Abstract
Background:
Older adults display wide individual variability (heterogeneity) in the effects of resistance exercise training on muscle strength. The mechanisms driving this heterogeneity are poorly understood. Understanding of these mechanisms could permit development of more targeted interventions and/or improved identification of individuals likely to respond to resistance training interventions. Thus, this study assessed potential physiological factors that may contribute to strength response heterogeneity in older adults: neural activation, muscle hypertrophy, and muscle contractility.
Methods:
In 24 older adults (72.3±6.8 years), we measured the following parameters before and after 12 weeks of progressive resistance exercise training: i) isometric leg extensor strength; ii) isokinetic (60°/sec) leg extensor strength; iii) voluntary (neural) activation by comparing voluntary and electrically-stimulated muscle forces (i.e., superimposed doublet technique); iv) muscle hypertrophy via dual-energy x-ray absorptiometry (DXA) estimates of regional lean tissue mass; and v) intrinsic contractility by electrically-elicited twitch and doublet torques. We examined associations between physiological factors (baseline values and relative change) and the relative change in isometric and isokinetic muscle strength.
Results:
Notably, changes in quadriceps contractility were positively associated with the relative improvement in isokinetic (r=0.37–0.46, p≤0.05), but not isometric strength (r=0.09–0.21). Change in voluntary activation did not exhibit a significant association with the relative improvements in either isometric or isokinetic strength (r=0.35 and 0.33, respectively; p>0.05). Additionally, change in thigh lean mass was not significantly associated with relative improvement in isometric or isokinetic strength (r=0.09 and −0.02, respectively; p>0.05). Somewhat surprising was the lack of association between exercise-induced changes in isometric and isokinetic strength (r=0.07).
Conclusions:
The strength response to resistance exercise in older adults appears to be contraction-type dependent. Therefore, future investigations should consider obtaining multiple measures of muscle strength to ensure that strength adaptations are comprehensively assessed. Changes in lean mass did not explain the heterogeneity in strength response for either contraction type, and the data regarding the influence of voluntary activation was inconclusive. For isokinetic contraction, the strength response was moderately explained by between-subject variance in the resistance-exercise induced changes in muscle contractility.
Keywords: Aging, Sarcopenia, Dynapenia, Resistance exercise, Variability
1. Introduction
The global population of older adults (defined by the United Nations as > 60 years and the National Institutes of Health as > 65 years) is expected to double to more than two billion by 2050 [1]. Forty-two percent of older adults have one or more physical limitations in performing daily tasks that are essential for independence [2]. One factor contributing to these physical limitations is age-related weakness (at times referred to as dynapenia; the Greek word for poverty of strength, power, or force) [3, 4].
Resistance exercise is considered the first-line therapy to manage age-related muscle weakness [5]. Nevertheless, changes in muscle strength in response to physical activity, including resistance training, exhibit wide individual variability (heterogeneity) [6–14]. For instance, following a five-month, twice/week progressive resistance exercise training (RET) program, roughly half of middle-aged and older adult participants demonstrated a robust (> 20%, and even up to 50%) increase in the maximal voluntary isometric strength of the leg extensors; however, one quarter of them exhibited a decrease or a negligible change [8].
While response heterogeneity presents a challenge for clinicians and many investigators, it also provides an excellent opportunity to explore the mechanistic basis of exercise-induced enhancement in strength. Most studies examining factors that determine individual responsivity to RET have primarily focused on variability in the anabolic muscle growth response (for review see [11, 15–17]). Relatively few studies examined physiological mechanisms contributing to the variability in the muscle strength response in older adults [9, 14, 18, 19]. Yet, muscle weakness is indisputably linked to poor health outcomes in older adults (e.g., mobility limitations [20, 21], falls [21], impairments in instrumental activities of daily living [22], premature death [22–24], and frailty [25]). This underscores the need to discern mechanisms underlying inter-individual variability in the strength response to exercise in older adultrs as better understanding these mechanisms could permit the development of more targeted interventions and/or better identify those who are most likely to respond to RET interventions.
Conceptually, three broad physiologic “factors” are likely associated with strength responsivity to RET: 1) the degree of adaptation in the capability of the nervous system to activate the musculature (voluntary activation; reflective of e.g., central neural drive, motor unit recruitment, rate coding, neuromuscular junction transmission), 2) the degree of adaptation in skeletal muscle hypertrophy (muscle mass; a proxy for contractile protein content), and 3) the degree of adaptation in contractility (the intrinsic-force-generating capacity of a muscle; reflective of integrity of the neuromuscular junction, excitation-contraction (E-C) coupling, and myosin isoform profile, to name a few ) [26]. Increased strength in response to RET would likely involve enhancement of at least one of these factors. In this study we sought to determine the relative contribution of neural activation, muscle hypertrophy, and/or muscle contractility to between-subject variability in the strength response associated with progressive RET in older adults.
2. Materials and Methods
2.1. General Overview of Study Design and the RET Program.
This study included baseline and post-intervention data of subsets of older adults from two different clinical trials conducted through the Ohio Musculoskeletal and Neurological Institute at Ohio University [the UNCODE study (NCT02505529) and the DART study (NCT03978572)]. The data presented herein is a secondary analysis from these studies, and as such should be considered preliminary in that it was not statistically powered for the these specific analyses. For other publications linked to these broader data sets, please see the following references: [27–32]. Both studies prescribed the same intervention of 12 weeks of total-body progressive resistance training in older adults. The only notable difference is that the UNCODE study utilized a twice-per-week training frequency, while DART study participants exercised three times per week.
Before and after training, we assessed voluntary activation by comparing voluntary and electrically-stimulated muscle forces using the superimposed doublet technique. Muscle hypertrophy was quantified via dual-energy x-ray absorptiometry (DXA)-derived estimates of regional lean tissue mass. We utilized electrically-elicited contractions as our index of intrinsic contractility. In addition to the commonly-reported twitch response to stimulation, we also assessed the response to doublet (100-Hz) stimulation, as differential responses to low- and high-frequency stimulation are thought to indicate the state of E-C coupling (e.g., impaired in low-frequency fatigue) [33–36]. We also assessed whether selected baseline measures (e.g., strength, pain, physical activity, self-reported and laboratory-based measures of physical function, and the aforementioned physiological factors) predicted the strength response to RET.
In general, within a ~45–60 min training session, participants performed 10–12 resistance exercises targeting the major muscle groups following a protocol published by our group [32]. Within a typical session, participants completed exercises using machine weights, free weights, or their own body weight that targeted the major muscle groups (i.e., chest, back, arms, shoulders, upper legs, and lower legs). The intensity of the exercise was ascertained based on each participant’s ability to complete the prescribed sets and repetitions, where the target intensity was designed to result in task failure, or near-failure, in the range of repetitions provided. In essence, the first two weeks served as a familiarization phase in which participants completed one set of 12–15 repetitions. In weeks 3–6, they progressed to two sets of 12–18 repetitions. The exercise prescription for weeks 7–10 consisted of three sets of 8–12 repetitions, and for weeks 11–12 it consisted of three sets of 6–10 repetitions where the intensity progressed accordingly. Exercise progression between and within phases was individualized based upon the participant’s exercise tolerance and adaptation to past sessions. Thus, the RET was pragmatic in nature. Primary and other study outcome measures were collected at baseline and after the 12-week progressive resistance training intervention. Study participants generally performed three exercises/session targeting the leg extensor muscles, which are the focus of the present study. These exercises included leg extensions on a MedX leg extension machine (MedX, Ocala, FL), leg presses on a Keiser pneumatic machine (A300 Leg Press, Keiser, Fresno, CA), and lunges.
2.2. Study Participants.
Twenty older adults from the UNCODE study (73.3 ± 6.7 years; 13 women and 7 men) and four older adults from the DART study (67.0 ± 4.8 years; 4 women) were included in these analyses. The age of the total sample was 72.3 ± 6.8 years. To be considered for the UNCODE study progressive resistance intervention, participants had to be ≥60 years of age, have a body mass index (BMI) between 18 and 40 kg/m2, answer “no” to questions 1–4 of the Physical Activity Readiness Questionnaire (PAR-Q), have a physician’s clearance to participate in exercise if answering “yes” to any of questions 5–7 of the PAR-Q, and have a “normal” or “abnormal, but clinically insignificant” electrocardiogram (ECG) result. To be considered for participation in the DART study, participants had to be between the ages of 60 and 75 years, have a BMI between 18 and 40 kg/m2, have a Short Physical Performance Battery (SPPB) score greater than or equal to 8, have a 6-minute walk (6MW) performance of 450–725 meters for a man or 400–675 meters for a woman, and have a “normal” or “abnormal, but clinically insignificant” ECG result.
For both UNCODE and DART, participants could not participate in a progressive resistance training program in the 24 weeks leading up to the study onset; had to be living independently; be free of major musculoskeletal, neurological, cardiac, pulmonary, renal, psychiatric, and cognitive diseases or disorders; and be able to perform traditional activities of daily living (e.g., toileting, showering). All participants had to be willing to undergo DXA scans, maintain their current diet, and adhere to the intervention program for its 12-week duration (study participants attended 94.4±7.8% of their prescribed exdercise sessions).
All participants were instructed to abstain from drinking caffeinated beverages for 4 hours before the testing session and from drinking alcohol for 24 hours before the testing session.
To characterize the study participants, we also measured: 6MW gait speed (on a 30-meter walkway with a left-hand turn around a cone) [29], SPPB score (composite score based on balance, 4-m walk gait speed, and 5x chair rise time) [37], body fat percentage using DXA [38], and moderate-to-vigorous intensity physical activity via accelerometry [39]. In the UNCODE study, we also utilized surveys to quantify baseline comorbidities via the Charlson Comorbidity Index [40], depression via the Center for Epidemiologic Studies Depression Scale (CES-D) [41], balance confidence via the Activities-Specific Balance Confidence Scale [42], community mobility via the Life-Space Questionnaire [43], fatigue and lower extremity function via the Quality of Life in Neurological Disorders (Neuro-QoL) surveys, pain interference and pain severity via the Brief Pain Inventory [44], pain resiliency via the Pain Resilience Scale [45], and pain catastrophizing via the Pain Catastrophizing Scale [46].
The Ohio University Institutional Review Board approved these studies, and all study participants provided informed written consent.
2.3. Primary Outcome Measures
2.3.1. Muscle Strength.
Leg extension maximal voluntary isometric and isokinetic strength measures were recorded utilizing a Biodex System 4 Dynamometer (Biodex Medical Systems Inc., Shirley, NY) as previously described [47]. For quantification of isometric maximal voluntary contraction (MVC) torque (Newton Meters, N-m), participants were seated with their non-dominant knee at 90° flexion and the knee axis of rotation in alignment with the rotational axis of the Biodex torque motor. A lap belt was applied to prevent movement at the hip, and the participants’ non-dominant lower extremities were affixed to a lower extremity lever arm, which was attached to the Biodex torque motor. We controlled for leg dominance given evidence of slight differences in strength capacity between the dominant and non-dominant limbs [48]. We elected to test the non-dominant limb due to radiographic evidence suggesting a higher incidence of knee osteoarthritis in the dominant leg [49]. The torque signal was scaled to maximize its resolution (208.7 mV/Nm; Biodex Researchers Tool Kit Software) and sampled at 625 Hz (MP150 Biopac Systems). Participants received visual feedback of torque on a monitor located 1 m in front of them. Provided with strong verbal encouragement, participants performed five isometric MVCs with 1 minute of rest between each effort; the peak value of these three trials was utilized as the isometric MVC for the analysis.
For maximal voluntary isokinetic torque, the non-dominant peak leg extension torque (N-m) was measured concentrically at 60° per second in isokinetic mode. Six trials were performed with 30 seconds rest between bouts; the maximum voluntary isokinetic strength was calculated as the mean of the highest three values of maximal isokinetic torque (N-m) produced between 90° and 30° of knee flexion. Rather than utilizing the single peak voluntary isokinetic torque value across all six trials as the maximum value, we used the mean of the top three maximal isokinetic torque values because this technique is consistent with the isokinetic leg extension strength measurement protocol utilized by Manini et al. (2007) to derive the sex-specific leg extension strength cut points for maintaining mobility [20].
2.3.2. Voluntary Activation and Contractility.
We applied large (e.g., 3×4- or 4×5-inch depending on the size of the quadriceps being tested) self-adhesive electrodes over the motor points of the rectus femoris and vastus medialis portions of the quadriceps muscles on the non-dominant leg. Electrodes were connected to a Digitimer DS7AH (Hertfordshire, UK) stimulator, which is specifically designed to generate the high currents needed to activate large muscle groups [50, 51]. With the knee positioned at 90°, we applied single, 200-μsec pulses at incrementally increasing current and constant voltage (400 V) until a plateau was reached in the evoked force output. The twitch torque (N-m) was determined from the maximum evoked torque output. Next, study participants were asked to perform two 4–5-second isometric MVCs, during which a 100-Hz supramaximal doublet was delivered followed by a second doublet to the resting muscle within 5 seconds of the first. From these data, we assessed voluntary activation utilizing a doublet interpolation technique similar to our previous study [52]. Here, the increase in torque immediately following the stimulation was expressed relative to a potentiated response evoked by the same doublet with the muscle at rest 1–2 s after the isometric MVC. Voluntary activation was calculated as follows:
where τd is the evoked torque during the MVC, and τf is the evoked torque following the MVC. A value of 100 is indicative of complete muscle activation. Additionally, both the twitch and 100-Hz doublet torques (τf) (N-m) were used to assess contractility. While both evoked responses are affected by intramuscular mechanisms of force production, the doublet may be more sensitive to factors such as calcium release and crossbridge cooperativity [36, 53–56]. We also report the twitch-to-doublet ratio because differential responses to high- and low-frequency stimulation are thought to indicate the state of E-C coupling, as in low-frequency fatigue [33–36]. Three study participants opted not to undergo doublet testing. Thus, for the doublet and twitch-to-doublet ratio, our sample size was 21. Of these 21 participants, two additional study participants’ data were excluded from the voluntary activation analysis because the superimposed doublet had not been delivered at the plateau portion of their MVC. Thus, our sample size for the voluntary activation was 19.
2.3.3. Lean Mass.
DXA scans (Hologic Discovery QDR model Series, Waltham, MA, USA) were performed to assess lean mass as we described previously [38, 47]. Following a whole-body scan, whole-body, appendicular, and thigh lean tissue mass were determined using the system’s software package (Hologic APEX, Version 4.0.2). Participants were advised to report to the laboratory in a hydrated state and were given scrubs to wear during the scan. Care was taken to follow The International Society for Clinical Densitometry guidelines for positioning during the scan [57].
2.4. Statistical Analysis.
We initially conducted a repeated-measures analysis of variance (ANOVA) to examine whether there was a sex difference in the strength response (time × sex interaction for isokinetic and isometric torque). The sex × time interactions were not significant (isometric torque p=0.09 and eta2=0.13; isokinetic torque p=0.49, eta2=0.02). We also performed a Fisher’s exact test to determine whether there was a statistically significant difference between the expected frequencies and the observed frequencies in the strength response (tertiles) by sex; however, no significant differences were found in either the isometric or isokinetic change response (p=0.13 and 0.42, respectively). Thus, we aggregated data across sex for all analyses. We used paired t-tests to examine changes over time (i.e., pre vs. post-intervention). Pearson and Spearman correlation analyses were used to examine associations between independent and dependent variables (IVs and DVs, respectively) at baseline (e.g., association between thigh lean mass (IV) and isometric torque (DV) as well as baseline IV measures and percent changes in strength, depending on parametric vs non-parametric distributions). Similarly, correlation analyses were used to examine the associations between the percent changes in the IVs and DVs following RET. For analyses where baseline electrically-stimulated twitch and doublet torque measures were the IV, we normalized the torque value for thigh lean mass to control for the inherent theoretical influence of muscle mass on these measures. For associations where percent changes in contractility vs. percent changes in strength were examined, we did not normalize for lean mass; theoretically, an increase in mass would impact both measures proportionally. A preset α-level of ≤ 0.05 (two-sided) was required for significance. The SPSS statistical package (version 19.0 for Mac, Chicago, IL) was used for data analysis. Data are presented as mean ± standard deviation (SD).
3. Results
At baseline (i.e., before RET), we observed strong associations between isometric and isokinetic torque measures of strength (r=0.892, p<0.001) (Figure 1). RET increased isometric torque 11.5±17.5% (p<0.005), but did not significantly increase isokinetic torque (4.3±11.1%, p=0.078). As expected, there was substantial heterogeneity in response to the RET program. Specifically, ~1/3 of subjects exhibited decreases or no change in both isometric and isokinetic torque, whereas 1/3 exhibited modest increases, and 1/3 exhibited robust increases (Figure 2A and 2B). Interestingly, we did not observe an association between the % change in isokinetic torque and % change in isometric torque (r=0.07, p=0.731) (Figure 2C); therefore, we performed separate association analyses for both indices of strength. Descriptive statistics of the effects of RET on other outcomes are shown in Table 1.
Figure 1.
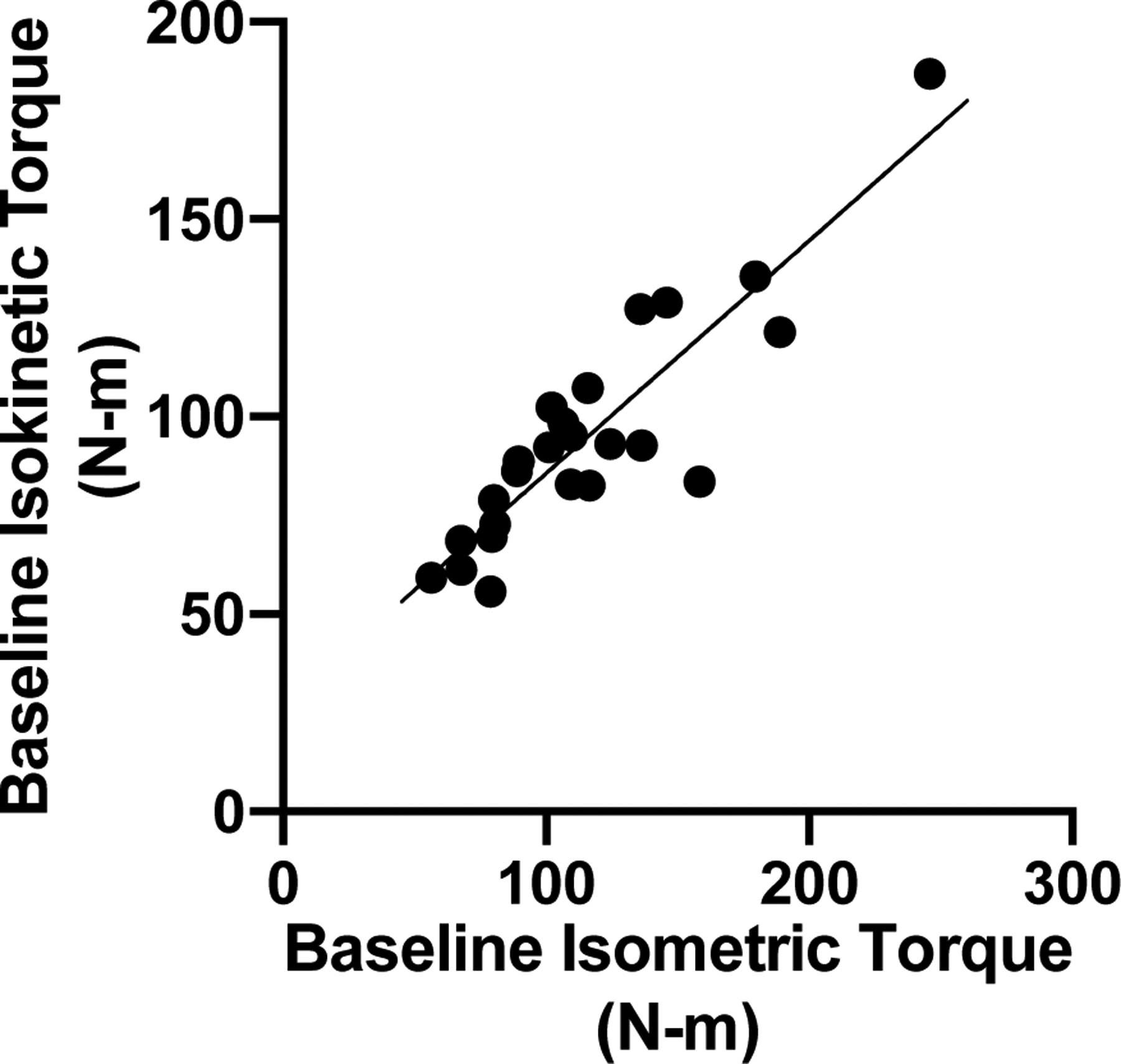
Before resistance exercise training, baseline isometric and isokinetic torque measures of leg extensor strength were strongly associated (r=0.892, p<0.001).
Figure 2.
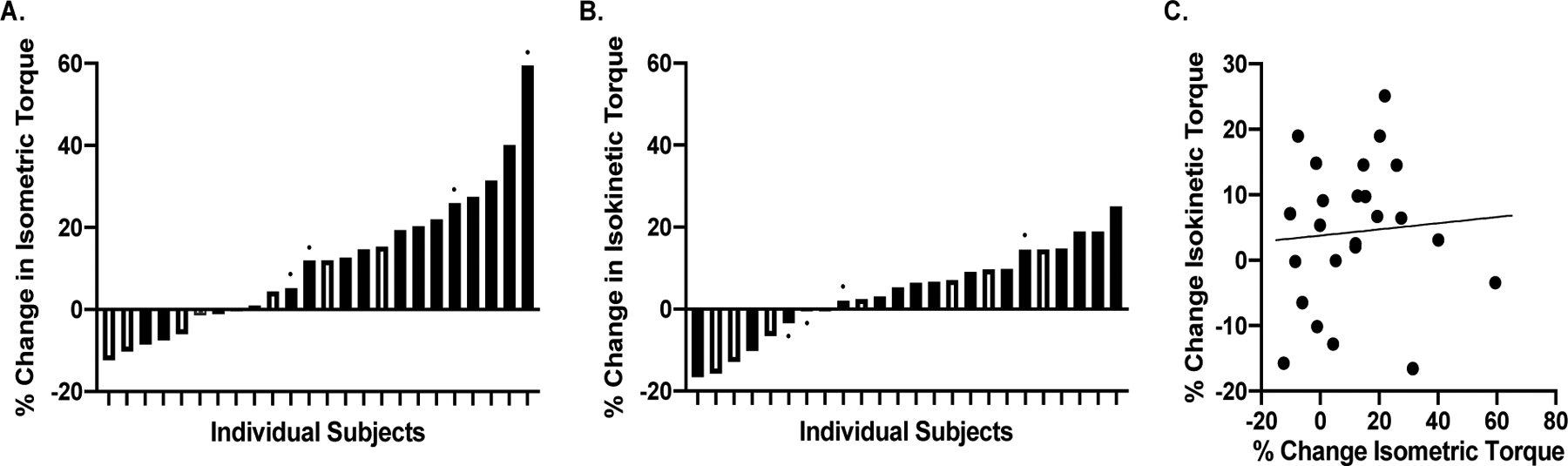
There was substantial heterogeneity in response to resistance exercise training in leg extensor isometric (A) and isokinetic (B) torque, respectively. Filled bars represent women, and open bars represent men. The small closed circle above selected individual data points represent data from participants in the DART Study. These are shown to illustrate that subjects across both studies demonstrated substantial variability in the strength response to resistance exercise training. C. The % change in isokinetic torque and % change in isometric torque were not associated (r=0.07, p=0.731).
Table 1.
Descriptive statistics of the effects of resistance exercise training on key outcomes.
| Outcome | Baseline | Post-training | % Change | P-value | N |
|---|---|---|---|---|---|
| Body Mass Index (kg/m2) | 26.36 (4.72) | 26.22 (4.48) | −0.36 (2.09) | 0.227 | 24 |
| Body Fat (%) | 34.69 (8.76) | 34.42 (8.37) | −0.48 (3.97) | 0.373 | 24 |
| Appendicular Lean Mass/Ht2 | 6.67 (1.16) | 6.71 (1.15) | 0.67 (3.78) | 0.419 | 24 |
| Accelerometry Min/Week of ≥Moderate Activity | 124.61 (48.82) | 101.25 (48.62) | −15.18 (35.34) | 0.019* | 24 |
| Short Physical Performance Battery Score | 11.62 (0.71) | 11.89 (0.45) | 2.39 (4.90) | 0.031* | 24 |
| Six-minute Walk Gait Speed (m/sec) | 1.48 (0.24) | 1.54 (0.26) | 4.04 (7.39) | 0.013* | 24 |
| Isometric Torque (N●m) | 115.26 (44.37) | 126.60 (46.83) | 11.51 (17.48) | 0.005* | 24 |
| Isokinetic Torque (N●m) | 94.66 (29.34) | 98.61 (32.64) | 4.31 (11.13) | 0.078 | 24 |
| Voluntary Activation (%) | 93.29 (4.91) | 89.92 (6.26) | −4.05 (7.52) | 0.033* | 19 |
| Thigh Lean Mass (Kg) | 4.73 (1.01) | 4.78 (0.93) | 1.17 (5.16) | 0.500 | 24 |
| Twitch Torque (N●m) | 20.99 (7.06) | 21.83 (7.49) | 5.30 (15.99) | 0.214 | 24 |
| Normalized Twitch Torque (N●m/Kg of Thigh Lean Mass) | 4.42 (1.07) | 4.54 (1.07) | 4.44 (17.43) | 0.358 | 24 |
| Doublet Torque (N●m) | 38.07 (13.02) | 39.99 (11.74) | 7.80 (17.14) | 0.147 | 21 |
| Normalized Doublet Torque (N●m/Kg of Thigh Lean Mass) | 8.13 (1.68) | 8.51 (1.30) | 6.33 (16.82) | 0.206 | 21 |
| Twitch to Doublet Ratio | 0.55 (0.09) | 0.58 (0.14) | 5.46 (17.45) | 0.156 | 21 |
Denotes statistically significant changes (p ≤ 0.05). Values are expressed as mean (SD).
Baseline values of isometric and isokinetic strength (both absolute and normalized to body weight) were not associated with their respective responses to RET (Table 2). Similarly, baseline levels of voluntary activation, thigh lean mass, twitch-to-doublet ratio, normalized twitch and doublet torque, physical activity (i.e., mins/week of moderate-to-vigorous activity), comorbidities, depression, fatigue, indices of pain severity, interference, resiliency, and catastrophizing were not associated with strength response (Table 2). Baseline values of self-reported balance confidence, lower extremity function, and community mobility were negatively associated with the % change in isokinetic torque following exercise (i.e., those with lower balance confidence, function and community mobility at baseline demonstrated greater gains), but no associations were observed for the % change in isometric strength (Table 2). In laboratory-based measures of physical function, we observed significant associations between baseline SPPB score and 5x Chair Rise time with % change in leg extensor isometric strength, such that participants with higher levels of baseline function demonstrated greater gains (Table 2). Baseline measures of 6MW and 4-meter walk velocities were not associated with changes in isometric or isokinetic strength; however, baseline 4-meter walk velocity did demonstrate a modest correlation (Table 2).
Table 2.
Association between baseline values and percent changes in isometric and isokinetic torque following resistance exercise training.
| Baseline Value | % Change in Isometric Torque | % Change in Isokinetic Torque |
|---|---|---|
| Isometric Torque | −0.26 | −0.08 |
| Normalized Isometric Torque (N●m/Kg of body weight) | −0.13 | −0.09 |
| Isokinetic Torque | −0.17 | −0.04 |
| Normalized Isokinetic Torque (N●m/Kg of body weight) | 0.03 | −0.08 |
| Short Physical Performance Battery (SPPB)† | 0.46* | 0.14 |
| Five Times Chair Rise (sec) | −0.45* | −0.13 |
| Four-meter Walk Velocity (m/sec) | 0.38 | −0.13 |
| Six-minute Walk Velocity (m/sec) | 0.23 | −0.24 |
| Voluntary Activation | −0.11 | −0.14 |
| Thigh Lean Mass | −0.34 | −0.15 |
| Normalized Twitch Torque (N●m/Kg of Thigh Lean Mass) | 0.07 | −0.19 |
| Normalized Doublet Torque (N●m/Kg of Thigh Lean Mass) | −0.16 | −0.18 |
| Twitch-to-Doublet Ratio | 0.15 | −0.01 |
| Accelerometry Mins/Week of Moderate-Vigorous Activity | 0.04 | 0.07 |
| Charlson Comorbidity Index† | −0.10 | 0.08 |
| Center for Epidemiologic Studies - Depression Scale (CES-D)† | 0.33 | 0.18 |
| Activities-specific Balance Confidence Scale† | 0.20 | −0.45* |
| Brief Pain History – Pain Severity† | −0.01 | 0.02 |
| Brief Pain History – Pain Interference† | 0.07 | 0.07 |
| Pain Resiliency Scale† | 0.16 | 0.03 |
| Pain Catastrophizing Scale† | 0.25 | 0.24 |
| Life Space Questionnaire† | 0.05 | −0.64* |
| Neuro-QoL Fatigue† | 0.12 | −0.06 |
| Neuro-QoL Lower Extremity Function (Mobility)† | −0.08 | −0.46* |
Denotes statistically significant associations (p ≤ 0.05).
Values represent Spearman’s rho correlation coefficients for non-parametric distributions.
3.1. Voluntary Activation.
The associations between % change in voluntary activation and % change in isometric and isokinetic strength were not statistically significant (r=0.35, p=0.145 and r=0.33, p=0.076, respectively) (Figure 3A and 3B).
Figure 3.
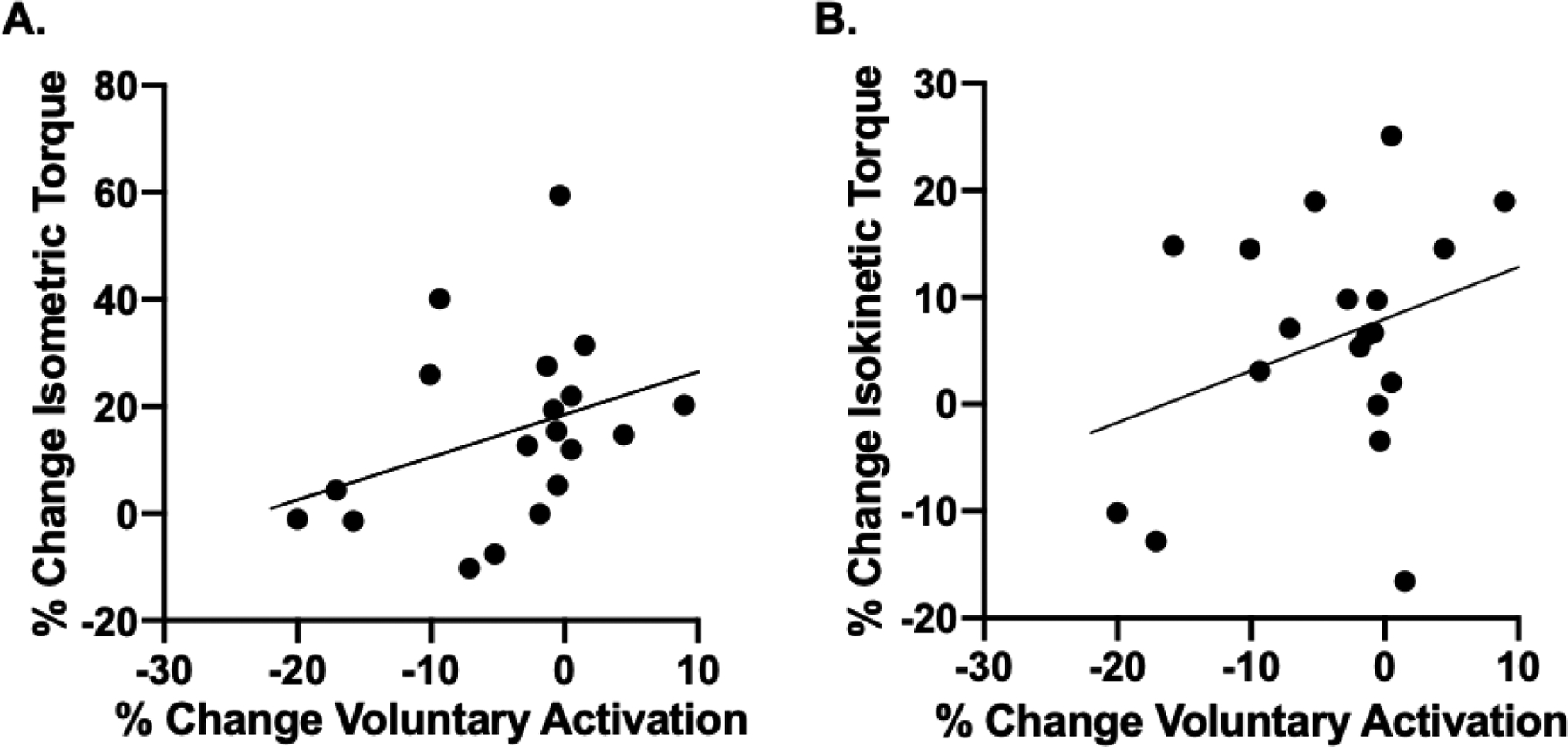
Associations between % change in voluntary activation and % change in isometric (A) and isokinetic (B) strength were not statistically significant (r=0.35, p=0.145 and r=0.33, p=0.076, respectively).
3.2. Lean Mass.
The associations between % change in thigh lean mass and % change in isometric and isokinetic strength were not statistically significant (r=0.09, p=0.671 and r=−0.02, p=0.937, respectively) (Figure 4A and 4B).
Figure 4.
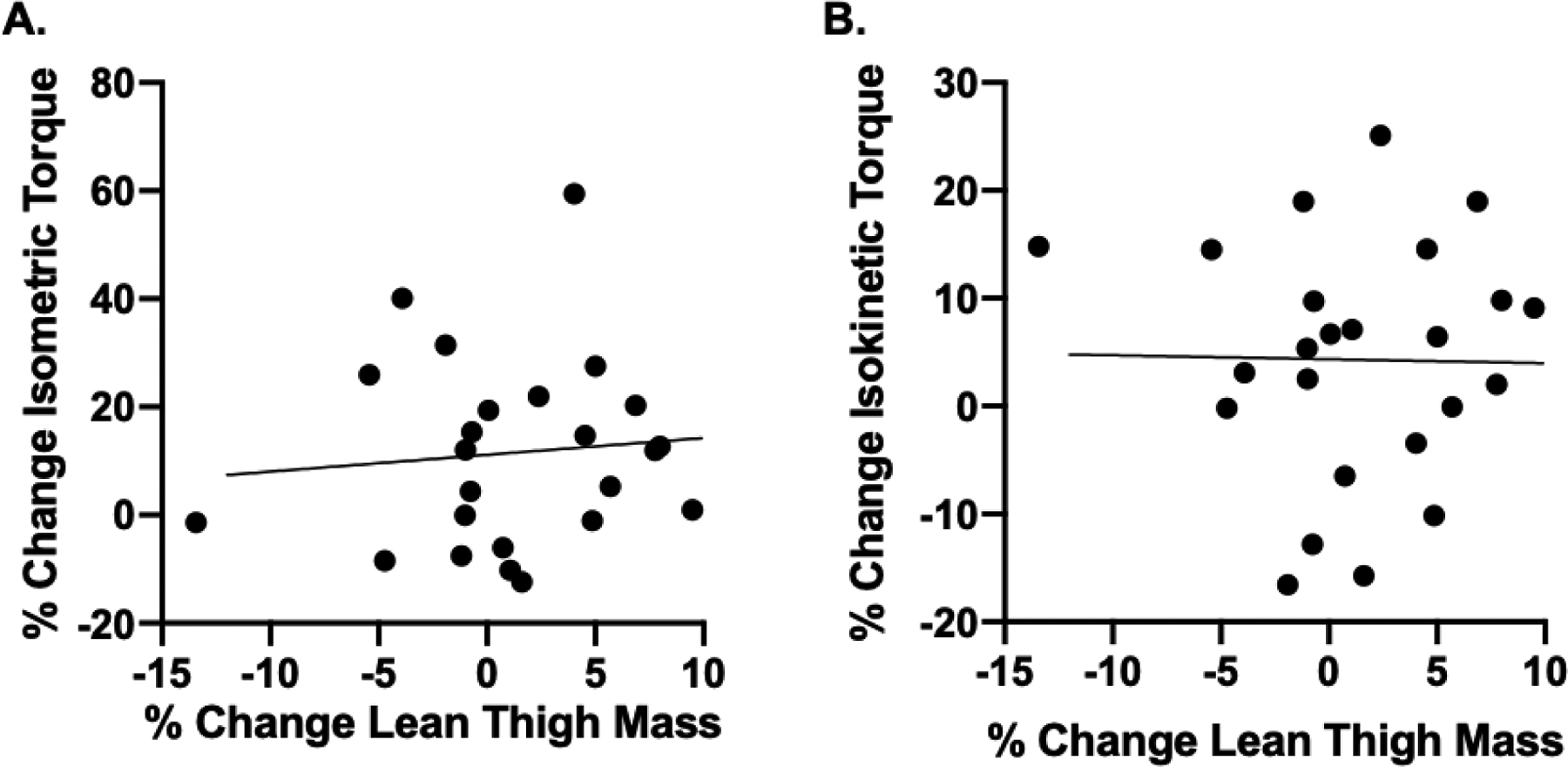
Associations between % change in thigh lean mass and % change in isometric (A) and isokinetic (B) strength were not statistically significant (r=0.09, p=0.671 and r=−0.02, p=0.937, respectively).
3.3. Contractility.
The associations between the % change in quadriceps twitch and doublet torque and the % change in isometric torque were not statistically significant (r=0.09, p=0.694, and r=0.21, p=0.359, respectively) (Figures 5A and 5B). Similarly, the association of % change in the twitch-to-doublet ratio with % change in isometric torque was not statistically significant (r=0.06, p=0.794) (Figure 5C). However, we observed a significant correlation between % change in twitch torque and % change in isokinetic torque (r=0.46, p=0.023) (Figure 5D). The correlation between % change in doublet torque and % change in isokinetic torque was not statistically significant (r=0.37; p=0.096) (Figure 5E). The association of % change in the twitch-to-doublet ratio with % change in isokinetic torque was statistically significant (r=0.48, p=0.031) (Figure 5F).
Figure 5.
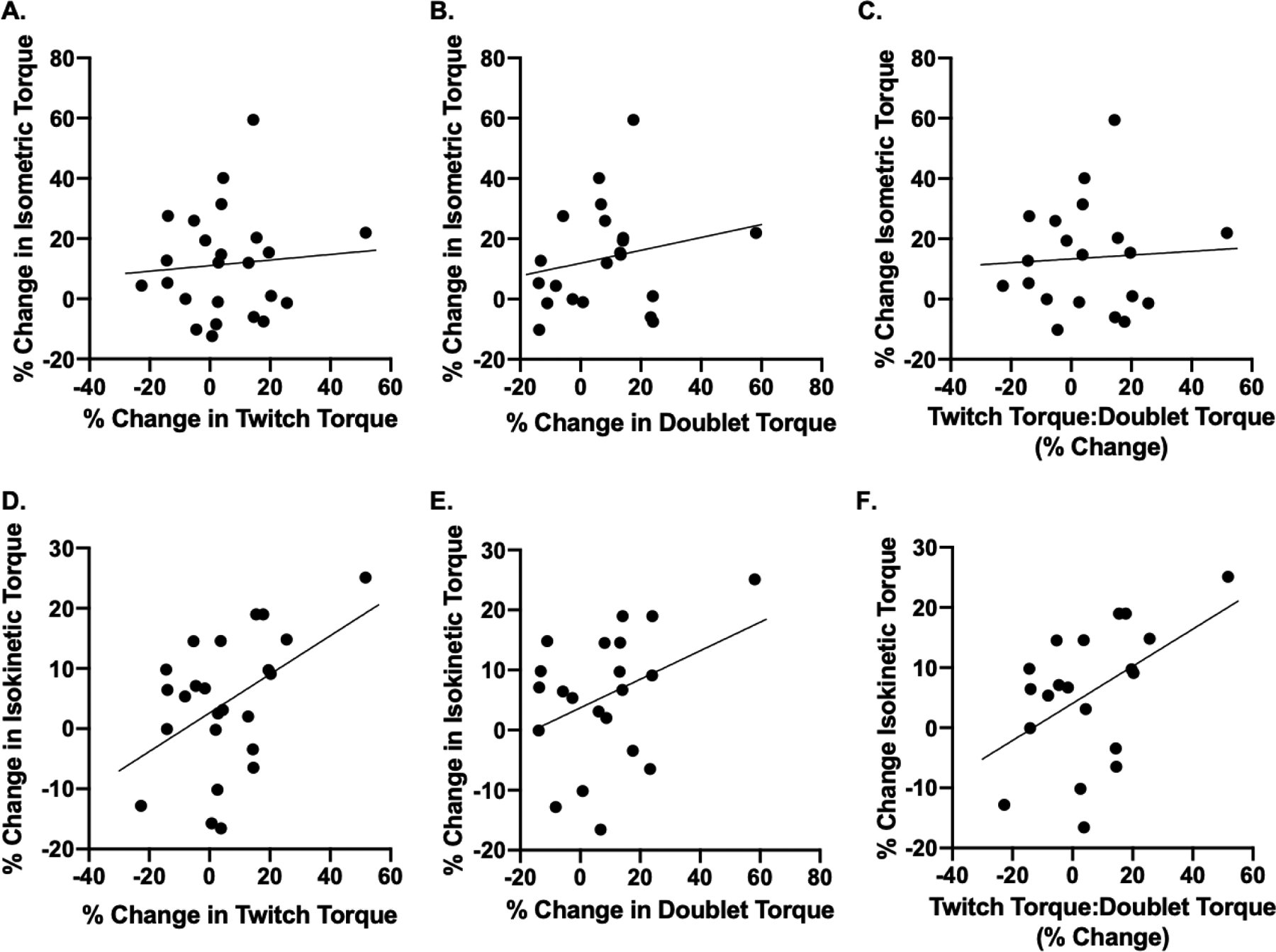
Associations between % change in quadriceps twitch (A) and doublet torque (B) and % change in isometric torque were not statistically significant (r=0.09, p=0.694, and r=0.21, p=0.359, respectively). C. The association of % change in the twitch-to-doublet ratio with % change in isometric torque was not statistically significant (r=0.06, p=0.794). D. The correlation between % change in twitch torque and % change in isokinetic torque was significant (r=0.46, p=0.023). E. The correlation of % change in doublet torque and % change in isokinetic torque was not statistically significant (r=0.37; p=0.096) F. The association of % change in the twitch-to-doublet ratio with % change in isokinetic torque was significant (r=0.48, p=0.031).
4. Discussion
Our most notable findings were that i) changes in quadriceps contractility were positively associated with the relative improvement in isokinetic, but not isometric, leg extensor strength; and ii) change in thigh lean mass was not associated with the relative improvement in isometric or isokinetic strength. A somewhat surprising finding was the lack of an association between the exercise-induced changes in isometric and isokinetic strength. Additionally, the change in neural activation was not associated with the relative improvements in either isometric or isokinetic strength; however, modest associations (i.e., modest effect sizes) were observed and are discussed in more detail below.
4.1. No Association Between the Exercise-induced Changes in Isometric and Isokinetic Strength.
Overall, the RET intervention resulted in modest mean increases in isometric strength (~12% explained variance) and a non-significant mean change in isokinetic strength (~4% explained variance). The extant literature clearly suggestes that the mean improvement in muscle strength in older adults following resistance training varies widely (e.g., [8]). In general, advanced age is believed to result in a blunting of the adaptive response to RET [58–62]. As supported by other studies in young adults and older adults [6–14], the observed mean increase in muscle strength is affected by a considerable degree of heterogeneity in the observed physiological response to progressive RET (Figure 2). Our finding of a dissociation between the exercise-induced relative changes in isometric and isokinetic strength was not anticipated. Others reported relatively strong associations between different measures of muscle strength under baseline conditions (i.e., not the degree of change following an intervention) [63, 64], similar to what we observed here (~80% explained variance between isometric and isokinetic strength). Thus, we expected that these changes would reasonably track each other over time.
We were only able to find one other study reporting the relationship between relative changes in isometric and isokinetic strength (although it is likely that additional reports exist). That study, which was conducted in a young adult population, reported that resistance exercise-induced relative change in isometric leg extension strength explained 44% of the variance in the relative change in isokinetic strength (at 60°/sec) [65]. Accordingly, we reached out to several authors of articles who measured isometric and isokinetic strength following resistance exercise and asked them to analyze their data for the relationship between the relative changes in isometric and isokinetic strength. Four authors shared their data with us. Data from Coratella and colleagues [66] indicated that the relative change in leg extensor isometric strength in young adults only explained 12% of the variance in relative change in isokinetic strength following eight weeks of training (personal communication with Giuseppe Coratella). Similarly, data from Jesse et al. [67] indicated that the relative change in leg extensor isometric strength explained 1–18% of the relative change in isokinetic strength in young adults following eight weeks of high-intensity RET depending on the speed (60°/sec vs. 180°/sec) of the isokinetic testing (personal communication with Jeremy Loenneke). Data from Heisterberg et al. [68] in older adults following 16 weeks of RET showed a slightly higher association with the relative change in leg extensor isometric strength, explaining 32% of the variance in relative change in isokinetic strength (personal communication with Abigail Mackey). Lastly, data from Da Boit et al. [69] indicated that the relative change in leg extensor isometric strength explained 33–40% of the relative change in isokinetic strength in older adults following eighteen weeks of RET depending on the speed (30°/sec vs. 240°/sec) of the isokinetic testing (personal communication with Mariasole Da Boit). Thus, it appears that our finding of no association between the relative change in isometric and isokinetic strength is reasonably consistent with the data referenced above, where the percent of explained variance between the two ranged from < 1% to only as high as 40%. Notably, our RET intervention utilized isotonic contractions, and we did not assess isotonic strength (e.g., 1-repetition maximum). Thus, it is possible, based on the known principles of specificity of training [70], that the isotonic strength response may have varied from our measures of isometric and isokinetic strength.
We closely examined our data on a subject-by-subject basis and noted that most subjects exhibited a positive adaptation in at least one type of muscle strength. In fact, 79% (19 of 24) of subjects demonstrated at least a 5% gain in either isometric or isokinetic leg extensor strength. These findings are consistent with those reported by Churchward-Venne et al., which demonstrated a large adaptive response to RET, and that nearly all subjects will demonstrate a positive response in at least some outcome variables if the duration of the exercise intervention is sufficient [7]. Findings of this nature suggest that it is critical for clinical trials examining the effectiveness of exercise interventions to obtain numerous measures of muscle strength, which arguably could be used to derive a composite score that is more broadly reflective of adaptation than a singular measure.
4.2. Physiological Underpinnings of the Resistance-Exercise-Induced Strength Response.
Our finding that the relative change in muscle contractility was a key predictor of the relative change in isokinetic strength is somewhat consistent with prior work in young adults [71]. In 2010, Erskine and colleagues reported that in young adult males (n=53, 20±3 years), the major determinant of the leg extensor isometric strength response to nine weeks of resistance training was the degree of training-induced adaptation in in vivo specific tension (assessed by normalizing an index of stimulated leg extensor torque to the quadriceps femoris physiological cross-sectional area of the muscle) [71]. Notably, Erskine et al. observed this association with isometric strength (isokinetic strength was not assessed), whereas we only observed it with isokinetic strength. To our knowledge, others have not specifically examined the role of contractility in explaining the between-subject variance in strength adaptations to resistance exercise, but our finding is congruent with findings demonstrating that RET increases in vivo whole muscle contractile-specific force more than other common physiological adaptations (e.g., muscle mass) in older adults [72]. The specific mechanisms accounting for changes in contractility are perhaps more difficult to discern. One potential explanation is that the training-induced improvements in the E-C coupling processes ultimately resulted in improved force output per unit mass (E-C coupling processes can be impaired by aging [26, 73]). There are, however, discrepant findings on whether exercise training alters individual myofiber specific tension (contractile output normalized to cell size) in older adults [61, 74–77]. It is possible that our findings are linked to changes in skeletal muscle fiber types/myosin heavy chain expression that alter the E-C coupling mechanisms [78, 79]. Interestingly, higher-velocity training paradigms elicit a slow-to-fast transcriptional response in myosin heavy chain transcript levels [80]. This could explain why we observed contractility to be more associated with the strength response during an isokinetic contraction than during an isometric contraction. However, further work is needed to confirm our interpretation.
We did not observe any notable relationship between the relative change in lean mass and the relative change in measures of muscle strength. While this may seem counterintuitive, this is not necessarily inconsistent with prior reports, where only modest to moderate associations were observed in both young adults and older adults [81–83]. For instance, in a large study of 287 adults, the relative changes in muscle size and muscle strength were weakly associated (r=0.16). However, a number of recent studies question the ability of DXA to accurately assess muscle mass [38, 84–86]. We discuss this further in section 4.4.
Enhanced neural activation has long been argued to be a potential mechanism of resistance training strength adaptations in both young adults and older adults [87–91]. Thus, we examined the relative changes in neural activation and muscle strength following exercise training. However, these findings are tenuous and inconclusive. While we did not find statistically significant associations (p-values ranged on the order of 0.07–0.14), the observed associaitons were modest (r=0.33–0.35) for both contraction types. It should be noted that there is a growing debate in the scienitifc community of the utility of the arbitrary threshold of p-values as they can force results into a binary context that lend to ignoring interesting but insignificant results [92]. Thus, we urge caution as it relates to interpreting our findings. We did observe a mean reduction in voluntary activation capacity following training (93% to 90%), which suggests that, on average, there was a reduction in neural activation following training. Anecdotally, we noted that some subjects expressed apprehension regarding the post-training voluntary activation assessment (i.e., anxiety or fear about the painful doublet electrical stimulation that they had experienced during the baseline test). While we cannot state for certain that this observation was associated with the slight mean reduction in voluntary activation following training, we do think it is a possibility.
4.3. Baseline Predictors of the Resistance-Exercise-Induced Strength Response.
For the most part, baseline characteristics did not predict the strength response. Our finding that sex did not influence the strength response is consistent with previously published findings [14]. Among older adults with osteoarthritis, prior studies indicate that higher pain severity and higher levels of pain catastrophizing are associated with greater physical disability [93] and avoidance of physical activity [94]. These findings suggest that pain and negative coping strategies may influence willingness to engage in exercise training, and hence could limit the potential benefits. In contrast, although the present sample was small, our study supports the notion that older adults who persist with training achieve gains in isometric and isokinetic strength regardless of their responses to baseline pain measures.
Baseline levels of strength influence the strength response in young adults [82]; however, we did not observe this association in older adults. Interestingly, we did observe that participants who had lower levels of self-reported physical function and mobility (e.g., lower community mobility based on the LifeSpace questionnaire and lower extremity function based on the Neuro-QoL survey) exhibited more robust isokinetic strength responses. Interestingly, we observed the opposite relationship with isometric strength responses (i.e., those with high physical function exhibited more robust isometric strength responses). These findings indicate that additional work is needed to examine these relationships with respect to differences in self-report vs. laboratory-based measures of physical function as well as contraction types.
4.4. Limitations.
We note several limitations of this work. First, while DXA is still widely used and regarded as a valid approach in the quantification of lean muscle mass, since data collection began for the present study, several papers, including one from our own group, have questioned whether DXA can accurately assess change in muscle mass [38, 84–86]. Indeed, we observed a strong association between magnetic resonance imaging-derived quadriceps muscle cross-sectional area and thigh lean mass in young adults before starting a 10-week RET program (r=0.89) [38]. However, the relative changes following RET did not robustly track each other (r=0.49) [38]. Thus, it is plausible that the DXA-derived estimate of lean mass in the current study has some inherent “noise” in the signal. Similarly, the assessment of voluntary activation likely has some inherent noise since it is dependent on the timing of the electrical stimulation occurring during the peak of the MVC. We visually inspected the mechanical signals and excluded two participants based on incorrect timing of the stimulation delivery, but potential timing error could still make this measurement less sensitive to detecting small changes. Additionally, apprehension could have influenced this measure. A second limitation is the relatively small sample size of this study, which makes the data prone to both type I and type II errors. In particular, we caution against the potential for outliers to drive associations. A third limitation, which we alluded to above, was that our resistance exercise training program consisted of a mix of machine weights, free weights, and body weight exercises, all in isotonic fashion, yet testing was performed before and after the training using isometric and isokinetic protocols. Thus, it is possible that the testing modalities were not specific for detecting changes resulting from the training. Similarly, it should be noted that the training program was gradually progressed towards higher intensity with the final 6-weeks focusing on intensities that resulted in task failure in the 6–12 repetition range. As such, it should be noted that the duration of the training protocol that is generally conceived of as a “strength” training paradigm was relatively short and that the mechanisms underlying the strength-response could vary based on nuances of the training program. Lastly, we are unable to determine the effect of certain drugs and nutritional supplements on the heterogeneity of strength response after RET. We asked subjects to maintain their diet throughout the duration of the study, but we did not have detailed medical or nutritional intake records. This is a potential limitation; vitamin D3 can have an additive effect with resistance training to improve strength in older adults [95]. Other physiological factors (e.g., muscle architecture, amount of intermuscular adipose tissue) for which we could not account could also have played a role in the strength response to RET [18, 96, 97].
5. Conclusions
The mechanisms underlying the large degree of heterogeneity in the muscle strength response to resistance exercise training in older adults are poorly understood. Our findings suggest that the strength response to resistance exercise in older adults is contraction-type dependent. We therefore encourage future investigations to obtain multiple measures of muscle strength to ensure that strength adaptations are comprehensively assessed. While changes in lean mass did not explain the heterogeneity in strength response for either the isometric or isokinetic contraction types, the strength response for isokinetic contraction was moderately explained by the between-subject variance in changes in muscle contractility.
Funding:
This work was supported, in part, by the National Institutes of Health [grant number NIA R01AG044424 to BC Clark] and the American Heart Association [grant number 19PRE34380496 to D Tavoian].
Declaration of Interest:
In the past 3 years, Brian Clark has received research funding from NMD Pharma and Astellas Pharma Global Development Inc. for contracted studies involving aging and muscle-related research. In the past 3 years, Brian Clark has received consulting fees from Regeneron Pharmaceuticals, Zev Industries, and the Gerson Lehrman Group for consultation specific to age-related muscle weakness. Brian Clark is a co-founder with equity of OsteoDx Inc.
Abbreviations:
- ANOVA
analysis of variance
- BMI
body mass index
- CES-D
Center for Epidemiology Studies Depression Scale
- DART
Dual-benefits of Aerobic and Resistance Training
- DV
dependent variable
- DXA
dual-energy x-ray absorptiometry
- E-C
excitation-contraction
- IV
independent variable
- MVC
maximal voluntary contraction
- PAR-Q
Physical Activity Readiness Questionnaire
- QoL
quality of life
- RET
resistance exercise training
- SD
standard deviation
- SPPB
Short Physical Performance Battery
- UNCODE
Unraveling the Neural Contributors of Dynapenia in Elders
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.U.N., United Nations World Population Aging 2017 P.D. Department of Economic and Social Affairs, Editor. 2017, United Nations: New York. [Google Scholar]
- 2.Seeman TE, et al. , Disability trends among older Americans: National Health And Nutrition Examination Surveys, 1988–1994 and 1999–2004. Am J Public Health, 2010. 100(1): p. 100–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Manini TM and Clark BC, Dynapenia and Aging: An Update. J Gerontol A Biol Sci Med Sci, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bhasin S, et al. , Sarcopenia Definition: The Position Statements of the Sarcopenia Definition and Outcomes Consortium. J Am Geriatr Soc, 2020. 68(7): p. 1410–1418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dent E, et al. , International Clinical Practice Guidelines for Sarcopenia (ICFSR): Screening, Diagnosis and Management. J Nutr Health Aging, 2018. 22(10): p. 1148–1161. [DOI] [PubMed] [Google Scholar]
- 6.Bouchard C and Rankinen T, Individual differences in response to regular physical activity. Med Sci Sports Exerc, 2001. 33(6 Suppl): p. S446–51; discussion S452–3. [DOI] [PubMed] [Google Scholar]
- 7.Churchward-Venne TA, et al. , There Are No Nonresponders to Resistance-Type Exercise Training in Older Men and Women. J Am Med Dir Assoc, 2015. 16(5): p. 400–11. [DOI] [PubMed] [Google Scholar]
- 8.Karavirta L, et al. , Individual responses to combined endurance and strength training in older adults. Med Sci Sports Exerc, 2011. 43(3): p. 484–90. [DOI] [PubMed] [Google Scholar]
- 9.Kostek MC, et al. , Muscle strength response to strength training is influenced by insulin-like growth factor 1 genotype in older adults. J Appl Physiol (1985), 2005. 98(6): p. 2147–54. [DOI] [PubMed] [Google Scholar]
- 10.Hubal MJ, et al. , Variability in muscle size and strength gain after unilateral resistance training. Med Sci Sports Exerc, 2005. 37(6): p. 964–72. [PubMed] [Google Scholar]
- 11.Lavin KM, et al. , The Importance of Resistance Exercise Training to Combat Neuromuscular Aging. Physiology (Bethesda), 2019. 34(2): p. 112–122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Burley SD, et al. , Positive, limited and negative responders: The variability in physical fitness adaptation to basic military training. J Sci Med Sport, 2018. 21(11): p. 1168–1172. [DOI] [PubMed] [Google Scholar]
- 13.Chmelo EA, et al. , Heterogeneity of physical function responses to exercise training in older adults. J Am Geriatr Soc, 2015. 63(3): p. 462–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ahtiainen JP, et al. , Heterogeneity in resistance training-induced muscle strength and mass responses in men and women of different ages. Age (Dordr), 2016. 38(1): p. 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Camera DM, Smiles WJ, and Hawley JA, Exercise-induced skeletal muscle signaling pathways and human athletic performance. Free Radic Biol Med, 2016. 98: p. 131–143. [DOI] [PubMed] [Google Scholar]
- 16.Camera DM, Anabolic Heterogeneity Following Resistance Training: A Role for Circadian Rhythm? Front Physiol, 2018. 9: p. 569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sparks LM, Exercise training response heterogeneity: physiological and molecular insights. Diabetologia, 2017. 60(12): p. 2329–2336. [DOI] [PubMed] [Google Scholar]
- 18.Marcus RL, Addison O, and LaStayo PC, Intramuscular adipose tissue attenuates gains in muscle quality in older adults at high risk for falling. A brief report. J Nutr Health Aging, 2013. 17(3): p. 215–8. [DOI] [PubMed] [Google Scholar]
- 19.Ivey FM, et al. , Effects of strength training and detraining on muscle quality: age and gender comparisons. J Gerontol A Biol Sci Med Sci, 2000. 55(3): p. B152–7; discussion B158–9. [DOI] [PubMed] [Google Scholar]
- 20.Manini TM, et al. , Knee extension strength cutpoints for maintaining mobility. J Am Geriatr Soc, 2007. 55(3): p. 451–7. [DOI] [PubMed] [Google Scholar]
- 21.Cawthon PM, et al. , Putative Cut-Points in Sarcopenia Components and Incident Adverse Health Outcomes: An SDOC Analysis. J Am Geriatr Soc, 2020. 68(7): p. 1429–1437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.McGrath R, et al. , Decreased Handgrip Strength is Associated With Impairments in Each Autonomous Living Task for Aging Adults in the United States. J Frailty Aging, 2019. 8(3): p. 141–145. [DOI] [PubMed] [Google Scholar]
- 23.Duchowny K, Do Nationally Representative Cutpoints for Clinical Muscle Weakness Predict Mortality? Results From 9 Years of Follow-up in the Health and Retirement Study. J Gerontol A Biol Sci Med Sci, 2019. 74(7): p. 1070–1075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Leong DP, et al. , Prognostic value of grip strength: findings from the Prospective Urban Rural Epidemiology (PURE) study. Lancet, 2015. 386(9990): p. 266–73. [DOI] [PubMed] [Google Scholar]
- 25.Syddall H, et al. , Is grip strength a useful single marker of frailty? Age Ageing, 2003. 32(6): p. 650–6. [DOI] [PubMed] [Google Scholar]
- 26.Russ DW, et al. , Evolving concepts on the age-related changes in “muscle quality”. J Cachexia Sarcopenia Muscle, 2012. 3(2): p. 95–109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Clark BC, et al. , Voluntary vs Electrically Stimulated Activation in Age-Related Muscle Weakness. JAMA Netw Open, 2019. 2(9): p. e1912052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wages NP, et al. , Relative contribution of muscle strength, lean mass, and lower extremity motor function in explaining between-person variance in mobility in older adults. BMC Geriatr, 2020. 20(1): p. 255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Riwniak C, et al. , Comparison of a multi-component physical function battery to usual walking speed for assessing lower extremity function and mobility limitation in older adults. J Nutr Health Aging, In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Moskowitz S, et al. , Is impaired dopaminergic function associated with mobility capacity in older adults? Geroscience, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Clark LA, et al. , Reduced Neural Excitability and Activation Contribute to Clinically Meaningful Weakness in Older Adults. J Gerontol A Biol Sci Med Sci, 2021. 76(4): p. 692–702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tavoian D, et al. , A Randomized Clinical Trial Comparing Three Different Exercise Strategies for Optimizing Aerobic Capacity and Skeletal Muscle Performance in Older Adults: Protocol for the DART Study. Front Med (Lausanne), 2019. 6: p. 236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Russ DW and Binder-Macleod SA, Variable-frequency trains offset low-frequency fatigue in human skeletal muscle. Muscle Nerve, 1999. 22(7): p. 874–82. [DOI] [PubMed] [Google Scholar]
- 34.Westerblad H, Duty S, and Allen DG, Intracellular calcium concentration during low-frequency fatigue in isolated single fibers of mouse skeletal muscle. J Appl Physiol (1985), 1993. 75(1): p. 382–8. [DOI] [PubMed] [Google Scholar]
- 35.Westerblad H, et al. , Functional significance of Ca2+ in long-lasting fatigue of skeletal muscle. Eur J Appl Physiol, 2000. 83(2–3): p. 166–74. [DOI] [PubMed] [Google Scholar]
- 36.Tanner BC, Daniel TL, and Regnier M, Filament compliance influences cooperative activation of thin filaments and the dynamics of force production in skeletal muscle. PLoS Comput Biol, 2012. 8(5): p. e1002506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Guralnik JM, et al. , A short physical performance battery assessing lower extremity function: association with self-reported disability and prediction of mortality and nursing home admission. J Gerontol, 1994. 49(2): p. M85–94. [DOI] [PubMed] [Google Scholar]
- 38.Tavoian D, et al. , Changes in DXA-derived lean mass and MRI-derived cross-sectional area of the thigh are modestly associated. Sci Rep, 2019. 9(1): p. 10028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Corbett DB, et al. , Evaluating Walking Intensity with Hip-Worn Accelerometers in Elders. Med Sci Sports Exerc, 2016. 48(11): p. 2216–2221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Charlson ME, et al. , A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis, 1987. 40(5): p. 373–83. [DOI] [PubMed] [Google Scholar]
- 41.Lewinsohn PM, et al. , Center for Epidemiologic Studies Depression Scale (CES-D) as a screening instrument for depression among community-residing older adults. Psychol Aging, 1997. 12(2): p. 277–87. [DOI] [PubMed] [Google Scholar]
- 42.Powell LE and Myers AM, The Activities-specific Balance Confidence (ABC) Scale. J Gerontol A Biol Sci Med Sci, 1995. 50A(1): p. M28–34. [DOI] [PubMed] [Google Scholar]
- 43.Stalvey B, et al. , The life space questionnaire: a measure of the extent of mobility of older adults. J Appl Gerontol, 1999. 18: p. 479–498. [Google Scholar]
- 44.Keller S, et al. , Validity of the brief pain inventory for use in documenting the outcomes of patients with noncancer pain. Clin J Pain, 2004. 20(5): p. 309–18. [DOI] [PubMed] [Google Scholar]
- 45.Slepian PM, et al. , Development and Initial Validation of the Pain Resilience Scale. J Pain, 2016. 17(4): p. 462–72. [DOI] [PubMed] [Google Scholar]
- 46.Sullivan MJL, Bishop SR, and Pivik J, The Pain Catastrophizing Scale: Development and validation. Psychol Assess, 1995. 7(4): p. 524–532. [Google Scholar]
- 47.Clark LA, et al. , Reduced neural excitability and activation contribute to clinically-meaningful weakness in older adults. J Gerontol A Biol Sci Med Sci, In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hunter SK, Thompson MW, and Adams RD, Relationships among age-associated strength changes and physical activity level, limb dominance, and muscle group in women. J Gerontol A Biol Sci Med Sci, 2000. 55(6): p. B264–73. [DOI] [PubMed] [Google Scholar]
- 49.Neame R, et al. , Distribution of radiographic osteoarthritis between the right and left hands, hips, and knees. Arthritis Rheum, 2004. 50(5): p. 1487–94. [DOI] [PubMed] [Google Scholar]
- 50.Adams GR, et al. , Mapping of electrical muscle stimulation using MRI. J Appl Physiol (1985), 1993. 74(2): p. 532–7. [DOI] [PubMed] [Google Scholar]
- 51.Rodriguez-Falces J, Maffiuletti NA, and Place N, Spatial distribution of motor units recruited during electrical stimulation of the quadriceps muscle versus the femoral nerve. Muscle Nerve, 2013. 48(5): p. 752–61. [DOI] [PubMed] [Google Scholar]
- 52.Russ DW, et al. , Development of a Neuromuscular Electrical Stimulation Protocol for Sprint Training. Med Sci Sports Exerc, 2012. 44(9): p. 1810–1819. [DOI] [PubMed] [Google Scholar]
- 53.Bakker AJ, et al. , Doublet stimulation increases Ca(2+) binding to troponin C to ensure rapid force development in skeletal muscle. J Gen Physiol, 2017. 149(3): p. 323–334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Chang YJ and Shields RK, Doublet electrical stimulation enhances torque production in people with spinal cord injury. Neurorehabil Neural Repair, 2011. 25(5): p. 423–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Duchateau J and Hainaut K, Nonlinear summation of contractions in striated muscle. II. Potentiation of intracellular Ca2+ movements in single barnacle muscle fibres. J Muscle Res Cell Motil, 1986. 7(1): p. 18–24. [DOI] [PubMed] [Google Scholar]
- 56.Binder-Macleod SA and Russ DW, Effects of activation frequency and force on low-frequency fatigue in human skeletal muscle. J Appl Physiol (1985), 1999. 86(4): p. 1337–46. [DOI] [PubMed] [Google Scholar]
- 57.Hangartner TN, et al. , The Official Positions of the International Society for Clinical Densitometry: acquisition of dual-energy X-ray absorptiometry body composition and considerations regarding analysis and repeatability of measures. J Clin Densitom, 2013. 16(4): p. 520–36. [DOI] [PubMed] [Google Scholar]
- 58.Kumar V, et al. , Age-related differences in the dose-response relationship of muscle protein synthesis to resistance exercise in young and old men. J Physiol, 2009. 587(1): p. 211–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Greig CA, et al. , Blunting of adaptive responses to resistance exercise training in women over 75y. Exp Gerontol, 2011. 46(11): p. 884–90. [DOI] [PubMed] [Google Scholar]
- 60.Hakkinen K, et al. , Changes in agonist-antagonist EMG, muscle CSA, and force during strength training in middle-aged and older people. J Appl Physiol (1985), 1998. 84(4): p. 1341–9. [DOI] [PubMed] [Google Scholar]
- 61.Raue U, et al. , Improvements in whole muscle and myocellular function are limited with high-intensity resistance training in octogenarian women. J Appl Physiol (1985), 2009. 106(5): p. 1611–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Welle S, Totterman S, and Thornton C, Effect of age on muscle hypertrophy induced by resistance training. J Gerontol A Biol Sci Med Sci, 1996. 51(6): p. M270–5. [DOI] [PubMed] [Google Scholar]
- 63.Lesnak JB, et al. , Ability of Isokinetic Dynamometer to Predict Isotonic Knee Extension 1-Repetition Maximum. J Sport Rehabil, 2020. 29(5): p. 616–620. [DOI] [PubMed] [Google Scholar]
- 64.Nunes JP, et al. , The Generality of Strength: Relationship between Different Measures of Muscular Strength in Older Women. Int J Exerc Sci, 2020. 13(3): p. 1638–1649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Cadore EL, et al. , Muscle conduction velocity, strength, neural activity, and morphological changes after eccentric and concentric training. Scand J Med Sci Sports, 2014. 24(5): p. e343–52. [DOI] [PubMed] [Google Scholar]
- 66.Coratella G, et al. , Sex-Related Responses to Eccentric-Only Resistance Training in Knee-Extensors Muscle Strength and Architecture. Res Q Exerc Sport, 2018. 89(3): p. 347–353. [DOI] [PubMed] [Google Scholar]
- 67.Jessee MB, et al. , Muscle Adaptations to High-Load Training and Very Low-Load Training With and Without Blood Flow Restriction. Front Physiol, 2018. 9: p. 1448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Heisterberg MF, et al. , Losartan has no additive effect on the response to heavy-resistance exercise in human elderly skeletal muscle. J Appl Physiol (1985), 2018. 125(5): p. 1536–1554. [DOI] [PubMed] [Google Scholar]
- 69.Da Boit M, et al. , Sex differences in the effect of fish-oil supplementation on the adaptive response to resistance exercise training in older people: a randomized controlled trial. Am J Clin Nutr, 2017. 105(1): p. 151–158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Kraemer WJ, Ratamess NA, and French DN, Resistance training for health and performance. Curr Sports Med Rep, 2002. 1(3): p. 165–71. [DOI] [PubMed] [Google Scholar]
- 71.Erskine RM, et al. , Inter-individual variability in the adaptation of human muscle specific tension to progressive resistance training. Eur J Appl Physiol, 2010. 110(6): p. 1117–25. [DOI] [PubMed] [Google Scholar]
- 72.Reeves ND, Narici MV, and Maganaris CN, Effect of resistance training on skeletal muscle-specific force in elderly humans. J Appl Physiol (1985), 2004. 96(3): p. 885–92. [DOI] [PubMed] [Google Scholar]
- 73.Delbono O, Expression and Regulation of Excitation-Contraction Coupling Proteins in Aging Skeletal Muscle. Curr Aging Sci, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Trappe S, et al. , Resistance training improves single muscle fiber contractile function in older women. Am J Physiol Cell Physiol, 2001. 281(2): p. C398–406. [DOI] [PubMed] [Google Scholar]
- 75.Trappe S, et al. , Effect of resistance training on single muscle fiber contractile function in older men. J Appl Physiol (1985), 2000. 89(1): p. 143–52. [DOI] [PubMed] [Google Scholar]
- 76.Aas SN, et al. , Musculoskeletal adaptations to strength training in frail elderly: a matter of quantity or quality? J Cachexia Sarcopenia Muscle, 2020. 11(3): p. 663–677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Harber MP, et al. , Aerobic exercise training induces skeletal muscle hypertrophy and age-dependent adaptations in myofiber function in young and older men. J Appl Physiol (1985), 2012. 113(9): p. 1495–504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Moreillon M, et al. , Hybrid fiber alterations in exercising seniors suggest contribution to fast-to-slow muscle fiber shift. J Cachexia Sarcopenia Muscle, 2019. 10(3): p. 687–695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Goodman C, Patterson M, and Stephenson G, MHC-based fiber type and E-C coupling characteristics in mechanically skinned muscle fibers of the rat. Am J Physiol Cell Physiol, 2003. 284(6): p. C1448–59. [DOI] [PubMed] [Google Scholar]
- 80.Englund DA, et al. , Resistance training performed at distinct angular velocities elicits velocity-specific alterations in muscle strength and mobility status in older adults. Exp Gerontol, 2017. 91: p. 51–56. [DOI] [PubMed] [Google Scholar]
- 81.Aas SN, et al. , Strength training and protein supplementation improve muscle mass, strength, and function in mobility-limited older adults: a randomized controlled trial. Aging Clin Exp Res, 2020. 32(4): p. 605–616. [DOI] [PubMed] [Google Scholar]
- 82.Erskine RM, Fletcher G, and Folland JP, The contribution of muscle hypertrophy to strength changes following resistance training. Eur J Appl Physiol, 2014. 114(6): p. 1239–49. [DOI] [PubMed] [Google Scholar]
- 83.Balshaw TG, et al. , Changes in agonist neural drive, hypertrophy and pre-training strength all contribute to the individual strength gains after resistance training. Eur J Appl Physiol, 2017. 117(4): p. 631–640. [DOI] [PubMed] [Google Scholar]
- 84.Clark BC, et al. , Comment on: “Pitfalls in the measurement of muscle mass: a need for a reference standard” by Buckinx et al. J Cachexia Sarcopenia Muscle, 2018. 9(7): p. 1269–1271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Cawthon PM, et al. , Strong Relation Between Muscle Mass Determined by D3-creatine Dilution, Physical Performance, and Incidence of Falls and Mobility Limitations in a Prospective Cohort of Older Men. J Gerontol A Biol Sci Med Sci, 2019. 74(6): p. 844–852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Evans WJ, et al. , D3 -Creatine dilution and the importance of accuracy in the assessment of skeletal muscle mass. J Cachexia Sarcopenia Muscle, 2019. 10(1): p. 14–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Moritani T and deVries HA, Neural factors versus hypertrophy in the time course of muscle strength gain. Am J Phys Med, 1979. 58(3): p. 115–30. [PubMed] [Google Scholar]
- 88.Sale DG, Neural adaptation to resistance training. Med Sci Sports Exerc, 1988. 20(5 Suppl): p. S135–45. [DOI] [PubMed] [Google Scholar]
- 89.Folland JP and Williams AG, The adaptations to strength training : morphological and neurological contributions to increased strength. Sports Med, 2007. 37(2): p. 145–68. [DOI] [PubMed] [Google Scholar]
- 90.Adkins DL, et al. , Motor training induces experience-specific patterns of plasticity across motor cortex and spinal cord. J Appl Physiol (1985), 2006. 101(6): p. 1776–82. [DOI] [PubMed] [Google Scholar]
- 91.Unhjem R, et al. , External Resistance Is Imperative for Training-Induced Efferent Neural Drive Enhancement in Older Adults. J Gerontol A Biol Sci Med Sci, 2021. 76(2): p. 224–232. [DOI] [PubMed] [Google Scholar]
- 92.Amrhein V, Greenland S, and McShane B, Scientists rise up against statistical significance. Nature, 2019. 567(7748): p. 305–307. [DOI] [PubMed] [Google Scholar]
- 93.Somers TJ, et al. , Pain catastrophizing and pain-related fear in osteoarthritis patients: relationships to pain and disability. J Pain Symptom Manage, 2009. 37(5): p. 863–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Zhaoyang R, Martire LM, and Darnall BD, Daily pain catastrophizing predicts less physical activity and more sedentary behavior in older adults with osteoarthritis. Pain, 2020. 161(11): p. 2603–2610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Antoniak AE and Greig CA, The effect of combined resistance exercise training and vitamin D3 supplementation on musculoskeletal health and function in older adults: a systematic review and meta-analysis. BMJ Open, 2017. 7(7): p. e014619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Reeves ND, Narici MV, and Maganaris CN, Musculoskeletal adaptations to resistance training in old age. Man Ther, 2006. 11(3): p. 192–6. [DOI] [PubMed] [Google Scholar]
- 97.Franchi MV, et al. , Architectural, functional and molecular responses to concentric and eccentric loading in human skeletal muscle. Acta Physiol (Oxf), 2014. 210(3): p. 642–54. [DOI] [PubMed] [Google Scholar]


