Abstract
Clinical studies indicate that obese individuals have an increased risk of developing co-morbid depressive illness and that these patients have reduced responses to antidepressant therapy, including selective serotonin reuptake inhibitors (SSRIs). Obesity, a condition of chronic mild inflammation including obesity-induced neuroinflammation, is proposed to contribute to decreases in synaptic concentrations of neurotransmitters like serotonin (5HT) by decreasing 5HT synthesis in the dorsal raphe nucleus (DRN) and/or affecting 5HT reuptake in DRN target regions like the hippocampus. In view of these observations, the goal of the current study was to examine inflammatory markers and serotonergic dynamics in co-morbid obesity and depression. Biochemical and behavioral assays revealed that high fat diet produced an obesity and depressive-like phenotype in one cohort of rats and that these changes were marked by increases in key pro-inflammatory cytokines in the hippocampus. In real time using fast scan cyclic voltammetry (FSCV), we observed no changes in basal levels of hippocampal 5HT, however responses to escitalopram were significantly impaired in the hippocampus of obese rats compared to diet resistant rats and control rats. Further studies revealed that these neurochemical observations could be explained by increases in SERT expression in the hippocampus driven by elevated neuroinflammation. Collectively, these results demonstrate that obesity-induced increases in neuroinflammation adversely affects SERT expression in the hippocampus of obese rats, thereby providing a potential synaptic mechanism for reduced SSRI responsiveness in obese subjects with co-morbid depressive illness.
Keywords: Serotonin, obesity, fast scan cyclic voltammetry, neuroinflammation
1. Introduction
The global rate of adult obesity has nearly tripled since 1975, now affecting a staggering 650 million people worldwide (World Health Organization, 2020). Obesity is defined as a body mass index (BMI) greater than 30 and is associated with a host of co-morbidities, including cardiovascular disease, type 2 diabetes mellitus (T2DM) and the metabolic syndrome (MetS). In addition to well described peripheral symptoms of obesity, the complications of obesity extend to the central nervous system (CNS) and may result in an increased risk for neurological comorbidities like depression. In support of this hypothesis, epidemiological studies indicate that there is an association between obesity and mood disorders (Stunkard et al., 2003;McElroy et al., 2004;Fabricatore and Wadden, 2006;Simon et al., 2006;Andersen et al., 2010;Luppino et al., 2010); this correlation is particularly strong for individuals with a BMI greater than 40 (Onyike et al., 2003). Animal studies support the concept that obesity induces depressive-like behaviors. For example, several genetic models of obesity (i.e. ob/ob mice and db/db mice) exhibit increased immobility time in the forced swim test (FST) compared to wild-type controls, a behavioral change that is indicative of behavioral despair in rodents (Collin et al., 2000;Sharma et al., 2010;Yamada et al., 2011). Diet-induced obesity (DIO) mice also exhibit decreased sucrose intake (i.e. anhedonia) and increased immobility in the FST (Yamada et al., 2011). While a number of factors have been suggested as mechanistic links between obesity and mood disorders (Raison et al., 2006;Reagan, 2012), neuroinflammation may be a key initiating factor in this co-morbidity.
Obesity is recognized as a state of chronic mild inflammation, which is proposed to play a critical role in the pathogenesis of co-morbid neuropsychiatric disorders, in part by disrupting neurotransmitters, such as serotonin (5HT); for reviews, see (Shelton and Miller, 2010;Soczynska et al., 2011). In this regard, obesity-induced neuroinflammation is proposed to decrease 5HT synthesis in the dorsal raphe nucleus (DRN) (Shelton and Miller, 2010). The raphe nucleus (RN) is located in the medial portion of the brainstem and is the primary site of 5HT synthesis in the CNS. The RN sends serotonergic projections throughout the brain, including to the hippocampus, and these serotonergic projections modulate a variety of brain functions, including mood. Indeed, decreased extracellular 5HT levels are the core feature of the monoamine hypothesis of depression. The efficacy of antidepressant drugs that increase brain levels of 5HT, such as the selective serotonin reuptake inhibitors (SSRIs), further supports a role for 5HT in the pathophysiology of depression. While obesity-induced increases in neuroinflammation in the RN have been proposed to reduce 5HT synthesis and thereby reduce synaptic concentrations of 5HT and SSRI responsiveness in RN projection sites like the hippocampus (Shelton and Miller, 2010), this hypothesis has not been directly examined. To address the roles of 5HT in obesity-induced neuroinflammation, in this work we apply innovative in vivo electrochemical tools for 5HT analysis in the rodent brain, namely fast scan cyclic voltammetry (FSCV) and fast scan cyclic adsorption voltammetry (FSCAV). FSCV and FCAV allow for assessment of basal synaptic concentrations of 5HT, as well as responsiveness to SSRIs, in real time. Accordingly, the current study combined these tools with biochemical analyses and microscopy to examine the nexus between obesity, inflammation, and 5HT neurochemistry underlying SSRI response. Taken together, these findings shed important light on functional changes in the rat brain in response to DIO and provide a mechanistic foundation for better understanding clinical SSRI therapy in obese individuals.
2. Materials and methods
2.1. Animals
All animal procedures and protocols were performed in accordance with regulations of the Columbia VA Health Care System Institutional Animal Care and Use Committee (IACUC), which operates with accreditation from the Association for Assessment and Accreditation of Laboratory Animal Care (AAALAC). Adult male Sprague Dawley rats were purchased from Envigo; rats were approximately 10 weeks of age at the start of the study. Animals were housed individually with enrichment on 12 hr light/dark cycles with ad libitum access to food and water. Animals were kept on a standard chow diet for one week while habituating upon arrival. Prior to dietary treatment, body weights were determined and rats were randomly assigned to the control diet group or the HFD group. This study used a 45 kcal% fat diet (Research Diets; cat# D12451) and the corresponding control diet (10 kcal% fat) that matches sucrose levels to the 45% fat diet (Research Diets cat# D12450H). Rats were maintained on the diet for at least 12 weeks and body weights were measured daily throughout treatment. Rats were maintained on their respective diets as they underwent behavioral and neurochemical studies. At sacrifice, trunk blood was collected and brains were harvested.
2.2. Chemicals and Reagents
Electrodes were calibrated in vitro using 5HT solutions of 10, 25, 50, and 100 nM of serotonin hydrochloride (Sigma-Aldrich Co., St. Louis, MO, USA) dissolved in tris buffer (15 nM H2NC(CH2OH)2 HCl, 140 mM NaCl, 3.25 mM KCl, 1.2 mM CaCl2, 1.25 mM NaH2PO4·H2O, 1.2 mM MgCl2, and 2.0 mM Na2SO4 (Sigma-Aldrich Co., St. Louis, MO, USA)). Escitalopram oxalate (≥98, HPLC, Sigma-Aldrich Co., St. Louis, MO, USA) at 10 mg kg−1 was dissolved in sterile saline (0.9% NaCl solution, Hospira, Lake Forest, IL, USA) and administered via intraperitoneal (i.p.) injections. Nafion™ (LQ-1105, 5% by weight, Ion Power Solutions, New Castle, DE, USA) was used to modify the microelectrodes.
2.3. Sucrose Preference
The sucrose preference test was performed as described previously (Grillo et al., 2011;Grillo et al., 2014;Macht et al., 2017). Rats were exposed for 24 hours to two identical bottles containing water and 1% sucrose solution. The next day the rats were water-deprived for 7 hours (12:00 -p.m.7:00 p.m.) before testing their preference for sucrose (1%) or water in a three-hour two-bottle choice beginning at the start of the dark cycle (7:00 pm).
2.4. Endocrine analyses
Plasma endocrine analyses were assessed as described in our previous studies (Grillo et al., 2015). Specifically, plasma triglycerides were determined using an enzymatic kit (modified Trinder; Pointe Scientific, Inc., Canton, MI, USA) and plasma leptin levels were determined by enzymelinked immunosorbent assay (ELISA; MilliporeSigma, Burlington, MA) according to the manufacturer’s instructions.
2.5. Neurochemical analysis
Stereotaxic surgeries (David Kopf Instruments, Tujunga, CA) were completed while animals were under isoflurane anesthesia. A deltaphase isothermal pad (Braintree Scientific, Braintree, MA) set at 37°C was used to maintain a healthy body temperature during surgery; coordinates were determined in reference to bregma. A nafion-modified carbon fiber microelectrode (fabricated as described in (Hashemi et al., 2009)) was placed into the hippocampus/CA2 (AP −5.5 mm, ML +5.0 mm, DV −4.0 mm). A stainless-steel stimulating electrode (diameter 0.2 mm, Plastics One, Roanoke, VA) was placed into the medial forebrain bundle (MFB) (AP −2.8 mm, ML +1.7 mm, DV −8.0 mm). A biphasic pulse (x120) was applied through a linear constant current stimulus isolator (NL800A, Neurolog, Medical Systems Corp., Great Neck, NY). Trains of 60 Hz were 350 μA at each phase (2 ms in width and 2 s in length). Additionally, an Ag/AgCl reference electrode was placed in the contralateral hemisphere. WCCV 2.0 (written by Christopher W. Atcherley and Michael L. Heien using LabVIEW 2012 (National Instruments, TX)) and a CHEM-CLAMP potentiostat (Dagan Corporation, MN) were used for FSCV to measure current. A waveform scanning from −0.1 V to 1.0 V, while holding at 0.2 V (Jackson et al., 1995) at 1000 V s−1 was employed. Calibrations for 5HT from current to concentration were used from previous work (Hashemi et al., 2009). Four baseline FSCV files of 5HT, each 10 min apart, were averaged for each animal to ensure signal stability, and then 10 mg/kg escitalopram was administered and 5HT was analyzed for two hours post administration. FSCAV was employed, prior to escitalopram administration, to determine basal measures of 5HT using methodology outlined in Abdulla et al., 2017. The 5HT waveform was applied at 100 Hz for 2 s, and then held at 0.2 V for 10 s to allow for controlled adsorption, then the 5HT waveform was then reapplied at 100 Hz, as described in Abdalla et al. 2017. Fifteen stable files (at one file per minute) were collected as baseline files. Post-surgery, electrodes underwent a post calibration of 10 files in solutions of 10, 25, 50 and 100 nM solutions of 5HT. Brain tissue was collected at the end of experiments after a high voltage was applied to the carbon fiber electrode to lesion the tissue and leave damage to determine electrode placement (as the electrode is too small for detection otherwise). Tissue was stored first in 4% paraformaldehyde and then in a 30% sucrose solution. The tissue was sliced on a cryostat and damage produced by the stimulating and carbon fiber electrodes could be visualized.
2.6. Cytokine analysis in dorsal raphe nucleus and hippocampus
Analysis of cytokine levels in the DRN and hippocampus was performed in a separate cohort of control, DR and DIO rats not used in FSCV experiments, as described in our earlier studies (Grillo et al., 2019). Briefly, dissected brain tissue was removed from −80° C and homogenized in lysis buffer (137mM NaCl, 20mM Tris-HCl, 10% Glycerol, 1% Tergitol-type NP-40) at a ratio of 1:5. After homogenization, each sample was sonicated for 30 seconds on ice and then centrifuged at 4° C for 15 minutes at 14,000g. One part supernatant was diluted with 4 parts DPBS (2.7mM KCl, 136.9mM NaCl, 1.5mM KH2PO4, 8.1mM Na2HPO4, 0.9mM CaCl2·2H20, 0.5mM MgCL2·6H20) and 1N HCL was added to each sample at a dilution of 1:50 (pH ≤ 3). Samples were quickly vortexed and were then neutralized with 1N NaOH at a dilution of 1:50 (pH 7.6). Prepared samples were stored at −20° C until use. Th1/Th2 rat cytokines were quantified using a Bio-Plex cytokine assay from Bio-Rad (#171k1002M) according to the manufacturer’s instructions. Brain lysates were prepared from DRN and hippocampus; a 1:3 dilution was used for RN extracts and hippocampal extracts were used neat. The plate was read on a Luminex plate reader using high photomultiplier voltage and analyzed with Bio-Plex manager software. Values were normalized for protein and expressed as pg/mg protein for each cytokine.
2.7. SERT Immunofluorescence and Intensity Analysis
Rats were transcardially perfused with 4% paraformaldehyde prepared in 0.1M phosphate buffer. Upon removal, brains were post-fixed for 1 hour in 4% paraformaldehyde at room temperature, dehydrated in 30% sucrose in 0.1M PB and frozen in 100% methyl butane on dry-ice. Brains were sectioned at 40μm on a sliding microtome and kept in cryoprotectant (25% glycerol, 25% propylene glycol/PBS) at −20°C until staining. Sections were washed in 0.1M PBS 3 times for 5 minutes each before being blocked in 4% normal horse serum with 0.4% Triton X-100 in 0.1M PBS for 1 hour. Sections were then incubated with primary antibody (Anti-serotonin transporter, (Neuromics RA24330; 1:500) and incubated overnight at 4°C. The samples were then washed and incubated in secondary antibody (goat anti-rabbit Alexafluor 546, Invitrogen A-11035 1:200; Invitrogen, Carlsbad, CA) for 4 hours at room temperature. Sections were washed, mounted on slides coated with 0.3% gelatin, and cover slipped with ProLong Gold antifade mounting medium (P36930; Invitrogen, Carlsbad CA). All images were taken on a Leica SP8 confocal microscope under the same conditions and settings. To compare fluorescent intensity, all z-stack images were collapsed into a maximum intensity projection. In ImageJ, the region of interest was outlined to focus on regions of SERT expression. Any regions that showed no staining were not selected for intensity comparison. Mean intensity was then measured from the remaining area. At least 3 images were measured for each animal.
2.8. Statistical analysis:
Statistical analysis was performed using a one-way ANOVA followed by a Tukey post hoc test. P ≤ 0.05 was set as set as the criterion for statistical significance. Basal and post escitalopram SERT reuptake (t1/2) was analyzed using a two-way ANOVA followed by a Tukey post hoc test.
3. Results
3.1. High fat diet elicits obesity in some but not all rats: DR versus DIO
To elicit an obesity phenotype, adult male Sprague Dawley rats were randomly assigned to a control diet group or a high fat diet (HFD) group. Rats were provided ad lib access to a 45 kcal% fat diet or the corresponding control diet. Previous seminal observations by Levin and coworkers illustrated that approximately 50% of rats provided a HFD will develop an obesity phenotype (i.e. diet-induced obesity; DIO), while the remaining rats will not (i.e. diet resistant; DR) (Levin et al., 1997). Rats segregated into DIO and DR groups after several weeks on the HFD; several metabolic and endocrine measures can be performed to differentiate between DIO and DR rats. In this regard, Figure 1 illustrates that increases in body weight are observed in DIO rats (n=6) compared to DR rats (n=7) and rats provided control chow (n=7) for the duration of the 16-week study (Figure 1A; [F(2,18)=22.44; p=0.0001]). An endocrine measure that many investigators use to distinguish between control, DR and DIO rats is plasma leptin, as leptin resistance is a hallmark feature of obesity. DIO rats exhibited the expected increases in plasma leptin levels compared to DR rats and control rats (Figure 1B; [F(2,18) = 8.754; p=0.002). Interestingly, rats provided a HFD did not exhibit changes in plasma triglycerides (Figure 1C; [F(2,18)=2.626; p=0.1). There are a variety of behavioral changes that have been described in DIO rats, ranging from changes in food intake, cognition, and most relevant to our study, anhedonia. In agreement with previous studies in obese rats (Grillo et al., 2011) and mice fed a HFD (Yamada et al., 2011), DIO rats exhibited significant decreases in one-hour [F(2,18)=22.56; p=0.0001], three-hour [F(2,18)=32.26; p=0.0001] and 12-hour sucrose consumption [F(2,18)=11.40; p=0.0007] (i.e. anhedonia) compared to DR rats and control chow rats (Figure 1). Interestingly, DR rats also exhibited anhedonia relative to control rats at the three-hour time point. Collectively, these results confirmed the ability of the HFD to induce an obesity phenotype and anhedonia in some (DIO) but not all (DR) rats provided access to a HFD.
Fig. 1.
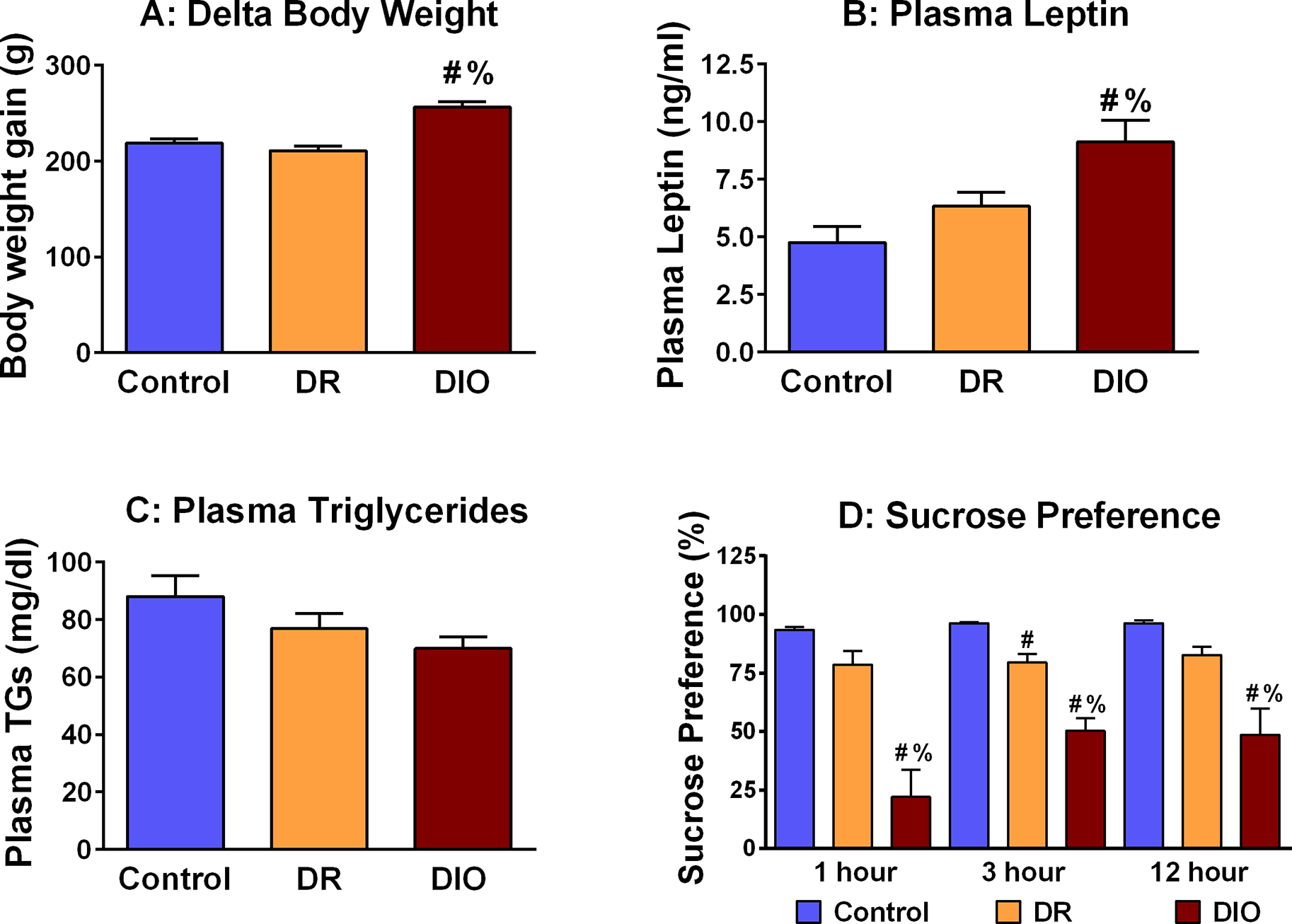
High fat diet exposure elicits an obesity and depressive-like phenotype in DIO, but not DR rats. (A) Cumulative body weight changes are significantly increased in DIO rats compared to DR rats and rats provided access to a control diet [F(2,18) = 22.44; p = 0.0001]. (B) DIO rats exhibit significant increases in plasma leptin levels compared to Control-chow rats and DR rats [F(2,18) = 8.754; p = 0.002]. (C) Plasma triglycerides are unchanged in Control rats, DR rats and DIO rats [F(2,18) = 2.626; p = 0.1]. (D) One-hour, three-hour and 12-hour sucrose preference was significantly decreased in DIO rats compared to Control rats and DR rats. Three-hour sucrose preference was also decreased in DR rats compared Control rats. One-hour [F(2,18) = 22.56; p = 0.001]; three-hour [F(2,18) = 32.26; p = 0.0001]; 12-hour [F(2,18) = 11.40; p = 0.0007] [# = p < 0.05 compared to control; % = p < 0.05 compared to DR]
3.2. Escitalopram is less chemically effective in the hippocampus of DIO rats
In view of clinical observations that obese subjects with co-morbid depression are more likely to exhibit treatment resistance to antidepressants (Kloiber et al., 2007;Rizvi et al., 2014), including SSRIs (Khan et al., 2007;Lin et al., 2014), we analyzed the serotonergic system in the hippocampus of live anesthetized rats using FSCV and FSCAV. Figure 2 shows confirmation of this methodology as well as traditional 5HT responses to the SSRI, escitalopram, marked by increases in amplitude of evoked release (Figure 2C) and slowing of reuptake in rats that were fed a ‘normal chow’ diet (Figure 2D). An example of stimulating electrode placement in the MFB and carbon fiber microelectrode placement in the CA2 region of the hippocampus are shown in Figure 3. Neurochemical studies showed that diet does not change baseline evoked release (FSCV) (Figure 4B–D) or extracellular levels of 5HT (FSCAV) (Figure 4E). However, we did see differences in stimulated release following i.p. administration of escitalopram (10 mgkg−1) (Figure 4B–D). Previous work by the Hashemi lab (Saylor et al. 2019) demonstrated that SSRI response is dose dependent but that 10 mg/kg escitalopram was sufficient to produce robust changes in hippocampal evoked serotonin (both increases in amplitude of release and slowing of reuptake). In particular, escitalopram produced more prominent slowing of reuptake (decrease in slope of the reuptake curve) in control and DR rats than in DIO rats (see Figure 4B–D).
Fig. 2. Response of hippocampal 5HT to escitalopram challenge.
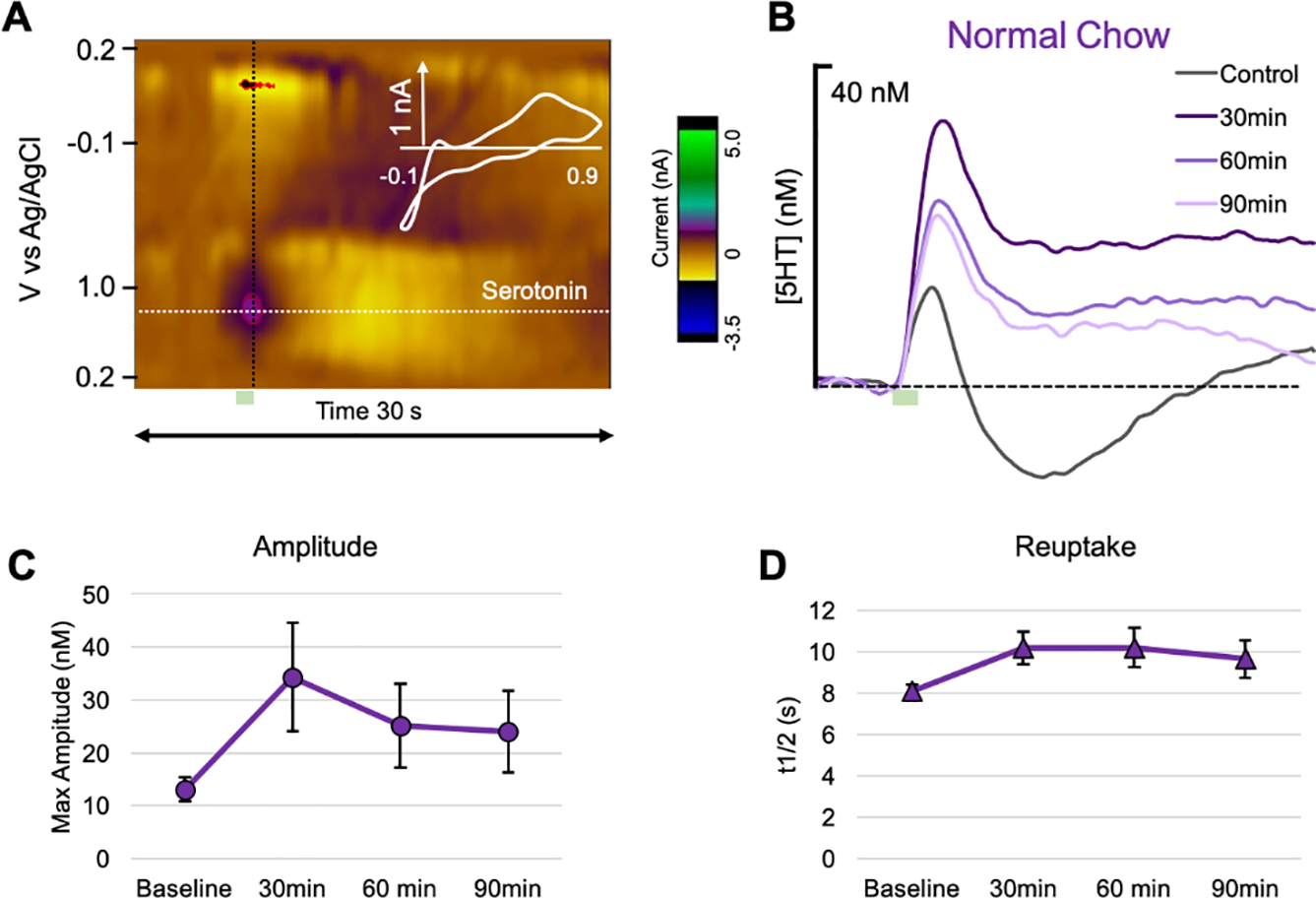
(A) An example color plot showing a 5HT event at 0.7 V. Stimulation is marked by a green box. (B) The average evoked 5HT release at baseline (gray), 30 (dark purple), 60 (medium purple), and 90 min post-escitalopram (10 mgkg−1, i.p., light purple) (n=5). Average (C) amplitude and (D) reuptake (t1/2) at baseline, 30, 60, and 90 min post-escitalopram.
Fig. 3. Tissue analysis of FSCV electrode placement.
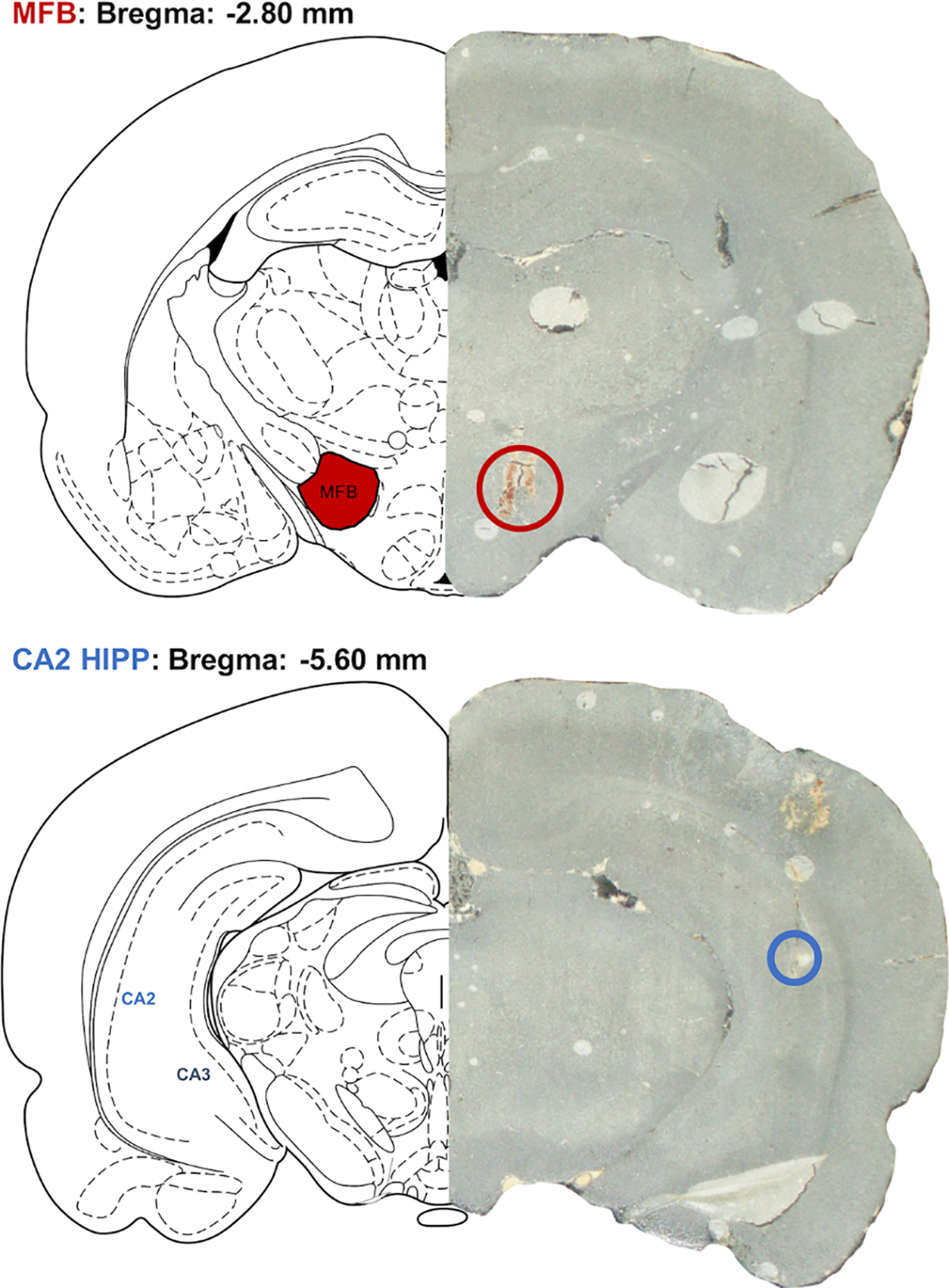
A graphic showing stimulating electrode placement in the MFB (top panel) and working electrode placement in the CA2 region of the hippocampus (bottom panel).
Fig. 4. Escitalopram is less effective at slowing hippocampal 5HT reuptake in obese rats.
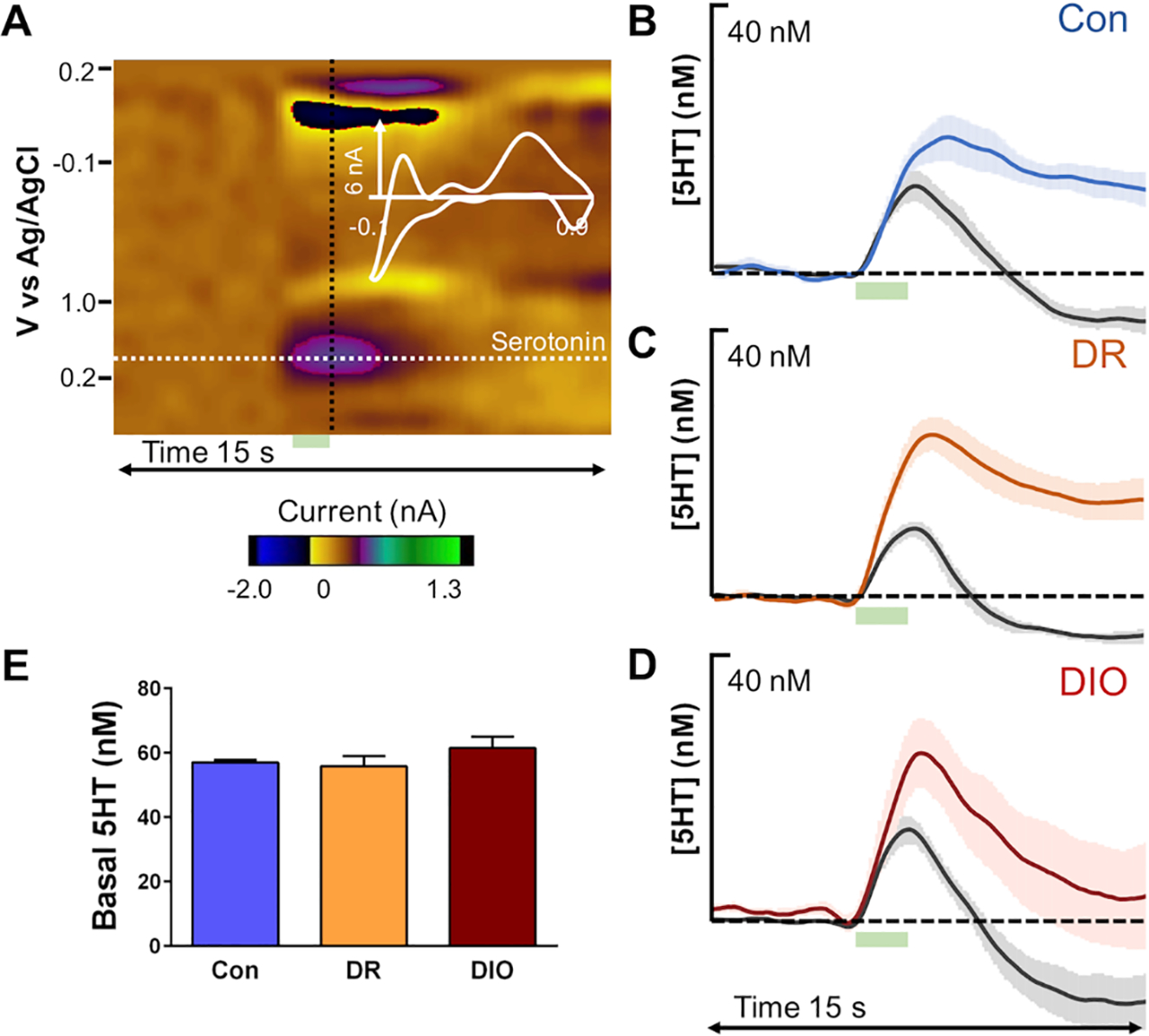
(A) An example of a color plot generated from rat hippocampal 5HT analysis is shown. The 5HT event is marked by a dotted white line at 0.7 V where the current vs. time trace was extracted and by a black dotted line where the current vs. voltage was extracted. Electrical stimulation at 5–7 s is denoted by the green box below. Average baseline hippocampal evoked release is shown for (B) control diet fed (n=7), (C) diet resistant high fat diet-fed (DR; n =7), and (D) diet induced obese (DIO) high fat-fed rats (n=6) in gray and 60 min post-escitalopram (10 mgkg−1, i.p.) in blue, orange and red respectively. (E) Basal hippocampal 5HT determined via FSCAV for each dietary group (control, DR, DIO; n=5 for each).
Additional analyses of these traces identified important functional changes in the rate of 5HT reuptake in the hippocampus of DIO rats. Rate of clearance following evoked release (determined via t1/2 or time required to decrease concentration by half) prior to administration of escitalopram did not differ between the groups (Figure 5A, Pre Rx). However, there was a significant effect of drug and a significant group effect in t1/2 activity 60 minutes following escitalopram administration (Figure 5A; [F(1, 34) = 30.11; p = 0.0001]). These changes are reflected in the percent inhibition of 5HT reuptake elicited by escitalopram (Pre Rx t1/2 + Post Rx escitalopram t1/2/Post Rx escitalopram t1/2), which was significantly decreased in the hippocampus of DIO rats compared to control rats (Figure 5B; [F(2,18) = 4.148; p = 0.03]). Collectively, these results demonstrate that responses to the SSRI escitalopram are impaired in the hippocampus of DIO rats.
Fig. 5.
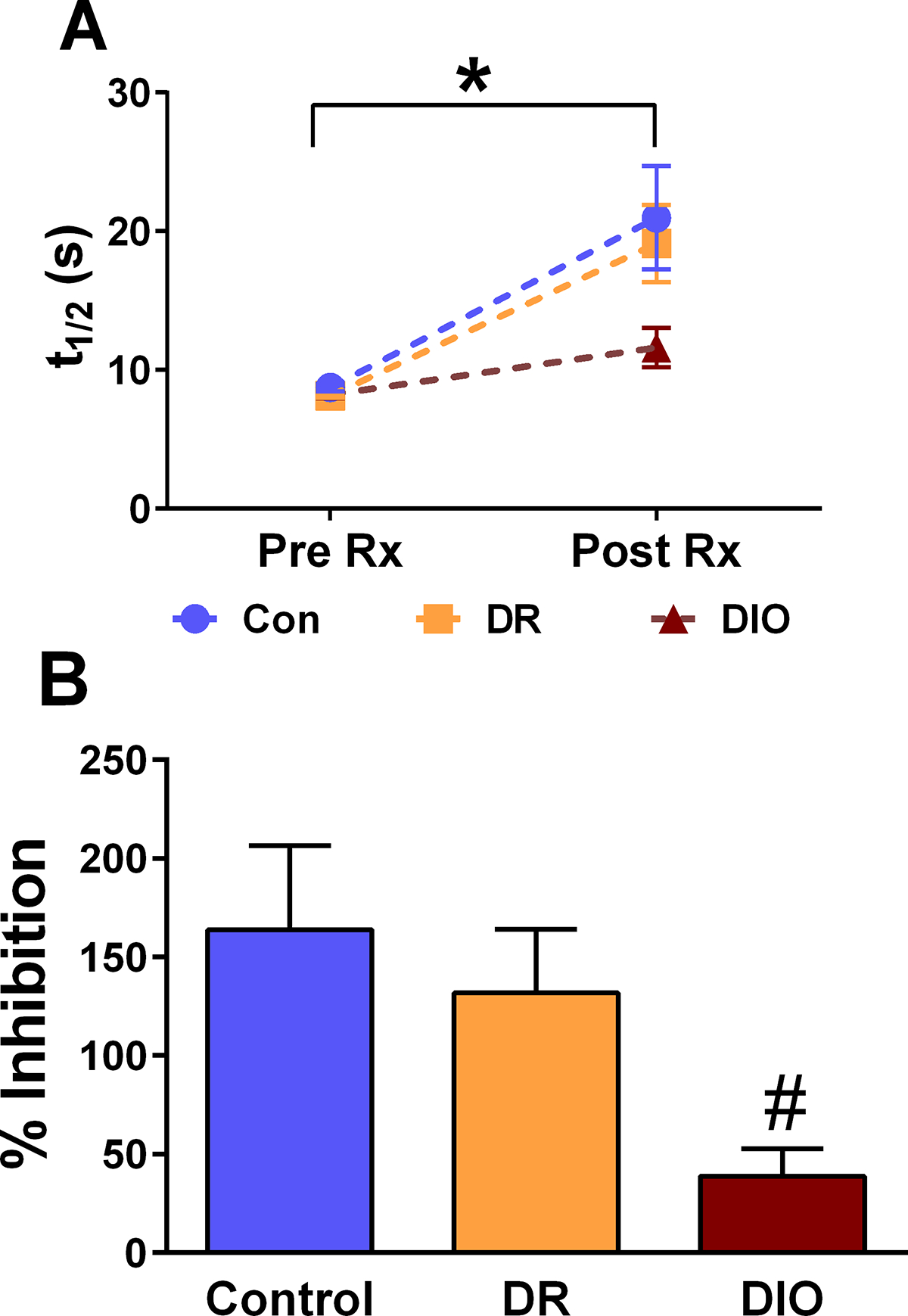
Neurochemical changes observed post-escitalopram in Control, DR and DIO rats. (A) The rate of basal 5HT reuptake prior to escitalopram administration (i.e. Pre Rx) is not different between Control, DR and DIO rats (n= 7, 7, and 6 respectively). Two-way ANOVA analysis revealed there was a significant effect of time [F(1, 34) = 30.11; p = 0.0001] and group [F(2,34) = 3.367; p = 0.04] of escitalopram administration on SERT reuptake. (B) Percent inhibition is significantly decreased in DIO rats compared to DR rats. [F(2,18) = 4.148; p = 0.03; *: p < 0.05; # = p < 0.05 compared to control]
3.3. Inflammatory markers are increased in the hippocampus, but not the DRN, of DIO rats
Since obesity-induced neuroinflammation is proposed to reduce 5HT synthesis in the CNS (Shelton and Miller, 2010), we examined cytokine levels in the DRN of control, DR, and DIO rats. Interestingly, we did not observe statistically-significant changes in DRN cytokine levels in these groups (Table 1). When combined with the absence of changes in basal hippocampal 5HT concentrations described above, these observations contrast with the hypothesis that obesity-induced neuroinflammation in the DRN reduces 5HT synthesis in obese rats. We also examined cytokine levels in the hippocampus of control, DR and DIO rats. The results indicate that protein expression of several cytokines is increased in the hippocampus of DIO rats relative to control-chow rats and/or DR rats (Table 2); Figure 6 provides representative examples of the cytokine results in DRN and hippocampus. Specifically, Figure 6 illustrates IL-1α, IL-1β, IL-4, IL-6, TNF-α and IFN-γ protein levels in the DRN (left column) and hippocampus (right column) of control-chow rats, DR rats and DIO rats. Statistical analysis revealed that compared to control chow rats and DR rats, DIO rats exhibited significant increases in hippocampal IL-1α [F(2,18)=1.268; p=0.01], IL-4 [F(2,18)=5.557; p=0.03], IL-10 [F(2,19)=4.020; p=0.03], IL-13 [F(2,19)=5.323; p=0.01] and IFN-γ [F(2,19)=7.436; p=0.004] levels. These results demonstrate that access to a HFD increases the expression of some, but not all, cytokines in the hippocampus of DIO rats.
Table 1.
Cytokine levels in the raphe nucleus of control rats, DR rats and DIO rats
| Cytokine | Control | DR | DIO | p value |
|---|---|---|---|---|
| IL-1α | 0.13 ± 0.02 | 0.10 ± 0.01 | 0.16 ± 0.04 | 0.26 |
| IL-1β | 0.16 ± 0.01 | 0.17 ± 0.02 | 0.20 ± 0.01 | 0.13 |
| IL-2 | 3.14 ± 0.37 | 2.70 ± 0.28 | 3.50 ± 0.44 | 0.33 |
| IL-4 | 0.09 ± 0.01 | 0.08 ± 0.01 | 0.11 ± 0.02 | 0.46 |
| IL-5 | 0.76 ± 0.07 | 0.61 ± 0.07 | 0.73 ± 0.05 | 0.31 |
| IL-6 | 0.74 ± 0.07 | 0.90 ± 0.08 | 0.99 ± 0.14 | 0.23 |
| IL-10 | ND | ND | ND | ---- |
| IL-13 | ND | ND | ND | ---- |
| TNF-α | 1.63 ± 0.15 | 1.33 ± 0.13 | 1.81 ± 0.23 | 0.18 |
| IFN-γ | 0.68 ± 0.06 | 0.57 ± 0.05 | 0.54 ± 0.08 | 0.28 |
| GM-CSF | 0.22 ± 0.03 | 0.17 ± 0.02 | 0.22 ± 0.03 | 0.24 |
Data expressed as pg of cytokine/mg of raphe nucleus extract. Lowest limit of detection (i.e. lowest standard) for each cytokine is as follows: IL-1α = 31.48 pg/ml; IL-1β = 10.54 pg/ml; IL-2 = 95.69 pg/ml; IL-4 = 1.5 pg/ml; IL-5 = 34.51 pg/ml; IL-6 = 15.49 pg/ml; IL-10 = 11.34 pg/ml; IL-12 = 4.19 pg/ml; IL-13 = 4.23 pg/ml; GM-CSF = 10.32 pg/ml; IFN-γ = 12.9 pg/ml; TNF-α = 16.68 pg/ml.
Table 2.
Cytokine levels in the hippocampus of control rats, DR rats and DIO rats
| Cytokine | Control | DR | DIO | p value |
|---|---|---|---|---|
| IL-1α | 4.0 ± 0.4 | 3.6 ± 0.3 | 8.2 ± 1.9 # % | 0.01 |
| IL-1β | 10.8 ± 1.6 | 9.8 ± 2.6 | 13.3 ± 4.2 | 0.72 |
| IL-2 | 275.0 ± 45.9 | 217.3 ± 50.5 | 380.3 ± 82.4 | 0.20 |
| IL-4 | 2.5 ± 0.3 | 2.8 ± 0.3 | 6.1 ± 1.2 # % | 0.01 |
| IL-5 | 24.3 ± 2.5 | 24.3 ± 2.3 | 38.7 ± 7.9 | 0.08 |
| IL-6 | 47.0 ± 5.4 | 38.6 ± 8.0 | 57.2 ± 11.3 | 0.32 |
| IL-10 | 4.7 ± 0.5 | 3.9 ± 0.5 | 7.5 ± 1.6 % | 0.04 |
| IL-13 | 19.7 ± 2.5 | 20.2 ± 1.9 | 41.3 ± 7.6 # % | 0.01 |
| TNF-α | 65.3 ± 16.3 | 58.5 ± 6.7 | 109.4 ± 31.0 | 0.19 |
| IFN-γ | 16.1 ± 3.0 | 17.7 ± 1.6 | 37.0 ± 8.1 # % | 0.004 |
| GM-CSF | 8.2 ± 1.3 | 7.7 ± 1.4 | 9.8 ± 2.6 | 0.71 |
Data expressed as pg of cytokine/mg of hippocampal extract.
significantly different from Control-chow rats
significantly different from DR rats.
Lowest limit of detection (i.e. lowest standard) for each cytokine is as follows: IL-1α = 31.48 pg/ml; IL-1β = 10.54 pg/ml; IL-2 = 95.69 pg/ml; IL-4 = 1.5 pg/ml; IL-5 = 34.51 pg/ml; IL-6 = 15.49 pg/ml; IL-10 = 11.34 pg/ml; IL-12 = 4.19 pg/ml; IL-13 = 4.23 pg/ml; GM-CSF = 10.32 pg/ml; IFN-γ = 12.9 pg/ml; TNF-α = 16.68 pg/ml.
Fig. 6.
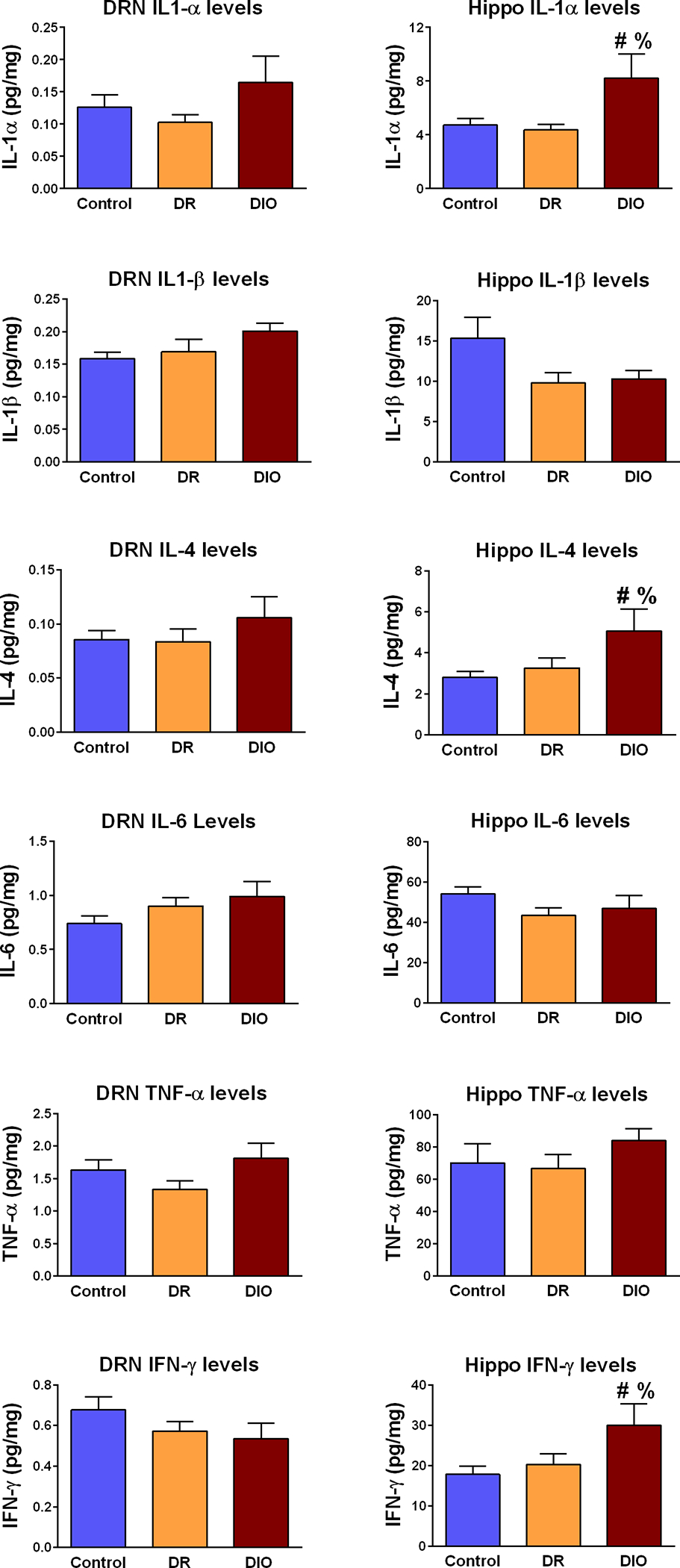
Analysis of cytokine levels in the dorsal raphe nucleus (DRN; left panel) and hippocampus (Hippo; right panel) in Control, DR and DIO rats. DRN expression of IL-1α, IL-1β, IL-4, IL-6, TNF-α and IFN-γ were similar in Control rats, DR rats and DIO rats. Conversely, IL-1α, IL-4 and IFN-γ levels were significantly increased in the hippocampus of DIO rats compared to Control rats and DR rats. Data expressed as mean (pg/mg protein) ± SEM. See Table 1 for complete list of DRN cytokines measured; see Table 2 for complete list of hippocampal cytokines measured. [# = p < 0.05 compared to control; % = p = 0.05 compared to DR]
3.4. SERT expression is increased in the hippocampus of DIO rats
Since previous studies suggest that SERTs may be regulated by cytokine exposure (Shelton and Miller, 2010), we examined SERT protein expression by fluorescence immunohistochemistry (fIHC) in the hippocampus of control, DR and DIO rats. SERT immunofluorescence exhibited the expected distribution in the hippocampus of all groups (Qian et al., 1995;Shigematsu et al., 2006). We examined SERT fIHC in the CA2 in approximately the same region where the carbon fiber microelectrodes were placed for 5HT neurochemical analysis. Representative images for control chow rats (Con CA2), DR rats (DR CA2) and DIO rats (DIO CA2) are shown in Figure 7, Panels A, B and C, respectively. Measurement of fluorescence intensity determined that SERT protein expression in the CA2 region was increased, although these increases did not achieve statistical significance; Panel D [F(2,9)=0.642; p=0.2]. We also examined SERT fIHC in the CA3 region of the hippocampus; representative images for control chow rats (Con CA3), DR rats (DR CA3) and DIO rats (DIO CA3) are shown in Figure 7, Panels E, F and G respectively. Measurement of fluorescence intensity determined that SERT protein expression was significantly increased in the CA3 region of DIO rats compared to DR rats (Panel H. [F(2,9)=0.619; p=0.008]). The increases in SERT levels suggest that the neurochemical changes we report are likely occurring in both the CA2 and CA3 regions. In support of this hypothesis, SERT immunofluorescence levels did not differ in the arcuate nucleus of the hypothalamus in control, DR and DIO rats; see Supplemental Figure 1. These results suggest that the increases in hippocampal SERT expression observed in DIO rats provide a potential mechanism for alterations in escitalopram responses in this group; see Figures 4 & 5).
Fig. 7.
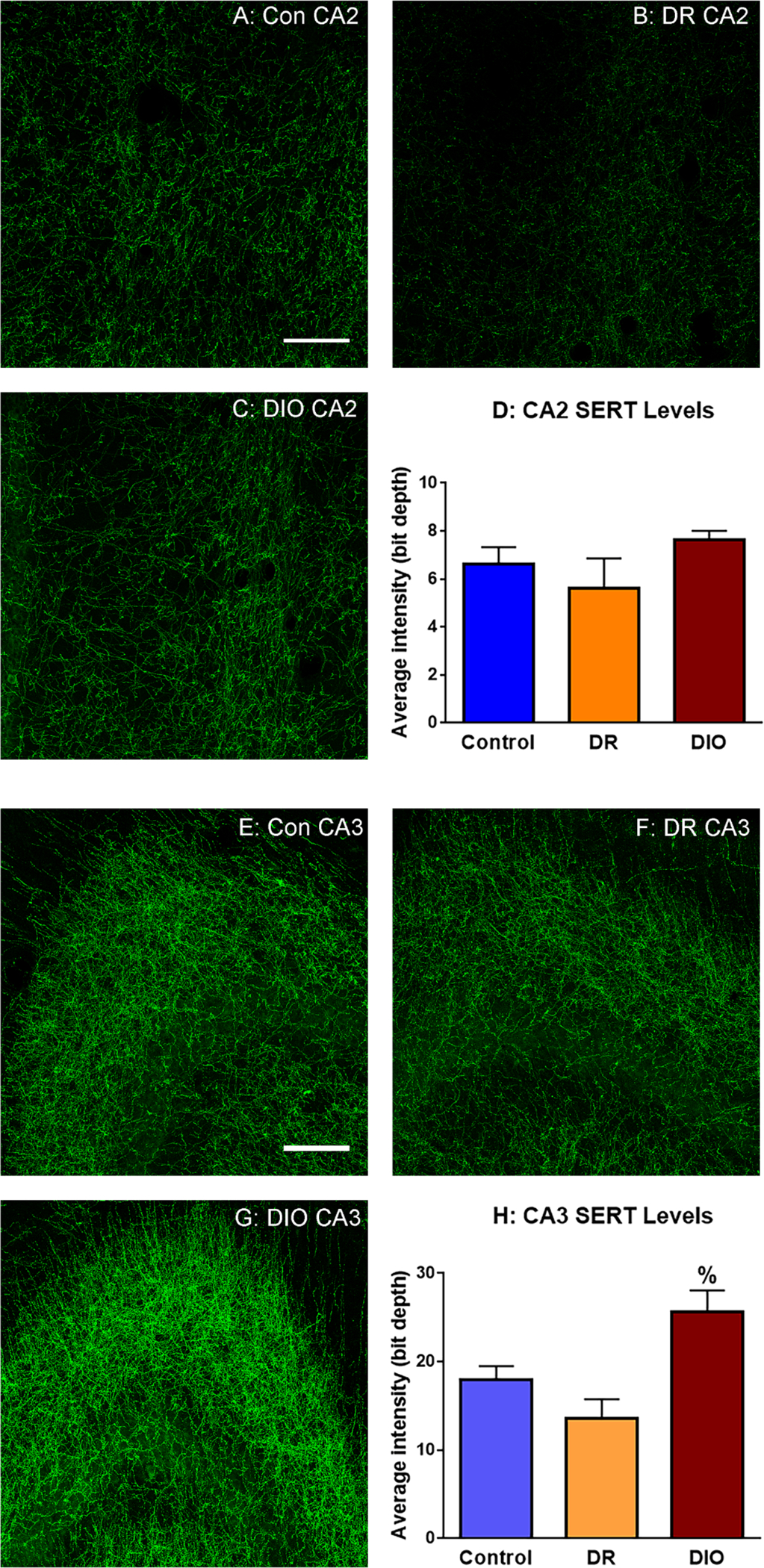
Fluorescence immunohistochemical analysis reveals that SERT expression levels are increased in the hippocampus of DIO rats. Representative images of SERT immunofluorescence in the CA2 region of the hippocampus of (A) Control rats (A: Con CA2), DR rats (B: DR CA2), and DIO (C: DIO CA2) rats. Fluorescence intensity analysis of confocal images determined that although SERT expression increased in the CA2 region of DIO rats (Panel D), these increases did not achieve statistical significance [F(2,9)=0.642; p=0.2]. Representative images of SERT immunofluorescence in the CA3 region of the hippocampus of Control rats (E: Con CA3), DR rats (F: DR CA3), and DIO (G: DIO CA3) rats. Fluorescence intensity analysis of confocal images determined that SERT expression is significantly increased in the hippocampus of DIO rats compared to DR rats (Panel H. [F(2,9)=0.619; p=0.008]. Results based on analysis of 3 images/rat and 4 rats per group; scale bar = 50 μm]
4. Discussion
4.1. High fat diet induced obesity increase SERT and impairs SSRI-induced slowing of 5HT reuptake
The results of the current study demonstrate that the synaptic responses to the SSRI escitalopram are significantly reduced in the hippocampus of DIO rats compared to DR rats and control-chow fed rats. In this study we performed FSCV, a unique technique that can probe the serotonergic system in vivo in real time. Electrical-evoked release via stimulation of the MFB allows for the analysis of serotonergic parameters including 5HT synthesis, packaging, and release, all of which were observed to be unchanged across the treatment groups (Wood et al., 2014). Moreover, FSCV can assess reuptake following a release event, thereby allowing for the analysis of the ability of SERT to rapidly remove released 5HT from the synaptic space, as marked by changes in the slope of the reuptake curve. Our results illustrate that at baseline this activity is unaltered by diet treatment group. This is perhaps not surprising as the serotonergic system is very tightly controlled to protect against the neurotoxic effects of surplus 5HT. However, a targeted inhibition of SERT through an SSRI challenge disproportionally affected 5HT reuptake in DIO rats suggesting alterations in SERT dynamics, such as increased SERT expression or activity in DIO rats. Indeed, the current study demonstrates that both 5HT reuptake and SERT expression are increased in the hippocampus of DIO rats. Our results also provide insight into the cellular mechanisms that drive these neurochemical alterations. For example, the current observations suggest that high-fat diet-induced increases in hippocampal cytokine levels in DIO rats elicit changes in hippocampal 5HT reuptake and expression, thereby providing a mechanistic basis for deleterious alterations in SSRI responsiveness in the hippocampus of obese rats.
The results of this study further highlight the important role of neuroinflammation in the CNS complications of obesity. While the cause and consequence relationships between neuroinflammation and obesity have been debated, the literature supports the hypothesis that hypothalamic neuroinflammation is a causative factor in the development and maintenance of obesity (Thaler and Schwartz, 2010;Thaler et al., 2013). Under these conditions, hypothalamic neuroinflammation initiates a cycle that induces the development of the hallmark endocrine and metabolic deficits in obesity (i.e. leptin resistance, increased food intake, body weight, adiposity, plasma triglyceride levels, etc.). Interestingly, studies by Levin and Hassanain illustrated that DIO-prone rats exhibit alterations in hypothalamic serotonergic tone while on standard lab chow, which may predispose these rats to an obesity phenotype when provided access to a high fat diet (Hassanain and Levin, 2002). Beyond the hypothalamus, obesity-induced increases in neuroinflammation in DRN have been proposed to reduce 5HT synthesis, which would reduce 5HT levels in target brain regions like the hippocampus (Shelton and Miller, 2010). However, our results demonstrate that cytokine levels are unchanged in the DRN of DIO rats compared to DR rats and control rats. Such observations are consistent with the lack of differences in basal hippocampal 5HT levels we observe in these groups. Taken together, these data do not support the hypothesis that obesity elicits neuroinflammation in the RN and thereby decreases 5HT levels in the hippocampus of obese rats.
In addition to effects on neurotransmitter synthesis, neuroinflammation has been proposed to contribute to obesity-depressive illness comorbidities by impacting SERT expression and activity (Shelton and Miller, 2010); our results provide substantial support for this hypothesis. In agreement with previous studies in mice (Morselli et al., 2014;Gladding et al., 2018), we report that cytokine levels are increased in the hippocampus of DIO rats compared to control and DR rats. These findings provide insights into the impaired responses to escitalopram in DIO rats and mechanistically extend beyond the traditional view of pro-inflammatory cytokines vis-à-vis anti-inflammatory cytokines, at least as it relates to SERT expression and activity. For example, IL-4 elicits dose-dependent decreases in SERT activity in B lymphocyte cell cultures (Mössner et al., 2001). Interestingly the effects of IL-10 on SERT expression and activity in intestinal epithelial cell cultures are also dose-dependent, with high doses of IL-10 increasing SERT expression and activity (Latorre et al., 2013). IFN-γ also increases SERT mRNA levels and activity in human placental choriocarcinoma cells (Morikawa et al., 1998). Another cell culture study determined that acute IL-1β treatment increased SERT expression and activity in human choriocarcinoma cells (Ramamoorthy et al., 1995). While performed in cultured cells of peripheral origin, these studies nonetheless provide important insights into the in vivo regulation of SERTs in the CNS. In this regard, while our study did not identify differences in hippocampal IL-1β levels, the ability of this cytokine to regulate SERT activity in neuronal cells may be mediated through activation of IL-1 receptors (IL-1Rs) (Zhu et al., 2006). In support of this concept, SB203580, an inhibitor of IL-R signaling, blocks LPS-induced increases in 5HT uptake in synaptosomes isolated from mouse midbrain (Zhu et al., 2010). This study also demonstrated that LPS-induced increases in 5HT uptake are not observed in synaptosomes prepared from IL-1R knockout mice. There are some important distinctions between the current results and the study by Zhu and coworkers. For example, the Zhu study examined the acute effects of LPS upon 5HT uptake in mouse midbrain synaptosomes while the current study examined the effects of high-fat diet induced neuroinflammation upon synaptic concentrations of 5HT in rat hippocampus in real time. Nonetheless, as both IL-1α and IL-1β activate IL-1Rs, these studies support the concept that activation of IL-1Rs regulate the expression and activity of hippocampal SERTs.
These changes in the expression and activity of SERTs may be linked to the inability of SSRIs to effectively inhibit SERTs in obesity and may thereby lead to exacerbated depressive-like behaviors. Indeed, these observations have important clinical implications since as noted above, obese individuals have increased risk of developing co-morbid depressive illness and are more likely to exhibit poorer treatment outcomes. Specifically, obese subjects are more likely to exhibit treatment resistant depression compared to non-obese individuals (Kloiber et al., 2007;Uher et al., 2009;Rizvi et al., 2014), including decreased therapeutic responses to SSRIs (Khan et al., 2007;Lin et al., 2014). In preclinical studies, SSRIs fail to modulate depressive-like behaviors in obese mice, illustrating that obese rodents also exhibit treatment resistance to SSRIs (Guo and Lu, 2014). When combined with the current results, these observations support the concepts that decreases in SSRI responsiveness is an important neurochemical component of depressive illness and that obese patients are more likely to exhibit treatment resistance to drugs that increase synaptic 5HT levels.
Collectively, the current findings reshape our understanding of the role of neuroinflammation in the development of co-morbid depressive illness in obesity. The absence of increased neuroinflammation in the DRN is consistent with the lack of changes in basal levels of 5HT in the hippocampus of DIO rats. However, hippocampal neuroinflammation, and perhaps more specifically IL-1α localized to the hippocampus, increases the expression of SERTs and also impairs SERT responses to escitalopram in the hippocampus of DIO rats. The functional outcome is that escitalopram cannot effectively increase synaptic concentrations of 5HT in the hippocampus of DIO rats, which may explain the reduced clinical efficacy of this SSRI in obese patients. Nonetheless, the current findings must be placed in a broader context since they cannot account for all of the potential consequences of obesity-induced neuroinflammation or the potential role of the metabolic and/or endocrine deficits in the CNS complications of obesity. In this regard, weight loss induced by dietary changes reverses depressive-like behaviors in obese rodents (Yamada et al., 2011;Grillo et al., 2014) and clinical studies have shown that mood is improved in patients following weight loss achieved through bariatric surgical procedures (Assimakopoulos et al., 2011;Burgmer et al., 2014;Gill et al., 2019). These results suggest that the entire metabolic and endocrine milieu, of which neuroinflammation is a part, contributes to the development of co-morbid depression and obesity. Under these conditions, even partial restoration of metabolic parameters may be adequate to restore SSRI sensitivity in obese patients. Therefore, from a broader perspective our studies may help inform pre-clinical treatment strategies to target neuroinflammation and SERT impairments to help alleviate depressive illness co-morbid with obesity.
Supplementary Material
Supplementary Figure: Fluorescence immunohistochemical analysis reveals that SERT expression levels are not different in the arcuate nucleus (ARC) of control, DR and DIO rats. Representative images of SERT immunofluorescence in the ARC of (A) Control rats (A: Con ARC), DR rats (B: DR ARC), and DIO (C: DIO ARC) rats. Fluorescence intensity analysis of confocal images determined that although SERT expression in the ARC is not different between the groups (Panel D) [F(2,9)=1.117; p=0.56].
Highlights.
Diet-induced obesity (DIO) elicits a depressive-like phenotype
DIO rats exhibit reduced hippocampal synaptic responses to escitalopram
Hippocampal SERT expression and activity is increased in DIO rats
Cytokines are increased in the hippocampus but not raphe nucleus of DIO rats
Results provide potential mechanism for reduced SSRI responsiveness in obesity
Acknowledgements:
The authors would like to acknowledge Srimal Samaranayake and Shane Berger for their assistance on surgeries and neurochemical analysis as well as Navid Tavakoli for his help with electrode fabrication and tissue analysis.
Funding and Disclosure:
This work was supported by the Department of Veterans Affairs grant numbers I21 BX002085 and IO1 BX001804 (LPR), the University of South Carolina School of Medicine Research Development Fund (LPR), the National Science Foundation grant number IOS-1656626 (CAG), the National Institutes of Health R01MH106563 (PH) and R21MH109959 (PH), and the University of South Carolina SPARC award (MH). Any opinions, findings, and conclusions or recommendations expressed in this material are those of the authors and do not necessarily reflect the views of the above stated funding agencies. The authors have no conflicts of interest to declare.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abdalla A, Atcherley CW, Pathirathna P, Samaranayake S, Qiang BD, Pena E, Morgan SL, Heien ML, and Hashemi P (2017). In Vivo Ambient Serotonin Measurements at Carbon-Fiber Microelectrodes. Analytical Chemistry 89, 9703–9711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andersen JR, Aasprang A, Bergsholm P, Sletteskog N, Våge V, and Natvig GK (2010). Anxiety and depression in association with morbid obesity: changes with improved physical health after duodenal switch. Health and quality of life outcomes 8, 52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Assimakopoulos K, Karaivazoglou K, Panayiotopoulos S, Hyphantis T, Iconomou G, and Kalfarentzos F (2011). Bariatric surgery is associated with reduced depressive symptoms and better sexual function in obese female patients: a one-year follow-up study. Obes.Surg 21, 362–366. [DOI] [PubMed] [Google Scholar]
- Burgmer R, Legenbauer T, Muller A, De Zwaan M, Fischer C, and Herpertz S (2014). Psychological outcome 4 years after restrictive bariatric surgery. Obes.Surg 24, 1670–1678. [DOI] [PubMed] [Google Scholar]
- Collin M, Håkansson-Ovesjö ML, Misane I, Ogren SO, and Meister B (2000). Decreased 5-HT transporter mRNA in neurons of the dorsal raphe nucleus and behavioral depression in the obese leptin-deficient ob/ob mouse. Brain Res Mol Brain Res 81, 51–61. [DOI] [PubMed] [Google Scholar]
- Fabricatore AN, and Wadden TA (2006). Obesity. Annu Rev Clin Psychol 2, 357–377. [DOI] [PubMed] [Google Scholar]
- Gill H, Kang S, Lee Y, Rosenblat JD, Brietzke E, Zuckerman H, and Mcintyre RS (2019). The long-term effect of bariatric surgery on depression and anxiety. J.Affect.Disord. 246, 886–894. [DOI] [PubMed] [Google Scholar]
- Gladding JM, Abbott KN, Antoniadis CP, Stuart A, and Begg DP (2018). The Effect of Intrahippocampal Insulin Infusion on Spatial Cognitive Function and Markers of Neuroinflammation in Diet-induced Obesity. Front Endocrinol (Lausanne) 9, 752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grillo CA, Mulder P, Macht VA, Kaigler KF, Wilson SP, Wilson MA, and Reagan LP (2014). Dietary restriction reverses obesity-induced anhedonia. Physiol Behav 128, 126–132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grillo CA, Piroli GG, Kaigler KF, Wilson SP, Wilson MA, and Reagan LP (2011). Downregulation of hypothalamic insulin receptor expression elicits depressive-like behaviors in rats. Behavioural brain research 222, 230–235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grillo CA, Piroli GG, Lawrence RC, Wrighten SA, Green AJ, Wilson SP, Sakai RR, Kelly SJ, Wilson MA, and Mott DD (2015). Hippocampal insulin resistance impairs spatial learning and synaptic plasticity. Diabetes 64, 3927–3936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grillo CA, Woodruff JL, Macht VA, and Reagan LP (2019). Insulin resistance and hippocampal dysfunction: Disentangling peripheral and brain causes from consequences. Experimental neurology 318, 71–77. [DOI] [PubMed] [Google Scholar]
- Guo M, and Lu XY (2014). Leptin receptor deficiency confers resistance to behavioral effects of fluoxetine and desipramine via separable substrates. Transl Psychiatry 4, e486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hashemi P, Dankoski EC, Petrovic J, Keithley RB, and Wightman RM (2009). Voltammetric detection of 5-hydroxytryptamine release in the rat brain. Anal Chem 81, 9462–9471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hassanain M, and Levin BE (2002). Dysregulation of hypothalamic serotonin turnover in diet-induced obese rats. Brain Res 929, 175–180. [DOI] [PubMed] [Google Scholar]
- Jackson BP, Dietz SM, and Wightman RM (1995). Fast-scan cyclic voltammetry of 5-hydroxytryptamine. Anal Chem 67, 1115–1120. [DOI] [PubMed] [Google Scholar]
- Khan A, Schwartz KA, Kolts RL, and Brown WA (2007). BMI, sex, and antidepressant response. J Affect Disord 99, 101–106. [DOI] [PubMed] [Google Scholar]
- Kloiber S, Ising M, Reppermund S, Horstmann S, Dose T, Majer M, Zihl J, Pfister H, Unschuld PG, Holsboer F, and Lucae S (2007). Overweight and obesity affect treatment response in major depression. Biol Psychiatry 62, 321–326. [DOI] [PubMed] [Google Scholar]
- Latorre E, Mendoza C, Matheus N, Castro M, Grasa L, Mesonero JE, and Alcalde AI (2013). IL-10 modulates serotonin transporter activity and molecular expression in intestinal epithelial cells. Cytokine 61, 778–784. [DOI] [PubMed] [Google Scholar]
- Levin BE, Dunn-Meynell AA, Balkan B, and Keesey RE (1997). Selective breeding for diet-induced obesity and resistance in Sprague-Dawley rats. Am J Physiol 273, R725–730. [DOI] [PubMed] [Google Scholar]
- Lin CH, Chen CC, Wong J, and Mcintyre RS (2014). Both body weight and BMI predicts improvement in symptom and functioning for patients with major depressive disorder. J Affect Disord 161, 123–126. [DOI] [PubMed] [Google Scholar]
- Luppino FS, De Wit LM, Bouvy PF, Stijnen T, Cuijpers P, Penninx BW, and Zitman FG (2010). Overweight, obesity, and depression: a systematic review and meta-analysis of longitudinal studies. Arch Gen Psychiatry 67, 220–229. [DOI] [PubMed] [Google Scholar]
- Macht VA, Vazquez M, Petyak CE, Grillo CA, Kaigler K, Enos RT, Mcclellan JL, Cranford TL, Murphy EA, Nyland JF, Solomon G, Gertler A, Wilson MA, and Reagan LP (2017). Leptin resistance elicits depressive-like behaviors in rats. Brain Behav Immun 60, 151–160. [DOI] [PubMed] [Google Scholar]
- Mcelroy SL, Kotwal R, Malhotra S, Nelson EB, Keck PE, and Nemeroff CB (2004). Are mood disorders and obesity related? A review for the mental health professional. J Clin Psychiatry 65, 634–651, quiz 730. [DOI] [PubMed] [Google Scholar]
- Morikawa O, Sakai N, Obara H, and Saito N (1998). Effects of interferon-alpha, interferongamma and cAMP on the transcriptional regulation of the serotonin transporter. Eur J Pharmacol 349, 317–324. [DOI] [PubMed] [Google Scholar]
- Morselli E, Fuente-Martin E, Finan B, Kim M, Frank A, Garcia-Caceres C, Navas CR, Gordillo R, Neinast M, Kalainayakan SP, Li DL, Gao Y, Yi CX, Hahner L, Palmer BF, Tschöp MH, and Clegg DJ (2014). Hypothalamic PGC-1α protects against high-fat diet exposure by regulating ERα. Cell Rep 9, 633–645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mössner R, Daniel S, Schmitt A, Albert D, and Lesch KP (2001). Modulation of serotonin transporter function by interleukin-4. Life Sci 68, 873–880. [DOI] [PubMed] [Google Scholar]
- Onyike CU, Crum RM, Lee HB, Lyketsos CG, and Eaton WW (2003). Is obesity associated with major depression? Results from the Third National Health and Nutrition Examination Survey. Am J Epidemiol 158, 1139–1147. [DOI] [PubMed] [Google Scholar]
- Qian Y, Melikian HE, Rye DB, Levey AI, and Blakely R (1995). Identification and characterization of antidepressant-sensitive serotonin transporter proteins using sitespecific antibodies. Journal of Neuroscience 15, 1261–1274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raison CL, Capuron L, and Miller AH (2006). Cytokines sing the blues: inflammation and the pathogenesis of depression. Trends Immunol 27, 24–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramamoorthy S, Ramamoorthy JD, Prasad PD, Bhat GK, Mahesh VB, Leibach FH, and Ganapathy V (1995). Regulation of the human serotonin transporter by interleukin-1β. Biochemical and biophysical research communications 216, 560–567. [DOI] [PubMed] [Google Scholar]
- Reagan LP (2012). Diabetes as a chronic metabolic stressor: causes, consequences and clinical complications. Experimental neurology 233, 68–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rizvi SJ, Grima E, Tan M, Rotzinger S, Lin P, Mcintyre RS, and Kennedy SH (2014). Treatment-resistant depression in primary care across Canada. Can J Psychiatry 59, 349–357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma AN, Elased KM, Garrett TL, and Lucot JB (2010). Neurobehavioral deficits in db/db diabetic mice. Physiology & behavior 101, 381–388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shelton RC, and Miller AH (2010). Eating ourselves to death (and despair): the contribution of adiposity and inflammation to depression. Progress in neurobiology 91, 275–299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shigematsu N, Yamamoto K, Higuchi S, and Fukuda T (2006). Novel non-uniform distribution of serotonin transporter in the mouse hippocampus and neocortex revealed by N-and C-terminal domain-specific immunohistochemistry. Brain research 1075, 110–116. [DOI] [PubMed] [Google Scholar]
- Simon GE, Von Korff M, Saunders K, Miglioretti DL, Crane PK, Van Belle G, and Kessler RC (2006). Association between obesity and psychiatric disorders in the US adult population. Arch Gen Psychiatry 63, 824–830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soczynska JK, Kennedy SH, Woldeyohannes HO, Liauw SS, Alsuwaidan M, Yim CY, and Mcintyre RS (2011). Mood disorders and obesity: understanding inflammation as a pathophysiological nexus. Neuromolecular medicine 13, 93–116. [DOI] [PubMed] [Google Scholar]
- Stunkard AJ, Faith MS, and Allison KC (2003). Depression and obesity. Biol Psychiatry 54, 330–337. [DOI] [PubMed] [Google Scholar]
- Thaler JP, Guyenet SJ, Dorfman MD, Wisse BE, and Schwartz MW (2013). Hypothalamic inflammation: marker or mechanism of obesity pathogenesis? Diabetes 62, 2629–2634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thaler JP, and Schwartz MW (2010). Minireview: Inflammation and obesity pathogenesis: the hypothalamus heats up. Endocrinology 151, 4109–4115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uher R, Mors O, Hauser J, Rietschel M, Maier W, Kozel D, Henigsberg N, Souery D, Placentino A, Perroud N, Dernovsek MZ, Strohmaier J, Larsen ER, Zobel A, Leszczynska-Rodziewicz A, Kalember P, Pedrini L, Linotte S, Gunasinghe C, Aitchison KJ, Mcguffin P, and Farmer A (2009). Body weight as a predictor of antidepressant efficacy in the GENDEP project. J Affect Disord 118, 147–154. [DOI] [PubMed] [Google Scholar]
- Wood KM, and Hashemi P (2013). Fast-scan cyclic voltammetry analysis of dynamic serotonin reponses to acute escitalopram. ACS Chem Neurosci 4, 715–720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood KM, Zeqja A, Nijhout HF, Reed MC, Best J, Hashemi P, 2014. Voltammetric and mathematical evidence for dual transport mediation of serotonin clearance in vivo. Journal of Neurochemistry 130, 351–359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- World Health Organization. (2020). Obesity and overweight fact sheet [Online]. Available: https://www.who.int/en/news-room/fact-sheets/detail/obesity-and-overweight [Accessed 2020].
- Yamada N, Katsuura G, Ochi Y, Ebihara K, Kusakabe T, Hosoda K, and Nakao K (2011). Impaired CNS leptin action is implicated in depression associated with obesity. Endocrinology 152, 2634–2643. [DOI] [PubMed] [Google Scholar]
- Zhu C-B, Blakely RD, and Hewlett WA (2006). The proinflammatory cytokines interleukin-1beta and tumor necrosis factor-alpha activate serotonin transporters. Neuropsychopharmacology 31, 2121. [DOI] [PubMed] [Google Scholar]
- Zhu CB, Lindler KM, Owens AW, Daws LC, Blakely RD, and Hewlett WA (2010). Interleukin-1 receptor activation by systemic lipopolysaccharide induces behavioral despair linked to MAPK regulation of CNS serotonin transporters. Neuropsychopharmacology 35, 2510–2520. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Figure: Fluorescence immunohistochemical analysis reveals that SERT expression levels are not different in the arcuate nucleus (ARC) of control, DR and DIO rats. Representative images of SERT immunofluorescence in the ARC of (A) Control rats (A: Con ARC), DR rats (B: DR ARC), and DIO (C: DIO ARC) rats. Fluorescence intensity analysis of confocal images determined that although SERT expression in the ARC is not different between the groups (Panel D) [F(2,9)=1.117; p=0.56].


