Abstract
Recent evidences have claimed that circular RNAs are deregulated in docetaxel (DTX) resistance in malignant tumors, including non-small-cell lung cancer (NSCLC). Hsa_circ_0014130 (circ_0014130) is a new biomarker in NSCLC. However, its role in DTX-resistant NSCLC remained to be annotated. In this study, real-time PCR was used to measure expression of circ_0014130, and circ_0014130 was upregulated in NSCLC tumors and DTX-resistant NSCLC cells (NCI-H1299/DTX and A549/DTX). MTT assay analyzed the half inhibitory concentration (IC50) of DTX, and it was lowered by circ_0014130 interference in DTX-resistant NSCLC cells. Moreover, colony formation assay, flow cytometry, transwell assays, and xenograft tumor model revealed that silencing circ_0014130 facilitated apoptosis rate of DTX-resistant NSCLC cells, suppressed the colony formation, migration and invasion, and retarded xenograft tumor growth in nude mice. Dual-luciferase reporter assay and RNA immunoprecipitation confirmed that circ_0014130 was one competing endogenous RNA (ceRNA) for miRNA (miR)-545-3p, and circ_0014130 modulated expression of yes-associated protein 1 (YAP1), a target gene for miR-545-3p. YAP1 upregulation and miR-545-3p downregulation were allied with circ_0014130 upregulation in NSCLC tumors and DTX-resistant NSCLC cells. Functionally, downregulating miR-545-3p could abate the effects of circ_0014130 knockdown in DTX-resistant NSCLC cells in vitro, whereas its overexpression exerted similar effects of circ_0014130 knockdown. Either, restoring YAP1 partially reversed miR-545-3p effects in DTX-resistant NSCLC cells. Collectively, there might be a novel circ_0014130-miR-545-3p-YAP1 ceRNA pathway in regulation of chemoresistance and malignant behaviors of DTX-resistant NSCLC cells, suggesting a potential therapeutic approach for DTX resistance.
Keywords: circ_0014130, miR-545-3p, YAP1, DTX resistance, NSCLC
Introduction
Docetaxel (DTX) is a derivative of taxanes that are frequently prescribed to be highly effective antineoplastic drug applied in human cancers including lung cancer (Kumar et al. 2016; Joshi et al. 2014). Moreover, DTX chemotherapy is especially effective to prevent malignant progression of non-small-cell lung cancer (NSCLC) and prolong survival of patients with advanced NSCLC (Fehrenbacher et al. 2018; Reck et al. 2018; Vokes et al. 2018). NSCLC is the most common subtype of lung cancer, ranking the first for male patients and the second for female patients in China (Chen et al. 2016). According to one investigation, 67.9% of NSCLC patients are diagnosed at advanced-stage (IIIB–IV) (Chen et al. 2013), and chemotherapy is practicable for those tumors. However, drug resistance is the main obstacle to exert curative effect to the maximum extent (Zhang et al. 2014). Therefore, it is imperative to elucidate the mechanism underlying chemoresistance and malignancy of DTX-resistant NSCLC.
Circular RNAs (circRNAs) are essentially noncoding RNAs with covalently closed loop structures (Drula et al. 2020), and circRNAs have been emerged as a novel focus in human cancer research (Jeyaraman et al. 2019). It is assured that circRNAs are characterized by the loss of 5′ cap and 3′ poly (A) tail, whereas the both are classic features of messenger RNAs (mRNAs). Very recently, there are evidences clarifying an involvement between circRNAs dysregulation and DTX resistance in malignant tumors including NSCLC (Hong et al. 2020; Shen et al. 2020; Yu et al. 2019). Moreover, cirRNAs have been identified to play a prominent role in tumor progression, dissemination, and drug response in lung cancer, and thereby they are considered as pivotal biomarkers for the diagnosis, prognosis and treatment (Di et al. 2019; Li et al. 2019). The hsa_circ_0014130 (also known as circPIP5K1A_019, circPIP5K1A) is a circRNA in 724 nucleotides back-spliced from exons 7-11 of PIP5K1A gene. Although circ_0014130 has been identified as a new biomarker in NSCLC (Zhang et al. 2018), the role of circ_0014130 in chemoresistance of NSCLC remains to be annotated.
Furthermore, circRNAs functioning as endogenous sponges for microRNAs (miRNAs) has been well-documented (Cheng et al. 2015), and circ_0014130 has been declared to sponge miRNAs in regulating the tumor development of NSCLC (Zhang et al. 2018). MiRNA (miR)-545-3p is a broad-spectrum tumor suppressor including in lung cancer (Jia et al. 2018; Yuan et al. 2019; Du et al. 2014). However, expression and function of miR-545-3p have not been expounded in DTX resistance, and we further wondered whether there was a crosslink between circ_0014130 and miR-545-3p in NSCLC.
In this study, we intended to investigate the expression and role of circ_0014130 and miR-545-3p in DTX-resistant NSCLC cells. Functionally, chemoresistance, colony formation, apoptosis, migration, and invasion in vitro and tumor growth in vivo were examined. Mechanically, the interaction between circ_0014130 and miR-545-3p was validated, and yes-associated protein 1 (YAP1), a master regulator in resistance to anti-cancer therapies (Kim and Kim 2017), was further validated as one target gene of miR-545-3p. These results suggested that circ_0014130, miR-545-3p and YAP1 might be novel targets in the treatment of DTX-resistant NSCLC.
Materials and methods
NSCLC tumor tissues and patients
Forty-three patients with NSCLC were recruited in Hebei General Hospital, and 43 pairs of tumor tissue and adjacent normal tissue were excised from these patients. The tumors were diagnosed by histopathological examination, and the exclusion criteria was NSCLC tumors received any anti-cancer therapy before. All tissue samples were restored in liquid nitrogen. The study protocols were approved by the Ethics Committee of Hebei General Hospital. Before clinical samples collection, written consent was received from each patient.
DTX-resistant NSCLC cells
Human NSCLC cell lines NCI-H1299 (Code. 0185) and A549 (Code. 0033) were from BCRJ (Duque de Caxias, Rio de Janeiro, Brazil). NCI-H1299 cells were cultured in RPMI-1640 medium (GIBCO-BRL, Grand Island, NY, USA) containing 2 mM L-glutamine, and A549 cells were cultivated in DMEM medium (GIBCO-BRL) supplemented with 4 mM L-glutamine. DTX-resistant NCI-H1299 and A549 (NCI-H1299/DTX and A549/DTX) cells were established depending on NCI-H1299 and A549 cells through continuous exposure in stepwise increased concentrations of DTX (Sigma-Aldrich, Milwaukee, WI, USA). Finally, NCI-H1299/DTX and A549/DTX cells were preserved in culture medium containing 10 μg/L of DTX to maintain DTX resistance. All cells were incubated in 10% of fetal bovine serum (GIBCO-BRL) in a humidified chamber with 5% CO2 and 37 °C.
Real-time polymerase chain reaction (real-time PCR)
Total RNA of tissues and cells was isolated using Trizol (Invitrogen, Carlsbad, CA, USA), and RNA samples of NCI-H1299/DTX and A549/DTX cells were treated with 3 U/μg of RNase R (Sigma-Aldrich) for 20 min at 37 °C. Then, RNA samples were subjected to reverse transcription using PrimeScript RT Reagent kit (Takara, Dalian, China) prior to real-time PCR with Universal SYBR Premix DimerEraser Kit (Takara). The semi-quantitation of real-time PCR depended on special primer pairs and ABI 7900 (Applied Biosystems, Carlsbad, CA, USA). The special sequence of primers was circ_0014130: 5′-TTCCCTAACCTCAACCAGAACC-3′ (forward) and 5′-AGGCTTCTCTCGCTCTTTCT-3′ (reverse), miR-545-3p: 5′-TCGGCAGGTCAGCAAACATTT-3′ (forward) and 5′-CAGTGCGTGTCGTGGAGT-3′ (reverse), and YAP1: 5′-CCCTCGTTTTGCCATGAACC-3′ (forward) and 5′-GTTGCTGCTGGTTGGAGTTG-3′ (reverse). The raw data of cycle threshold (Ct) was obtained from ABI 7900 system, and ΔCt was acquired by subtracting Ct of glyceraldehyde-phosphate dehydrogenase (GAPDH) from Ct of circ_0014130 or YAP1, and subtracting Ct of U6 small nuclear RNA (RNU6) from Ct of miR-545-3p; ΔΔCt was calculated by subtracting ΔCt of the control group from ΔCt of the experimental group. The relative gene expression was presented as fold change using the 2−ΔΔCt equation.
Cell transfection
The siRNA and shRNA pools for circ_0014130 (si/sh-circ_0014130), miR-545-3p mimic and miR-545-3p inhibitor (anti-miR-545-3p) were synthesized by GenePharma (Shanghai, China), as well as the negative controls. YAP1 overexpression vector (pcDNA-YAP1, YAP1) and circ_0014130 overexpression vector (pcDNA-circ_0014130, circ_0014130) was established through inserting the full-length of YAP1 or circ_0014130 into pcDNA3.1 vector (Thermo Fisher Scientific, Waltham, MA, USA). NCI-H1299/DTX and A549/DTX cells were transiently transfected with these oligonucleotides (40 nM) or vectors (1.5 μg) using Lipofectamine 3000 (Invitrogen). For stable transfection, shRNAs were inserted into the shRNA expression vector pGPU6/Neo (GenePharma) after annealing. The sequence of special oligonucleotides was si-circ_0014130: 5′-AAUUUAGGCAGCAAAGUCCGA-3′ (guide) and 5′-GGACUUUGCUGCCUAAAUUCU-3′ (passenger), miR-545-3p mimic: 5′-UCAGCAAACAUUUAUUGUGUGC-3′, anti-miR-545-3p: 5′-mGmCmAmCmAmCmAmAmUmAmAmAmUmGmUmUmUmGmCmUmGmA-3′, sh-circ_0014130: 5′-TTGTTCATCACCACAATCC-3′ (guide) and 5′-GGATTGTGGTGATGAACAA-3′ (passenger).
MTT assay
After transfection for 30 h, NCI-H1299/DTX and A549/DTX cells were transferred in 96-well plate at a cell density of 5 000 cells per well, followed with treatment of DTX (20, 40, 60, 80, and 100 μg/L) for 48 h. Next, 2 mg/mL of MTT reagent (Sigma-Aldrich) was added into each well for another 4 h. Finally, the formazan generated in living cells was resolved in 100 μL of dimethylsulfoxide, followed with the determination of optical density at 570 nm on microplate reader. Relative cell viability (%) was presented with normalization to control group (without DTX treatment), and cell viability curve was drawn to determine the half inhibitory concentration (IC50) of DTX.
Colony formation assay
After transfection for 30 h, NCI-H1299/DTX and A549/DTX cells were re-seeded in 24-well plate at a cell density of 500 cells per well. The cells were incubated in complete culture medium for another 15 days; then colonies formed were fixed with 4% paraformaldehyde for 15 min, and stained with 0.1% crystal violet for 30 min. The colonies were photographed and counted under microscope. Relative colony formation (%) was presented with normalization to corresponding control group (100%).
Flow cytometry
After transfection for 30 h, NCI-H1299/DTX and A549/DTX cells were harvested by Trypsin without EDTA (Axygen, Hangzhou, China). The cells were stained with Annexin V-fluorescein isothiocyanate (FITC) Apoptosis Detection Kit (Beyotime, Shanghai, China) and sorted by flow cytometry. Apoptotic cells (%) were analyzed by accuri C6 flow cytometer (BD Biosciences, San Jose, CA, USA).
Transwell assay
After transfection for 30 h, NCI-H1299/DTX and A549/DTX cells were harvested in culture medium without serum. Transwell chamber (8 μm; Corning, New York, NY, USA) for 24-well plate was utilized to measure transwell migration and invasion. For migration, a total of 1 × 105 cells were inoculated into the upper chamber, and the complement culture medium (containing 10% fetal bovine serum) was loaded in the lower chamber. This system was incubated at 5% CO2 in 37 °C for another 48 h. Then, the migrated cells into the lower surface of chamber were fixed with 4% paraformaldehyde for 15 min, and stained with 0.1% crystal violet for 30 min. The migrated cells were photographed and counted under microscope at 100 ×, and the migratory cell number was calculated according to 5 random fields. For invasion, the chamber was pre-coated with matrigel, and a total of 5 × 105 cells were inoculated into the upper chamber; the other procedures were the same to migration assay.
Dual-luciferase reporter assay and RNA immunoprecipitation (RIP)
The sequences of circ_0014130 and the 3′ untranslated region of YAP1 (YAP1 3′UTR) were cloned into pmiR-REPORTTM vectors (RiboBio, Guangzhou, China), thus generating wild-type reporter vectors circ_0014130-wt and YAP1-wt, respectively. The intact binding sites of miR-545-3p on circ_0014130 and YAP1 3′UTR were mutated using Q5 Site-Directed Mutagenesis Kit (New England Biolabs, Ipswich, MA, USA), and mutant-type reporter vectors circ_0014130-mut and YAP1-mut were established, respectively. NCI-H1299/DTX and A549/DTX cells were co-transfected with reporter vectors and miR-545-3p mimic or its negative control NC for another 48 h, followed by the determination of luciferase activities on Dual-Glo Luciferase Assay System (Promega, Madison, WI, USA).
RIP assay was conducted using Magna RIP RNA-Binding Protein Immunoprecipitation Kit (Millipore, Billerica, MA, USA) according to the manufacturer’s instructions. Briefly, NCI-H1299/DTX and A549/DTX cells were lysed in RIP buffer, and then incubated with magnetic beads coupled with anti-Ago2 or anti-IgG. The total RNA in immunoprecipitated contents was extracted by Trizol prior to real-time PCR analyzing the enrichment of circ_0014130, miR-545-3p and YAP1 mRNA in input and RIPs mediated by anti-Ago2 or anti-IgG.
Western blotting
The total proteins in tissues and cells were extracted in RIPA buffer (Beyotime) in the presence of protease inhibitors (Roche, Basel, Switzerland). After concentration determination using Pierce BCA protein assay kit (Thermo Fisher Scientific), protein samples (20 μg) were subjected to normal western blotting procedures. The antibodies including anti-YAP1 (sc-376830, 1:500), anti-GAPDH (sc-365062, 1:1000), anti-proliferating cell nuclear antigen (anti-PCNA; sc-25280, 1:2500), and goat anti-mouse IgG-HRP (sc-2005, 1:10,000) were from Santa cruz (Shanghai, China). Ultimately, protein blots were visualized using enhanced-chemiluminescence reagent (Millipore), and band densities were analyzed using Image Lab software (Bio-Rad, Hercules, CA, USA).
Xenograft tumor model
Male athymic nude mice (BALB/c-nu/nu, 4–6 weeks old) were purchased from Nanjing Biomedical Research Institute of Nanjing University (Nanjing, China). The xenograft mice were divided into four groups (n = 4): sh-NC, sh-circ_0014130, sh-NC + DTX, and sh-circ_0014130 + DTX. A549/DTX cells stably expressed sh-circ_0014130 or sh-NC were subcutaneously injected at a density of 5 × 106 cells per mouse. After cell transplantation for 6 days, DTX was intraperitoneally administered into the latter two groups of mice at the dose of 5 mg DTX/kg of mice, and DTX administration was performed once every 3 days for eight times. And, the former two groups of mice were intraperitoneally injected with equal volume of saline. The long (a) and short diameters (b) of tumors were recorded from the 6th day after transplantation, and every time before DTX treatment. The tumor volume was calculated using 0.5 × a × b × b equation, and tumor weight was recorded after tumor excision from mice. This animal experiment was approved by the Institutional Animal Care and Use Committee of Hebei General Hospital.
Statistical analysis
Statistical data was presented as the mean ± standard deviation from three independent experiments. The differences were analyzed on GraphPad Prism 7 software (GraphPad, San Diego, CA, USA), and intergroup differences were confirmed by the Student′s t-test or analysis of variance. Pearson′s correlation coefficient test determined the relationship among circ_0014130, miR-545-3p and YAP1 in NSCLC tumor tissues.
Results
Expression of circ_0014130 was upregulated in DTX-resistant NSCLC cells
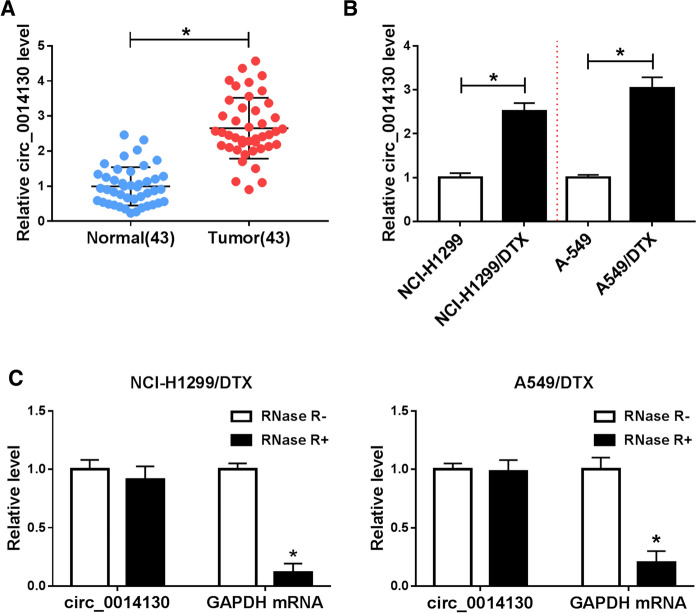
Among these enrolled NSCLC patients (n = 43), expression level of circ_0014130 was apparently higher in tumor tissues than paracancerous normal tissues (Fig. 1a). Moreover, its expression was upregulated in NCI-H1299/DTX and A549/DTX cells, paralleled with the parental cells (Fig. 1b). In addition, circ_0014130 level was resistant to RNase R treatment in NCI-H1299/DTX and A549/DTX cells, which was not similar to GAPDH mRNA (Fig. 1c). These data demonstrated a stable upregulation of circ_0014130 in NSCLC tumors and DTX-resistant NSCLC cells.
Fig. 1.
Expression of hsa_circ_0014130 (circ_0014130) in non-small-cell lung cancer (NSCLC) tissues and docetaxel (DTX)-resistant cells. a Real-time PCR detected circ_0014130 expression level in NSCLC tumor tissues (Tumor; n = 43) and normal tissues (Normal; n = 43). b Real-time PCR detected circ_0014130 level in DTX-resistant NCI-H1299 and A549 (NCI-H1299/DTX and A549/DTX) cells, and the parental cells. c Real-time PCR compared the expression levels of circ_0014130 and the message RNA of glyceraldehyde-phosphate dehydrogenase (GAPDH mRNA) in NCI-H1299/DTX and A549/DTX cells with and without RNase R treatment (RNase R ±). Data of each panel were from three independent experiments, and *P < 0.05
Silencing of circ_0014130 suppressed chemoresistance, colony formation, migration and invasion of DTX-resistant NSCLC cells in vitro
To investigate the role of circ_0014130 in DTX resistance of NSCLC cells, NCI-H1299/DTX and A549/DTX cells were transfected with si-circ_0014130. Real-time PCR data showed a distinctive decrease of circ_0014130 level in si-circ_0014130-administrated NCI-H1299/DTX and A549/DTX cells than the control cells (Fig. 2a). According to MTT assay, cell viability of NCI-H1299/DTX and A549/DTX cells was gradually inhibited by DTX with the increase of concentration (Fig. 2b, c), and IC50 of DTX was lowered by si-circ_0014130 transfection in NCI-H1299/DTX cells (from 73 to 35 μg/L) and A549/DTX cells (from 78 to 37 μg/L). This prompted a suppressive role of circ_0014130 silencing in chemoresistance of DTX-resistant NSCLC cells. Subsequently, cell behaviors of these resistant cells were further explored. Colony formation assay indicated that relative colony formation of NCI-H1299/DTX and A549/DTX cells was dramatically descended due to circ_0014130 knockdown (Fig. 2d), which was accompanied with ascended rate of apoptotic cells (Fig. 2e). Interfering circ_0014130 inhibited transwell migratory cell number and invasive cell number in NCI-H1299/DTX and A549/DTX cells (Fig. 2f, g). These results showed that blocking circ_0014130 suppressed DTX resistance, colony formation, migration and invasion in DTX-resistant NSCLC cells in vitro.
Fig. 2.
Effect of circ_0014130 silencing in DTX-resistant NSCLC cells in vitro. a–g NCI-H1299/DTX and A549/DTX cells were exogenously administrated with siRNA against circ_0014130 (si-circ_0014130) or the negative control si-NC. a Real-time PCR measured circ_0014130 level after transfection. b, c MTT assay evaluated cell viability of transfected cells after DTX (0–100 μg/L) treatment for 48 h, and the half inhibitory concentration (IC50) of DTX was presented. d Colony formation assay examined relative colony formation (%) after transfection. e Flow cytometry measured the percentage of apoptotic cells after transfection. f, g Transwell assays detected numbers of migratory cells and invasive cells after transfection. Data of each panel were from three independent experiments, and *P < 0.05
MiR-545-3p was a target of circ_0014130 in NSCLC cells
Bioinformatic analysis predicted the existence of putative binding sites between circ_0014130 and miR-545-3p. Therefore, the expression of miR-545-3p in NSCLC was validated using real-time PCR, and miR-545-3p was downregulated in NSCLC tumor tissues (n = 43) and DTX-resistant NCI-H1299/DTX and A549/DTX cells (Fig. 3a, b). Additionally, miR-545-3p expression in NSCLC tumors was mildly correlated with circ_0014130 according to Pearson’s correlation coefficient (r = − 0.5506 and P = 0.0001; Fig. 3c). As shown in Fig. 3d, the putative miR-545-3p-binding sequence UUUGCUG in circ_0014130-wt was site-directed mutated into AAACGAC according to CircInteractome tool (https://circinteractome/hsa_circ_0014130-hsa-mir-545-3p). Afterwards, dual-luciferase reporter assay manifested that relative luciferase activity of circ_0014130-wt was markedly declined in NCI-H1299/DTX and A549/DTX cells with transfection of miR-545-3p mimic, compared to NC mimic transfection (Fig. 3e). Furthermore, RIP assay testified a simultaneous enrichment of circ_0014130 and miR-545-3p in immunoprecipitated RNAs mediated by anti-Ago2 than anti-IgG-mediated RIP (Fig. 3f). These outcomes indicated a direct interaction between circ_0014130 and miR-545-3p in NCI-H1299/DTX and A549/DTX cells. Besides, miR-545-3p was negatively regulated by circ_0014130 in NCI-H1299/DTX and A549/DTX cells, as evidenced by low level of miR-545-3p in circ_0014130-overexpressed cells, and high level of miR-545-3p in circ_0014130-silenced cells (Fig. 3g).
Fig. 3.
The interaction of circ_0014130 and miR-545-3p. a Real-time PCR detected miR-545-3p expression level in NSCLC tumors (n = 43) and normal tissues (n = 43). b Real-time PCR detected miR-545-3p level in NCI-H1299/DTX and A549/DTX cells, and the parental cells. c Pearson’s correlation coefficient assay analyzed the correlation between circ_0014130 and miR-545-3p levels in 43 NSCLC tumor tissues. d CircInteractome computational tool predicted the binding site between wild-type of circ_0014130 (circ_0014130-wt) and miR-545-3p. e Dual-luciferase reporter assay measured luciferase activity of circ_0014130-wt and its mutant (circ_0014130-mut) in NCI-H1299/DTX and A549/DTX cells administrated with miR-545-3p mimic (miR-545-3p) or its negative control (NC). f RNA immunoprecipitation (RIP) assessed the enrichment of circ_0014130 and miR-545-3p in immunoprecipitated RNAs using Ago2 antibody (Anti-Ago2) or IgG antibody (Anti-IgG). g Real-time PCR measured miR-545-3p expression level in NCI-H1299/DTX and A549/DTX cells administrated with circ_0014130 overexpression vector (circ_0014130), si-circ_0014130, or the controls (circ-NC and si-NC). Data of each panel were from three independent experiments, and *P < 0.05
The deletion of miR-545-3p attenuated the effects of circ_0014130 silencing in DTX-resistant NSCLC cells in vitro
To further discover the part of miR-545-3p in suppressive role of circ_0014130 in DTX resistance and malignant progression of DTX-resistant NSCLC cells, circ_0014130-silenced NCI-H1299/DTX and A549/DTX cells were exogenously introduced with anti-miR-545-3p. Real-time PCR data depicted that the upregulation of miR-545-3p induced by si-circ_0014130 transfection was attenuated due to anti-miR-545-3p introduction (Fig. 4a). What’s more, miR-545-3p ablation via transfection rescued cell viability and IC50 value of DTX in circ_0014130-silenced NCI-H1299/DTX and A549/DTX cells under DTX treatment (Fig. 4b, c). Relative colony formation was decreased and apoptotic cell rate was descended by blocking circ_0014130, and these impacts were diminished by inhibiting miR-545-3p (Fig. 4d, e). Transwell migration and invasion of NCI-H1299/DTX and A549/DTX cells were inhibited by si-circ_0014130 transfection, which was abolished by anti-miR-545-3p addition (Fig. 4f, g). These data demonstrated that miR-545-3p downregulation could reverse the effects of circ_0014130 knockdown on DTX resistance, colony formation, apoptosis, migration and invasion in DTX-resistant NSCLC cells, hinting a circ_0014130-miR-545-3p axis.
Fig. 4.
The inhibitory effect of miR-545-3p deletion on circ_0014130 silencing in DTX-resistant NSCLC cells in vitro. a–g NCI-H1299/DTX and A549/DTX cells were exogenously administrated with si-NC, si-circ_0014130 alone or combined with miR-545-3p inhibitor (anti-miR-545-3p) or its negative control anti-NC. a Real-time PCR measured miR-545-3p level after transfection. b, c MTT assay evaluated IC50 of DTX in transfected cells. d Colony formation assay examined relative colony formation (%) after transfection. e Flow cytometry measured the percentage of apoptotic cells after transfection. f, g Transwell assays detected numbers of migratory cells and invasive cells after transfection. Data of each panel were from three independent experiments, and *P < 0.05
MiR-545-3p sponged by circ_0014130 directly regulated YAP1 expression
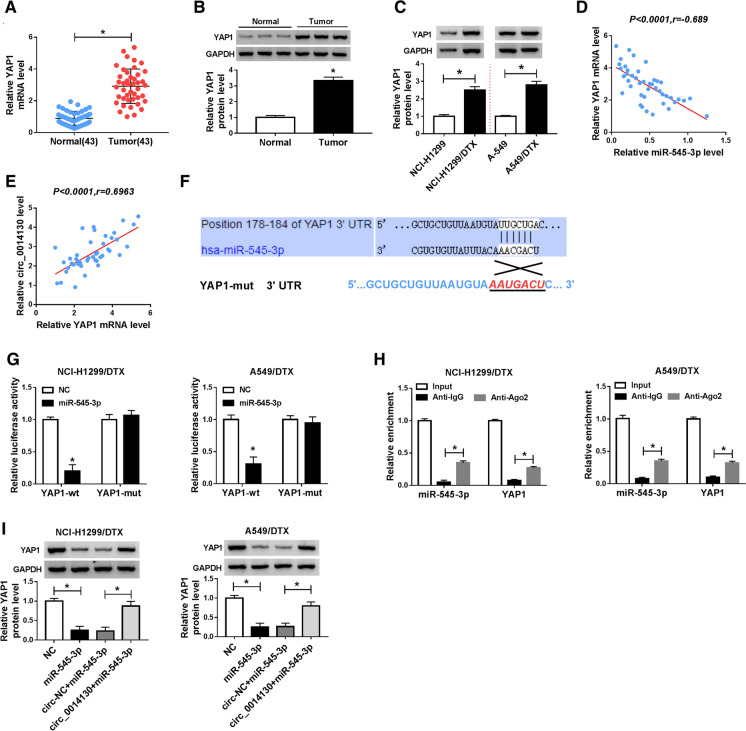
Furthermore, the in-depth molecular mechanism of circ_0014130 was following validated. Real-time PCR and western blotting data disclosed that YAP1 expression was upregulated in NSCLC tumor tissues (n = 43; Fig. 5a, b); notably, YAP1 mRNA level in NSCLC tumors was negatively correlated with miR-545-3p level (Fig. 5d), and positively correlated with circ_0014130 level (Fig. 5e). The protein expression of YAP1 was consistently higher in NCI-H1299/DTX and A549/DTX cells than NCI-H1299 and A549 cells (Fig. 5c). TargetScan computational tool (http://www.targetscan.org/ENST00000282441.5-miR-545-3p) predicted the binding site between YAP1 3′UTR and miR-545-3p (Fig. 5f), and dual-luciferase reporter assay identified this target relationship. As shown in Fig. 5g, NCI-H1299/DTX and A549/DTX cells exhibited a significant decline of luciferase activity after co-transfection with YAP1-wt and miR-545-3p mimic. Moreover, YAP1 and miR-545-3p were concurrently enriched in Ago2-mediated RIP contents (Fig. 5h). Thus, we considered YAP1 as a target gene for miR-545-3p. MiR-545-3p overexpression via transfection led to low protein expression of YAP1 in NCI-H1299/DTX and A549/DTX cells, whereas this downregulation was diminished with circ_0014130 vector introduction (Fig. 5i). This suggested that circ_0014130 could modulate YAP1 expression via sponging miR-545-3p.
Fig. 5.
Identification of yes-associated protein 1 (YAP1) as a target of miR-545-3p. a Real-time PCR detected YAP mRNA expression level in NSCLC tumors (n = 43) and normal tissues (n = 43). b Western blotting examined YAP1 protein level in three NSCLC patients. c Western blotting examined YAP1 protein level in NCI-H1299/DTX and A549/DTX cells, and the parental cells. d, e Pearson’s correlation coefficient assay analyzed the correlation among miR-545-3p, YAP1 mRNA and circ_0014130 levels in 43 NSCLC tumor tissues. f TargetScan computational tool predicted the binding site between the wild-type of YAP1 3′UTR (YAP1-wt) and miR-545-3p. g Dual-luciferase reporter assay measured luciferase activity of YAP1-wt and its mutant (YAP1-mut) in NCI-H1299/DTX and A549/DTX cells administrated with miR-545-3p or NC. h RIP assessed the enrichment of miR-545-3p and YAP1 mRNA in immunoprecipitated RNAs using Anti-Ago2 or Anti-IgG. i Western blotting measured YAP1 protein level in NCI-H1299/DTX and A549/DTX cells administrated with NC, miR-545-3p alone or combined with circ_0014130 or circ-NC. Data of each panel were from three independent experiments, and *P < 0.05
Downregulation of YAP1 conferred the inhibitory effect of miR-545-3p overexpression on chemoresistance and malignant development of DTX-resistant NSCLC cells in vitro
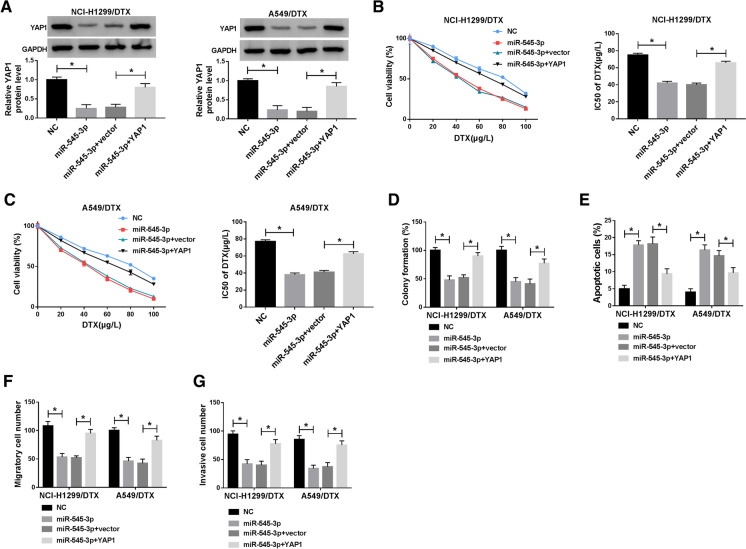
The role of miR-545-3p and YAP1 in DTX-resistant NSCLC cells was next detected. NCI-H1299/DTX and A549/DTX cells were forcedly overexpressed miR-545-3p via mimic transfection, and allied with this was YAP1 depression (Fig. 6a). Cell viability and IC50 of DTX were inhibited by pre-transfection of miR-545-3p mimic in DTX-treated NCI-H1299/DTX and A549/DTX cells (Fig. 6b, c). Relative colony formation of NCI-H1299/DTX and A549/DTX cells was dramatically descended due to miR-545-3p overexpression (Fig. 6d); contrarily, rate of apoptotic cells was elevated in NCI-H1299/DTX and A549/DTX cells in the presence of miR-545-3p mimic alone (Fig. 6e). Transwell migration and invasion were reduced in miR-545-3p mimic-transfected NCI-H1299/DTX and A549/DTX cells (Fig. 6f, g). These results presented a suppressive role of miR-545-3p overexpression in DTX-resistant NSCLC cells; however, YAP1 overexpression vector administration could not only restore YAP1 expression in miR-545-3p-overexpressed NCI-H1299/DTX and A549/DTX cells (Fig. 6a), but also result in a significant rescue of DTX resistance, colony formation, migration and invasion (Fig. 6b–g). These outcomes indicated that miR-545-3p overexpression conferred chemoresensitivity and anti-tumor activity in DTX-resistant NSCLC cells through inhibiting YAP1.
Fig. 6.
The role of miR-545-3p and YAP1 in DTX-resistant NSCLC cells in vitro. a–g NCI-H1299/DTX and A549/DTX cells were exogenously administrated with miR-545-3p or NC, and co-treated with miR-545-3p and YAP1 overexpression vector (YAP1) or control vector. a Western blotting measured YAP1 level after transfection. b, c MTT assay evaluated IC50 of DTX in transfected cells. d Colony formation assay examined relative colony formation (%) after transfection. e Flow cytometry measured the percentage of apoptotic cells after transfection. f, g Transwell assays detected numbers of migratory cells and invasive cells after transfection. Data of each panel were from three independent experiments, and *P < 0.05
Knockdown of circ_0014130 restrained DTX resistance and tumor growth of NSCLC cells in vivo through regulating miR-545-3p and YAP1
The in vivo experiment was conducted by injection of A549/DTX cells into nude mice. As a result, tumor volume and weight were lowered by sh-circ_0014130 transfection under DTX treatment or not than sh-NC transfection (Fig. 7a, b). Essentially, expression of circ_0014130, YAP1 and PCNA (cell proliferation marker) was silenced, and miR-454-3p was promoted in xenograft tumor tissues in sh-circ_0014130 transfection groups (Fig. 7c, d). These results suggested a similar suppressive role of circ_0014130 knockdown in DTX resistance and tumor growth of DTX-resistant NSCLC cells in vivo by regulating miR-545-3p and YAP1.
Fig. 7.
The role of circ_0014130 knockdown in DTX resistance of NSCLC cells in vivo. a–d Male nude mice were subcutaneously injected with A549/DTX cells transfected with shRNA against circ_0014130 (sh-circ_0014130) or sh-NC, followed with or without DTX (5 mg/kg) treatment every three days for 8 times. a, b Tumor volume and tumor weight in each group (n = 4) were measured after implantation. c Real-time PCR detected expression levels of circ_0014130 and miR-545-3p in tumor tissues from mice. d Western blotting examined protein expression of YAP1 and proliferating cell nuclear antigen (PCNA) in tumor tissues from mice. Data of each panel were from three independent experiments, and *P < 0.05
Discussion
Circ_0014130 was an oncogene, and had been widely documented to participate in the complex biological courses of diverse tumors, such as gastric cancer, colon cancer and ovarian cancer (Ma et al. 2020; Sun et al. 2019; Zhang et al. 2019), as well as NSCLC (Zhang et al. 2018). In NSCLC, cell proliferation and invasion were markedly inhibited by circ_0014130 downregulation in NCI-H1299 and A549 cells, meanwhile apoptosis was greatly induced (Geng et al. 2020; Wang et al. 2020); endothelial-mesenchymal transition in vitro and pulmonary metastasis in vivo were also suppressed due to circ_0014130 silence (Chi et al. 2019). Notably, Chi et al. observed that circ_0014130 was predominantly localized in the cytoplasm (Chi et al. 2019), thus serving as competing endogenous RNA (ceRNA) for multiple miRNAs, including miR-136-5p, miR-142-5p and miR-600 (Geng et al. 2020; Wang et al. 2020; Chi et al. 2019). In this study, we supported the stable upregulation of circ_001430 in NSCLC tumor tissues, and discovered a new circ_0014130-miR-545-3p interaction underlying the development of NSCLC cells acquired DTX resistance. Zhang et al. (Zhang et al. 2018) noticed a correlation between circ_0014130 expression and clinical features of NSCLC patients such as TNM stage, lymphatic metastasis and diagnostic potential, suggesting it as a new biomarker for the diagnosis of NSCLC. Here, our data indicated that circ_0014130 was highly expressed in DTX-resistant NSCLC NCI-H1299 and A549 cells, and its knockdown could attenuated IC50 of DTX, colony formation, migration and invasion of NCI-H1299/DTX and A549/DTX in vitro, as well as the tumor growth in vivo no matter with DTX treatment or not. These findings prompted that blocking circ_0014130 might be an effective approach to reverse DTX resistance in the therapy of DTX-resistant NSCLC patients.
MiR-545-3p was a tumor suppressor in NSCLC by inhibiting the malignant cell behaviors such as cell proliferation, cell cycle progression, migration and invasion (Du et al. 2014; Cui et al. 2019). Here, we declared that miR-545-3p was implicated in DTX resistance in NSCLC cells via being sponged by circ_0014130. Similarly, miR-545-3p upregulation was accompanied with the suppressive role of circ_0072083 knockdown in cisplatin resistance of NSCLC H522 and A549 cells by regulating colony formation, apoptosis, cell cycle and metastasis (Du et al. 2014). Furthermore, silenced miR-545-3p could reverse radiosensitivity of Lewis lung tumor both in vitro and in vivo (Liao et al. 2015a,2015b). Taken these data together, we suspected that miR-545-3p might be a chemoradiotherapy-related miRNA in NSCLC. Molecularly, diverse functional genes were directly regulated by this miRNA, including cyclin D1, CDK4, ZEB2, Ku80 and CBLL1 (Du et al. 2014; Cui et al. 2019; Liao et al. 2015a; Li et al. 2020), and these genes were closely involved in cell proliferation, metastasis and DNA repair. In this study, we identified a novel target of miR-545-3p, namely YAP1 which was an essential negative regulator of Hippo pathways in guiding cell growth and differentiation (Nishio et al. 2017).
YAP1 had been documented to contribute to resistance to anti-cancer therapies including cytotoxic chemotherapy and radiotherapy (Kim and Kim 2017). The expression of YAP1 was mainly localized in the nucleus of NSCLC cells in association with advanced TNM stage, nodal metastasis and unfavorable prognosis of NSCLC patients (Wang et al. 2010; Zhu et al. 2019a). In terms of chemoresistance, YAP1 could affect NSCLC cells to gefitinib, erlotinib, and EGFR-TKI (Zhu et al. 2019b; Hsu et al. 2016; Lee et al. 2016). However, the role of YAP1 in DTX resistance in lung cancers remained to be illuminated, even though this had been already reported in other types of cancer (Heywood et al. 1990; Lai et al. 2019; Wang et al. 2016). Luckily, we discovered that YAP1 upregulation was contributing to DTX resistance and malignant development of NSCLC, and further identified that YAP1 functioning in DTX-resistant NSCLC cells via serving as a downstream target for circ_0014130-miR-545-3p crosslink, at least.
By the way, there were several limitations of this research, such as not investigating role of circ_0014130 depletion in metastasis in vivo, not further exploring the underlying signaling pathway of the circ_0014130/miR-545-3p-YAP1 ceRNA axis, and not further confirming the marker proteins for drug resistance, cell survival, migration and invasion. Even so, we demonstrated that circ_0014130 expression was heterogeneous in NSCLC cells, namely lower in DTX-sensitive cells and higher in DTX-resistant cells; downregulation of circ_0014130 could sensitize DTX resistance-acquired NSCLC cells to DTX and suppressed malignant behaviors in vitro and in vivo via regulating miR-545-3p-YAP1 axis. This finding might suggest a potential biomarker for DTX-based chemotherapy, and a therapeutic approach to interfere the acquired chemoresistance of DTX in NSCLC patients.
Abbreviations
- DTX
Docetaxel
- NSCLC
Non-small-cell lung cancer
- NCI-H1299/DTX
DTX-resistant NCI-H1299 cells
- A549/DTX
DTX-resistant A549 cells
- circ_0014130
Hsa_circ_0014130
- YAP1
Yes-associated protein 1
- GAPDH
Glyceraldehyde-phosphate dehydrogenase
- IC50
Inhibitory concentration
- PCNA
Proliferating cell nuclear antigen
- anti-miR-545-3p
miR-545-3p inhibitor
- RIP
RNA immunoprecipitation
Funding
There is no funding to report.
Data availability
The data that support the findings of this study are available on request from the corresponding author.
Declarations
Conflict of interest
The authors declare that they have no financial conflicts of interest.
Ethics Approval
The design of this protocol follows the tenets of the Declaration of Helsinki, approved by the Ethics Committee of Hebei General Hospital.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- Chen Y, Han S, Zheng MJ, Xue Y, Liu WC. Clinical characteristics of 274 non-small cell lung cancer patients in China. Onkologie. 2013;36:248–254. doi: 10.1159/000350301. [DOI] [PubMed] [Google Scholar]
- Chen W, Zheng R, Baade PD, et al. Cancer statistics in China, 2015. CA Cancer J Clin. 2016;66:115–132. doi: 10.3322/caac.21338. [DOI] [PubMed] [Google Scholar]
- Cheng DL, Xiang YY, Ji LJ, Lu XJ. Competing endogenous RNA interplay in cancer: mechanism, methodology, and perspectives. Tumour Biol. 2015;36:479–488. doi: 10.1007/s13277-015-3093-z. [DOI] [PubMed] [Google Scholar]
- Chi Y, Luo Q, Song Y, et al. Circular RNA circPIP5K1A promotes non-small cell lung cancer proliferation and metastasis through miR-600/HIF-1alpha regulation. J Cell Biochem. 2019;120:19019–19030. doi: 10.1002/jcb.29225. [DOI] [PubMed] [Google Scholar]
- Cui J, Pan G, He Q, Yin L, Guo R, Bi H. MicroRNA-545 targets ZEB2 to inhibit the development of non-small cell lung cancer by inactivating Wnt/beta-catenin pathway. Oncol Lett. 2019;18:2931–2938. doi: 10.3892/ol.2019.10619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di X, Jin X, Li R, Zhao M, Wang K. CircRNAs and lung cancer: biomarkers and master regulators. Life Sci. 2019;220:177–185. doi: 10.1016/j.lfs.2019.01.055. [DOI] [PubMed] [Google Scholar]
- Drula R, Braicu C, Harangus A, et al. Critical function of circular RNAs in lung cancer. Wiley Interdiscip Rev RNA. 2020;11:e1592. doi: 10.1002/wrna.1592. [DOI] [PubMed] [Google Scholar]
- Du B, Wang Z, Zhang X, et al. MicroRNA-545 suppresses cell proliferation by targeting cyclin D1 and CDK4 in lung cancer cells. PLoS ONE. 2014;9:e88022. doi: 10.1371/journal.pone.0088022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fehrenbacher L, von Pawel J, Park K, et al. Updated efficacy analysis including secondary population results for OAK: a randomized phase III study of atezolizumab versus docetaxel in patients with previously treated advanced non-small cell lung cancer. J Thorac Oncol. 2018;13:1156–1170. doi: 10.1016/j.jtho.2018.04.039. [DOI] [PubMed] [Google Scholar]
- Geng Y, Bao Y, Zhang W, Deng L, Su D, Zheng H. Circular RNA hsa_circ_0014130 INHIBITS APOPTOSIS IN NON-SMALL CELL LUNG CANCER BY Sponging miR-136-5p and upregulating BCL2. Mol Cancer Res. 2020;18:748. doi: 10.1158/1541-7786.MCR-19-0998. [DOI] [PubMed] [Google Scholar]
- Heywood BR, Sparks NH, Shellis RP, Weiner S, Mann S. Ultrastructure, morphology and crystal growth of biogenic and synthetic apatites. Connect Tissue Res. 1990;25:103–119. doi: 10.3109/03008209009006985. [DOI] [PubMed] [Google Scholar]
- Hong X, Liu N, Liang Y, et al. Circular RNA CRIM1 functions as a ceRNA to promote nasopharyngeal carcinoma metastasis and docetaxel chemoresistance through upregulating FOXQ1. Mol Cancer. 2020;19:33. doi: 10.1186/s12943-020-01149-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsu PC, You B, Yang YL, et al. YAP promotes erlotinib resistance in human non-small cell lung cancer cells. Oncotarget. 2016;7:51922–51933. doi: 10.18632/oncotarget.10458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeyaraman S, Hanif EAM, Ab Mutalib NS, Jamal R, Abu N. Circular RNAs: potential regulators of treatment resistance in human cancers. Front Genet. 2019;10:1369. doi: 10.3389/fgene.2019.01369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jia X, Liu X, Li M, et al. Potential tumor suppressing role of microRNA-545 in epithelial ovarian cancer. Oncol Lett. 2018;15:6386–6392. doi: 10.3892/ol.2018.8130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joshi M, Liu X, Belani CP. Taxanes, past, present, and future impact on non-small cell lung cancer. Anticancer Drugs. 2014;25:571–583. doi: 10.1097/CAD.0000000000000080. [DOI] [PubMed] [Google Scholar]
- Kim MH, Kim J. Role of YAP/TAZ transcriptional regulators in resistance to anti-cancer therapies. Cell Mol Life Sci. 2017;74:1457–1474. doi: 10.1007/s00018-016-2412-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar P, Raza K, Kaushik L, Malik R, Arora S, Katare OP. Role of colloidal drug delivery carriers in taxane-mediated chemotherapy: a review. Curr Pharm Des. 2016;22:5127–5143. doi: 10.2174/1381612822666160524144926. [DOI] [PubMed] [Google Scholar]
- Lai CJ, Lin CY, Liao WY, Hour TC, Wang HD, Chuu CP. CD44 promotes migration and invasion of docetaxel-resistant prostate cancer cells likely via induction of hippo-yap signaling. Cells. 2019;8:295. doi: 10.3390/cells8040295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee JE, Park HS, Lee D, et al. Hippo pathway effector YAP inhibition restores the sensitivity of EGFR-TKI in lung adenocarcinoma having primary or acquired EGFR-TKI resistance. Biochem Biophys Res Commun. 2016;474:154–160. doi: 10.1016/j.bbrc.2016.04.089. [DOI] [PubMed] [Google Scholar]
- Li C, Zhang L, Meng G, et al. Circular RNAs: pivotal molecular regulators and novel diagnostic and prognostic biomarkers in non-small cell lung cancer. J Cancer Res Clin Oncol. 2019;145:2875–2889. doi: 10.1007/s00432-019-03045-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li H, Liu F, Qin W. Circ_0072083 interference enhances growth-inhibiting effects of cisplatin in non-small-cell lung cancer cells via miR-545-3p/CBLL1 axis. Cancer Cell Int. 2020;20:78. doi: 10.1186/s12935-020-1162-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liao C, Xiao W, Zhu N, et al. MicroR-545 enhanced radiosensitivity via suppressing Ku70 expression in Lewis lung carcinoma xenograft model. Cancer Cell Int. 2015;15:56. doi: 10.1186/s12935-015-0207-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liao C, Xiao W, Zhu N, et al. Radiotherapy suppressed tumor-specific recruitment of regulator T cells via up-regulating microR-545 in Lewis lung carcinoma cells. Int J Clin Exp Pathol. 2015;8:2535–2544. [PMC free article] [PubMed] [Google Scholar]
- Ma Y, Cong X, Zhang Y, Yin X, Zhu Z, Xue Y. CircPIP5K1A facilitates gastric cancer progression via miR-376c-3p/ZNF146 axis. Cancer Cell Int. 2020;20:81. doi: 10.1186/s12935-020-1122-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishio M, Maehama T, Goto H, et al. Hippo vs. Crab: tissue-specific functions of the mammalian Hippo pathway. Genes Cells. 2017;22:6–31. doi: 10.1111/gtc.12461. [DOI] [PubMed] [Google Scholar]
- Reck M, Taylor F, Penrod JR, et al. Impact of nivolumab versus docetaxel on health-related quality of life and symptoms in patients with advanced squamous non-small cell lung cancer: results from the CheckMate 017 Study. J Thorac Oncol. 2018;13:194–204. doi: 10.1016/j.jtho.2017.10.029. [DOI] [PubMed] [Google Scholar]
- Shen Z, Zhou L, Zhang C, Xu J. Reduction of circular RNA Foxo3 promotes prostate cancer progression and chemoresistance to docetaxel. Cancer Lett. 2020;468:88–101. doi: 10.1016/j.canlet.2019.10.006. [DOI] [PubMed] [Google Scholar]
- Sun Y, Li X, Chen A, et al. circPIP5K1A serves as a competitive endogenous RNA contributing to ovarian cancer progression via regulation of miR-661/IGFBP5 signaling. J Cell Biochem. 2019;120:19406–19414. doi: 10.1002/jcb.29055. [DOI] [PubMed] [Google Scholar]
- Vokes EE, Ready N, Felip E, et al. Nivolumab versus docetaxel in previously treated advanced non-small-cell lung cancer (CheckMate 017 and CheckMate 057): 3-year update and outcomes in patients with liver metastases. Ann Oncol. 2018;29:959–965. doi: 10.1093/annonc/mdy041. [DOI] [PubMed] [Google Scholar]
- Wang Y, Dong Q, Zhang Q, Li Z, Wang E, Qiu X. Overexpression of yes-associated protein contributes to progression and poor prognosis of non-small-cell lung cancer. Cancer Sci. 2010;101:1279–1285. doi: 10.1111/j.1349-7006.2010.01511.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Lieberman R, Pan J, et al. miR-375 induces docetaxel resistance in prostate cancer by targeting SEC23A and YAP1. Mol Cancer. 2016;15:70. doi: 10.1186/s12943-016-0556-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang M, Shi J, Jiang H, Xu K, Huang Z. Circ_0014130 participates in the proliferation and apoptosis of nonsmall cell lung cancer cells via miR-142-5p/IGF-1 axis. Cancer Biother Radiopharm. 2020;35:233. doi: 10.1089/cbr.2019.2965. [DOI] [PubMed] [Google Scholar]
- Yu W, Peng W, Sha H, Li J. Hsa_circ_0003998 Promotes chemoresistance via modulation of miR-326 in lung adenocarcinoma cells. Oncol Res. 2019;27:623–628. doi: 10.3727/096504018X15420734828058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan G, Wu H, Du Y, He F. Tumor suppressor role of microRNA-545 in oral squamous cell carcinoma. Oncol Lett. 2019;17:2063–2068. doi: 10.3892/ol.2018.9820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang W, Lei P, Dong X, Xu C. The new concepts on overcoming drug resistance in lung cancer. Drug Des Dev Ther. 2014;8:735–744. doi: 10.2147/DDDT.S60672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang S, Zeng X, Ding T, et al. Microarray profile of circular RNAs identifies hsa_circ_0014130 as a new circular RNA biomarker in non-small cell lung cancer. Sci Rep. 2018;8:2878. doi: 10.1038/s41598-018-21300-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Q, Zhang C, Ma JX, Ren H, Sun Y, Xu JZ. Circular RNA PIP5K1A promotes colon cancer development through inhibiting miR-1273a. World J Gastroenterol. 2019;25:5300–5309. doi: 10.3748/wjg.v25.i35.5300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu L, Ma G, Liu J, et al. Prognostic significance of nuclear Yes-associated protein 1 in patients with nonsmall cell lung cancer: a systematic review and meta-analysis. Medicine (baltimore) 2019;98:e15069. doi: 10.1097/MD.0000000000015069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu J, Tao L, Jin L. MicroRNA5063p reverses gefitinib resistance in nonsmall cell lung cancer by targeting Yesassociated protein 1. Mol Med Rep. 2019;19:1331–1339. doi: 10.3892/mmr.2018.9710. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available on request from the corresponding author.