Abstract
Introduction
Dry eye is a multifactorial condition of the eye caused by insufficient tear production and imbalance in tear composition leading to faster evaporation of tear fluid. It is also associated with inflammation that often leads to ocular surface damage. Symptoms of dry eyes include itchiness, soreness, red eyes, a burning sensation, eye fatigue and blurred vision. The objective of this study was to evaluate the efficacy and safety of our multi-ingredient supplement in subjects with dry eye syndrome (DES).
Methods
We recruited 60 subjects with mild to moderate DES who were randomized in a 1:1 ratio in a single-center study to receive LCD (lutein 20 mg, zeaxanthin 4 mg, curcumin 200 mg curcuminoids, vitamin D3 600 IU) or placebo (soybean oil) capsules for 8 weeks. The primary outcomes evaluated were changes in tear volume by Schirmer’s test and ocular symptoms by the Ocular Surface Disease Index (OSDI); secondary outcomes included evaluation of changes in Standard Patient Evaluation of Eye Dryness (SPEED) questionnaire, tear film break-up time (TBUT), corneal and conjunctival staining, tear osmolarity, matrix metalloproteinase-9 (MMP-9), artificial tear use and safety assessments. The outcomes were compared between the LCD and placebo groups at baseline and day 56 of supplementation.
Results
Fifty-nine subjects, 30 from LCD and 29 from placebo group, completed the study. The LCD group showed significant improvements (P < 0.0001) for Schirmer’s test, OSDI, TBUT, SPEED, ocular staining scores, tear osmolarity (P = 0.0005), MMP-9 (P = 0.0017) and reduced artificial tear use (P = 0.0004) and its frequency of use (P < 0.0001) in subjects compared to placebo from baseline to day 56. No safety issues were observed in the study.
Conclusion
The LCD supplement showed significant improvements in the production, stability and quality of tears by reducing ocular surface damage and tear inflammation and can be used as an adjuvant to artificial tears in subjects with DES.
Trial Registration
Clinical Trials Registry of India (http://ctri.nic.in/) identifier: CTRI/2021/01/030493.
Keywords: Artificial tears, Curcumin, Dry eye syndrome, Lutein, Ocular damage, Oral nutritional supplement, Vitamin D3, Zeaxanthin
Key Summary Points
| Why carried out this study? |
| Dry eye syndrome (DES) is a multifactorial disease of the ocular surface associated with reduced tear secretion, increased osmolarity of the tears and inflammation that may lead to ocular surface damage. DES is one of the most common reasons for seeking eye care with a projected direct cost to the US economy of $3.8 billion. |
| There is a high prevalence of DES (5–50%) globally and increased risk of DES with aging, female gender and the younger population that increasingly uses digital devices. |
| Artificial tears and topical corticosteroids used as first-line treatments usually improve the symptoms of dry eyes but are associated with adverse effects with long-term use. Many nutritional supplements have been investigated to manage the symptoms of DES. This study was conducted to evaluate efficacy of a multi-ingredient supplement containing lutein, zeaxanthin, curcumin and vitamin D3 in subjects with dry eye syndrome (DES). |
| What was learned from the study? |
| Results from our study indicate that 8 weeks of supplementation of our formulation helps in improving tear production, stabilizes tear film, and eventually reduces hyperosmolarity of the tears in subjects suffering from DES. This further results in decreased inflammation as measured by reduced MMP-9 levels in the tear fluid and decreased ocular surface damage as measured by corneal and conjunctival staining during the supplementation. |
| Overall, we have demonstrated statistically significant clinical improvements in dry eye symptoms as compared to the placebo in subjects validated through well-established subjective questionnaire-based approaches such as OSDI and SPEED. |
Digital Features
This article is published with digital features, including [list digital features available e.g. a summary slide], to facilitate understanding of the article. To view digital features for this article go to https://doi.org/10.6084/m9.figshare.14696787.
Introduction
Dry eye syndrome (DES) is a multifactorial disease of the ocular surface associated with reduced tear secretion, increased osmolarity of the tears and inflammation that may lead to ocular surface damage. DES is marked by tear film instability, irritation or burning sensation of the corneal surface with significant pain and discomfort to the eye [1]. Prevalence of DES ranges from 5 to 50% globally with an estimated 25–30 million people affected worldwide with a substantial effect on vision and quality of life [2]. The risk of DES increases with age, female gender [3, 4] and the younger population who increasingly uses digital devices [5]. It is estimated that 16 million Americans have been diagnosed with dry eyes, and nearly half of all US adults experience dry eye signs and symptoms [6]. Furthermore, DES is one of the most common reasons for seeking eye care [5] with a projected direct cost to the US economy of $3.8 billion [2, 7].
Increased tear film evaporation and reduced tear production, resulting in tear film hyperosmolarity, are recognized as triggering factors for initiation of DES [8]. Increased tear osmolarity is believed to activate the inflammatory cascades leading to inflammatory protein production [8], which along with inflammatory cells disrupt the integrity of the cornea and conjunctival epithelium, further amplifying the chronic inflammatory response and exacerbating tear film instability, which is a characteristic feature of DES [9, 10]. Matrix metalloproteinase-9 (MMP-9) is one of the inflammatory mediators that is consistently elevated in the tears of individuals with DES and known to further escalate inflammation; it is hence used as a reliable clinical biomarker to assess the efficacy of DES therapeutic measures [10]. Approval of the anti-inflammatory agent cyclosporin for treating DES by the US Food and Drug Administration further underlines the role of inflammation in understanding the pathogenesis of DES [11].
Traditionally, artificial tears have been used as the first-line treatment for DES followed by topical corticosteroids in severe conditions, which usually improve the symptoms of dry eyes but are associated with adverse effects with long-term use [12]. Use of artificial tear drops requires multiple applications per day to get continuous relief but is often associated with low compliance over long-term use due to inconvenience. Hence, there has been an interest in evaluating the role of oral nutritional supplements that are considered safe particularly when used over an extended period. Many nutraceutical ingredients have been investigated, such as omega-3 fatty acids [13, 14], carotenoids [15, 16], anthocyanins [17–19] and antioxidants [20], to manage the symptoms of DES. Although omega-3 fatty acid products have been widely used in the treatment of dry eyes, a recent large-scale multi-center trial of 3000 mg omega-3 fatty acids for 12 months did not show any benefit in patient symptoms or signs compared with the placebo [13].
Recently, we reported the role of a novel multi-ingredient formulation containing lutein, zeaxanthin, curcumin and vitamin D3 (LCD) in ameliorating DES by inhibiting inflammation and oxidative stress and enhancing glycosylated phosphoproteins in rats. It is well known that both lutein and zeaxanthin are major carotenoids present in the human macula and known to protect the retina from photo-oxidative damage by absorbing blue light [21]. Curcumin is a natural polyphenol from the plant Curcuma longa, which is extensively used as a dietary supplement for its anti-inflammatory and antioxidant properties [22–24]. Vitamin D3 is an essential nutrient widely studied for immunomodulatory activities and known to improve tear secretion and tear break-up time [19, 20]. We optimized our formulation to enhance the oral bioavailability of ingredients by reducing the particle size through micronization and incorporating multiple functional oils, both of which are known to improve absorption from the small intestine. Furthermore, we demonstrated the protective effect of our formulation with 4 weeks of oral supplementation in a rat model with experimentally induced DES. The formulation helped to ameliorate DES by increasing the production of tears and improving tear film stability, with reduced corneal inflammation and ocular surface damage. We also observed reduced oxidative stress, suppressed molecular markers of the inflammatory response and restored expression of tear proteins such as mucins [25]. The present study was conducted to further assess the efficacy and safety of the above oral formulation in human subjects with mild to moderate DES.
Methods
Study Design and Procedures
This was a prospective, randomized, double-blind, parallel, placebo-controlled, clinical interventional study with 56 days of investigational product administration.
The study was initiated after obtaining written approval from an institutional ethics committee, LPR (LifePoint Research) Ethics Committee, Pune, India, on 6 August 2019. The study was carried out as per the requirements of the Indian Council of Medical Research (ICMR) ethical guidelines, International Council for Harmonization (ICH) Guidance on Good Clinical Practice (E6R2) and the Declaration of Helsinki. The study was registered with the Clinical Trials Registry of India (CTRI/2021/01/030493).
Voluntary informed consent was obtained from every participant before enrolling in the study. Subjects were randomly assigned in a 1:1 ratio to receive the LCD or placebo. The LCD was a soft gel capsule of ~ 670 mg weight comprising lutein (20 mg) and zeaxanthin (4 mg) from marigold flower extract prepared at OmniActive Health Technologies (Pune, India) by saponification and thermal isomerization of marigold flower oleoresin, curcumin (200 mg total curcuminoids) using the turmeric extract containing 95% total curcuminoids from Synthite (Kolenchery, India), algal source vitamin D3 (600 IU) from Avlaan Inc. (Plymouth, MN, USA), medium-chain triglyceride (MCT) oil and linseed oil from AAK Kamani (Mumbai, India), olive oil from Cargill India Pvt. Ltd. (Haryana, India), sunflower lecithin from VAV lipids (Mumbai, India), tocopherol from Matrix Fine Sciences (Aurangabad, India) and thyme oil from Sigma Aldrich (St. Louis, MO, USA). Lutein, zeaxanthin, curcuminoids and vitamin D3 in the formulation were analyzed by high-performance liquid chromatography using reference standards. The placebo soft gel capsule was prepared using soybean oil to look like the LCD capsule to keep the study double-blinded. The randomization schedule was generated by a non-study assigned, independent expert ensuring the treatment balance by using SAS® statistical software. After randomization, subjects were instructed to consume one capsule every morning after breakfast at the same time every day for 56 days (8 weeks). The total duration of the clinical study was a maximum of 67 days including the screening period of 8 days. Artificial tears (hydroxypropyl methylcellulose, 0.70% w/v) were prescribed as rescue medication if the subject was not able to tolerate the symptoms of dry eyes, and the same was documented.
Information about gender, age, body weight, height, BMI, medical history, concomitant medication history, digital screen exposure, presence of allergic problems and drug reactions, contact lens use and oral contraceptives was obtained during the screening visit.
Study Population and Inclusion/Exclusion Criteria
The study participants were selected based on the inclusion and exclusion criteria provided in Table 1.
Table 1.
Inclusion and exclusion criteria
| Subjects with below criteria were included in the study |
| a. Aged between 18 and 65 years (both limits inclusive) |
| b. Clinical diagnosis of symptomatic dry eye syndrome confirmed at least in any one of the eyes |
| c. Free from any clinically significant disease, other than dry eye syndrome, that might interfere with the study evaluations |
|
d. Following results at least in any one of the eyes Schirmer’s test without anesthesia ≤ 10 mm Ocular Surface Disease Index (OSDI) test symptoms between 12 and 40 Tear film break up time ≤ 10 s Tear osmolarity ≥ 316 milliosmole/l Fluorescein corneal staining ≥ 1 and < 3 |
| Subjects with the following criteria were excluded from the study |
| a. Pregnant, nursing or planning a pregnancy within the study participation period |
| b. With a history of allergy or sensitivity to lutein, zeaxanthin, curcumin or vitamin D3, related compounds or any component of the formulation |
| c. With the presence of severe dry eye syndrome with complications such as perforated corneal ulcer, uveitis, glaucoma, that may interfere with the evaluation of the subject’s dry eye syndrome in the investigator’s opinion |
| d. With current evidence of ocular infections or inflammatory conditions such as acute conjunctivitis or other medical condition that, in the investigator’s opinion, would place the subject at undue risk by participating or compromise the integrity of the study data |
| e. With poorly controlled diabetes mellitus, rheumatoid arthritis, systemic lupus erythematosus, which cause dry eye syndrome |
| f. With herpetic eye disease, chronic infection of the lacrimal gland, laser in situ keratomileusis, poorly controlled hypertension (> 140/96 mmHg), evidence of malignancy |
| g. Suffering from major systemic illness necessitating long-term drug treatment (psycho-neuro-endocrinal disorders, etc.) |
| h. With concurrent serious hepatic dysfunction or renal dysfunction, uncontrolled pulmonary dysfunction (asthmatic and chronic obstructive pulmonary disease subjects) or other concurrent severe diseases |
| i. With any ocular trauma or surgery that may affect corneal sensitivity and/or normal tear distribution (e.g., cataract surgery, refractive surgery) within the 6 previous months to study inclusion |
| j. Not willing to stop the use of ocular topical medications and contact lens during the intervention |
| k. With an inability to swallow soft gel capsules |
| l. With use of lutein, zeaxanthin, curcumin or vitamin D3 on prescription for other medical indications or health-conscious reasons |
| m. With use of steroids, oral contraceptive pills, estrogens replacement therapy or any other medication that may adversely affect the outcome of the study |
| n. On aspirin or anticoagulant therapy |
| o. With any other medical condition that might adversely impact the safety of the study participants or confound the study results |
| p. Treated with any investigational drug or investigational device within 3 months before study entry |
Safety and Efficacy Parameters
Safety assessments included monitoring of adverse events, physical examination, vital signs measurements, laboratory assessments and urine pregnancy tests for females of childbearing potential.
Primary efficacy endpoints in the study were Schirmer’s test and Ocular Surface Disease Index (OSDI). Secondary efficacy endpoints included tear film break-up time (TBUT), Standard Patient Evaluation of Eye Dryness (SPEED), corneal and conjunctival staining, tear osmolarity, matrix metalloproteinase-9 (MMP-9) biomarker and information on artificial tear use.
Statistical Analysis
A sample size of 50 subjects (25 subjects for the LCD group and 25 subjects for the placebo group) was required to achieve statistically significant results for the LCD group compared to the placebo (two-sided, α = 0.05, continuity corrected Z-test) with 80% power, 40 as a standard deviation and difference in mean of 0.70. Considering treatment allocation of 1:1 with a 20% drop-out rate, 60 subjects were to be enrolled in the study (i.e., LCD: 30; placebo: 30).
Data are presented as an average of both eyes in the study and summarized for demographic and baseline characteristics, efficacy variables and safety variables. For categorical variables, the number and percentage of each category within a parameter were calculated for non-missing data. For continuous variables with non-missing values, statistics included the number of observations, mean, standard deviation, median, minimum and maximum values. Percent change was calculated for each visit from baseline to present the standardized data. All statistical analyses were performed using SAS®, version 9.4. Subjects with missing data were excluded only from analyses for which data were not available.
The interactions were evaluated by a one-way ANOVA (Analysis of Variance) model using PROC ANOVA in SAS® for evaluations involving the primary and secondary efficacy endpoints. Kruskal-Wallis test was executed for the ordinal datasets. For the dichotomous variable Z-test was performed using SAS®. The criterion for a significant test by treatment was set at a P < 0.05.
Primary Efficacy Endpoint Evaluation
The primary endpoints included evaluation of the tear volume as measured by the length of wetting of Schirmer’s test strips and ocular symptoms measured by using OSDI scores from baseline visit to visit 5 (day 56). Sterile Schirmer’s strips were placed into the subjects’ lower temporal lid margin of both eyes. The subjects were asked to close their eyes for 5 min. The strips were removed, and the length of the moisture area was measured. OSDI questionnaires were completed by the study coordinators based on the responses of the subjects at the respective visit. The analyses were performed between groups (i.e., LCD and placebo) and within groups (changes from baseline and respective visits). Primary efficacy endpoints were evaluated at baseline, day 14, day 28 and day 56 of the investigational product consumption.
Secondary Efficacy Endpoint Evaluation
TBUT and SPEED were evaluated at baseline, day 14, day 28 and day 56, while corneal and conjunctival staining, tear osmolarity and MMP-9 marker were evaluated at baseline and day 56 of the consumption of investigational products. The stain impregnated part of 1 mg fluorescein sodium sterile paper strips was instilled with a small drop of sterile saline solution and gently touched into the subjects’ lower eyelid; the subject was asked to blink several times. The eyes were examined under a blue light with a yellow filter using a slit-lamp. The first appearance of the distinct break-up in the tear film from the moment the subject stopped blinking was noted. The corneal and conjunctival damages were also observed and noted. The study coordinators completed the SPEED questionnaire based on the responses provided by the subjects at the respective visits. I-PEN® osmolarity system (I-MED Pharma, QC, Canada) was used to evaluate tear osmolarity as per the instructions provided in the user manual. InflammaDry® MMP-9 test kits (QUIDEL®, CA, USA) were used for qualitative determination of MMP-9 presence in both eyes. A positive MMP-9 result indicates the presence of MMP-9 ≥ 40 ng/ml, and a negative one indicates the presence of MMP-9 < 40 ng/ml. Artificial tears use was evaluated throughout the study duration. The subjects were prescribed hydroxypropyl methylcellulose 0.7% w/v at the randomization visit as a rescue medication. The subjects had the right to discontinue the use of artificial tears on their own if they felt that their dry eye symptoms improved during the study.
Safety Analysis
Safety analyses were performed using hematology, biochemistry, urinalysis, the incidence of adverse events, physical examination and vital sign measurements for all the randomized subjects who received at least one dose of the study supplement. Descriptive statistics [n (number of subjects), mean, standard deviation, median, minimum and maximum] for continuous safety variables and frequency and the percentage for categorical safety variables such as adverse events were summarized by treatment.
Results
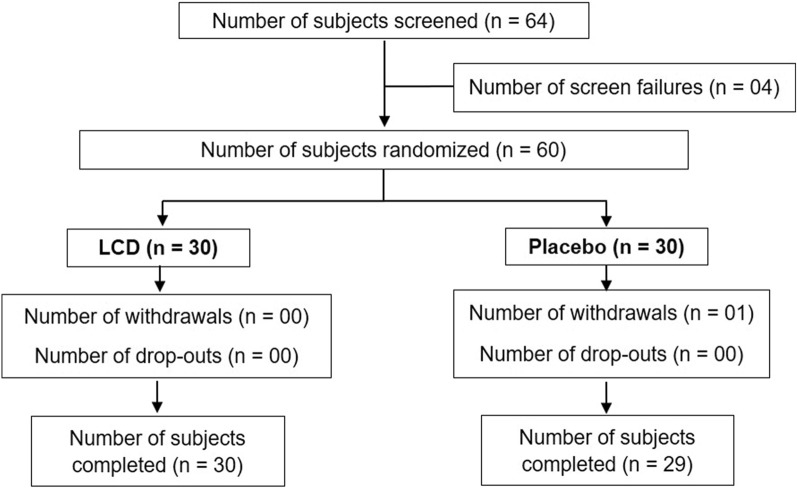
A total of 64 subjects were screened, of which 4 did not fulfil the eligibility criteria and were considered screen failures (Fig. 1). Of the 60 randomized subjects, 30 were allocated to the LCD group and 30 to the placebo group. At the end of the study, 59 subjects completed the study as per protocol requirements: 30 subjects in the LCD group (17 males and 13 females) and 29 in the placebo group (18 males and 11 females). One subject from the placebo group withdrew consent. The demographic characteristics are shown in Table 2. The mean age of subjects in the LCD group was 47.15 ± 12.12 years and in the placebo group was 45.43 ± 12.32 years. The mean body mass index (BMI) was 24.50 ± 2.86 for the LCD group and 24.06 ± 3.40 for the placebo group, and screen exposure time for subjects in the LCD group was 7.75 ± 2.76 h and in the placebo group was 7.86 ± 2.77 h at the screening visit. There was no statistical significance between the two groups for age, sex, BMI and screen exposure time at the baseline.
Fig. 1.
Consolidated Standards of Reporting Trials (CONSORT) diagram for efficacy and safety of lutein, zeaxanthin, curcumin and vitamin D3 oral supplement in a randomized clinical interventional study for dry eye syndrome, showing subject disposition including screening, randomization, withdrawals and completion
Table 2.
Subject demographics
| Demographics characteristics | LCD (N = 30) | Placebo (N = 29) |
|---|---|---|
| Age (years) | ||
| N | 30 | 29 |
| Mean ± SD | 47.15 ± 12.12 | 45.43 ± 12.32 |
| Median | 49 | 46 |
| Min, max | 20, 64 | 22, 65 |
| P-value | 0.5922 | – |
| Sex, n (%) | ||
| Male | 17 (56.67) | 18 (62.07) |
| Female | 13 (43.33) | 11 (37.93) |
| P-value | 0.1521 | - |
| Height (cm) | ||
| N | 30 | 29 |
| Mean ± SD | 155.1 ± 5.40 | 156.6 ± 4.80 |
| Median | 155 | 157 |
| Min, max | 143, 166 | 147, 169 |
| P-value | 0.2584 | – |
| Weight (kg) | ||
| N | 30 | 29 |
| Mean ± SD | 59.13 ± 9.09 | 59.12 ± 9.77 |
| Median | 58 | 57 |
| Min, max | 47, 81 | 46, 97 |
| P-value | 0.9970 | – |
| BMI (kg/m2) | ||
| N | 30 | 29 |
| Mean ± SD | 24.50 ± 2.86 | 24.06 ± 3.40 |
| Median | 24 | 24 |
| Min, max | 20, 30 | 19, 36 |
| P-value | 0.5941 | – |
N number of subjects in the specified treatment, n number of subjects in the specified category
Percentages are based on the number of subjects in the specified treatment. Note 2: For continuous variables P-value calculated using Analysis of Variance (ANOVA) and for categorical variables using chi-square test/Fisher exact test if cell frequency is < 5
Efficacy
The efficacy analysis was performed for 59 subjects who completed the study. The missing observations were imputed using the LOCF approach (the last observation carried forward).
Primary Efficacy Analyses
Schirmer’s Test In the LCD group, the baseline mean Schirmer’s strip wetness length as a measure of tear volume for the average of both eyes was 6.62 ± 1.73 mm, which increased to 9.90 ± 4.21 mm (↑51.95 ± 56.59%) on day 14, 12.23 ± 3.18 mm (↑92.60 ± 57.44%) on day 28 and 14.32 ± 2.51 mm (↑127.2 ± 59.70%) on day 56 when compared to the baseline value. In the placebo group, the baseline mean Schirmer’s strip wetness length for the average of both eyes was 7.09 ± 2.45 mm, which increased to 8.14 ± 2.55 mm (↑20.49 ± 35.92%) on day 14, 8.69 ± 2.90 mm (↑27.75 ± 35.47%) on day 28 and 7.43 ± 3.29 mm (↑7.70 ± 38.07%) on day 56 compared to the baseline value (Table 3a).
Table 3.
Summary of efficacy endpoints between LCD and placebo groups
| Visit | LCD (N = 30) | Placebo (N = 29) | P-value by ANOVA for LCD vs. placebo |
|---|---|---|---|
| Mean ± SD | Mean ± SD | ||
| a. Schirmer’s test—average of both eyes | |||
| Baseline | 6.62 ± 1.73 | 7.09 ± 2.45 | 0.3979 |
| Day 14 | 9.90 ± 4.21 | 8.14 ± 2.55 | 0.0580 |
| Day 28 | 12.23 ± 3.18 | 8.69 ± 2.90 | < 0.0001** |
| Day 56 | 14.32 ± 2.51 | 7.43 ± 3.29 | < 0.0001** |
| b. Ocular Surface Disease Index (OSDI) scores | |||
| Baseline | 30.10 ± 4.88 | 32.54 ± 5.06 | 0.0642 |
| Day 14 | 25.24 ± 4.74 | 30.68 ± 5.48 | 0.0001** |
| Day 28 | 19.16 ± 4.40 | 30.60 ± 5.91 | < 0.0001** |
| Day 56 | 17.09 ± 4.85 | 31.00 ± 7.18 | < 0.0001** |
| c. Tear film break-up time (TBUT)—average of both eyes | |||
| Baseline | 8.18 ± 1.18 | 7.86 ± 1.25 | 0.3129 |
| Day 14 | 9.97 ± 1.80 | 7.97 ± 1.48 | < 0.0001** |
| Day 28 | 11.03 ± 1.32 | 7.93 ± 1.18 | < 0.0001** |
| Day 56 | 12.13 ± 1.38 | 6.86 ± 1.51 | < 0.0001** |
| d. Standardized patient evaluation of eye dryness (SPEED) score | |||
| Baseline | 11.63 ± 1.25 | 12.21 ± 1.78 | 0.1559 |
| Day 14 | 8.97 ± 1.30 | 11.03 ± 2.23 | < 0.0001** |
| Day 28 | 7.07 ± 1.36 | 10.31 ± 2.16 | < 0.0001** |
| Day 56 | 6.07 ± 1.86 | 12.41 ± 2.90 | < 0.0001** |
| e. Corneal staining—average of both eyes | |||
| Baseline | 1.14 ± 0.17 | 1.10 ± 0.17 | 0.3965 |
| Day 56 | 0.79 ± 0.25 | 1.24 ± 0.20 | < 0.0001** |
| f. Conjunctival staining—average of both eyes | |||
| Baseline | 0.88 ± 0.22 | 0.86 ± 0.20 | 0.7775 |
| Day 56 | 0.58 ± 0.32 | 1.03 ± 0.27 | < 0.0001** |
| g. Tear osmolarity—average of both eyes | |||
| Baseline | 327.23 ± 12.42 | 327.34 ± 11.06 | 0.9711 |
| Day 56 | 320.42 ± 12.24 | 331.72 ± 11.20 | 0.0005** |
N number of subjects in specified treatment, SD standard deviation. Between-group analysis with ANOVA
*P < 0.05
**P < 0.01
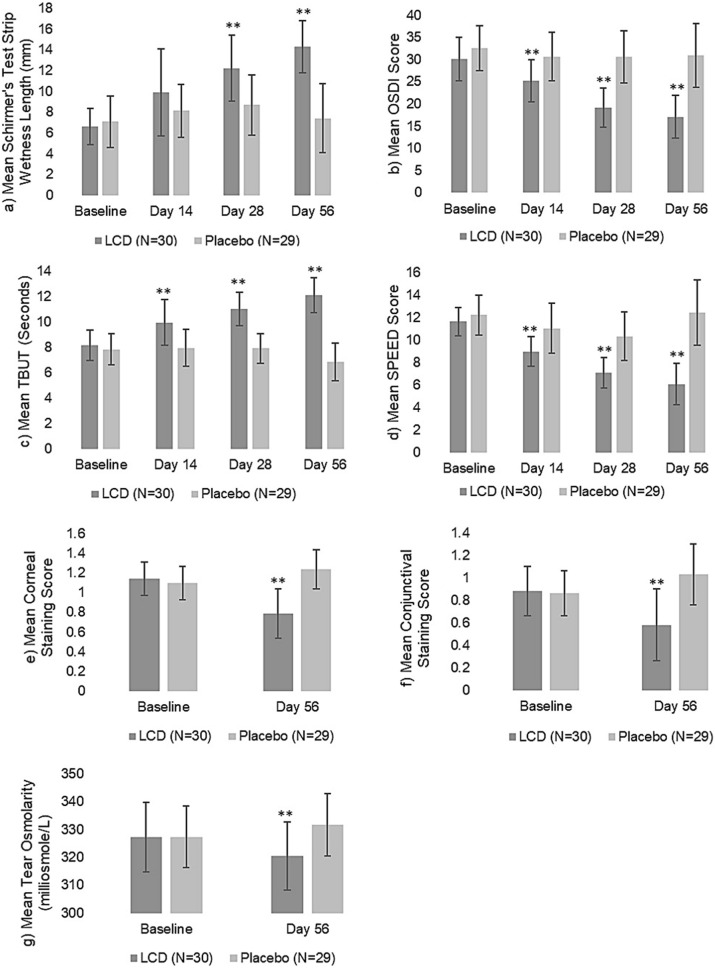
There were no significant differences between the LCD and placebo groups before supplementation, suggesting homogeneity of subjects in the two groups at baseline as evaluated by between-group analysis using ANOVA. However, the LCD group showed a statistically significant increase in the tear volume over placebo on days 28 and 56 (P < 0.0001) while nearing significance on day 14 (P = 0.0580) (Fig. 2a).
Fig. 2.
Summary of efficacy endpoint results—LCD vs. placebo. Schirmer’s test (a), Ocular Surface Disease Index (OSDI) (b), tear film break-up time (TBUT) (c), and Standard Patient Evaluation of Eye Dryness (SPEED) (d) results of LCD group showed a statistically significant increase in mean over placebo group on days 14, 28 and 56. Corneal staining (e) and conjunctival staining (f) scores and tear osmolarity (g) mean of the LCD group showed a statistically significant decrease over the placebo group on day 56. *P < 0.05. **P < 0.01
OSDI The baseline mean OSDI scores for LCD and placebo groups were 30.10 ± 4.88 and 32.54 ± 5.06, respectively, and were not significantly different from each other. In the LCD group, the mean OSDI score reduced to 25.24 ± 4.74 (↓15.8 ± 9.94%), 19.16 ± 4.40 (↓35.5 ± 13.93%) and 17.09 ± 4.85 (↓42.7 ± 14.42%) on days 14, 28 and 56, respectively. In the placebo group, mean OSDI scores were reduced to 30.68 ± 5.48 (↓4.93 ± 13.69%) on day 14, 30.60 ± 5.91 (↓5.21 ± 14.71%) on day 28 and 31.00 ± 7.18 (↓3.93 ± 19.28%) on day 56 (Table 3b).
The LCD group showed a statistically significant decrease (P = 0.0001) in the mean OSDI score over the placebo group on days 14, 28 and 56 as per between-group analysis using ANOVA test (Fig. 2b).
Secondary Efficacy Analyses
TBUT In the LCD group, the baseline mean TBUT score for the average of both eyes was 8.18 ± 1.18 s, which increased to 9.97 ± 1.80 s (↑22.21 ± 17.33%) on day 14, 11.03 ± 1.32 s (↑36.56 ± 18.93%) on day 28 and 12.13 ± 1.38 s (↑51.10 ± 26.53%) on day 56. In the placebo group, the baseline mean TBUT score for the average of both eyes was 7.86 ± 1.25 s, which increased to 7.97 ± 1.48 s (↑2.60 ± 19.30%) on day 14, 7.93 ± 1.18 s (↑2.55 ± 17.66%) on day 28 and decreased to 6.86 ± 1.51 s (↓12.1 ± 16.53%) on day 56 (Table 3c).
No significant differences were observed between the LCD and placebo groups before supplementation as analyzed by between-group analysis using ANOVA test, suggesting homogeneity of subjects across the groups at baseline. However, the LCD group showed a statistically significant increase in the mean TBUT score over the placebo group on days 14, 28 and 56 (P < 0.0001) (Fig. 2c).
SPEED The baseline mean SPEED scores for the LCD and placebo groups were 11.63 ± 1.25 and 12.21 ± 1.78, respectively, and therefore were not significantly different from each other. The mean SPEED score gradually reduced to 8.97 ± 1.30 (↓22.4 ± 11.36%) on day 14, 7.07 ± 1.36 (↓38.9 ± 11.28%) on day 28 and 6.07 ± 1.86 (↓47.3 ± 16.39%) on day 56 in the LCD group. However, in the placebo group, the mean SPEED scores were 11.03 ± 2.23 (↓8.72 ± 17.13%) on day 14, 10.31 ± 2.16 (↓14.1 ± 19.87%) on day 28 and 12.41 ± 2.90 (↑3.64 ± 28.06%) on day 56 (Table 3d).
The LCD group showed a statistically significant decrease in mean SPEED score over the placebo group on days 14, 28 and 56 (P < 0.0001) (Fig. 2d) as per between-group analysis using ANOVA test.
Corneal Staining Scores There was a decrease in the mean corneal staining score for the average of both eyes from 1.14 ± 0.17 to 0.79 ± 0.25 with 31.1 ± 20.06% reduction for the LCD group whereas it increased from 1.10 ± 0.17 to 1.24 ± 0.20 with 13.98 ± 16.74% increase in the placebo group on day 56 (Table 3e).
In the between-group analysis using ANOVA test, data showed no significant differences between the LCD and placebo groups before supplementation, but the LCD group showed a statistically significant decrease (P < 0.0001) in mean corneal staining score compared to the placebo group on day 56 (Fig. 2e).
Conjunctival Staining Scores There was a decrease in the mean conjunctival staining score for the average of both eyes from 0.88 ± 0.22 at baseline to 0.58 ± 0.32 with 35.5 ± 33.34% reduction on day 56 for the LCD group whereas an increase in the mean conjunctival staining score was observed for the placebo group from 0.86 ± 0.20 at baseline to 1.03 ± 0.27 with 19.53 ± 18.84% increase on day 56 (Table 3f). While between-group analysis using ANOVA test showed no significant differences between the LCD and placebo groups before supplementation at baseline, it showed a statistically significant decrease (P < 0.0001) in the mean conjunctival staining score for the LCD group compared to the placebo group on day 56 (Fig. 2f).
Tear Osmolarity Mean tear osmolarity for the average of both eyes for the LCD group was 327.23 ± 12.42 mOsms/l at baseline, which decreased by 2.07 ± 1.87% to 320.42 ± 12.24 mOsms/l on day 56. However, mean tear osmolarity was increased in the placebo group from 327.34 ± 11.06 mOsms/l at baseline to 331.72 ± 11.20 mOsms/l at day 56 with a slight increase of 1.35 ± 1.71% (Table 3g).
The between-group analysis using ANOVA test showed no significant differences between LCD and placebo groups at baseline but the LCD group showed a statistically significant decrease (P < 0.0005) in tear osmolarity compared to the placebo group on day 56 (Fig. 2g).
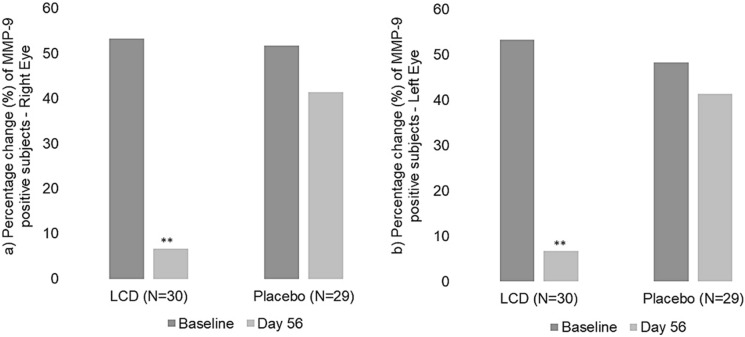
MMP-9 Biomarker MMP-9 levels were measured at baseline and end of the study in the tears of the subjects using the InflammaDry® test. Sixteen subjects (53.33%) out of 30 from the LCD group and 15 subjects (51.72%) out of 29 from the placebo group showed positive MMP-9 results in the right eye at the baseline visit. Sixteen subjects (53.33%) out of 30 from the LCD group and 14 (48.28%) out of 29 subjects from the placebo group showed positive MMP-9 results in the left eye at the baseline visit (Table 4a, b; Fig. 3a, b). However, on day 56, only two subjects from the LCD group showed positive MMP-9 results for both eyes, which was a reduction of 87.50% from baseline. In the placebo group, 12 subjects showed positive MMP-9 results, which was a reduction of only 20% for the right eye and 14.29% for the left eye from the baseline.
Table 4.
Number of subjects positive for MMP-9 biomarker between LCD and placebo groups
| Visit | LCD (N = 30) | Placebo (N = 29) | P-value by Z-test for LCD vs. placebo | ||
|---|---|---|---|---|---|
| Negative | Positive | Negative | Positive | ||
| n (%) | n (%) | n (%) | n (%) | ||
| a. Right eye | |||||
| Baseline | 14 (46.67) | 16 (53.33) | 14 (48.28) | 15 (51.72) | 0.9015 |
| Day 56 | 28 (93.33) | 2 (6.67) | 17 (58.62) | 12 (41.38) | 0.0017** |
| b. Left eye | |||||
| Baseline | 14 (46.67) | 16 (53.33) | 15 (51.72) | 14 (48.28) | 0.6977 |
| Day 56 | 28 (93.33) | 2 (6.67) | 17 (58.62) | 12 (41.38) | 0.0017** |
N number of subjects in specified treatment, n number of subjects in specified category, % n (number of subjects in specified category)/N (number of subjects in specified treatment) × 100
*P < 0.05
**P < 0.01
Fig. 3.
Percentage change of MMP-9 biomarker presence—LCD vs. placebo (a right eye; b left eye). A significant reduction in the number of subjects with MMP-9 biomarker-positive results for tear inflammation was observed on day 56 for the LCD group compared to the placebo group. P < 0.05. **Statistically significant, P < 0.01
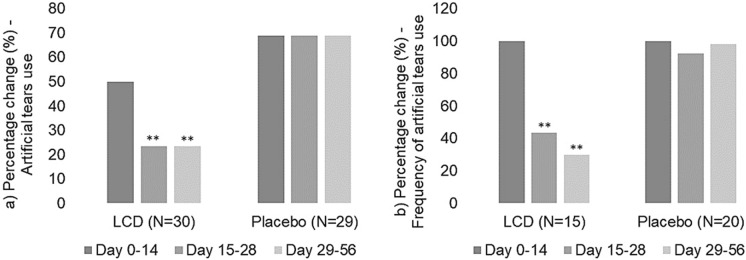
Artificial Tear Use and Frequency Under ethical guidelines, the subjects were allowed to use artificial tears during the study period as a rescue medication. Fifteen (50%) subjects in the LCD group and 20 (68.97%) subjects in the placebo group used artificial tears between day 0 and 14. However, only 7 (23.33%) subjects in the LCD group and 20 (68.97%) subjects in the placebo group continued using artificial tears from day 15 to 56. There was a 53.33% reduction in the use of artificial tears from baseline to day 56 for the LCD group, and no change was observed for the placebo group (Table 5; Fig. 4a).
Table 5.
Summary of the number of subjects who used artificial tears between LCD and placebo groups
| Visit | LCD (N = 30) | Placebo (N = 29) | P-value by Z-test for LCD vs. placebo | ||
|---|---|---|---|---|---|
| No | Yes | No | Yes | ||
| n (%) | n (%) | n (%) | n (%) | ||
| Day 0–14 | 15 (50.00) | 15 (50.00) | 9 (31.03) | 20 (68.97) | 0.1382 |
| Day 15–28 | 23 (76.67) | 7 (23.33) | 9 (31.03) | 20 (68.97) | 0.0004** |
| Day 29–56 | 23 (76.67) | 7 (23.33) | 9 (31.03) | 20 (68.97) | 0.0004** |
N number of subjects in specified treatment, n number of subjects in specified category, % n (number of subjects in specified category)/N (number of subjects in specified treatment) × 100
*P < 0.05
**P < 0.01
Fig. 4.
Percentage change of artificial tear usage (a) and frequency during the study (b)—LCD vs. placebo. The LCD group showed a significant reduction in both the number of subjects and the frequency of using artificial tears from day 15 to 28 and day 29 to 56 compared to the placebo group. *P < 0.05. **P < 0.01
The between-group analysis using Z-test indicated a significant reduction in the number of subjects using artificial tears from day 15 to 28 (P = 0.0004) and day 29 to 56 (P = 0.0004) for the LCD group compared to the placebo group (Fig. 4a).
Furthermore, we observed that artificial tears were used 2.47 times/day in the LCD group and 2.65 times/day in the placebo group between day 0 to 14, which reduced to 1.07 times/day, a 64.40% reduction from baseline between day 15 to 28 and 0.73 times/day, and a reduction of 75.60% from baseline between day 29 to 56 in the LCD group. However, in the placebo group the use of artificial tears remained largely unchanged between baseline to day 56 with 2.45 times/day and 2.60 times/day, respectively, from day 15 to 28 and 29 to 56 (Table 6). The between-group analysis using the Kruskal-Wallis test showed a significant reduction in the frequency of artificial tears use from day 15 to 28 (P = 0.0009) and day 29 to 56 (P < 0.0001) in the LCD group compared to the placebo group (Fig. 4b).
Table 6.
Summary of the mean frequency of artificial tears use between LCD and placebo groups
| Visit | LCD (N = 15) | Placebo (N = 20) | P-value by Kruskal–Wallis test for LCD vs. placebo | |
|---|---|---|---|---|
| Mean (%) | Mean (%) | |||
| Day 0–14 | 2.47 (100.0) | 2.65 (100.0) | 0.2851 | |
| Day 15–28 | 1.07 (43.24) | 2.45 (92.45) | 0.0009** | |
| Day 29–56 | 0.73 (29.73) | 2.60 (98.11) | < 0.0001** | |
N number of subjects in specified treatment
*P < 0.05
**P < 0.01
Safety
The safety analysis was conducted for all the subjects who were randomized for the trial and consumed at least one dose of the study product. We found that the study product was safe and well tolerated with no incidence of supplementation-related adverse events. Though a total of four subjects reported adverse events with one subject from the LCD group reporting fever and three subjects from the placebo group reporting events such as body pain, headache and paresthesia, none of these adverse events were found to be related to the study supplements. No adverse events were severe; they were of mild intensity. There were no notable changes in urine analysis parameters, vital signs and physical examination. Hematology and biochemical laboratory results remained relatively unchanged.
Discussion
DES is a disease of the ocular surface and the tear film [26] associated with decreased tear production, tear-film instability, tear hyperosmolarity, ocular inflammation, and ocular surface injury and morbidity [26–28]. Current treatment approaches for DES are palliative in nature and do not address the underlying cause of the condition [29]. Earlier, we reported a novel and optimized oral multi-ingredient formulation containing lutein, zeaxanthin, curcumin and vitamin D3 that ameliorated the symptoms of dry eye condition in benzalkonium chloride-induced DES in rats by reducing inflammation and oxidative stress and promoting tear production. The current study was undertaken to further explore the efficacy and safety of our multi-ingredient supplement in human subjects with mild to moderate DES. We used multiple clinically validated endpoints and subjective clinical and laboratory parameters to measure the efficacy of our formulation in this clinical study. We observed clinically and statistically significant improvements in all the endpoints measured during the study including tear production, tear stability, tear quality, tear osmolarity and reduced ocular inflammation, symptoms and ocular surface damage after 56 days of supplementation with our formulation. Furthermore, we also observed that the formulation is safe and well tolerated in study subjects and can be used to decrease the symptoms in subjects with DES.
We assessed the effect of the test formulation, LCD, on tear production using Schirmer’s test, which measures tear production from the lacrimal gland [30, 31]. Schirmer’s test is a validated method of evaluating aqueous-deficient dry eyes under clinical conditions [26] and has been used for approval of topical cyclosporine in the treatment of patients with DES [32]. We observed a 127.2% increase in Schirmer’s strip wetness length from baseline in the LCD group at day 56 and a statistically significant (P < 0.0001) increase in tears in the LCD group compared to placebo. Furthermore, we assessed tear function using the TBUT test, which measures the amount of time it takes for tears to break up after blinking [30]. TBUT of < 10 s is abnormal, indicating tear film instability [33]. We observed a 51.10% increase in TBUT score on day 56 of the supplementation compared to baseline and a statistically significant (P < 0.0001) increase in TBUT score at all the time points measured compared to the placebo group.
Symptom questionnaires such as OSDI score and SPEED score that explore DES in depth including diagnosis and impact on the quality of life are used to track the progression of dry eye symptoms of the subjects over time. We observed statistically significant improvements (P < 0.0001) in both OSDI score and SPEED score at all the time points measured for the LCD group compared to the placebo. Similarly, we observed a significant reduction (P < 0.0001) in the ocular surface damage as measured by corneal and conjunctival staining in the LCD group compared to the placebo on day 56 of the supplementation. Corneal and conjunctival damage is a common consequence of DES triggered by ocular surface inflammation, which is usually measured by the staining methods [26].
Increased tear film evaporation associated with reduced tear production in DES results in tear film hyperosmolarity, which in turn stimulates the inflammatory response. We observed a statistically significant decline (P < 0.0005) in tear hyperosmolarity in the LCD group compared to the placebo group on day 56 of supplementation. MMPs are endopeptidases involved in extracellular matrix remodeling after corneal surface damage and play an important role in the pathogenesis of dry eye with elevated levels in tears of dry eye patients [11, 34–36]. The InflammaDry® is a rapid, non-invasive, clinically validated kit that measures MMP-9 levels in the tear fluid and helps in the diagnosis and monitoring of subjects during treatment response evaluation [37]. We found that > 50% of the subjects in both groups were positive for MMP-9 at the beginning of the study. The placebo group showed 41.38% of the subjects positive for MMP-9 after 56 days of the supplementation, whereas the LCD group showed only 6.67% of the subjects positive for MMP-9 at the end of the study, thus clearly demonstrating the reduction of inflammatory response due to the nutritional supplementation.
All the subjects were allowed to use artificial tears for ethical reasons in the current study. We observed that for subjects who were administered LCD, the number of subjects and frequency of use of artificial tears were significantly reduced whereas the same number of subjects in the placebo group continued to use artificial tears and the frequency of use was not reduced.
Collectively, the results from our study indicate that 8 weeks of supplementation with our formulation helps in improving tear production, stabilizes tear film and eventually reduces hyperosmolarity of the tears in subjects suffering from DES. This further results in decreased inflammation as measured by reduced MMP-9 levels in the tear fluid and decreased ocular surface damage as measured by corneal and conjunctival staining during supplementation. Overall, we have demonstrated statistically significant clinical improvements in dry eye symptoms compared to the placebo in subjects, which was further validated through well-established subjective questionnaire-based approaches such as OSDI and SPEED with statistically significant improvements in the scores throughout the study. Our results further validate our previous study [26] done using a rat dry eye model where we demonstrated that a combination of lutein, zeaxanthin, curcumin and vitamin D3 is effective in managing the symptoms of dry eye condition with a multi-modal mechanism of action.
Conclusions
The formulation was found to be safe and well tolerated in both humans as observed in the current study and in an animal model as published earlier [26]. In conclusion, our study demonstrated that our multi-ingredient supplement made of traditional and well-established nutritional ingredients significantly improved the symptoms of dry eye after 8 weeks of oral administration as compared to placebo and may be a safer alternative to reduce the symptoms of dry eye condition.
One of the limitations was that the study supplementation was provided only for 8 weeks, and additional studies may be required to evaluate the potential of prolonged benefits with a longer study duration of 6 months. Second, we used soybean oil for the placebo as we wanted to use an oil that lacked high omega 3 content to avoid any clinical efficacy from the oil in the placebo group. Soybean oil is the most consumed vegetable oil in the world including the US and likely to not interfere with the study results [38, 39].
Acknowledgements
We thank the participants of the study. The authors acknowledge the guidance and support provided by Dr. Arun Balakrishnan, Dr. Muralidhara Padigaru and Mr. Abhijeet Morde from OmniActive Health Technologies Ltd., Prof. Govindasamy Kumaramanickavel, Independent Consultant, and the staff of Lifepoint Multispecialty Hospital, Pune, Maharashtra, India, for conducting this study.
Funding
The study was supported by OmniActive Health Technologies Limited (Mumbai, India). The sponsor also funded the journal’s Rapid Service Fees and Open Access Fees.
Medical Writing, Editorial, and Other Assistance
Editorial assistance in the preparation of this article was provided by Dr. Prabhu Shankar Lakshmanan of G7 Synergon Private Limited, Bengaluru, India.
Authorship
All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this article, take responsibility for the integrity of the work as a whole, and have given their approval for this version to be published.
Authorship Contributions
All authors contributed to the study conception and design. Study product was supplied by OmniActive Health Technologies Limited. Study conduct, subject recruitment and data collection were performed by Dr. Pranav Radkar and Dr. Sunil Chaudhary. Statistical analysis and study report were completed by Ms. Jenet Jemila Mary, Dr. Durairaj Sathish Kumar and Dr. Prabhu Shankar Lakshmanan. The first draft of the manuscript was written by Dr. Prabhu Shankar Lakshmanan and Dr. Pranav Radkar, and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Disclosures
The entire study, including the journal’s publication fee, was sponsored by OmniActive Health Technologies Limited (Mumbai, India).
Compliance with Ethics Guidelines
Institutional ethics approval was obtained from LPR (LifePoint Research) Ethics Committee, Pune, India (EC Re-Registration Number ECR/751/Inst/MH/2015/RR-18). This study was conducted in compliance with the ethical principles originating in or derived from the Declaration of Helsinki and in compliance with the International Conference on Harmonization (ICH), Good Clinical Practice (GCP) Guidelines, as well as in strict compliance with the “New Drugs and Clinical Trial Rules- 2019,” the Ministry of Health and the Government of India, at all stages of the trial for adherence to protocol and compliance with ethical and regulatory guidelines. The EC was duly apprised of the progress and updates of the trial at regular intervals as per prescribed guidelines.
Data Availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
- 1.Bron AJ, Abelson MB, Ousler G, Pearce E, Tomlinson A, Yokoi N, et al. Methodologies to diagnose and monitor dry eye disease: report of the Diagnostic Methodology Subcommittee of the International Dry Eye WorkShop (2007) Ocul Surf. 2007;5(2):108–152. doi: 10.1016/S1542-0124(12)70083-6. [DOI] [PubMed] [Google Scholar]
- 2.Bielory L, Syed BA. Pharmacoeconomics of anterior ocular inflammatory disease. Curr Opin Allergy Clin Immunol. 2013;13(5):537–542. doi: 10.1097/ACI.0b013e328364d843. [DOI] [PubMed] [Google Scholar]
- 3.Nazrana N, Jain T, Verma S. Role of nutrition in maintaining normal eyesight-a review. Int J Med Biomed Stud. 2020;4(3):194–198. doi: 10.32553/ijmbs.v4i3.1068. [DOI] [Google Scholar]
- 4.Tiffany JM. The normal tear film. In: Geerling G, Brewitt H, editors. Surgery for the dry eye. Karger Publishers; 2008:1–20.
- 5.Stapleton F, Alves M, Bunya VY, Jalbert I, Lekhanont K, Malet F, et al. Tfos dews ii epidemiology report. Ocul Surf. 2017;15(3):334–365. doi: 10.1016/j.jtos.2017.05.003. [DOI] [PubMed] [Google Scholar]
- 6.Farrand KF, Fridman M, Stillman IÖ, Schaumberg DA. Prevalence of diagnosed dry eye disease in the United States among adults aged 18 years and older. Am J Ophthalmol. 2017;182:90–98. doi: 10.1016/j.ajo.2017.06.033. [DOI] [PubMed] [Google Scholar]
- 7.Farrand K, Stillman I, Fridman M, Schaumberg D. Impact of dry eye disease on quality of life, work productivity, daily activities, and health care resource use in a survey of 74,095 American adults. Value Health. 2016;19(3):A127. doi: 10.1016/j.jval.2016.03.521. [DOI] [Google Scholar]
- 8.Bron AJ, de Paiva CS, Chauhan SK, Bonini S, Gabison EE, Jain S, et al. Tfos dews ii pathophysiology report. Ocul Surf. 2017;15(3):438–510. doi: 10.1016/j.jtos.2017.05.011. [DOI] [PubMed] [Google Scholar]
- 9.Stern ME, Schaumburg CS, Pflugfelder SC. Dry eye as a mucosal autoimmune disease. Int Rev Immunol. 2013;32(1):19–41. doi: 10.3109/08830185.2012.748052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sambursky R, Davitt WF, Latkany R, Tauber S, Starr C, Friedberg M, et al. Sensitivity and specificity of a point-of-care matrix metalloproteinase 9 immunoassay for diagnosing inflammation related to dry eye. JAMA Ophthalmol. 2013;131(1):24–28. doi: 10.1001/jamaophthalmol.2013.561. [DOI] [PubMed] [Google Scholar]
- 11.Pflugfelder SC. Antiinflammatory therapy for dry eye. Am J Ophthalmol. 2004;137(2):337–342. doi: 10.1016/j.ajo.2003.10.036. [DOI] [PubMed] [Google Scholar]
- 12.Yang C, Sun W, Gu Y. A clinical study of the efficacy of topical corticosteroids on dry eye. J Zhejiang Univ Sci B. 2006;7(8):675–678. doi: 10.1631/jzus.2006.B0675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Asbell PA, Maguire MG, Pistilli M, Ying G, Szczotka-Flynn LB, Hardten DR, et al. n-3 Fatty acid supplementation for the treatment of dry eye disease. N Engl J Med. 2018;378(18):1681–1690. doi: 10.1056/NEJMoa1709691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Deinema LA, Vingrys AJ, Wong CY, Jackson DC, Chinnery HR, Downie LE. A randomized, double-masked, placebo-controlled clinical trial of two forms of omega-3 supplements for treating dry eye disease. Ophthalmology. 2017;124(1):43–52. doi: 10.1016/j.ophtha.2016.09.023. [DOI] [PubMed] [Google Scholar]
- 15.Chao S-C, Nien C-W, Iacob C, Hu D-N, Huang S-C, Lin H-Y. Effects of lutein on hyperosmoticity-induced upregulation of IL-6 in cultured corneal epithelial cells and its relevant signal pathways. J Ophthalmol. 2016;2016:1–7. doi: 10.1155/2016/8341439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chao S-C, Vagaggini T, Nien C-W, Huang S-C, Lin H-Y. Effects of lutein and zeaxanthin on LPS-induced secretion of IL-8 by uveal melanocytes and relevant signal pathways. J Ophthalmol. 2015;2015:1–7. doi: 10.1155/2015/152854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Reins RY, Baidouri H, McDermott AM. Vitamin D activation and function in human corneal epithelial cells during TLR-induced inflammation. Invest Ophthalmol Vis Sci. 2015;56(13):7715–7727. doi: 10.1167/iovs.15-17768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Xue M-L, Zhu H, Thakur A, Willcox M. 1α, 25-Dihydroxyvitamin D3 inhibits pro-inflammatory cytokine and chemokine expression in human corneal epithelial cells colonized with Pseudomonas aeruginosa. Immunol Cell Biol. 2002;80(4):340–345. doi: 10.1046/j.1440-1711.80.4august.1.x. [DOI] [PubMed] [Google Scholar]
- 19.Zhang J, Dai Y, Wu D, Xu J. Calcitriol, the active metabolite of Vitamin D3, inhibits dry eye related corneal inflammation in vivo and in vitro. Ocul Immunol Inflamm. 2019;27(2):257–265. doi: 10.1080/09273948.2017.1372486. [DOI] [PubMed] [Google Scholar]
- 20.Yoon SY, Bae SH, Shin YJ, Park SG, Hwang S-H, Hyon JY, et al. Low serum 25-hydroxyvitamin D levels are associated with dry eye syndrome. PLoS ONE. 2016;11(1):e0147847. doi: 10.1371/journal.pone.0147847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bernstein PS, Li B, Vachali PP, Gorusupudi A, Shyam R, Henriksen BS, et al. Lutein, zeaxanthin, and meso-zeaxanthin: The basic and clinical science underlying carotenoid-based nutritional interventions against ocular disease. Prog Retin Eye Res. 2016;50:34–66. doi: 10.1016/j.preteyeres.2015.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Liu X-F, Hao J-L, Xie T, Mukhtar NJ, Zhang W, Malik TH, et al. Curcumin, a potential therapeutic candidate for anterior segment eye diseases: a review. Front Pharmacol. 2017;8:66. doi: 10.3389/fphar.2017.00066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chung S-H, Choi SH, Choi JA, Chuck RS, Joo C-K. Curcumin suppresses ovalbumin-induced allergic conjunctivitis. Mol Vis. 2012;18:1966. [PMC free article] [PubMed] [Google Scholar]
- 24.Chen M, Hu D-N, Pan Z, Lu C-W, Xue C-Y, Aass I. Curcumin protects against hyperosmoticity-induced IL-1β elevation in human corneal epithelial cell via MAPK pathways. Exp Eye Res. 2010;90(3):437–443. doi: 10.1016/j.exer.2009.12.004. [DOI] [PubMed] [Google Scholar]
- 25.Morde A, Muz OE, Orhan C, Erten F, Tuzcu M, Ozercan IH, et al. Efficacy of a novel integrated active herbal formulation in experimentally induced rat model for dry eye disease. Curr Dev Nutr. 2020;4(Supplement_2):441–441. doi: 10.1093/cdn/nzaa045_074. [DOI] [Google Scholar]
- 26.Lemp MA, Foulks GN. The definition and classification of dry eye disease. Ocul Surf. 2007;5(2):75–92. doi: 10.1016/S1542-0124(12)70081-2. [DOI] [PubMed] [Google Scholar]
- 27.Phadatare SP, Momin M, Nighojkar P, Askarkar S, Singh KK. A Comprehensive review on dry eye disease: diagnosis, medical management, recent developments, and future challenges [Internet], Vol. 2015. Advances in Pharmaceutics. Hindawi; 2015: p. e704946. https://www.hindawi.com/journals/ap/2015/704946/. Accessed 10 Aug 2020.
- 28.Aragona P, Giannaccare G, Mencucci R, Rubino P, Cantera E, Rolando M. Modern approach to the treatment of dry eye, a complex multifactorial disease: a PICASSO board review. Br J Ophthalmol. 2021;105(4):446–453. doi: 10.1136/bjophthalmol-2019-315747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lee BS, Kabat AG, Bacharach J, Karpecki P, Luchs J. Managing dry eye disease and facilitating realistic patient expectations: a review and appraisal of current therapies. Clin Ophthalmol Auckl NZ. 2020;14:119. doi: 10.2147/OPTH.S228838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Dohlman TH, Ciralsky JB, Lai EC. Tear film assessments for the diagnosis of dry eye. Curr Opin Allergy Clin Immunol. 2016;16(5):487–491. doi: 10.1097/ACI.0000000000000307. [DOI] [PubMed] [Google Scholar]
- 31.Downie LE, Keller PR. A pragmatic approach to dry eye diagnosis: evidence into practice. Optom Vis Sci. 2015;92(12):1189–1197. doi: 10.1097/OPX.0000000000000721. [DOI] [PubMed] [Google Scholar]
- 32.Allergan . Restasis package insert. Irvine: Allergan; 2013. [Google Scholar]
- 33.Milner MS, Beckman KA, Luchs JI, Allen QB, Awdeh RM, Berdahl J, et al. Dysfunctional tear syndrome: dry eye disease and associated tear film disorders–new strategies for diagnosis and treatment. Curr Opin Ophthalmol. 2017;28(Suppl 1):3. doi: 10.1097/01.icu.0000512373.81749.b7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Messmer EM, von Lindenfels V, Garbe A, Kampik A. Matrix metalloproteinase 9 testing in dry eye disease using a commercially available point-of-care immunoassay. Ophthalmology. 2016;123(11):2300–2308. doi: 10.1016/j.ophtha.2016.07.028. [DOI] [PubMed] [Google Scholar]
- 35.Acera A, Rocha G, Vecino E, Lema I, Durán JA. Inflammatory markers in the tears of patients with ocular surface disease. Ophthalmic Res. 2008;40(6):315–321. doi: 10.1159/000150445. [DOI] [PubMed] [Google Scholar]
- 36.Solomon A, Dursun D, Liu Z, Xie Y, Macri A, Pflugfelder SC. Pro-and anti-inflammatory forms of interleukin-1 in the tear fluid and conjunctiva of patients with dry-eye disease. Invest Ophthalmol Vis Sci. 2001;42(10):2283–2292. [PubMed] [Google Scholar]
- 37.Tesón M, González-García MJ, López-Miguel A, Enríquez-de-Salamanca A, Martín-Montañez V, Benito MJ, et al. Influence of a controlled environment simulating an in-flight airplane cabin on dry eye disease. Invest Ophthalmol Vis Sci. 2013;54(3):2093–2099. doi: 10.1167/iovs.12-11361. [DOI] [PubMed] [Google Scholar]
- 38.Su H, Liu R, Chang M, Huang J, Wang X. Dietary linoleic acid intake and blood inflammatory markers: a systematic review and meta-analysis of randomized controlled trials. Food Funct. 2017;8(9):3091–3103. doi: 10.1039/C7FO00433H. [DOI] [PubMed] [Google Scholar]
- 39.US consumption of edible oils by type [Internet]. Statista. 2019. https://www.statista.com/statistics/301044/edible-oils-consumption-united-states-by-type/. Accessed 8 Mar 2021.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.