Abstract
Objective
To assess the concordance of high-risk HPV (HR-HPV) testing with the Alinity assay on cervical samples collected with diverse collection/storage protocols (ThinPrep, SurePath, Cervicollect) and to assess inter-assay concordance of HR-HPV testing of cervical cell specimens with Alinity m HR HPV assay (Alinity) vs cobas® 4800 HPV assay (cobas).
Methods
Specimens were obtained from 560 women attending a Women's Health clinic. Two specimens were obtained from each woman with combinations of two of the three collection devices and aliquots were tested by the two assays.
Results
Alinity showed an agreement of 93.9%, Kappa = 0.89 (263/280) between ThinPrep and SurePath specimens; 97.5%, Kappa = 0.95 (347/356) and 92.9%, Kappa = 0.85 (104/112) between ThinPrep and SurePath aliquots taken before or after cytology processing, respectively. Cervi-Collect specimens showed an agreement of 94.6%, Kappa = 0.89 (265/280) with ThinPrep specimens. Compared to cobas, Alinity showed agreements of 94.3%, Kappa = 0.88 (395/419) and 91.8%, Kappa = 0.82 (257/280) between ThinPrep and SurePath specimens, respectively. Alinity and cobas detected genotypes 16/18 and other high-risk HPV types at similar rates and showed similar correlations with cytology grades.
Conclusions
Compared to cobas, Alinity performed equally well for detecting HPV in cervical specimens obtained with ThinPrep and SurePath. The Cervi-Collect device compared well to the other collection methods. Alinity is a reliable assay for simultaneous detection of HPV-16/18 and other high-risk genotypes in cervical specimens.
Keywords: Alinity m HR HPV assay, ThinPrep, SurePath and cervi-collect cervical specimens, HPV genotype Simultaneous detection, cobas 4800, HPV testing In cervical cancer screening
Highlights
-
•
Alinity m HR HPV assay demonstrated very good agreement with ThinPrep and SurePath pre- and post-cytology cervical specimens.
-
•
Alinity m HR HPV had very good agreement with ThinPrep and Cervi-Collect specimens.
-
•
Alinity m HR HPV and cobas 4800 HPV had very good agreement and identified HPV-16/18 and other HR types.
1. Introduction
Persistent infection with oncogenic high-risk human papillomaviruses (HR-HPV) is the underlying cause of cervical cancer [1,2]. Testing for HR-HPV is more sensitive and efficient than Papanicolaou cytology for detecting pre-cancer lesions and cancer [[3], [4], [5], [6]]. HR-HPV testing has been recommended as a standalone test, or in conjunction with cytology in primary cervical cancer screening and for triaging women with atypical squamous cells of undetermined significance (ASC-US) cytology [[7], [8], [9], [10]]. HR-HPV tests are used routinely in some countries and many are transitioning to HPV-based testing in cervical cancer screening strategies. There are numerous commercial HPV tests currently marketed and used by clinical laboratories for cervical screening. However, only a few of them have been adequately validated with documented clinical performance characteristics per international guidelines, and those without such validations are considered unreliable [[11], [12], [13]].
Many commercial HR-HPV assays target ≤14 high-risk oncogenic types associated with cervical cancer, with some platforms allowing the simultaneous partial or extended genotyping, especially targeting genotypes 16/18 [8,14,15]. This is valuable from the standpoint of clinical management of HPV-positive women as HPV-16/18 cause the majority of cervical cancers worldwide [[16], [17], [18]]. Testing for HPV-16/18 allows the determination of a genotype-specific risk threshold in primary cervical cancer screening and in triage of low-grade cytological abnormalities, and has led to the US guidelines recommending direct referral to colposcopy for women testing positive for HPV-16/18 in primary screening [19]. This underscores the importance of choosing HR-HPV tests that are validated and provide genotype-specific results simultaneously in a single analysis in cervical cancer screening.
The Alinity m System (Abbott Molecular, Des Plaines, USA) is a continuous random-access platform that automatically performs extraction, amplification and analysis. The Alinity m HR HPV assay (Alinity) is a real-time PCR assay, performed on the Alinity m System, and detects DNA from 14 HR-HPV genotypes: 16, 18, 31, 33, 35, 39, 45, 51, 52, 56, 58, 59, 66, and 68. In a single analysis, Alinity allows the detection of genotypes 16, 18 and 45 individually, with concurrent detection of 11 other high-risk (OHR) genotypes in pools of two groups: genotypes 31, 33, 52, 58 in one group and genotypes 35, 39, 51, 56, 59, 66, 68 in another group.
Alinity was designed to detect HPV DNA in cervical cells collected in Thin Prep PreservCyt (Hologic Inc., San Diego, USA) or SurePath Preservative Fluid (Becton, Dickinson and Company (BD), Franklin Lakes, USA). This enables testing for both cytology and HPV either in a co-testing mode or adjunctively for triage in risk stratification. Alinity m Cervi-Collect (Cervi-Collect) is a new companion cervical specimen collection and transport medium, originally developed for use with the Abbott RealTime HR HPV assay [20] and has been evaluated in comparison with ThinPrep [21].
The clinical performance of Alinity has been validated against the Hybrid Capture 2 (HC2; Qiagen, Hilden, Germany) assay, and the cobas® 4800 HPV assay (cobas, Roche Diagnostics, Penzberg, Germany) [13]. The cobas assay targets the same 14 HR-HPV genotypes as Alinity and detects genotypes 16/18 individually and 12 OHR genotypes collectively in a single analysis. The cobas assay has been extensively validated and widely used in cervical screening strategies and is a well-suited comparator to assess Alinity [8,22].
The objectives of this study were: i. To assess the performance of Alinity for the detection of HR-HPV in cervical specimens collected in ThinPrep and SurePath media; ii. To determine the compatibility of Cervi-Collect with Alinity; iii. To compare the performance of Alinity with cobas for the detection of HR-HPV.
2. Methods
2.1. Study protocol
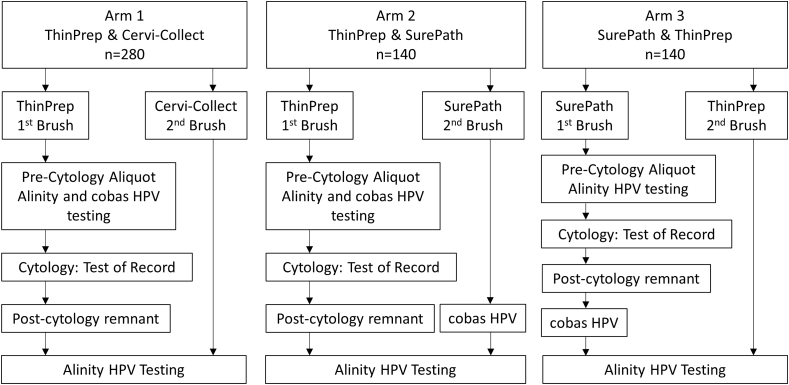
Cervical specimens were collected from 560 women attending the Women's Health clinic which also specializes in colposcopy referrals at the Juravinski Hospital, Hamilton, Canada. There were no age limits, but pregnant women and women without a cervix were excluded. ThinPrep, SurePath, and Cervi-Collect brushes were used to obtain cervical specimens per standard practice and manufactures' instructions. Two cervical specimens were obtained from each woman using two of the three devices in accordance with a study specimen collection scheme (Fig. 1). The enrolled women were divided into 3 arms: 1. Women (n = 280) from whom cervical specimens were obtained first with a ThinPrep brush, followed by a Cervi-Collect brush; 2. Women (n = 140) from whom cervical specimens were obtained first with a ThinPrep brush, followed by a SurePath brush; 3. Women (n = 140) from whom cervical specimens were obtained first with a SurePath brush followed by a ThinPrep brush. Patients were enrolled consecutively from January 9, 2018 to May 30, 2018. Study arm 1 was performed first and after completion new patents were enrolled into arm 2 followed by arm 3.
Fig. 1.
Study arms, cervical sample collection and testing scheme.
Cervical specimens were tested either by Alinity alone or with both Alinity and cobas (Fig. 1). Aliquots of ThinPrep first brush specimens were removed prior to cytology for testing by Alinity and cobas (Pre-cytology specimens; Arms, 1 and 2). Aliquots of SurePath first brush specimens were removed prior to cytology for testing with Alinity (Arm 3). Cytology was performed per standard practice using ThinPrep and SurePath first brush specimens (Test of record; Arms, 1, 2 and 3). The residual post cytology specimens remaining in ThinPrep and SurePath first brush vials were tested with Alinity (Post-cytology specimens; Arms, 1, 2 and 3). Specimens obtained with the second brush of SurePath (Arm 2) were tested by Alinity and cobas. In Arm 3, the SurePath post-cytology remnant samples remaining in the vial were tested by cobas. Specimens obtained with the ThinPrep second brush (Arm 3) were tested with Alinity. Cervical specimens were obtained with Cervi-Collect as a second brush samples (Arm 1) and tested only with Alinity. All specimens were refrigerated or frozen until testing by Alinity and cobas.
Results obtained from Alinity and cobas testing, using cervical specimens obtained with the three collection devices were compared to determine the relative performance and agreements, and were also correlated with cytological grades.
2.2. Ethics
The study was approved by the Hamilton Integrated Research Ethics Board (HiREB). All women were informed verbally and in writing about the study and use of their cervical specimens for HPV testing using the Alinity and cobas assays. Women providing written informed consent were enrolled into the study.
2.3. Cytology
Cytology test of record was carried out as part of routine patient care per standard practice using ThinPrep specimens at St. Josephs Healthcare Hamilton and SurePath cytology was performed per standard practice at LifeLabs®.
2.4. Alinity HPV testing
Aliquots of cervical specimens collected by all three collection and transportation devices were shipped on dry ice weekly to Abbott Molecular, Des Plaines, USA for Alinity testing on the Alinity m System. The fully automated procedure consisted of sample preparation, real-time PCR and result reporting. During sample preparation, 0.4 mL of the sample was processed by the system, where it was pretreated and lysed with chaotropic reagents, allowing DNA to be captured on magnetic microparticles. The bound purified DNA was washed and eluted. A lyophilized amplification master mix consisting of polymerase, primers, probes and dNTPs was rehydrated using the eluate and activation reagent. The resultant PCR mixture was then transferred to a reaction vessel, which was subsequently capped and transferred to the amplification and detection unit. Upon completion of real-time PCR, results were automatically reported. In addition to HPV signals, β-globin was detected as an internal control for sample adequacy, DNA recovery, and PCR efficiency. The results were reported for the 14 HR-HPV genotypes, HPV-16, 18, 45 and 11 OHR genotypes.
Two investigators of the study team made an onsite visit to Abbott Molecular to test the study specimens on Alinity independently. They received orientation on the features and operation of the Alinity m platform, and tested random specimens collected in ThinPrep, SurePath and Cervi-Collect devices on Alinity per the manufacturer's instructions.
2.5. cobas HPV testing
Aliquots of cervical specimens collected in ThinPrep and SurePath devices were shipped at ambient temperature on a monthly basis to the Newfoundland Public Health Laboratory, St. John's, NL, Canada for cobas testing. This test was performed on the cobas 4800 automated platform (Roche Diagnostics) per the manufacturer's instructions. Results were reported as positive or negative for HPV-16/18 and 12 OHR types.
2.6. Data analysis
The percent positive, negative and overall agreements with Cohen's kappa coefficient were calculated between ThinPrep, SurePath and Cervi-Collect specimens tested with Alinity. Additionally, the percent agreements and Kappa were also calculated between Alinity and cobas for ThinPrep and SurePath specimens.
3. Results
A total of 1120 cervical specimens were collected from 560 women between 21 and 76 years of age (median 34 years). A series of tests were performed on Alinity alone or in combination with cobas, using cervical specimens obtained in the three collection devices per the testing scheme shown in Fig. 1.
In the first series of testing (Table 1), the performance of Alinity was assessed using a total of 280 paired ThinPrep and SurePath specimens from study Arms 2 and 3. The results showed 103/280 (36.8%) ThinPrep specimens testing positive vs. 100/280 (35.7%) SurePath specimens with an overall agreement of 93.9% (Kappa = 0.89).
Table 1.
Agreement of Alinity m HR HPV assay results between ThinPrep and SurePath specimens (n = 280).
| SurePath |
Agreement | ||||
|---|---|---|---|---|---|
| Positive | Negative | Total | |||
| ThinPrep | Positive | 93 | 10 | 103 | Positive agreement: 93/103 = 90.3% |
| Negative | 7 | 170 | 177 | Negative agreement: 170/177 = 96.0% |
|
| Total | 100 | 180 | 280 | Overall agreement: 263/280 = 93.9% Kappa: 0.89 |
|
In the second series (Table 2), the performance of Alinity was determined using first brush pre- and post-cytology ThinPrep specimens (Arms, 1 and 3) and SurePath (Arm 3) specimens. Mainly due to the need to repeat cytology there were 64 ThinPrep and 28 SurePath samples unavailable for HPV testing. Thus 356 of 420 paired ThinPrep specimens and 112 of 140 SurePath specimens were available for Alinity testing. For ThinPrep samples 140 pre-cytology vs. 139 post-cytology specimens tested positive for an overall agreement of 97.5% (Kappa = 0.95). A total of 112 paired SurePath specimens were available for Alinity testing: 45 pre-cytology vs. 39 post-cytology specimens showed an overall agreement of 92.9% (Kappa = 0.85).
Table 2.
Agreement of Alinity m HR HPV assay results between ThinPrep and SurePath pre- and post-cytology specimens.
| ThinPrep Specimens (n = 356a) |
SurePath Specimens (n = 112a) |
||||||||
|---|---|---|---|---|---|---|---|---|---|
| Post-cytology |
Post-cytology |
||||||||
| Positive | Negative | Total | Positive | Negative | Total | ||||
| Pre-cytology | Positive | 135 | 5 | 140 | Pre-cytology | Positive | 38 | 7 | 45 |
| Negative | 4 | 212 | 216 | Negative | 1 | 66 | 67 | ||
| Total | 139 | 217 | 356 | 39 | 73 | 112 | |||
| Positive agreement: 135/139 = 97.1% Negative agreement: 212/217 = 97.7% Overall agreement: 347/356 = 97.5%. Kappa: 0.95 |
Positive agreement: 38/39 = 97.4% Negative agreement: 66/73 = 90.4% Overall agreement: 104/112 = 92.9%. Kappa: 0.85 |
||||||||
Due to repeat cytology there were 64 ThinPrep and 28 SurePath samples unavailable for HPV testing.
The suitability of Cervi-Collect specimens for Alinity testing was assessed by comparing agreements with ThinPrep specimens (Table 3). A total of 280 specimens were collected using each device. Cervi-Collect specimens were positive in 117 (41.8%) vs. 118 (42.1%) ThinPrep specimens with an overall agreement of 94.6% (Kappa = 0.89).
Table 3.
Agreement of Alinity m HR HPV assay results between Cervi-Collect and ThinPrep specimens (n = 280).
| ThinPrep |
Agreement | ||||
|---|---|---|---|---|---|
| Positive | Negative | Total | |||
| Cervi-Collect | Positive | 110 | 7 | 117 | Positive agreement: 110/117 = 94.0% |
| Negative | 8 | 155 | 163 | Negative agreement: 155/163 = 95.1% |
|
| Total | 118 | 162 | 280 | Overall agreement: 265/280 = 94.6% Kappa: 0.89 |
|
The comparative performance of Alinity and cobas was determined using cervical specimens collected in ThinPrep and SurePath (Table 4). Of 420 cervical specimens collected with the ThinPrep first brush (Arms, 1 and 2), 419 were available for testing by both assays. Alinity was positive in 171 (40.8%) and cobas in 175 (41.8%) specimens for an overall agreement of 94.3% (Kappa = 0.88). A total of 280 SurePath specimens comprised of 140 s brush (Arm, 2) and 140 first brush post-cytology (Arm, 3) were available for testing with the two assays. Alinity was positive in 100 (35.7%) and cobas in 105 (37.5%) specimens and showed an overall agreement of 91.8% (Kappa = 0.82).
Table 4.
Comparative performance of Alinity m HR HPV assay with cobas HPV test using ThinPrep and SurePath specimens.
| cobas HPV |
|||||||
|---|---|---|---|---|---|---|---|
| ThinPrep specimens (n = 419a) |
SurePath specimens (n = 280) |
||||||
| Positive | Negative | Total | Positive | Negative | Total | ||
| Alinity m HR HPV | Positive | 161 | 10 | 171 | 91 | 9 | 100 |
| Negative | 14 | 234 | 248 | 14 | 166 | 180 | |
| Total | 175 | 244 | 419 | 105 | 175 | 280 | |
| Positive agreement Negative agreement Overall agreement |
161/175 = 92.0% 234/244 = 95.9% 395/419 = 94.3% Kappa: 0.88 |
91/105 = 86.7% 166/175 = 94.9% 257/280 = 91.8% Kappa: 0.82 |
|||||
Due to repeat cytology there was 1 ThinPrep sample unavailable for HPV testing.
Alinity and cobas detected 57 specimens with genotypes 16/18 with only 3 discordant results. Of 118 OHR positive specimens detected by cobas, 114 were positive by Alinity. Positivity rates for Alinity and cobas according to cytology scoring is shown in Table 5 for 413 ThinPrep results. As expected, patients with high-grade squamous intraepithelial lesions (HSIL) had the highest HPV rates in both tests. For each cytology category differences between Alinity and cobas were minimal.
Table 5.
Association of Alinity m HR HPV and cobas HPV assay results with ThinPrep cytology grades.
|
Cytology grades |
Number of specimens testing positive in |
||
|---|---|---|---|
| Alinity m HR HPV | cobas HPV | P-value | |
| Negative, n = 207 | 45 (21.7%) | 43 (20.8%) | 0.480 |
| ASC-US, n = 83 | 33 (39.8%) | 34 (41.0%) | 1.000 |
| LSIL, n = 85 | 56 (65.9%) | 61 (71.8%) | 0.074 |
| HSIL, n = 38 | 36 (94.7%) | 37 (97.4%) | 1.000 |
ASC-US, Atypical squamous cells of undetermined significance.
LSIL, Low-grade squamous intraepithelial lesion.
HSIL, High-grade squamous intraepithelial lesion.
4. Discussion
Commercial HPV tests have typically been designed to make use of cervical specimens obtained in liquid-based cytology collection and transport media developed for cytology platforms, as this allows for both cytology screening and HPV testing to be performed using the same specimen, thus aiding co-and reflex testing and ensuring efficiency. This operational efficiency is important both in routine clinical as well as laboratory practices. This approach has played an important role in a seamless widespread transition of HPV tests for ASC-US triage in cytology-based cervical screening or HPV primary screening [[7], [8], [9], [10],23,24]. ThinPrep and SurePath cervical collection and transport media are commonly used on their respective cytology platforms, which have been preferentially chosen by major manufacturers of HPV tests.
Alinity was designed to use cervical specimens collected in either ThinPrep or SurePath collection media. A recent study assessing the performance of Alinity using cervical specimens collected in ThinPrep showed similar results between Hybrid Capture 2, cobas HPV and Abbott RealTime HR-HPV assays [13]. In the present study, we compared cervical specimens collected in ThinPrep and SurePath for testing with Alinity and showed excellent overall agreement of 93.9% (Kappa = 0.89) between the specimen types (Table 1). We demonstrated minimal difference between the pre- and post-cytology leftover samples by showing an agreement of 97.5% (Kappa = 0.95) between pre- and post-cytology ThinPrep and 92.9% (Kappa = 0.85) between pre- and post cytology SurePath specimens (Table 2). This indicates that either pre- or post cytology specimens in either media could be reliably used for Alinity testing.
The Cervi-Collect brush and transport medium kit was initially developed for collection and transportation of cervical specimens for testing in an Abbott RealTime HR-HPV assay with a view to achieving efficient sample collection and optimal stability, and compatibility with the Abbott m2000sp, an automated sample preparation instrument. We previously validated the Cervi-Collect device showing a high-performance rating for the detection of HPV with the RealTime HR-HPV assay as well as other kit characteristics such as package insert, usability, safety etc., with a high favourable rating through a healthcare collector questionnaire [21]. The Alinity Cervi-Collect specimen collection device in the current study is similar to the original Cervi-Collect device, with the exception that the brush length is longer. In the present study, we assessed the suitability of cervical specimens collected in Alinity Cervi-Collect in comparison with ThinPrep specimens for testing with Alinity, with the results showing an overall agreement of 94.6% (Kappa = 0.89). This is similar to the agreement of 93.1% (Kappa = 0.86) we previously observed between Cervi-Collect and ThinPrep specimens tested with the RealTime HR-HPV assay [21]. This is the first study to evaluate the performance of the Alinity Cervi-Collect device for the detection of HPV with the Alinity assay.
Our study has some limitations. We could not perform cytology on Cervi-Collect because it is designed for HPV testing, and not for cytology. Thus, any need to perform subsequent cytology on patients that are HR HPV-positive in a Cervi-Collect specimen would require additional specimen collection with ThinPrep or SurePath. A limitation of the agreement analysis between two specimens from each subject is unavoidably confounded for the collection device and the order of collection thus potentially lowering the agreement results. Examination of the 17 discordant samples in Table 1 revealed that 80% (8/10) of samples only positive in ThinPrep and 71.4% (5/7) of samples only positive in SurePath were collected first and may be a reflection of collection order. More SurePath specimens for measuring pre- and post-cytology agreements (Table 2) would have allowed a stronger Kappa statistical calculation.
We determined the comparative performance of Alinity with cobas, including genotype characterization using both ThinPrep and SurePath specimens and showed that Alinity performed similarly to cobas regardless of specimen types (Table 4) with an overall agreement of 94.3% (Kappa = 0.88) for ThinPrep and 91.8% (Kappa = 0.82) for SurePath specimens. In terms of the ability to simultaneously identify genotypes 16/18, Alinity identified the same genotypes in total as cobas and performed similarly in identifying 12 OHR types with ThinPrep specimens. In addition, the similarity of Alinity performance with cobas is attested in terms of their close and consistent association with cytological grades using ThinPrep specimens (Table 5).
Validation of cervical specimens collected in multiple collection and transport media allows for flexibility and choice in clinical laboratory preference, practices and cost consideration in the use of HPV testing in cervical cancer screening strategies. In this regard, while there are also numerous commercial HPV tests available only some are clinically validated and considered reliable for routine use in primary cervical screening and for adjunctive testing in triage. In a recent study, the Alinity assay was validated per international consensus guideline criteria and reported to meet the criteria for HPV test requirements in cervical cancer settings [13,25]. This study also indicated the extended genotyping capability of Alinity might also be of value in improving patient risk stratification. Alinity has been indicated in a variety of clinical applications such as: ASC-US triage to determine the need for referral to colposcopy, to use in conjunction with cervical cytology as an adjunct test to screen for HR-HPV genotypes, and as a first-line primary cervical screening test to detect genotypes 16/18 for identifying women at increased risk for the development of cervical cancer or prediction of the presence of high-grade disease with or without cervical cytology. The Alinity assay has European Conformity Mark for in vitro diagnostics (CE-IVD). Further to the above recent validation study of Alinity [13], our study provides additional useful data for the performance of this new test for detecting HR-HPV in cervical specimens obtained in multiple collection and transport media.
5. Conclusions
Alinity performed equally well to detect HR-HPV in cervical specimens obtained in ThinPrep and SurePath devices as well as in the newly developed Alinity Cervi-Collect medium. The performance of Alinity was similar to that of cobas, including its ability to simultaneously identify genotypes 16/18 and OHR types in a single analysis using ThinPrep medium.
Funding support
The study was funded solely as a research grant by Abbott Molecular Inc.
Declaration of competing interest
Sam Ratnam received honorarium from Abbott Molecular Inc. Max Chernesky received a research grant from Abbott Molecular Inc. Shihai Huang, Erika Herrero-Garcia, Ajith M. Joseph and Hao Jiang are employees of Abbott Molecular Inc. Other members of the study team declare no conflict of interest.
CRediT authorship contribution statement
Dan Jang: Project administration, Supervision, Data curation, Writing – original draft, Writing – review & editing. Sam Ratnam: Methodology, Supervision, Formal analysis, Writing – original draft, Writing – review & editing. Marek Smieja: Project administration, Investigation, Methodology, Data curation, Formal analysis, Writing – review & editing. David J. Speicher: Writing – review & editing. Manuel Arias: Writing – review & editing. Avery Clavio: Writing – review & editing. Dustin Costescu: Resources, Supervision, Writing – review & editing. Laurie Elit: Supervision, Writing – review & editing. Shihai Huang: Resources. Erika Herrero-Garcia: Resources. Ajith M. Joseph: Resources. Hao Jiang: Resources. Robert Needle: Data curation, Writing – review & editing. Max Chernesky: Methodology, Supervision, Data curation, Writing – review & editing.
Acknowledgments
We wish to acknowledge the staff (clinicians/nurses/clerks) at the Hamilton Health Sciences Juravinski Colposcopy clinic.
References
- 1.Doorbar J., Quint W., Banks L., Bravo I.G., Stoler M., Broker T.R. The biology and life-cycle of human papillomaviruses. Vaccine. 2012;30(Suppl 5):F55–F70. doi: 10.1016/j.vaccine.2012.06.083. [DOI] [PubMed] [Google Scholar]
- 2.Castle P.E., Rodriguez A.C., Burk R.D., Herrero R., Wacholder S., Hildesheim A. Long-term persistence of prevalently detected human papillomavirus infections in the absence of detectable cervical precancer and cancer. J. Infect. Dis. 2011;203:814–822. doi: 10.1093/infdis/jiq116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dillner J., Rebolj M., Birembaut P., Petry K.U., Szarewski A., Munk C. Long term predictive values of cytology and human papillomavirus testing in cervical cancer screening: joint European cohort study. BMJ. 2008;337:a1754. doi: 10.1136/bmj.a1754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kitchener H.C., Gilham C., Sargent A., Bailey A., Albrow R., Roberts C. A comparison of HPV DNA testing and liquid based cytology over three rounds of primary cervical screening: extended follow up in the ARTISTIC trial. Eur. J. Canc. 2011;47:864–871. doi: 10.1016/j.ejca.2011.01.008. [DOI] [PubMed] [Google Scholar]
- 5.Ronco G., Dillner J., Elfstrom K.M., Tunesi S., Snijders P.J., Arbyn M. Efficacy of HPV-based screening for prevention of invasive cervical cancer: follow-up of four European randomised controlled trials. Lancet. 2014;383:524–532. doi: 10.1016/S0140-6736(13)62218-7. [DOI] [PubMed] [Google Scholar]
- 6.Arbyn M., Ronco G., Anttila A., Meijer C.J., Poljak M., Ogilvie G. Evidence regarding human papillomavirus testing in secondary prevention of cervical cancer. Vaccine. 2012;30(Suppl 5):F88–F99. doi: 10.1016/j.vaccine.2012.06.095. [DOI] [PubMed] [Google Scholar]
- 7.Wright T.C., Jr., Cox J.T., Massad L.S., Twiggs L.B., Wilkinson E.J., Conference A.S.-S.C. Consensus Guidelines for the management of women with cervical cytological abnormalities. J. Am. Med. Assoc. 2001;287:2120–2129. doi: 10.1001/jama.287.16.2120. 2002. [DOI] [PubMed] [Google Scholar]
- 8.Wright T.C., Stoler M.H., Behrens C.M., Sharma A., Zhang G., Wright T.L. Primary cervical cancer screening with human papillomavirus: end of study results from the ATHENA study using HPV as the first-line screening test. Gynecol. Oncol. 2015;136:189–197. doi: 10.1016/j.ygyno.2014.11.076. [DOI] [PubMed] [Google Scholar]
- 9.Solomon D., Schiffman M., Tarone R., group A.S. Comparison of three management strategies for patients with atypical squamous cells of undetermined significance: baseline results from a randomized trial. J. Natl. Cancer Inst. 2001;93:293–299. doi: 10.1093/jnci/93.4.293. [DOI] [PubMed] [Google Scholar]
- 10.Fontham E.T.H., Wolf A.M.D., Church T.R., Etzioni R., Flowers C.R., Herzig A. Cervical cancer screening for individuals at average risk: 2020 guideline update from the American Cancer Society. CA A Cancer J. Clin. 2020;70:321–346. doi: 10.3322/caac.21628. [DOI] [PubMed] [Google Scholar]
- 11.Arbyn M., Simon M., Peeters E., Meijer C., Berkhof J., Cuschieri K. List of human papillomavirus assays Suitable for primary cervical cancer screening. Clin. Microbiol. Infect. 2020 doi: 10.1016/j.cmi.2021.04.031. https://pubmed.ncbi.nlm.nih.gov/33975008/ 2021. [DOI] [PubMed] [Google Scholar]
- 12.Dhillon S.K., Ostrbenk Valencak A., Xu L., Poljak M., Arbyn M. Clinical and analytical evaluation of the alinity m HR HPV assay within the VALGENT-3 framework. J. Clin. Microbiol. 2021;59 doi: 10.1128/JCM.00286-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ostrbenk Valencak A., Sterbenc A., Seme K., Poljak M. Alinity m HR HPV assay fulfills criteria for human papillomavirus test requirements in cervical cancer screening settings. J. Clin. Microbiol. 2019;58 doi: 10.1128/JCM.01120-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Poljak M., Ostrbenk A., Seme K., Sterbenc A., Jancar N., Vrtacnik Bokal E. Three-year longitudinal data on the clinical performance of the Abbott RealTime High Risk HPV test in a cervical cancer screening setting. J. Clin. Virol. 2016;76(Suppl 1):S29–S39. doi: 10.1016/j.jcv.2015.11.021. [DOI] [PubMed] [Google Scholar]
- 15.Bonde J.H., Pedersen H., Quint W., Xu L., Arbyn M., Ejegod D.M. Clinical and analytical performance of the BD onclarity HPV assay with SurePath screening samples from the Danish cervical screening program using the VALGENT framework. J. Clin. Microbiol. 2020;58 doi: 10.1128/JCM.01518-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Munoz N., Bosch F.X., de Sanjose S., Herrero R., Castellsague X., Shah K.V. Epidemiologic classification of human papillomavirus types associated with cervical cancer. N. Engl. J. Med. 2003;348:518–527. doi: 10.1056/NEJMoa021641. [DOI] [PubMed] [Google Scholar]
- 17.Khan M.J., Castle P.E., Lorincz A.T., Wacholder S., Sherman M., Scott D.R. The elevated 10-year risk of cervical precancer and cancer in women with human papillomavirus (HPV) type 16 or 18 and the possible utility of type-specific HPV testing in clinical practice. J. Natl. Cancer Inst. 2005;97:1072–1079. doi: 10.1093/jnci/dji187. [DOI] [PubMed] [Google Scholar]
- 18.de Sanjose S., Quint W.G., Alemany L., Geraets D.T., Klaustermeier J.E., Lloveras B. Human papillomavirus genotype attribution in invasive cervical cancer: a retrospective cross-sectional worldwide study. Lancet Oncol. 2010;11:1048–1056. doi: 10.1016/S1470-2045(10)70230-8. [DOI] [PubMed] [Google Scholar]
- 19.Huh W.K., Ault K.A., Chelmow D., Davey D.D., Goulart R.A., Garcia F.A. Use of primary high-risk human papillomavirus testing for cervical cancer screening: interim clinical guidance. Gynecol. Oncol. 2015;136:178–182. doi: 10.1016/j.ygyno.2014.12.022. [DOI] [PubMed] [Google Scholar]
- 20.Huang S., Erickson B., Tang N., Mak W.B., Salituro J., Robinson J. Clinical performance of Abbott RealTime High Risk HPV test for detection of high-grade cervical intraepithelial neoplasia in women with abnormal cytology. J. Clin. Virol. 2009;45(Suppl 1):S19–S23. doi: 10.1016/S1386-6532(09)70004-6. [DOI] [PubMed] [Google Scholar]
- 21.Chernesky M., Huang S., Jang D., Erickson B., Salituro J., Engel H. Performance of a new HPV cervi-collect collection and transportation kit. J Oncol. 2012;2012:503432. doi: 10.1155/2012/503432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wright T.C., Jr., Stoler M.H., Behrens C.M., Apple R., Derion T., Wright T.L. The ATHENA human papillomavirus study: design, methods, and baseline results. Am. J. Obstet. Gynecol. 2012;206:46 e1–e11. doi: 10.1016/j.ajog.2011.07.024. [DOI] [PubMed] [Google Scholar]
- 23.Clarke M.A., Cheung L.C., Castle P.E., Schiffman M., Tokugawa D., Poitras N. Five-Year risk of cervical precancer following p16/ki-67 dual-stain triage of HPV-positive women. JAMA Oncol. 2019;5:181–186. doi: 10.1001/jamaoncol.2018.4270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wright T.C., Jr., Behrens C.M., Ranger-Moore J., Rehm S., Sharma A., Stoler M.H. Triaging HPV-positive women with p16/Ki-67 dual-stained cytology: results from a sub-study nested into the ATHENA trial. Gynecol. Oncol. 2017;144:51–56. doi: 10.1016/j.ygyno.2016.10.031. [DOI] [PubMed] [Google Scholar]
- 25.Meijer C.J., Berkhof J., Castle P.E., Hesselink A.T., Franco E.L., Ronco G. Guidelines for human papillomavirus DNA test requirements for primary cervical cancer screening in women 30 years and older. International journal of cancer Journal international du cancer. 2009;124:516–520. doi: 10.1002/ijc.24010. [DOI] [PMC free article] [PubMed] [Google Scholar]