Abstract
Objective
Since the approval of pembrolizumab for advanced or recurrent PD-L1 positive (CPS > 1%) cervical cancer, the clinical characteristics associated with response have remained undefined. We sought to characterize the clinicopathologic features of patients with advanced cervical cancer at our institution who derived durable clinical benefit from treatment with pembrolizumab.
Methods
We conducted a retrospective cohort study of 14 patients with recurrent or metastatic cervical cancer who received pembrolizumab monotherapy from August 2017 to November 2019 and were followed until November 1, 2020. Reviewed clinical data included age, histology, tumor molecular profiling results, stage at diagnosis, treatment history, baseline pattern of metastatic disease at initiation of anti-PD-1 therapy, and outcomes. Treatment response was evaluated by computed tomography using RECIST v1.1 criteria.
Results
The objective response rate was 21% (n = 3), including two partial responses and one complete response. Two patients (14%) had stable disease of six months or greater, for an observed durable clinical benefit rate of 36%. When stratified by those who derived clinical benefit, metastatic spread to lung and/or lymph node only at baseline was associated with improved response to pembrolizumab (n = 7, p = 0.02) and associated with significantly improved PFS and OS. Tumor mutational burden was higher in those with durable clinical benefit compared to non-responders (median 12.7 vs. 3.5 mutations/megabase, p = 0.03).
Conclusions
Our findings highlight clinical features that may select for a population most likely to benefit from pembrolizumab monotherapy and underscores the need for identification of additional biomarkers of response.
Keywords: Immune checkpoint blockade, Cervical cancer, PD-1 resistance, Tumor microenvironment
1. Introduction
Over the last decade, while patients with early-stage cervical cancer have benefited from impressive strides made in prevention, detection, and treatment, prognosis remains poor for those that present with advanced or recurrent disease. Chemoradiation is the standard of care for management of locally advanced disease, but even with its demonstrated benefit, 4-year overall survival barely exceeds 50% (Rose et al., 1999, Vale et al., 2006). Those patients that present with recurrent or metastatic disease fare far worse, with a median survival of only 17 months (Pfaendler and Tewari, 2016, Tewari et al., 2014). Treatment response rates in this setting are disappointing and range from 13% with single-agent cisplatin to 48% with platinum or topotecan plus taxane-based therapy plus bevacizumab (Tewari et al., 2014, Moore et al., 2004, Monk et al., 2009, Long et al., 2005, Tewari et al., 2017).
Immunotherapy has emerged as a promising strategy in the effort to address this unmet therapeutic need. Immune checkpoint inhibitors (ICI), designed to counteract immunologic tolerance to cancer cells in the tumor microenvironment, have been developed against cytotoxic T-lymphocyte associated protein 4 (CTLA-4) and the programmed cell death protein 1 (PD-1) (Ferris et al., 2016, Brahmer et al., 2015, Brahmer et al., 2012, Robert et al., 2015). PD-1 is a protein receptor expressed in both the innate and adaptive immune system that when bound to its ligands (PD-L1 and PD-L2), results in down-regulation of cytotoxic T cell activation. PD-L1 expression has been reported in up to 54% of squamous cell carcinomas (SCC) and up to 16.7% of endocervical carcinomas (ECC) (Reddy et al., 2017, Heeren et al., 2016). Given this rate of positivity, as well as the fact that the majority of cervical cancer cases are secondary to human papilloma virus infection (HPV), it was hypothesized that this malignancy may be susceptible to ICI treatment.
Pembrolizumab was developed as a highly selective monoclonal antibody against PD-1. Two studies, the phase Ib KEYNOTE 028 and phase II KEYNOTE 158, confirmed the acceptable safety profile of pembrolizumab and demonstrated promising monotherapy antitumor activity in PD-L1 positive recurrent or metastatic cervical cancer with an objective response rate of 14.6% (Frenel et al., 2017, Chung et al., 2019). Based on these results, pembrolizumab received accelerated approval by the Food and Drug Administration in June 2018 for use in patients with metastatic or recurrent PD-L1 positive cervical cancer who progressed during or following chemotherapy. Since its approval, the clinical characteristics of patients that best respond to this immunotherapy have largely remained undefined. Here, we sought to describe patients with advanced cervical cancer that derived durable clinical benefit from treatment with pembrolizumab at our institution.
2. Materials and Methods
2.1. Patient selection
Patients with recurrent or metastatic cervical cancer who initiated pembrolizumab from August 2017 to November 2019 at Memorial Sloan Kettering Cancer Center (MSKCC) were retrospectively analyzed. Identified patients were followed until November 1, 2020. Patient demographics, treatment history including prior chemotherapy and radiation, baseline pattern of metastatic disease at initiation of anti-PD-1 treatment, and outcomes were recorded. Treatment response was evaluated by computed tomography as assessed by Response Evaluation Criteria in Advanced Solid Tumors (version 1.1; RECIST 1.1) (Eisenhauer et al., 2009). Timing of scans was at physician discretion. Outcomes with pembrolizumab including progression-free survival (PFS) and overall survival (OS) were collected. The study was approved by the Institutional Review Board at MSKCC.
2.2. Statistical analysis
Baseline clinical and disease characteristics were summarized as medians and ranges for continuous variables and as numbers and percentages for categorical variables. Fisher's exact test or Mann-Whitney U test was used for analysis as appropriate. A two-tailed p-value of<0.05 was considered statistically significant. Kaplan-Meier survival analysis was used to determine PFS and OS. Time was calculated from initiation of pembrolizumab to progression per RECIST 1.1 criteria for PFS and from initiation of pembrolizumab to death due to any cause for OS. Durable clinical benefit (DCB) was defined as a best response of complete response (CR), partial response (PR), or stable disease (SD) for 6 months or greater. All statistical analyses were performed using SPSS (version 14.0; SPSS, Inc, Chicago, Ill, USA).
2.3. PD-L1 expression
All PD-L1 IHC at MSKCC was performed using Cell Signaling Technology’s PD-L1 (E1L3N®) XP® Rabbit mAB (n = 11). Two patients whose PD-L1 testing was performed through an outside institution utilized Dako® PD-L1 IHC 22C3 pharmDx.
2.4. Next generation sequencing
Formalin-fixed, paraffin-embedded (FFPE) tissue specimens from either the primary tumor or a metastatic site of twelve patients were utilized for DNA extraction. The samples underwent genomic mutational profiling of 468 mutations using the MSKCC IMPACT™ (Integrated Mutation Profiling of Actionable Cancer Targets) assay as previously described (Cheng et al., 2015). One patient underwent genomic testing through FoundationOne®. One patient did not consent for genetic testing.
3. Results
3.1. Patient demographics
In total, 14 patients with recurrent, previously treated cervical cancer initiated pembrolizumab during the study period. Patient demographics and clinical profiles are described in Table 1. The median age at initiation of therapy was 59 years (range 22–77) and 79% (n = 11) had squamous cell carcinoma (SCC) on histology. The three non-SCC histologies included endocervical adenocarcinoma, high-grade adenocarcinoma with clear cell and endometrioid features, and mesonephric adenocarcinoma. All tested tumors expressed PD-L1 with combined positive score (CPS) ≥ 1, (n = 13); one patient whose tumor was microsatellite unstable (MSI-H) by NGS did not undergo PD-L1 testing. At initiation of therapy, three patients (14%) had lung-only metastases, two (14%) had lymph node-only metastases, two (14%) had a combination of lymph node and lung metastases, five (36%) had multi-site disease (three or more organ systems), one (7%) had liver metastases, and one (7%) had an isolated mesenteric mass.
Table 1.
Patient demographics and clinical profiles (n = 14).
| Characteristic | No. (%) |
|---|---|
| Age at initiation of pembrolizumab | |
| Median age, years (range) | 59 (22–77) |
| Initial FIGO stage | |
| IB | 3 (21%) |
| II | 2 (14%) |
| III | 5 (36%) |
| IV | 4 (29%) |
| PD-L1 status (CPS > 1%) | |
| Positive | 13 (93%) |
| Unknown | 1 (7%) |
| Tumor Mutational Burden (TMB) score | |
| Median, mutations/Mb (range) | 5.3 (1.8–20.2) |
| Site of disease | |
| Lung only | 3 (21%) |
| Lymph node only | 2 (14%) |
| Lymph node + Lung | 2 (14%) |
| Liver | 1 (7%) |
| Multi-site | 5 (36%) |
| Isolated mass | 1 (7%) |
| Histology | |
| Endocervical adenocarcinoma | 1 (7%) |
| Squamous cell carcinoma | 11 (79%) |
| Mixed adenocarcinoma (Clear cell + endometrioid) | 1 (7%) |
| Mesonephric | 1 (7%) |
| Previous radiotherapy | |
| 13 (93%) | |
| Prior lines of metastatic therapy | |
| Cis/RT only | 2 (14%) |
| 1 | 3 (21%) |
| 2 | 7 (50%) |
| 3 | 0 (0%) |
| 4 | 2 (14%) |
3.2. Treatment
All patients received pembrolizumab monotherapy as a 200 mg intravenous infusion every three weeks. Thirteen patients (93%) had received prior radiation. Two patients (14%) received pembrolizumab as first-line therapy after recurrence, three (21%) as second-line, seven (50%) as third-line, and two (14%) as fifth-line. The median number of doses received was 7 (range 3–19) and the median duration of treatment was 4.8 months (range 1.4–15.2). Table 2 provides a summary of treatment duration, best response, and outcomes.
Table 2.
Clinicopathologic, treatment, and response details.
| Patient | Histology | Pattern of Disease | Prior Lines of Therapy for Recurrent Disease | Best Response | Duration of Treatment (Months) | Progression Free Survival (Months) | Overall Survival (Months) | Subsequent therapy |
|---|---|---|---|---|---|---|---|---|
| Non-responders | ||||||||
| 1 | SCC | Multi-site | 1 | PD | 1.35 | 0.53 | 3.29* | None |
| 2 | Endocervical adenocarcinoma w/ endometrioid/ clear cell features | Multi-site | 4 | PD | 1.38 | 1.87 | 11.41 | Paclitaxel/Topotecan |
| 3 | SCC | Multi-site | 2 | PD | 3.45 | 3.65 | 10.55* | Paclitaxel, Topotecan, Gemcitabine |
| 4 | SCC | Mesenteric mass | 2 | PD | 4.18 | 2.63 | 13.81 | Topotecan/Paclitaxel/Bevacizumab, Bevacizumab, Pemetrexed |
| 5 | SCC | Multi-site | 0 | PD | 4.31 | 2.56 | 6.71* | None |
| 6 | SCC | Multi-site | 4 | PD | 4.37 | 2.04 | 11.28* | Carboplatin, Paclitaxel, Topotecan |
| 7 | SCC | Liver | 1 | PD | 4.7 | 3.29 | 16.54* | Atezolizumab, Carboplatin, Topotecan, Paclitaxel, Gemcitabine |
| 8 | Endocervical adenocarcinoma, mesonephric-type | Lung | 0 | PD | 13.28 | 3.45 | 14.33 | Remains on Pembrolizumab |
| 9 | Endocervical adenocarcinoma | Lung | 2 | PD | 15.02 | 8.38 | 38.99 | Vinorelbine, Topotecan, Ipilimumab/Nivolumab |
| Responders | ||||||||
| 10 | SCC | Lymph node | 2 | SD | 7.82 | 17.92 | 18.38 | None |
| 11 | SCC | Lung + lymph node | 2 | SD | 12.66 | 15.29 | 25.12 | Gemcitabine |
| 12 | SCC | Lung | 1 | PR | 14.5 | 12.20 | 14.5 | Remains on Pembrolizumab |
| 13 | SCC | Lymph node | 2 | CR | 20.38 | 17.39 | 20.38 | Remains on Pembrolizumab |
| 14 | SCC | Lung + lymph node | 2 | PR | 22.72 | 19.46 | 22.72 | Remains on Pembrolizumab |
Abbreviations: SCC, squamous cell carcinoma; PD, progression of disease; PR, partial response; CR, complete response; SD, stable disease.
Deceased
3.3. Outcomes
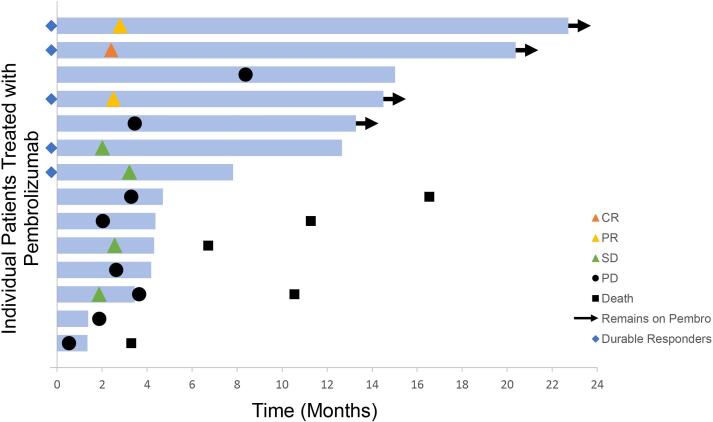
The median time to first radiologic assessment was 2.5 months (range 0.53–8.38). The overall response rate (ORR) was 21% (n = 3), including two partial responses and one complete response. The ORR in adenocarcinoma was 0%. Two patients (14%) had stable disease of six months or greater for an observed DCB of 36%. Of the five patients with DCB, three remain on pembrolizumab with observed antitumor activity of up to 19.5 months. One patient continues to have stable disease 10 months after discontinuation of pembrolizumab, despite progression on two prior lines of therapy. Three patients continued pembrolizumab despite disease progression per RECIST 1.1 criteria due to perceived clinical benefit (range 2.4–9.8 months); one of these remains on pembrolizumab to the present day at 9.8 months post-progression. Duration of therapy and radiologic outcomes are presented in Fig. 1.
Fig. 1.
Time to and duration of response assessed by RECIST v1.1 in the total patient cohort (n = 14). Length of bars represents time from initiation of therapy to last imaging assessment. CR, complete response; PR, partial response; SD, stable disease; PD, progressive disease.
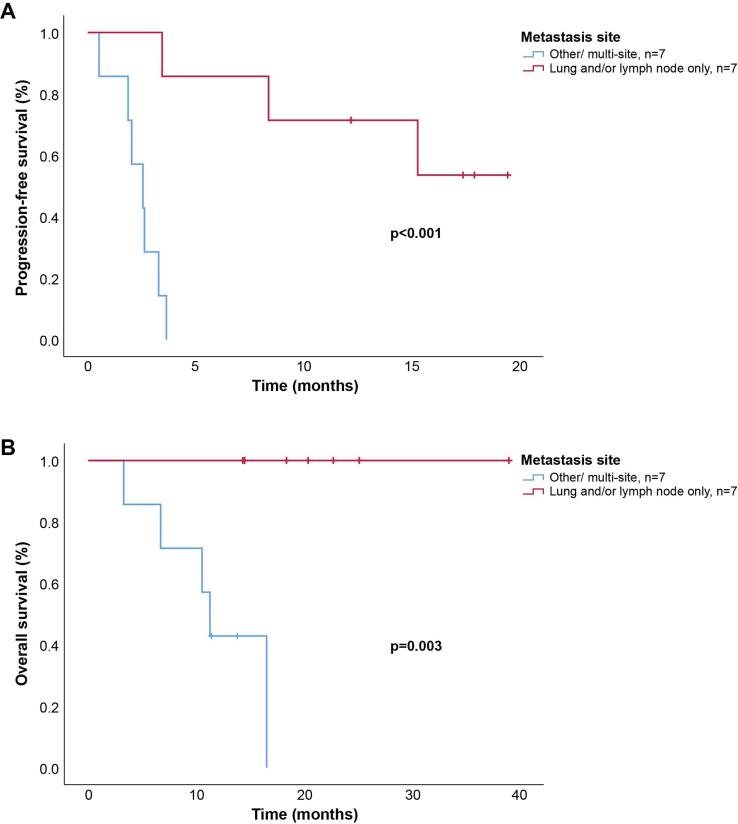
On univariable analysis, metastases restricted to lymph nodes and/or lung at baseline were associated with improved response to pembrolizumab (n = 7, p = 0.02). With a median follow-up time of 14.4 months (range 3.3–39.0), the 12-month PFS in those with a lymph node and/or lung pattern of disease was 71% vs. 0% in those with multi-site or visceral disease (p < 0.001). Median OS was 11.2 months (95% CI 9.4–14.2) vs. not reached in the lymph node and/or lung metastasis-only cohort (p = 0.003). Kaplan-Meier survival curves are displayed in Fig. 2.
Fig. 2.
Kaplan-Meier estimates of survival as stratified by disease location. (A) Progression-free survival (PFS) assessed by RECIST v1.1. (B) Overall survival (OS).
3.4. Genomic profiling
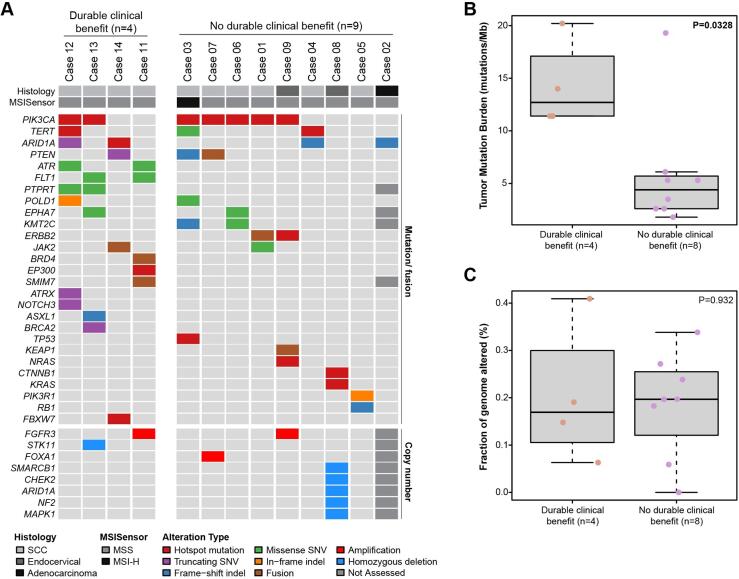
Tumor molecular profiling data are outlined in Fig. 3. The median tumor mutational burden (TMB) was 5.3 mutations/Mb (range 1.8–20.2). The most frequent genetic alterations were mutations in PIK3CA (58%, n = 7), ARID1A (33%, n = 4), PTEN (25%, n = 3), and TERT promoter (17%, n = 2). TMB was significantly higher in those with DCB than in non-responders (median 12.7, range 11.3–20.2 vs. 3.5, range 1.8–19.3 mutations/Mb, p = 0.03). There were no significant differences in mutational profiles, MSIsensor scores, or fraction genome altered between the two groups.
Fig. 3.
Genomic analysis of recurrent cervical cancer patients treated with pembrolizumab. (A) Non-synonymous somatic mutations and copy number alterations identified on targeted next generation sequencing of primary or metastatic tumors. Histology, MSI type, and genetic alteration as according to color-coded legend. (B) Tumor mutational burden (TMB) stratified by response to therapy. (C) Fraction genome altered stratified by response to therapy. Note: Case 13 underwent a distinct sequencing panel on which specific genes, TMB and fraction genome altered were not assessed, and Case 9 did not undergo genomic analysis.
4. Discussion
In our cohort of heavily pretreated patients with advanced cervical cancer, pembrolizumab demonstrated excellent activity with an ORR of 21% and DCB of 36%. This is comparable with published data (Frenel et al., 2017, Chung et al., 2019). In our cohort, those patients that derived clinical benefit demonstrated consistent clinical criteria, including TMB score > 10 mutations/Mb, squamous cell histology, and a lung and/or lymph node only pattern of metastatic disease.
Despite the demonstrated anti-tumor activity of PD-1 inhibitors in cervical cancer, response rates in the recurrent setting remain largely equivalent to conventional therapies. Therefore, an urgent but unmet need exists to define the optimal treatment population and identify prognostic biomarkers of response to immunotherapy. Beyond PD-L1 expression, other biomarkers such as DNA mismatch repair deficiency and high TMB have been shown to increase likelihood of immunotherapy response in other malignancies (Le et al., 2015, Howitt et al., 2015, Hu-Lieskovan et al., 2019, Ribas et al., 2016). In our cohort, elevated TMB was associated with response. The single MSI-H patient who did not respond to pembrolizumab had retained tumor mismatch repair immunohistochemical staining, suggesting intact protein activity. Next generation sequencing in other solid tumors has led to identification of molecular profiles that enrich for non-response to immunotherapy, including genetic alterations in JAK1, CTNNB1, STK11 and KEAP1 (Frank et al., 2018, Skoulidis et al., 2018, Trujillo et al., 2019, Shin et al., 2017). Notably, alterations in these genes were uncommon or absent in our cohort and were not associated with lack of response to pembrolizumab.
Our results additionally suggest that there may be an underlying pathophysiology of metastatic patterns that confer improved response to anti-PD-1 therapy. This observation builds upon results from previous studies, particularly in urothelial, breast, and colon cancer, that have explored the association between sites of metastatic disease to chemotherapy response (Wyld et al., 2003, Ye et al., 2013, Zarour et al., 2017). Specifically, we found that those with visceral or multi-site disease had significantly poorer outcomes, with a 12-month PFS of 0% compared with 71% in those with metastases restricted to lung and/or lymph nodes (p < 0.001), and decreased OS (11.2 months vs. not reached, p = 0.003).
Our findings are consistent with those of Pires da Silva et al., showing that the location of metastatic disease, and specifically the presence of liver metastases, impacts survival in melanoma patients receiving combination immunotherapy (Silva, 2018). It is hypothesized that metastases may interfere with the immune-regulatory behavior of the liver, in turn negatively impacting the response of disease to immunotherapy (Bilen et al., 2019). Other studies have shown that the total burden of disease may also impact the likelihood of response; in one study of melanoma patients treated with pembrolizumab, Huang and colleagues found that there was a relationship between exhausted T-cell reinvigoration and tumor burden, where those patients with a higher tumor burden were less likely to respond (Huang et al., 2017). This raises the possibility that even robust reinvigoration by anti-PD-1 therapy may be clinically ineffective if the tumor burden is high.
Limitations of our study include its retrospective nature, small sample size, and selective enrollment of patients with PD-L1–positive tumors. Additionally, our cohort was heavily pretreated with a majority having received three or more prior lines of therapy before pembrolizumab.
In sum, while pembrolizumab has shown activity as monotherapy for cervical cancer patients who have limited therapeutic options and poor prognoses, the percentage of patients who derive clinical benefit remains stubbornly low. Our findings highlight clinical features that may select for a population most likely to respond to pembrolizumab monotherapy, including lung or lymph node-only metastases with high TMB. In contrast, those with more extensive metastatic disease or visceral metastases may benefit from more aggressive combinatorial strategies and should be strongly considered for clinical trial enrollment. Currently accruing trials seek to establish the most effective combination therapies in the recurrent or advanced setting by combining immunotherapy with other immune targets, traditional systemic agents, and radiation. There must be an ongoing parallel effort to define biomarkers in this population beyond PD-L1 expression (Dyer et al., 2019).
Funding
This study was supported in part by the Memorial Sloan Kettering Cancer Center Support Grant P30 CA008748. B.W. is funded in part by Breast Cancer Research Foundation and Cycle for Survival grants. R.E.O. is supported in part by the NIH/NCI P01 CA190174 grant.
Disclosures
C.F.F. reports personal/consultancy fees from AstraZeneca, as well as participation in scientific steering committees (compensation waived) for Merck and Genentech. These are outside the scope of the submitted work. She also reports institutional research funding from Genentech, Merck, Bristol Myers Squibb, and AstraZeneca. N.R.A. reports grants from Stryker/Novadaq, Olympus, and GRAIL, all paid to the institution, outside the scope of the submitted work. Memorial Sloan Kettering Cancer Center (MSK) has financial interests relative to GRAIL. As a result of these interests, MSK could ultimately potentially benefit financially from the outcomes of this research. C.A. reports personal fees from Tesaro, personal fees from Immunogen, grants and personal fees from Clovis, personal fees from Mateon Therapeutics, personal fees from Cerulean Pharma, grants from Genentech, grants from AbbVie, and grants from Astra Zeneca. These are outside the scope of the submitted work. C.K. reports research funding from Bristol Myers Squibb, Merus, and Gritstone Oncology, outside the scope of the submitted work. R.E.O. reports personal fees from Tesaro, GlaxoSmithKline, Regeneron, Genentech USA, Genmab Therapeutics outside the scope of the submitted work. She is a non-compensated steering committee member for the PRIMA, Moonstone (Tesaro/GlaxoSmithKline) and DUO-O (AstraZeneca) studies, outside the scope of the submitted work. D.Z. reports personal/consultancy fees from Merck, Synlogic Therapeutics, Biomed Valley Discoveries, Trieza Therapeutics, Tesaro, and Agenus, outside the scope of the submitted work. He also reports institutional research support from AstraZeneca, Plexxikon, and Genentech, outside the scope of the submitted work. B.W. reports ad hoc membership in REPARE Therapeutics, outside the scope of the submitted work. M.M.L. is a consultant for Intuitive Surgical, Inc., outside the scope of the submitted work.
CRediT authorship contribution statement
Kathryn M. Miller: Conceptualization, Investigation, Formal analysis, Writing - original draft, Writing - review & editing, Visualization. Olga T. Filippova: Formal analysis, Writing - review & editing, Visualization. Sara A. Hayes: Conceptualization, Investigation, Writing - review & editing. Nadeem R. Abu-Rustum: Resources, Writing - review & editing. Carol Aghajanian: Resources, Writing - review & editing. Vance Broach: Resources, Writing - review & editing. Lora H. Ellenson: Resources, Writing - review & editing. Pier Selenica: Resources, Formal analysis, Visualization. Elizabeth L. Jewell: Resources, Writing - review & editing. Chrisann Kyi: Resources, Writing - review & editing. Yuliya Lakhman: Conceptualization, Investigation, Writing - review & editing. Jennifer J. Mueller: Resources, Writing - review & editing. Roisin E. O’Cearbhaill: Resources, Writing - review & editing. Kay J. Park: Resources, Writing - review & editing. Yukio Sonoda: Resources, Writing - review & editing. Dmitriy Zamarin: Resources, Writing - review & editing. Britta Weigelt: Conceptualization, Formal analysis, Writing - original draft, Visualization. Mario M. Leitao: Conceptualization, Formal analysis, Resources, Writing - original draft, Writing - review & editing. Claire F. Friedman: Conceptualization, Resources, Writing - original draft, Writing - review & editing, Visualization, Supervision, Funding acquisition.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Rose P.G., Bundy B.N., Watkins E.B. Concurrent cisplatin-based radiotherapy and chemotherapy for locally advanced cervical cancer. The New England journal of medicine. 1999;340(15):1144–1153. doi: 10.1056/NEJM199904153401502. [DOI] [PubMed] [Google Scholar]
- Vale C., Tierney J., Stewart L. Concomitant chemoradiotherapy for cervical cancer: a systematic review and meta-analysis of individual patient data. Gynecologic oncology. 2006;100(2):442–443. doi: 10.1016/j.ygyno.2005.09.018. [DOI] [PubMed] [Google Scholar]
- Pfaendler K.S., Tewari K.S. Changing paradigms in the systemic treatment of advanced cervical cancer. Am J Obstet Gynecol. 2016;214(1):22–30. doi: 10.1016/j.ajog.2015.07.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tewari K.S., Sill M.W., Long H.J., 3rd Improved survival with bevacizumab in advanced cervical cancer. The New England journal of medicine. 2014;370(8):734–743. doi: 10.1056/NEJMoa1309748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore D.H., Blessing J.A., McQuellon R.P. Phase III study of cisplatin with or without paclitaxel in stage IVB, recurrent, or persistent squamous cell carcinoma of the cervix: a gynecologic oncology group study. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2004;22(15):3113–3119. doi: 10.1200/JCO.2004.04.170. [DOI] [PubMed] [Google Scholar]
- Monk B.J., Sill M.W., McMeekin D.S. Phase III trial of four cisplatin-containing doublet combinations in stage IVB, recurrent, or persistent cervical carcinoma: a Gynecologic Oncology Group study. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2009;27(28):4649–4655. doi: 10.1200/JCO.2009.21.8909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Long H.J., 3rd, Bundy B.N., Grendys E.C., Jr Randomized phase III trial of cisplatin with or without topotecan in carcinoma of the uterine cervix: a Gynecologic Oncology Group Study. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2005;23(21):4626–4633. doi: 10.1200/JCO.2005.10.021. [DOI] [PubMed] [Google Scholar]
- Tewari K.S., Sill M.W., Penson R.T. Bevacizumab for advanced cervical cancer: final overall survival and adverse event analysis of a randomised, controlled, open-label, phase 3 trial (Gynecologic Oncology Group 240) Lancet (London, England). 2017;390(10103):1654–1663. doi: 10.1016/S0140-6736(17)31607-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferris R.L., Blumenschein G., Jr., Fayette J. Nivolumab for Recurrent Squamous-Cell Carcinoma of the Head and Neck. The New England journal of medicine. 2016;375(19):1856–1867. doi: 10.1056/NEJMoa1602252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brahmer J.R., Hammers H., Lipson E.J. Nivolumab: targeting PD-1 to bolster antitumor immunity. Future Oncol. 2015;11(9):1307–1326. doi: 10.2217/fon.15.52. [DOI] [PubMed] [Google Scholar]
- Brahmer J.R., Tykodi S.S., Chow L.Q. Safety and activity of anti-PD-L1 antibody in patients with advanced cancer. The New England journal of medicine. 2012;366(26):2455–2465. doi: 10.1056/NEJMoa1200694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robert C., Long G.V., Brady B. Nivolumab in previously untreated melanoma without BRAF mutation. The New England journal of medicine. 2015;372(4):320–330. doi: 10.1056/NEJMoa1412082. [DOI] [PubMed] [Google Scholar]
- Reddy O.L., Shintaku P.I., Moatamed N.A. Programmed death-ligand 1 (PD-L1) is expressed in a significant number of the uterine cervical carcinomas. Diagnostic Pathology. 2017;12(1):45. doi: 10.1186/s13000-017-0631-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heeren A.M., Punt S., Bleeker M.C. Prognostic effect of different PD-L1 expression patterns in squamous cell carcinoma and adenocarcinoma of the cervix. Mod Pathol. 2016;29(7):753–763. doi: 10.1038/modpathol.2016.64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frenel J.S., Le Tourneau C., O'Neil B. Safety and Efficacy of Pembrolizumab in Advanced, Programmed Death Ligand 1-Positive Cervical Cancer: Results From the Phase Ib KEYNOTE-028 Trial. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2017;35(36):4035–4041. doi: 10.1200/JCO.2017.74.5471. [DOI] [PubMed] [Google Scholar]
- Chung H.C., Ros W., Delord J.P. Efficacy and Safety of Pembrolizumab in Previously Treated Advanced Cervical Cancer: Results From the Phase II KEYNOTE-158 Study. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2019;37(17):1470–1478. doi: 10.1200/JCO.18.01265. [DOI] [PubMed] [Google Scholar]
- Eisenhauer E.A., Therasse P., Bogaerts J. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1) Eur J Cancer. 2009;45(2):228–247. doi: 10.1016/j.ejca.2008.10.026. [DOI] [PubMed] [Google Scholar]
- Cheng D.T., Mitchell T.N., Zehir A. Memorial Sloan Kettering-Integrated Mutation Profiling of Actionable Cancer Targets (MSK-IMPACT): A Hybridization Capture-Based Next-Generation Sequencing Clinical Assay for Solid Tumor Molecular Oncology. J Mol Diagn. 2015;17(3):251–264. doi: 10.1016/j.jmoldx.2014.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le D.T., Uram J.N., Wang H. PD-1 Blockade in Tumors with Mismatch-Repair Deficiency. The New England journal of medicine. 2015;372(26):2509–2520. doi: 10.1056/NEJMoa1500596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howitt B.E., Shukla S.A., Sholl L.M. Association of Polymerase e-Mutated and Microsatellite-Instable Endometrial Cancers With Neoantigen Load, Number of Tumor-Infiltrating Lymphocytes, and Expression of PD-1 and PD-L1. JAMA Oncol. 2015;1(9):1319–1323. doi: 10.1001/jamaoncol.2015.2151. [DOI] [PubMed] [Google Scholar]
- Hu-Lieskovan S., Lisberg A., Zaretsky J.M. Tumor Characteristics Associated with Benefit from Pembrolizumab in Advanced Non-Small Cell Lung Cancer. Clin Cancer Res. 2019;25(16):5061–5068. doi: 10.1158/1078-0432.CCR-18-4275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ribas A., Hamid O., Daud A. Association of Pembrolizumab With Tumor Response and Survival Among Patients With Advanced Melanoma. Jama. 2016;315(15):1600–1609. doi: 10.1001/jama.2016.4059. [DOI] [PubMed] [Google Scholar]
- Frank R., Scheffler M., Merkelbach-Bruse S. Clinical and Pathological Characteristics of KEAP1- and NFE2L2-Mutated Non-Small Cell Lung Carcinoma (NSCLC) Clin Cancer Res. 2018;24(13):3087–3096. doi: 10.1158/1078-0432.CCR-17-3416. [DOI] [PubMed] [Google Scholar]
- Skoulidis F., Goldberg M.E., Greenawalt D.M. STK11/LKB1 Mutations and PD-1 Inhibitor Resistance in KRAS-Mutant Lung Adenocarcinoma. Cancer discovery. 2018;8(7):822–835. doi: 10.1158/2159-8290.CD-18-0099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trujillo J.A., Luke J.J., Zha Y. Secondary resistance to immunotherapy associated with β-catenin pathway activation or PTEN loss in metastatic melanoma. Journal for ImmunoTherapy of Cancer. 2019;7(1):295. doi: 10.1186/s40425-019-0780-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shin D.S., Zaretsky J.M., Escuin-Ordinas H. Primary Resistance to PD-1 Blockade Mediated by JAK1/2 Mutations. Cancer discovery. 2017;7(2):188. doi: 10.1158/2159-8290.CD-16-1223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wyld L., Gutteridge E., Pinder S.E. Prognostic factors for patients with hepatic metastases from breast cancer. British journal of cancer. 2003;89(2):284–290. doi: 10.1038/sj.bjc.6601038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye L.C., Liu T.S., Ren L. Randomized controlled trial of cetuximab plus chemotherapy for patients with KRAS wild-type unresectable colorectal liver-limited metastases. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2013;31(16):1931–1938. doi: 10.1200/JCO.2012.44.8308. [DOI] [PubMed] [Google Scholar]
- Zarour L.R., Anand S., Billingsley K.G. Colorectal Cancer Liver Metastasis: Evolving Paradigms and Future Directions. Cell Mol Gastroenterol Hepatol. 2017;3(2):163–173. doi: 10.1016/j.jcmgh.2017.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ines Esteves Domingues Pires Da Silva SL, Maria Gonzalez, Alexander Guminski, Georgina V. Long, and Alexander M. Menzies. Distinct patterns of response and toxicity (tox) by sites of metastases (mets) in patients (pts) treated with ipilumumab combined with PD-1 antibodies (ipi+PD1). Journal of Clinical Oncology. 2018;36(15_suppl):9553.
- Bilen M.A., Shabto J.M., Martini D.J. Sites of metastasis and association with clinical outcome in advanced stage cancer patients treated with immunotherapy. BMC Cancer. 2019;19(1):857. doi: 10.1186/s12885-019-6073-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang A.C., Postow M.A., Orlowski R.J. T-cell invigoration to tumour burden ratio associated with anti-PD-1 response. Nature. 2017;545(7652):60–65. doi: 10.1038/nature22079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dyer B.A., Zamarin D., Eskandar R.N., Mayadev J.M. Role of Immunotherapy in the Management of Locally Advanced and Recurrent/Metastatic Cervical Cancer. J Natl Compr Canc Netw. 2019;17(1):91–97. doi: 10.6004/jnccn.2018.7108. [DOI] [PubMed] [Google Scholar]