Abstract
Background:
Heterogeneity exists in triple negative breast cancer (TNBC) response to standard anthracycline/taxane based neoadjuvant systemic therapy (NAST) with 40–50% of patients having pathologic complete response to therapy (pCR). Early assessment of imaging response during NAST may identify a subset of TNBCs that are likely to have pCR upon completion of treatment. We aimed to evaluate the performance of early US after 2 cycles of neoadjuvant NAST in identifying excellent responders to NAST in TNBC patients.
Materials and Methods:
215 patients with TNBC enrolled in the ongoing ARTEMIS clinical trial. The patients were divided into a discovery cohort (n=107) and a validation cohort (n=108). Receiver operating characteristic analysis with 95% confidence intervals (CIs) and multivariate logistic regression analysis were performed to model the probability of pCR using the percentage of tumor volume reduction by ultrasound from baseline to after 2 cycles of AC.
Results:
Overall, 39.3% (42/107) of patients achieved pCR. Positive predictive value (PPV) analysis identified a cutoff point of 80% TVR after 2 cycles; the pCR rate was 77% (17/22) in patients with TVR ≥ 80%, AUC was 0.84 (95% CI, 0.77 to 0.92, P < .0001). In the validation cohort, the pCR was 44%. The PPV for pCR with a TVR ≥ 80% after two cycles was 76% (95% CI: 55%−91%) and AUC was 0.79 (95% CI, 0.70 to 0.87, P<0.0001).
Conclusions:
Percentage TVR by US evaluation after 2 cycles of NAST may be a cost-effective early imaging biomarker for pCR to AC/taxane-based NAST.
Keywords: Breast Cancer, TNBC, NAST, Imaging, Ultrasound
Precis:
Early Ultrasound imaging identifies excellent responders to NAST on TNBC patients. Percentage of tumor volume reduction by ultrasound may be a cost-effective imaging biomarker to predict pCR.
INTRODUCTION
TNBC comprises approximately 12% to 24% of breast cancers and is immunohistochemically characterized by a lack of expression of estrogen receptor and progesterone receptor and absence of overexpression of human epidermal growth factor receptor 2 (HER2).1, 2 TNBC encompasses a heterogeneous, often aggressive group of tumors with a wide range of clinical outcomes, including variable response to NAST.3 Although genomic profiling has expanded our knowledge regarding the different molecular subtypes of TNBC, to date there are no clinically available profiling strategies to predict response of TNBC to NAST.4
In TNBC, patients commonly receive a combination of an anthracycline and cyclophosphamide followed by a taxane as standard NAST.5 Known advantages of NAST are decreases in tumor burden, increases in the rate of breast-conserving surgery, assessment in vivo of treatment efficacy, and the opportunity to modify treatment in the uncommon event of tumor progression. Additionally, use of NAST can identify patients requiring additional adjuvant therapy if residual disease is found at the time of surgical resection.6 The rate of pCR to anthracycline-and-taxane-based NAST regimens in TNBC ranges from 18% to 60% and differs by TNBC subtype.7, 8 pCR has become an early surrogate endpoint for long-term overall survival and disease-free survival.9, 10
Notably, a feature of most clinical trials of NAST in TNBC is escalation of therapy—i.e., the addition of experimental drugs to standard therapy. However, a substantial proportion of patients have pCR with standard NAST; thus, most patients treated in randomized trials of escalation of NAST do not benefit from trial participation. They are unnecessarily exposed to the associated toxicities and costs from escalation of therapy while in fact they could benefit from less intensive chemotherapy. Clearly, there is an unmet clinical need to develop non-invasive imaging biomarkers that can identify TNBCs that are likely to have pCR to standard NAST early in the course of therapy, thus identifying a group of patients where treatment escalation is unnecessary.
The use of ultrasound imaging findings as an early surrogate biomarker for pathologic response is appealing in a clinical trial since imaging is noninvasive and allows a window of opportunity to modify systemic therapy if imaging shows that the current systemic therapy is ineffective. Findings on conventional breast imaging, such as mammography and US, correlate moderately well with residual tumor after NAST, with US being more accurate (59.6% versus 31.7%).11, 12 Breast magnetic resonance imaging (MRI) is the most sensitive modality for monitoring treatment response in patients undergoing NAST.13–15 Multiple breast MRI quantitative parameters are currently under active investigation as early predictors of response to NAST,16, 17 among which reduction in tumor volume has been suggested as the most reliable.18, 19 However, breast MRI has several limitations, including patient convenience, high cost, lack of standardization in image acquisition/processing, and clinicians reluctance to undertake additional complex investigations.
The use of conventional B-mode US as an early imaging predictor of excellent response in TNBC patients has not been reported. We hypothesized that detection of early response during NAST would indicate a chemotherapy sensitive biology and aimed to evaluate the performance of early US after two cycles of neoadjuvant AC in identifying excellent responders to standard NAST in TNBC patients.
Material and Methods
Patient selection
This prospective study was approved by the Institutional Review Board of The University of Texas MD Anderson Cancer Center and was part of an ongoing clinical trial of patients with stage I-III TNBC (“A Robust TNBC Evaluation FraMework to Improve Survival” [ARTEMIS]; NCT02276442) who were prospectively monitored for response to NAST. Informed consent was obtained from all patients before enrollment in this trial. The 114 patients for the primary cohort who had baseline US before treatment and after 2 cycles of neoadjuvant AC from November 2015 to March 2018 were analyzed. One hundred twelve patients with baseline US before treatment and after two cycles of AC from April 2018 to February 2020 were selected as a validation cohort.
Chemotherapy
NAST consisted of dose-dense AC for four cycles followed by paclitaxel every two weeks for four cycles or weekly for 12 doses. US response after the first 2 doses of AC was examined in the study reported here. Patients evaluated by ultrasound after 4 cycles of AC that showed no response to therapy or progression were offered therapy on clinical trials as the second phase of their NAST (Figure 1). After completing NAST, patients underwent surgical resection with assessment of residual disease by pathologic evaluation. Patients who had a pCR after undergoing targeted therapy due to chemo-resistant disease after AC treatment (n=7 for the primary cohort and n=4 for the validation cohort) were considered confounders and excluded from this study. As all the patients on targeted therapy underwent a first phase of AC, patients of this group who did not achieve pCR were included, thus leaving 107 patients for the primary cohort and 108 patients for the validation cohort available for our analyses.
Figure 1.
Imaging schema in patients with triple negative breast cancer according to the ARTEMIS study protocol.
Imaging
Patients were monitored with sequential US for each phase of NAST (Figure 1). Whole-breast US was performed on all patients using Epiq 5G scanners with 12- to 18-MHz high-frequency linear array transducers (Philips Healthcare, Andover, MA, USA) by eight dedicated breast sonographers, and findings were evaluated by fourteen breast fellowship-trained radiologists.
The transverse and longitudinal images of the index lesions were captured. The measurements of the index lesions in three dimensions were obtained. The volume (V) of the index lesion was calculated using the three greatest tumor dimensions (referred to as W, L, and H) in the following equation:
The percentage change in the tumor volume between pretreatment baseline and after two cycles of AC was calculated with the following equation:
The regional nodal basins, including the axillary level I-III nodes, and the internal mammary chain, were examined by US. For patients with abnormal findings in the axillary region, the supraclavicular lymph nodes were assessed. The index lymph node in the highest involved nodal chain was targeted for fine-needle aspiration biopsy using a 21-gauge needle under sonographic guidance. The disease was staged according to the AJCC Cancer Staging Manual, 8th edition.20 The number of lymph nodes at baseline and after two cycles of AC was recorded.
Histopathology review
Before treatment, core needle biopsy specimens of the primary tumors were obtained for immunohistochemical assessment and assessment of histological type. Estrogen receptor, progesterone receptor, HER2, and Ki-67 results were reported as the percentage of cells with positive nuclear staining. TNBC was defined as a tumor in which < 10% of invasive tumor cells had positive nuclear staining for estrogen receptor and < 10% of cells had positive nuclear staining for progesterone receptor.21 HER2 negativity was defined according to the American Society of Clinical Oncology/College of American Pathologists guidelines.22
Surgical specimens were assessed by dedicated breast pathologists. pCR was defined as no residual invasive disease in the breast (ypT0/Tis) or in the resected nodal tissue (ypN0).
Statistical analysis
Receiver operating characteristic analysis with 95% confidence intervals (CIs) and multivariate logistic regression analysis were performed to model the probability of pCR using the percentage of tumor volume reduction from baseline to after 2 cycles of AC. Positive predictive value (PPV) curve analysis was performed to identify an exploratory cutoff of percentage reduction in tumor volume between baseline and after 2 cycles of AC that predicted pCR. Wilcoxon rank-sum test was applied for the comparison of continuous variables, for example, age, between the discovery and validation cohorts. Fisher’s exact test was used for comparison of categorical variables should any cell count in a contingency table be less than 5; otherwise, Chi-squared test was applied to compare categorical variables. P-value ≤ 0.05 was considered as nominally significant for all analyses. All statistical analyses were performed using R version 3.6.1.
RESULTS
The clinical characteristics of the patients from the primary and the validation cohorts in this analysis are summarized in Table 1. Eighty-six (80.4%) and 92 (85.2%) patients in the primary and validation cohorts respectively had invasive ductal carcinoma respectively, 65 (60.7%) and 74 (68.5%) patients had clinical T2 disease, and 62 (57.9%) and 75 (69.4) patients had N0 disease. Forty-two patients (39.3%) of the primary cohort and 48 (44.4%) of the validation cohort achieved pCR according to pathologic evaluation at surgery.
TABLE 1.
Patient and Tumor Characteristics of 215 TNBC patients
| CHARACTERISTIC |
DISCOVERY COHORT (N=107) |
VALIDATION COHORT (N=108) |
P-VALUE |
|---|---|---|---|
| MEDIAN AGE (RANGE), YEARS | 56.0 (26–77) | 49 (28–79) | 0.003* |
| HISTOLOGIC TYPE, N (%) | 0.77** | ||
| IDC | 86 (80.4) | 92 (85.2) | |
| ILC | 2 (1.9) | 1 (0.9) | |
| METAPLASTIC | 15 (14) | 12 (11.1) | |
| OTHER | 4 (3.7) | 3 (2.8) | |
| CLINICAL T STAGE, N (%) | 0.62** | ||
| 1 | 23 (21.6) | 20 (18.5) | |
| 2 | 65 (60.7) | 74 (68.5) | |
| 3 | 15 (14.0) | 12 (11.1) | |
| 4 | 4 (3.7) | 2 (1.9) | |
| CLINICAL N STAGE, N (%) | 0.10** | ||
| 0 | 62 (57.9) | 75 (69.4) | |
| 1 | 27 (25.3) | 22 (20.4) | |
| 2 | 1 (0.9) | 3 (2.8) | |
| 3 | 17 (15.9) | 8 (7.4) | |
| TYPE OF SURGERY1, N (%) | 0 47*** | ||
| BCS | 68 (63.6) | 74 (68.5) | |
| MASTECTOMY | 39 (36.4) | 33 (30.6) | |
| PCR AT SURGERY, N (%) | 0.53*** | ||
| YES | 42 (39.3) | 48 (44.4) | |
| NO | 65 (60.7) | 60 (55.6) |
One patient not available in the validation cohort due to development of metastatic disease
BCS, breast-conserving surgery; IDC, invasive ductal carcinoma; ILC, invasive lobular carcinoma; pCR, pathologic complete response.
Wilcoxon rank-sum test
Fisher’s exact test
Chi-squared test.
Univariate logistic regression analysis of the relationship between pCR and percentage reduction in tumor volume between baseline and after 2 cycles of AC showed that greater reduction in tumor volume was associated with a higher probability of pCR (OR = 1.06 per 1% increase in tumor volume reduction; 95% CI, 1.03–1.09; p<0.0001) (Figure 2). Multivariate logistic regression including age at diagnosis, baseline tumor volume and tumor stage as covariates, resulted in a very similar effect size estimate for tumor volume reduction (OR=1.05; 95% CI, 1.03–1.08; p<0.0001).
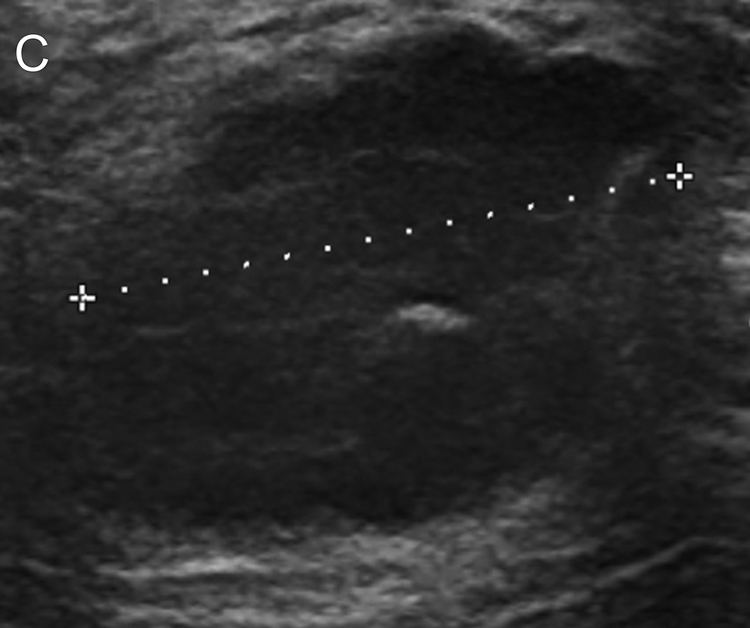
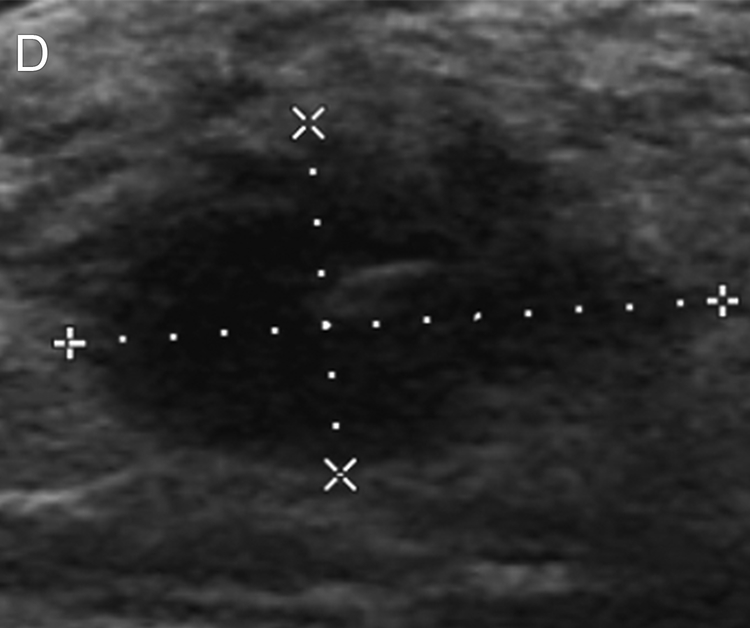
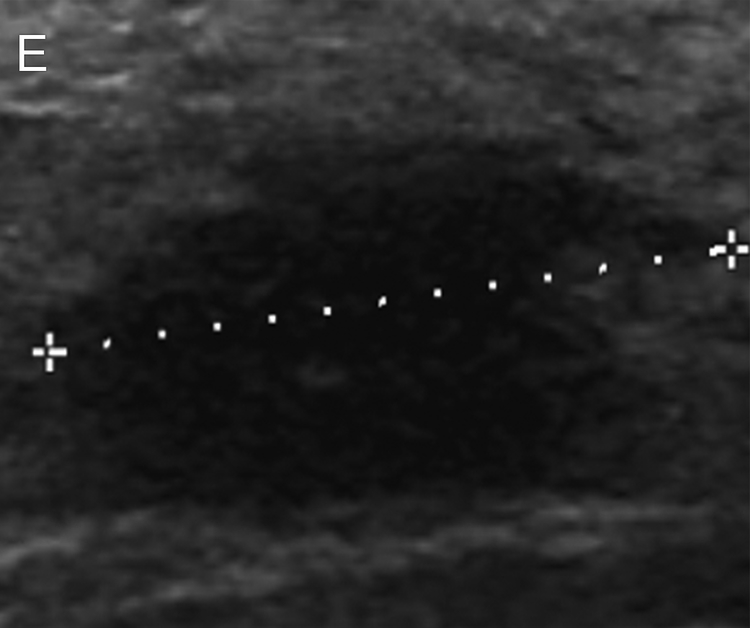
Figure 2.
Images of a 52-year-old woman with triple negative breast cancer in the right breast. (a) Craniocaudal mammogram shows an oval mass (arrow) correlating with the palpable abnormality (triangle). (b, c) Longitudinal and transverse gray-scale ultrasound images of the tumor at baseline evaluation show a hypoechoic mass measuring 2.9 × 2.6 × 1.9 cm. (d, e) Longitudinal and transverse gray-scale ultrasound images of the tumor after 2 cycles of neoadjuvant doxorubicin and cyclophosphamide show the tumor measuring 1.8 × 1.7 × 0.9 cm, with an 80% calculated reduction in tumor volume, suggestive of treatment response predictive of pathologic complete response (pCR). The patient completed neoadjuvant systemic therapy and underwent breast-conserving surgery, and surgical pathology evaluation showed a pCR.
PPV curve analysis showed that an exploratory cutoff point of 77% for tumor volume reduction (TVR) after 2 cycles led to the highest PPV of 81% (Figure 3a). To facilitate the ease of clinical practice, we rounded up the cutoff point to 80%. Specifically, the pCR rate was 77% (17/22) in patients with a percentage reduction in tumor volume ≥ 80% and 29% (25/85) in patients with a percentage reduction < 80% (Figure 3a). The AUC for the TVR after 2 cycles predicting pCR was 0.84 (95% CI, 0.77 to 0.92, P < .0001). In the validation cohort of 108 patients (Figure 3b) after excluding 4 patients who had pCR after experimental therapy, the overall pCR rate was 44%. The PPV for TVR ≥ 80% after 2 cycles was 76% (95% CI: 55%−91%) (19 out of 25 patients with TVR ≥ 80% had pCR). The pCR rate was 35% (29/83) in patients with TVR < 80% after 2 cycles. The AUC for TVR was 0.79 (95% CI, 0.70 to 0.87, P<0.0001).
Figure 3.
Positive predictive value (PPV) for pathologic complete response (pCR) by percentage reduction in tumor volume after 2 cycles of neoadjuvant doxorubicin and cyclophosphamide in the discovery cohort (a) and the validation cohort (b). The graph shows a PPV of 77% and 76% for pCR prediction at 80% volume reduction (vertical dotted lines) in the discovery and the validation cohorts, respectively. PPV is shown in red solid line and its 95% exact confident intervals are shown in blue dashed lines.
Additional analysis of interval change between the number of lymph nodes at baseline US and US after two cycles of neoadjuvant AC did not improve pCR prediction for this model.
DISCUSSION
This is the first study showing the potential of conventional breast US to predict pCR to NAST in TNBC patients. We found that early volumetric change after two cycles of AC identified patients with a high probability of achieving a pCR. Our study showed that a cut-point of tumor volume reduction of ≥80% after two cycles of AC had a PPV of 77% and an AUC of 0.84 in the primary cohort, and a PPV of 76 % and an AUC of 0.79 in the validation cohort. These findings suggest that conventional breast US may be used as an inexpensive imaging biomarker to select the fraction of TNBC patients in whom NAST is likely to produce a pCR. Sparing these patients the higher cost and adverse effects of adding drugs to standard NAST would be a paradigm shift in the treatment of TNBC excellent responders.
Also, if a new escalated regimen for TNBC becomes FDA approved in an unselected patient population, the number of TNBC patients receiving unnecessary therapy is considerably magnified, and over time, the cost of delivering these drugs and treating the additional toxic effects they cause can become astronomical. For example, if immunotherapy gains approval for NAST for TNBC on the basis of the KEYNOTE-522 trial, 51% of patients treated (the pCR rate in the control arm) will be exposed to a drug that substantially increases the likelihood of toxic effects but most likely does not improve their survival.23
Assessment of response to NAST by using conventional imaging such as mammography and US is based on changes in tumor dimensions. Dobruch-Sobczack et al assessed several US parameters to predict treatment response in patients with breast cancer undergoing NAST.24 They found a statistically significant difference in the mean change in tumor volume between responders and non-responders after the first and the fourth courses of NAST. However, the study was performed in 13 patients with 19 tumors, which is a very small cohort of patients.
The known limitations of breast US include high intraobserver and interobserver variability and challenges in measuring infiltrative lesions. However, in contrast to some other subtypes of breast cancer, TNBC is associated with a mass in 70% to 86% of cases.25, 26 In addition, tumor shrinkage patterns in NAST have been closely related to molecular subtypes and pathologic tumor response.27, 28 Eom et al reported a concentric pattern of shrinkage in TNBC in 59% (38/64) of patients, and all 16 patients in their study who underwent pCR had concentric tumor shrinkage.29 These specific imaging characteristics can increase the accuracy of US in the evaluation of treatment response in TNBC, facilitating the selection of TNBC patients with excellent response to NAST. Baumgartner et al evaluated the accuracy of breast US for prediction of pCR after NAST in 124 breast cancer patients, 36 of whom had TNBC.30 US was more accurate in the prediction of residual tumor in patients with TNBC, with a PPV of 83.3%, a negative predictive value of 75%, and a false-negative rate of 37.5%. Although these predictive values are not high enough to predict pCR after NAST, US might be used as an imaging modality to select early exceptional responders to NAST in TNBC. Our study shows that a reduction of more or equal than 80% in tumor volume between baseline and after 2 cycles of AC on US can predict pCR in TNBC patients with an AUC of 0.84 in the discovery cohort and an AUC of 0.79 in the validation cohort, similar to the MRI AUC values of 0.75 to 0.77 reported for early treatment response.31, 32 Therefore, a significant reduction in tumor volume on US after 2 cycles of NAST can serve as an excellent low-cost predictor to select the subset of exceptional responders in TNBC patients. More complex assessments using shear wave US for TNBC have failed to demonstrate benefit beyond the use of gray-scale US employed here.33–35
Although breast MRI has been proposed to be the imaging modality of choice for early evaluation of early treatment response to NAST, the use of MRI for this purpose in clinical practice has been limited, owing to the lack of consistency and standardization of specific MRI parameters that hold greater potential for early prediction of pCR to NAST and the cost and the complexity of breast MRI.36–38 In contrast, conventional breast US is widely accessible, facilitating the implementation of serial US examinations to monitor treatment response.
This study has several strengths. First, to our knowledge, this is the first study to evaluate and validate the performance of B-mode breast US to predict early response in a relatively large cohort of TNBC patients. Second, all patients in the primary and the validation cohorts underwent the same chemotherapeutic regimen. TNBC patients who achieved pCR on targeted therapy after dose-dense AC were excluded from this study.
A weakness of this study is that the analysis was performed in a single institution, which means that validation in a multi-institutional setting would be required to demonstrate generalizability of the approach. In addition, breast US is associated with high intraobserver and interobserver variability, even among experienced breast radiologists. However, the concentric shrinkage pattern of TNBC in the majority of patients makes this variability of less concern in TNBC.
In conclusion, ≥ 80 % reduction in tumor volume between baseline and after two cycles of NAST as evaluated by breast US demonstrated an association with excellent response to NAST. The use of breast US for early identification of excellent TNBC responders would allow clinicians to tailor treatment strategies, for example, by shortening and de-escalating chemotherapy for excellent responders, sparing them from genomic profiling and the toxicity of prolonged chemotherapy or investigational agent therapy, and offering patients with chemotherapy-insensitive TNBC clinical trials with novel agents. Further multi-institutional studies are needed to validate these results.
ACKNOWLEDGEMENT
We thank Stephanie P. Deming, senior scientific editor, Research Medical Library, at The University of Texas MD Anderson Cancer Center, for editing the article.
FUNDING
This study has received funding by the National Institutes of Health/National Cancer Institute (Cancer Center Support Grant P30 CA016672); specifically, resources from the Biostatistics Resource Group were used.
This work was supported by The University of Texas MD Anderson Cancer Center Breast Cancer Moonshot Program and a Cancer Prevention and Research Institute of Texas Multi-Investigator Research Award (MIRA): [RP160710-C1-CPRIT].
DISCLOSURES
TM is a medical consultant for Hologic and Merit Medical, JL has research support from Novartis, Medivation/Pfizer, GSK, EMD-Serono, Astrazeneca, Medimmune, Zenith, Jounce. She is in the advisory committee (uncompensated) of Astrazeneca, Ayala, Pfizer. WY receives royalties from Elsevier for a textbook. She is an uncompensated consultant for Braid Health. All remaining authors have no declared conflict of interest.
Contributor Information
Beatriz E. Adrada, Department of Breast Imaging, The University of Texas MD Anderson Cancer Center, 1515 Holcombe Blvd., Unit 1350, Houston, TX 77030, USA.
Rosalind Candelaria, Department of Breast Imaging, The University of Texas MD Anderson Cancer Center, 1515 Holcombe Blvd., Unit 1350, Houston, TX 77030, USA
Stacy Moulder, Department of Breast Oncology, The University of Texas MD Anderson Cancer Center, 1515 Holcombe Blvd., Unit 1354, Houston, TX 77030, USA
Alastair Thompson, Department of Breast Surgery, The University of Baylor College of Medicine, Lester and Sue Smith Breast Cancer, Houston, TX 77030, USA
Peng Wei, Department of Biostatistics, The University of Texas MD Anderson Cancer Center, 1515 Holcombe Blvd., Unit 1411, Houston, TX 77030, USA
Gary J. Whitman, Department of Breast Imaging, The University of Texas MD Anderson Cancer Center, 1515 Holcombe Blvd., Unit 1350, Houston, TX 77030, USA
Vicente Valero, Department of Breast Medical Oncology, The University of Texas MD Anderson Cancer Center, 1515 Holcombe Blvd., Unit 1354, Houston, TX 77030, USA
Jennifer K. Litton, Department of Breast Medical Oncology, The University of Texas MD Anderson Cancer Center, 1515 Holcombe Blvd., Unit 1354, Houston, TX 77030, USA.
Lumarie Santiago, Department of Breast Imaging, The University of Texas MD Anderson Cancer Center, 1515 Holcombe Blvd., Unit 1350, Houston, TX 77030, USA
Marion E. Scoggins, Department of Breast Imaging, The University of Texas MD Anderson Cancer Center, 1515 Holcombe Blvd., Unit 1350, Houston, TX 77030, USA
Tanya Moseley, Department of Breast Imaging and Surgical Oncology, The University of Texas MD Anderson Cancer Center, 1515 Holcombe Blvd., Unit 1350, Houston, TX 77030, USA
Jason B White, Department of Breast Medical Oncology, The University of Texas MD Anderson Cancer Center, 1515 Holcombe Blvd., Unit 1354, Houston, TX 77030, USA
Elizabeth. E Ravenberg, Department of Breast Medical Oncology, The University of Texas MD Anderson Cancer Center, 1515 Holcombe Blvd., Unit 1354, Houston, TX 77030, USA
Wei T. Yang, Department of Breast Imaging, The University of Texas MD Anderson Cancer Center, 1515 Holcombe Blvd., Unit 1350, Houston, TX 77030, USA
Gaiane M. Rauch, Department of Breast Imaging, The University of Texas MD Anderson Cancer Center, 1515 Holcombe Blvd., Unit 1473, Houston, TX 77030, USA
REFERENCES
- 1.Dawson SJ, Provenzano E, Caldas C. Triple negative breast cancers: clinical and prognostic implications. Eur J Cancer 2009; 45 Suppl 1: 27–40. [DOI] [PubMed] [Google Scholar]
- 2.Schmadeka R, Harmon BE, Singh M. Triple-negative breast carcinoma: current and emerging concepts. Am J Clin Pathol 2014; 141: 462–477. [DOI] [PubMed] [Google Scholar]
- 3.Lebert JM, Lester R, Powell E, et al. Advances in the systemic treatment of triple-negative breast cancer. Curr Oncol 2018; 25: S142–S150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Masuda H, Baggerly KA, Wang Y, et al. Differential response to neoadjuvant chemotherapy among 7 triple-negative breast cancer molecular subtypes. Clin Cancer Res 2013; 19: 5533–5540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Palma G, Frasci G, Chirico A, et al. Triple negative breast cancer: looking for the missing link between biology and treatments. Oncotarget 2015; 6: 26560–26574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Masuda N, Lee SJ, Ohtani S, et al. Adjuvant capecitabine for breast cancer after preoperative chemotherapy. N Engl J Med 2017; 376: 2147–2159. [DOI] [PubMed] [Google Scholar]
- 7.Gamucci T, Pizzuti L, Sperduti I, et al. Neoadjuvant chemotherapy in triple-negative breast cancer: A multicentric retrospective observational study in real-life setting. J Cell Physiol 2018; 233: 2313–2323. [DOI] [PubMed] [Google Scholar]
- 8.Sikov WM, Berry DA, Perou CM, et al. Impact of the addition of carboplatin and/or bevacizumab to neoadjuvant once-per-week paclitaxel followed by dose-dense doxorubicin and cyclophosphamide on pathologic complete response rates in stage II to III triple-negative breast cancer: CALGB 40603 (Alliance). J Clin Oncol 2015; 33: 13–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cortazar P, Zhang L, Untch M, et al. Pathological complete response and long-term clinical benefit in breast cancer: the CTNeoBC pooled analysis. Lancet 2014; 384: 164–172. [DOI] [PubMed] [Google Scholar]
- 10.von Minckwitz G, Untch M, Blohmer JU et al. Definition and impact of pathologic complete response on prognosis after neoadjuvant chemotherapy in various intrinsic breast cancer subtypes. J Clin Oncol 2012; 30: 1796–1804. [DOI] [PubMed] [Google Scholar]
- 11.Croshaw R, Shapiro-Wright H, Svensson E et al. Accuracy of clinical examination, digital mammogram, ultrasound, and MRI in determining postneoadjuvant pathologic tumor response in operable breast cancer patients. Ann Surg Oncol 2011; 18: 3160–3163. [DOI] [PubMed] [Google Scholar]
- 12.Keune JD, Jeffe DB, Schootman M, et al. Accuracy of ultrasonography and mammography in predicting pathologic response after neoadjuvant chemotherapy for breast cancer. Am J Surg 2010; 199: 477–484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yeh E, Slanetz P, Kopans DB, et al. Prospective comparison of mammography, sonography, and MRI in patients undergoing neoadjuvant chemotherapy for palpable breast cancer. AJR Am J Roentgenol 2005; 184: 868–877. [DOI] [PubMed] [Google Scholar]
- 14.Li H, Yao L, Jin P, et al. MRI and PET/CT for evaluation of the pathological response to neoadjuvant chemotherapy in breast cancer: A systematic review and meta-analysis. Breast 2018; 40: 106–115. [DOI] [PubMed] [Google Scholar]
- 15.Choi WJ, Kim HH, Cha JH et al. Comparison of pathologic response evaluation systems after neoadjuvant chemotherapy in breast cancers: correlation with computer-aided diagnosis of MRI features. AJR Am J Roentgenol 2019; 213: 944–952. [DOI] [PubMed] [Google Scholar]
- 16.Michishita S, Kim SJ, Shimazu K, et al. Prediction of pathological complete response to neoadjuvant chemotherapy by magnetic resonance imaging in breast cancer patients. Breast 2015; 24: 159–165. [DOI] [PubMed] [Google Scholar]
- 17.Marino MA, Helbich T, Baltzer P, Pinker-Domenig K. Multiparametric MRI of the breast: a review. J Magn Reson Imaging 2018; 47: 301–315. [DOI] [PubMed] [Google Scholar]
- 18.Partridge SC, Gibbs JE, Lu Y, et al. MRI measurements of breast tumor volume predict response to neoadjuvant chemotherapy and recurrence-free survival. AJR Am J Roentgenol 2005; 184: 1774–1781. [DOI] [PubMed] [Google Scholar]
- 19.Hylton NM, Blume JD, Bernreuter WK, et al. Locally advanced breast cancer: MR imaging for prediction of response to neoadjuvant chemotherapy--results from ACRIN 6657/I-SPY TRIAL. Radiology 2012; 263: 663–672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hortobagyi GN, Connolly JL, Edge SB, et al. AJCC Cancer Staging Manual. 8th ed. New York, NY: Springer International Publishing, 2016. [Google Scholar]
- 21.Hammond ME, Hayes DF, Dowsett M et al. American Society of Clinical Oncology/College of American Pathologists guideline recommendations for immunohistochemical testing of estrogen and progesterone receptors in breast cancer. J Clin Oncol 2010; 28: 2784–2795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wolff AC, Hammond ME, Hicks DG, et al. Recommendations for human epidermal growth factor receptor 2 testing in breast cancer: American Society of Clinical Oncology/College of American Pathologists clinical practice guideline update. J Clin Oncol 2013; 31: 3997–4013. [DOI] [PubMed] [Google Scholar]
- 23.Schmid P, Cortes J, Pusztai L, et al. Pembrolizumab for early triple-negative breast cancer. N Engl J Med 2020; 382: 810–821. [DOI] [PubMed] [Google Scholar]
- 24.Dobruch-Sobczak K, Piotrzkowska-Wroblewska H, Klimonda Z, et al. Ultrasound echogenicity reveals the response of breast cancer to chemotherapy. Clin Imaging 2019; 55: 41–46. [DOI] [PubMed] [Google Scholar]
- 25.Boisserie-Lacroix M, Macgrogan G, Debled M, et al. Triple-negative breast cancers: associations between imaging and pathological findings for triple-negative tumors compared with hormone receptor-positive/human epidermal growth factor receptor-2-negative breast cancers. Oncologist 2013; 18: 802–811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dogan BE, Gonzalez-Angulo AM, Gilcrease M, et al. Multimodality imaging of triple receptor-negative tumors with mammography, ultrasound, and MRI. AJR Am J Roentgenol 2010; 194: 1160–1166. [DOI] [PubMed] [Google Scholar]
- 27.Kim TH, Kang DK, Yim H, et al. Magnetic resonance imaging patterns of tumor regression after neoadjuvant chemotherapy in breast cancer patients: correlation with pathological response grading system based on tumor cellularity. J Comput Assist Tomogr 2012; 36: 200–206. [DOI] [PubMed] [Google Scholar]
- 28.Goorts B, Dreuning KMA, Houwers JB, et al. MRI-based response patterns during neoadjuvant chemotherapy can predict pathological (complete) response in patients with breast cancer. Breast Cancer Res 2018; 20: 34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Eom HJ, Cha JH, Choi WJ, et al. Predictive clinicopathologic and dynamic contrast-enhanced MRI findings for tumor response to neoadjuvant chemotherapy in triple-negative breast cancer. AJR Am J Roentgenol 2017; 208: W225–W230. [DOI] [PubMed] [Google Scholar]
- 30.Baumgartner A, Tausch C, Hosch S, et al. Ultrasound-based prediction of pathologic response to neoadjuvant chemotherapy in breast cancer patients. Breast 2018; 39: 19–23. [DOI] [PubMed] [Google Scholar]
- 31.Li X, Arlinghaus LR, Ayers GD, et al. DCE-MRI analysis methods for predicting the response of breast cancer to neoadjuvant chemotherapy: pilot study findings. Magn Reson Med 2014; 71: 1592–1602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.O’Flynn EA, Collins D, D’Arcy J et al. Multi-parametric MRI in the early prediction of response to neo-adjuvant chemotherapy in breast cancer: value of non-modelled parameters. Eur J Radiol 2016; 85: 837–842. [DOI] [PubMed] [Google Scholar]
- 33.Savaridas SL, Sim YT, Vinnicombe SJ, et al. Are baseline ultrasound and mammographic features associated with rates of pathological completes response in patients receiving neoadjuvant chemotherapy for breast cancer? Cancer Imaging 2019; 19: 67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Evans A, Whelehan P, Thompson A et al. Prediction of pathological complete response to neoadjuvant chemotherapy for primary breast cancer comparing interim ultrasound, shear wave elastography and MRI. Ultraschall Med 2018; 39: 422–431. [DOI] [PubMed] [Google Scholar]
- 35.Evans A, Armstrong S, Whelehan P, et al. Can shear-wave elastography predict response to neoadjuvant chemotherapy in women with invasive breast cancer? Br J Cancer 2013; 109: 2798–2802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Jun W, Cong W, Xianxin X, Daqing J. Meta-analysis of quantitative dynamic contrast-enhanced MRI for the assessment of neoadjuvant chemotherapy in breast cancer. Am Surg 2019; 85: 645–653. [PubMed] [Google Scholar]
- 37.Prevos R, Smidt ML, Tjan-Heijnen VC, et al. Pre-treatment differences and early response monitoring of neoadjuvant chemotherapy in breast cancer patients using magnetic resonance imaging: a systematic review. Eur Radiol 2012; 22: 2607–2616. [DOI] [PubMed] [Google Scholar]
- 38.Marinovich ML, Sardanelli F, Ciatto S, et al. Early prediction of pathologic response to neoadjuvant therapy in breast cancer: systematic review of the accuracy of MRI. Breast 2012; 21: 669–677. [DOI] [PubMed] [Google Scholar]