Graphical abstract
Keywords: Foreign accent syndrome, Larynx phonation area, Lesion network mapping
Highlights
-
•
Foreign accent syndrome (FAS) is marked by accent changes perceived as foreign.
-
•
While about 100 cases have been reported, the anatomical substrate is unknown.
-
•
Using lesion network mapping, we identified disruptions in speech networks in FAS.
-
•
Disrupted networks involved the middle precentral gyrus (the larynx motor area).
-
•
This study highlights the potential role of these regions in FAS.
Abstract
Background
Foreign accent syndrome (FAS) is a rare acquired speech disorder wherein an individual’s spoken accent is perceived as “foreign.” Most reported cases involve left frontal brain lesions, but it is known that various other lesions can also cause FAS. To determine whether heterogeneous FAS-causing lesions are localized to a common functional speech network rather than to a single anatomical site, we employed a recently validated image analysis technique known as “lesion network mapping.”
Methods
We identified 25 published cases of acquired neurogenic FAS without aphasia, and mapped each lesion volume onto a reference brain. We next identified the network of brain regions functionally connected to each FAS lesion using a connectome dataset from normative participants. Network maps were then overlapped to identify common network sites across the lesions.
Results
Classical lesion overlap analysis showed heterogeneity in lesion anatomical location, consistent with prior reports. However, at least 80% of lesions showed network overlap in the bilateral lower and middle portions of the precentral gyrus and in the medial frontal cortex. The left lower portion of the precentral gyrus is suggested to be the location of lesions causing apraxia of speech (AOS), and the middle portion is considered to be a larynx-specific motor area associated with the production of vowels and stop/nasal consonants and with the determination of pitch accent.
Conclusions
The lesions that cause FAS are anatomically heterogeneous, but they share a common functional network located in the bilateral posterior region of the frontal lobe. This network specifically includes not only the lower portion of the central gyrus, but also its middle region, which is referred to as the larynx motor cortex and is known to be associated with phonation. Our findings suggest that disrupted networks in FAS might be anatomically different from those in AOS.
1. Introduction
Foreign accent syndrome (FAS) is a rare speech disorder, usually caused by neurological disorders, that is characterized by the emergence of a foreign accent. The most common etiology of FAS is stroke, followed by head trauma (Lippert-Gruener et al., 2005, Monrad-Krohn, 1947, Perkins et al., 2010, Liu et al., 2015); metastatic brain tumor (Abel et al., 2009); multiple sclerosis (Bakker et al., 2004, Chanson et al., 2009); progressive degenerative brain disease, including primary progressive aphasia (Luzzi et al., 2008, Paolini et al., 2013); learning disorders (Mariën et al., 2009, Keulen et al., 2016); and psychogenic disorders (Reeves et al., 2007, Reeves and Norton, 2001). Accent change is thought to result from a combination of segmental deficits, i.e., phonetic distortions and phonemic paraphasias (Berthier et al., 1991, Blumstein et al., 1987, Graff-Radford et al., 1986, Gurd et al., 1988, Ingram et al., 1992, Kurowski et al., 1996), and suprasegmental changes, i.e., stress, pitch, and rhythm variation known as dysprosody (Monrad-Krohn, 1947, Blumstein et al., 1987, Ladefoged and Johnson, 2006, Takayama et al., 1993). Because of the overlaps in speech features in FAS, it is still controversial whether this condition should be considered a distinct syndrome as opposed to a subtype of apraxia of speech (AOS), a motor speech disorder characterized by slow speech rate, articulatory distortions, and distorted sound substitutions, as well as segmentation of syllables, articulatory groping, and trial and error articulatory movements (Duffy, 2013, Josephs et al., 2012).
Since Pierre Marie first descried the Parisian French patient who developed a distinct Alsatian accent in 1907, and Arnold Pick described a 26-year-old Czech butcher who spoke with a Polish accent after a stroke in 1919 (Pick, 1919), >100 FAS cases have been reported in the academic literature. Most patients had lesions in the left frontal lobe, especially in the motor and premotor areas, which help coordinate complex articulatory movements. (Takayama et al., 1993, Berthier et al., 1991, Blumstein et al., 1987, Graff-Radford et al., 1986, Sakurai et al., 2015, Ardila et al., 1988, Nakano et al., 1996); however, no brain region has consistently been reported to be responsible for pure FAS without other neurological symptoms. Lesion locations have varied widely, including the left basal ganglia (Gurd et al., 1988, Ingram et al., 1992, Fridriksson et al., 2005), left corona radiata (Tani et al., 2002), left internal capsule (Ryalls and Whiteside, 2006), right precentral and postcentral gyri (Berthier et al., 1991), right middle cerebral artery region (Dankovičová et al., 2001), right frontal area (Miller et al., 2006), brain stem (Tran and Mills, 2013, Keulen et al., 2017), and cerebellum (Keulen et al., 2017, Mariën et al., 2013).
Accordingly, FAS is hypothesized to be caused by the disruption of widely distributed speech networks rather than a single anatomical site. To verify this hypothesis and to identify the neuroanatomical substrate for FAS, we adopted a recently validated technique known as “lesion network mapping” (Boes et al., 2015). Based on the concept of diaschisis and the finding that symptoms are not attributed solely to the lesion itself but also to regions functionally connected to the lesion (Fasano et al., 2017), this technique utilizes normative connectome data to identify the networks associated with focal brain lesions without the need for special imaging sequences. This approach has been increasingly used to investigate the networks responsible for various neurological symptoms, including visual/auditory hallucinations, central post-stroke pain, subcortical aphasia (Boes et al., 2015), hemi-chorea-hemiballismus (Laganiere et al., 2016), Capgras syndrome (Darby et al., 2017), coma (Fischer et al., 2016), impaired decision making (Sutterer et al., 2016), freezing of gait (Fasano et al., 2017), and the rubber hand illusion (Wawrzyniak et al., 2018). Employing this unique methodology, we tested the hypothesis that lesions causing FAS would be connected to a common network of sites involved in motor speech function.
2. Materials & methods
2.1. Case selection
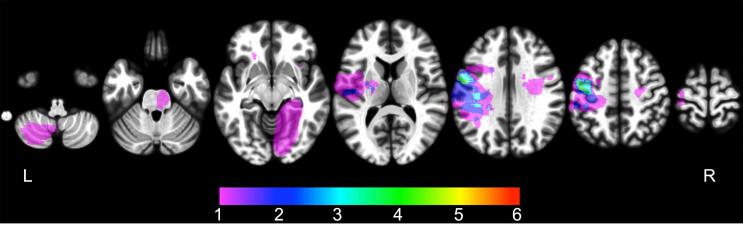
Cases of neurogenic FAS were identified through systematic searches of the PubMed and Ichushi-Web (Japanese) databases using the term “foreign accent syndrome” and performed in August 2017. Additional cases were identified by manual searches of reference lists in previous review articles on FAS (Jonkers et al., 2017). This literature search identified a total of 88 neurogenic FAS cases from 79 studies. Inclusion criteria were the emergence of a foreign accent according to Whitaker's operational definition (i.e., the accent was judged by the patient, by acquaintances, and by the investigator, to sound foreign and to be unlike the patient’s native dialect before cerebral insult Whitaker et al., 1982), following focal regional neurologic disorders such as stroke, head trauma, or brain tumor resection, and the clear appearance of affected lesions on at least one CT or MRI slice. Exclusion criteria included apparent aphasia (i.e., cases with language symptoms other than speech motor symptoms lasting longer than 3 months) and poor image resolution. Based on these criteria, we included 24 papers describing 25 cases of neurogenic FAS without aphasia in our analysis (Abel et al., 2009, Berthier et al., 1991, Gurd et al., 1988, Takayama et al., 1993, Sakurai et al., 2015, Tran and Mills, 2013, Keulen et al., 2017, Nakano et al., 1996, Fridriksson et al., 2005, Tani et al., 2002, Ryalls and Whiteside, 2006, Seliger et al., 1992, Gurd et al., 2001, Avila et al., 2004, Marien et al., 2006, Scott et al., 2006, Verhoeven and Mariën, 2010, Akhlaghi et al., 2011, Bhandari, 2011, van der Scheer et al., 2014, Moreno-Torres et al., 2013, Tomasino et al., 2013) (Fig. 1; Table 1).
Fig. 1.
Lesion locations in each case.
Table 1.
Demographic data of the identified FAS cases.
| Lesion # | Author, year | Age/gender | Lesion site | Etiology | Language / speech impairment | FAS duration |
|---|---|---|---|---|---|---|
| 1 | (Gurd et al., 1988) | 41/F | Left basal ganglia | infarction | total muteness at onset | > 8 months |
| 2 | (Berthier et al., 1991) | 70/M | Middle portion of the left precentral gyrus | infarction | muteness within 4 weeks | > 5 years |
| 3 | 58/F | Right frontoparietal lobe | infarction | muteness within 2 months | > 2 months | |
| 4 | (Seliger et al., 1992, Wawrzyniak et al., 2018) | 65/F | Subcortical region of deep left hemisphere | infarction | N/A | < 4 months |
| 5 | (Takayama et al., 1993, Takayama et al., 1993) | 44/F | Middle fifth of the posterior lateral aspect of the left precentral gyrus | infarction | muteness within hours | > 1 month |
| 6 | (Nakano et al., 1996, Sakurai et al., 2015) | 55/M | Left precentral gyrus-middle frontal gyrus | infarction | transient muteness | < 2 weeks |
| 7 | 37/M | Middle portion of the left precentral gyrus | infarction | transient muteness (<11 days) | N/A | |
| 8 | (Gurd et al., 2001, Jonkers et al., 2017) | 47/F | Both frontal lobes, left inferior frontal corona radiata, left thalamus | infarction | aphasia within a month | > 5 months |
| 9 | (Tani et al., 2002, Nakano et al., 1996) | 54/F | Left putamen-corona radiata | infarction | N/A | < 7 months |
| 10 | (Avila et al., 2004, Whitaker et al., 1982) | 51/F | Left corona radiata, right temporal lobe | infarction | total muteness, progressively recovered | > 2 years |
| 11 | (Fridriksson et al., 2005, Ardila et al., 1988) | 45/M | Left basal ganglia, left putamen | infarction | severely slurred speech for 2 h | > 6 months |
| 12 | (Marien et al., 2006, Seliger et al., 1992) | 53/F | Left frontoparietal lobe | infarction | short period of muteness | < 1–3 years |
| 13 | (Ryalls and Whiteside, 2006, Fridriksson et al., 2005) | 57/F | Left internal capsule | infarction | muteness within 2 months | > 3 years |
| 14 | (Scott et al., 2006, Gurd et al., 2001) | 54/F | Left white matter underneath the precentral sulcus, dorsal and medial to the anterior insula | infarction | initially unable to speak | > 2 years |
| 15 | (Abel et al., 2009) | 60/F | Left anterior parietal lobe | tumor | dysarthria within 2 weeks post-operatively | > 2 weeks post-operatively |
| 16 | (Verhoeven and Mariën, 2010, Avila et al., 2004) | 53/F | Left inferior frontal gyrus, left precentral gyrus, left anterior insular cortex, left postcentral gyrus, left supramarginal gyrus | infarction | muteness within 11 days | > 27 days |
| 17 | (Akhlaghi et al., 2011, Marien et al., 2006) | 40/M | Left temporo-occipital lobe | infarction | muteness, followed by a few days of meaningless and incoherent speech | > 4 months |
| 18 | (Bhandari, 2011, Scott et al., 2006) | 55/M | Left parieto-occipital region, left middle frontal gyrus | infarction | N/A | transient (a day) |
| 19 | (van der Scheer et al., 2014, Verhoeven and Mariën, 2010) | 59/M | Left posterior, precentral, and postcentral gyri | infarction | muteness on a single day | N/A (>2 weeks) |
| 20 | (Moreno-Torres et al., 2013, Akhlaghi et al., 2011) | 44/F | Bilateral deep frontal lobe | infarction | muteness and minor writing spelling errors for a week | > 17 months |
| 21 | (Tomasino et al., 2013, Bhandari, 2011) | 50/F | Left deep frontal lobe | tumor | five brief episodes of speech arrest | < 1 month (until the surgery) |
| 22 | Tran and Mills, 2013, Dankovičová et al., 2001) | 60/F | Left hemi-pons | infarction | N/A | < 1 month (until the second stroke) |
| 23 | (Sakurai et al., 2015, Josephs et al., 2012) | 42/F | Left precentral and premotor cortices around the inferior frontal sulcus | infarction | agrammatism in the acute phase | 15 months ~ 2 years |
| 24 | (Keulen et al., 2017, Miller et al., 2006) | 44/M | Right hemi-pons | infarction | apraxic agraphia | N/A |
| 25 | 72/M | Left posterior inferior brainstem, postero-inferior portion of the left cerebellar hemisphere | infarction | very mild word-finding difficulties, aphasia in non-native languages (differential polyglot aphasia) | N/A |
Lesion locations in 25 cases of pure FAS without aphasia. Each lesion was overlaid onto the ICBM-152 brain template. All lesions were traced true to their laterality, and right/left orientation is shown on the upper left. Axial coordinates refer to MNI space.
2.2. Lesion (functional) network mapping
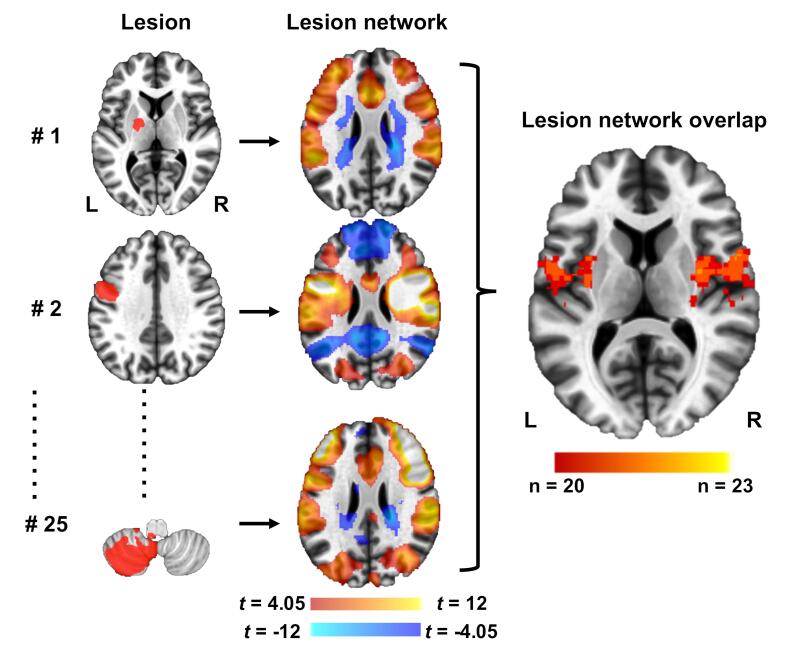
To investigate the networks associated with FAS lesions, we applied the lesion (functional) network mapping technique in reference to a previous report (Boes et al., 2015) (Fig. 2). This method involved 3 steps: (1) the volume of each lesion was transferred to a reference brain (i.e.; lesion mapping); (2) the network of brain regions functionally connected to each lesion was computed using resting-state functional connectivity MRI (rs-fc MRI) data from a large normative cohort; and (3) the resulting lesion (functional) network maps were thresholded and overlaid to identify common network sites across the lesions. The normative rs-fc MRI dataset was the same as that used in the previous lesion (functional) network mapping study (Boes et al., 2015, Laganiere et al., 2016, Darby et al., 2017), and consisted of 104 healthy participants (41 males, mean age 21.4 ± 2.8 years) from part of a publicly available dataset (The Brain Genomics Superstruct Project; http://neuroinformatics.harvard.edu/gsp/) (Buckner).
Fig. 2.
Lesion network mapping technique (Boes et al., 2015, Laganiere et al., 2016, Darby et al., 2017).
Twenty-five lesions resulting in pure FAS were manually traced onto a reference template (left column). Functional connectivity maps for each lesion volume were derived from a large connectome dataset of healthy controls (middle column). The 25 functional connectivity maps were overlapped to identify common networks across the lesions (right column). Positive correlations with the lesion are shown in hot colors while negative correlations are shown in cool colors.
2.3. Lesion mapping
We manually traced the lesion locations onto a reference brain template (MNI152 brain, 1 × 1 mm; http://fsl.fmrib.ox.ac.uk/fsldownloads) using MRIcron software (www.mccauslandcenter.sc.edu/mricro/mricron). Lesions were traced in 2D planes using neuroanatomical landmarks by 2 independent investigators (Y.H. and T.H.) to ensure accurate transfer onto the template brain. To identify areas of lesion overlap, 2D lesions from figures were extended by 2 mm perpendicular to the plane in which they were displayed to more closely approximate natural 3D lesion contours, in accordance with previous lesion network mapping studies (Boes et al., 2015). In cases where multiple lesions were displayed, lesions were mapped together and treated as a single lesion for subsequent analyses. The cohort of 25 lesions is displayed in Fig. 1.
2.4. Normative resting-state functional connectivity MRI dataset
The normative rs-fcMRI dataset consisted of 104 healthy participants (41 males, mean age 21.4 ± 2.8 years) who were part of a publicly available dataset (Buckner; Thomas Yeo et al., 2011). The study was conducted with the written consent of each subject and approved by the Partners’ Institutional Review Board. Imaging was performed on a 3 T Siemens whole-body MRI System with a phased-array head coil. Each subject completed two 6.2-min (124 frames) resting-state functional magnetic resonance imaging (fMRI) scans (TR = 3000 ms, TE = 30 ms, FA = 85°, 3 × 3 × 3 mm voxels, FOV = 216, 47 axial slices with interleaved acquisition and no gap). Participants were asked to rest in the scanner with their eyes open. rs-fcMRI data were processed in accordance with the strategy of Fox et al. (Fox et al., 2005) as implemented by Van Dijk et al. (Van Dijk et al., 2010). Structural data, including a high-resolution, multi-echo, T1-weighted, magnetization-prepared, gradient-echo image (TR = 2200 ms, TI = 1100 ms, TE = 1.54 ms for image 1 to 7.01 ms for image 4, FA = 7°, 1.2 × 1.2 × 1.2 mm voxels, FOV = 230), were also downloaded from the same dataset as rs-fc MRI.
2.5. rs-fc MRI data processing and statistical analysis
Based on the normative rs-fc MRI dataset consisting of two runs per participant, rs-fc MRI maps were created for each lesion using a standard seed-based approach with SPM12 software (http://www.fil.ion.ucl.ac.uk/spm/) and the CONN 17.f toolbox (http://www.nitrc.org/projects/conn).
First, for each normative subject we removed the first four volumes, then the functional images were corrected for slice time and motion, co-registered with a high-resolution anatomical scan, normalized into the Montreal Neurological Institute coordinate (MNI) space, resampled at 2 mm (Perkins et al., 2010), and smoothed with 6-mm full width at half-maximum Gaussian blur. In addition, Artifact Detection Tools (ART: http://www.nitrc.org/projects/artifact_detect) were used to measure motion artifacts in all subjects and to detect outliers (ART-based scrubbing).
Following the pre-processing steps, the blood oxygenated level-dependent (BOLD) signal data were temporally band-pass filtered (0.009–0.08 Hz) and the signals from the cerebrospinal fluid and the white matter were removed from the data through linear regression. A motion parameter was also included in the linear regression to minimize BOLD signal artifacts caused by head motion. Then, a general linear model was applied to examine significant BOLD signal correlations with respect to time between each seed and each voxel. For subsequent t-tests, the toolbox converted the resulting correlation coefficients to Z scores using Fisher’s Z transformation.
2.6. Functional connectivity of the region of maximum overlap
Each of the 25 lesion-seeded rs-fc MRI network maps was thresholded at a t value of positive or negative 4.05 (p < 0.00005, uncorrected), with reference to the previous lesion (functional) network mapping studies (Boes et al., 2015, Laganiere et al., 2016, Darby et al., 2017). After applying this threshold, the resulting network maps were binarized and overlapped to identify regions of shared positive or negative correlation. A threshold for the group analysis was set at 80% (20 of the 25 cases). Peak coordinates in these maps were identified using FSL's clustering algorithm (minimum distance between local maxima of 10 mm, minimum cluster size of 20 voxels). For comparison, we also conducted a traditional lesion overlap-mapping analysis using MRIcron.
3. Results
3.1. Patient characteristics
We identified 25 cases of FAS without aphasia (Fig. 1, Table 1). There were 16 females, and ages ranged from 37 to 72 (mean 52 ± 9.2) years. The etiology of most lesions was ischemic stroke, while 2 cases were caused by brain tumor. Lesion sites were anatomically heterogeneous, with primary locations in the frontal cortex, temporal cortex, parietal cortex, posterior cortex, subcortical white matter, midbrain, and cerebellum. Three lesions were in the right cerebrum, 18 were in the left cerebrum, and one each was in the bilateral cerebrum, left cerebellum, left pons, and right pons.
3.2. Lesion (functional) network mapping
Traditional lesion overlapping analysis detected the maximum overlap in only 5 of 25 cases (20%), indicating marked heterogeneity in lesion location (Fig. 3). The maximum overlap was identified in the lower portion of the left precentral gyrus near the insular cortex.
Fig. 3.
Traditional lesion mapping results.
In contrast, lesion (functional) network mapping analysis revealed that the overlap ratio of lesion-derived functional networks was quite high (~92%), and network overlap was observed specifically within the motor speech area involving the bilateral middle portion of the precentral gyrus ([48, 2, 43] and [−37, −10, 43] in the MNI space), the bilateral lower portion of the precentral gyrus extending to the insular cortex ([32, −5, 14] and [−24, 2, −18] in the MNI space), and the medial frontal cortex corresponding to the supplementary motor area (SMA) ([-8, 11, 31] in the MNI space) (Table 2, Fig. 4). Twenty of 25 FAS lesions were also functionally connected with the left parietal opercular cortex, the bilateral thalamus, and the bilateral cerebellum (lobule VI), in which the cluster sizes were relatively small (Table 2). All lesions were positively correlated with these brain sites, and no other areas of network overlap (positive or negative) met our threshold (80% overlap).
Table 2.
Overlapping clusters in lesion (functional) network mapping.
| Cluster # | Max overlap | Cluster size (voxels) | MNI |
Brain region | ||
|---|---|---|---|---|---|---|
| X | Y | Z | ||||
| 1 | 23 | 16,608 | 32 | −5 | 14 | Right precentral gyrus to insular cortex |
| 2 | 22 | 10,139 | −24 | 2 | −18 | Left precentral gyrus to insular cortex |
| 3 | 21 | 6094 | −8 | 11 | 31 | Bilateral medial frontal cortex (SMA) |
| 4 | 22 | 1538 | 48 | 2 | 43 | Right precentral gyrus (middle portion) |
| 5 | 21 | 826 | −37 | −10 | 43 | Left precentral gyrus (middle portion) |
| 6 | 20 | 383 | −52 | −38 | 15 | Left parietal operculum cortex |
| 7 | 20 | 377 | −9 | −21 | −2 | Left thalamus |
| 8 | 20 | 29 | 28 | −57 | −26 | Right cerebellum (lobule VI) |
| 9 | 20 | 25 | 12 | −18 | 3 | Right thalamus |
| 10 | 20 | 21 | −9 | −62 | −17 | Left cerebellum (lobule VI) |
SMA: supplementary motor area.
MNI: Montreal Neurological Institute coordinates.
Fig. 4.
Lesion (functional) network mapping results.
Although network overlap in the frontal areas was high for FAS lesions, 3 lesions failed to show functional connectivity with this site (lesions of cases #8, 14, and 22; Fig. 1, Table 1).
Regions of overlap among 25 FAS lesions are shown mainly in the left precentral sulcus. The color scale indicates the number of overlapping lesions. The maximum number of overlapping cases was only 5 (20%), indicating marked heterogeneity in lesion location.
Regions of overlap among 25 FAS lesion-seeded networks are shown in red. The main cluster is located in the bilateral insula, lower portion of the prefrontal gyrus, medial frontal cortex, and upper middle portion of the prefrontal gyrus. The maximum number of overlapping cases was 23 (92%), which was higher than with the traditional lesion overlapping method. These results are illustrated using the Mango visualization software (http://ric.uthscsa.edu/mango/). Axial coordinates refer to MNI space.
4. Discussion
Because FAS is a relatively rare neurological symptom and lesions occur at various sites, traditional lesion overlap analyses have so far failed to identify the relevant neuroanatomical substrate. Adopting the lesion (functional) network mapping approach, we showed that anatomically heterogeneous lesions causing FAS without aphasia were located within a single network with shared functional connectivity with the bilateral frontal lobe, thus providing new insights into the anatomical framework and the pathophysiology of FAS.
The overlap ratio of lesion (functional) networks was quite high (~92%), and the main overlapping regions involved the bilateral middle portion of the precentral gyrus, the bilateral lower portion of the precentral gyrus extending to the insular cortex, and the medial frontal cortex. It is notable that these brain areas correspond to the key anatomical locations in the Directions Into Velocities of Articulators (DIVA) model, a widely used broader speech motor network model (Golfinopoulos et al., 2010). According to this model, production of a speech sound begins with activation of a “Speech Sound Map” in the left ventral premotor cortex, followed by excitatory feedforward commands projecting to the “Articulator Velocity and Position Maps” in the bilateral ventral primary motor cortex. Finally, the commands from the maps are released to the articulators when the activity of the appropriate cell in the “Initiation Map” becomes active. The “Initiation Map” is hypothesized to lie bilaterally within the SMA, which is connected with the basal ganglia, including the bilateral caudate, putamen, pallidum, and thalamus. The medial frontal overlapping region in our analysis might correspond with the anatomical location of the “Initiation Map,” and the lower and middle precentral gyrus with the “Articulator Velocity and Position Maps.”
4.1. The middle portion of the precentral gyrus
The middle portion of the precentral gyrus was the most commonly overlapping brain region, functionally connecting with 22 (right) or 21 (left) lesions (88% and 84%, respectively). Interestingly, this region was close to the site reported to be activated by repeated glottal stops (i.e., forced closure of the glottis in the absence of vocalizing) or vowel phonation in a functional MRI study (peak activation, Brodmann areas 4 [−38, −14, 32], [44, −10, 34] and 6 [−53, 0, 42], [53, 4, 42] on glottal stops, and Brodmann areas 4 [−40, −10, 30], [44, −8, 34] and area 6 [−51, 0, 44], [50, −2, 37] on phonation, in the MNI space) (Brown et al., 2008). Thus, this area is regarded as the area of the motor cortex that controlled the intrinsic muscles of the larynx, and is referred to as the “larynx/phonation area.”
The larynx is the organ of phonation, and consists of 2 muscles controlling the vocal folds, namely the intrinsic and extrinsic laryngeal muscles. The intrinsic muscles control 2 dimensions of vocal-fold movement to modify the positioning and tension of the vocal folds. One dimension involves the opening (abduction) and closing (adduction) of the glottal space, while the other involves tensing and relaxing of the vocal folds for the purpose of altering vocal pitch (F0 variation) and vocal intensity (Brown et al., 2008). Control of vocal pitch, corresponding to the vocal fold eigenfrequencies, is achieved mainly by varying the stiffness and tension of the vocal folds through the activation of the intrinsic laryngeal muscles, especially the cricothyroid muscle (Zhang, 2016). The intrinsic muscles also increase vocal intensity, either elevating the subglottal pressure, which increases vibration amplitude, or increasing vocal fold adduction (Zhang, 2016, Seikel et al., 2009).
Taking these factors into account, the abnormal prosody characterized by disrupted normal pitch and intensity may correspond to intrinsic laryngeal muscle dysfunction regulated by the middle portion of the precentral gyrus. In accordance with this notion, a relatively large number of FAS patients with a localized lesion in this area have been reported thus far (Blumstein et al., 1987, Graff-Radford et al., 1986, Takayama et al., 1993, Sakurai et al., 2015, Nakano et al., 1996, Berthier et al., 2015). This brain region has been suggested to be related to dysprosody (Takayama et al., 1993, Sakurai et al., 2015) and production of regional accent features (Berthier et al., 2015) in FAS patients. By contrast, Tomasino et al. reported a patient with a tumor of the left precentral gyrus who showed increased fMRI activity in the left laryngeal area during tasks involving counting, sentence production, and pseudo-word pronunciation (Tomasino et al., 2013). They argued that this FAS case should be thought of as a disorder of the feedforward control commands, in particular involving the articulator velocity and position maps, which are hypothesized to lie along the caudo-ventral portion of the precentral gyrus.
4.2. The lower portion of the precentral gyrus
The lower portion of the precentral gyrus was also a common overlapping region (88% to 92%) in this study. The Rolandic operculum, the lower portion of the precentral and postcentral gyrus, was reported to be activated by lip and tongue movements (peak activation, Brodmann area 43 [−60, −13, 19], [57, −15, 19] on lip movement, and area 43 [−63, −9, 15], [65, −7, 21] on tongue movement) in a functional MRI study (Brown et al., 2008). These brain regions have also been reported to be critical areas for the intra- and inter-syllabic coordination of complex articulatory movements (Baldo et al., 2011). Although the precise anatomical brain regions associated with AOS remain controversial (Dronkers, 1996, Hillis et al., 2004), sophisticated analytical methods, including voxel-based lesion-symptom mapping, have indicated an association between AOS and the anterior insula (Dronkers, 1996, Ogar et al., 2006) or the lower portion of the precentral gyrus (Graff-Radford et al., 2014). In accordance with the results of these reports and our finding that the lower portion of the precentral gyrus is involved in FAS, a relationship has been suggested between FAS and AOS. Several researchers have hypothesized that FAS is a subtype or mild form of AOS, because AOS and FAS share many common characteristics such as increased variability in sound production (Miller et al., 2006, Marien et al., 2006, Whiteside and Varley, 1998), and some patients actually have both AOS and FAS (Ingram et al., 1992, Katz et al., 2008, Laures‐Gore et al., 2006).
However, other researchers have suggested that FAS is qualitatively different from peripheral AOS (Takayama et al., 1993, Scott et al., 2006) because in several cases FAS has been accompanied by other speech disorders, such as peripheral dysarthria, pseudo-bulbar palsy (Berthier et al., 1991, Blumstein et al., 1987, Graff-Radford et al., 1986), and cerebellar ataxic speech (Marien et al., 2006), and furthermore, cases of FAS with cerebellar or brain stem lesions that are not responsible for AOS have been reported (Marien et al., 2006, Ackermann et al., 1992).
Alternatively, it is proposed that FAS represents a compensatory response to impaired motor regulation of speech, including that caused by AOS and ataxic speech (Fridriksson et al., 2005, Jonkers et al., 2017). It is difficult to determine which of the above hypotheses is correct based on the findings of our study, because we did not exclude patients who had both FAS and AOS. Therefore, investigation of cases of pure FAS without aphasia or AOS will help clarify whether FAS is distinguishable from dysarthria, aphasia, and AOS.
4.3. The supplementary motor area
The SMA was another overlapping region with high prevalence (up to 84%) in this study. The SMA, located on the medial aspect of the frontal lobe anterior to the leg representation of the primary motor cortex, is known to play a crucial role in controlling various degrees of actions or behaviors. For example, previous studies in monkeys and humans, including functional imaging studies, demonstrated that the SMA is responsible for “self-initiated” or internally driven actions, sequential actions, the learning of new tasks, and the cognitive control of actions, including task switching (Nachev et al., 2008). Furthermore, SMA lesions in humans can lead to several behavioral disorders, including the following: utilization behavior, in which the patient is unable to resist the impulsive utilization of an object, even when it is not needed (Boccardi et al., 2002); alien-limb syndrome, distinguished by involuntary actions such as grasping objects without the intention to do so (Feinberg et al., 1992); and motor neglect, characterized by abolishment of spontaneous movement or underutilization of the affected limb when it would be appropriate to move (Laplane et al., 1977, Krainik et al., 2001).
Regarding language and speech processing, Berthier et al. suggested that lesions of the SMA could be involved in the development of FAS (Berthier et al., 2015). In addition, damages to the SMA, especially on the left side, is known to cause a peculiar type of aphasia, termed SMA aphasia, characterized by a lack of spontaneous initiation of speech with well-preserved articulation once speech has begun (Masdeu et al., 1978, Alexander and Schmitt, 1980, Pai, 1999). Furthermore, stimulation of the SMA and pre-SMA were also reported to produce both vocalization and arrest of speech (Penfield and Rasmussen, 1950). More recently, damage to the left frontal “aslant tract” (FAT), a fiber pathway that connects the posterior region of the inferior frontal gyrus with the SMA and pre-SMA (Catani et al., 2012), correlated with verbal fluency performance in primary progressive aphasia (Catani et al., 2013). The diffusion measures of the bilateral FAT were also reported to be altered in persistent developmental stuttering (Kronfeld-Duenias et al., 2016).
As mentioned above, disruption of the SMA could result in various speech motor disorders. Therefore, we supposed that SMA disruption might contribute to FAS, but not be necessary or sufficient for its development. The lack of reported FAS cases with a single lesion in the SMA supports this notion.
4.4. Other brain regions
The bilateral cerebellum and thalamus were also common overlapping regions, but the cluster sizes were relatively small. Regarding the cerebellum, the overlaps were located bilaterally in medial lobule VI, which is known to somatotopically represent articulatory apparatuses such as the lips and tongue (Callan et al., 2007, Marien et al., 2014). Functional imaging studies in humans showed bilateral activation in medial lobule VI during an articulation task (Thürling et al., 2011). Clinical investigations also revealed that patients with cerebellar stroke developed dysarthria, termed “ataxic dysarthria” when the lesion involved rostral paravermal regions such as vermal lobule VI (Ackermann et al., 1992, Urban et al., 2003). These data suggest that overt speech is mediated by lobule VI, one of the common overlapping regions in our study. The cerebellum has been hypothesized to be involved in precise processing of temporal information related to speech (Ackermann et al., 2004). This process is thought to be carried out by a feedforward control system mediated by a trans-cerebellar pathway, including the bilateral thalamus and the SMA (Golfinopoulos et al., 2010). Accordingly, our overlap in the bilateral thalamus and the cerebellum might be related to a speech feedforward network disorder linked to the lower and middle precentral gyrus and the SMA.
The left parietal operculum cortex, known as the secondary somatosensory cortex, is generally not considered to be involved in the motor aspects of speech. However, Tian et al. reported activation of this brain region during an articulation task and an articulation-imagery task using fMRI in healthy volunteers (Tian et al., 2016). They argued that this area was elicited by the perception of somatosensory feedback used to estimate the somatosensory consequences of overt speech (Tian et al., 2016). Based on this notion, the overlap in the parietal operculum might indicate that impairments of this feedback system affect FAS development.
4.5. Lesions lacking functional connectivity to common frontal regions
Although our lesion (functional) network mapping results demonstrated remarkable overlap of lesion-derived functional networks, the lesions of 3 patients showed no functional connectivity to the precentral gyrus or the SMA, the most common overlapping sites (lesions in cases #8, 14, and 22; Fig. 1, Table 1). First, the lesions in a 47-year-old female patient were small infarctions located in both frontal lobes, the left inferior frontal corona radiata, and the left thalamus (Gurd et al., 2001). Second, the lesion in a 59-year-old male was a small infarction located in the white matter underneath the left precentral sulcus (Scott et al., 2006). The lesions of both patients were smaller than those in the other patients, and were located mainly in the deep white matter, and not in the cortex. The lesion (functional) network mapping method uses functional MRI as a normative connectome dataset, and meaningful BOLD signals can be expected mainly in the grey matter, and not in the white matter (Buxton, 2013, Logothetis et al., 2001). Therefore, these two patients harboring localized white matter lesions are likely to be inappropriate subjects for this analytical method, and the use of an alternate structural connectome, such as diffusion-weighted MRI, might solve this problem. The third patient was a 60-year-old female whose lesion was an infarction in the left pons (Tran and Mills, 2013). While the lesion did not show functional connectivity to the common overlapping brain sites, the area of its connectivity was located slightly adjacent to the voxels with maximal overlap in the left precentral gyrus (supplementary Fig. 1).
4.6. Duration of FAS
The duration of FAS was heterogeneous in our patient cohort, and it would be meaningful to know if patients who recover from FAS have a different neural correlate than those who do not. Although symptom duration was often not fully described, we selected cases in which FAS persisted for at least 1 year (cases #2, 10, 12, 13, 14, 20, and 23) or for <1 year (cases #3, 6, 9, and 18), and found no significant differences between the two groups in terms of FAS etiology or lesion / lesion (functional) network distribution. To clarify the relationships of these factors with FAS duration, future studies should be performed with larger numbers of patients for whom detailed information is available.
4.7. Limitations
There are several limitations to the present study. First, our results are based on the analysis of a highly select group of cases chosen by excluding those lacking imaging data or complications of aphasia. However, we believe that “pure” FAS samples defined in this way may be optimal for investigating the neural substrate of FAS because patients with FAS who also have aphasia tend to have larger lesions, and their analysis often detects brain areas that are irrelevant to FAS. Additionally, while most of the analyzed patients had experienced ischemic stroke, we also included 2 patients with brain tumors. Because of the different trajectories of disease progression following stroke, we conducted an additional analysis that excluded the patients with tumors. However, the results were similar to our primary findings, except for a smaller overlap size due to the smaller number of cases (see supplementary table and supplementary Fig. 2). Second, all lesions were published 2D figures that we analyzed retrospectively and did not examine directly. This may have led to diagnostic inaccuracy or heterogeneity, and limited the available clinical information. Similarly, the analysis could not rule out the potential contributions of chronic lesions or lesions that were not highlighted in the cited article. Thus, prospective studies based on common diagnostic criteria and precise neuroimaging information are required to overcome these limitations. Third, we used a 2D figure to approximate a 3D lesion, and adopted a connectome dataset from a younger cohort. In a prior validation study, however, 2D representations like those used in our study were compared to actual 3D lesions, and younger connectome cohorts were compared to older ones, and the results were nearly identical in each condition. These results justify our analysis using 2D lesions and younger connectome cohort (Boes et al., 2015). Therefore, we believe that our analysis yielded valuable results despite partial distortions in lesion representation. Fourth, this technique, as with classical lesion overlap analysis and voxel lesion symptom mapping, does not account for the compensatory effect on the damaged region. FAS can also result from a compensatory response to abnormal regulation of speech (Fridriksson et al., 2005), involving functional compensation for one or more damaged components of the speech production network by other nodes of this network. Our results might have been modified by such compensatory effects. Similarly, our analysis did not incorporate any information about the hierarchical organization in each overlapping region. Therefore, in future studies it might be valuable to precisely investigate the mechanisms underlying FAS by using electrophysiological stimulation methods such as transcranial magnetic stimulation or by targeting only the acute stage of stroke.
5. Conclusion
Although anatomically heterogeneous lesions can cause FAS, our lesion (functional) network mapping analysis of FAS without aphasia suggested the importance of disruption in the speech motor network, including not only the lower part of the precentral gyrus, known to be associated with AOS, but also the bilateral middle portion of the precentral gyrus, considered to be the larynx/phonation area. These data suggest that the chief characteristics of FAS, including changes in stress, pitch, or rhythm variation, might be associated with disrupted motor control of the larynx. Our conclusions are based on the analysis of a limited number of biased cases, and therefore large-scale studies are needed to determine whether FAS is a subtype of AOS or a condition distinguishable from AOS.
6. Availability of data and materials
The datasets used and analyzed during the present study are available from the corresponding author on reasonable request and with approval of our ethics committee.
CRediT authorship contribution statement
Yuichi Higashiyama: Conceptualization, Methodology, Formal analysis, Investigation, Data curation, Writing - original draft, Visualization. Tomoya Hamada: Conceptualization, Methodology, Validation, Formal analysis, Investigation, Writing - original draft, Visualization. Asami Saito: Methodology, Formal analysis, Investigation, Visualization. Keisuke Morihara: Formal analysis, Investigation, Visualization. Mitsuo Okamoto: Methodology, Formal analysis, Investigation, Visualization. Katsuo Kimura: Methodology, Validation. Hideto Joki: Methodology, Validation. Hitaru Kishida: Methodology, Validation. Hiroshi Doi: Validation, Writing - review & editing, Supervision. Naohisa Ueda: Validation, Writing - review & editing. Hideyuki Takeuchi: Validation, Writing - review & editing, Supervision. Fumiaki Tanaka: Methodology, Writing - review & editing, Supervision, Project administration.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
Acknowledgments
Data were provided by the Brain Genomics Superstruct Project of Harvard University and the Massachusetts General Hospital (Principal Investigators: Randy Buckner, Joshua Roffman, and Jordan Smoller), with support from the Center for Brain Science Neuroinformatics Research Group, the Athinoula A. Martinos Center for Biomedical Imaging, and the Center for Human Genetic Research. Twenty individual investigators at Harvard and Massachusetts General Hospital generously contributed data to the overall project.
This work was supported by JSPS KAKENHI Grant Number JP16H06280, a Grant-in-Aid for Scientific Research on Innovative Areas – Platforms for Advanced Technologies and Research Resources “Advanced Bioimaging Support.”
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.nicl.2021.102760.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
Supplementary Fig. 1.
Supplementary Fig. 2.
References
- Lippert-Gruener M., Weinert U., Greisbach T., Wedekind C. Foreign accent syndrome following traumatic brain injury. Brain Inj. 2005;19(11):955–958. doi: 10.1080/02699050500109506. [DOI] [PubMed] [Google Scholar]
- Monrad-Krohn G.H. Dysprosody or altered melody of language. Brain. 1947;70(4):405–415. doi: 10.1093/brain/70.4.405. [DOI] [PubMed] [Google Scholar]
- Perkins R.A., Ryalls J.H., Carson C.K., Whiteside J.D. Acoustic analyses of two recovered cases of foreign accent syndrome. Aphasiology. 2010;24(10):1132–1154. [Google Scholar]
- Liu H.E., Qi P., Liu Y.L., Liu H.X., Li G. Foreign accent syndrome: two case reports and literature review. Eur. Rev. Med. Pharmacol. Sci. 2015;19:81–85. [PubMed] [Google Scholar]
- Abel T.J., Hebb A.O., Silbergeld D.L. Cortical stimulation mapping in a patient with foreign accent syndrome: case report. Clin. Neurol. Neurosurg. 2009;111(1):97–101. doi: 10.1016/j.clineuro.2008.07.014. [DOI] [PubMed] [Google Scholar]
- Bakker J.I., Apeldoorn S., Metz L.M. Foreign accent syndrome in a patient with multiple sclerosis. Can. J. Neurol. Sci. 2004;31(2):271–272. doi: 10.1017/s0317167100053956. [DOI] [PubMed] [Google Scholar]
- Chanson J.B., Kremer S., Blanc F., Marescaux C., Namer I.J., de Seze J. Foreign accent syndrome as a first sign of multiple sclerosis. Mult. Scler. 2009;15(9):1123–1125. doi: 10.1177/1352458509106611. [DOI] [PubMed] [Google Scholar]
- Luzzi S., Viticchi G., Piccirilli M., Fabi K., Pesallaccia M., Bartolini M., Provinciali L., Snowden J.S. Foreign accent syndrome as the initial sign of primary progressive aphasia. J. Neurol. Neurosurg. Psychiatry. 2008;79(1):79–81. doi: 10.1136/jnnp.2006.113365. [DOI] [PubMed] [Google Scholar]
- Paolini S., Paciaroni L., Manca A., Rossi R., Fornarelli D., Cappa S.F., Abbatecola A.M., Scarpino O. Change of accent as an atypical onset of non fluent primary progressive aphasia. Behav. Neurol. 2013;27(2):221–227. doi: 10.3233/BEN-120299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marien P., Verhoeven J., Wackenier P., Engelborghs S., De Deyn P.P. Foreign accent syndrome as a developmental motor speech disorder. Cortex. 2009;45:870–878. doi: 10.1016/j.cortex.2008.10.010. [DOI] [PubMed] [Google Scholar]
- Keulen S., Marien P., Wackenier P., Jonkers R., Bastiaanse R., Verhoeven J. Developmental Foreign Accent Syndrome: Report of a New Case. Front. Hum. Neurosci. 2016;10:65. doi: 10.3389/fnhum.2016.00065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reeves R.R., Burke R.S., Parker J.D. Characteristics of psychotic patients with foreign accent syndrome. J. Neuropsychiatry Clin. Neurosci. 2007;19(1):70–76. doi: 10.1176/jnp.2007.19.1.70. [DOI] [PubMed] [Google Scholar]
- Reeves R.R., Norton J.W. Foreign accent-like syndrome during psychotic exacerbations. Neuropsychiatry Neuropsychol. Behav. Neurol. 2001;14:135–138. [PubMed] [Google Scholar]
- Berthier M.L., Ruiz A., Massone M.I., Starkstein S.E., Leiguarda R.C. Foreign accent syndrome: behavioural and anatomical findings in recovered and non-recovered patients. Aphasiology. 1991;5(2):129–147. [Google Scholar]
- Blumstein S.E., Alexander M.P., Ryalls J.H., Katz W., Dworetzky B. On the nature of the foreign accent syndrome: a case study. Brain Lang. 1987;31(2):215–244. doi: 10.1016/0093-934x(87)90071-x. [DOI] [PubMed] [Google Scholar]
- Graff-Radford N.R., Cooper W.E., Colsher P.L., Damasio A.R. An unlearned foreign “accent” in a patient with aphasia. Brain Lang. 1986;28(1):86–94. doi: 10.1016/0093-934x(86)90093-3. [DOI] [PubMed] [Google Scholar]
- Gurd J.M., Bessell N.J., Bladon R.A.W., Bamford J.M. A case of foreign accent syndrome, with follow-up clinical, neuropsychological and phonetic descriptions. Neuropsychologia. 1988;26(2):237–251. doi: 10.1016/0028-3932(88)90077-2. [DOI] [PubMed] [Google Scholar]
- Ingram J.C.L., McCormack P.F., Kennedy M. Phonetic analysis of a case of foreign accent syndrome. J. Phonet. 1992;20(4):457–474. [Google Scholar]
- Kurowski K.M., Blumstein S.E., Alexander M. The foreign accent syndrome: a reconsideration. Brain Lang. 1996;54(1):1–25. doi: 10.1006/brln.1996.0059. [DOI] [PubMed] [Google Scholar]
- Ladefoged, P., Johnson, K., 2006. A Course in Phonetics, 5th ed. Boston, MA.
- Takayama Y., Sugishita M., Kido T., Ogawa M., Akiguchi I. A case of foreign accent syndrome without aphasia caused by a lesion of the left precentral gyrus. Neurology. 1993;43:1361–1363. doi: 10.1212/wnl.43.7.1361. [DOI] [PubMed] [Google Scholar]
- Duffy J.R. and management; 2013. Motor speech disorders: substrates, differential diagnosis. [Google Scholar]
- Josephs K.A., Duffy J.R., Strand E.A. Characterizing a neurodegenerative syndrome: primary progressive apraxia of speech. Brain. 2012;135:1522–1536. doi: 10.1093/brain/aws032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pick A. Uber Anderungen des Sprachcarakters als Begleiterscheinung aphasicher Storungen. Zeitschrift fur gesamte Neurologie und Psychiatrie. 1919;45:230–241. [Google Scholar]
- Sakurai Y., Itoh K., Sai K., Lee S., Abe S., Terao Y., Mannen T. Impaired laryngeal voice production in a patient with foreign accent syndrome. Neurocase. 2015;21(3):289–298. doi: 10.1080/13554794.2014.892622. [DOI] [PubMed] [Google Scholar]
- Ardila A., Rosselli M., Ardila O. Foreign accent: an aphasic epiphenomenon? Aphasiology. 1988;2(5):493–499. [Google Scholar]
- Nakano A., Tsukahara Y., Yokoyama E., Sayama I. Two cases of “foreign accent syndrome” without aphasia. Shinkei Shinrigaku. 1996;12:244–250. [Google Scholar]
- Fridriksson J., Ryalls J., Rorden C., MORGAN P.S., George M.S., Baylis G.C. Brain damage and cortical compensation in foreign accent syndrome. Neurocase. 2005;11(5):319–324. doi: 10.1080/13554790591006302. [DOI] [PubMed] [Google Scholar]
- Tani T., Amada M., Shimizu N., Izuka Y., Araki R. Analysis of nonverbal oral movement in a case with foreign accent syndrome. Shitsugosho Kenkyu. 2002;22(2):153–163. [Google Scholar]
- Ryalls J., Whiteside J. An atypical case of Foreign Accent Syndrome. Clin. Linguist. Phon. 2006;20(2-3):157–162. doi: 10.1080/02699200400026900. [DOI] [PubMed] [Google Scholar]
- Dankovičová J., Gurd J.M., Marshall J.C., MacMahons M.K.C., Stuart-Smith J., Coleman J.S. Aspects of non-native pronunciation in a case of altered accent following stroke (foreign accent syndrome) Clin. Linguist. Phonet. 2001;15:195–218. [Google Scholar]
- Miller N., Lowit A., O’Sullivan H. What makes acquired foreign accent syndrome foreign? J. Neurolinguist. 2006;19(5):385–409. [Google Scholar]
- Tran A.X., Mills L.D. A case of foreign accent syndrome. J. Emerg. Med. 2013;45(1):26–29. doi: 10.1016/j.jemermed.2012.11.015. [DOI] [PubMed] [Google Scholar]
- Keulen S., Mariën P., van Dun K., Bastiaanse R., Manto M., Verhoeven J.o. The Posterior Fossa and Foreign Accent Syndrome: Report of Two New Cases and Review of the Literature. Cerebellum. 2017;16(4):772–785. doi: 10.1007/s12311-017-0849-6. [DOI] [PubMed] [Google Scholar]
- Mariën P., Verslegers L., Moens M., Dua G., Herregods P., Verhoeven J.o. Posterior fossa syndrome after cerebellar stroke. Cerebellum. 2013;12(5):686–691. doi: 10.1007/s12311-013-0478-7. [DOI] [PubMed] [Google Scholar]
- Boes A.D., Prasad S., Liu H., Liu Q.i., Pascual-Leone A., Caviness V.S., Fox M.D. Network localization of neurological symptoms from focal brain lesions. Brain. 2015;138(10):3061–3075. doi: 10.1093/brain/awv228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fasano A., Laganiere S.E., Lam S., Fox M.D. Lesions causing freezing of gait localize to a cerebellar functional network. Ann. Neurol. 2017;81(1):129–141. doi: 10.1002/ana.24845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laganiere S., Boes A.D., Fox M.D. Network localization of hemichorea-hemiballismus. Neurology. 2016;86(23):2187–2195. doi: 10.1212/WNL.0000000000002741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darby R.R., Laganiere S., Pascual-Leone A., Prasad S., Fox M.D. Finding the imposter: brain connectivity of lesions causing delusional misidentifications. Brain. 2017;140(2):497–507. doi: 10.1093/brain/aww288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischer D.B., Boes A.D., Demertzi A., Evrard H.C., Laureys S., Edlow B.L., Liu H., Saper C.B., Pascual-Leone A., Fox M.D., Geerling J.C. A human brain network derived from coma-causing brainstem lesions. Neurology. 2016;87(23):2427–2434. doi: 10.1212/WNL.0000000000003404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutterer M.J., Bruss J., Boes A.D., Voss M.W., Bechara A., Tranel D. Canceled connections: lesion-derived network mapping helps explain differences in performance on a complex decision-making task. Cortex. 2016;78:31–43. doi: 10.1016/j.cortex.2016.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wawrzyniak M., Klingbeil J., Zeller D., Saur D., Classen J. The neuronal network involved in self-attribution of an artificial hand: A lesion network-symptom-mapping study. Neuroimage. 2018;166:317–324. doi: 10.1016/j.neuroimage.2017.11.011. [DOI] [PubMed] [Google Scholar]
- Jonkers R., van der Scheer F., Gilbers D. The common denominator in the perception of accents in cases with foreign accent syndrome. Aphasiology. 2017;31(9):1021–1043. [Google Scholar]
- Whitaker H.A. Levels of impairment in disorders of speech. In: Malatesha R.N., Hartlage L.C., editors. Neuropsychology and Cognition: NATO Advanced Study Institute Series: The Hague. Martinus Nijhoff Publishers; 1982. pp. 168–207. [Google Scholar]
- Seliger G.M., Abrams G.M., Horton A. Irish brogue after stroke. Stroke. 1992;23(11):1655–1656. doi: 10.1161/01.str.23.11.1655. [DOI] [PubMed] [Google Scholar]
- Gurd J.M., Coleman J.S., Costello A., Marshall J.C. Organic or functional? A new case of foreign accent syndrome. Cortex. 2001;37(5):715–718. doi: 10.1016/s0010-9452(08)70622-1. [DOI] [PubMed] [Google Scholar]
- Avila C., González J., Parcet M.-A., Belloch V. Selective alteration of native, but not second language articulation in a patient with foreign accent syndrome. NeuroReport. 2004;15(14):2267–2270. doi: 10.1097/00001756-200410050-00025. [DOI] [PubMed] [Google Scholar]
- Marien P., Verhoeven J., Engelborghs S., Rooker S., Pickut B.A., De Deyn P.P. A role for the cerebellum in motor speech planning: evidence from foreign accent syndrome. Clin. Neurol. Neurosurg. 2006;108:518–522. doi: 10.1016/j.clineuro.2005.06.006. [DOI] [PubMed] [Google Scholar]
- Scott Sophie K., Clegg Frances, Rudge Peter, Burgess Paul. Foreign accent syndrome, speech rhythm and the functional neuronatomy of speech production. J. Neurolinguist. 2006;19(5):370–384. [Google Scholar]
- Verhoeven Jo, Mariën Peter. Neurogenic foreign accent syndrome: articulatory setting, segments and prosody in a Dutch speaker. J. Neurolinguist. 2010;23(6):599–614. [Google Scholar]
- Akhlaghi, A., Jahangiri, N., Azarpazhooh, M., Elyasi, M., 2011. Foreign accent syndrome: neurolinguistic description of a new case. In: 2011 International Conference on language, literature and linguistics (ICLLL 2011).
- Bhandari HS. Transient foreign accent syndrome. BMJ Case Rep 2011;2011. [DOI] [PMC free article] [PubMed]
- van der Scheer Fennetta, Jonkers Roel, Gilbers Dicky. Foreign accent syndrome and force of articulation. Aphasiology. 2014;28(4):471–489. [Google Scholar]
- Moreno-Torres Ignacio, Berthier Marcelo L., Mar Cid Maria del, Green Cristina, Gutiérrez Antonio, García-Casares Natalia, Froudist Walsh Seán, Nabrozidis Alejandro, Sidorova Julia, Dávila Guadalupe, Carnero-Pardo Cristóbal. Foreign accent syndrome: a multimodal evaluation in the search of neuroscience-driven treatments. Neuropsychologia. 2013;51(3):520–537. doi: 10.1016/j.neuropsychologia.2012.11.010. [DOI] [PubMed] [Google Scholar]
- Tomasino Barbara, Marin Dario, Maieron Marta, Ius Tamara, Budai Riccardo, Fabbro Franco, Skrap Miran. Foreign accent syndrome: a multimodal mapping study. Cortex. 2013;49(1):18–39. doi: 10.1016/j.cortex.2011.10.007. [DOI] [PubMed] [Google Scholar]
- Buckner RL, Roffman JL, Smoller JW. Brain Genomics Superstruct Project (GSP) [online]. Available at: http://dx.doi.org/10.7910/DVN/25833.
- Yeo B.T., Krienen F.M., Sepulcre J. The organization of the human cerebral cortex estimated by intrinsic functional connectivity. J. Neurophysiol. 2011;106:1125–1165. doi: 10.1152/jn.00338.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox M.D., Snyder A.Z., Vincent J.L., Corbetta M., Van Essen D.C., Raichle M.E. The human brain is intrinsically organized into dynamic, anticorrelated functional networks. PNAS. 2005;102:9673–9678. doi: 10.1073/pnas.0504136102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Dijk K.R., Hedden T., Venkataraman A., Evans K.C., Lazar S.W., Buckner R.L. Intrinsic functional connectivity as a tool for human connectomics: theory, properties, and optimization. J. Neurophysiol. 2010;103:297–321. doi: 10.1152/jn.00783.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golfinopoulos E., Tourville J.A., Guenther F.H. The integration of large-scale neural network modeling and functional brain imaging in speech motor control. Neuroimage. 2010;52(3):862–874. doi: 10.1016/j.neuroimage.2009.10.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown S., Ngan E., Liotti M. A larynx area in the human motor cortex. Cereb. Cortex. 2008;18(4):837–845. doi: 10.1093/cercor/bhm131. [DOI] [PubMed] [Google Scholar]
- Zhang Zhaoyan. Mechanics of human voice production and control. J. Acoust. Soc. Am. 2016;140(4):2614–2635. doi: 10.1121/1.4964509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seikel J.A., King D.W., Drumright D.G. 4th ed. Cengage Learning; 2009. Anatomy & Physiology for Speech, Language, and Hearing. [Google Scholar]
- Berthier Marcelo L., Dávila Guadalupe, Moreno-Torres Ignacio, Beltrán-Corbellini Álvaro, Santana-Moreno Daniel, Roé-Vellvé Núria, Thurnhofer-Hemsi Karl, Torres-Prioris María José, Massone María Ignacia, Ruiz-Cruces Rafael. Loss of regional accent after damage to the speech production network. Front. Hum. Neurosci. 2015;9 doi: 10.3389/fnhum.2015.00610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baldo Juliana V., Wilkins David P., Ogar Jennifer, Willock Sharon, Dronkers Nina F. Role of the precentral gyrus of the insula in complex articulation. Cortex. 2011;47(7):800–807. doi: 10.1016/j.cortex.2010.07.001. [DOI] [PubMed] [Google Scholar]
- Dronkers Nina F. A new brain region for coordinating speech articulation. Nature. 1996;384(6605):159–161. doi: 10.1038/384159a0. [DOI] [PubMed] [Google Scholar]
- Hillis A.E., Work M., Barker P.B., Jacobs M.A., Breese E.L., Maurer K. Re-examining the brain regions crucial for orchestrating speech articulation. Brain. 2004;127(7):1479–1487. doi: 10.1093/brain/awh172. [DOI] [PubMed] [Google Scholar]
- Ogar Jennifer, Willock Sharon, Baldo Juliana, Wilkins David, Ludy Carl, Dronkers Nina. Clinical and anatomical correlates of apraxia of speech. Brain Lang. 2006;97(3):343–350. doi: 10.1016/j.bandl.2006.01.008. [DOI] [PubMed] [Google Scholar]
- Graff-Radford Jonathan, Jones David T., Strand Edythe A., Rabinstein Alejandro A., Duffy Joseph R., Josephs Keith A. The neuroanatomy of pure apraxia of speech in stroke. Brain Lang. 2014;129:43–46. doi: 10.1016/j.bandl.2014.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whiteside S.P., Varley R.A. A reconceptualisation of apraxia of speech: a synthesis of evidence. Cortex. 1998;34(2):221–231. doi: 10.1016/s0010-9452(08)70749-4. [DOI] [PubMed] [Google Scholar]
- Katz William F., Garst Diane M., Levitt June. The role of prosody in a case of foreign accent syndrome (FAS) Clin. Linguist. Phon. 2008;22(7):537–566. doi: 10.1080/02699200802106284. [DOI] [PubMed] [Google Scholar]
- Laures‐Gore Jacqueline, Henson Janice Contado, Weismer Gary, Rambow Mary. Two cases of foreign accent syndrome: An acoustic-phonetic description. Clin. Linguist. Phon. 2006;20(10):781–790. doi: 10.1080/02699200500391105. [DOI] [PubMed] [Google Scholar]
- Ackermann Hermann, Vogel Matthias, Petersen Dirk, Poremba Michael. Speech deficits in ischaemic cerebellar lesions. J. Neurol. 1992;239(4):223–227. doi: 10.1007/BF00839144. [DOI] [PubMed] [Google Scholar]
- Nachev Parashkev, Kennard Christopher, Husain Masud. Functional role of the supplementary and pre-supplementary motor areas. Nat. Rev. Neurosci. 2008;9(11):856–869. doi: 10.1038/nrn2478. [DOI] [PubMed] [Google Scholar]
- Boccardi Edoardo, Sala Sergio Della, Motto Cristina, Spinnler Hans. Utilisation behaviour consequent to bilateral SMA softening. Cortex. 2002;38(3):289–308. doi: 10.1016/s0010-9452(08)70661-0. [DOI] [PubMed] [Google Scholar]
- Feinberg T.E., Schindler R.J., Flanagan N.G., Haber L.D. Two alien hand syndromes. Neurology. 1992;42:19–24. doi: 10.1212/wnl.42.1.19. [DOI] [PubMed] [Google Scholar]
- Laplane D., Talairach J., Meininger V., Bancaud J., Orgogozo J.M. Clinical consequences of corticectomies involving the supplementary motor area in man. J. Neurol. Sci. 1977;34(3):301–314. doi: 10.1016/0022-510x(77)90148-4. [DOI] [PubMed] [Google Scholar]
- Krainik A., Lehericy S., Duffau H., Vlaicu M., Poupon F., Capelle L., Cornu P., Clemenceau S., Sahel M., Valery C.-A., Boch A.-L., Mangin J.-F., Le Bihan D., Marsault C. Role of the supplementary motor area in motor deficit following medial frontal lobe surgery. Neurology. 2001;57(5):871–878. doi: 10.1212/wnl.57.5.871. [DOI] [PubMed] [Google Scholar]
- Masdeu J.C., Schoene W.C., Funkenstein H. Aphasia following infarction of the left supplementary motor area: a clinicopathologic study. Neurology. 1978;28:1220–1223. doi: 10.1212/wnl.28.12.1220. [DOI] [PubMed] [Google Scholar]
- Alexander M.P., Schmitt M.A. The aphasia syndrome of stroke in the left anterior cerebral artery territory. Arch. Neurol. 1980;37(2):97–100. doi: 10.1001/archneur.1980.00500510055010. [DOI] [PubMed] [Google Scholar]
- Pai Ming-Chyi. Supplementary motor area aphasia: a case report. Clin. Neurol. Neurosurg. 1999;101(1):29–32. doi: 10.1016/s0303-8467(98)00068-7. [DOI] [PubMed] [Google Scholar]
- Penfield W., Rasmussen T. Macmillan; New York: 1950. National Institute on Drug A. The cerebral cortex of man : a clinical study of localization of function. [Google Scholar]
- Catani Marco, Dell’Acqua Flavio, Vergani Francesco, Malik Farah, Hodge Harry, Roy Prasun, Valabregue Romain, Thiebaut de Schotten Michel. Short frontal lobe connections of the human brain. Cortex. 2012;48(2):273–291. doi: 10.1016/j.cortex.2011.12.001. [DOI] [PubMed] [Google Scholar]
- Catani M., Mesulam M.M., Jakobsen E. A novel frontal pathway underlies verbal fluency in primary progressive aphasia. Brain. 2013;136:2619–2628. doi: 10.1093/brain/awt163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kronfeld-Duenias Vered, Amir Ofer, Ezrati-Vinacour Ruth, Civier Oren, Ben-Shachar Michal. The frontal aslant tract underlies speech fluency in persistent developmental stuttering. Brain Struct. Funct. 2016;221(1):365–381. doi: 10.1007/s00429-014-0912-8. [DOI] [PubMed] [Google Scholar]
- Callan Daniel, Kawato Mitsuo, Parsons Lawrence, Turner Robert. Speech and song: the role of the cerebellum. Cerebellum. 2007;6(4):321–327. doi: 10.1080/14734220601187733. [DOI] [PubMed] [Google Scholar]
- Marien P., Ackermann H., Adamaszek M. Consensus paper: Language and the cerebellum: an ongoing enigma. Cerebellum. 2014;13:386–410. doi: 10.1007/s12311-013-0540-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thürling M., Küper M., Stefanescu R., Maderwald S., Gizewski E.R., Ladd M.E., Timmann D. Activation of the dentate nucleus in a verb generation task: a 7T MRI study. Neuroimage. 2011;57(3):1184–1191. doi: 10.1016/j.neuroimage.2011.05.045. [DOI] [PubMed] [Google Scholar]
- Urban Peter Paul, Marx Juergen, Hunsche Stefan, Gawehn Joachim, Vucurevic Goran, Wicht Susanne, Massinger Claudia, Stoeter Peter, Hopf Hanns Christian. Cerebellar speech representation: lesion topography in dysarthria as derived from cerebellar ischemia and functional magnetic resonance imaging. Arch. Neurol. 2003;60(7):965. doi: 10.1001/archneur.60.7.965. [DOI] [PubMed] [Google Scholar]
- Ackermann Hermann, Mathiak Klaus, Ivry Richard B. Temporal organization of “internal speech” as a basis for cerebellar modulation of cognitive functions. Behav. Cogn. Neurosci. Rev. 2004;3(1):14–22. doi: 10.1177/1534582304263251. [DOI] [PubMed] [Google Scholar]
- Tian Xing, Zarate Jean Mary, Poeppel David. Mental imagery of speech implicates two mechanisms of perceptual reactivation. Cortex. 2016;77:1–12. doi: 10.1016/j.cortex.2016.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buxton Richard B. The physics of functional magnetic resonance imaging (fMRI) Rep. Prog. Phys. 2013;76(9):096601. doi: 10.1088/0034-4885/76/9/096601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Logothetis Nikos K., Pauls Jon, Augath Mark, Trinath Torsten, Oeltermann Axel. Neurophysiological investigation of the basis of the fMRI signal. Nature. 2001;412(6843):150–157. doi: 10.1038/35084005. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and analyzed during the present study are available from the corresponding author on reasonable request and with approval of our ethics committee.