Abstract
Natural killer (NK) cells are known to play a role in mediating innate immunity and have been implicated in mediating anti-tumor responses via antibody-dependent cell-mediated cytotoxicity (ADCC) based on the reactivity of CD16 with the Fc region of human IgG1 antibodies. The NK-92 cell line, devoid of CD16 and derived from a lymphoma patient, has been well characterized. The adoptive transfer of irradiated NK-92 cells demonstrated safety and showed preliminary evidence of clinical benefit for cancer patients. The molecules 41BB and CD3 are commonly used as stimulators in the CAR structure, and their expression in NK cells can promote the activation of NK cells, leading to the enhanced perforin- and granzyme-mediated lysis of tumor cells. This study showed that genetically modified NK-92 cells combined with antibody-mediated ADCC using rituximab and trastuzumab monoclonal antibodies lysed tumor cells more efficient than the NK-92 cell lines. It also showed that the anti-tumor activity of chimeric stimulator molecules of the CAR-modified CD16 receptor was stronger than that of CD16 (allotype V158). These studies provide a rationale for the use of genetically modified NK-92 cells in combination with IgG1 anti-tumor monoclonal antibodies. We also provide a rationale for the chimeric modified CD16 receptor that can improve the anti-tumor effect of NK92 cells via ADCC.
Supplementary Information
The online version of this article (10.1007/s10616-021-00476-1) contains supplementary material, which is available to authorized users.
Keywords: Natural killer cells, NK-92, CD16, CAR, ADCC, Chimeric receptor
Introduction
Natural killer (NK) cells play a pivotal role in mediating innate immunity, which defends against virus-infected and malignant cells (Weiner et al. 2009; Paust et al. 2010; Gras Navarro et al. 2015). Unlike cytotoxic T lymphocytes, NK cells target tumors, and virus-infected cells circumvent major histocompatibility complex (MHC) (Gladow et al. 2004). NK cells are activated without stimulation (Carson et al. 2001). However, a variety of cytokines, including interleukin (IL)-2 and IL12, can enhance NK cell cytotoxic activity. In addition to these mechanisms, NK cells play a role in anti-tumor immunity alone or in combination with specific antibodies via antibody-dependent cell-mediated cytotoxicity (ADCC). For adoptive immunotherapy, NK cells provide a effector cell type with the capacity of recognizing antigen-positive and MHC-I-negative or MHC-I-low tumor cells (Yokoyama and Kim 2006). However, NK cells can malfunction due to the lack of adhesion molecules presented on the cell surface, for example, the HLA-G molecule, which can bind to immunoglobulin-like receptors (KIRs), or the production of molecules by lymphoblasts, such as MICA and MICB that binding to NK cell activating receptors, such as NKG2D (Fernandez-Messina et al. 2012; Yang et al. 2013). Allogeneic NK cells are used for manipulation unless the T cell is depleted to prevent graft versus host disease (GVHD). By contrast, the activated NK cell line NK-92, which continuously grows and is IL-2 dependent, can easily be expanded in vitro and is highly cytotoxic against a variety of malignant cells. Homologous NK cells from patients are often poor, but the safety of NK92 cells has been studied in phase I trials (Scott et al. 2001). Preclinical studies showed that NK-92 cells do not form tumors when transplanted into severe combined immunodeficiency (SCID) or athymic mice. Several clinical studies show repeated infusions of irradiated NK-92 cells were harmless. Clinical responses were observed in patients with melanoma, lung cancer, Merkel cell carcinoma, lymphoma, and kidney cancer (Klingemann et al. 2016). Despite the allogeneic nature of NK-92 cells, the formation of anti-human leukocyte antigen (HLA) antibodies was observed in less than half of the patients. Moreover, the pharmacodynamics of NK-92 clearance did not differ upon repeated doses. NK-92 cells do not express the CD16 Fc receptor, which is necessary for the NK-mediated ADCC lysis of tumor cells.
Many patients benefit from adoptive immunotherapy of some mAbs, such as the rituximab (Rituxan) and the trastuzumab (Herceptin), and cell-mediated immunity is recognized as one of the mechanisms responsible for their clinical efficiency (Koene et al. 1997; Cartron et al. 2002). In addition to lysing malignant and virus-infected cells, ADCC, which is mediated by the CD16 (FccRIIIa) receptor present on the NK cells surface, can primarily be activated in NK cells. Therefore some clinical studies employing the IgG1 isotype mAbs trastuzumab (Herceptin) or rituximab (Rituxan) showed that breast and lymphoma NSG mice, respectively, whose NK-92 cells express CD16 have improved overall survival compared to NK-92 cell lines (Bibeau et al. 2009). Similarly, after transduction with the FcIIIγRa/FεcRI (referred to as CD16/γ) receptor fusion gene, CD4+ and CD8+ cytotoxic T lymphocytes stably expressed the CD16/γ receptor on their surface and mediated ADCC. Thus, associating a therapeutic mAb and CD16/γ-transduced T cells could combine the advantages associated with the functional potential of cytotoxic lymphocytes and recognition of target cells unrestricted by the major histocompatibility complex (Clemenceau et al. 2015). This strategy can significantly improve NK92 cell killing cell activity. However, the single activation signal factor can only cause a weak reaction.
NK-92 cells have now been engineered to express the CD16 high-affinity FcγRIIIa (158 V) receptor (Brown et al. 2017). The study showed that single nucleotide polymorphisms (SNPs) at the FccRIIIa amino acid position 158 regulated ADCC. NK cells can have two CD16 valine (V) alleles with 158 V (V/V genotype), which creates the high-affinity Fc receptor FcγRIIIa, two alleles of 158 F (F/F genotype), which is lower affinity, or one of each (V/F genotype). IL-2 can replenish the granular stock of NK cells, leading to the enhanced perforin- and granzyme-mediated lysis of “exhausted” NK cells (Vanherberghen et al. 2013). NK cells could against some types of tumor because of its nonspecific.
Adoptive T cells or NK cells effectively eliminate tumors in vivo and vitro (Barrett et al. 2014). CAR generally contains a single-chain variable fragment from a conventional monoclonal antibody (mAb) in its extracellular antigen-binding domain, a hinge, such as CD8 IgD domain hinge, which can improve antigen-antibody binding efficiency, its transmembrane domains and it′s cell signaling endodomain (Qin et al. 2017). Intracellular costimulation domains, such as 41BB (CD137) and CD28, play important roles in adoptive cell therapy. Previous studies revealed that 41BB can enhance NK cell activation, reproduction and antileukemic responses (Kruschinski et al. 2008). Thus, we used CD16 extracellular domains (affinity antibody segment) in which the affinity antigenic segment on the CAR structure was replaced. We constructed the recombinant plasmids CD16/CAR and transfected it into the engineered cell line NK92.
The aim of the present study was to directly compare the single signal structure Fc receptor (CD16V158) with the common stimulus signal receptor (CD16/CAR) in combination with the antibody ADCC-mediated specific anti-tumor effect. To this end, as a first step in designing a model, we equipped the same cytotoxic lymphocyte line (the human NK cell line NK-92) with either FcγIIIa (referred to as NK92-CD16) or FcγIIIa/CAR (referred to as NK92-41BB) and compared their efficiencies in killing HER2- and CD20-positive tumor target cells in vitro. The study showed that the genetically modified NK92 cells did not affect the cancer cell killing activity. Simultaneously, chimeric cells (NK92- 41BB) significantly improved the antitumor activity. Similarly, the presence of the 41BB signal factor increased the NK92 cell secretion of perforin and granzyme.
Materials and methods
Cells and culture medium
NK-92 cells, the human NK cell line (ATCC, Rockville, MD), were grown in RPMI 1640 culture media (Gibco, Cergy Pontoise, France) supplemented with 10% FBS (PAA Laboratories, Les Mureaux, France), 100 IU/mL IL-2 (Proleukin) (Chiron Corporation, Emeryville, US), 2 mM L-glutamine (Gibco), penicillin (100 IU/mL), and streptomycin (0.1 µg/mL) (Gibco). The HER2-negative MDA-MB-468 and HER2-positive BT-474, SKBR3, SKOV3 and MCF7 breast cancer cell lines were obtained from ATCC. Cell lines were cultured in complete media consisting of DMEM (Sigma Aldrich, St. Quentin Fallavier, France), 10% heat-inactivated fetal calf serum, 2 mM glutamine (Sigma Aldrich), 100 U/mL penicillin, and 10 µg/mL streptomycin (Sigma Aldrich).
Lentivirus vector production
Transient retroviral supernatants were produced using PEI precipitation with 15 µg plasmid. Two million HEK 293T cells were seeded onto 10 cm diameter dishes 24 h prior to transfection. The transfection was performed with 15 µg CD713B-CD16 and CD713B-CD16/CAR plasmids DNA using PEI precipitation (Invitrogen). The media (10 mL) were replaced 6 h after transfection. The conditioned medium was collected at 48 and 72 h after transfection, filtered through 0.45 μm pore-sized filters, and stored at 4 °C. The viral titer was determined by the transduction of 293T cells (1 × 106 cells per well in 6-well plates) with serial dilutions of virus and analyzed for eGFP expression 4 days after infection. The retroviral supernatant titers were typically 1–5 × 107 IU (infectious units)/mL.
NK-92 cell line transduction using lentivirus supernatant
The NK-92 cell line was resuspended in RPMI 1640 culture medium supplemented with 10% FBS and 100 IU/mL recombinant IL-2, seeded in 6-well plates at 1 × 106 cells in 1 mL per well, and exposed to 2 × 2 mL of lentivirus supernatant purified (overnight at 4 °C with PEG800 and 4000×g, 40 min) in the presence of 4 µg/mL polybrene (Sigma, St. Quentin Fallavier, France). The culture media were changed 24 h after infection. Mock (untransduced) controls, in which the supernatant of untransfected packaging cells was added to NK-92 cells, were utilized in parallel. The transduction efficiencies were assessed 2 days later by flow cytometry and screened with puromycin for flow detection at 7 and 14 days.
Flow cytometry
The state of the cells was adjusted in advance and cultured in complete culture media at 37 °C in a humidified incubator with 5% CO2. Logarithmic growth phase cells were counted and resuspended in fresh complete media. Next, 1 × 106 cells were placed into a 1.5 mL EP tube. The cells were centrifuged at 800×g for 5 min and washed twice with 1× PBS. The cells were resuspended in 100 µL of PBS. In the dark, 5 µL of the flow antibody anti-CD16-PE was added to the cell suspension. The sample was mix thoroughly at 37 °C in the dark for 30 min or at 4 °C overnight. The sample was centrifuged at 800×g for 5 min and washed twice with 1× PBS. The cells were resuspended in 500 µL of PBS and examined using flow cytometry.
Cytotoxicity and ADCC assay
We adjusted the cell state in advance. The target cells were digested with trypsin, and 2 mL of media were used to stop the digestion. Next, 10 µL of cell suspension was stained with trypan blue and counted. When the cell viability reached at least 90%, the cell density was adjusted to 105 cells/mL. The cell suspension was added to a 96-well cell culture plate at 90 µL/well. The plate was incubated overnight at 37 °C in a humified incubator saturated at 5% CO2. The next day, the antibody was diluted in 1×PBS at a concentration of 100 µg/mL. Then, 20 µL of antibody solution was added to the experimental group, and 20 µL of 1× PBS was added to the control group. We placed the samples in at 37 °C in a humidified incubator saturated at 5% CO2 for 30 min. The effector cells were grown in the logarithmic phase to ensure that the cell viability reached more than 90%. The cell density was adjusted, and the effector cell suspension was prepared. Effector cells were added to the target cell wells (90 µL/well) at effect/target ratios of 1:1; 4:1; 8:1; 16:1. The final volume was 200 µL/well. We set a blank control group, effector cell control group, target cell control group and antibody control group. Each group had 3 replicates. We tapped the 96-well plate and allowed the cells to mix thoroughly. The plate was incubated at 37 °C in a humified incubator saturated at 5% CO2 for 8 h. After the reaction, CCK-8 solution was added to the 96-well plate (20 µL/well) and placed in a humidified incubator at 37 °C saturated with 5% CO2 for 1.5 h. The absorbance was measured at 450 nm and 630 nm with a microplate reader. The cell killing efficiency (%) = [experimental group-effector cell control group]/[control group-blank control group] ×100%.
Statistical analysis
Data were expressed as the mean of triplicates ± S.D. unless stated otherwise. Data were compared by SPSS; P < 0.05 was considered significant.
Results
Expression of CD16 and the fusion gene CD16/CAR on the surface of NK-92 cells
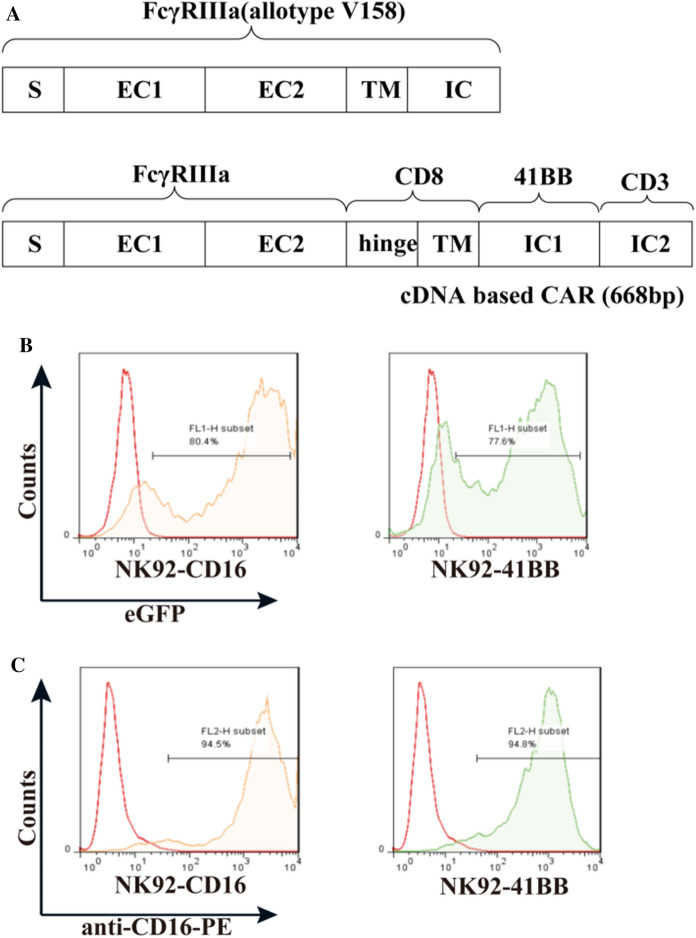
The chimeric cDNAs were synthesized by GeneCust. CD16 cDNA comprises the leader (S), two extracellular domains (EC1 and EC2) of the human V/V genotype, the intact transmembrane (TM) domain, and the intracellular (IC) domain (Fig. 1A). CD16/CAR chimeric cDNA comprises the leader (S), two extracellular domains (EC1 and EC2) of human CD16, the intact transmembrane (TM) domain and the intracellular (IC) domain with CAR (Fig. 1A). The gene was linked to the lentiviral vector pCDH-CMV, and the lentivirus particles were expressed in 293T cells (Sup 1). The purified particles were measured by titer, which was 107TU/ml. After transduction, NK-92 cells were engineered to express the high-affinity CD16 Fc receptor CD16 (FcγRIIIa 158 V) and CD16/CAR (FcγRIIIa 158 V/CAR); 80.4% of the NK-92 expressed CD16, and 77.6% expressed CD16/CAR via GFP detection (Fig. 1B). After 7 days of infection, the positive rate was above 90% when using puromycin for screening. After 14 days, the positive rate was stably above 95% (Sup1). The transduced NK-92 cells were screened with 10 mg/ml puromycin. The expression of CD16 and CD16/CAR was confirmed using FACS analysis with the human anti-CD16-PE, and the positive rate was up to about 95% (Fig. 1C). As described in the results, we obtained the Fc high-affinity NK-92 engineered cell lines NK92-CD16 (transduced CD16) and NK92-41BB (transducted CD16/CAR).
Fig. 1.
A Recombinant plasmid design and infection of NK92 cell lines. CD16 and a schematic representation of the chimera is shown. The CD16/CAR chimeric cDNA comprised the leader (S), two extracellular domains (EC1 and EC2), and the intact transmembrane (TM) domain of human CD16; however, intracellular segments were replaced by CAR molecules. B Recombinant lentiviral plasmid transiently infects 293T to express lentivirus particles and NK92 cells. Green fluorescent protein (eGFP) expression was assessed to determine the infection efficiency. C Anti-CD16-PE was used to determine CD16 expression in the CD16-transduced NK-92 cell line
Protein expression and activity analysis of genetic modification of NK92 cells
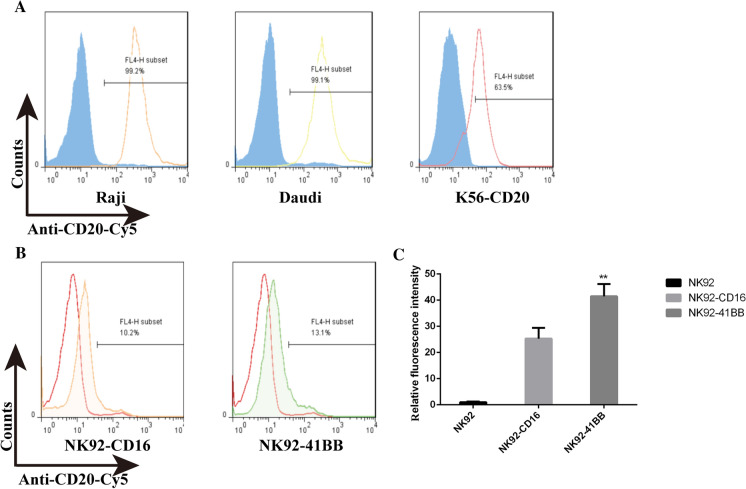
CD16 activity is its combination with the antibody Fc, and CD16 activity can be verified by detecting the genetic modification of NK92 cell affinity with an antibody. The CD20 antibody rituximab has an enormous profile in non-Hodgkin’s lymphoma (NHL). As shown in Fig. 2A, the CD20 antibody rituximab was predetermined by the fluorescein Cy5 and the combination reaction with the CD20 high-expression cell strains Raji and Daudi. The antibody was active, as the combined positive rate with Raji was 98.7%, and that with Daudi was 98%. Genetically engineering a modification of the constructed K562-CD20 cells were also associated with a positive of 45%. Because the fluorescein-Cy5 antibodies had a detectable activity, anti-CD20-Cy5 was combined with NK92-CD16 and NK92-41BB. As shown in Fig. 2B, combining rate of 13% for NK92-41BB was statistically significantly higher than the NK92-CD16 with binding rate of 10%. The antibody and cellular combination efficiencies were calculated using enzyme-linked immunoassay (ELISA), as shown in Fig. 2C; compared with those in NK92 cells, NK92-41BB and NK92-CD16 cells were significantly higher. The genetically modified cells stabilized the expression of the carried gene, and the expressed protein had a biological activity that was combined with Fc.
Fig. 2.
CD16 expression and activity detection. A The antibody-fluorescein (Cy5)-coupled protein was detected using the Raji strain highly expressing the CD20 protein, the Daudi cell strain, and the exogenously constructed K562-CD20 cell line. B The CD16 affinity antibody Fc was detected with rituximab-Cy5. C The antibody and cellular combination efficiency was calculated using ELISA
Comparison of tumor cell lysis using NK92 cells with modified or wild-type genes
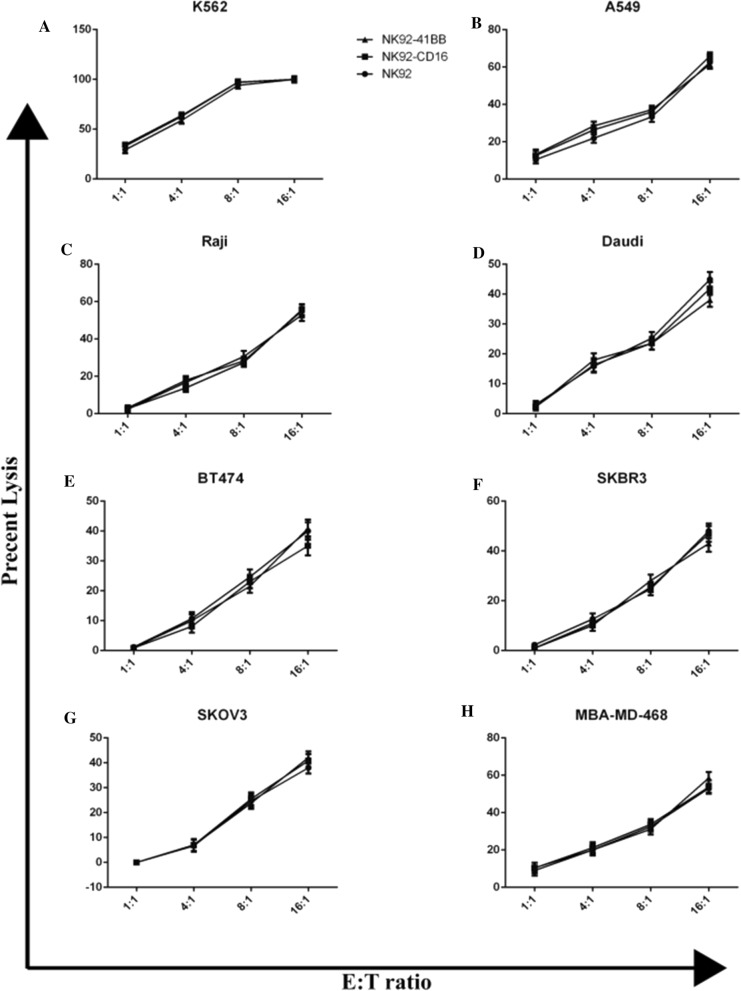
To investigate the cytotoxic activity of NK92-41BB cells against tumors without antibody, non-Hodgkin’s B cell lymphoma, breast cancer cells and ovarian cancer SKOV3 and adenocarcinoma A549 cells were selected. For all the target cells, the effects of lysis increased as the effect target ratio increased. As shown in Fig. 3, at an E:T ratio of 8:1, the K562 cells were nearly lysed. However, nearly 30% of the other target cells were lysed. At the efficiency target ratio of 16:1, the anti-tumor activity of these cells lysed only 40–60% of the target cells This indicated that K562 is sensitive to NK92 cells at low E:T ratios. They did not lyse the Raji, Daudi, BT474, SKBR3, SKOV3, or MBA-MD-468 cells at E:T ratios of 1:1 even when NK92-41BB and NK92-CD16 overexpressed CD16. This result illustrated that CD16 could not improve the cytotoxic activity against tumors. For the tumors we selected, there was no difference in the abilities of the NK92, NK92-41BB and NK92-CD16 to eliminate the tumors. We hypothesized that the cytotoxic activity of NK92 cells overexpressing CD16 could be improved when the corresponding antibody was present.
Fig. 3.
NK92 lysis of human tumor cell lines. NK cells were co-cultured with different target tumor cells to an 8-h lysis assay at different E:T ratios. Analysis of blood (A), lung (B), lymphoma (C and D), breast (E and F) and uterine tumors (G and H) using the CCK-8 assay. The results shown are the means (SD) of triplicate measurements from one of at least three comparable replicates
ADCC of modified NK92 cells were evaluated with CCK-8 assays
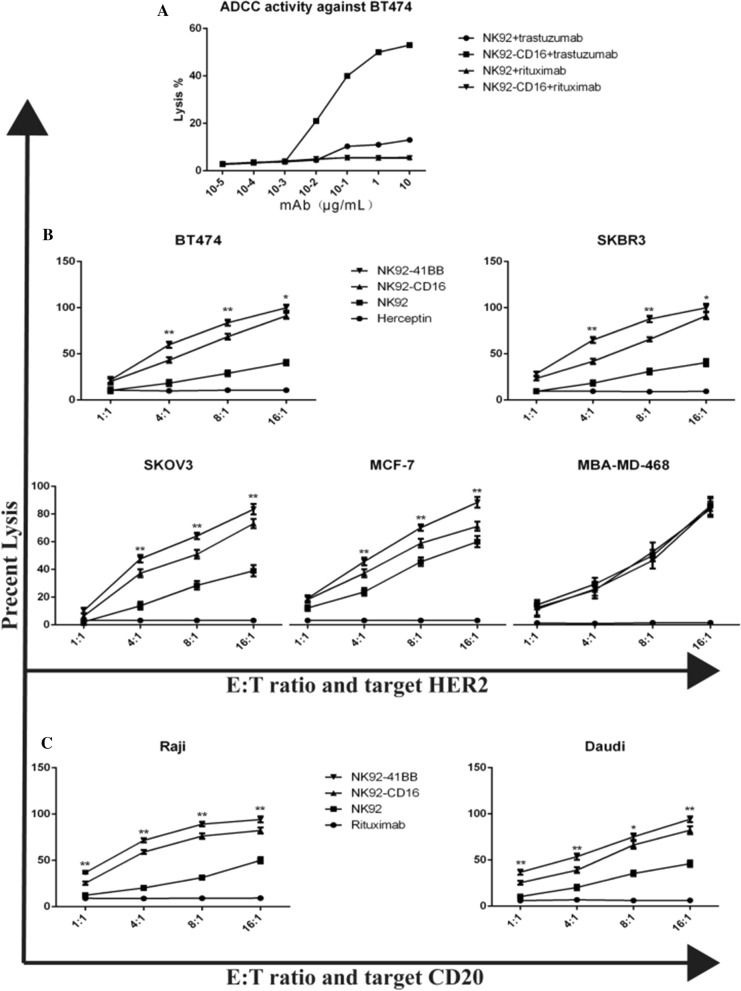
Antibody-dependent cellular cytotoxicity (ADCC) was mediated by the Fc receptor CD16. The ADCC activity of NK92-41BB cells against the BT474 cell line was tested in the presence of serial concentrations of trastuzumab and rituximab. This demonstrated a plateau (close to 60% of specialized lysis) at 12 µg/mL (E/T ratio: 8/1) (Fig. 4A). The ADCC effect was not observed in NK92-CD16 or NK92 cells in the presence of rituximab. Noted that a background level of lysis was observed for NK-92 cells with trastuzumab (compared to NK-92 with rituximab), likely due to the background expression of CD16 in the NK-92 cell line. For all other in vitro experiments, trastuzumab was used at a concentration of 10 µg/ml. Next, we compared the efficiencies of target cell lysis induced either after direct recognition of the HER2 by the NK-92 cells or after indirect recognition by the NK92, NK92-CD16 and NK92- 41BB cells in the presence of trastuzumab. To this end, these target cells (HER2-negative MDA-MB-468 cells and HER2-positive BT474 cells) were coated or not coated with trastuzumab, and NK-92 was used as a negative control. The cytotoxic activities of NK92-41BB and NK92-CD16 cells against trastuzumab coated or not coated with MDA-MB-468 and BT474 were summarized in Fig. 4B. Neither CD16 nor recombined CD16 (NK92-CD16 or NK92-41BB) presented significant levels of cytotoxicity against the HER2-negative MDA-MB-468 cell line. When tested against BT474, NK92- 41BB showed a high level of cytotoxic activity (25.81 ± 4.03% at 1:1, p < 0.05; 52.02 ± 3.66% at 4:1, p < 0.01; 72.18 ± 3.20% at 8:1, p < 0.01; 92.00 ± 4.27% at 16:1, p < 0.01) in the presence of trastuzumab. To confirm the differences in cytotoxic performance among NK92, NK92-CD16 and NK92- 41BB, further comparison was performed against 3 different HER2-positive cell lines: SKOV3, MCF-7, MBA-MD-468 (Fig. 4B). These data showed that under these experimental conditions in vitro, the co-stimulation of signaling pathways by NK92-41BB was always more effective than the single pathway by NK92 and NK92-CD16, which was based on 8 h cytotoxicity assays. Similarly, the cell lysis of Raji and Daudi also exhibited these results when combined with rituximab (Fig. 4C). This also indicated that 41BB in NK92-41BB could induce a more intense immune reaction with antigen stimulation in the presence of the corresponding antibody.
Fig. 4.
NK92 cells ADCC-mediated by trastuzumab and cetuximab were evaluated with 8 h CCK-8 assays. A The effector cells NK92-CD16 were tested against the HER2-positive BT474 cell line preincubated in the presence of increasing concentrations of trastuzumab (mean of two experiments). B Comparison of the killing effects of different modified NK92 cells in combination with trastuzumab on HER2-expressing cells. The highly-expressing cells BT474 and SKBR3, media-expressing cells SKOV3, low-expressing cells MCF-7, and negative-expressing cells MDA-MB-468 were used with the antibody at 10 µg/mL. C Comparing rituximab (10 µg/mL) in CD20-expressing cells of the Raji and Daudi cell strains. The results shown are the means (SD) of triplicate measurements from one of at least three comparable repeat experiments
Cell apoptosis were detected for the antitumor effect of NK92 cells mediated by ADCC
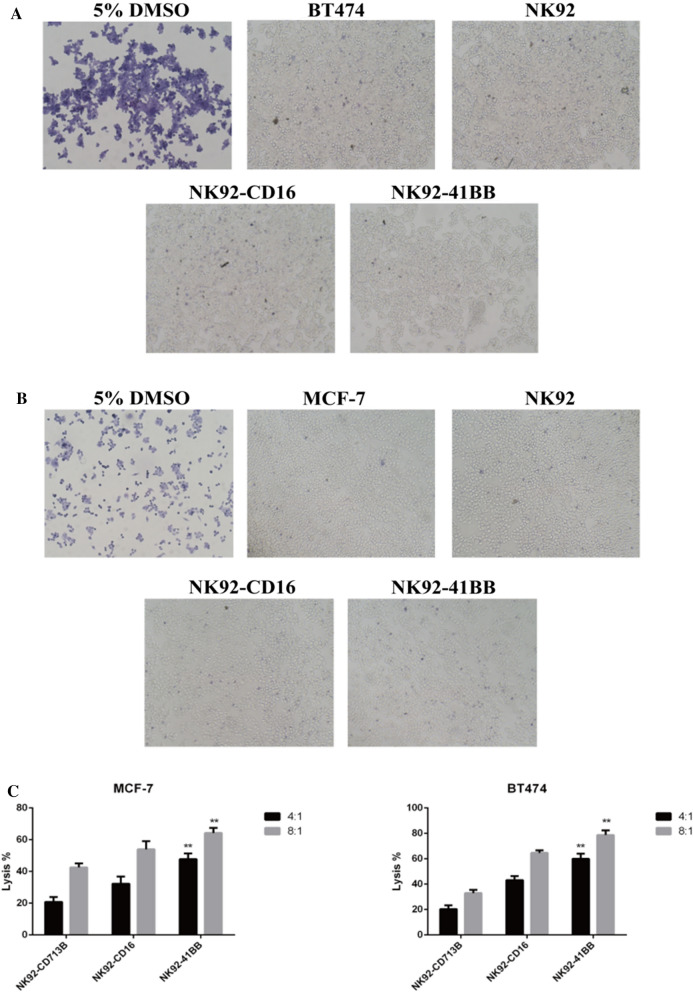
Dead cells were stainable with trypan blue solution because the cell membrane permeability was changed to more intuitively respond to the NK92 combined antibody-mediated ADCC targeting tumor cell killing effect. The NK-92 cells were at the target effect ratio of 8:1, and the 10 µg/mL corresponding antibody was used. After 8 h of incubating the effector and target cells, the effector cells were washed away, the target cells were stained with trypan blue, and the target cell viability was observed under a microscope. The dead cells with poor adherence were easy remove using PBS. NK92-41BB had a stronger effect on antagonizing BT474 and MCF-7 with the corresponding concentration of trastuzumab (Fig. 5A and B). To further examine the ADCC effect of genetically modified NK92 cells, apoptotic cells were counted using flow cytometry. Effector cells were incubated with HER2-positive BT474 cells, MCF-7 cells and HER2-negative MBA-MD-468 cells in the presence of trastuzumab. As shown in Fig. 5C, the HER2-positive cell line BT474 showed a significantly higher lysis efficiency at 8 h with the NK9241BB combined antibody (E/T ratio: 4/1 and 8/1). However, there was no significant difference in the apoptotic effect in the HER2-negative cell line MBA-MD-468. This result was consistent with the measurement of trypan and CCK-8. At low target effect ratios, the effects of effectors incubated with the antibody against tumors were higher than those with no antibody. This also illustrated that ADCC played a pivotal role in adoptive cellular immunotherapy.
Fig. 5.
Trypan blue staining to observe killing activity. A BT474 was killed by effector cells in combination with trastuzumab (10 µg/mL) for 10 h. B MCF-7 was killed by effector cells in combination with trastuzumab (10 µg/mL) for 10 h. Positive controls were treated with 5% DMSO. C The effects of highly expressed BT474 cells and low-expression MCF-7 cells were evaluated using the V-PE/7-AAD Apoptosis Kit after 10 h of incubation with trastuzumab (10 µg/mL). The data represented the means from three independent experiments
Detection of granzyme-associated protein CD107a
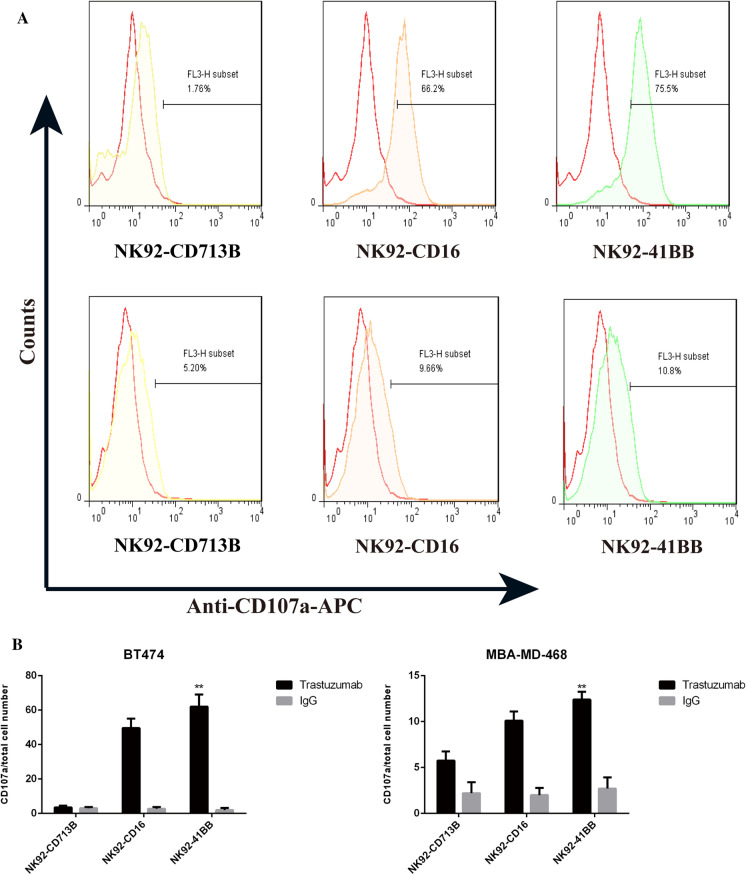
One of the main ways for NK cells to combat tumors is using ADCC. The killing response is mainly caused by NK cells releasing granzymes, perforin disrupting the morphologies of target cells, and the induction of target cell apoptosis. Upon degranulation, lysosome-associated membrane protein-1 (CD107a) and CD107a molecules are transported to the surface of the cell membrane, and the up-regulation of CD107a molecules is consistent with perforin secretion. As shown in Fig. 6A, BT474 cells were targeted with a combination of HER2 antibodies, and NK92- 41BB cells detected higher amounts of CD107a than other cells and negatively expressing MBA-MD-468 cells (Fig. 6B). Higher HER2 expression was correlated with more CD107a expression. The NK cell activating receptor (NKG2D) and the apoptosis related factor ligand (FASL) were also detected by qPCR (Sup 2). There was no difference in the expression of NKG2D and FASL among these three effectors.
Fig. 6.
Detection of granzyme-associated protein CD107a. A BT474 and MDA-MB-468 were killed by effector cells NK92-CD713B, NK92-CD16 and NK92-41BB in combination with trastuzumab (10 µg/mL) for 10 h. Flow cytometry was used to detect CD107a expression using anti-CD107a-APC. B Calculation of the percentage of apoptosis using SPSS for statistical analysis. The results shown are the means (SD) of triplicate measurements from one of at least three comparable repeat experiments
Discussion
In this study, we compared two constructs, allowing us to implement two strategies relying on an adoptive transfer of cytotoxic lymphocytes to improve the targeting of HER2- and CD20-positive tumors. In the first strategy, lymphocytes were equipped with a human CD16 receptor to permit ADCC in the presence of trastuzumab. In the second strategy, we modified the CD16 gene using chimerism and replaced its intracellular signal region with a CAR construct (Fig. 1A).
After transducing with CD16 and a recombinant gene, the human NK cell line NK-92 displayed stable cell surface expression of CD16 and the chimeric CD16/CAR receptor (Fig. 1B). Analyzing the protein expression and activity of the stable strain cells showed that the genetically modified cell line stably expressed the CD16 protein and had the biological activity to bind to the Fc fragment of the antibody (Fig. 2). In this study, different human tumor cell lines, including lung, colon, breast, blood and lymphoma, cervical, and ovarian carcinoma lines, were shown to be lysed by NK92 cells in vitro. A range of lysis was observed among the cell lines (Fig. 3). These results were similar to those observed with the parental NK-92 line.
Specific cytotoxic activity against HER2-positive target cells was demonstrated in both cases. When we compared their potencies against four HER2-positive target cell lines, NK92-41BB always performed better than NK92-CD16. Similar results were obtained for CD20-positive cells. CCK-8 experiments, target cell apoptosis experiments, and trypan blue experiments all demonstrated this result (Figs. 4 and 5). The lytic target cell protein granzyme perforin-related protein CD107a in the ADCC pathway was also examined.
NK cells kill the target cell pathway mainly via surface expression of the apoptotic ligand FasL, which binds to the apoptosis receptor Fas on the target cell and promotes apoptosis of the target cell (Bradley et al. 1998). Secreted cytokines include IFN-γ, TNF-α, etc. Target cells (Tam et al. 1999); release granzymes and perforin, lyse target cells (Campbell and Hasegawa 2013); and kill tumor cells by CD16 (FcγRIII) binding to the anti-tumor antibody. After the combined antibody reaction, the activation of NKG2D, pro-apoptotic ligand FasL, and tumor necrosis factor TNF-α expression levels were examined to assess whether the modified NK92 cells enhance these pathways for anti-tumor responses. The results showed that in the presence of antibodies, the modified cells acted mainly via ADCC (Sup 2).
In recent years, two methods of genetically modified NK92 cell, CD16 modification and CAR modification, anti-tumor activity have been studied. Some researchers compared the two methods. Williams et al. demonstrated the CD16 + NK-92 cell with CD123 antibody improved survival in xenografted mice model of AML, compared with NK-92 cell (Williams et al. 2018). Tassev et al. detailed the relocalization of NK-92 cells using HLAA2-restricted EBNA3C-specific chimeric receptors (TCR-like antibodies) and directly compared the two systems. They showed that CAR-mediated methods more effective to kill target cells than ADCC (Tassev et al. 2012). Beatrice et al. compared their potencies against four HER2-positive target cell lines. NK92-CAR always performed better than NK92-CD16 (Uchiyama et al. 2010). Overall, the effects of NK92-CAR tend to be better than NK92-CD16, but most of these results are for hematological cancer. In solid tumor experiments, NK92-CARs also present the efficiency and safety in antitumor (Fabian et al. 2020; Montagner et al. 2020). However, Fabian et al. suggested lower ADCC-mediated signal than CAR signaling when PD-L1 t-haNK cells combined with avelumab monoclonal antibody to kill tumor cells. Herein the NK92-CD16 cells we constructed to kill tumor cells with monoclonal antibody through ADCC mainly. Plus, another vivo experiments showed that NK92-CD16 cells could enter into the inside of tumor tissue more than NK-92-CAR (Clemenceau et al. 2015). Those studies revealed that CD16-modified NK92 cells have a good prospect for treating solid tumors.
To what extent these observations can be promoted remains in question. For ADCC, the effector/target cell interaction depends on the number of CD16 receptors, their affinity for the antibody used to recognize the target, the number of target antigens, and non-specific interactions between the target and the influencer (such as LFA-1/ICAM1), time, concentration of the Abs in the liquid phase, and other factors. Like CAR, CAR density, CAR affinity, and antigen expression levels all affect the outcome of the interaction. In addition, for the CAR, other variables are also important, such as selecting the sensor chain and additional signals (such as 41BB). Both the ADCC effect and the CAR structure effect have different advantages and disadvantages. In this study, the ADCC receptor protein CD16 intracellular signal region was replaced with the intracellular signal region of the CAR structure. Utilizing the advantages of ADCC and CAR effects, an efficient, specific anti-tumor cell line was proposed.
Jochems et al. engineered a NK cell line which co-expressing the high affinity CD16 allele and IL-2 and testified the antitumor activity in the way of secretion of granzyme (Jochems et al. 2016). As previously described, we used a CD8 hinge to help to eliminate off-target activation, make the extracellular segment more flexible, and exert optimal cytotoxicity potentially. In despite of the different E/T ratios, Jochems et al. reached the maximal kill ratio at 18 h, however we often at 8 h. Chen et al. modified the NK-92MI, an IL-2-independent cell line, expressing a CD16 or CD64 CAR with CD28 and 41-BB two co-stimulation factors (Chen et al. 2017). The cytotoxicity was similar as our study in vitro. However, there is only one co-stimulation factor we used in our CD16/CAR. It might be more secure because the more co-stimulation factors and continuous secretion of IL-2 could induce the cytokine storm.
In conclusion, NK92 cells modified with the CD16/CAR gene had a higher efficiency, specific tumor cell killing activity and higher antibody affinity than NK92 cells. In the anti-tumor response with the combined antibody, intracellular segment signal molecules 41BB and CD3 were used to achieve cell proliferation and activation. Our research proposes a new strategy for improving anti-tumor effects by modifying NK92 cells. In the future, we are going to carry out the mouse model to test the activity of antitumor via ADCC in vivo. Furthermore, we will construct a corresponding CAR NK-92 cell for contrast. What’s more, we will add one more monoclonal antibody, such as anti-PD-L1 or anti-CTLA4, to verify the activity of CD16-NK92 cells in vitro and vivo in the future. And we also will consider knocking out the endogenous FcRs to enhance the efficiency of CD16/CAR binding to antibodies.
Supplementary Information
Below is the link to the Supplementary Information.
Supplementary Information 1 (PNG 199 kb)
Acknowledgements
This work was supported by a grant from the Institute of Biomedicine & National Engineering Research Center of Genetic Medicine, China.
Authors’ contributions
ZH and ZZl have contributed equally to the research. CW designed and provided the T-CD19-CAR. LGm, ZH and LSy analyzed the data. LGm, ZH, and LG wrote the paper.
Data availability
All the cell lines, raw experimental data, data on statistical analysis and/or detailed explanations regarding Materials and methods are available from the corresponding author on reasonable request.
Declarations
Conflict of interest
All authors have no conflicts of interest to declare.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- Barrett DM, Teachey DT, Grupp SA. Toxicity management for patients receiving novel T-cell engaging therapies. Curr Opin Pediatr. 2014;26:43–49. doi: 10.1097/mop.0000000000000043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bibeau F, Lopez-Crapez E, Di Fiore F, et al. Impact of Fc{gamma}RIIa-Fc{gamma}RIIIa polymorphisms and KRAS mutations on the clinical outcome of patients with metastatic colorectal cancer treated with cetuximab plus irinotecan. J Clin Oncol. 2009;27:1122–1129. doi: 10.1200/JCO.2008.18.0463. [DOI] [PubMed] [Google Scholar]
- Bradley M, Zeytun A, Rafi-Janajreh A, et al. Role of spontaneous and interleukin-2-induced natural killer cell activity in the cytotoxicity and rejection of Fas+ and Fas- tumor cells. Blood. 1998;92:4248–4255. doi: 10.1182/blood.V92.11.4248. [DOI] [PubMed] [Google Scholar]
- Brown TL, Tucci J, Dyson ZA, et al. Dynamic interactions between prophages induce lysis in Propionibacterium acnes. Res Microbiol. 2017;168:103–112. doi: 10.1016/j.resmic.2016.09.004. [DOI] [PubMed] [Google Scholar]
- Campbell KS, Hasegawa J. Natural killer cell biology: an update and future directions. J Allergy Clin Immunol. 2013;132:536–544. doi: 10.1016/j.jaci.2013.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carson WE, Parihar R, Lindemann MJ, et al. Interleukin-2 enhances the natural killer cell response to Herceptin-coated Her2/neu-positive breast cancer cells. Eur J Immunol. 2001;31:3016–3025. doi: 10.1002/1521-4141(2001010)31:10<3016::AID-IMMU3016>3.0.CO;2-J. [DOI] [PubMed] [Google Scholar]
- Cartron G, Dacheux L, Salles G, et al. Therapeutic activity of humanized anti-CD20 monoclonal antibody and polymorphism in IgG Fc receptor FcgammaRIIIa gene. Blood. 2002;99:754–758. doi: 10.1182/blood.V99.3.754. [DOI] [PubMed] [Google Scholar]
- Chen Y, You F, Jiang L, et al. Gene-modified NK-92MI cells expressing a chimeric CD16-BB-ζ or CD64-BB-ζ receptor exhibit enhanced cancer-killing ability in combination with therapeutic antibody. Oncotarget. 2017;8:37128–37139. doi: 10.18632/oncotarget.16201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clemenceau B, Valsesia-Wittmann S, Jallas AC, et al. In vitro and in vivo comparison of lymphocytes transduced with a human CD16 or with a chimeric antigen receptor reveals potential off-target interactions due to the IgG2 CH2-CH3 CAR-spacer. J Immunol Res. 2015 doi: 10.1155/2015/482089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fabian KP, Padget MR, Donahue RN, et al. PD-L1 targeting high-affinity NK (t-haNK) cells induce direct antitumor effects and target suppressive MDSC populations. J Immunother Cancer. 2020 doi: 10.1136/jitc-2019-000450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandez-Messina L, Reyburn HT, Vales-Gomez M. Human NKG2D-ligands: cell biology strategies to ensure immune recognition. Front Immunol. 2012;3:299. doi: 10.3389/fimmu.2012.00299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gladow M, Uckert W, Blankenstein T. Dual T cell receptor T cells with two defined specificities mediate tumor suppression via both receptors. Eur J Immunol. 2004;34:1882–1891. doi: 10.1002/eji.200425041. [DOI] [PubMed] [Google Scholar]
- Gras Navarro A, Bjorklund AT, Chekenya M. Therapeutic potential and challenges of natural killer cells in treatment of solid tumors. Front Immunol. 2015;6:202. doi: 10.3389/fimmu.2015.00202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jochems C, Hodge JW, Fantini M, et al. An NK cell line (haNK) expressing high levels of granzyme and engineered to express the high affinity CD16 allele. Oncotarget. 2016;7:86359–86373. doi: 10.18632/oncotarget.13411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klingemann H, Boissel L, Toneguzzo F. Natural killer cells for immunotherapy: advantages of the NK-92 cell line over blood NK cells. Front Immunol. 2016;7:91. doi: 10.3389/fimmu.2016.00091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koene HR, Kleijer M, Algra J, et al. Fc gammaRIIIa-158V/F polymorphism influences the binding of IgG by natural killer cell Fc gammaRIIIa, independently of the Fc gammaRIIIa-48L/R/H phenotype. Blood. 1997;90:1109–1114. doi: 10.1182/blood.V90.3.1109. [DOI] [PubMed] [Google Scholar]
- Kruschinski A, Moosmann A, Poschke I, et al. Engineering antigen-specific primary human NK cells against HER-2 positive carcinomas. Proc Natl Acad Sci USA. 2008;105:17481–17486. doi: 10.1073/pnas.0804788105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montagner IM, Penna A, Fracasso G, et al. Anti-PSMA CAR-engineered NK-92 cells: an off-the-shelf cell therapy for prostate cancer. Cells. 2020 doi: 10.3390/cells9061382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paust S, Senman B, von Andrian UH. Adaptive immune responses mediated by natural killer cells. Immunol Rev. 2010;235:286–296. doi: 10.1111/j.0105-2896.2010.00906.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qin L, Lai YX, Zhao RC, et al. Incorporation of a hinge domain improves the expansion of chimeric antigen receptor T cells. J Hematol Oncol. 2017 doi: 10.1186/s13045-017-0437-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott MP, Neese STT, et al. Changes in biosynthesis and degradation of juvenile hormone during breeding by burying beetles: a reproductive or social role? J Insect Physiol. 2001;47:295–302. doi: 10.1016/S0022-1910(00)00116-5. [DOI] [PubMed] [Google Scholar]
- Tam YK, Maki G, Miyagawa B, et al. Characterization of genetically altered, interleukin 2-independent natural killer cell lines suitable for adoptive cellular immunotherapy. Hum Gene Therapy. 1999;10:1359–1373. doi: 10.1089/10430349950018030. [DOI] [PubMed] [Google Scholar]
- Tassev DV, Cheng M, Cheung NKV. Retargeting NK92 cells using an HLA-A2-restricted, EBNA3C-specific chimeric antigen receptor. Cancer Gene Ther. 2012;19:84–100. doi: 10.1038/cgt.2011.66. [DOI] [PubMed] [Google Scholar]
- Uchiyama S, Suzuki Y, Otake K, et al. Development of novel humanized anti-CD20 antibodies based on affinity constant and epitope. Cancer Sci. 2010;101:201–209. doi: 10.1111/j.1349-7006.2009.01392.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanherberghen B, Olofsson PE, Forslund E, et al. Classification of human natural killer cells based on migration behavior and cytotoxic response. Blood. 2013;121:1326–1334. doi: 10.1182/blood-2012-06-439851. [DOI] [PubMed] [Google Scholar]
- Weiner LM, Dhodapkar MV, Ferrone S. Monoclonal antibodies for cancer immunotherapy. Lancet. 2009;373:1033–1040. doi: 10.1016/S0140-6736(09)60251-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams BA, Wang X-H, Leyton JV, et al. CD16 (+)NK-92 and anti-CD123 monoclonal antibody prolongs survival in primary human acute myeloid leukemia xenografted mice. Haematologica. 2018;103:1720–1729. doi: 10.3324/haematol.2017.187385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang F, Shao Y, Yang F, et al. Valproic acid upregulates NKG2D ligand expression and enhances susceptibility of human renal carcinoma cells to NK cell-mediated cytotoxicity. Arch Med Sci. 2013;9:323–331. doi: 10.5114/aoms.2013.34413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yokoyama WM, Kim S. Licensing of natural killer cells by self-major histocompatibility complex class I. Immunol Rev. 2006;214:143–154. doi: 10.1111/j.1600-065X.2006.00458.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Information 1 (PNG 199 kb)
Data Availability Statement
All the cell lines, raw experimental data, data on statistical analysis and/or detailed explanations regarding Materials and methods are available from the corresponding author on reasonable request.