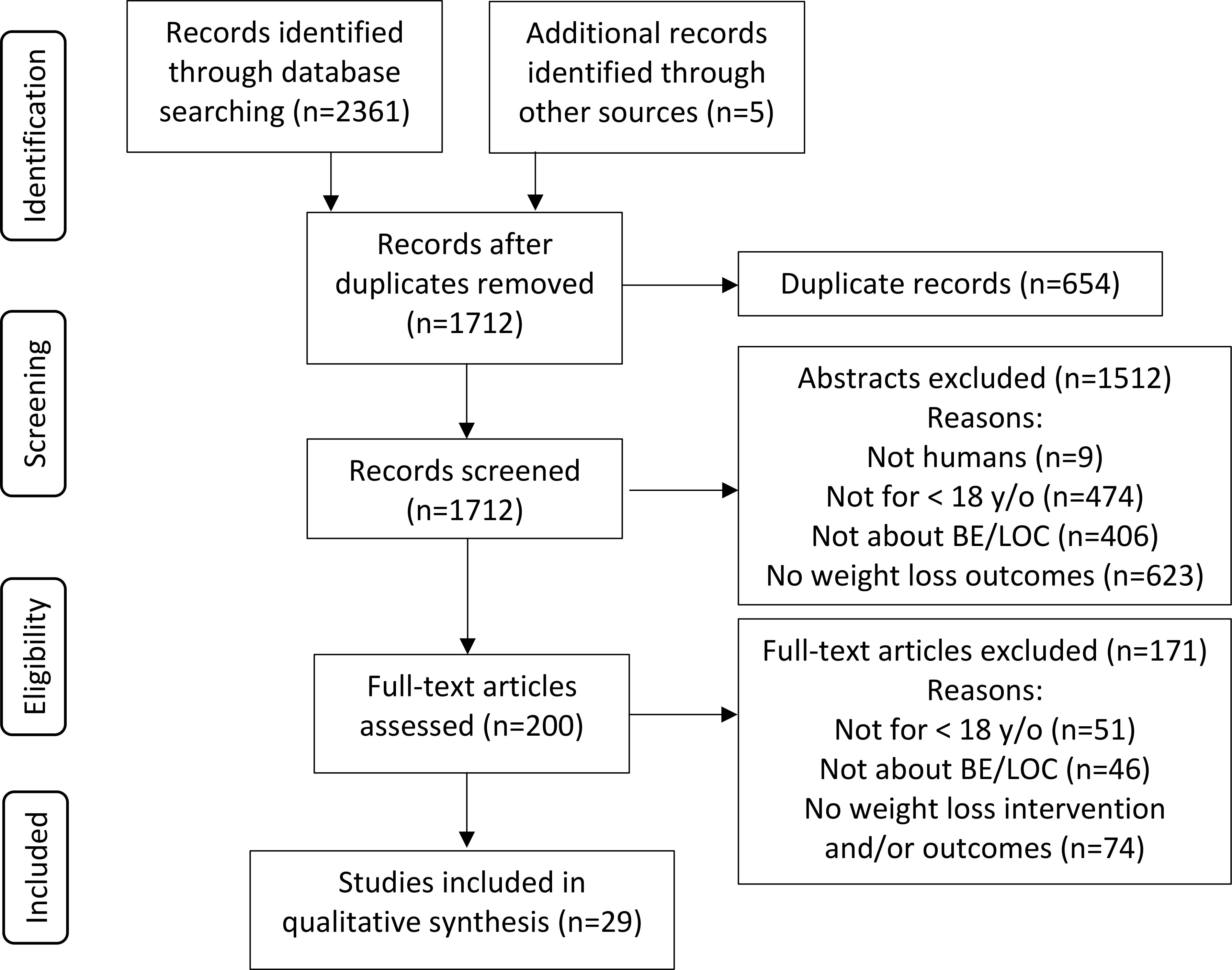
Flow Diagram for Study Selection.
To ensure that no pertinent studies were missed by one reviewer, the 200 studies were assessed individually by AFM and KMQ, resulting in 171 excluded articles due to: a) no weight loss intervention or data on weight loss outcomes (n=74); b) no data on patients under 18 years old (n=51); or c) no data on BE/LOC (n=46). This resulted in the inclusion of 29 articles.
Data Extraction/Synthesis
Data extracted were recorded in a Microsoft Excel file by one author (AFM). These data included study population characteristics, study design, measures, and interventions used, and results (Tables S2.1, S2.2, S2.3). Regarding study population, data were extracted on sample size, sample population and inclusion criteria, age, gender, race/ethnicity, and baseline weight assessment. Data were extracted on country of study, study design, study duration, study aims, intervention, measures used to assess BE/LOC, and weight change. Data extracted regarding study results included baseline weight variables (e.g. BMI, z-BMI, BMI percentile), baseline BE/LOC, follow-up (F/U) BE/LOC (when available), weight change (e.g. BMI change, z-BMI change, weight change), and overall study findings.
Risk of Bias
The risk of bias was assessed for the randomized clinical trials (n=8) using the Cochrane Collaboration’s Tool for Randomized Clinical Trials, the non-randomized studies (n=3) using the Cochrane Tool for Non-Randomized Case-Control Studies, and the cohort studies (n=18) using the RIT Tool for Cohort Studies. Independent reviewers (authors AFM, KMQ) assessed the risk of bias of each report using these tools, with agreement on 91% of individual assessments (Tables S3.1, S3.2, S3.3). Discordance in reviewers’ assessments were resolved through discussion.
Quality Assessment
The quality of the 29 reports included was determined in accordance with the NIH Quality Assessment Tools for Controlled Intervention Studies, for Before-After (Pre-Post) Studies with No Control Group, and for Case-Control Studies. Independent reviewers (authors AFM and KMQ) assessed the quality of each report using these tools, with agreement on 89% of individual assessments (Tables S4.1, S4.2, S4.3). Discordance in reviewers’ assessments were resolved through discussion.
Results
Study Population Characteristics
All studies were published between January 2004 and March 2020. The majority of studies were conducted in the USA (n=16), with the remaining studies conducted in Belgium (n=5), Brazil (n=4), Sweden (n=3), and Germany (n=1) (Table S2.2). There was also a wide range of sample sizes across studies. Studies such as Tanofsky-Kraff et al. 2010 were pilot studies with smaller sample sizes (n=38) (31). Sample size ranged from 22 to 234 (32, 33).
Many studies’ samples had similar demographics. Twenty-four studies (82.8%) had majority female samples, and only five had a majority of males (34–38). Additionally, race/ethnicity information on study samples was available for 18 studies. Of these studies, 83.3% (n=15) had racial/ethnic minorities make up at least 20% of their study sample. Many of the studies that had a smaller percentage of participants from racial/ethnic minority groups or did not report racial/ethnic demographics were conducted outside of the USA in racially homogenous countries. One study specifically intended to recruit solely racial/ethnic minorities into its study sample, resulting in a breakdown of 88% African-American, 6% Hispanic, and 6% other minorities (38).
While all studies focused exclusively on children and adolescents, the studies ranged in the age of participants. A majority of included studies (n=16) exclusively recruited teenagers between the age brackets of 13–17, 13–18, 14–17, 14–18, or 15–19 years, or recruited solely high school students (Table S2.1). Otherwise, studies included youth between the ages of 7–17, 8–15, or 8–17 years (n=7). The remaining studies recruited only younger children, between the ages of 7–11, 8–12, or 8–13 years (n=6). Across all studies, the mean sample age ranged from 9.93 to 17.1 years.
The primary measures used to identify overweight and obesity in the 29 studies were BMI (n=17) and weight percentile (n=12). Some studies reported adjusted BMI (adjusted for age and gender) or z-BMI. Of those that used BMI to measure overweight/obesity, the average baseline BMI ranged from 25.1kg/m2 to 51.6 kg/m2 (31, 39). Participants with BMIs above 30, 35, or 40 kg/m2 were selected for various studies. Of the studies that used weight percentile requirements, participants ranged from above 85th, 90th, 95th, or 97th percentile, or between 75th to 97th percentiles.
Research Methods
Of the 29 studies included, RCTs accounted for 28% (n=8), case-control studies made up 10% (n=3), and longitudinal cohort studies comprised 62% (n=18). While all the studies involved a weight loss intervention, there was considerable variation in the types of interventions. 21% (n=6) reported on specific surgical interventions such as Roux-en-Y gastric bypass, laparoscopic adjustable band, sleeve gastrectomy, or they included all forms of bariatric surgeries. Another 21% (n=6) reported on in-patient weight loss treatments. 21% (n=6) assessed family-based behavioral interventions, 17% (n=5) assessed interdisciplinary therapies, and 14% (n=4) assessed interpersonal therapy alone. One study utilized a virtual CBT program, and another assessed family-based lifestyle modification with pharmaceutical intervention (sibutramine) or placebo, followed by lifestyle modification visits and open label pharmacotherapy. Study intervention period also ranged from 16 weeks to 2 years (not including surgical interventions). Similarly, study follow-up period varied from no follow-up (n=9), to follow-up of up to 6 years.
These studies also varied in the measures used for assessing BE/LOC and weight loss. A majority of the studies utilized a self-report assessment to determine BE/LOC (n=16). These 16 studies used the following interventions: surgical (n=6), inpatient behavioral (n=2), and outpatient behavioral (n=8). Of note, all the studies utilizing behavioral interventions assessed self-reported BE/LOC. Of these studies that used self-report assessments, 43.7% (n=7) measured BE or LOC using the Binge Eating Scale (BES), 12.5% (n=2) with the Children’s Eating Disorder Examination Questionnaire (ChEDE-Q), 12.5% (n=2) with the Questionnaire on Eating and Weight Patterns (QEWP), and the remaining studies (n=5) each utilized one of the following: the Eating Behaviors Inventory (EBI), the Children’s Eating Attitudes Test (ChEAT), the Eating Disorder Diagnostic Scale (EDDS), the Eating Disorder Examination Questionnaire (EDE-Q) and a single-item questionnaire reporting binges per month. These self-report questionnaires varied in the length of assessment, with a range of 1- to 64-items assessing BE and associated behaviors, and nearly all used more than one self-report assessment for confirmatory purposes. The remaining studies assessed BE or LOC clinically through a semi-structured interview (n=13). The interventions of these 13 studies include pharmacotherapy (n=1), CBT (n=1), inpatient behavioral therapy (n=4), and outpatient behavioral interventions (n=7). The majority of these studies (n=12) utilized validated semi-structured interviews: Eating Disorder Examination (EDE) (n=5), Children’s Eating Disorder Examination (ChEDE) (n=6), and Eating Behaviors Inventory (EBI) (n=1). One study utilized a non-validated confirmatory interview following the QEWP to determine presence of BE. The assessed outcomes included binges per month; prevalence of BE, BED, or subthreshold BE; SBEs, OBEs, OOEs; BE episodes in prior 3 months; LOC episodes per month; LOC-OBE, LOC-C (continuous LOC); BE days per month and BE times per week; and BES score. Similarly, weight loss measures varied, either by change in z-BMI, BMI, BMI percentile, weight, percent weight change, excess BMI percent loss, and/or BMI adjusted for age/gender.
All studies included data on BE/LOC and weight loss, but there was additional variation in the analysis of these outcomes. All studies assessed baseline BE/LOC, but only 72% of studies (n=21) assessed BE/LOC following treatment, allowing for assessment of change in BE/LOC. Of these 21 studies that assessed this change, only nine of them directly analyzed the relationship between change in BE/LOC and weight loss outcomes. Meanwhile, 15 studies directly analyzed the relationship between baseline BE/LOC and weight loss (Table 2). The remaining 14 studies, though included in this review, did not directly analyze the relationship between BE/LOC and weight loss, but did include data on both these measures.
Table 2:
Main Findings regarding BE/LOC and Weight Loss Outcomes (WLO) for all 29 studies
| Study | Intervention Type | Duration(s) measured | Change in weight from baseline1 (↑,↓,=) | Relationship between WLO and baseline BE/LOC1 (↑,↓,,=, or N/A)* | Relationship b/w WLO & change in BE/LOC1 (↑,↓,,=, or N/A)** | Change in BE/LOC from baseline1 (↑,↓,=, or N/A) |
|---|---|---|---|---|---|---|
| Bishop-Gilyard et al. 2011 (61) | Pharmacotherapy + behavioral | 6 month F/U | ↓ | = | = | ↓ |
| 12 month F/U | ↓ | = | = | ↓ | ||
| Goldschmidt et al. 2018 (33) | Bariatric Surgery | 6-month F/U | ↓ | = | = | ↓ |
| 1-year F/U | ↓ | = | ↓ | ↓ | ||
| 2-year F/U | ↓ | = | ↓ | ↓ | ||
| 3-year F/U | ↓ | = | ↓ | ↓ | ||
| Jarvholm et al. 2020 (26) | Bariatric Surgery | 5-year F/U | ↓ | = | BE: ↓ LOC: = |
↓ |
| Jarvholm et al. 2018 (53) | Bariatric Surgery | 1-year F/U | ↓ | N/A | N/A | ↓ |
| 2-year F/U | ↓ | = | ↓ | ↓ | ||
| Mackey et al. 2018 (44) | Bariatric Surgery | 12-month F/U | ↓ | ↓ | N/A | N/A |
| Sysko et al. 2014 (49) | Bariatric Surgery | 12-month F/U | ↓ | ↓ | N/A | N/A |
| Hunsaker et al. 2018 (39) | Bariatric Surgery | 24-month F/U | ↓ | N/A | N/A | N/A |
| Jones et al. 2008 (40) | Online CBT for obesity | EOT | ↓ | N/A | = | ↓OBEs; ↓ SBEs; = OOEs |
| 5-month F/U | ↓ | N/A | = | ↓OBEs; ↓ SBEs; = OOEs | ||
| Goossens et al. 2011 (47) | Inpatient behavioral | 6 year F/U | = | N/A | N/A | ↓SBE; = OBE |
| Goossens et al. 2009 (43) | Inpatient behavioral | 1 month of Tx | ↓ | = | N/A | N/A |
| 4 months of Tx | ↓ | = | N/A | N/A | ||
| End of Tx (EOT) | ↓ | =3 | N/A | N/A | ||
| Braet et al. 2004 (46) | Inpatient behavioral | EOT | ↓ | = | N/A | ↓ |
| 14-month F/U | ↓ | = | N/A | ↓ | ||
| Braet et al. 2006 (75) | Inpatient behavioral | EOT | ↓ | N/A | N/A | ↓ |
| 2-year F/U | ↓ | N/A | N/A | ↓ | ||
| Braet et al. 2009 (22) | Inpatient behavioral | EOT | ↓ | N/A | N/A | N/A |
| 1-year F/U | ↓ | N/A | N/A | N/A | ||
| 2-year F/U | ↓ | N/A | N/A | N/A | ||
| Van Vlierberghe et al. 2009 (48) | Inpatient behavioral | EOT | ↓ | N/A | N/A | ↓ |
| Balantekin et al. 2017 (76) | Behavioral therapy | EOT | ↓ | N/A | N/A | = |
| Teder et al. 2013 (34) | Behavioral therapy | EOT | N/A2 | N/A | = | ↓ |
| 1-year F/U | N/A2 | N/A | N/A | N/A | ||
| Wildes et al. 2010 (51) | Behavioral therapy | EOT | ↓ | ↓ | N/A | N/A |
| 6-month F/U | ↓ | (not sig) | N/A | N/A | ||
| 12-month F/U | ↓ | (not sig) | N/A | N/A | ||
| Levine et al. 2006 (35) | Behavioral therapy | 8-month F/U | N/A2 | =3 | N/A | N/A |
| Albayrak et al. 2019 (36) | Behavioral therapy | EOT | ↓ | (not sig) | N/A | N/A |
| Tanofsky-Kraff et al. 2014 (45) | Behavioral therapy | EOT | z-BMI: ↓ Adj-BMI: ↓ BMI: = |
N/A | = | LOC: ↓ OBE: ↓ |
| 6-month F/U | z-BMI: ↓ Adj-BMI: ↓ BMI: = |
N/A | = | LOC: ↓ OBE: ↓ (IPT); ↓ (control) |
||
| 12-month F/U | z-BMI: ↓ Adj-BMI: ↓ BMI: = |
N/A | = | LOC: ↓ OBE: ↓ |
||
| Tanofsky-Kraff et al. 2017 (41) | Behavioral therapy | 3-year F/U | ↓ | = | = | ↓ |
| Antunes et al. 2009 (50) | Behavioral therapy | EOT | ↓ | ↓ | ↓ | ↓ |
| Carnier et al. 2010 (77) | Behavioral therapy | EOT | ↓ | N/A | N/A | ↓ |
| Carnier et al. 2008 (32) | Behavioral therapy | EOT | ↓ | N/A | N/A | ↓ |
| Damaso et al. 2013 (42) | Behavioral therapy | EOT | ↓ | N/A | N/A | ↓ |
| Eichen et al. 2019 (78) | Behavioral therapy | EOT | ↓ | N/A | N/A | ↓ |
| 6-month F/U | ↓ | N/A | N/A | N/A | ||
| 18-month F/U | ↓ | N/A | N/A | ↓ | ||
| Shomaker et al. 2017 (37) | Behavioral therapy | EOT | = | N/A | N/A | ↓ |
| 6-month F/U | = | N/A | N/A | ↓ | ||
| 1-year F/U | = | N/A | N/A | ↓ | ||
| Tanofsky-Kraff et al. 2010 (31) | Behavioral therapy | 1-year F/U | ↓ | N/A | N/A | ↓ |
| Germann et al. 2006 (38) | Behavioral therapy | 11-month F/U | = | = | N/A | N/A |
↑ = higher baseline BE associated with more weight loss and ↓ = higher baseline BE associated with less weight loss
where ↑ = greater increase in BE associated with more weight loss and ↓ = greater decrease in BE associated with more weight loss
: ↑ and ↓ each denote statistical significance in this association
: Although authors reported successful weight loss outcomes at these time points, they did not directly provide this data or state if it was statistically significant
: No significant association was found, but there notably was a significant negative correlation between baseline SBE and program completion
Study Results
Baseline BE/LOC.
While all studies assessed baseline BE/LOC, the level of BE/LOC across study samples varied significantly (Table S2.3), as these studies varied in their inclusion criteria and participant demographics. Per the inclusion criteria, all participants in Jones et al. 2008, which utilized an online CBT intervention, reported some level of BE or overeating behaviors (40). Similarly, two studies only included participants that reported at least 1 LOC episode in the previous month at baseline (27, 41). The remaining 26 studies had no inclusion criteria regarding BE/LOC behaviors. Of these studies, BE/LOC prevalence ranged greatly. Damaso et al. exhibited the lowest BE prevalence at 6%, while Goossens et al. 2009 reported the highest rate of baseline LOC at 71.4% (42, 43). Studies on surgical interventions had a relatively small range of baseline BE and LOC symptoms. BE symptoms were present in 15.0% to 15.4% of participants, and LOC symptoms were present in 23.5% to 27.8% of participants across all studies utilizing surgical interventions that assessed each BE and LOC baseline symptoms (26, 33, 44). In the studies that utilized behavioral therapy, BE and LOC symptoms at baseline were present in a broader range across studies: BE prevalence ranged from 6% to 56%, and LOC prevalence ranged from 6.2% to 71.4% of patients (42, 43, 45, 46).
Mean weight loss.
Nearly all studies displayed statistically significant weight loss outcomes following weight loss interventions, with the exception of five studies (Table 2). Three studies found that their mean BMI, adjusted BMI, and z-BMI changes were not statistically significant from baseline or from the control groups (37, 38, 47). Two studies reportedly resulted in successful weight loss but did not directly display these data or state if they were statistically significant, likely due to their small sample sizes (34, 35). Additionally, Tanofksy-Kraff et al. 2014 showed no significant change in BMI at End-of-Treatment (EOT) and each follow-up, although decreases in z-BMI and adjusted BMI were found to be significant. Though these studies’ interventions resulted in weight losses that were not statistically significant, multiple studies indicated large weight losses (45). These significant weight loss outcomes were assessed at a range of time frames, from as short as immediately following the first month of treatment, to follow-ups 5-years after EOT. Studies of surgical therapies ranged in weight loss outcomes. The study with the lowest BMI reduction, Sysko et al. 2013, resulted in a mean 4.66 BMI decrease from baseline. Contrastingly, Hunsacker et al. 2018 had the greatest mean BMI decrease of 15.6 units (36, 45). In comparison, of the studies on behavioral therapy (n=15), the largest mean decrease in BMI was 4.1 units at the end of 1 year of treatment, while two of these studies found no significant decrease (42). Inpatient treatment had significant variation in weight loss. Goossens et al. 2011 showed no significant difference in weight loss, with a decrease of mean BMI of only 0.31. The most successful inpatient behavioral intervention resulted in mean percent weight losses of 13.4%, 36.9%, and 52.5% of adjusted BMI after 1 month, 4 months, and 10 months of treatment, respectively (48). On average, bariatric surgery interventions had larger weight reductions when compared to inpatient and behavioral interventions.
Baseline BE/LOC and weight loss.
Of the 14 studies that assessed the relationship between weight loss and BE/LOC at baseline, ten found no significant association between baseline BE/LOC and WL (Table 2). All four that found a significant association exhibited that higher baseline BE/LOC was associated with less weight loss (44, 49–51). These studies varied in most respects, including the intervention used (two utilized bariatric surgery and two utilized behavioral therapy), the BE/LOC measures used (ChEAT, EDDS, EDE-Q, and BES respectively), the outcomes asessed (days of BE per month, frequency of SBEs, score on BES above or below 18, EDE-Q assessment of OBEs and SBEs), and the follow-up period, ranging from no follow-up to a 15-month follow-up (50, 52). Furthermore, the samples of these four studies varied in their mean ages (10.2 to 16.6 years old), sample sizes (66 to 192 participants), and nationalities (three were conducted in the USA, one in Brazil). These four studies also include one study which did find a significant association with weight loss at EOT, but the correlation was no longer significant at either follow-up assessment (51). Additionally, the ten studies with no significant associations includes a study by Albayrak et al. which found that higher baseline BE/LOC was associated with less weight loss, but results were not strong enough to be significant.
Two of the studies that found no significant association between baseline BE/LOC and weight loss outcomes (WLO) did assess the relationship between reported SBEs and program completion (35, 43). These two studies each came to opposite conclusions: while Goossens et al. found that baseline SBE had a positive correlation with program completion, Levine et al. found that baseline SBE had a negative correlation with program completion.
Change in BE/LOC and Weight Loss.
Several studies assessed change in BE/LOC, yet only a few (n=9) directly analyzed the relationship between weight loss success and change in BE/LOC (Table 2). Of these, a majority of the studies found no significant association (n=5). The other studies (n=4) all illustrated that a greater decrease in BE/LOC was significantly associated with more weight loss (26, 33, 50, 53).
These nine studies included one pharmacologic intervention; multidisciplinary, family-based, or interpersonal therapy intervention; and three bariatric surgical interventions. All three studies assessing surgical outcomes found a positive association between decreased BE/LOC and improved weight loss, while only one of the four studies of multidisciplinary therapy displayed this association. There was also an association between the BE/LOC measure used and significant results. All four studies that found a significant association between change in BE/LOC and weight loss utilized a self-report questionnaire to assess BE/LOC, either BES to assess BE (n=3) or QEWP-R to assess LOC and OBEs (n=1). Contrastingly, four of the five studies that found no significant association used validated interviews assess BE/LOC.
The association between change in BE/LOC and weight did not appear to be moderated by racial, ethnic, gender, or nationality demographics of the samples. Instead, there is some suggestion that the relationship between BE/LOC and impaired weight loss may be strongest among adolescents. The four studies that showed a significant association between a greater decrease in BE/LOC and more weight loss had inclusion criteria of either 13–19 or 13–18 years old, with a mean age between 16.5 and 17.1. Whereas the other five studies had a mean age range of 10.9 to 15.1, with a total age range of 8–17 years old. Further studies are necessary to investigate the potential influence of age on these relationships. Another distinction between these groups of studies is that nearly all the studies that found no significant association had relatively short follow-up times, either 5 months, 9 months, 1 year, 2 years, or no follow-up. The four studies that found a significant association had, on average, longer follow-ups of 2 years, 4 years, 5 years, with only one study including no follow-up.
Change in BE/LOC across study.
Twenty-one studies assessed change in BE/LOC (Table 2). All except one indicated a significant decrease in at least one measure of BE/LOC behavior (54). While the results of Balantekin et al. 2017 were not statistically significant, a decrease in number of LOC episodes per month was present, from 1.28 at baseline to 0.57 at EOT.
A decrease in BE/LOC was found across studies that used a variety of measures. Damaso et al. assessed prevalence of BE and found a decline from 6% to 2% (42). Carnier et al. 2008 assessed BES score, BE prevalence, severe BED and moderate BE, and found significant decreases in all these measure – mean BES score deceased from 18 to 11.61, BE prevalence decreased from 40% to 17%, severe BED prevalence declined from 10.12% to 5.88%, and moderate BE decreased from 30.33% to 11.49% (32). Mean LOC episodes per month deceased in the intervention group of Tanokfsy-Kraff et al. 2010 from 3.5 to 0.53 (31). Similarly, participants reporting LOC-OBE in Goldschmidt et al. deceased from 15.4% to 3.8% (33). Some studies that assessed BE/LOC using multiple measures found that not all measures resulted in significant change. For example, Goossens et al. 2011 found a significant decrease in SBEs but no significant change in OBEs (43). Similarly, Jones et al. found a significant decrease in SBEs and OBEs but not OOEs at both EOT and at follow-up (40).
Risk of Bias
Overall, the risk of bias was low across the studies as assessed by the Cochrane and RIT Tools for Risk of Bias Assessments (Tables S3.1, S3.2, S3.3). Specifically, each study had consistent inclusion criteria, exclusion criteria, and methods of recruiting participants. Additionally, the majority of studies (n=23, 79%) reported either low loss to follow-up (below 20%) or assessed the impact of loss to follow-up through some statistical adjustment method. Despite these strengths, one of the most consistent sources of potential bias was that the majority of studies (n=24, 83%) did not report any blinding of study staff delivering interventions and assessing outcomes. The main outcomes that were analyzed in this review, in particular weight, and self-reported BE/LOC measures, were likely minimally impacted by a lack of blinding, but it is possible that the lack of blinding may have biased study findings. Future studies should include masked outcome assessors when possible, to help reduce the potential for bias.
Quality Assessment
Overall study quality was moderate, as assessed by the NIH Quality Assessment Tools (Tables S4.1, S4.2, S4.3). The included randomized clinical trials (n=8) and case-control studies (n=3) had higher study quality than the Before-After/Pre-Post studies (n=18). This is largely due to the fact that 82% (n=9) of included RCTs and case-control studies (n=11) reported no statistically significant differences between groups at baseline. Additionally, all 29 studies utilized clearly defined, valid, and reliable measures to assess outcomes, and had clearly described and consistently delivered interventions across the study population. While this displayed strong quality overall, the quality of many studies suffered due to their sample sizes. Only 45% of studies (n=13) either reported sample size justifications, reported sample sizes large enough to be confident in their findings, or reported sample sizes large enough to detect a difference in outcomes with at least 80% power. This is a commonly occurring limitation in long-term clinical studies such as those included in this review. This still detracted from overall study quality, and thus we consider these studies to be of moderate, but not high, quality.
Discussion
These 29 articles show the effects of pharmacotherapy, surgical interventions, or behavioral therapy on weight outcomes and BE/LOC. Nearly all included studies presented statistically significant weight loss outcomes, and high baseline BE/LOC rates. Among children and adolescents, weight loss interventions resulted in significant declines in BE/LOC. Evidence was found that higher baseline BE and LOC measures could hinder weight loss success in children and adolescents, and that greater decreases in BE and LOC are association with improved weight losses.
While BE/LOC prevalence in adolescents is thought to be relatively low, between 0.3% and 3.1% (55), previous research has estimated BE/LOC prevalence among adolescents seeking weight loss interventions to be upwards of 30%, and was even as high as 70% in some included studies (7, 56, 57). Baseline BE/LOC in included studies ranged from 6% to 71%, not including studies which listed BE/LOC in their inclusion criteria. There are a multitude of explanations for this large range. Firstly, several different BE and LOC assessments were utilized, from a single-item questionnaire to structured clinical interviews, which may result in inconsistencies in prevalence across studies. Additionally, the age range of participants varied across studies, and research has indicated that the prevalence of BE/LOC increases in late adolescence (58). Furthermore, the prevalence of BE/LOC differed across demographic groups and across countries, and thus the differences in gender, racial, and ethnic breakdown could account for this range (59).
We found declines in BE/LOC that are consistent with weight loss research on adults; previous studies have found pharmacological, surgical, CBT, and interpersonal therapy interventions to be successful in decreasing BE/LOC in adults seeking weight management (18, 60). Of the studies that assessed change in BE/LOC, 95% (n=21) demonstrated a decrease. While this was the general trend, these studies were limited in their lack of reporting of changes in BE/LOC on an individual level. The one study that assessed this found that BE prevalence decreased from 24% to 3%, but four patients developed new BE behaviors during the study (61). Thus, continued monitoring for BE/LOC is important for youth in weight loss programs. Additionally, continued research could allow for further assessment of development of disordered eating during weight loss interventions in children and adolescents.
In addition to an overall decrease in BE/LOC, the studies analyzed assessed that a greater decrease in BE/LOC may be associated with larger weight loss. Nine studies assessed this change, four of these studies denoted a statistically significant relationship. Three of the four studies utilized bariatric surgery interventions. These results are similarly consistent with research on adult populations, which found that the persistence of post-bariatric BE/LOC is associated with less weight loss (60, 62, 63). Though previous research has confirmed the importance of screening for these behaviors in adults following bariatric surgery, this review establishes that similar screening is necessary in youth following bariatric surgery (62). Further research addressing the association between the change in BE/LOC and weight loss outcomes with non-surgical interventions is needed. Most non-surgical interventions did not assess this relationship, but research with adults illustrate that this association may be significant. Multiple studies have found that adults with overweight who reported greater decrease in BE following non-surgical weight loss interventions were more successful than adults who had less significant change in BE (56, 64).
Though the included studies involved numerous types of interventions, only one included study utilized pharmacotherapy (61). It is important to note the limited literature on medications for BE/LOC and weight loss in adults and adolescents alike, as most studies of weight loss medications do not include data on BE/LOC (65).
There was significant variability in the measures used to assess BE/LOC. Most studies utilized self-report assessments. Adolescents are more likely to report elevated BE on a questionnaire when compared to an interview (66). Adolescents may find it difficult to assess if their food intake is objectively large, possibly leading to overreporting of OBE (67). Delineating SBEs/OBEs on self-report measures may be confounded across time as participants learn what is considered an appropriate amount of food as they undergo treatment (35, 47). However, adolescents may experience shame or guilt related to BE and may be more likely to disclose this behavior on a questionnaire compared to an interview. Nonetheless, most studies utilized validated self-report or interview measures such as EDE-Q or EDE, ChEDE-Q or ChEDE, and EDI. Not all studies used validated measures. For example, Teder et al. 2013 assessed BE/LOC via a single-item. Furthermore, there is concern regarding the validity of LOC, compared to BE, in adolescents. Specifically, multiple studies noted that measuring SBE is likely less predictive than OBE, possibly due to the difficulty adolescents have in defining experiences of LOC. The ChEDE was the most frequently used interview measure in the included studies. It is also validated for use in children and adolescents and is able to assess multiple facets of LOC, including OBEs, SBEs, and OOEs. While time and labor intensive, we suggest the use of the ChEDE to assess BE and LOC in future studies, when feasible.
Concerns have been expressed about using weight loss interventions in youth, particularly that they may promote disordered eating and restrictive diets (4). It is important to emphasize the distinction between restrictive, disordered dieting and the weight loss interventions used in the included studies. The studies included followed specific medical interventions, such as bariatric surgeries or FDA-approved medications, as well as interdisciplinary behavioral therapies. These behavioral therapies focus on psycho-education, building communication skills, resolving family conflict, identifying triggers, interpersonal therapy, and cognitive-behavioral therapy techniques. Though the primary intention of these therapies in these studies was weight loss, they have been found to decrease, not increase, disordered eating behaviors, including but not limited to BE/LOC, while also improving general psychopathology. For example, Braet et al. 2006, which utilized an inpatient weight loss intervention, found that following treatment, patients showed significant improvement in global self-worth, and decreased symptoms of eating disorders. Shomaker et al 2017 assessed the impact of family-based interpersonal therapy, an intervention used by six of the included studies. Their results found that this intervention, in adolescents, may be effective for weight loss, and also for depression, mood symptoms, and disordered eating. Though concerns regarding promotion of diet culture in weight loss interventions have an important role in the discussion on weight management, these interventions which have been shown to improve general mood and decrease psychopathology are distinct from restrictive, disordered dieting that may be inaccurately associated with such therapies (68). This aligns with research in adults as well (69), as a recent meta-analysis found an overall improvement in mental health outcomes following weight management interventions (70). However, the longest study to assess this issue was two years following treatment. Further longer term, well-powered studies are necessary to examine disordered eating as well as increases in weight, shape, and eating concerns that may occur during weight loss treatments. There is a particular need for studies that include a non-intervention follow-up to look at changes in BE/LOC after treatment and support have ceased, when individuals may be vulnerable to weight regain. Additionally, studies are necessary to identify subtypes or phenotypes of individuals who may particularly at risk for developing BE/LOC as a result of weight loss interventions.
Depression and other co-morbid mental illnesses are common in youth with BE/LOC and further research is needed to examine how this influences BE/LOC and weight loss (71, 72). One included study found that the presence of at least one psychological disorder showed negative predictive value for weight loss four months after the start of the program (48). Additionally, some studies hypothesized that disordered psychological symptoms may predict risk for BE/LOC behavior, although others postulate that BE/LOC behaviors may predict these symptoms. One study found that overweight adolescents who report more symptoms of depression are more at risk of developing LOC in the future (47). Similarly, pediatric LOC often predicts both worsening disordered eating and disordered mood symptoms (45). Additionally, the results of one included study found that the decrease noted in BE can be partly attributed to a decrease in anxiety scores, “since an anxious individual is more likely to develop binge eating disorders” (50).
This review’s strengths include its systematic research strategy, use of multiple databases, multiple reviewers assessing study eligibility, and a review structure following PRISMA guidelines (30). Additionally, two reviewers separately determined that risk of bias in each study was low on average, and study quality was high. Nevertheless, there are still some limitations in the applications of the study results. One significant limitation is a result of the range of BE/LOC measures used across studies. While this allowed for analysis of both BE and LOC behaviors, it is important to recognize that these are different concepts, and thus could benefit from separate analysis. This review, and most of the included studies, did not assess related eating disorder pathology, such as weight or shape concerns, bulimia nervosa or other purging behaviors. However, some studies did report this, including Braet et al. 2004 which found that those who reported BE at baseline, when compared to those who did not, had greater improvement in bulimia, body dissatisfaction, and shape concerns following treatment. Similarly, Carnier et al. 2008 found that short-term therapy was effective in significantly reducing the prevalence of both BE and bulimia nervosa symptoms in their sample, attributing this to the encouragement of normal eating behaviors.
BE/LOC prevalence is high in children and adolescents seeking weight loss interventions. Weight loss interventions proved successful in achieving weight loss outcomes in these studies and also resulted in significant declines in BE/LOC. This held true for nearly all studies regardless of weight loss intervention. While some studies also demonstrated a significant correlation between decreases in BE/LOC over time and greater weight loss, further research is warranted. These findings can hopefully guide future treatment for adolescents seeking weight loss, particularly those struggling with BE/LOC behaviors.
Supplementary Material
Study Importance Questions.
- What is already known about this subject? What major reviews have already been published on this subject?
- Binge eating (BE) is reported by 20–35% of youth seeking weight loss, and loss of control eating (LOC) is estimated to be prevalent in 31% in youth with overweight.
- BE and LOC typically decrease with weight loss interventions in adults, but the relationship with BE and LOC in children and adolescents is unclear.
- A previous systematic review examined eating disorder risk in children and adolescents who received weight loss treatments of in-person health education or a diet regimen, but included limited data on BE/LOC.
- What are the new findings in your manuscript?
- We identified 29 articles on behavioral therapy, bariatric surgery, and pharmacotherapy.
- Among children and adolescents, weight loss interventions resulted in significant declines in BE/LOC.
- There was some evidence that higher baseline BE/LOC may be associated with less weight loss, and that greater declines in BE/LOC over time may be associated with improved weight loss.
- How might your results change the direction of research or the focus of clinical practice?
- This review demonstrates that weight loss interventions can be useful both in increasing weight loss and improving BE/LOC in children and adolescents.
- Further research is needed regarding the associations between both baseline BE/LOC and change in BE/LOC and weight loss in youth.
Acknowledgments
Funding: AMC was supported, in part, by the National Institute of Nursing Research of the National Institutes of Health under Award Number K23NR017209.
Disclosure: AMC reports grants and consulting fees from WW International, Inc., outside the submitted work. TAW discloses serving on advisory boards for Novo Nordisk and WW International, Inc. The other authors declare no conflicts of interest.
References
- 1.Rao DP, Kropac E, Do MT, Roberts KC, Jayaraman GC. Childhood overweight and obesity trends in Canada. Heal Promot chronic Dis Prev Canada Res policy Pract 2016;36:194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Radman M, McGuire J, Zimmerman J. Childhood Obesity, Endothelial Cell Activation, and Critical Illness. Front Pediatr 2020;8:441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fidelix YL, Farias Júnior JC, Lofrano-Prado MC, Guerra RL, Cardel M, Prado WL. Multidisciplinary intervention in obese adolescents: predictors of dropout. Einstein (Sao Paulo) 2015;13:388–394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sole-Smith V A Weight Watchers App for Kids Raises Concerns. New York Times 2020. [Google Scholar]
- 5.Fairburn CG. Cognitive behavior therapy and eating disorders. Guilford Press; 2008. [Google Scholar]
- 6.Eddy KT, Tanofsky-Kraff M, Thompson-Brenner H, Herzog DB, Brown TA, Ludwig DS. Eating disorder pathology among overweight treatment-seeking youth: Clinical correlates and cross-sectional risk modeling. Behav Res Ther 2007;45:2360–2371. [DOI] [PubMed] [Google Scholar]
- 7.Tanofsky-Kraff M, Schvey NA, Grilo CM. A developmental framework of binge-eating disorder based on pediatric loss of control eating. Am Psychol 2020;75:189–203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Marzilli E, Cerniglia L, Cimino S. A narrative review of binge eating disorder in adolescence: prevalence, impact, and psychological treatment strategies. Adolesc Health Med Ther 2018;9:17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Saunders JF, Frazier LD, Nichols-Lopez KA. Self-esteem, diet self-efficacy, body mass index, and eating disorders: modeling effects in an ethnically diverse sample. Eat Weight Disord 2016;21:459–468. [DOI] [PubMed] [Google Scholar]
- 10.Stunkard AJ. Eating patterns and obesity. Psychiatr Q 1959;33:284–295. [DOI] [PubMed] [Google Scholar]
- 11.Berkowitz R, Stunkard AJ, Stallings VA. Binge-eating disorder in obese adolescent girls. Ann N Y Acad Sci 1993;699:200. [DOI] [PubMed] [Google Scholar]
- 12.Goldschmidt AB, Aspen VP, Sinton MM, Tanofsky-Kraff M, Wilfley DE. Disordered eating attitudes and behaviors in overweight youth. Obesity 2008;16:257–264. [DOI] [PubMed] [Google Scholar]
- 13.Goossens L, Braet C, Decaluwé V. Loss of control over eating in obese youngsters. Behav Res Ther 2007;45:1–9. [DOI] [PubMed] [Google Scholar]
- 14.Tanofsky-Kraff M, Goossens L, Eddy KT, et al. A multisite investigation of binge eating behaviors in children and adolescents. J Consult Clin Psychol 2007;75:901–913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Theim KR, Tanofsky-Kraff M, Salaita CG, et al. Children’s descriptions of the foods consumed during loss of control eating episodes. Eat Behav 2007;8:258–265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fitzsimmons-Craft EE, Ciao AC, Accurso EC, et al. Subjective and objective binge eating in relation to eating disorder symptomatology, depressive symptoms, and self-esteem among treatment-seeking adolescents with bulimia nervosa. Eur Eat Disord Rev 2014;22:230–236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.He J, Huang F, Yan J, Wu W, Cai Z, Fan X. Prevalence, demographic correlates, and association with psychological distress of night eating syndrome among chinese college students. Psychol Heal Med 2018;23:578–584. [DOI] [PubMed] [Google Scholar]
- 18.Peckmezian T, Hay P. A systematic review and narrative synthesis of interventions for uncomplicated obesity: Weight loss, well-being and impact on eating disorders. J Eat Disord 2017;5:15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Devlin MJ, Goldfein JA, Petkova E, Liu L, Walsh BT. Cognitive behavioral therapy and fluoxetine for binge eating disorder: two-year follow-up. Obesity (Silver Spring) 2007;15:1702–1709. [DOI] [PubMed] [Google Scholar]
- 20.Murphy R, Straebler S, Cooper Z, Fairburn CG. Cognitive behavioral therapy for eating disorders. Psychiatr Clin 2010;33:611–627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Castelnuovo G, Pietrabissa G, Manzoni GM, et al. Cognitive behavioral therapy to aid weight loss in obese patients: current perspectives. Psychol Res Behav Manag 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Braet C, Beyers W. Subtyping Children and Adolescents Who Are Overweight: Different Symptomatology and Treatment Outcomes. J Consult Clin Psychol 2009;77:814–824. [DOI] [PubMed] [Google Scholar]
- 23.Burton AL, Abbott MJ. Conceptualising binge eating: a review of the theoretical and empirical literature. Behav Chang 2017;34:168–198. [Google Scholar]
- 24.Schulte SJ. Predictors of binge eating in male and female youths in the United Arab Emirates. Appetite 2016;105:312–319. [DOI] [PubMed] [Google Scholar]
- 25.Schulte EM, Grilo CM, Gearhardt AN. Shared and unique mechanisms underlying binge eating disorder and addictive disorders. Clin Psychol Rev 2016;44:125–139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Järvholm K, Bruze G, Peltonen M, et al. 5-year mental health and eating pattern outcomes following bariatric surgery in adolescents: a prospective cohort study. Lancet Child Adolesc Heal 2020;4:210–219. [DOI] [PubMed] [Google Scholar]
- 27.Vannucci A, Shomaker LB, Field SE, et al. History of weight control attempts among adolescent girls with loss of control eating. Heal Psychol Off J Div Heal Psychol Am Psychol Assoc 2014;33:419–423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jebeile H, Gow ML, Baur LA, Garnett SP, Paxton SJ, Lister NB. Treatment of obesity, with a dietary component, and eating disorder risk in children and adolescents: A systematic review with meta-analysis. Obes Rev an Off J Int Assoc Study Obes 2019;20:1287–1298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nicholls D, Barrett E. Eating disorders in children and adolescents. BJPsych Adv 2015;21:206–216. [Google Scholar]
- 30.Moher D, Liberati A, Tetzlaff J, Altman DG, Group TP. Preferred Reporting Items for Systematic Reviews and Meta-Analyses: The PRISMA Statement. PLOS Med 2009;6:e1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tanofsky-Kraff M, Wilfley DE, Young JF, et al. A pilot study of interpersonal psychotherapy for preventing excess weight gain in adolescent girls at-risk for obesity. Int J Eat Disord 2010;43:701–706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Carnier J, Lofrano MC, Prado WL, et al. Hormonal Alteration in Obese Adolescents with Eating Disorder: Effects of Multidisciplinary Therapy. Horm Res Paediatr 2008;70:79–84. [DOI] [PubMed] [Google Scholar]
- 33.Goldschmidt AB, Khoury J, Jenkins TM, et al. Adolescent Loss-of-Control Eating and Weight Loss Maintenance After Bariatric Surgery. Pediatrics 2018;141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Teder M, Mörelius E, Nordwall M, et al. Family-based behavioural intervention program for obese children: an observational study of child and parent lifestyle interpretations. PLoS One 2013;8:e71482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Levine MD, Ringham RM, Kalarchian MA, Wisniewski L, Marcus MD. Overeating among seriously overweight children seeking treatment: Results of the children’s eating disorder examination. Int J Eat Disord 2006;39:135–140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Albayrak Ö, Pott W, Hebebrand J, De Zwaan M, Pauli-Pott U. Baseline dietary restraint predicts negative treatment outcomes after 12 months in children and adolescents with obesity participating in a lifestyle intervention. Obes Facts 2019;12:179–189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Shomaker LB, Tanofsky-Kraff M, Matherne CE, et al. A randomized, comparative pilot trial of family-based interpersonal psychotherapy for reducing psychosocial symptoms, disordered-eating, and excess weight gain in at-risk preadolescents with loss-of-control-eating. Int J Eat Disord 2017;50:1084–1094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Germann JN, Kirschenbaum DS, Rich BH, O’Koon JC. Long-term Evaluation of Multi-disciplinary Treatment of Morbid Obesity in Low-income Minority Adolescents: La Rabida Children’s Hospital’s FitMatters Program. J Adolesc Heal 2006;39:553–561. [DOI] [PubMed] [Google Scholar]
- 39.Hunsaker SL, Garland BH, Rofey D, et al. A Multisite 2-Year Follow Up of Psychopathology Prevalence, Predictors, and Correlates Among Adolescents Who Did or Did Not Undergo Weight Loss Surgery. J Adolesc Heal Off Publ Soc Adolesc Med 2018;63:142–150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Jones M, Luce KH, Osborne MI, et al. Randomized, controlled trial of an internet-facilitated intervention for reducing binge eating and overweight in adolescents. Pediatrics 2008;121:453–462. [DOI] [PubMed] [Google Scholar]
- 41.Tanofsky-Kraff M, Shomaker LB, Wilfley DE, et al. Excess weight gain prevention in adolescents: Three-year outcome following a randomized controlled trial. J Consult Clin Psychol 2017;85:218–227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Dâmaso AR, de Piano A, Campos RM da S, et al. Multidisciplinary approach to the treatment of obese adolescents: effects on cardiovascular risk factors, inflammatory profile, and neuroendocrine regulation of energy balance. Int J Endocrinol 2013;2013:541032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Goossens L, Braet C, Van Vlierberghe L, Mels S. Weight parameters and pathological eating as predictors of obesity treatment outcome in children and adolescents. Eat Behav 2009;10:71–73. [DOI] [PubMed] [Google Scholar]
- 44.Mackey ER, Olson A, Merwin S, Wang J, Nadler EP. Perceived Social Support for Exercise and Weight Loss in Adolescents Undergoing Sleeve Gastrectomy. Obes Surg 2018;28:421–426. [DOI] [PubMed] [Google Scholar]
- 45.Tanofsky-Kraff M, Shomaker LB, Wilfley DE, et al. Targeted prevention of excess weight gain and eating disorders in high-risk adolescent girls: a randomized controlled trial. Am J Clin Nutr 2014;100:1010–1018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Braet C, Tanghe A, Decaluwé V, Moens E, Rosseel Y. Inpatient treatment for children with obesity: Weight loss, psychological well-being, and eating behavior. J Pediatr Psychol 2004;29:519–529. [DOI] [PubMed] [Google Scholar]
- 47.Goossens L, Braet C, Verbeken S, Decaluwé V, Bosmans G. Long-term outcome of pediatric eating pathology and predictors for the onset of loss of control over eating following weight-loss treatment. Int J Eat Disord 2011;44:397–405. [DOI] [PubMed] [Google Scholar]
- 48.Van Vlierberghe L, Braet C, Goossens L, Rosseel Y, Mels S. Psychological disorder, symptom severity and weight loss in inpatient adolescent obesity treatment. Int J Pediatr Obes 2009;4:36–44. [DOI] [PubMed] [Google Scholar]
- 49.Sysko R, Hildebrandt TB, Kaplan S, Brewer SK, Zitsman JL, Devlin MJ. Predictors and correlates of follow-up visit adherence among adolescents receiving laparoscopic adjustable gastric banding. Surg Obes Relat Dis 2014;10:914–920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Antunes HKM, do Prado WL, de Piano A, et al. Quality of life in Brazilian obese adolescents: Effects of a long-term multidisciplinary lifestyle therapy. Health Qual Life Outcomes 2009;7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wildes JE, Marcus MD, Kalarchian MA, Levine MD, Houck PR, Cheng Y. Self-reported binge eating in severe pediatric obesity: impact on weight change in a randomized controlled trial of family-based treatment. Int J Obes (Lond) 2010;34:1143–1148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Sysko R, Devlin MJ, Hildebrandt TB, Brewer SK, Zitsman JL, Walsh BT. Psychological outcomes and predictors of initial weight loss outcomes among severely obese adolescents receiving laparoscopic adjustable gastric banding. J Clin Psychiatry 2012;73:1351–1357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Järvholm K, Olbers T, Peltonen M, et al. Binge eating and other eating-related problems in adolescents undergoing gastric bypass: results from a Swedish nationwide study (AMOS). Appetite 2018;127:349–355. [DOI] [PubMed] [Google Scholar]
- 54.Balantekin KN, Birch LL, Savage JS. Eating in the absence of hunger during childhood predicts self-reported binge eating in adolescence. Eat Behav 2017;24:7–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Sonneville KR, Horton NJ, Micali N, et al. Longitudinal Associations Between Binge Eating and Overeating and Adverse Outcomes Among Adolescents and Young Adults: Does Loss of Control Matter? JAMA Pediatr 2013;167:149–155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Gorin AA, Niemeier HM, Hogan P, et al. Binge Eating and Weight Loss Outcomes in Overweight and Obese Individuals With Type 2 Diabetes: Results From the Look AHEAD Trial. Arch Gen Psychiatry 2008;65:1447–1455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Decaluwé V, Braet C. Prevalence of binge-eating disorder in obese children and adolescents seeking weight-loss treatment. Int J Obes 2003;27:404–409. [DOI] [PubMed] [Google Scholar]
- 58.Bohon C Binge Eating Disorder in Children and Adolescents. Child Adolesc Psychiatr Clin N Am 2019;28:549–555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.He J, Cai Z, Fan X. Prevalence of binge and loss of control eating among children and adolescents with overweight and obesity: An exploratory meta-analysis. Int J Eat Disord 2017;50:91–103. [DOI] [PubMed] [Google Scholar]
- 60.Devlin MJ, King WC, Kalarchian MA, et al. Eating pathology and experience and weight loss in a prospective study of bariatric surgery patients: 3-year follow-up. Int J Eat Disord 2016;49:1058–1067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Bishop-Gilyard CT, Berkowitz RI, Wadden TA, Gehrman CA, Cronquist JL, Moore RH. Weight reduction in obese adolescents with and without binge eating. Obesity (Silver Spring) 2011;19:982–987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Chao AM, Wadden TA, Faulconbridge LF, et al. Binge-eating disorder and the outcome of bariatric surgery in a prospective, observational study: Two-year results. Obesity (Silver Spring) 2016;24:2327–2333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Sheets CS, Peat CM, Berg KC, et al. Post-operative psychosocial predictors of outcome in bariatric surgery. Obes Surg 2015;25:330–345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Mason C, de Dieu Tapsoba J, Duggan C, Wang C-Y, Alfano CM, McTiernan A. Eating behaviors and weight loss outcomes in a 12-month randomized trial of diet and/or exercise intervention in postmenopausal women. Int J Behav Nutr Phys Act 2019;16:113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Kelly AS, Auerbach P, Barrientos-Perez M, et al. A randomized, controlled trial of liraglutide for adolescents with obesity. N Engl J Med 2020;382:2117–2128. [DOI] [PubMed] [Google Scholar]
- 66.Decaluwé V, Braet C, Fairburn CG. Binge eating in obese children and adolescents. Int J Eat Disord 2003;33:78–84. [DOI] [PubMed] [Google Scholar]
- 67.Birgegård A, Norring C, Clinton D. Binge eating in interview versus self-report: different diagnoses show different divergences. Eur Eat Disord Rev 2014;22:170–175. [DOI] [PubMed] [Google Scholar]
- 68.Butryn ML, Wadden TA. Treatment of Overweight in Children and Adolescents: Does Dieting Increase the Risk of Eating Disorders? Int J Eat Disord 2005;37:285–293. [DOI] [PubMed] [Google Scholar]
- 69.Wadden TA, Foster GD, Sarwer DB, et al. Dieting and the development of eating disorders in obese women: results of a randomized controlled trial. Am J Clin Nutr 2004;80:560–568. [DOI] [PubMed] [Google Scholar]
- 70.Jones RA, Lawlor ER, Birch JM, et al. The impact of adult behavioural weight management interventions on mental health: A systematic review and meta-analysis. Obes Rev 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Elliott CA, Tanofsky-Kraff M, Mirza NM. Parent report of binge eating in Hispanic, African American and Caucasian youth. Eat Behav 2013;14:1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Sonneville KR, Calzo JP, Horton NJ, et al. Childhood hyperactivity/inattention and eating disturbances predict binge eating in adolescence. Psychol Med 2015;45:2511–2520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Goldschmidt AB, Doyle AC, Wilfley DE. Assessment of binge eating in overweight youth using a questionnaire version of the Child Eating Disorder Examination with Instructions. Int J Eat Disord 2007;40:460–467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Limburg K, Shu CY, Watson HJ, Hoiles KJ, Egan SJ. Implications of DSM-5 for the diagnosis of pediatric eating disorders. Int J Eat Disord 2018;51:392–400. [DOI] [PubMed] [Google Scholar]
- 75.Braet C Patient Characteristics as Predictors of Weight Loss after an Obesity Treatment for Children. Obesity 2006;14:148–155. [DOI] [PubMed] [Google Scholar]
- 76.Balantekin KN, Hayes JF, Sheinbein DH, et al. Patterns of eating disorder pathology are associated with weight change in family‐based behavioral obesity treatment. Obesity 2017;25:2115–2122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Carnier J, De Piano A, De Lima Sanches P, et al. The role of orexigenic and anorexigenic factors in an interdisciplinary weight loss therapy for obese adolescents with symptoms of eating disorders. Int J Clin Pract 2010;64:784–790. [DOI] [PubMed] [Google Scholar]
- 78.Eichen DM, Strong DR, Rhee KE, et al. Change in eating disorder symptoms following pediatric obesity treatment. Int J Eat Disord 2019;52:299–303. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


