Abstract
Microbial fermentation for enzyme production and then whole-cell catalysis for l-2-aminobutyric acid (l-ABA) production have huge potential for industrial application, but the catalytic capacities of cells are directly related to the fermentation process. Using a 50 L fermenter, the effects of initial glycerol concentration in the medium and rotating speed on cell catalytic capacity were investigated. Fermentation cells showed the best catalytic activity when the initial glycerol concentration was 12 g/L and the rotating speed was 250 rpm. Furthermore, we studied the difference between glycerol and glycerol mixtures as fed-batch media in pH–stat fed-batch fermentation. Results showed that glycerol had better catalytic activity than the glycerol mixture, and the effect of fed-batch fermentation was better than batch fermentation. Meanwhile, the enzyme activities of leucine dehydrogenase and formate dehydrogenase reached 129.87 U/g DCW and 437.02 U/g DCW, respectively, and the intracellular NAD(H) concentration reached 14.94 μmol/g DCW. Using the optimized fermentation parameters, amplified fermentation was then carried out in a 5000 L fermenter to demonstrate the industrial production of l-ABA by Escherichia coli BL21.
Keywords: l-2-Aminobutyric acid, Optimization, Enzyme activity, Glycerol, Amplified fermentation
Introduction
l-2-Aminobutyric acid (l-ABA) is an essential precursor as an unnatural chiral amino acid in pharmaceutical manufacturing (Taylor et al. 1998). It can serve as an intermediate for the manufacture of the anti-tuberculosis drug ethambutol and the anti-epilepsy drug levetiracetam (Nugent and Feaster 1998; Sasa 2016). l-ABA can be synthesized via chemical and biological methods. However, the disadvantages of chemical synthesis strategies are obvious and include poor selectivity, harsh reaction conditions, various by-products, and difficulties in separation and purification (Xu et al. 2019). Biological synthesis strategies have been extensively studied and mainly include microbial fermentation and enzyme catalysis. In contrast to natural amino acids, a microbial fermentation process for commercial production of unnatural amino acids has not been efficiently exploited to date because complicated metabolic mechanisms lead to by-products and low yields (Schmid et al. 2001; Zhang et al. 2010).
In recent years, the demand for l-ABA as a key drug intermediate has greatly increased, and there is considerable motivation to establish an effective and pollution-free method. Not surprisingly, enzymatic conversion is being studied by many research groups. To eliminate the catalytic reaction of remnant l-alanine, alanine racemase of Bacillus subtilis in combination with d-amino acid oxidase of Rhodotorula gracilis or Trigonopsis variabilis was introduced into the reaction system for l-ABA synthesis (Zhu et al. 2011). The amino acid dehydrogenase uses reduced nicotinamide adenine dinucleotide (NADH) as coenzyme, so the ability to regenerate NADH in the biosynthesis of l-ABA is important (Tao et al. 2014). l-Threonine deaminase (l-TD) from Escherichia coli K12 was modified by directed evolution and rational design to achieve a higher productivity of l-ABA and total turnover number of coenzyme (Wang et al. 2018). Therefore, industrial production of l-ABA is possible through the construction of suitable strains and fermentation culture.
In our previous study, optimization of a 5 L batch fermentation was carried out on the recombinant strain E. coli BL21 containing LeuDH/FDH (LeuDH gene from Thermoactinomyces intermedius and FDH gene from Fusarium graminearum) constructed in our laboratory. The optimum results were obtained at an agitation rate of 450 rpm, induction temperature of 28 °C, and medium pH of 7.0. In this study, we aimed to obtain high enzyme activity and conversion rate by optimizing appropriate fermentation conditions in a 50 L fermentation tank. This process was also conducted on a 5000 L scale to verify the feasibility of l-ABA industrial production.
Materials and methods
Materials
l-ABA standard, 2,4-dinitrofluorobenzene (DNFB), and l-threonine were purchased from J&K Scientific (Beijing, China). Methanol and acetonitrile were HPLC grade and were purchased from Tedia (Fairfield, OH, USA). Ammonium formate, lactose, kanamycin (Kan), and streptomycin (Sm) were obtained from Sangon Biotech (Shanghai, China). NAD+ and NADH were purchased from Bontac Bio-Engineering (Shenzhen, China) and an NAD/NADH assay kit was purchased from BioAssay Systems (Hayward, CA, USA). All other chemicals and reagents were purchased from commercial sources.
Bacterial strain and plasmid
The expression vector pCDFDuet Leudh encoding l Leucine dehydrogenase (LeuDH) was amplified from Thermoactinomyces intermedius. The expression vector pET-28b-fdh encoding FDH was amplified from Candida boidinii. As a result, the genetically engineered E. coli BL21 harbored the recombinant plasmids pCDFDuet Leudh and pET-28b-fdh. The strain was constructed and preserved in our laboratory, and was used as the fermentation strain in this study.
Culture conditions
Luria–Bertani (LB) medium (yeast extract, 5 g/L; tryptone, 10 g/L; NaCl, 10 g/L) was used to culture the recombinant E. coli BL21 with 30 µg/mL kanamycin (Kan) 30 µg/mL and streptomycin sulfate (Sm). The first stage of seed broth was inoculated into a 500-mL shake flask containing 100 mL of LB medium, and cells were grown at 37 °C for 12 h with agitation (150 rpm). Subsequently, the second stage of seed broth was inoculated at 10% (v/v) into 900 mL of LB medium. After the cells were grown for 8 h at 37 °C with agitation (150 rpm), the seed medium was ready for use.
The optimum fermentation conditions were studied using a 50 L fermenter (Shanghai Baoxing Biological Equipment Engineering, Shanghai, China) with a working volume of 30 L for batch fermentation. The basal fermentation medium consisted of: tryptone, 15 g/L; yeast extract, 12 g/L; glycerol, 12 g/L; NaCl, 10 g/L; (NH4)2SO4, 5 g/L; KH2PO4, 1.36 g/L; K2HPO4, 2.28 g/L; MgSO4·7H2O, 0.375 g/L. The cultures were inoculated at 6% (v/v) into the 50 L fermenter with Kan (30 µg/mL) and Sm (30 µg/mL). The 50 L fermenter was equipped with pH, dissolved oxygen (DO), and temperature probes, and cells were cultured in the fermenter at 37 °C with agitation at 250 rpm and ventilation at 3.5 L/min. The pH of the medium was adjusted to pH 7.0 by addition of acid [50% phosphoric acid (v/v)] or base [50% ammonia (v/v)]. Lactose was added to the culture (8 g/L final concentration) for gene induction when the optical density at 600 nm (OD600) reached 8–10 and the culture temperature was lowered to 28 °C. After 17 h of induction culture, the fermentation was stopped and centrifuged at 7500 g (Sorvall LYNX6000; Thermo Fisher Scientific, Waltham, MA, USA) for 10 min to collect the cells, which were then stored at − 20 °C. The strain was fermented at least three times under the same culture conditions.
Optimization of initial glycerol concentration and rotating speed
The growth and protein expression of the strain under different initial glycerol concentrations were examined with initial glycerol concentrations of 12, 15, 18, 21, and 24 g/L. The enzyme activity and intracellular NAD(H) concentrations of the cells were then determined. These experimental data determined the catalytic capacity of the cells and the appropriate glycerol concentration was selected according to the conversion rate. In the fermentation process, excessively high or low DO was detrimental to cell growth and protein expression. In this experiment, constant air flow (3.5 L/min) was used in the fermentation process, and the DO level was adjusted by selecting different rotating speeds. Trials were conducted using different rotating speeds of 200, 250, 300, 350, and 400 rpm.
pH–Stat fed-batch fermentation
Fed-batch fermentation is widely used for recombinant protein production by E. coli and glycerol in the culture can be controlled at low levels with an appropriate glycerol feeding strategy that meets the requirements for cell growth (Nelofer et al. 2013). The pH–stat is a simple indirect feedback control strategy that couples nutrient feeding with pH monitoring. This concept is based on the rise of pH that occurs because of the excretion of ammonium ions when the principal carbon source is depleted (Kim et al. 2004). The medium pH exerts complex effects because it influences the solubility of nutrients and trace elements, and the cellular metabolism in general (Scherholz and Curtis 2013). Homeostasis of pH is important for the function and stability of all cellular enzymes (Liu et al. 2018). Low pH is known to inhibit cell growth and a similar phenomenon was observed at high pH (Hong et al. 2010). In the pH–stat fed-batch fermentation, the pH was controlled at pH 7.0 according to the previous optimization. The fermentation broth was slightly acidic in the early and middle stages of the E. coli BL21 fermentation process (Barbirato et al. 1997), and the pH was controlled with the addition of 50% (v/v) ammonia water. When the glycerol was exhausted, the pH rose rapidly, and feed-batch medium was injected into the broth. The volume of medium added was controlled by the pH response. Two feed-batch media were studied: pure glycerol and a glycerol mixture (tryptone, 75 g/L; yeast extract, 50 g/L; glycerol, 500 g/L; NaCl, 5 g/L; (NH4)2SO4, 20 g/L; KH2PO4, 3 g/L; K2HPO4, 3.94 g/L; MgSO4·7H2O, 16.26 g/L). The parameters of the cells cultured under these two feeding conditions were measured, the differences between the two supplements were compared, and the appropriate means to amplify fermentation were selected.
Amplifying fermentation and industrial production of l-ABA
To achieve industrial-scale production of L-ABA, expanded fermentation of E. coli BL21 can provide more enzymes for the catalytic reaction. Taking the previously optimized conditions as a reference, the magnification test was then carried out. Stored seeds were inoculated into a shaking flask containing 100 mL of LB medium as the first stage of shaking bottle seed liquid and cells were grown at 30 °C for 12 h with agitation (150 rpm). The second stage of shaking bottle seed liquid was inoculated with seed liquid at 2% (v/v) and cultured at 37 °C and 150 rpm. When the OD600 reached of 4–5, 1000 mL of superior seed liquid was inoculated into a 50 L fermentation tank containing 30 L of fermentation liquid. The mixture was cultured at 37 °C with agitation (500 rpm). When the OD600 of the cell suspension reached 4–6, all of the cultured fermentation liquid was added to a 500 L fermentation tank (Shanghai Baoxing Biological Equipment Engineering) containing 300 L of fermentation liquid. The fermentation continued at 37 °C with agitation at 240 rpm until the OD600 reached 4–6 when 150 L of superior seed liquid was inoculated into a 5000 L fermentation tank (Shanghai Baoxing Biological Equipment Engineering) containing 3000 L of fermentation liquid supplemented with Kan (30 µg/mL) and Sm (30 µg/mL). The pH of the medium was adjusted to pH 7.0 ± 0.5 by addition of 50% (v/v) ammonia and fermentation continued at 37 °C with agitation (180 rpm) and ventilation (270 m3/h). When OD600 reached 10–12, the culture temperature was lowered to 28 °C and lactose was added (final concentration 8 g/L) to the culture medium for gene induction. After fermentation induction for 16 h, cells were collected and stored at − 20 °C.
The whole-cell catalytic synthesis of l-ABA was achieved using the coenzyme recycling system and l-threonine as the substrate, ammonium formate was the co-substrate, and NAD+ provided the initiation factor of coenzyme recycling. The 3000 L reaction system used 540 kg of l-threonine, 285 kg of ammonium formic, 0.45 kg of NAD+, 15 kg of l-TD wet cells, 45 kg of LeuDH/FDH wet cells, and 2105 kg of tap water. The whole system was fermented for 8 h at 35 °C with agitation. The reaction liquid was separated using a ceramic membrane, and the purified liquid was concentrated by reverse osmosis. Water in the concentrated solution was removed by rotary evaporation until complete dehydration was achieved. Two mass equivalents of methanol was added to the solid for recrystallization, and the product was obtained by centrifugation and crystal drying.
Analytical methods and enzyme assays
l-ABA synthesis was catalyzed by l-TD and LeuDH/FDH, and the reaction system was as follows: l-threonine, 180 g/L; ammonium formate, 95.3 g/L; NAD+, 0.15 g/L; l-TD wet cells, 5 g/L (cells cultured and preserved in our laboratory); LeuDH/FDH wet cells, 15 g/L (cells cultured and preserved in our laboratory). The 100-mL reaction system was based on tap water (neutral pH), and whole-cell catalysis was performed at 35 °C for 8 h with agitation at 600 rpm. The system was regularly sampled, and the reaction was terminated with concentrated hydrochloric acid. The reaction mixture was analyzed by high-performance liquid chromatography (HPLC) after sample treatment. The concentration of l-ABA in the reaction mixture was determined using an UltiMate™ 3000 HPLC system (Thermo Fisher Scientific) fitted with a C18 separation column (5 µm, 4.6 × 250 mm; Welch, Shanghai, China) after pre-column derivatization with DNFB. The analysis is based on the nucleophilic aromatic ring substitution reaction that occurs under basic conditions between the free end of amino acid NH2 and DNFB to produce a yellow dinitrobenzene amino acid derivative that can be quantitatively determined by HPLC (Wang et al. 2003). The mobile phase was 0.02 mM Na2HPO4 (pH 7.2 adjusted by phosphoric acid) and acetonitrile (70:30, v/v). The flow rate was 1.0 mL/min with the column temperature was 30 °C. The conversion rate of l-ABA formation from l-threonine was calculated and analyzed based on the HPLC data.
A Biophotometer® D30 (Eppendorf, Framingham, MA, USA) was used to measure the OD600 as an indicator of biomass. The fermentation broth was centrifuged at 8000 rpm for 10 min as wet cell weight, and dry cell weight (DCW) and wet cell weight were related through a calibration curve. A known number of wet cells were dissolved in buffer solution, ultrasonicated in an ice water bath, and the supernatant was centrifuged to detect the enzyme activity of crude enzymes. The activity of FDH was determined by measuring the increase in absorbance of NADH at 340 nm with an enzyme Labeled instrument (SpectraMax M5; Molecular Devices, San Jose, CA, USA) and using formate (167 mmol/L), NAD+ (1.67 mmol/L), and phosphate buffer (200 mmol/L, pH 8.0) (Park et al. 2010). LeuDH activity was determined by the same method as above and using NH3/NH4Cl buffer (200 mol/L, pH 7.5), phosphate buffer (200 mmol/L, pH 8.0), NADH (0.25 mmol/L), and 2-oxobutyric acid (50 mmol/L) (Ansorge and Kula 2000). An enzyme activity unit was defined as the amount of enzyme produced at 1 µmol of NADH/min.
Determination of NAD+/NADH was based on the lactate dehydrogenase cycle reaction, and through the change of color intensity of the reduced product. The absorbance of the product was measured at 565 nm with an enzyme Labeled instrument, which was proportional to the NAD+/NADH concentration in the sample (Zhao et al. 1987; Matsumura and Hisako 1980; Vilcheze et al. 2005). The cell suspension was configured with a DCW of 1 g/L and the operational process for determining NAD+/NADH was based on the instructions of the EnzyChrom NAD+/NADH Assay Kit (E2ND-100) (BioAssay Systems, Hayward, CA, USA).
Results and discussion
Screening of appropriate glycerol concentration in the medium
The carbon source is one of the most important components in the growth and metabolism of microbial cells, and influences the formation of the carbon skeletons of nucleic acids, various amino acids, and other carbohydrates (Lou et al. 2016). To determine the appropriated initial glycerol concentration for fermentation production, we tested the effects of different initial glycerol concentrations on cell growth and enzyme co-expression of E. coli BL21. As shown in Fig. 1, as the concentration of glycerol in the fermentation medium increased, the biomass was basically the same, and then the biomass decreased significantly once the glycerol concentration rose above 18 g/L. However, the conversion rate decreased with increased glycerol concentration and low glycerol concentration gave better catalytic activity. To further investigate the factors affecting catalytic capacity, we investigated the role of enzyme activity and intracellular NAD(H). Test results demonstrated that the activities of LeuDH and FDH decreased with increased glycerol concentration and followed patterns that were similar to that of the conversion rate. Base on triplicate tests, the activities of LeuDH and FDH reached 129.17 U/g DCW and 339.56 U/g DCW in E. coli BL21, respectively, after cultivation in the medium with a glycerol concentration of 12 g/L. A possible explanation for these results is that in the presence of a high glycerol concentration, the engineered bacteria grew so rapidly and accumulated 3-hydroxypropionaldehyde such that the rate of plasmid replication was less than the rate of bacterial growth, resulting in premature cessation of growth (Wang et al. 2015). At the same time, an excessive glycerol concentration may have caused the production of acidic substances, which would inhibit the growth of the bacteria and expression of the protein. Thus, we considered that a relatively low concentration of glycerol could improve protein yield, but that an excessively high concentration of glycerol was not conducive to the production of protein (Feng et al. 2011).
Fig. 1.

Effect of glycerol concentration on fermentation process
The concentrations of intracellular NAD(H) were at a higher level when the glycerol concentration was less than 18 g/L. However, the concentration of intracellular NAD(H) decreased significantly when the glycerol concentration was greater than 18 g/L (Table 1). This result indicates that high glycerol concentration was not conducive to the accumulation of NAD(H). Therefore, we chose 12 g/L as the optimal initial glycerol concentration to explore the effects of other factors on enzyme activity and cell growth.
Table 1.
Effect of different glycerol concentrations on intracellular NAD(H)
| Glycerol concentrations (g/L) | NAD+ (μmol/g DCW) | NADH (μmol/g DCW) | NAD(H) (μmol/g DCW) |
|---|---|---|---|
| 12 | 11.54 ± 0.13 | 1.95 ± 0.05 | 13.48 ± 0.10 |
| 15 | 12.91 ± 0.11 | 1.84 ± 0.04 | 14.75 ± 0.15 |
| 18 | 11.03 ± 0.09 | 2.01 ± 0.07 | 13.04 ± 0.16 |
| 21 | 7.15 ± 0.18 | 1.51 ± 0.09 | 8.66 ± 0.13 |
| 24 | 6.95 ± 0.16 | 0.88 ± 0.08 | 7.83 ± 0.11 |
Effect of rotating speed on cell catalytic capacity
Dissolved oxygen is one of the important environmental factors in aerobic microbial fermentation and plays an important role in cell growth, substance metabolism, and protein synthesis. To further improve the catalytic ability of E. coli BL21, we therefore investigated the effect of different rotational speeds on fermentation. As shown Fig. 2, the biomass increased with increased rotating speed, but there was no significant increase in biomass when the rotating speed was higher than 350 rpm. The catalytic ability of cells was significantly worse when the rotating speed was too high or too low while relatively good catalytic capacity of the cell was observed at 250 rpm. Increased rotating speed would be expected to increase the DO in the fermentation broth, and lead to rapid cell growth and increased biomass. However, higher rotating speeds may also produce high shear force that can damage the cells, while rapid growth may be detrimental to the protein expression and the accumulation of intracellular NAD(H) (Oliva et al. 2003). Thus, a rotating speed of 250 rpm was selected to explore the influence of other factors on enzyme activity.
Fig. 2.
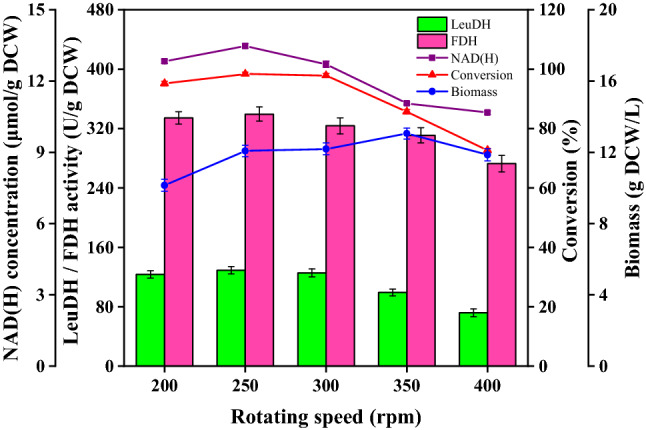
Effect of rotating speed on fermentation process
Comparison of two feed media
To remove the effect of high concentration of glycerol, the fed-batch fermentation with pH–stat was conducted by controlling a suitable carbon source. The pH in the culture medium is a comprehensive index of cell metabolic activity under certain environmental conditions, is an important parameter in the fermentation process, and has a great influence on cell growth and protein accumulation. The fed-batch fermentation was started after the pH increased, 316 mL glycerin feeding medium was injected, 400 mL glycerol mixed feeding medium was injected and this shows that the amount of added glycerol was significantly less than that of glycerol mixture. This indicated that the glycerol mixture provided more adequate nutrients than glycerol alone. Thus, the biomass of the glycerol-fed fermentation was less than the biomass of the fermentation fed with glycerol mixture (Fig. 3a). However, the conversion of the glycerol mixture was not significantly better than that of glycerol, and, on the contrary, glycerol showed better catalytic performance. Figure 3b, c shows that the enzyme activity of the glycerol-fed fermentation was better than that of the fermentation fed with glycerol mixture. The concentration of intracellular NAD(H) in the glycerol-fed fermentation was also higher (Fig. 3b). Basically, the enzyme activity and intracellular NAD(H) gradually increased with increased induction time, and the best enzymatic activity was observed after 16 h. It is likely that high growth rate leads to the overflow of the central metabolic pathway, saturation of the tricarboxylic acid cycle, and/or the electron transport chain might be detrimental to protein expression (Kweon et al. 2001). Therefore, feeding with glycerol is likely to maintain a relatively slow growth rate and maintain good protein activity under such conditions.
Fig. 3.

Effect of different feeding medias on fermentation process; a biomass and catalytic efficiency, b enzyme activity and intracellular NAD(H)
Compared with batch fermentation, fed-batch fermentation has obvious advantages. However, phosphoric acid is not suited to cell-based fermentation and can cause significant phosphorus pollution during mass production. Therefore, the use of a feed medium to maintain pH stability can be used as a substitute for phosphoric acid. At the same time adding a feeding medium to the fermented liquid can supplement nutrients and control the concentration of the carbon source, which not only improves the biomass, but also improves the catalytic ability of the cells. Therefore, we chose glycerol as the feeding medium for fed-batch fermentation and fermentation was stopped at 16 h after induction.
Amplifying production of L-ABA
To achieve industrial-scale production of l-ABA, we carried out amplification fermentation of E. coli BL21 according to the results of previous experiments, and the expanded fermentation was carried out in a 5000 L fermentation tank. The parameters of the fed-batch fermentation process are shown in Fig. 4a. DO remained at a low level after fermentation for 4 h, and gradually increased after 15 h. The addition of ammonium hydroxide stopped at about 8 h and was replaced with glycerol for pH control. Table 2 shows that the enzyme activity of LeuDH and FDH were 129.38 U/g DCW and 436.25 U/g DCW, respectively, and the enzyme activity was similar to that observed for cells cultured in the 50 L fermenter. This indicated that the protein expression of cells was satisfactory under these fermentation conditions and was not greatly affected by the scaled-up process. Therefore, it further showed that a lower glycerol concentration was conducive to protein folding and maintained a more stable enzyme activity. Then, continuously provide a carbon source for the culture medium by means of feeding, ensuring the continuous growth of cells and accumulating enzyme protein. For NAD(H), although the intracellular NAD(H) concentration was lower than observed in laboratory-scale fermentations, the conversion rate of the cells reached 99.51%, which would meet the requirements of industrial production.
Fig. 4.

Time course of thepH-stat fed-batch in a 5000 L fermentation tank (a) and the industrial production (b)
Table 2.
Comparison the performance of cells cultivated by 50 L fermenter and 5000 L fermenter
| Fermentation scale | Conversion rate (%) | Biomass (g DCW/L) | LeuDH (U/g DCW) | FDH (U/g DCW) | NAD(H) (μmol/g DCW) |
|---|---|---|---|---|---|
| 50 L | 99.87 ± 0.55 | 13.60 ± 0.21 | 129.87 ± 5.18 | 437.02 ± 11.21 | 14.94 ± 0.10 |
| 5000 L | 99.51 ± 0.64 | 13.42 ± 0.11 | 129.38 ± 6.29 | 436.25 ± 9.36 | 14.32 ± 0.12 |
Essentially, the fermentation cells were used as catalysts for the catalytic reaction, and the reaction process is shown in Fig. 4b. As the catalytic reaction proceeded, the l-threonine was quickly depleted and converted to the intermediate 2-oxobutyric acid and l-ABA. The concentration of l-ABA reached a maximum at about 6 h and remained unchanged for the remainder of the fermentation. l-ABA is highly soluble in water, but is insoluble in organic reagents, so the product was effectively extracted by dehydration of the reaction solution firstly, and then by recrystallization from methanol to give a final yield of 93.96 ± 3.38%.
Conclusions
The fermentation conditions of the genetically engineered E. coli BL21 were optimized to achieve industrial-scale fermentation production. Since glycerol has a huge impact on the catalytic efficiency of cells, this study had explored a suitable method to ensure the catalytic activity of cells. Besides, the l-ABA pilot test verification was realized through experimental optimization, which laid the foundation for industrialized production. The effects of different initial glycerol concentrations and rotating speeds on batch fermentation were studied. The optimal initial glycerol concentration was 12 g/L and the optimal rotating speed was 250 rpm to give a conversion rate of 98.39%. It is worth noting that higher glycerol concentration was not conducive to cell growth and protein accumulation, thus affecting the catalytic ability of cells. Therefore, we adopted the strategy of pH–stat fed-batch fermentation, and glycerol was used as the feeding medium. The LeuDH and FDH enzyme activities were 129.87 U/g DCW and 437.02 U/g DCW, respectively, the intracellular NAD(H) concentration was 14.94 μmol/g DCW, and the conversion rate was increased to 99.87%. This strategy maintained good cellular catalytic capacity. According to the conditions established in a laboratory-scale fermentation, the culture was expanded to a 5000 L fermenter. This process achieved a conversion rate of 99.51%, which demonstrated the effectiveness of the process for industrial production of l-ABA.
Author contributions
JMX, ZQL and YGZ designed the research. JMX, MW, YHJ and ZQL performed the research and analyzed the data. ZQL and YGZ supervised the research. JMX and MW wrote the paper. JMX and MW revised the paper. All authors read and approved the final manuscript.
Funding
This work was supported by the National Key R&D Program of China (2018YFA0901400).
Declarations
Conflict of interest
The authors have no conflicts of interest to declare that are relevant to the content of this article.
Contributor Information
Jian-Miao Xu, Email: xujianmiao@zjut.edu.cn.
Ming Wang, Email: wm318106lrn@163.com.
Yi-Hua Jin, Email: 18852865243@163.com.
Zhi-Qiang Liu, Email: microliu@zjut.edu.cn.
Yu-Guo Zheng, Email: zhengyg@zjut.edu.cn.
References
- Ansorge MB, Kula MR. Investigating expression systems for the stable large-scale production of recombinant L Leucine-dehydrogenase from Bacillus cereus in Escherichia coli. Appl Microbiol Biot. 2000;53(6):668–673 . doi: 10.1007/s002539900290. [DOI] [PubMed] [Google Scholar]
- Barbirato F, Larguier A, Conte T, Astruc S, Bories A. Sensitivity to pH, product inhibition, and inhibition by NAD+ of 1,3-propanediol dehydrogenase purified from Enterobacter agglomerans CNCM 1210. Arch Microbiol. 1997;168(2):160–163. doi: 10.1007/s002030050482. [DOI] [PubMed] [Google Scholar]
- Feng LL, Zhang JF, Luo H, Li Z, Zhang HJ. Study on optimization of fermentation condition for nitrilase-producer Escherichia coli BL21 (DE3)/pET-Nit. Adv Mater Res. 2011;1091:192–196. doi: 10.4028/www.scientific.net/AMR.175-176.192. [DOI] [Google Scholar]
- Hong AA, Cheng KK, Peng F, Zhou S, Sun Y, Liu CM, Liu DH. Strain isolation and optimization of process parameters for bioconversion of glycerol to lactic acid. J Chem Technol Biot. 2010;84(10):1576–1581. doi: 10.1002/jctb.2209. [DOI] [Google Scholar]
- Kim BS, Lee SC, Lee SY, Chang YK, Chang HN. High cell density fed-batch cultivation of Escherichia coli using exponential feeding combined with pH-stat. Bioproc Biosyst Eng. 2004;26(3):147–150 . doi: 10.1007/s00449-004-0361-5. [DOI] [PubMed] [Google Scholar]
- Kweon DH, Han NS, Park KM, Seo JH. Overproduction of Phytolacca insular is protein in batch and fed-batch culture of recombinant Escherichia coli. Process Biochem. 2001;36(6):537–542 . doi: 10.1016/s0032-9592(00)00237-5. [DOI] [Google Scholar]
- Liu J, Li S, Xia X, Hu H, Fu Q, Xiao Y, Qu G, Shen Z, Cheng L. Optimization of culture conditions for high cell-density fermentation of bovine Escherichia coli. Kafkas Univ Vet Fak Derg. 2018;24(5):735–742. [Google Scholar]
- Lou F, Li N, Zhao Y, Guo S, Chen T. Effects of overexpression of carboxylation pathway genes and inactivation of malic enzymes on malic acid production in Escherichia coli. Chin J Biotechnol. 2016;32(11):1539. doi: 10.13345/j.cjb.160101. [DOI] [PubMed] [Google Scholar]
- Matsumura H, Miyachi S. Cycling assay for nicotinamide adenine dinucleotides. Method Enzymol. 1980;69:465–470 . doi: 10.1016/s0076-6879(80)69045-4. [DOI] [Google Scholar]
- Nelofer R, Noor ZRARR, Basri M, Ariff AB. Optimization of fed-batch fermentation for organic solvent tolerant and thermostable lipase production from recombinant E. coli. Turk J Biochem. 2013;38(3):299–307. doi: 10.5505/tjb.2013.81994. [DOI] [Google Scholar]
- Nugent WA, Feaster JE. Practical synthesis of methyl Z-2-(N-acetylamino) but-2-enoate. An intermediate to D- and L-2-aminobutyric acid. Synthetic Commun. 1998;28:1617–1623. doi: 10.1080/00397919808006866. [DOI] [Google Scholar]
- Oliva A, SantoveñaA FJ, Llabrés M. Effect of high shear rate on stability of proteins: kinetic study. J Pharmaceut Biomed. 2003;33(2):145–155. doi: 10.1016/S0731-7085(03)00223-1. [DOI] [PubMed] [Google Scholar]
- Park SE, Lee JE, Kweon DH, Koo HM, Park SM, Park JC, Park YC, Seo JH. Effects of ALD6 overexpression in saccharomyces cerevisiae on bioethanol production in presence of furan-derivatives. J Biotechnol. 2010;147:0168–1656 . doi: 10.1016/j.jbiotec.2010.08.381. [DOI] [Google Scholar]
- Sasa M. A new frontier in epilepsy: novel antiepileptogenic drugs. J Pharmacol Sci. 2006;100:487–494 . doi: 10.1254/jphs.cpj06010x. [DOI] [PubMed] [Google Scholar]
- Scherholz ML, Curtis WR. Achieving pH control in microalgal cultures through fed-batch addition of stoichiometrically-balanced growth media. BMC Biotechnol. 2013;13(1):39. doi: 10.1186/1472-6750-13-39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmid A, Dordick JS, Hauer B, Kiener A, Wubbolts M, Witholt B. Industrial biocatalysis today and tomorrow. Nature. 2001;409(6817):258–268. doi: 10.1038/35051736. [DOI] [PubMed] [Google Scholar]
- Tao RS, Jiang Y, Zhu FY, Yang S. A one-pot system for production of L-2-aminobutyric acid from L-threonine by L-threonine deaminase and a NADH regeneration system based on L Leucine dehydrogenase and formate dehydrogenase. Biotechnol Lett. 2014;36(4):835–841 . doi: 10.1007/s10529-013-1424-y. [DOI] [PubMed] [Google Scholar]
- Taylor PP, Pantaleone DP, Senkpeil RF. Novel biosynthetic approaches to the production of unnatural amino acids using transaminases. Trends Biotechnol. 1998;16:412. doi: 10.1016/s0167-7799(98)01240-2. [DOI] [PubMed] [Google Scholar]
- Vilcheze C, Weisbrod TR, Chen B, Kremer L, Hazbon MH, Wang F, Alland D, Sacchettini JC, Jacobs WR. Altered NADH/NAD+ ratio mediates coresistance to isoniazid and ethionamide in mycobacteria. Antimicrob Agents Ch. 2005;49(2):708 . doi: 10.1128/aac.49.2.708-720.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J, Zhan XY, Teng YK, Zhang DZ. Determination of the content of amino acid by pre-column derivatization and RP-HPLC. J Shenyang Pharm Univ. 2003;20(6):428. [Google Scholar]
- Wang Y, Zhang L, Zhang W, Wu H, Zhu XM, Xu YJ, Yan JQ, Yu JY. Increasing plasmid-based DNA vaccine construct (16 kb pSVK-HBVA) production in Escherichia coli XL10-Gold through optimization of media component. Biotechnol Biotec Eq. 2015;29(1):164–174 . doi: 10.1080/13102818.2014.989103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Li GS, Qiao P, Lin L, Yang LR. Increased productivity of L-2-aminobutyric acid and total turnover number of NAD+/NADH in a one-pot system through enhanced thermostability of L-threonine deaminase. Biotechnol Lett. 2018 doi: 10.1007/s10529-018-2607-3. [DOI] [PubMed] [Google Scholar]
- Xu JM, Li JQ, Zhang B, Liu ZQ, Zheng YG. Fermentative production of the unnatural amino acid L-2-aminobutyric acid based on metabolic engineering. Microb Cell Fact. 2019;18(1):43 . doi: 10.1186/s12934-019-1095-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang K, Li H, Cho KM, Liao JC. Expanding metabolism for total biosynthesis of the nonnatural amino acid L-homoalanine. Proc Natl Acad Sci USA. 2010;107:6234–6239. doi: 10.1073/pnas.0912903107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao Z, Hu X, Ross CW. Comparison of tissue preparation methods for assay of nicotinamide coenzymes. Plant Physiol. 1987;84:987–988 . doi: 10.1104/pp.84.4.987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu L, Tao RS, Wang Y, Jiang Y, Lin X, Yang YL, Zheng HB, Jiang WH, Yang S. Removal of L-alanine from the production of L-2-aminobutyric acid by introduction of alanine racemase and D-amino acid oxidase. Appl Microbiol Biot. 2011;90(3):903–910 . doi: 10.1007/s00253-011-3127-4. [DOI] [PubMed] [Google Scholar]


