Abstract
Voltage dependent anion channel 2 (VDAC2) is an outer mitochondrial membrane porin known to play a significant role in apoptosis and calcium signaling. Abnormalities in calcium homeostasis often leads to electrical and contractile dysfunction and can cause dilated cardiomyopathy and heart failure. However, the specific role of VDAC2 in intracellular calcium dynamics and cardiac function is not well understood. To elucidate the role of VDAC2 in calcium homeostasis, we generated a cardiac ventricular myocyte-specific developmental deletion of Vdac2 in mice. Our results indicate that loss of VDAC2 in the myocardium causes severe impairment in excitation-contraction coupling by altering both intracellular and mitochondrial calcium signaling. We also observed adverse cardiac remodeling which progressed to severe cardiomyopathy and death. Reintroduction of VDAC2 in 6-week-old knock-out mice partially rescued the cardiomyopathy phenotype. Activation of VDAC2 by efsevin increased cardiac contractile force in a mouse model of pressure-overload induced heart failure. In conclusion, our findings demonstrate that VDAC2 plays a crucial role in cardiac function by influencing cellular calcium signaling. Through this unique role in cellular calcium dynamics and excitation-contraction coupling VDAC2 emerges as a plausible therapeutic target for heart failure.
Subject terms: Molecular biology, Cardiology, Cardiomyopathies
The authors found that VDAC2 plays a crucial role in influencing mitochondrial calcium dynamics and cellular calcium signalling. A VDAC2 agonist, efsevin, rescued the heart failure phenotype, identifying a new potential therapeutic target for heart failure.
Introduction
Non-ischemic dilated cardiomyopathy (DCM) is one of the most common causes that lead to the syndrome of chronic heart failure (HF) which is currently a growing global epidemic1,2. DCM is a condition in which the cardiac ventricular chambers enlarge and have impaired systolic and diastolic function. The impaired myocardial function has been partly attributed to alterations in the function of contractile proteins and excitation–contraction coupling (ECC)3. Calcium plays a crucial role in ECC and influences cardiac rhythmicity and cellular contraction. During systole, electrical excitation of the membrane causes L-type calcium channels (LTCC) to open and a small amount of calcium enters the cell which binds to ryanodine receptor 2 (RyR2) and triggers the sarcoplasmic reticulum (SR) to release some of its calcium reserve. This release results in an overall increase in the cytosolic calcium which binds to troponin thereby facilitating cellular contraction. The excess cytosolic calcium is pumped out of the cytoplasm through three main processes. Part of the calcium is pumped back into the SR through sarcoplasmic endoplasmic reticulum calcium ATPase 2a (SERCA2a) and the rest of the calcium is extruded out of the cell via the sodium–calcium exchanger (NCX1) and the sarcolemma calcium pump. The mitochondria also appear to play a role in calcium uptake during this process and its importance is under investigation4,5.
The voltage-dependent anion channel 2 (VDAC2) is a 32 kDa porin present on the mitochondrial outer membrane (MOM) and contributes to apoptosis, steroidogenesis, metabolite flux, and calcium homeostasis6. VDAC2 is known to interact with pro-apoptotic proteins such as Bcl2 family proteins. While VDAC2–BAK (Bcl2-antagonist/killer protein) interaction is reported to be highly important to control cellular apoptosis, it is still controversial if this interaction promotes or inhibits apoptosis. Studies show that VDAC2–BAK complex was absent during death stimulus and deletion of VDAC2 promotes apoptosis7. In contrast, other studies have shown that truncated-Bid (BH3-interacting domain death agonist) induced MOM permeabilization and cell death in wild-type (WT) but not in VDAC2 knock-out (KO) mouse embryonic fibroblasts8.
Calcium flux into the mitochondrial matrix takes place primarily through the mitochondrial calcium uniporter (MCU), present in the mitochondrial inner membrane (MIM). However, the initial calcium import into the inter-membrane space is through VDACs. The interaction between MOM and SR is crucial for this mitochondrial calcium signaling9. Evidence suggests that coupling of VDAC2 with sub-sarcolemmal RyR2 is essential for calcium transfer from SR to the mitochondria10. Mitochondrial calcium dynamics have mostly been associated with the MIM and primarily, the MCU. However, it has been reported that MCU-KO mice do not show any adverse cardiac phenotypes11,12. This suggests an important role of VDACs and MOM in mitochondrial calcium signaling.
Increasing evidence suggests the importance of VDAC2 in physiologic cardiac function and in this manuscript, we hypothesize that mitochondrial calcium dynamics have a significant influence in this process13,14. Increasing the calcium uptake through VDAC2 has been shown to reverse the arrhythmia phenotype observed in the tremblor zebrafish model (NCX1h mutant) and the tachycardia phenotype in mice with catecholaminergic polymorphic ventricular tachycardia (RYR2 mutant)13,14. Both these arrhythmic models have irregular cellular calcium signaling. Enhancing mitochondrial calcium uptake through VDAC2 using a small molecule called efsevin, pharmacologically helped restore the rhythmic phenotype in these models. VDAC2 knock-down in HL-1 cell line has shown to have specific effects on calcium homeostasis such as increased diastolic calcium and restricted calcium spark expansion15. However, after a global knockdown of VDAC2 was shown to be embryonic lethal7, no further studies have investigated the effects of cardiac VDAC2-KO in animal models. Advancing our understanding of VDAC2’s role in calcium signaling and ECC may have significant implications. In this study, in an effort to address this gap in knowledge we performed cardiac-specific Vdac2 deletion and studied its effects on calcium cycling. Our results indicate a strong dependency on VDAC2 and cellular calcium dynamics to the ECC. VDAC2-KO mice showed significantly slower mitochondrial calcium uptake and altered cellular calcium signaling with smaller calcium transient, a slower rate of decay, and a slower rate of rising in cytosolic calcium. Adverse cardiac remodeling and severe cardiomyopathy led to the death of these KO mice. Reintroduction of VDAC2 in 6-week-old KO mice using an adeno-associated virus 9 (AAV9) vector seemed to partially rescue the cardiomyopathy phenotype suggesting a plausible role of VDAC2 as a therapeutic target in clinical HF. To evaluate this hypothesis, we took a gain-of-function approach and measured the effects of VDAC2 agonist efsevin in murine tissue from failing hearts: in line with the HF phenotype induced by VDAC2-KO, efsevin enhanced contractile force in failing myocardium from a murine pressure-overload model establishing VDAC2 as a promising target for HF.
Results
Cardiac-specific developmental deletion of VDAC2 causes DCM and leads to increased mortality
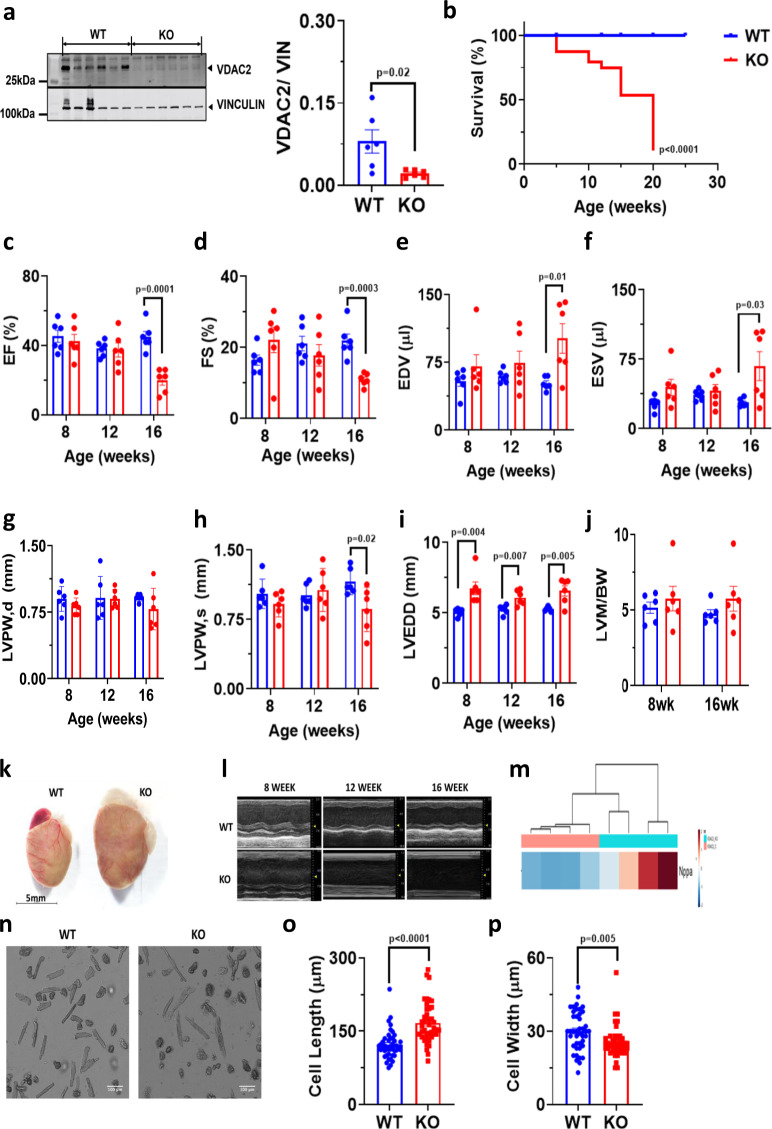
Cardiac ventricle-specific developmental Vdac2 KO and littermate WT were used for this study. Mice with either flox or Cre were used as WT and echocardiography data on flox or Cre only mice shows no significant differences (Supplementary Fig. 1a–g). Deletion of VDAC2 was confirmed using western blot (Fig. 1a). We observed no difference in VDAC1 and VDAC3 protein levels in VDAC2-KO mice (Supplementary Fig. 2a–c), suggesting a pure VDAC2-dependent phenotype. Increased mortality was observed in the KO between 5 and 20-weeks postpartum. Most of these events occurred during 20- weeks postpartum (Fig. 1b). Serial echocardiographic analysis, showed progressive deterioration of cardiac function in VDAC2-KO mice as reflected by significant reductions around 16 weeks in left ventricular (LV) ejection fraction (EF), fractional shortening (FS), and posterior wall thickness at systole (LVPW,s) and a significant increase in LV end-diastolic diameter (LVEDD), LV end-diastolic and end-systolic volumes (EDV and ESV, respectively). No difference in LV posterior wall thickness at diastole (LVPW,d) and LV mass (normalized to body weight) was observed (Fig. 1c–j). We are also providing Supplementary Table 1 which describes the cardiac phenotype during the entire development, including intra-utero echocardiograms performed in pregnant mice16. We also found increased gene expression of NPPA in KO mice (Fig. 1m), all of which are hallmarks of DCM. Increased dilation was also evident from echocardiography and the KO hearts were significantly larger than WT hearts (Fig. 1k, l). VDAC2-KO mice also had significantly longer and thinner cardiomyocytes compared to WT mice at 16-weeks of age (Fig. 1n–p). Taken together, our results suggest that deletion of VDAC2 in the myocardium during development causes DCM and eventually leads to increased mortality.
Fig. 1. Cardiac-specific deletion of VDAC2 causes dilated cardiomyopathy and leads to increased mortality.
a Western blot image of VDAC2 and lane-loading control VINCULIN and quantification in WT (Wild-type) and KO (Knock-out) using Image Studio Lite (version5.2.5) (p = 0.02) (n = 6); b Kaplan–Meier survival curve (n = 10) (p < 0.0001); c–j Echocardiography analysis of left ventricular ejection fraction (EF) (p = 0.000143), fractional shortening (FS) (0.000376), left ventricular end-diastolic and end-systolic volumes (EDV and ESV) (p = 0.0132 and p = 0.0311), left-ventricular posterior wall thickness at diastole and systole (LVPW,d and LVPW,s) (p = ns and p = 0.0244), left ventricular end-diastolic diameter (LVEDD) (8-week p = 0.004824; 12-week p = 0.007670 and 16-week p = 0.005986) (n = 6) and j left ventricular mass to body weight ratio (LVM/BW), respectively (n = 6); k Representative heart image of 16-week-old mice (scale bar, 5 mm); l Representative m-mode image from echocardiography (n = 1); m Differential expression of NPPA gene (n = 4); n Representative ventricular cardiomyocyte image using confocal microscopy (scale bar, 100 μm ); o, p Cardiomyocyte cell length (p < 0.0001) and width (p = 0.0058) measured using Zen5 imaging software, respectively (n = 42, N = 3). p-value: unpaired two-tailed t-test performed in panels a, o, and p, Mantel–Cox test performed in panel b and two-stage set-up multiple t-test (Benjamini, Krieger, and Yekutieli) performed for longitudinal echocardiographic data. Data are represented as differences between mean ± SEM.
Altered gene and protein expression profiles indicate altered calcium signaling and regulation in VDAC2-KO
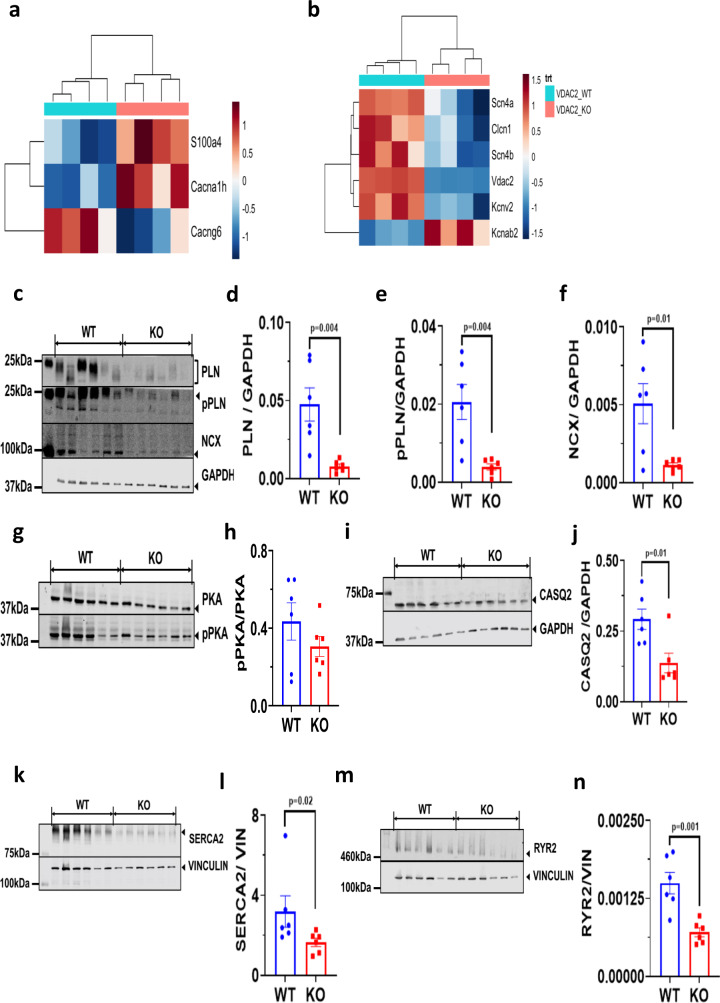
Gene expression profile from ventricular RNA of 16-week-old KO mice showed a number of differentially expressed genes involved in calcium signaling and regulation. Calcium channel genes such as Cacna1h, a T-type calcium channel (Cav 3.2) was upregulated whereas Cacng6, a subunit of the LTCC was significantly downregulated (Fig. 2a). Other ion channels were also differentially expressed between the two groups. Specifically, a significant downregulation of sodium and potassium ion channels was observed in VDAC2-KO mice, including Scn4a, Scn4b, and Kcnv2, all of which have been reported to play significant roles in normal cardiac function (Fig. 2b). Proteins involved in ECC such as RYR2, SERCA2, NCX1, total and phosphorylated phospholamban (PLN), and calsequestrin (CASQ2, the SR calcium-binding protein) were significantly decreased in the KO (Fig. 2c–n). Collectively, these results suggest that loss of VDAC2 leads to abnormalities in cellular calcium signaling pathways.
Fig. 2. Altered gene and protein expression profiles indicate altered calcium signaling and regulation in VDAC2-KO.
a, b Differential gene expression data derived from 16-week old cardiac ventricular tissue (n = 4); c–f Western blot image of PLN (p = 0.0042), pPLN (p = 0.0045), and NCX (p = 0.0121) along with lane-loading control GAPDH and respective quantification using Image Studio Lite (version 5.2.5) (n = 6); g, h Western blot image of PKA and pPKA and quantification using Image Studio Lite (version 5.2.5) (p = ns) (n = 6); i, j Western blot image of CASQ2 and lane-loading control GAPDH and respective quantification using Image Studio Lite (version 5.2.5) (p = 0.0111) (n = 6); k, l Western blot image of SERCA2 and lane-loading control VINCULIN and quantification using Image Studio Lite (version 5.2.5) (Mann–Whitney: p = 0.0260) (n = 6); m, n Western blot image of RYR2 and lane-loading control VINCULIN and quantification using Image Studio Lite (version 5.2.5) (p = 0.0018) (n = 6). p-value: unpaired two-tailed t-test and two-tailed Mann–Whitney test performed. Data are represented as mean ± SEM.
Impaired calcium cycling in VDAC2-KO cardiac myocytes
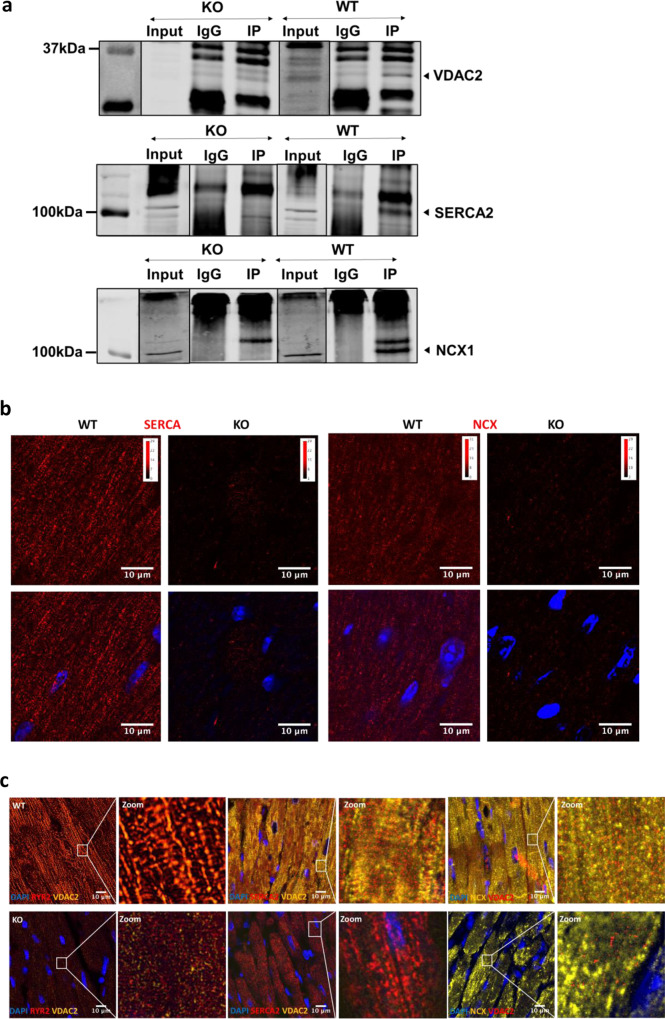
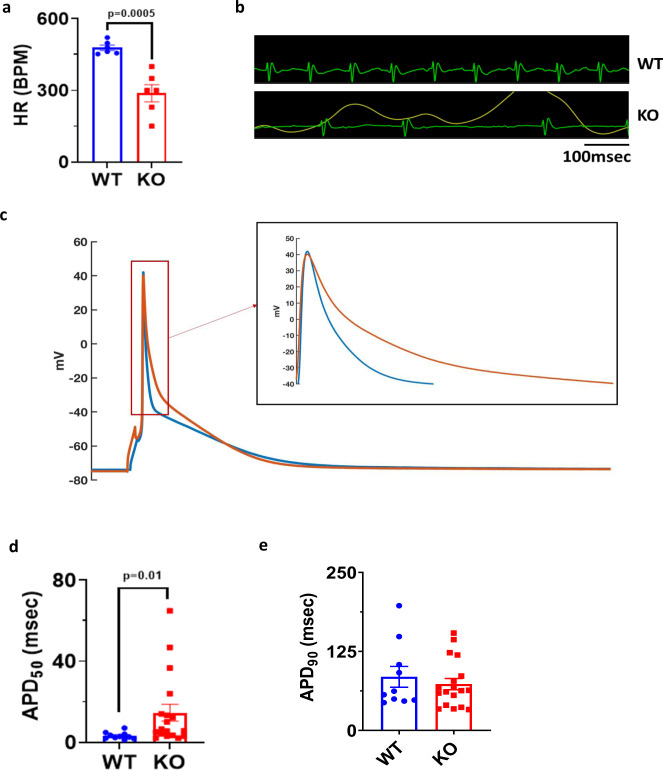
To further assess calcium handling in these mice, we recorded calcium transients in 16-week-old cardiac myocytes. Compared to WT, VDAC2-KO myocytes had smaller calcium transients (F/F0) with a slower rate of decay (tau) and slower maximum rate of rising (F/F0/s) (Fig. 3a–e). To investigate if the decreased calcium transients are directly attributable to the lack of VDAC2 we tested the effects of VDAC2 agonist efsevin14 on calcium transients in WT cardiomyocytes and found the opposite effect, namely an increase in calcium transient amplitude and an acceleration of the decay phase (Supplementary Fig. 3a–c). SERCA2a and NCX1 are the two main channels involved in cytosolic calcium clearance during diastole and we showed that these protein levels are significantly reduced in VDAC2-KO (Fig. 2f and l). Co-immunoprecipitation (Co-IP) studies using whole-heart lysate and pull-down using VDAC2 antibody revealed VDAC2–SERCA2 interaction and VDAC2–NCX1 interaction, respectively. VDAC2-KO heart was used as a negative control for co-IP (Fig. 4a). This interaction was also confirmed by immunofluorescence and proximity ligation assays (PLA) (Fig. 4b, c). We hypothesize that these interactions, similar to the previously discovered VDAC2–RyR2 interaction10, are crucial for calcium uptake by the mitochondria and indirectly by the SR. As a result, a disruption of this interaction caused drastic changes in the calcium signaling in the cardiac myocytes. This altered calcium signaling may explain bradyarrhythmia observed in VDAC2-KO (Fig. 5a, b)17. Additionally, in agreement with the altered gene expression of the above-mentioned ion channels, the action potential (AP) of the KO was different from that of WT with a significantly longer AP duration at 50% repolarization (APD50) and a normal 90% repolarization (APD90) (Fig. 5c–e). Collectively, we found significant differences in most aspects of calcium cycling in the VDAC2-KO, which presumably contributes to the progressive decline in myocardial function.
Fig. 3. Impaired calcium cycling in VDAC2-KO cardiomyocytes.
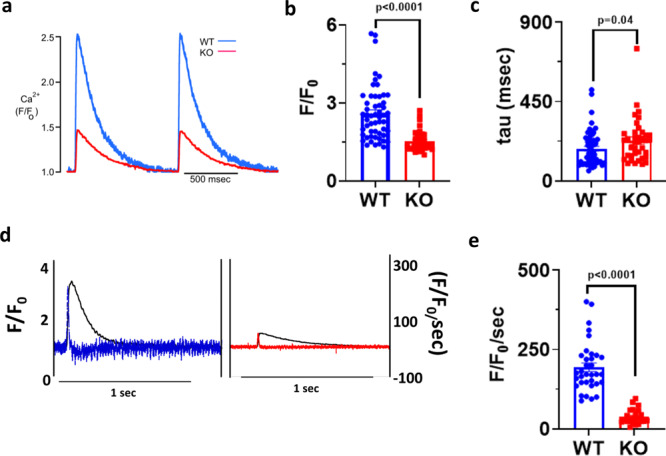
a Representative calcium transient image of 16-week-old mice (Time frame, 500 ms); b Amplitude (F/F0) data (p < 0.0001) (WT n = 54, KO n = 38, N = 3); c Rate of calcium uptake (tau) (ms) (p = 0.0492) (WT n = 54, KO n = 38, N = 3); d Representative image of rate of calcium release of 16-week-old mice (Time frame, 1 s); e Rate of calcium release (F/F0/s) (p < 0.0001) (WT n = 34, KO n = 23, N = 3). p-value: unpaired two-tailed t-test performed in all comparisons. Data are represented as mean ± SEM.
Fig. 4. Cross-talk between mitochondria and ER/SR facilitates mitochondrial calcium cycling.
a Representative co-immunoprecipitation image from a cardiac ventricular sample of 8-week-old mice (n = 3) in WT and KO (Input—non-IP sample; IgG—control antibody; IP—VDAC2 protein pull-down); b Representative proximity ligation assay image for VDAC2–SERCA2 and VDAC2–NCX1 interaction in 8-week-old mice (scale bar, 10 μm) (n = 3); c Representative immunofluorescence image from 16-week-old mice heart (scale bar, 10 μm) (n = 3).
Fig. 5. Altered action potential in VDAC2-KO cardiomyocytes.
a Heart rate (HR), beats per minute (BPM) (p = 0.0005) (n = 6); b Representative ECG of 16-week-old mice (time frame, 100 ms); c Representative action potential image of 16-week-old mice cardiomyocyte (time frame, 100 ms); d, e Action potential repolarization duration at 50% and 90%, respectively (two-tailed Welch test: APD90 p = ns, APD50 p = 0.0148) (WT n = 10, KO n = 18, N = 3). p-value: unpaired two-tailed t-test and two-tailed Welch test performed. Data are represented as mean ± SEM.
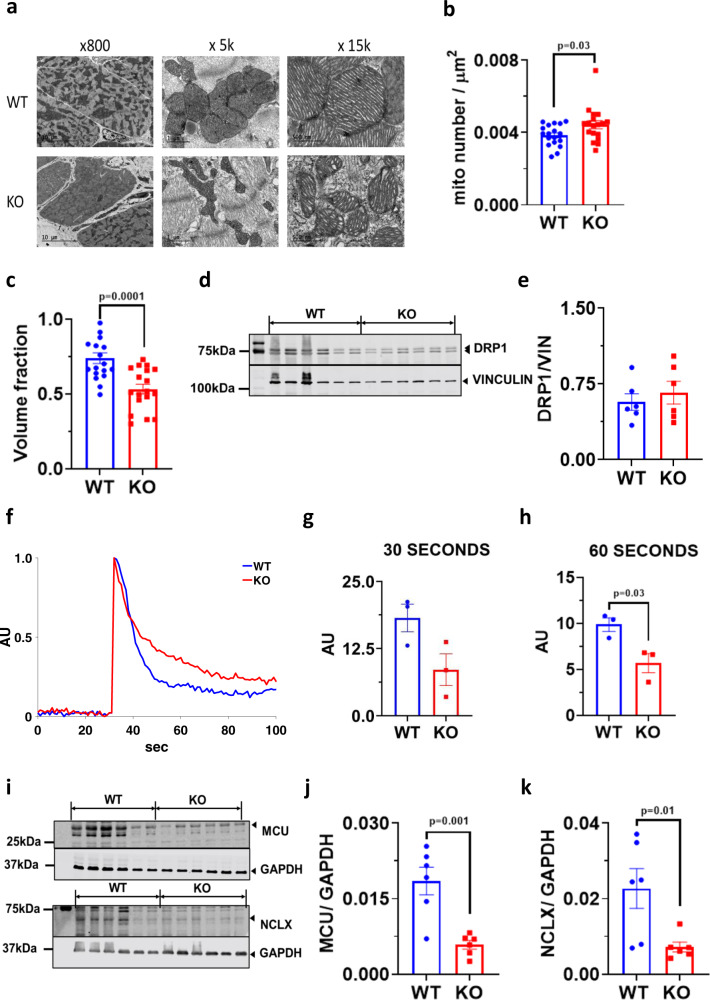
Altered mitochondrial structure and function in VDAC2-KO
Transmission electron microscopy (TEM) performed on 16-week-old KO and WT hearts showed substantial alterations in mitochondrial distribution and structure (Fig. 6a–c). KO mitochondria were significantly smaller, disoriented, and less dense compared to WT mitochondria. We did not observe any significant difference in dynamin-related protein 1 (DRP1) levels (a marker for mitochondrial fission) although it showed a trend towards an increase in KO mice (Fig. 6d, e). We also observed a significant reduction in the rate of calcium uptake in KO mitochondria suggesting VDAC2 plays an important role in mitochondrial calcium transport (Fig. 6f–h). A significant decline in MCU and NCLX protein levels was also observed in the KO (Fig. 6i–k). These proteins are known to play an important role in mitochondrial calcium transport and signaling and the decreased protein level in the KO is consistent with the slower rate of calcium uptake.
Fig. 6. Altered mitochondrial structure and function in VDAC2-KO.
a Representative transmission electron microscopy image at ×800, ×5000 and ×15,000 magnifications (scale bar, 10 μm, 1 μm, and 500 nm, respectively) (n = 3); b Number of mitochondria per unit area measured using Adobe Photoshop CC 2019 (p = 0.0365) (n = 18 images, N = 3); c Mitochondrial volume fraction measured using Adobe Photoshop CC 2019 using 22 × 14 grid (p = 0.0001) (n = 18 images, N = 3); d, e Western blot image of DRP1 and lane-loading control VINCULIN and quantification using Image Studio Lite (version 5.2.5) (p = ns) (n = 6); f Representative mitochondrial calcium uptake image produced using MATLAB (n = 3); g, h Mitochondrial calcium uptake in the first 30 s (p = ns) and 60 s of calcium pulse (p = 0.0300) (n = 3), respectively; i–k Western blot image of MCU (p = 0.0014) and NCLX (p = 0.0169) along with respective lane-loading control GAPDH and quantification using Image Studio Lite (version 5.2.5) (n = 6). p-value: unpaired two-tailed t-test performed in all comparisons. Data are represented as ±SEM.
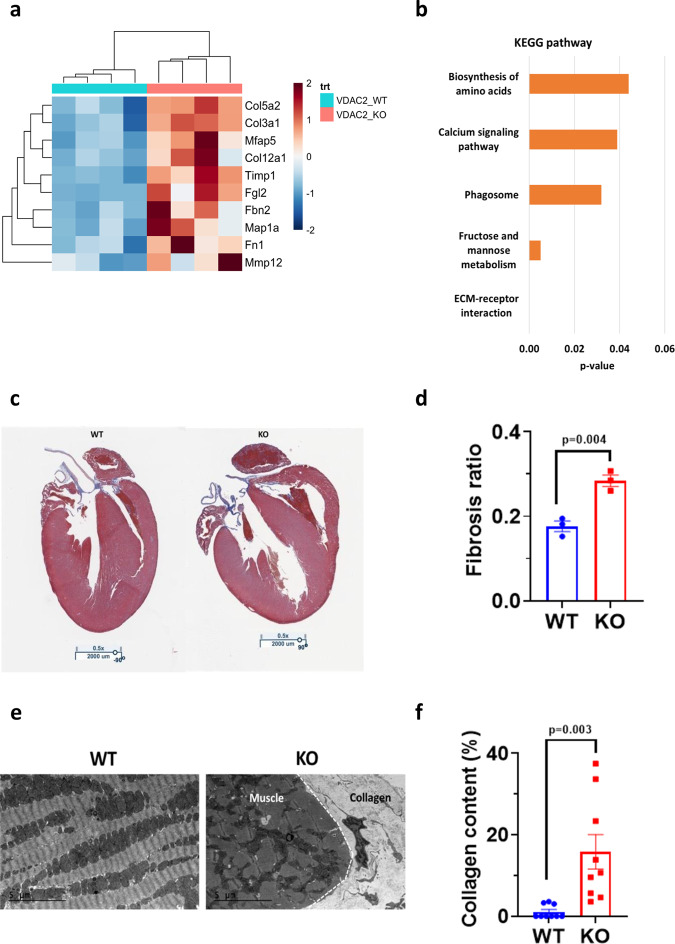
Increased cardiac fibrosis observed in VDAC2-KO
Gene expression profile showed a significant upregulation of extracellular matrix (ECM) proteins and collagen encoding genes including Col3a1, Col5a2, and Mmp12 in the KO that suggests increased collagen deposition and fibrosis (Fig. 7a). Kyoto Encyclopedia of Genes and Genomes (KEGG) pathway analysis in KO mice indicated that the ECM production pathway changes reached the highest statistical significance (Fig. 7b). Masson’s trichrome stain and TEM on 16-week-old mice hearts showed significantly higher collagen content in the KO (Fig. 7c–f). The observed increased myocardial fibrosis is consistent with adverse structural and functional myocardial remodeling and cardiomyopathy in the KO mice.
Fig. 7. Increased cardiac fibrosis observed in VDAC2-KO.
a Differential gene expression data from 16-week-old mice ventricular sample (n = 4); b KEGG pathway analysis of differentially expressed genes in KO indicates ECM production pathway as highest statistical significance (n = 4); c Representative Masson’s trichrome stain image (×0.5 magnification; scale bar, 2000 μm) (n = 3); d Fibrosis quantification using Aperio software and colocalization v9 algorithm (p = 0.0043) (n = 3); e Representative transmission electron microscopy image (scale bar, 5 μm) (n = 3); f Collagen quantification using ImageJ software (p = 0.0033) (n = 9 images, N = 3). p-value: unpaired two-tailed t-test performed in all comparisons. One-tail Fisher Exact test performed for KEGG pathway analysis. Data are represented as ±SEM.
Metabolic alterations observed in VDAC2-KO
Since VDAC2 is known to interact with a number of key enzymes like phosphofructokinase (PFK) and hexokinase (HK) that are involved in glycolysis and is known to transport a number of metabolites and ATP, we also measured the RNA and protein levels of specific enzymes in key metabolic pathways. The volcano plot from RNA sequencing data showed significant differences in enzymes involved in metabolism and we observed an overall downregulation of enzyme levels involved in glycolysis, tricarboxylic acid (TCA) cycle, and glycolytic accessory pathways. Specifically, enzymes including transketolase (TKT), succinyl-CoA ligase (SUCLA2), pyruvate dehydrogenase (PDH), and pyruvate carboxylase (PC). However, no significant difference was observed in fatty acid oxidation (FAO) pathways (Supplementary Fig. 4a–o). VINCULIN and GAPDH were used as lane-loading controls and we observed no difference in these protein levels between WT and KO (Supplementary Fig. 4p).
No significant difference in mitochondrial ROS production
VDAC2-KO did not show a significant difference (compared to WT) in reactive oxygen species (ROS) production upon inhibition of complex I and complex III using rotenone and antimycin, respectively (Supplementary Fig. 5a–d). However, we found a trend (p = 0.08) towards a decrease in complex II in the KO (Supplementary Fig. 5e–i).
Unaltered mitochondrial respiration
Oxidative phosphorylation (OXPHOS) measurements with LV myocardial tissue showed no difference in mitochondrial oxygen consumption between the WT and KO under normal conditions (Supplementary Fig. 6a–i). This suggests that VDAC2 is not essential for normal mitochondrial respiration despite its role in calcium signaling. These results are similar to those observed in previous publications with MCU-KO11,18.
Partial rescue of HF phenotype upon VDAC2 re-introduction
Cardiac ventricular myocyte-specific reintroduction of VDAC2 in KO and WT mice was assessed using western blot and qRT-PCR (Fig. 8a, b). Serial echocardiography was performed on these mice up to 10 weeks post-injection (16-week-old mice) to get comparable results. Cardiac structural and functional improvement was observed in KO mice injected with AAV9-αMHC-VDAC2-GFP vector evident from relative improvement in EF, FS, and LV volumes and from a decrease in LV diameter compared to KO mice injected with control AAV9-αMHC-GFP vector (Fig. 8c–g). Complete rescue of the phenotype was not observed, however, the re-introduction of VDAC2 aided in better cardiac functioning.
Fig. 8. Partial rescue of HF phenotype upon VDAC2 reintroduction.
a Western blot image of VDAC2-GFP and lane-loading control VINCULIN and quantification using Image Studio Lite (version 5.2.5) (KO GFP vs. KO VDAC2-GFP p = 0.0568) (n = 3); b qRT-PCR of Vdac2 normalized to Vcl (Vinculin) (KO GFP vs. KO VDAC2-GFP p = 0.0044) (n = 3); c–g Serial echocardiography parameter including ejection fraction (EF) (week 6: WT GFP vs. KO GFP p = 0.0036, week 10: WT GFP vs. KO GFP p = 0.0008, WT VDAC2-GFP vs. KO VDAC2-GFP p = 0.0456, KO GFP vs. KO VDAC2-GFP p = 0.0359), fractional shortening (FS) (week 6: WT GFP vs. KO GFP p = 0.0091; week 10: WT GFP vs. KO GFP p = 0.0043, WT VDAC2-GFP vs. KO VDAC2-GFP p = 0.0652), left ventricular end diastolic diameter (LVEDD) (pre: WT GFP vs. KO GFP p = 0.0169, WT VDAC2-GFP vs. KO VDAC2-GFP p = 0.0407; week 10: WT GFP vs. KO GFP p = 0.0107, KO GFP vs. KO VDAC2-GFP p = 0.0444), end diastolic and end systolic volumes (EDV (pre: WT GFP vs. KO GFP p = 0.0032; week 10: WT GFP vs. KO GFP p = 0.0104, KO GFP vs. KO VDAC2-GFP p = 0.0378) and ESV (week6: WT GFP vs. KO GFP p = 0.03, week 10: WT GFP vs. KO GFP p = 0.0008, KO GFP vs. KO VDAC2-GFP p = 0.0066)) respectively (n = 3). p-value: unpaired t-test performed in all comparisons between KO GFP and KO VDAC2-GFP in panels a and b. Ordinary one-way ANOVA was performed for echocardiographic data and panel b. Data are represented as ±SEM.
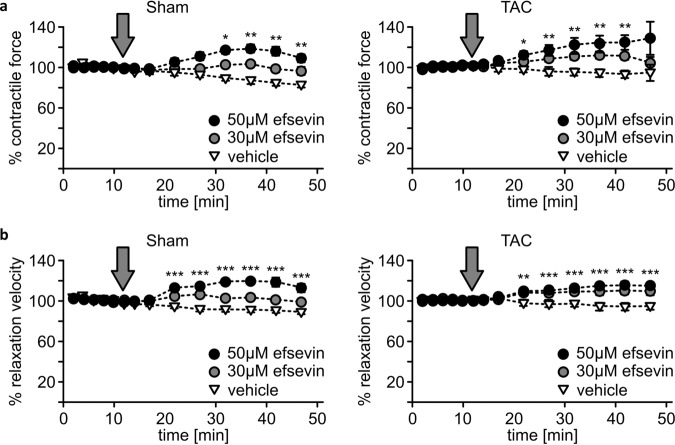
VDAC2 as a pharmacological target for HF
We show that VDAC2 regulates cardiac calcium signaling without having major effects on bioenergetics and VDAC2 deletion causes DCM, making it a candidate structure for HF therapy. We therefore, addressed the question of increasing VDAC2s calcium uptake activity could enhance cardiac contractile force in HF. Therefore, we used the established model of pressure overload-induced HF by transverse aortic constriction (TAC). Since the administration of efsevin to mice is difficult for more than a few days due to its short plasma half-life time and efsevin is not orally bioavailable, we took advantage of organotypic heart slices and investigated the effect of efsevin on myocardial contractile strength. Strikingly, efsevin enhanced contractile strength and accelerated relaxation in both control and failing hearts (Fig. 9a, b).
Fig. 9. Enhanced contractile force and accelerated relaxation speed in myocardial tissue upon VDAC2 activation.
a Measurement of relative contractile force in organotypic myocardial slices from both Sham- (left panel) and TAC-operated mice (right panel) after superfusion with either 30 or 50 µM of the VDAC2 agonist efsevin. Each dot represents the quantification of twitch amplitudes over 20 s at indicated time points. The gray arrow indicates the start of efsevin superfusion (left panel: DMSO vs. 30 μM Efsevin: 10 min p = 0.016, 32 min p = 0.010, 37 min p = 0.0004, 42 min p = 0.0012 and 47 min p = 0.0062; DMSO vs. 50 μM Efsevin: 22 min p = 0.0007, 27–47 min p < 0.0001, respectively) (right panel: DMSO vs. 30 μM Efsevin: 17 min p = 0.0038, 22 min p = 0.0009, 27 min p = 0.00031, 32 min p = 0.00029, 37 min p = 0.00015, 42 min p = 0.00073; DMSO vs. 50 μM Efsevin: 22 min p = 0.0218, 27 min p = 0.0044, 32 min p = 0.0037, 37 min p = 0.0031, 42 min p = 0.0034). b Relaxation speed of twitches of myocardial slices shown in a (N = 8, n = 22 slices for TAC, N = 6, n = 14 slices for Sham) (left panel: DMSO vs. 30 μM Efsevin: 22 min p = 0.0072, 27 min p = 0.00019, 32 min p = 0.044, 37 min p = 0.0125, 42 min p = 0.0145, 47 min p = 0.0308; DMSO vs. 50 μM Efsevin: 22–47 min p < 0.0001 respectively) (right panel: DMSO vs. 30 μM Efsevin: 22 min p < 0.0001, 27 min p = 0.0001, 32 min p < 0.0001, 37 min p < 0.0001, 42 min p = 0.0001, 47 min p = 0.0002; DMSO vs. 50 μM Efsevin: 22 min p = 0.002, 27 min p = 0.0001, 32–42 min p < 0.0001, respectively, 47 min p = 0.0001). p-value: unpaired t-test performed in all comparisons. *<0.05, **<0.01 and ***<0.001. Data are represented as ±SEM.
Discussion
In this study, we investigated the role of VDAC2 in the heart by creating a cardiac ventricular myocyte-specific deletion of VDAC2 using α-Myosin heavy chain (αMHC) promoter. We observed increased postnatal mortality along with severe impairment in cardiac structure and function consistent with DCM. Our gene and protein studies suggested altered calcium signaling in the KO mice. In agreement with these data, the KO showed smaller calcium transients, a slower decay time, and a slower rate of calcium release. Substantial adverse cardiac remodeling and mitochondrial disorganization along with slower mitochondrial calcium uptake were also observed in the KO indicating that VDAC2 plays a crucial role in cardiac functioning by maintaining mitochondrial and cellular calcium homeostasis. Additionally, reintroduction of VDAC2 in young-KO mice showed a partial rescue of the cardiomyopathy phenotype suggesting the importance of VDAC2 in normal cardiac functioning.
Mitochondria–ER/SR interactions have long been studied and it is commonly believed that this interaction is crucial for mitochondrial calcium dynamics9,19,20. Mitochondria are known to recognize calcium-rich niches near SERCA2, RYR2, and NCX1 where major calcium transport happens21. We show in this study that the crosstalk of VDAC2 with both calcium release through—RYR2 and diastolic calcium removal by SERCA2 and NCX1 plays an important role in determining cellular calcium homeostasis and when this crosstalk is lost, there is a significant impairment in both mitochondrial and cytosolic calcium signaling. We also observed a significant reduction in most calcium handling proteins like RYR2, SERCA2, NCX1, and CASQ2 in VDAC2-KO mice. While these results are unexpected, they might be explained by different hypotheses: It is feasible that the lack of mitochondrial calcium uptake specifically through the loss of RYR2–VDAC2 connection results in a lack of energy demand which was shown to depend on mitochondrial calcium uptake22. In this scenario the basic metabolism of VDAC2-KO cells would be comparable to WT (as demonstrated in our results), while they are unable to adapt to higher workload under stress situations and might consecutively down-regulate calcium handling proteins. Alternatively, the down-regulation might be a result of compensatory mechanism to the slower rate of calcium uptake observed in the KO. Additionally, a down-regulation due to loss of structural integrity of the myocardium as observed in our experiment is feasible. Also, our data stands in contrast to previous observations that knock-down of VDAC2 in HL-1cells did not alter expression levels of calcium handling proteins10. However, major differences between the model systems might explain these discrepancies: Partial inhibition of VDAC2 (~75%) was achieved using viral transduction and cells were cultured in vitro for limited time. It is thus feasible that the down-regulation of these proteins is masked either by the remaining VDAC2 expression, the lack of triggers present in vivo-like pressure or adrenergic stimulation, or that these translational changes only manifest after a longer time.
The significant impairment in SR-mediated calcium release and uptake in VDAC2-KO mice can be associated with reduced RYR2 and SERCA2 activities, respectively. We also observed a significant downregulation of total and phosphorylated PLN in VDAC2-KO which may account for the reduced SERCA2 activity and slower rate of decline in calcium transient. Additionally, the varying molecular weights seen in total PLN protein is likely due to post-translational modification of PLN by phosphorylation at different sites or different combination of sites including Ser10, Ser16, and/or Thr17. Therefore, an average intensity of the bands was taken and normalized with GAPDH23–25. Our co-IP studies suggest an interaction of VDAC2 with SERCA2. We propose that this interaction might be important for facilitating mitochondrial calcium uptake and also indirectly influencing cytosolic calcium signaling. Another pathway for calcium extrusion from the cytoplasm is through NCX1 and we observed that VDAC2 interacts with NCX1. This interaction, similar to that with SERCA2 and RyR2, is also likely to influence cellular calcium signaling. Sub-cellular regions near RyR2, SERCA2, and NCX1 act as calcium compartments with markedly different calcium concentration than the bulk cytosol and both the interaction of VDAC2 with calcium handling proteins and the localization of mitochondria near these regions indicates a substantial role of mitochondrial calcium uptake for cellular calcium handling. We propose that upon disruption of this interaction, there is less overall mitochondrial calcium uptake and reduced cytosolic calcium clearance which leads to impaired intracellular calcium signaling (Supplementary Fig. 7).
In agreement with other studies, we showed that VDAC2 is crucial for cellular calcium cycling and normal cardiac functioning, thereby making it a promising therapeutic target for DCM and chronic HF6,15. To test this hypothesis, we investigated the potency of efsevin as a therapeutic agent for the treatment of HF. Indeed, efsevin enhanced contractile force in organotypic tissue slices of failing murine myocardium, establishing VDAC2 as a promising target for HF.
HF is associated with an increased risk for arrhythmia and efsevin was previously suggested to suppress arrhythmogenesis in cardiomyocytes13,14 suggesting a role for VDAC2 also as a protective pathway against arrhythmia. This data is backed up by our findings, that VDAC2-KO mice also experienced arrhythmias. We hypothesize that the altered calcium signaling (reduced CASQ2, RYR2, and SERCA2 activities) has the potential to cause these arrhythmias26,27. The reduced CASQ2 activity suggests reduced RyR2 sensitivity to cytosolic calcium which in turn explains the reduced gene expression of L-type calcium channel28. Additionally, the expression of T-type calcium channels in the adult KO myocardium is indicative of disease states such as cardiomyopathies and HF where increased expression of T-type channels in adult ventricular myocytes leads to increased susceptibility to arrhythmias29,30. The cellular patch-clamp experiments in the VDAC2-KO showed a tendency towards increased early after depolarizations perhaps due to reactivation of these calcium channels31. We also observed a significantly longer APD50 in the KO which can be explained by the genetic downregulation of the transient outward channel thereby making VDAC2 influence on the cardiomyocyte even more prominent.
VDAC2–tubulin interaction and VDAC2–PFKP interaction have been studied in cancer metabolism research were specifically targeting VDAC2 has shown to down-regulate glycolysis thereby preventing cancer cell growth and proliferation32,33. In agreement with this work, we also observed significant alterations in a number of enzymes involved in glucose metabolism, its accessory pathways, and the TCA cycle but no difference was observed in FAO or electron transport chain (ETC). This unaltered FAO and ETC may help explain the relatively unaltered mitochondrial respiration since these metabolic pathways provide the necessary ATP.
VDAC2 plays an important role in apoptosis by interacting with BAK, a proapoptotic protein, and keeping it inactive34. Essentially, VDAC2–BAK interaction prevents cellular apoptosis, and Vdac2 deletion results in free (or active) BAK thus causing excessive apoptosis35. We hypothesize that this increased apoptosis during late cardiac development might contribute to the increased fibrosis observed in our VDAC2-KO mice.
Calcium release into the cytoplasm is mainly mediated by the RyR2 and previous publications have established the importance of physical interaction between VDAC2 and RyR2 for normal calcium transport into the mitochondria10. Mitochondrial calcium uptake was significantly impaired in our VDAC2-KO mice indicating the importance of VDAC2 in mitochondrial calcium transport. A similar reduction in mitochondrial calcium uptake was observed in MCU-KO mice, however, no other phenotypical difference was observed between the MCU-WT and MCU-KO36, indicating differences in the role of these two transporters incellular calcium handling. One explanation derived from this work is that VDAC might act as a scaffolding protein to link the MCU to other calcium handling proteins and thus creating cellular calcium microdomains, while MCU might serve as the controller of mitochondrial calcium uptake. This is in line with observations that VDAC2 was described to be a vividly interacting channel20.
Overall, our findings highlight the importance of VDAC2 in influencing ECC and cardiac disease progression. Previous research has shown that increased calcium uptake through VDAC2 (using efsevin) reverses arrhythmic phenotypes. Here, we demonstrate the crucial role of VDAC2 in ECC and the pathogenesis of DCM and provide reasonable evidence for VDAC2 to serve as a therapeutic target for HF. Thus, drugs targeting VDAC2 might fulfill a double role by suppressing cardiac arrhythmia and enhancing cardiac function.
Methods
Animals and animal care
All animal studies were performed in accordance with the University of Iowa Animal Care and Use Committee (IACUC). All procedures involving animals were approved by the Animal Care and Use Committee of the University of Utah and complied with the American Physiological Society’s Guiding Principles in the Care and Use of Animals and the UK Animals (Scientific Procedures) Act 1986 guidelines. The mice were housed in 12 h dark/light cycle at 70 °F and 40% humidity.
Generation of cardiac-specific knockout
C57BL6J mice were used for all experiments. Both male and female mice were used for all experiments. Vdac2 flox/flox mice were obtained from Ren et al. 35. The founder mice were cross-bred with transgenic αMHC-Cre (Jackson Laboratory#009074)37 and the resulting heterozygous (Vdac2 flox/+; αMHC-Cre) mice were crossed to obtain a cardiac ventricular myocyte-specific deletion of VDAC2.
Echocardiographic analysis
Mice were anesthetized with 1.5% Isoflurane (Vet One, NDC13985-046-60) during echocardiography. Echocardiographic images were taken on the Vivo system. Echoes were performed serially from embryonic age 17 to 16 weeks post-natal. 2D long-axis and short-axis views were obtained and used for analysis using Vivo strain software (version 3.1.1). Two consecutive cardiac cycles were used for all the measurements. Limb leads were used to record an electrocardiogram (ECG).
Collagen content evaluation/Masson’s trichrome stain
Mice were euthanized using sodium pentobarbital (NDC76478-501-50). Hearts were harvested and formalin-fixed for 48 h followed by paraffin embedding. 5 μm thick sections were cut and stained with Trichrome for fibrosis analysis using the Dako automated special strainer. The slides for fibrosis analysis were scanned under ×20 and analyzed using Aperio Image Scope software (version 12.3.2.8013) (using the colocalization v9 algorithm)38. A ratio of the total stained area to the collagen-stained area was reported.
Cardiomyocyte isolation
Adult mice were anesthetized with sodium pentobarbital (50 mg kg−1) and the excised heart was attached to an aortic cannula and perfused with solutions gassed with 100% O2 and held at 37 °C, pH 7.3. Perfusion with a 0 mM Ca2+ solution for 5 min was followed by 15 min of perfusion with the same solution containing 1 mg ml−1 collagenase (type II, Worthington Biochemical, Freehold, NJ, USA) and 0.1 mg/ml protease (type XIV, Sigma Chemical, catalog #P5147). The heart was then perfused for 1 min with a stopping solution (the same solution containing 20% serum and 0.2 mM CaCl2). All perfusions were performed at a flow rate of 2 ml min−1. The atria were removed and the ventricles were minced and shaken for 10 min, and then filtered through a nylon mesh. Cells were stored at 37 °C in a normal HEPES buffered solution. All myocytes used in this study were rod-shaped, had well-defined striations, and did not spontaneously contract. Experiments were performed within 7 h of isolation.
Cell super-fusion chamber
The Plexiglas cell bath had a clear glass bottom and was mounted on the stage of an inverted microscope (Diaphot, Nikon, Japan). The temperature of the solutions in the super-fusion chamber was 36 ± 0.3 °C. Bathing solutions flowed continuously through the bath at 4 ml min−1, and solution depth was held at ~2–3 mm. Exchange of the bath solution required ~5 s. The bottom of the bath was coated with laminin (Collaborative Research, Bedford, MA, USA) to improve cell adhesion.
Cell bathing solutions and pipette filling solutions
The normal control bathing solution contained (mM): 126.0 NaCl, 11.0 dextrose, 4.4 KCl, 1.0 MgCl2, 1.08 CaCl2, and 24.0 HEPES titrated to pH 7.4 with 1 M NaOH. The pipette solution used for recording APs contained (mM): 110.0 KCl, 5.0 NaCl, 5.0 MgATP, 5.0 phosphocreatine, 1.0 NaGTP, 10.0 HEPES titrated to pH 7.2 with 1 M KOH.
AP measurement
Transmembrane potential (Vm) was measured with borosilicate glass suction pipettes (resistance 1–2 MΩ when filled) connected to an Axoclamp 2B amplifier system (Axon Instruments/Molecular Devices) in bridge mode39. The Vm signal was filtered at 5 kHz, digitized at 50 kHz with a 16-bit A/D converter (Digidata 1322A), and analyzed using PCLAMP 8 software (Molecular Devices). APs were triggered with brief (2–3 ms) square pulses of depolarizing intracellular current (~2 nA). APD50 and APD90 were measured.
Intracellular calcium concentration measurement
Calcium transients were detected in single myocytes with an epifluorescence system using the fluorescent indicator, Fluo-4. Cells were incubated in the normal control solution containing 12.5 µM Fluo4-AM (Invitrogen, catalog#F14201) and 0.3 mM probenecid (Sigma, catalog #P8761) at 30 °C for 20 min. The cells were then continuously bathed in the same solution containing no indicator. Probenecid (0.3 mM) was included in the bathing solutions to help retard fluo-4 loss from the cells. Fluorescence emission (535 nm, bandpass filter) was collected with a photomultiplier tube via a ×40 objective during continuous excitation at 485 nm. Calcium transients were elicited with field stimulation at a cycle length of 1 s.
TEM and analysis
LV transmural samples from 16-week-old mice were used for this study. Tissue samples were fixed overnight at 4 °C in 0.1 M sodium cacodylate buffer containing 1% paraformaldehyde and 2.5% glutaraldehyde. Samples were washed with the same buffer and post-fixed for 2 h in 2% osmium tetroxide buffered with cacodylate buffer. Samples were then rinsed in nano-pure water and stained with en-bloc stain for 1 h at room temperature in uranyl acetate. Tissues were then dehydrated through a graded series of ethanol: 10 min in 50%, 3 × 10 min in 70%, 2 × 10 min in 95%, 4 × 10 min in absolute ethanol, and 3 × 10 min with absolute acetone. Samples were incubated at room temperature in a gradually increasing concentration of epoxy resin (Electron Microscopy Science, Hatfield, PA) to facilitate infiltration. Samples were then transferred to 50% resin in acetone for 1 h and overnight in 75% resin in acetone. Samples were then transferred to 100% resin for 8 h with three fresh resin changes. Samples were then embedded and polymerized at 60 °C for 48 h. Ultrathin sections (70 nm) were obtained with a diamond knife (Diatome) using Leica UC6 (Leica Microsystems, Vienna, Austria). Sections were post-stained with saturated uranyl acetate for 10 min followed by Reinold stain for 5 min. Sections were imaged at 120 kV with JEOL 1400 Plus40. Mitochondrial density and number analysis were performed using Adobe Photoshop tool using ×1500 images. A 22 × 14 grid was used for density measurements. The same images were used for collagen quantification using Fiji software. The ratio of collagen area to total area was reported.
Mitochondria isolation and calcium uptake
Mice were euthanized using carbondioxide for 3 min and the heart was excised. The atria were removed and the ventricles were used for mitochondria isolation using differential centrifugation41. Protein estimation was performed using Pierce Assay. 100 μg of protein was used per trial, resuspended in 100 µL of a solution containing (in mM): 125.0 KCl, 20.0 HEPES, 10.0 K2HPO4, 5.0 Glutamate, 5.0 Malate, and 1 μM Oregon Green BAPTA/6F (pH 7.2 with 1 M KOH). Imaging was performed on a Biotek Cytation 5 instrument in 96-well flat-bottomed plates. Baseline measurements were taken for 30 s and 12 μM calcium chloride was injected and the fluorescence (excitation 490 nm, emission 517 nm) was measured for 3 min to obtain the calcium uptake slope.
Retro-orbital injections
6-week-old WT and KO mice were randomly chosen to receive either AAV9-αMHC-VDAC2-GFP vector or control AAV9-αMHC-GFP vector. N = 3 mice were used in each group and a total of four groups were used (WT-AAV and KO-AAV received the vector with VDAC2 and WT-GFP and KO-GFP received control vector). A concentration of 3.1 × 10e12 VG kg−1 was used for each injection and the mice were serially echoed for 10 weeks post-injection.
RNA extraction, sequencing, and qRT-PCR
miRNeasy Mini kit (Qiagen) was used for RNA extraction from the LV transmural sample. The extracted RNA was used for total RNA sequencing (RNA Seq). Agilent RNA Screen Tape Assay was used for QC experiments. Illumina TruSeq Stranded RNA kit was used for library preparation and Ribo-Zero Gold was used to remove rRNA and the sequencing was performed on an Illumina HiSeq 2500 with 50 bp single-end reads. The same RNA was used for cDNA synthesis (NEB #E3010S) and qRT-PCR was performed using Vdac2 and Vcl (VINCULIN) primers (Table 1).
Table 1.
List of antibodies used.
| Resource | Concentration | Identifier | Source |
|---|---|---|---|
| Western blot-antibodies | |||
| Voltage-dependent anion-selective channel protein 2 (VDAC2) | 1:1000 | 9412S | Cell Signaling Technology |
| Voltage-dependent anion-selective channel protein 1 (VDAC1) | 1:1000 | ab14734 | Abcam |
| Voltage-dependent anion-selective channel protein 3 (VDAC3) | 1:1000 | ab130561 | Abcam |
| Vinculin | 1:1000 | 13901S | Cell Signaling Technology |
| Glyceraldehyde 3-phosphate dehydrogenase (GAPDH) | 1:1000 | 5174S | Cell Signaling Technology |
| Transketolase (TKT) | 1:500 | 8616S | Cell Signaling Technology |
| Methylenetetrahydrofolate dehydrogenase 1 (MTHFD1) | 1:500 | sc-271412 | Santa Cruz Biotech |
| Aldolase B (ALDOB) | 1:200 | sc-365449 | Santa Cruz Biotech |
| Serine hydroxymethyltransferase 1 (SHMT1) | 1:500 | 80715 | Cell Signaling Technology |
| Isocitrate dehydrogenase (IDH1/2) | 1:500 | sc-373816 | Santa Cruz Biotech |
| Phosphofructokinase 1 (PFK1) | 1:500 | sc-166722 | Santa Cruz Biotech |
| Pyruvate carboxylase (PC) | 1:1000 | NBP1-49536 | Novus Bio |
| Pyruvate dehydrogenase (PDH) | 1:500 | 3205S | Cell Signaling Technology |
| Lactate dehydrogenase (LDH) | 1:1000 | 3582S | Cell Signaling Technology |
| Hexokinase 1 (HK1) | 1:500 | 2024S | Cell Signaling Technology |
| Calsequestrin 2 (CASQ2) | 1:500 | ab3516 | Abcam |
| Citrate synthase (CS) | 1:1000 | 14309S | Cell Signaling Technology |
| Succinyl Co-A synthetase (SUCLG2) | 1:1000 | 8071S | Cell Signaling Technology |
| Succinate dehydrogenase A (SDHA) | 1:1000 | 5839S | Cell Signaling Technology |
| Sodium–calcium exchanger 1 (NCX1) | 1:500 | ab151608 | Abcam |
| Sarcoplasmic endoplasmic reticular calcium ATPase 2 (SERCA2) | 1:500 | 4388S | Cell Signaling Technology |
| Ryanodine receptor 2 (RYR2) | 1:500 | MA3-916 | Thermofisher Scientific |
| Phospholamban (PLN) | 1:1000 | 14562S | Cell Signaling Technology |
| Phospho-phospholamban (pPLN) | 1:1000 | 8496S | Cell Signaling Technology |
| Protein kinase A (PKA) | 1:500 | SAB4502337 | Sigma Aldrich |
| Phospho-protein kinase A (pPKA) | 1:500 | SAB4503969 | Sigma Aldrich |
| Mitochondrial calcium uniporter (MCU) | 1:500 | 14997S | Cell Signaling Technology |
| Mitochondrial sodium calcium exchanger (NCLX) | 1:500 | SAB2102181 | Sigma Aldrich |
| Dynamin-related protein 1 (DRP1) | 1:500 | 14647S | Cell Signaling Technology |
| Oxidative phosphorylation (OXPHOS) | 1:1000 | ab110413 | Abcam |
| IRDye 680RD Donkey anti-Mouse Secondary | 1:10,000 | 926-68072 | LI-COR Biosciences |
| IRDye 800CW Donkey anti-Rabbit Secondary | 1:10,000 | 926-32212 | LI-COR Biosciences |
| Co-immunoprecipitation-antibodies | |||
| Voltage-dependent anion-selective channel protein 2 (VDAC2) | 5 μg | PA5-28106 | Thermofisher Scientific |
| Immunoglobulin G (IgG) | 5 μg | AC005 | AbClonal |
| Immunofluorescence/proximity ligation assay—antibodies/stain | |||
| 4′,6-diamidino-2-phenylindole (DAPI) | 1:1000 | D3571 | Thermofisher Scientific |
| Voltage-dependent anion-selective channel protein 2 (VDAC2) | 1:1000 | 66388-1-IG | Thermofisher Scientific |
| Sodium–calcium exchanger 1 (NCX1) | 1:500 | ab151608 | Abcam |
| Sarcoplasmic endoplasmic reticular calcium ATPase 2 (SERCA2) | 1:500 | 4388S | Cell Signaling Technology |
| Ryanodine receptor 2 (RYR2) | 1:500 | MA3-916 | Thermofisher Scientific |
| Quantitative reverse transcription-PCR-primers | |||
| Voltage-dependent anion-selective channel protein 2 (VDAC2) | Mm00834279_m1 | Thermofisher Scientific | |
| Vinculin | Mm00447745_m1 | Thermofisher Scientific | |
RNA Seq analysis
RNA Seq analysis was performed with the High-Throughput Genomics and Bioinformatics Analysis Shared Resource at Huntsman Cancer Institute at the University of Utah. mm10, M_musculus_Dec_2011, GRCm38 genome build was used for sequence alignment. Sample outliers were checked by summarizing the output files from cutadapt, FastQC, Picard CollectRnaSeqMetrics, STAR, and feature counts by using the previously described method42. 5% false discovery rate (FDR) with DeSeq2 (version 1.24.0) was used to identify differentially expressed genes43. Genes were filtered using the following criteria: adjusted p-value < 0.05, absolute log2 fold change > 0.585 and normalized base mean >3043. Heat maps were produced using the R library p heatmap. KEGG44 and Ingenuity pathway analysis (IPA) software was used for gene ontology analysis.
Protein extraction and Western blotting
WT and KO mice hearts (n = 6 each) were excised. 30 μg of transmural LV sample was homogenized using metal beads for 3 min in RIPA buffer with 2× protease and phosphatase inhibitor (Thermo-Scientific #1861281). The homogenate was transferred to a new tube containing 10 μl of 1× PMSF and allowed to rotate for 30 min at 4 °C and centrifuged at 11,000 × g for 10 min at 4 °C. The supernatant was used for protein estimation using the Pierce BCA Protein Assay kit (Thermo-Scientific #23225). An equal volume of 2× Laemmli buffer (with 10% DTT) was added to the sample and boiled for 10 min at 98 °C.
30 μg protein was used for SDS–PAGE. The gel was run at constant volts (50 V) and proteins were transferred to nitrocellulose membrane at constant current (350 mA). For RYR2, proteins were transferred at constant volt (40 V) overnight. The membranes were blocked for 1 h with 5% non-fat milk and probed with primary antibodies overnight in the cold room. Antibodies and the concentration used are listed in Table 1. Blots were washed with 1× PBST thrice and probed with a secondary antibody for 1 h. Blots were washed with 1× PBST thrice prior to scanning using LI-COR. Image Studio Lite software was used to analyze the protein blots. Each blot had its own lane loading control and the values were normalized accordingly.
Co-immunoprecipitation
30 μg of LV transmural sample from 8-week-old WT and KO mice were used for protein extraction and quantification was done using a BCA assay kit. Protein A magnetic beads (Bio-Rad, catalog#161-4011) were washed thrice with 1× PBST. 4 mg ml−1 of lysate was precleared by rotating with beads for 30 min at 4 °C and 10% of the precleared lysate was saved as input. 5 μg of antibody (Table 1) was used for conjugation with the beads by rotating at room temperature for 10 min, followed by washing twice with 1× PBST and once with the lysis buffer (Cell Signaling, catalog #9806). The precleared lysate and antibody were kept for rotation at room temperature for 1 h. The magnet was then washed thrice with 1× PBST, magnetized and the elute was boiled at 70 °C for 10 min with 40 μl of 1× Laemmli buffer (10% β-mercaptoethanol) and used for western blot.
Immunofluorescence
16-week-old mice hearts were fixed in 10% paraformaldehyde for 4–6 h followed by paraffin embedding. Samples were cut at 5 μm thickness and deparaffinized and antigen retrieval was performed. The antibodies used are listed in Table 1. Secondary anti-mouse, anti-rabbit, or anti-goat were used appropriately. All images for each protein were acquired using the same laser setting and processed using Fiji software.
Proximity ligation assay
16-week-old mice heart samples embedded in paraffin were used for this assay. Duolink In Situ PLA probe anti-mouse, anti-rabbit, and anti-goat were used (DUO92001, DUO92005, and DUO92015). The antibodies used are listed in Table 1. Samples were processed using the manufacturer’s protocol. All images for each combination were acquired using the same laser settings and processed using Fiji software.
Force measurements in murine organotypic slices
Hearts from adult mice were excised after cervical dislocation and flushed in ice-cold preparation buffer (in mM): 136.0 NaCl, 5.4 KCl, 1.0 MgHPO4, 0.9 CaCl2, 30.0 2,3-Butanedionemonoxim, 5.0 HEPES, pH 7.4. For sectioning, hearts were embedded in low melting agarose and cut into 300 µm-thick coronary plane slices using a LeicaVT1200S vibratome and glued (Histoacryl, B. Brain Melsungen AG, Germany) onto small plastic triangles cut from 0.1 mm-thick polyester copier clear before being mounted in an organ bath chamber (Mayflower, Hugo Sachs Elektronic; HSE, Germany). Contractility was measured under a constant flow-through (4 ml min−1) in prewarmed measuring buffer (in mM): 136.0 NaCl, 5.4 KCl, 1.0 MgCl2, 0.33 NaH2PO4, 10.0 Glucose, 1.8 CaCl2, 23.0 NaHCO3, pH 7.4, 37 °C under electrical pacing at 3 Hz and force was recorded by a force transducer connected to an amplifier (Hugo Sachs, Germany) using WinEDR (University of Strathclyde, UK). Kinetics of single twitches was analyzed using LabChart Reader (ADInstruments).
TAC surgery
TAC45 was performed on RyR2R4496C+/− knock-in mice46, which were previously described to develop a severe form of HF47. In brief, 8–14-week-old mice were anesthetized by medetomidine/midazolam/fentanyl (0.5, 5.0, and 0.05 mg kg−1 body weight intraperitoneally, respectively) and the aorta was constricted around a 27-gauge needle. Sham mice underwent the same procedure without aortic constriction. 6 weeks after surgery, mice were sacrificed by cervical dislocation, hearts were isolated and flushed (in mM): 136.0 NaCl, 5.4 KCl, 1.0 MgCl2, 0.33 NaH2PO4, 10.0 Glucose, 0.9 CaCl2, 30.0 2,3-butanedionemonooxime, 5.0 HEPES, pH 7.4, 4 °C and success of TAC surgery was confirmed by measuring the heart weight to tibia length ratio.
Statistics and reproducibility
All data were summarized as ±SEM. GraphPad Prism (version8.2.1) was used for all statistical analyses. A two-tailed test was performed in all comparisons. Unpaired Student’s t-test was used for all analyses with WT and KO. Multiple t-test and one-way ANOVA were used for longitudinal echocardiographic data and while comparing multiple groups, respectively. p-value < 0.05 was considered statistically significant. All experiments were repeated independently (at least three biological replicates, and three technical replicates wherever applicable) with reproducible results. Samples used for western blot quantification were from the same gel and when unavoidable, were processed in parallel.
Reporting summary
Further information on research design is available in the Nature Research Reporting Summary linked to this article.
Supplementary information
Acknowledgements
This work was supported by American Heart Association-Heart Failure Strategically Focused Research Network, Grant 16SFRN29020000 (Dr. Drakos); National Heart, Lung, and Blood Institute (NHLBI) RO1 Grant HL135121-01 (Dr. Drakos); NHLBI RO1 Grant HL132067-01A1 (Dr. Drakos). National Heart, Lung, and Blood Institute (NHLBI) R01 Grant GM071779 (Dr. Kwon). Additional support was provided by the Nora Eccles Treadwell Foundation to Dr. Drakos.
Source data
Author contributions
T.S.S. designed and performed experiments, analyzed data, and drafted the manuscript. D.K.A.R., K.S., S.S., R.B., A.T.K. and D. Ca performed experiments and helped to analyze the data. S.N. helped to analyze the data, edited the manuscript, and revised it critically for intellectual content. P.S., O.S.K., A.A., A.D. performed experiments, edited the manuscript, and revised it critically for intellectual content. J.L. and C.X. edited the manuscript and revised it critically for intellectual content. O.K. and C.X. synthesized efsevin and provided the required quantities to perform the requested experiments. K.S., P.S., A.D. and J.S. performed all experiments on efsevin, analyzed data, and edited the manuscript. O.K., E.H.Y.C., K.J.W., T.G., R.S.R., F.B.S., K.W.S., J.S. and D.Ch. helped with the data analysis, edited the manuscript, and revised it critically for intellectual content. S.G.D. provided overall guidance, conceived the research concept, supervised the study and data analysis, and critically revised the manuscript for intellectual content.
Data availability
Source data are provided within this manuscript as a source data file. RNA sequencing is uploaded to NCBI’s GEO database under accession code GSE168487. Source data are provided with this paper.
Competing interests
The authors declare no competing interests.
Footnotes
Peer review information Nature Communications thanks Walter Koch and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. Peer reviewer reports are available.
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
The online version contains supplementary material available at 10.1038/s41467-021-24869-0.
References
- 1.Luscher TF. Heart failure: the cardiovascular epidemic of the 21st century. Eur. Heart J. 2015;36:395–397. doi: 10.1093/eurheartj/ehv004. [DOI] [PubMed] [Google Scholar]
- 2.van der Meer P, Gaggin HK, Dec GW. ACC/AHA versus ESC guidelines on heart failure: JACC guideline comparison. J. Am. Coll. Cardiol. 2019;73:2756–2768. doi: 10.1016/j.jacc.2019.03.478. [DOI] [PubMed] [Google Scholar]
- 3.Hasenfuss G, et al. Alteration of contractile function and excitation–contraction coupling in dilated cardiomyopathy. Circ. Res. 1992;70:1225–1232. doi: 10.1161/01.RES.70.6.1225. [DOI] [PubMed] [Google Scholar]
- 4.Bers DM. Cardiac excitation–contraction coupling. Nature. 2002;415:198–205. doi: 10.1038/415198a. [DOI] [PubMed] [Google Scholar]
- 5.Eisner DA, Caldwell JL, Kistamas K, Trafford AW. Calcium and excitation–contraction coupling in the heart. Circ. Res. 2017;121:181–195. doi: 10.1161/CIRCRESAHA.117.310230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Naghdi S, Hajnoczky G. VDAC2-specific cellular functions and the underlying structure. Biochim. Biophys. Acta. 2016;1863:2503–2514. doi: 10.1016/j.bbamcr.2016.04.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cheng EH, Sheiko TV, Fisher JK, Craigen WJ, Korsmeyer SJ. VDAC2 inhibits BAK activation and mitochondrial apoptosis. Science. 2003;301:513–517. doi: 10.1126/science.1083995. [DOI] [PubMed] [Google Scholar]
- 8.Roy SS, Ehrlich AM, Craigen WJ, Hajnoczky G. VDAC2 is required for truncated BID-induced mitochondrial apoptosis by recruiting BAK to the mitochondria. EMBO Rep. 2009;10:1341–1347. doi: 10.1038/embor.2009.219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Eisner V, Csordas G, Hajnoczky G. Interactions between sarco-endoplasmic reticulum and mitochondria in cardiac and skeletal muscle—pivotal roles in Ca(2)(+) and reactive oxygen species signaling. J. Cell Sci. 2013;126:2965–2978. doi: 10.1242/jcs.093609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Min CK, et al. Coupling of ryanodine receptor 2 and voltage-dependent anion channel 2 is essential for Ca(2)+ transfer from the sarcoplasmic reticulum to the mitochondria in the heart. Biochem. J. 2012;447:371–379. doi: 10.1042/BJ20120705. [DOI] [PubMed] [Google Scholar]
- 11.Pan X, et al. The physiological role of mitochondrial calcium revealed by mice lacking the mitochondrial calcium uniporter. Nat. Cell Biol. 2013;15:1464–1472. doi: 10.1038/ncb2868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Murphy E, et al. Unresolved questions from the analysis of mice lacking MCU expression. Biochem. Biophys. Res. Commun. 2014;449:384–385. doi: 10.1016/j.bbrc.2014.04.144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shimizu, H. et al. Mitochondrial Ca(2+) uptake by the voltage-dependent anion channel 2 regulates cardiac rhythmicity. Elife4, 04801 (2015). [DOI] [PMC free article] [PubMed]
- 14.Schweitzer MK, et al. Suppression of arrhythmia by enhancing mitochondrial Ca(2+) uptake in catecholaminergic ventricular tachycardia models. JACC Basic Transl. Sci. 2017;2:737–747. doi: 10.1016/j.jacbts.2017.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Subedi KP, et al. Voltage-dependent anion channel 2 modulates resting Ca(2)+ sparks, but not action potential-induced Ca(2)+ signaling in cardiac myocytes. Cell Calcium. 2011;49:136–143. doi: 10.1016/j.ceca.2010.12.004. [DOI] [PubMed] [Google Scholar]
- 16.Navankasattusas S, et al. The netrin receptor UNC5B promotes angiogenesis in specific vascular beds. Development. 2008;135:659–667. doi: 10.1242/dev.013623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Landstrom AP, Dobrev D, Wehrens XHT. Calcium signaling and cardiac arrhythmias. Circ. Res. 2017;120:1969–1993. doi: 10.1161/CIRCRESAHA.117.310083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kwong JQ, et al. The mitochondrial calcium uniporter selectively matches metabolic output to acute contractile stress in the heart. Cell Rep. 2015;12:15–22. doi: 10.1016/j.celrep.2015.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sharma VK, Ramesh V, Franzini-Armstrong C, Sheu SS. Transport of Ca2+ from sarcoplasmic reticulum to mitochondria in rat ventricular myocytes. J. Bioenerg. Biomembr. 2000;32:97–104. doi: 10.1023/A:1005520714221. [DOI] [PubMed] [Google Scholar]
- 20.Sander, P., Gudermann, T. & Schredelseker, J. A Calcium guard in the outer membrane: is VDAC a regulated gatekeeper of mitochondrial calcium uptake? Int. J. Mol. Sci.22, 22020946 (2021). [DOI] [PMC free article] [PubMed]
- 21.Dewenter M, von der Lieth A, Katus HA, Backs J. Calcium signaling and transcriptional regulation in cardiomyocytes. Circ. Res. 2017;121:1000–1020. doi: 10.1161/CIRCRESAHA.117.310355. [DOI] [PubMed] [Google Scholar]
- 22.Morkin E. Control of cardiac myosin heavy chain gene expression. Microsc. Res. Tech. 2000;50:522–531. doi: 10.1002/1097-0029(20000915)50:6<522::AID-JEMT9>3.0.CO;2-U. [DOI] [PubMed] [Google Scholar]
- 23.Imagawa T, Watanabe T, Nakamura T. Subunit structure and multiple phosphorylation sites of phospholamban. J. Biochem. 1986;99:41–53. doi: 10.1093/oxfordjournals.jbchem.a135478. [DOI] [PubMed] [Google Scholar]
- 24.Wegener AD, Jones LR. Phosphorylation-induced mobility shift in phospholamban in sodium dodecyl sulfate–polyacrylamide gels. Evidence for a protein structure consisting of multiple identical phosphorylatable subunits. J. Biol. Chem. 1984;259:1834–1841. doi: 10.1016/S0021-9258(17)43484-3. [DOI] [PubMed] [Google Scholar]
- 25.Colyer J. Phosphorylation states of phospholamban. Ann. N. Y. Acad. Sci. 1998;853:79–91. doi: 10.1111/j.1749-6632.1998.tb08258.x. [DOI] [PubMed] [Google Scholar]
- 26.Anderson ME. Multiple downstream proarrhythmic targets for calmodulin kinase II: moving beyond an ion channel-centric focus. Cardiovasc. Res. 2007;73:657–666. doi: 10.1016/j.cardiores.2006.12.009. [DOI] [PubMed] [Google Scholar]
- 27.Federico, M., Valverde, C. A., Mattiazzi, A. & Palomeque, J. Unbalance between sarcoplasmic reticulum Ca2+ uptake and release: a first step toward Ca2+ triggered arrhythmias and cardiac damage. Front. Physiol.10, 2019.01630 (2020). [DOI] [PMC free article] [PubMed]
- 28.Chen X, et al. Sorcin ablation plus beta-adrenergic stimulation generate an arrhythmogenic substrate in mouse ventricular myocytes. J. Mol. Cell. Cardiol. 2018;114:199–210. doi: 10.1016/j.yjmcc.2017.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Xu M, Welling A, Paparisto S, Hofmann F, Klugbauer N. Enhanced expression of L-type Cav1.3 calcium channels in murine embryonic hearts from Cav1.2-deficient mice. J. Biol. Chem. 2003;278:40837–40841. doi: 10.1074/jbc.M307598200. [DOI] [PubMed] [Google Scholar]
- 30.Cribbs L. T-type calcium channel expression and function in the diseased heart. Channels. 2010;4:447–452. doi: 10.4161/chan.4.6.12870. [DOI] [PubMed] [Google Scholar]
- 31.Kistamas K, et al. Calcium handling defects and cardiac arrhythmia syndromes. Front. Pharm. 2020;11:72. doi: 10.3389/fphar.2020.00072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Maldonado EN. VDAC-tubulin, an anti-Warburg pro-oxidant switch. Front. Oncol. 2017;7:4. doi: 10.3389/fonc.2017.00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zhou K, et al. VDAC2 interacts with PFKP to regulate glucose metabolism and phenotypic reprogramming of glioma stem cells. Cell Death Dis. 2018;9:988. doi: 10.1038/s41419-018-1015-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chin HS, et al. VDAC2 enables BAX to mediate apoptosis and limit tumor development. Nat. Commun. 2018;9:4976. doi: 10.1038/s41467-018-07309-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ren D, et al. The VDAC2-BAK rheostat controls thymocyte survival. Sci. Signal. 2009;2:ra48. doi: 10.1126/scisignal.2000274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Harrington JL, Murphy E. The mitochondrial calcium uniporter: mice can live and die without it. J. Mol. Cell. Cardiol. 2015;78:46–53. doi: 10.1016/j.yjmcc.2014.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Oka T, et al. Cardiac-specific deletion of Gata4 reveals its requirement for hypertrophy, compensation, and myocyte viability. Circ. Res. 2006;98:837–845. doi: 10.1161/01.RES.0000215985.18538.c4. [DOI] [PubMed] [Google Scholar]
- 38.Drakos SG, et al. Impact of mechanical unloading on microvasculature and associated central remodeling features of the failing human heart. J. Am. Coll. Cardiol. 2010;56:382–391. doi: 10.1016/j.jacc.2010.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Warren M, et al. Blockade of CaMKII depresses conduction preferentially in the right ventricular outflow tract and promotes ischemic ventricular fibrillation in the rabbit heart. Am. J. Physiol. Heart Circ. Physiol. 2017;312:H752–H767. doi: 10.1152/ajpheart.00347.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Cluntun AA, et al. The pyruvate-lactate axis modulates cardiac hypertrophy and heart failure. Cell Metab. 2020 doi: 10.1016/j.cmet.2020.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sommakia S, et al. Mitochondrial cardiomyopathies feature increased uptake and diminished efflux of mitochondrial calcium. J. Mol. Cell. Cardiol. 2017;113:22–32. doi: 10.1016/j.yjmcc.2017.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ewels P, Magnusson M, Lundin S, Kaller M. MultiQC: summarize analysis results for multiple tools and samples in a single report. Bioinformatics. 2016;32:3047–3048. doi: 10.1093/bioinformatics/btw354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Love MI, Huber W, Anders S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol. 2014;15:550. doi: 10.1186/s13059-014-0550-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kanehisa M, Goto S. KEGG: Kyoto Encyclopedia of Genes and Genomes. Nucleic Acids Res. 2000;28:27–30. doi: 10.1093/nar/28.1.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Muhlfeld C, et al. Hypoinnervation is an early event in experimental myocardial remodelling induced by pressure overload. J. Anat. 2013;222:634–644. doi: 10.1111/joa.12044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Cerrone M, et al. Bidirectional ventricular tachycardia and fibrillation elicited in a knock-in mouse model carrier of a mutation in the cardiac ryanodine receptor. Circ. Res. 2005;96:e77–e82. doi: 10.1161/01.RES.0000169067.51055.72. [DOI] [PubMed] [Google Scholar]
- 47.Sedej S, et al. Subclinical abnormalities in sarcoplasmic reticulum Ca(2+) release promote eccentric myocardial remodeling and pump failure death in response to pressure overload. J. Am. Coll. Cardiol. 2014;63:1569–1579. doi: 10.1016/j.jacc.2013.11.010. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Source data are provided within this manuscript as a source data file. RNA sequencing is uploaded to NCBI’s GEO database under accession code GSE168487. Source data are provided with this paper.