Highlights
-
•
Pharmaceutical removal using biological digesters, sonication and membrane filtration.
-
•
Biotransformation of pharmaceutical in biological and ultrasound treatment.
-
•
Hybrid ultrasound coupled treatment processes with biological reactor and membrane filtration.
Keywords: Pharmaceutical waste, Activated sludge, Membrane filtration, Ultrasound, Biotransformation
Abstract
Contaminants of emerging concern (CEC) such as pharmaceuticals commonly found in urban and industrial wastewater are a potential threat to human health and have negative environmental impact. Most wastewater treatment plants cannot efficiently remove these compounds and therefore, many pharmaceuticals end up in aquatic ecosystems, inducing problems such as toxicity and antibiotic-resistance. This review reports the extent of pharmaceutical removal by individual processes such as bioreactors, advanced oxidation processes and membrane filtration systems, all of which are not 100% efficient and can lead to the direct discharge of pharmaceuticals into water bodies. Also, the importance of understanding biotransformation of pharmaceutical compounds during biological and ultrasound treatment, and its impact on treatment efficacy will be reviewed. Different combinations of the processes above, either as an integrated configuration or in series, will be discussed in terms of their degradation efficiency and scale-up capabilities. The trace quantities of pharmaceutical compounds in wastewater and scale-up issues of ultrasound highlight the importance of membrane filtration as a concentration and volume reduction treatment step for wastewater, which could subsequently be treated by ultrasound.
1. Introduction
Freshwater, a precious natural resource essential for agriculture, energy generation and life, only makes up less than 1% of the world's water. With the global population projected to reach 9.8 billion by 2050 [1], there is an urgent concern on freshwater demand and availability. This impending disaster is heightened with a rise in water pollution and stricter wastewater discharge limits.
Currently the biggest threat to the global freshwater reserve and, ecological and public health is the rising of emerging contaminants in the environment such as pharmaceutical drugs [2], [3]. Wastewater treatment plants (WWTPs) are considered the primary source of pharmaceuticals in the environment, although a higher concentration of certain compounds can be found in hospital effluents [4]. Current water treatment methods implemented in WWTPs are inefficient for the complete removal of most pharmaceuticals [2], [5], leading to the discharge of these compounds directly into water bodies. The concentration of pharmaceuticals in hospital effluents [4], [6], [7], pharmaceutical industry effluents [8], [9] and WWTPs [10], [11] range from ng L−1 to µg L−1. The concentration of these compounds in the environment depends on discharge, geographical location, climate conditions and consumption [12]. Compared to Europe and the USA, the concentration of pharmaceuticals in Indian and Japanese waters have been found 40 times higher [13] and one order of magnitude lower [14], respectively, ascribed to the higher and lower consumption of pharmaceuticals in each country.
The increase in the human life expectancy coupled with the rise in population is expected to increase the use of pharmaceuticals [12], [15] and thus, intensify the presence of antibiotics, analgesics, anti-inflammatory, antihistaminic, antiepileptic and other type of drugs in aquatic environments. This contributes to the increase in the ecotoxicology of lakes and rivers that can be harmful to aquatic organisms [16], [17]. Additionally, the presence of pharmaceuticals can lead to the development of antibiotic resistance genes potentially causing antibiotic-resistant bacteria or so-called superbugs [18]. Some pharmaceuticals such as diclofenac (DCF) and erythromycin have been incorporated into the watch list included in the Decision 2015/495/EU of 20 March 2015 and in accordance with the Directive 2013/39/EU to monitor and gather data to address the risk these pharmaceuticals may entail [19]. However, currently there are no discharge standards and regulations at EU level for most of the pharmaceuticals found in WWTPs. Similarly, The United States Environmental Protection Agency catalogued emerging pollutants as hazardous substances that lack regulatory standards [20].
Pharmaceuticals are thereby recognized as contaminants of emerging concern (CEC) because they are compounds of continuous use; recalcitrant to conventional processes; detected at very low concentrations (ng L−1 - µg L−1) in water bodies; their environmental effects are not completely known; and in most cases are unregulated in wastewaters [21].
Biological digesters are well developed and frequently adopted methods for treating industrial wastewaters and have shown to effectively remove some pharmaceuticals such as ibuprofen (IBP) and paracetamol (PCT) but ineffective in the elimination of many of them e.g. carbamazepine (CBZ) and DCF [22], [23]. Due to the inefficiency of biological processes to eliminate pharmaceuticals, the combination of these processes with alternative treatment systems is an option to treat these pollutants.
Advanced oxidation processes (AOPs) are alternative treatments capable of producing and utilizing radical species (such as HO•) to degrade CEC [24]. The combination of AOPs have been successfully combined with biological treatments to treat recalcitrant pollutants in aqueous matrices and the compounds generated during the preliminary chemical oxidative process can be biologically mineralised [25], [26], [27]. However, it is important to mention that photochemical AOPs such as photo-Fenton, UVC/H2O2 and UVC/Persulphate have some drawbacks such as iron precipitation, UVC-lamps fouling and the need to store and transport hydrogen peroxide or persulphate [26].
An interesting alternative to overcome some of those drawbacks is the coupling of photochemical AOPs with Ultrasound (US) and US-based AOPs. For instance, the combination of US with the photo-Fenton process can (I) take advantage of the hydrogen peroxide produced from hydroxyl radical recombination during the ultrasonic treatment to promote Fenton-type reactions, (II) increase the degradation kinetics of CEC by each process (hydrophobic and hydrophilic pollutants by US and photo-Fenton, respectively), (III) enhance the homogenization and mass transfer, and (IV) minimize the use of reagents (iron and H2O2), limiting the secondary pollution and costs of storing and transportation [21], [28].
It is important to mention that US and US-based AOPs are effective in the removal of pharmaceuticals from water streams as tertiary treatment methods [29], [30]. However, the low mineralisation percentages achieved with US-based AOPs [29], [30] leads to the formation of intermediates that can increase water toxicity [31], [32]. As above mentioned, US can be coupled with other AOPs (e.g., photochemical systems) to increase degradation effects. However, large scale US processing is currently limited [33], [34], [35].
Membrane filtration processes can provide high separation and concentration of particulates, and ultrafiltration (UF) and microfiltration (MF) membranes have been effectively coupled with biological treatments, known as membrane bioreactors (MBR)[36], [37]. However, these membranes do not provide an absolute barrier against pharmaceuticals. Nanofiltration (NF), forward osmosis (FO) and reverse osmosis (RO) can offer high rejection and concentration of pharmaceuticals but the highly concentrated retentates then require further treatment. There is potential for NF, FO or RO to be used to preconcentrate and reduce the volume of wastewater before the application of tertiary US-based AOP treatments, but this hybrid system has not been widely investigated in the literature [38].
Several review papers have been published discussing the performance of US on pharmaceuticals removal, either as the main topic of the paper [39], [40], [41], [42], [43], [44] or integrated into a more general analysis on advanced oxidation processes [9], [45], [46], [47], however there is very little literature incorporating membrane filtration systems. Therefore, the objective of the present review is to showcase biological, US-based AOPs and membrane filtration, individually and discuss the potential benefits in combining these three processes for the removal of pharmaceuticals from water and wastewater, highlighting the potential of the hybrid systems and underlining research gaps for future studies.
2. Biological treatment
Biological treatments are well-established water treatment methods currently implemented in WWTPs all over the world. Biological reactors can be aerobic or anaerobic. In aerobic reactors, sufficient dissolved oxygen (DO) is introduced (usually > 2 mg L−1) by injecting air or pure O2 (or by mechanical stirring) to provide an environment for aerobic bacteria to grow. In anaerobic reactors, no oxygen is supplied to the system, and the removal of organic matter and nutrients from the influent water are mainly by anaerobic bacteria. Anoxic reactors have DO < ~0.2 mg L−1 whereas anaerobic reactors are free of oxygen molecules with only bonded oxygen can be found, e.g. nitrates (NO3−) [48], [49]. Depending on water composition, removal needs and water discharge or reuse regulations, aerobic and anaerobic methods can be used individually or combined with the main purpose of reducing organic load and nutrients (nitrogen, N, and phosphorus, P) present in the influent water [50], [51].
Aerobic reactors are used to treat waters with low organic loading (chemical oxygen demand (COD) < 1000 mg L−1) and when the treatment requires the presence of oxygen, whereas anaerobic reactors are typically applied to treat wastewater with high organic loading (COD > 4000 mg L-1). For an increase in organic matter and nutrient removal, biological reactors are compartmentalised into anaerobic-anoxic-aerobic zones achieving effluents with low presence of organic matter (measured as biological oxygen demand (BOD), or chemical oxygen demand (COD)), nitrogen and phosphorus [48], [49]. Although biological treatment methods have shown > 90% removal in COD, BOD, N and P [52], [53], they are not that effective at removing CEC such as pharmaceuticals [4], [22], [54], [55]. The treatment efficiencies of pharmaceuticals in the biological process of WWTPs vary from 20 to 99% [10], [56], [57], showing removal percentages below 20% for some compounds such as CBZ [10], [57], [58], [59]. Thus, CBZ has been proposed as a possible pharmaceutical marker in WWTPs [60], [61], [62]. The energy requirements for aerobic processes are generally higher because of the additional aeration required and the need to further treat the sludge (biomass), however aerobic treatment has some distinct advantages over the anaerobic treatment process. These include reduced odor (due to non-production of hydrogen sulfide or methane) and better nutrient removal efficacy (facilitating direct discharge into surface waters or disinfection). Therefore, this review will focus on the activated sludge system, the most common aerobic treatment method, for the biological system.
2.1. Activated sludge process
2.1.1. Biodegradation and sorption
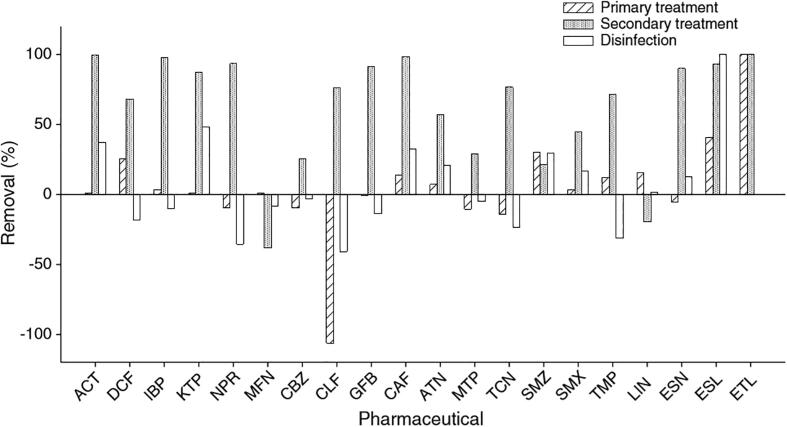
The removal of pharmaceuticals entering a WWTP mainly occurs in the biological treatment stage (Fig. 1) [57], [63], [64]. The removal mechanism depends on the nature of the pharmaceutical (e.g., hydrophobicity and biodegradability), characteristics of mixed liquor suspend solids (MLSS) such as type of sludge, and WWTP operational parameters (e.g., hydraulic retention time (HRT), sludge retention time (SRT), pH and temperature) [65]. Reports on removal mechanisms for pharmaceuticals vary with either: a limited contribution from biological degradation [55], [66], and removal of organic pollutants controlled by sorption processes [10]; removal of pharmaceuticals mainly attributed to biotransformation [67] and negligible contributions from hydrolysis, volatilisation and photodegradation [66], [68], [69]; or both biodegradation and sorption as key removal mechanisms for pharmaceuticals [65], [68], [70], showing different removal mechanisms for pharmaceuticals within the same therapeutic group (e.g., antibiotics, analgesics, anti-inflammatory) [65], [71].
Fig. 1.
Average removal of pharmaceuticals in WWTP processes. Acetaminophen (ACT), diclofenac (DCF), ibuprofen (IBP), ketoprofen (KTP), naproxen (NPR), mefenamic acid (MFN), carbamazepine (CBZ), clofibric acid (CLF), gemfibrozil (GFB), caffeine (CAF), atenolol (ATN), metoprolol (MTP), triclosan (TCN), sulfamethazine (SMZ), sulfamethoxazole (SMX), trimethoprim (TMP), lincomycin (LIN), estrone (ESN), estriol (ESL), estradiol (ETL). Reprinted from [57], Copyright (2011), with permission from Elsevier.
Waters entering a WWTP also carry metabolites/conjugates substances from pharmaceutical metabolism in humans or animals. Such metabolites/conjugates act as a reservoir because they can be enzymatically cleaved, releasing the parent pharmaceuticals. Additionally, the gradual release of pharmaceuticals from faeces particles can occur [72]. Consequently, the concentration of the parent drug increases in the effluents [54], [73], as illustrated in Fig. 1(case of those pharmaceuticals having negative removals; i.e., their concentrations increased after passing through the processes in WWTP).
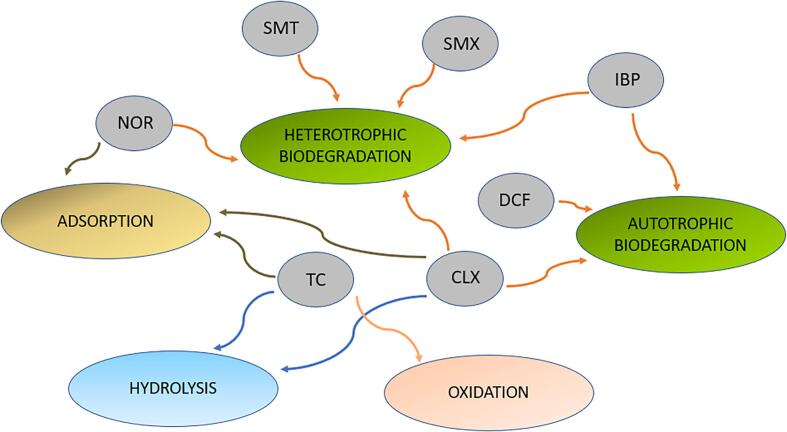
Pharmaceuticals can be removed via autotrophic biodegradation (bacteria present in nitrification processes, i.e. conversion of ammonium (NH4+) to nitrate (NO3−), that use inorganic carbon as substrate) and via heterotrophic biodegradation (bacteria that use organic carbon as substrate) [70]. Both processes occur simultaneously in the activated sludge process (ASP) and the main degradation route principally depends on the characteristics of the pharmaceutical (Fig. 2) [58], [70]. However, some researchers disagree on the relationship between biodegradability and pharmaceutical structure [22], showing no correlation between biological removal and compound structure [74]. For some pharmaceuticals faster biotransformation rates have been found under aerobic conditions compared to anaerobic and anoxic conditions [75], [76], whereas an increase in the removal of CBZ, atenolol and triclosan (TRC), among others, have been reported in anoxic–oxic conditions [57]. The increase in biodegradation was attributed to the anoxic–oxic dual zones provided by the sludge.
Fig. 2.
Pharmaceutical removal routes of sulfamethazine (SMT), sulfamethoxazole (SMX), ibuprofen (IBP), diclofenac (DCF), norfloxacin (NOR), cephalexin (CLX) and tetracycline (TC) in the ASP. Adapted after Peng et al. [70].
Biodegradation rate constant (kbio) is employed in the literature to quantify the biodegradability of a compound, for pharmaceuticals showing no biodegradable characteristics, their sorption (absorption and adsorption [23]) into sludge is assumed to be fast compared to their biological degradation [79], where sorption equilibrium is reached after 0.5–1 h [66], [80], [81]. Sorption can be considered as a removal mechanism for compounds with mid-low biodegradation rates (<2.5 L gss−1d−1 such as erythromycin [22], [55] or tetracycline (TC) [67], [75]. The sorption coefficient (Kd) will depend on electrostatic interactions (electrostatic force) [68], [70], [75] and compound hydrophobicity, expressed as the octanol–water distribution coefficient (Dow, also referred as octanol–water partition coefficient, log P), where an increase in removal efficiency by increasing hydrophobicity (increase in Dow) has been observed for a given pharmaceutical [10]. For compounds with high-water solubility (low hydrophobicity) such as SMX, removal by sorption is expected to be low [70]. Taking this into account, several authors have highlighted the importance of looking into the specific interaction between pharmaceuticals and flocs, and biodegradation mechanism to understand and improve the performance of bioreactors [54], [65], [67], [70].
2.1.2. Biotransformation
Biodegradation of pharmaceuticals can occur by co-metabolism (in which other substances are the carbon or energy sources during the pollutant transformation) or by substrate consumption (called catabolism, in which the pharmaceutical is the carbon and energy source during the transformation). For example, ciprofloxacin (CIP) and 17β-estradiol have been biodegraded under anaerobic conditions by both co-metabolism and substrate consumption [82].
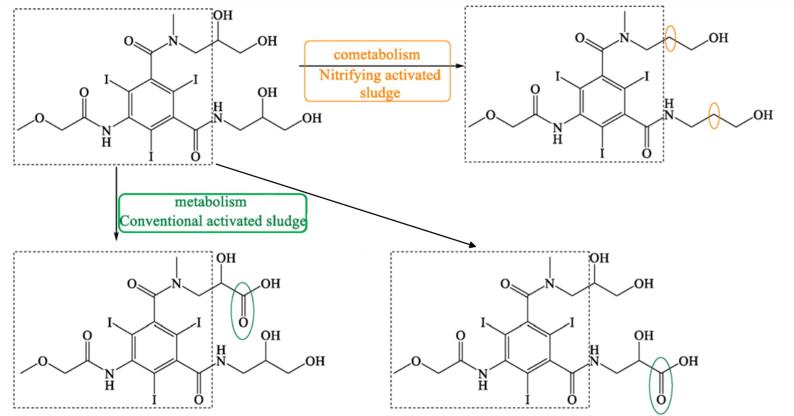
Biological treatment of pharmaceutical products can result in mineralisation (i.e., the transformation of the pollutant to carbon dioxide, water and inorganic ions), degradation to smaller/shorter chain products, or minor structural changes [72]. The formation of metabolites or biotransformation in the biological treatment of pharmaceuticals is highly dependent on the specific conditions e.g., inherent nature of the pharmaceutical, and microorganisms type. From a general point of view, biotransformations involve oxidative, reductive and lytic mechanistic pathways (Table 1). For instance, the biodegradation of the X-ray contrast media iopromide leads to the oxidation of the primary alcohols (forming carboxylates) on the side chains of the pharmaceutical during the treatment by conventional activated sludge, while dehydroxylation at the two side chains occurred in the nitrifying activated sludge, which was associated to a co-metabolism pathway (Fig. 3) [83].
Table 1.
Examples of biotransformations of pharmaceuticals.
| Pharmaceutical | Biotransformation product | Primary transformations | References |
|---|---|---|---|
| Analgesics and anti-inflammatories | |||
Ibuprofen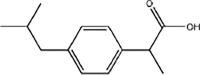
|
 hydroxy-ibuprofen (OH-Ibu) |
Hydroxylation of alkyl group | [85] |
 carboxy-ibuprofen (CA-Ibu) |
Alkyl/alcohol oxidation | ||
 carboxy-hydratropic acid (CA-HA) |
β-oxidation | ||
Naproxen
|
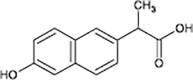 |
O-demethylation | [86], [87] |
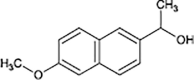 |
Decarboxylation | ||
| Antihypertensives | |||
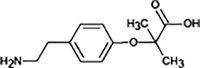
Atenolol
|
 ATE-268 |
Primary amide hydrolysis | |
Bezafibrate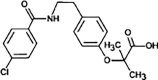
|
 BEZ-224 |
Secondary amide hydrolysis | [91], [93] |
 |
Dechlorination | ||
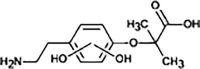 BEZ-256 |
Hydroxylation | ||
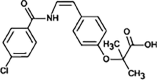 BEZ-360 |
dehydrogenation | ||
Valsartan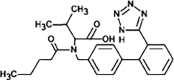
|
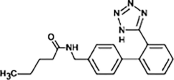 VAL-336 |
Tertiary amide hydrolysis | [91], [93] |
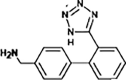 VAL-252 |
Secondary amide hydrolysis | ||
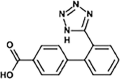 VAL-267 |
Amine oxidation | ||
| Antimicrobials | |||
Trimethoprim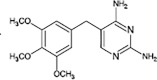
|
 |
Hydroxylations | [95] |
Oseltamivir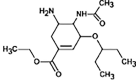
|
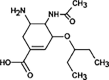 OSE-285 |
Dealkylation | [91], [93] |
| Psychiatric | |||
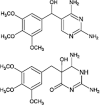
Carbamazepine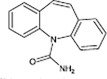
|
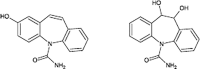 |
Hydroxylations | [95] |
Diazepam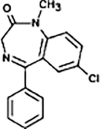
|
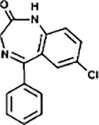 DIA-271 |
N-dealkylation | [91], [93] |
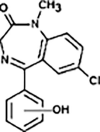 |
Hydroxylation | ||
Levetiracetam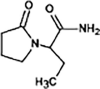
|
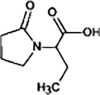 LEV-172 |
Primary amide hydrolysis | [91], [93] |
| Contrast media | |||
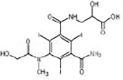
Iohexol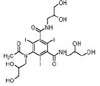
|
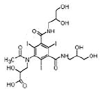 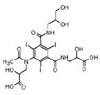
|
Alcohol oxidation | [84] |
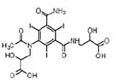 |
Amide hydrolysis | ||
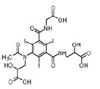 |
Decarboxylation | ||
Iomeprol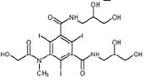
|
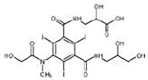 |
Alcohol oxidation | [84] |
 |
Secondary amide hydrolysis | ||
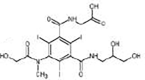 |
Decarboxylation | ||
| Oestrogens | |||
17β-estradiol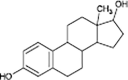
|
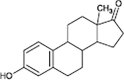 |
Alcohol oxidation | [87] |
Mestranol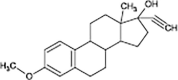
|
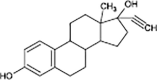 |
O-demethylation | |
| Others | |||
Diphenhydramine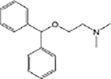
|
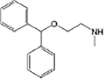 |
N-demethylation | [88] |
Guaifenesin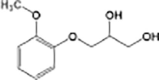
|
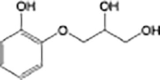 |
O-demethylation | |
Oxybenzone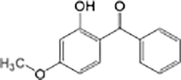
|
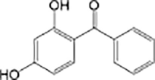 |
O-demethylation | |
Fig. 3.
Biodegradation products and pathways of iopromide by conventional activated sludge and nitrifying activate sludge. Reprinted from [83], Copyright (2016), with permission from Elsevier.
Biodegradation pathway determination is based on the elucidation of transformation products, fundamental metabolic logic, and the time sequence of appearance of such products [47]. For example, for pharmaceuticals such as iohexol, the analyses of the biotransformation products indicate that the microorganisms induce oxidation of the primary or secondary alcohol groups, decarboxylation, and cleavage of N-C bond (Table 1). Oxidation of primary alcohol groups to carboxylates is induced by alcohol and aldehyde dehydrogenases. Decarboxylation (which followed the oxidation of the alcohols) can be promoted by different thiamine pyrophosphate dependent enzymes. Meanwhile, the cleavage of N-C bonds involves the action of monooxygenases (i.e., cytochrome P-450 monooxygenases) [84].
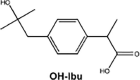
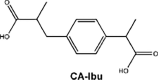
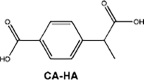
In the case of IBP, the formation of hydroxy-ibuprofen, carboxy-ibuprofen and carboxy-hydratropic acid has been reported (Table 1). Experiments in both biofilm reactors (BFR) and batch activated sludge demonstrated that hydroxyl-ibuprofen was the main biotransformation product under oxic conditions, and carboxy-hydratropic acid under anoxic conditions. Meanwhile, carboxy-ibuprofen was found under oxic and anoxic conditions almost only in the batch activated sludge [85]. In turn, the biotransformation of naproxen induces the demethylation and decarbonylation of this pharmaceutical.
Anaerobic systems are also able to promote O-demethylation paths on pharmaceuticals such as guaifenesin, naproxen, oxybenzone and mestranol (Table 1) [86], [87]. N-demethylation is another plausible mechanism for pharmaceuticals, this has been found in the biodegradation of diphenhydramine [88]. O-demethylation and N-demethylation are very common metabolic pathways in nature for the degradation of biologically active compounds as pharmaceuticals. Such pathways are promoted by cytochrome P450 enzymes [68], [89].
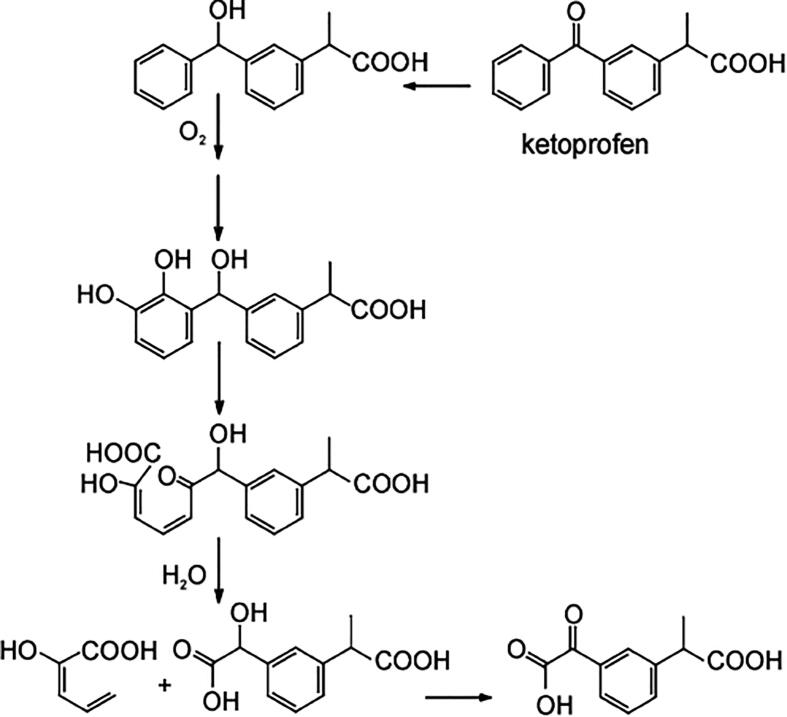
On the other hand, a reduction pathway is reported as the initial step in the biotransformation of ketoprofen. Such transformation comprises a reduction of its ketone group (to increase the electron density of the aromatic rings, rendering these more reactive), followed by hydroxylation forming a catechol structure. A subsequent oxidative ring-opening of catechol by meta-cleavage, plus hydrolysis leads to the generation of 3-(hydroxy-carboxymethyl) hydratropic acid product. As a final step, alcohol is oxidised to produce the 3-(keto-carboxymethyl) hydratropic acid (see Fig. 4) [90]. For the antibiotic trimethoprim, hydroxylation by microorganisms from activated sludge leads to the generation of α-hydroxy-trimethoprim and hydroxylated-trimethoprim [87]. Meanwhile, the antiviral oseltamivir exhibited an ester hydrolysis during biotreatments [91]. Biotransformations of sartan antihypertensives such as candesartan, eprosartan, irbesartan, olmesartan, and valsartan have been investigated by Letzel et al. [92]. For valsartan (which has been widely studied), the first biotransformation pathway is an N-dealkylation reaction. Such product is subsequently transformed by an amide hydrolysis reaction and oxidation [91].
Fig. 4.
Proposed pathways for biotransformation of ketoprofen. Reprinted from [90], Copyright (2005), with permission from Elsevier.
Some researches focus on the amide transformation pathway since the amide moiety is a common functional group in pharmaceutical structures and it is susceptible to biodegradation [91], [93]. Helbling et al. [91] researched on the biological treatment of atenolol, bezafibrate, diazepam, levetiracetam, oseltamivir and valsartan, and found that the pharmaceuticals with primary amides, as atenolol and levetiracetam, are hydrolysed to form the respective acids as primary transformation products. Compounds such as bezafibrate, having secondary amides, can also undergo hydrolysis. In such a case, the pathway is strongly dependent on the chain attached to N (amide hydrolysis by enzymes is influenced by both the amine protonation and steric hindrance from N-substituents). In turn, tertiary amides on pharmaceuticals (e.g., diazepam or valsartan) show N-dealkylation resulting in the formation of secondary amides, which is catalyzed by a large set of bacterial enzymes (e.g., cytochrome p-450 superfamily) [91], [93].
Fig. 5.
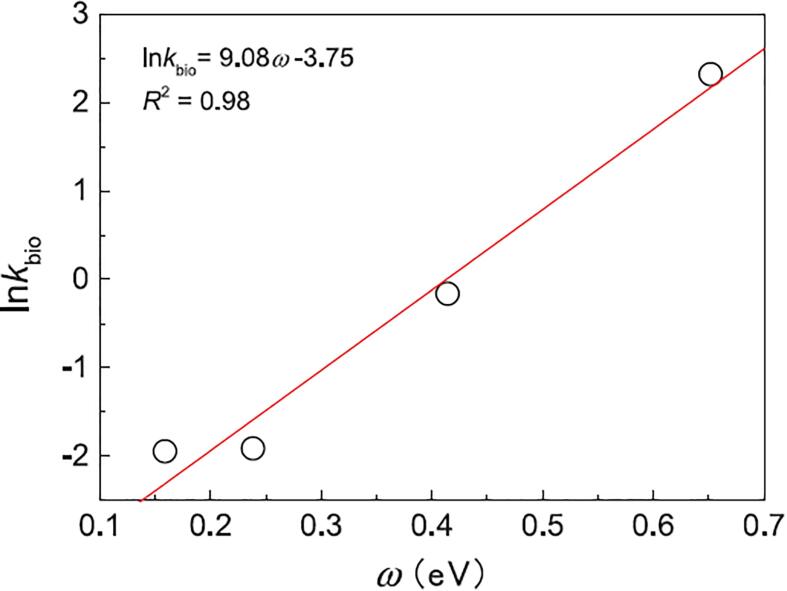
Correlation between electrophilic index (ω) and the natural logarithm of biodegradation constant rate (ln kbio) for metronidazole, sulfamethoxazole, bezafibrate and ibuprofen. Reprinted from [68], Copyright (2018), with permission from Elsevier.
In the hydrolysis pathways (catalyzed by hydrolases), water is a nucleophile. Similarly, cytochrome P450 enzymes can act as nucleophiles. Cytochrome P450 enzymes, are associated with the metabolism of many pharmaceuticals, through reactions such as hydroxylation, epoxidation, desulphurization, peroxidation, deamination, sulphur oxidation, dehalogenation, N–oxide reduction, and N/O/S-dealkylation. Particularly, iron (III)-peroxo porphyrin acts as a nucleophile adding either an epoxide or hydroxyl group to some pharmaceuticals. For example, sulfamethoxazole undergoes desulphurisation and deamination mediated by cytochrome P450 derivatives, producing 4-aminothiophenol, 3-amino-5-methylisoxazole, aniline, sulfanilamide, hydroxy-N-(5-methyl-1,2-oxazol-3-yl) benzene-1-sulfonamide and N-acetyl-sulfamethoxazole [68], [89]. A correlation between the biodegradation and quantum chemical molecular descriptors for pharmaceuticals was reported [68]. The electrophilic index (ω) presented a direct correlation with the biodegradation constants (kbio) for SMX, metronidazole, bezafibrate, and IBP (Fig. 5). Thus, ω could act as a predictive parameter for the biodegradability of pharmaceuticals. The electrophilicity index is an indicator of the ability of a molecule to add electron density (i.e., the molecule acts as an electrophile, adding electron density from a nucleophile). Some biochemical transformations follow this mechanism [68]. The information provided by ω is consistent with experimental evidence.
As the experimental determination of pharmaceutical biotransformation is a very complex process, several tools are currently available to predict the mechanistic pathways and the structures of most probable transformation products [93]. There are several internet sites relevant for the topics of biodegradation pathways that use of metabolic rules to predict plausible transformation products and enzymatic catalysis [94]. These tools have been successfully used to corroborate and predict biotransformation pathways and products. For instance, the application of the UM-BBD tool (a Biocatalysis/Biodegradation Database from the University of Minnesota) to amide-containing pharmaceuticals, predicted the formation of most transformation products determined experimentally [91], [93]. Another example of a biodegradation pathway prediction system utilisation was reported by Kosjek et al. [95], to identify biotransformation products of vincristine (an antimitotic and antineoplastic pharmaceutical). In such a work, the use of EAWAG BBD/PPS (the considered prediction system) resulted in a list of fourteen possible transformation products and three likely pathways for the first three stages of aerobic biotransformation were reported. Out of the fourteen suspects, four were found positive experimentally.
The EAWAG BBD/PPS system in combination with EPI Suite was also used by Letzel et al. for studying the biodegradation processes of five pharmaceuticals from the sartan group [92], starting with the theoretical predictions of transformation products, the authors developed an efficient workflow for the identification of the products. Similarly, predictors were used as a first step in the identification of biotransformation products of citalopram (a worldwide highly consumed antidepressant) formed in activated sludge [96].
Prediction systems have higher accuracy for compounds with similar structures to compounds with known biodegradation pathways, hence have limitations in applicability [97]. However, the use of predictive tools can generate valuable information for understanding the biotransformation of pharmaceutical CECs and can decrease money and time spent on experimental research [97].
2.1.3. Effect of operational parameters on biodegradation
2.1.3.1. Hydraulic and sludge retention time
Changes in SRT and HRT primarily affect intermediate biodegradable compounds. Increasing HRT allows longer contact time between the bacterial community and pharmaceuticals, increasing the removal percentage [98], [99]. Processes with high HRT provide more time for slow reactions such as biodegradation and sorption to occur [63]. A positive correlation between SRT and removal efficiency has also been reported for intermediate biodegradable pharmaceuticals [99], [100], [101], [102], and attributed to the heterotrophic bacteria community [78]. At higher SRT a more diverse microbial population with stronger biodegradation capabilities can be found considering enough time is provided to slow-growing microbes to develop [99], [100]. For readily biodegradable substances such as IBP and PCT, increasing SRT and HRT, or changing the type of microbes, have little effect on the removal efficiency [11], [98], [103] if a minimum SRT is adopted. The required minimum SRT depends on the type of pharmaceutical and for readily biodegradable pharmaceuticals a low minimum SRT can be expected (e.g., 5 days for IBP, Fig. 6) [100], [103]. When the bioreactor operates below a minimum SRT value, no removal due to biodegradation can be expected as a result of an insufficient microbial population. Under these circumstances, if a substance is not adsorbed by the sludge, the pharmaceutical will by-pass the WWTP with no or minor removal [100]. For persistent pharmaceuticals such as CBZ with low sorption and biodegradation rates, SRT and HRT do not play a major role in the degradation efficiency due to the little interaction between the compounds and microbes and/or sludge [98], [103]. However, contradictory results have been published for the slowly biodegradable DCF, where increasing SRT has caused both increasing [100] and decreasing [98] removal efficiency. According to Majewsky et al. [98], a reduction in SRT would increase the active heterotrophic biomass fraction, increasing degradation efficiency. Nevertheless, optimisation of pharmaceutical removal in existing plants requires altering SRT and HRT, which is difficult to implement [98].
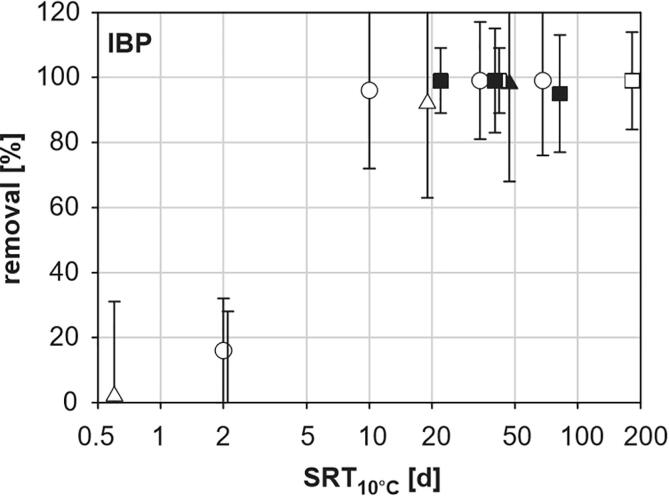
Fig. 6.
Removal percentage of ibuprofen (IBP) as a function of the sludge retention time (SRT). Reprinted from [100], Copyright (2005), with permission from Elsevier.
2.1.3.2. Temperature and pH
The working temperature in WWTPs usually ranges between 10 and 30 °C depending on location and seasonal variations [58], [104], [105]. Increasing temperature from 10 to 20 to 25–30 °C increased the degradation of pharmaceuticals such as TRC [99] and SMX [59], and was attributed to the increase in microbial activity during warmer conditions (e.g., summer season) [59], [106]. An average of 25% increase in the removal of pharmaceuticals has been presented in summer compared to winter [107]. Some studies, however, reported that the removal of micro-pollutants showed no clear dependency on the temperature measured in winter (12–20 °C) and summer (20–26 °C) [58], [74]. The concentration of pharmaceuticals in WWTPs also changes with higher concentrations in winter than summer, attributed to higher consumption of pharmaceuticals in the cold season [108]. At a given reactor temperature (winter or summer), pharmaceutical concentrations determined the structural shifts of the bacterial community in the ASP, showing a more diverse microbial community in reactors with a higher concentrations of pharmaceuticals potentially leading to a lower removal efficiency [109].
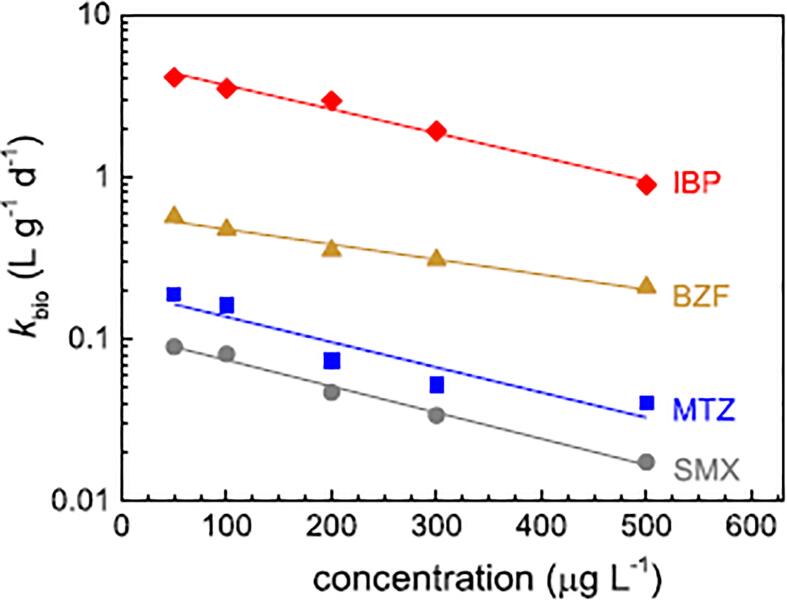
In wastewater, pharmaceuticals are often present at trace levels. They should not be considered as a source of carbon or energy for the metabolic activity of microorganisms. In contrast to typical substrates or nutrients (e.g., glucose), many pharmaceuticals and their metabolites in wastewater are potentially toxic or recalcitrant to microorganisms. Thus, high concentrations of pharmaceutical induce inhibitory effects on biodegradation (reflected as a decreasing of biodegradation rate constant, kbio) (Fig. 7)[89].
Fig. 7.
Relationship between initial concentration and biodegradation rate constant (kbio) for metronidazole (MTZ), sulfamethoxazole (SMX), bezafibrate (BZF) and ibuprofen (IBU). Reprinted from [89], Copyright (2019) with permission from Elsevier.
The removal of pharmaceuticals with acidic functional groups, (e.g. IBP, DCF and SMX) can be higher in acidic conditions due to the increased hydrophobicity of the substances [110]. The increase in pH speeds up the limiting step in their removal, which is the transfer from the water to sludge phase [77]. For non-ionisable compounds (containing no functional groups that can be protonated and de-protonated) such as CBZ, the removal is independent of the pH since compound hydrophobicity does not change significantly with pH [77], [81], [110].
3. Ultrasound (US) processing
US has been widely employed for the removal of pharmaceuticals in real (hospital wastewater, pharmaceutical industry effluent and municipal wastewater) [54], [111], [112] and synthetic samples [113], [114]. The concentration of pharmaceuticals such as DCF, CBZ, IBP and SMX vary in the range of ng L−1 to µg L−1 in real effluents after a biotreatment [6], [54], [111], [115]. For synthetic wastewater, the concentration varies from µg L−1 to mg L−1 [116], [117], [118], [119].
The degrading action by the US on pharmaceuticals is mainly associated with the acoustic cavitation phenomenon, which is the creation, growth and violent adiabatic collapse of bubbles [120]. Upon collapse, high temperatures and pressures are generated inside the bubble which dissociate water molecules to generate reactive radicals that can degrade the organic compounds [120]. The degradation location of these compounds can occur in three regions [121], [122]: (i) inside the bubble (ii) at the bubble-solution interface and (iii) in the bulk solution. The exact location would depend on the volatility, hydrophobicity and hydrophilicity of the organic compound, which can vary depending on the solution conditions.
Acoustic cavitation can be generated by employing plate transducers (20 kHz to 2 MHz) and horn sonicators (<100 kHz) for the removal of pharmaceuticals [111], [112], [113], [114], [116], [118], [119], [123], [124]. Lab-scale reactors are principally used with treatment volumes below 1 L [54], [113], [117], even though the treatment performance of a US pilot plant combined with ozone (O3) has also been assessed (reactor volume 2300 L) [125]. The extent of degradation depends on the sonication parameters (applied frequency, calorimetric power density and reactor type, irradiation mode [126]) and solution conditions (solution pH, temperature, the nature and concentration of the pollutant, organic matter, inorganics, suspended solids) [111], [117], [118], [119], [127], [128].
3.1. Solution conditions
3.1.1. pH
A change in the pH can alter the molecular structure of some pharmaceuticals and affect degradation under ultrasonic treatment depending on the functional groups of the compounds and their pKa value [116], [129]. The hydrophobicity of DCF (pKa = 4.2 [10], [102]) increases at pH < pKa because DCF remains at the molecular form that is more hydrophobic than the ionic molecules. This then allows DCF to move towards the cavitation bubbles where the concentration of radicals is higher, increasing the DCF degradation rate [118]. Similar results have been reported for CIP [116], CBZ [130] and IBP [30] with experiments conducted at low (~3), medium (~7) and high (~11) pH values. Nevertheless, Guyer and Ince [131] suggested that the pH value should be significantly higher than the pKa to see a relevant reduction in DCF hydrophobicity, as similar degradation rates were observed at pH 3 and 5.7.
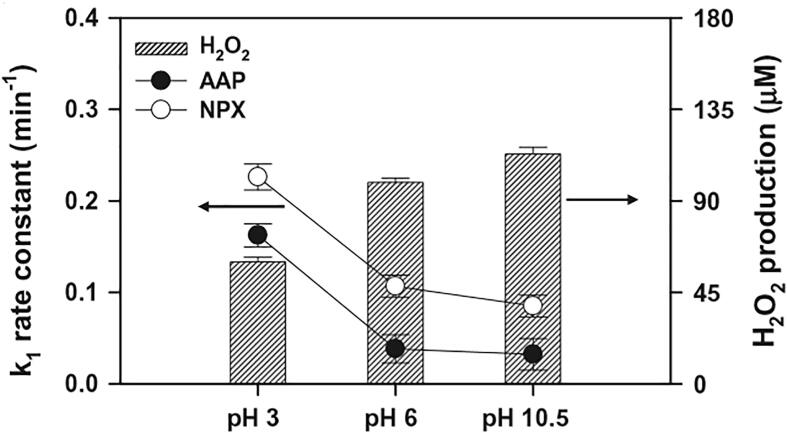
Although the relationship between pH and pKa seems to be the most reported explanation to interpret the abovementioned results, a different approach was taken by Rahmani et al. [113] to justify a reduction in tinidazole (TNZ) removal efficiency by increasing pH with the external addition of H2O2. According to the authors, a reduction in the oxidation potential of hydroxyl radicals (HO•) is observed at pH ≥ 3 along with the production of hydroperoxyl radicals (HO2•) by the reaction of H2O2 with HO•. The HO2• poses a low oxidation ability and further reacts with HO•, reducing the amount of hydroxyl radical available and thus, decreasing the removal efficiency of TNZ. Accordingly, a much larger production of H2O2 due to US irradiation has been reported at pH 3 than at pH 9, suggesting that the enhancement in degradation rate at low pH could also be due to the higher concentration of radicals accumulated in the liquid bulk upon H2O2 dissociation [129]. Additionally, H2O2 shows a stronger oxidation potential in the acidic medium than in the alkaline medium [132]. Hence for sono-degradation of pharmaceuticals the production rate of H2O2 at specific pH conditions (Fig. 8 [123] alongside the pKa values and logarithm of octanol–water distribution coefficients for different pharmaceutical compounds (Table 2) need to be considered.
Fig. 8.
Effect of solution pH on the degradation of acetaminophen (AAP) and naproxen (NPX) and the production of H2O2 at 580 kHz and 15 ± 1 °C. Reprinted from [123], Copyright (2014) with permission from Elsevier.
Table 2.
Properties of pharmaceuticals [123].
| Acetaminophen (AAP) | Naproxen (NPX) | |
|---|---|---|
| pKa | 9.4 | 4.2 |
| Log DOW (pH 3, 6, 10.5) | 0.91, 0.91, −0.28 | 3.18, 1.22, 0.05 |
| Henry’s law constant (KH) (atm m3 mol−1) | 6.42 × 10−13 | 3.39 × 10−10 |
The effect of pH on pharmaceutical removal by US changes when sonication is combined with other AOPs such as O3. O3 decomposition rate increases with increasing pH, which subsequently increases HO• production [133]. This effect then dominates when US is combined with O3, and an increase in pharmaceutical degradation rates with increasing pH is then reported [134], [135]. US (45 kHz) has also been coupled to electrochemical oxidation for the treatment of pharmaceutical wastewater after an aerobic-anoxic–oxic treatment. The reduction in pH was beneficial due to a higher production of hypochlorous acid (HOCl) compared to hypochlorite (ClO−) in chloride-containing wastewater, where the former, HOCl, is a stronger oxidant than the latter. US also enhances the diffusion of electrochemically generated HO• into the solution that would otherwise be attached to the electrode, resulting in an increase in HO• concentration [32].
3.1.2. Temperature
Increasing solution temperature can increase pharmaceutical removal by reducing cavitation threshold and thus, increase HO• production [40], [136]. That is, increasing solution temperature is expected to increase reaction rates, increasing removal rates of e.g. DCF [118], SMX and IBP [40]. However, Sutar et al. [137] showed using DCF that there exists a temperature threshold of 50 °C, above which increasing solution temperature reduced the degradation efficiency from 100% to below 20%. This decrease in sonochemical effect at elevated temperatures is caused by the increase in the vapour that enters the cavitation bubble which reduces the cavitation collapse intensity by the “cushion” effect [136], [138].
3.1.3. Type and concentration of pharmaceutical
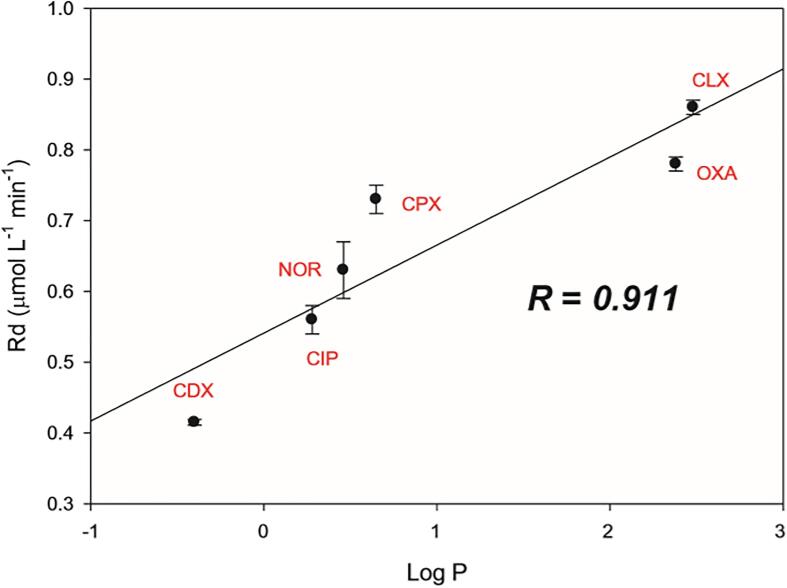
Sonochemical degradation efficiency of pharmaceuticals found in real [54] and synthetic [139] hospital wastewater is strongly dependent on their chemical structure. As mentioned before, the principal degradation mechanism is attributed to hydroxyl radical attack [113], [124], [140], [141] and thus, to the hydrophobic nature of the compound [139]. For pharmaceuticals with low volatility (Table 2), the higher the hydrophobicity of a given pharmaceutical, indicated by the logarithm of octanol–water partition coefficient (Log P, also named Log Kow), the higher the degradation rate (Fig. 9) [124], [128], [139], [142].
Fig. 9.
Degradation rate of cefadroxil (CDX), ciprofloxacin (CIP), norfloxacin (NOR), cephalexin (CPX), oxacillin (OXA) and cloxacillin (CLX) as a function of octanol–water partition coefficient (log P), which is correlated with hydrophobicity [139]. Note: Distribution coefficient and partition coefficient are different (but related) concepts. The first one refers to the concentration ratio of all species of the compound (ionized plus un-ionized). Meanwhile, the partition coefficient refers to the concentration ratio of non-ionized species of the compound [143].
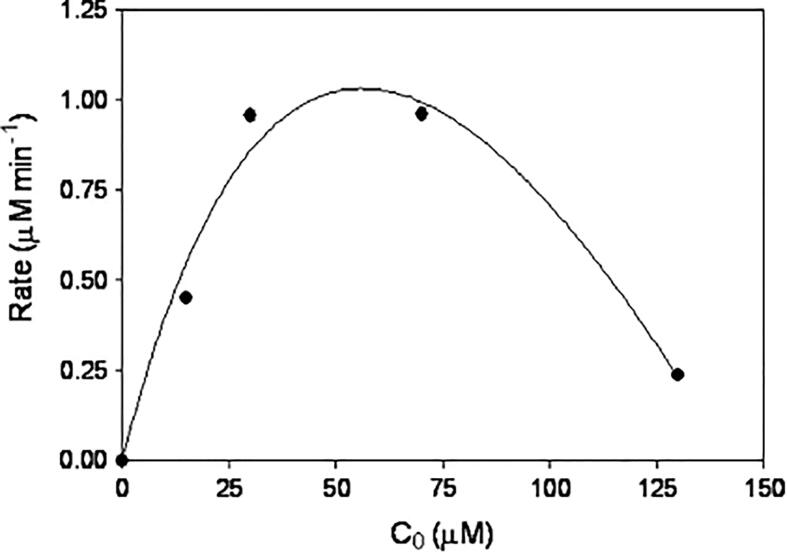
When the initial concentration of a single compound is low (i.e. below 10 mg L-1 for DCF), the degradation rate increases with increasing concentration (Fig. 10) [118], [131], [141], [144]. However, there exists a certain threshold concentration above which degradation rate decreases with increasing concentration and reduces removal efficiency (Fig. 10) [113], [117], [119], [131], [144]. This optimum concentration varies depending on the hydrophobicity of the pollutant [145]. At concentrations below the concentration threshold and provided that sufficient HO• are produced (dependent on applied frequency and power), increasing initial concentration of the pharmaceutical leads to an increase in the probability of HO• attack and hence the observed increase in the degradation rate [118], [131], [146]. This behaviour is also demonstrated by the reduction in H2O2 concentration with increasing initial solute concentration, underlining more HO• are consumed by increasing initial concentration of a given pharmaceutical and thus, less HO• are available for recombination into H2O2 [118]. When the concentration of pharmaceuticals is above the concentration threshold, the pharmaceuticals would be in excess compared to the amount of HO• present in solution [118]. This, coupled with the saturation of the pharmaceuticals at the bubble–liquid interface would be responsible for the decrease in removal efficiency at high initial solute concentrations [131], [145]. This shows the importance of the nature of the pharmaceutical (volatility, hydrophobicity, diffusivity, etc.) under study to achieve an optimum degradation efficiency with US in single pharmaceutical mixtures.
Fig. 10.
The impact of concentration on the initial rate of DCF degradation by ultrasound irradiation. 861 kHz, pH 5.7 and 40 min treatment. Reprinted from [131], Copyright (2011) with permission from Elsevier.
When a mixture of different pharmaceuticals is sonicated (e.g., AMX, DCF and CBZ) and even though the concentration of each of the tested pharmaceutical is low (2.5–10 mg/L) the high TOC value of the mixed solution and the competitiveness among the pharmaceuticals have shown to lead to degradation rates that were independent of the pharmaceutical concentration (zero-order kinetics) [147]. On the contrary, when the concentration of the mixed solution increased from 4 to 10 mg/L after up to 30 min of US treatment a reduction in the removal percentage of a mixture of SMX, DCF and CBZ was observed suggesting a dependence on the type of pharmaceuticals in the mixture [114].
3.2. Sonication parameters
Most of the studies found in the literature show results at one specific frequency: 275 kHz [22], 375 kHz [41], [115], 520 kHz [103], [106], 20 kHz [105], 354 kHz [127]. Considering solution and sonication conditions, reactor geometry and transducer type play an important role in sonochemical [148] and acoustic efficiency (the ratio of electric power to calorimetric power) [149], it is difficult to evaluate frequency effects across different studies. The acoustic efficiency among different transducers may vary considerably even though similar frequencies are employed. For example, to produce an acoustic calorimetric power of 11 W at frequencies of 520 and 400 kHz, the respective acoustic efficiencies were 58.2% (from electrical power of 20 W) [119] and 11.4% (from electrical power of 100 W) [130] despite similar treatment time and sample volume. Therefore, care needs to be taken when interpreting data from different frequencies and powers from different reports. Similarly, when reporting ultrasound-induced pharmaceutical pollutant degradation, it is important to report the sonication and solution conditions. Nevertheless, there are some general trends that can be drawn from reported ultrasound processes
3.2.1. Frequency
Frequency has a direct impact on the size of cavitation bubbles (larger at a lower frequency) and cavitation activity (higher at a higher frequency, but can decrease if frequency is too high)[150]. Thus, the collapse intensity of the bubbles (mechanical effects) and the production of radicals (chemical effects) have shown to depend on the frequency [126], leading to changes in the final degradation efficiency of pharmaceuticals. In the low-frequency range (<160 kHz), increasing frequency tends to increase the degradation percentages [111], [113], [151] for both horn reactor [151] and ultrasonic bath [113]. For the latter, the tinidazole degradation percentage plateaued between 120 and 180 kHz. This increase in the removal rate with increasing frequency is attributed to the increase in the production of radicals (HO• principally), suggesting radical attack as the main degradation mechanism.
In the mid-high frequency range (300–700 kHz) a significant increase in removal efficiencies have been reported compared to low-frequency ranges [123]. Although the reported optimum frequency (Table 3) to yield the highest degradation percentage differs from study to study, these frequencies were reported to produce the most HO• [124], [131], highlighting HO• as the principal removal mechanism. However, the relative differences in the degradation obtained between different frequencies were shown to be significantly smaller than the differences in the HO• production i.e. increasing frequency from 216 to 617 kHz resulted in a three-fold increase in H2O2 production rate, but the percentage degradation only increased by 3% [152]. This suggests that HO• production alone cannot explain the observed pharmaceutical degradation. Hence other factors such as bubble dynamics, mechanical effects and pyrolysis within the bubble core may contribute towards the degradation mechanism.
Table 3.
Frequency performance on the degradation percentage of different pharmaceuticals. For each of the studies, different concentrations, treatment times and power densities have been used and therefore, only the relative trend of the degradation percentages as a function of frequency should be compared across the different studies rather than the absolute values.
| Pharmaceutical |
Frequency (kHz) |
||||
|---|---|---|---|---|---|
| <300 | 300–500 | 500–700 | 700–900 | >900 | |
| Losartan [124] | – | 70% | 60% | 15% | – |
| Diclofenac [131]* | – | – | 94% | 96% | 89% |
| Diclofenac [152] | – | 92% | 95% | 25% | – |
| Atenolol [153] | 65% | 95% | 90% | – | 90% |
| Levodopa [117] | – | – | 91% | 90% | 66% |
| Paracetamol [117] | – | – | 95% | 92% | 67% |
| Ibuprofen [140] | 50% | – | – | – | 85% |
| Sulfamethoxazole [140] | 40% | – | – | – | 75% |
*Pseudo-first order rate constants and 60 min treatment.
3.2.2. Power
The applied power needs to exceed a certain power threshold to induce acoustic cavitation as below this limit, the amplitude of the soundwave is too small to cause bubble nucleation [126]. There is also an upper power limit, where further increase in acoustic power contributes to an increase in coalescence and degassing, thereby limiting the number of active cavitation bubbles and reduces removal efficiency. Independent of frequency and reactor type, increasing applied power density have shown to increase cavitation activity [150], [154], the production of H2O2 [152] and radicals [119], and therefore the observed increase in the degradation rate with power for compounds such as losartan [124], DCF and CBZ [111], [118], [130], levodopa and PCT [117], and IBU [141].
3.2.3. Pulsing
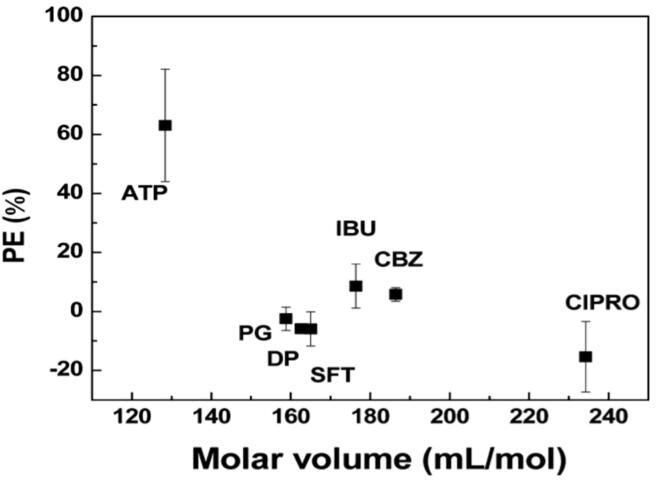
The importance of the irradiation pattern has been demonstrated not only for an increase in electrical and cavitation efficiency of ultrasonic reactors [154], but also for an improvement in pharmaceutical removal [137], [142], [155], [156], [157]. During ultrasound pulse off-times, pollutants accumulate in the liquid-bubble interface, and upon bubble collapse during ultrasound pulse on-times, degradation of pollutants increases [155], [157]. The diffusion to the cavitation bubble surface results in degradation enhancement, showing higher diffusivity for small-sized compounds [156]. This was supported by a strong correlation between the size of several pharmaceuticals (molar volume) and pulse enhancement which is a measure used to compare the difference in the rate of degradation between pulsed and continuous sonication (Fig. 11) (a positive pulse enhancement indicates that pulse sonication is more efficient than continuous pulsing). Pulsing mode would also benefit the degradation of pharmaceuticals with high diffusivity and/or hydrophobicity by providing time during the silent cycles for compounds to diffuse and accumulate on the liquid-bubble interfaces [142].
Fig. 11.
Pulse enhancement (PE) as a function of molar volume of five pharmaceuticals: acetaminophen (ATP), ibuprofen (IBU), carbamazepine (CBZ), ciprofloxacin (CIPRO) and sulfamethoxazole (SFT). Reprinted with permission from [156] Copyright 2013 American Chemical Society.
3.3. Transformations of pharmaceuticals under ultrasound action
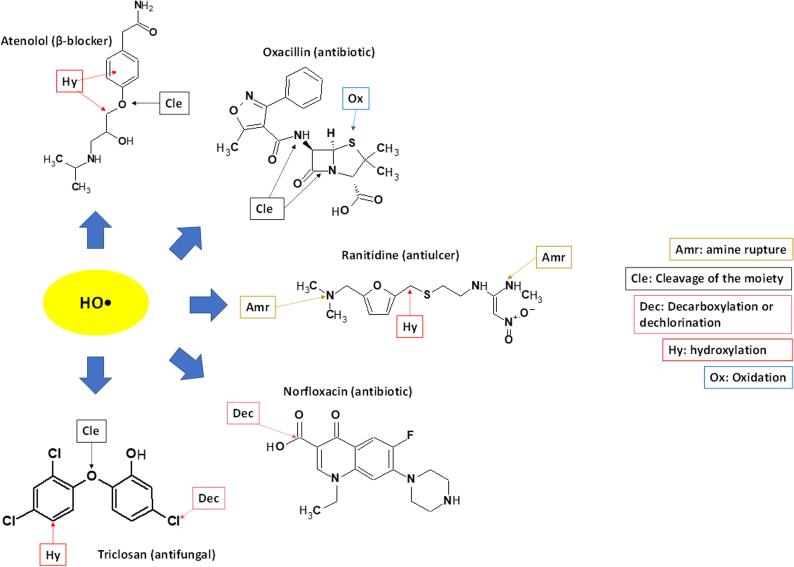
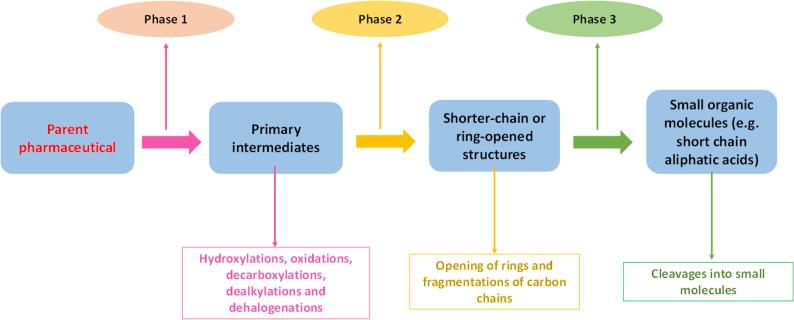
Structural identification of pharmaceutical transformation products shows that the US treatment initially induces modifications such as hydroxylations, oxidations, fragmentations, decarboxylations, dealkylations or dehalogenations (Fig. 12) [47]. In the case of the antibiotic cephalexin, the opening of its β-lactam ring by the action of sonogenerated HO• is an initial transformation route; whereas for norfloxacin, a decarboxylation pathway has been suggested [139]. Hydroxylation of aromatic rings is a typical transformation induced by HO•. This is observed during treatment by ultrasound of acetaminophen [158]. Also, atenolol presents hydroxylations of its aromatic ring and propoxyl group, in addition to a rupture of the central ether [159]. Pathways of opening of β-lactam and fragmentation of the central amide, plus oxidation of the thioether group, have been found in the sonodegradation of oxacillin [29].
Fig. 12.
Typical primary transformations induced by the sonogenerated hydroxyl radical (HO•) on pharmaceuticals.
The treatment of CBZ by ultrasound leads to hydroxylations and epoxidation at the double bond in the middle of its structure, with the subsequent fragmentations of the initial products [119]. Meanwhile, the antiulcer ranitidine is transformed by HO• through attacks to N,N-dimethylamine, N-methylamine and α-carbons to the sulphur on its structure [160]. In turn, the antihypertensive losartan is degraded by means of an imidazole ring rupture, hydroxylations of the biphenyl-tetrazole nucleus and alkyl moiety, besides oxidation of its alcohol group [124].
Breakdown of the sulfonamide moiety (i.e., the S-N bond) and hydroxylation of the aniline group are the primary modifications informed for sulfamethazine treatment by sonochemical action [161]. The degradation of antifungal TRC leads to hydroxylated and dechlorinated products, in addition to cleavages through its ether moiety, when subjected to ultrasound [162].
Overall, sonochemical transformations of the pharmaceuticals follow a succession of three phases (Fig. 13): phase 1) generation of initial degradation products from parent pharmaceutical; phase 2) cleavage of the initial intermediates to produce shorter-chain or ring-opened substances; and phase 3) degradation of molecules from phase 2 into small compounds such as aliphatic carboxylic acids [39].
Fig. 13.
Schematic sequence of phases during pharmaceutical degradation by sonochemistry.
3.4. Toxicity assessment of intermediates produced by US
US is effective in pharmaceutical degradation but lacks evidence for total mineralisation; i.e. the process shows low TOC removal [29], [118], [130], [147], [163]. This is because most primary degradation products are hydrophilic, which limits their proximity to the cavitation bubble where most sonogenerated HO• exist. The intermediates produced can also be toxic to aquatic ecosystems, in some cases more toxic than the parent compounds [163]. This is the case of DCF, where upon sonication the toxicity of the samples increases within the first ~ 20–30 min of the reaction. By prolonging sonication time, toxic by-products gradually degrade (mineralise) and toxicity reduces [31], [118]. A similar behaviour has been reported for CBZ [130], amoxicillin [163], NOR [54] and CIPRO [116] in distilled water (synthetic waters). Likewise, an initial increase and a continuous decrease in toxicity have also been reported for US-based AOPs such as sonoelectrochemistry [32], sonophotocatalysis [164] and US-enhanced catalytic ozonation [165]. When real and more complex wastewater are tested, a small variation in wastewater characteristics may show different behaviours in the evolution of toxicity under ultrasonic irradiation [147]. Thus, toxicity determination on pharmaceutical containing effluents and studies on the behaviour and evolution of intermediates in sonicated waters [54], [111], [147] and WWTPs [108], [166], [167] are required alongside US treatment.
Although US has a low mineralising capability, when applied to antibiotics some by-products without antimicrobial activity are formed. This is because the sonogenerated HO• modifies the moieties on antibiotics responsible for such activity [139], [29]. Sonochemical treatment also generates smaller molecules that are more biodegradable than the parent compounds [39], indicating the positive changes induced by the ultrasound action in water contaminated with pharmaceuticals.
4. Membrane filtration
Membrane filtration systems differ in terms of pore size and surface properties. In decreasing pore sizes, these membranes include microfiltration (MF), ultrafiltration (UF), nano filtration (NF), reverse osmosis (RO), and forward osmosis (FO). The selection of the type of membrane filtration then depends on the property of the contaminant to be separated in terms of size, charge and hydrophobicity and hydrophilicity.
4.1. Membrane filtration operations
Membrane filtration of liquid effluent feed will generate two different streams, namely retentate and permeate. The retentate is a concentrated stream containing components that are rejected by the membrane and the permeate stream is the water that passes through the membrane. The performance or efficiency of the filtration process is governed by the water permeate flux and the quality of this water permeate stream depends on the membrane’s ability to rejection the targeted component. Membrane filtration is a pressure driven process and the relationship between the water permeate flux and pressure is described by Eq. (1):
| (1) |
where Jw is the water permeate flux, is the transmembrane pressure (TMP) across the membrane, the is the osmotic pressure difference between the feed and the permeate, and the A is the water permeability characteristic of the membrane. The membrane’s apparent rejection factor (R) is calculated according to the concentration of the targeted component in the feed solution (Cf) and the permeate (Cp) according to Eq. (2):
| (2) |
The required TMP to achieve a certain desired water permeate flux will depend on the effluent feed characteristics in terms of osmotic pressure and components that could foul the membrane surface such as suspended solids. However, increasing TMP will increase the cost associated with the filtration process, and the propensity for the membrane to foul will also increase [168].
For forward osmosis, rather than the application of a hydraulic pressure across the membrane, a draw solution with a high osmotic pressure compared to the feed is used on the other side of the membrane [169]. The osmotic difference between the feed and the draw solution then forces water to flow from the feed to the draw solution side without the need to apply an external hydraulic pressure. The water permeate flux is then described by Eq. (3):
| (3) |
where are the osmostic pressures of the draw solution and feed, respectively. Although FO is an attractive form of recovering water without the expensive high hydraulic pressure, this will only be viable if the low cost draw solution with high osmotic pressure are available and does not need to be regenerated to recover the water and recycled back to the system [169], [170].
4.1.1. Concentration polarisation
It is clear from Eqs. (1), (3) that the osmotic pressure of the feed, permeate and draw solution is important. For processes such as NF and RO, the TMP needs to overcome the osmotic pressure of the feed, which is why high TMP is required in the desalination of sea water. Similarly, for FO, the osmotic pressure in the draw solution must also overcome the osmotic pressure of the feed. However, often the osmotic pressure of the feed is lower than the actual osmotic pressure at the membrane surface. This is due to the development of concentration polarization, which is the buildup of a solute concentration gradient at a membrane surface brought about by the convective flow of solutes towards the membrane surface and the high membrane surface reject properties. This causes the concentration of the solute on the membrane surface facing the feed side to be higher than that in the bulk, and the consequence of this is a much higher osmotic pressure on the membrane surface than in the bulk (concentrative concentration polarization). Therefore, if the bulk osmotic pressure was used in Eq. (1) and Eq. (3), this would underestimate the net driving pressure for the system and result in a lower water permeate flux (Eq (1)) than expected. The TMP must be increased to compensate for the concentration polarization effect to achieve the desired water permeate flux. This concentration polarization increases as the feed becomes more concentrated, which decreases the rejection efficiency as well as filtration performance and increases operational cost. Concentration polarization is more severe for forward osmosis because a high concentration of salt is usually used as the draw solution, causing a dilutive concentration polarization on the draw solution side that further decreases the effective TMP across the membrane (Eq. (3)).
4.1.2. Membrane fouling
One of the major challenges facing membrane filtration processes is fouling. This is a process whereby colloidal particulates, organic matter and bacteria in solution is deposited or adsorbed onto the surface of the membrane, forming a surface layer that can significantly reduce the water permeate flux as well as rejection factor [171], [172] and subsequently increase operational cost.
4.2. Pharmaceutical separation
The molecular weight cutoff of most of microfiltration (MF) and ultrafiltration (UF) membranes is larger than the size and molecular weight of most pharmaceuticals [173]. Therefore, efficient separations of pharmaceuticals from water and wastewater can be achieved with NF, RO and FO membranes.
When NF/RO are employed for pharmaceutical removal, rejection can happen by hydrophobic and electrostatic interactions, as well as by size exclusion [174]. It is important to highlight that rejection by size exclusion is only achieved for non-hydrophobic neutral and positively charged pharmaceuticals [173]. Hydrophobic compounds can interact with the hydrophobic membrane surface, adsorb onto and diffuse through it, while negatively charged solutes get rejected by electrostatic interaction (negatively charged membrane surface) [173]. Taking this into account, the rejection of pharmaceuticals in NF/RO is mainly influenced by feedwater characteristics (pH, type and amount of organic matter, suspended solids and ionic strength), nature of the pharmaceutical and membrane properties [173], [175], leading to rejection values above 95% [176], [177], [178]. FO membranes have achieved a similar removal efficiency for pharmaceuticals such as DCF, CBZ and IBP, showing a strong correlation between compound hydrophobicity and rejection percentage (≥93%) [179].
High rejection factors will lead to a cleaner permeate effluent, but at the same time, will produce a concentrated stream (retentate) that will require further treatment. Pharmaceutical concentration in the retentate depends on the concentration factor (ratio of the concentration of the compound in the retentate compared to the feed [175], [180]), which varies from 3 to 5 [181], [182] and up to 40 with RO if the compound is completely retained and the recovery of the system is high [175]. This retentate with high pharmaceutical concentration needs to be further treated before discharge.
The development of a fouling layer has shown to severely increase [183], [184] or decrease [185], [186] the removal of pharmaceutical by NF and RO membranes. The retention of six pharmaceutical compounds was observed to be higher when real wastewater effluent (>60%) was filtered compared to a synthetic solution containing just the pharmaceuticals and water (<60%) [183]. This was attributed to alterations of the membrane surface properties by the organic fouling layer and the interaction between the organic macromolecules with the pharmaceuticals. The characteristic of the organic macromolecules, which differs depending on the pretreatment prior to NF/RO process, was shown to be important [183]. Conversely, for biofouling, a decrease in the retention of pharmaceutical compounds was observed for positively charged pharmaceuticals. This effect was attributed to the development of a negatively charged biofoulant layer attracting the accumulation of the positively charged pharmaceuticals within the fouling layer, thereby reducing the rejection efficiency.
5. Combined systems
As outlined in the above sections biological treatment methods are ineffective in the removal of compounds such as CBZ due to the little interaction of this compound with the MLSS [22], [23]. Ultrasound has proven effective in the degradation of pharmaceuticals from water and wastewater, however, despite reaching 100% degradation for certain pharmaceuticals, sonication alone is not sufficient to achieve high or complete mineralisation [29], [30]. Sonication has shown to lead to the formation of intermediates with a higher toxicity than the parent compounds [31], [32], but in some cases these intermediates can increase biodegradability or reduce antimicrobial resistance. NF, FO and RO have shown high pharmaceutical rejection but do not serve as an absolute barrier against all pharmaceuticals [38], producing concentrated solutions that need to be further treated prior to discharge. The optimisation of the abovementioned treatment methods could therefore be obtained by combining filtration systems with bioreactors and/or ultrasound, or combining ultrasound with other AOPs.
5.1. Biological systems coupled with ultrasound
For treating water/wastewater containing pharmaceuticals, the combination of a biological process with ultrasound can be performed sequentially and simultaneously. Within the former, two configurations are possible: ultrasound as a pre-treatment to a biological system or ultrasound as a post-treatment to the bioprocess.
Considering few toxic and/or non-biodegradable pharmaceuticals are present in simple water matrices (i.e., “pure” water or water with few components), the application of sonochemical action first is recommended. For instance, fluoxetine in deionised water was not degraded by aerobic microorganisms; however, the pre-treatment of this pharmaceutical using ultrasound improved biodegradability [187]. Similarly, oxacillin in simulated wastewater from a pharmaceutical industry (water containing the target pollutant, mannitol and calcium carbonate) was pre-treated sonochemically, leading to biodegradable compounds (with no antibiotic activity) that were mineralised by a conventional biological process [29]. A recent research on the sequential combination of ultrasound with unadapted activated sludge to degrade IBP also showed that the pre-treatment with ultrasound positively changes the biodegradability of water polluted with such pharmaceutical, indicating that ultrasonication is a promising technology to assist the conventional biological treatments [188].
The strategy of biological treatment prior to ultrasonic process is more convenient for complex matrices having high amounts of biodegradable substances in addition to pharmaceuticals (e.g., hospital or municipal wastewater). This is because the biological system acts as a “filter”, diminishing organic matter that can compete with the pharmaceuticals for the US-generated radicals [54], [147]. Biological treatment followed by US was applied to treat hospital effluents [54] and the degradation of fifteen pharmaceuticals evaluated. Biological and sonochemical systems were complementary as biodegradable organic matter (e.g., macro-components) in the hospital effluent was removed by the biological system, but most pharmaceuticals were not biodegraded. Moreover, the concentrations of some pharmaceuticals (norfloxacin, ciprofloxacin and clarithromycin) increased, and only acetaminophen and valsartan were significantly diminished by the biotreatment. Then, the ultrasonic action decreased the concentrations of pharmaceuticals except for acetaminophen and valsartan, suggesting that these substances were released from active sludge flocs coming from the biological process. When the sorbed pharmaceuticals were released, they also are susceptible to the chemical effects of ultrasound. Furthermore, the sonogenerated HO• led to ~ 59% of pondered removal of pharmaceuticals [54]. Similarly, municipal wastewater previously biotreated in WWTP and loaded with pharmaceuticals was subjected to US. In this regard, an illustrative work on the sonication of a mixture of three pharmaceuticals (DCF, amoxicillin and CBZ) spiked on an effluent from an urban wastewater treatment plant was reported by Naddeo et al. [147]. The ultrasound application produced solutions susceptible to be discharged into natural aqueous media.
The above-presented information was focused on the sequential combination of biological systems with ultrasound; nonetheless, the simultaneous combination could be considered. Although information about the degradation of pharmaceuticals by such combination is still scarce, it is widely reported that the sonication enhances the performance of biological treatments, especially for the nutrient substrate removals. The ultrasonic process increases the enzymatic activity, and thus decreases the detention time and time of hydrolysis (which is a limiting factor in digestion step), also this may limit the sludges accumulation [189], [190]. Additionally, ultrasound increases the removal of pollutants by improving the biological activity sludges, changing the cell membrane permeability, enhancing cell growth and biosynthesis [191].
5.2. Biological system coupled with membranes (Membrane bioreactors, MBR)
A MBR is the combination of activated sludge treatment with MF or UF membranes, and therefore provides a solid–liquid separation and avoids the use of secondary clarifiers. The membrane can either be integrated and submerged inside the bioreactor or external to the bioreactor as a side stream. The major drawback this system poses is membrane fouling by the formation of a biofilm on the membrane surface [192]. The structural integrity and physical properties of this biofilm is governed by the extracelluar polymeric substances (EPSs), natural polymers of high molecular weight, secreted by microorganisms in the bioreactor.
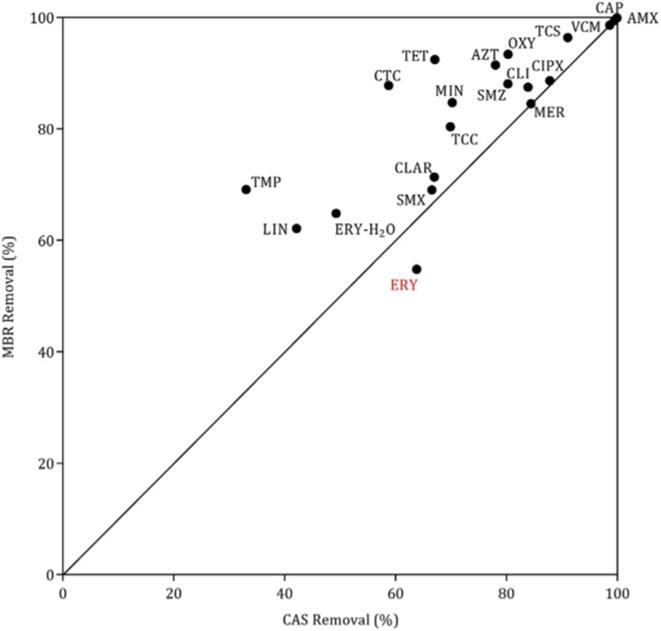
MBR have been recognised as promising current and future biotreatment methods [192] due to the possibility of achieving higher SRT and MLSS within compact reactors compared to ASP [100], [193], [194]. As observed in ASP, longer SRT would generate more diverse microbial population (e.g. nitrifying bacteria) [36] and higher concentration of MLSS can derive into low food to microorganism ratio (F:M ratio), where the lack of biodegradable organic matter may cause microbes to use poorly degradable compounds as substrate. Therefore, MBR were reported to give higher pharmaceutical removal efficiencies (Fig. 14) [36], [37] and are capable of meeting more sensitive discharge water standards compared to ASP [192]. However, treatment efficiencies have shown to be similar when ASP and MBR are operated with comparable operational parameters such as SRT [100]. ASP and MBR had the same removal efficiency for easy to degrade pharmaceuticals such as IBP [194], whereas for poorly biodegradable compounds such as CBZ, MBR also showed a low removal efficiency [195]. Thus, MBR would be advantageous over ASP for the elimination of moderately removed pharmaceuticals [194] such as ketoprofen and naproxen [90]. However, it is difficult to conclude whether the removal of pharmaceuticals is better in MBR as biodegradation is affected by many other factors not directly related to reactor configuration. In order to increase the removal efficiency of conventional MBR, these bioreactors can be coupled to NF [196] or RO [38] membranes and obtain removal efficiencies > 90%.
Fig. 14.
Removal of antibiotics and antimicrobials in membrane bioreactor (MBR) and conventional activated sludge (CAS) processes. Trimethoprim (TMP), lincomycin (LIN), erythromycin (ERY), sulfamethoxazole (SMX), clarithromycin (CLAR), triclocarban (TCC), minocycline (MIN), tetracycline (TET), sulfamethazine (SMZ), chlortetracycline (CTC), meropenem (MER), clindamycin (CLI), azithromycin (AZT), ciprofloxacin (CIPX), oxytetracycline (OXY), triclosan (TCS), vancomycin (VCM), chloramphenicol (CAP), amoxicillin (AMX). Reprinted from [36], Copyright (2016) with permission from Elsevier.
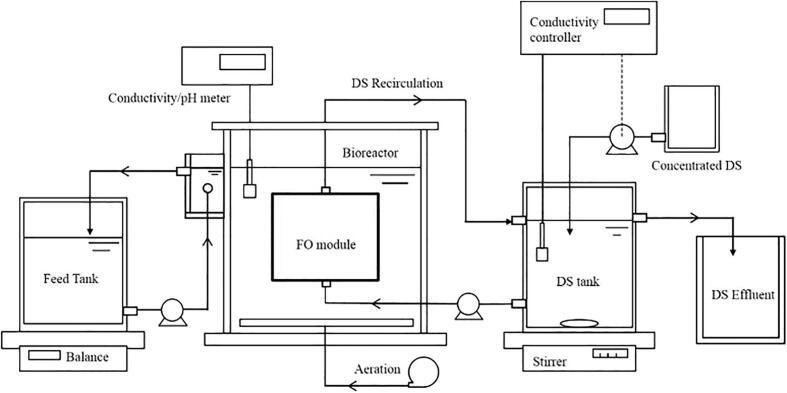
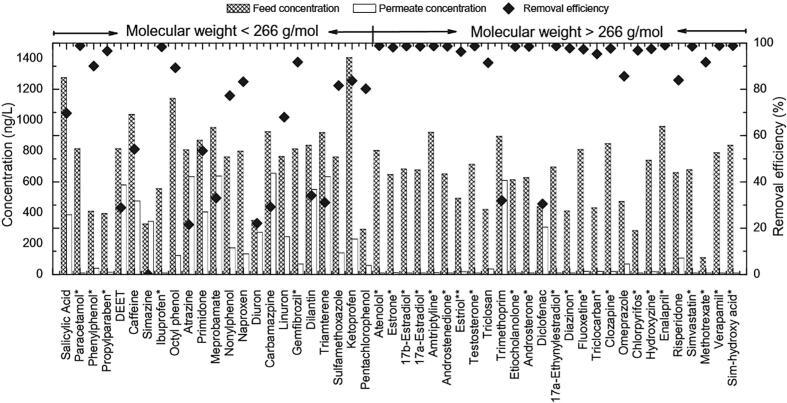
FO membrane bioreactors also referred to as osmotic membrane bioreactors (OMBR) is a recently developed treatment process (Fig. 15) [197], [198], [199] applied to pharmaceutical removal [200], [201]. A good removal percentage (≥80%) was obtained for high molecular weight compounds (>266 g/mol). The physical separation provided by FO membrane contributed to an increase in the retention time of these high molecular weight compounds within the bioreactor (higher retention time than the actual HRT) and thus, allowing high pharmaceutical removal through biodegradation. Considering physical rejection of low molecular weight compounds by FO is expected to be minimal, the removal of low molecular weight compounds, therefore, relies both on the rate of the biodegradation during the HRT and on the rate the compound is permeating through the membrane (Fig. 16) [71], [200]. The principal advantage of FO compared to RO is the lower operating cost due to the need of high external hydraulic pressure applied with the latter [202], although regeneration of the draw solutions in FO systems is necessary [203]. Despite high removal rate (>90%) is achieved with MBRs, they do not provide an absolute barrier to these type of micropollutants and thus, additional or coupled treatment methods should be considered [38], such as the combination of US and membranes or US-based AOPs.
Fig. 15.
Schematic diagram of the OMBR setup. Reprinted from [71], Copyright (2018) with permission from Elsevier.
Fig. 16.
Concentration of organic contaminants in the feed and the permeate, as well as the removal efficiencies by the OMBR system. Reprinted from [200], Copyright (2012) with permission from Elsevier.
5.3. Membrane filtration and ultrasound
The coupling of ultrasound and membrane filtration systems have been reported, but mainly for the physical effects of ultrasound brought about by the pressure gradient and the violent oscillations and collapse of cavitation bubbles. These effects have been covered in reviews focused on ultrasound enhancement of permeate flux, removal or prevention in the development of fouling, and membrane surface cleaning [204], [205]. More recently, ultrasound has been applied to FO process to reduce internal concentration polarization and increase water permeate flux [206]. These studies have mainly focused on frequencies below 100 kHz.
Membrane filtration was coupled with ultrasound for the treatment of pharmaceutical compounds, in the presence of activated carbon as key adsorbent [207]. Secondes et al. [207] simultaneously combined ultrafiltration (UF), activated carbon (AC) and US (35 and 130 kHz) for the removal of DCF, CBZ and SMX in deionised water (Fig. 17). The lowest frequency was shown to increase the adsorption of the pharmaceuticals into the AC compared to the highest frequency and this improved adsorption was attributed to the increase in the collapse intensity of the cavitating bubbles at 35 kHz. No changes were observed in the trans-membrane pressure (TMP) throughout the process (i.e. no fouling). This study did not report the effect of the ultrasound on the degradation of the pharmaceuticals and filtration without the activated carbon. However, in a later study [112], the same system was applied to a real effluent containing the same three pharmaceuticals. With the real effluent, fouling was observed in the UF-only study but this fouling layer improved the removal by>20%. Coupling US to UF only slightly improved the removal percentage (5–10% for SMX, 20–30% for DCF and CBZ), with the 130 kHz performing slightly better than the 20 kHz. This improvement by the higher frequency was attributed to the milder collapse intensity of cavitation bubbles that would not dislodge the fouling layer. When AC was added to the membrane filtration system, the removal increased to 90%, which was further improved by US to 99% with no differences the two frequencies. Although the degradation of the pharmaceutical compounds by US was not evaluated, this was used to explain the improved removal.
Fig. 17.
Schematic diagram of the ultrasound, activated carbon and ultrafiltration membrane treatment unit. Reprinted from [207], Copyright (2014) with permission from Elsevier.
5.4. Biological-Membrane-Ultrasound hybrid system.
There are only a few reports on the hybrid biological-membrane-ultrasound (BMUS) systems for treating waste effluents. One study [208] used BMUS for the digestion of waste activated sludge where the US coupled membrane filtration system was external to the anaerobic MBR (Fig. 18). In that study, ultrasound was found to not have altered the physical properties of the digestion broth to enhance the filtration permeation but was able to selectively remove the outer lose fouling layer of the membrane and slightly improve permeation.
Fig. 18.
A schematic diagram of US coupled with an anaerobic membrane bioreactor. Reprinted from [208], Copyright (2013) with permission from Elsevier.
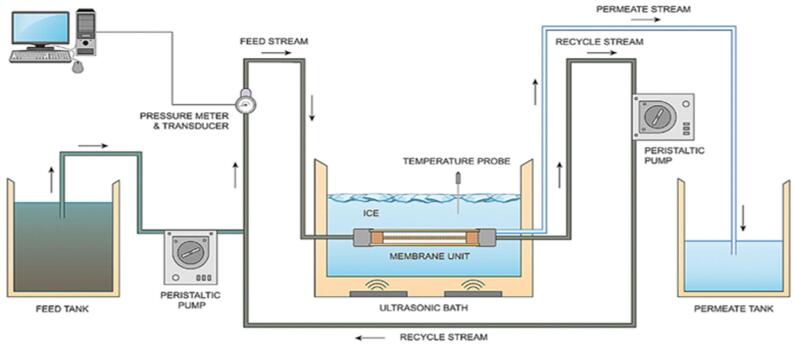
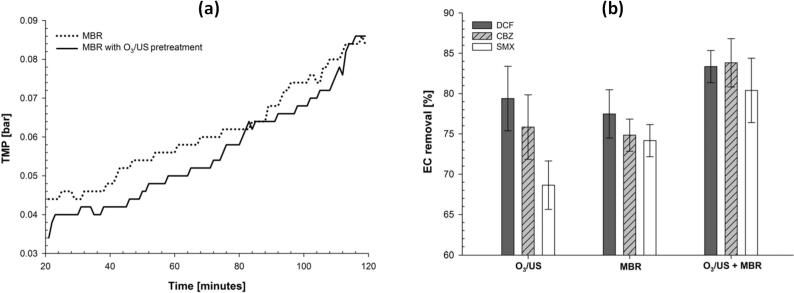
Another study [209] applied the BMUS system on pharmaceutical waste (DCF, SMX and CBZ) and used ultrasound coupled with O3 to pretreat the effluent prior to an MBR system in a batch mode. The US-O3 pretreatment reduced slightly the fouling in the MBR (Fig. 19a) under 180 min of treatment and enhanced the pharmaceutical removal (Fig. 19b). This was attributed to the effect of US-O3 on the microbial metabolism products (reduced EPS concentration by 50%) on the MBR. US-O3 pretreatment did not lower the toxicity of the wastewater prior to the MBR, presumably caused by the formation of more toxic intermediates by US-O3. However, the toxicity of the permeate from the MBR was much lower than the MBR influent stream, suggesting that the intermediates were more biologically oxidizable in the MBR.
Fig. 19.
(a) TMP of the MBR for a given permeate flux as a function of time with and without O3/US pre-treatment prior to MBR and (b) The removal of pharmaceutical compounds (DCF, CBZ and SMX) after different treatments: O3/US treatment, MBR process and O3/US followed by MBR in series [209].
In both of the above mentioned (BMUS) studies, low frequency ultrasound (20 kHz [209] and 28 kHz [208]) were used which would have low sono-degradation efficiencies. To the best of the authors’ knowledge, no BMUS systems using high frequency ultrasound and their potential to further improve the degradation efficiency have not been reported yet.
6. Summary and future perspectives
This review has highlighted the performance of the individual treatments (biological ASP, ultrasound and membrane filtration) and the benefits of hybrid combined systems.
In the ASP, the two main removal mechanisms have been identified as biodegradation and sludge sorption. High removal (>90%) could be obtained for easily degradable compounds such as IBP or PCT, while the removal of CBZ is often found below 20% due to the little interaction of CBZ with microbes and/or sludge present in the bioreactor. Biodegradation of some pharmaceuticals can be associated with its electrophilicity index, while the sorption coefficient depends on compoundś hydrophobicity. More hydrophobic and electrophilic pharmaceuticals tend to achieve a better removal percentage. While intermediate biodegradable compounds would be primarily affected by changes in SRT and HRT, and higher temperatures and low pH (increase in hydrophobicity) lead to increasing removal performance. However, as identified in this review, the biotransformation of pharmaceutical products is important and should not be assumed to be equivalent to bioremoval because the transformed products can be more toxic than the parent compound. Therefore, research into biological degradation should also evaluate the biotransformation intermediates.
Ultrasound has shown to be effective at degrading pharmaceuticals, however the reported degradation efficiency varies depending on the sonication and solution conditions. Studies often employ one frequency and it is often difficult to compare across studies to evaluate the effect of frequency due to the differences in reactor geometry and ultrasound transducer efficiency. Nevertheless, general observations are that frequencies between 300 and 600 kHz usually result in higher sonochemical activity, leading to the highest compound removal. For small-sized pharmaceuticals (high diffusivity), ultrasound pulsing has shown to give higher removal percentages compared to continuous sonication. However, more research is needed to capitalize on the benefit of pulsing, which is more energy efficient compared to continuous sonication. As with biodegradation processes, biotransformation of pharmaceuticals under sonication cannot be neglected as studies have shown sonication can lead to more toxic intermediates.
Membrane filtration systems such as RO and FO, although not absolute, have shown high rejection for typical pharmaceuticals found in WWTPs, with the ability to concentrate these compounds up to 40 folds. This is important when concentration or volume reduction is required. Pharmaceutical compounds in effluents are usually in trace quantities, and therefore the concentration of the effluent would be important for detection and treatment. However, industries with real effluents often face challenges such as concentration polarization and fouling which limits the filtration efficiency and increases operational costs. In addition, the concentrated retentate with high concentration of pharmaceutics would have low biodegradability or high bactericidal toxic effect that limits the treatment using biological processes.
This review has highlighted the advantages of combining the above treatments in a hybrid system. MBRs, the combination of the biological digester and membrane filtration, are widely implemented in wastewater treatment plants, however, there is a lack of evidence that MBRs are better than removing pharmaceuticals compared to conventional bioreactors. The combination of ultrasound with bioreactors or filtration membranes have been reported, but these studies have used low frequency ultrasound to capitalise on the stronger physical impact on removing and preventing membrane fouling. The combination of bioreactor, membrane and ultrasound have shown to demonstrate potential whether as integrated or in series, but the number of published studies on these hybrid BMUS systems are limited. There is also scope to investigate the order in which ultrasound is applied as pre-treatment, post-treatment or integrated system, as well as synergistic effects when coupled with other AOPs and adsorption processes. There is a clear research gap on the benefits of high frequency ultrasound (200–600 kHz) for the hybrid BMUS systems that needs to be explored to further improve the removal of pharmaceuticals.
CRediT authorship contribution statement
Pello Alfonso-Muniozguren: Conceptualization, Writing - original draft, Writing - review & editing, Visualization. Efraím A. Serna-Galvis: Conceptualization, Writing - review & editing, Visualization. Madeleine Bussemaker: Writing - review & editing. Ricardo A. Torres-Palma: Writing - review & editing, Supervision, Funding acquisition. Judy Lee: Conceptualization, Writing - review & editing, Visualization, Supervision, Project administration, Funding acquisition.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
The authors thank the financial support provided by the Royal Society (UK) through the project: “Sound” methods of remediating emerging contaminants in hospital wastewater (ICA\R1\191053). The authors also acknowledge the support provided by MINCIENCIAS COLOMBIA (before named COLCIENCIAS) through the project No. 111577757323; as well as Universidad de Antioquia UdeA by means “Programa de Sostenibilidad”.
Contributor Information
Ricardo A. Torres-Palma, Email: ricardo.torres@udea.edu.co.
Judy Lee, Email: j.y.lee@surrey.ac.uk.
References
- 1.Nations, U. World Population Prospects: The 2017 Revision, Key Findings and Advance Tables; Departement of Economic and Social Affaire: New York City, NY, USA, 2017.
- 2.Luo Y., Guo W., Ngo H.H., Nghiem L.D., Hai F.I., Zhang J., Liang S., Wang X.C. A review on the occurrence of micropollutants in the aquatic environment and their fate and removal during wastewater treatment. Sci. Total Environ. 2014;473–474:619–641. doi: 10.1016/j.scitotenv.2013.12.065. [DOI] [PubMed] [Google Scholar]
- 3.Salimi M., Esrafili A., Gholami M., Jafari A.J., Kalantary R.R., Farzadkia M., Kermani M., Sobhi H.R. Contaminants of emerging concern: a review of new approach in AOP technologies. Environ. Monit. Assess. 2017;189:414. doi: 10.1007/s10661-017-6097-x. [DOI] [PubMed] [Google Scholar]
- 4.Wiest L., Chonova T., Bergé A., Baudot R., Bessueille-Barbier F., Ayouni-Derouiche L., Vulliet E. Two-year survey of specific hospital wastewater treatment and its impact on pharmaceutical discharges. Environ. Sci. Pollut. Res. Int. 2018;25:9207–9218. doi: 10.1007/s11356-017-9662-5. [DOI] [PubMed] [Google Scholar]
- 5.Michael I., Rizzo L., McArdell C.S., Manaia C.M., Merlin C., Schwartz T., Dagot C., Fatta-Kassinos D. Urban wastewater treatment plants as hotspots for the release of antibiotics in the environment: A review. Water Res. 2013;47(3):957–995. doi: 10.1016/j.watres.2012.11.027. [DOI] [PubMed] [Google Scholar]
- 6.Kovalova L., Siegrist H., von Gunten U., Eugster J., Hagenbuch M., Wittmer A., Moser R., McArdell C.S. Elimination of micropollutants during post-treatment of hospital wastewater with powdered activated carbon, ozone, and UV. Environ. Sci. Technol. 2013;47:7899–7908. doi: 10.1021/es400708w. [DOI] [PubMed] [Google Scholar]
- 7.Al Qarni H., Collier P., O'Keeffe J., Akunna J. Investigating the removal of some pharmaceutical compounds in hospital wastewater treatment plants operating in Saudi Arabia. Environ. Sci. Pollut. Res. Int. 2016;23:13003–13014. doi: 10.1007/s11356-016-6389-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Guo X., Yan Z., Zhang Y., Kong X., Kong D., Shan Z., Wang N. Removal mechanisms for extremely high-level fluoroquinolone antibiotics in pharmaceutical wastewater treatment plants. Environ. Sci. Pollut. Res. Int. 2017;24:8769–8777. doi: 10.1007/s11356-017-8587-3. [DOI] [PubMed] [Google Scholar]
- 9.Gadipelly C., Pérez-González A., Yadav G.D., Ortiz I., Ibáñez R., Rathod V.K., Marathe K.V. Pharmaceutical Industry Wastewater: Review of the Technologies for Water Treatment and Reuse. Ind. Eng. Chem. Res. 2014;53:11571–11592. [Google Scholar]
- 10.Rosal R., Rodríguez A., Perdigón-Melón J.A., Petre A., García-Calvo E., Gómez M.J., Agüera A., Fernández-Alba A.R. Occurrence of emerging pollutants in urban wastewater and their removal through biological treatment followed by ozonation. Water Res. 2010;44:578–588. doi: 10.1016/j.watres.2009.07.004. [DOI] [PubMed] [Google Scholar]
- 11.Kreuzinger N., Clara M., Strenn B., Kroiss H. Relevance of the sludge retention time (SRT) as design criteria for wastewater treatment plants for the removal of endocrine disruptors and pharmaceuticals from wastewater. Water Sci. Technol. 2004;50:149–156. [PubMed] [Google Scholar]
- 12.Santos J.L., Aparicio I., Alonso E. Occurrence and risk assessment of pharmaceutically active compounds in wastewater treatment plants. A case study: Seville city (Spain) Environ. Int. 2007;33:596–601. doi: 10.1016/j.envint.2006.09.014. [DOI] [PubMed] [Google Scholar]
- 13.Balakrishna K., Rath A., Praveenkumarreddy Y., Guruge K.S., Subedi B. A review of the occurrence of pharmaceuticals and personal care products in Indian water bodies. Ecotoxicol. Environ. Saf. 2017;137:113–120. doi: 10.1016/j.ecoenv.2016.11.014. [DOI] [PubMed] [Google Scholar]
- 14.Nakada N., Tanishima T., Shinohara H., Kiri K., Takada H. Pharmaceutical chemicals and endocrine disrupters in municipal wastewater in Tokyo and their removal during activated sludge treatment. Water Res. 2006;40:3297–3303. doi: 10.1016/j.watres.2006.06.039. [DOI] [PubMed] [Google Scholar]
- 15.Daughton C.G., Ternes T.A. Pharmaceuticals and personal care products in the environment: agents of subtle change? Environ. Health Perspect. 1999;107(suppl 6):907–938. doi: 10.1289/ehp.99107s6907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.S.A. Ortiz de García, G. Pinto Pinto, P.A. García-Encina, R. Irusta-Mata, Ecotoxicity and environmental risk assessment of pharmaceuticals and personal care products in aquatic environments and wastewater treatment plants, Ecotoxicology (London, England), 23 (2014) 1517-1533. [DOI] [PubMed]
- 17.Kołodziejska M., Maszkowska J., Białk-Bielińska A., Steudte S., Kumirska J., Stepnowski P., Stolte S. Aquatic toxicity of four veterinary drugs commonly applied in fish farming and animal husbandry. Chemosphere. 2013;92:1253–1259. doi: 10.1016/j.chemosphere.2013.04.057. [DOI] [PubMed] [Google Scholar]
- 18.Ahmed M.B., Zhou J.L., Ngo H.H., Guo W. Adsorptive removal of antibiotics from water and wastewater: Progress and challenges. Sci. Total Environ. 2015;532:112–126. doi: 10.1016/j.scitotenv.2015.05.130. [DOI] [PubMed] [Google Scholar]
- 19.Barbosa M.O., Moreira N.F.F., Ribeiro A.R., Pereira M.F.R., Silva A.M.T. Occurrence and removal of organic micropollutants: An overview of the watch list of EU Decision 2015/495. Water Res. 2016;94:257–279. doi: 10.1016/j.watres.2016.02.047. [DOI] [PubMed] [Google Scholar]
- 20.Dharupaneedi S.P., Nataraj S.K., Nadagouda M., Reddy K.R., Shukla S.S., Aminabhavi T.M. Membrane-based separation of potential emerging pollutants. Sep. Purif. Technol. 2019;210:850–866. doi: 10.1016/j.seppur.2018.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Prada-Vásquez M.A., Estrada-Flórez S.E., Serna-Galvis E.A., Torres-Palma R.A. Developments in the intensification of photo-Fenton and ozonation-based processes for the removal of contaminants of emerging concern in Ibero-American countries. Sci. Total Environ. 2021;765:142699. doi: 10.1016/j.scitotenv.2020.142699. [DOI] [PubMed] [Google Scholar]
- 22.Radjenović J., Petrović M., Barceló D. Fate and distribution of pharmaceuticals in wastewater and sewage sludge of the conventional activated sludge (CAS) and advanced membrane bioreactor (MBR) treatment. Water Res. 2009;43:831–841. doi: 10.1016/j.watres.2008.11.043. [DOI] [PubMed] [Google Scholar]
- 23.Martínez-Alcalá I., Guillén-Navarro J.M., Fernández-López C. Pharmaceutical biological degradation, sorption and mass balance determination in a conventional activated-sludge wastewater treatment plant from Murcia, Spain. Chem. Eng. J. 2017;316:332–340. [Google Scholar]
- 24.Miklos D.B., Remy C., Jekel M., Linden K.G., Drewes J.E., Hübner U. Evaluation of advanced oxidation processes for water and wastewater treatment–A critical review. Water Res. 2018;139:118–131. doi: 10.1016/j.watres.2018.03.042. [DOI] [PubMed] [Google Scholar]
- 25.Oller I., Malato S., Sánchez-Pérez J., Gernjak W., Maldonado M., Pérez-Estrada L., Pulgarín C. A combined solar photocatalytic-biological field system for the mineralization of an industrial pollutant at pilot scale. Catal. Today. 2007;122:150–159. [Google Scholar]
- 26.Oller I., Malato S., Sanchez-Perez J.A. Combination of Advanced Oxidation Processes and biological treatments for wastewater decontamination–a review. Sci. Total Environ. 2011;409:4141–4166. doi: 10.1016/j.scitotenv.2010.08.061. [DOI] [PubMed] [Google Scholar]
- 27.Cai Q., Wu M., Li R., Deng S., Lee B., Ong S., Hu J. Potential of combined advanced oxidation–Biological process for cost-effective organic matters removal in reverse osmosis concentrate produced from industrial wastewater reclamation: Screening of AOP pre-treatment technologies. Chem. Eng. J. 2020;389 [Google Scholar]
- 28.Papoutsakis S., Miralles-Cuevas S., Gondrexon N., Baup S., Malato S., Pulgarin César. Coupling between high-frequency ultrasound and solar photo-Fenton at pilot scale for the treatment of organic contaminants: An initial approach. Ultrason. Sonochem. 2015;22:527–534. doi: 10.1016/j.ultsonch.2014.05.003. [DOI] [PubMed] [Google Scholar]
- 29.Serna-Galvis E.A., Silva-Agredo J., Giraldo-Aguirre A.L., Flórez-Acosta O.A., Torres-Palma R.A. High frequency ultrasound as a selective advanced oxidation process to remove penicillinic antibiotics and eliminate its antimicrobial activity from water. Ultrason. Sonochem. 2016;31:276–283. doi: 10.1016/j.ultsonch.2016.01.007. [DOI] [PubMed] [Google Scholar]
- 30.Adityosulindro S., Barthe L., González-Labrada K., Jáuregui Haza U.J., Delmas H., Julcour C. Sonolysis and sono-Fenton oxidation for removal of ibuprofen in (waste)water. Ultrason. Sonochem. 2017;39:889–896. doi: 10.1016/j.ultsonch.2017.06.008. [DOI] [PubMed] [Google Scholar]
- 31.Ziylan A., Dogan S., Agopcan S., Kidak R., Aviyente V., Ince N.H. Sonochemical degradation of diclofenac: byproduct assessment, reaction mechanisms and environmental considerations. Environ. Sci. Pollut. Res. Int. 2014;21:5929–5939. doi: 10.1007/s11356-014-2514-7. [DOI] [PubMed] [Google Scholar]
- 32.Yang B., Zuo J., Li P., Wang K., Yu X., Zhang M. Effective ultrasound electrochemical degradation of biological toxicity and refractory cephalosporin pharmaceutical wastewater. Chem. Eng. J. 2016;287:30–37. [Google Scholar]
- 33.Cui M., Jang M., Ibrahim S., Park B., Cho E., Khim J. Arsenite removal using a pilot system of ultrasound and ultraviolet followed by microfiltration. Ultrason. Sonochem. 2014;21:1527–1534. doi: 10.1016/j.ultsonch.2014.01.001. [DOI] [PubMed] [Google Scholar]
- 34.Gole V.L., Fishgold A., Sierra-Alvarez R., Deymier P., Keswani M. Treatment of perfluorooctane sulfonic acid (PFOS) using a large-scale sonochemical reactor. Sep. Purif. Technol. 2018;194:104–110. [Google Scholar]
- 35.Manickam S., Parthasarathy S., Alzorqi I., Ng E.H., Tiong T.J., Gomes R.L., Ali A. Role of H2O2 in the fluctuating patterns of COD (chemical oxygen demand) during the treatment of palm oil mill effluent (POME) using pilot scale triple frequency ultrasound cavitation reactor. Ultrason. Sonochem. 2014;21:1519–1526. doi: 10.1016/j.ultsonch.2014.01.002. [DOI] [PubMed] [Google Scholar]
- 36.Tran N.H., Chen H., Reinhard M., Mao F., Gin K.Y. Occurrence and removal of multiple classes of antibiotics and antimicrobial agents in biological wastewater treatment processes. Water Res. 2016;104:461–472. doi: 10.1016/j.watres.2016.08.040. [DOI] [PubMed] [Google Scholar]
- 37.Kruglova A., Kråkström M., Riska M., Mikola A., Rantanen P., Vahala R., Kronberg L. Comparative study of emerging micropollutants removal by aerobic activated sludge of large laboratory-scale membrane bioreactors and sequencing batch reactors under low-temperature conditions. Bioresour. Technol. 2016;214:81–88. doi: 10.1016/j.biortech.2016.04.037. [DOI] [PubMed] [Google Scholar]
- 38.Sahar E., David I., Gelman Y., Chikurel H., Aharoni A., Messalem R., Brenner A. The use of RO to remove emerging micropollutants following CAS/UF or MBR treatment of municipal wastewater. Desalination. 2011;273:142–147. [Google Scholar]
- 39.Rayaroth M.P., Aravind U.K., Aravindakumar C.T. Degradation of pharmaceuticals by ultrasound-based advanced oxidation process. Environ. Chem. Lett. 2016;14(3):259–290. [Google Scholar]
- 40.Camargo-Perea A.L., Rubio-Clemente A., Peñuela G.A. Use of Ultrasound as an Advanced Oxidation Process for the Degradation of Emerging Pollutants in Water. Water. 2020;12:1068. [Google Scholar]
- 41.Chu K.H., Al-Hamadani Y.A.J., Park C.M., Lee G., Jang M., Jang A., Her N., Son A., Yoon Y. Ultrasonic treatment of endocrine disrupting compounds, pharmaceuticals, and personal care products in water: A review. Chem. Eng. J. 2017;327:629–647. [Google Scholar]
- 42.Anandan S., Kumar Ponnusamy V., Ashokkumar M. A review on hybrid techniques for the degradation of organic pollutants in aqueous environment. Ultrason. Sonochem. 2020;67 doi: 10.1016/j.ultsonch.2020.105130. [DOI] [PubMed] [Google Scholar]
- 43.Ince N.H. Ultrasound-assisted advanced oxidation processes for water decontamination. Ultrason. Sonochem. 2018;40:97–103. doi: 10.1016/j.ultsonch.2017.04.009. [DOI] [PubMed] [Google Scholar]
- 44.Yap H.C., Pang Y.L., Lim S., Abdullah A.Z., Ong H.C., Wu C.-H. A comprehensive review on state-of-the-art photo-, sono-, and sonophotocatalytic treatments to degrade emerging contaminants. Int. J. Environ. Sci. Technol. 2019;16(1):601–628. [Google Scholar]
- 45.Lee W.J., Goh P.S., Lau W.J., Ismail A.F. Removal of Pharmaceutical Contaminants from Aqueous Medium: A State-of-the-Art Review Based on Paracetamol. Arabian Journal for Science and Engineering. 2020 [Google Scholar]
- 46.Ahmed M.B., Zhou J.L., Ngo H.H., Guo W., Thomaidis N.S., Xu J. Progress in the biological and chemical treatment technologies for emerging contaminant removal from wastewater: A critical review. J. Hazard. Mater. 2017;323:274–298. doi: 10.1016/j.jhazmat.2016.04.045. [DOI] [PubMed] [Google Scholar]
- 47.E.A. Serna-Galvis, J. Lee, F. Hernández, A.M. Botero-Coy, R.A. Torres-Palma, Sonochemical Advanced Oxidation Processes for the Removal of Pharmaceuticals in Wastewater Effluents, (2020).
- 48.Henze M., van Loosdrecht M.C.M., Ekama G.A., Brdjanovic D. Biological wastewater treatment: principles. Modelling and Design. 2008 [Google Scholar]
- 49.C.P. Leslie Grady. Jr, Glen T. Daigger, Nancy G. Love, C.D.M. Filipe, Biological wastewater treatment, Third edition ed., IWA publishing, CRC Press2011.
- 50.G. Garuti, M. Dohanyos, A. Tilche, Anaerobic-Aerobic Combined Process for the Treatment of Sewage with Nutrient Removal: The Ananox® Process, Water Science and Technology, 25 (1992) 383-394.
- 51.Callado N.H., Foresti E. Removal of organic carbon, nitrogen and phosphorus in sequential batch reactors integrating the aerobic/anaerobic processes. Water Sci. Technol. 2001;44:263–270. [PubMed] [Google Scholar]
- 52.Alfonso-Muniozguren P., Lee J., Bussemaker M., Chadeesingh R., Jones C., Oakley D., Saroj D. A combined activated sludge-filtration-ozonation process for abattoir wastewater treatment. J. Water Process Eng. 2018;25:157–163. [Google Scholar]
- 53.Kovalova L., Siegrist H., Singer H., Wittmer A., McArdell C.S. Hospital wastewater treatment by membrane bioreactor: performance and efficiency for organic micropollutant elimination. Environ. Sci. Technol. 2012;46:1536–1545. doi: 10.1021/es203495d. [DOI] [PubMed] [Google Scholar]
- 54.Serna-Galvis E.A., Silva-Agredo J., Botero-Coy A.M., Moncayo-Lasso A., Hernández F., Torres-Palma R.A. Effective elimination of fifteen relevant pharmaceuticals in hospital wastewater from Colombia by combination of a biological system with a sonochemical process. Sci. Total Environ. 2019;670:623–632. doi: 10.1016/j.scitotenv.2019.03.153. [DOI] [PubMed] [Google Scholar]
- 55.Joss A., Zabczynski S., Göbel A., Hoffmann B., Löffler D., McArdell C.S., Ternes T.A., Thomsen A., Siegrist H. Biological degradation of pharmaceuticals in municipal wastewater treatment: Proposing a classification scheme. Water Res. 2006;40:1686–1696. doi: 10.1016/j.watres.2006.02.014. [DOI] [PubMed] [Google Scholar]
- 56.Carballa M., Omil F., Lema J.M., Llompart M.A., García-Jares C., Rodríguez I., Gómez M., Ternes T. Behavior of pharmaceuticals, cosmetics and hormones in a sewage treatment plant. Water Res. 2004;38:2918–2926. doi: 10.1016/j.watres.2004.03.029. [DOI] [PubMed] [Google Scholar]
- 57.Behera S.K., Kim H.W., Oh J.-E., Park H.-S. Occurrence and removal of antibiotics, hormones and several other pharmaceuticals in wastewater treatment plants of the largest industrial city of Korea. Sci. Total Environ. 2011;409:4351–4360. doi: 10.1016/j.scitotenv.2011.07.015. [DOI] [PubMed] [Google Scholar]
- 58.Suarez S., Lema J.M., Omil F. Removal of Pharmaceutical and Personal Care Products (PPCPs) under nitrifying and denitrifying conditions. Water Res. 2010;44:3214–3224. doi: 10.1016/j.watres.2010.02.040. [DOI] [PubMed] [Google Scholar]
- 59.Castiglioni S., Bagnati R., Fanelli R., Pomati F., Calamari D., Zuccato E. Removal of pharmaceuticals in sewage treatment plants in Italy. Environ. Sci. Technol. 2006;40:357–363. doi: 10.1021/es050991m. [DOI] [PubMed] [Google Scholar]
- 60.Tran N.H., Li J., Hu J., Ong S.L. Occurrence and suitability of pharmaceuticals and personal care products as molecular markers for raw wastewater contamination in surface water and groundwater. Environ. Sci. Pollut. Res. 2014;21:4727–4740. doi: 10.1007/s11356-013-2428-9. [DOI] [PubMed] [Google Scholar]
- 61.Hai F., Yang S., Asif M.B., Sencadas V., Shawkat S., Sanderson-Smith M., Gorman J., Xu Z.Q., Yamamoto K. Carbamazepine as a Possible Anthropogenic Marker in Water: Occurrences, Toxicological Effects, Regulations and Removal by Wastewater Treatment Technologies. Water. 2018;10 [Google Scholar]
- 62.Clara M., Strenn B., Kreuzinger N. Carbamazepine as a possible anthropogenic marker in the aquatic environment: investigations on the behaviour of Carbamazepine in wastewater treatment and during groundwater infiltration. Water Res. 2004;38:947–954. doi: 10.1016/j.watres.2003.10.058. [DOI] [PubMed] [Google Scholar]
- 63.Miège C., Choubert J.M., Ribeiro L., Eusèbe M., Coquery M. Removal efficiency of pharmaceuticals and personal care products with varying wastewater treatment processes and operating conditions – conception of a database and first results. Water Sci. Technol. 2008;57:49–56. doi: 10.2166/wst.2008.823. [DOI] [PubMed] [Google Scholar]
- 64.Park J., Yamashita N., Park C., Shimono T., Takeuchi D., Tanaka H. Removal characteristics of pharmaceuticals and personal care products: Comparison between membrane bioreactor and various biological treatment processes. Chemosphere. 2017;179:347–358. doi: 10.1016/j.chemosphere.2017.03.135. [DOI] [PubMed] [Google Scholar]
- 65.Tiwari B., Sellamuthu B., Ouarda Y., Drogui P., Tyagi R.D., Buelna G. Review on fate and mechanism of removal of pharmaceutical pollutants from wastewater using biological approach. Bioresour. Technol. 2017;224:1–12. doi: 10.1016/j.biortech.2016.11.042. [DOI] [PubMed] [Google Scholar]
- 66.Deng Y., Li B., Yu K., Zhang T. Biotransformation and adsorption of pharmaceutical and personal care products by activated sludge after correcting matrix effects. Sci. Total Environ. 2016;544:980–986. doi: 10.1016/j.scitotenv.2015.12.010. [DOI] [PubMed] [Google Scholar]
- 67.Samaras V.G., Stasinakis A.S., Mamais D., Thomaidis N.S., Lekkas T.D. Fate of selected pharmaceuticals and synthetic endocrine disrupting compounds during wastewater treatment and sludge anaerobic digestion. J. Hazard. Mater. 2013;244–245:259–267. doi: 10.1016/j.jhazmat.2012.11.039. [DOI] [PubMed] [Google Scholar]
- 68.Min X., Li W., Wei Z., Spinney R., Dionysiou D.D., Seo Y., Tang C.-J., Li Q., Xiao R. Sorption and biodegradation of pharmaceuticals in aerobic activated sludge system: A combined experimental and theoretical mechanistic study. Chem. Eng. J. 2018;342:211–219. [Google Scholar]
- 69.Kim M., Guerra P., Shah A., Parsa M., Alaee M., Smyth S.A. Removal of pharmaceuticals and personal care products in a membrane bioreactor wastewater treatment plant. Water Sci. Technol. 2014;69:2221–2229. doi: 10.2166/wst.2014.145. [DOI] [PubMed] [Google Scholar]
- 70.Peng J., Wang X., Yin F., Xu G. Characterizing the removal routes of seven pharmaceuticals in the activated sludge process. Sci. Total Environ. 2019;650:2437–2445. doi: 10.1016/j.scitotenv.2018.10.004. [DOI] [PubMed] [Google Scholar]
- 71.Srinivasa Raghavan D.S., Qiu G., Ting Y.-P. Fate and removal of selected antibiotics in an osmotic membrane bioreactor. Chem. Eng. J. 2018;334:198–205. [Google Scholar]
- 72.Kagle J., Porter A.W., Murdoch R.W., Rivera-Cancel G., Hay A.G. Biodegradation of pharmaceutical and personal care products. Adv. Appl. Microbiol. 2009;67:65–108. doi: 10.1016/S0065-2164(08)01003-4. [DOI] [PubMed] [Google Scholar]
- 73.Botero-Coy A.M., Martínez-Pachón D., Boix C., Rincón R.J., Castillo N., Arias-Marín L., Manrique-Losada L., Torres-Palma R., Moncayo-Lasso A., Hernández F. An investigation into the occurrence and removal of pharmaceuticals in Colombian wastewater. Sci. Total Environ. 2018;642:842–853. doi: 10.1016/j.scitotenv.2018.06.088. [DOI] [PubMed] [Google Scholar]
- 74.Joss A., Keller E., Alder A.C., Göbel A., McArdell C.S., Ternes T., Siegrist H. Removal of pharmaceuticals and fragrances in biological wastewater treatment. Water Res. 2005;39:3139–3152. doi: 10.1016/j.watres.2005.05.031. [DOI] [PubMed] [Google Scholar]
- 75.Inyang M., Flowers R., McAvoy D., Dickenson E. Biotransformation of trace organic compounds by activated sludge from a biological nutrient removal treatment system. Bioresour. Technol. 2016;216:778–784. doi: 10.1016/j.biortech.2016.05.124. [DOI] [PubMed] [Google Scholar]
- 76.Stadler L.B., Su L., Moline C.J., Ernstoff A.S., Aga D.S., Love N.G. Effect of redox conditions on pharmaceutical loss during biological wastewater treatment using sequencing batch reactors. J. Hazard. Mater. 2015;282:106–115. doi: 10.1016/j.jhazmat.2014.08.002. [DOI] [PubMed] [Google Scholar]
- 77.Urase T., Kikuta T. Separate estimation of adsorption and degradation of pharmaceutical substances and estrogens in the activated sludge process. Water Res. 2005;39:1289–1300. doi: 10.1016/j.watres.2005.01.015. [DOI] [PubMed] [Google Scholar]
- 78.Falås P., Andersen H.R., Ledin A., Jansen J. Impact of solid retention time and nitrification capacity on the ability of activated sludge to remove pharmaceuticals. Environ. Technol. 2012;33:865–872. doi: 10.1080/09593330.2011.601764. [DOI] [PubMed] [Google Scholar]
- 79.Wang X., Grady C.P.L., Jr. Effects of biosorption and dissolution on the biodegradation of di-n-butyl phthalate. Water Environ. Res. 1995;67:863–871. [Google Scholar]
- 80.Yang S.F., Lin C.F., Wu C.J., Ng K.K., Lin A.Y., Hong P.K. Fate of sulfonamide antibiotics in contact with activated sludge–sorption and biodegradation. Water Res. 2012;46:1301–1308. doi: 10.1016/j.watres.2011.12.035. [DOI] [PubMed] [Google Scholar]
- 81.Ternes T.A., Herrmann N., Bonerz M., Knacker T., Siegrist H., Joss A. A rapid method to measure the solid–water distribution coefficient (Kd) for pharmaceuticals and musk fragrances in sewage sludge. Water Res. 2004;38:4075–4084. doi: 10.1016/j.watres.2004.07.015. [DOI] [PubMed] [Google Scholar]
- 82.Martins M., Sanches S., Pereira I.A. Anaerobic biodegradation of pharmaceutical compounds: new insights into the pharmaceutical-degrading bacteria. J. Hazard. Mater. 2018;357:289–297. doi: 10.1016/j.jhazmat.2018.06.001. [DOI] [PubMed] [Google Scholar]
- 83.Xu Y., Yuan Z., Ni B.-J. Biotransformation of pharmaceuticals by ammonia oxidizing bacteria in wastewater treatment processes. Sci. Total Environ. 2016;566:796–805. doi: 10.1016/j.scitotenv.2016.05.118. [DOI] [PubMed] [Google Scholar]
- 84.Kormos J.L., Schulz M., Kohler H.-P.-E., Ternes T.A. Biotransformation of selected iodinated X-ray contrast media and characterization of microbial transformation pathways. Environ. Sci. Technol. 2010;44:4998–5007. doi: 10.1021/es1007214. [DOI] [PubMed] [Google Scholar]
- 85.Zwiener C., Seeger S., Glauner T., Frimmel F. Metabolites from the biodegradation of pharmaceutical residues of ibuprofen in biofilm reactors and batch experiments. Anal. Bioanal. Chem. 2002;372:569–575. doi: 10.1007/s00216-001-1210-x. [DOI] [PubMed] [Google Scholar]
- 86.Lahti M., Oikari A. Microbial transformation of pharmaceuticals naproxen, bisoprolol, and diclofenac in aerobic and anaerobic environments. Arch. Environ. Contam. Toxicol. 2011;61:202–210. doi: 10.1007/s00244-010-9622-2. [DOI] [PubMed] [Google Scholar]
- 87.Kosjek T., Heath E., Petrović M., Barceló D. Mass spectrometry for identifying pharmaceutical biotransformation products in the environment. TrAC, Trends Anal. Chem. 2007;26:1076–1085. [Google Scholar]
- 88.A.W. Porter, S.J. Wolfson, M. Häggblom, L.Y. Young, Microbial transformation of widely used pharmaceutical and personal care product compounds, F1000Research, 9 (2020). [DOI] [PMC free article] [PubMed]
- 89.Wei Z., Li W., Zhao D., Seo Y., Spinney R., Dionysiou D.D., Wang Y., Zeng W., Xiao R. Electrophilicity index as a critical indicator for the biodegradation of the pharmaceuticals in aerobic activated sludge processes. Water Res. 2019;160:10–17. doi: 10.1016/j.watres.2019.05.057. [DOI] [PubMed] [Google Scholar]
- 90.Quintana J.B., Weiss S., Reemtsma T. Pathways and metabolites of microbial degradation of selected acidic pharmaceutical and their occurrence in municipal wastewater treated by a membrane bioreactor. Water Res. 2005;39:2654–2664. doi: 10.1016/j.watres.2005.04.068. [DOI] [PubMed] [Google Scholar]
- 91.Helbling D.E., Hollender J., Kohler H.-P.-E., Singer H., Fenner K. High-throughput identification of microbial transformation products of organic micropollutants. Environ. Sci. Technol. 2010;44:6621–6627. doi: 10.1021/es100970m. [DOI] [PubMed] [Google Scholar]
- 92.Letzel T., Bayer A., Schulz W., Heermann A., Lucke T., Greco G., Grosse S., Schüssler W., Sengl M., Letzel M. LC–MS screening techniques for wastewater analysis and analytical data handling strategies: Sartans and their transformation products as an example. Chemosphere. 2015;137:198–206. doi: 10.1016/j.chemosphere.2015.06.083. [DOI] [PubMed] [Google Scholar]
- 93.Helbling D.E., Hollender J., Kohler H.-P.-E., Fenner K. Structure-based interpretation of biotransformation pathways of amide-containing compounds in sludge-seeded bioreactors. Environ. Sci. Technol. 2010;44:6628–6635. doi: 10.1021/es101035b. [DOI] [PubMed] [Google Scholar]
- 94.Wackett L.P. Microbial biocatalysis databases: An annotated selection of World Wide Web sites relevant to the topics in microbial biotechnology. Microb. Biotechnol. 2018;11:429. doi: 10.1111/1751-7915.13257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Kosjek T., Negreira N., Heath E., de Alda M.L., Barceló D. Aerobic activated sludge transformation of vincristine and identification of the transformation products. Sci. Total Environ. 2018;610:892–904. doi: 10.1016/j.scitotenv.2017.08.061. [DOI] [PubMed] [Google Scholar]
- 96.Beretsou V.G., Psoma A.K., Gago-Ferrero P., Aalizadeh R., Fenner K., Thomaidis N.S. Identification of biotransformation products of citalopram formed in activated sludge. Water Res. 2016;103:205–214. doi: 10.1016/j.watres.2016.07.029. [DOI] [PubMed] [Google Scholar]
- 97.Sivakumar S., Anitha P., Ramesh B., Suresh G. Analysis of EAWAG-BBD pathway prediction system for the identification of malathion degrading microbes. Bioinformation. 2017;13:73. doi: 10.6026/97320630013073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Majewsky M., Gallé T., Yargeau V., Fischer K. Active heterotrophic biomass and sludge retention time (SRT) as determining factors for biodegradation kinetics of pharmaceuticals in activated sludge. Bioresour. Technol. 2011;102:7415–7421. doi: 10.1016/j.biortech.2011.05.032. [DOI] [PubMed] [Google Scholar]
- 99.Armstrong D.L., Lozano N., Rice C.P., Ramirez M., Torrents A. Degradation of triclosan and triclocarban and formation of transformation products in activated sludge using benchtop bioreactors. Environ. Res. 2018;161:17–25. doi: 10.1016/j.envres.2017.10.048. [DOI] [PubMed] [Google Scholar]
- 100.Clara M., Kreuzinger N., Strenn B., Gans O., Kroiss H. The solids retention time-a suitable design parameter to evaluate the capacity of wastewater treatment plants to remove micropollutants. Water Res. 2005;39:97–106. doi: 10.1016/j.watres.2004.08.036. [DOI] [PubMed] [Google Scholar]
- 101.Kim S., Eichhorn P., Jensen J.N., Weber A.S., Aga D.S. Removal of antibiotics in wastewater: Effect of hydraulic and solid retention times on the fate of tetracycline in the activated sludge process. Environ. Sci. Technol. 2005;39:5816–5823. doi: 10.1021/es050006u. [DOI] [PubMed] [Google Scholar]
- 102.Ejhed H., Fång J., Hansen K., Graae L., Rahmberg M., Magnér J., Dorgeloh E., Płaza G. The effect of hydraulic retention time in onsite wastewater treatment and removal of pharmaceuticals, hormones and phenolic utility substances. The Science of the total environment. 2017;618:250–261. doi: 10.1016/j.scitotenv.2017.11.011. [DOI] [PubMed] [Google Scholar]
- 103.Strenn B., Clara M., Gans O., Kreuzinger N. Carbamazepine, diclofenac, ibuprofen and bezafibrate–investigations on the behaviour of selected pharmaceuticals during wastewater treatment. Water Sci. Technol. 2004;50:269–276. [PubMed] [Google Scholar]
- 104.Bowen E.J., Dolfing J., Davenport R.J., Read F.L., Curtis T.P. Low-temperature limitation of bioreactor sludge in anaerobic treatment of domestic wastewater. Water Sci. Technol. 2013;69:1004–1013. doi: 10.2166/wst.2013.821. [DOI] [PubMed] [Google Scholar]
- 105.Huang Z., Gedalanga P.B., Asvapathanagul P., Olson B.H. Influence of physicochemical and operational parameters on Nitrobacter and Nitrospira communities in an aerobic activated sludge bioreactor. Water Res. 2010;44:4351–4358. doi: 10.1016/j.watres.2010.05.037. [DOI] [PubMed] [Google Scholar]
- 106.Dordio A., Carvalho A.J.P., Teixeira D.M., Dias C.B., Pinto A.P. Removal of pharmaceuticals in microcosm constructed wetlands using Typha spp. and LECA. Bioresour. Technol. 2010;101:886–892. doi: 10.1016/j.biortech.2009.09.001. [DOI] [PubMed] [Google Scholar]
- 107.Vieno N.M., Tuhkanen T., Kronberg L. Seasonal Variation in the Occurrence of Pharmaceuticals in Effluents from a Sewage Treatment Plant and in the Recipient Water. Environ. Sci. Technol. 2005;39:8220–8226. doi: 10.1021/es051124k. [DOI] [PubMed] [Google Scholar]
- 108.Yu Y., Wu L., Chang A.C. Seasonal variation of endocrine disrupting compounds, pharmaceuticals and personal care products in wastewater treatment plants. Sci. Total Environ. 2013;442:310–316. doi: 10.1016/j.scitotenv.2012.10.001. [DOI] [PubMed] [Google Scholar]
- 109.Kraigher B., Kosjek T., Heath E., Kompare B., Mandic-Mulec I. Influence of pharmaceutical residues on the structure of activated sludge bacterial communities in wastewater treatment bioreactors. Water Res. 2008;42:4578–4588. doi: 10.1016/j.watres.2008.08.006. [DOI] [PubMed] [Google Scholar]
- 110.Tadkaew N., Sivakumar M., Khan S.J., McDonald J.A., Nghiem L.D. Effect of mixed liquor pH on the removal of trace organic contaminants in a membrane bioreactor. Bioresour. Technol. 2010;101:1494–1500. doi: 10.1016/j.biortech.2009.09.082. [DOI] [PubMed] [Google Scholar]
- 111.Naddeo V., Landi M., Scannapieco D., Belgiorno V. Sonochemical degradation of twenty-three emerging contaminants in urban wastewater. Desalin. Water Treat. 2013;51:6601–6608. [Google Scholar]
- 112.M. Secondes, L. Borea, V. Naddeo, F. Ballesteros Jr, V. Belgiorno, Combined application of membrane ultrafiltration, adsorption, and ultrasound irradiation for the removal of pharmaceutical compounds from real wastewater, 2017.
- 113.Rahmani H., Gholami M., Mahvi A.H., Alimohammadi M., Azarian G., Esrafili A., Rahmani K., Farzadkia M. Tinidazole removal from aqueous solution by sonolysis in the presence of hydrogen peroxide. Bulletin of Environmental Contamination and Toxicology. 2014;92:341–346. doi: 10.1007/s00128-013-1193-2. [DOI] [PubMed] [Google Scholar]
- 114.Fraiese A., Naddeo V., Uyguner-Demirel C.S., Prado M., Cesaro A., Zarra T., Liu H., B. V., B.J. F., Removal of emerging contaminants in wastewater by sonolysis, photocatalysis and ozonation. Global NEST Journal. 2019;21:98–105. [Google Scholar]
- 115.Ort C., Lawrence M.G., Reungoat J., Eaglesham G., Carter S., Keller J. Determining the fraction of pharmaceutical residues in wastewater originating from a hospital. Water Res. 2010;44:605–615. doi: 10.1016/j.watres.2009.08.002. [DOI] [PubMed] [Google Scholar]
- 116.De Bel E., Dewulf J., Witte B.D., Van Langenhove H., Janssen C. Influence of pH on the sonolysis of ciprofloxacin: Biodegradability, ecotoxicity and antibiotic activity of its degradation products. Chemosphere. 2009;77:291–295. doi: 10.1016/j.chemosphere.2009.07.033. [DOI] [PubMed] [Google Scholar]
- 117.Isariebel Q.-P., Carine J.-L., Ulises-Javier J.-H., Anne-Marie W., Henri D. Sonolysis of levodopa and paracetamol in aqueous solutions. Ultrason. Sonochem. 2009;16:610–616. doi: 10.1016/j.ultsonch.2008.11.008. [DOI] [PubMed] [Google Scholar]
- 118.Naddeo V., Belgiorno V., Kassinos D., Mantzavinos D., Meric S. Ultrasonic degradation, mineralization and detoxification of diclofenac in water: Optimization of operating parameters. Ultrason. Sonochem. 2010;17(1):179–185. doi: 10.1016/j.ultsonch.2009.04.003. [DOI] [PubMed] [Google Scholar]
- 119.Tran N., Drogui P., Zaviska F., Brar S.K. Sonochemical degradation of the persistent pharmaceutical carbamazepine. J. Environ. Manage. 2013;131:25–32. doi: 10.1016/j.jenvman.2013.09.027. [DOI] [PubMed] [Google Scholar]
- 120.Lee J. Importance of Sonication and Solution Conditions on the Acoustic Cavitation Activity. In: Ashokkumar M., editor. Handbook of Ultrasonics and Sonochemistry. Spring Sicence + Business Media; Singapore: 2016. pp. 137–175. [Google Scholar]
- 121.Nie E., Yang M., Wang D., Yang X., Luo X., Zheng Z. Degradation of diclofenac by ultrasonic irradiation: kinetic studies and degradation pathways. Chemosphere. 2014;113:165–170. doi: 10.1016/j.chemosphere.2014.05.031. [DOI] [PubMed] [Google Scholar]
- 122.Nasseri S., Mahvi A.H., Seyedsalehi M., Yaghmaeian K., Nabizadeh R., Alimohammadi M., Safari G.H. Degradation kinetics of tetracycline in aqueous solutions using peroxydisulfate activated by ultrasound irradiation: effect of radical scavenger and water matrix. J. Mol. Liq. 2017;241:704–714. [Google Scholar]
- 123.Im J.-K., Boateng L.K., Flora J.R.V., Her N., Zoh K.-D., Son A., Yoon Y. Enhanced ultrasonic degradation of acetaminophen and naproxen in the presence of powdered activated carbon and biochar adsorbents. Sep. Purif. Technol. 2014;123:96–105. [Google Scholar]
- 124.Serna-Galvis E.A., Isaza-Pineda L., Moncayo-Lasso A., Hernández F., Ibáñez M., Torres-Palma R.A. Comparative degradation of two highly consumed antihypertensives in water by sonochemical process. Determination of the reaction zone, primary degradation products and theoretical calculations on the oxidative process. Ultrason. Sonochem. 2019;58 doi: 10.1016/j.ultsonch.2019.104635. [DOI] [PubMed] [Google Scholar]
- 125.Ibanez M., Gracia-Lor E., Bijlsma L., Morales E., Pastor L., Hernandez F. Removal of emerging contaminants in sewage water subjected to advanced oxidation with ozone. J. Hazard. Mater. 2013;260:389–398. doi: 10.1016/j.jhazmat.2013.05.023. [DOI] [PubMed] [Google Scholar]
- 126.Wood R.J., Lee J., Bussemaker M.J. A parametric review of sonochemistry: Control and augmentation of sonochemical activity in aqueous solutions. Ultrason. Sonochem. 2017;38:351–370. doi: 10.1016/j.ultsonch.2017.03.030. [DOI] [PubMed] [Google Scholar]
- 127.Suri R., Nayak M., Devaiah U., Helmig E. Ultrasound assisted destruction of estrogen hormones in aqueous solution: Effect of power density, power intensity and reactor configuration. J. Hazard. Mater. 2007;146:472–478. doi: 10.1016/j.jhazmat.2007.04.072. [DOI] [PubMed] [Google Scholar]
- 128.Serna-Galvis E.A., Botero-Coy A.M., Martínez-Pachón D., Moncayo-Lasso A., Ibáñez M., Hernández F., Torres-Palma R.A. Degradation of seventeen contaminants of emerging concern in municipal wastewater effluents by sonochemical advanced oxidation processes. Water Res. 2019;154:349–360. doi: 10.1016/j.watres.2019.01.045. [DOI] [PubMed] [Google Scholar]
- 129.Ince N.H., Tezcanli-Güyer G. Impacts of pH and molecular structure on ultrasonic degradation of azo dyes. Ultrasonics. 2004;42:591–596. doi: 10.1016/j.ultras.2004.01.097. [DOI] [PubMed] [Google Scholar]
- 130.Rao Y., Yang H., Xue D., Guo Y., Qi F., Ma J. Sonolytic and sonophotolytic degradation of Carbamazepine: Kinetic and mechanisms. Ultrason. Sonochem. 2016;32:371–379. doi: 10.1016/j.ultsonch.2016.04.005. [DOI] [PubMed] [Google Scholar]
- 131.Güyer G.T., Ince N.H. Degradation of diclofenac in water by homogeneous and heterogeneous sonolysis. Ultrason. Sonochem. 2011;18:114–119. doi: 10.1016/j.ultsonch.2010.03.008. [DOI] [PubMed] [Google Scholar]
- 132.Campos-Martin J.M., Blanco-Brieva G., Fierro J.L.G. Hydrogen Peroxide Synthesis: An Outlook beyond the Anthraquinone Process. Angew. Chem. Int. Ed. 2006;45(42):6962–6984. doi: 10.1002/anie.200503779. [DOI] [PubMed] [Google Scholar]
- 133.Alfonso-Muniozguren P., Hazzwan Bohari M., Sicilia A., Avignone-Rossa C., Bussemaker M., Saroj D., Lee J. Tertiary treatment of real abattoir wastewater using combined acoustic cavitation and ozonation. Ultrason. Sonochem. 2020;64 doi: 10.1016/j.ultsonch.2020.104986. [DOI] [PubMed] [Google Scholar]
- 134.Guo W.-Q., Yin R.-L., Zhou X.-J., Du J.-S., Cao H.-O., Yang S.-S., Ren N.-Q. Sulfamethoxazole degradation by ultrasound/ozone oxidation process in water: kinetics, mechanisms, and pathways. Ultrason. Sonochem. 2015;22:182–187. doi: 10.1016/j.ultsonch.2014.07.008. [DOI] [PubMed] [Google Scholar]
- 135.Somensi C.A., Simionatto E.L., Dalmarco J.B., Gaspareto P., Radetski C.M. A comparison between ozonolysis and sonolysis/ozonolysis treatments for the degradation of the cytostatic drugs methotrexate and doxorubicin: Kinetic and efficiency approaches. J. Environ. Sci. Health A Tox Hazard. Subst. Environ. Eng. 2012;47(11):1543–1550. doi: 10.1080/10934529.2012.680414. [DOI] [PubMed] [Google Scholar]
- 136.Thompson L.H., Doraiswamy L.K. Sonochemistry: science and engineering. Ind. Eng. Chem. Res. 1999;38:1215–1249. [Google Scholar]
- 137.Sutar R.S., Rathod V.K. Ultrasound assisted enzymatic degradation of diclofenac sodium: Optimization of process parameters and kinetics. J. Water Process Eng. 2016;9:e1–e6. [Google Scholar]
- 138.Adewuyi Y.G. Sonochemistry: Environmental Science and Engineering Applications. Ind. Eng. Chem. Res. 2001;40:4681–4715. [Google Scholar]
- 139.Serna-Galvis E.A., Montoya-Rodríguez D., Isaza-Pineda L., Ibáñez M., Hernández F., Moncayo-Lasso A., Torres-Palma R.A. Sonochemical degradation of antibiotics from representative classes-Considerations on structural effects, initial transformation products, antimicrobial activity and matrix. Ultrason. Sonochem. 2019;50:157–165. doi: 10.1016/j.ultsonch.2018.09.012. [DOI] [PubMed] [Google Scholar]
- 140.Al-Hamadani Y.A.J., Chu K.H., Flora J.R.V., Kim D.H., Jang M., Sohn J., Joo W., Yoon Y. Sonocatalytical degradation enhancement for ibuprofen and sulfamethoxazole in the presence of glass beads and single-walled carbon nanotubes. Ultrason. Sonochem. 2016;32:440–448. doi: 10.1016/j.ultsonch.2016.03.030. [DOI] [PubMed] [Google Scholar]
- 141.Madhavan J., Grieser F., Ashokkumar M. Combined advanced oxidation processes for the synergistic degradation of ibuprofen in aqueous environments. J. Hazard. Mater. 2010;178:202–208. doi: 10.1016/j.jhazmat.2010.01.064. [DOI] [PubMed] [Google Scholar]
- 142.Xiao R., Wei Z., Chen D., Weavers L.K. Kinetics and Mechanism of Sonochemical Degradation of Pharmaceuticals in Municipal Wastewater. Environ. Sci. Technol. 2014;48:9675–9683. doi: 10.1021/es5016197. [DOI] [PubMed] [Google Scholar]
- 143.Kwon Y. Springer Science & Business Media; 2001. Handbook of essential pharmacokinetics, pharmacodynamics and drug metabolism for industrial scientists. [Google Scholar]
- 144.Madhavan J., Kumar P.S.S., Anandan S., Zhou M., Grieser F., Ashokkumar M. Ultrasound assisted photocatalytic degradation of diclofenac in an aqueous environment. Chemosphere. 2010;80:747–752. doi: 10.1016/j.chemosphere.2010.05.018. [DOI] [PubMed] [Google Scholar]
- 145.Panda D., Sethu V., Manickam S. Removal of hexabromocyclododecane using ultrasound-based advanced oxidation process: Kinetics, pathways and influencing factors. Environ. Technol. Innovation. 2020;17 [Google Scholar]
- 146.Naddeo V., Belgiorno V., Ricco D., Kassinos D. Degradation of diclofenac during sonolysis, ozonation and their simultaneous application. Ultrason. Sonochem. 2009;16:790–794. doi: 10.1016/j.ultsonch.2009.03.003. [DOI] [PubMed] [Google Scholar]
- 147.Naddeo V., Meriç S., Kassinos D., Belgiorno V., Guida M. Fate of pharmaceuticals in contaminated urban wastewater effluent under ultrasonic irradiation. Water Res. 2009;43:4019–4027. doi: 10.1016/j.watres.2009.05.027. [DOI] [PubMed] [Google Scholar]
- 148.Klíma J., Frias-Ferrer A., González-García J., Ludvík J., Sáez V., Iniesta J. Optimisation of 20kHz sonoreactor geometry on the basis of numerical simulation of local ultrasonic intensity and qualitative comparison with experimental results. Ultrason. Sonochem. 2007;14:19–28. doi: 10.1016/j.ultsonch.2006.01.001. [DOI] [PubMed] [Google Scholar]
- 149.Koda S., Kimura T., Kondo T., Mitome H. A standard method to calibrate sonochemical efficiency of an individual reaction system. Ultrason. Sonochem. 2003;10:149–156. doi: 10.1016/S1350-4177(03)00084-1. [DOI] [PubMed] [Google Scholar]
- 150.Lee J., Yasui K., Tuziuti T., Kozuka T., Towata A., Iida Y. Spatial distribution enhancement of sonoluminescence activity by altering sonication and solution conditions. J. Phys. Chem. B. 2008;112:15333–15341. doi: 10.1021/jp8060224. [DOI] [PubMed] [Google Scholar]
- 151.A. Roudbari, M. Rezakazemi, Hormones removal from municipal wastewater using ultrasound, AMB Express, 8 (2018) 91-91. [DOI] [PMC free article] [PubMed]
- 152.Hartmann J., Bartels P., Mau U., Witter M., Tümpling W.v., Hofmann J., Nietzschmann E. Degradation of the drug diclofenac in water by sonolysis in presence of catalysts. Chemosphere. 2008;70(3):453–461. doi: 10.1016/j.chemosphere.2007.06.063. [DOI] [PubMed] [Google Scholar]
- 153.Nejumal K.K., Manoj P.R., Aravind U.K., Aravindakumar C.T. Sonochemical degradation of a pharmaceutical waste, atenolol, in aqueous medium. Environ. Sci. Pollut. Res. 2014;21(6):4297–4308. doi: 10.1007/s11356-013-2301-x. [DOI] [PubMed] [Google Scholar]
- 154.Lee J., Ashokkumar M., Yasui K., Tuziuti T., Kozuka T., Towata A., Iida Y. Development and optimization of acoustic bubble structures at high frequencies. Ultrason. Sonochem. 2011;18(1):92–98. doi: 10.1016/j.ultsonch.2010.03.004. [DOI] [PubMed] [Google Scholar]
- 155.Darvishi Cheshmeh Soltani R., Mashayekhi M. Decomposition of ibuprofen in water via an electrochemical process with nano-sized carbon black-coated carbon cloth as oxygen-permeable cathode integrated with ultrasound. Chemosphere. 2018;194:471–480. doi: 10.1016/j.chemosphere.2017.12.033. [DOI] [PubMed] [Google Scholar]
- 156.Xiao R., Diaz-Rivera D., Weavers L.K. Factors Influencing Pharmaceutical and Personal Care Product Degradation in Aqueous Solution Using Pulsed Wave Ultrasound. Ind. Eng. Chem. Res. 2013;52:2824–2831. [Google Scholar]
- 157.Xiao R., Diaz-Rivera D., He Z., Weavers L.K. Using pulsed wave ultrasound to evaluate the suitability of hydroxyl radical scavengers in sonochemical systems. Ultrason. Sonochem. 2013;20:990–996. doi: 10.1016/j.ultsonch.2012.11.012. [DOI] [PubMed] [Google Scholar]
- 158.Jagannathan M., Grieser F., Ashokkumar M. Sonophotocatalytic degradation of paracetamol using TiO2 and Fe3+ Sep. Purif. Technol. 2013;103:114–118. [Google Scholar]
- 159.Nejumal K., Manoj P., Aravind U.K., Aravindakumar C. Sonochemical degradation of a pharmaceutical waste, atenolol, in aqueous medium. Environ. Sci. Pollut. Res. 2014;21:4297–4308. doi: 10.1007/s11356-013-2301-x. [DOI] [PubMed] [Google Scholar]
- 160.Elias M.T., Jisha C., Aravind U.K., Aravindakumar C. Oxidative Degradation of Ranitidine Induced by UV and Ultrasound: Identification of Transformation Products using LC-Q-ToF-MS. Environ. Chem. 2019;16:41–54. [Google Scholar]
- 161.Gao Y.-Q., Gao N.-Y., Deng Y., Gu J.-S., Gu Y.-L., Zhang D. Factors affecting sonolytic degradation of sulfamethazine in water. Ultrason. Sonochem. 2013;20:1401–1407. doi: 10.1016/j.ultsonch.2013.04.007. [DOI] [PubMed] [Google Scholar]
- 162.Vega L.P., Soltan J., Peñuela G.A. Sonochemical degradation of triclosan in water in a multifrequency reactor. Environ. Sci. Pollut. Res. 2019;26:4450–4461. doi: 10.1007/s11356-018-1281-2. [DOI] [PubMed] [Google Scholar]
- 163.Kıdak R., Doğan Ş. Medium-high frequency ultrasound and ozone based advanced oxidation for amoxicillin removal in water. Ultrason. Sonochem. 2018;40:131–139. doi: 10.1016/j.ultsonch.2017.01.033. [DOI] [PubMed] [Google Scholar]
- 164.Hapeshi E., Fotiou I., Fatta-Kassinos D. Sonophotocatalytic treatment of ofloxacin in secondary treated effluent and elucidation of its transformation products. Chem. Eng. J. 2013;224:96–105. [Google Scholar]
- 165.Wang Y., Zhang H., Chen L. Ultrasound enhanced catalytic ozonation of tetracycline in a rectangular air-lift reactor. Catal. Today. 2011;175:283–292. [Google Scholar]
- 166.Muñoz I., José Gómez M., Molina-Díaz A., Huijbregts M.A.J., Fernández-Alba A.R., García-Calvo E. Ranking potential impacts of priority and emerging pollutants in urban wastewater through life cycle impact assessment. Chemosphere. 2008;74:37–44. doi: 10.1016/j.chemosphere.2008.09.029. [DOI] [PubMed] [Google Scholar]
- 167.Noguera-Oviedo K., Aga D.S. Lessons learned from more than two decades of research on emerging contaminants in the environment. J. Hazard. Mater. 2016;316:242–251. doi: 10.1016/j.jhazmat.2016.04.058. [DOI] [PubMed] [Google Scholar]
- 168.Artemi A., Chen G.Q., Kentish S.E., Lee J. The relevance of critical flux concept in the concentration of skim milk using forward osmosis and reverse osmosis. J. Membr. Sci. 2020;611:118357. doi: 10.1016/j.memsci.2020.118357. [DOI] [Google Scholar]
- 169.Artemi A., Chen G.Q., Kentish S.E., Lee J. Pilot scale concentration of cheese whey by forward osmosis: A short-cut method for evaluating the effective pressure driving force. Sep. Purif. Technol. 2020;250 [Google Scholar]
- 170.L. Francis, O. Ogunbiyi, J. Saththasivam, J. Lawler, Z. Liu, A Comprehensive review of forward osmosis and niche applications, Environmental Science: Water Research & Technology, (2020).
- 171.Crossley O.P., Thorpe R.B., Peus D., Lee J. Phosphorus recovery from process waste water made by the hydrothermal carbonisation of spent coffee grounds. Bioresour. Technol. 2020;301 doi: 10.1016/j.biortech.2019.122664. [DOI] [PubMed] [Google Scholar]
- 172.Enfrin M., Lee J., Le-Clech P., Dumée L.F. Kinetic and mechanistic aspects of ultrafiltration membrane fouling by nano-and microplastics. J. Membr. Sci. 2020;601 [Google Scholar]
- 173.Ganiyu S.O., van Hullebusch E.D., Cretin M., Esposito G., Oturan M.A. Coupling of membrane filtration and advanced oxidation processes for removal of pharmaceutical residues: A critical review. Sep. Purif. Technol. 2015;156:891–914. [Google Scholar]
- 174.Nghiem L.D., Schäfer A.I., Elimelech M. Pharmaceutical retention mechanisms by nanofiltration membranes. Environ. Sci. Technol. 2005;39:7698–7705. doi: 10.1021/es0507665. [DOI] [PubMed] [Google Scholar]
- 175.Al-Rifai J.H., Gabelish C.L., Schäfer A.I. Occurrence of pharmaceutically active and non-steroidal estrogenic compounds in three different wastewater recycling schemes in Australia. Chemosphere. 2007;69:803–815. doi: 10.1016/j.chemosphere.2007.04.069. [DOI] [PubMed] [Google Scholar]
- 176.Snyder S.A., Adham S., Redding A.M., Cannon F.S., DeCarolis J., Oppenheimer J., Wert E.C., Yoon Y. Role of membranes and activated carbon in the removal of endocrine disruptors and pharmaceuticals. Desalination. 2007;202:156–181. [Google Scholar]
- 177.Alturki A.A., Tadkaew N., McDonald J.A., Khan S.J., Price W.E., Nghiem L.D. Combining MBR and NF/RO membrane filtration for the removal of trace organics in indirect potable water reuse applications. J. Membr. Sci. 2010;365:206–215. [Google Scholar]
- 178.Košutić K., Dolar D., Ašperger D., Kunst B. Removal of antibiotics from a model wastewater by RO/NF membranes. Sep. Purif. Technol. 2007;53:244–249. [Google Scholar]
- 179.Jin X., Shan J., Wang C., Wei J., Tang C.Y. Rejection of pharmaceuticals by forward osmosis membranes. J. Hazard. Mater. 2012;227–228:55–61. doi: 10.1016/j.jhazmat.2012.04.077. [DOI] [PubMed] [Google Scholar]
- 180.Van der Bruggen B., Lejon L., Vandecasteele C. Reuse, Treatment, and Discharge of the Concentrate of Pressure-Driven Membrane Processes. Environ. Sci. Technol. 2003;37:3733–3738. doi: 10.1021/es0201754. [DOI] [PubMed] [Google Scholar]
- 181.Radjenović J., Petrović M., Ventura F., Barceló D. Rejection of pharmaceuticals in nanofiltration and reverse osmosis membrane drinking water treatment. Water Res. 2008;42:3601–3610. doi: 10.1016/j.watres.2008.05.020. [DOI] [PubMed] [Google Scholar]
- 182.Nghiem L.D., Schäfer A.I. Critical risk points of nanofiltration and reverse osmosis processes in water recycling applications. Desalination. 2006;187:303–312. [Google Scholar]
- 183.Kimura K., Iwase T., Kita S., Watanabe Y. Influence of residual organic macromolecules produced in biological wastewater treatment processes on removal of pharmaceuticals by NF/RO membranes. Water Res. 2009;43:3751–3758. doi: 10.1016/j.watres.2009.05.042. [DOI] [PubMed] [Google Scholar]
- 184.Li C., Li H., Yang Y., Hou L.-A. Removal of pharmaceuticals by fouled forward osmosis membranes: Impact of DOM fractions, Ca2+ and real water. Sci. Total Environ. 2020;738 doi: 10.1016/j.scitotenv.2020.139757. [DOI] [PubMed] [Google Scholar]
- 185.Bellona C., Marts M., Drewes J.E. The effect of organic membrane fouling on the properties and rejection characteristics of nanofiltration membranes. Sep. Purif. Technol. 2010;74:44–54. [Google Scholar]
- 186.Vogel D., Simon A., Alturki A.A., Bilitewski B., Price W.E., Nghiem L.D. Effects of fouling and scaling on the retention of trace organic contaminants by a nanofiltration membrane: the role of cake-enhanced concentration polarisation. Sep. Purif. Technol. 2010;73(2):256–263. [Google Scholar]
- 187.Serna-Galvis E.A., Silva-Agredo J., Giraldo-Aguirre A.L., Torres-Palma R.A. Sonochemical degradation of the pharmaceutical fluoxetine: effect of parameters, organic and inorganic additives and combination with a biological system. Sci. Total Environ. 2015;524:354–360. doi: 10.1016/j.scitotenv.2015.04.053. [DOI] [PubMed] [Google Scholar]
- 188.Abdelhay A., Allafi A., Albsoul A. Optimization of ibuprofen degradation in water using high frequency ultrasound-assisted biological reactor. Water Sci. Technol. 2020;81:2250–2259. doi: 10.2166/wst.2020.291. [DOI] [PubMed] [Google Scholar]
- 189.Mehrdadi N., Kootenaei F.G. An investigation on effect of ultrasound waves on sludge treatment. Energy Procedia. 2018;153:325–329. [Google Scholar]
- 190.Romero-Pareja P., Aragon C., Quiroga J., Coello M. Evaluation of a biological wastewater treatment system combining an OSA process with ultrasound for sludge reduction. Ultrason. Sonochem. 2017;36:336–342. doi: 10.1016/j.ultsonch.2016.12.006. [DOI] [PubMed] [Google Scholar]
- 191.Zhang Q.-Q., Jin R.-C. The Application of Low-Intensity Ultrasound Irradiation in Biological Wastewater Treatment: A Review. Critical Reviews in Environmental Science and Technology. 2015;45(24):2728–2761. [Google Scholar]
- 192.Besha A., Gebreyohannes A., Tufa R.A., Bekele D., Curcio E., Giorno L. Removal of Emerging Micropollutants by Activated Sludge Process and Membrane Bioreactors and the Effects of Micropollutants on Membrane Fouling: A Review. J. Environ. Chem. Eng. 2017;5 [Google Scholar]
- 193.Clara M., Strenn B., Gans O., Martinez E., Kreuzinger N., Kroiss H. Removal of selected pharmaceuticals, fragrances and endocrine disrupting compounds in a membrane bioreactor and conventional wastewater treatment plants. Water Res. 2005;39:4797–4807. doi: 10.1016/j.watres.2005.09.015. [DOI] [PubMed] [Google Scholar]
- 194.Sipma J., Osuna B., Collado N., Monclús H., Ferrero G., Comas J., Rodriguez-Roda I. Comparison of removal of pharmaceuticals in MBR and activated sludge systems. Desalination. 2010;250:653–659. [Google Scholar]
- 195.Radjenovic J., Petrovic M., Barceló D. Analysis of pharmaceuticals in wastewater and removal using a membrane bioreactor. Anal. Bioanal. Chem. 2007;387:1365–1377. doi: 10.1007/s00216-006-0883-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Wang L., Albasi C., Faucet-Marquis V., Pfohl-Leszkowicz A., Dorandeu C., Marion B., Causserand C. Cyclophosphamide removal from water by nanofiltration and reverse osmosis membrane. Water Res. 2009;43:4115–4122. doi: 10.1016/j.watres.2009.06.007. [DOI] [PubMed] [Google Scholar]
- 197.Achilli A., Cath T., Marchand E., Childress A. The forward osmosis membrane bioreactor for domestic wastewater treatment. Proceedings of the Water Environment Federation. 2007, 2007,:6520–6530. [Google Scholar]
- 198.Achilli A., Cath T., Marchand E., Childress A. The novel osmotic membrane bioreactor for wastewater treatment. Proceedings of the Water Environment Federation. 2008, 2008,:6210–6221. [Google Scholar]
- 199.Achilli A., Cath T.Y., Marchand E.A., Childress A.E. The forward osmosis membrane bioreactor: A low fouling alternative to MBR processes. Desalination. 2009;239:10–21. [Google Scholar]
- 200.Alturki A., McDonald J., Khan S., Hai F., Price W., Nghiem L. Performance of a novel osmotic membrane bioreactor (OMBR) system: Flux stability and removal of trace organics. Bioresour. Technol. 2012;113:201–206. doi: 10.1016/j.biortech.2012.01.082. [DOI] [PubMed] [Google Scholar]
- 201.Alturki A.A., McDonald J.A., Khan S.J., Price W.E., Nghiem L.D., Elimelech M. Removal of trace organic contaminants by the forward osmosis process. Sep. Purif. Technol. 2013;103:258–266. [Google Scholar]
- 202.J. Heo, S. Kim, N. Her, C.M. Park, M. Yu, Y. Yoon, Chapter 5 - Removal of contaminants of emerging concern by FO, RO, and UF membranes in water and wastewater, in: A.J. Hernández-Maldonado, L. Blaney (Eds.) Contaminants of Emerging Concern in Water and Wastewater, Butterworth-Heinemann2020, pp. 139-176.
- 203.Van der Bruggen B., Luis P. Forward osmosis: Understanding the hype. Rev. Chem. Eng. 2015;31:1–12. [Google Scholar]
- 204.Kyllönen H.M., Pirkonen P., Nyström M. Membrane filtration enhanced by ultrasound: a review. Desalination. 2005;181(1-3):319–335. [Google Scholar]
- 205.Muthukumaran S., Kentish S.E., Stevens G.W., Ashokkumar M. Application of ultrasound in membrane separation processes: a review. Rev. Chem. Eng. 2006;22:155–194. [Google Scholar]
- 206.Choi Y., Hwang T.-M., Jeong S., Lee S. The use of ultrasound to reduce internal concentration polarization in forward osmosis. Ultrason. Sonochem. 2018;41:475–483. doi: 10.1016/j.ultsonch.2017.10.005. [DOI] [PubMed] [Google Scholar]
- 207.Secondes M.F.N., Naddeo V., Belgiorno V., Ballesteros F. Removal of emerging contaminants by simultaneous application of membrane ultrafiltration, activated carbon adsorption, and ultrasound irradiation. J. Hazard. Mater. 2014;264:342–349. doi: 10.1016/j.jhazmat.2013.11.039. [DOI] [PubMed] [Google Scholar]
- 208.Xu M., Wen X., Huang X., Yu Z., Zhu M. Mechanisms of membrane fouling controlled by online ultrasound in an anaerobic membrane bioreactor for digestion of waste activated sludge. J. Membr. Sci. 2013;445:119–126. [Google Scholar]
- 209.Prado M., Borea L., Cesaro A., Liu H., Naddeo V., Belgiorno V., Ballesteros F., Jr Removal of emerging contaminant and fouling control in membrane bioreactors by combined ozonation and sonolysis. Int. Biodeterior. Biodegrad. 2017;119:577–586. [Google Scholar]