Abstract
Major Depressive Disorder is a major public health problem and has a high rate of treatment resistance. Fear conditioning has been proposed as a potential mechanism sustaining negative affect in mood disorders. With the aim of exploring cognitive effects of rapid-acting antidepressant treatments as a potential mechanism of action that can be targeted by neuromodulation, we performed a narrative review of the extant literature on effects of electroconvulsive therapy, ketamine or esketamine, and sleep deprivation on emotional/fear memory retrieval-reconsolidation. We explore interference with reconsolidation as a potential common pathway that explains in part the efficacy of rapid-acting antidepressant treatments with disparate mechanisms of action. We propose the testable hypothesis that fear learning circuits can be specifically targeted by neuromodulation to attempt rapid amelioration of depressive symptoms (especially repetitive negative thinking) while limiting unspecific, untoward cognitive side effects.
Keywords: Depression, Memory Reconsolidation, Neuroimaging, Neuromodulation
1. Introduction
Depression (MDD) is the main cause of disability worldwide (Friedrich 2017). Most patients respond to available pharmacological antidepressant treatments, but remission rates are unsatisfactory, and over 10% of patients fail to improve to any clinically significant degree with regular pharmacological measures alone (Rush et al 2006, Nemeroff 2018). Unfortunately, the development of antidepressant pharmacotherapies with novel mechanisms of action has stalled and therefore there is an utter need to understand the pathophysiology of MDD, define treatment targets, and the optimal strategies to engage the latter, leading to meaningful clinical changes. This is especially true for those methods which have demonstrated efficacy and a rapid onset of action among treatment-refractory patients (Hyman 2012). The mechanisms of action of rapid-acting treatments such as electroconvulsive therapy (ECT), ketamine, and sleep deprivation (SD) are unsettled. As mentioned below, most theories seek to explain efficacy in terms of modifications in neurotransmitter systems, neurohormonal changes and their associated neural plasticity changes, brain neurotrophic factor levels, and even systemic changes in inflammation; ultimately, all such mechanisms are purported to exert their effects by modification of the function or structure of specific brain circuits underlying clinical manifestations (e.g. Duman et al 2016). However, prevailing theories have not been entirely successful in providing consistent mechanisms of action of heuristic value, which would in turn assist the development of new, more efficacious and better tolerated treatments.
Cognitive effects of available rapid-acting treatments, especially anterograde episodic memory deficits (Steif et al 1986, de Souza et al 2019), are almost universally conceptualized as undesirable collateral consequences compounding cognitive deficits characteristic of MDD, to be avoided when better rapid-acting treatments become available (Andrade et al 2016, Carreno et al 2020). On the other hand, rapid acting antidepressants also induce emotional learning impairments, and in the present review we propose such effects are one of a number of possible overlapping mechanisms by which these treatments effect change, and suggest that, if properly understood, new methods can be designed to optimize antidepressant effect while decreasing the intensity of adverse cognitive symptoms (especially episodic memory consolidation problems). We have chosen to address cognitive effects because,
Diverse cognitive effects are common to treatments for resistant depression with demonstrated clinical efficacy, and have been used in similar ways in experiments of interference with emotional learning, and
Such effects fulfill the requirement of a rapid mechanism of action (i.e., less than 24h for onset of action).
Specifically, we comment on three rapid acting treatment modalities for severe, refractory depression which have widely accepted efficacy. ECT is the most efficacious treatment for patients with treatment-resistant MDD (Hermida et al 2018). More recently, ketamine and its s-enantiomer esketamine have been incorporated into the available armamentarium (Correia-Melo et al 2020) in the treatment of this disorder. Neither of these treatments displays long-lasting effects (Kellner 2013, Perez-Esparza et al 2018). Last, sleep deprivation (SD) has long been recognized as an acute antidepressant, whose clinical use is limited by the fact its effect unfortunately disappears even faster than those of ECT and ketamine-usually after the first subsequent night (or even nap) of uninterrupted sleep, unless it is combined with other interventions (Sikkens et al 2019). In the present review, we propose that these three methods of disparate nature (i.e., neuromodulatory, pharmacological, and behavioral respectively) may act at least in part by inhibition of mechanisms of persistent emotional/visceral memory reconsolidation, whose observable, untreated clinical correlate in patients with depression is repetitive negative thinking (RNT, usually referred to in the depression literature as rumination; Nolen-Hoeksema et al., 2008; McEvoy et al., 2010). Whereas in all probability each of these methods operate through alternative mechanisms unique to each of them (e.g., ketamine has recently been suggested to affect endogenous opioid neurotransmission and prefrontal plasticity via rTORC intracellular signaling, Williams et al 2018; Klein et al 2020), the characterization of a partially overlapping mechanism of action would suggest a shared treatment target for rapidly acting antidepressant therapies and facilitate the development of additional interventions able to engage such biomarker, possibly focal novel neuromodulation approaches, addressed at nodal points in brain circuits that participate in the formation of some of the most clinically significant depressive symptoms, and their maintenance in the course of a severe and treatment-refractory depressive episode. Alternatively, investigational or approved drugs affecting cognition (e.g., NMDA neuromodulation or surface-acting steroids) may also share in part this mechanism of action (Machado-Vieira et al 2017, Scott 2019). Specifically, we set out to explore literature evidence on the potential impact of these three types of antidepressant treatments useful in refractory depression, on their interference with the consolidation and reconsolidation of emotional memories.
In general, learning and memory are usually conceptualized as going through a series of phases, including a learning or encoding stage, a stabilization stage (in which the initially unstable new information is consolidated, possibly via synaptic consolidation), a maintenance stage where the consolidated memory is preserved, sometimes for the lifetime of the organism, and then a retrieval stage where the memory is conjured up through specific mechanisms (McGaugh 1966, Spear 1973, Kandel 2001, Dudai 2004, Nader 2015). Other than acquisition and stabilization, these phases of learning and memory have been traditionally considered passive phenomena. However, maintenance of memory is currently considered an active process as well, so that long-term memories of various modalities are susceptible to manipulation in the form of disruption or restoration, a process originally known as “cued amnesia” (Lewis et al 1972), but currently commonly referred to as “reconsolidation” (Nader et al 2000, Sara 2000, Sara 2015). In essence, reconsolidation implies that a previously stored (consolidated) memory can be rendered labile by retrieval, and in this setting, it can be either reconsolidated or attenuated. Whereas the original descriptions of this phenomenon involved fear conditioning, the phenomenon has been demonstrated for a large variety of species, types of memories, and disruptive treatments (see Nader 2015 for a detailed review). Importantly, the original evidence on the possibility to produce selective retrograde amnesia in animals, employed electroconvulsive shock, which is the gold standard for the treatment of resistant depression (Misanin et al 1968). As illustrated by Figure 1, in humans and animals, retrieval-reconsolidation is typically defined according to three-staged studies encompassing several days, involving encoding, retrieval, manipulation of the retrieved memory, and memory test where the effect of manipulation is measured (Agren 2014, Post and Kegan 2017).
Figure 1:
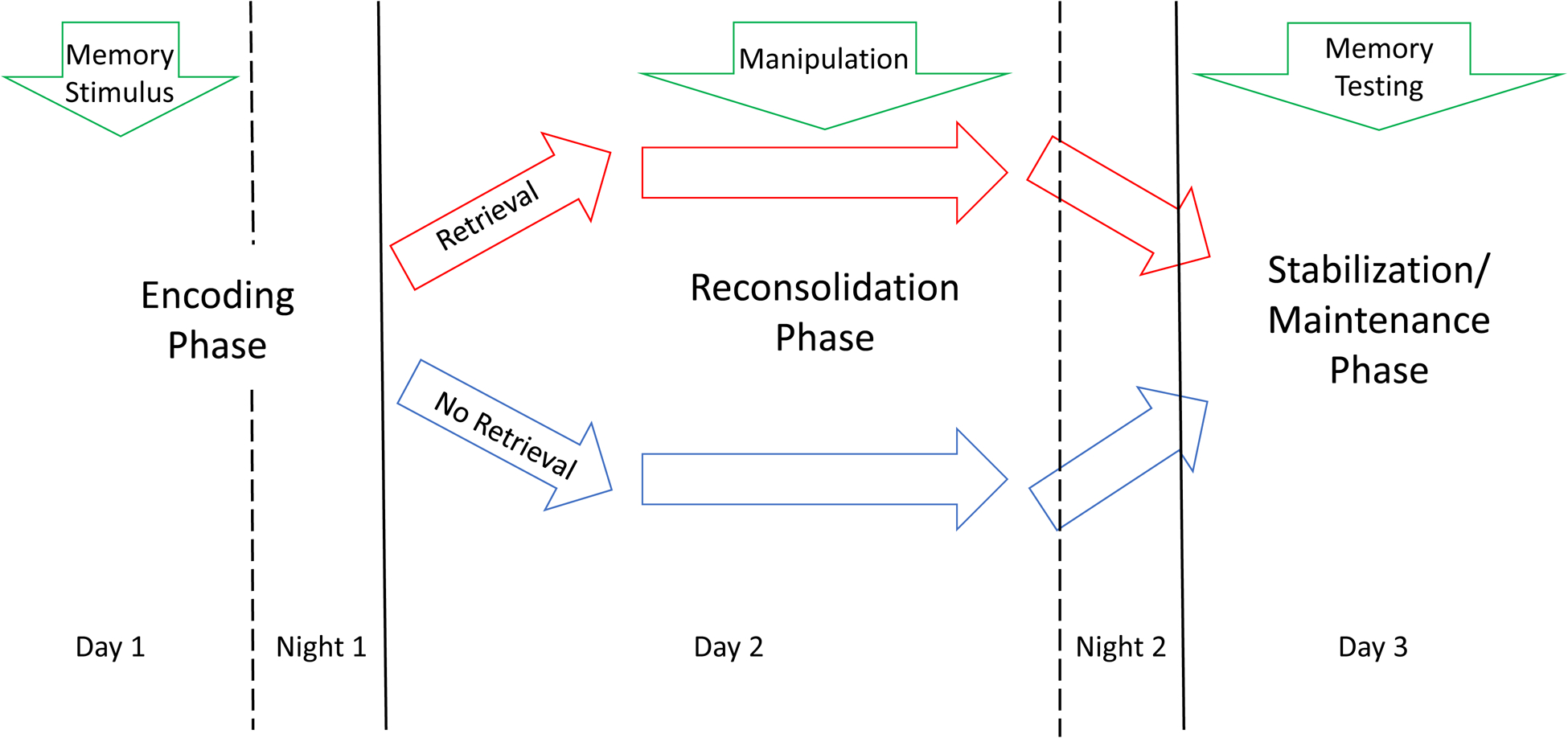
Typical sequence of memory stimulus, retrieval, manipulation, and testing in a reconsolidation experiment. Night periods after encoding and retrieval/manipulation are shown to emphasize the role of sleep in engram stabilization in general, and in reconsolidation of retrieved memories in particular. Please see the text for details.
Implicit in this scheme, is the presence of sleep periods during which the memory is first encoded, and then reconsolidated (Sara 2017). Whereas both slow-wave sleep (SWS) and rapid eye movement, or paradoxical sleep (REM) are relevant to the consolidation of recently encoded memories (or reconsolidation of retrieved memories), most evidence suggests that fear and emotional learning consolidation occurs during REM, once relevant information to be remembered has been selected during SWS (Cairney et al 2015, Sara 2017). This is relevant for the interpretation of the mechanism of action of sleep deprivation (SD) as hypothesized below, involving either the whole night, or the second half of the night, where REM sleep tends to concentrate. Anatomic circuits involved in the general phenomenon of retrieval and reconsolidation of memory engrams depends, of course, on the type of learning being considered (Nader 2000, Agren 2014, Post and Kegan 2017). In the present review we will discuss only those circuits presumably related to fear and emotional memory retrieval and reconsolidation, hypothesized to be related to depression symptom generation, and potentially amenable to modulation.
2. Method
We performed a PubMed search employing the words ketamine OR esketamine OR electroconvulsive therapy OR sleep deprivation AND reconsolidation. We report on studies available as of January 19 2021. In addition, we included a ScienceDirect search until March 30 2021. Figure 2 summarizes the PRISMA flow diagram for study inclusion. A total of 31 studies, without duplications, was screened for inclusion. Five articles did not focus on the effect of rapid-acting antidepressant methods on memory reconsolidation after retrieval. We included 31 additional articles referenced in searched communications. Results include 17 animal studies.
Figure 2:
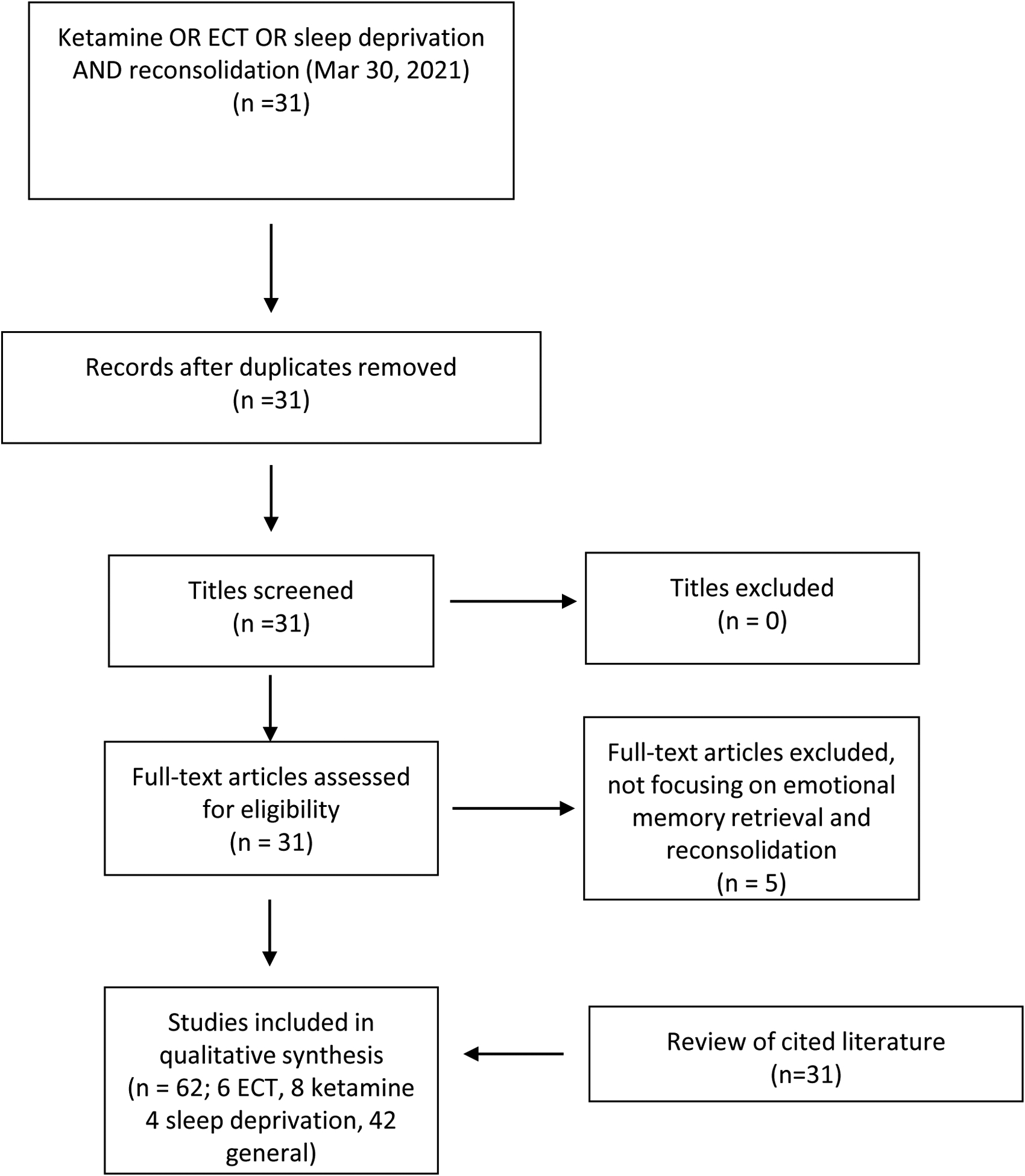
PRISMA flow diagram for reviewed literature. Please see the text for details.
Most studies were case reports and small case series without control groups, or animal studies, so we did not conduct hypothesis-testing statistical analyses. Outcomes were therefore summarized in a narrative manner.
3. Results
ECT, SD, and ketamine have all been demonstrated in experimental and clinical paradigms to interfere with emotional/fear learning and reconsolidation. The latter specifically involves reactivation of emotional memories (which then resume a labile neurobiological status), and then interfere with their consolidation. Experiments testing reconsolidation memory phenomena therefore should comply with the following criteria (Nader et al 2000, Agren 2014):
Consolidated memories have to be reactivated through a reminder cue
The intervention on the reactivated memory has to be delivered at a specific time after reactivation, and
Detection of the influence of the intervention on the reconsolidated memory has to be tested after a certain period to allow the latter to take place - usually 24 h including a sleep period, rather than immediately, to avoid confounding short-term memory effects.
3.1. Electroconvulsive Therapy (ECT)
ECT has been demonstrated to impair emotional memory reconsolidation in animals and humans, and in such cases, this has been suggested to exert a beneficial clinical effect. In fact, the first evidence for the existence of memory consolidation was produced in the late 1960s with the help of electroconvulsive shock, when a small series of studies employed this method to demonstrate amnesia for a fear conditioning memory (Misanin et al 1968, Schneider and Sherman 1968, Lewis 1969), although interest in this topic immediately waned, until it reemerged in the 2000s (Nader et al 2000, Dunbar and Taylor 2017). As ECT is well known for its broad amnestic effects (Bergfeld et al 2017), and given the effect on retrieved emotional memories in the late 1960s, Kroes et al (2014) have used a single ECT session in 42 patients with unipolar depression, to test the effect of it on reconsolidation of trauma-related episodic memories. They exposed the participants to two emotionally aversive stories initially, and memory was reactivated one week later for one of the stories only. Immediately after memory retrieval, and discovered a significant effect on reactivated, but not on non-reactivated memories. In fact, retrieval in the control, non-ECT group, resulted in an enhancement of memory performance for the retrieved story, confirming that ECT disrupts a previously consolidated memory, which has been rendered labile due to retrieval (Kores et al 2014). Gahr et al (2014) report on a single patient with PTSD whom they exposed to eight successive ECT applications after narrative reactivation of a traumatic experience. They observed not only improvement of depression, as expected, but also decreased ability of the patient to remember the traumatic event specifically associated with clinical onset of symptoms, retrieved before each right unilateral ECT session. Scheepens et al. (2020) have recently published a randomized clinical controlled trial testing a hypothesis conceptually related to the one advanced herein, namely, that ECT might be rendered more effective in the acute setting, and in the longer term (six months), by activating autobiographic episodic memories that are negatively laden, and therefore attached to negative cognitive schema. They randomized patients clinically eligible for ECT (older individuals and those with psychosis were excluded) to receive either a brief emotional memory reactivation, or a control memory reactivation. For the emotional memory reactivation, three negative autobiographical memories were chosen by each participant, and such memories were read out loud to the patient in three minutes, a few minutes before the ECT session (only one memory was read after each session, alternating between the three memories evoked by each patient). The control intervention was the exposure to positive reinforcers, such as the importance of attention to items such as sleep, physical exercise, and substance use, in mental health. The study incorporated 32 patients exposed to autobiographical memories and 34 patients exposed to health-related memories. Recruitment was terminated before the target number of participants because of a change in Dutch mental health care policy. Six biweekly sessions were planned initially, but the ECT course was discontinued in an unspecified number of patients if no clinical improvement was seen after two weeks (4 sessions; over a quarter of paticipants did not respond or remit). Average number of ECT (SD) sessions was 15.2 ± 7.5 for the group as a whole. Right unilateral placement was used in about two thirds of participants in all patients. The authors did not observe differences between both memory interventions in regard to efficacy, or likelihood of relapse (Scheepens et al 2020). This trial is, to the extent of our knowledge, the only one to have addressed the hypothesis that interference with reconsolidation can play a role in ECT efficacy, and offers important insights into this subject, to be addressed in the following section.
3.2. Ketamine
Ketamine has received extensive attention in regards to its effects on memory function in general, and emotional memory reconsolidation in particular, in both experimental settings and clinical samples of depressed, traumatized, and addicted individuals (Becker et al 2017, Veen et al 2018, Das et al 2019, Piva et al 2020). N-mehtyl-D-aspartate receptors (NMDAR) are widely distributed in the brain, but are particularly dense at frontal and limbic regions involved in emotion and memory regulation. Ketamine is one of a number of NMDAR antagonists that transiently disrupt both emotional memory acquisition and reconsolidation, with consistent animal and human cognitive effects (Becker et al 2017, Das et al 2013, Das et al 2019, Piva et al 2020). Ketamine impairs emotional memory consolidation in a valence-specific pattern, apparently modulating mOFC-parahipoccampal connectivity in humans, which are areas consistently involved in rumination, fear conditioning, and the regulation of visceral function (Becker et al 2017, Cheng et al 2018). Becker et al (2017) studied 21 healthy persons in a randomized, controlled crossover design on the effect of exposure to ketamine or placebo, and observed that this agent produces general impairment of emotional memory encoding irrespective of valence, which is more intense for positive stimuli. During encoding, however, ketamine showed maximum effects on right parahippocampal and orbitofrontal cortices, and left amygdala (Becker et al 2017). The effect of ketamine on memory reconsolidation has also been explored in the context of substance use and reward in both humans (Das et al 2019) and experimental animals (Piva et al 2020). Das et al (2019) exposed 90 alcohol use disorder individuals to either placebo + retrieved maladaptive reward memories, ketamine + control memories, or ketamine + maladaptive reward memories, and observed that pretreatment with ketamine immediately before maladaptive memories (but not before control memories, or maladaptive memories followed by placebo) resulted in a significantly decreased number of drinking days in the 10 days following the experiment, suggesting a “rewriting” of the reward quality of maladaptive drinking memories (Das et al 2019). Piva et al (2020) showed that ketamine impairs contextual memories associated to sucrose availability, seemingly via plastic effects, i.e. tissue-specific increases or decreases in the number of NMDARs of postsynaptic cells at the nucleus accumbens, hippocampus, and amygdala. In the only negative observation available on the ability of ketamine to impair reconsolidation, these authors did not observe behavioral changes in their reconsolidation experiment, possibly because retrieval occurred after the expected window for ketamine effect (i.e., ketamine was administered >24 h before retrieval). By means of a similar mechanism of action, ketamine has been proposed as a potential blocker of traumatic memories in PTSD patients, when such memories are retrieved in trauma-focused psychotherapy, and thereby brought back to a neurobiologically labile state (Veen et al 2018); this research group has recently provided supportive evidence for this view in a preclinical study, where they showed that ketamine in a single clinically meaningful dose (0.5 mg/kg) decreases fear memory in marmoset monkeys when paired with retrieval (Philippens et al 2021). These findings confirm the ability of ketamine to disrupt established fear memories when administered during contextual fear reconsolidation in rats divided into groups with high and low behavioral response to a novelty context, which also displayed different sensitivity to fear conditioning and extinction (Duclot et al 2016); in this experiment, ketamine was associated to an up regulation of BDNF expression in nucleus accumbens and different cortical areas including the hippocampus, and a down-regulation in the amygdala, providing a synaptic plasticity explanation for the result of the behavioral experiments. Last, in a recent, open-label trial on three patients, Pradhan and Rossi (2020) observed that a single dose of ketamine administered before repetitive transcranial magnetic stimulation and mindfulness focusing on extinction, improved the behavioral outcome, which was interpreted as reflecting the ability of ketamine to impair reconsolidation (Pradhan and Rossi 2020).
3.3. Sleep Deprivation (SD)
Sleep is critical in the consolidation of different types of memory, and in particular emotional memories that are either new or recently retrieved/reconsolidated within the 24 h prior to the sleep cycle (Sara 2000, Krause et al 2017). Whereas SD before the acquisition of an emotional memory enhances its negative aspects, which relates to the worsening effect of sleep problems on depression (Feng et al 2018, Riemann et al 2019), and adverse cognitive/memory effects of both insomnia and purposeful sleep deprivation (Riemann et al 2019), normal sleep is necessary to achieve memory consolidation during a critical 24 h period after emotional learning has taken place. Therefore, not surprisingly SD interferes with reconsolidation of emotional memories or emotional memories acquired during daytime immediately prior to the SD cycle, probably by decreasing ventromedial prefrontal cortex control of amygdala activity (McCormick et al 2018), and its connectivity with insular cortex (Feng et al 2018). Remarkably, this seems to be particularly valid for 1) emotional stimuli with negative or neutral valence, but not those with positive valence, and 2) selected individuals with prominent depressive symptoms, but not to a similar extent in those persons reporting few or no symptoms of depression (Harrington et al 2018). These investigators studied 56 healthy participants with varying degrees of current depressive symptomatology in the Beck Depression Inventory-II. Participants with high- or low BDI-II scores were exposed to 360 images from the International Affective Picture System (divided into “positive,” “negative,” and “neutral” pictures of equal numbers) and an immediate recognition test immediately before sleep. The effect of complete sleep deprivation for one night was assessed both immediately after the night of wake (12 h after learning) and then 7 d after learning. Approximately half of patients had a normal sleep night for comparison. The authors found a specific effect of sleep deprivation in memory performance for neutral and negative emotional pictures, in the group with higher BDI-II score only (Harrington et al 2018), SD also has consistent, robust, and acute antidepressant effects, with response rates of up to 50% even in treatment-refractory patients, when either total-sleep or late-night (second half of the night) deprivation is used (Boland et al 2017). The effect is immediate but, unfortunately, it lasts until the next night of sleep (or even the next short nap, Riemann et al 2019), suggesting that a sleep-related phenomenon perpetuates depressive symptomatology among responders to this treatment. Apart from synaptic plasticity and neurotransmitter changes, cognitive consequences of sleep deprivation are immediate after each sleep cycle (Boland et al 2017, Riemann et al 2019) and therefore constitute a good candidate to explain the immediate antidepressant effect of sleep deprivation, and its immediate disappearance after the first, recovery sleep episode (as stated, even a daytime nap seems to have this effect; Riemann et al 2019).
An important limitation of the reviewed literature is the lack of randomized, double-blind trials attesting to the efficacy of the treatment methods to interfere with emotional memory reconsolidation, with the exception of the negative study by Scheepens et al (2020). Therefore, from a clinical perspective, level of evidence for these procedures is anecdotal at this time.
4. Discussion
4.1. Repetitive Negative Thinking (RNT) as a Modifiable Emotional Memory Retrieval and Reconsolidation Mechanism
As stated above, whereas new emotional memories are sensitive to disruption before they are consolidated into stable engrams, emotional memories already consolidated can resume a labile state when reactivated during retrieval, and such memories then become susceptible to disruption by the very same agents that impair initial consolidation of new memories (Nader et al 2000, Kroes et al 2014). In particular, protein synthesis blockade in components of emotional circuits impairs reconsolidation after retrieval of such memories, suggesting plastic -rather than immediate, neurophysiologic changes- are necessary for consolidation or reconsolidation of emotional memories (Nader et al 2000). As we have seen, ECT, ketamine, and SD, which are well-established rapid acting treatments for refractory depression, powerfully interfere with emotional memory reconsolidation as well.
The question arises, could any symptom dimension of clinical depression represent a cycle of emotional memory retrieval and reconsolidation that is being targeted by current effective, rapid acting treatments?
A cycle of retrieval-reconsolidation in affective disorders has been previously proposed to explain recurrent mood episodes that become progressively less dependent upon environmental factors (especially stress) to appear (Post and Kegan 2017). They propose that with each new environmental stressor, triggering of mood episodes is more likely to occur, and that early in the natural history of mood disorders, the stressful stimulus opens a window of labile retrieved traumatic memories, amenable to be accessed and disrupted with the appropriate psychotherapeutic measures (Post and Kegan 2017). We propose herein that pessimistic, fearful persistent daytime RNT, a prominent and classic clinical hallmark of chronic, severe depression (Kraepelin, 1921) could be conceptualized as an ever-increasing, persistent loop of emotional memory retrieval and reconsolidation (with a sleep period being a necessary condition for the latter) that increases and then perpetuates clinical depression severity. RNT is characterized by recurring, maladaptive thinking about overvalued (or even delusional) ideas that ultimately have to do with fear of imminent personal failure, doom, death, financial bankrupt, divine justice, etc. Not surprisingly, RNT as a symptom of depression bears important treatment and prognostic implications. It has been associated with longer duration of episodes (Nolen-Hoeksema et al 2008, Zhang et al in press), persistent residual symptoms of depression among treatment responders (Peters et al 2016), more depressed mood, greater risk of recurrence after recovery (Moberly and Watkins 2008, Schwert et al 2017), suicidality (Arwert and Sizoo 2020), and has also been proposed as a mechanistic link between environmental stress and depressive symptoms (Michel et al 2013). In particular, the role of RNT in recurrence after recovery, and the link between stress and depressive symptoms, are in line with the interesting theory put forward by Post and Kegan (2017), referred to above. Moreover, Corlett et al (2009) have proposed that delusional thinking in schizophrenia could be interpreted as continuously reconsolidated and strengthened memories, persistently recalled, and therefore destabilized, updated, and reinforced; in this case, the authors propose the origin might be an aberrant dopaminergic input that erroneously codes for prediction error, thus inducing memory destabilization, when it should not occur (Corlett et al 2009). In the case of treatment resistant depression in particular, the pattern of maladaptive thinking tends to focus on the same, recurring fearful theme in each individual patient, and is accompanied by prominent visceral manifestations indistinguishable from trauma-related cognitions, or actual environmental stress cues (increased sympathetic and decreased parasympathetic output; sleep difficulties; lack of appetite; hyperarousal; and psychomotor agitation). Not surprisingly, RNT has been shown to lead to preferential encoding of negative self-referent material (Moulds, Kandris, Starr, & Wong, 2007), the retrieval of overgeneral memories (Watkins & Teasdale, 2001), and poor emotional processing of negative events (Watkins, 2004). A classical description of its cognitive and interoceptive features was given by Kraepelin (1921, p 25, p 75):
The torment of the states of depression, which is nearly unbearable, according to the perpetually recurring statements by the patients, engenders almost in all, at least from time to time, weariness of life, only too frequently also a great desire to put an end to life at any price. (…).
At the same time, complaints are heard that the patient must meditate so much that fresh thoughts are always coming to him, that he has too much in his head, that he has no rest (…).
These cognitive symptoms are uniformly accompanied by prominent interoceptive visceral phenomena that feed into dysfunctional cognitions in a recurrent loop (p 19–20):
His body has taken on a quite different form; his nerves are dried up, his organs withered; his brain is obstructed with mucus, everything internal is dead, his voice is like tin; his blood does not circulate (…), his genitals are shriveled, (…) in his body everything is sewn up and entangled (…).
4.2. Hypothesis on cognitive mechanisms of action of rapid-acting treatments for depression
We propose that a persistent cognitive loop of RNT accompanied by interoceptive manifestations, reconsolidated on a daily basis during sleep, is an important dimension of unresponsive major depression. Paulus and Stein (2010) have proposed a model of integration of actual vs. predicted interoceptive, bottom-up information about internal status with anterior insula playing a major integrative role, resulting in abnormal cognitive appraisal of visceral sensations by anterior cingulate and PFC, then feeding back into affective symptoms. Importantly, Makovack et al. (2020) have elaborated on this model and incorporated the idea that RNT elicits the same physiological response as an actual external stressor, which permits to infer that fearful emotion learning and re-learning can be applied not only to experimental fear stimuli but also to internally-generated stimuli as those of the fearful cognitions characteristic of RNT. As stated below, this and other burgeoning evidence points to activation of fear circuits during RNT, which in theory could be targeted by focused neuromodulation techniques. Lability of the emotional memory engram associated to such repetitive cycles of retrieval and reconsolidation of negative emotions (in both its cognitive and interoceptive dimensions) would represent, in this perspective, the therapeutic window for rapid-acting antidepressants among treatment-refractory patients. Acting in different ways, each of the treatments discussed in the previous section would owe their efficacy, at least in part, to interference with recent retrieval and reconsolidation of emotionally-laden depressive cognitions and associated visceral sensations occurring in the form of RNT (suppression of REM sleep in SD, NMDA blockade in ketamine, and probably direct and strong interference with the molecular sequence of events taking place in the circuitry involved in stabilization of the memory trace in the case of ECT, Sutherland et al 2010). Thus, in theory, once the brain circuits subserving emotional memory reconsolidation are defined, they can be targeted to specifically interfere with the reexperiencing-reconsolidation cycle. This approach has already received some support in experimental and clinical settings, especially in the realms of addiction and posttraumatic stress.
ECT and ketamine have been proposed as treatments for PTSD and addiction, and we propose that such treatments are effective in depression in part because they can interfere with emotional reconsolidation in this disorder as well. In fact, pessimistic RNT and its associated visceral sensations in depression involve similar fear-processing brain circuits as PTSD (Paulus and Stein 2010). Moreover, PTSD and depression frequently coexist, and similarly to PTSD, early and recent trauma/stress is a prominent characteristic of depression. PTSD has also been proposed by some authors, as a dimension of mood disorders given its overlap with, and ubiquity in the latter; burgeoning genetic evidence points to considerable overlap of risk for PTSD and depression (Smoller 2016, Maul et al 2020). Last, there is evidence, both recent and old, that ECT and ketamine specifically target RNT (Charney and Nelson 1981, Lehmann et al 2016, Vidal et al 2020).
As stated above, the proposed mechanism of action cannot account for all clinically relevant effects of all three treatment modalities reviewed herein. For example, both ECT and ketamine are known to show incipient effects within the initial 24 h of the first treatment application, sometimes before the first sleep cycle. Whereas this view represents an oversimplification of the effects of three clinically established treatments for rapid-acting amelioration of refractory patients, the hypothesis provides a common conceptual framework that can be relatively easy to test with available interventional and diagnostic instruments, as described below. If demonstrated accurate to explain in part the efficacy of all three methods, it would in turn permit designing targeted neuromodulation strategies that circumvent their well-known cognitive adverse effects, and their short-lived efficacy.
4.3. Potential targets for the neuromodulation of emotional memory and learning
In theory, neuromodulation of brain circuit nodes directly involved in emotional memory retrieval and reconsolidation would bring about the desired antidepressant effect, while preserving other types of memory –most importantly anterograde episodic memory-, and thus limiting the functional impact of the antidepressant procedure. Moreover, if this was the case, the antidepressant effect could be maintained over time by frequent, focused stimulation (noninvasive or invasive) of the appropriate targets. We propose three potential targets on the basis of recent views of circuits subserving emotional learning, memory retrieval, and reconsolidation: anterior insular cortex, dorsal anterior cingulate cortex, and posterior cingulate cortex.
4.4. Brain circuits underlying RNT in depression overlap with those involved in emotional memory retrieval and reconsolidation.
As stated above, emotional memory is one of a variety of cognitive paradigms that are subjected to reconsolidation upon retrieval (Nader 2015). In general, emotion-memory interaction occurs at various stages in the processing of sensory information, and emotional information has been studied for the most part by its effects on episodic memory, where information with an emotional valence is encoded advantageously in relation to emotionally neutral information (Sharot and Phelps 2004, LaBar and Cabeza 2006) and in the paradigm of fear learning and conditioning, in which contextual information is associated with a fear response, characterized by a specific pattern of autonomic output (Critchley et al 2002, Knight et al 2004, see Fullana et al 2015 for a systematic review of human studies). Saarimaki and coworkers (2016) have applied multivariate pattern analysis to the experiencing of a series of basic emotions. In these studies, a network involving anterior insula, orbitofrontal cortex, anterior cingulate cortex, anterior thalamus, and amygdala, are found to participate in the experiencing and encoding of emotional states; in particular sadness and fear show significant overlap in the brain structures involved (Saarimaki et al 2016). Regarding RNT, as we have seen this symptom involves a series of cognitive and interoceptive processes, including self-referential thinking, autobiographical account, and emotion regulation (Cooney et al 2010; Sin et al 2018). In fact, in clinical and experimental settings RNT activates partially overlapping circuits underlying such processes, and in particular emotion processing and encoding. Default mode network (DMN) activation (including medial prefrontal cortex [mPFC], anterior cingulum [ACC], posterior cingulum [PCC] and precuneus), a hallmark of self-referential thinking, has been described as overactive during rumination in depressive patients compared to healthy subjects (Cooney et al 2010, Zhou et al 2020). Rumination in depression is also associated with increased amygdala activation in different paradigms, but not uniformly (Cooney et al 2010, Burkhouse et al 2017, but see also Makovac et al 2020), and is negatively correlated with resting state activity in the ACC, which has reciprocal connections with amygdala and modulates its activity during emotion processing (Kuhn et al 2012). In a recent connectome study (Zhang et al 2020), amygdala appeared as a major site of connectivity dysfunction during rumination in patients with major depression. Most importantly, from a clinical standpoint, in partially remitted patients with depression (who are the clinical group ultimately eligible for rapid active antidepressant treatments), connectivity of both the subgenual cingulate cortex (Jacobs et al 2014) and amygdala (Peters et al 2016) with the posterior cingulate cortex is related to RNT and residual clinical symptoms, respectively. Posterior cingulate cortex has been defined as a potential target for neurofeedback of pathological rumination in a large functional connectivity study (Misaki et al 2020). Also, insula, temporal pole, orbitofrontal cortex, and hippocampus have all been implicated in RNT in MDD (Rolls 2016, Cheng et al 2018, Apazoglu et al 2019, Jacob et al 2020). Recently, Makovac et al (2020), based upon a large metaanalysis of functional imaging during perseverative thinking, have proposed a heuristically valid model whereby involvement of prefrontal, insular, and cingulate regions during perseverative cognition accompanied by physiological arousal, may support the link between cognitive control and autonomic arousal characteristic of emotional learning paradigms (Makovac et al 2020). Importantly, this recent metaanalysis did not reveal significant amygdala involvement, whereas on the other hand revealed anterior or “visceral” insular cortex as a major player in RNT, which is important in view of the anterior insular cortex playing a pivotal role in depression and anxiety (Paulus and Stein 2010) and self-sentience in normal conditions (Craig 2003). Critical involvement of insular connectivity in RNT has been supported by other groups (Kaiser et al. 2016, Kaiser et al. 2019). These authors consider insular involvement as the possible vehicle linking aberrant cognition and the autonomic disturbances characteristic of depression, i.e., relative parasympathetic withdrawal, and preeminence of sympathetic output (Guinjoan et al 1995, Craig 2003, Berger et al 2012, Critchley and Harrison 2013).
Most animal and human experimental paradigms of emotional learning target fear conditioning, and specifically classical Pavlovian fear conditioning, which we propose might be at play during RNT in depression. This might be a reason for the negative result of Scheepens et al. (2020), who employed a brief reactivation of episodic, autobiographical memories with a negative emotional bias, rather than an emotional learning paradigm. Other reasons for the negative result might include the active, potentially therapeutic control situation, early termination of the trial, and a less assertive treatment approach, including right unilateral electrode placement, sparse ECT sessions (i.e., two weekly instead of three weekly), and early termination after just 4 biweekly session with no clinical improvement) (Hermida et al. 2018; Scheepens et al, 2020). Moreover, the authors do not comment on measures of RNT in the experimental groups. The fear conditioning experimental model is relevant to the issue discussed herein, because as described above, fear circuits significantly overlap with circuits active during RNT in patients with depression (Figure 3; see Fullana et al 2015 for a detailed review and metaanalysis), providing a reasonably consistent framework to define potential neuromodulation targets specifically addressing rumination in treatment-resistant patients with depression. In their large meta-analysis of human studies, Fullana et al. (2015) observed that comparison of significant brain activation during conditioned stimulus exposure and non-conditioned stimulus exposure (i.e., an indication of the presence of emotional learning) produced significant results in areas that show a remarkable overlap with those active during rumination in depression (as described above). Most important, several such areas also coincide with major current neuromodulatory targets for the treatment of this condition. These include anterior insular cortex, dorsal ACC (Schoene-Bake et al 2010, Yang et al 2014), precunei, dorsolateral prefrontal cortex (Lefaucher et al 2014), secondary somatosensory cortex/temporoparietal junction (Misaki et al 2020), and ventral striatum (Bergfeld et al 2016, Mithani et al 2020) (Figure 3, reproduced from Fullana et al 2015). Thus, a potential interpretation of this overlap might be that several invasive and noninvasive neuromodulatory treatments for depression owe their efficacy, at least in part, to interference with emotional learning.
Figure 3:
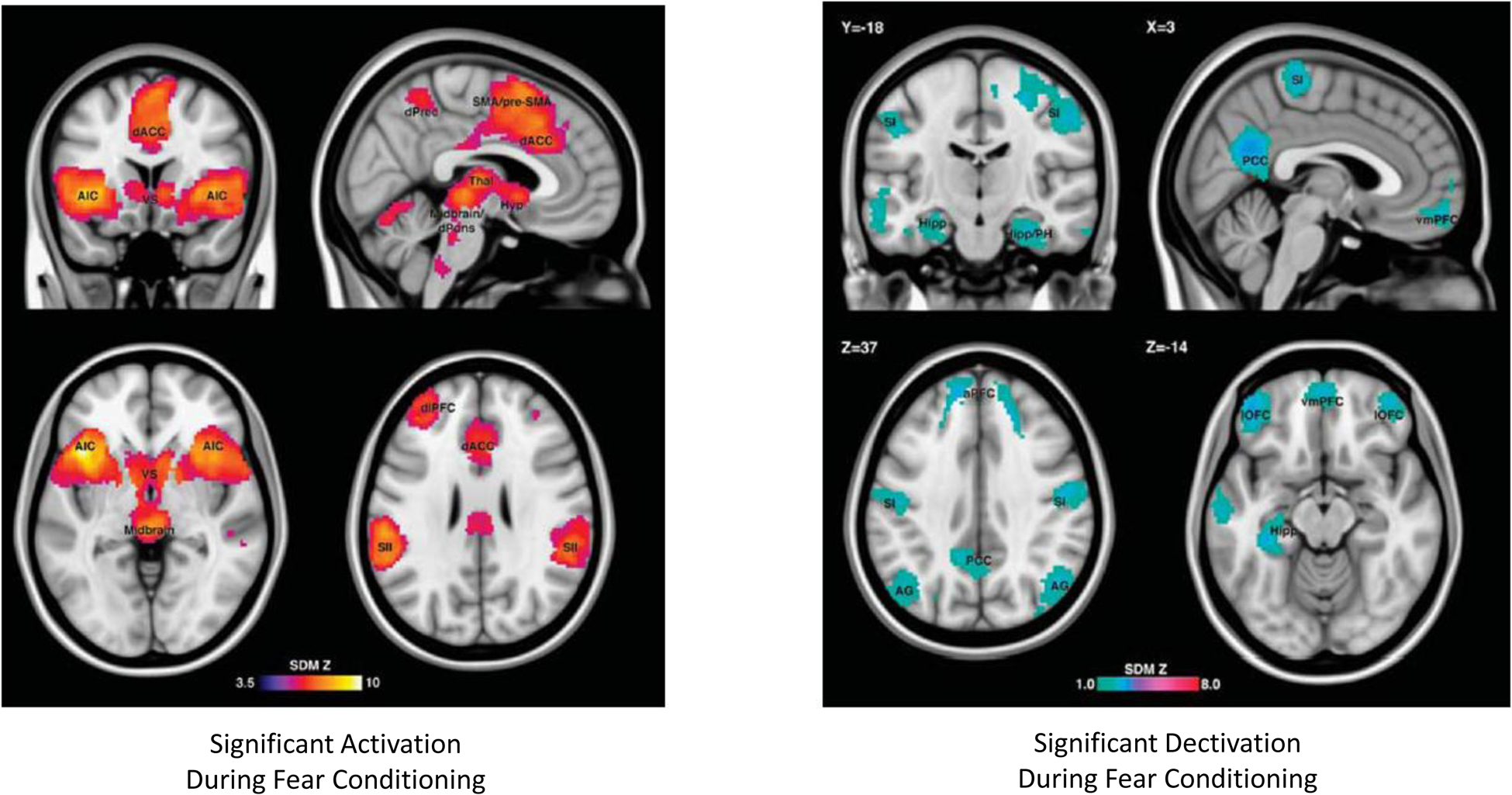
Brain areas with significant activation (left panel) or deactivation (right panel) during fear conditioning in human studies (as per meta-analysis by Fullana et al., 2015). Please see the text for details.
4.5. A conceptual framework to target emotional learning circuits with invasive or noninvasive neuromodulation
Several new methods for neuromodulation are being considered for the treatment of refractory depression, some well-established (e.g., repetitive transcranial magnetic stimulation) and others that hold promise but are in an early stage of development (e.g., ultrasound neuromodulation, deep brain stimulation).
Brain circuits underlying emotional learning and rumination offer a series of potential neuromodulation target nodes to ameliorate rumination and establish if any beneficial effect occurs via interference with memory reconsolidation. Any such method should achieve clinical efficacy while maintaining indemnity of episodic memory. Emotional memory experiments have mostly targeted fear circuits, which overlap with depressive clinical phenomenology, as already mentioned. Metaanalyses of functional brain imaging studies of reconsolidation reveal a consistent circuit involving both activated and de-activated nodes during emotional memory consolidation (Figure 3, Fullana et al 2015). Based on the reviewed literature a parsimonious, step-wise approach to targeting emotional learning circuits, could include the demonstration of the efficacy of noninvasive neuromodulatory inhibition (vs sham neuromodulation) of anterior insula, posterior cingulate cortex, or dorsal anterior cingulate cortex for inhibition of emotional learning in a well-established paradigm of fear conditioning (Klucken et al. 2012).
If such experiments demonstrated efficacy to inhibit emotional learning and to achieve symptom improvement, the direct modulation of nodes displaying maximal efficacy at interference with fear conditioning would probably be warranted in an efficacy trial. This would incorporate a series of potential stimulation paradigms, either invasive (i.e. DBS) or noninvasive (especially ultrasound, recently purported to permit focused, deep neuromodulation). Focused ultrasound (fUS), a recent promising procedure that, in contrast with other noninvasive techniques, permits targeted, reversible neuromodulation of subcortical brain tissue (Tufail et al. 2010, Beisteiner et al. 2020, Sanguinetti et al. 2020, Schafer et al. 2021). Also, neuromodulation of the reviewed deep brain structures could be attained with deep-brain stimulation (Mayberg et al 2005). In this case, a traditional open-loop, continuous stimulation could be used, but a closed-loop DBS could be alternatively employed to target either daytime RNT (retrieval), or nighttime reconsolidation if REM sleep episodes can be identified and then trigger DBS.
4.6. Conclusion
The hypothesis that RNT exerts its deleterious effect on depression prognosis and treatment-resistance via repetitive cycles of retrieval-reconsolidation of negative affect-laden cognitions is speculative but can be put to test by acting on the brain circuit nodes proposed above. It is fully compatible with previous suggestions that abnormal processes of retrieval and reconsolidation of memories can have a role in the natural history of mood disorders (Post and Kegel 2017). In fact, RNT as a cycle of retrieval and reconsolidation might be in part the mechanism that explains its role in persisting residual symptoms, treatment resistance, and proneness to recurrence in depression, as proposed herein. Moreover, at an individual psychopathological level, this view is compatible with previous propositions that seek to explain the persistence of delusional thinking (Corlett et al 2009). Its potential effect on depression prognosis and applicability to the clinical setting would derive from the testing of such hypothesis. We believe safe and effective alternatives are utterly needed for treatment-refractory depressive patients, and would warrant the testing of this hypothesis.
Highlights.
Electroconvulsive therapy, ketamine, and sleep deprivation interfere with reconsolidation of emotional memories after retrieval.
Repetitive negative thinking is an important symptom of depression that can be conceptualized as a cycle of emotional memory retrieval-reconsolidation.
Brain circuits involved in emotional memory reconsolidation can be a direct target of neuromodulation in depression.
Acknowledgments
This work was supported in part by grants from the National Institutes of Health (R01 MH112737) and the AE Foundation (to JAC), and Agencia de Promocion, FONCYT, Ministry of Health, Argentina (PICT-2019-02328) (to SMG). The authors wish to acknowledge the three anonymous reviewers for their valuable input and comments on this review.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest
JAC serves in the scientific advisory board for Hyka Therapeutics and Feelmore Labs, and has been a consultant for Neuronetics. The other authors have nothing to disclose relevant to the content of the present communication.
References
- Agren T (2014): Human reconsolidation: A reactivation and update. Brain Res Bull 105: 70–82. [DOI] [PubMed] [Google Scholar]
- Andrade C, Arumugham SS, Thirthalli J (2016): Adverse Effects of Electroconvulsive Therapy Psychiatr Clin North Am. 39(3):513–530. [DOI] [PubMed] [Google Scholar]
- Arwert TG, Sizoo BB (2020): Self-reported suicidality in male and female adults with autism spectrum disorders: rumination and self-esteem. J Autism Devel Disord. 10.1007/s10803-020-04372-z. [DOI] [PubMed] [Google Scholar]
- Becker B, Steffens M, Zhao Z, Kendrick KM, Neumann C, Weber B, Schultz J, Mehta MA, Ettinger U, Hurlemann R (2017): General and emotion-specific neural effects of ketamine during emotional memory formation. NeuroImage 150: 308–317. [DOI] [PubMed] [Google Scholar]
- Berger S, Kliem A, Yeragani V, Bär KJ (2012): Cardio-respiratory coupling in untreated patients with major depression. J Affect Disord 139(2):166–171. [DOI] [PubMed] [Google Scholar]
- Bergfeld IO, Mantione M, Hoogendoorn ML, Ruhé HG, Notten P, van Laarhoven J, Visser I, Figee M, de Kwaasteniet BP, Horst F, Schene AH, van den Munckhof P, Beute G, Schuurman R, Denys D (2016): Deep brain stimulation of the ventral anterior limb of the internal capsule for treatment-resistant depression: a randomized clinical trial. JAMA Psychiatry. 73(5):456–464. [DOI] [PubMed] [Google Scholar]
- Bergfeld IO, Mantione M, Hoogendoorn MLC, Horst F, Notten P, Schuurman PR, Denys D (2017): Episodic memory following deep brain stimulation of the ventral anterior limb of the internal capsule and electroconvulsive therapy. Brain Stimulation 10: 959–966. [DOI] [PubMed] [Google Scholar]
- Boland EM, Rao H, Dinges DF, Smith RV, Goel N, Detre JA, Basner M, Sheline YI, Thase ME, Gehrman PR (2017): Meta-analysis of the antidepressant effects of acute sleep deprivation. J Clin Psychiatry 78:e1020–e1030. [DOI] [PubMed] [Google Scholar]
- Burkhouse KL, Jacobs RH, Peters AT, Ajilore O, Watkins ER, Langenecker SA (2017): Neural correlates of rumination in adolescents with remitted major depressive disorder and healthy controls. Cognitive Affective Behav Neurosci 17(2): 394–405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cairney SA, Ashton JE, Roschchupkina AA, Sobczak JM (2015): A dual role for sleep spindles in sleep-dependent memory consolidation? J Neurosci 35: 12328–12330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carreno FR, Lodge DJ, Frazer A (2020): Ketamine: Leading us into the future for development of antidepressants. Behav Brain Res. doi: 10.1016/j.bbr.2020.112532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charney DS, Nelson JC (1981): Delusional and nondelusional unipolar depression: further evidence for distinct subtypes. Am J Psychiatry 138(3):328–333. [DOI] [PubMed] [Google Scholar]
- Cheng W, Rolls ET, Qiu J, Xie X, Wei D, Huang C-C, Yang AC, Tsai SJ, Li Q, Meng J, Lin C-P, Xie P, Feng J (2018): Increased functional connectivity of the posterior cingulate cortex with the lateral orbitofrontal cortex in depression. Transl Psychiatry 8:90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole EJ, Stimpson KH, Bentzley BS, Gulser M, Cherian K, Tischler C, Nejad R, Pankow H, Choi E, Aaron H, Espil FM, Pannu J, Xiao X, Duvio D, Solvason HB, Hawkins J, Guerra A, Jo B, Raj KS, Phillips AL, Barmak F, Bishop JH, Coetzee JP, DeBattista C, Keller J, Schatzberg AF, Sudheimer KD, Williams NR (2020): Stanford Accelerated Intelligent Neuromodulation Therapy for Treatment-Resistant Depression. Am J Psychiatry. doi: 10.1176/appi.ajp.2019.19070720. [DOI] [PubMed] [Google Scholar]
- Cooney RE, Joorman J, Eugene F, Dennis EL, Gotlib LH (2010): Neural correlates of rumination in depression. Cognitive Affective Behav Neurosci 10(4): 470–478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corlett PR, Krystal JH, Taylor JR, Fletcher PC (2009): Why do delusions persist? Front Hum Neurosci 3:12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Correia-Melo FS, Leal GC, Vieira F, Jesus-Nunes AP, Mello RP, Magnavita G, Caliman-Fontes AT, Echegaray MVF, Bandeira ID, Silva SS, Cavalcanti DE, Araújo-de-Freitas L, Sarin LM, Tuena MA, Nakahira C, Sampaio AS, Del-Porto JA, Turecki G, Loo C, Lacerda ALT, Quarantini LC (2020): Efficacy and safety of adjunctive therapy using esketamine or racemic ketamine for adult treatment-resistant depression: A randomized, double-blind, non-inferiority study. J Affect Disord 264:527–534. [DOI] [PubMed] [Google Scholar]
- Critchley HD, Mathias CJ, Dolan RJ (2002): Fear conditioning in humans: the influence of awareness and autonomic arousal on functional neuroanatomy. Neuron 33: 653–663. [DOI] [PubMed] [Google Scholar]
- Das RK, Freeman TP, Kamboj SK (2013): The effects of N-methyl-d-aspartate and beta-adrenergic receptor antagonists on the reconsolidation of reward memory: a meta-analysis. Neurosci Biobehav Rev 37: 240–255. [DOI] [PubMed] [Google Scholar]
- Das RK, Gale G, Walsh K, Hennessy VE, Iskandar G, Mordecai LA, Brandner B, Kindt M, Curran V, Kamboj SK (2019): Ketamine can reduce harmful drinking by pharmacologically rewriting drinking memories. Nature Communications 10: 5187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Souza IBMB, Meurer YSR, Macedo Tavares P, Pugliane KC, Lima RH, Silva RH, Freitas Barbosa F (2019): Episodic-like memory impairment induced by sub-anesthetic doses of ketamine. Behav Brain Res 359: 165–171. [DOI] [PubMed] [Google Scholar]
- Duclot F, Perez-Taboada I, Wright KN, Kabbaj M (2016): Prediction of individual differences in fear response by novelty seeking, and disruption of contextual fear memory reconsolidation by ketamine. Neuropharmacology 109: 293–305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duman RS, Aghajanian GK, Sanacora G, Krystal JH (2016): Synaptic plasticity and depression: new insights from stress and rapid-acting antidepressants. Nat Med. 22(3):238–249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunbar AB, Taylor JR (2017): Reconsolidation and psychopathology: Moving towards reconsolidation-based treatments. Neurobiol Learn Mem 142: 162–171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng P, Becker B, Zheng Y, Feng T (2018): Sleep deprivation affects fear memory consolidation: bi-stable amygdala connectivity with insula and ventromedial prefrontal cortex. SCAN 2018: 145–155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedrich MJ (2017): Depression is the leading cause of disability around the world. JAMA 317:1517. [DOI] [PubMed] [Google Scholar]
- Fullana MA, Harrison BJ, Soriano-Mas C, Vervillet B, Cardoner N, Avila-Parcet A, Radua J (2015): Neural signatures of human fear conditioning: an updated and extended meta-analysis of fMRI studies. Mol Psychiatry doi: 10.1038/mp.2015.88. [DOI] [PubMed] [Google Scholar]
- Gahr M, Schoenfeldt-Lecuona C, Spitzer M, Graf H (2014): Electroconvulsive therapy and post-traumatic stress disorders: first experience with conversation-based reactivation of traumatic memory content and subsequent ECT-mediated impairment of reconsolidation. J Neuropsychiatry Clin Neurosci 26:3. [DOI] [PubMed] [Google Scholar]
- Harrington MO, Nedberge KM, Durrant SJ (2018): The effect of sleep deprivation on emotional memory consolidation in participants reporting depressive symptoms. Neurobiol Learn Mem 152: 10–19. [DOI] [PubMed] [Google Scholar]
- Hermida AP, Glass OM, Shafi H, McDonald WM (2018): Electroconvulsive Therapy in Depression: Current Practice and Future Direction. Psychiatr Clin North Am 41(3):341–353. [DOI] [PubMed] [Google Scholar]
- Hyman SE (2012): Revolution stalled. Sci Transl Med 4: 155 cm 11. [DOI] [PubMed] [Google Scholar]
- Jacob Y, Morris LS, Huang KH, Schneider M, Rutter S, Verma G, Murrough JW, Balchandani P (2020): Neural correlates of rumination in major depressive disorder: a brain network analysis. Neuroimage Clin. 25:102142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobs RH, Jenkins LM, Gabriel LB et al. (2014): Increased coupling of intrinsic networks in remitted depressed youth predicts rumination and cognitive control. PLoS One 9(8): e104366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaiser RH, Whitfield-Gabrieli S, Dillon DG, Goer F, Beltzer M, Minkel J, Smoski M, Dichter G, Pizzagalli DA (2016): Dynamic resting-state functional connectivity in major depression. Neuropsychopharmacology 41: 1822–1830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaiser RH, Kang MS, Lew Yechan, van der Feen J, Aguirre B, Clegg R, Goer F, Esposito E, Auerbach RP, Hutchison RM, Pizzagalli DA (2019): Abnormal frontoinsular-default network dynamics in adolescent depression and rumination: a preliminary resting-state co-activation pattern analysis. Neuropsychopharmacology 44: 1604–1612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kandel ER (2001): The molecular biology of memory storage: a dialoque between genes and synapses. Science 294: 1030–1038. [DOI] [PubMed] [Google Scholar]
- Kellner CH (2013): Relapse after electroconvulsive therapy (ECT). J ECT 29(12): 1–2. [DOI] [PubMed] [Google Scholar]
- Klein ME, Chandra J, Sheriff S, Malinow R (2020): Opioid system is necessary but not sufficient for antidepressive actions of ketamine in rodents. PNAS 117: 2656–2662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klucken T, Schweckendiek J, Koppe G, Merz CJ, Kagerer S, Walter B et al. Neural correlates of disgust- and fear-conditioned responses. Neuroscience 2012; 201: 209–218. [DOI] [PubMed] [Google Scholar]
- Knight DC, Cheng DT, Smith CN, Stein EA, Helmstetter FJ (2004): Neural substrates mediating human delay and trace fear conditioning. J Neurosci 24: 218–228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krause AJ, Simon EB, Mander BA, Greer SM, Saletin JM, Goldstein-Piekarski AN, Walker MP (2017): The sleep-deprived human brain. Nature Rev Neurosci 18: 404–418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kroes MCW, Tendolkar I, van Wingen GA, van Waarde JA, Strange BA, Fernandez G (2014): An electroconvulsive therapy procedure impairs reconsolidation of episodic memories in humans. Nature Neuroscience 17: 204–206. [DOI] [PubMed] [Google Scholar]
- Kühn S, Vanderhasselt MA, De Raedt R, Gallinat J (2012): Why ruminators wońt stop: the structural and resting state correlates of rumination and its relation to depression. J Affect Dis 141(2): 352–360. [DOI] [PubMed] [Google Scholar]
- LaBar KS, Cabeza R (2006): Cognitive neuroscience of emotional memory. Nat Rev Neurosci 7: 54–64. [DOI] [PubMed] [Google Scholar]
- Lefaucher JP et al. (2014): Evidence-based guidelines on the therapeutic use of repetitive transcranial magnetic stimulation (rTMS).Clin Neurophysiol. 125(11):2150–2206. [DOI] [PubMed] [Google Scholar]
- Lehmann M, Seifritz E, Henning A, Walter M, Böker H, Scheidegger M, Grimm S (2016): Differential effects of rumination and distraction on ketamine induced modulation of resting state functional connectivity and reactivity of regions within the default-mode network. Soc Cogn Affect Neurosci 11(8):1227–1235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis DJ (1969): Sources of experimental amnesia. Psychol Rev 76: 461–472. [DOI] [PubMed] [Google Scholar]
- Lewis DJ, Bregman NJ, Mahan JJ Jr (1972): Cue-dependent amnesia in rats. J Comp Physiol Psychol 81: 243–247. [DOI] [PubMed] [Google Scholar]
- Machado-Vieira R, Henter ID, Zarate CA Jr (2017): New targets for rapid antidepressant action. Prog Neurobiol 152:21–37.9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macovak E, Fagioi S, Rae CL, Critchley HD, Ottaviani C (2020): Cańt get it off my brain: meta-analysis of neuroimaging studies on perseverative cognition. Psychiatry Res: Neuroimaging. In press. [DOI] [PubMed] [Google Scholar]
- Maul S, Giegling I, Fabbri C, Corponi F, Serretti A, Rujescu D (2020): Genetics of resilience: Implications from genome-wide association studies and candidate genes of the stress response system in posttraumatic stress disorder and depression. Am J Med Genet B Neuropsychiatr Genet. 183(2):77–94. [DOI] [PubMed] [Google Scholar]
- McCormick C, Ciaramelli E, De Luca F, Maguire EA (2018): Comparing and contrasting the cognitive effects of hippocampal and ventromedial prefrontal cortex damage: a review of human lesion studies. Neuroscince 374: 295–318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McEvoy PM, Mahoney AEJ, Moulds ML (2010): Are worry, rumination, and post-event processing one and the same? Development of the repetitive thinking questionnaire. J Anxiety Disorders 24: 509–519. [DOI] [PubMed] [Google Scholar]
- McGaugh JL (1966): Time-dependent processes in memory storage. Science 153: 1351–1358. [DOI] [PubMed] [Google Scholar]
- Michel LC, McLaughlin KA, Shepherd K, Nolen-Hoeksema S (2013): Rumination as a mechanism linking stressful life events to sympoms of depression and anxiety: longitudinal evidence in early adolescents and adults. J Abnormal Psychol 122(2): 339–352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Misaki M, Tsuchiyagaito A, Al Zoubi O, Paulus M, Bodurka J, Tulsa 1000 investigators (2020): Connectome-wide search for functional connectivity locus associated with pathological rumination as a target for real-time fMRI neurofeedback intervention. NeuroImage: Clinical. 26:102244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Misanin JR, Miller RR, Lewis DJ (1968): Retrograde amnesia produced by electroconvulsive shock after reactivation of a consolidated memory trace. Science 160:554–555. [DOI] [PubMed] [Google Scholar]
- Mithani K, Davison B, Meng Y, Lipsman N. (2020): The anterior limb of the internal capsule: Anatomy, function, and dysfunction. Behav Brain Res. 387:112588. [DOI] [PubMed] [Google Scholar]
- Moberly NJ, Watkins ER (2008): Processing mode influences the relationship between trait rumination and emotional vulnerability. Behav Ther 37: 281–291. [DOI] [PubMed] [Google Scholar]
- Moulds ML, Kandris E, Starr S, &Wong ACM (2007). The relationship between rumination, avoidance and depression in a non-clinical sample. Behaviour Research and Therapy, 45, 251–261. [DOI] [PubMed] [Google Scholar]
- Nader K, Schafe GE, Le Doux JE (2000): Fear memories require protein synthesis in the amygdala for reconsolidation after retrieval. Nature 406: 722–726. [DOI] [PubMed] [Google Scholar]
- Nader K (2015): Reconsolidation and the dynamic nature of memory. Cold Spring Harb Perspect Biol 7:a021782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nemeroff CB (2018): The search for treatments for veterans with major depression. Of paramount important, yet still elusive. JAMA Psychiatry 75: 877–878. [DOI] [PubMed] [Google Scholar]
- Nolen-Hoeksema S, Wisco B, Lyubomirsky S (2008): Rethinking rumination. Perpect Psychol Sci 3: 400–424. [DOI] [PubMed] [Google Scholar]
- Pérez-Esparza R, Corona T, Ruiz-García RG, Oñate-Cadena N, de la Fuente-Sandoval C, Ramírez-Bermúdez J (2018): Time until relapse after augmentation with single-dose ketamine in treatment-resistant depression. Psychiatry Clin Neurosci 72(8): 623. [DOI] [PubMed] [Google Scholar]
- Peters AD, Burkhouse K, Feldhaus CC, Langenecker SA, Jacobs RH (2016): Aberrant resting-state functional connectivity in limbic and cognitive control networks relates to depressive rumination and mindfulness: a pilot study among adolescents with a history of depression. J Affect Disord 200: 178–181. [DOI] [PubMed] [Google Scholar]
- Philippens IHCHM, Draaisma L, Baarends, Krugers HJ, Vermetten E (2021): Ketamine treatment upon memory retrieval reduces fear memory in marmoset monkeys. Eur Neuropsychopharm 50: 1–11. [DOI] [PubMed] [Google Scholar]
- Piva A, Caffino L, Padovani L, Pintori N, Mottarlini F, Sferrazza G, Paolone G, Fumagalli F, Chiamulera C (2020): The metaplastic effects of ketamine on sucrose renewal and contextual memory reconsolidation in rats. Behav Brain Res 379: 112347. [DOI] [PubMed] [Google Scholar]
- Post RM, Kegan R (2017): Prevention of recurrent affective episodes using extinction training in the reconsolidation window: A testable psychotherapeutic strategy. Psychiatry Res 249: 327–336. [DOI] [PubMed] [Google Scholar]
- Pradhan B, Rossi G (2020): Combining ketamine, brain stimulation (rTMS) and mindfulness therapy (TIMBER) for opioid addiction. Cureus 12(11):e11798. doi: 10.7759/cureus.11798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riemann D, Krone LB, Wulff K, Nissen C (2019): Sleep, insomnia, and depression. Neuropsychopharmacology 0:1–16; 10.1038/s41386-019-0411-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rolls ET (2016): A non-reward attractor theory of depression. Neurosci Biobehav Rev 68: 47–58. [DOI] [PubMed] [Google Scholar]
- Rush AJ, Trivedi MH, Wisnieski SR, et al. (2006): Acute and longer-term outcomes in depressed outpatients requiring one or several treatment steps: a STAR*D report. Am J Psychiatry 163: 1905–1907. [DOI] [PubMed] [Google Scholar]
- Saarimaki H, Gotsopoulos A, Jaaskelainen IP, Lampinen J, Vuilleumier P, Hari R, Sams M, Nummenmaa L (2016): Discrete neural signatures of basic emotions. Cerebral Cortex 26: 2563–2573. [DOI] [PubMed] [Google Scholar]
- Sara SJ (2000): Retrieval and reconsolidation: Toward a neurobiology of remembering. Learn Mem 7: 73–84. [DOI] [PubMed] [Google Scholar]
- Sara SJ (2015): Reactivation, retrieval, replay and reconsolidation in and out of sleep: connecting the dots. Front Behav Neurosci 4: 185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sara SJ (2017): Sleep to remember. J Neurosci 37(3): 457–463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheepens DS, van Waarde JA, ten Doesschate F, Westra M, Kroes MCW, Schene AH, Bockting CLH, Schoevers RA, Denys DAJP, Ruhe HG, van Wingen GA (2020): Effectiveness of emotional memory reactivation vs control memory reactivation before electroconvulsive therapy in adult patients with depressive disorder: A randomized clinical trial. JAMA Nework Open 3(8):e2012389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider AM, Sherman W (1968): Amnesia: A function of the temporal relation of footshock to electroconvulsive shock. Science 159: 219–221. [DOI] [PubMed] [Google Scholar]
- Schoene-Bake JC, Parpaley Y, Weber B, Panksepp J, Hurwitz TA, Coenen VA (2010): Tractographic analysis of historical lesion surgery for depression. Neuropsychopharmacology. 35(13):2553–2563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwert C, Aschenbrenner S, Weisbrod M, Schröder A (2017): Cognitive impairments in unipolar depression: the impact of rumination. Psychopathology DOI: 10.1159/000478785. [DOI] [PubMed] [Google Scholar]
- Scott LJ (2019): Brexanolone: First Global Approval. Drugs 79(7):779–783. [DOI] [PubMed] [Google Scholar]
- Sharot T, Phelps EA (2004): How arousal modulates memory: disentangling the effects of attention and retention. Cogn Affect Behav Neurosci 4: 294–306. [DOI] [PubMed] [Google Scholar]
- Sikkens D, Riemersma-Van der Lek RF, Meesters Y, Schoevers RA, Haarman BCM (2019): Combined sleep deprivation and light therapy: Clinical treatment outcomes in patients with complex unipolar and bipolar depression. J Affect Disord 246:727–730. [DOI] [PubMed] [Google Scholar]
- Smoller JW (2016): The Genetics of StressRelated Disorders: PTSD, Depression, and Anxiety Disorders. Neuropsychopharmacology. 41(1):297–319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spear N (1973): Retrieval of memory in animals. Psychol Rev 80: 163–194. [Google Scholar]
- Steif BL, Sackeim HA, Portnoy S, Decina P, Malitz S (1986): Effects of depression and ECT on anterograde memory. Biol Psychiatry 21 (10): 921–930. [DOI] [PubMed] [Google Scholar]
- Sutherland RJ1, Sparks FT, Lehmann H (2010): Hippocampus and retrograde amnesia in the rat model: a modest proposal for the situation of systems consolidation. Neuropsychologia. 48(8):2357–2369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veen C, Jacobs G, Philippens I, Vermetten E (2018) Subanesthetic Dose Ketamine in Posttraumatic Stress Disorder: A Role for Reconsolidation During Trauma-Focused Psychotherapy?. In: Vermetten E, Baker D, Risbrough V (eds) Behavioral Neurobiology of PTSD. Current Topics in Behavioral Neurosciences, vol 38. Springer, Cham. 10.1007/7854_2017_34 [DOI] [PubMed] [Google Scholar]
- Vidal S, Jermann F, Aubry JM, Richard-Lepouriel H, Kosel M (2020): Effect of Ketamine on Rumination in Treatment-Resistant Depressive Patients. J Clin Psychopharmacol 40(6):607–610. [DOI] [PubMed] [Google Scholar]
- Watkins E (2004). Adaptive and maladaptive ruminative self-focus during emotional processing. Behaviour Research and Therapy, 42, 1037–1052. [DOI] [PubMed] [Google Scholar]
- Watkins E, & Teasdale JD (2001). Rumination and overgeneral memory in depression: Effects of self-focus and analytic thinking. Journal of Abnormal Psychology, 110, 353–357. [DOI] [PubMed] [Google Scholar]
- Williams NR, Heifets BD, Blasey C, Sudheimer K, Pannu J, Pankow H, Hawins J, Birnbaum J, Lyons DM, Rodriguez CI, Schatzberg AF (2018): Attenuation of antidepressant effects of ketamine by opioid receptor antagonism. Am J Psychiatry 175: 1205–1215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang JC, Ginat DT, Dougherty DD, Makris N, Eskandar EN (2014): Lesion analysis for cingulotomy and limbic leucotomy: comparison and correlation with clinical outcomes. J Neurosurg. 120(1):152–163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang R, Kranz GS, Zou , Deng Y, Huan X, Lin K, Lee TMC (2020): Rumination network dysfunction in major depression: a brain connectome study. Progr Neuropsychopharm Biol Psychiatry 98: 109819. [DOI] [PubMed] [Google Scholar]
- Zhou HX, Chen X, Shen YQ, Li L, Chen NX, Zhu ZC, Castellanos FX, Yan CG (2020): Rumination and the default mode network: meta-analysis of brain imaging studies and implications for depression. NeuroImage 206: 116287. [DOI] [PubMed] [Google Scholar]


