Abstract
Objective:
We sought to describe clinicopathologic and surgical factors associated with oncologic outcomes in patients undergoing tertiary cytoreduction and to present a clinical model to identify patients with high-grade serous ovarian cancer (HGSOC) who may benefit most from tertiary cytoreduction.
Methods:
We retrospectively identified patients with recurrent epithelial ovarian, fallopian tube, or primary peritoneal cancer who underwent tertiary cytoreduction at our institution from 1/1/1990-1/1/2019. Kaplan-Meier curves were used to estimate survival and compared using the log-rank test. Cox-proportional hazards regression was used to detect variables associated with survival.
Results:
Of 114 patients who met inclusion criteria, 79 (69.2%) had high-grade serous tumors. Of patients with available genetic testing (n=66), 22 (33%) harbored germline or somatic BRCA mutations. Fifty-eight women (50.9%) died of disease. Complete gross resection (CGR) at tertiary cytoreduction, treatment-free interval (TFI), and platinum sensitivity were all significantly associated with disease-specific survival (DSS) and maintained significance on multivariate analysis (HR 3.71, 95%CI: 1.59-8.70; HR 0.49, 95%CI: 0.28-0.85; and HR 2.94, 95%CI: 1.22-7.07, respectively). Adjuvant treatment was not associated with a survival difference. Patients with HGSOC and a single site of recurrence who were ≥2 years from secondary cytoreduction had the greatest survival after tertiary cytoreduction (median DSS, 79.5 months).
Conclusions:
Proper patient selection for tertiary cytoreduction is essential. Those who achieve CGR likely derive the greatest benefit from tertiary surgery. Platinum sensitivity and prolonged TFI are also associated with improved DSS. Patients with HGSOC and single-site recurrence who were ≥2 years out from secondary cytoreduction had the longest DSS.
Keywords: ovarian cancer, high-grade serous ovarian carcinoma, tertiary cytoreduction, surgery
Introduction
Approximately 60-85% of patients with epithelial ovarian carcinoma will experience recurrence (1). Treatment in the recurrent setting is typically focused on optimizing quality of life and prolonging survival, rather than cure, and may include chemotherapeutics, hormonal management, immunotherapy, targeted therapies, surgery, or a clinical trial. Secondary surgical cytoreduction can be considered at the time of first recurrence, depending on the individual patient, as well as tumor and disease characteristics (1). The role of secondary cytoreduction has been evaluated in three randomized trials: Gynecologic Oncology Group (GOG 213), DESKTOP III/ENGOT-ov20, and SOC-1. Although the outcomes of the trials varied, patients who achieved a complete gross resection (CGR) at the time of secondary cytoreduction had improved survival outcomes across all three studies (2-4). After secondary cytoreduction, the vast majority of patients will develop a subsequent recurrence, at which point non-surgical management is most often preferred (4). In select patients, however, additional surgical resection may be beneficial. Although the role of tertiary cytoreduction in this patient population is debatable, more research in this area is warranted (5-10).
We previously reported on patients who underwent tertiary cytoreduction at our institution between 1/1/1990 and 7/31/2008 (5). Our findings showed a 46.8-month survival benefit associated with CGR compared to gross residual disease of >0.5 cm. Additional evaluation of clinicopathologic factors associated with oncologic outcomes were limited by the sample size. In this study, we sought to update outcomes from our original series and further expand our number of cases, describe the oncologic outcomes of a relatively large cohort of patients undergoing tertiary cytoreduction, and assess clinicopathologic and surgical factors associated with oncologic outcomes. We also sought to present a clinical model to help guide in the identification and selection of patients with high-grade serous ovarian cancer (HGSOC) who may benefit from tertiary cytoreduction.
Methods
After Institutional Review Board approval, we identified all patients with epithelial ovarian, fallopian tube, or primary peritoneal cancer who underwent surgery with cytoreductive intent, after a prior secondary cytoreductive surgery, at our institution between 1/1/1990 and 1/1/2019. We excluded patients who underwent surgery for reasons other than intended cytoreduction, such as correction of malignant bowel obstruction or fistula repair; patients with non-epithelioid histologic disease; patients whose secondary or tertiary surgery was a second-look laparoscopy or laparotomy unprompted by evidence of recurrent disease on exam or imaging; and patients whose tertiary surgery was performed at an outside institution.
Patients were identified from two databases using a data abstraction cut-off date of 11/15/2020. The medical records of patients who underwent tertiary cytoreduction and were included in our prior report were reviewed, and data were updated. We then used the Memorial Sloan Kettering Institutional Database (MSK-IDB) to identify all patients with epithelial ovarian, fallopian tube, or primary peritoneal cancer diagnosed after 7/31/2008—the cut-off date in our prior publication (5). The MSK-IDB is the primary data enterprise warehouse and is sourced from most of the institution’s primary clinical and operational systems. Abstraction from the MSK-IDB began with patients who underwent secondary cytoreduction, identified as those who underwent an additional surgery with cytoreductive intent at least 6 months after the date of initial diagnosis, excluding those who underwent second-look laparoscopy or laparotomy without radiographic or physical evidence of recurrence, those with diagnoses of tubal intraepithelial carcinoma, and those with surgeries coded as “primary.” The remaining patient charts were then manually reviewed to exclude patients who did not undergo a tertiary cytoreduction.
Multiple data points were abstracted from the electronic medical records. Stage at initial diagnosis was assigned following the International Federation of Gynecology and Obstetrics (FIGO) 2014 staging system. Tumor grade, histology, and BRCA mutational status were obtained from pathology and genetic testing reports, when available. Preoperative CA-125 levels were obtained from preoperative laboratory testing performed within 1 month of surgery. The number of sites of tumor recurrence and volume of residual disease were classified based on their description from operative reports. Optimal cytoreduction was defined as maximal residual tumor diameter ≤1 cm for initial cytoreduction based on Gynecologic Oncology Group (GOG) definitions, and CGR was defined as resection of all macroscopic residual disease. Surgical complications included events within 30 days of tertiary cytoreduction and were graded according to a previously validated institutional grading system (11). Grade 1 complications were those requiring oral medications or bedside intervention; grade 2 complications included those requiring intravenous medications or a blood transfusion in response to an adverse event; grade 3 complications were those requiring interventional radiology, endoscopy, intubation, or surgery to treat an event; grade 4 complications were those resulting in lasting disability requiring major rehabilitation or organ resection; and grade 5 complications were those resulting in patient death. Platinum sensitivity was reported at the time of tertiary cytoreduction and at any time during the patient’s treatment. Platinum-resistant disease was defined as progression of disease while on platinum therapy and/or progression or recurrence within 6 months of completion of last platinum therapy. After tertiary cytoreduction, adjuvant therapies were defined as those given immediately following surgery and included cytotoxic therapy, hormonal treatment, Poly (ADP-ribose) polymerase inhibition (PARPi), and/or radiation. Adjuvant cytotoxic therapies were further stratified by regimen and included: platinum doublets, single-agent platinum, non-platinum doublets, and single-agent non-platinum. Maintenance regimens were also stratified by regimen and included: hormonal therapy, bevacizumab, PARPi, cytotoxic agents, and targeted therapies.
Progression-free survival (PFS), in months, was measured from the date of tertiary cytoreduction to the date of either disease progression or death from disease. Disease-specific survival (DSS) was measured from the date of tertiary cytoreduction to the date of death from disease. Patients who experienced death due to reasons not attributable to cancer were censored for follow-up at date of death. Patients alive and disease-free or alive with disease were censored for PFS and DSS, respectively, at date of last follow-up. Survival curves were estimated using the Kaplan-Meier method and compared using log-rank tests. Significant factors associated with improved outcome on univariate analysis were then evaluated using a Cox proportional hazard regression to examine independent association. Associations were shown as hazard ratios (HRs) with 95% confidence intervals (95% CIs). All statistical analyses were performed using SPSS version 26.0 (Armonk, NY).
Results
Patient population and operative characteristics
One hundred fourteen patients met the study inclusion criteria. Our cohort’s baseline clinicopathologic characteristics, prior to tertiary cytoreduction, are described in Table 1. The majority of patients had serous (75.4%) and high-grade (87.7%) tumors. Of patients with available genetic testing (n=66), 22 (33%) harbored a germline (n=19) or somatic (n=3) BRCA mutation. The majority of patients underwent optimal cytoreduction during their primary cytoreductive surgery (91.1%) or CGR during their secondary cytoreductive surgery (67.6%). Overall, 38 patients (33.3%) experienced a postoperative complication: 16 (14.0%) experienced a grade 3 complication and 1 patient (0.9%) experienced a grade 4 complication. There were no grade 5 complications.
Table 1:
Clinicopathologic characteristics prior to tertiary cytoreduction (N=114)
| Variable | n (%) |
|---|---|
| Age at initial diagnosis, years | |
| Median (range) | 52.4 (23.1-79.2) |
| Origin of disease | |
| Fallopian Tube | 9 (7.9%) |
| Ovarian | 103 (90.4%) |
| Peritoneal | 2 (1.8%) |
| Stage | |
| I | 10 (8.8%) |
| II | 16 (14.0%) |
| III | 76 (66.7%) |
| IV | 12 (10.5%) |
| Grade | |
| Low grade | 14 (12.3%) |
| High grade | 100 (87.7%) |
| Histology | |
| Serous | 86 (75.4%) |
| Endometrioid | 10 (8.8%) |
| Clear Cell | 4 (3.5%) |
| Carcinosarcoma | 3 (2.6%) |
| Mucinous | 5 (4.4%) |
| Other | 2 (1.8%) |
| Mixed | 4 (3.5%) |
| BRCA status (n=66) | |
| Negative | 44 (67%) |
| Any BRCA | 22 (33%) |
| NACT | |
| Received NACT | 4 (3.5%) |
| Underwent PDS | 110 (96.5%) |
| Initial cytoreduction (n=101) | |
| Optimal | 92 (91.1%) |
| Suboptimal | 9 (8.9%) |
| Secondary cytoreduction (n=111) | |
| Complete Gross Resection | 75 (67.6%) |
| Any residual | 36 (32.4%) |
| Time to first recurrence, months | |
| Median (range) | 26.9 (5.2-326.6) |
NACT, neoadjuvant chemotherapy; PDS, primary debulking surgery
Table 2 describes clinicopathologic and select operative data at the time of tertiary cytoreduction. Median patient age was 57.5 years (range, 30.0-83.0 years). The median treatment-free interval prior to tertiary cytoreduction was 16.1 months (range, 0.36-152.8 months). One hundred seven patients had preoperative imaging results available for review. On imaging, 56 (52.3%) of these patients had a single site of disease, 31 (29.0%) had two sites of disease, and 20 (18.7%) had three or more sites of disease. Fifty-two patients with preoperative imaging suggestive of single-site disease also had intraoperative descriptions of recurrent disease distribution. Among these 52 patients, intraoperative findings revealed 36 patients (69.2%) with single-site disease, 12 (23.1%) with two sites of disease, and 4 (7.7%) with three or more sites of disease.
Table 2:
Clinicopathologic characteristics at tertiary cytoreduction (N=114)
| Variable | n (%) |
|---|---|
| Age at tertiary cytoreduction, years | |
| Median (range) | 57.5 (30.0-83.0) |
| Treatment-free interval, months | |
| Median (range) | 16.1 (0.36-152.8) |
| Time from secondary cytoreduction, months | |
| Median (range) | 24.7 (5.6-111.3) |
| Platinum sensitivity | |
| Sensitive | 101 (88.6%) |
| Resistant | 13 (11.4%) |
| CA-125 at tertiary cytoreduction, U/mL (n=89) | |
| Median (range) | 31 (4.0-2475.0) |
| Preoperative imaging (n=107) | |
| CT | 70 (65.4%) |
| PET-CT | 33 (47.1%) |
| MRI | 4 (5.7%) |
| Sites of recurrence on imaging (n=107) | |
| 1 site | 56 (52.3%) |
| 2 sites | 31 (29.0%) |
| 3 or more sites | 20 (18.7%) |
| Residual disease at tertiary cytoreduction | |
| CGR | 102 (89.5%) |
| Residual disease | 12 (10.5%) |
| Sites of recurrence (n=106) | |
| Median (range) | 2 (1-10) |
| Sites of recurrence | |
| Single | 51 (44.7%) |
| Multiple | 63 (55.3%) |
| Surgical procedure performed | |
| Open non-visceral resection (abdominopelvic) | 24 (21.1%) |
| MIS non-visceral resection (abdominopelvic) | 1 (0.9%) |
| Large bowel resection | 32 (28.1%) |
| Small bowel resection | 16 (14.0%) |
| Partial liver resection | 12 (10.5%) |
| Open pelvic and/or paraaortic lymph node resection | 20 (17.5%) |
| MIS pelvic and/or paraaortic lymph node resection | 3 (2.6%) |
| Inguinal lymph node resection | 4 (3.5%) |
| Diaphragm resection | 7 (6.1%) |
| Open visceral resection (spleen, pancreas, adrenal, kidney) | 11 (9.6%) |
| MIS visceral resection (spleen) | 1 (0.9%) |
| Cardiothoracic resection (supradiaphragmatic/cardiophrenic/mediastinal lymph nodes, lung) | 6 (5.3%) |
| Adjuvant therapy after tertiary cytoreduction (n=113) | |
| Cytotoxic | 72 (63.7%) |
| Hormonal | 12 (10.6%) |
| Radiation | 12 (10.6%) |
| PARPi | 2 (1.8%) |
| Observation | 15 (13.3%) |
| Status at last follow-up | |
| AWD | 28 (24.6%) |
| NED | 24 (21.1%) |
| DOD | 60 (52.6%) |
| DOO | 2 (1.8%) |
CT, computed tomography; PET, positron emission tomography; MRI, magnetic resonance imaging; CGR, complete gross resection; MIS, minimally invasive surgery; PARPi, Poly (ADP-ribose) polymerase inhibition; AWD, alive with disease; NED, no evidence of disease; DOD, died of disease; DOO, died of other cause
The most common procedures were large bowel resections (28.1%), open pelvic and/or paraaortic lymph node resections (21.1%), and open non-visceral resections of abdominopelvic disease (17.5%). Five patients (4.4%) underwent a minimally invasive procedure. Of 103 patients who underwent open tertiary cytoreduction of abdominopelvic recurrence, 14 (13.6%) first underwent diagnostic laparoscopy. A CGR was achieved in 102 patients (89.5%), including 54 (96.4%) with single-site recurrence, 28 (90.3%) with two sites of recurrence, and 18 (90.0%) with three or more sites of recurrence on preoperative imaging. All patients undergoing diagnostic laparoscopy prior to open abdominopelvic resection of recurrence achieved a CGR. The median length of hospital stay for all patients was 6 days (range, 0-74 days).
Following surgery, 72 (63.7%) of 113 patients received cytotoxic chemotherapy, 12 (10.6%) radiation, 12 (10.6%) hormonal therapy, and 15 (13.3%) underwent observation. Cytotoxic regimens included 49 platinum doublets, 2 single-agent platinums, 2 non-platinum doublets, and 17 single-agent non-platinums. Two patients received chemotherapy at an outside institution, and the regimens used were unknown. Sixteen patients received maintenance therapy with hormonal agents (n=1), bevacizumab (n=9), PARPi (n=2), cytotoxic agents with bevacizumab (n=2), or targeted therapies (n=2). Most of the patients who underwent radiation had non-serous histologic disease (n=5), were treated prior to 2000 (n=3), or had recurrent disease at a similar site of prior resection (n=3). For the 15 patients observed after surgery, 12 ultimately underwent treatment. For these 12 patients, the median time to additional therapy was 7.0 months (range, 3.0-141.5 months).
Progression-free survival
The median follow-up time after tertiary cytoreduction for the entire cohort was 35.4 months (range, 0.2-272.9 months). Eighty-five patients (75%) recurred during follow-up. The median time to recurrence was 10.2 months among those who recurred (range, 1.7-263.2 months).
Table 3 details our analyses of the association of various factors with PFS. Time interval of ≥2 compared with <2 years between primary and secondary surgery was associated with a 10.5-month improvement in PFS (HR 0.54; 95% CI: 0.34-0.84). An interval of ≥2 compared with <2 years from secondary to tertiary cytoreduction was associated with a 14-month improvement in PFS (HR 0.51; 95% CI: 0.33-0.79). A treatment-free interval prior to tertiary cytoreduction of ≥1 compared with <1 year was associated with a 9.2-month improvement in PFS (HR 0.51; 95% CI: 0.33-0.81). Platinum-sensitive vs. platinum-resistant cases had a 7.3-month longer median PFS (HR 2.45; 95% CI: 1.45-5.28). Serous compared with non-serous histology was associated with worse PFS (HR 1.94; 95% CI: 1.12-3.34). There were no other factors, including BRCA mutational status and residual disease at tertiary cytoreduction, statistically associated with PFS.
Table 3.
Factors associated with progression-free survival
| Variable | Total (n) |
Median PFS (mo) |
Univariate HR (95% Cl) |
Univariate p-value |
Multivariate HR (95% CI) |
Multivariate p-value |
|---|---|---|---|---|---|---|
| Age at tertiary surgery | ||||||
| <55 years | 42 | 10.5 | ref | |||
| ≥55 years | 72 | 17.4 | 0.87 (0.55-1.38) | 0.56 | ||
| Stage | ||||||
| I | 10 | 13.2 | ref | |||
| II | 16 | 14.3 | 1.60 (0.60-4.29) | 0.35 | ||
| III | 76 | 12.0 | 1.94 (0.82-4.59) | 0.13 | ||
| IV | 12 | 17.4 | 2.23 (0.75-6.61) | 0.15 | ||
| Grade | ||||||
| Low grade | 14 | 18.9 | ref | |||
| High grade | 100 | 12.0 | 0.50 (0.24-1.04) | 0.06 | ||
| Histology | ||||||
| Serous | 86 | 11.3 | ref | |||
| Endometrioid | 10 | 11.1 | 0.52 (0.22-1.22) | 0.13 | ||
| Clear Cell | 4 | 14.3 | 0.47 (0.12-1.93) | 0.30 | ||
| Carcinosarcoma | 3 | 2.5 | 6.07 (1.83-20.12) | 0.003* | ||
| Mucinous | 5 | - | 0.20 (0.04-0.82) | 0.03* | ||
| Other | 2 | 8.5 | 0.59 (0.08-4.26) | 0.60 | ||
| Mixed | 4 | 29.9 | 0.52 (0.16-1.65) | 0.26 | ||
| Serous | ||||||
| Non-serous | 28 | 29.9 | ref | 0.007 | ||
| Serous | 86 | 11.3 | 1.94 (1.12-3.34) | 0.02* | 2.12 (1.22-3.68) | |
| BRCA status | ||||||
| Negative | 44 | 12.0 | ref | |||
| Any BRCA | 22 | 21.0 | 0.77 (0.40-1.47) | 0.42 | ||
| Initial cytoreduction | ||||||
| ≤1 cm residual | 92 | 12.0 | ref | |||
| ≥1 cm residual | 9 | 8.1 | 1.27 (0.61-2.65) | 0.53 | ||
| Secondary cytoreduction | ||||||
| CGR | 75 | 14.6 | ref | |||
| Any residual | 36 | 11.1 | 1.20 (0.75-1.91) | 0.45 | ||
| Time to first recurrence | ||||||
| <2 years | 44 | 9.2 | ref | |||
| ≥2 years | 64 | 19.7 | 0.54 (0.34-0.84) | 0.006* | ||
| Treatment-free interval | ||||||
| ≤1 year | 40 | 8.2 | ref | 0.005 | ||
| >1 year | 73 | 17.4 | 0.51 (0.33-0.81) | 0.004* | 0.52 (0.33-0.82) | |
| Time from secondary cytoreduction | ||||||
| <2 years | 53 | 9.4 | ref | |||
| ≥2 years | 61 | 23.7 | 0.51 (0.33-0.79) | 0.003* | ||
| Platinum sensitivity | ||||||
| Sensitive | 101 | 15.0 | ref | 0.002 | ||
| Resistant | 13 | 7.7 | 2.76 (1.45-5.28) | 0.002* | 2.80 (1.46-5.38) | |
| CA-125 prior to tertiary cytoreduction (continuous) | 1.000 (1.000-1.001) | 0.27 | ||||
| Residual disease at tertiary | ||||||
| CGR | 102 | 13.3 | ref | |||
| Any residual | 12 | 11.1 | 1.61 (0.82-3.16) | 0.17 | ||
| Sites of recurrence | ||||||
| Single | 51 | 20.9 | ref | |||
| Multiple | 63 | 11.1 | 1.29 (0.83-2.01) | 0.25 | ||
| Cytotoxic therapy after tertiary cytoreduction | ||||||
| Cytotoxic | 72 | 14.3 | ref | |||
| No cytotoxic | 12 | 10.2 | 1.01 (0.64-1.58) | 0.98 | ||
PFS, progression-free survival; CGR, complete gross resection
statistically significant
Given the availability of three time-interval variables possibly reflecting a more favorable disease biology (time from primary to secondary surgery, secondary to tertiary surgery, treatment to tertiary surgery), only treatment-free interval prior to tertiary surgery was selected for inclusion in the multivariable model. The final multivariate model included treatment-free interval prior to tertiary surgery (≥1 year vs. <1 year), platinum sensitivity, and serous versus non-serous histology. All variables maintained significance in the multivariable model (Table 3).
Disease-specific survival
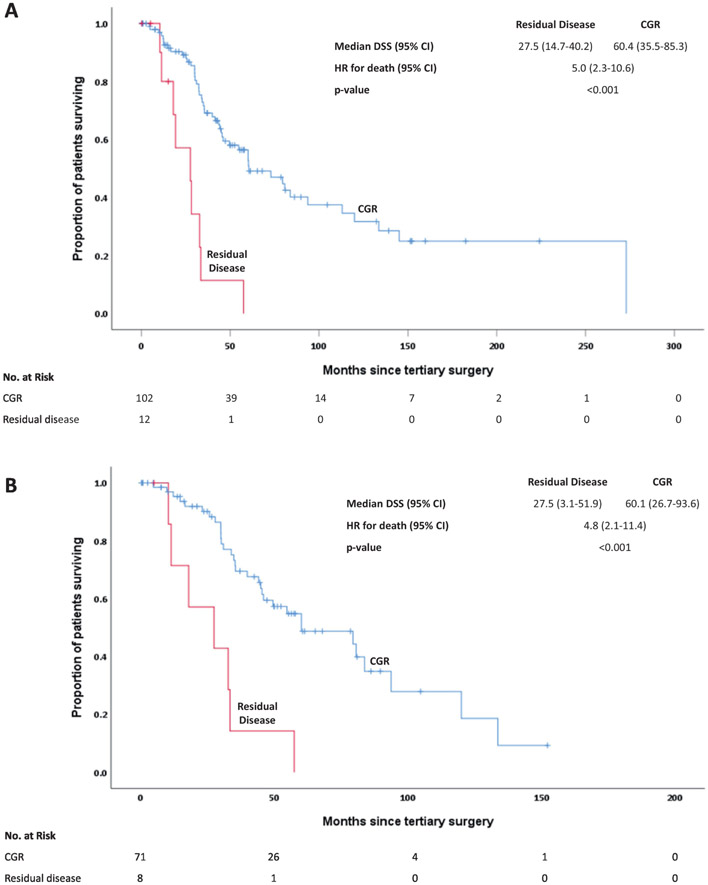
Table 4 details our DSS analyses. Fifty-eight patients (50.9%) died from their disease. CGR at tertiary cytoreduction was significantly associated with DSS, with a median DSS of 60.3 months for those who achieved a CGR compared to 27.5 months for those left with any macroscopic residual disease (HR 4.99; 95% CI: 2.35-10.60) (Figure 1A). Time to first recurrence, time from secondary cytoreduction, and treatment-free interval were also associated with DSS. Patients with platinum-sensitive disease had a median DSS of 60.3 months, compared with 18.0 months for those with platinum-resistant disease (HR 4.41; 95% CI: 2.04-9.52).
Table 4.
Factors associated with disease-specific survival
| Variable | Total (n) |
Median DSS (mo) |
Univariate HR (95% CI) |
Univariate p-value |
Multivariable HR (95% CI) |
Multivariable p-value |
|---|---|---|---|---|---|---|
| Age at tertiary cytoreduction | ||||||
| <55 years | 42 | 46.0 | ref | |||
| ≥55 years | 72 | 72.9 | 0.79 (0.46-1.35) | 0.38 | ||
| Stage at diagnosis | ||||||
| I | 10 | 113.0 | ref | |||
| II | 16 | 80.7 | 1.73 (0.51-5.95) | 0.38 | ||
| III | 76 | 46.0 | 2.60 (0.92-7.33) | 0.07 | ||
| IV | 12 | 83.8 | 2.11 (0.52-8.61) | 0.30 | ||
| Grade | ||||||
| Low grade | 14 | 272.9 | ref | 0.08 | ||
| High grade | 100 | 54.7 | 3.55 (1.25-10.1) | 0.017* | 2.61 (0.89-7.62) | |
| Histology | ||||||
| Serous | 86 | 57.5 | ref | |||
| Endometrioid | 10 | 32.3 | 1.02 (0.45-2.34) | 0.96 | ||
| Clear Cell | 4 | 72.9 | 0.50 (0.07-3.68) | 0.50 | ||
| Carcinosarcoma | 3 | 12.4 | 23.45 (5.78-95.1) | <0.001* | ||
| Mucinous | 5 | - | 0.17 (0.02-1.26) | 0.082 | ||
| Other | 2 | 41.4 | 1.53 (0.21-11.24) | 0.68 | ||
| Mixed | 4 | 60.4 | 0.66 (0.16-2.75) | 0.57 | ||
| Serous | ||||||
| Non-serous | 28 | 60.4 | ref | |||
| Serous | 86 | 57.5 | 1.19 (0.65-2.19) | 0.58 | ||
| BRCA status | ||||||
| Negative | 44 | 72.9 | ref | |||
| Any BRCA | 22 | - | 0.59 (0.21-1.64) | 0.31 | ||
| Initial cytoreduction | ||||||
| ≤1 cm residual | 92 | 60.1 | ref | |||
| >1 cm residual | 9 | 46.0 | 1.34 (0.57-3.17) | 0.50 | ||
| Secondary cytoreduction | ||||||
| CGR | 75 | 57.5 | ref | |||
| Any residual | 36 | 60.3 | 0.98 (0.57-1.68) | 0.93 | ||
| Time to first recurrence | ||||||
| <2 years | 44 | 43.5 | ref | |||
| ≥2 years | 64 | 83.8 | 0.52 (0.30-0.90) | 0.019* | ||
| Treatment-free interval | ||||||
| ≤1 year | 40 | 44.2 | ref | <0.02 | ||
| >1 year | 73 | 60.4 | 0.49 (0.29-0.84) | 0.009* | 0.49 (0.28-0.85) | |
| Time from secondary cytoreduction | ||||||
| <2 years | 53 | 43.5 | ref | |||
| ≥2 years | 61 | 79.5 | 0.54 (0.31-0.92) | 0.022* | ||
| Platinum sensitivity | ||||||
| Sensitive | 101 | 60.3 | ref | <0.02 | ||
| Resistant | 13 | 18.0 | 4.41 (2.04-9.52) | <0.001* | 2.94 (1.22-7.07) | |
| CA-125 prior to tertiary cytoreduction (continuous) | 89 | 1.001 (1.000-1.001) | 0.193 | |||
| Residual disease at tertiary cytoreduction | ||||||
| CGR | 102 | 60.3 | ref | 0.003 | ||
| Any residual | 12 | 27.5 | 4.84 (2.05-11.43) | 0.02* | 3.71 (1.59-8.70) | |
| Sites of recurrence | ||||||
| Single | 51 | 79.5 | ref | |||
| Multiple | 63 | 46.0 | 1.45 (0.85-2.49) | 0.18 | ||
| Cytotoxic therapy after tertiary cytoreduction | ||||||
| Cytotoxic | 72 | 57.5 | ref | |||
| No cytotoxic | 12 | 60.1 | 1.14 (0.66-1.94) | 0.64 | ||
DSS, disease-specific survival; CGR, complete gross resection
statistically significant
Figure 1.
A: Disease-specific survival (DSS) by cytoreductive status
B: Disease-specific survival for high-grade serous ovarian cancers (HGSOCs) by cytoreductive status
As with PFS, we chose to include only treatment-free interval prior to tertiary surgery in the multivariate model for DSS. The final model included treatment-free interval prior to tertiary surgery (≥1 year vs. <1 year), platinum sensitivity, tumor grade, and residual disease at tertiary cytoreduction. Three of the four variables were found to maintain significance in this multivariable model: treatment-free interval, platinum sensitivity, and residual disease at tertiary cytoreduction (Table 4).
Progression-free and disease-specific survival in high-grade serous carcinoma
We analyzed PFS and DSS specifically among the 79 patients with HGSOC (Supplementary Tables 1 and 2). There were no factors significantly associated with PFS, although the median PFS was 13.1 months for those who achieved a CGR compared to 9.5 months for those who did not (HR 2.03; 95% CI, 0.94-4.40). Furthermore, PFS was 13.3 months for those with platinum-sensitive disease compared to 7.4 months for those with platinum-resistant disease (HR 1.95; 95% CI: 0.83-4.56). A multivariate model for PFS was not performed considering the lack of significance for all factors on univariate analysis.
CGR at time of tertiary cytoreduction was significantly associated with improved DSS (median DSS, 60.1 months compared to 27.5 months (HR 4.84; 95% CI: 2.05-11.43) (Figure 1B). While only CGR at tertiary cytoreduction reached significance for DSS, several other variables demonstrated a positive association. For example, patients who were ≥2 years out from secondary debulking (n=38) had a 34.1-month higher median DSS compared to patients who were <2 years out from secondary debulking (n=41). Despite some possible absolute differences in DSS for other factors, none were statistically significant.
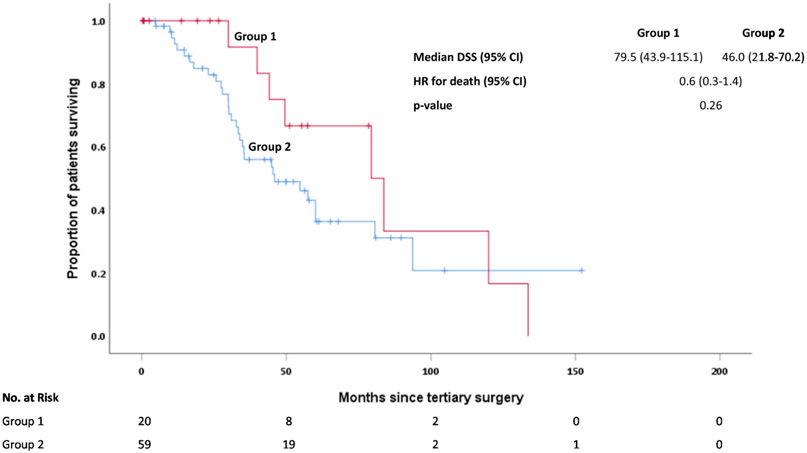
We sought to identify a group of patients with HGSOC who may derive the greatest benefit from tertiary cytoreduction. We grouped cases into one of two groups. Group 1 included patients who had a single site of recurrence and were ≥2 years out from secondary debulking (n=20). Group 2 included all other patients (n=59). The median DSS for Group 1 was 79.5 months (95% CI: 43.9-115.1 months) compared to 46.0 months for Group 2 (95% CI: 21.8-70.2 months). This was not statistically significant (p=0.26) despite the separation in the curves and an absolute difference of 33.5 months in median DSS (Figure 2).
Figure 2:
Disease-specific survival (DSS) for high-grade serous ovarian cancers (HGSOCs) by group (Group 1 includes patients who had a single site of recurrence and were ≥2 years out from secondary cytoreduction. Group 2 includes all other patients).
Discussion
Surgery is a mainstay of primary ovarian cancer treatment and a viable, albeit controversial, treatment option in select cases of recurrence (4, 12, 13). There are limited data on the value of further surgical cytoreduction in patients who recur after a secondary cytoreduction, as tertiary cytoreduction is an uncommon clinical scenario (8).
Careful patient selection is key in the decision process for any intervention, and more so for surgical interventions in recurrent disease. In our study, all patients were offered surgery with the intent for CGR, and 90% of our patients selected for tertiary cytoreduction achieved a CGR. High fidelity between preoperative imaging and intraoperative findings demonstrated the value of skilled radiology in accurately identifying patients likely to achieve a CGR. For example, radiology accurately predicted single-site recurrent disease; only 18.7% of patients with predicted single-site disease on imaging ultimately had three or more sites of recurrence. High rates of CGR (≥90%) across patients with single-to multi-site disease is also suggestive of careful interpretation of imaging and appropriate triage of patients to tertiary cytoreduction. We also observed that patients who underwent tertiary cytoreduction were more likely to have more “indolent” disease. The majority were platinum sensitive (88.6%), had been optimally cytoreduced at initial diagnosis (91.1%), and achieved a CGR at secondary cytoreduction (67.6%). The median CA-125 of 31 units/mL was quite low, and the rate of BRCA mutations was quite high (33%).
The importance of CGR at tertiary cytoreduction was still evident and strongly associated with outcomes, even in this highly selected cohort of patients. For patients who achieved a CGR, the median DSS was 5 years, compared to just over 2 years for those in whom a CGR was not achieved. The association between CGR and improved survival was maintained when specifically looking at HGSOC, where CGR over any residual disease was associated with a 32.8-month increase in median DSS (60.3 vs. 27.5 months, respectively). Among all patients, the relationship between CGR and DSS maintained its significance (p<0.001) on multivariable analysis, including tumor grade, platinum sensitivity, and treatment-free interval. These findings are consistent with our prior study, the Multicenter Italian Trials in Ovarian Cancer and Gynecologic Malignancies group study, and one other multi-institutional retrospective study, which all reported a significant association between residual disease following tertiary cytoreduction and survival (5, 7, 8). For example, in the 406-patient multi-institutional study, 54.1% of patients achieved a CGR, and the median OS following tertiary cytoreduction was 37.0 months longer for these patients (49.0 vs. 12.0 months) (8). The study differed from ours in that our rates of CGR approached 90%; however, the relationship between improved survival and CGR remained.
Our group previously described an algorithm that incorporated variables of treatment interval and sites of recurrence for triaging patients to secondary cytoreduction (1). High fidelity use of this model has been associated with CGR rates of 86% among patients undergoing secondary cytoreduction for platinum-sensitive recurrent ovarian cancer (14). Arbeitsgemeinschaft Gynaekologische Onkologie (AGO) score may also be a valuable tool in the selection of patients who may best benefit from secondary cytoreduction (12, 15). Given the demonstrated utility of secondary cytoreduction selection models and scores, we sought to create such a predictive model to assist in the selection of cases for tertiary cytoreduction specific to HGSOC. Our data showed that low-grade carcinomas did exceedingly well after surgery and were a smaller group of cases. Non-serous histologies were rare. Thus, we concluded that an algorithm/model was most useful for patients with HGSOC.
It was difficult to develop a robust clinical model from our data, as 90% of our patients achieved a CGR and did quite well. In developing a model in a purely hypothesis-generating fashion, the subgroups became smaller, thus limiting statistical significance. The best DSS outcomes were in patients who had a single site of disease recurrence and were ≥2 years removed from secondary cytoreduction. In these patients, there was an absolute median DSS improvement of 33 months compared to all other patients, with survival curves that remained separate. However, no statistical significance was noted. This is likely due to our sample sizes and could possibly be assessed in larger cohorts. It seems to make sense that patients with a long disease course and a single site of recurrence amenable to CGR would be ideal candidates for a tertiary cytoreduction. It also seems that others may also benefit, as the median DSS was still a very reasonable 46 months. The key is to achieve a CGR without excessive morbidity.
Our analyses are limited by the retrospective nature of this study. Selection to proceed with surgery was at the provider’s discretion and not randomly assigned. While selection is clearly a potential bias, it can also be a reflection of careful clinical decision-making when deciding whether to offer surgical intervention. Preoperative imaging data suggest that patients were carefully selected for tertiary cytoreduction, with greater than 80% of patients having radiographic evidence of two or fewer sites of recurrence. This careful patient selection and 90% CGR rate limited our ability to propose a robust predictive model. A randomized controlled trial would be the usual recommendation to properly address this intervention, but it may not be feasible. Furthermore, the rates of optimal cytoreduction and CGR in both the primary and secondary settings at our institution have clearly changed over this time. Also, the surgeons performing these surgeries have not always been the same. This may or may not be a limitation. The study is further limited by its span of three decades and the inherent evolution of all aspects of ovarian cancer management during that time period, including adapted definitions of optimal cytoreduction, advancements in radiographic imaging, the increasing use of minimally invasive surgery (MIS), and innovation in targeted therapy, such as PARPi, for recurrent ovarian cancer (16). In this study, BRCA mutation was not associated with improved outcome, possibly due to the relatively good survival among all patients; however, this finding has its limitation, as before a certain time period, genetic testing was not broadly performed and/or available in the medical record, expanded panel testing was infrequent, and there was no homologous recombination deficiency testing. Furthermore, the role of PARPi could not be assessed given that the majority of patients underwent tertiary cytoreduction prior to the introduction of PARPi for patients with BRCA mutations or homologous recombination deficiency (17).
Despite its limitations, we believe these data will provide some assistance in guiding clinical decision making and patient counseling in the recurrent ovarian cancer setting. Our tertiary cytoreduction “response rate” (the CGR rate), which is used as an important surrogate in many therapeutic studies in the recurrent setting, was 89.5%. This demonstrates that surgical intervention can still play a significant role in the management of carefully selected patients. Here, we have identified that patients likely to achieve a CGR have the potential to benefit most from tertiary cytoreduction, as do patients with prolonged treatment-free intervals and platinum-sensitive disease. In select cases like these, tertiary surgery should still be considered. And, as perioperative care and MIS approaches advance, the toxicity of surgery will be further minimized to maximize patient benefit.
Supplementary Material
Supplementary Table 1. Factors associated with progression-free survival for high-grade serous ovarian cancers
Supplementary Table 2. Factors associated with disease-specific survival for high-grade serous ovarian cancers
Highlights.
Patients who undergo complete gross resection derive the greatest survival benefit from tertiary surgery
Platinum sensitivity and prolonged treatment-free interval are associated with improved survival after tertiary surgery
HGSOC patients with single-site recurrence who are ≥2 years from secondary surgery may benefit most from tertiary surgery
Acknowledgments
Funding: Funded in part through the NIH/NCI Cancer Center Support Grant P30 CA008748.
Footnotes
Conflict of Interest Statement
Outside the submitted work, Dr. Leitao reports personal fees from JnJ/Ethicon and Intuitive Surgical, Inc. Dr. O'Cearbhaill reports personal fees from Tesaro, GlaxoSmithKline, Regeneron, and Seattle Genetics; she is also a non-compensated steering committee member for the PRIMA, Moonstone (Tesaro/GSK) and DUO-O (AstraZeneca) studies. Her institution receives funding for clinical research from Bayer/Celgene/Juno, Tesaro/GSK, Ludwig Cancer Institute, Abbvie/StemCentrx, Regeneron, TCR2 Therapeutics, Atara Biotherapeutics, MarkerTherapeutics, Syndax Pharmaceuticals, Genmab Therapeutics, Sellas Therapeutics, Genentech, Kite Pharma, and the Gynecologic Oncology Foundation. Dr. Abu-Rustum reports grants from Stryker/Novadaq, Olympus, and GRAIL. Dr. Chi reports personal fees from Bovie Medical Co., Verthermia Inc. (now Apyx Medical Corp.), Biom ‘Up, and C Surgeries, as well as other from Intuitive Surgical, Inc., and TransEnterix, Inc. The other authors have nothing to disclose.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Chi DS, McCaughty K, Diaz JP, Huh J, Schwabenbauer S, Hummer AJ, et al. Guidelines and selection criteria for secondary cytoreductive surgery in patients with recurrent, platinum-sensitive epithelial ovarian carcinoma. Cancer. 2006;106(9):1933–9. [DOI] [PubMed] [Google Scholar]
- 2.Du Bois A SJ, Vergote I, Ferron G, Reuss A, Meier W, Greggi S, Jensen PT, Selle F, Guyon F, Pomel C, Lecuru F, Zang R, Avall-Lundqvist E, Kim JW, Ponce J, Raspagliesi F, Ghaem-Maghami S, Reinthaller A, Harter P. Randomized phase III study to evaluate the impact of secondary cytoreductive surgery in recurrent ovarian cancer: Final analysis of AGO DESKTOP III/ENGTO-ov20. Journal of Clinical Oncology. 2020;38(38). [Google Scholar]
- 3.Shi T, Zhu J, Feng Y, Tu D, Zhang Y, Zhang P, et al. Secondary cytoreduction followed by chemotherapy versus chemotherapy alone in platinum-sensitive relapsed ovarian cancer (SOC-1): a multicentre, open-label, randomised, phase 3 trial. Lancet Oncol. 2021. [DOI] [PubMed] [Google Scholar]
- 4.Coleman RL, Spirtos NM, Enserro D, Herzog TJ, Sabbatini P, Armstrong DK, et al. Secondary Surgical Cytoreduction for Recurrent Ovarian Cancer. N Engl J Med. 2019;381(20):1929–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Shih KK, Chi DS, Barakat RR, Leitao MM Jr., Tertiary cytoreduction in patients with recurrent epithelial ovarian, fallopian tube, or primary peritoneal cancer: an updated series. Gynecol Oncol. 2010;117(2):330–5. [DOI] [PubMed] [Google Scholar]
- 6.Karam AK, Santillan A, Bristow RE, Giuntoli R 2nd, Gardner GJ, Cass I, et al. Tertiary cytoreductive surgery in recurrent ovarian cancer: selection criteria and survival outcome. Gynecol Oncol. 2007;104(2):377–80. [DOI] [PubMed] [Google Scholar]
- 7.Falcone F, Scambia G, Benedetti Panici P, Signorelli M, Cormio G, Giorda G, et al. Tertiary cytoreductive surgery in recurrent epithelial ovarian cancer: A multicentre MITO retrospective study. Gynecol Oncol. 2017;147(1):66–72. [DOI] [PubMed] [Google Scholar]
- 8.Fotopoulou C, Zang R, Gultekin M, Cibula D, Ayhan A, Liu D, et al. Value of tertiary cytoreductive surgery in epithelial ovarian cancer: an international multicenter evaluation. Ann Surg Oncol. 2013;20(4):1348–54. [DOI] [PubMed] [Google Scholar]
- 9.Hirakawa T, Minaguchi T, Itani Y, Kasamatsu Y, Murase S, Sakurada S, et al. Current status of tertiary debulking surgery and prognosis after secondary debulking surgery for recurrent Mullerian epithelial cancer in Japan: a retrospective analysis of 164 patients (KCOG-G1402). World J Surg Oncol. 2017;15(1):132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Arvas M, Salihoglu Y, Sal V, Gungor T, Sozen H, Kahramanoglu I, et al. Tertiary Cytoreduction for Recurrent Epithelial Ovarian Cancer: a Multicenter Study in Turkey. Asian Pac J Cancer Prev. 2016;17(4):1909–15. [DOI] [PubMed] [Google Scholar]
- 11.Strong VE, Selby LV, Sovel M, Disa JJ, Hoskins W, Dematteo R, et al. Development and assessment of Memorial Sloan Kettering Cancer Center's Surgical Secondary Events grading system. Ann Surg Oncol. 2015;22(4):1061–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dubois A VI, Ferron G, Reuss A, Meier W, Greggi S. . Randomized controlled phase III study evaluating the impact of secondary cytoreductive surgery in recurrent ovarian cancer: AGO DESKTOP III/ENGOT ov20. J Clin Oncol. 2017;35(15). [Google Scholar]
- 13.Zang R ZJ, Shi T, Liu J, Tu D, Yin S, Jiang R, Zhang P, Jia H, Luan Y, Zhang Y, Chen X, Huang X, Tian W, Gao W, Feng Y, Yang H, Cheng X, Cai Y. A randomized phase III trial of secondary cytoreductive surgery in later recurrent ovarian cancer: SOC1/SGOG-OV2. J Clin Oncol. 2020;38(15). [Google Scholar]
- 14.Cowan RA, Eriksson AGZ, Jaber SM, Zhou Q, Iasonos A, Zivanovic O, et al. A comparative analysis of prediction models for complete gross resection in secondary cytoreductive surgery for ovarian cancer. Gynecol Oncol. 2017;145(2):230–5. [DOI] [PubMed] [Google Scholar]
- 15.Harter P, du Bois A, Hahmann M, Hasenburg A, Burges A, Loibl S, et al. Surgery in recurrent ovarian cancer: the Arbeitsgemeinschaft Gynaekologische Onkologie (AGO) DESKTOP OVAR trial. Ann Surg Oncol. 2006;13(12):1702–10. [DOI] [PubMed] [Google Scholar]
- 16.Eisenhauer EA. Real-world evidence in the treatment of ovarian cancer. Annals of Oncology. 2017;28:61–5. [DOI] [PubMed] [Google Scholar]
- 17.Kaufman B, Shapira-Frommer R, Schmutzler RK, Audeh MW, Friedlander M, Balmana J, et al. Olaparib monotherapy in patients with advanced cancer and a germline BRCA1/2 mutation. J Clin Oncol. 2015;33(3):244–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Table 1. Factors associated with progression-free survival for high-grade serous ovarian cancers
Supplementary Table 2. Factors associated with disease-specific survival for high-grade serous ovarian cancers