Abstract
Many depressed individuals experience difficulties in executive functioning that contribute substantially to functional impairment. It is unknown whether a subtype of depression characterized by chronic inflammation is differentially associated with worse executive functioning. This study examined whether the combination of depression and higher C reactive protein (CRP) is differentially associated with worse executive functioning and whether this association is stronger in older adults. This cross-sectional study analyzed data collected from population-representative sample of 43,896 adults aged 44.13 years (SD = 13.52) who participated in the baseline assessment of a cohort study (LifeLines). A multivariate regression model tested whether depressed individuals (established via structured interview) exhibiting higher levels of inflammation (indexed via high-sensitivity CRP assay following an overnight fast) performed worse on a behavioral test of executive functioning. Depression (B = −3.66, 95% CI: −4.82,−2.49, p <.001) and higher log-transformed CRP (B = −0.67, 95% CI: −0.87,−0.47, p <.001) were associated with worse executive functioning, after adjustment for age, sex, educational attainment, body mass index, smoking status, exposure to stressful life events and chronic stressors, sedentary behavior, and number of chronic medical conditions. Depressed individuals with higher log-transformed CRP exhibited differentially poorer executive functioning (B = −1.09, 95% CI: −2.07,−0.11, p <.001). This association did not differ based on age (B = 0.01, 95% CI: −0.08, 0.10, p =.82). Executive functioning is poorer in depressed individuals with higher CRP, even in early adulthood. Interventions that reduce inflammation may improve cognitive functioning in depression.
1.1. Introduction
Depression is characterized by an early onset, recurrent course, and high prevalence – all factors contributing to its severe disease burden.1–3 Although the cardinal symptoms of depression are low mood and anhedonia,4 it is typically accompanied by a wide range of symptoms, including cognitive dysfunction. In fact, 227 unique symptom profiles exist by which an individual meets criteria for a depression diagnosis5 and this heterogeneous presentation suggests that multiple subtypes of depression may exist that are characterized by distinct etiologies, risk factors, and disrupted neurobiological systems.6 There is considerable evidence that one depressive subtype may be characterized by a dysregulated immune system.7, 8
The immune system’s rapid and non-specific response to antigens (innate immune system) and the slower, antibody-generating, specific response (adaptive immune system) are dysregulated in depression.9 Research has predominantly focused on the role of the innate immune system in depression because it is known to induce “sickness behaviors” (e.g., anhedonia, fatigue) reminiscent of depression,10 although there is growing appreciation that multiple inflammatory subtypes may exist.11 Activation of the innate immune response, whether through administration of a potent inflammatory cytokine (interferon-α), a purified endotoxin, or vaccination, reliably induce depression.10, 12 Further, depressed individuals exhibit higher inflammatory biomarkers that index activation of the innate immune system, such as interleukin-6 (IL-6) and C-reactive protein (CRP).13 and both IL-6 and CRP prospectively predict future depressive symptoms in observational studies (although the reverse also is observed).14 A subgroup of depressed individuals (25–30%) display higher inflammatory biomarkers and, additionally, respond to medication designed to dampen inflammatory activity.15, 16 These studies provide compelling evidence that an inflammatory subtype of depression exists.
A subset of depressed individuals also exhibit cognitive dysfunction (particularly in episodic memory, attention, and executive functions).17 which is observable at first onset of depression,18, 19 when depression is in remission,17, 20 and is more severe following repeated depressive episodes.21 In addition, cognitive dysfunction in late-onset depression is associated with more pronounced deficits in verbal memory, processing speed, and executive functioning when compared to early onset depression.22 The underlying mechanism(s) by which cognitive functioning is disrupted in depression is unknown; however, inflammation has been hypothesized to play a causal role.23 Chronic inflammation disrupts neuronal processes (e.g., synaptic plasticity/neurogenesis) and affects brain regions and their respective cognitive associates (e.g., hippocampus: episodic memory; dorsolateral prefrontal cortex/anterior cingulate cortex: executive function) – thereby linking inflammation with abnormal brain structure and function associated with both depression and cognitive dysfunction.23, 24 Inflammation is associated with impaired cognition in medical disorders25–27 and in population-based samples.28–31 It also is associated with worse cognitive functioning (primarily psychomotor speed/executive functioning) in depressed groups and non-depressed controls32–35 as well as in community samples of both depressed and non-depressed youth/youth with more or less severe depressive symptoms.36, 37 However, despite this evidence, results typically involve small, clinical samples of depressed adults and rarely included a wide age range.
Older depressed individuals with heightened inflammation may experience worse deficits in cognitive functioning. Although the prevalence of depression is lower in older adults, its occurrence is still common38 and older individuals are at greater risk of experiencing repeated depressive episodes across the lifespan, which is associated with lower hippocampal and prefrontal cortex volumes39–41 as well as worse episodic memory, processing speed, and executive functioning.42 Moreover, many older individuals experience “inflammageing”, a process characterized by higher, basal levels of circulating inflammatory cytokines,43 which in turn is associated with cognitive impairment and dementia in older individuals.44 Indeed, the association of CRP with future depressive symptoms is stronger in older samples.14 Together these findings suggest that the relationship between depression, inflammation and cognitive functioning may become stronger with age, but no study has investigated this across the lifespan.
As there is strong evidence that i) a subset of depressed individuals exhibit higher inflammatory biomarkers, ii) a subset of depressed individuals exhibit cognitive dysfunction, and iii) higher inflammatory biomarkers are associated with worse cognitive functioning, the current study examines whether it is the depressed individuals who exhibit higher values on an inflammatory biomarker (CRP) that perform worse on a test of executive functioning, above and beyond individuals with either depression or higher CRP alone. Moreover, the study will examine whether these associations become stronger in older adults, given the increase in depression, cognitive dysfunction, and ‘inflammaging’ later in life. Data were drawn from the first wave of a prospective, population-based, Dutch, cohort study of 152,728 adults. We tested whether individuals with higher CRP and a current depression diagnosis (major depression or dysthymia assessed via a structured diagnostic interview) performed worse on a behavioral measure of executive functioning when controlling for important confounds, such as sex, adiposity, stress, and substance use. Specifically, we hypothesized that:
Hypothesis One:
Depressed individuals who exhibit higher CRP values will perform worse on a test of executive functioning than individuals with either current depression or higher CRP alone, after adjustment for relevant covariates.
Hypothesis Two:
Older individuals who meet criteria for depression and exhibit higher CRP values will perform worse on a test of executive functioning than individuals who are younger, meet criteria for depression, or exhibit higher CRP alone, after adjustment for relevant covariates.
2.1. Materials and Methods
2.2. Participants
Data were drawn from a 152,728 adults aged 18–93 years who participated in a multi-disciplinary, prospective, population-based cohort study (LifeLines Study) investigating the biological, behavioral, and environmental determinants of health. Between 2006 and 2013, 167,782 participants living in the three northern provinces of The Netherlands (Friesland, Groningen and Drenthe) were recruited for a baseline assessment through the practices of the general practitioner (GP), of whom 152,728 were aged 18 years or older. All inhabitants in The Netherlands are registered with a general practitioner and 73% of invited GPs (n = 562/812) agreed to take part. Initially, GP practices invited patients aged between 25 and 50 years to participate, unless the participating GP considered the patient ineligible based on severe psychiatric or physical illness, limited life expectancy (<5 years), or insufficient knowledge of the Dutch language to complete a Dutch questionnaire. From 333,307 potential participants who were contacted via mail, 81,652 completed a consent form. Following consent, participants received a baseline questionnaire as well as an invitation to complete a comprehensive health assessment at a LifeLines research site. Subsequently, participants were asked to indicate whether their family members (e.g., partners, parents, parents-in-law, children etc.) would be willing to participate in the study. An additional 64,489 participants (38%) were recruited via participating family members and a final 21,588 participants (13%) self-registered on the LifeLines website. Further information on study design, recruitment, and participants has been published.45
From the 152,728 adults assessed at baseline, the current study utilized data from a sub-set of individuals who had completed a behavioral assessment of executive functioning (n = 87,567; 57.34%), a diagnostic interview assessing mental health (n = 146,614; 96.00%), and who had completed a blood draw (n = 56,849; 37.22%). From the 47,017 individuals who had data on all three measures, we excluded a further 3,121 (6.63%) individuals who reported a medical diagnosis (it should be noted that certain individuals possessed comorbid conditions) characterized by (i) chronic alterations in immune functioning (Diabetes: 1,924; Rheumatoid Arthritis 2; Crohn’s Disease: 134; Blood Clotting Disorder: 254; Multiple Sclerosis: 100), (ii) cognitive dysfunction (Epilepsy: 535; Stroke: 254; Dementia: 3; Schizophrenia: 40), or disrupted hepatic functioning (Hepatitis: 469; Liver Cirrhosis: 33) so that we could exclude the possibility that observed associations were attributable to individuals with these chronic conditions or that changes in CRP values or cognitive functioning were attributable primarily to underlying medical/psychiatric/neurological conditions. Exclusion criteria were broadly based on published recommendations.46, 47 From the remaining 43,896 participants, a small number of individuals had missing data on specific variables (e.g., educational attainment was missing in 505 individuals) and the minimum N for primary analyses was 42,222. Missing data analyses are presented in detail as supplementary information and meaningfully differences were not observed, although statistically significant differences were observed when comparing the analytic sample with the excluded sample. Within the analytic sample, 17,482 individuals were not related to other participants, 4,704 had one other relative included in the study, 1,772 had two or more relatives included, 809 had four or more relatives included, 408 had five or more relatives included, 229 had six or more relatives included, 148 had seven or more relatives included, and the remaining 3% had eight or more relatives included in the study.
2.3. Measures
2.3.1. Depressive Disorder
The Mini International Neuropsychiatric Interview (MINI) is a brief structured interview designed to screen for anxiety and depressive disorders.48 Lifelines used an adapted version of a Dutch translation of the MINI that was administered by trained interviewers – details on the version used in LifeLines has been previously published.49 Participants were considered to meet criteria for a depressive disorder if they met DSM-IV criteria for major depression or dysthymia at the time of the interview: namely endorsing at least five of nine depressive symptoms, with at least one symptoms consisting of sadness or anhedonia, over the last two weeks. Impairment was assessed in the MINI for dysthymia but not depression and consequently, impairment was not used as a criterion for major depression. The MINI has demonstrable reliability and validity.50
2.3.2. Executive functioning
The Ruff Figural Fluency Test (RFFT) is a reliable and valid51 executive functioning measure that primarily assesses figural fluency, although performance also is likely reliant on other executive functions.52 Respondents are asked to draw as many unique designs as possible within 60 seconds by connecting dots in different patterns. Dots are presented in an array of five-dot patterns arranged in five columns and seven rows; the arrangement of five-dot patterns dots is the same on each array. Participants complete five trials, with each trial either using different distractors or different patterns. The total number of unique designs was used as the dependent variable in the analyses, in line with previous LifeLine studies.53 In LifeLines, the RFFT was administered to all participants until April, 2012, and subsequently in a random half of the sample. Data from participants who failed to generate a single unique design per trial (n = 181) were removed.
2.3.3. C-Reactive Protein
Blood samples were obtained from participants before 10AM via venipuncture following an overnight fast. Complete details on blood specimen data collection are outlined in a methodological paper describing the Lifelines Cohort.45 C-Reactive Protein was quantified using three separate methods over the course of baseline assessment [(84.58% of total CRP values; assessed in serum; CardioPhase hsCRP, Siemens Healthcare Diagnostics, Marburg, Germany), (12.90% of total CRP values; assessed in plasma; CardioPhase hsCRP, Siemens Healthcare Diagnostics, Marburg, Germany), and (2.52% of total CRP values; assessed in plasma; CRPL3, Roche Diagnostics, Mannheim, Germany)]. Assay method 2 and 3 were identical and only differed in terms of the manufacturer. A conversion formula (new = 0.92 x old - 0.01) was derived from an internal validation using 39 samples, according to the AMC (alternative method comparison, Deming Regression) protocol in order that Method 1 could be compared with Method 2 and 3. For CardioPhase hsCRP, the intra-assay coefficient of variability was 3.45% and the inter-assay coefficient of variability was 3.15%. For CRPL3, the intra-assay coefficient of variability was 4.15% and the inter-assay coefficient of variability was 5.8%.
2.3.4. Covariates
Age, sex, and educational attainment were reported by participants. Educational attainment was determined using a single-item question and categorized as: low [no education, primary education, lower/preparatory vocational education, lower general secondary education (leaving secondary school aged >16 years)], ‘moderate’ (intermediate vocational education/apprenticeship, higher secondary education), and ‘high’ (higher vocational education, university).
Height was measured to the closest 0.1 cm and body weight was measured without shoes to 0.1 kg precision to estimate body mass index (BMI). Smokers were identified as individuals who have smoked over the last month. The List of Threatening Experiences (LTE) assesses (Yes/No) whether participants have experienced 12 major categories of stressful life events 54. The Long-term Difficulties Inventory (LDI) is a self-report questionnaire assessing chronic stress and evaluates whether individuals experience aspects of life (e.g., finances/social relationships) with difficulty/stress using a three-point scale (not stressful/slightly stressful/very stressful) 55. Sedentary behavior was estimated as the average number of minutes spent watching television, with impossible values (i.e., >1,440 minutes, n = 36) removed. A composite measure was created counting the number of chronic medical conditions participants reported. Participants reported by questionnaire whether they were diagnosed with any of the following conditions: cardiovascular disease (n = 3,495; e.g., aortic aneurysm, arrhythmia, heart attack), chronic obstructive pulmonary disease (n = 2,255), asthma (n = 3,635), fibromyalgia (n = 1), or irritable bowel syndrome (n = 4,134). Of 43,896 participants, 8,823 individuals reported having one condition, 1,858 endorsed two conditions, and 315 endorsed three or more conditions with 30 individuals reporting four conditions and three individuals reported five conditions; due to the small number of individuals with more than three conditions, this variable was scored as: no conditions, one condition, two conditions, or three or more conditions.
2.4. Analyses
Analyses were conducted in R 3.5.2.56 Multivariable regression models were estimated using ‘lmer4’57 and graphed using ‘ggplot2’.58 CRP was log-transformed to impose a normal distribution and continuous predictor variables were mean-centered. Four iterative models were tested: Model 1 included depression diagnosis, CRP and demographic variables; Model 2 included Model 1 variables and added health-related variables; Model 3 added an interaction term (depression diagnosis x log-transformed CRP) to the variables included in Model 2. This model tested hypothesis 1 that, after adjustment for known relevant covariates (incorporated in models 1 and 2), the combination of being depressed and high CRP values would be associated with poorer executive functioning than their separate associations. In model 4, we also included a three-way interaction term (in addition to lower-order interaction terms) to test whether a potential interaction of depression diagnosis and log-transformed CRP differed by age. This model tested that the hypothesized association of hypothesis 1 would be stronger in older than younger respondent. CRP values greater than 10mg/L were retained in analyses because there is accumulating evidence that 10mg/L is not a sensitive cut-off of acute illness or injury;59 however, sensitivity analyses evaluated whether removing these values substantially influenced results. Results were replicated (i) when missing data for covariates were imputed using the ‘mice’ package60 and (ii) including individuals with medical/psychiatric conditions that were removed from the analytic sample. Full details on the multiple imputation methodology are outlined in the ‘mice’ package documentation.60 Random intercept hierarchical linear modelling was additionally performed using the ‘lmer’ package to estimate whether parameters changed when clusters of observation were nested within families (e.g., children, parents, grandparents, sons, siblings, grandparents, aunts, uncles, cousins, etc.). The threshold for statistical significance was set so alpha is equal to .05 and hypothesis tests were two-sided. Unstandardized beta coefficients were reported given that the metric of the dependent variable is inherently meaningful and intuitive.
3.1. Results
The characteristics of the sample are described in Table 1. Due to the large sample size, almost all correlations between the study variables meet the criteria for statistical significance (Table 2). Notably, poorer executive functioning was associated with depression and higher CRP values. Lower levels of education were more substantially associated with poorer executive functioning, as were older age, more chronic stress, and higher levels of sedentary behaviors. Depression was most substantially associated with experience of stressful life events and chronic stress. Higher CRP values were most substantially associated with lower educational attainment, being female, higher levels of sedentary behavior, and depression.
Table 1.
Sample Characteristics
| Measures | Analytic Sample (n = 43,896) |
|---|---|
| Age [Mean (SD)] | 44.13 (13.52) |
| Sex (% Female) | 58% |
| Education (N %) | |
| - Lower | 13,406 (31%) |
| - Moderate | 17,296 (40%) |
| - Higher | 12,666 (29%) |
| RFFT (Unique designs) | 81.81 (26.04) |
| Depression diagnosis, Major Depression/Dysthymia, Current | 3% |
| C-reactive protein (mg/L), [Median (Interquartile range); Mean (SD)] | 1.2 (.60, 2.80); 2.58 (4.70) |
| Body mass index | 26.11 (4.55) |
| Stressful life events (LTE) | 1.03 (1.27) |
| Chronic stress (LDI) | 2.45 (2.50) |
| Sedentary behavior [minutes spent watching television; Mean (SD)] | 145.1 (87.58) |
| Chronic medical conditions | .30 (.48) |
Lower = no education, primary education, lower/preparatory vocational education, lower general secondary education; Moderate = intermediate vocational education/apprenticeship, higher secondary education; Higher = higher vocational education, university
Table 2.
Bivariate Correlations of Study Variables for 42,396 Participants
| Measure | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1. Executive functioning (RFFT) | −.04a | −.07a | .36a | −.32a | .03a | −.05a | .12a | −.16a | −.06a | −.10a | −.02a |
| 2. Depression diagnosis | - | .04a | −.07a | −.02a | .04a | .16a | .24a | .06a | .08a | .04a | .07a |
| 3. C-reactive protein (log-transformed) | - | −.10a | .01b | .14a | .05a | .02a | .12a | .06a | .36a | .07a | |
| 4. Educational attainment | - | −.23a | −.02a | −.08a | .10a | −.25a | −.05a | −.15a | −.11a | ||
| 5. Age | - | −.03a | .01 | −.25a | .13a | .07a | .16a | −.12a | |||
| 6. Sex | - | .02a | .10a | .03a | .07a | −.07a | −.04a | ||||
| 7. Stressful life events (LTE) | - | .33a | .08a | .10a | .06a | .10a | |||||
| 8. Chronic stress (LDI) | - | −.04a | .12a | .00 | .09a | ||||||
| 9. Sedentary behavior | - | .06a | .16a | .08a | |||||||
| 10. Chronic medical conditions | - | .06a | .01c | ||||||||
| 11. Body mass index | - | −.03a | |||||||||
| 12. Smoking Status | - |
Probability
= P <.001;
= P = .005;
= P = .01;
RFFT = Ruff Figural Fluency Test; LTE = List of Threatening Experiences; LDI = Long-term Difficulties Inventory
Regression Analyses
Multivariable regression analysis (Table 3) indicated that depression and higher CRP values were both associated with worse executive functioning when controlling for demographic characteristics (Model 1) and health-related variables (Model 2). The magnitude of the association was modest for both variables. In Model 3, depression interacted with CRP such that individuals with a depression diagnosis and higher CRP exhibited worse executive functioning, although again the magnitude of the association was modest – see Figure 1 for a visual depiction and see Supplementary Table 1 for standardized coefficients. Test of simple slopes were conducted in order to probe and report the magnitude of the difference between the depressed and non-depressed participants at different levels of CRP (−1 SD CRP = −2.11, t(42196) = −2.3, p =.02; Mean CRP = −3.45, t(42196) = −5.7, p < .001; +1 SD CRP = −4.79, t(42196) = −6.1, p < .001; +2 SD CRP = −6.14, t(42196) = −4.8, p < .001) – note that due to a floor (not detectable CRP values, values were not observed at 2 standard deviations below the mean). In an additional model that included a three-way interaction term (depression diagnosis x CRP x age, in addition to all relevant lower-order interactions), no interaction terms other than depression by CRP was significant. In sensitivity analyses where CRP values ≥10mg/L were excluded, results were comparable and the only notable difference was a small increase in the size of the interaction term (B = −1.80, SE = .58). No notable differences were observed when missing covariate data were handled using multivariate imputation for chained equations nor when individuals with medical/psychiatric conditions who were excluded from primary analyses were included in the analytic sample. In a random intercept hierarchical linear model when family is modelled as a random effect, no substantial differences in any of the parameter estimates were observed – see Supplementary Table 2 for complete information on parameter estimates.
Table 3.
Regression models reporting unstandardized coefficients standard errors, and confidence intervals predicting executive functioning estimated using the Ruff Figural Fluency Test (RFFT).
| Predictor Variables | Dependent variable: Ruff Figural Fluency Test (RFFT) |
|||||
|---|---|---|---|---|---|---|
| Model 1 | Model 2 | Model 3 | ||||
| Unstandardized beta coefficient (standard error) | 95% Confidence Interval | Unstandardized beta coefficient (standard error) | 95% Confidence Interval | Unstandardized beta coefficient (standard error) | 95% Confidence Interval | |
| Depression Diagnosis | −3.50(0.56)a | −4.59, −2.38 | −3.70(0.59)a | −4.83, −2.50 | −3.40(0.60)a | −4.63, −2.27 |
| C-reactive protein (log-transformed) | −0.78(0.09)a | −0.97, −0.60 | −0.67(0.10)a | −0.83, −0.43 | −0.63(0.10)a | −0.83, −0.43 |
| Age | −0.47(0.01)a | −0.48, −0.45 | ‒0.44(0.01)a | −0.46, −0.42 | −0.44(0.01)a | −0.46, −0.42 |
| Sex (Female) | 1.70(0.20)a | 1.29, 2.09 | 1.62(0.21)a | 1.22, 2.04 | 1.60(0.21)a | 1.22, 2.04 |
| Education attainment (Reference = Low) | ||||||
| Moderate | 8.50(0.25)a | 8.01, 8.98 | 7.90(0.25)a | 7.39, 8.37 | 7.90(0.25)a | 7.39, 8.37 |
| High | 18.00(0.26)a | 17.05, 18.09 | 16.00(0.28)a | 15.92, 17.01 | 16.00(0.28)a | 15.92, 17.01 |
| Body mass index | 0.02(0.03) | −0.03, 0.08 | 0.03(0.03) | −0.03, 0.08 | ||
| Stressful life events | −0.43(0.09)a | −0.60, −0.26 | −0.43(0.09)a | −0.60, −0.26 | ||
| Chronic stress | 0.46(0.05)a | 0.37, 0.56 | 0.46(0.05)a | 0.37, 0.56 | ||
| Sedentary behavior | −0.015(0.001)a | −0.017, −0.012 | −0.015(0.001)a | −0.017, −0.012 | ||
| Smoking status | −0.34(0.25) | −0.83, 0.14 | −0.34(0.25) | −0.83, 0.14 | ||
| Health-related medical conditions | −0.95(0.18)a | −1.30, −0.61 | −0.95(0.18)a | −1.30, −0.61 | ||
| Interaction (Depression diagnosis × C-reactive protein) | −1.10(0.50)b | −2.08, −0.12 | ||||
| Intercept | 73.00(0.22)a | 72.50, 73.38 | 74.00(0.24)a | 73.19, 74.15 | 74.00(0.24)a | 73.19, 74.15 |
Probability:
=P < .001
=P = 0.03.
Figure 1.
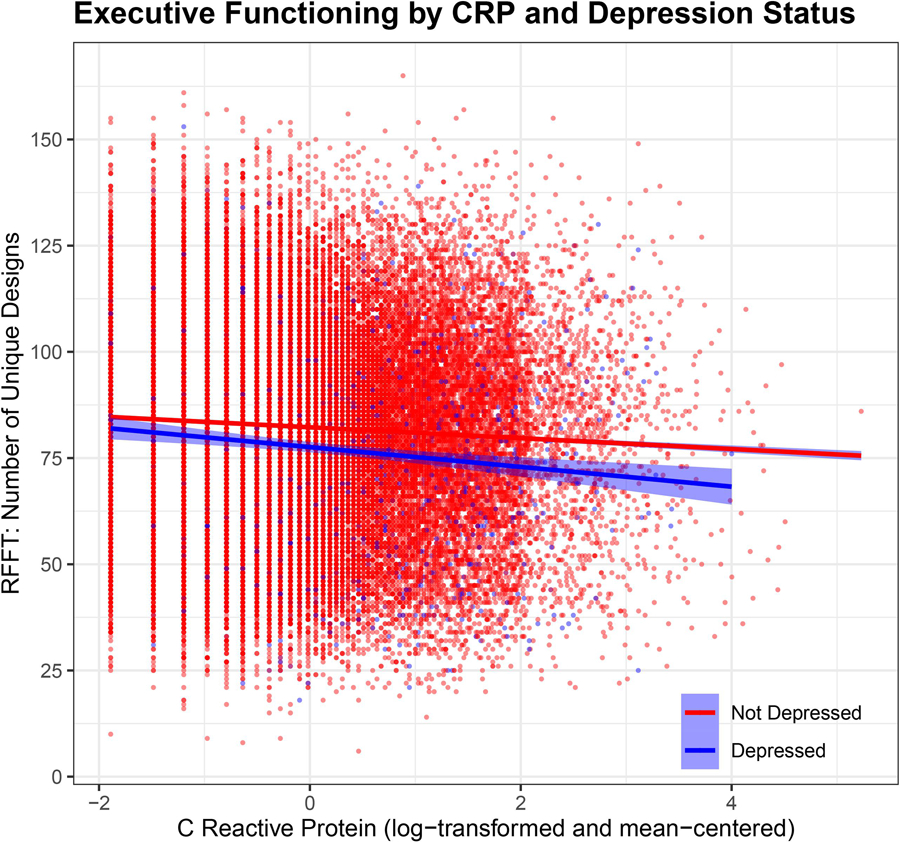
Interaction of Depression Status and Log-Transformed C-Reactive Protein Predicting Executive Functioning.
4.1. Discussion
This is the first study to investigate whether the combination of depression and higher C reactive protein is differentially associated with worse executive functioning across the lifespan. Based on a Dutch, population-representative sample of 43,896 people, not only was depression and higher CRP values associated with worse executive functioning, but those with depression and higher CRP values performed differentially worse, although the size of the cumulative association was small. Importantly, in this sample of adults aged 18 to 93 years, and therefore representing a broad spectrum of the human lifespan, there was no evidence that the cumulative association of depression and CRP on executive functioning was stronger later in life. These associations were independent of a broad range of demographic and health-related factors associated with depression, CRP, and executive functioning, and importantly, could be observed in the general population, as opposed to clinical studies which constitute the majority of previous studies.
This is the first study to demonstrate that depressed individuals with higher CRP performed worse on a behavioral assessment of executive functioning compared to those who were either depressed or exhibited higher CRP alone. These findings agree with prior clinical32–35 and population-based studies61 reporting that higher inflammatory biomarkers are associated with worse cognitive functioning in depressed individuals and in non-depressed controls or population-based samples. However, it goes further to suggest that the combination of depression and higher CRP amplify this deleterious association. These results cannot speak to whether cognitive deficits are generalized in nature or driven by deficits in specific cognitive functions, such as psychomotor speed.37, 62 However, it should be noted that the magnitude of the cumulative association was very small and should be considered alongside more substantial associations of demographic and somatic health-related variables. When considered as a whole, these results suggest that inflammatory processes are implicated in cognitive dysfunction in depression, but the relationship is unlikely to be unique to depression and further that other pathways exist that lead from depression to cognitive dysfunction.
There is compelling evidence that cognitive deficits are more pronounced in late-onset depression22 and inflammatory biomarkers also are associated with cognitive dysfunction in middle-aged and elderly samples, although there is substantial variability in the magnitude of observed associations and in the domains of cognitive impairment.28, 63, 64 The predominance of studies investigating inflammation in older samples reflects interest in the role of ‘inflammageing’ in senescence.43 Surprisingly, this study did not find evidence that the combination of depression and higher CRP were differentially associated with worse executive dysfunction later in life. Instead, these results contribute to a growing body of research suggesting that both depression19, 65 and inflammatory biomarkers37 are linked with worse cognitive functioning across the lifespan. Indeed, in a study of adolescents, BMI was prospectively associated with increases in depressive symptoms and decreases in executive functioning, with the association of BMI and executive functioning mediated by an inflammatory cytokine (interleukin-6).66 Further work is needed to characterize the pathways leading from activated inflammatory physiology to cognitive dysfunction in depression. Whereas a common genetic liability for adiposity and higher inflammatory biomarkers may lead to cognitive dysfunction and depression for some, for others it may be health-related factors associated with low socioeconomic status (e.g., poor diet) that leads to adiposity and higher inflammatory biomarkers.67 In other cases, it is likely that behaviors (e.g., sedentary behaviors, poor diet) that follow depression lead to a pro-inflammatory state, and resultant cognitive dysfunction.68 Indeed, each of these explanations are probably partially true, which may account for the bidirectional association of depression and inflammatory biomarkers reporting by Mac Giollabhui, Ng 14 in their comprehensive meta-analysis. Thus, it is likely that different mechanisms are at play in different individuals linking depression, inflammation and cognitive dysfunction, and importantly, only a subgroup of depressed individuals (approximately 25–30%) exhibit indicators inflammation, which likely contributes to discrepant findings.16
Depression and inflammation were both associated with worse executive functioning; however, the magnitude of the associations were small, and although this is generally characteristic of effect sizes in psychological science,69 results should be considered within a broader context. To provide a helpful point of reference for readers, normative data from a Dutch adult sample suggest that performance on the RFFT declines steadily from early adulthood onwards and that five years of aging is associated with a performance decline of 4–4.5 designs generated on the RFFT.70 The associations of depression (3.7), inflammation (0.7 per SD increase in CRP), and their combination (depressed individuals with CRP values at the mean/+1 SD/+2 SD) generated, respectively, 3.5, 4.8, and 6.1 fewer designs. Therefore, the strength of the association of these factors with executive functioning was modest in size, particularly when compared to the associations of educational level, aging and sedentary behavior with executive functioning.
Nonetheless, readers should consider the degree to which depression can lead to worse cognitive functioning via stress generation, sedentary behavior, and even worse educational attaintment.71, 72 Similarly caution should be exercised in the case of CRP because, for instance, there is considerable overlap between CRP and BMI – in fact, it is estimated that adipose tissue is responsible for 30% of circulating interleukin-6 (a pro-inflammatory cytokine that directly stimulates CRP production and plays a critical role in activation of the innate immune system).73 Indeed, there is evidence of a common genetic liability for adiposity, increased inflammatory biomarkers, and depression.74, 75 Consequently, caution should be exercised when interpreting the association of depression independent of chronic stress, or of CRP independent of BMI. Moreover, there is a growing need to characterize the temporal sequence and causal relationships between overlapping constructs, such as: diet, stress, sedentary behaviors, adiposity, inflammatory biomarkers, and depression.76, 77
This study possesses notable strengths as well as limitations. Although CRP is a crude measure of inflammatory physiology,78 it is a widely-used and reliable. Moreover, the precision of our estimate was improved through assessment in fasting participants within a similar window of time (before 10AM). Utilizing a very large, population-representative sample increases power and generalizability; however, a cross-sectional study design precludes causal inference. Nonetheless, despite the cross-sectional nature of these data, we believe that examining the relationship between depression, inflammation, and cognitive functioning in a population-representative sample that uses reliable and well-validated measures is important, given the dearth of high-quality data examining this question. Importantly, LifeLines recruited a substantial proportion of northern Netherlands (10%) and implemented a recruitment strategy designed to incorporate multiple generations of the same family. As such, over 67% of participants in LifeLines have at least one family member enrolled in the study. As such, the assumption that all observations are independent has been violated, which reduces confidence in the parameters estimated because contributory factors have not been modelled (e.g., genetic commonalities, living in same home, etc.). Finally, although we used a reliable and valid measure of executive functioning, it is possible that differences in executive functioning actually reflect generalized cognitive dysfunction, rather than specific difficulties in executive functioning.
This paper showed that low-grade inflammation and depression are both independently associated with worse executive functioning and their combination exerts a small cumulative association. There is urgent need for stronger theory describing the role that inflammatory processes play in the etiology of cognitive dysfunction in depression and for well-powered, prospective studies in youth to establish the causal relationships between related risk factors, such as poor diet, adiposity, and stress so that we can better understand and maintain brain health at a population level.
Supplementary Material
Highlights.
Depression status and higher C-reactive protein (CRP) values were associated with worse performance on a test of executive functioning.
The combination of depression and higher CRP was differentially associated with worse executive functioning than depression or CRP alone, although the magnitude of this association was modest.
There was no evidence that the association of higher C-reactive protein and poorer executive functioning in depressed individuals differed across the adult lifespan.
Acknowledgments
This research was supported by National Institute of Mental Health Grants MH079369 and MH101168 to Lauren Alloy and National Institute of Mental Health National Research Service Award F31MH118808 as well as an American Psychological Foundation grant to Naoise Mac Giollabhui.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest
No disclosures or conflicts of interest to report.
References
- 1.Erskine HE, Moffitt TE, Copeland WE, Costello EJ, Ferrari AJ, Patton G et al. A heavy burden on young minds: the global burden of mental and substance use disorders in children and youth. Psychol Med 2015; 45(07): 1551–1563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Burcusa SL, Iacono WG. Risk for recurrence in depression. Clin Psychol Rev 2007; 27: 959–985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kessler RC, Berglund P, Demler O, Jin R, Koretz D, Merikangas KR et al. The epidemiology of major depressive disorder: results from the National Comorbidity Survey Replication (NCS-R). JAMA 2003; 289(23): 3095–3105. [DOI] [PubMed] [Google Scholar]
- 4.American Psychiatric Association. Diagnostic and statistical manual of mental disorders (5th ed.). 2013.
- 5.Zimmerman M, Ellison W, Young D, Chelminski I, Dalrymple K. How many different ways do patients meet the diagnostic criteria for major depressive disorder? Compr Psychiatry 2015; 56: 29–34. [DOI] [PubMed] [Google Scholar]
- 6.Kunugi H, Hori H, Ogawa S. Biochemical markers subtyping major depressive disorder. Psychiatry Clin Neurosci 2015; 69(10): 597–608. [DOI] [PubMed] [Google Scholar]
- 7.Maes M, Scharpe S, Bosmans E, Vandewoude M, Suy E, Uyttenbroeck W et al. Disturbances in acute phase plasma proteins during melancholia: Additional evidence for the presence of an inflammatory process during that illness. Prog Neuropsychopharmacol Biol Psychiatry 1992; 16(4): 501–515. [DOI] [PubMed] [Google Scholar]
- 8.Smith RS. The macrophage theory of depression. Med Hypotheses 1991; 35(4): 298–306. [DOI] [PubMed] [Google Scholar]
- 9.Irwin MR, Miller AH. Depressive disorders and immunity: 20 years of progress and discovery. Brain Behav Immun 2007; 21(4): 374–383. [DOI] [PubMed] [Google Scholar]
- 10.Dantzer R, O’Connor JC, Freund GG, Johnson RW, Kelley KW. From inflammation to sickness and depression: when the immune system subjugates the brain. Nat Rev Neurosci 2008; 9(1): 46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lynall M-E, Turner L, Bhatti J, Cavanagh J, de Boer P, Mondelli V et al. Peripheral Blood Cell–Stratified Subgroups of Inflamed Depression. Biol Psychiatry 2020; 88(2): 185–196. [DOI] [PubMed] [Google Scholar]
- 12.Schedlowski M, Engler H, Grigoleit J-S. Endotoxin-induced experimental systemic inflammation in humans: A model to disentangle immune-to-brain communication. Brain Behav Immun 2014; 35: 1–8. [DOI] [PubMed] [Google Scholar]
- 13.Dowlati Y, Herrmann N, Swardfager W, Liu H, Sham L, Reim EK et al. A meta-analysis of cytokines in major depression. Biol Psychiatry 2010; 67(5): 446–457. [DOI] [PubMed] [Google Scholar]
- 14.Mac Giollabhui N, Ng TH, Ellman LM, Alloy LB. The longitudinal associations of inflammatory biomarkers and depression revisited: systematic review, meta-analysis, and meta-regression. Mol Psychiatry 2020. [DOI] [PMC free article] [PubMed]
- 15.Raison CL, Miller AH. Is depression an inflammatory disorder? Curr Psychiatry Rep 2011; 13(6): 467–475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Osimo EF, Baxter LJ, Lewis G, Jones PB, Khandaker GM. Prevalence of low-grade inflammation in depression: a systematic review and meta-analysis of CRP levels. Psychol Med 2019; 49(12): 1958–1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rock PL, Roiser JP, Riedel WJ, Blackwell AD. Cognitive impairment in depression: a systematic review and meta-analysis. Psychol Med 2014; 44(10): 2029–2040. [DOI] [PubMed] [Google Scholar]
- 18.Lee R, Hermens DF, Porter Ma, Redoblado-Hodge MA. A meta-analysis of cognitive deficits in first-episode Major Depressive Disorder. J Affect Disord 2012; 140: 113–124. [DOI] [PubMed] [Google Scholar]
- 19.Ahern E, Semkovska M. Cognitive functioning in the first-episode of major depressive disorder: A systematic review and meta-analysis. Neuropsychology 2017; 31(1): 52. [DOI] [PubMed] [Google Scholar]
- 20.Semkovska M, Quinlivan L, O’Grady T, Johnson R, Collins A, O’Connor J et al. Cognitive function following a major depressive episode: a systematic review and meta-analysis. The Lancet Psychiatry 2019; 6(10): 851–861. [DOI] [PubMed] [Google Scholar]
- 21.Hammar A, Ardal G. Cognitive functioning in major depression--a summary. Front Hum Neurosci 2009; 3: 26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hasselbalch BJ, Knorr U, Kessing LV. Cognitive impairment in the remitted state of unipolar depressive disorder: a systematic review. J Affect Disord 2011; 134: 20–31. [DOI] [PubMed] [Google Scholar]
- 23.Carvalho AF, Miskowiak KK, Hyphantis TN, Kohler CA, Alves GS, Bortolato B et al. Cognitive dysfunction in depression - pathophysiology and novel targets. CNS Neurol Disord Drug Targets 2014; 13(10): 1819–1835. [DOI] [PubMed] [Google Scholar]
- 24.McAfoose J, Baune BT. Evidence for a cytokine model of cognitive function. Neurosci Biobehav Rev 2009; 33: 355–366. [DOI] [PubMed] [Google Scholar]
- 25.Crisan AF, Oancea C, Timar B, Fira-Mladinescu O, Crisan A, Tudorache V. Cognitive impairment in chronic obstructive pulmonary disease. PLoS One 2014; 9(7): e102468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Huang YS, Guilleminault C, Hwang FM, Cheng C, Lin CH, Li HY et al. Inflammatory cytokines in pediatric obstructive sleep apnea. Medicine 2016; 95(41): e4944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Li X, Robertson CM, Yu X, Cheypesh A, Dinu IA, Li J. Early postoperative systemic inflammatory response is an important determinant for adverse 2-year neurodevelopment-associated outcomes after the Norwood procedure. J Thorac Cardiovasc Surg 2014; 148(1): 202–206. [DOI] [PubMed] [Google Scholar]
- 28.Noble JM, Manly JJ, Schupf N, Tang MX, Mayeux R, Luchsinger JA. Association of C-reactive protein with cognitive impairment. Arch Neurol 2010; 67(1): 87–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Baune B, Ponath G, Golledge J, Varga G. Association between IL-8 cytokine and cognitive performance in an elderly general population—the MEMO-Study. Neurobiol Aging 2008; 29(6): 937–944. [DOI] [PubMed] [Google Scholar]
- 30.Reichenberg A, Yirmiya R, Schuld A, Kraus T, Haack M, Morag A et al. Cytokine-associated emotional and cognitive disturbances in humans. Arch Gen Psychiatry 2001; 58(5): 445–452. [DOI] [PubMed] [Google Scholar]
- 31.Singh-Manoux A, Dugravot A, Brunner E, Kumari M, Shipley M, Elbaz A et al. Interleukin-6 and C-reactive protein as predictors of cognitive decline in late midlife. Neurology 2014; 83(6): 486–493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Krogh J, Benros ME, Jørgensen MB, Vesterager L, Elfving B, Nordentoft M. The association between depressive symptoms, cognitive function, and inflammation in major depression. Brain Behavior and Immunity 2014; 35: 70–76. [DOI] [PubMed] [Google Scholar]
- 33.Chang HH, Lee IH, Gean PW, Lee SY, Chi MH, Yang YK et al. Treatment response and cognitive impairment in major depression: association with C-reactive protein. Brain Behavior and Immunity 2012; 26(1): 90–95. [DOI] [PubMed] [Google Scholar]
- 34.Goldsmith DR, Haroon E, Woolwine BJ, Jung MY, Wommack EC, Harvey PD et al. Inflammatory markers are associated with decreased psychomotor speed in patients with major depressive disorder. Brain Behavior and Immunity 2016; 56: 281–288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Grassi-Oliveira R, Bauer ME, Pezzi JC, Teixeira AL, Brietzke E. Interleukin-6 and verbal memory in recurrent major depressive disorder. Neuro Endocrinol Lett 2011; 32(4): 540–544. [PubMed] [Google Scholar]
- 36.Cullen AE, Tappin BM, Zunszain PA, Dickson H, Roberts RE, Nikkheslat N et al. The relationship between salivary C-reactive protein and cognitive function in children aged 11–14years: Does psychopathology have a moderating effect? Brain Behav Immun 2017; 66: 221–229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mac Giollabhui N, Alloy LB, Hartman CA. Investigating whether depressed youth exhibiting elevated C reactive protein perform worse on measures of executive functioning, verbal fluency and episodic memory in a large, population based sample of Dutch adolescents. Brain Behav Immun 2020. [DOI] [PMC free article] [PubMed]
- 38.Hasin DS, Sarvet AL, Meyers JL, Saha TD, Ruan WJ, Stohl M et al. Epidemiology of Adult DSM-5 Major Depressive Disorder and Its Specifiers in the United States. JAMA Psychiatry 2018; 75(4): 336–346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Videbech P, Ravnkilde B. Hippocampal volume and depression: a meta-analysis of MRI studies. The American journal of psychiatry 2004; 161: 1957–1966. [DOI] [PubMed] [Google Scholar]
- 40.Sexton CE, Mackay CE, Ebmeier KP. A Systematic Review and Meta-Analysis of Magnetic Resonance Imaging Studies in Late-Life Depression. The American journal of geriatric psychiatry 2013; 21(2): 184–195. [DOI] [PubMed] [Google Scholar]
- 41.Treadway MT, Waskom ML, Dillon DG, Holmes AJ, Park MTM, Chakravarty MM et al. Illness progression, recent stress, and morphometry of hippocampal subfields and medial prefrontal cortex in major depression. Biol Psychiatry 2015; 77(3): 285–294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Talarowska M, Zajączkowska M, Gałecki P. Cognitive functions in first-episode depression and recurrent depressive disorder. Psychiatria Danubina 2015; 27(1): 0–43. [PubMed] [Google Scholar]
- 43.Franceschi C, Bonafè M, Valensin S, Olivieri F, De Luca M, Ottaviani E et al. Inflamm‐aging: an evolutionary perspective on immunosenescence. Ann N Y Acad Sci 2000; 908(1): 244–254. [DOI] [PubMed] [Google Scholar]
- 44.Gorelick PB. Role of inflammation in cognitive impairment: results of observational epidemiological studies and clinical trials. Ann N Y Acad Sci 2010; 1207(1): 155–162. [DOI] [PubMed] [Google Scholar]
- 45.Scholtens S, Smidt N, Swertz MA, Bakker SJ, Dotinga A, Vonk JM et al. Cohort Profile: LifeLines, a three-generation cohort study and biobank. Int J Epidemiol 2014; 44(4): 1172–1180. [DOI] [PubMed] [Google Scholar]
- 46.Ligthart GJ, Corberand JX, Fournier C, Galanaud P, Hijmans W, Kennes B et al. Admission criteria for immunogerontological studies in man: the SENIEUR protocol. Mech Ageing Dev 1984; 28(1): 47–55. [DOI] [PubMed] [Google Scholar]
- 47.O’Connor MF, Bower JE, Cho HJ, Creswell JD, Dimitrov S, Hamby ME et al. To assess, to control, to exclude: effects of biobehavioral factors on circulating inflammatory markers. Brain Behav Immun 2009; 23(7): 887–897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sheehan DV, Lecrubier Y, Sheehan KH, Amorim P, Janavs J, Weiller E et al. The Mini-International Neuropsychiatric Interview (M.I.N.I.): the development and validation of a structured diagnostic psychiatric interview for DSM-IV and ICD-10. J Clin Psychiatry 1998; 59 Suppl 20: 22–33;quiz 34–57. [PubMed] [Google Scholar]
- 49.Wanders RB, van Loo HM, Vermunt JK, Meijer RR, Hartman CA, Schoevers RA et al. Casting wider nets for anxiety and depression: disability-driven cross-diagnostic subtypes in a large cohort. Psychol Med 2016; 46(16): 3371–3382. [DOI] [PubMed] [Google Scholar]
- 50.Lecrubier Y, Sheehan D, Weiller E, Amorim P, Bonora I, Sheehan K et al. The Mini International Neuropsychiatric Interview (MINI). A short diagnostic structured interview: Reliability and validity according to the CIDI. Eur Psychiatry 1997; 12(5): 224–231. [Google Scholar]
- 51.Ross TP. The reliability and convergent and divergent validity of the Ruff Figural Fluency Test in healthy young adults. Arch Clin Neuropsychol 2014; 29(8): 806–817. [DOI] [PubMed] [Google Scholar]
- 52.Ruff RM, Light RH, Evans RW. The Ruff Figural Fluency Test: a normative study with adults. Dev Neuropsychol 1987; 3(1): 37–51. [Google Scholar]
- 53.Gulpers B, Lugtenburg A, Zuidersma M, Verhey F, Voshaar RO. Anxiety disorders and figural fluency: A measure of executive function. J Affect Disord 2018; 234: 38–44. [DOI] [PubMed] [Google Scholar]
- 54.Brugha TS, Cragg D. The List of Threatening Experiences: the reliability and validity of a brief life events questionnaire. Acta Psychiatr Scand 1990; 82(1): 77–81. [DOI] [PubMed] [Google Scholar]
- 55.Hendriks A, Ormel J, van de Willige G. Long-term difficulties measured by a self-report questionnaire and semi-structured interview: a comparison of methods. Gedrag en Gezondheid 1990; 18: 273–283. [Google Scholar]
- 56.Team RC. R: A Language and Environment for Statistical Computing R Foundation for Statistical Computing: Vienna, Austria, 2018. [Google Scholar]
- 57.Bates D, Mächler M, Bolker B, Walker S. Fitting linear mixed-effects models using lme4. arXiv:1406.58232014. [Google Scholar]
- 58.Wickham H ggplot2 – Elegant Graphics for Data Analysis (2nd Edition). Springer-Verlag: New York, NY, 2016. [Google Scholar]
- 59.Mac Giollabhui N, Ellman LM, Coe CL, Byrne ML, Abramson LY, Alloy LB. To exclude or not to exclude: Considerations and recommendations for C-reactive protein values higher than 10 mg/L. Brain Behav Immun 2020; 87: 898–900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Sv Buuren, Groothuis-Oudshoorn K. mice: Multivariate imputation by chained equations in R. Journal of statistical software 2010: 1–68.
- 61.Zheng F, Xie W. High-sensitivity C-reactive protein and cognitive decline: the English Longitudinal Study of Ageing. Psychol Med 2018; 48(8): 1381–1389. [DOI] [PubMed] [Google Scholar]
- 62.Miller AH, Raison CL. The role of inflammation in depression: from evolutionary imperative to modern treatment target. Nat Rev Immunol 2016; 16(1): 22–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Jenny NS, French B, Arnold AM, Strotmeyer ES, Cushman M, Chaves PH et al. Long-term assessment of inflammation and healthy aging in late life: the Cardiovascular Health Study All Stars. The Journals of Gerontology: Series A 2012; 67(9): 970–976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Teunissen C, Van Boxtel M, Bosma H, Bosmans E, Delanghe J, De Bruijn C et al. Inflammation markers in relation to cognition in a healthy aging population. J Neuroimmunol 2003; 134(1–2): 142–150. [DOI] [PubMed] [Google Scholar]
- 65.Mac Giollabhui N, Olino TM, Nielsen J, Abramson LY, Alloy LB. Is Worse Attention a Risk Factor for or a Consequence of Depression, or Are Worse Attention and Depression Better Accounted for by Stress? A Prospective Test of Three Hypotheses. Clin Psychol Sci 2019; 7(1): 93–109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Mac Giollabhui N, Swistun D, Murray S, Moriarity DP, Kautz MM, Ellman LM et al. Executive dysfunction in depression in adolescence: the role of inflammation and higher body mass. Psychol Med 2020; 50(4): 683–691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Muscatell KA, Brosso SN, Humphreys KL. Socioeconomic status and inflammation: a meta-analysis. Mol Psychiatry 2018; 25(9): 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Berk M, Williams LJ, Jacka FN, O’Neil A, Pasco Ja, Moylan S et al. So depression is an inflammatory disease, but where does the inflammation come from? BMC Med 2013; 11: 200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Button KS, Ioannidis JP, Mokrysz C, Nosek BA, Flint J, Robinson ES et al. Power failure: why small sample size undermines the reliability of neuroscience. Nat Rev Neurosci 2013; 14(5): 365–376. [DOI] [PubMed] [Google Scholar]
- 70.Izaks GJ, Joosten H, Koerts J, Gansevoort RT, Slaets JP. Reference data for the Ruff Figural Fluency Test stratified by age and educational level. PLoS One 2011; 6(2): e17045–e17045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Hammen C. Generation of stress in the course of unipolar depression. J Abnorm Psychol 1991; 100(4): 555. [DOI] [PubMed] [Google Scholar]
- 72.Dalsgaard S, McGrath J, Ostergaard SD, Wray NR, Pedersen CB, Mortensen PB et al. Association of Mental Disorder in Childhood and Adolescence With Subsequent Educational Achievement. JAMA Psychiatry 2020; 77(8): 797–805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Mohamed-Ali V, Goodrick S, Rawesh A, Katz D, Miles J, Yudkin J et al. Subcutaneous adipose tissue releases interleukin-6, but not tumor necrosis factor-α, in vivo. The Journal of Clinical Endocrinology & Metabolism 1997; 82(12): 4196–4200. [DOI] [PubMed] [Google Scholar]
- 74.Khandaker GM, Zuber V, Rees JM, Carvalho L, Mason AM, Foley CN et al. Shared mechanisms between coronary heart disease and depression: findings from a large UK general population-based cohort. Mol Psychiatry 2019: 1–10. [DOI] [PMC free article] [PubMed]
- 75.Milaneschi Y, Lamers F, Peyrot WJ, Baune BT, Breen G, Dehghan A et al. Genetic Association of Major Depression With Atypical Features and Obesity-Related Immunometabolic Dysregulations. JAMA Psychiatry 2017; 74(12): 1214–1225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Michigan A, Johnson TV, Master VA. Review of the Relationship between C-Reactive Protein and Exercise. Mol Diagn Ther 2011; 15(5): 265–275. [DOI] [PubMed] [Google Scholar]
- 77.Minihane AM, Vinoy S, Russell WR, Baka A, Roche HM, Tuohy KM et al. Low-grade inflammation, diet composition and health: current research evidence and its translation. Br J Nutr 2015; 114(7): 999–1012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Del Giudice M, Gangestad SW. Rethinking IL-6 and CRP: Why they are more than inflammatory biomarkers, and why it matters. Brain Behav Immun 2018; 70: 61–75. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


