Abstract
Pathogenesis of endometriosis is still unclear and a role of both innate and adaptive immune system has been postulated. Some recent findings have revealed an increased risk to have concomitant autoimmune disease in women with endometriosis, but no study so far has investigated whether this association could affect endometriosis severity and stage. We retrospectively reviewed medical patients’ notes of women with a confirmed diagnosis of endometriosis who referred to our endometriosis outpatient clinic between January 2015 and December 2019. Cases (endometriosis and an autoimmune disease) were matched in a 1:3 ratio by age and study period with controls (endometriosis without history of autoimmunity). At univariate logistic analysis, concomitant autoimmunity (OR 2.63, 95% CI 1.64–4.21, p < 0.001) and the number of laparoscopic procedures performed (OR 2.81, 95% CI 1.45–5.43, p = 0.002) emerged as factors significantly associated with the likelihood of stage IV endometriosis. In the multivariate logistic regression model, concomitant autoimmunity remained a significant predictor of stage IV endometriosis (OR 2.54, 95% CI 1.57–4.10, p = 0.004), whereas the association between the number of laparoscopic procedures performed and stage IV endometriosis was found to be of borderline-significance (OR 2.70, 95% 1.37–5.30, p = 0.050). Our findings suggest that endometriosis is more severe in patients who are also affected by autoimmune disturbances after controlling for relevant confounders.
Subject terms: Adaptive immunity, Autoimmunity, Innate immunity
Introduction
Endometriosis, traditionally defined as the presence of endometrial-like tissue outside the uterine cavity, is a chronic gynaecological disease which affects 2–22% of reproductive age women1. It can be asymptomatic or associated with infertility, chronic pelvic pain and dyspareunia2. Although its pathogenesis has not been completely clarified, endometriosis is known to be a hormone dependent chronic inflammatory disease3 characterized by activation of both innate and adaptive immune system4,5. Indeed, researches made during the last two decades have found plenty of immunological abnormalities. An increased production of pro-inflammatory cytokines/chemokines, a higher concentration of peritoneal macrophages4, alterations in B cell activation, and immunological abnormalities in T/B cell function6 are only few examples of this immunological dysfunction. Also, some genes involved in the immune response were found to be differently expressed in peripheral leukocytes of women with endometriosis (stage III-IV) similarly to other non-gynaecologic and chronic inflammatory conditions7.
A recent systematic review and meta-analysis by Shigesi and coworkers8 has found an increased risk of comorbidity of autoimmune diseases including systemic lupus erythematosus (SLE), Sjögren’s syndrome (SS), rheumatoid arthritis (RA), autoimmune thyroid disorders (ATD), celiac disease (CLD), multiple sclerosis (MS), inflammatory bowel disease (IBD), and Addison’s disease in women with endometriosis, despite a high risk of bias related to the low quality of included studies exists and was reported by the authors. Indeed, endometriosis seems to share features characteristic of autoimmune diseases such as an increased presence of auto-antibodies9 Serum of endometriosis patients contains a high level of anti-macrophage colony stimulating factor antibodies (anti-GM-CSF Ab) which correlates with severity and number of lesions10. GM-CSF, which is a hematopoietic growth factor produced by many different cells including endometrial cells, plays a crucial role in linking innate and adaptive immunity10,11.
Some of genetic polymorphisms in the autoimmunity genes have also been investigated in endometriosis, although with inconsistent results12. These findings have led to the idea of endometriosis as a unique immunological scenario outlining the plausible association between endometriosis and immunity/autoimmunity6. Nonetheless, no study has so far investigated whether the observed association between endometriosis and autoimmunity might be related to the severity of endometriosis.
To shed more light into the possible link between endometriosis and autoimmunity, we designed a matched case–control study to investigate whether the concomitant presence of autoimmune diseases is associated with different stages of endometriosis.
Results
Baseline characteristics of the whole cohort of women included is shown in Table 1.
Table 1.
Baseline characteristics of patients (n = 384).
| Baseline characteristics | |
|---|---|
| Age at evaluation (years) | 38.8 ± 6.1 |
| Age at diagnostic LS (years) | 30.9 ± 5.6 |
| Age at symptoms onset (years) | 17 [13–25] |
| Time from LS (years) | 7.9 ± 5.7 |
| Number of surgical procedures | 1 [1–1] |
| Number of clinical evaluations | 2 [1–2] |
| r-AFS stage | |
| I | 12 (3.1%) |
| II | 37 (9.7%) |
| III | 182 (47.5%) |
| IV | 152 (39.7%) |
| Type of endometriosis | |
| OMA | 268 (69.9%) |
| SPE | 220 (57.4%) |
| DIE | 140 (36.6%) |
| Hormone therapy | |
| Any | 180 (47%) |
| None | 124 (32.2%) |
| Not known | 80 (20.8%) |
Values are mean ± SD or median [25%-75%] or n (%).
LS, laparoscopic surgery; r-AFS, revised American Fertility Society; OMA, ovarian endometrioma; SPE, superficial peritoneal endometriosis; DIE, deep infiltrating endometriosis.
Comparison between women with endometriosis and concomitant autoimmune disease (cases, n = 96) and women with endometriosis and negative autoimmune status (controls, n = 268) is shown in Table 2, where also the specific autoimmune diseases found in our population are shown.
Table 2.
Baseline characteristics of patients according with the autoimmunity status (n1 = 96; n2 = 288).
| Baseline characteristics | Cases (n1 = 96) |
Controls (n2 = 288) |
p-value |
|---|---|---|---|
| Age at evaluation (years) | 38.7 ± 6.1 | 38.7 ± 6.1 | 0.987 |
| Age at diagnostic LS (years) | 31.1 ± 6.2 | 30.1 ± 5.4 | 0.761 |
| Age at symptoms onset (years) | 16 [14–20] | 18 [13–25] | 0.960 |
| Time from LS (years) | 7.7 ± 5.9 | 8.0 ± 5.7 | 0.703 |
| Number of surgical procedures | 1 [1–1] | 1 [1–1] | 0.166 |
| Number of clinical evaluations | 2 [2–3] | 2 [1–2] | 0.0001 |
| r-AFS stage | |||
| I | 2 (2.1%) | 10 (3.5%) | 0.495 |
| II | 7 (7.3%) | 30 (10.5%) | 0.364 |
| III | 32 (33.3%) | 150 (52.3%) | 0.001 |
| IV | 55 (57.3%) | 97 (25.3%) | 0.000 |
| Type of endometriosis | |||
| OMA | 75 (78.1%) | 193 (67.2%) | 0.096 |
| SPE | 45 (46.9%) | 175 (61%) | 0.016 |
| DIE | 43 (44.8%) | 97 (33.8%) | 0.053 |
| Hormone therapy | |||
| Any | 43 (44.8%) | 137 (47.7%) | 0.617 |
| None | 32 (33.3%) | 92 (32.1%) | 0.817 |
| Not known | 21 (21.9%) | 58 (20.2%) | 0.727 |
| Concomitant autoimmune disease | 0.000 | ||
| None | 287 (100%) | ||
| ATD | 57 (59.4%) | ||
| T1D | 10 (10.4%) | ||
| IBD | 9 (9.4%) | ||
| CLD | 1 (1%) | ||
| LES/APS | 5 (5.2%) | ||
| RA | 6 (6.3%) | ||
| Fibromyalgia | 2 (2.1%) | ||
| SS | 1 (1%) | ||
| MS | 4 (4.2%) | ||
| Psoriasis | 1 (1%) | ||
| Multiple | 15 (15.6%) | ||
Values are mean ± SD or median [IQR] or n (%).
Bold values denote statistical significance at the p < 0.05 level.
LS, laparoscopic surgery; r-AFS, revised American Fertility Society; OMA, ovarian endometrioma; SPE, superficial peritoneal endometriosis; DIE, deep infiltrating endometriosis; ATD, autoimmune thyroid disorders; T1D, type 1 diabetes; IBD, inflammatory bowel diseases; CLD, coeliac disease; SLE, systemic lupus erythematosus; APS, antiphospholipid syndrome, RA, rheumatoid arthritis; SS, systemic sclerosis; MS, multiple sclerosis.
The two groups were comparable in terms of age at laparoscopy (31.1 ± 6.2 versus 30.1 ± 5.4 in cases and controls respectively, p = 0.761) and time interval between laparoscopy and inclusion in the study (7.7 ± 5.9 versus 8.0 ± 5.7 years in cases and controls respectively, p = 0.703).The proportion of women using hormonal treatment for endometriosis at the time of inclusion in the study was also comparable between the two groups (44.8% versus 47.7% in cases and controls respectively, p = 0.617). Compared to women without autoimmunity, women with concomitant autoimmune disease had a higher mean ASRM score (51 versus 39, p = 0.001, Fig. 1) and presented more often with stage IV endometriosis (25.3% versus 57.3%, p < 0.001) and less frequently with stage III disease (33.3% vs 52.3%, p = 0.001).
Figure 1.
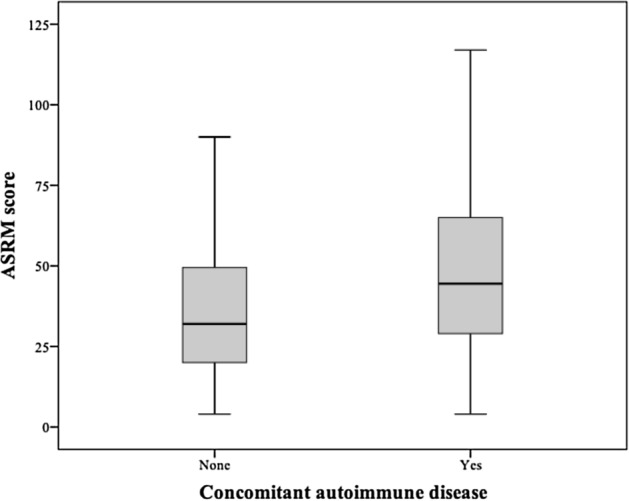
Boxplots of ASRM score according with autoimmunity status. We compared mean ASRM score between women without autoimmunity and women with concomitant autoimmune disease. ASRM score, American Society for Reproductive Medicine.
At univariate logistic analysis, concomitant autoimmunity (OR 2.63, 95% CI 1.64–4.21, p < 0.001) and the number of laparoscopic procedures performed (OR 2.81, 95% CI 1.45–5.43, p 0.002) emerged as factors significantly associated with the likelihood of stage IV endometriosis (Table 3).
Table 3.
Logistic regression analysis of independent risk factors for stage IV of endometriosis disease.
| Parameters | Univariate logistic regression | Multiple logistic regression | ||||
|---|---|---|---|---|---|---|
| Odds ratio | 95% CIa | p-value | Odds ratio | 95% CIa | p-value | |
| Age at evaluation (years) | 1.01 | 0.97–1.04 | 0.65 | |||
| Age at diagnostic LS (years) | 0.97 | 0.94–1.01 | 0.23 | |||
| Age at symptoms onset (years) | 0.97 | 0.94–1.01 | 0.19 | |||
| Time from LS (years) | 1.01 | 0.97–1.05 | 0.48 | |||
| Number of LS procedures* | 2.81 | 1.45–5.43 | 0.002 | 2.70 | 1.37–5.30 | 0.050 |
| Number of clinical evaluations | 0.91 | 0.79–1.05 | 0.20 | |||
| Autoimmunity* | 2.63 | 1.64–4.21 | 0.000 | 2.54 | 1.57–4.10 | 0.004 |
| Hormone therapy | 1.26 | 0.95–1.67 | 0.11 | |||
LS, laparoscopic surgery.
*Variables included in the multiple logistic regression analysis.
Bold values denote statistical significance at the p ≤ 0.05 level.
a95% confidence intervals.
In the multivariate logistic regression model, concomitant autoimmunity remained a significant predictor of stage IV endometriosis (OR 2.54, 95% CI 1.57–4.10, p = 0.004), whereas the association between the number of laparoscopic procedures performed and the diagnosis of stage IV endometriosis was found to be borderline-significant (OR 2.70, 95% 1.37–5.30, p = 0.050, Table 3).
Discussion
To the best of our knowledge, our study has been the first that investigated whether the presence of concomitant autoimmunity is associated with advanced stage of disease in women with endometriosis, and suggests that endometriosis is more aggressive in patients who are also affected by autoimmune disturbances after controlling for relevant confounders. The coexistence of endometriosis and autoimmunity is a well-known occurrence. Based on previous studies and meta-analyses, women with endometriosis are at higher risk for autoimmune diseases including SLE, SS, RA, CD, MS, or IBD compared to healthy controls8. Nonetheless, none of the previous studies have addressed whether the coexistence of the two conditions is associated with a more severe endometriosis. Our results are thus the first to suggest that comorbid autoimmune conditions in patients with endometriosis might be considered as a risk factor for stage IV disease.
While our novel results might outline an interplay between endometriosis progression and autoimmunity, causal relationships cannot be inferred and conclusions about whether more severe endometriosis is a consequence of concomitant autoimmunity cannot be drawn. Common pathological mechanisms such as macrophage dysfunction and impaired clearance of apoptotic cells13 might underlie the two conditions, or systemic and local tissue damage resulting from autoimmune diseases might have untoward effects on endometriosis progression. Nonetheless, our results might have several clinical implications: on one hand, closer surveillance or different treatment strategies for endometriosis might be needed in patients with concomitant autoimmunity. The diagnosis of a concomitant autoimmune disease might for example indicate that the patient is at higher risk for fibrotic progression—a common and highly pathogenic feature of both stage IV endometriosis14,15 and autoimmune diseases. Fibrosis prevention is a putative target for early immune-modulatory therapies16. Thus, the identification of concomitant autoimmune disease as an early risk factor for fibrotic progression in patients with endometriosis would be of outmost clinical importance. On the other hand, if our results were confirmed by larger studies, screening for autoimmunity might become indicated in patients with endometriosis and targeted studies focused on immune-modulatory therapies in this subgroup of patients might become valuable.
Our study also presents some limitations. First, its retrospective nature. While only women with a both surgical and histological diagnosis of endometriosis were included, medical patients’ notes regarding concomitant autoimmune diseases severity and stage were less detailed and for i.e. did not comprise information about age of onset. In addition, our study has a moderate sample size in this setting (100–1000 patients8) and confirmation of our novel results in investigations with a large sample size is needed.
Conclusions
Our findings suggest that the presence of concomitant autoimmune disease in patients with endometriosis might be considered as a risk factor for stage IV disease severity.
Methods
This was a retrospective case–control study carried out at the department of Obstetrics and Gynecology, IRCCS San Raffaele Scientific Institute, Milan. We reviewed medical notes of all women of reproductive age and surgically/histopathologically confirmed diagnosis of endometriosis who referred to our endometriosis clinic from January 2015 to February 2019. Among them, we selected women with concomitant autoimmunity (cases), whose presence was assessed by medical interview at the time of visit. Whenever a patient self-reported a diagnosis of autoimmune disease, the presence of such condition was further investigated by retrieving previous blood tests for auto-antibodies or previous rheumatological records. We considered any of the following autoimmune diseases: autoimmune thyroiditis, celiac disease (CD), systemic lupus erythematous (SLE), inflammatory bowel disease (IBD), multiple sclerosis (MS), systemic sclerosis (SS), sjogren syndrome, rheumatoid arthritis (RA), Type 1 diabetes mellitus (T1D), Addison’s disease (AD), Behcet syndrome (BD), antiphospholipid syndrome, autoimmune hepatitis, vasculitis, polymyositis/dermatomyositis and myasthenia gravis. Cases were matched to controls in a 1:3 ratio by age and study period (the following 3 age-matched women with endometriosis and without history of autoimmune diseases).
For all included patients, we recorded age, medical present/past history, previous pelvic surgery, hormonal therapy at the time of inclusion in the study, smoking status, number of visits, time/type of surgery performed, histopathological diagnosis of endometriosis. Endometriotic lesions were classified according to their phenotype as ovarian endometrioma (OMA), deep infiltrating endometriosis (DIE) and superficial peritoneal endometriosis (SPE)17. Stage was assigned by medical surgery reports according to the revised American Society for Reproductive Medicine (ASRM)18,19 classification into minimal-mild endometriosis (stage 1–2) and moderate-severe endometriosis (stage 3–4). All participating patients gave an informed consent for their anonymized data to be used for research purposes (EndoGWA1, approved on 14th October 2014 by the Ethical Committee of the Ospedale San Raffaele in Milan, Istituto di Ricovero e Cura a Carattere Scientifico). Data collection followed the principles outlined in the Declaration of Helsinki.
Statistical analysis
Data were analyzed using IBM SPSS Statistics (Version 24.0, Chicago, IL, USA). A p-value < 0.05 was considered to be statistically significant. A Shapiro–Wilk test was used to ascertain whether continuous variables had normal distribution. Assumption of homogeneity of variances was tested and satisfied based on Levene’s test when appropriate. Continuous and normally distributed variables were presented as mean ± standard deviation (SD), while continuous not normally distributed variables were expressed as median [25th–75th percentile] and categorical variables were presented as absolute values and percentages (%). The patients’ characteristics were compared between the group with a concomitant autoimmune disease (cases) and the group with a negative autoimmune status (controls) by use of a Person χ2 test or a Fisher’s exact test for qualitative variables and an independent Student’s t-test or a Mann–Whitney U test for quantitative variables as appropriate.
A logistic regression analysis was performed to determine the variables that could be independently associated with the presence of stage IV disease in endometriosis patients. Confounding factors were determined to be statistically significant at the threshold of p ≤ 0.05 by univariate analysis and were tested in a multiple logistic regression model. Odds ratios (OR) and their 95% confidence intervals (95% CI) were reported.
Author contributions
Conceptualization, V.S.V.; Methodology, V.S.V.; Software, N.S.; Validation, V.S.V., P.V., E.P., F.S., J.O. and M.C.; Formal analysis, N.S. and V.S.V.; Investigation, V.S.V. and R.V.; Resources, R.V. and N.S.; Data curation, R.V. and N.S.; Writing—original draft preparation, R.V., N.S. and V.S.V.; Writing—review and editing, V.S.V., P.V., E.P., F.S., J.O., D.P., R.Q.P and M.C; Visualization, V.S.V., P.V., E.P., F.S., J.O., D.P., R.Q.P. and M.C; Supervision, V.S.V., P.V. and M.C.; Project administration, V.S.V. All authors have read and agreed to the published version of the manuscript.
Data availability
The datasets generated during and/or analysed during the current study are available from the corresponding author on reasonable request.
Competing interests
The authors declare no competing interests.
Footnotes
The original online version of this Article was revised: The original version of this Article contained errors in the spelling of the authors Valeria Stella Vanni, Roberta Villanacci, Noemi Salmeri, Enrico Papaleo, Diana Delprato, Jessica Ottolina, Patrizia Rovere-Querini, Stefano Ferrari, Paola Viganò & Massimo Candiani which were incorrectly given as Vanni Valeria Stella, Villanacci Roberta, Salmeri Noemi, Papaleo Enrico, Delprato Diana, Ottolina Jessica, Rovere-Querini Patrizia, Ferrari Stefano, Viganò Paola & Candiani Massimo.
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Change history
8/31/2021
A Correction to this paper has been published: 10.1038/s41598-021-97506-x
References
- 1.Guo SY, Wang Y. Sources of heterogeneities in estimating the prevalence of endometriosis in infertile and previously fertile women. Fertil. Steril. 2006;86:1584–1595. doi: 10.1016/j.fertnstert.2006.04.040. [DOI] [PubMed] [Google Scholar]
- 2.Giudice LC, Kao L. Endometriosis. Lancet. 2004;364:1789–1799. doi: 10.1016/S0140-6736(04)17403-5. [DOI] [PubMed] [Google Scholar]
- 3.Giudice LC. Clinical practice endometriosis. N. Engl. J. Med. 2010;362:2389–2398. doi: 10.1056/NEJMcp1000274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Eisenberg VH, Zolti M, Soriano D. Is there an association between autoimmunity and endometriosis? Autoimmun. Rev. 2012;11:806–814. doi: 10.1016/j.autrev.2012.01.005. [DOI] [PubMed] [Google Scholar]
- 5.Riccio LGC, Baracat EC, Chapron C, Batteux F, Abrão MS. The role of the B lymphocytes in endometriosis: A systematic review. J. Reprod. Immunol. 2017;123:29–34. doi: 10.1016/j.jri.2017.09.001. [DOI] [PubMed] [Google Scholar]
- 6.Zhang T, De Carolis C, Man GCW, Wang CC. The link between immunity, autoimmunity and endometriosis: A literature update. Autoimmun. Rev. 2018;17:945–955. doi: 10.1016/j.autrev.2018.03.017. [DOI] [PubMed] [Google Scholar]
- 7.Gentilini D, et al. Gene expression profiling of peripheral blood mononuclear cells in endometriosis identifies genes altered in non-gynaecologic chronic inflammatory diseases. Hum. Reprod. 2011;26:3109–3117. doi: 10.1093/humrep/der270. [DOI] [PubMed] [Google Scholar]
- 8.Shigesi N, et al. The association between endometriosis and autoimmune diseases: A systematic review and meta-analysis. Hum. Reprod. Update. 2019;25:486–503. doi: 10.1093/humupd/dmz014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lebovic DI, Mueller MD, Taylor RN. Immunobiology of endometriosis. Fertil. Steril. 2001;75:1–10. doi: 10.1016/S0015-0282(00)01630-7. [DOI] [PubMed] [Google Scholar]
- 10.Toullec L, et al. High levels of Anti-GM-CSF antibodies in deep infiltrating endometriosis. Reprod. Sci. 2020;27:211–217. doi: 10.1007/s43032-019-00021-8. [DOI] [PubMed] [Google Scholar]
- 11.Shi Y, et al. Granulocyte-macrophage colony-stimulating factor (GM-CSF) and T-cell responses: What we do and don’t know. Cell Res. 2006;16:126–133. doi: 10.1038/sj.cr.7310017. [DOI] [PubMed] [Google Scholar]
- 12.Bianco B, et al. The possible role of genetic variants in autoimmune-related genes in the development of endometriosis. Hum. Immunol. 2012;73:306–315. doi: 10.1016/j.humimm.2011.12.009. [DOI] [PubMed] [Google Scholar]
- 13.Capobianco A, Rovere-Querini P. Endometriosis, a disease of the macrophage. Front. Immunol. 2013;4:9. doi: 10.3389/fimmu.2013.00009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Vigano P, Candiani M, Monno A, Giacomini E, Vercellini P, Somigliana E. Time to redefine endometriosis including its pro-fibrotic nature. Hum. Reprod. 2018;33:347–352. doi: 10.1093/humrep/dex354. [DOI] [PubMed] [Google Scholar]
- 15.Viganò P, et al. Cellular components contributing to fibrosis in endometriosis: A literature review. J. Minim. Invasive Gynecol. 2020;27:287–295. doi: 10.1016/j.jmig.2019.11.011. [DOI] [PubMed] [Google Scholar]
- 16.Taskin MI, Gungor AC, Adali E, Yay A, Onder GO, Inceboz U. A humanized anti-interleukin 6 receptor monoclonal antibody, tocilizumab, for the treatment of endometriosis in a rat model. Reprod. Sci. 2016;23:662–669. doi: 10.1177/1933719115612134. [DOI] [PubMed] [Google Scholar]
- 17.Nisolle M, Donnez J. Peritoneal endometriosis, ovarian endometriosis, and adenomyotic nodules of the rectovaginal septum are three different entities. Fertil. Steril. 1997;68:585–596. doi: 10.1016/S0015-0282(97)00191-X. [DOI] [PubMed] [Google Scholar]
- 18.Guzick DS, Silliman NP, Adamson GD, Buttram VC, Jr, Canis M, Malinak LR, Schenken RS. Revised American Society for Reproductive Medicine classification of endometriosis: 1996. Fertil. Steril. 1997;67:817–821. doi: 10.1016/S0015-0282(97)81392-1. [DOI] [PubMed] [Google Scholar]
- 19.Johnson NP, et al. World Endometriosis Society Sao Paulo Consortium. World Endometriosis Society consensus on the classification of endometriosis. Hum. Reprod. 2017;32:315–324. doi: 10.1093/humrep/dew293. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets generated during and/or analysed during the current study are available from the corresponding author on reasonable request.


