Abstract
Purpose:
The neuropsychological complications of temporal lobe epilepsy are characterized by a spectrum of reproducible cognitive phenotypes that vary in the presence, type and degree of impairment. The nature of the disruptions to the neuropsychological networks that underlie these phenotypes remain to be characterized and represent the subject of this investigation.
Methods:
Participants included 30 healthy controls and 104 patients with temporal lobe epilepsy who fell into three cognitive phenotypes (intact, focal impairment, generalized impairment). Eighteen neuropsychological measures representing multiple cognitive domains (language, memory, executive function, visuoperception, motor speed) were examined by graph theory techniques within the control and each epilepsy cognitive phenotype group to characterize their global and local network properties.
Results:
Across the control and epilepsy cognitive phenotype groups (intact to focal to generalized impairment), there was: 1) an orderly breakdown in the positive manifold reflected by a stepwise reduction of positive associations among the neuropsychological tests, 2) stepwise abnormal increases in global measures including the normalized clustering coefficient and modularity index, 3) stepwise abnormal decreases in normalized global efficiency, 4) a community structure demonstrating well organized modules within the control group while each epilepsy group showed deviations from controls, and 5) lower strength, compared to controls, across 8 nodes in the focal and generalized impairment groups compared to only 3 nodes in the no-impairment epilepsy group, pointing to the superior integration of local connections in controls.
Discussion:
The cognitive phenotypes of temporal lobe epilepsy are characterized by orderly abnormalities in their underlying neuropsychological networks. These findings inform the network perturbations that underlie the taxonomy of cognitive abnormality in temporal lobe epilepsy and provide a model for examination of similar issues in other focal and generalized epilepsies.
Keywords: Temporal lobe epilepsy, Cognition, Phenotypes, Graph theory, Network analysis
1. Introduction
Temporal lobe epilepsy is not associated with an invariant impairment in anterograde memory as classically proposed, but is instead associated with a heterogeneous neuropsychological profile reflected in reproducible cognitive phenotypes. These range from a phenotype expected for the disorder including abnormal anterograde memory (that may be accompanied by concomitant language and/or executive dysfunction), to less expected phenotypes that include grossly intact performance as well as a generally impaired cognitive profile (Hermann et al., 2007; Baxendale and Thompson, 2020; Rodriguez-Cruces et al., 2018, 2020; Reyes et al., 2019, 2020). One group found two focal phenotypes (learning/memory and executive function/speed) in addition to the intact and generalized impaired groups (Elverman et al., 2019). Neuroimaging research has revealed concomitant stepwise abnormalities consistent with the presence and degree of impairment across the cognitive phenotypes, these relationships reflected in brain structure, connectivity (diffusion and resting state fMRI), and large scale covariance analyses of cortical/subcortical gray and white matter (Dabbs et al., 2009; Reyes et al., 2019; Rodríguez-Cruces et al., 2020; Hermann et al., 2020)—findings that support the hypothesis that disrupted networks rather than focal pathology underlie the heterogeneous cognitive presentations of temporal lobe epilepsy.
Unexplored, however, is how the underlying cognitive networks themselves are impacted across the various phenotypes, that is, what is the pattern of altered associations among the neuropsychological tests themselves and how are these altered associations reflected across the cognitive phenotypes of temporal lobe epilepsy compared to controls? This question can be addressed by the application of graph theory analytics to the administered neuropsychological measures. Cognitive networks in epilepsy have been examined infrequently in this fashion. Three previous studies examined baseline and prospective neuropsychological networks in children with new onset idiopathic epilepsies (Garcia-Ramos et al., 2015, 2016) and adults with chronic temporal lobe epilepsy compared to controls (Kellermann et al., 2016). The results revealed that children with epilepsy exhibited a less structured global development of their cognitive network, as shown by both lower global integration and segregation compared to healthy controls, and an altered regional organization reflected by the different nature of employed hubs among groups. Regarding chronic temporal lobe epilepsy, the modular structure of their cognitive graph was found to be poorly structured compared to controls, with the domains of memory and executive function more separated in the epilepsy network compared to controls.
The purpose of this investigation is to examine the neuropsychological networks underlying the three epilepsy cognitive phenotypes identified in the Epilepsy Connectome Project (ECP) using graph theory analytics. In that previous analysis, unsupervised machine learning was applied to summary metrics (deviation from controls z-scores) representing five cognitive domains (language, visuospatial, memory, executive function/processing speed, motor speed). Cluster analysis showed the taxonomy of cognitive impairment to include three latent groups: 1) Generalized-cognitive impairment (Gen-CI) characterized by impairment across all cognitive domains, Focal-cognitive impairment (Focal-CI) characterized by impairment in language, memory and executive function domains, and No-cognitive impairment (No-CI) characterized by unimpaired and generally commensurate performance compared to controls across all cognitive domains (Hermann et al., 2020).
While informative, the presence, nature, and quantitative features of disruptions to the neuropsychological networks themselves within each cognitive phenotype remain to be examined. That is, what is the relationship among the 18 specific test measures in the controls and how are those relationships altered within and across the intact, focal impaired, and generalized impaired cognitive phenotypes? What are the underlying neuropsychological modules and how are they altered across groups, what are the cognitive hubs of those modules, and how efficiently are the modules organized and segregated? It is cognitive network typology, defined using the neuropsychological tests themselves, informed by the application of graph theory, that is the focus here.
We hypothesize that, similar to prior examinations of morphological networks (i.e., cortical and subcortical volumes) associated with cognitive phenotypes that have revealed a stepwise increase in both global clustering and global efficiency linked to increasing cognitive impairment, similar relationships would characterize the neuropsychological networks.
2. Methods
We report how we determined our sample size, all data exclusions, all inclusion/exclusion criteria, whether inclusion/exclusion criteria were established prior to data analysis, all manipulations and all measures in the study.
2.1. Participants
Participants included 104 temporal lobe epilepsy patients and 30 healthy control volunteers prospectively enrolled in the Epilepsy Connectome Project (ECP) (See Table 1) (Cook et al., 2019; Hwang et al., 2019). ECP is a two-site research project involving the Medical College of Wisconsin and the University of Wisconsin–Madison, reviewed and approved by the IRB (Institutional Review Board) at the Medical College of Wisconsin. All participants provided written informed consent, with all procedures consistent with the Declaration of Helsinki.
Table 1 –
Demographic and clinical characteristics.
| HC (n = 30) | Generalized-CI (n = 20) | Focal-CI (n = 31) | No-CI (n = 53) | |
|---|---|---|---|---|
| Age (mean ± SD) | 35.4 ± 11.6 | 38.2 ± 13.5 | 36.0 ± 11.2 | 42.0 ± 10.5 |
| Gender (F/M) | 14/16 | 12/8 | 17/14 | 36/17 |
| Education (mean ± SD) | 16.3 ± 2.4 | 12.3 ± 2.0 | 13.6 ± 1.6 | 16.3 ± 2.4 |
| FSIQ (WASI-2)a (mean ± SD) | 112.9 ± 14.5 | 84.5 ± 8.6 | 96.7 ± 8.5 | 109.8 ± 9.7 |
| Recurring Seizure Onset Age (years: mean ± SD) | – | 16.8 ± 11.9 | 23.9 ± 11.4 | 29.3 ± 15.1 |
| Number of AEDs (mean ± SD) | – | 2.4 ± .7 | 1.7 ± .9 | 1.8 ± .9 |
HC: Healthy Controls, SD: Standard deviation, WASI-2: Weschler Abbreviated Scale of Intelligence (2nd Ed)-2 subtest, AED: antiepileptic drugs.
Statistically significant between groups.
Eligible patients were between the ages of 18 and 60, had estimated full-scale IQ (Intelligence Quotient) at or above 70, spoke English fluently, with no medical contraindications to MRI. The diagnosis of temporal lobe epilepsy was supported by two or more of the following: 1) described or observed clinical semiology consistent with seizures of temporal lobe origin, 2) EEG evidence of either Temporal Intermittent Rhythmic Delta Activity or temporal lobe epileptiform discharges, 3) temporal lobe onset of seizures captured on video EEG monitoring, or 4) MRI evidence of mesial temporal sclerosis or hippocampal atrophy. Patients with any of the following were excluded: 1) lesions other than mesial temporal sclerosis causative for seizures, and 2) an active infectious/autoimmune/inflammatory etiology of seizures. The temporal lobe epilepsy cohort composing the Epilepsy Connectome Project is not a pure presurgical cohort. A modest proportion (34%) of participants underwent ictal monitoring. This in turn has limitations, primarily in regard to unequivocal seizure lateralization. However, the proportion of patients who underwent ictal monitoring is quite similar to the prevalence of medication refractory epilepsy (Kwan & Brodie, 2000). In this regard the cohort is less biased toward medication-refractory/surgical temporal lobe epilepsy and, therefore, more representative of temporal lobe epilepsy in general.
Adjusted (age, ICV) hippocampal volumes were derived and at a conservative threshold (z ≤ −1.5), 23% of the temporal lobe epilepsy group exhibited unilateral (12.4%) or bilateral (10.3%) hippocampal atrophy. Using a more liberal threshold (z < −1.0), 43% of the temporal lobe epilepsy sample exhibited hippocampal atrophy pointing to the presence but less severe nature of hippocampal atrophy in this temporal lobe epilepsy group. As expected, the rate of hippocampal atrophy (z ≤ −1.5) in the temporal lobe epilepsy group compared to controls was significantly elevated (p = .037). Of those temporal lobe epilepsy patients with hippocampal atrophy, 55% were unilateral and 45% were bilateral (using z ≤ −1.5 threshold).
Control participants were healthy adults between the ages of 18 and 60. Exclusion criteria included: Edinburgh Laterality (Handedness) Quotient less than +50; primary language other than English; history of any learning disability, brain injury or illness, substance abuse, or major psychiatric illness (major depression, bipolar disorder, or schizophrenia); current use of vasoactive medications; and medical contraindications to MRI. The controls were volunteers recruited through IRB-approved publicly posted announcements. All participants were compensated for participation in the study. There were 104 temporal lobe epilepsy patients and, per protocol, 30 controls with complete neuropsychological datasets. The specific data from ECP used in this investigation (cognition) (https://osf.io/4z5bd/).
2.2. Neuropsychological assessment
The healthy control and epilepsy participants underwent comprehensive neuropsychological evaluation. A total of 18 cognitive tests were administered (Table 2) and participants with complete datasets were included. The battery comprised measures of intelligence (Wechsler Abbreviated Scale of Intelligence-2 Vocabulary and Block Design subtests) (Wechsler, 2011), verbal learning and memory (Rey Auditory Verbal Learning Test) including total words learned across trials and delayed recall (Rey, 1964), object naming (Boston Naming Test) (Kaplan et al., 1983), letter fluency (Controlled Oral Word Association Test) (Heaton et al., 2004), semantic fluency (Animal Naming) (Heaton et al., 2004; Strauss et al., 2006), spatial orientation (Judgement of Line Orientation) (Benton et al., 1983), face recognition (Facial Recognition Test) (Benton et al., 1983), speeded fine motor dexterity (Grooved Pegboard, dominant and non-dominant hands) (Klove, 1963), and selected subtests from the National Institutes of Health Toolbox-Cognitive Battery including the Pattern Comparison Processing Speed (Carlozzi et al., 2014, 2015), Dimensional Change Card Sort, List Sorting Working Memory, Flanker Inhibitory Control and Attention, Picture Vocabulary, Oral Reading Recognition, and Picture Sequence Memory tests. Legal copyright restrictions prevent public archiving of the various neuropsychological tests used in the study. These can be obtained from the copyright holders in the cited references accompanying each test.
Table 2 –
Neuropsychological tests.
| Abbreviation | Name | Cognitive ability | Cognitive Domain |
|---|---|---|---|
| WASI_BLCK | WASI Block Design | Nonverbal fluid reasoning | Visuospatial/Intelligence |
| WASI_VOC | WASI Vocabulary | Word knowledge | Language/Intelligence |
| WMEM* | List Sorting Working Memory | Working memory | Executive |
| RAVLT_DELAY | Rey Auditory Verbal Learning Test | Delayed verbal memory | Memory |
| RAVLT_TOTAL | Rey Auditory Verbal Learning Test | Verbal learning and memory (total words learned trials 1–5) | Memory |
| SQMEM* | Picture Sequence Memory Test | Acquisition, storage and retrieval of new information | Memory |
| FACREC | Facial Recognition Test | Face recognition/matching | Visuospatial |
| JOLO | Judgement of Line Orientation | Spatial orientation | Visuospatial |
| BNT | Boston Naming Test | Visual naming | Language |
| ORAL* | Oral Reading Recognition | Word reading | Language |
| VOCAB* | Picture Vocabulary | Receptive vocabulary | Language |
| COWA | Controlled Oral Word Association Test | Letter fluency | Language/Executive |
| SEMNTFL | Animal naming | Semantic fluency | Language/Executive |
| DCCS* | Dimensional Change Card Sort | Plan, organize and monitor | Executive/processing speed |
| FLANK* | Flanker Inhibitory Control and Attention | Attentional control | Executive/processing speed |
| GROVPEGD | Grooved Pegboard (dominant hand) | Speeded fine motor dexterity | Motor speed |
| GROVPEGND | Grooved Pegboard (non-dominant hand) | Speeded fine motor dexterity | Motor speed |
| PSPEED* | Pattern Comparison Processing Speed | Speed | Processing Speed |
Note.
Indicates NIH Toolbox tests.
Each raw test score was regressed on age for the control data. Gender and education were not adjusted, as those and other sociodemographic variables were of interest as predictors of phenotype membership. Scores were normalized to z-scores with the regression parameters and standard error of the estimate from the control group used to compute z-scores for the patients. Regression assumptions were checked using both plots and statistical tests. No obvious patterning or deviations from linearity were seen in a residual versus fitted value plot using the control data. Residual normality was investigated both visually using a quantile–quantile comparison plot as well as statistically using Shapiro–Wilks test.
2.3. Graph theory (GT) measures
Symmetric matrices of 18 tests (nodes) were calculated for the healthy controls and each temporal lobe epilepsy cluster group based on the z-scores from the cognitive tests (Table 2) controlling for gender. Weighted-symmetric adjacency matrices were created for each group based on the partial correlation coefficient between each pair of nodes. Subsequently, the diagonal elements and the negative correlations were removed from graphs, and both global and local measures were calculated. Given that graphs only have 18 nodes, we decided to be conservative and preserve all positive correlations for the analyses. The MATLAB-based Brain Connectivity Toolbox (http://www.brain-connectivity-toolbox.net/) was used to calculate GT measures.
To statistically investigate group differences, each group matrix was resampled by replacement (i.e., bootstrapped) a total of 250 times for each group. Since results from GT measures can occur by chance, each graph measure was also calculated on 250 random matrices with the same number of nodes and degree distribution as the pertinent graphs. In this way, the null hypothesis could be tested. P-values were corrected for multiple comparisons for each of the measures. Specifically, Bonferroni correction for the global analyses was based on the standard alpha level of .05 divided by the number of tests (three in total) multiplied by the permuted matrices of all four groups (2504). Graph theory measures were obtained from each resampled matrix, and averages of the GT measures were used for evaluations.
The global measures investigated included normalized average clustering coefficient (CC), normalized global efficiency, and modularity index (Q), using the modularity Louvain algorithm. These measures have been thoroughly described in previous work (Garcia-Ramos et al., 2016). In short, global efficiency examines network integration (Wang et al., 2010); having a high global efficiency means that nodes are interconnected efficiently since there are many paths that connect different pairs of nodes. The average CC characterizes the level of segregation of the network, since it quantifies the number of connections that exist between the nearest neighbors of a node as a proportion of the maximum number of possible connections (Sporns et al., 2004). Since the investigated networks have a small number of nodes, we decided to be conservative and calculate normalized values for the average CC and global efficiency in order to avoid the influence of other network characteristics. This was done by dividing the given measure to the same measure calculated in 250 random graphs, for each group.
The community structure of a network indexes the sub-division of such a network into segregated communities or modules that contribute to the same processes while also allowing for a visual inspection of the network, while Q speaks to how easily the communities are identified by the algorithm. Given that the modularity algorithm provides a statistical estimate for each output (Blondel et al., 2008), we calculated modularity 1,000 times for each group, and the highest proportion was chosen as the number of modules in that group. Furthermore, in order to be certain regarding the module assignment for each node, we created a script that calculated the proportion of module assignment for each node in order to have each node assigned to the module with the highest probability. The Force Atlas 2 algorithm of the open source software Gephi 9.2 (https://gephi.org) was used for the 2D visualization of modularity and community structures (scaling = 100).
To investigate the network hubs, which are the most fundamental regions for the configuration of a network (Sporns et al., 2007), the node degree was the centrality measure used. The degree of a node is the sum of the edges or connections that a given node has. Nodes with high degree (>average + SD) are considered hubs of the network. The strength of a node denotes the strength of the correlation between a node and the rest of the nodes in the graph. The node strength was also investigated for each cognitive phenotype group with respect to controls by calculating Z-scores.
3. Results
Examining demographic and clinical characteristics of the groups (Table 1), the epilepsy and controls groups were comparable in age (p = .54) and gender (p = .28), but differed significantly in FSIQ (p < .001); and the groups with temporal lobe epilepsy were not significantly different in age of first seizure (p = .097) and number of antiepileptic drugs (AED) (p = .052) but differed significantly in age of onset of recurrent seizures (p = .009).
3.1. Phenotype groups
Figure 1 represents the three identified cognitive phenotype groups from the ECP (Hermann et al., 2020). As noted, 18 neuropsychological test metrics were examined in all participants, and the test metrics were assigned to 5 cognitive domains that were then subjected to cluster analysis. Three cognitive phenotypes were identified that ranged from performance comparable to controls (No-CI), performance impaired across all cognitive domains (Generalized-CI), and performance characterized by leading impairment in language, memory and executive function (Focal-CI). The network typology of the 18 neuropsychological metrics within and across the control and epilepsy cognitive phenotype groups follows below.
Fig. 1 –
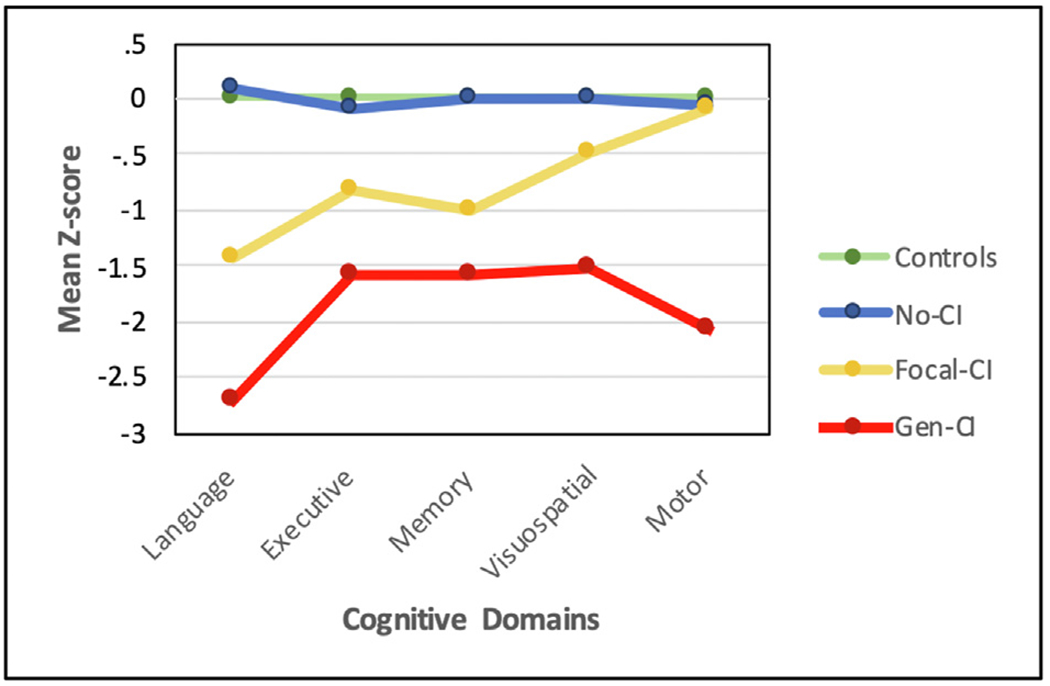
Cognitive phenotype groups from the Epilepsy Connectome Project. Controls (green, N = 30), No Cognitive Impairment (No-CI) being the most intact (blue, N = 57), Focal Cognitive Impairment (Focal-CI) (yellow, N = 34) with leading impairments in language, executive function and memory; and Generalized Cognitive Impairment (Gen-CI) (red, N = 20) being the most impaired overall. Plotted are mean domain scores for each phenotype. Reprinted from Hermann et al. (2020).
Details regarding construction of the overarching cognitive phenotypes and their correlates are provided in Hermann et al. (2020) including the association of hippocampal atrophy (none, unilateral, bilateral) with cognitive phenotype distribution which was significant (Chi Square = 20.7, df = 9, p < .014) indicating that bilateral hippocampal atrophy was associated with the generalized cognitive impairment cluster (No-CI, Focal-CI, Generalized-CI = 10%, 10%, 26%). There was no significant association between cognitive phenotype and laterality defined by interictal EEG (left, right, bilateral) (p = .55) or ictal monitoring (p = .23). That said, only a subset of temporal lobe epilepsy participants (34%) underwent ictal monitoring, another indication of the less severe nature of the epilepsy of this cohort. The presence versus absence of ictal monitoring (a potential indication of medication resistant seizures) was not associated with cognitive phenotype membership (p = .24).
3.2. Adjacency matrices
Figure 2 shows the adjacency matrices of each group without the diagonal elements. It can be observed that negative correlations increase with increasing cognitive impairment. Specifically, controls show 8 negative correlations, No-CI show 33, Focal-CI show 52, and Generalized-CI show 60. These deviations from positive associations infer a breakdown of the so-called positive manifold (positive correlations among the test measures). Since this investigation removed negative links from the matrices, such a reduction of positive associations resulted in a reduction in the number of edges with increased cognitive impairment. To be exact, controls preserved 95% of their edges, No-CI preserved 68%, Focal-CI 60%, and Generalized-CI preserved 57%.
Fig. 2 –
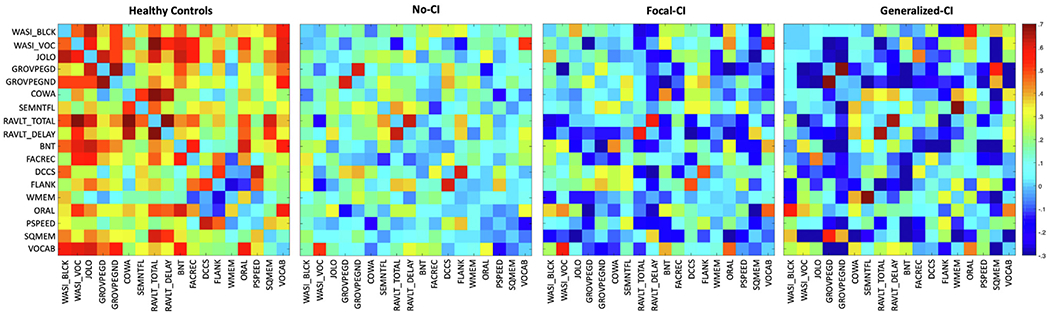
Adjacency matrices. Adjacency matrices of (from left to right): healthy controls, temporal lobe epilepsy with No-CI, temporal lobe epilepsy with Focal-CI, and temporal lobe epilepsy with Generalized-CI. Diagonal elements were set to zero.
3.3. Modularity and network hubs
The community structure of the controls appeared highly integrated compared to the temporal lobe epilepsy groups (Fig. 3), reflected in the node separation, which was more pronounced in all three temporal lobe epilepsy groups. Although the No-CI group did not show the same level of integration as controls, its network appeared highly segregated with well-defined modules, followed by the Focal-CI group. In the Generalized-CI phenotype the modules were less organized as reflected in the mixture of same-color nodes. Although controls have a highly integrated network and modules are separated, they are not as segregated as in the temporal lobe epilepsy groups. This can be observed in the closeness of different modules (same-color nodes) in controls. The three modules of the controls reflected well organized systems characterized predominantly but not exclusively by verbal and visual memory metrics (red), executive function/processing speed (green), and language and visuospatial and motor dexterity measures (yellow). Deviations from this network organization were clearly evident across the epilepsy cognitive phenotype groups.
Fig. 3 –
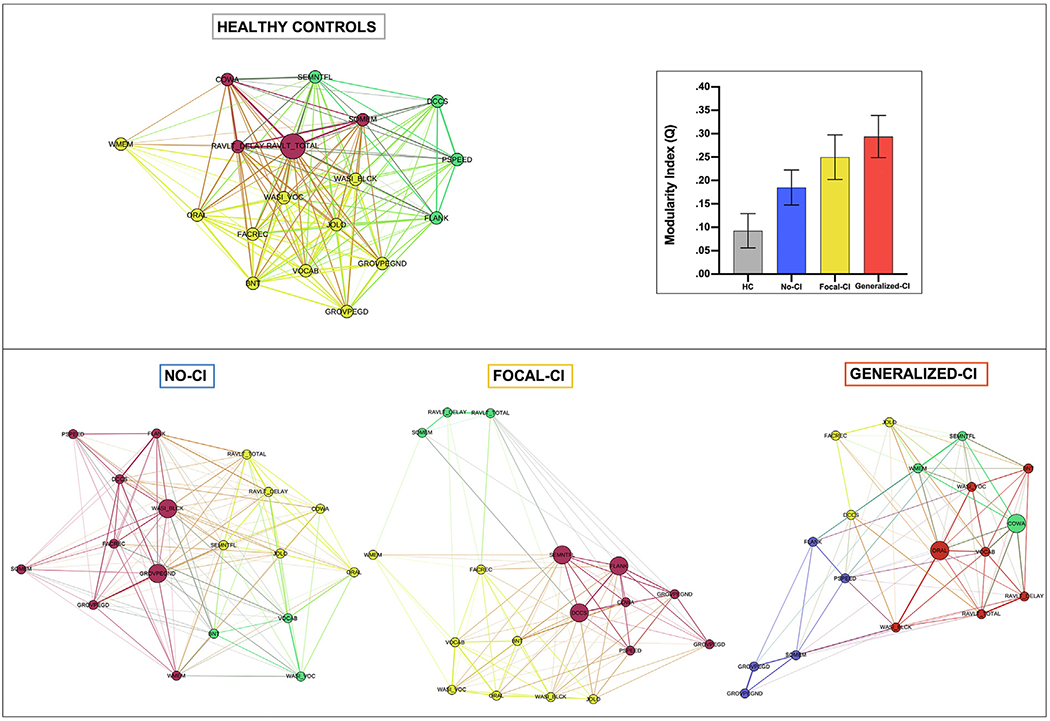
Community Structure and Modularity Index. (top) Community structure of healthy controls and the modularity index graph, with healthy controls (gray), No-CI (blue), Focal-CI (yellow), and Generalized-CI (orange). Error bars represent the standard deviation. (Bottom) Community structure in No-CI, Focal-CI, and Generalized-CI.
ANOVA testing revealed the groups were significantly different in Q (inset within Fig. 3), and post-hoc analyses revealed that each group was significantly different from each other (F1,3 = 1086, p < .001). The lack of segregation that is observed in the community structure of controls was confirmed with their low Q. Starting with controls at the bottom, the cognitive phenotype groups exhibited a stepwise increase in Q, with the No-CI phenotype higher than controls, the Focal-CI phenotype higher than No-CI phenotype, and the Generalized-CI higher than all phenotype groups. Lower Q appears to reflect benefit in terms of the integrity of the cognitive networks.
Regarding the hubs of the networks, the controls showed only one, the No-CI and Generalized-CI phenotype groups showed 2, and Focal-CI phenotype group revealed 3 hubs; based on node degree. However, most of the nodes in controls showed high degree: the node with the lowest degree had a degree of 11, while in the other groups the comparable figure was 9.7 (No-CI), 6 (Focal-CI), and 5 (Generalized-CI). In order to know which nodes serve as a hub, we calculated the average + one SD of the degree for each group. The threshold in controls was higher compared to the other groups (HC = 16.5, No-CI = 13.6, Focal-CI = 11.5, and Generalized-CI = 10.7), and 17/18 nodes in controls had a degree higher than 13.6 (the threshold for No-CI). The hub in controls was a verbal learning metric [total words recalled over learning trials (RAVLT_TOTAL)], while the hubs in No-CI were tests of visuospatial-construction ability (WASI_BLCK) and speeded fine motor dexterity (GROVPEGND). Focal-CI exhibited two hubs involving executive/processing speed (DCCS, FLANK) and one language/executive metric (SEMNTFL); and Generalized-CI exhibited two hubs reflected by metrics of language/executive function (COWA) and language (ORAL).
3.4. Global measures
In terms of normalized average CC, the Generalized-CI group exhibited the highest values, followed by Focal-CI, then No-CI, and lastly healthy controls (Fig. 4). Normalized global efficiency showed the healthy controls to have the highest value, followed by No-CI, then Focal-CI and lastly Generalized-CI. Both global measures seem to be sensitive to increasing impairment when examining the neuropsychological networks across cognitive phenotypes; normalized CC increased with increasing cognitive impairment while normalized Global efficiency decreased with increasing cognitive impairment. ANOVA revealed that groups differed significantly in global efficiency (F1,3 = 2260, p < .001) but not average CC (F1,3 = .152, p > .9).
Fig. 4 –
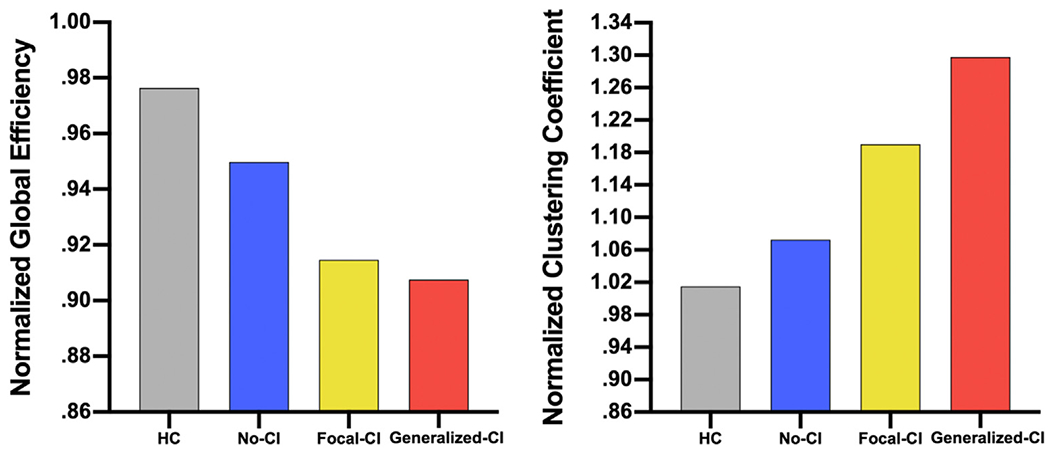
Normalized Global Measures. (left) Normalized average clustering coefficient, and (right) normalized global efficiency in controls (gray), patients with No-CI (blue), patients with Focal-CI (yellow), and patients with Generalized-CI (orange).
The higher Q associated with Generalized-CI seemed to be due to high CC and low global efficiency. Since both controls and No-CI were those with the highest cognitive integrity, and both showed higher normalized global efficiency, lower normalized CC, and lower Q, the combination of high normalized global efficiency with low normalized CC and Q infers a more intact cognitive network.
3.5. Node strength
The node strength was calculated for each group, and z-scores were calculated based on the average and standard deviation of the node strength in controls. Fig. 5 shows the No-CI group presented only three nodes with a strength lower than controls, based on one SD below controls, while both Focal-CI and Generalized-CI showed 8 nodes with lower strength than controls. None of the groups showed a node with significantly higher strength than controls.
Fig. 5 –
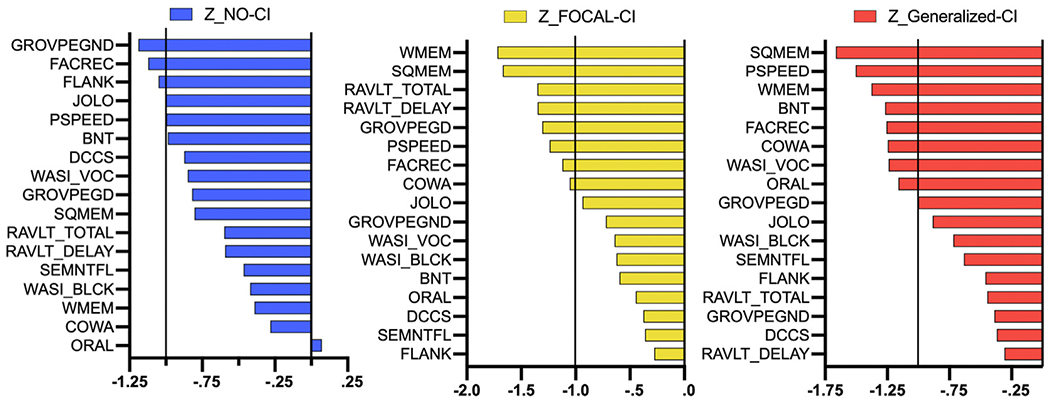
Node Strength Z-scores. Z-scores regarding the node strength in No-CI (left), Focal-CI (middle), and Generalized-CI (right).
4. Discussion
While the neuropsychological presentations of temporal lobe epilepsy are heterogeneous across individual patients, this heterogeneity has been captured by unsupervised machine learning and other analytic approaches that have demonstrated the existence of a core set of distinct cognitive phenotypes that are reproducible across centers and robust to the methods used to identify them. These phenotypes range from surprisingly cognitively unaffected, to predominantly memory impaired with/without language and executive dysfunction, to unexpected pervasive cognitive impairment in this focal epilepsy (Hermann et al., 2007; Elverman et al., 2019; Baxendale and Thompson, 2020; Rodríguez-Cruces et al., 2018, 2020; Reyes et al., 2019, 2020).
Fundamental to this approach, and rarely considered, is the fact that a large set of individual neuropsychological tests and metrics form the basic building blocks for these phenotypic approaches. Typically, a substantial number of tests and metrics are grouped into a much smaller number of widely appreciated cognitive domains (e.g., language, visuospatial function, memory, executive function, motor speed) where-upon latent profiles of these cognitive domains, or phenotypes, are identified. While there is regularity in the identified phenotypic profiles of temporal lobe epilepsy, the nature of the “neuropsychological networks” that underlie them, defined by the patterns of association, integration and organization of the neuropsychological tests themselves across the cognitive phenotypes, has remained an open question and one that was addressed here using a graph theory approach.
In this investigation the “neuropsychological networks” of controls and three temporal lobe epilepsy cognitive phenotype groups were investigated using the 18 basic neuropsychological tests/metrics. Three main findings emerged. First, across increasingly impaired cognitive phenotype groups there was an increasing breakdown in the positive manifold of the neuropsychological network reflected in a reduction of the number of positive associations. Second, global measures including normalized CC and the modularity index showed a stepwise increase across the increasingly abnormal temporal lobe epilepsy phenotype groups, while normalized global efficiency showed the opposite stepwise behavior. Third, regarding community structure, controls exhibited well organized modules while each epilepsy group showed substantial deviations from controls. Finally, node strength calculations again showed a superior integration in controls now in terms of local connections. These findings will be discussed below.
4.1. Adjacency matrices
Regarding the correlation or adjacency matrices, it was evident that the underlying neuropsychological networks increasingly “lost” positive correlations between the tests and an associated reduced number of edges as a function of membership in increasingly impaired cognitive phenotype groups (Controls > No-CI > Focal-CI > Gen-CI). These findings infer a stepwise breakdown in the positive manifold across increasingly abnormal cognitive phenotype groups, pointing to a unique and uncommonly documented correlate of cognitive impairment.
4.2. Global measures and community structure
Across increasingly abnormal epilepsy cognitive phenotype groups, global measures of the underlying neuropsychological networks demonstrated a stepwise increase in Q and normalized CC; with a concomitant stepwise reduction in normalized global efficiency. Therefore, with worsening cognitive phenotypes, network segregation (global CC) was higher and network integration (global efficiency) was lower, pointing to more segregated and less integrated neuropsychological networks in a stepwise and orderly pattern of abnormality across groups (Controls to No-CI to Focal CI to Generalized CI).
Controls also demonstrated a highly integrated network with low segregation which was evident in the closeness of nodes regardless of their module association. Controls showed one module composed predominantly of verbal and visual memory metrics, a second comprised primarily of executive function/speed-related tests, and a third module characterized predominantly but not exclusively by a mix of language, visuospatial, and motor dexterity measures. Controls appeared to demonstrate good integration within and between modules, a configuration not unexpected for a healthy cognitive network. The epilepsy phenotype groups deviated considerably from this network configuration.
The No-CI phenotype exhibited one module containing only language-based tests, with the second module containing a mixture of verbal memory, language/executive, language, and visuospatial skills. The rest of the nodes were together in a module. Like controls, nodes within modules were highly integrated, however, but showing high segregation from the other modules.
In the Focal-CI phenotype most tests from both the visuospatial and verbal domains were together in the same module (yellow) with the addition of a metric of working memory. A second module, containing only memory tests, appeared as a peripheral module, almost not connected to the rest of the graph. Similar to the No-CI phenotype, nodes within a module were well-integrated with the exception of the yellow module, which had working memory almost disconnected from the network as a peripheral node (i.e., almost not connected to its module and the network in general); therefore, barely contributing to the network topology.
In the Generalized-CI phenotype, node configuration seemed completely opposite to controls. This group demonstrated poorly integrated nodes within modules, which were also weakly integrated to the rest of the network. Specifically, there was a module comprised of tests from the processing speed domain, speeded fine motor dexterity, and one memory measure. Furthermore, two of the three visuospatial tests were together with one executive/processing speed measure in another module where nodes were not well-integrated together (yellow module). Another module contained the two language/executive tests along with working memory that also showed a lack of within-module integration. Finally, a fourth module contained the measures of language and verbal learning and memory, as well as the one remaining visuospatial measure.
In summary, the cognitive networks of all three temporal lobe epilepsy phenotypes demonstrated an overall and within-module reduction in integration with increasing cognitive impairment, which ultimately was substantially different from controls.
4.3. Network hubs and node strength
There were differences across groups in both the number and type of hubs represented, with all epilepsy groups exhibiting more hubs than controls, and also differing from controls in the neuropsychological abilities represented by those hubs. These findings suggest that controls do not require multiple central regions to demonstrate typical cognitive ability, while temporal lobe epilepsy phenotypes appear to actively rely on a greater number of hubs. Furthermore, since most of the nodes in controls showed high degree (>11), it appears that nodes in controls contribute equally to the configuration of the network, appearing greatly integrated to each other and resulting in almost no node overshadowing another.
When investigating node strength using Z-scores, none of the epilepsy groups showed a node with a significantly higher strength than the control group. The No-CI group showed 3 nodes with lower strength than controls, and both Focal-CI and Generalized-CI groups presented 8 nodes with lower strength than controls, suggesting that the strength of associations between neuropsychological tests might be playing a role in the neuropsychological networks undergirding the epilepsy phenotypes.
4.4. The broader utility and potential implications of a network analysis of neuropsychological data
Clearly a major finding is that the positive correlative manifold for cognitive tests breaks down in temporal lobe epilepsy. The comparison of the adjacency matrices (Fig. 2) provides a clear visual representation of this finding. Conceiving of these relationships between tests as a network of cognitive abilities it then follows that the use of graph theory may be a natural analytical tool to quantify and visualize network features. The modularity results (Fig. 3) serve as a useful example of how graph theory can quantify a general finding into a single metric (Q) that allows for statistical comparison and was able to highlight the monotonic relationship between increasing Q from controls through the generalized cognitive impairment phenotype. Similar results follow from examination of the clustering coefficient and global efficiency metrics (Fig. 4). These results expand upon the adjacency matrix gestalt by providing specific established graph theory metrics with explanatory power. Increased modularity, increased clustering coefficient and decreased normalized global efficiency associated with increasingly abnormal cognitive phenotypes of temporal lobe epilepsy suggest that the cognitive domains are increasing less integrated—inferring that that the primary abnormality in temporal lobe epilepsy may not be domain specific but rather related to disrupted interactions (networks) of cognitive abilities and domains and that these graph theory metrics provide an established and arguably useful method for quantifying this result.
Might these results have implications for interventions such as cognitive therapy? The decrease in the positive manifold across cognitive phenotype groups clearly indicates that treatment of one ability or one test deficit may have less generalized benefit across cognitive abilities. The implication is that cognitive rehabilitation may be more challenging in the more impaired cognitive phenotypes. From a speculative perspective, neurostimulatory techniques may be better suited to attempt to re-establish normal cognitive network architecture than typical epilepsy treatments such as anti-seizure medications or resection. This could be a testable hypothesis through examining surgical cohorts with similar baseline cognitive dysfunction and comparing outcomes for those treated with responsive neurostimulation (RNS) (Skarpaas et al., 2019) adjusting for response to treatment (seizure freedom).
Could particularly salient nodes/hubs be a useful consideration for therapies for cognitive impairment? It would be interesting if specific cognitive domains could be targeted in each subgroup that could result in focal or generalized improvement. Unfortunately, we do not believe these data would support such a strategy. Shown in the results (Fig. 3) is that the cognitive domains become more isolated in temporal lobe epilepsy and the hubs are in general the areas that are better preserved within particular subgroups. In general, we would advocate for therapies related to the patient specific deficits, though as above we are interested in the possibility that neurostimulation might have broader network effects, though this remains to be studied.
One evident value of the approach presented here is that differences between groups or within individuals over time can be visualized in a way that reveals patterns that would otherwise be very difficult to discern from tables or simple bar graphs. Relationships between nodes are obvious and colorization can be used to bring into view proposed modules. Whether the visualization of the patterns that emerge can have practical significance remains to be seen, but of course it is possible especially in the case of longitudinal research.
4.5. Conclusion and future directions
Heterogeneity inherent in the breakdown of cognitive function in temporal lobe epilepsy has been demonstrated through investigation of individual tests, broader cognitive domains, and recently identified cognitive phenotypes. The latter is a useful approach to capturing the neuropsychological heterogeneity inherent in the epilepsies and ultimately the similarities and differences across epilepsy syndromes. To date, the identified cognitive phenotypes have not been tethered to the perturbations that may be occurring in the interrelationships among the individual tests themselves which, as shown here through application of graph theory, are informative in and of themselves. It is uncommon to apply graph theory to neuropsychological tests themselves (Garcia-Ramos et al., 2016; Tosi et al., 2020; van der Maas et al., 2017), but this may merit more consideration in the future. Specifically, future studies could focus on how or whether cognitive cluster classification can provide evidence about the trajectory or prognosis of epilepsy, drug resistance, and prognosis for psychiatric co-morbidity.
Acknowledgements
This study was supported by grant number U01NS093650 (Epilepsy Connectome Project) from the National Institutes of Health. Cole J Cook was supported in part by grant number T32CA009206 from the National Cancer Institute of the National Institutes of Health. The authors would like to thank all the participants and their families. Additionally, the authors would like to thank Dace Almane, Megan Rozman, Taylor McMillan, Elizabeth Awe, Courtney Forseth, Peter Kraegel, Anna Freiberg, Neelima Tellapragada for project oversight, recruitment and data collection.
No part of the study procedures and/or analyses were pre-registered prior to the research being conducted.
List of Abbreviations
- GT
graph theory
- Q
modularity index
- CC
clustering coefficient
- Gen-CI
Generalized-cognitive impairment = characterized by impairment across all cognitive domains
- Focal-CI
Focal-cognitive impairment = characterized by impairment in language, memory and executive function domains
- No-CI
No-cognitive impairment = characterized by unimpaired and generally commensurate performance compared to controls across all cognitive domains
Footnotes
Open practices
The study in this article earned an Open Data badge for transparent practices. This manuscript used data from the Epilepsy Connectome Project (ECP). All ECP data (cognition, imaging, clinical) will be available in the public domain in 2021.
REFERENCES
- Baxendale S, & Thompson P (2020. September 7). The association of cognitive phenotypes with postoperative outcomes after epilepsy surgery in patients with temporal lobe epilepsy. Epilepsy & Behavior: E&b, 112, 107386. 10.1016/j.yebeh.2020.107386. [DOI] [PubMed] [Google Scholar]
- Benton AL, Hamsher KD, Varney NR, & Spreen O (1983). Contributions to neuropsychological assessment: A clinical manual. New York, NY: Oxford University Press. [Google Scholar]
- Blondel VD, Guillaume JL, Lambiotte R, & Lefebvre E (2008). Fast unfolding of communities in large networks. Journal of Statistical Mechanics: Theory and Experiment, IOP Publishing, P10008, 1–12. [Google Scholar]
- Carlozzi NE, Beaumont JL, Tulsky DS, & Gershon RC (2015). The NIH toolbox pattern comparison processing speed test: Normative data. Archives of Clinical Neuropsychology: the Official Journal of the National Academy of Neuropsychologists, 30(5), 359–368. 10.1093/arclin/acv031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlozzi NE, Tulsky DS, Chiaravalloti ND, Beaumont JL, Weintraub S, Conway K, & Gershon RC (2014). NIH toolbox Cognitive Battery (NIHTB-CB): The NIHTB pattern comparison processing speed test. Journal of the International Neuropsychological Society: JINS, 20(6), 630–641. 10.1017/S1355617714000319 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cook CJ, Hwang G, Mathis J, Nair VA, Conant LL, Allen L, & Meyerand ME (2019). Effective connectivity within the default mode network in left temporal lobe epilepsy: Findings from the epilepsy connectome project. Brain Connectivity, 9(2), 174–183. 10.1089/brain.2018.0600 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dabbs K, Jones J, Seidenberg M, & Hermann B (2009. August). Neuroanatomical correlates of cognitive phenotypes in temporal lobe epilepsy. Epilepsy & Behavior: E&b, 15(4), 445–451. 10.1016/j.yebeh.2009.05.012. Epub 2009 Jun 26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elverman KH, Resch ZJ, Quasney EE, Sabsevitz DS, Binder JR, & Swanson SJ (2019. July). Temporal lobe epilepsy is associated with distinct cognitive phenotypes. Epilepsy & Behavior: E&b, 96, 61–68. 10.1016/j.yebeh.2019.04.015. Epub 2019 May 9. [DOI] [PubMed] [Google Scholar]
- Garcia-Ramos C, Lin JJ, Kellermann TS, Bonilha L, Prabhakaran V, & Hermann BP (2016. November). Graph theory and cognition: A complementary avenue for examining neuropsychological status in epilepsy. Epilepsy & Behavior: E&b, 64(Pt B), 329–335. 10.1016/j.yebeh.2016.02.032. Epub 2016 Mar 24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia-Ramos C, Lin JJ, Prabhakaran V, & Hermann BP (2015. October 27). Developmental reorganization of the cognitive network in pediatric epilepsy. Plos One, 10(10), e0141186. 10.1371/journal.pone.0141186. eCollection 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heaton RK, Miller SW, Taylor MJ, & Grant I (2004). Revised comprehensive norms for an expanded Halstead-Reitan battery: Demographically adjusted neuropsychological norms for African American and Caucasian adults. Lutz, Fla: Psychological Assessment Resources. [Google Scholar]
- Hermann B, Conant LL, Cook CJ, Hwang G, Garcia-Ramos C, Dabbs K, Nair VA, Mathis J, Bonet CNR, Allen L, Almane DN, Arkush K, Birn R, DeYoe EA, Felton E, Maganti R, Nencka A, Raghavan M, Shah U, … Meyerand ME (2020). Network, clinical and sociodemographic features of cognitive phenotypes in temporal lobe epilepsy. Neuroimage Clinical, 27, 102341. 10.1016/j.nicl.2020.102341. Epub 2020 Jul 10.Neuroimage Clin. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hermann B, Seidenberg M, Lee EJ, Chan F, & Rutecki P (2007. January). Cognitive phenotypes in temporal lobe epilepsy. Journal of the International Neuropsychological Society: JINS, 13(1), 12–20. 10.1017/S135561770707004X [DOI] [PubMed] [Google Scholar]
- Hwang G, Dabbs K, Conant L, Nair VA, Mathis J, Almane DN, & Hermann B (2019). Cognitive slowing and its underlying neurobiology in temporal lobe epilepsy. Cortex; a Journal Devoted To the Study of the Nervous System and Behavior, 117, 41–52. 10.1016/j.cortex.2019.02.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaplan EF, Goodglass H, & Weintraub S (1983). The boston naming test (2nd ed.). Philadelphia, PA: Lea & Febiger. [Google Scholar]
- Kellermann TS, Bonilha L, Eskandari R, Garcia-Ramos C, Lin JJ, & Hermann BP (2016. October). Mapping the neuropsychological profile of temporal lobe epilepsy using cognitive network topology and graph theory. Epilepsy & Behavior: E&b, 63, 9–16. 10.1016/j.yebeh.2016.07.030. Epub 2016 Aug 15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klove H (1963). Clinical neuropsychology. The Medical Clinics of North America, 47, 1647–1658. [PubMed] [Google Scholar]
- Kwan P, & Brodie MJ (2000. February 3). Early identification of refractory epilepsy. The New England Journal of Medicine, 342(5), 314–319. 10.1056/NEJM200002033420503. [DOI] [PubMed] [Google Scholar]
- Rey A (1964). L’Examen clinique en psychologie, par André Rey., 2e édition. Paris: Presses universitaires de France; (Vend^me Impr. des P.U.F.). [Google Scholar]
- Reyes A, Kaestner E, Bahrami N, Balachandra A, Hegde M, Paul BM, Hermann B, & McDonald CR (2019. April 23). Cognitive phenotypes in temporal lobe epilepsy are associated with distinct patterns of white matter network abnormalities. Neurology, 92(17), e1957–e1968. 10.1212/WNL.0000000000007370. Epub 2019 Mar 27.Neurology. 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reyes A, Kaestner E, Ferguson L, Jones JE, Seidenberg M, Barr WB, Busch RM, Hermann BP, & McDonald CR (2020. June). Among authors: Hermann BP. Cognitive phenotypes in temporal lobe epilepsy utilizing data and clinically driven approaches: Moving toward a new taxonomy. Epilepsia, 61(6), 1211–1220. 10.1111/epi.16528. Epub 2020 May 4.Epilepsia. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodríguez-Cruces R, Bernhardt BC, & Concha L (2020. June). Multidimensional associations between cognition and connectome organization in temporal lobe epilepsy. Neuroimage, 213, 116706. 10.1016/j.neuroimage.2020.116706. Epub 2020 Mar 6. [DOI] [PubMed] [Google Scholar]
- Rodríguez-Cruces R, Velázquez-Pérez L, Rodríguez-Leyva I, Velasco AL, Trejo-Martínez D, Barragán-Campos HM, Camacho-Téllez V, & Concha L (2018. February). Association of white matter diffusion characteristics and cognitive deficits in temporal lobe epilepsy. Epilepsy & Behavior: E&b, 79, 138–145. 10.1016/j.yebeh.2017.11.040. Epub 2018 Jan 4. [DOI] [PubMed] [Google Scholar]
- Skarpaas TL, Jarosiewicz B, & Morrell MJ (2019. July). Brain-responsive neurostimulation for epilepsy (RNS® System). Epilepsy Research, 153, 68–70. 10.1016/j.eplepsyres.2019.02.003. Epub 2019 Feb 20. [DOI] [PubMed] [Google Scholar]
- Sporns O, Chialvo D, Kaiser M, & Hilgetag CC (2004). Organization, development and function of complex brain networks. Trends in Cognitive Sciences, 8, 418–425. [DOI] [PubMed] [Google Scholar]
- Sporns O, Honey CJ, & Kotter R (2007). Identification and classification of hubs in brain networks. Plos One, 2(10). 10.1371/journal.pone.0001049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strauss E, Sherman E, & Spreen O (2006). A compendium of neuropsychological tests (3rd ed.). New York: Oxford University Press. [Google Scholar]
- Tosi G, Borsani C, Castiglioni S, Daini R, Franceschi M, & Romano D (2020. March). Complexity in neuropsychological assessments of cognitive impairment: A network analysis approach. Cortex; a Journal Devoted to the Study of the Nervous System and Behavior, 124, 85–96. 10.1016/j.cortex.2019.11.004 [DOI] [PubMed] [Google Scholar]
- Van Der Maas HLJ, Kan KJ, Marsman M, & Stevenson CE (2017). Network models for cognitive development and intelligence. Journal of Intelligence, 5(2), 16. 10.3390/jintelligence5020016. Published 2017 Apr 20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J, Zuo X, & He Y (2010. June 7). Graph-based network analysis of resting-state functional MRI. Frontiers in Systems Neuroscience, 4, 16. 10.3389/fnsys.2010.00016. eCollection 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wechsler D (2011). Wechsler Abbreviated Scale of Intelligence-second edition (WASI-II). San Antonio, TX: NCS Pearson. [Google Scholar]


