Abstract
Characterization of indigenous sheep breeds using morphological traits is essential for designing rational conservation and improvement strategies. This study was conducted to check the morphological diversity of three fat-tailed and three thin-tailed indigenous sheep breeds of Ethiopia. The phenotypic traits such as live body weight and linear body measurements (body length, wither height, chest girth, chest depth, rump height, rump length, ear length, tail length, and pelvic width) were measured and used for analysis. The statistical analysis was done using different procedures of SAS 9.4. Analysis of variance showed significant variation between breeds. Multivariate analyses clearly assigned the studied sheep breeds into distinct populations. Mahalanobis distance showed significant (p < 0.01) difference between breeds. The present morphometric information obtained could support future decision-making on the management, conservation, and improvement of the studied sheep genetic resources.
Keywords: Breed classification, Fat-tail sheep, Multivariate analysis, Mahalanobis distance, Thin-tail sheep
Breed classification; Fat-tail sheep; Multivariate analysis; Mahalanobis distance; Thin-tail sheep.
1. Introduction
Sheep (Ovis aries) have become important farm animals across the world through adaptation to a diverse range of environments and varied production systems. Indigenous African sheep genetic resources have been classified into two main groups, fat-tailed and thin-tailed sheep. The fat-tailed sheep are the most widely distributed, being found in a large part of North Africa and in Eastern and Southern [1]. The thin-tailed sheep are present mainly in Morocco, Sudan, West Africa, and in west Ethiopia along the border of Sudan. African sheep were domesticated outside Africa. They share a common ancestry with European and Asian sheep. The Eastern African sheep are classified as either fat-tailed or fat-rumped [1].
The sheep population in Ethiopia is estimated to be about 31.1 million and out of this 99.8 % of the population is indigenous breeds [2]. Ethiopia has diverse sheep populations of 14 traditionally classified sheep breed types and nine distinct breeds [3]. The existence of this diversity is due largely to the geographical location being near the historical entry point of many livestock populations from Asia, its diverse topographic and climatic conditions; the huge livestock populations size, and a wide range in production systems [4,5]. Ethiopian sheep are described as fat-tailed, fat-rumped, and thin-tailed based on tail type. The fat-tailed sheep type is widespread in the country [3].
Characterization of livestock breeds based on their morphological trait variations is the first step towards the available Animal Genetic Resources (AnGR) [6]. Morphological characterization involves the description and documentation of the physical traits of a breed [7]. Characterization of animal genetic resources (AnGR) encompasses all activities associated with the identification, qualitative, and quantitative description of breed productions and natural habitat and production systems to which they are or not adapted. Morphological characterization has been an accessible and easy-to-use tool in conservation and breeding programs. Description of the physical characteristics of livestock breeds is very important for developing a breeding strategy in a particular production system. Sheep biodiversity has been studied using morphological traits and DNA based markers [4, 8, 9, 10, 11].
The phenotypic variation in a population arises due to evolutionary forces such as mutation, drift, selection, and migration that give to change in allelic frequency in space and time. Their magnitude in phenotypic variability among deems could differ under different environmental conditions and farming practices of sheep. The morphometric variation between populations can offer a basis for understanding flock structure and maybe more applicable for studying, environmentally, and human-induced variation. According to [4], the morphological description is an essential part of breed characterization that can physically identify, describe, and recognize a breed, and to classify livestock breeds into broad categories [12]. reported that morphological measurements such as heart girth, height at withers, and body length can be used for the rapid choice of large size individuals in the field to enable the establishment of élite flocks.
This study focuses on sheep populations in the northwestern part and northeastern (Rift Valley regions) of Ethiopia, where morphologically undescribed populations exist and admixtures are suspected due to two or more breeds reared together. The populations represent all the thin-tailed populations and the fat-tailed populations in the northwestern and northeastern regions including the Rift Valley region of Ethiopia, respectively. Therefore, the aim of this study was to check the morphological diversity of both the fat-tailed and thin-tailed indigenous sheep populations based on morphological traits for future conservation and breeding improvement purpose.
2. Material and method
2.1. Description of the study area
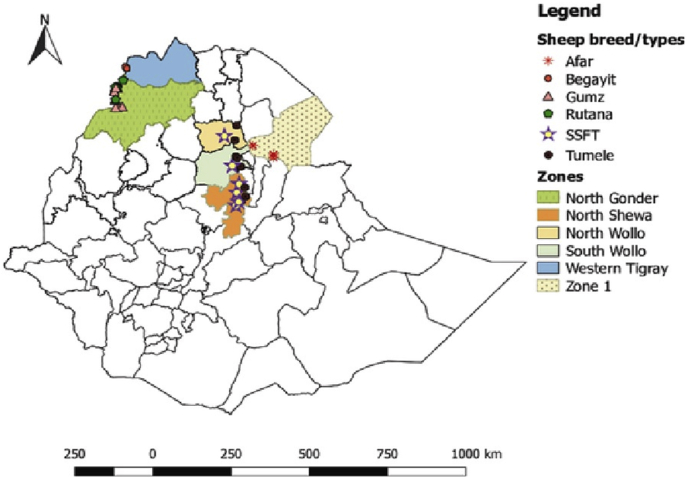
The study was conducted in two major areas of Ethiopia. The first area of study includes the Amhara region of North Gonder (Metema and AbraJira woredas) and Western Administrative zones (Kafta Humera Woreda) of the Tigray regional state. These areas were selected because they are the main production area of the thin-tailed sheep breeds/types, especially (Gumz, Rutana, and Begayit). This area is characterized by hot to warm, moist, and sub-moist tropical climate, a vast area of plains lowlands suitable for large-scale and subsistence agriculture including crop and livestock production systems. The altitude range from 550-1680 m above sea level [4, 13, 14].
The other main study area includes North Shewa, South Wollo, and North Wollo administrative zones of Amhara regional state and zone one of the Afar regional state (Figure 1). These areas were also selected to characterized fat-tailed sheep breeds (Tumele, Afar and SSFT).
Figure 1.
Map of Ethiopia showing the present study areas.
The fat-tailed sheep breeds/types found in from the sub-alpine cool highland to hot to humid semi-arid and arid climate in northeastern Ethiopia. Northeastern highland area of North Shewa, South Wollo, and North Wollo zones are breeding areas of small short fat-tailed sheep breeds (SSFT). The altitude ranges from 1700-3500 m above sea level in which sheep production is a major practice and the mainstay for people.
The mid-altitude and lowland area of these zone up to the border of the Afar region is the main breeding tract for 'Tumele' sheep populations. The altitude ranges from 1200 to 2200 m above sea level and a mixed crop-livestock production system is predominant [15]. Afar region is the main breeding tract of Afar sheep breed which characterized by arid to a semi-arid climate with low and erratic rainfall with annual average rainfall less than 555 mm. They rear multiple species including cattle, goats, sheep, camel, and donkeys.
2.2. Data collection
The zone, woreda, and kebele which have fat-tailed and thin-tailed sheep populations were selected using a purposive sampling technique. The individual animals in the village were selected randomly from smallholder farmers and private farms. Phenotypic observation and measurement were done on 660 mature female sheep (ewe) based on the phenotypic characterization descriptive format recommended by [16]. The experiment aproved by ethical committee Addis Ababa university, cellular, microbial and molecular biology department and Ethiopian biodiversity institute.
Morphological traits like body length (BL), chest girth (CG), chest depth (CD), Rump height (RH), rump length (RL), wither height (WH), tail length (TL), pelvic width (PW), Ear Length (EL), horn length (HL) and body weight (BWT) were measured and recorded. The live body weights of the sheep were measured using the Salter scale (50 kg capacity with 200 g precision) and other linear body measurements were taken after restraining and holding the animals in a natural position using measuring tape calibrated in centimeter (cm). Adult sheep were classified into three age groups based on the number of pairs of permanent incisors (PPI) following the finding of [17] for African sheep breed: 2 PPI = 22.5–27.0 months, 3 PPI = 28.0–38 months and 4 PPI = above 39.0 months. Litter size and parity of female animals were collected using the farmer's recalling method. Body measurments were taken as seen in Table 1.
Table 1.
Body measurements were made using measuring tape calibrated in centimeters (cm).
| Traits | Description |
|---|---|
| Body length (BL) | It was measured as the diagonal distance from the tip of the sternum to the base of the tail |
| Chest girth (CG) | It was taken as the circumference of the body immediately behind the shoulder blades in a vertical plane perpendicular to the long axis of the body |
| Chest depth (CD) | The distance measured from the backbone at the shoulder to the brisket between the front legs (cm). |
| Rump height (RH) | Height from ground to the spina illiaca (cm). |
| Rump length (RL) | Distance from the anterior point to the posterior extremity of the pin bone (cm). |
| Wither height (WH) | It was measured from the bottom of the front foot to the highest point of the shoulder between the withers |
| Pelvic width (PW) | It was measured as the distance between pelvic bones across the dorsum |
| Horn length (HL) | It was taken as the length of the horn on its exterior side from its root at the poll of the tip |
| Scrotum circumference (SC) | It was measured by pushing the testicles to the bottom of the scrotum and on the greatest circumference |
| Tail length (TL) | It was taken as the distance from the base to the tip of the tail on the outer side of the tail |
2.3. Statstical analysis
The least mean square, standard error of the morphometric traits of each population were analyzed using the PROC GLM procedure of SAS 9.4. Ten quantitative traits (body weight, body length, height at wither, chest girth, chest depth, rump length, rump height, pelvic width, ear length, and tail length) were submitted to principal component analysis (PCA) (PROC PRINCOMP) to reduce data dimensionality and enable facilitates analysis by grouping the data into smaller sets as first differentiation between subpopulations. The discriminant analysis, which describes the variation among groups and identifies variables with greater discriminatory power between groups, was examined by using the Stepwise discriminant procedure (PROC STEPDISC) of SAS 9.4. The relative importance of the morphometric variables in discriminating between the breeds was assessed using the level of significance, partial R2, and F-statistic. The CANDISC procedure was used to enable differentiation between the breeds, to estimate Mahalanobis distances, and to derive canonical functions. The DISCRIM procedure was used to compute canonical functions to assign each individual sheep to its sampling breed and to calculate the percent of the correct assignment.
3. Results and discussion
The value of the least square means and standard error of body weight and linear body measurements were presented in Table 2 and Table 3. The coefficient of variation indicated that variability ranged from 5.16% (rump height) to 20.2% (ear length) which were the lowest and highest variability, respectively. Ear length, tail length, and body weight had a high coefficient of variation. All variables have shown a significant difference (P < 0.001) between breeds. Most of the linear body measurements have similar value for Begayit and Rutana sheep except for body weight, tail length, and chest girth. The body weight and chest girth were higher for Rutana than Begayit sheep. The tail length was longer for Begayit than Rutana and the other breeds.
Table 2.
Number of observations, overall mean, CV, significant level and least square mean (±SE) of body weight and linear body measurement of fat-tailed and thin-tailed female sheep breeds/types.
| N | BWT (LSM±SE) | N | TL (LSM±SE) | N | CD (LSM±SE) | N | RH (LSM±SE) | N | RL (LSM±SE) | |
|---|---|---|---|---|---|---|---|---|---|---|
| Overall | 659 | 31.2 ± 0.00 | 660 | 33.6 ± 0.00 | 590 | 36.3 ± 0.00 | 590 | 68.4 ± 0.00 | 590 | 21.5 ± 0.00 |
| cv | 16.41 | 15.64 | 7.6 | 5.16 | 9.29 | |||||
| breed | ∗∗∗ | ∗∗∗ | ∗∗∗ | ∗∗∗ | ∗∗∗ | |||||
| Afar | 111 | 18.9 ± 0.49e | 112 | 17.3 ± 0.50f | 42 | 27.6 ± 0.43d | 42 | 58.4 ± 0.55d | 42 | 13.8 ± 0.31d |
| Tumele | 122 | 26.3 ± 0.47d | 122 | 25.8 ± 0.48d | 122 | 34.4 ± 0.25c | 122 | 64.9 ± 0.32c | 122 | 21.1 ± 0.18bc |
| SSFT | 112 | 24.5 ± 0.49d | 112 | 21.2 ± 0.51e | 112 | 35.2 ± 0.27b | 112 | 64.9 ± 0.34bc | 112 | 21.8 ± 0.19b |
| Gumz | 104 | 35.9 ± 0.50c | 104 | 38.7 ± 0.52c | 104 | 36.3 ± 0.27b | 104 | 68.8 ± 0.35b | 104 | 20.9 ± 0.20c |
| Rutana | 115 | 43.1 ± 0.49a | 115 | 49.9 ± 0.50b | 115 | 39.5 ± 0.26a | 115 | 74.3 ± 0.34a | 115 | 23.2 ± 0.19a |
| Begayit | 95 | 39.7 ± 0.53b | 95 | 52.1 ± 0.54a | 95 | 39.3 ± 0.28a | 95 | 73.7 ± 0.36a | 95 | 23.3 ± 0.21a |
| dentation | ∗∗∗ | NS | ∗∗∗ | NS | ∗ | |||||
| 2PPI | 206 | 29.6 ± 0.36c | 206 | 33.9 ± 0.37 | 179 | 34.6 ± 0.22b | 179 | 67.1 ± 0.28 | 179 | 20.4 ± 0.16 |
| 3PPI | 208 | 31.8 ± 0.36b | 209 | 34.3 ± 0.37 | 179 | 35.6 ± 0.21a | 179 | 67.6 ± 0.27 | 179 | 20.7 ± 0.15 |
| 4PPI | 245 | 32.9 ± 0.33a | 245 | 34.3 ± 0.34 | 232 | 36.0 ± 0.19a | 232 | 67.9 ± 0.24 | 232 | 21.0 ± 0.14 |
N = number of observations, CV = Coefficient of variation ∗∗∗ = significant at P < 0.001, ∗ = significant at P < 0.05 and NS = non-significant, a,b,c,d,e,f means different letters within the same column and class are statistically significant, BWT = body weight, TL = tail length, CD = chest depth, RH = rump height, and RL = rump length; SE = Standard error, 2PPI = two pair of permanent incisors, 3PPI = three pair of permanent incisor and 4PPI = four and above pair of permanent incisors.
Table 3.
Number of observations, overall mean, CV, significant level and least square mean (±SE) of EL, BL, CG, WH and PW of fat-tailed and thin-tailed female sheep breeds/types.
| N | EL (LSM±SE) | N | BL (LSM±SE) | N | CG (LSM±SE) | N | WH (LSM±SE) | N | PW (LSM±SE) | |
|---|---|---|---|---|---|---|---|---|---|---|
| overall | 651 | 10.5 ± 0.00 | 660 | 53.2 ± 0.00 | 660 | 75.3 ± 0.00 | 660 | 67.8 ± 0.00 | 660 | 15.5 ± 0.00 |
| cv | 20.2 | 5.25 | 5.41 | 5.34 | 9.09 | |||||
| breed | ∗∗∗ | ∗∗∗ | ∗∗∗ | ∗∗∗ | ∗∗∗ | |||||
| Afar | 112 | 4.4 ± 0.20e | 112 | 46.6 ± 0.27d | 112 | 66.6 ± 0.39e | 112 | 59.6 ± 0.34d | 112 | 13.1 ± 0.13c |
| Tumele | 116 | 7.4 ± 0.20d | 122 | 51.3 ± 0.26c | 122 | 72.7 ± 0.37d | 122 | 64.1 ± 0.33c | 122 | 15.4 ± 0.13b |
| SSFT | 109 | 10.2 ± 0.21c | 112 | 51.6 ± 0.27c | 112 | 71.6 ± 0.39d | 112 | 64.6 ± 0.35c | 112 | 15.8 ± 0.14b |
| Gumz | 104 | 12.6 ± 0.21b | 104 | 54.5 ± 0.25b | 104 | 77.4 ± 0.40c | 104 | 70.0 ± 0.36b | 104 | 15.6 ± 0.14b |
| Rutana | 115 | 14.8 ± 0.20a | 115 | 58.2 ± 0.27a | 115 | 82.7 ± 0.39a | 115 | 75.0 ± 0.34a | 115 | 16.7 ± 0.13a |
| Begayit | 95 | 14.5 ± 0.22a | 95 | 57.8 ± 0.29a | 95 | 81.4 ± 0.42b | 95 | 74.7 ± 0.37a | 95 | 16.5 ± 0.15a |
| dentation | NS | ∗∗∗ | ∗∗∗ | ∗∗∗ | ∗∗∗ | |||||
| 2PPI | 204 | 10.5 ± 0.15 | 206 | 52.4 ± 0.20b | 206 | 73.1 ± 0.29c | 206 | 67.2 ± 0.26b | 206 | 15.2 ± 0.10b |
| 3PPI | 205 | 10.8 ± 0.15 | 209 | 53.9 ± 0.20a | 209 | 75.9 ± 0.29b | 209 | 68.3 ± 0.25a | 209 | 15.7 ± 0.10a |
| 4PPI | 242 | 10.7 ± 0.14 | 245 | 53.8 ± 0.18a | 245 | 77.2 ± 0.27a | 245 | 68.5 ± 0.24a | 245 | 15.6 ± 0.09a |
N = number of observations, CV = Coefficient of variation ∗∗∗ = significant at P < 0.001 and NS = non-significant, a,b,c,d,e, means different letters within the same column and class are statistically significant, EL = ear length, BL = body length, CG = chest girth, WH = wither height, PW = pelvic width; SE = Standard error, 2PPI = two pair of permanent incisors, 3PPI = three pair of permanent incisor and 4PPI = four and above pair of permanent incisors.
The least-square means and standard error of body weight of fat-tailed sheep Afar, SSFT, and Tumele female sheep were 18.9 ± 0.49, 24.5 ± 0.49, and 26.3 ± 0.47kg, respectively. While the least square means and standard error of body weight of thin-tailed female sheep were 43.1 ± 0.49, 39.7 ± 0.53, and 35.9 ± 0.50kg for Rutana, Begayit, and Gumz, respectively. The body weight and linear body measurement of fat-tailed female lower than the thin-tailed sheep breed in the current study. The lower body weight and linear body measurements of fat-tailed sheep might be related to small body size to adapt to harsh environments (cool to very high temperature, recurrent drought, and shortage of feeds) of their habitats.
The value of body weight and linear body measurement within fat-tailed sheep breeds were showed variation. The value of body weight and linear body measurement for SSFT and Tumele sheep breeds were similar except tail length, chest depth, and ear length. Afar female sheep breed is lower body weight and linear body measurement as compared to the other populations. The current results of Afar sheep were lower than the value reported for the same breed [18] and for Habru and Gubalafto sheep population. It is also lower than the value reported for Menz sheep (20.6 ± 0.15kg) [20] and the value reported for yearling Farta sheep (20.08 ± 0.73kg) [21]. The lower body weight of Afar sheep breed in the current study might be related to drought and feed shortage of the area during the time of data collection.
The body weight found in the current study of SSFT and Tumele sheep was higher than the value reported for Menz, Afar, and Farta sheep [21]. The current value of bodyweight of SSFT and Tumele female sheep were lower than the value reported for sheep populations of Sidama-Gedeo (27.6 ± 2.3kg), Kenbata, Tembaro-Hadiya (27.5 ± 6.0kg) Gamogofa (28.0 ± 4.0kg), Wolaita (32.0 ± 5.3kg) and Gurage-Silte (30.8 ± 4.5kg) and [22] and the value reported for Washera [21]. These variations might be explained by the differences in sheep type and management and/or production environments in which the animals were kept.
All traits had a significant difference between age group except tail length, rump height, and ear length. The body weight was higher for the 4PPI age group followed by 3PPI and then 2PPI. The chest depth, body length, wither height and pelvic width value were similar for 3PPI and 4PPI and significantly different (p < 0.001) from the 2PPI age group. The chest girth value was increased as age increases. Gumz and SSFT sheep breed had similar values of chest depth, rump height, and pelvic width.
The value of body length in the present study in Afar sheep was lower than the value reported for the same breed and for Menz sheep [3, 20]. It was also less than the value reported for the sheep population of the southern regional state (Sidama-Gedeo, Kenbata, Tembaro-Hadiya, Gamogofa, Wolaita, and Gurage-Silte) [22]. The value of body length of SSFT and Tumele was lower than the value reported for Gubalafto and Habru sheep population [19] and the value reported for Gum, Washera, Farta, Menz, Afar, and Tikur sheep [3, 21, 23, 24]. This value is higher than that of Afar sheep in the present study and also comparable with the value reported for Menz. The body size and shape are the most dominant morphological characteristics influencing the adaptation of animals in a harsh environment [25]. Animals with longer legs have higher kinetic capacity being more adapted to plain and long tracks with bodies further from the ground to avoid heat radiation and animals’ body close to the ground which may correspond to its adaptations to mountain terrain.
The chest girth value of Afar sheep was lower than SSFT and Tumele sheep in the present study and also lower than the value reported for the same breed in a previous study [3] and comparable to the value reported for the same breed.
The value of body weight, chest depth, wither height, and chest girth of Begayit sheep were higher than the value reported for Gumz, SSFT, Tumele, and Afar in the current study and higher than the previous result (34.1kg, 26.8cm, 64.4cm, and 68.6cm), respectively reported by [28] for the same breed. But the value of the body length and pelvic width was lower than the previous report (63.7 ± 0.5 cm and 18.3cm), respectively. This might be related to the age difference of animals used for data collection.
Litter size is defined as the number of offspring born per parturition. It is one of the most important reproductive parameters affecting the productivity of a dam and thereby the profitability of a farm. The litter size of fat-tailed sheep was lower than the thin-tailed sheep breeds. The litter size of fat-tailed sheep breeds ranged from 1.00 to 2.55 with an average value of 1.02 ± 0.02. Whereas litter size of thin-tailed sheep breeds ranging from 1.00 to 3.5 with an average of 1.47 ± 0.02 (Table 4). The lower litter size of fat-tailed sheep might be related to the shortage of feed in high land, semi-arid and arid lowland area of northeastern as compared to moist and humid lowland area of the northwestern area of the country. The litter size of SSFT sheep found in the ranges of the result reported for the same breed [3] and it is in agreement with the result reported for Menz sheep [29, 30], and lower than the result reported for the Menz sheep [31]. The Litter size of Afar sheep breed found in the present study is in agreement with the result reported for sheep breeds in pastoral and agro-pastoral production systems [32].
Table 4.
Least mean square and standard error of litter size of fat-tailed and thin-tailed sheep breeds of northeastern and northwestern Ethiopia.
| Traits | N | Min | MAX | Litter size (LSM±SE) |
|---|---|---|---|---|
| overall | 624 | 1.00 | 1.23 ± 0.01 | |
| Tail type | ∗∗∗ | |||
| Fat-tailed | 332 | 1.00 | 2.25 | 1.02 ± 0.02b |
| Thin-tailed | 292 | 1.00 | 3.50 | 1.47 ± 0.02a |
| breed | ∗∗∗ | |||
| Afar | 113 | 1.00 | 1.33 | 1.03 ± 0.03d |
| Tumele | 113 | 1.00 | 2.25 | 1.10 ± 0.03d |
| SSFT | 106 | 1.00 | 2.00 | 1.04 ± 0.03d |
| Gumz | 93 | 1.00 | 2.83 | 1.47 ± 0.04b |
| Rutana | 97 | 1.00 | 3.50 | 1.76 ± 0.03a |
| Begayit | 102 | 1.00 | 2.00 | 1.26 ± 0.04c |
| parity | ∗∗∗ | |||
| 1 | 125 | 1.00 | 2.00 | 1.13 ± 0.03c |
| 2 | 145 | 1.00 | 3.00 | 1.22 ± 0.03b |
| 3 | 134 | 1.00 | 3.00 | 1.25 ± 0.03b |
| 4 | 105 | 1.00 | 3.50 | 1.28 ± 0.03b |
| 5 | 72 | 1.00 | 3.60 | 1.25 ± 0.04b |
| ≥6 | 43 | 1.00 | 2.83 | 1.51 ± 0.05a |
∗∗∗ = significant at P < 0.001, a,b,c,d, different letters with the same column with the same class indicates significant differences.
The litter size of Gumz sheep breeds in the present study was higher than the result reported for the same breeds [3, 23]. In the current study higher litter size was observed in Rutana sheep breeds and followed by Gumz sheep breeds. The litter size found in thin-tailed sheep breeds was higher than the result reported for Menz (1.14 ± 0.01) and Horro (1.16 ± 0.01) sheep breeds [33]. Parity had effects on the mean litter size. There was a general increasing trend in litter size with increasing parity of ewe. This might be explained that the lower litter size of younger ewes might be associated with an underdeveloped state of the reproductive futures required successive litter bearing compared with older ewes that have reached physiological maturity.
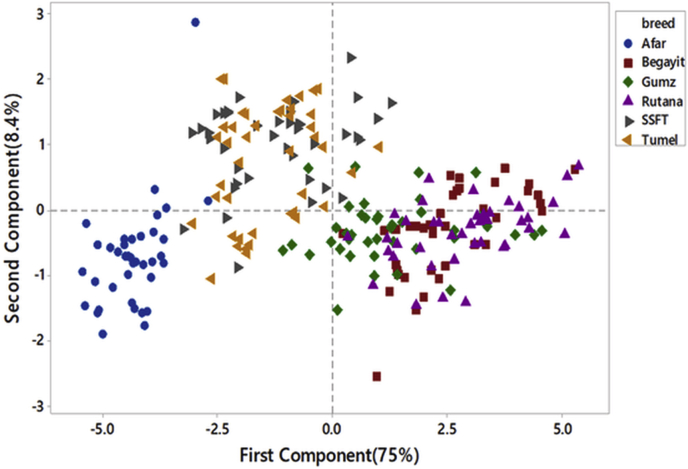
The principal component analysis has shown the first two PC explained more than 83 % of the total variation (Table 5). The first principal component accounted for 75% of the total variation and the second PC explained 8% of the total variation [34]. found similar results that the first factor explained 57.03% of the generalized variance and about 11% of the total variance was explained by the second factor for the Yankasa sheep age group between 15.5 to 28.3 months. All traits were contributing to PC1 and had a positive value. Rump length, pelvic width, and chest depth were the most contributing traits to PC2 and had a positive value. According to [35], the first PC almost always has positive coefficients for all variables and simply reflects overall ‘size’ of the individuals but the later PCs usually contrast some of the measurements with others, and can often be interpreted as defining certain aspects of ‘shape’ that are important for the breeds. These may be related to the different associations of each measurement with bone, environmental components. According to [34], the grouping of conformation traits into PCs might be related to the different associations of each measurement with skeletal growth, environmental influence. Similarly [36], extracted two components with a total variance of 66.85% in young sheep and four components in adult sheep which explained a total variance of 62.13% using morphometric traits and explained that the first factor (PC1) in each case had high loadings for variables relating to body size, whilst PC2 had a high association with traits reflecting body shape. [37] also found that the first and second principal components explained 67.6 and 11.03% of the generalized variance in body measurements and gave approximately equal emphasis to each variable.
Table 5.
Eigenvalue, the proportion of variance, eigenvectors and cumulative variance of qualitative traits of the northeastern and northwestern sheep population.
| Variable | PC1 | PC2 |
|---|---|---|
| Body weight | 0.34 | -0.22 |
| Tail Length | 0.33 | -0.3 |
| Chest depth | 0.33 | 0.24 |
| Rump Height | 0.33 | -0.03 |
| Rump Length | 0.27 | 0.58 |
| Ear Length | 0.31 | -0.27 |
| Body Length | 0.33 | -0.01 |
| Chest Girth | 0.34 | -0.15 |
| Height At Wither | 0.34 | -0.17 |
| Pelvic Width | 0.25 | 0.59 |
| Eigenvalue | 7.50 | 0.84 |
| Proportion of variance | 0.75 | 0.08 |
| Cumulative | 0.75 | 0.83 |
The plot of principal components (Figure 2) showed that PC1 classified thin tailed sheep breeds from fat-tailed sheep breeds. And further sub-classification within fat-tailed and thin-tailed sheep were observed. More overlap was observed in thin-tailed sheep breeds indicated that the presence of a high level of admixture between populations within groups. The Afar sheep breed had a low level of admixture in the fat-tailed sheep breed. This indicated that gene flow from other breeds of the nearby areas is low. The importance of PCA as a multivariate statistical tool was evident in the reduction of a large number of explanatory variables into components that gave a better description of size and shape.
Figure 2.
Score plot of quantitative traits northeastern fat-tailed and northwestern thin-tailed sheep breeds/types.
The discriminate analysis encompasses procedures for classifying observations into groups and describing the relative importance of variables for distinguishing among groups [38]. The summary result of the stepwise discriminant analysis is shown in Table 6. The analysis of variance revealed that there were significant differences in morphometric measurements among studied sheep populations. Out of ten variables subjected to the analysis, eight were selected by the stepwise discriminant procedure. Tail length chronologically followed by rump length, ear length, body weight, chest depth, chest girth, wither height and rump height were the most discriminating variables in separating the studied sheep breeds. Tail length, rump length, ear length, and body weight were the most discriminating variables among the studied sheep breeds. Their respective partial R2 were 0.84, 0.44, 0.34, and 0.13 and F values 640.52, 91.18, 60.01, and 17.02 with high significant values (P < 0.0001). The variables pelvic width and body length were similar among the studied sheep breeds. Similarly reports from the stepwise discriminant analysis indicated tail length, rump height, chest girth, ear length, and chest depth as the most discriminating variables for classification of indigenous sheep as examined [39]. The tail length, rump height, ear length, and body weight are more important in differentiating between the sheep population than acquiring numerous additional measurements.
Table 6.
Summary of a stepwise selection of traits.
| Step | Entered | PartialR-Square | F Value | Pr > F | Wilks' Lambda | Pr < Lambda | ASCC | Pr > ASCC |
|---|---|---|---|---|---|---|---|---|
| 1 | TL | 0.84 | 640.52 | <.0001 | 0.16 | <.0001 | 0.17 | <.0001 |
| 2 | RL | 0.44 | 91.18 | <.0001 | 0.09 | <.0001 | 0.26 | <.0001 |
| 3 | EL | 0.34 | 60.01 | <.0001 | 0.06 | <.0001 | 0.30 | <.0001 |
| 4 | bwt | 0.13 | 17.02 | <.0001 | 0.05 | <.0001 | 0.32 | <.0001 |
| 5 | CD | 0.03 | 3.92 | 0.002 | 0.05 | <.0001 | 0.32 | <.0001 |
| 6 | CG | 0.03 | 3.29 | 0.006 | 0.05 | <.0001 | 0.33 | <.0001 |
| 7 | RH | 0.02 | 2.48 | 0.031 | 0.05 | <.0001 | 0.33 | <.0001 |
| 8 | WH | 0.03 | 3.11 | 0.009 | 0.05 | <.0001 | 0.34 | <.0001 |
ASCC = Average Squared Canonical Correlation.
All pairwise distances were significant (P < 0.0001) that showed the populations had different measurements value and distinct genetic group (Table 7.) The largest Mahalanobis distance was observed between Afar and Begayit (58.24) and followed by between Afar and Rutana (57.44). The small Mahalanobis distance was observed between Rutana and Begayit (1.08) and between small short fat-tailed (SSFT) and "Tumele" sheep breeds (3.15).
Table 7.
Mahalanobis distance between fat-tailed and thin-tailed sheep breeds.
| Breeds/type | Begayit | Gumz | Rutana | Afar | SSFT | Tumele |
|---|---|---|---|---|---|---|
| Begayit | 6.62 | 1.08 | 58.24 | 33.86 | 30.65 | |
| Gumz | <.0001 | 5.16 | 29.44 | 12.97 | 11.45 | |
| Rutana | <.0001 | <.0001 | 57.44 | 32.16 | 29.84 | |
| Afar | <.0001 | <.0001 | <.0001 | 10.24 | 5.16 | |
| SSFT | <.0001 | <.0001 | <.0001 | <.0001 | 3.15 | |
| Tumele | <.0001 | <.0001 | <.0001 | <.0001 | <.0001 |
The largest distance between Begayit and Afar sheep and between Rutana and Afar sheep might be geographically distant, differences in management practices, agroclimatic conditions, and biophysical resources. According to [41, 42], the might be partly associated with the differences in management practices, biophysical resources, and relative breeding objectives practiced between populations [43]. stated that phenotypic differences are maintained in part by the reduction of gene flow among populations separated by large distances. [42]also stated that phenotypic divergence between breeds/populations might be partly attributed to differences in management practices, agro-climatic conditions, and biophysical resources. [44] also described that the significant differences in the distance indicated that differences among sheep breed populations are important for classification. The smallest distance observed between Begayit and Rutana possibly due to high gene flow between two breeds due to the proximity of their breeding tract and contained similar genes.
The number of observation and proportion of correct classification and misclassified observations were recorded as shown in Table 8 and also Table 9 presents the proportion of correct classification and misclassification of the cross-validated observations. Classification of sheep breeds into their correct source genetic group under field conditions is important for their effective management and conservation. In the present study, the proportion of Begayit, Gumz, Rutana, Afar, SSFT and Tumele sheep that were correctly classified into their true source breed 64.7%, 73.4%, 63.8%, 91.5%, 82.1% and 76.4%, respectively. The respective cross-validation classification results are 60.8%, 62.4%, 51.7%, 88%, 76.8%, and 68.5% for Begayit, Gumz, Rutana, Afar, SSFT and Tumele were correctly assigned into their true genetic source group.
Table 8.
Number of observations and percent classified into breeds.
| Rutana | SSFT | Afar | Begayit | Gumz | Tumele | Total | |
|---|---|---|---|---|---|---|---|
| Rutana | 74 (63.79) | 0 (0.00) | 0 (0.00) | 24 (20.69) | 18 (15.52) | 0 (0.00) | 116 (100) |
| SSFT | 0 (0.00) | 92 (82.14) | 6 (5.36) | 0 (0.00) | 3 (2.68) | 11 (9.82) | 112 (100) |
| Afar | 0 (0.00) | 4 (3.42) | 107 (91.45) | 0 (0.00) | 0 (0.00) | 6 (5.13) | 117 (100) |
| Begayit | 30 (29.41) | 0 (0.00) | 0 (0.00) | 66 (64.71) | 6 (5.88) | 0 (0.00) | 102 (100) |
| Gumz | 18 (16.51) | 1 (0.92) | 0 (0.00) | 5 (4.59) | 80 (73.39) | 5 (4.59) | 109 (100) |
| Tumele | 0 (0.00) | 16 (12.60) | 6 (4.72) | 0 (0.00) | 8 (6.30) | 97 (76.38) | 127 (100) |
| Total | 122 (17.86) | 113 (16.54) | 119 (17.42) | 95 (13.91) | 115 (16.84) | 119 (17.42) | 683 (100) |
| Error rate | 0.36 | 0.18 | 0.09 | 0.35 | 0.27 | 0.24 | 0.25 |
| Priors | 0.17 | 0.16 | 0.17 | 0.15 | 0.16 | 0.19 |
Table 9.
Cross validation table that shows the number of observations and percent classified into breeds.
| Rutana | SSFT | Afar | Begayit | Gumz | Tumele | Total | |
|---|---|---|---|---|---|---|---|
| Rutana | 60 (51.72) | 0 (0.00) | 0 (0.00) | 34 (29.31) | 22 (18.97) | 0 (0.00) | 116 (100) |
| SSFT | 0 (0.00) | 86 (76.79) | 8 (7.14) | 0 (0.00) | 3 (2.68) | 15 (13.39) | 112 (100) |
| Afar | 0 (0.00) | 6 (5.13) | 103 (88.03) | 0 (0.00) | 0 (0.00) | 8 (6.84) | 117 (100) |
| Begayit | 33 (32.35) | 0 (0.00) | 0 (0.00) | 62 (60.78) | 7 (6.86) | 0 (0.00) | 102 (100) |
| Gumz | 21 (19.27) | 2 (1.83) | 0 (0.00) | 7 (6.42) | 68 (62.39) | 11 (10.09) | 109 (100) |
| Tumele | 0 (0.00) | 23 (18.11) | 7 (5.51) | 0 (0.00) | 10 (7.87) | 87 (68.50) | 127 (100) |
| Total | 114 (16.69) | 117 (17.13) | 118 (17.28) | 103 (15.08) | 110 (16.11) | 121 (17.72) | 683 (100) |
| Error rate | 0.48 | 0.23 | 0.12 | 0.39 | 0.38 | 0.32 | 0.32 |
| Priors | 0.1698 | 0.1640 | 0.1713 | 0.1493 | 0.1596 | 0.1859 |
The overall error level recorded was 0.32 that indicated 68% of the population assigned correctly into their genetic groups. The lower error rate (0.12) was observed in Afar sheep populations while the highest error rate (0.48) observed in the Rutana sheep breed. The lower error rate in Afar breed reared by afar community might be isolated from other breeds because of isolation of communities due to cultural barriers. But in Rutana sheep breed the high error rate indicates the presence of gene flow with neighboring sheep populations [45] observed that most (61%) of the Sudanese (Djallonke) individuals were classified as being Sudan Sahel (Mossi) individuals but most (89.5%) Burkina-Sahel individuals were classified into their correct environmental area. Results from the discriminant analysis in the present study showed that most Begayit sheep (64.7%) were correctly classified as their true breeds but the remaining 20.7% and 4.6% were classified as Rutana and Gumz sheep, respectively. After applying discriminant analysis [46], also identified 83.5% of the goats they studied were correctly classified into their source breed. The higher error rate observed in thin-tailed sheep indicates the presence of intermixing between neighboring sheep population.
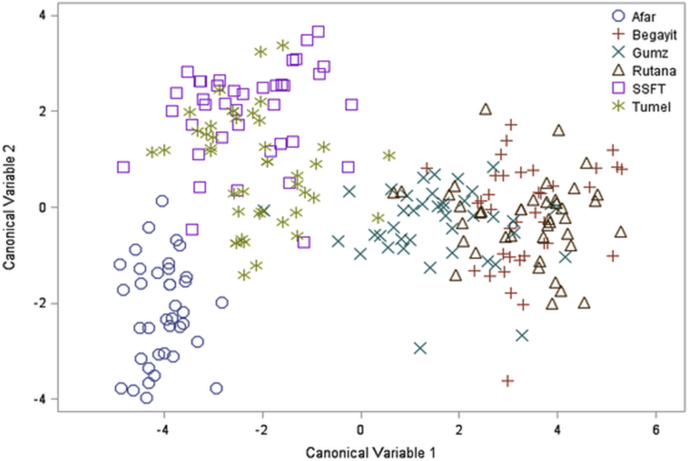
Similar to the principal component analysis the scatter plot of the six populations built based on the morphometric measurements using multivariate discriminant analysis was showed two main clusters. The first one was formed by fat-tailed sheep and the second one by thin-tailed sheep populations. The first group further classified into two groups and Afar sheep purely isolated from fat-tailed group while SSFT and Tumele had some intermix. Similarly, from thin-tailed group Gumz sheep isolated with few intermix with Rutana. Rutana and Begayit sheep were highly intermixed as shown in Figure 3.
Figure 3.
The scattered plot showed the classification of sheep breeds based on the discriminant analysis of quantitative traits of fat-tailed in the right and thin-tailed sheep breeds in the left.
4. Conclusion
Analysis of variance showed significant differences between breeds. The larger variability was observed in ear length, tail length and body weight and lower variability was observed in rump height. The thin-tailed sheep breed had larger body size as compared to fat-tailed sheep populations. PCA's analysis using morphological traits revealed two PCs isolated based on tailed type indicated that sheep populations had larger phentypic distance between fat-tailed population and thin-tailed sheep populations than within fat-tailed or thin-tailed group. Multivariate analyses clearly assigned the studied sheep breeds into distinct populations. All pairwise mahalanobis distances were significant that showed the populations had different measurements value and distinct genetic group. Populations that were found in the similar geographical region had low pairwise Mahalanobis distance. While populations that are geographically isolated had larger pairwise Mahalanobis distance. These might be partly associated with the differences in management practices, biophysical resources, and relative breeding objectives practiced between populations. The high level of admixture observed in thin-tailed sheep in the present study indicated the needs conservation for sustainable utilization of the genetic resources. Low level of genetic admixture in Afar and SSFT sheep breeds would create an opportunity for future genetic improvement in line with conserving the pure breed in the study area. The present morphometric information obtained could support a future decision-making on the management, conservation, and improvement of the studied sheep genetic resources.
Declarations
Author contribution statement
Belay Deribe: Conceived and designed the experiments; Performed the experiments; Analyzed and interpreted the data; Contributed reagents, materials, analysis tools or data; Wrote the paper.
Dereje Beyene, Kifle Dagne, Tesfaye Getachew, Solomon Gizaw: Conceived and designed the experiments; Wrote the paper.
Ayele Abebe: Contributed reagents, materials, analysis tools or data; Wrote the paper.
Funding statement
This work was supported by Amhara Agricultural Research Institute (AARI), Ethiopian Institute of Agriculture Research (EIAR) sheep Project and Addis Ababa University.
Data availability statement
Data will be made available on request.
Declaration of interests statement
The authors declare no conflict of interest.
Additional information
No additional information is available for this paper.
References
- 1.Muigai A.W.T., Hanotte O. The origin of African sheep: archaeological and genetic perspectives. Afr. Archaeol. Rev. 2013;30(1):39–50. doi: 10.1007/s10437-013-9128-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.National C.S.A. Addis Ababa: CSA; 2017. Integrated Household Survey Agricultural Sample Survey. [Google Scholar]
- 3.Solomon Gizaw, van Arendonk J.A.M., Hanotte O., Komen H. Wageningen University; The Netherlands: 2008. Sheep Resources of Ethiopia: Genetic Diversity and Breeding Strategy. [Google Scholar]
- 4.Solomon Gizaw, Van Arendonk J.A., Komen H., Windig J., Hanotte O. Population structure, genetic variation and morphological diversity in indigenous sheep of Ethiopia. Anim. Genet. 2007;38(6):621–628. doi: 10.1111/j.1365-2052.2007.01659.x. [DOI] [PubMed] [Google Scholar]
- 5.Ayalew Workneh, Getahun Ephrem, Tibbo Markos, Mamo Yetnayet, Rege J., editors. Proceedings of the 11th Annual Conference of the Ethiopian Society of Animal Production (ESAP) 2004. Current state of knowledge on characterization of farm animal genetic resources in Ethiopia. 28–30 August 2003. [Google Scholar]
- 6.Lanari M.R., Taddeo H., Domingo E., Pérez Centeno M., Gallo L. Phenotypic differentiation of exterior traits in local criollo goat population in patagonia (Argentina) Arch. Anim. Breed. 2003;46(4):347–356. [Google Scholar]
- 7.Scherf B.D., Pilling D. 2015. The Second Report on the State of the World's Animal Genetic Resources for Food and Agriculture. [Google Scholar]
- 8.Michailidou S., Tsangaris G., Fthenakis G.C., Tzora A., Skoufos I., Karkabounas S.C. Genomic diversity and population structure of three autochthonous Greek sheep breeds assessed with genome-wide DNA arrays. Mol. Genet. Genom. 2018;293(3):753–768. doi: 10.1007/s00438-018-1421-x. [DOI] [PubMed] [Google Scholar]
- 9.Tesfaye Getachew Genetic Diversity and Admixture Analysis of Ethiopian Fat-Tailed and Awassi Sheep Using SNP Markers for Designing Crossbreeding Schemes. BOKU Natural Resources and Life Sciences; Vienna: 2015. [PhD Thesis] [Google Scholar]
- 10.Edea Z., Bhuiyan M., Dessie T., Rothschild M., Dadi H., Kim K. Genome-wide genetic diversity, population structure and admixture analysis in African and Asian cattle breeds. Animal. 2015;9(2):218–226. doi: 10.1017/S1751731114002560. [DOI] [PubMed] [Google Scholar]
- 11.Kijas J., Lenstra J., Hayes B., Boitard S., Porto Neto L., San Cristobal M. Genome-wide analysis of the world's sheep breeds reveals high levels of historic mixture and strong recent selection. PLoS Biol. 2012;10(2) doi: 10.1371/journal.pbio.1001258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dossa L.H., Wollny C., Gauly M., Gbégo I. Community-based management of farm animal genetic resources in practice: framework for focal goats in two rural communities in Southern Benin. Anim. Genet. Resour. Inf. 2009;44:11–31. [Google Scholar]
- 13.Abegaz S., Hegde B., Taye M. Fundación CIPAV; Colombia: 2011. Growth and Physical Body Characteristics of Gumuz Sheep under Traditional Management Systems in Amhara Regional State, Ethiopia Cali.http://www.lrrd.org/lrrd23/lrrd23.htm [Available from: [Google Scholar]
- 14.Ftiwi Mulugeta, Tamir Berhan. Phenotypic characterization of indigenous cattle in western Tigray, northern Ethiopia. Indian J. Dairy Sci. 2015;68(2) [Google Scholar]
- 15.Hurni H. Explanatory notes on three maps at a scale of. Vol. 1. 1998. Agroecological belts of Ethiopia. (1,000,000) [Google Scholar]
- 16.FAO . 2012. Phenotypic Characterization of Animal Genetic Resources. Rome. [Google Scholar]
- 17.Wilson R.T., Durkin J.W. Age at permanent incisor eruption in indigenous goats and sheep in semi-arid Africa. Livest. Prod. Sci. 1984;11(4):451–455. [Google Scholar]
- 18.Getachew T., Haile A., Tibbo M., Sharma A., Kifle A., Terefe E. Morphological characters and body weight of Menz and Afar sheep within their production system. Ethiop. J. Anim. Prod. 2009;9(1):99. [Google Scholar]
- 19.Mohammed T., Kebede K., Mekasha Y., Abera B. Herd management and breeding practices of sheep owners in North Wollo Zone, Northern Ethiopia. Middle East J. Sci. Res. 2014;21(9):1570–1578. [Google Scholar]
- 20.Getachew Tesfaye. Haramaya University; Haramaya, Ethiopia: 2008. Characterization of Menz and Afar Indigenous Sheep Breeds of Smallholders and Pastoralists for Designing Community-Based Breeding Strategies in ETHIOPIA. [Google Scholar]
- 21.Mekuriaw Shigdaf, Mekuriaw Zeleke, Taye Mengiste, Mekuriaw Getenet, Amane A., Bimrewa T. Growth performance and linear body measurements of Washera, Farta and their crossbreed sheep under farmers management system in Western Highland of Amhara Region. Sci. J. Vet. Adv. 2013;2(9):132–143. [Google Scholar]
- 22.Melesse A., Banerjee S., Lakew A., Mersha F., Hailemariam F., Tsegaye S. Variations in linear body measurements and establishing prediction equations for live weight of indigenous sheep populations of southern Ethiopia. Sci. J. Anim. Sci. 2013;2(1):15–25. [Google Scholar]
- 23.Solomon Abegaz, Hegde B., Taye Mengistie. Growth and physical body characteristics of Gumuz sheep under traditional management systems in Amhara Regional State, Ethiopia. Livest. Res. Rural Dev. 2011;23(5) [Google Scholar]
- 24.Bimerow T., Yitayew A., Taye M., Mekuriaw S. Morphological characteristics of Farta sheep in Amhara region, Ethiopia. Online J. Anim. Feed Res. 2011;1(6):299–305. [Google Scholar]
- 25.Berihulay H., Abied A., He X., Jiang L., Ma Y. Adaptation mechanisms of small ruminants to environmental heat stress. Animals. 2019;9(3):75. doi: 10.3390/ani9030075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Amare B., Kefyalew A., Zeleke M. Typical features, characterization and breeding objectives of Begait sheep in Ethiopia. Anim. Genet. Resour. /Resources génétiques animales/Recursos genéticos animales. 2012;51:117–123. [Google Scholar]
- 29.Niftalem D. Alemaya University of Agriculture; Dire Dawa, and Ethiopia: 1990. On-farm Study of Reproductive and Growth Performance of the Menze Sheep in Debre Berhan-Ethiopia. An M. Sc: Thesis. [Google Scholar]
- 30.Mekoya A. Alemaya University; Ethiopia: 1999. Husbandry Practices and Productivity of Sheep in Lallo-Mama Mider Woreda of central highlands of Ethiopia. MSc thesis. [Google Scholar]
- 31.Mukasa-Mugerwa E., Lahlou-Kassi A. Reproductive performance and productivity of Menz sheep in the Ethiopian highlands. Small Rumin. Res. 1995;17(2):167–177. [Google Scholar]
- 32.Wilson R.T. 1986. Livestock Production in central Mali: Long-Term Studies on Cattle and Small Ruminants in the Agropastoral System: ILRI (Aka ILCA and ILRAD) [Google Scholar]
- 33.Berhan A., Van Arendonk J. Reproductive performance and mortality rate in Menz and Horro sheep following controlled breeding in Ethiopia. Small Rumin. Res. 2006;63(3):297–303. [Google Scholar]
- 34.Yakubu A. Characterisation of the local Muscovy duck in Nigeria and its potential for egg and meat production. World Poultry Sci. J. 2013;69(4):931–938. [Google Scholar]
- 35.Jolliffe I. Principal component analysis. Technometrics. 2003;45(3):276. [Google Scholar]
- 36.Mavule B., Muchenje V., Bezuidenhout C., Kunene N. Morphological structure of Zulu sheep based on principal component analysis of body measurements. Small Rumin. Res. 2013;111(1-3):23–30. [Google Scholar]
- 37.Yunusa A., Salako A., Oladejo O. Principal component analysis of the morphostructure of Uda and Balami sheep of Nigeria. Int. Res. J. Agric. Sci. 2013;1(3):45–51. [Google Scholar]
- 38.Lix L., Sajobi T. Discriminant analysis for repeated measures data: a review. Front. Psychol. 2010;1:146. doi: 10.3389/fpsyg.2010.00146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Agaviezor B.O., Peters S.O., Adefenwa M.A., Yakubu A., Adebambo O.A., Ozoje M.O. Morphological and microsatellite DNA diversity of Nigerian indigenous sheep. J. Anim. Sci. Biotechnol. 2012;3(1):38. doi: 10.1186/2049-1891-3-38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Effa Delesa K. Haramaya University; 2012. Phenotypic and Molecular Characterization of Ethiopian Equines: Their Genetic Diversities and Geograpphical Districutions. [Google Scholar]
- 42.Yadav D.K., Jain A., Kulkarni V.S., Govindaiah M.G., Aswathnarayan T., Sadana D.K. Classification of four ovine breeds of southern peninsular zone of India: morphometric study using classical discriminant function analysis. SpringerPlus. 2013;2(1):1–8. doi: 10.1186/2193-1801-2-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Yakubu A., Ibrahim I.A. Multivariate analysis of morphostructural characteristics in Nigerian indigenous sheep. Ital. J. Anim. Sci. 2011;10(2):e17. [Google Scholar]
- 44.Yakubu A., Peters S., Ilori B., Imumorin I., Adeleke M., Takeet M. Multifactorial discriminant analysis of morphological and heat-tolerant traits in indigenous, exotic and cross-bred turkeys in Nigeria. Anim. Genet. Resour. /Resources génétiques animales/Recursos genéticos animales. 2012;50:21–27. [Google Scholar]
- 45.Traoré A., Tamboura H.H., Kaboré A., Royo L., Fernandez I., Álvarez I. Multivariate characterization of morphological traits in Burkina Faso sheep. Small Rumin. Res. 2008;80(1-3):62–67. [Google Scholar]
- 46.Ebegbulem V., Ibe S., Asuquo B. Morphometric differentiation of West African dwarf goats in Southeastern Nigeria using discriminant analysis. J. Agric. Vet. Sci. 2011;3:29–34. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data will be made available on request.