Abstract
The uninjured murine heart contains a heterogeneous population of macrophages with disparate ontogenies and functions. These macrophages are often associated with blood vessels and can be subclassified based on the expression of CC chemokine receptor 2 (CCR2) and major histocompatibility complex class II (MHC-II). The biological cues that modulate these macrophage pool subpopulations have not been completely identified. It has been recently shown that a sub-population of circulating naïve B cells adheres to the myocardial microvasculature. We hypothesized that B cells might modulate the phenotype of myocardial macrophages. To test this hypothesis, we analyzed both the relative location of B cells and macrophages in myocardial histological section and the prevalence of myocardial macrophage subsets in hearts from B cell–deficient mice (μMT) and mice depleted of B cells through administration of an anti-CD20 antibody. We found that B cells pause in the microvasculature in proximity of macrophages and modulate the number of myocardial CCR2−MHC-IIhigh cells. Through in vitro studies we found that this is likely the result of a paracrine effect of B cells on the expression of MHC-II in CCR2− cells. These results reveal an unexpected relationship between B cells and resident macrophages and, highlighting a direct intramyocardial effect of circulating B cells, challenge the currently held belief that naïve recirculating B lymphocytes merely shuttle between lymphoid stations.
Keywords: Tissue-associated B cells, resident macrophages, naïve B cells
Introduction
Current models of B cell biology posit that naïve B cells merely recirculate between primary and secondary lymphoid stations until meeting their cognate antigen [1, 2]. However, recent studies have highlighted that: 1) the naïve myocardium contains a sizeable population of naïve circulating B cells that pause on the myocardial microvascular endothelium; 2) B cell-deficient and B cell-depleted mice have alterations in myocardial mass, myocardial contractility and number of myocardial T cells [3, 4]. These studies pointed to an unexpected biological relationship between B cells and the heart but did not investigate whether B cells exhibit any local effects in the myocardium [3]. The demonstration of a local B cell effect within the myocardium would broaden our understanding of the relationship between circulating immune cells and the myocardium and challenge current paradigms of B cell biology.
The murine myocardium contains four distinct subsets of CD64+ macrophages defined by different expression levels of CC chemokine receptor 2 (CCR2) and major histocompatibility complex class II (MHC-II) [5]. The vast majority of macrophages in the naïve heart are CCR2− resident macrophages (MHC-IIhigh or MHC-IIlow) of embryonic origin. CCR2+ cells are bone marrow-derived monocytic cells that acquire higher levels of MHC-II expression as they fully differentiate into tissue macrophages [5–7]. These macrophage subsets have several important roles in the context of myocardial physiology and pathology [6, 8–11]. Recently, a population of intra parenchymal myocardial macrophages has been identified in close association with the vasculature [8]. The factors that modulate the myocardial macrophage pool composition in the naïve heart have not been completely understood.
Here, we used histology, flow cytometric analysis of myocardial macrophages from B cell–deficient mice and B cell–depleted mice, and primary cardiac macrophage cultures to investigate the hypothesis that B cells modulate the phenotype of myocardial macrophages through a paracrine effect.
Materials and Methods
Mice
To investigate the effects of B cell deficiency, we used 4-5 week-old male and female wild-type (WT) (background C57BL/6J; Stock No. 000664) and μMT mice (B cell deficient) (background C57BL/6J; B6.129S2-lghmtm1Cgn/J; Stock No. 002288) age and sex matched. These strains were purchased from The Jackson Laboratory and bred in house. The CD19 reporter model was generated as previously reported [3]. Mice were bred and maintained at the Washington University School of Medicine Animal Care Facility. Experiments were performed according to protocols approved by the Institutional Animal Care and Use Committee of Washington University School of Medicine.
Antibody-mediated B cell depletion
Wild-type (WT) mice were injected once intraperitoneally with anti-CD20 antibody (BioLegend; Clone: SA271G2; Cat.#152104; 1 mg/mL). Mice were injected between 21 and 28 days of age (100 μL/mouse). Littermate control mice were injected with isotype control antibody (BioLegend; Clone: RTK4530; Cat# 400644). All mice were analyzed 7 days post-injection. B cell depletion was assessed by flow cytometry (Sup. Figure 1).
Flow cytometry
Cardiac tissue was digested and stained as described previously [5, 12]. Murine samples were stained with the following fluorescently conjugated antibodies (BioLegend; dilution 0.5μL/100μL): CD45 (30-F11, PerCP-Cy5); CD19 (1D3/CD19, APC); Ly6G (1A8, FITC); CD11b (M1/70, BV605); CCR2 (SA203G11, BV421); I-A/I-E (MHC-II) (M5/144.15.2) and CD64 (X54-5/7.1, PE). Flow cytometric analysis was performed on a BD LSR Fortessa X-20. Macrophages were gated as CD45+CD11b+Ly6G−CD64+CCR2+/−MHC-IIhigh/low (Sup. Figure 2).
Cell isolation
Heart cell suspensions and splenocytes were prepared as previously described [12, 13]. Cardiac macrophages were gated as CD45+Aqua−Ly6G−CD64+, splenic neutrophils were gated as CD45+Aqua−Ly6G+ and splenic B cells were gated as CD45+Aqua−CD19+. Antibody dilutions and specifications are described in “Flow cytometry” section. Cell viability was assessed by Zombie Aqua staining (BioLegend; Cat#423102; dilution 1:100). Cells were isolated through FACS sorting on a BD FACS Aria-III (nozzle 100μm).
For culture of primary macrophages in B cell conditioned media experiment, discrimination of live vs dead cells was achieved using Vibrant DyeCycle Violet Ready Flow dye (ThermoFisher) to mark viable cells and Propidium Iodide to stain dead cells. CD45+Aqua−Ly6G−CD64+ cells and CD45+CD19+ cells were isolated with a MoFlow sorter.
Macrophage/ B cell culture or co-culture
Cardiac CD45+Aqua−Ly6G−CD64+ cells from μMT mice and splenic CD45+Aqua−CD19+ cells from WT mice were cultured in 96-well plates (5000 cells/100 μL) for 40 hours at 37°C as described previously [12]. Splenic neutrophils CD45+Aqua−Ly6G+ were used as a negative control. Cultured cells were stained for CD45, CD64, CCR2, MHC-II and CD19 or Ly6G (antibody specifications are shown in “Flow cytometry” section; dilution 0.2μL/100μL). Flow cytometric analysis was performed on a BD LSR Fortessa X-20 (gating strategy is shown in Sup. Figure 3).
Cardiac CD45+PI−DyeCycle+Ly6G−CD64+ cells from μMT or WT mice were culture in 96 well plates at about 7000 cells/well. 14 million splenic B cells were sorted and plated in 4 mL of media in 2 C6 wells. After over-night incubation, primary macrophages were rinsed in HBSS. 700 μl of media were collected from the B cell cultures (without disturbing B cells on the bottom) and 125 μL/well of B cell condition media or control media were added to the macrophages. After 10 hours, additional 700 μL of media was collected from the B cell cultures and additional 125μL of B cell conditioned media or culture media were added to the macrophages. After additional 12 hours, flow cytometric analysis was performed on a BD LSR Fortessa X-20 (gating strategy is shown in Sup. Figure 3).
BrdU incorporation
Cells were plated as described in “Cell Culture” section and then allowed to rest overnight. Cell replication was determined by the rate of BrdU incorporation (APC BrdU Flow Kit, Cat#552598, BD Pharmingen). BrdU was added to each well at 10 μM the morning after plating. BrdU incorporation was assessed by flow cytometry 30-36 hours after BrdU administration.
Immunofluorescence
CD19-reporter mouse hearts were fixed and processed as previously described [3, 13]. Heart sections were stained with the primary antibodies anti-CD68 (Bio-Rad; Cat#MCA1957T; 1:400) and anti-CD31 (R&D Systems; Cat#AF3628; 1:15), overnight at 4°C, followed by incubation with donkey anti-rat Alexa Fluor 488 (ThermoFisher; Cat#A-21208; 1:200) and NL637 (R&D Systems; Cat#NL002; NorthernLights anti-goat IgG-NL637; 1:200) for 1–4 h at room temperature. DAPI was used to stain the nucleus. Fluorescence was visualized using a Zeiss LSM 880 Confocal microscope at the Washington University Center for Cellular Imaging (WUCCI).
Statistical analyses
All results are presented as mean ± SEM. Statistical comparisons between two groups were performed using two-tailed Student’s t-test, correcting for multiple comparisons with the Holm-Sidak method. Outliers defined as experimental points two standard deviations away from the mean were excluded. All data were analyzed with Graph-Pad Prism Version 8.
Results and Discussion
We began investigating the relationship between B cells and myocardial macrophages by immunostaining cryosections of naïve murine hearts and analyzing the relative positions of B cells and CD68+ macrophages. We found that myocardial-associated B cells were almost exclusively intravascular and myocardial macrophages were frequently perivascular (Figure 1A and Sup. Movies 1–2). Intravascular myocardial associated B cells and perivascular myocardial macrophages were typically in close proximity (Figure 1A and Sup. Movies 1–2). We did not observe any contact between B cells and macrophages. The intravascular location of myocardial B cells and the perivascular location of resident macrophages are consistent with existing literature [3, 8, 14]. However, the proximity between intravascular B cells and perivascular resident macrophages was a novel and unexpected observation that supported the possibility of a cross-talk between macrophages and B cells in the naive heart.
Figure 1. B cells are in proximity of parenchymal macrophages and they modulate the resident myocardial macrophage pool composition in the naïve heart.
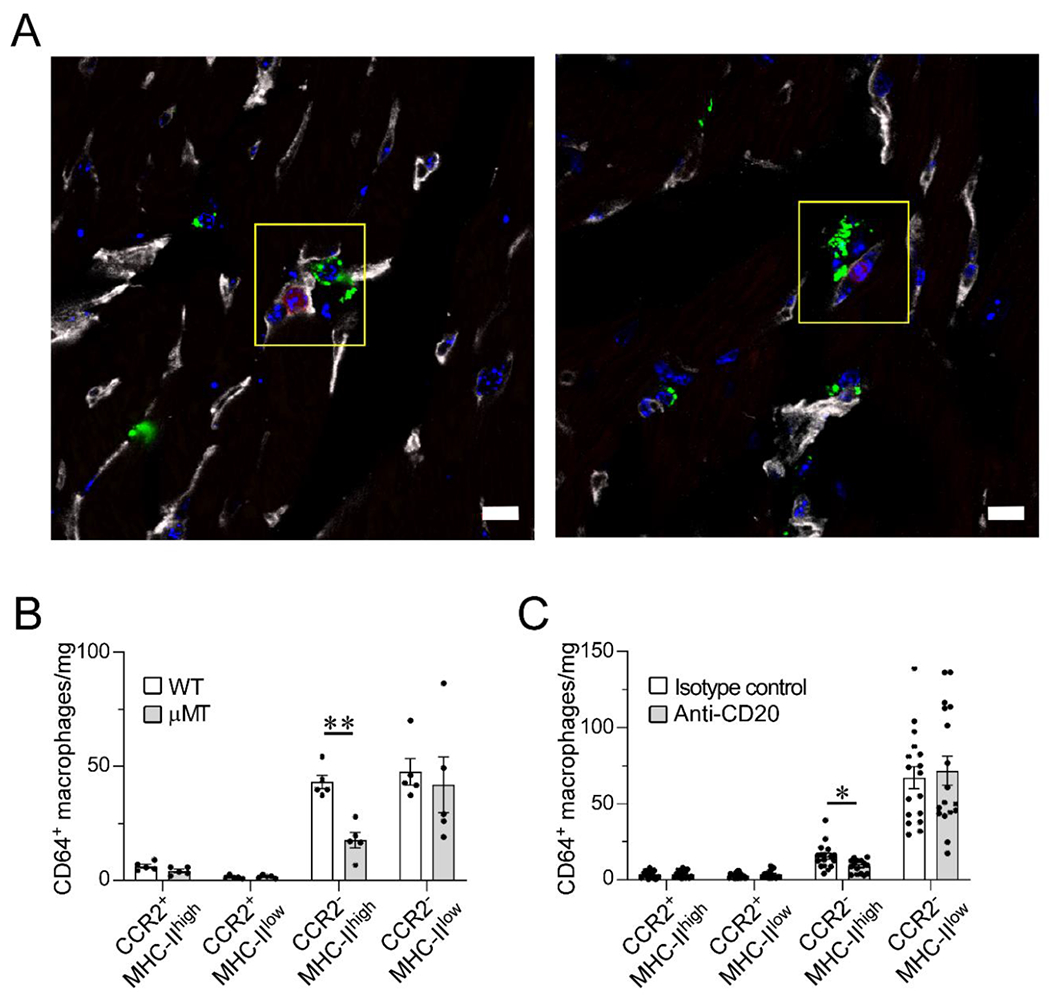
(A) Confocal images of immunostained myocardial sections from a CD19-tdTomato reporter mouse. CD19+ B cells (red) are intravascular (CD31, gray); CD68+ macrophages (green) are intraparenchymal and perivascular in the naïve heart. CD68+ macrophages are closely associated to the microvasculature and in proximity to intracapillary B cells (yellow box). Blue stain, DAPI. Scale bar=10 μm. Data collected from 3 hearts (2 slices per heart). (B) Flow cytometric analysis of the cardiac macrophage pool as defined by CCR2 and MHC-II expression in wild-type (WT) and B cell-deficient (μMT) mice. Hearts from B cell-deficient mice display significantly lower numbers of CCR2−MHC-IIhigh macrophages than WT hearts. 5 hearts per condition. (C) Flow cytometric analysis of cardiac macrophage subpopulations in littermate mice treated with anti-CD20 B cell–depleting antibody or isotype control. B cell depletion was associated with reduced numbers of CCR2−MHC-IIhigh macrophages. Isotype control: 17 hearts; Anti-CD20: 17 hearts, except for CCR2−MHC-IIhi (15 hearts) and CCR2+MHC-IIlow (16 hearts). Outliers defined as experimental points two standard deviations away from the mean were excluded. Statistical comparisons between two groups were performed using two-tailed Student’s t-test, correcting for multiple comparisons with the Holm-Sidak method. Bars represent mean ± SEM. *p<0.05, **p<0.01 (B-C).
Next, we analyzed CCR2 and MHC-II expression on cardiac CD64+ macrophages in B cell–deficient and B cell–depleted mice (see full gating strategy in Sup. Figure 2). We compared wild-type mice (WT, C57BL/6J) to syngeneic, age- and sex-matched B cell–deficient mice (μMT, C57BL/6J). The hearts of B cell–deficient mice displayed a significant decrease in CCR2−MHC-IIhigh macrophages compared to WT hearts (Figure 1B). CCR2−MHC-IIhigh macrophages are resident cardiac macrophages [5, 7]. To confirm these results, we depleted B cells in WT mice by administering an anti-CD20 antibody (Sup. Figure 1). Consistent with the findings using the μMT mouse model, antibody-mediated B cell depletion reduced the number of myocardial CCR2−MHC-IIhigh macrophages (Figure 1C). These combined results indicated that B cells modulate the myocardial macrophage pool in the naïve murine heart by modulating the number of resident myocardial CCR2−MHC-IIhigh cells.
We hypothesized that B cells might affect the myocardial macrophage pool directly. To test this hypothesis, we established an in vitro macrophage/B cell coculture system. We isolated primary cardiac CD64+ macrophages from μMT mice and cultured them in the presence or absence of splenic CD19+ B cells from WT mice. Primary CD64+ macrophages are almost exclusively CCR2− cells (Sup. Figure 3). 40 hours of co-culture with B cells resulted in a higher prevalence of CCR2−MHC-IIhigh macrophages (Figure 2A). As a negative control, we co-cultured cardiac CD64+ macrophages with splenic neutrophils, which did not change the prevalence of CCR2−MHC-IIhigh macrophages (Sup. Figure 4). These in vitro results supported our in vivo data, and suggested that B cells directly modulate resident myocardial macrophage subpopulations.
Figure 2. B cells directly modulate the MHC-II expression on CCR2− myocardial macrophages.
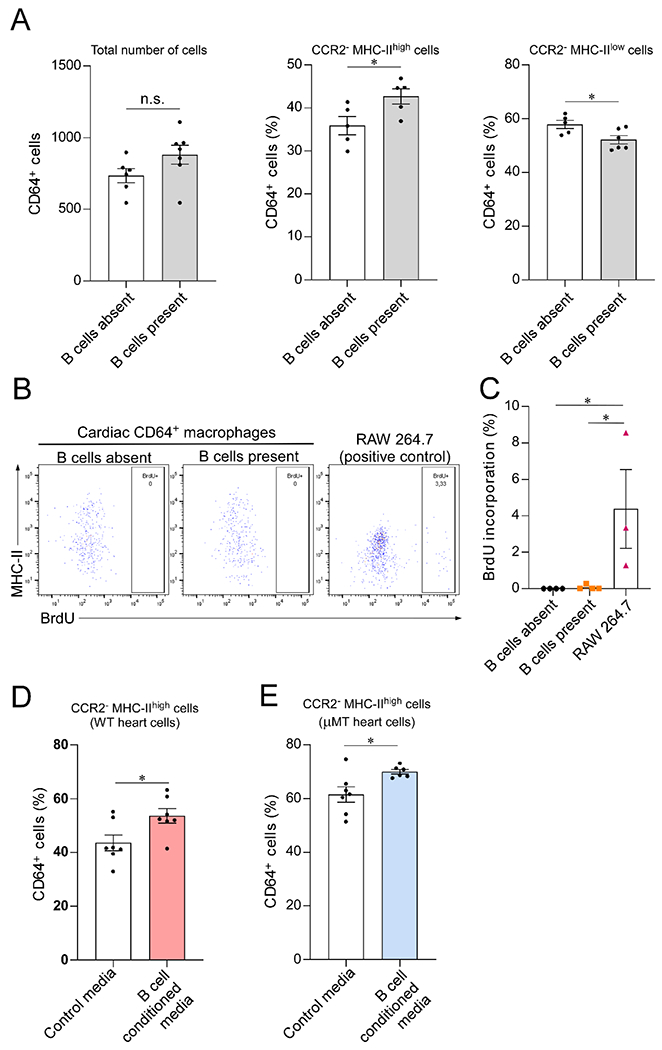
(A) Cardiac CD64+ macrophages isolated from μMT hearts cultured alone or in the presence of WT CD19+ splenic B cells. Coculture with B cells increased the prevalence of CCR2−MHC-IIhigh macrophages. Left, total number of CD64+ macrophages analyzed by flow cytometry after 40 hours in culture (n= 6 and 7 samples/condition; p=0.1). Middle, CCR2−MHC-IIhigh macrophages in relation to the total number of cells (n= 5 samples/condition). Right, CCR2− MHC-IIlow macrophages in relation to the total number of cells (n= 5 and 6 samples/condition). (B) Representative flow cytometry plots representing the expression of MHC-II and BrdU incorporation by cardiac CD64+ macrophages cocultured with B cells (n= 4 samples/condition). RAW 264.7 macrophage-derived cells were used as a positive control for BrdU incorporation (n=3 samples/condition). (C) Bar graph showing that BrdU incorporation by cultured primary cardiac CD64+ macrophages is close to zero and is not affected by the presence of B cells. (D-E) Exposure to B cell conditioned media increased the percentage of CCR2−MHC-IIhigh cells in primary cardiac macrophages isolated from both WT (n = 7 samples per condition) and μMT animals (n = 7 and 6 samples/condition), suggesting that the effect of B cells is, at least in part, mediated by a secreted molecules. Outliers defined as experimental points two standard deviations away from the mean were excluded. Statistical comparisons between two groups were performed using two-tailed Student’s t-test (A, D and E) or One-Way ANOVA followed by Tukey’s test for multiple comparison (C). Bars represent mean ± SEM. n.s. = not significant; *p<0.05.
We performed proliferation studies via BrdU incorporation to investigate whether the B cell-mediated increase in CCR2−MHC-IIhigh macrophages observed in our in vitro system was secondary to proliferation of CCR2−MHC-IIhigh macrophages or maturation of CCR2−MHC-IIlow cells. Figure 2B shows expression of MHC-II and BrdU incorporation in myocardial macrophages cultured in the presence or absence of B cells. We did not observe any BrdU incorporation in cultured primary myocardial macrophages (Figure 2C), suggesting that the B cell mediated increase in CCR2−MHC-IIhigh macrophages observed is likely the result of B cell mediated upregulation of MHC-II on CCR2−MHC-IIlow cells. This is consistent with the notion that the pool of CCR2−MHC-IIhigh macrophages in the heart largely results from the maturation of CCR2−MHC-IIlow cells into CCR2−MHC-IIhigh macrophages [7].
Since secreted factors can mediate local communication between cells [15], even across endothelial monolayers [16], we hypothesized that B cells might affect the myocardial macrophage pool through a paracrine effect. To test this hypothesis, we cultured myocardial CD64+ macrophages in the presence of B cell conditioned media or regular media (Figure 2D–E). Additionally, to investigate whether there was a difference in the response of WT or μMT macrophages to B cells, we tested the effect of B cell conditioned media in both WT and μMT derived heart macs. Figures 2D–E show that exposure to B cell conditioned media was associated with an increase in the percentage of CCR2−MHC-IIhigh macrophages. B cell conditioned media induced an increase in the percentage of CCR2−MHC-IIhigh macrophages of approximately 23% in WT macrophages and 14% in μMT macrophages. These data support the notion that B cells modulate the phenotype of myocardial macrophages through secreted factors. In these in vitro experiments, we observed that the proportion of MHC-IIhigh cultured macrophages was higher in macrophages isolated from uMT hearts than WT hearts. This difference could be explained by batch to batch variations or by macrophage plasticity differences in vivo versus in vitro.
In summary, our in vivo findings indicate that B cells modulate the number of myocardial CCR2−MHC-IIhigh resident macrophages. The findings of our histological analysis together with our in vitro studies suggest that this is likely the result of a direct paracrine effect of B cells on MHC-II expression in myocardial CCR2− resident cells. Since the vast majority of B cells in the naïve murine heart are intravascular [3], this effect is most likely mediated by intravascular B cells. However, we cannot exclude a contribution of the few intraparenchymal B cells present in the naïve murine heart or a communication between cardiac macrophages and extracardiac B cells.
Our data unveils a previously unappreciated crosstalk between unstimulated B cells and tissue-resident macrophages. Moreover, since myocardial associated B cells are naïve circulating B cells that pause within the myocardial microvasculature [3], our findings challenge the widely believed assumption that naive B cells merely recirculate between lymphoid stations until meeting their cognate antigen. Further work will be needed to understand the functional implications of our observations and to explore the mechanistic basis of the effect of B cells on CCR2− macrophages.
Supplementary Material
Highlights.
Intravascular myocardial associated B cells are found in proximity to cardiac macrophages.
B cell depletion leads to a reduction in the number of CCR2−MHC-IIhigh resident macrophages in the heart.
B cell modulate MHC-II expression on cardiac macrophages through a paracrine effect.
Acknowledgments
This study was supported by NIH grant 1K08HL145108-01A1 and institutional funds from the Washington University in St. Louis Division of Cardiology. The authors acknowledge Dr. Doug Mann for critical discussions of this work. The authors acknowledge Dr. Erica Lantelme, Dr. Hao Zhang, the Washington University Flow Cytometry & Fluorescence Activated Cell Sorting Core and the Bloomberg School of Public Health Cell Sorting Facility for assistance with FACS analysis and cell sorting, as well as Dr. Dennis Oakley and the Washington University Center for Cellular Imaging (WUCCI) for assistance with confocal imaging. The authors acknowledge Servier Medical Art by Servier, licensed under a Creative Commons Attribution 3.0 Unported License.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosures
LA is cofounder of i-Cordis LLC, which is focused on the development of B cell–modulating therapies for the treatment of heart failure, and coinventor on patent WO2019028062, “Pirfenidone derivatives for modulation of B lymphocyte activity and organ protection,” owned by Washington University. CRR and FP have no conflict of interest to disclose.
References
- [1].Alberts B Molecular biology of the cell. New York: Garland Science,; 2002. [Google Scholar]
- [2].Cooper MD. The early history of B cells. Nat Rev Immunol. 2015;15(3):191–7. [DOI] [PubMed] [Google Scholar]
- [3].Adamo L, Rocha-Resende C, Lin CY, Evans S, Williams JW, Dun H, et al. Myocardial B cells are a subset of circulating lymphocytes with delayed transit through the heart. JCI Insight. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Adamo L, Rocha-Resende C, Mann DL. The Emerging Role of B Lymphocytes in Cardiovascular Disease. Annu Rev Immunol. 2020;38:99–121. [DOI] [PubMed] [Google Scholar]
- [5].Lavine KJ, Epelman S, Uchida K, Weber KJ, Nichols CG, Schilling JD, et al. Distinct macrophage lineages contribute to disparate patterns of cardiac recovery and remodeling in the neonatal and adult heart. Proc Natl Acad Sci U S A. 2014;111(45):16029–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Bajpai G, Bredemeyer A, Li W, Zaitsev K, Koenig AL, Lokshina I, et al. Tissue Resident CCR2− and CCR2+ Cardiac Macrophages Differentially Orchestrate Monocyte Recruitment and Fate Specification Following Myocardial Injury. Circ Res. 2019;124(2):263–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Epelman S, Lavine KJ, Beaudin AE, Sojka DK, Carrero JA, Calderon B, et al. Embryonic and adult-derived resident cardiac macrophages are maintained through distinct mechanisms at steady state and during inflammation. Immunity. 2014;40(1):91–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Chakarov S, Lim HY, Tan L, Lim SY, See P, Lum J, et al. Two distinct interstitial macrophage populations coexist across tissues in specific subtissular niches. Science. 2019;363(6432). [DOI] [PubMed] [Google Scholar]
- [9].Dick SA, Macklin JA, Nejat S, Momen A, Clemente-Casares X, Althagafi MG, et al. Self-renewing resident cardiac macrophages limit adverse remodeling following myocardial infarction. Nat Immunol. 2019;20(1):29–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Leid J, Carrelha J, Boukarabila H, Epelman S, Jacobsen SE, Lavine KJ. Primitive Embryonic Macrophages are Required for Coronary Development and Maturation. Circ Res. 2016;118(10):1498–511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Wang Y, Sano S, Yura Y, Ke Z, Sano M, Oshima K, et al. Tet2-mediated clonal hematopoiesis in nonconditioned mice accelerates age-associated cardiac dysfunction. JCI Insight. 2020;5(6). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Adamo L, Staloch LJ, Rocha-Resende C, Matkovich SJ, Jiang W, Bajpai G, et al. Modulation of subsets of cardiac B lymphocytes improves cardiac function after acute injury. JCI Insight. 2018;3(11). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Rocha-Resende C, Weinheimer C, Bajpai G, Adamo L, Matkovich SJ, Schilling J, et al. Immunomodulatory role of non-neuronal cholinergic signaling in myocardial injury. JCI Insight. 2019;5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Rocha-Resende C, Yang W, Li W, Kreisel D, Adamo L, Mann D. Developmental changes in myocardial B cells mirror changes in B cells associated with different organs. JCI Insight. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Oyler-Yaniv A, Oyler-Yaniv J, Whitlock BM, Liu Z, Germain RN, Huse M, et al. A Tunable Diffusion-Consumption Mechanism of Cytokine Propagation Enables Plasticity in Cell-to-Cell Communication in the Immune System. Immunity. 2017;46(4):609–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Randolph GJ, Furie MB. A soluble gradient of endogenous monocyte chemoattractant protein-1 promotes the transendothelial migration of monocytes in vitro. J Immunol. 1995;155(7):3610–8. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


