Abstract
The epidemiology of human malaria differs considerably between and within geographic regions due, in part, to variability in mosquito species behaviours. Recently, the WHO emphasised stratifying interventions using local surveillance data to reduce malaria. The usefulness of vector surveillance is entirely dependent on the biases inherent in the sampling methods deployed to monitor mosquito populations. To understand and interpret mosquito surveillance data, the frequency of use of malaria vector collection methods was analysed from a georeferenced vector dataset (> 10,000 data records), extracted from 875 manuscripts across Africa, the Americas and the Asia-Pacific region. Commonly deployed mosquito collection methods tend to target anticipated vector behaviours in a region to maximise sample size (and by default, ignoring other behaviours). Mosquito collection methods targeting both host-seeking and resting behaviours were seldomly deployed concurrently at the same site. A balanced sampling design using multiple methods would improve the understanding of the range of vector behaviours, leading to improved surveillance and more effective vector control.
Subject terms: Malaria, Entomology
Introduction
Substantial progress has been made to reduce the global incidence of human malarias. As malaria transmission diminishes, malaria cases become more spatially heterogenous1,2. The latest World Health Organisation (WHO) guidance to national malaria programs encourages the use of local evidence to select interventions by transmission stratum, rather than utilising a one-size-fits-all approach3. Vector surveillance data is thus increasingly critical to support this informed decision-making process, with surveillance, including vector surveillance, now considered a core intervention4. The WHO recommends monitoring a set of vector surveillance indicators based on transmission intensity and vector control measures deployed5–7. These indicators are species identification, abundance, peak biting times, resting and biting locations, insecticide susceptibility and infection rates6. However, national programme capacity limitations (e.g. inadequate strategic frameworks, logistics shortfalls, limitations in human resources or financial constraints) often prohibit monitoring the complete set of indicators8. Guidance on which surveillance tools should be used to measure the recommended indicators are provided by the WHO. Each of these surveillance tools has associated biases and limitations9.
Globally, there are 41 dominant malaria vector species (DVS) responsible for most human malaria transmission. Each DVS has unique combinations of behaviours. In Africa, seven DVS are recognised10. Nine DVS are found in the Americas (North, Central and South America)11 and a staggering nineteen DVS are recognised in the Asia-Pacific region12. Additionally, species complexes containing cryptic species are found across all regions. One consequence of the regional variability in mosquito fauna is that the epidemiology of human malaria transmission and the effectiveness of vector control measures differs considerably between Africa, the Americas and the Asia-Pacific region13,14.
Representative vector surveillance requires unbiased sampling of mosquito populations. The high variability in biting and resting behaviours between regions and by vector species may impact the efficacy of sampling methods and potentially delay detecting changes in vector behaviours that reduce the efficacy of malaria control methods and thereby delay changing strategies to better control the vectors. Due to these differences in vector bionomics, certain patterns of behaviours are commonly associated with geographic areas. Many DVS in Africa are described as being distributed rurally, anthropophagic (preferring to bite humans) and predominantly biting late at night and indoors. Contrastingly, while also predominantly rural in distribution, many American and Asian DVS are considered to predominantly bite and rest outdoors and are opportunistic (more zoophagic) in their blood feeding preferences10–12,15,16. Exceptions abound: for example, An. culicifacies is an urban, indoor biting and resting mosquito found in Asia17 and An. arabiensis is an African vector that can exhibit opportunistic, outdoor blood feeding habits18. We hypothesise that these behavioural differences will impact collection method efficacy (i.e., the number of mosquitoes captured). Hence, collection methods might be selected on the basis of efficacy to maximise numbers captured. Thus, the use of traps might vary in the frequency with which they are deployed by geographic area. Consequently, the predominant vector behaviours reported in an area may also reflect the biases associated with the collection method used.
Here, the frequency of use of the most commonly deployed malaria vector collection methods in Africa, the Americas and the Asia-Pacific region between 1981 and 2015 was analysed from an extensive database of spatially defined information on anopheline vector bionomics19. Geographical patterns of collection method use from the three regions were compared with requirements for accurately monitoring malaria vectors.
Results
The database contained 5678 data records from 450 publications from Africa, 1346 data records from 134 publications from the Americas and 3898 data records from 291 publications from the Asia-Pacific region. Of these 875 publications, 51 (9 from Africa, 15 from the Americas, 27 from the Asia-Pacific region) did not contain specific information on collection methods. The majority of publications reported one (n = 312) or two (n = 340) collection methods used in the study, while three different collection methods were used in 155 publications (see Fig. 1). More than three collection methods were reported in only a few publications.
Figure 1.
Number of publications per continent. For each continent, the number of publications is shown, as well as the number of collection methods that were reported in each publication. Zero collection methods used means that there was no specific information available about the type of collection method(s) used in a publication.
Individual data records (rows in the database) showed unique site-collection period-species combinations (see Supplementary Material 1 for an example). The count of collection methods used in Africa was 9824 with a mean number of 1.74 collection methods per data record. The count of collection methods used in the Americas was 1925 with a mean number of 1.47 collection methods per data record. The count of collection methods used in Asia-Pacific was 5819 with a mean number of 1.75 collection methods per data record. The different collection methods recorded in the database targeting host-seeking or resting mosquitoes were categorised as by Farlow et al.9, which showed that the same seven collection method groups were used in Africa, the Americas and the Asia-Pacific region (Tables 1, 2).
Table 1.
Sampling methods for host-seeking mosquitoes.
| Sampling method group | Definition/tool used | Sampling method (indoor) | Sampling method (outdoor) |
|---|---|---|---|
| Human landing catch | Mosquitoes attracted to collectors are captured by aspiration on exposed lower legs |
HLC (Human landing catch) MB (Man biting, location unspecified) MBI (Man biting indoors) |
MBO (Man biting outdoors) |
| Light trap | CO2-baited CDC trap with light bulb | ILT (Indoor light trap) | OLT (Outdoor light trap) |
| Human-baited double net trap | A collection method composed of two mesh tents with one inside the other. The inner tent contains and protects a human that acts as bait for host-seeking mosquitoes. The outer tent has a gap between the mesh wall bottom and the ground or open tent doors to allow mosquitoes to enter. Mosquitoes are trapped and retained between the tent walls and are periodically collected by aspiration |
HBT (Human-baited tent) HBN (Human-baited net) |
|
| Animal-baited trap | As human-baited trap, but containing an animal (cow/goat/pig/monkey) instead of human |
ABI (Animal-baited inside, in animal shelter) AB (Animal-baited, location unspecified) |
CBT (Cow-baited trap) ABT (Animal-baited trap) ABO (Animal-baited outside) ABN (Animal-baited net trap) |
| Odour trap | A mechanical trap releasing CO2 and/or host odours while capturing attracted mosquitoes | Odour-bait |
Table 2.
Sampling methods for resting and other behaviours.
| Sampling method group | Definition/tool used | Sampling method (indoor) | Sampling method (outdoor) |
|---|---|---|---|
| Natural resting site collections | Capture of mosquitoes by oral, battery-powered and backpack aspirators; includes knockdown spray catches targeting resting mosquitoes in which an insecticide fog immobilises mosquitoes that fall to the floor and are collected |
HRI (House resting indoors) RO (shelter)/RO (ani-shelter): resting in natural or animal shelters |
RO (Resting outdoors) |
| Artificial resting site collections | Includes capture of mosquitoes in constructed shelters and Window Exit traps | WinExit: wire mesh (glue optional) covering the windows, which traps mosquitoes trying to exit houses | RO (pit): resting outdoors in pit traps and other man-made shelters like clay pots, resting boxes, barrier traps and Malaise traps |
The different sampling methods recorded in Massey et al. (2016)19 targeting resting mosquitoes were grouped into categories of techniques/tools used as proposed by Farlow et al. (2020)9. Artificial resting sites are specifically constructed to lure and catch resting mosquitoes, while Natural resting sites are not, i.e. a house is not specifically constructed to lure and catch resting mosquitoes so it is considered a Natural resting site.
Sampling host-seeking anophelines
Global analysis of the data showed that indoor and outdoor human landing catches (HLCs) were by far the most frequently used methods to collect anophelines. The proportional use of HLCs in the Asia-Pacific region was 0.597 (nASIA-PACIFIC = 3475) which was not significantly different to Africa at 0.604 (nAFRICA = 5934) (Table 3). Contrastingly, the proportion of HLCs in the Americas was much higher, at 0.890 (nAMERICAS = 1713). Comparing within continents, statistically there was no discernible preference for indoor or outdoor use of HLCs in Africa (HLCOUT = 25.82%, HLCIN = 31.03%; Mann–Whitney U, p = 0.28, n.s.), the Americas (HLCOUT = 47.48%, HLCIN = 33.82%; Mann–Whitney U, p = 0.85, n.s.) and the Asia-Pacific region (HLCOUT = 29.20%, HLCIN = 25.90%; Mann–Whitney U, p = 0.71, n.s.). However, when comparing between continents, outdoor HLCs tended to be more common in the Americas as well as the Asia-Pacific region when compared to Africa where there was a tendency to perform indoor HLCs, reflecting perceived vector biting characteristics (Table 3). Sampling location was not recorded for a small number of the HLCs (Africa: 3.55%, Americas: 7.69%, Asia-Pacific: 4.62%).
Table 3.
The number of data records for each collection method used in Africa, the Americas and the Asia-Pacific region, categorised by 5-year period.
| HLC | Human-baited trap | Animal-baited trap | Light trap | Odour trap | Natural resting site collection | Resting (other) | Total | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Indoor | Outdoor | Unknown | Indoor | Outdoor | Indoor | Outdoor | ||||||
| Africa | ||||||||||||
| 1981–1985 | 71 | 22 | 1 | 0 | 0 | 2 | 1 | 0 | 12 | 0 | 2 | 111 |
| 1986–1990 | 512 | 351 | 61 | 19 | 16 | 124 | 6 | 0 | 332 | 35 | 84 | 1540 |
| 1991–1995 | 535 | 385 | 81 | 11 | 0 | 144 | 4 | 2 | 428 | 41 | 59 | 1690 |
| 1996–2000 | 545 | 541 | 58 | 15 | 0 | 85 | 1 | 9 | 762 | 17 | 24 | 2057 |
| 2001–2005 | 796 | 762 | 77 | 8 | 2 | 127 | 19 | 4 | 497 | 71 | 72 | 2435 |
| 2006–2010 | 537 | 448 | 38 | 4 | 5 | 156 | 69 | 1 | 344 | 7 | 95 | 1704 |
| 2011–2015 | 13 | 14 | 0 | 0 | 0 | 14 | 8 | 0 | 17 | 0 | 3 | 69 |
| NA | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 1 |
| Total | 3048 | 2537 | 349 | 57 | 24 | 670 | 108 | 20 | 2489 | 173 | 349 | 9824 |
| Prop. | 31.03% | 25.82% | 3.55% | 0.58% | 0.24% | 6.82% | 1.10% | 0.20% | 25.34% | 1.76% | 3.55% | 100% |
| Americas | ||||||||||||
| 1981–1985 | 20 | 20 | 0 | 0 | 9 | 0 | 0 | 0 | 2 | 0 | 4 | 55 |
| 1986–1990 | 143 | 146 | 3 | 0 | 8 | 2 | 1 | 0 | 19 | 27 | 0 | 349 |
| 1991–1995 | 134 | 140 | 16 | 0 | 18 | 1 | 53 | 0 | 1 | 10 | 0 | 373 |
| 1996–2000 | 44 | 160 | 23 | 0 | 0 | 11 | 0 | 0 | 2 | 3 | 0 | 243 |
| 2001–2005 | 99 | 185 | 99 | 8 | 0 | 0 | 1 | 0 | 1 | 12 | 0 | 405 |
| 2006–2010 | 209 | 222 | 7 | 0 | 0 | 6 | 0 | 8 | 0 | 3 | 0 | 455 |
| 2011–2015 | 2 | 2 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 6 |
| NA | 0 | 39 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 39 |
| Total | 651 | 914 | 148 | 9 | 35 | 20 | 55 | 8 | 25 | 55 | 5 | 1925 |
| Prop. | 33.82% | 47.48% | 7.69% | 0.46% | 1.82% | 1.04% | 2.86% | 0.42% | 1.29% | 2.86% | 0.26% | 100% |
| Asia-Pacific region | ||||||||||||
| 1981–1985 | 204 | 233 | 0 | 4 | 91 | 0 | 8 | 0 | 98 | 22 | 0 | 660 |
| 1986–1990 | 450 | 366 | 152 | 23 | 10 | 43 | 44 | 0 | 308 | 72 | 5 | 1473 |
| 1991–1995 | 187 | 286 | 28 | 18 | 41 | 16 | 51 | 7 | 535 | 2 | 4 | 1175 |
| 1996–2000 | 418 | 494 | 8 | 0 | 27 | 31 | 19 | 1 | 195 | 47 | 1 | 1241 |
| 2001–2005 | 114 | 162 | 10 | 8 | 8 | 14 | 11 | 0 | 186 | 54 | 8 | 575 |
| 2006–2010 | 131 | 147 | 57 | 29 | 3 | 51 | 8 | 0 | 169 | 11 | 10 | 616 |
| 2011–2015 | 0 | 2 | 0 | 0 | 0 | 3 | 1 | 0 | 2 | 3 | 0 | 11 |
| NA | 3 | 9 | 14 | 15 | 6 | 4 | 0 | 0 | 14 | 0 | 3 | 68 |
| Total | 1507 | 1699 | 269 | 97 | 186 | 162 | 142 | 8 | 1507 | 211 | 31 | 5819 |
| Prop. | 25.90% | 29.20% | 4.62% | 1.67% | 3.20% | 2.78% | 2.44% | 0.14% | 25.90% | 3.63% | 0.53% | 100% |
The data frame was manipulated to summarise the combination of collection methods used per data record, ensuring that the location (indoors or outdoors) for each sampling effort was noted. When individual data records were examined in further detail, HLC collections were used in three sampling strategies: indoor-only, outdoor only and simultaneously indoor and outdoor. Simultaneous indoor and outdoor HLCs were common practice in all regions. In the analysed data, 77.4% of HLCs in Africa, 50.1% of HLCs in the Americas and 67.5% of HLCs in the Asia-Pacific region were deployed indoors and outdoors simultaneously. Interestingly, the proportion of outdoor-only HLC collections is much larger in the Americas and the Asia-Pacific region than in Africa (Americas: 21.1%, Asia-Pacific: 21.1%, Africa: 3.5%). The opposite is true for indoor-only HLC collections, which occurred more often in Africa (Africa: 19.1%, Americas: 0.6%, Asia-Pacific: 11.4%).
Indoor and outdoor light trap collections in Africa showed large differences (Table 3). Indoor light trap collections were deployed 6-times more frequently than outdoor light trap collections (light trapIN = 6.82%, light trapOUT = 1.10%, Mann–Whitney U, p = 0.03). Indoor and outdoor light trap collections in the Asia-Pacific region accounted for only 2.78% and 2.44% of total sampling effort, respectively, and did not significantly differ (Mann–Whitney U, p = 0.42, n.s.) (Table 3). In the Americas, indoor and outdoor light trap collections were deployed infrequently as well (light trapIN = 1.04%, light trapOUT = 2.86%, Mann–Whitney U, p = 0.28, n.s.). Indoor light trap collections were more often deployed in Africa than in the Asia-Pacific region, although the difference was not statistically significant (nAFRICA = 670, nASIA = 162; Mann–Whitney U, p = 0.086).
Alternative sampling methods to HLCs designed to collect host-seeking mosquitoes—human-baited tent traps (HBTs) and animal-baited tent traps (ABTs)—were more commonly used in the Asia-Pacific region than in Africa and the Americas, albeit infrequently (Table 3). Animal-baited trap use accounted for 3.2% (n = 186), while HBT use accounted for only 2.0% (n = 97) of the total sampling effort in the Asia-Pacific region. The use of ABTs in the Asia-Pacific region decreased strongly after the year 2000, while relative HBT use varied strongly between the 5-year periods. In Africa, HBTs were rarely used (n = 57). Their use peaked at 0.6% in 1986–1990 and decreased thereafter with no data records of HBT use in the period 2011–2015. ABT deployment in Africa was even less common, accounting for only 0.24% of data records (n = 24), of which 16 occurred between 1986 and 1990. ABT and HBT use was also uncommon in the Americas (nABT = 35, nHBT = 9), where the deployment of ABTs was not recorded in the database after 1995.
Sampling resting anophelines
The collection of anophelines from natural indoor resting sites (defined as structures, including houses and animal shelters, not constructed specifically to lure resting mosquitoes20) was the second most commonly used method in both the Asia-Pacific region (29.5%, n = 1718) and Africa (27.1%, n = 2662; Table 3). While the collection of anophelines from natural resting sites was also the second most frequently used method in the Americas, it was infrequently used (4.15%, n = 80). The ratio between indoor and outdoor collections was highly skewed in Africa and the Asia-Pacific region, with indoor resting collections exceeding outdoor resting collections from vegetation sevenfold in the Asia-Pacific region (nat. restingOUT = 3.63%, nat. restingIN = 25.90%; Mann–Whitney U, p = 0.01) to almost 15-fold in Africa (nat. restingOUT = 1.76%, nat. restingIN = 25.34%; Mann–Whitney U, p = 0.03). In the Americas, outdoor resting collections amongst vegetation exceeded indoor resting collections twofold but this was not significant (nat. restingOUT = 2.86%, nat. restingIN = 1.29%; Mann–Whitney U, p = 0.82). It is important to note that the small proportion of outdoor resting collections generally reflected the difficulty of collecting mosquitoes outdoors and not the absence of outdoor resting mosquitoes.
When sampling location per data record was taken into account, natural resting collections showed a sharp contrast in both Africa and the Asia-Pacific region, with indoor-only resting collections (Africa: n = 2248, 92.9%; Asia-Pacific region: n = 1325, 86.3%) far outnumbering simultaneous indoor and outdoor resting collections (Africa: n = 144, 5.9%; Asia-Pacific region: n = 168, 10.9%) as well as outdoor-only resting collections (Africa: n = 27, 1.1%; Asia-Pacific region: n = 43, 2.8%). The Americas contrasted with the other two geographic regions analysed, because outdoor-only resting collections from vegetation (n = 43, 63.2%) outnumbered indoor-only resting collections (n = 13, 19.1%) and simultaneous indoor and outdoor resting collections (n = 12, 17.6%).
Pit traps and other artificial resting sites (e.g. clay pots, box traps etc.) can be used as alternatives to vegetation aspiration to increase mosquito numbers in outdoor resting collections21. Only information on the use of pit traps was found in the dataset for artificial resting site collections. Pit traps were rarely used in the Americas (n = 4) and the Asia-Pacific region (n = 15), where aspiration of natural vegetation was more common. In Africa however, pit trap use was more frequent (n = 156) and comparable to the aspiration of vegetation (n = 173). Pit traps were used in 27 African studies included in the database and, in most of these studies, used in lieu of the aspiration of vegetation.
Temporal patterns in sampling methods
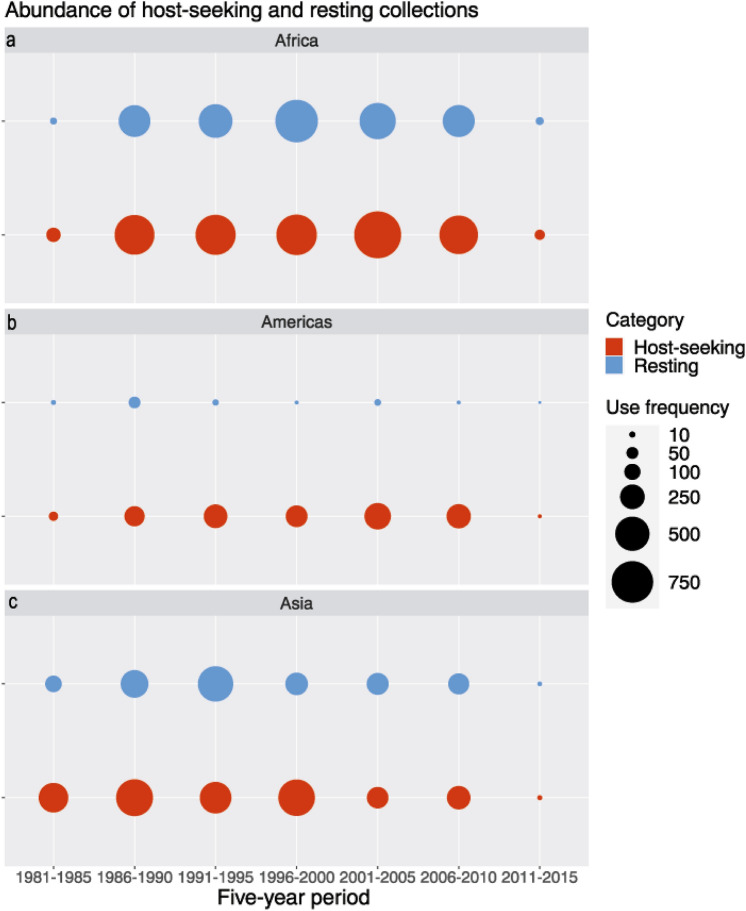
In Africa, malaria vector sampling increased in the late 1980s for both host-seeking as well as resting collections (Fig. 2a). Total sampling effort remained quite stable in the following years to 2010. In the Americas (Fig. 2b), the abundance of host-seeking collections followed a similar pattern to Africa: between 1986 and 2010 the abundance of host-seeking collections remained quite stable. Resting collection sampling frequency, on the other hand, decreased in the 1990s and remained a very infrequently deployed sampling method until 2011–2015 (the final recorded period of the database). In the Asia-Pacific region, the abundance of host-seeking and resting collections varied more than in the other continents (Fig. 2c). Remarkably, the total sampling effort in the Asia-Pacific region was much lower in the twenty-first century than it was in the twentieth century, while the total sampling effort in Africa and the Americas did not show such a decrease. Between 2011 and 2015, sampling effort declined sharply in the three analysed regions, which is most likely due to the small number of published records.
Figure 2.
Number of data records. The number of data records for the two categories of collection methods, host-seeking and resting, presented per 5-year time period. (a) Africa, (b) Americas, (c) Asia-Pacific region.
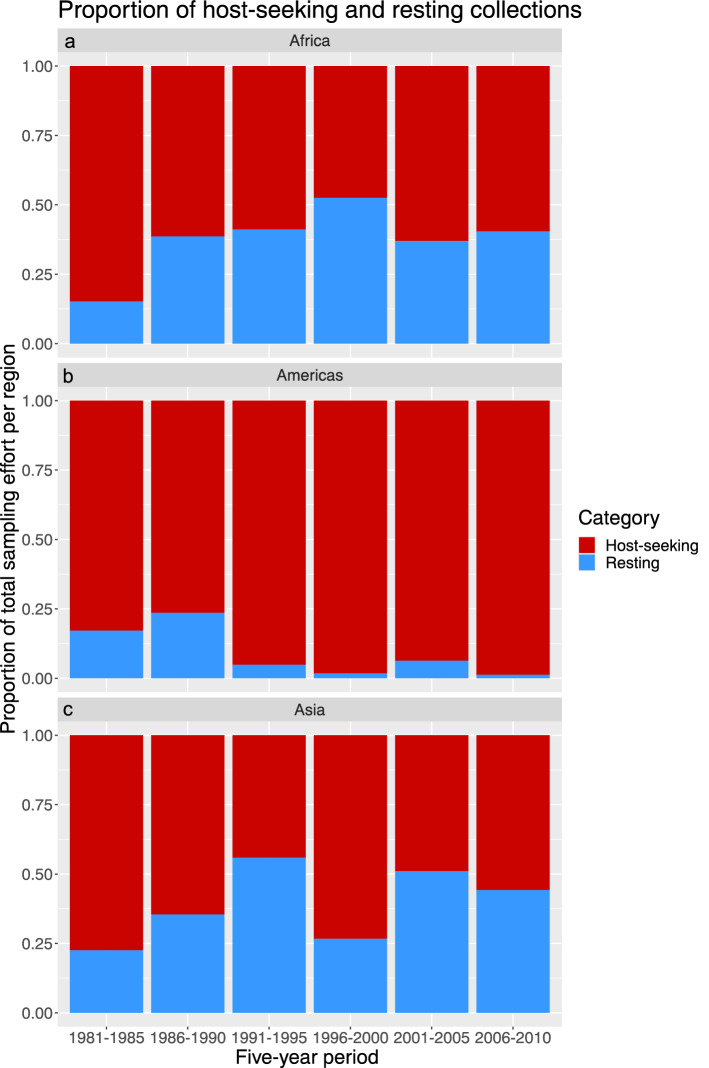
Although a slight upward trend can be seen in the proportion of resting collections, relative to host-seeking collections in Africa, this was by no means significant (Fig. 3a). The ratio between host-seeking and resting collections in Africa was the most stable of the three geographic areas analysed. The Americas was the only region in this analysis which showed a highly skewed ratio between host-seeking and resting collections (Fig. 3b). Here, resting collections were being used regularly in the 1980s, but the sampling effort in the Americas consisted almost entirely of host-seeking collections since the 1990s. In the Asia-Pacific region, the proportions of host-seeking and resting collections showed a slight upward trend but was not as stable as in Africa (Fig. 3c). The more varying ratios in the Asia-Pacific were not significantly different from the ratios observed in Africa (GLM, Tukey’s Post-hoc comparison, p > 0.99). However, the clear decrease in the proportion of resting collections since the 1990s in the Americas was significantly different from Africa (GLM, Tukey’s Post-hoc comparison, p = 0.0014) and the Asia-Pacific region (GLM, Tukey’s Post-hoc comparison, p = 0.0022). The variation in resting collections among the analysed periods was not significant (GLM, Tukey’s Post-hoc comparison, p > 0.65).
Figure 3.
Proportion of host-seeking and resting collections. The proportion of host-seeking (red) and resting (blue) collections presented per 5 year time period for each region analysed. (a) Africa, (b) Americas, (c) Asia-Pacific region.
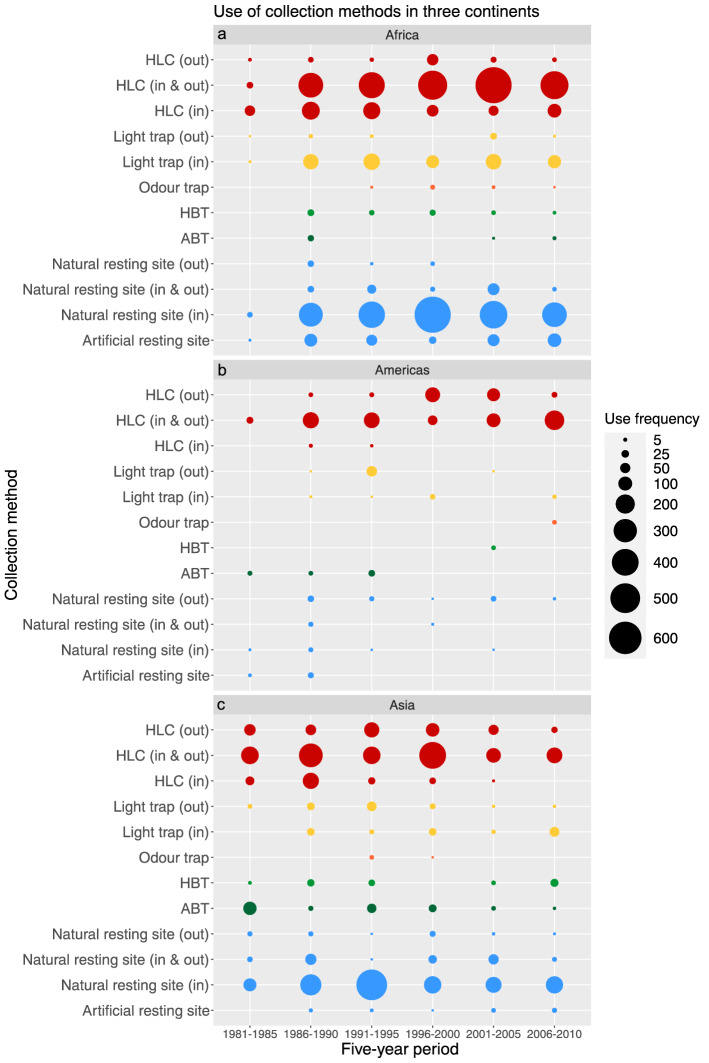
The total sampling effort for each region is displayed in Fig. 4. In Africa, HLC (simultaneously indoors and outdoors) and indoor resting collections dominated the entire database time frame. Light traps were consistently deployed indoors, but were almost never deployed outdoors. Other alternative methods to HLCs, specifically HBTs, ABTs and odour traps, were used infrequently in Africa. In the Americas, HLCs made up the bulk of host-seeking mosquito collections. Sampling of natural resting sites occurred mainly outdoors since the 1990s, which contrasts with the indoor natural resting site collections in Africa and the Asia-Pacific region. Alternatives to HLC were rarely deployed. Together with the infrequent use of resting collections, this creates a highly singular dependence on surveillance data from HLCs. The Asia-Pacific region, like Africa, showed that surveillance data depended largely on HLCs and indoor resting collections. However, the deployment location of light traps was more evenly divided between indoors and outdoors than in Africa. Additionally, HBTs and ABTs were more often used as an alternative or complementary method to HLCs in the Asia-Pacific region than either in Africa or the Americas. Animal-baited traps were consistently used since 1980, whereas HBTs were used less frequently, and not at all in 1996–2000. What is also remarkable is that the overall mosquito sampling effort decreased in the periods 2001–2005 and 2006–2010 in the Asia-Pacific region, while sampling effort in Africa and the Americas remained stable.
Figure 4.
Number of data records per collection method. The number of data records for each collection method used in (a) Africa, (b) the Americas, (c) the Asia-Pacific region, presented per 5-year time period. ABT = animal-baited trap, HLC = human landing catch, HBT = human-baited double net trap.
Geospatial patterns in sampling method use
Visual inspection of the plotted locations of collection methods in the three analysed regions suggested that there was a clustering of collection method use in specific areas (Fig. 5). In Africa, the locations of indoor/outdoor HLCs and indoor natural resting collections were confined to West and East Africa, with very limited sampling in Central Africa (Fig. 5a). However, the data did not immediately show a geospatial pattern in the spread of both methods. 30 and 48 density-based clusters were detected of HLC (indoor/outdoor) and indoor natural resting collections, respectively. The detected clusters for both methods were spread across the continent, not being confined to one country and were mostly of small size, indicating that the sampling effort in these few areas was high. It is possible that these clusters roughly reflect the locations of research facilities. Computing Moran’s I for all the locations in Africa where surveys occurred supported the absence of continent-wide clustering of any sampling method (Moran’s I = 0.09, p > 0.95, n.s.).
Figure 5.
Geographical distributions. Geographical distribution of the different collection methods used in (a) Africa, (b) the Americas and (c) the Asia-Pacific region to collect malaria vectors. ABT = animal-baited trap, HLC = human landing catch, HBT = human-baited double net trap. Maps were made with R statistical software (R version 4.0.2), packages ‘tidyverse’ and ‘maps’45.
In the Americas, the locations of indoor/outdoor HLCs were widespread across the region, with only a few locations of natural resting collections (Fig. 5b). The density-based cluster calculation detected six clusters of HLC (indoor/outdoor) locations, but these were not country-specific and additionally, five of the six clusters overlapped each other. Density-based cluster analyses were unable to detect any significant spatial clustering in other sampling methods due to the limited data. However, computing Moran’s I for all locations where sampling occurred indicated that there was spatial autocorrelation of sampling methods (Moran’s I = 0.19, p < 0.001).
In the Asia-Pacific region, a distinction could be seen in the spread of the sampling methods most often used (Fig. 5c). Indoor/outdoor HLCs were deployed across the entire Asia-Pacific region with the exception of India, where indoor natural resting collections were mainly deployed while sparsely used in the rest of the region. This was also shown by the density-based cluster detection, which detected 13 clusters of HLC (indoor/outdoor), of which the largest were not country-specific and were all found in the Greater Mekong Subregion (Myanmar, Thailand, Vietnam, Lao PDR and Cambodia). Smaller, country-specific clusters of indoor/outdoor HLCs were detected in the Philippines, Papua New Guinea and the Solomon Islands. Sixteen clusters of indoor natural resting collections were detected by density-based clustering, fourteen of which were (partially) in India. One large cluster was found in Myanmar and the final cluster was in Sri-Lanka. Outside these countries, indoor natural resting collections were too few in number and too wide-spread to fall into a cluster. Clustering of methods was supported by Moran’s I (Moran’s I = 0.27, p < 0.001), which showed that similar methods were more often deployed near each other than randomly spread.
Discussion
This investigation analysed data from 875 distinct studies to examine collection methods use in Africa, the Americas and the Asia-Pacific region. Differences among the methods used to collect anophelines were observed among the regions and the potential causes for these differences explored. All analyses performed in this research were based on a precompiled bionomics dataset, which collated data from published studies. While the dataset was very rich, it did not contain data from national malaria control programmes (NMCPs) and other routine surveillance programs. Such documents are rarely publicly available and are often not published in English, which made finding these publications hard and data extraction unreliable. Therefore, we assumed that the dataset used for our analyses was a representative sample of the existing data on malaria vectors in Africa, the Americas and the Asia-Pacific region.
The HLC was the most commonly used method to sample mosquitoes in the three regions analysed. The HLC is considered the ‘gold standard’ in mosquito collections, because it gives the most accurate estimate of the human exposure to mosquito bites9. However, biting rates from HLCs are crude and likely overestimate true human biting rates since collectors, in contrast to the general public, do not make any attempts to ward off mosquitoes21,22. While concerns are often raised that collectors may be bitten by a mosquito before they can collect it, human landing collectors actually have a reduced risk of acquiring malaria due to prophylaxis23. Despite the reduced malaria risk, collectors are still vulnerable to arbovirus exposure and thus many attempts have been made to find an alternative to HLCs24–30. Despite its drawbacks, HLC still remains the most frequently used method for collecting malaria vectors.
The use of CDC light traps did not differ significantly between Africa, the Americas and the Asia-Pacific region. Outdoor light traps were not commonly deployed in any region, but indoor light traps were more often deployed in Africa than in the Americas or the Asia-Pacific region. Although this difference is not statistically significant, sampling more frequently indoors in Africa is consistent with the current dogma that most of the African DVS are endophagic10,15. The fact that the African DVS are perceived as endophagic and anthropophilic is consistent with the very limited use of animal-baited traps (24 data records from 6 studies) in this region. Additionally, bionomics data on African anophelines shows that their indoor/outdoor biting ratio is around 50/5019. By focussing on sampling indoors on human hosts, researchers might miss potentially important shifts in mosquito behaviours toward increased rates of opportunistic feeding or an increase in outdoor biting31. This might overestimate the perceived vulnerability of vectors to indoor interventions and thus overestimate the effectiveness of the currently recommended control methods, long-lasting insecticidal nets (LLINs) and indoor residual spraying of insecticides (IRS), that protect people from mosquito bites when they are indoors32–34.
Animal-baited traps were more commonly used in Asia-Pacific than in Africa or the Americas. However, this collection method was not as popular as expected when considering the perceived more opportunistic biting behaviour of many Asian vectors12, being only used in 25 out of 292 studies. The limited use of ABTs may reflect their significant operational constraints of being labour intensive and requiring substantial training for efficient deployment7,9. Hence, animal-baited traps were mainly used as a complementary method to HLC, instead of an alternative, thus covering a wider range of mosquito biting behaviours. Increasing the amount of entomological data from a wider array of surveillance methods for different vector behaviours is recommended for both researchers and NMCPs6. Animal-baited collection methods can play an important role in understanding vector behaviours more completely.
Natural resting site collection of anophelines was the second-most commonly used mosquito sampling method. This method encompasses indoor and outdoor handheld aspirators, backpack aspirator collections and pyrethrum spray collections (PSC). While indoor collections of resting mosquitoes are easier to conduct and yield reasonable sample numbers, outdoor collections of resting mosquitoes are notoriously difficult due to mosquitoes’ wide and heterogeneously dispersed resting sites35,36. This is consistent with observations in this study that showed that a large majority of resting collections (> 85% in Africa and the Asia-Pacific region) were conducted indoors. Although some inference on outdoor resting can be made by examining abdominal status of mosquitoes collected indoors37–39, the tendency to preferably collect resting mosquitoes by indoor sampling may have generated a bias towards indoor resting in our understanding of resting behaviours. Additionally, the tendency towards indoor resting collections may have delayed detection of changes in resting behaviours (e.g. behavioural resistance) following scale-up of indoor residual spraying. The current assumption, especially in Asia, is that the majority of malaria vectors rest outdoors12. Hence, surveillance strategies that only sample resting mosquitoes indoors will maintain the present knowledge gap on (outdoor) resting behaviours of malaria vectors.
Artificial resting sites (pit traps) were used in Africa as an alternative collection method to sample outdoor-resting anophelines. Other ways to create artificial resting sites for outdoor mosquito collections (clay pots, box shelters, etc.21) were not documented in the dataset analysed, so direct comparisons between respective sampling efforts of pit traps and other methods could not be made. However, Odiere et al. (2007)35 reported that clay pots were more successful and practical than pit traps in collecting both the more endophilic An. gambiae s.s. and the more exophilic An. arabiensis. In contrast, more recent research showed that clay pots were somewhat less productive than pit traps in collecting An. gambiae s.l.40,41. However, clay pots yielded a comparable relative abundance of anopheline species and were more practical in many situations, thereby offsetting their lower productivity. In areas with traditionally high or increased (e.g., resulting from high IRS coverage) levels of exophily, outdoor resting surveillance is crucial in understanding the ecology of malaria vectors and the effectivity of applied interventions. In such areas, not only in Africa but also in the Americas and the Asia-Pacific region, artificial resting site collections could be a valuable asset in vector surveillance.
The geospatial pattern of published mosquito collections in the Asia-Pacific region shows that HLCs, both indoor and outdoor, are the main surveillance method in the entire region except India where indoor resting collections are common practice. The astonishing number of data records from India (almost 50% of the data from the Asia-Pacific region comes from India) were almost exclusively acquired by indoor resting collections and may reflect an emphasis on one DVS, Anopheles culicifacies, which rests and bites traditionally indoors. However, expert opinions and bionomics data show that An. culicifacies can also be found resting outdoors12,19.
The use of a single method or insufficient sampling sites used to define a vector’s resting behaviours may limit the capacity to detect potential changes in its resting behaviour in response to selective pressure from indoor residual spraying or LLINs (e.g. resting indoors or outdoors, duration of resting at one location, peak biting time, endophagy/exophagy or choice of blood host)12,42,43. Most resting collections (mechanical aspirator collections, PSCs) are conducted in the early morning21 and cannot detect shifts in temporal or spatial resting patterns. Furthermore, data on host-seeking or biting behaviours cannot be derived from resting collections. A comparable argument can be made when HLCs alone are used to define biting behaviours. HLCs can detect spatio-temporal shifts in host-seeking and biting behaviours when contemporaneous indoor and outdoor collections are made, but cannot be analysed for changes in blood host choice43.
The use of one sampling method, specifically the method which results in the highest vector numbers, is a cost-effective way of acquiring large numbers of mosquitoes to define a limited number of vector indicators. In contrast, collecting mosquitoes by using multiple sampling methods for biting and resting, indoors and outdoors (and by representative sampling), yields more epidemiologically relevant data for researchers and policy makers. Therefore, entomological surveillance should utilise multiple complementary collection methods across different micro-habitats to sample different behaviours. Concurrent use of complementary collection methods will enable a more comprehensive characterisation of vector behaviours, will better define vector species richness and community composition, as well as enable the early detection of behavioural shifts that may threaten the effectiveness of malaria vector control34.
Conclusions
We observed a tendency towards using collection methods to potentially maximise the number of mosquitoes captured based on anticipated vector behaviours in a geographic region by targeting specific vector behaviours (and by default, ignoring other behaviours). Although similar malaria vector collection methods were used in the three regions, their frequency of use varied between Africa, the Americas and the Asia-Pacific region. Their frequency of use may have resulted from biases in the perceived behaviours of the DVS in each region in order to maximise the numbers captured. Adherence to current dogmas and expert opinions to design vector sampling strategies may reinforce biases in surveillance data and can delay the detection of behavioural shifts of vectors which could lead to reduced vector control. A more varied, tailor-made surveillance effort integrating multiple collection methods for specific regions can provide better insights in vector behaviour and changes in vector behaviours.
Methods
All analyses in this study were based on the bionomics data extracted and collated from literature published between 1981 and 2015 on the global DVS19. A detailed structure of the database as well as original references are in Supplementary File 1 and the original publication. The database contained 10,922 data records from 875 publications. Information on Anopheles species included the (georeferenced) study location, starting month and year of the study, mosquito collection methods used, biting and resting locations, preferred blood hosts and peak biting times. The methods used to collect malaria vectors in the WHO regions ‘Africa’, ‘Americas’ and ‘Asia-Pacific’ were analysed because 96.4% of the global malaria burden is found in these regions5, where malaria vector behaviours differ.
Individual data records (rows in the database) show unique site-collection period-species combinations. A single study or publication could therefore comprise multiple data records (rows), depending on: (1) the number of sites studied, (2) the number of sampling events included in the study, (3) the commencement date and (4) the species collected. Collection sites were recorded with geographical latitude and longitude, without differentiating between the area size, which included “point” (≤ 10 km2), “wide-area” (> 10 and ≤ 25 km2), “small polygon” (> 25 and ≤ 100 km2) and “large polygon” (> 100 km2). While month and year defined the start as well as the end of an experiment (two data records could have similar start dates but different end dates), only the study starting year was used to categorise the time of the study. Accurate estimates of the number of collection nights for each individual study (the sampling effort) could not be extracted because only start and end month were recorded and the number of surveys, traps and work-hours were not recorded in the database.
Collection methods were only analysed if they captured species of interest. In the construction of the dataset, mosquito collection methods were categorised as: ‘vector biology sampling’, ‘infection sampling’, ‘human biting rate collection’ and ‘resting collection’ depending on the bionomic metric the data were informing. These categories were not mutually exclusive, so for each data record a collection method could be recorded twice (see also Supplementary File 1). The data frame was manipulated to summarise the combination of collection methods used per data record, ensuring that the location (indoors or outdoors) for each sampling effort was noted. The different collection methods recorded in the database targeting host-seeking or resting mosquitoes were categorised as by Farlow et al.9 (Tables 1, 2).
All geo-referenced data records were compiled, summarised by continent, stratified by year and method, and mapped in R Studio. The Mann–Whitney U test analysed within-continent differences between indoor and outdoor sampling methods. To explore trends in the proportional use of host-seeking and resting collections over the entire database time period, a generalised linear model was constructed with ‘proportion host-seeking collections’ as dependent variable and ‘start year of the research’ as independent variable. Running the model with a quasibinomial distribution accounted for overdispersion of the data. The data were summarised by 5-year periods for visualisation. Potential geospatial patterns of collection method use were first studied visually in each of the three regions analysed. Consequently, the two sampling methods used most often that showed at least some degree of clustering were analysed by density-based clustering following the OPTICS algorithm, described in detail by Hahsler et al. (2019)44. OPTICS starts with a random data point and provides the order in which new points are explored and added to a cluster. Follow-up ξ-extraction is required to detect clusters of variable density and provide the cluster hierarchy. This means that the OPTICS method can detect clusters within clusters. Afterwards, Moran’s I for spatial autocorrelation tested whether the detected clustering of the analysed sampling methods was significant, or the locations where a sampling method was deployed were spread randomly across a continent/region. Data analyses were performed in R Studio with R statistical software (R version 4.0.2) using the packages ‘tidyverse’, ‘maps’, ‘gganimate’, ‘geosphere’, ‘ape’, ‘psych’, ‘dbscan’ and ‘sjmisc’45.
Supplementary Information
Abbreviations
- ABT
Animal-baited trap
- Data record
A unique site-collection period-species combination which corresponds with a single row in the original database
- DVS
Dominant vector species
- HLC
Human landing catch
- HBT
Human-baited double net trap
- IRS
Indoor residual spraying
- LLIN
Long-lasting insecticidal bed net
- NMCP
National Malaria Control Program
- PSC
Pyrethrum spray collection
- WHO
World Health Organisation
Author contributions
B.v.d.S., T.L.R. and T.R.B. conceived the research. B.v.d.S. analysed the data and wrote the original draft of the manuscript. T.L.R., K.S. and M.E.S. provided input on the data analyses. All authors reviewed and approved the final manuscript.
Funding
This work was supported, in whole or in part, by the Bill and Melinda Gates Foundation, Contract No. 18931 to James Cook University, and supported by ZOOMAL project (‘Evaluating zoonotic malaria and agricultural land use in Indonesia’; #LS-2019-116), Australian Centre for International Agricultural Research, Australian Government. Under the grant conditions of the Foundation, a Creative Commons Attributions 4.0 Generic License has already been assigned to the Author Accepted Manuscript that might arise from this submission. BvdS was supported by a James Cook University Postgraduate Research Scholarship.
Data availability
All data generated or analysed during this study are included in this published article and its supplementary information files. Original databases can also be found in Massey NC, Garrod G, Wiebe A, Henry AJ, Huang Z et al. Sci Data. A global bionomic database for the dominant vectors of human malaria. 2016;3:1–13. https://doi.org/10.1038/sdata.2016.14.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
The online version contains supplementary material available at 10.1038/s41598-021-94656-w.
References
- 1.Mogeni P, et al. Effect of transmission intensity on hotspots and micro-epidemiology of malaria in sub-Saharan Africa. BMC Med. 2017;15:1–11. doi: 10.1186/s12916-017-0887-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bousema T, et al. Hitting hotspots: Spatial targeting of malaria for control and elimination. PLoS Med. 2012;9:1–7. doi: 10.1371/journal.pmed.1001165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.World Health Organization . WHO Technical Brief for Countries Preparing Malaria Funding Requests for the Global Fund (2020–2022) WHO; 2020. [Google Scholar]
- 4.World Health Organization . Guidelines for malaria vector control. Geneva: World Health Organization; 2019. [PubMed] [Google Scholar]
- 5.World Health Organization . World Malaria Report 2019. WHO; 2019. [Google Scholar]
- 6.Health Organization, W . Malaria Surveillance, Monitoring and Evaluation: A Reference Manual. Health Organization; 2018. [Google Scholar]
- 7.Burkot TR, et al. A global analysis of National Malaria Control Programme vector surveillance by elimination and control status in 2018. Malar. J. 2019;18:1–12. doi: 10.1186/s12936-019-3041-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Russell, T. L., Farlow, R., Min, M., Espino, E., Mnzava, A. & Burkot, T. R. Capacity of National Malaria Control Programmes to implement vector surveillance: a global analysis. Malar J.19, 422 (2020). [DOI] [PMC free article] [PubMed]
- 9.Farlow R, Russell TL, Burkot TR. Nextgen Vector Surveillance Tools: Sensitive, specific, cost-effective and epidemiologically relevant. Malar. J. 2020;19:1–13. doi: 10.1186/s12936-020-03494-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sinka ME, et al. The dominant Anopheles vectors of human malaria in Africa, Europe and the Middle East: Occurrence data, distribution maps and bionomic précis. Parasit. Vectors. 2010;3:1–34. doi: 10.1186/1756-3305-3-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sinka ME, et al. The dominant Anopheles vectors of human malaria in the Americas: Occurrence data, distribution maps and bionomic précis. Parasit. Vectors. 2010;3:1–26. doi: 10.1186/1756-3305-3-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sinka ME, et al. The dominant Anopheles vectors of human malaria in the Asia-Pacific region: Occurrence data, distribution maps and bionomic précis. Parasit. Vectors. 2011;4:1–46. doi: 10.1186/1756-3305-4-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nkumama IN, O’Meara WP, Osier FHA. Changes in malaria epidemiology in africa and new challenges for elimination. Trends Parasitol. 2017;33:128–140. doi: 10.1016/j.pt.2016.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bhatia R, Rastogi RM, Ortega L. Malaria successes and challenges in Asia. J. Vector Borne Dis. 2013;50:239–247. [PubMed] [Google Scholar]
- 15.White GB. Anopheles gambiae complex and disease transmission in Africa. Trans. R. Soc. Trop. Med. Hyg. 1974;68:278–298. doi: 10.1016/0035-9203(74)90035-2. [DOI] [PubMed] [Google Scholar]
- 16.Manguin S, Garros C, Dusfour I, Harbach RE, Coosemans M. Bionomics, taxonomy, and distribution of the major malaria vector taxa of Anopheles subgenus Cellia in Southeast Asia: An updated review. Infect. Genet. Evol. 2008;8:489–503. doi: 10.1016/j.meegid.2007.11.004. [DOI] [PubMed] [Google Scholar]
- 17.Barik TK, Sahu B, Swain V. A review on Anopheles culicifacies: From bionomics to control with special reference to Indian subcontinent. Acta Trop. 2009;109:87–97. doi: 10.1016/j.actatropica.2008.09.017. [DOI] [PubMed] [Google Scholar]
- 18.Takken W, Verhulst NO. Host preferences of blood-feeding mosquitoes. Annu. Rev. Entomol. 2013;58:433–453. doi: 10.1146/annurev-ento-120811-153618. [DOI] [PubMed] [Google Scholar]
- 19.Massey NC, et al. A global bionomic database for the dominant vectors of human malaria. Sci. Data. 2016;3:1–13. doi: 10.1038/sdata.2016.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.World Health Organization . WHO Malaria Terminology. WHO; 2016. [Google Scholar]
- 21.Silver JB. Mosquito Ecology—Field Sampling Methods. Springer; 2008. [Google Scholar]
- 22.Burkot TR, Graves PM. The value of vector-based estimates of malaria transmission. Ann. Trop. Med. Parasitol. 1995 doi: 10.1080/00034983.1995.11812943. [DOI] [PubMed] [Google Scholar]
- 23.Gimnig JE, et al. Incidence of malaria among mosquito collectors conducting human landing catches in Western Kenya. Am. J. Trop. Med. Hyg. 2013;88:301–308. doi: 10.4269/ajtmh.2012.12-0209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hawkes FM, Dabiré RK, Sawadogo SP, Torr SJ, Gibson G. Exploiting Anopheles responses to thermal, odour and visual stimuli to improve surveillance and control of malaria. Sci. Rep. 2017;7:1–9. doi: 10.1038/s41598-017-17632-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tangena JAA, Thammavong P, Hiscox A, Lindsay SW, Brey PT. The human-baited double net trap: An alternative to human landing catches for collecting outdoor biting mosquitoes in Lao PDR. PLoS ONE. 2015;10:1–13. doi: 10.1371/journal.pone.0138735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hiscox A, et al. Development and optimization of the Suna trap as a tool for mosquito monitoring and control. Malar. J. 2014;13:257. doi: 10.1186/1475-2875-13-257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Burkot TR, et al. Barrier screens: a method to sample blood-fed and host-seeking exophilic mosquitoes. Malar. J. 2013;12:49. doi: 10.1186/1475-2875-12-49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.St Laurent B, et al. Host attraction and biting behaviour of Anopheles mosquitoes in South Halmahera, Indonesia. Malar. J. 2017;16:1–9. doi: 10.1186/s12936-017-1950-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Williams CR, Long SA, Russell RC, Ritchie SA. Field efficacy of the BG-Sentinel compared with CDC Backpack Aspirators and CO2-baited EVS traps for collection of adult Aedes aegypti in Cairns, Queensland, Australia. J. Am. Mosq. Control Assoc. 2006;22:296–300. doi: 10.2987/8756-971X(2006)22[296:FEOTBC]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 30.Ritchie SA, et al. A simple non-powered passive trap for the collection of mosquitoes for arbovirus surveillance. J. Med. Entomol. 2013;50:185–194. doi: 10.1603/ME12112. [DOI] [PubMed] [Google Scholar]
- 31.Sinka ME, et al. Modelling the relative abundance of the primary African vectors of malaria before and after the implementation of indoor, insecticide-based vector control. Malar. J. 2016;15:1–10. doi: 10.1186/s12936-016-1187-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gatton ML, et al. The importance of mosquito behavioural adaptations to malaria control in Africa. Evolution (N. Y.) 2013;67:1218–1230. doi: 10.1111/evo.12063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chareonviriyaphap T, et al. Review of insecticide resistance and behavioral avoidance of vectors of human diseases in Thailand. Parasit. Vectors. 2013;6:1–28. doi: 10.1186/1756-3305-6-280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Russell TL, Beebe NW, Cooper RD, Lobo NF, Burkot TR. Successful malaria elimination strategies require interventions that target changing vector behaviours. Malar. J. 2013;12:56. doi: 10.1186/1475-2875-12-56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Odiere M, et al. Sampling outdoor, resting Anopheles gambiae and other mosquitoes (Diptera: Culicidae) in Western Kenya with clay pots. J. Med. Entomol. 2007;44:14–22. doi: 10.1093/jmedent/41.5.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Burkot TR, Graves PM, Paru R, Lagog M. Mixed blood feeding by the malaria vectors in the Anopheles punctulatus Complex (Diptera: Culicidae) J. Med. Entomol. 1988;25:205–213. doi: 10.1093/jmedent/25.4.205. [DOI] [PubMed] [Google Scholar]
- 37.Ould Lemrabott MA, et al. Seasonal abundance, blood meal sources and insecticide susceptibility in major anopheline malaria vectors from southern Mauritania. Parasit. Vectors. 2018;11:1–9. doi: 10.1186/s13071-018-2819-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.World Health Organization. Manual on practical entomology in malaria—Part II methods and techniques. In Manual on practical entomology in malaria 1–197 (World Health Organization, 1975).
- 39.Ribeiro H, Janz J. Exophagy and exophily in malaria vectors. Bull. Soc. Vector Ecol. 1990;15:185–188. [Google Scholar]
- 40.Degefa T, et al. Evaluation of the performance of new sticky pots for outdoor resting malaria vector surveillance in western Kenya. Parasit. Vectors. 2019;12:1–14. doi: 10.1186/s13071-019-3535-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Machani MG, et al. Resting behaviour of malaria vectors in highland and lowland sites of western Kenya: Implication on malaria vector control measures. PLoS ONE. 2020;15:55–66. doi: 10.1371/journal.pone.0224718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Carrasco D, et al. Behavioural adaptations of mosquito vectors to insecticide control. Curr. Opin. Insect Sci. 2019;34:48–54. doi: 10.1016/j.cois.2019.03.005. [DOI] [PubMed] [Google Scholar]
- 43.Taylor B. Changes in the feeding behaviour of a malaria vector, Anopheles farauti Lav., following use of DDT as a residual spray in houses in the British Solomon Islands Protectorate. Trans. R. Entomol. Soc. Lond. 1975;127:277–292. doi: 10.1111/j.1365-2311.1975.tb00576.x. [DOI] [Google Scholar]
- 44.Hahsler M, Piekenbrock M, Doran D. dbscan: Fast density-based clustering with R. J. Stat. Softw. 2019;91:1–30. doi: 10.18637/jss.v091.i01. [DOI] [Google Scholar]
- 45.R Core Team. R: A Language and Environment for Statistical Computing (R Foundation for Statistical Computing, 2020). https://www.R-project.org/.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data generated or analysed during this study are included in this published article and its supplementary information files. Original databases can also be found in Massey NC, Garrod G, Wiebe A, Henry AJ, Huang Z et al. Sci Data. A global bionomic database for the dominant vectors of human malaria. 2016;3:1–13. https://doi.org/10.1038/sdata.2016.14.