Abstract
Intra-articular injection of mesenchymal stem cells has shown benefit for the treatment of osteoarthritis (OA).However, MSCs at the origin of these clinical results are heterogenous cell populations with limited cellular characterization. Here, two transgenic reporter mice were used to examine the differential effects of two precisely defined perivascular cell populations (Pdgfrα+ and Pdgfrβ+cells) from white adipose tissue for alleviation of OA. Perivascular mesenchymal cells were isolated from transgenic Pdgfrα-and Pdgfrβ-CreERT2 reporter animals and delivered as a one-time intra-articular dose to C57BL/6J mice after destabilization of the medial meniscus (DMM). Both Pdgfrα+ and Pdgfrβ+ cell preparations improved metrics of cartilage degradation and reduced markers of chondrocyte hypertrophy. While some similarities in cell distribution were identified within the synovial and perivascular spaces, injected Pdgfrα+ cells remained in the superficial layers of articular cartilage, while Pdgfrβ+ cells were more widely dispersed. Pdgfrβ+ cell therapy prevented subchondral sclerosis induced by DMM, while Pdgfrα+ cell therapy had no effect. In summary, while both cell therapies showed beneficial effects in the DMM model, important differences in cell incorporation, persistence, and subchondral sclerosis were identified.
Keywords: Pdgfrα, Pdgfrβ, cell therapy, adventitial cell, synovium, osteoarthritis, DMM
Introduction
Osteoarthritis (OA) is a common chronic joint disease, typifiedby cartilage degeneration and subchondral bone thickening1. The current treatmentsare largely limited to pain control with negligible effects on cartilage maintenance.Autologous chondrocyte transplantation has been used to repair cartilage, buttheir tendency to dedifferentiate haslimited therapeutic use2. Mesenchymal stem/stromal cells (MSCs) reside in bone marrow and many adult tissues, andhave been widely explored as a cell based therapeutic for OA3; 4.Wide variability in outcomes after conventional MSC implantation have also been reported, including fibrocartilage formation and inappropriate ossification5; 6. Nevertheless, MSCs are known to be of variable cellular composition 7. The cellular heterogeneity resides amid donors, tissue sources and within the MSCs populations8;9. Because the most common MSC-based cell therapies are used in bulk, the effects of cell population heterogeneity in terms of therapeutic effects are often masked 10.
Mesenchymal progenitor cells have long been known to reside in a perivascular niche11;12, and have been well typified within the adipose tissue from mouse13, human14; 15and canine sources12. Intra-tissue heterogeneity in perivascular cells has been an emerging area of focus, and expression of receptors for PDGF (platelet derived growth factor) has been shown to define functional relevant perivascular cell subsets in human and mouse tissues16–18.For example, subsets of pericytes expressing different combinations of PDGFRα and PDGFRβ can mediate tissue‐specific regeneration 18; 19. Two transgenic perivascular reporter mice have been previously characterized, including Pdgfrα (Platelet derived growth factor receptor alpha)13 and Pdgfrβ reporter animals20; 21. Adipose derived Pdgfrα+cells areenriched in the tunica adventitiaof large blood vessels, and are less common in microvessels13.Pdgfrβ+ cells are enriched among microvascular pericytes as well as vascular smooth muscle cells21; 22. In this study, we compared the precise effects of intra-articular injection of Pdgfrα+ andPdgfrβ+perivascular cellson OA after joint destabilization.
Materials and methods
Animals
All animal studies were performed with institutional Animal Care and Use Committee (ACUC) approval within Johns Hopkins University (JHU), complying with all relevant ethical regulations.Pdgfrα-CreERT2mice were a kind gift from the Bergles laboratory at JHU and are commercially available (stock No.018280, Jackson lab, Bar Harbor, ME).mT/mG mice were purchased from Jackson lab (stock No.007576). Pdgfrβ-CreERT2 mice were purchased from Jackson lab (stock No. 029684). Pdgfrα-CreERT2or Pdgfrβ-CreERT2 mice were crossed with mT/mG mice to generate Pdgfrα-CreERT2; mT/mGor Pdgfrβ-CreERT2; mT/mGmice which were used for all experiments. Tamoxifen (TM, T5648, Sigma, St Louis, MO) was provided by intraperitoneal injection as per previously validated protocols to mixed gender, seven week old Pdgfrα-CreERT2 ;mT/mG mice or Pdgfrβ-CreERT2; mT/mG mice 2 weeks prior to cell isolation13; 23. TM was dissolved in sunflower seed oil. Tail genomic DNA was used for genotyping.
Cell isolation
Pdgfra and orPdgfrβ‐CreERT2‐mT/mG mice were administered TM two weeks in advance of cell isolation. Inguinal fat pads from 10 mice per genotype were dissected, pooled, minced, and digested with collagenase Type 2 (Worthington,Lakewood, NJ), 0.5% bovine serum albumin and α-MEM (minimum essential medium; Gibco, Grand Island, NY) for 1.5 h at 37℃. mGFP+ cells were isolated with LSR II (BD Biosciences) using FACS Diva software (BD Biosciences) and Flow Jo v10 software (Tree Star, Inc, OR).Cell debris was excluded based on size characterizationand mGFP+cells were collected.Cells were cultured for 2–3 passages prior in vivo administration. Cells were cultured in DMEM at 37°C in a humidified atmosphere containing 95% air and 5% CO2in DMEM (ThermoFisher Scientific, Inc, Waltham, Massachusetts), with 1% fetal bovine serum (FBS, ThermoFisher Scientific), and 1% penicillin/streptomycin. Medium was changed every 3 days unless otherwise stated.Cells were characterized by flow cytometry after 2 weeks culture expansion. 1*106 trypsinized cells were incubated with anti-αSMA-Alexa Fluor 647, anti-Nestin-Cy7, anti‐Sca‐1‐Alexa Fluor 647, CD146-Alexa Fluor 647 and CD34-BV421.
Destabilization of the medial meniscus
Destabilization of the medial meniscus (DMM) or sham surgery was performed at the age of 10 weeks in mixed gender C57BL/6J mice, in similarity to prior reports24. Equal numbers of male and female animals were distributed across treatment groups. Briefly, left hindlimbs were disinfected with povidone-iodine and 70% ethanol, and buprenorphine SR (1mg/kg) was injected subcutaneously. A 1 cm longitudinal incision was made on the medial aspect of the knee joint under general anesthesia using 2% isoflurane. Blunt dissection of the joint capsule along the medial side of the patellar ligament was performed to expose the medial menisco-tibial ligament (MMTL). The IFP was exposed, displaced laterally, and the MMTL was transected using a 11-blade scalpel to destabilize the medial meniscus. The medial joint capsule and the skin were next closed with 5–0 prolene suture (Ethicon, Bridgewater, NJ). Sham surgery was performed with a similar surgical approach with visualization, but without transection of the MMTL. One week after surgery,either 1*106Pdgfra+orPdgfrβ+ cells were injected into the intra-articular space. Equal volume of PBS was injected as a control. In total, 40 mice,5 males and 5 females pertreatment group, underwent surgery. Groups included (1) sham operated, (2) DMM operated, PBS treated (control), (3) DMM operated, Pdgfra+ cell treated, and (4) DMM operated, Pdgfrβ+ cell treated.Eight weeks after DMM or sham surgery, animals were sacrificed andhindlimbs were harvested for analysis.
Histology and histomorphometry
Stifle joints were fixed in 4% paraformaldehyde for 24 h, decalcified with 14% ethylenediaminetetraacetic acid (EDTA) for 14 d, and embedded in OCT compound (Sakura, Torrance, California).Sagittal sections of the stifle joint were prepared at 6 µm thickness with a Cryofilm type 3c (SECTION-LAB, Hiroshima, Japan). Inguinal fat pads from donor Pdgfra orPdgfrβ‐CreERT2‐mT/mG mice were also dissected, embedded in OCT, and cryosectioned at 30 μm thickness. Routine safranin O / fast greenstaining of stifle joints section was performed with methods adopted from past work25–27.
For immunofluorescent immunohistochemistry, sections were washed in 1× PBS, blocked in 5% normal goat serum (S-1000, Vector Labs, CA, USA) for 30 min, and incubated with primary antibodies specific for ACAN (1:100, ab3778, Abcam, MA, USA), COL10(1:100, ab58632, Abcam), CD31 (1:100, ab 28364, Abcam)and scleraxis (1:100, ab58655, Abcam) at 4 °C overnight. Sections were then incubated with Alexa Fluor® 647-conjugated secondary antibodies (1:200, ab150083, Abcam) or DyLight® 594 (1:100, DI-1594, Vector Labs), and mounted with mounting medium containing DAPI (H-1500, Vector Labs, Burlingame, CA)(Supplemental Table 1). Immunofluorescence images were acquired on a Leica DM 6B or LSM 880 FCS confocal microscope (Carl Zeiss, Oberkochen, Germany).
Microcomputed tomography (µCT) imaging
μCT analysis of the tibial and femoral subchondral bone was performed using a SkyScan1175 system (Bruker, MA)with the following settings: voltage, 65 kVp; current, 153 μA; resolution, 1.0‐mm aluminum filter 10 μm/pixel. The images were reconstructed and analyzed usingNRecon v1.7 and CTAn v1.20, respectively. XYZ axes were adjusted by Dataviewer V1.5.Three-dimensional histomorphometric analysis was performed on cross-sectional images of the tibial and the femoral subchondral bone. We defined the region of interest as the whole subchondral bone medial and lateral compartments and used 15 consecutive images from the medial and lateral tibial plateau for 3-dimensional reconstruction and analysis.Two 3-dimensional structural parameters were analyzed; bone volume density (BV/TV) and bone mineral density(BMD).
OARSI scoring of the knee joints
Six frozen semi-serial sagittal sections with 36 µm intervals were prepared from the medial compartment of each knee joint to represent the weight-bearing area of the distal femur and proximal tibia28. Safranin O / fast green stained sections were analyzed for cartilage injury using the OARSI scoring system 29. A range of 0 – 24 was determined according to the formula: score = grade (G1 to G6) x stage (S1 to S4). The scoring was done by three different authors (TS, SN and MC). The average scores of both femur and tibia were summed.
Statistical analysis
Statistical analyses were performed in IBM SPSS Statistics 16.0. Quantitative data are expressed at mean ± SD.A two tailed Student’s t test was used for two-group comparisons, and a one-way ANOVA test was used for comparisons of three or more groups, followed by Tukey’s post hoc test. *P<0.05 and **P<0.01 were considered significant.
Results
Isolation of Pdgfrα+ and Pdgfrβ+ cellsfrom mouse subcutaneous adipose tissue
In order to precisely define the identity of perivascular cell subsets, we used inducible mouse reporter systems for Pdgfrα and Pdgfrβ, and validated cell identity. Each Cre line was crossed with mT/mG reporter animals, which express membranous TdTomato in all cells untilCre recombination occurs when the TdTomato cassette is excised, and membranous mGFP is expressed30–32. Perivascular cell identity was assessed in the resulting PdgfrαmT/mGand PdgfrβmT/mGreporter animals after exposure to Tamoxifen (TM)13; 33. Consistent with prior reports, PdgfrαmT/mGreporter activity highlighted perivascular cells within the tunica adventitia, along with a portion of cells within a pericytic location13, while PdgfrβmT/mG reporter activity highlighted all pericytes and vascular smooth muscle cells22. After tissue dissociation, by flow cytometry Pdgfrα+ cells comprised 14.5% of total viable cells, while Pdgfrβ+ cells comprised on average 11% of total viable cells (Fig. 1).Pericyte and stem cell markers were assessed amongPdgfrα+ and Pdgfrβ+ cell fractions (Supplementary Fig. 1).The expression of Sca-1 (Stem cell antigen 1) expression was high across both cell preparations (90.1% and 89.4%, respectively). In contrast, αSMA and CD146 expression was enriched among the Pdgfrβ+ cell fraction (89.91 and 71.88% among Pdgfrβ+cells and 12.75 and 4.06% among Pdgfrα+cells).
Figure 1.
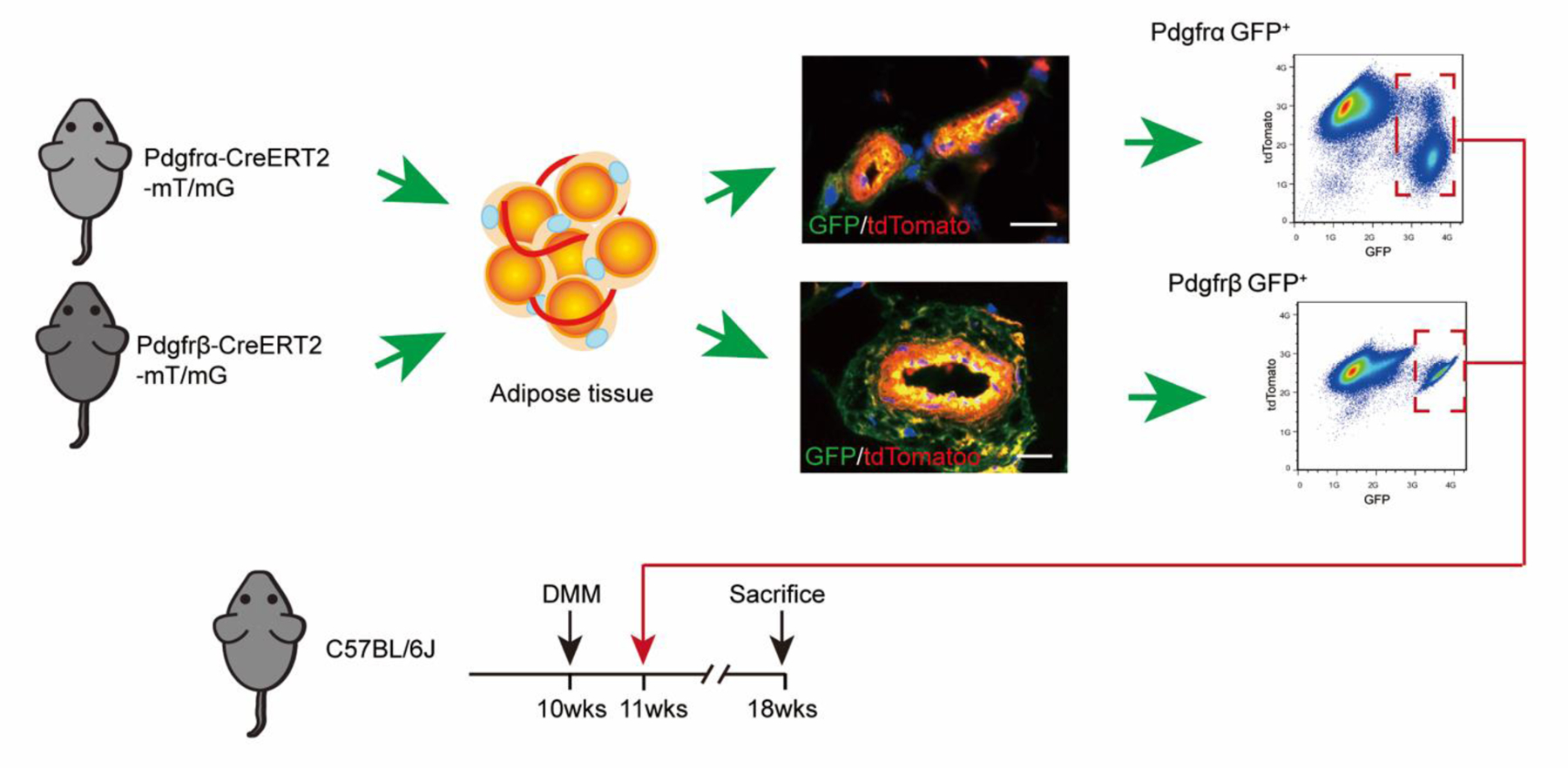
Pdgfrα+ and Pdgfrβ+cell isolation and experimental outline. Pdgfrα-CreERT2 and Pdgfrβ CreERT2 ; mT/mG reporter mice were injected with tamoxifen (TM), followed by isolation of inguinal white adipose tissue, enzymatic dissociation and FACS isolation of GFP+ cells, isolating either Pdgfrα+ and Pdgfrβ+cells. Representative in situ location of Pdgfrα+ and Pdgfrβ+cells and FlowJo plots are shown. 1 M purified Pdgfrα+ and Pdgfrβ+cells were administered by intra-articular injection to C57BL/6J recipient mice on week after DMM surgery. N=10 mice per group. DMM: Destabilization of the medial meniscus. Scale bars: 10 µm
To address the therapeutic potential of either Pdgfrα+ or Pdgfrβ+expressing perivascular cells, we performed intra-articular injection of either Pdgfrα+ or Pdgfrβ+ perivascular cell subsets (Figs. 1–5). Briefly, we injected either FACS purified Pdgfrα+ or Pdgfrβ+ adipose tissue perivascular cells into the stifle joint after DMM (Fig. 1). Cells were prepared from inguinal fat pads of either PdgfrαmT/mGor PdgfrβmT/mG reporter animals. Cells were injected as a one-time dose in equal numbers (1M total cells) and compared to PBS control injection. All knee joints were analyzed at 8 weeks after DMM.
Figure 5.

Cartilage appearance and OARSI scoring after Pdgfrα+ or Pdgfrβ+ cell therapy. (A) Representative images of Safranin O / fast green stained sections in sagittal orientation among sham operated or DMM operated animals. Animals treated with a one-time dose of 1M Pdgfrα+ or Pdgfrβ+cells, or PBS control. Sections shown at 8 wks post-operative. (B) OARSI scoring (0–24) among treatment groups. N=10 animals per group. Mean values and one SD are reported, with each dot representing an individual animal. DMM: Destabilization of the medial meniscus. Scale bars: 100 µm. *P<0.05.
Pdgfrα+ and Pdgfrβ+ cell engraftment in the stifle joint.
Differences in Pdgfrα+ or Pdgfrβ+ cell engraftment and persistence were next evaluated in our model (Fig. 2). Pdgfrα+ cells were found superficially within the articular cartilage as well as menisci, while Pdgfrβ+ cells were more widely distributed within all layers of cartilage as well as subchondral bone (Fig. 2, upper rows). As expected, no GFP+ cells were identified in control tissues (Fig. 2, lower rows).
Figure 2.

Cell engraftment and persistence within cartilage of Pdgfrα+ and Pdgfrβ+ cells after intra-articular injection. Representative images of GFP expression among DMM-operated with Pdgfrα+ and Pdgfrβ+cell therapy. Animals treated with a one-time dose of 1M Pdgfrα+ and Pdgfrβ+cells, or PBS control. High-magnification images show the location of GFP expression cells in Pdgfrα+ and Pdgfrβ+ cell treated group among the meniscus (M), subchondral bone (SB), or superficial articular cartilage (AC). As a negative control, sham-operated or DMM-operated / PBS injected stifle joints are also shown. Thick and thin white dashed lines indicate edges of articular cartilage and meniscus, respectively. N=10 animals per group. Scale bars: 100 µm.
We further examined the distribution of Pdgfrα+ or Pdgfrβ+ cells within the injected joint, examining in detail the meniscus, medial cruciate ligament, infrapatellar fat pad (IFP) and joint capsule (Fig. 3). After DMM surgery, mGFP+ cells were found in the meniscus with relatively similar frequency and distribution across both Pdgfrα+ and Pdgfrβ+ cell treated joints. Interestingly, ACAN immunostaining of the meniscus showed significant changes after cell therapy. Consistent with prior reports 34; 35, DMM operated animals showed a reduction in meniscal ACAN immunostaining in comparison to sham operatd controls (Fig. 3A, left). Conversely, both Pdgfrα+ and Pdgfrβ+ cell treated joints showed a relative increase in ACAN immunoreactivity in areas around cell incorporation within the meniscus (Fig. 3A, right).
Figure 3.

Cell persistence within joint-associated tissues of Pdgfrα+ and Pdgfrβ+ cells after intra-articular injection. Animals treated with a one-time dose of 1 Pdgfrα+ and Pdgfrβ+cells, or PBS control. (A) Aggrecan (ACAN) immunofluorescent staining in combination with GFP + cells within the meniscus. (B) Scleraxis (SCX) immunofluorescent staining in combination with GFP + cells within the medial cruciate ligament. (C) CD31 immunofluorescent staining in combination with GFP + cells within the infrapatellar fat pad (IFP) and (D) GFP + cells within synovium. N=10 animals per group. DMM: Destabilization of the medial meniscus. Scale bars: 100 µm.
Incorporation of injected cells into the medial cruciate ligament (MCL) was also observed (Fig. 3B). Here, DMM ligamentocytes changed from a consistent spindled shape with elongated nucleus to a more rounded cell shape (Fig. 3B, left). This change in morphology was associated with a reduction in scleraxis (SCX) immunoreactivity, consistent with prior reports 36. Significant incorporation of injected Pdgfrβ+ but not Pdgfrα+ cells was found within and around the MCL (Fig. 3B, right). As well, SCX expression was partially restored among ligamentocytes upon either Pdgfrα+orPdgfrβ+ cell treatment.
Incorporation of injected cells was also found within perivascular areas of the infrapatellar fat pad (IFP) (Fig. 3C). This was most conspicuous among larger microvessels of the IFP, and seen among both Pdgfrα+ and Pdgfrβ+cell injected joints. Finally, incorporation of both Pdgfrα+ and Pdgfrβ+ fluorescent cells was found within the synovial membrane of treated joints (Fig. 3D).
Subchondral bone sclerosis after Pdgfrα+ or Pdgfrβ+ cell therapy
Changes in subchondral bone after cell therapy were next examined by microcomputed tomography (microCT) (Fig. 4). MicroCT reconstructions of femoral and tibial subchondral bone demonstrated a clear, qualitative increase in bone after DMM, which was most apparent in the medial compartment (Fig. 4A,B,D). Cell therapies, especially Pdgfrβ+ cell therapy, showed a diminution of this effect. These changes were quantified separately in both the medial (Fig. 4B–E) and lateral aspects of the joint (Fig. 4G–J), and separately for both femoral and tibial subchondral bone. As expected, DMM induced a significant increase in bone mineral density (BMD) within femoral and tibial subchondral bone on both medial and lateral aspects. DMM surgery led to increase in BV/TVs, which was limited to the medial tibial subchondral bone (Fig. 4E). Remarkably, Pdgfrβ+ cell therapy completely prevented subchondral bone changes associated with DMM, including a complete reversal of any increase in BMD or BV/TV across all regions of interest. In contrast, no significant change in subchondral bone was found with Pdgfrα+ cell therapy in comparisons to PBS control injection. All analyses were stratified by gender, with similar results (Supplementary Fig. 2).Thus, Pdgfrβ+ but not Pdgfrα+cell therapy led to improved quantitative metrics of subchondral bone sclerosis after DMM.
Figure 4.
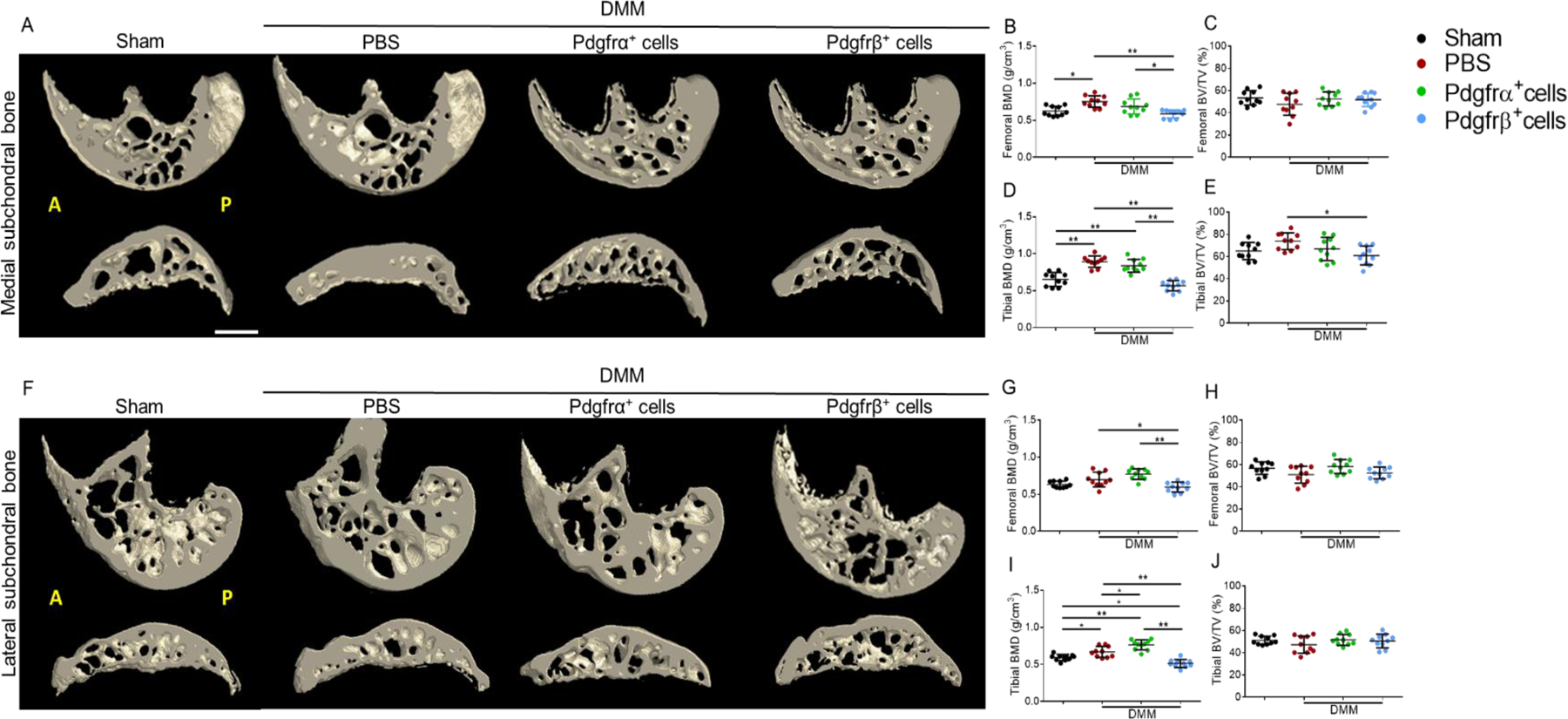
Subchondral sclerosis after Pdgfrα+ or Pdgfrβ+cell therapy. Animals treated with a one-time dose of 1M Pdgfrα+ or Pdgfrβ+ cells, or PBS control. (A,B) Representative 3D reconstructions of micro computed tomography (microCT) imaging of the medial and lateral compartments of the femoral and tibial subchondral bone. (C-J) Quantification of subchondral bone within the (C,D) medial femur, (E,F) medial tibia, (G,H) lateral femur, and (I,J) lateral tibia. Quantification include Bone mineral density (BMD) and Bone volume density (bone volume per tissue volume, BV/TV). N=10 animals per group. Mean values and one SD are reported, with each dot representing an individual animal. A: anterior; P: posterior; DMM: Destabilization of the medial meniscus. Scale bars: 100 µm. *P<0.05, **P<0.01.
Histologic appearance of articular cartilage after Pdgfrα+ or Pdgfrβ+ cell therapy
Sagittal histologic sections of the knee joint were stained with safranin O/fast Green(Fig.5).Typical degenerative changes to the femoral and tibial articular cartilage were observed among PBS control-treated animals after DMM in comparison to sham surgery (Fig. 5A, left hand images). Importantly, a cell dependent effect on arthritis progression was found among either Pdgfrα+ or Pdgfrβ+ perivascular cell therapy (Fig. 5A, right hand images). Pdgfrβ+ cell injection led to a significant improvement in the appearance of the femoral and tibial articular cartilage, with less prominent loss of safranin O staining among superficial articular cartilage. In contrast, Pdgfrα+ cell therapy led to a less dramatic improvement in histologic appearance at 8 weeks after intra-articular injection. Using the OARSI histopathology scoring system,cartilage damage and matrix proteoglycan depletionwere measured (Fig. 5B). The average OARSI scores (0–24) were determined across treatment groups. As expected, a significant increase in OARSI score was found across DMM-operated mice in comparison to sham-operated animals (10.78±1.97 vs 2.44±1.89).Pdgfrβ+ cell therapy led to the largest improvement in OARSI score (8.18±2.35), while the Pdgfra+ cell therapy also showed a significant reduction in OARSI score in comparison to PBS control (9.02±1.99). Results were stratified by gender, with similar findings (Supplementary Fig. 3).
Immunohistochemical staining of articular cartilage after Pdgfrα+ or Pdgfrβ+ cell therapy
Cartilage antigen expression was next assayed after Pdgfrα+ or Pdgfrβ+ cell therapy by immunohistochemistry, including aggrecan (ACAN), and type X collagen (ColX) (Fig. 6). As expected, DMM induced a significant loss of ACAN immunoreactivity among superficial articular cartilage in comparison to sham-operated conditions (Fig. 6A, upper rows). DMM-operated animals treated with either Pdgfrα+ or Pdgfrβ+ cell therapy showed partial restoration of Acan immunoreactivity (Fig. 6A, lower rows).
Figure 6. Cartilage antigen immunohistochemistry after Pdgfrα+ or Pdgfrβ+ cell therapy.

Representative images of (A) Aggrecan (ACAN), and (B) Type X Collagen (Col10) among sham operated or DMM operated animals. Animals treated with a one-time dose of 1.0 M Pdgfrα+ or Pdgfrβ+cells, or PBS control. Sections shown at 8 wks post-operative. N=10 animals per group. Scale bars: 100 µm.
Conversely, the expression of ColX, a marker of chondrocyte hypertrophy and severity of OA, showed the opposite expression patterns (Fig. 6B). Here, and as expected, DMM induced a significant increase in ColX immunoreactivity (Fig. 6B, upper rows). Both Pdgfrα+ and Pdgfrβ+ cell therapy reversed this increase in ColX expression among DMM-operated stifle joints (Fig. 6B, lowerrows).
Discussion
We compared the effects of intra-articular injection of subcutaneous adipose tissue derived Pdgfrα+ and Pdgfrβ+ perivascular cells subsets on OA after joint destabilization. Eight weeks after, we evaluated the engraftment and persistence on both Pdgfrα+ and Pdgfrβ+ cells in the stifle joint tissues and their therapeutic effects.The surface of articular cartilage was a preferred site of engraftment and persistence for Pdgfrα+cells. Pdgfrβ+ cells were more widely engrafted in all layers of cartilage and in the subchondral bone. OARSI scoring and cartilage antigen immunohistochemistry showed an improvement from both cell therapies compared to the PBS treated group after DMM. Interestingly, Pdgfrβ+ cells had a remarkable beneficial effect on the cartilage; The proteoglycan content, ACAN, was more marked while the OA marker for hypertrophic chondrocytes, colX, was reduced. Pdgfrβ+ cells are known for their chondrogenic differentiation ability37. They can also mediate tissue repair via the activation of the PDGF pathway that promotes chondrocyte differentiation, migration, and proteoglycan production38. Together with their intrinsic homing into the different cartilage layers showed here, they might play an important role in enhancing cartilage regeneration and repair.
The subchondral bone was also preferred tissue of engraftment and persistence for Pdgfrβ+ cells. The subchondral bone sclerosis was remarkably reduced by the Pdgfrβ+ cells. In OA, the sclerosis of the subchondral may be a biomechanical compensational adaptation to the articular cartilage damage39. Together with their beneficial effect on the articular cartilage and their ability to engraft, persist and stimulate bone repair40, the Pdgfrβ+cells may have alleviated the pathologic compensational adaptation response of the osteoarthritic subchondral bone and reduce the sclerosis. Paradoxically, Su et al described that an excessive amount of PDGF-BB, which activates Pdgfrβ signaling after DMM, may result in a pathologic subchondral bone with a higher bone volume fraction41. The injected and engrafted Pdgfrβ+cells may play a role in PDGF-BB regulation and hence reduce its effect on the subchondral bone.
Pdgfrβ+cells not only engrafted and persisted in the articular cartilage and bone, but they were also observed in the medial cruciate ligament, meniscus, infrapatellar fat pad (IFP) and joint capsule42. The meniscus is known to host a pool of perivascular stem cells in both the inner avascular region and the vascularized region 43. We saw that the meniscus was remarkably engrafted with both Pdgfra+andPdgfrβ+cells, possibly contributing to meniscal regeneration marked by increasing proteoglycan content after DMM. Significant incorporation of injected Pdgfrβ+ but not Pdgfra+cells was found within and around the MCL. Scx is a distinct marker for tendon and ligament progenitors and differentiated cells44. Here, SCX expression was partially restored after DMM among ligamentocytes upon either Pdgfra+orPdgfrβ+ cell treatment.
In this study, we injected perivascular cells obtained from the white subcutaneous adipose tissue. Most remarkable effects were observed in the articular cartilage and the subchondral bone and induced by Pdgfrβ+cells. Once implanted in OA pathologic microenvironment the perivascular cells ameliorated several key radiologic and histologic parameters of OA. Several mechanisms can be involved in the way perivascular progenitor cells mediate their effects after intra-articular injection. In contrast, perivascular cells inhibit osteoclast formation via non-EV derived secondary messengers 45. Future studies must define the extent to which an injectiablePdgfrβ+cell therapy plays direct or paracrine roles in OA prevention, and the extent to which anti-inflammatory and/or immunosuppressive actions play a contributory role 11.
Several limitations exist toward the broader extrapolation of our results. Firstly, patients with (OA) primarily seek treatment due to pain and disability, yet the primary endpoints we utilized were histological and radiographic measures of joint destruction. Applying behavioral analyses would provide information about gait abnormalities seen in humans and in rodent OA models reflecting similar compensatory behaviors that protect an injured limb from loading46. A logical next step would be to assess pain levels, motor function and gait after injectable perivascular cell therapies. Second, human and mouse pericytes have analogous antigen expression, and a follow up study should determine if a Pdgfrβ+cell therapy derived from human fat demonstrates comparable cellular characteristics. Finally, future studies would determine the downstream molecular mediators of a Pdgfrβ+cell therapy andexpand on whether purified cell therapies with a high pericyte content would be most beneficial for the amelioration or prevention of OA.
Supplementary Material
Acknowledgments
The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute of Health, Department of Defense, or U.S. Army. A.W.J. is a paid consultant for Novadip and LifeSprout LLC. This arrangement has been reviewed and approved by the Johns Hopkins University in accordance with its conflict of interest policies.
Funding:
AWJ was supported by the NIH/NIAMS (R01 AR070773, K08 AR068316), NIH/NIDCR (R21 DE027922), USAMRAA (W81XWH-180109121, W81XWH-18–1–0336, W81XWH-18–10613), American Cancer Society (Research Scholar Grant, RSG-18–027–01-CSM), the Maryland Stem Cell Research Foundation, and MTF Biologics.
Reference
- 1.Chen D, Shen J, Zhao W, et al. 2017. Osteoarthritis: toward a comprehensive understanding of pathological mechanism. Bone Res 5:16044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wang AT, Feng Y, Jia HH, et al. 2019. Application of mesenchymal stem cell therapy for the treatment of osteoarthritis of the knee: A concise review. World J Stem Cells 11:222–235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Im GI. 2018. Tissue Engineering in Osteoarthritis: Current Status and Prospect of Mesenchymal Stem Cell Therapy. BioDrugs 32:183–192. [DOI] [PubMed] [Google Scholar]
- 4.Kong L, Zheng LZ, Qin L, et al. 2017. Role of mesenchymal stem cells in osteoarthritis treatment. J Orthop Translat 9:89–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hwang NS, Elisseeff J. 2009. Application of stem cells for articular cartilage regeneration. J Knee Surg 22:60–71. [DOI] [PubMed] [Google Scholar]
- 6.Oldershaw RA. 2012. Cell sources for the regeneration of articular cartilage: the past, the horizon and the future. Int J Exp Pathol 93:389–400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wilson A, Hodgson-Garms M, Frith JE, et al. 2019. Multiplicity of Mesenchymal Stromal Cells: Finding the Right Route to Therapy. Front Immunol 10:1112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Xu J, Li D, Hsu CY, et al. 2020. Comparison of skeletal and soft tissue pericytes identifies CXCR4(+) bone forming mural cells in human tissues. Bone Res 8:22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Krieger T, Simons BD. 2015. Dynamic stem cell heterogeneity. Development 142:1396–1406. [DOI] [PubMed] [Google Scholar]
- 10.McLeod CM, Mauck RL. 2017. On the origin and impact of mesenchymal stem cell heterogeneity: new insights and emerging tools for single cell analysis. Eur Cell Mater 34:217–231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Meyers CA, Xu J, Zhang L, et al. 2018. Early Immunomodulatory Effects of Implanted Human Perivascular Stromal Cells During Bone Formation. Tissue Eng Part A 24:448–457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.James AW, Zhang X, Crisan M, et al. 2017. Isolation and characterization of canine perivascular stem/stromal cells for bone tissue engineering. PLoS One 12:e0177308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wang Y, Xu J, Meyers CA, et al. 2020. PDGFRalpha marks distinct perivascular populations with different osteogenic potential within adipose tissue. Stem Cells 38:276–290. [DOI] [PubMed] [Google Scholar]
- 14.Crisan M, Corselli M, Chen WC, et al. 2012. Perivascular cells for regenerative medicine. J Cell Mol Med 16:2851–2860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.James AW, Zara JN, Corselli M, et al. 2012. Use of human perivascular stem cells for bone regeneration. J Vis Exp:e2952. [DOI] [PMC free article] [PubMed]
- 16.Wang Y, Xu J, Meyers CA, et al. 2020. PDGFRα marks distinct perivascular populations with different osteogenic potential within adipose tissue. Stem Cells 38:276–290. [DOI] [PubMed] [Google Scholar]
- 17.Böhm A-M, Dirckx N, Tower RJ, et al. 2019. Activation of Skeletal Stem and Progenitor Cells for Bone Regeneration Is Driven by PDGFRβ Signaling. Developmental cell 51:236–254. e212. [DOI] [PubMed] [Google Scholar]
- 18.Jensen AR, Kelley BV, Mosich GM, et al. 2018. Neer Award 2018: Platelet-derived growth factor receptor alpha co-expression typifies a subset of platelet-derived growth factor receptor beta-positive progenitor cells that contribute to fatty degeneration and fibrosis of the murine rotator cuff. J Shoulder Elbow Surg 27:1149–1161. [DOI] [PubMed] [Google Scholar]
- 19.Murray IR, Gonzalez ZN, Baily J, et al. 2017. alphav integrins on mesenchymal cells regulate skeletal and cardiac muscle fibrosis. Nat Commun 8:1118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cuttler AS, LeClair RJ, Stohn JP, et al. 2011. Characterization of Pdgfrb-Cre transgenic mice reveals reduction of ROSA26 reporter activity in remodeling arteries. Genesis 49:673–680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Eilken HM, Dieguez-Hurtado R, Schmidt I, et al. 2017. Pericytes regulate VEGF-induced endothelial sprouting through VEGFR1. Nat Commun 8:1574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Olson LE, Soriano P. 2011. PDGFRbeta signaling regulates mural cell plasticity and inhibits fat development. Dev Cell 20:815–826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Park DY, Lee J, Kim J, et al. 2017. Plastic roles of pericytes in the blood-retinal barrier. Nat Commun 8:15296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Glasson SS, Blanchet TJ, Morris EA. 2007. The surgical destabilization of the medial meniscus (DMM) model of osteoarthritis in the 129/SvEv mouse. Osteoarthritis Cartilage 15:1061–1069. [DOI] [PubMed] [Google Scholar]
- 25.Lee S, Shen J, Pan HC, et al. 2016. Calvarial Defect Healing Induced by Small Molecule Smoothened Agonist. Tissue Eng Part A 22:1357–1366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Levi B, James AW, Nelson ER, et al. 2011. Acute skeletal injury is necessary for human adipose-derived stromal cell-mediated calvarial regeneration. Plast Reconstr Surg 127:1118–1129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Siu RK, Zara JN, Hou Y, et al. 2012. NELL-1 promotes cartilage regeneration in an in vivo rabbit model. Tissue Eng Part A 18:252–261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yahara Y, Takemori H, Okada M, et al. 2016. Pterosin B prevents chondrocyte hypertrophy and osteoarthritis in mice by inhibiting Sik3. Nat Commun 7:10959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pritzker KP, Gay S, Jimenez SA, et al. 2006. Osteoarthritis cartilage histopathology: grading and staging. Osteoarthritis Cartilage 14:13–29. [DOI] [PubMed] [Google Scholar]
- 30.Kang SH, Fukaya M, Yang JK, et al. 2010. NG2+ CNS glial progenitors remain committed to the oligodendrocyte lineage in postnatal life and following neurodegeneration. Neuron 68:668–681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chen Q, Zhang H, Liu Y, et al. 2016. Endothelial cells are progenitors of cardiac pericytes and vascular smooth muscle cells. Nat Commun 7:12422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Muzumdar MD, Tasic B, Miyamichi K, et al. 2007. A global double-fluorescent Cre reporter mouse. Genesis 45:593–605. [DOI] [PubMed] [Google Scholar]
- 33.Sono T, Hsu CY, Wang Y, et al. 2020. Perivascular Fibro-Adipogenic Progenitor Tracing during Post-Traumatic Osteoarthritis. Am J Pathol 190:1909–1920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Fang H, Huang L, Welch I, et al. 2018. Early Changes of Articular Cartilage and Subchondral Bone in The DMM Mouse Model of Osteoarthritis. Sci Rep 8:2855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Miller RE, Ishihara S, Tran PB, et al. 2018. An aggrecan fragment drives osteoarthritis pain through Toll-like receptor 2. JCI Insight 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hasegawa A, Nakahara H, Kinoshita M, et al. 2013. Cellular and extracellular matrix changes in anterior cruciate ligaments during human knee aging and osteoarthritis. Arthritis Res Ther 15:R29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hindle P, Khan N, Biant L, et al. 2017. The infrapatellar fat pad as a source of perivascular stem cells with increased chondrogenic potential for regenerative medicine. Stem Cells Translational Medicine 6:77–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ellman MB, Yan D, Ahmadinia K, et al. 2013. Fibroblast growth factor control of cartilage homeostasis. J Cell Biochem 114:735–742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Li G, Yin J, Gao J, et al. 2013. Subchondral bone in osteoarthritis: insight into risk factors and microstructural changes. Arthritis Res Ther 15:223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Xu J, Wang Y, Hsu CY, et al. 2019. Human perivascular stem cell-derived extracellular vesicles mediate bone repair. Elife 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Su W, Liu G, Liu X, et al. 2020. Angiogenesis stimulated by elevated PDGF-BB in subchondral bone contributes to osteoarthritis development. JCI Insight 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sono T, Hsu CY, Wang Y, et al. 2020. Perivascular Fibro-Adipogenic Progenitor Tracing during Post-Traumatic Osteoarthritis. Am J Pathol. [DOI] [PMC free article] [PubMed]
- 43.Lee KI, Olmer M, Baek J, et al. 2018. Platelet-derived growth factor-coated decellularized meniscus scaffold for integrative healing of meniscus tears. Acta Biomater 76:126–134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lui PP. 2015. Markers for the identification of tendon-derived stem cells in vitro and tendon stem cells in situ - update and future development. Stem Cell Res Ther 6:106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Negri S, Wang Y, Sono T, et al. 2020. Human perivascular stem cells prevent bone graft resorption in osteoporotic contexts by inhibiting osteoclast formation. Stem Cells Transl Med. [DOI] [PMC free article] [PubMed]
- 46.Ruan MZ, Patel RM, Dawson BC, et al. 2013. Pain, motor and gait assessment of murine osteoarthritis in a cruciate ligament transection model. Osteoarthritis Cartilage 21:1355–1364. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


