Abstract
Objective:
Recent research has revealed that cognitively unimpaired older adults who are at higher risk for developing Alzheimer’s disease (AD) dementia often exhibit subtle cognitive alterations in their neuropsychological profiles. Emerging evidence suggests that autobiographical memory, which is memory for personal events and knowledge, may be sensitive to early AD-related cognitive alterations. In the present study, we investigated whether the rapid generation of autobiographical memory category exemplars, a retrieval process that taxes the neural network that is vulnerable to early AD, is compromised in cognitively unimpaired middle-aged and older carriers of the e4 allele of the apolipoprotein E gene (APOE4), which increases risk for AD dementia.
Methods:
In addition to standard neuropsychological tests, we administered a fluency task that requires generating exemplars for two types of autobiographical memory, namely episodic memories and personal semantics, to a group of cognitively unimpaired middle-aged and older adults (n = 45) that was enriched with APOE4 carriers (n = 20).
Results:
While no APOE4 deficits were found on standard neuropsychological tests, episodic and personal semantic exemplar generation was reduced in the APOE4 group.
Discussion:
Autobiographical memory aberrations associated with higher risk for AD are evident in fluency and affect both episodic memory and personal semantics.
Keywords: Autobiographical memory, Apolipoprotein E, Alzheimer’s disease, Cognitive aging, medial temporal lobe, episodic memory, semantic memory
Introduction
Alzheimer’s disease (AD) causes a protracted period of pathological changes that may begin years before a clinical diagnosis of cognitive impairment (Jack et al., 2013; Sperling et al., 2011; Vos et al., 2013). Treatment effectiveness, and the prospect of prevention therapies, depend on improving the detection of individuals who, while cognitively unimpaired, are at increased risk for AD-related clinical cognitive decline (i.e., mild cognitive impairment or dementia). For an early detection approach to be feasible, there is need for widely available, affordable, and minimally invasive tools that can support diagnostic procedures.
According to some conceptual models, cognitive assessment, which is relatively affordable and non-invasive, can help with the early detection of higher AD risk (Caselli & Reiman, 2013; Han et al., 2017; Edmonds et al., 2015). Numerous studies have indicated that cognitive alterations do not arise abruptly, but rather slowly accumulate following a similar trajectory as the neuropathological processes that underlie AD. For instance, cognitively unimpaired middle-aged and older carriers of the e4 allele of the apolipoprotein E gene (APOE4), which increases risk for clinical AD at least two- to three-fold (Corder et al., 1993), show more rapid cognitive decline when followed over several years (Bretsky et al., 2003; Caselli et al., 2004; 2009; 2011). Recent longitudinal studies have also revealed that accelerated cognitive decline is detectable up to 20 years before a diagnosis of mild cognitive impairment (Caselli et al., in press) and is predictive of clinical conversion in individuals who are amyloid beta (Aβ) positive (Papp et al., in press). Studies that have measured subtle cognitive alterations cross-sectionally with neuropsychological profiles have shown a similar link to later AD-related clinical cognitive impairment (Edmonds et al., 2015; Osuna et al., 2019).
In light of the evidence linking cognitive alterations to increased risk for developing clinical AD, there has been much interest in identifying cognitive tests that are sensitive to early AD neuropathology, with the goal of creating more precise composites for diagnosis and clinical trials (Papp et al., in press; Parra et al., 2011; Rentz et al., 2013). We recently proposed that one potentially effective approach is to focus on episodic memory tests that place heavy computational demands on interaction between the medial temporal lobe and its cortical connections (Grilli et al., 2018). Our hypothesis is based on two key points. The first is that the early trajectory of hallmark AD neuropathological processes, namely Aβ and tau accumulation, affect much of the distributed medial temporal lobe-cortical network that supports episodic memory (Benoit & Schacter, 2015; Ranganath & Ritchey, 2012; Reagh & Ranganath, 2018; Vilberg & Rugg, 2013). Aβ is focused in anterior and posterior cortical regions, including medial and lateral prefrontal cortex and medial regions of the parietal cortex (e.g., precuneus, posterior cingulate cortex), and tau accumulation and neurodegeneration begins in the medial temporal lobes (entorhinal/perirhinal, parahippocampal, and hippocampal regions) (Masters et al., 2015; Sperling et al., 2011). The second point is that while the amyloid hypothesis proposes that Aβ proceeds tau accumulation, both pathologies are believed to progress for years before a clinical cognitive diagnosis is warranted (Jack et al., 2013; Sperling et al., 2011), and in some individuals, tau accumulation/neurodegeneration can outpace Aβ (Edmonds et al., 2015). Given the localization of these pathologies, and heterogeneity in trajectories, we therefore suggest that cognitive tests that tax medial temporal lobe-cortical interaction may be well suited to amplify the signal of subtle cognitive alterations that arise from distributed AD pathologies.
The retrieval of episodic autobiographical memories (EAMs), meaning memories for real-world, personal events, has been strongly linked to the medial temporal lobe-cortical network that is vulnerable to early AD pathology (Martinelli et al., 2013; Svoboda et al., 2006). According to several theories, interaction between these cortical regions and the medial temporal lobe is essential for the retrieval of detailed EAMs (Eichenbaum et al., 2007; Moscovitch et al., 2016; Ranganath & Ritchey, 2012). Both natural lesion studies (Berryhill et al., 2007; Bertossi et al., 2016; Grilli & Verfaellie, 2014; Phillippi et al., 2015; Rosenbaum et al., 2008), and research using transcranial magnetic stimulation (Bonnici et al., 2018; Thakral et al., 2017), have supported a medial temporal lobe-cortical interaction viewpoint, showing that the ability to retrieve EAMs, or episodic details of singular events, is compromised by disruption to the medial temporal lobe, or its anterior and posterior cortical connections. In fact, task-based fMRI findings suggest that EAMs may uniquely tax interaction within this network relative to other types of episodic memory (Chen et al., 2017; Monge et al., 2018). Similarly, the results from some neuropsychological studies of individuals with anterior or posterior cortical lesions have indicated that EAM appears to be more severely affected than performance on many other episodic memory tests (Berryhill et al., 2007; Bertossi et al., 2016; Davidson et al., 2008). Thus, compared to other means for assessing episodic memory, EAM may be particularly sensitive to subtle changes in a neural network that if compromised, increases risk for conversion to clinical AD.
We recently provided initial support for a link between EAM and subtle cognitive alterations by comparing autobiographical memory in cognitively unimpaired middle-aged and older adults who varied in their APOE4 status (Grilli et al., 2018). Notably, cognitively unimpaired middle-aged and older APOE4 carriers are at greater risk for a number of AD suggestive alterations that affect the medial temporal lobe-cortical network underlying episodic memory. For instance, clinically normal APOE4 carriers in this age group tend to have higher Aβ burden (Jack et al., 2015). Cognitively unimpaired APOE4 carriers also show greater medial temporal lobe volume loss over several years, independent of Aβ (Montandon et al., 2020). Additional structural and functional alterations in the medial temporal lobe-cortical network that supports episodic memory also have been reported in cognitively unimpaired, middle-aged and older adult APOE4 carriers (Reiman et al., 2005; Ryan et al., 2011; Sheline et al., 2010) in ways that are consistent with broader indicators of the preclinical progression of AD (Sheline & Raichle, 2013). Therefore, while comparing APOE4 carriers to non-carriers cross-sectionally cannot predict individual conversion, such an approach can evaluate whether autobiographical memory is linked to higher risk for clinical AD.
In Grilli and colleagues (Grilli et al., 2018), we had participants narrate autobiographical events, and we scored their narratives for their episodic and non-episodic detail make-up, using a well-established autobiographical interview approach and scoring protocol (Levine et al., 2002). We found that the APOE4 carrier group showed a reduction in episodic specificity across remote and recent time periods, meaning that when they described autobiographical events, the APOE4 group generated fewer perceptual, action, scene, and related details that added to the vividness and uniqueness of the events. In comparison, the APOE4 carrier group did not generate fewer non-episodic details in their narratives, including semantic and other language-based details, nor were they reduced on a battery of neuropsychological tests. Taken together, we interpreted these findings as evidence that reduced EAM may be a sign of higher risk for clinical AD.
Present study
In the present study, we asked two follow-up questions about the EAM hypothesis. Our initial study of autobiographical memory in APOE4 middle-aged and older adults showed reduced episodic detail generation while narrating unique events, reflecting a deficit in EAM elaboration. According to cognitive models of autobiographical memory, before a memory can be elaborated, one’s autobiographical memory knowledge base must be mentally searched and an EAM selected (Addis et al., 2012; Conway, 2005). Therefore, the first objective of the present study was to evaluate whether autobiographical memory selection is compromised in cognitively unimpaired APOE4 carriers. The autobiographical interview approach that we used in our initial study also explicitly directed participants to retrieve episodic details to describe the events. While semantic details are nonetheless often generated, they are far less frequent and may not reflect an individual’s capacity to access semantic details for events. In fact, according to Conway’s (2005) model of autobiographical memory organization, reduced access to episodic memory may limit how efficiently a person can mentally search for “personal semantics,” meaning semantic knowledge about the self. Also, prior research has shown that some personal semantics have episodic qualities that may translate to shared dependence on the medial temporal lobe - cortical network that supports episodic memory (Renoult et al., 2012). For instance, autobiographical facts attached to a spatiotemporal context, such as a repeated event (e.g., social gatherings after work) or lifetime period (e.g., college years), are dependent on the medial temporal lobe (Grilli & Verfaellie, 2014; 2016). Collectively, reduced episodic memory, along with lower access to knowledge related to repeated events and lifetime periods, may have consequences for retrieval of a wide range of personal semantics. Therefore, our second objective was to evaluate whether cognitively unimpaired APOE4 middle-aged and older adults can show an alteration in personal semantic selection.
To address these questions, we built on prior studies that have modified traditional category fluency tasks to target rapid and repeated autobiographical memory selection, or autobiographical fluency (Dritschel et al., 1992). These studies have shown that autobiographical fluency depends on medial temporal lobe-cortical interaction (Greenberg et al., 2008; Ryan et al., 2008; Sheldon et al., 2012; 2016), and is sensitive to aging (Piolino et al., 2010) and clinical AD, including in individuals who have Aβ positive mild cognitive impairment (Tomadesso et al., 2019). In the present study we adopted a well-established autobiographical fluency task that evaluates both episodic memory and personal semantic category exemplar generation (Dritschel et al., 1992) and has been used with clinical AD (Addis & Tippett, 2004). In this particular task, the retrieval of personal semantics is targeted by recalling the names of personally known people. Prior fMRI and neuropsychological studies have shown that fluent retrieval from seemingly abstract autobiographical categories, like names of familiar people, is related to medial temporal and connected cortical regions (Greenberg et al., 2009; Sheldon & Moscovitch, 2012). This particular autobiographical fluency task also attaches the category of names to specific time periods (e.g., early adulthood), which may further increase demands on episodic mental search and retrieval strategies that depend on the medial temporal lobe. Therefore, while the task targets an abstract type of semantic knowledge (i.e., names of personally known people), it can be viewed as a good assessment of the possibility that personal semantics are reduced in older APOE4 carriers, at least under high autobiographical fluency retrieval demands. To contextualize our autobiographical memory findings, participants also completed standard neuropsychological fluency tasks targeting non-autobiographical categories.
We hypothesized that if individuals at higher risk of clinical AD tend to exhibit reduced episodic specificity selection, the APOE4 group would generate fewer unique events on the autobiographical fluency task. Given that personal semantic fluency may be linked to medial temporal lobe-cortical interaction, we also hypothesized that the APOE4 group may show reduced fluency for generating personally familiar names from identified lifetime periods.
Methods
Participants
Forty-five middle-aged and older adults (ages 53–84) were included in the present study, which was approved by the Institutional Review Board at University of Arizona. All participants were characterized as cognitively unimpaired according to a neuropsychological profile approach based on eight neuropsychological test scores, which are reported in Table 1. Consistent with past studies (Bondi et al., 2014, 2008; Grilli et al., 2018), two neuropsychological test scores were selected from multiple cognitive domains, namely learning and memory, attention/executive functioning, language, and visuospatial functioning, and individuals were considered cognitively unimpaired if neither of the following were met: (1) they performed more than 1 standard deviation below the age-corrected (and education-corrected if available) normative mean on both scores in one cognitive domain, or (2) they performed more than 1 standard deviation below the age-corrected (and education-corrected if available) normative mean on one test in three cognitive domains. In addition, to be included participants had to score below the ≥ 16 cutoff for depression on the Center for Center for Epidemiological Studies Depression Scale (Radloff, 1977), they had to deny a remarkable history of neurologic conditions that could have cognitive effects, and they had to report being independent in activities of daily living. Self-reported race/ethnicity was Non-Hispanic White for 44 participants and Hispanic for 1 participant.
Table 1.
Means and standard deviations (in parenthesis) for demographics and neuropsychological test scores (z scores) for APOE4 carriers and non-carriers.
| APOE4 Carriers | Non-Carriers | |
|---|---|---|
| Age | 70.7(6.6) | 70.8(7.3) |
| Education | 17.3(2.0) | 17.4(1.7) |
| Gender | 13F/7M | 18F/7M |
| VCI | 118.8(10.0) | 121.0(11.7) |
| Learning and Memory | ||
| CVLT-II LDFR | 0.83(.78) | 0.62(1.06) |
| RCFTLDFR | 0.69 (.97) | −0.1(.83) |
| Attention/Executive Functioning | ||
| Trails A | 0.09(1.13) | 0.73(1.2) |
| Trails B | 0.07(.64) | 0.3(.7) |
| Language | ||
| BNT | 1.1(.96) | 1.1(.82) |
| Animals | 0.01(1.04) | −0.05(.99) |
| Visuospatial Functioning | ||
| WAIS-IV Block Design | 1.0(.81) | 0.8(.84) |
| RCFT Copy | 0.5(1.34) | −0.2(.95) |
Notes: The APOE4 carriers were matched to the non-carriers on the group level for age, education, gender, and verbal comprehension (VCI). To be eligible for the study, participants had to perform largely within normal limits on a battery of neuropsychological tests (see Participants section). Data represent means with standard deviations in parenthesis. VCI = Verbal Comprehension Index; CVLT LDFR = California Verbal Learning Test Long Delay Free Recall; RCFT LDFR = Rey Complex Figure Test Long Delay Free Recall; BNT = Boston Naming Test; WAIS = Weschler Adult Intelligence Scale; For the neuropsychological tests, VCI scores are scaled scores and the remaining scores are age (and education if available) corrected z-scores.
Table 1 shows that as a group, the APOE4 carriers were matched to the APOE4 non-carriers on age, gender, education, and intelligence, as measured by the Verbal Comprehension Index from the Wechsler Adult Intelligence Scale (WAIS-IV; Wechsler, 2008), p’s ≥ .50.
For APOE genotyping, we collected saliva samples from each participant using the Oragene DNA Collection Kit (DNA Genotek, Ottawa, ON, Canada). APOE rs429358 and APOE rs7412 genotyping, which was completed at The Translational Genomics Research Institute in Phoenix, Arizona, was carried out using TaqMan allelic discrimination (Applied Biosystems, Foster City, CA, United States) and ABI Prism 7000 sequence detection (Applied Biosystems, Foster City, CA, United States), as described in Corneveaux and colleagues (Corneveaux et al., 2010). Some participants were recruited from our existing database and others were newly recruited for the present study. Twenty of the participants were APOE4 carriers (all e3/e4 heterozygotes), and 25 were APOE4 non-carriers (23 e3/e3 homozygotes and 2 e2/e3 heterozygotes).
Power analysis
We used G*Power 3.1 for our power analysis (Faul et al., 2007). We assumed that an APOE effect on autobiographical fluency would be similar in magnitude to the effect that was found in Grilli and colleagues (Grilli et al., 2018), which was d = .87 for the difference in episodic detail generation in autobiographical event narratives. We also assumed that if there is a significant interaction between group and episodic memory vs. personal semantic fluency, the magnitude would be similar to that of Grilli and colleagues (Grilli et al., 2018), which was medium to large, partial η2= .19. With these effects, and α = .05 and power (1 - β) = .80, our estimated required sample size was 44.
Procedures
Participants were drawn from a multi-session project of cognitive aging and AD risk, which included neuropsychological testing and experimental behavioral testing.
Standard neuropsychological testing
As mentioned, participants underwent standard neuropsychological testing as part of our screening for study eligibility. Our neuropsychological battery consists of the subtests for the Verbal Comprehension Index on the WAIS-IV (Weschler, 2008), the California Verbal Learning Test (CVLT-II; Delis et al., 2000), which is a auditory word-list learning task, the Rey-Osterrieth Complex Figure Test (RCFT; Rey, 1941), which is a complex figure visuospatial memory task, Trail Making Test A and B (Reitan & Wolfson, 1993), which measures mental processing speed and executive functioning, the Boston Naming Test (BNT; Goodglass et al., 2001), which assesses confrontation naming, and the Controlled Oral Word Association Test (COWAT; Benton, 1969), which requires generating exemplars to letters and general semantic categories. In regard to the COWAT, participants generated word exemplars for three letters, namely “F,” “A,” and “S.” They also generated exemplars to the categories “animals” and “fruits and vegetables.” Notably, while animal fluency is part of our battery for assessing cognitive status, we added the additional categories for additional information about participants’ performance on non-autobiographical category/semantic fluency abilities.
Autobiographical fluency task
For the present study, the experimental behavioral task of interest was an autobiographical fluency task, which we adapted from Addis and Tippett (Addis & Tippett, 2004) and builds on the initial autobiographical fluency task by Dritschel and colleagues (Dritschel et al., 1992). Consistent with Addis and Tippett (Addis & Tippett, 2004), our autobiographical fluency task required generating exemplars from an episodic category (i.e., unique events) and a personal semantic category (i.e., names of family/friends/other personally known people). For each category, participants were asked to generate exemplars from three time periods presented in chronological order: childhood (i.e., up to age 18), early adulthood (i.e., 18–35), and recent life (i.e., the past 15 years). For each time period, participants first completed the personal semantic category followed by the episodic memory category. For the personal semantic category, participants were instructed to “name people that you knew” during each time period. For the episodic category, participants were instructed to “recall unique events that happened” during each time period. They were further told that “a unique event is an experience that never happened twice in the same way. For example, recalling the one time your parents forgot to pick you up from soccer practice would be a unique event. However, recalling participating in soccer practices would not be a unique event, because that sounds like something that happened many times.” Similar to prior research (Addis & Tippett, 2004), we instructed participants to provide a short title for each event, rather than describe the events in great detail. We gave participants an example of how to report a unique event with a short title for each time period (e.g., childhood: “the one time I was left at soccer practice”). Consistent with Addis and Tippett (2004), for each category at each time period, participants were given 90 seconds to generate exemplars. Responses were audio recorded for later scoring.
In regard to scoring, first, we tallied each response provided for the time periods, separated by category. Consistent with standard category fluency tasks, from the total tallied responses, we separated “correct” responses from repetitions and “intrusions,” meaning responses that were not names or unique events. Unique events were defined as events that would unfold in less than a day and made reference to a unique feature (e.g., action) that would separate the description from one that could be a repeated experience (Levine et al., 2002). Our primary outcome was total “correct” in each category, whether that be unique events or names. We are not using “correct” in the sense that memories were verified, but rather that we judged them as within the rules of the task (e.g., they were a person’s name). However, we also analyzed correct summed with intrusions, addressing concern about scoring. Finally, we scored for repetitions within a time period. We did not score for repetitions across time periods, because we assumed that some names would be relevant to more than one time period, and we did not want to uniquely alter the task demands or the dependence between time periods for the personal semantic fluency task.
Analyses
To contextualize our autobiographical memory results, we compared APOE4 carriers and non-carriers on the eight neuropsychological test scores used in our screening battery, contrasting raw scores with independent samples t-tests. We also investigated whether there were APOE4 differences on two more neuropsychological fluency tests that were not part of our screener, namely fruits/vegetables exemplar generation and FAS letter exemplar generation.
Our primary model of interest was a 2 (Group: APOE4 carrier vs. non-carrier) × 2 (Autobiographical category: episodic vs. personal semantic) mixed-design analysis of variance (ANOVA) examining total correct responses across all time periods. As reported below, we found main effects without an interaction and followed-up with independent samples t-tests to better understand the effect of APOE4 on both categories. We repeated our ANOVA and follow-up t-tests replacing total correct with total correct plus total intrusions, to evaluate whether our exclusion of some exemplars on the fluency task altered the outcomes. We also used an independent samples t-test to evaluate whether there were differences in repetitions.
We conducted a few follow-up analyses. We compared the word count per response in the episodic fluency task with an independent samples t-test to determine whether APOE4 carriers and non-carriers were similar in their adherence to our instructions to provide short titles for events. Our intention for using multiple time points for each category was to increase our sampling rate and improve reliability of our estimates for each category. Notably, in Grilli and colleagues (Grilli et al., 2018), we did not find a relationship between the APOE4 effect and remoteness, nor did Addis and Tippett (2004) in their study of AD dementia. Yet, for each autobiographical fluency category, we also followed-up with an exploratory 2 (Group: APOE carrier vs. non-carrier) × 3 (Time period: childhood vs. early adulthood vs. recent life) mixed-design ANOVA. Significant interactions were further examined with independent samples t-tests of relevant autobiographical categories.
We used JASP for our analyses (JASP Team, 2020). All visualizations of the data were created in R (R Core Team, 2019).
Data availability
All data are deposited here: https://osf.io/4c8dr/?view_only=4c3f80d5c40f46a08eb2fb2ab0e8110f
Results
Standard neuropsychological tests
The APOE4 carrier group was not significantly reduced on any of the 10 neuropsychological test scores. The APOE4 carriers actually performed better than the non-carriers on the RCFT copy and recall trials. Table 2 shows the raw scores for the 7 non-fluency based neuropsychological test scores along with t scores and associated p values.
Table 2.
Means and standard deviations (in parenthesis) for raw scores on the neuropsychological tests for the APOE4 carriers and non-carriers, along with results from independent samples t-tests.
| APOE4 Carriers | Non-Carriers | t score | p value | |
|---|---|---|---|---|
| CVLT LDFR | 11.9(3.3) | 11.7(3.1) | 0.2 | 0.85 |
| RCFT LDFR | 18.7(6.0) | 14.3(6.1) | 2.4 | 0.02 |
| Trails A | 32.0”(9.6) | 28.7”(9.2) | 1.1 | 0.26 |
| Trails B | 73.1”(20.3) | 64.1”(18.2) | 1.6 | 0.13 |
| BNT | 58.2(2.3) | 58.4(1.8) | 0.4 | 0.69 |
| WAIS-IV Block Design | 43.9(9.8) | 40.7(9.4) | 1.1 | 0.27 |
| RCFT Copy | 31.8(3.6) | 28.2(6.4) | 2.2 | 0.03 |
Notes: As a group, the APOE4 carriers were not significantly lower than the non-carriers for raw scores on any of the standard neuropsychological tests that were included in the cognitive screening battery. Rather, the APOE4 carriers performed better than non-carriers for our RCFT measures. CVLT LDFR = California Verbal Learning Test Long Delay Free Recall; RCFT LDFR = Rey Complex Figure Test Long Delay Free Recall; BNT = Boston Naming Test; WAIS-IV = Weschler Adult Intelligence Scale Fourth Edition
In Figure 1, we show performance on the three standard neuropsychological fluency tests, which assess general, non-autobiographical categories. As noted, APOE4 carriers and non-carriers did not significantly differ on our standard neuropsychological tests of category fluency for letters (i.e., FAS), t(43) < 1, animals, t(43) < 1, or fruits and vegetables, t(43) = 1.7, p = .11.
Figure 1. APOE4 carriers were not significantly reduced on general semantic fluency.
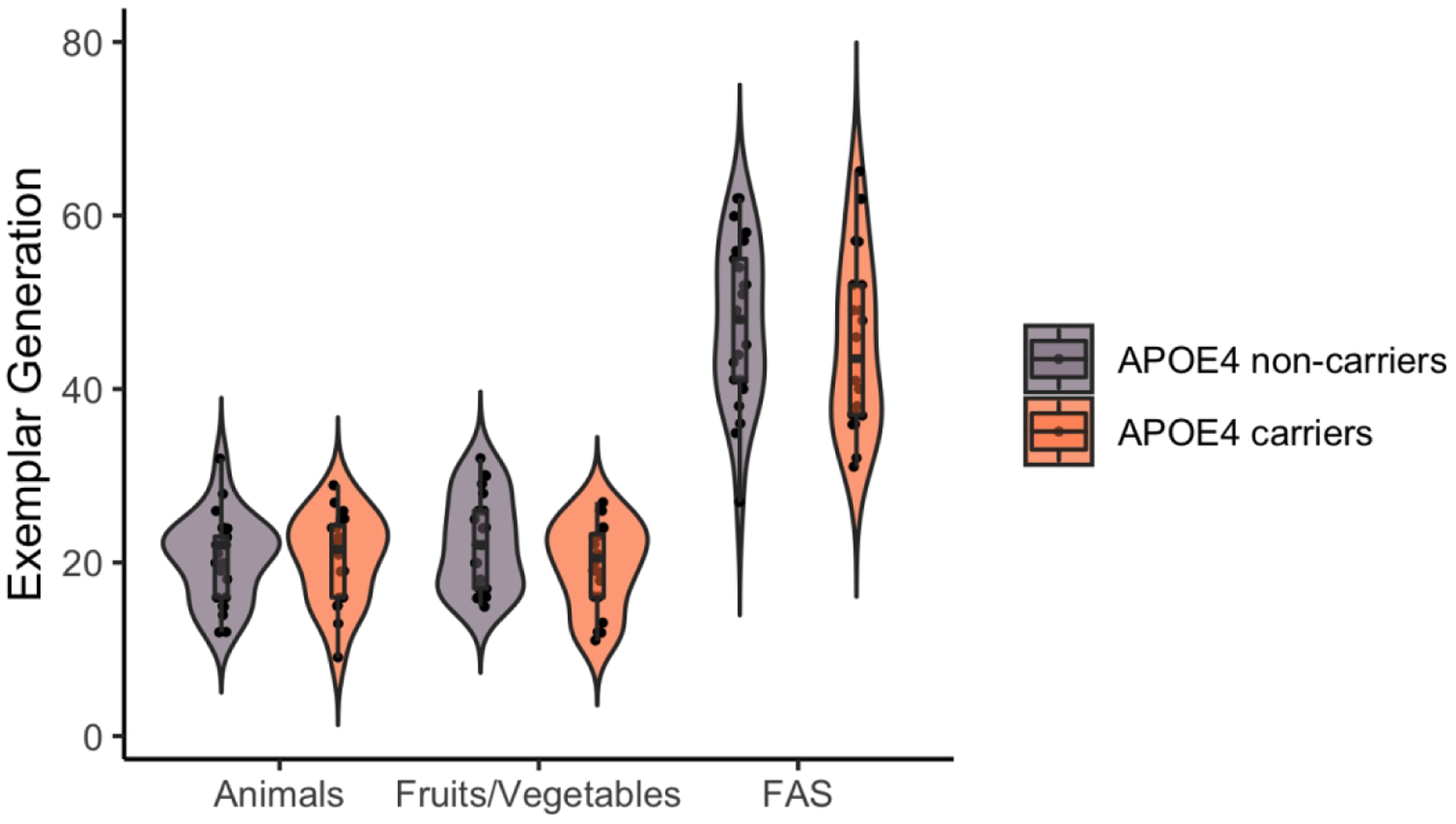
Figure 1 shows a hybrid violin/box/dot plot of exemplar generation for APOE4 carriers and non-carrier groups on the standard neuropsychological tests of category fluency, which required generating category exemplars of animals, fruits/vegetables, and the letters “F”, “A”, and “S”. APOE4 carriers were not significantly reduced on these general semantic fluency tasks. The contour of the violin plot represents the distribution of the data, and the dots are jittered. For the boxplot, the line within the box represents the medians, horizontal edges represent first and third quartiles, and whiskers represent 1.5 standard deviations above the upper quartile and below the lower quartile. Figure created using RStudio and the ggplot2 package (Wickham, 2016).
Episodic and personal semantic autobiographical memory fluency
On average APOE4 carriers generated a total of 73.5 correct names (range: 34 – 119) and 23.7 correct episodic memories (range: 10 – 41) on the autobiographical fluency tasks. In comparison, on average APOE4 non-carriers generated a total of 87.4 correct names (range: 50 – 118) and 31.0 correct episodic memories (range: 12 – 63) on the fluency task. As shown in Figure 2, our 2 (Group: APOE4 carrier vs. non-carrier) × 2 (Autobiographical category: episodic vs. personal semantic) mixed-design ANOVA revealed a main effect of group, F(1,43) = 6.34, p = .02, η2= .13, such that APOE4 carriers generated fewer exemplars overall on the autobiographical fluency task. There also was a main effect of category, F(1,43) = 334.88, p < .001, partial η2= .89, with participants generating more exemplars for personal semantic relative to episodic memory fluency. However, there was not a significant interaction between group and category, F(1,43) = 1.31, p =.26. Critically, APOE4 carriers exhibited a significant reduction for episodic memory fluency, t(43) = 2.4, p = .02, d = .71, 95% CI [.1, 1.31], and personal semantic fluency, t(43) = 2.1, p = .04, d = .64, 95% CI [.03, 1.24].1
Figure 2. APOE4 carriers were significantly reduced for both personal semantic and episodic memory fluency.
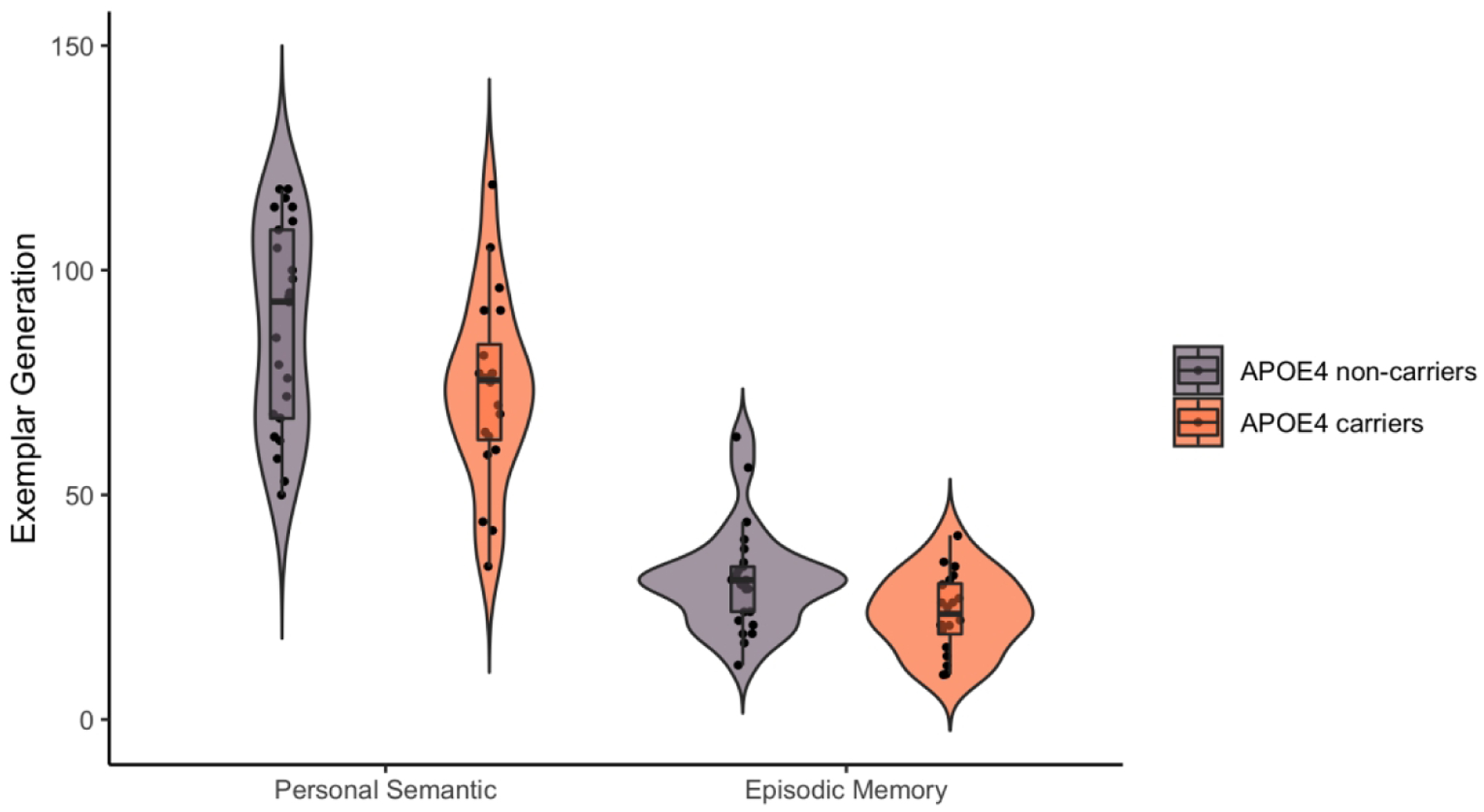
Figure 2 shows a hybrid violin/box/dot plot of exemplar generation for APOE4 carriers and non-carrier groups on the autobiographical category fluency tasks. The APOE4 carriers, as a group, generated fewer familiar name (personal semantic) and episodic memory exemplars. The contour of the violin plot represents the distribution of the data, and the dots are jittered. For the boxplot, the line within the box represents the medians, horizontal edges represent first and third quartiles, and whiskers represent 1.5 standard deviations above the upper quartile and below the lower quartile. Figure created using RStudio and the ggplot2 package (Wickham, 2016).
On average, both APOE4 carriers and non-carriers generated few intrusions on the episodic memory fluency trials (Mcarrier = 3.3; Mnon-carrier = 3.4), and only one APOE4 non-carrier generated any intrusions on the personal semantic fluency trials. As such, not surprisingly, replacing total correct with total correct plus total intrusions did not significantly alter the outcomes. APOE4 carriers generated fewer exemplars, as evidenced by a main effect of group, F(1,43) = 6.2, p = .02, η2= .13, and fluency was higher for personal semantics exemplars versus episodic memory exemplars, as shown by a main effect of category, F(1,43) = 295.51, p < .001, partial η2= .87. The magnitude of the APOE4 fluency reduction also did not significantly differ for the autobiographical categories, as shown by the lack of a significant interaction between group and category, F(1,43) = 1.36, p = .25, η2= .03, with APOE4 carriers showing a significant reduction for both, t’s ≥ 2.2, p’s ≤ .04, d’s ≥ .65.
Total repetitions across the entire fluency task also were quite low per person (Mcarrier = 1.1; Mnon-carrier = 0.8) and did not significantly differ by APOE4 group, t(43) < 1.
Follow-up autobiographical memory analyses
Although participants were instructed to provide short titles for events on the episodic fluency task, one possibility is that APOE4 carriers were more descriptive in their reports, which would limit their ability to generate as many events under time constraints. However, this was not the case, as the number of words used to describe each event by APOE4 carriers (M = 6.1, SD = 1.9) did not significantly differ from non-carriers (M = 6.7, SD = 2.3), t = .88, p = .38.
As mentioned, we followed-up our main analyses with exploratory analyses of the relationship between remoteness and the APOE fluency reduction. For personal semantic fluency, there was a main effect of remoteness, F(2,86) = 9.97, p < .001, partial η2= .19, such that childhood exemplar generation (M = 30, SD = 8.1) was significantly greater than both early adulthood (M = 24.6, SD = 8.9) and recent life (M = 26.7, SD = 9.5), t’s ≥ 2.54, p’s ≤ .01, d’s ≥ .38, which did not differ, t = 2.00, p = .052. However, there was not a significant interaction between group and remoteness, F(2,86) < 1.
In contrast, for episodic memory fluency, while there was not a main effect of remoteness, F(2,86) < 1, there was a significant interaction between remoteness and APOE status, F(2,86) = 4.6, p = .01, partial η2= .10. APOE4 carriers demonstrated reduced fluency for childhood (Mcarrier = 7.3, SD = 3.1; Mnon-carrier = 10.6, SD = 4.0) and recent time periods (Mcarrier = 7.2, SD = 3.6; Mnon-carrier = 10.6, SD = 4.5), t’s ≥ 2.8, p’s ≤ .004, but not early adulthood (Mcarrier = 9.3, SD = 3.6; Mnon-carrier = 9.8, SD = 4.7), t < 1.
Discussion
Autobiographical memory fluency and APOE4
On an autobiographical fluency task, a group of cognitively unimpaired middle-aged and older APOE4 carriers generated fewer category exemplars for unique events relative to a group of APOE4 non-carriers who were comparable on age, verbal intelligence, and a host of neuropsychological tests, including general category fluency. The present study, therefore, revealed that APOE4 status not only affects EAM elaboration (Grilli et al., 2018), but also the ability to rapidly search for and repeatedly select EAMs. Given prior fMRI (Ryan et al., 2008; Sheldon et al., 2012; 2016) and neuropsychological (Greenberg et al., 2009) evidence of medial temporal lobe-cortical involvement in category fluency for episodic/spatial autobiographical memory, we interpret our findings as consistent with the notion that tasks placing heavy computational demands on the neural network that is most vulnerable to clinical AD can amplify signs of subtle cognitive alteration in higher risk individuals.
Our episodic autobiographical memory category fluency findings build on recent work by Tomadesso and colleagues (Tomadesso et al., 2019). In their study, EAM exemplar generation on a fluency task was compared across three groups: cognitively normal older adults, older adults with Aβ positive mild cognitive impairment, and older adults with Aβ negative mild cognitive impairment. Both mild cognitive impairment groups demonstrated reduced autobiographical fluency for remote and recent time periods, and the Aβ positive group showed a trend for more severe reductions relative to the Aβ negative group, despite comparable performance between clinical groups on a host of standard neuropsychological tests. Although we do not have Aβ or tau biomarkers in our sample, the results of Tomadesso and colleagues (Tomadesso et al., 2019) raise the possibility that non-clinical levels of AD pathology, for which APOE4 carriers are at increased risk for at late middle and older ages (Caselli & Reiman, 2013), may alter autobiographical fluency.
In addition to reduced episodic memory fluency, as a group, the APOE4 carriers also generated fewer exemplars for the personal semantic category (i.e., names). In fact, the APOE4-related reduction in personal semantic fluency was comparable to that of episodic memory fluency, as measured by their similar effect sizes, which were medium in magnitude. These findings suggest that autobiographical memory deficits in cognitively unimpaired older adults at higher risk for developing AD dementia can extend to personal semantics, at least when such knowledge is assessed under high autobiographical retrieval fluency demands. This conclusion is consistent with fMRI and neuropsychological studies showing that fluent retrieval of exemplars from seemingly abstract autobiographical categories is supported by the medial temporal lobe (Greenberg et al., 2009; Sheldon & Moscovitch, 2012). Medial temporal lobe involvement in this sort of fluency task could reflect autobiographical memory organization (Conway, 2005) and the possibility that episodic memories and repeated event/lifetime period knowledge may be used to cue the retrieval of more abstract personal semantic category exemplars, including names of people. Relatedly, our personal semantic fluency task required that participants search for exemplars from particular lifetime periods. This added spatiotemporal retrieval demand strikes another commonality with the way episodic autobiographical memories may be searched for and retrieved. Therefore, while a familiar person’s name is a type of abstract personal semantic knowledge, the retrieval strategies used to fluently generate exemplars from this category may draw on episodic mental search routes and content.
Why personal semantic fluency, but not general semantic fluency, was compromised in the group of APOE4 carriers could be related to a few differences in task demands. For instance, the mental search strategies used to generate personally known names may be more episodic relative to the strategies used to fluently generate exemplars from general semantic categories. Relatedly, the fact that our personal semantic fluency task had an added spatiotemporal context, namely lifetime periods, could have further increased demands on medial temporal lobe involvement relative to the general semantic fluency tasks. Another difference is that the personal semantic fluency task required retrieving unique names, whereas the general semantic fluency tasks probed basic level category knowledge. The greater specificity of the personal semantic fluency task may have placed higher cognitive demands on anterior lateral and medial temporal lobe regions that have been implicated in specific concepts (e.g., items) and naming (Clarke & Tyler, 2014; Tranel, 2009). Notably, remoteness of knowledge acquisition seems like an unlikely explanation for the difference between autobiographical fluency and general semantic fluency. While the basic level general categories that we assessed reflect knowledge that was likely acquired remotely, APOE4 carriers as a group showed reduced personal semantics and episodic memory for remote and recent time periods.
Overall, the findings suggest that cognitive assessments that track fluency of autobiographical memory generation may be valuable tools for early detection of increased risk for clinical AD. In this regard, it is noteworthy that our exploratory analyses revealed that while remoteness did not have a significant effect on personal semantic fluency, the APOE4 reduction for episodic memory fluency was not detected for the early adulthood period. Interestingly, the early adulthood period overlaps with the reminiscence bump in autobiographical memory, which is a period consisting of highly accessible episodic memories (Conway & Rubin, 1993; Rubin et al., 1986). We suggest that the reminiscence bump, which contains many firsts and self-defining moments (Rathbone et al., 2008; Thomsen et al., 2011), may account for the lack of an APOE4 effect in this time period. However, future research will need to examine the effect of remoteness more closely, because we used relatively coarse time periods.
Limitations and future directions
The present study has a few limitations that will need follow-up. First, while APOE4 is associated with increased risk for clinical AD, not all APOE4 carriers develop mild cognitive impairment or dementia. As shown in Figure 1, not surprisingly there is overlap in fluency performance between the APOE4 carrier and non-carrier groups, possibly reflecting individual risk profiles. However, a longitudinal study is required to evaluate the degree to which reduced fluency predicts later conversion to mild cognitive impairment or dementia. Second, future research will need to investigate whether reduced autobiographical fluency is sensitive to biomarkers of amyloid or tau, given the need for more sensitive cognitive tools for assessing these pathologies and cognitive endpoints for clinical trials. Third, although prior fMRI and neuropsychological research has shown that autobiographical category fluency tasks are associated with medial temporal lobe and cortical involvement (Ryan et al., 2008; Sheldon et al., 2012; 2016), it will be important to reveal the neural underpinnings of the APOE4 associated reduction that was captured here. Fourth, consistent with prior research (Addis & Tippett, 2004; Dritschel et al., 1992), we always administered the personal semantic category before the episodic memory category within each time period. A future study could evaluate whether probing episodic memories first affects the sensitivity of APOE4 status to personal semantic fluency, or if the magnitude of the episodic memory fluency reduction is altered. Finally, the demographics of our sample are notably high for education and IQ, and the sample is almost entirely Non-Hispanic White. Future research will need to examine the degree to which reduced autobiographical memory is associated with higher risk for AD in more diverse groups.
Conclusions
We found that middle-aged and older APOE4 carriers, despite being cognitively unimpaired, tended to show reduced autobiographical fluency relative to age and cognitively similar APOE4 non-carriers. Overall, these findings indicate that there may be broad autobiographical memory alterations associated with increased risk for clinical AD.
Acknowledgements
We have no conflicts of interest to report. Research reported in this publication was supported by the Arizona Department of Health Services/Arizona Alzheimer’s Consortium (MDG) and the National Institute on Aging of the National Institutes of Health under Award Number R03AG060271 (MDG). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
Adding age, education, and normalized general semantic fluency (Animal z-scores) as covariates did not change the significant APOE group effect on autobiographical fluency, whether we used total correct or total correct plus intrusions as the dependent variable.
References
- Addis DR, Knapp K, Roberts RP, & Schacter DL (2012). Routes to the past: Neural substrates of direct and generative autobiographical memory retrieval. NeuroImage, 59(3), 2908–2922. 10.1016/j.neuroimage.2011.09.066 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Addis DR, & Tippett LJ (2004). Memory of myself: Autobiographical memory and identity in Alzheimer’s disease, Memory 12(1), 56–74. 10.1080/09658210244000423 [DOI] [PubMed] [Google Scholar]
- Benoit RG, & Schacter DL (2015). Specifying the core network supporting episodic simulation and episodic memory by activation likelihood estimation. Neuropsychologia, 75, 450–457. 10.1016/j.neuropsychologia.2015.06.034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benton AL (1969). Development of a multilingual aphasia battery: Progress and problems. Journal of the Neurological Sciences, 9, 39–48. [DOI] [PubMed] [Google Scholar]
- Berryhill ME, Phuong L, Picasso L, Cabeza R, & Olson IR (2007). Parietal lobe and episodic memory: Bilateral damage causes impaired free recall of autobiographical memory. Journal of Neuroscience, 27(52), 14415–14423. 10.1523/JNEUROSCI.4163-07.2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bertossi E, Tesini C, Cappelli A, & Ciaramelli E (2016). Ventromedial prefrontal damage causes a pervasive impairment of episodic memory and future thinking. Neuropsychologia, 90, 12–24. 10.1016/j.neuropsychologia.2016.01.034 [DOI] [PubMed] [Google Scholar]
- Bondi MW, Edmonds EC, Jak AJ, Clark LR, Delano-Wood L, McDonald CR, Nation DA, Libon DJ, Au R, Galasko D, Salmon DP (2014). Neuropsychological criteria for mild cognitive impairment improves diagnostic precision, biomarker associations, and progression rates. Journal of Alzheimer’s Disease, 42(1), 275–289. 10.3233/JAD-140276 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bondi MW, Jak AJ, Delano-Wood L, Jacobson MW, Delis DC, & Salmon DP (2008). Neuropsychological contributions to the early identification of Alzheimer’s disease. Neuropsychology Review, 18(1), 73–90. 10.1007/s11065-008-9054-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonnici HM, Cheke LG, Green DAE, Fitzgerald THMB, & Simons JS (2018). Specifying a causal role for angular gyrus in autobiographical memory. Journal of Neuroscience, 38(49), 10438–10443. 10.1523/JNEUROSCI.1239-18.2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bretsky P, Guralnik JM, Launer L, Albert M, & Seeman TE (2003). The role of APOE-ε4 in longitudinal cognitive decline: MacArthur studies of successful aging. Neurology, 60(7), 1077–1081. 10.1212/01.WNL.0000055875.26908.24 [DOI] [PubMed] [Google Scholar]
- Caselli RJ, Dueck AC, Locke DEC, Hoffman-Synder CR, Woodruff BK, Rapcsak SZ, & Reiman EM (2011). Longitudinal modeling of frontal cognition in APOE e4 homozygotes, heterozygotes, and noncarriers. Neurology, 76(16), 1383–1388. doi: 10.1212/WNL.0b013e3182167147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caselli RJ, Dueck AC, Osborne D, Sabbagh MN, Connor DJ, Ahern GL, … Reiman EM (2009). Longitudinal modeling of age-related memory decline and the APOE ε4 effect. New England Journal of Medicine, 361(3), 255–263. 10.1056/NEJMoa0809437 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caselli RJ, Langlais BT, Dueck AC, Chen Y, Su Y, Locke DEC, … Reiman EM (in press). Neuropsychological decline up to 20 years before incident mild cognitive impairment. Alzheimer’s and Dementia. 10.1016/j.jalz.2019.09.085 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caselli RJ, Reiman EM, Osborne D, Hentz JG, Baxter LC, Hernandez JL, & Alexander GG (2004). Longitudinal changes in cognition and behavior in asymptomatic carriers of the APOE e4 allele. Neurology, 62(11), 1990–1995. 10.1212/01.WNL.0000129533.26544.BF [DOI] [PubMed] [Google Scholar]
- Caselli RJ, & Reiman EM (2013). Characterizing the preclinical stages of Alzheimer’s disease and the prospect of presymptomatic intervention, Journal of Alzheimer’s Disease, 33(1), 405–416. 10.3233/JAD-2012-129026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen HY, Gilmore AW, Nelson SM, & McDermott KB (2017). Are there multiple kinds of episodic memory? An fMRI investigation comparing autobiographical and recognition memory tasks. Journal of Neuroscience, 37(10), 2764–2775. 10.1523/JNEUROSCI.1534-16.2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarke A, & Tyler LK (2014). Object-specific semantic coding in human perirhinal cortex. Journal of Neuroscience, 34(14), 4766–4775. doi: 10.1523/JNEUROSCI.2828-13.2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conway MA (2005). Memory and the self. Journal of Memory and Language, 53(4), 594–628. 10.1016/j.jml.2005.08.005 [DOI] [Google Scholar]
- Conway MA, & Rubin DC (1993). The structure of autobiographical memory. In Collins AE, Gathercole SE, Conway MA, & Morris PEM (Eds.), Theories of memory (pp. 103–137). Hove, UK: Lawrence Erlbaum. [Google Scholar]
- Corder EH, Saunders AM, Strittmatter WJ, Schmechel DE, Gaskell PC, Small GW, … Pericak-Vance MA (1993). Gene dose of apolipoprotein E type 4 allele and the risk of Alzheimer’s disease in late onset families. Science, 261(5123), 921–923. 10.1126/science.8346443 [DOI] [PubMed] [Google Scholar]
- Corneveaux JJ, Liang WS, Reiman EM, Webster JA, Myers AJ, Zismann VL, … Huentelman MJ (2010). Evidence for an association between KIBRA and late-onset Alzheimer’s disease. Neurobiology of Aging, 31(6), 901–909. 10.1016/j.neurobiolaging.2008.07.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davidson PSR, Anaki D, Ciaramelli E, Cohn M, Kim ASN, Murphy KJ, … Levine B (2008). Does lateral parietal cortex support episodic memory? Evidence from focal lesion patients. Neuropsychologia, 46(7), 1743–1755. 10.1016/j.neuropsychologia.2008.01.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delis DC, Kramer JH, Kaplan E, and Ober BA (2000). California Verbal Learning Test Second Edition. The Psychological Corporation. New York, NY. [Google Scholar]
- Dritschel BH, Williams JMG, Baddeley AD, & Nimmo-Smith I (1992). Autobiographical fluency: A method for the study of personal memory. Memory & Cognition, 20(2), 133–140. 10.3758/BF03197162 [DOI] [PubMed] [Google Scholar]
- Han DS, Nguyen CP, Stricker NH, & Nation DA (2017). Detectable Neuropsychological Differences in Early Preclinical Alzheimer’s Disease: A Meta-Analysis. Neuropsychology Review, 27(4), 305–325. 10.1007/s11065-017-9345-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edmonds EC, Delano-Wood L, Galasko DR, Salmon DP, & Bondi MW (2015). Subtle Cognitive Decline and Biomarker Staging in Preclinical Alzheimer’s Disease. Journal of Alzheimer’s Disease, 47(1), 231–242. 10.3233/JAD-150128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eichenbaum H, Yonelinas AP, & Ranganath C (2007). The Medial Temporal Lobe and Recognition Memory. Annual Review of Neuroscience, 30(1), 123–152. 10.1146/annurev.neuro.30.051606.094328 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faul F, Erdfelder E, Lang AG, & Buchner A (2007). G*Power 3: A flexible statistical power analysis program for the social, behavioral, and biomedical sciences. Behavior Research Methods, 39(2), 175–191. 10.3758/BF03193146 [DOI] [PubMed] [Google Scholar]
- Goodglass H, Kaplan E, & Barresi B (2001). Boston diagnostic aphasia examination (3rd ed.). Philadelphia: Lippincott Williams & Wilkins. [Google Scholar]
- Greenberg DL, Keane MM, Ryan L, & Verfaellie M (2009). Impaired category fluency in medial temporal lobe amnesia: The role of episodic memory. Journal of Neuroscience, 29(35), 10900–10908. 10.1523/JNEUROSCI.1202-09.2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grilli MD, & Verfaellie M (2016). Experience-near but not experience-far autobiographical facts depend on the medial temporal lobe for retrieval: Evidence from amnesia. Neuropsychologia, 81, 180–185. 10.1016/j.neuropsychologia.2015.12.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grilli MD, & Verfaellie M (2014). Personal semantic memory: Insights from neuropsychological research on amnesia. Neuropsychologia, 61(1), 56–64. 10.1016/j.neuropsychologia.2014.06.012 [DOI] [PubMed] [Google Scholar]
- Grilli MD, Wank AA, Bercel JJ, & Ryan L (2018). Evidence for Reduced Autobiographical Memory Episodic Specificity in Cognitively Normal Middle-Aged and Older Individuals at Increased Risk for Alzheimer’s Disease Dementia. Journal of the International Neuropsychological Society, 24(10), 1073–1083. 10.1017/S1355617718000577 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heaton RK, Miller SW, Taylor MJ, & Grant I (2004). Revised comprehensive norms for an expanded Halstead-Reitan Battery: Demographically adjusted neuropsychological norms for African American and Caucasian adults. Lutz, FL: Psychological Assessment Resources. [Google Scholar]
- Jack CR, Knopman DS, Jagust WJ, Petersen RC, Weiner MW, Aisen PS, … Trojanowski JQ (2013). Tracking pathophysiological processes in Alzheimer’s disease: An updated hypothetical model of dynamic biomarkers. The Lancet Neurology, 12(2), 207–216. 10.1016/S1474-4422(12)70291-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jack CR, Wiste HJ, Weigand SD, Knopman DS, Vemuri P, Mielke MM, … Petersen RC (2015). Age, sex, and APOE ϵ4 effects on memory, brain structure, and β-Amyloid across the adult life Span. JAMA Neurology, 72(5), 511–519. 10.1001/jamaneurol.2014.4821 [DOI] [PMC free article] [PubMed] [Google Scholar]
- JASP Team (2020). JASP (Version 0.12.2)[Computer software]
- Levine B, Svoboda E, Hay JF, Winocur G, & Moscovitch M (2002). Aging and autobiographical memory: Dissociating episodic from semantic retrieval. Psychology and Aging, 17(4), 677–689. 10.1037/0882-7974.17.4.677 [DOI] [PubMed] [Google Scholar]
- Masters CL, Bateman R, Blennow K, Rowe CC, Sperling RA, & Cummings JL (2015). Alzheimer’s Disease: Nat Rev Dis Primers, 1, 1–18. 10.1038/nrdp.2015.56 [DOI] [PubMed] [Google Scholar]
- Monge ZA, Wing EA, Stokes J, & Cabeza R (2018). Search and recovery of autobiographical and laboratory memories: Shared and distinct neural components. Neuropsychologia, 110, 44–54. 10.1016/j.neuropsychologia.2017.07.030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montandon ML, Herrmann FR, Garibotto V, Rodriguez C, Haller S, & Giannakopoulos P (2020). Determinants of mesial temporal lobe volume loss in older individuals with preserved cognition: a longitudinal PET amyloid study. Neurobiology of Aging, 87, 108–114. 10.1016/j.neurobiolaging.2019.12.002 [DOI] [PubMed] [Google Scholar]
- Moscovitch M, Cabeza R, Winocur G, & Nadel L (2016). Episodic memory and beyond: The hippocampus and neocortex in transformation. Annual Review of Psychology, 67(1), 105–134. 10.1146/annurev-psych-113011-143733 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osuna J, Thomas K, Edmonds E, Bangen K, Weigand A, Wong C, … Bondi M (2019). Subtle cognitive decline predicts progression to mild cognitive impairment above and beyond Alzheimer’s disease risk factors. Archives of Clinical Neuropsychology, 34(6), 846, 10.1093/arclin/acz035.14 [DOI] [Google Scholar]
- Papp KV, Buckley R, Mormino E, Maruff P, Villemagne VL, Masters CL, … Amariglio RE (in press). Clinical meaningfulness of subtle cognitive decline on longitudinal testing in preclinical AD. Alzheimer’s and Dementia. 10.1016/j.jalz.2019.09.074 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parra MA, Della Sala S, Abrahams S, Logie RH, Méndez LG, & Lopera F (2011). Specific deficit of colour-colour short-term memory binding in sporadic and familial Alzheimer’s disease. Neuropsychologia, 49(7), 1943–1952. 10.1016/j.neuropsychologia.2011.03.022 [DOI] [PubMed] [Google Scholar]
- Philippi CL, Tranel D, Duff M, & Rudrauf D (2013). Damage to the default mode network disrupts autobiographical memory retrieval. Social Cognitive and Affective Neuroscience, 10(3), 318–326. 10.1093/scan/nsu070 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piolino P, Coste C, Martinelli P, Macé AL, Quinette P, Guillery-Girard B, & Belleville S (2010). Reduced specificity of autobiographical memory and aging: Do the executive and feature binding functions of working memory have a role? Neuropsychologia, 48(2), 429–440. 10.1016/j.neuropsychologia.2009.09.035 [DOI] [PubMed] [Google Scholar]
- R Core Team (2019). R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria, URL https://www.R-project.org/. [Google Scholar]
- Radloff LS (1977). The CES-D scale: A self-report depression scale for research in the general population. Applied Psychological Measurements, 1, 385–401. [Google Scholar]
- Ranganath C, & Ritchey M (2012). Two cortical systems for memory-guided behaviour. Nature Reviews Neuroscience, 13(10), 713–726. 10.1038/nrn3338 [DOI] [PubMed] [Google Scholar]
- Rathbone CJ, Moulin CJA, & Conway MA (2008). Self-centered memories: The reminiscence bump and the self. Memory and Cognition, 36(8), 1403–1414. 10.3758/MC.36.8.1403 [DOI] [PubMed] [Google Scholar]
- Reagh ZM, & Ranganath C (2018). What does the functional organization of cortico-hippocampal networks tell us about the functional organization of memory? Neuroscience Letters, 680, 69–76. 10.1016/j.neulet.2018.04.050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reiman EM, Chen K, Alexander GE, Caselli RJ, Bandy D, Osborne D, … Hardy J (2005). Correlations between apolipoprotein E ε4 gene dose and brain-imaging measurements of regional hypometabolism. Proceedings of the National Academy of Sciences of the United States of America, 102(23), 8299–8302. 10.1073/pnas.0500579102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reitan RM, & Wolfson D (1993). The Haldstead-Reitan Neuropsychological Test Battery: Theory and clinical interpretation. Tucson: Neuropsychology Press. [Google Scholar]
- Renoult L, Davidson PSR, Palombo DJ, Moscovitch M, & Levine B (2012). Personal semantics: At the crossroads of semantic and episodic memory, Trends in Cognitive Sciences, 16(11), 550–558. 10.1016/j.tics.2012.09.003 [DOI] [PubMed] [Google Scholar]
- Rentz DM, Parra Rodriguez MA, Amariglio R, Stern Y, Sperling R, & Ferris S (2013). Promising developments in neuropsychological approaches for the detection of preclinical Alzheimer’s disease: A selective review. Alzheimer’s Research and Therapy, 5(6): 58. 10.1186/alzrt222 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rey A (1941). L’examen psychologique dans les cas d’encephalopathie traumatique. Archives de Psychologie, 28, 286–340. [Google Scholar]
- Rosenbaum RS, Moscovitch M, Foster JK, Schnyer DM, Gao F, Kovacevic N, … Levine B (2008). Patterns of autobiographical memory loss in medial-temporal lobe amnesic patients. Journal of Cognitive Neuroscience, 20(8), 1490–1506. 10.1162/jocn.2008.20105 [DOI] [PubMed] [Google Scholar]
- Rubin DC, Wetzler SE, & Nebes RD (1986). Autobiographical memory across the adult lifespan. In Rubin DC (Ed.), Autobiographical memory (pp. 202–221). Cambridge, UK: Cambridge University Press. [Google Scholar]
- Rugg MD, & Vilberg KL (2013). Brain networks underlying episodic memory retrieval. Current Opinion in Neurobiology, 23(2), 255–260. 10.1016/J.CONB.2012.11.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryan L, Cox C, Hayes SM, & Nadel L (2008). Hippocampal activation during episodic and semantic memory retrieval: Comparing category production and category cued recall. Neuropsychologia, 46(8), 2109–2121. 10.1016/j.neuropsychologia.2008.02.030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryan L, Walther K, Bendlin BB, Lue LF, Walker DG, & Glisky EL (2011). Age-related differences in white matter integrity and cognitive function are related to APOE status. NeuroImage, 54(2), 1565–1577. 10.1016/j.neuroimage.2010.08.052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheldon S, McAndrews MP, Pruessner J, & Moscovitch M (2016). Dissociating patterns of anterior and posterior hippocampal activity and connectivity during distinct forms of category fluency. Neuropsychologia, 90, 148–158. 10.1016/j.neuropsychologia.2016.06.028 [DOI] [PubMed] [Google Scholar]
- Sheldon S, & Moscovitch M (2012). The nature and time-course of medial temporal lobe contributions to semantic retrieval: An fMRI study on verbal fluency. Hippocampus, 22(6), 1451–1466. 10.1002/hipo.20985 [DOI] [PubMed] [Google Scholar]
- Sheline YI, Morris JC, Snyder AZ, Price JL, Yan Z, D’Angelo G, … Mintun MA (2010). APOE4 allele disrupts resting state fMRI connectivity in the absence of amyloid plaques or decreased CSF Aβ42. Journal of Neuroscience, 30(50), 17035–17040. 10.1523/JNEUROSCI.3987-10.2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheline YI, & Raichle ME (2013). Resting state functional connectivity in preclinical Alzheimer’s disease. Biol Psychiatry, 74(5), 340–347. doi: 10.1016/j.biopsych.2012.11.028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sperling RA, Aisen PS, Beckett LA, Bennett DA, Craft S, Fagan AM, … Phelps CH (2011). Toward defining the preclinical stages of Alzheimer’s disease: Recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimer’s & Dementia, 7(3), 280–292. 10.1016/J.JALZ.2011.03.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thakral PP, Madore KP, & Schacter DL (2017). A Role for the Left Angular Gyrus in Episodic Simulation and Memory. The Journal of Neuroscience: The Official Journal of the Society for Neuroscience, 37(34), 8142–8149. 10.1523/JNEUROSCI.1319-17.2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomsen DK, Pillemer DB, & Ivcevic Z (2011). Life story chapters, specific memories and the reminiscence bump. Memory, 19(3), 267–279. 10.1080/09658211.2011.558513 [DOI] [PubMed] [Google Scholar]
- Tomadesso C, Gonneaud J, Egret S, Perrotin A, Pélerin A, de Flores R, … La Joie R (2019). Is there a specific memory signature associated with Aβ-PET positivity in patients with amnestic mild cognitive impairment? Neurobiology of Aging, 77, 94–103. 10.1016/j.neurobiolaging.2019.01.017 [DOI] [PubMed] [Google Scholar]
- Tranel D (2009). The left temporal pole is important for retrieving words for unique concrete entities. Aphasiology, 23(7), 867. doi: 10.1080/02687030802586498 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vos SJB, Xiong C, Visser PJ, Jasielec MS, Hassenstab J, Grant EA, … Fagan AM (2013). Preclinical Alzheimer’s disease and its outcome: A longitudinal cohort study. The Lancet Neurology, 12(10), 957–965. 10.1016/S1474-4422(13)70194-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wechsler D (2008) WAIS-IV Manual. Psychological Corporation. New York, NY. [Google Scholar]
- Wickham H(2016). ggplot2: Elegant graphics for data analysis. Springer-Verlag; New York. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data are deposited here: https://osf.io/4c8dr/?view_only=4c3f80d5c40f46a08eb2fb2ab0e8110f


