Abstract
Immune activation complicates HIV despite antiretroviral therapy (ART). Indoleamine 2,3 dioxygenase (IDO) catabolizes tryptophan (T) to kynurenine (K), regulating immune activity, and IDO activity increases with age. This study examines the relationship of IDO activity, bacterial translocation, and aging in people living with HIV (PLWH) on ART. Samples and data from PLWH on ART from the Centers for AIDS Research Network of Integrated Clinical Systems and from matched HIV-uninfected patients (controls) from the Multicenter AIDS Cohort Study and the Women’s Interagency HIV Study were analyzed. The ratio of K to T (K:T) and neopterin were indicators of inflammation; 16S ribosomal DNA (16S rDNA) and lipopolysaccharide (LPS) were markers of bacterial translocation. Samples and data from 205 PLWH and 99 controls were analyzed. PLWH had higher K:T values across all ages, with a significant relationship between age and K:T for both groups. CD4 count or CD4 nadir had no association with K:T. There was no positive association between level of 16S rDNA or LPS detection and K:T. K:T and neopterin were associated. PLWH had elevated IDO activity, at younger ages, despite ART. This study suggests K:T ratio increases with age in both groups and is elevated in PLWH at all ages compared with age-matched controls.
INTRODUCTION
HIV causes lifelong infection typified by immune activation and dysfunction that worsens as the infection progresses. Despite modern antiretroviral therapy (ART), older people living with HIV (PLWH) are at increased risk of cardiovascular disease or non-AIDS-associated malignancies, in part due to chronic inflammation and declining immune system function.1,2 Several studies have suggested PLWH be considered to be of advanced age beginning at age 50.3,4 The immune mechanisms of chronic inflammation and the impact on the cardiovascular system and risk for malignancy are still poorly understood. Measures of chronic inflammation have been shown to be associated with overall mortality and cardiovascular events in PLWH despite ART, even with CD4 cell recovery to >500 cells/mm3.1,5,6
Reduction in plasma tryptophan in patients with HIV was described in 1988; this was later learned to be due to increased degradation of tryptophan, not from low intake. L-tryptophan (2,3)-dioxygenase (TDO) and indoleamine 2,3 dioxygenase (IDO) both catabolize tryptophan (T) to kynurenine (K) in the first step of the kynurenine biosynthetic pathway, which is essential to the production of the neurotransmitter serotonin (5-hydroxytryptamine) and nicotinamide adenine dinucleotide (NAD/NADH). TDO is primarily active in the liver for dietary tryptophan metabolism.7 IDO is produced by dendritic cells and regulatory T cells, and influences T cell differentiation toward T regulatory and away from helper cells (Th-17) by creating a K-rich and T-depleted environment. The ratio of kynurenine to tryptophan (K:T) also directly impairs T cell function.8,9 IDO has been shown to be induced by inteferon gamma (INF-γ), tumor necrosis factor alpha (TNF-α), transforming growth factor beta (TGF-β), and lipopolysaccharide (LPS).7,8 Its activity can be measured by quantification of the K:T ratio in plasma or other tissues.5 IDO plays a critical role in orchestrating immune tolerance in malignancy, tolerance of the gut microbiota, fetal tolerance during pregnancy, and various chronic infections. Chronic IDO activation has been implicated in multiple infectious diseases such as tuberculosis, influenza, leishmaniasis, and listeriosis.10 IDO has also been shown to be the primary driver of peripheral tryptophan levels in PLWH.11–14 Increased IDO activity has been associated with detectable levels of microbial translocation from the gastrointestinal tract, gut microbiota changes, as well as HIV disease progression and mortality rates in PLWH.15
IDO activity level increased with age in a general population cohort without known infections.16 Additionally, higher levels of IDO activity have been associated with increased mortality in PLWH.17 IDO promotes HIV-associated immune pathogenesis in humanized mice and macaques and is directly induced by HIV infection.18 IDO activity along with neopterin, an indicator of inflammation, has been shown to decline in PLWH treated with ART.19 Elevated IDO activity is associated with advancing HIV disease in humans.8 Continuous bacterial translocation from the gastrointestinal tract of PLWH has been theorized to be a major driver of chronic inflammation and progression of comorbidities that persist despite ART.8,20,21
This study examines the effect of age on IDO activity in PLWH who are stable on ART. Immune activation and bacterial translocation are also examined as possible associated factors with IDO activity level in PLWH. The goal of the study was to explore the associations between these parameters and their impact on the hypothesis that IDO activity associated with age or bacterial translocation is a key factor in chronic inflammation in PLWH.
METHODS
In this study, samples of plasma collected between 2000 and 2012, frozen and kept at −80°C, and de-identified data from PLWH who were virally suppressed, defined as viral load <50 copies/mL for at least 1 year, were obtained from the Centers for AIDS Research Network of Integrated Clinical Systems. Samples of plasma collected between 2003 and 2015, frozen and kept at −80°C, and data from age-matched and sex-matched HIV-uninfected (control) patients were obtained from the Multicenter AIDS Cohort Study and the Women’s Interagency HIV Study.
These cohorts were used to ensure an adequate number of samples were obtainable for both PLWH and HIV-uninfected individuals at the extremes of age. In order to ensure adequate representation of ages, 40 PLWH and 20 control patients from each age strata (30–39, 40–49, 50–59, 60–69 and 70–79 years) were requested. Information about age, sex, race, nadir CD4 count, concurrent viral load, concurrent CD4 count and complete blood cell count was obtained. Exclusion criteria included renal disease (characterized as estimated glomerular filtration rate <60 cc/min or a serum creatinine >1.5 mg/dL), nephrotic syndrome, chronic hepatitis C virus, active hepatitis B, autoimmune disease, documented concurrent infection and receipt of immunosuppressive medications such as mycophenolate, ciclosporin, tacrolimus, chemotherapy, immunotherapy, intravenous immunoglobulin, or prednisone.
IDO activity (HPLC)
IDO activity was assessed by de-proteinizing serum for high performance liquid chromatography (HPLC) analysis to detect K and T as described.22
Markers of chronic inflammation, immune status and bacterial translocation
Samples were stored at −80°C until thawing for analysis to minimize changes in neopterin levels prior to measurement.23 Plasma samples were tested for neopterin using commercially available ELISA (IBL International, Mannedorf, Switzerland). Bacterial translocation was evaluated by detection of bacterial 16S ribosomal DNA (16S rDNA) by real-time PCR directly from plasma samples. The quantification of bacteria was based on a standard curve constructed from samples prepared with known quantities of Campylobacter. Bacterial translocation was evaluated by detection of LPS by commercial kit limulus amebocyte lysate assay (Lonza QCL-1000).
Statistical analysis
Descriptive statistics were performed for all subjects. Log transformations were used when needed if continuous data had a skewed distribution. Comparisons between controls and PLWH were made using χ2 tests for categorical data and two sample t-tests with a Satterthwaite adjustment for continuous data. Analysis of variance was used to determine the differences between gender and race for K:T ratio. The association between K:T and covariates (age, CD4, CD4 nadir, levels of bacterial DNA, LPS, and neopterin) for control and PLWH subjects was determined using analysis of covariance homogeneity of slopes model separately for each covariate. A significant interaction between a covariate and group membership would indicate that the relationships were different. A sensitivity analysis was performed to determine the effect of removing PLWH subjects with undetectable viral loads. SAS V.9.4 was used for all analyses. Significance was determined at a type I error rate of 5%.
RESULTS
Samples and data from 205 PLWH on ART and 99 HIV-uninfected (control) patients were analyzed. The groups did not differ by gender, race, or age. The groups were predominantly male (81% for both); 45% of PLWH and 58% of controls self-identified as white, respectively. The prespecified age ranges for assessment were represented; the mean age was 52 in both groups (range 35–83 and 35–87) (table 1).
Table 1.
Demographics for controls and PLWH
| PLWH |
Control |
||
|---|---|---|---|
| n=205 | n=99 | P value | |
| Gender, n (column %) | |||
| Male | 166 (81) | 80 (81) | 0.97 |
| Female | 39 (19) | 19 (19) | |
| Race, n (column %)* | |||
| White | 92 (45) | 46 (58) | 0.17 |
| Black/African American | 79 (39) | 21 (26) | |
| Hispanic/Latino | 25 (12) | 11 (14) | |
| Other | 9 (4) | 2 (3) | |
| Age, median (min, max) | 52 (35, 87) | 52 (35, 83) | 0.39 |
19 control women were missing race.
PLWH, people living with HIV.
Renal function in PLWH was comparable with control patients. As expected, CD4 and CD4 nadir were significantly higher for control subjects (table 2).
Table 2.
Laboratory comparison of controls and PLWH
| PLWH n=205 |
Control n=99 |
P value | |
|---|---|---|---|
| CD4+ cells, median (min, max) Control, n=77 | 512 (30, 2135) | 859 (360, 1717) | <0.0001 |
| CD4+ nadir, median (min, max) Control, n=80 | 143 (0, 668) | 581 (242, 1198) | <0.0001 |
| K:T, median (min, max)* | 0.065 (0.025, 0.361) | 0.047 (0.016, 0.124) | <0.0001 |
| Neopterin, median (min, max)* | 6.4 (0.1, 79.5) | 3.9 (0.5, 58.7) | <0.0001 |
| LPS, median (min, max)* | 0.17 (0, 1.02) | 0.27 (0, 2.0) | <0.0001 |
| 16S rDNA PCR Ct, median (min, max) | n=172 26.9 (21.5, 28.5) |
n=96 27.5 (24.8, 31.3) |
<0.0001 |
Log10 transformation prior to analysis.
K, kynurenine; LPS, lipopolysaccharide; PLWH, people living with HIV; 16S rDNA PCR Ct, PCR cycle threshold for 16S ribosomal DNA; T, tryptophan.
Of note, 12% of the PLWH for whom we received samples had viral loads above the undetectable range at the designated time point. Removing their data in a sensitivity analysis did not change the final results and thus these subjects were retained in order to maximize power. The distribution of K:T for both control and PLWH subjects was no longer skewed after the log transformation. Outliers were not considered to be influential due to the small number present in relation to the total sample size for each group (figure 1). PLWH had higher K:T ratio values across all ages (p<0.0001; figure 2), reflecting higher IDO activity. Even younger (age <50 years) PLWH had median K:T ratio values greater than older (age ≥50 years) control patients (median (min, max): 0.047 (0.016, 0.124) vs 0.065 (0.025, 0.361), respectively). Age was associated with significant increase in K:T, as hypothesized (p<0.0001; figure 2), and this increase was not different depending on status of HIV infection (p=0.92, r2=0.18 for the interaction). K:T ratios did not differ by gender or by race or ethnicity.
Figure 1.
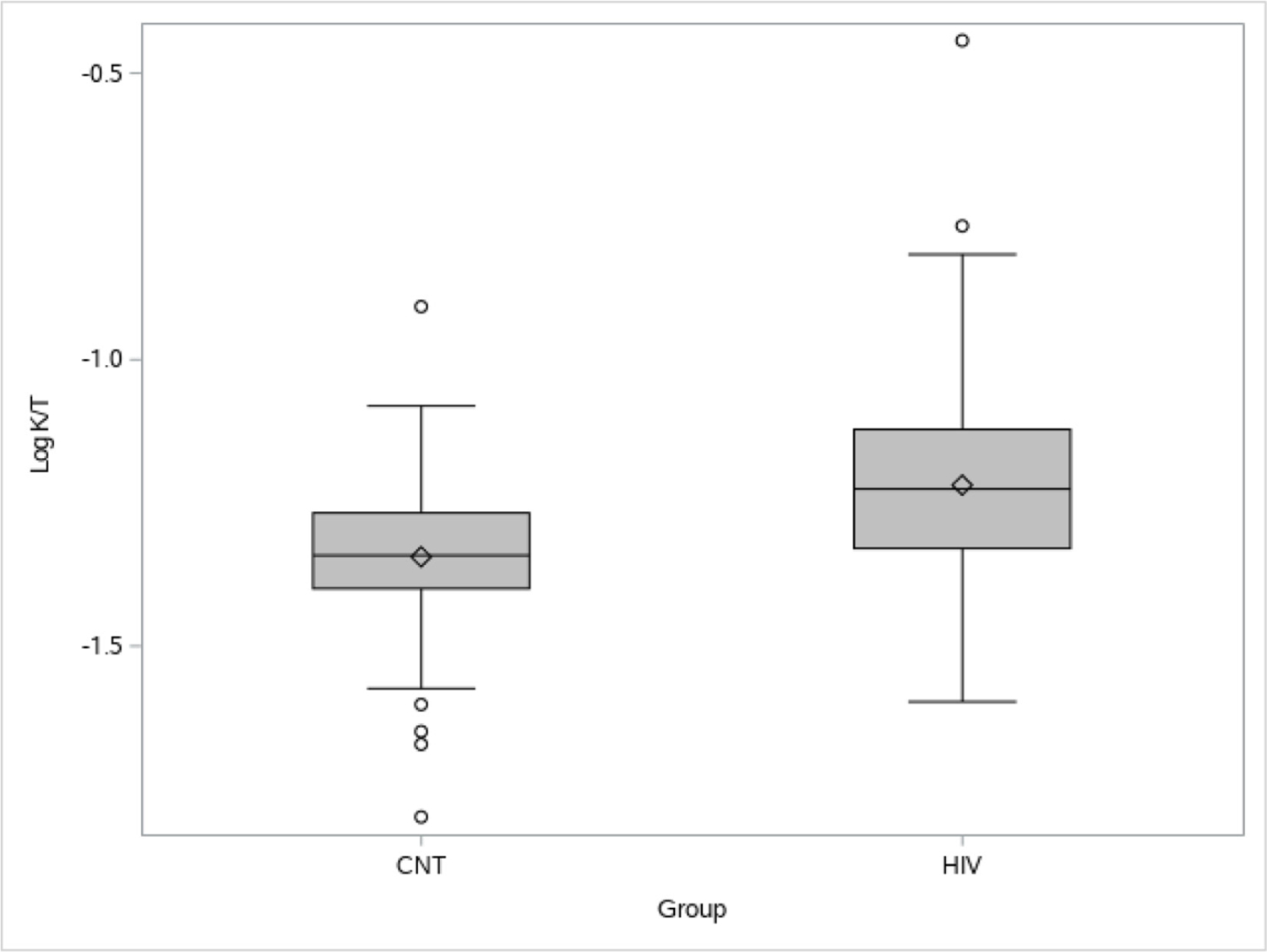
Distribution of log K:T for control (CNT) and people living with HIV (PLWH). PLWH had higher K:T ratios than HIV-uninfected CNT subjects. A log10 transformation (log K:T) was used to minimize the influence of the outlying higher K:T values, especially in PLWH. K, kynurenine; T, tryptophan.
Figure 2.
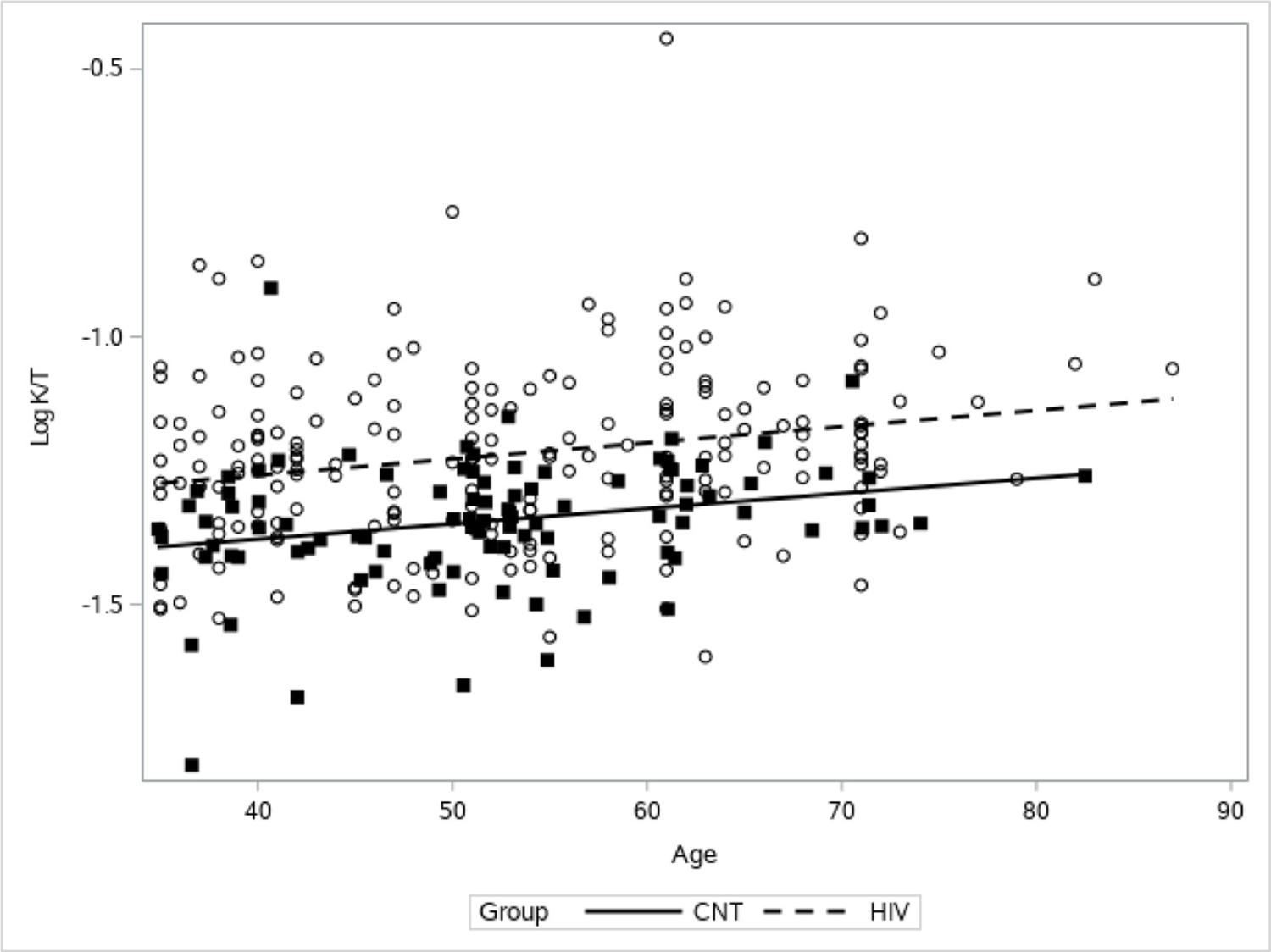
Association between age and K:T ratio. There was a significant positive association between age and K:T (p<0.0001) for both people living with HIV (PLWH) (dotted line and open circles) and control (CNT) (solid line and filled squares). PLWH had higher K:T across ages (p<0.0001). K, kynurenine; T, tryptophan.
Current CD4+ cell count or CD4+ nadir cell count did not appear to have a relationship with K:T ratio (online supplemental digital content figure 1). For PLWH, as LPS increased, K:T decreased, whereas there was no association between K:T and LPS for controls (p=0.0071 for the interaction; figure 3). For both PLWH and controls there was no association between bacterial 16S rDNA PCR cycle threshold detection and K:T ratio (figure 4). PLWH did have a lower cycle threshold for 16S rDNA PCR, indicating more detection of 16S rDNA compared with controls (PLWH mean=26.6, 95% CI 26.5 to 26.8; control=27.5, 95% CI 27.3 to 27.6). As expected, both groups had a positive association between K:T ratio and neopterin (p<0.0001; online supplemental digital content figure 2). Neopterin levels were significantly higher for PLWH subjects compared with controls (table 1).
Figure 3.
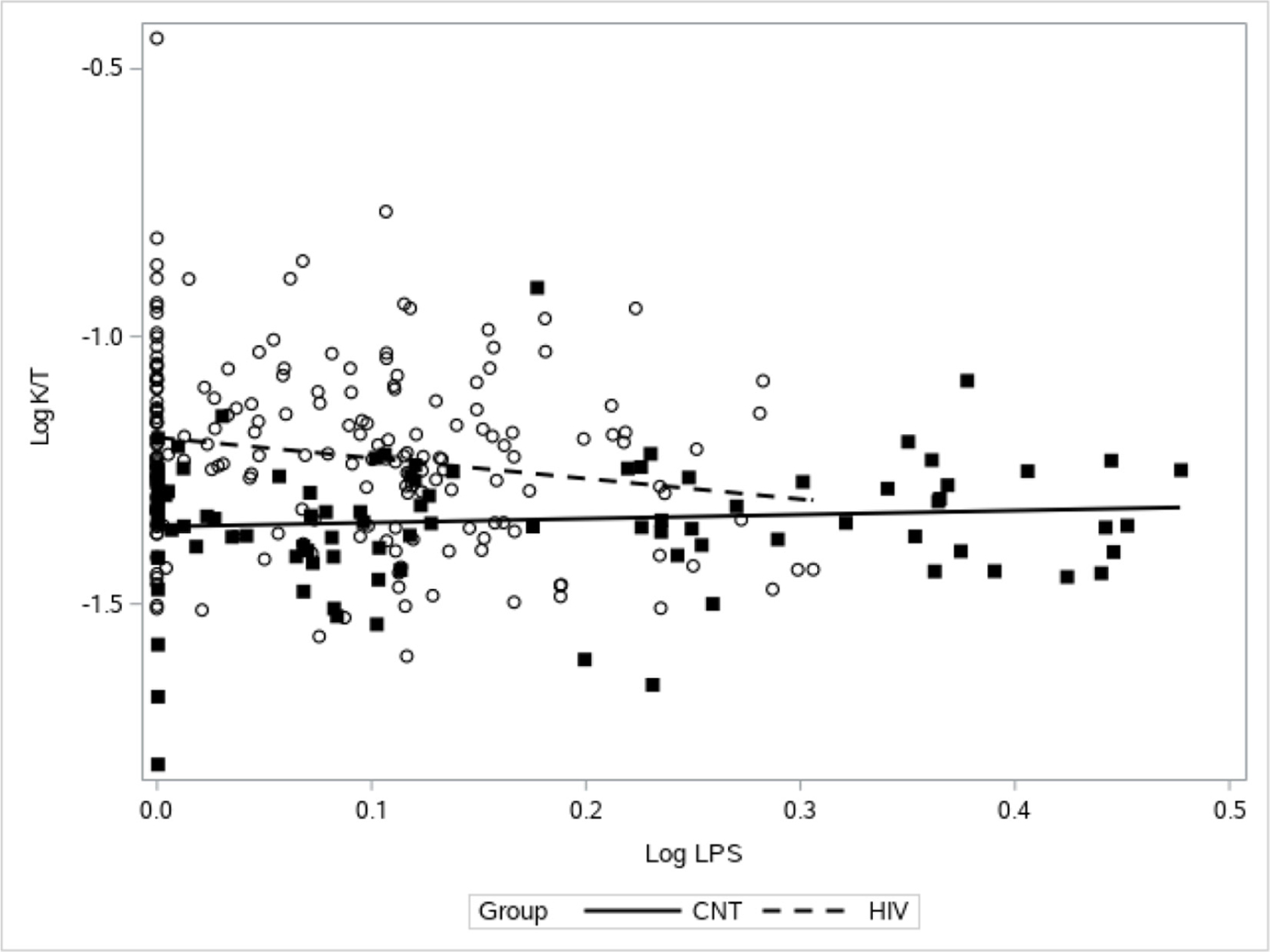
Association between LPS and K:T. The relationship between LPS and K:T is significantly different for HIV-infected and HIV-uninfected (CNT) subjects (p=0.0071 for the interaction). There is no association for HIV-uninfected subjects (solid line and filled squares) and a significant negative relationship for HIV-infected subjects (dotted line and open circles). As LPS increases in HIV-infected subjects, K:T decreases. K, kynurenine; LPS, lipopolysaccharide; T, tryptophan.
Figure 4.
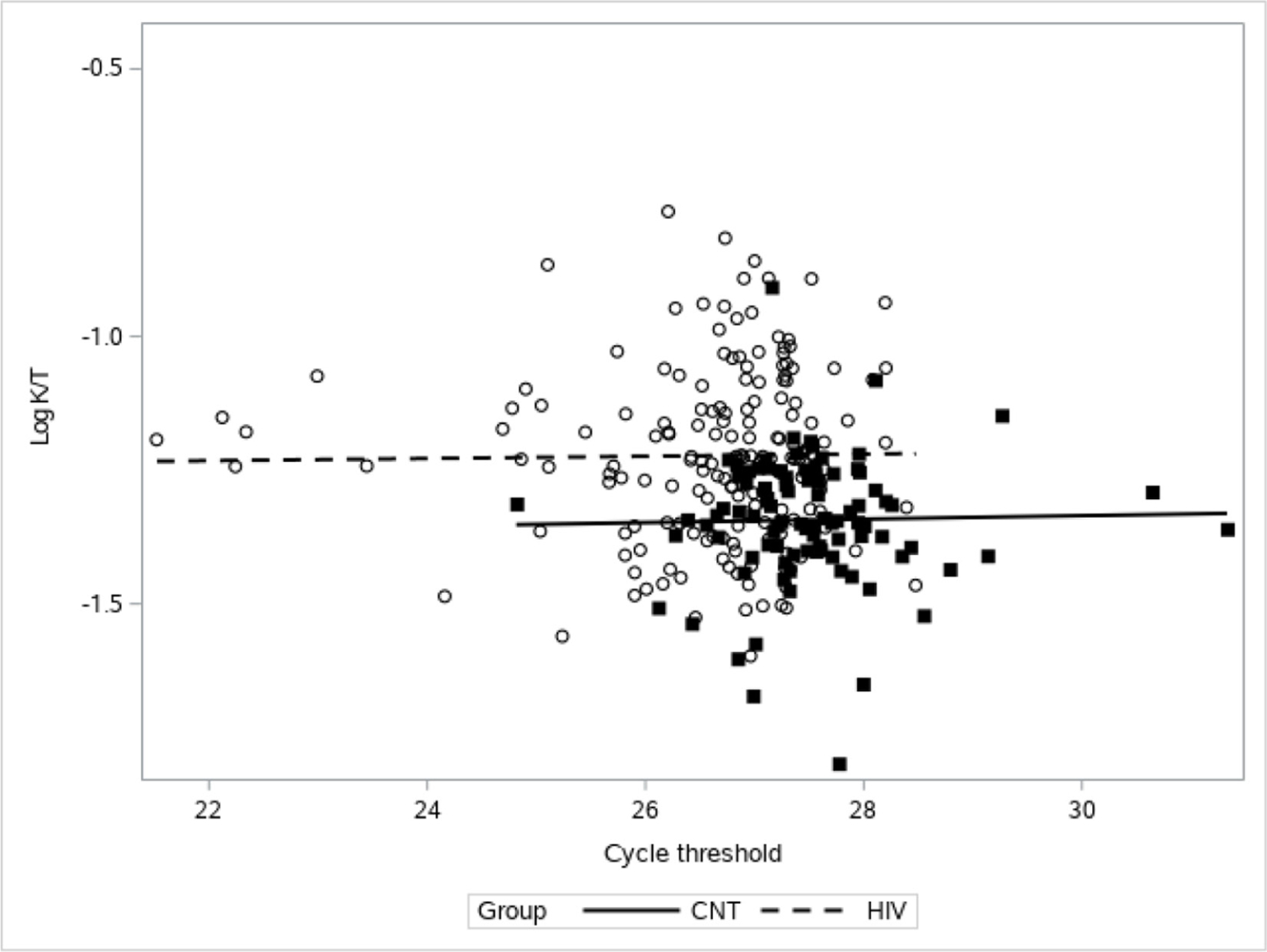
Association between 16S rDNA PCR and K:T. There is not a significant association between 16S rDNA PCR cycle threshold gene expression and K:T levels (p=0.28) for both people living with HIV (dotted line and open circles) and control (CNT) (solid line and filled squares). 16S rDNA PCR, PCR for 16S ribosomal DNA; K, kynurenine; T, tryptophan.
DISCUSSION
This study analyzed samples and data from similar groups of PLWH subjects on ART and HIV-uninfected controls drawn from two major national specimen and data repositories to examine the effect of aging on IDO activity, as measured by K:T ratio, according to HIV status. Large, national cohorts were used to maximize the age range able to be assessed, which is one of the strengths of this study. Ages were requested from specific age strata to ensure that extremes of age were represented, and the groups were well matched. It was found that PLWH on ART have elevated K:T compared with all controls, even at younger ages. This supports previous studies’ findings that PLWH do have a persistent chronic inflammatory state despite measured virologic suppression.6,14,24,25 This study did support the main hypothesis that K:T would increase with advancing age in both groups, in keeping with previously published literature.16,17 This study was the largest, multisite cohort study with sufficient numbers for statistical power in age strata to focus on the effect of both HIV and age on immune regulation. The absolute differences between K:T ratio in the PLWH and control groups were small but statistically significant. This difference may have clinical implications which are yet unknown as most studies that showed increased mortality in PLWH or controls with higher K:T ratios did not consider age.4,5
Similar to our finding of no relationship between IDO activity and microbial translocation, a recent study by Chen and colleagues25 also found no relationship between IDO and markers of translocation, but did find IDO correlated with total HIV DNA in peripheral blood, immune activation, and T cell exhaustion.25 These findings of no association of K:T and microbial translocation are unlike previously published literature which proposed a link between translocation and chronic inflammation in HIV.8,20,21,26 This may reflect advancements in HIV care, specifically earlier implementation of ART, which appears to influence the residual HIV DNA in peripheral blood.25 The CD4 nadir also did not associate with K:T ratio in this cohort, which might also be an effect of earlier ART initiation or more potent modern regimens limiting the depopulation of gut-associated lymphatics or the innate immune system. In our study, the K:T ratio was still indicative of ongoing inflammation in PLWH, as noted by the associated higher levels of neopterin27; however, in this cohort of PLWH, the current assays were unable to detect microbial translocation from the gastrointestinal tract as measured by plasma levels of bacterial 16S rDNA or LPS.
Limitations
The limitations of this study are numerous due to the inherent challenges of using specimen and data repositories. (1) There may have been laboratory limitations due to the storage and freezing/unfreezing of specimens. (2) Data were not available from all repositories regarding whether subjects were fasting at the time of specimen collection, and non-fasting state could lead to elevated tryptophan levels. Whenever possible, fasting specimens were used. (3) There was an inadvertent deviation from the study’s protocol such that 12% of ‘well controlled’ PLWH had detectable viral loads. However, every attempt was made statistically to determine whether inclusion of those subjects would impact the study results. The results were not significantly different regardless of their inclusion. An effort was made to control for the impact of duration or stage of HIV infection by including the CD4 nadir in the analysis. (4) The timing of sample collection and transmitted laboratory data were requested to be the same; however, it is unknown if that was always the case. (5) The samples from PLWH and HIV-negative controls were collected from separate cohorts and thus may have been subject to differences in specimen collection, handling or storage, which may have impacted the results.19 (6) Coexisting infections were excluded where possible, but it is likely that there may have been subclinical or unreported infections at the time of specimen collection. We attempted to minimize the impact of unaccounted for coinfections by deriving controls from cohorts identified as being at increased risk for HIV and thus having a heightened likelihood of having similar comorbidities and exposures as the PLWH cohort.28 (7) Medications have the potential to activate or suppress IDO function29; the attributable impact of specific ART regimens, duration on those regimens, and concomitant medications on IDO function was beyond the scope of this study. (8) Furthermore, the LPS results should be interpreted with caution due to the known limitations of the Lonza assay.
In conclusion, PLWH were observed to have K:T and neopterin levels indicative of increased inflammation at all age strata when compared with age-matched controls. This cohort also demonstrated advancing age to be associated with an increase in the K:T ratio levels, indicating increased IDO activity with age for the first time in PLWH. Also, markers of microbial translocation (16S rDNA and LPS) did not appear to correlate positively with K:T ratio for PLWH or control subjects. This study suggests K:T ratio increases with age in both groups and is elevated in PLWH at all ages compared with age-matched controls.
Supplementary Material
Significance of this study.
What is already known about this subject?
People living with HIV (PLWH) often experience the complications of aging earlier.
PLWH, despite virologic control, have measurable increased levels of chronic inflammation.
Evidence of immune regulation by indoleamine 2,3 dioxygenase (IDO) is more active in PLWH.
What are the new findings?
With aging, PLWH have elevated IDO activity when compared with age-matched control patients without HIV.
Even younger PLWH have higher IDO activity than all control patients without HIV.
IDO activity, thought to be driven by microbial translocation from the gastrointestinal tract, may be driven by multiple factors in PLWH.
How might these results change the focus of research or clinical practice?
Chronic inflammation in PLWH could be from stimulation from the microbiome, but further studies need to be done to demonstrate the mechanism.
In clinical practice, premature aging of PLWH should encourage vigilance for complications of aging in this population.
Acknowledgements
We would like to thank the patients who participated in the specimen repositories and the repositories for their support of this project. Special thanks to Elias Manavathu for laboratory support. WIHS Principal Investigators: UAB-MS WIHS (Mirjam-Colette Kempf and Deborah Konkle-Parker), U01-AI-103401; Atlanta WIHS (Ighovwerha Ofotokun, Anandi Sheth, and Gina Wingood), U01-AI-103408; Bronx WIHS (Kathryn Anastos and Anjali Sharma), U01-AI-035004; Brooklyn WIHS (Deborah Gustafson and Tracey Wilson), U01-AI-031834; Chicago WIHS (Mardge Cohen and Audrey French), U01-AI-034993; Metropolitan Washington WIHS (Seble Kassaye and Daniel Merenstein), U01-AI-034994; Miami WIHS (Maria Alcaide, Margaret Fischl, and Deborah Jones), U01-AI-103397; UNC WIHS (Adaora Adimora), U01-AI-103390; Connie Wofsy Women’s HIV Study, Northern California (Bradley Aouizerat and Phyllis Tien), U01-AI-034989; WIHS Data Management and Analysis Center (Stephen Gange and Elizabeth Golub), U01-AI-042590; Southern California WIHS (Joel Milam), U01-HD-032632 (WIHS I–WIHS IV).
Funding
This work was supported by an Augusta University Department of Medicine Research Initiative Grant, an Augusta University Immunotherapy Discovery Institute Seed Grant, P30 AI027763, and R24 AI067039. The WIHS is funded primarily by the National Institute of Allergy and Infectious Diseases (NIAID), with additional co-funding from the Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD), the National Cancer Institute (NCI), the National Institute on Drug Abuse (NIDA), and the National Institute of Mental Health (NIMH). Targeted supplemental funding for specific projects is also provided by the National Institute of Dental and Craniofacial Research (NIDCR), the National Institute on Alcohol Abuse and Alcoholism (NIAAA), the National Institute on Deafness and other Communication Disorders (NIDCD), and the NIH Office of Research on Women’s Health. WIHS data collection is also supported by UL1-TR000004 (UCSF CTSA), UL1-TR000454 (Atlanta CTSA), P30-AI-050410 (UNC CFAR), and P30-AI-027767 (UAB CFAR).
Footnotes
The results presented in this paper were presented at IDWeek October 2018, San Francisco, California (poster #639).
Publisher's Disclaimer: Disclaimer The funding organizations had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication. The contents of this publication are the sole responsibility of the authors and do not necessarily reflect the views, opinions or policies of Uniformed Services University of the Health Sciences (USUHS), The Henry M Jackson Foundation for the Advancement of Military Medicine, the Department of Defense (DoD), the Department of Veterans Affairs, or the Departments of the Army, Navy, or Air Force. Mention of trade names, commercial products, or organizations does not imply endorsement by the US Government. Investigator time was supported with resources from the Charlie Norwood VA Medical Center. The contents of this publication are solely the responsibility of the authors and do not represent the official views of the National Institutes of Health (NIH), the federal government, or affiliated institutions. The authors assume full responsibility for analyses and interpretations of these data. Data in this manuscript were collected by the CFAR Network of Integrated Clinical Systems (CNICS) and MACS/WIHS Combined Cohort Study (CCS).
Competing interests SB is an Editorial Board Member of the Journal of Investigative Medicine. ALM is a shareholder in NewLink Genetics and receives licensing income from this source. All other authors declare no conflicts.
Patient consent for publication Not required.
Ethics approval This study was deemed to not require ethics approval as it was judged to be non-human subject research by the Augusta University Institutional Review Board as only de-identified data and specimens were used and no prospective data or specimens were collected (Human Subjects Determination IRBHSD00000001). Informed consent was obtained prior to participation for all subjects by each of the respective cohort studies. Both repositories are approved by their respective institutional review boards and have consent for samples and data to be used for subsequent studies.
Provenance and peer review Not commissioned; externally peer reviewed.
Supplemental material This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.
Data availability statement
Data are available upon reasonable request.
REFERENCES
- 1.Freiberg MS, Chang C-CH, Kuller LH, et al. Hiv infection and the risk of acute myocardial infarction. JAMA Intern Med 2013;173:614–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Shiels MS, Pfeiffer RM, Engels EA. Age at cancer diagnosis among persons with AIDS in the United States. Ann Intern Med 2010;153:452–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Desai S, Landay A. Early immune senescence in HIV disease. Curr HIV/AIDS Rep 2010;7:4–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Effros RB, Fletcher CV, Gebo K, et al. Aging and infectious diseases: workshop on HIV infection and aging: what is known and future research directions. Clin Infect Dis 2008;47:542–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hunt PW, Cao HL, Muzoora C, et al. Impact of CD8+ T-cell activation on CD4+ T-cell recovery and mortality in HIV-infected Ugandans initiating antiretroviral therapy. AIDS 2011;25:2123–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lederman MM, Funderburg NT, Sekaly RP, et al. Residual immune dysregulation syndrome in treated HIV infection. Adv Immunol 2013;119:51–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Boasso A, Shearer GM. How does indoleamine 2,3-dioxygenase contribute to HIV-mediated immune dysregulation. Curr Drug Metab 2007;8:217–23. [DOI] [PubMed] [Google Scholar]
- 8.Favre D, Mold J, Hunt PW, et al. Tryptophan catabolism by indoleamine 2,3-dioxygenase 1 alters the balance of Th17 to regulatory T cells in HIV disease. Sci Transl Med 2010;2:32ra36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jenabian M-A, Patel M, Kema I, et al. Distinct tryptophan catabolism and Th17/Treg balance in HIV progressors and elite controllers. PLoS One 2013;8:e78146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mellor AL, Munn DH. Creating immune privilege: active local suppression that benefits friends, but protects foes. Nat Rev Immunol 2008;8:74–80. [DOI] [PubMed] [Google Scholar]
- 11.Fuchs D, Hausen A, Reibnegger G, et al. Interferon-Gamma concentrations are increased in sera from individuals infected with human immunodeficiency virus type 1. J Acquir Immune Defic Syndr 1989;2:158–62. [PubMed] [Google Scholar]
- 12.Fuchs D, Forsman A, Hagberg L, et al. Immune activation and decreased tryptophan in patients with HIV-1 infection. J Interferon Res 1990;10:599–603. [DOI] [PubMed] [Google Scholar]
- 13.Fuchs D, Möller AA, Reibnegger G, et al. Increased endogenous interferon-gamma and neopterin correlate with increased degradation of tryptophan in human immunodeficiency virus type 1 infection. Immunol Lett 1991;28:207–11. [DOI] [PubMed] [Google Scholar]
- 14.Murray MF. Tryptophan depletion and HIV infection: a metabolic link to pathogenesis. Lancet Infect Dis 2003;3:644–52. [DOI] [PubMed] [Google Scholar]
- 15.Jenabian M-A, El-Far M, Vyboh K, et al. Immunosuppressive tryptophan catabolism and gut mucosal dysfunction following early HIV infection. J Infect Dis 2015;212:355–66. [DOI] [PubMed] [Google Scholar]
- 16.Frick B, Schroecksnadel K, Neurauter G, et al. Increasing production of homocysteine and neopterin and degradation of tryptophan with older age. Clin Biochem 2004;37:684–7. [DOI] [PubMed] [Google Scholar]
- 17.Pertovaara M, Raitala A, Lehtimäki T, et al. Indoleamine 2,3-dioxygenase activity in nonagenarians is markedly increased and predicts mortality. Mech Ageing Dev 2006;127:497–9. [DOI] [PubMed] [Google Scholar]
- 18.Boasso A, Vaccari M, Fuchs D, et al. Combined effect of antiretroviral therapy and blockade of IDO in SIV-infected rhesus macaques. J Immunol 2009;182:4313–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zangerle R, Widner B, Quirchmair G, et al. Effective antiretroviral therapy reduces degradation of tryptophan in patients with HIV-1 infection. Clin Immunol 2002;104:242–7. [DOI] [PubMed] [Google Scholar]
- 20.Routy J-P, Mehraj V, Vyboh K, et al. Clinical relevance of kynurenine pathway in HIV/AIDS: an immune checkpoint at the crossroads of metabolism and inflammation. AIDS Rev 2015;17:96–106. [PubMed] [Google Scholar]
- 21.Hunt PW, Sinclair E, Rodriguez B, et al. Gut epithelial barrier dysfunction and innate immune activation predict mortality in treated HIV infection. J Infect Dis 2014;210:1228–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Brandacher G, Margreiter R, Fuchs D. Clinical relevance of indoleamine 2,3-dioxygenase for alloimmunity and transplantation. Curr Opin Organ Transplant 2008;13:10–15. [DOI] [PubMed] [Google Scholar]
- 23.Aziz N, Detels R, Quint JJ, et al. Stability of cytokines, chemokines and soluble activation markers in unprocessed blood stored under different conditions. Cytokine 2016;84:17–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Somsouk M, Estes JD, Deleage C, et al. Gut epithelial barrier and systemic inflammation during chronic HIV infection. AIDS 2015;29:43–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chen J, Xun J, Yang J, et al. Plasma indoleamine 2,3-dioxygenase activity is associated with the size of the human immunodeficiency virus reservoir in patients receiving antiretroviral therapy. Clin Infect Dis 2019;68:1274–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Brenchley JM, Price DA, Schacker TW, et al. Microbial translocation is a cause of systemic immune activation in chronic HIV infection. Nat Med 2006;12:1365–71. [DOI] [PubMed] [Google Scholar]
- 27.Schroecksnadel K, Zangerle R, Bellmann-Weiler R, et al. Indoleamine-2, 3-dioxygenase and other interferon-gamma-mediated pathways in patients with human immunodeficiency virus infection. Curr Drug Metab 2007;8:225–36. [DOI] [PubMed] [Google Scholar]
- 28.García-Álvarez M, Berenguer J, Guzman-Fulgencio M, et al. Bacterial DNA translocation and liver disease severity among HIV-infected patients with chronic hepatitis C. J Acquir Immune Defic Syndr 2012;61:552–6. [DOI] [PubMed] [Google Scholar]
- 29.Maneechotesuwan K, Ekjiratrakul W, Kasetsinsombat K, et al. Statins enhance the anti-inflammatory effects of inhaled corticosteroids in asthmatic patients through increased induction of indoleamine 2, 3-dioxygenase. J Allergy Clin Immunol 2010;126:754–62. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data are available upon reasonable request.


