Abstract
Anaplastic astrocytoma, a diffusely infiltrating, malignant, astrocytic, primary brain tumor, is most commonly observed between 30 and 50 years of age. Anaplastic astrocytomas are now classified as WHO grade III lesions, with imaging characteristics and prognosis between diffuse low-grade astrocytomas (WHO grade II) and glioblastomas (WHO IV). Anaplastic astrocytoma can appear mostly in the cerebrum followed by cerebellum. However, it is rarely observed in the fourth ventricle. In this article, we aimed to describe an uncommon case of a pediatric, fourth-ventricular, anaplastic astrocytoma. A 9-year-old male who underwent MRI brain then adopted gross-total tumor eradication. The final histopathology findings were consistent with an anaplastic astrocytoma.
Keywords: Anaplastic astrocytoma, Intraventricular, Extraparenchymal, Children
Introduction
Anaplastic astrocytoma is a malignant, astrocytic, diffusely infiltrating primary brain tumor that develops at a median age of 41 [1]. Nuclear atypia, enhanced cellularity, considerable proliferative activity as shown by mitoses, and the absence of either endothelial proliferation or necrosis, the 2 pathologic hallmarks of glioblastoma, are currently employed to diagnose anaplastic astrocytoma [1]. A quarter of all anaplastic astrocytoma cases are thought to be de novo tumors, whereas the other three quarters are thought to be the result of transition from a lower-grade astrocytoma [2]. Anaplastic astrocytoma account for 4% of all malignant CNS tumors and 10% of all gliomas [3]. The cerebrum is the most common site of anaplastic astrocytoma, followed by the cerebellum. It is, however, uncommon in the fourth ventricle.
We purposed to discuss an unusual example of a pediatric fourth-ventricular anaplastic astrocytoma in this article.
Case presentation
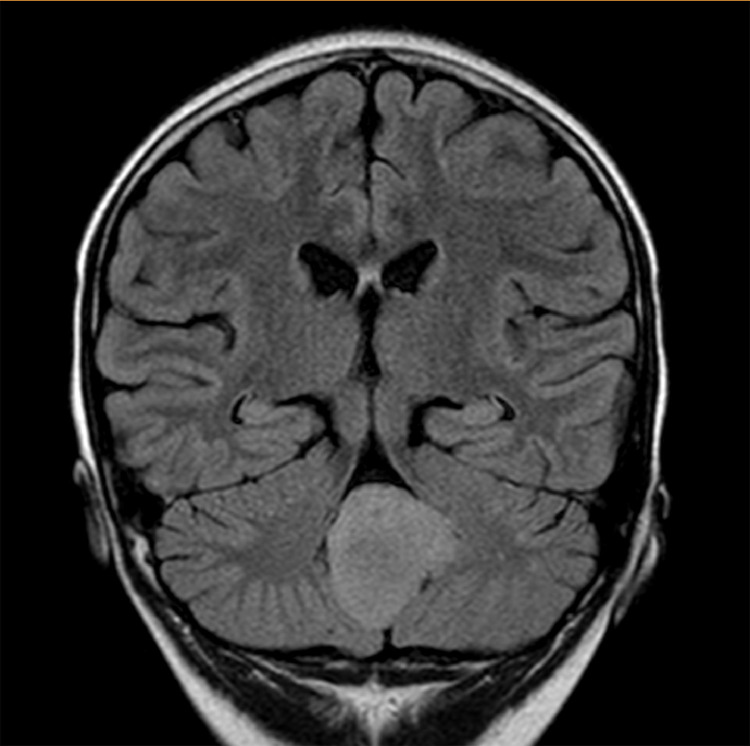
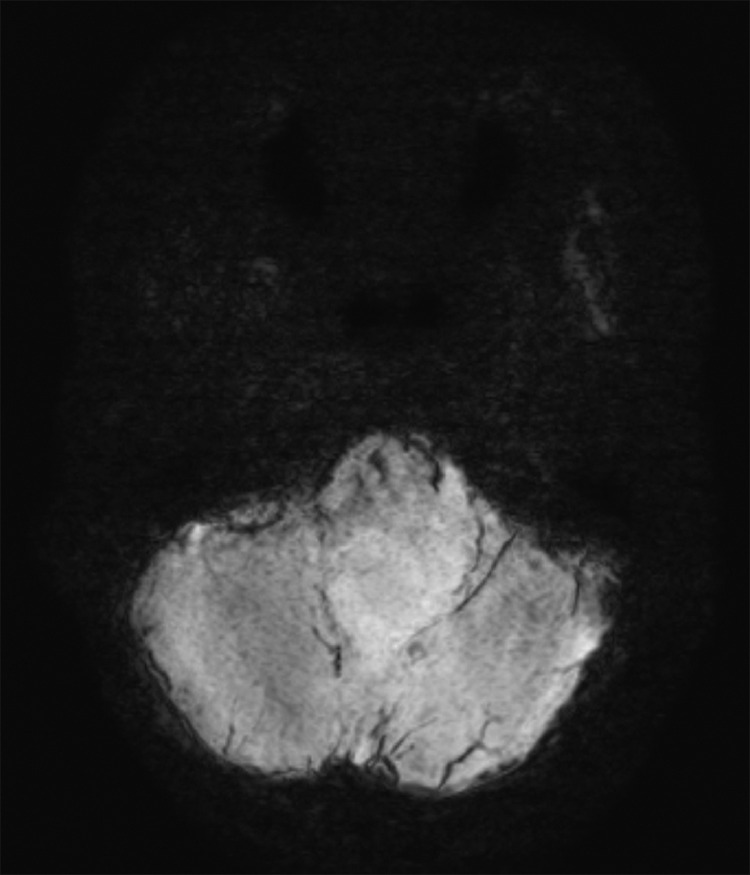
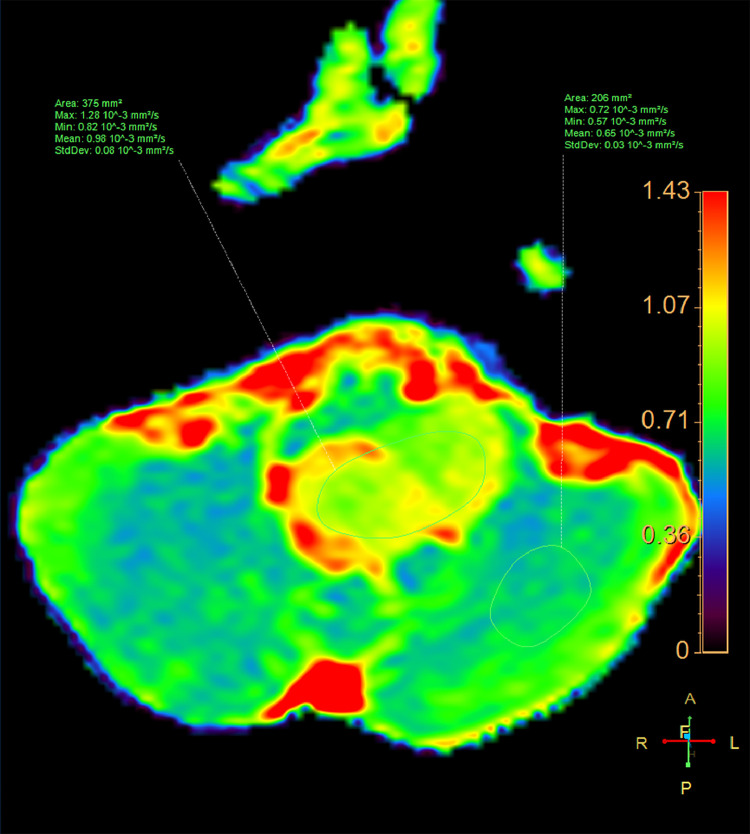
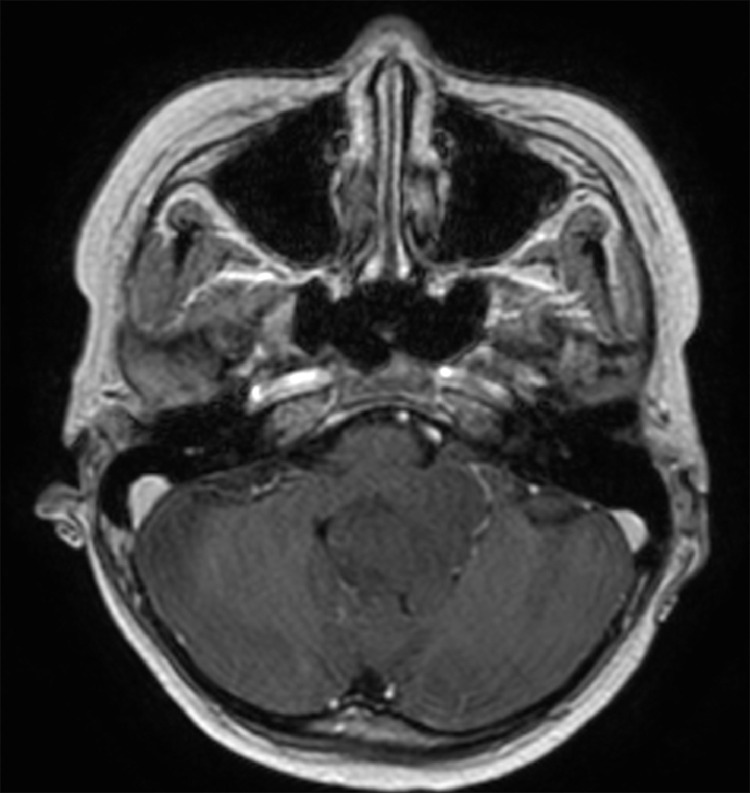
Due to headache and nausea, lasting 3 months, a 9-year-old male was transferred to Children's Hospital 2. His medical profile revealed no abnormalities. Routine blood test results were within normal ranges. Head magnetic resonance imaging (MRI), with contrast agent, revealed no lesions in the supratentorial region and the lack of hydrocephalus. A homogeneous hyperintense mass (37 × 20 × 31 mm3), without surrounding edematous parenchyma, was identified in the fourth ventricle, on T2-weighted imaging (Fig. 1) and fluid-attenuated inversion recovery imaging (Fig. 2). The tumor tended to develop through the left Luschka foramen, and no signs of hemorrhage or calcification were identified within the tumor (Fig. 3). The mean apparent diffusion coefficient (ADC) values for the parenchyma and tumor were 0.65 and 0.98 × 10−3 mm2/s, respectively (Fig. 4). On T1-weighted imaging, with contrast agent, the tumor showed very weak enhancement (Fig. 5). The preliminary diagnosis was ependymoma, and the patient underwent gross-total tumor resection. Paraffin sections of tumoral tissues were consistent with anaplastic astrocytoma (Fig. 6) accompanied by appropriate immunohistochemistry findings including positive glial fibrillary acidic protein, negative epithelial membrane antigen, negative isocitrate dehydrogenase-1, and negative isocitrate dehydrogenase-2 (IDH-2). The patient was released at 10 days post-treatment, without complications. The symptoms of headache and nausea were resolved after 7 days of surgery. Nevertheless, this patient was loss to follow-up eventually.
Fig. 1.
A homogeneous, high-signal-intensity mass, located in the fourth ventricle, on axial T2-weighted.
Fig. 2.
A homogeneous, high-signal-intensity mass, located in the fourth ventricle, on coronal FLAIR imaging.
Fig. 3.
No hemosiderin or ossification indicators were observed within the mass on susceptibility-weighted imaging.
Fig. 4.
Axial ADC map of the lesion and the cerebellar parenchyma.
Fig. 5.
Axial T1-weighted imaging, with contrast enhancement.
Fig. 6.
Histopathological sections showed the predominant infiltration of small astrocytic glial cells, with elongated nuclei and nuclear atypia, accompanied by glassy eosinophilic cytoplasm and increased mitotic activity (Hematoxylin and eosin staining, × 100).
Discussion
According to 2016 WHO classification guidelines for tumors of the central nervous system, an anaplastic astrocytoma is a grade III tumor that primarily occurs in adulthood, with a peak age of incidence ranging from 40 to 50 years [1]. Thus, anaplastic astrocytoma in children is relatively uncommon. Anaplastic astrocytoma is characterized by histopathological features, including nuclear atypia, elevated cellularity, strong mitotic activity, and substantial proliferative behavior, and the absence of 2 classical histopathological signs associated with glioblastoma: necrosis and endothelial proliferation [1].
Both anaplastic astrocytoma and glioblastoma, which are predominantly intraparenchymal brain tumors, are classified as high-grade gliomas, with dismal prognoses. In previous reports, glioblastomas have rarely been located in the ventricles [4], [5], [6], [7], [8]. Yamashita et al. [9] described a congenital, intra-right-lateral-ventricular anaplastic astrocytoma. To the extent of our knowledge, no reports exist describing infratentorial, intraventricular, anaplastic astrocytoma in children. Thus, our pediatric, intra-fourth-ventricular, anaplastic astrocytoma case may represent the first such report in the world.
Diffuse brainstem glioma, a term used to describe infiltrating astrocytomas, encompassed a variety of tumors, ranging from WHO grade II to WHO grade IV tumors. While diffuse brainstem glioma accounts for 25% of all posterior cranial fossa tumors, anaplastic astrocytoma in this region is very uncommon [1]. Diffuse brainstem glioma is usually located in the pons also known as diffuse intrinsic pontine glioma. The appearance of diffuse midline brainstem glioma is diffusely enlarged encasing the basilar artery and exhibits low signal intensity on T1-weighted image but dramatically high signal intensity on T2-weighted image. In a previous by Thong et al. [10], the mean ADC value of midline brainstem glioma was 1.39 × 10−3 mm2/s. In addition, in another study by Duc et al. [11], the mean diffusivity value of midline brainstem glioma was 1.28 × 10−3 mm2/s. In comparison with typical diffuse midline brainstem glioma, the lesion in this case report was non-midline and focal along with low tumoral ADC value of 0.98 × 10−3 mm2/s.
Conventionally, intraventricular tumors have been classified into 2 distinct forms, depending on their origins: primary and secondary [6,12,13]. Tumors that originate from the ventricular wall, ventricle lining cells, or intraventricular components are referred to as primary ventricular neoplasms, which include meningioma, ependymoma, and choroid plexus tumors. Tumors in which more than two-thirds of the neoplasm derives from structures that are contiguous with the ventricular system and that progressively expand into the ventricular system are referred to as transependymally formed, secondary, ventricular neoplasms [6,12,13].
These secondary ventricular tumors derive primarily from distinct cerebral and cerebellar tissues, including pilocytic astrocytomas, subependymal giant cell astrocytomas, and other less frequent types, such as anaplastic astrocytomas and glioblastomas [6,12,13].
According to Doetsch et al. [12], the subventricular region below the ependymal lining of the ventricular system consists primarily of 4 extensive cell groups, including ependymal cells, type B astrocytic cells, type C astrocytic cells, and oligodendrocyte precursor cells. Thus, unregulated proliferation associated with astrocytic cell mutation may result in the development of anaplastic astrocytomas in uncommon positions, such as in the fourth ventricle [12,13]. Anaplastic astrocytoma, once established, might eventually penetrate the ventricular system, via transependymal invasion. Our case report provided evidence to support this transependymal secondary invasion theory.
Conclusion
To sum up, fourth ventricular anaplastic astrocytoma is exceedingly rare in the general population and even rarer in the pediatric population. In the present report, an unusual presentation of a pediatric anaplastic astrocytoma in the fourth ventricle triggered the misdiagnosis of ependymoma. Neuroradiologists should consider that anaplastic astrocytoma can appear with atypical imaging characteristics, which might imitate other common primary brain neoplasms; thus, anaplastic astrocytoma should be included in the differential diagnosis, to attain better treatment strategies and prognosis.
Statement of authorship
Dang VH and Nguyen MD contributed equally to this work as co-first authors.
Statement of ethics
The institutional review board of Children's Hospital 2 approved this study (Ref: 352/NĐ2-CĐT). Written informed consent from the patient's legal guardian was obtained for the publication of this case report and any accompanying images.
Patient consent statement
Informed consent for patient information to be published in this article was obtained.
Footnotes
Competing interests: The authors do not report any conflicts of interest.
References
- 1.Louis D.N., Perry A., Reifenberger G., von Deimling A., Figarella-Branger D., Cavenee W.K. The 2016 World Health Organization classification of tumors of the central nervous system: a summary. Acta Neuropathol. 2016;131(6):803–820. doi: 10.1007/s00401-016-1545-1. [DOI] [PubMed] [Google Scholar]
- 2.Mechtler L. Neuroimaging in neuro-oncology. Neurol Clin. 2009;27(1):171–201. doi: 10.1016/j.ncl.2008.09.015. [DOI] [PubMed] [Google Scholar]
- 3.Ostrom Q.T., Gittleman H., Liao P., Rouse C., Chen Y., Dowling J. CBTRUS statistical report: primary brain and central nervous system tumors diagnosed in the United States in 2007-2011. Neuro Oncol. 2014;16(Suppl 4) doi: 10.1093/neuonc/nou223. (Suppl 4): iv1-63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kim Y.J., Lee S.K., Cho M.K., Kim Y.J. Intraventricular glioblastoma multiforme with previous history of intracerebral hemorrhage: a case report. J Korean Neurosurg Soc. 2008;44(6):405–408. doi: 10.3340/jkns.2008.44.6.405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hariri O.R., Quadri S.A., Farr S., Gupta R., Bieber A.J., Dyurgerova A. Third ventricular glioblastoma multiforme: case report and literature review. J Neurol Surg Rep. 2015;76(2):e227–e232. doi: 10.1055/s-0035-1560048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Patnaik A., Mishra S.S., Senapati S.B. Intraventricular glioblastoma multiforme mimicking meningioma and review of the literature. Asian J Neurosurg. 2017;12(1):75–77. doi: 10.4103/1793-5482.145104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sarsilmaz A., Gelal F., Apaydin M., Varer M., Bezircioglu H., Rezanko T. Intraventricular glioblastoma multiforme: a pediatric case report. J Pediatr Hematol Oncol. 2010;32(6):519–522. doi: 10.1097/MPH.0b013e3181e34138. [DOI] [PubMed] [Google Scholar]
- 8.Klein O., Marchal J.C. Intraventricular glioblastoma: a paediatric case report. Br J Neurosurg. 2007;21(4):411–413. doi: 10.1080/02688690701452776. [DOI] [PubMed] [Google Scholar]
- 9.Yamashita S., Ryu S., Miyata S., Uchinokura S., Yokogami K., Uehara H. A huge intraventricular congenital anaplastic astrocytoma: case report with histopathological and genetic consideration. Brain Tumor Pathol. 2012;29(2):107–112. doi: 10.1007/s10014-011-0071-z. [DOI] [PubMed] [Google Scholar]
- 10.Minh Thong P., Minh Duc N. The role of apparent diffusion coefficient in the differentiation between cerebellar medulloblastoma and brainstem glioma. Neurol Int. 2020;12(3):34–40. doi: 10.3390/neurolint12030009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Duc N.M. The role of diffusion tensor imaging metrics in the discrimination between cerebellar medulloblastoma and brainstem glioma. Pediatr Blood Cancer. 2020;67(9):e28468. doi: 10.1002/pbc.28468. [DOI] [PubMed] [Google Scholar]
- 12.Doetsch F., Caillé I., Lim D.A., García-Verdugo J.M., Alvarez-Buylla A. Subventricular zone astrocytes are neural stem cells in the adult mammalian brain. Cell. 1999;97(6):703–716. doi: 10.1016/s0092-8674(00)80783-7. [DOI] [PubMed] [Google Scholar]
- 13.Germano I., Swiss V., Casaccia P. Primary brain tumors, neural stem cell, and brain tumor cancer cells: where is the link? Neuropharmacology. 2010;58(6):903–910. doi: 10.1016/j.neuropharm.2009.12.019. [DOI] [PMC free article] [PubMed] [Google Scholar]