Abstract
Background
Quality indicators (QIs) for the management of breast cancer (BC) have been published in Europe and internationally. In Belgium, a task force was established to select measurable process indicators of systemic treatment for BC, focusing on appropriateness of delivered care. The objective of this study was to evaluate the results of the selected QIs, both nationally and among individual centres.
Patients and Methods
Female Belgian residents with unilateral primary invasive BC diagnosed between 2010 and 2014 were selected from the Belgian Cancer Registry database. The national number enabled linkage with the national reimbursement database, which contains information on all reimbursed medical procedures. A total of 12 process indicators were measured on the population and hospital level. Intercentre variability was assessed by median results and interquartile ranges.
Results
A total of 48 872 patients were included in the study. QIs concerning specific BC subtypes only applied to patients diagnosed in 2014 (n = 9855). Clinical stage (cStage) I patients (n = 17 116) were staged with positron emission tomography/computed tomography. Among patients who were pT1aN0 human epidermal growth factor receptor 2 (HER2) positive (n = 47), 25.5% (n = 12) received adjuvant trastuzumab. Among patients with de novo metastatic luminal A/B-like HER2-negative BC (n = 295), 17.3% (n = 51) received upfront chemotherapy. (Neo)adjuvant chemotherapy was administered in 52.4% (n = 12 592) of operated women with cStage I-III, in 37.0% (n = 1270) of operated women with cStage I-III luminal A/B-like HER2-negative BC, and in 19.1% of operated women with cStage I luminal A/B-like HER2-negative BC. In the population of operated patients with cStage I-III, of those younger than 70 years that started adjuvant endocrine therapy (n = 3591), 81.7% (n = 2932) continued treatment for ≥4.5 years. Among patients in cStage I-III older than 70 years (n = 8544), 19.0% (n = 1622) received (neo)adjuvant chemotherapy, whereas among patients with cStage I-III luminal A/B-like HER2-negative BC (n = 1388), 13.0% (n = 181) received (neo)adjuvant chemotherapy. In patients with cStage I-II luminal A/B-like HER2-negative BC older than 70 years (n = 1477), 11.6% (n = 171) were not operated and received upfront endocrine treatment.
Conclusion
Well-considered QIs using population-based data can evaluate quality of care and expose disparities among treatment centres. Their use in daily practice should be implemented in all centres treating BC.
Key words: quality indicators, breast cancer, population-based data, quality of care, systemic treatment, national cancer registry, overtreatment
Highlights
-
•
Coupling Belgian Cancer Registry data with administrative data allows population-based calculation of QIs.
-
•
Monitoring QIs by real-world data enables evaluation of national results and intercentre differences.
-
•
Substantial differences among treatment centres were noted for several process indicators of systemic treatment.
-
•
Chemotherapy administration to patients with early luminal BC was remarkably variable among treatment centres.
-
•
The evaluation of endocrine therapy intake in adjuvant setting on a national level was reassuring.
Introduction
Quality indicators (QIs) are specific tools to measure the quality of provided health care. They must be reliable, clinically relevant, interpretable, actionable, and measurable.1, 2, 3 In relation to the specific aspect of quality that is being assessed, QIs are classified as process-, structure-, or outcome indicators.3 Quality assessment through QIs tries to monitor performance to evaluate whether it meets acceptable standards, also detecting underuse and overuse of technologies or treatments. Berwick and Cassel4 recently reported about the problem of ‘inappropriate care’, the problems of ‘overuse, underuse and misuse’ and the need to substitute ‘volume-based payment’ by ‘value-based payment’. In the United States, the Quality Oncology Practice Initiative published its quality measures in 2013.5 Their indicators were created to enhance patient-centred decisions, supply information to providers and institutions, drive transparency and care improvement, and enable comparative research.
QIs for the management of breast cancer (BC) have been published in Europe by the European Society of Breast Cancer Specialists (EUSOMA) in 2010, and in Belgium a set of measurable indicators was validated on national cancer registry data in 2011.6,7 The EUSOMA guidelines were updated in 2017, identifying five extra QIs focused on systemic treatment, and the requirements of a specialist breast centre were renewed in 2020.8,9 Recently, a collaboration between national and international experts released a comprehensive report on global quality care, focusing on value-based care for BC.10,11 On this basis, a task force within the Belgian Society of Medical Oncology (BSMO) defined a set of measurable process indicators for patients with BC, with an essential intent to improve the patient's quality of care.12 Particularly relevant is the challenge of overtreatment, as it burdens patients with unnecessary toxicity and society with unnecessary costs.13 In addition, an important element in the assessment of quality of care is the definition of an appropriate standard, which sometimes is a major challenge.14
The current study reports on the resulting set of clinically relevant process QIs based on population-based data. The aim is to evaluate the results on a national and centre level. The ultimate intent is to improve quality of care and reduce disparities between treatment centres in Belgium.15
Patients and methods
Data sources
Data on patients diagnosed with cancer were obtained from the Belgian Cancer Registry (BCR) database, which has a national coverage since 2004.16 The patient's national number enabled linkage with the national administrative reimbursement database (called IMA) that was used to obtain information on medical procedures (e.g. imaging, diagnostic or therapeutic procedures) and pharmaceuticals reimbursed for patients with cancer by health insurance.
Through linkage with the Crossroads Bank for Social Security, the BCR can perform active follow-up on vital status and date of death of the patients. Follow-up for this study was considered up to 1 April 2020.
Study population
All female patients with unilateral primary invasive BC diagnosed between 1 January 2010 and 31 December 2014 and with an official residence in Belgium at the moment of diagnosis were selected (N = 55 801; Supplementary Figure S1, available at https://doi.org/10.1016/j.esmoop.2021.100207). BC was defined based on the International Statistical Classification of Diseases and Related Health Problems 10th revision (ICD-10).17 Patients deceased or lost to follow-up at the incidence date (n = 70) were excluded because there is not enough information in the IMA database regarding treatment for these patients. Patients with prior BC (n = 5508) were excluded, because there is no direct link between the IMA data and specific cancer diagnoses, and therefore information on treatment of patients with multiple breast tumours cannot be evaluated in a reliable way. Patients who were diagnosed with phyllodes breast tumours (n = 88) were excluded, because the treatment of these breast tumours differs substantially from breast carcinomas. Only Belgian residents for whom a national number was available were considered, as the national number is necessary to link the patients to the IMA data (on this basis, we excluded 61). Patients who were treated in a foreign hospital were excluded because for these patients no IMA data are available (n = 161). Finally, patients for whom no IMA data were available were also excluded (n = 1041). A total of 48 872 patients was finally included in this study.
Belgian hospitals and breast clinics
Belgian hospitals are subsidized by the public health authorities and could have the following characteristics: general hospital, university hospital, or general hospital with university affiliation. From 2007 onwards, official conditions were included in the Belgian legislation that defined certified coordinate or satellite oncological care programs for BC (i.e. Breast Clinic).
Patient allocation
A preliminary analysis showed that 94% of the patients had breast surgery and chemotherapy in the same centre. Therefore, the hospital that performed the surgical procedure was regarded as the centre of main treatment. If there was no centre of surgery, priority rules designed by expert opinion were applied to allocate the remaining patients to a specific centre. According to these priority rules, patients were allocated in the decreasing order of priority to the centre where chemotherapy was administered, the centre where trastuzumab was administered, the centre where the multidisciplinary team discussion took place, the centre where endocrine therapy was prescribed, the centre of radiotherapy, the centre that registered the cancer diagnosis at the cancer registry, and lastly the centre where the diagnostic biopsy was performed.
Patient and tumour characteristics
Baseline patient and tumour characteristics were accessible from the BCR database. Tumours were staged by the respective registering hospitals, following the Union for International Cancer Control (UICC) TNM classification in use in the year of diagnosis (7th and 8th editions).
A recent population-based study calculated the incidence of BC subtypes in Belgium according to the 2011 St Gallen surrogate classification using combinations of estrogen receptor (ER), progesterone receptor, and human epidermal growth factor receptor 2 (HER2) status and tumour differentiation grade.18,19 The results of this national study, restricted to incidence year 2014, were used for the indicators selecting particular BC subtypes, as shown in Table 1. The proliferation marker Ki-67, which is generally included in the molecular classification for BC, is not available in a structured way in the BCR database. Therefore, the surrogate classification was applied, using tumour differentiation grade as a surrogate for Ki-67. Subsequently, luminal BC subtypes are named ‘-like’, to stress that a surrogate classification was applied.
Table 1.
Patient, tumour, and treatment characteristics for the study population (N = 48 872)
| Characteristic | Cohort incidence years 2010-2014, n (%) | Cohort incidence year 2014 n (%) |
|---|---|---|
| All patients | 48 872 (100.0) | 9855 (100.0) |
| Age at diagnosis | ||
| Mean (SD) | 62.2 (14.1) | 62.4 (14.1) |
| Age categories | ||
| <50 years | 9961 (20.4) | 1972 (20.0) |
| 50-69 years | 23 642 (48.4) | 4750 (48.2) |
| ≥70 years | 15 269 (31.2) | 3133 (31.8) |
| Clinical stage | ||
| 0 | 442 (0.9) | 97 (1.0) |
| I | 17 116 (35.0) | 3621 (36.7) |
| II | 14 751 (30.2) | 3075 (31.2) |
| III | 3116 (6.4) | 609 (6.2) |
| IV | 2628 (5.4) | 545 (5.5) |
| Unknown | 10 819 (22.1) | 1908 (19.4) |
| Histological subtype | ||
| Ductal | 36 928 (75.6) | 7420 (75.3) |
| Lobular | 6669 (13.7) | 1425 (14.5) |
| Mixed ductal/lobular | 2821 (5.8) | 558 (5.7) |
| Other | 2454 (5.0) | 452 (4.6) |
| Molecular subtype | ||
| Luminal A-like | — | 5367 (54.5) |
| Luminal B-like HER2 negative | — | 1465 (14.9) |
| Luminal B-like HER2 positive | — | 1220 (12.4) |
| HER2-positive nonluminal | — | 454 (4.6) |
| Triple negative | — | 845 (8.6) |
| Unknown | — | 504 (5.1) |
| Treatment | ||
| Breast surgery | 43 012 (88.0) | 8633 (87.6) |
| Breast-conserving surgery | 26 926 (55.1) | 5517 (56.0) |
| Mastectomy | 16 086 (32.9) | 3116 (31.6) |
| Endocrine therapy | 38 742 (79.3) | 7810 (79.3) |
| (Neo)adjuvant | 34 767 (71.1) | 6996 (71.0) |
| Without surgery | 3975 (8.1) | 814 (8.3) |
| Chemotherapy | 19 759 (40.4) | 3984 (40.4) |
| (Neo)adjuvant | 18 236 (37.3) | 3643 (37.0) |
| Without surgery | 1523 (3.1) | 341 (3.5) |
| Trastuzumab | 5419 (11.1) | 1197 (12.2) |
| (Neo)adjuvant | 4972 (10.2) | 1085 (11.0) |
| Without surgery | 447 (0.9) | 112 (1.1) |
| Radiotherapy | 36 444 (74.6) | 7235 (73.4) |
| (Neo)adjuvant | 35 192 (72.0) | 6972 (70.8) |
| Without surgery | 1252 (2.6) | 263 (2.7) |
| No active reimbursed treatmenta | 993 (2.0) | 204 (2.1) |
HER2, human epidermal growth factor receptor 2; SD, standard deviation.
No breast surgery, no endocrine therapy, no chemotherapy, no trastuzumab, no radiotherapy, according to IMA data.
Staging and treatment characteristics
Staging procedures and treatments were obtained from the IMA database. Specific timeframes were applied, during which the medical intervention should have taken place in order to be included in the analyses (Supplementary Table S1, available at https://doi.org/10.1016/j.esmoop.2021.100207). The respective timeframes were defined based on expert opinion as well as data driven (e.g. breast surgery within −1/+9 months around the incidence date was considered as treatment for BC, chemotherapy administered within 4 months after the surgery date was regarded as adjuvant chemotherapy).
Identification of quality indicators
QIs of interest were identified by literature review and national expert opinion after consulting the BSMO members by survey.12,20 The selection of indicators was both driven and limited by the availability of data in the BCR/IMA databases.
Statistical analysis
All analyses were performed with SAS version 9.4 (SAS Institute Inc., Cary, NC, USA).
Representation of the results
The QIs were measured on a national and centre level. For every indicator, the average national result was calculated, as well as several parameters to evaluate the variability between the different treatment centres. These parameters include the median centre result and interquartile range (IQR). Funnel plots visualize the unadjusted results for all the centres treating BC and allow displaying the variability of the indicator results among centres, with certified specialist breast centres being indicated in red. The indicator result (y-axis) is plotted against its precision (x-axis). The precision value on the x-axis is related to the centre size. A reference value equalling the national average is added to the plot, accompanied by a 95% and 99% prediction limit around the reference value for all possible values of the precision (x-axis). A hospital situated outside the prediction limits could be considered an outlier (positive or negative); however, an outlier does not automatically imply (sub)optimal quality of care. Differences in case-mix between the centres were not taken into account.
Results
Study population
A total of 48 872 patients were included in this observational cohort study (Supplementary Figure S1, available at https://doi.org/10.1016/j.esmoop.2021.100207). For indicators selecting a specific subtype, only patients diagnosed in the year 2014 (n = 9855) were included. Table 1 provides patient and tumour characteristics as well as treatment specifications for the 2010-2014 cohort.
Centres treating patients with breast cancer and centre volume distribution
All Belgian hospitals treating patients with BC were included (n = 102), among which 53 centres were certified specialist breast centres (comprising three satellite centres). All included patients were allocated to a specific centre according to the algorithm mentioned before, allowing the calculation of an average annual treatment volume for each centre. During the study period (2010-2014), 28 centres treated at least 125 patients per year, and were considered high-volume centres (HVCs) as per Belgian regulation, 49 centres treated on average <60 patients per year [low-volume centres (LVCs)], and the remaining 25 centres were medium-volume centres (MVCs; annual treatment volume between 60 and 125 patients). The mentioned treatment volumes apply to the selected study population as described earlier. Therefore these must be regarded as artificial volumes and do not reflect the actual individual treatment volumes in Belgian hospitals.
Identification of quality indicators
A total of 12 process (sub)indicators were identified.
-
1.
Proportion of patients with clinical stage (cStage) I BC that received positron emission tomography/computed tomography (PET/CT) during staging.
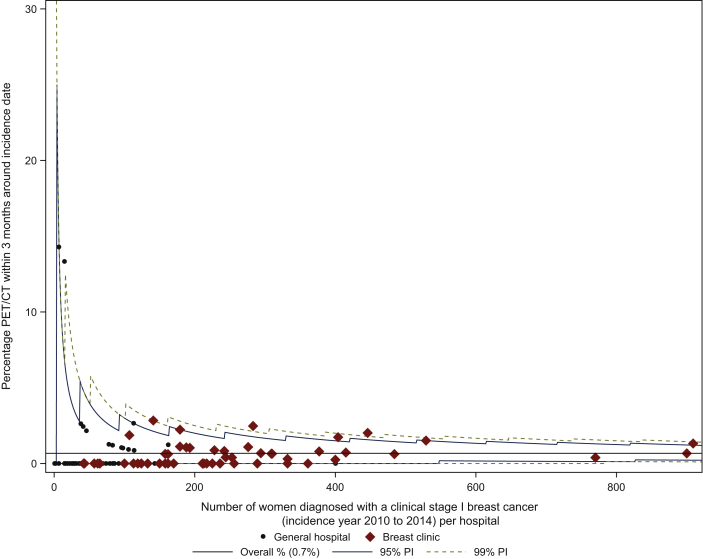
A total of 115 out of 17 116 (0.7%) cStage I patients were staged with PET/CT in a 3-month timeframe around the incidence date. Figure 1 depicts the intercentre variability, with a median centre result of 0.0% (IQR 0.0% to 0.9%). The median centre results and IQR according to treatment centre volumes (LVC versus MVC versus HVC) were comparable.
-
2.
Proportion of patients with BC that received intravenous chemotherapy during the last 2 weeks of life.
Out of all deceased patients (n = 8433), 5.4% received chemotherapy during the last 2 weeks of life (n = 452). This proportion was comparable between all centres, with a median centre result of 5.1% (IQR 2.9% to 6.7%). The median centre results and IQR according to the different treatment centre volumes were comparable.
-
3.
Proportion of stage pT1apN0 HER2-positive patients that received adjuvant trastuzumab.
Of the HER2-positive patients diagnosed in 2014 who underwent primary surgery and were staged pT1aN0 (n = 47), 25.5% received adjuvant trastuzumab (n = 12; median centre result 0.0%, IQR 0.0% to 50.0%). In patients younger than 70 years the average proportion was 27.5%, as opposed to 14.3% in patients older than 70 years.
-
4.
Proportion of patients with metastatic luminal A/B-like HER2-negative BC that received chemotherapy within 3 months after diagnosis.
Patients diagnosed in 2014 with cStage IV luminal A/B-like HER2-negative BC (n = 295) were administered chemotherapy and received no surgery (regardless of endocrine therapy) within the first 3 months after the incidence date in 17.3% (n = 51; Supplementary Figure S2, available at https://doi.org/10.1016/j.esmoop.2021.100207). The overall median centre result was 0.0% (IQR 0.0% to 28.6%). In LVC, MVC, and HVC, the median centre results were 0.0% (IQR 0.0% to 0.0%), 0.0% (IQR 0.0% to 20.0%), and 14.3% (IQR 0.0% to 33.3%), respectively. In patients younger than 70 years, the average proportion was 25.4%, as opposed to 9.8% in patients older than 70 years.
-
5.Proportion of patients with BC younger than 70 years that received (neo)adjuvant chemotherapy.
-
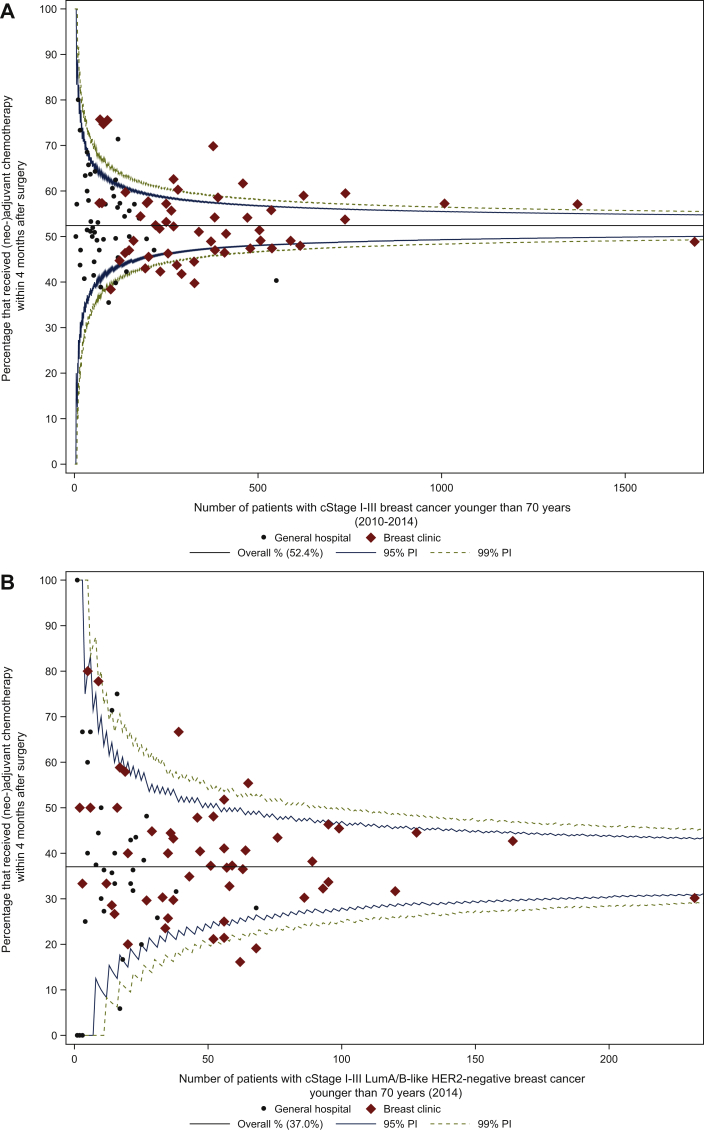
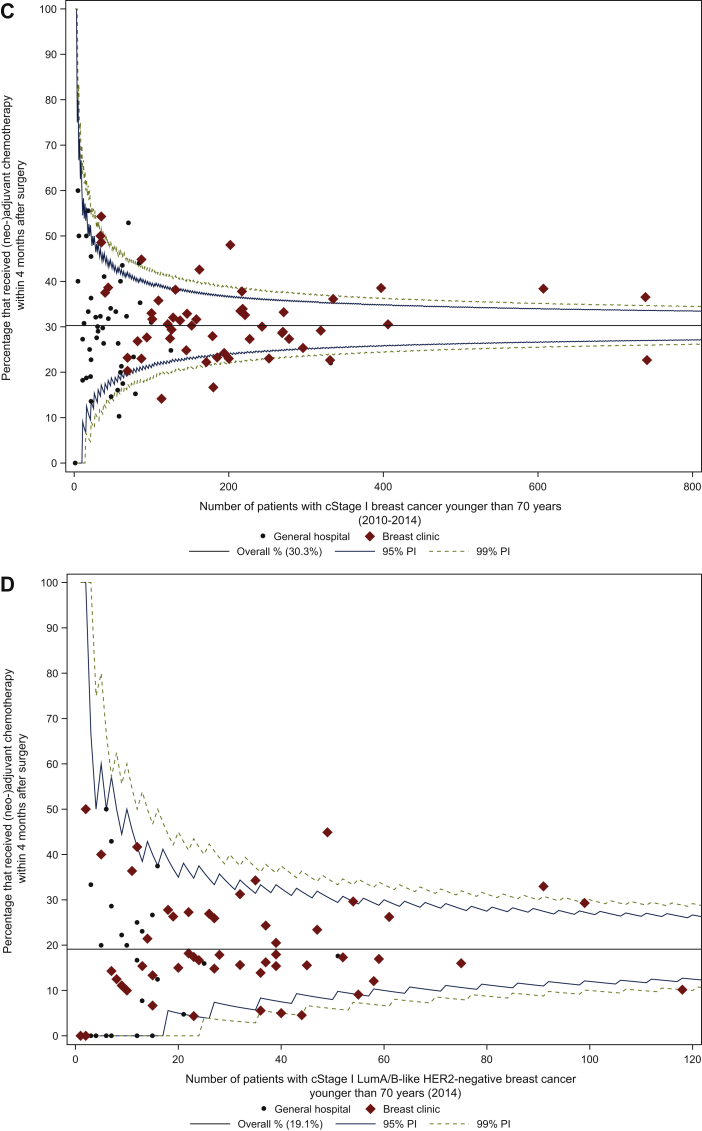
a.In cStage I-III (2010-2014), of 24 030 women who were operated, 12 592 received either neoadjuvant or adjuvant chemotherapy within the first 4 months after surgery (52.4%). This proportion varied between the centres, with a median centre result of 52.1% (IQR 47.0% to 57.5%; Figure 2A). Inter-centre variability according to treatment centre volume was comparable.
-
b.In patients with cStage I-III luminal A/B-like HER2-negative BC (2014): of 3429 operated women, 1270 received (neo)adjuvant chemotherapy within the first 4 months after surgery (37.0%). The median centre result was 37.3% (IQR 30.0% to 46.3%; Figure 2B). The median centre result in HVC only (40.3%, IQR 31.0% to 45.9%) was slightly higher compared with both LVC (36.4%, IQR 30.0% to 50.0%) and MVC (36.4%, IQR 29.6% to 44.8%).
-
c.In cStage I (2010-2014), 30.3% of the patients were administered (neo)adjuvant chemotherapy (n = 3972), with a variable proportion among centres (median centre result of 30.2%, IQR 23.3% to 36.2%; Figure 2C). Intercentre variability according to treatment centre volume was comparable.
-
d.In patients with cStage I luminal A/B-like HER2-negative BC (2014): 19.1% of the patients received (neo)adjuvant chemotherapy (n = 407), with a variable proportion among centres (median centre result of 16.1%, IQR 7.7% to 26.2%; Figure 2D). The median centre result in HVC only (17.3%, IQR 14.3% to 26.3%) was higher compared with both LVC and MVC.
-
a.
-
6.
Proportion of patients with cStage I-III BC younger than 70 years that completed at least 4.5 years of adjuvant endocrine therapy.
Figure 1.
Proportion of patients with clinical stage I breast cancer that received PET/CT during staging.
PET/CT, positron emission tomography/computed tomography; PI, prediction interval.
Figure 2.
Proportion of patients with breast cancer younger than 70 years that received (neo)adjuvant chemotherapy in (A) cStage I-III, (B) cStage I-III luminal A/B-like HER2-negative, (C) cStage I, and (D) cStage I luminal A/B-like HER2-negative breast cancer.
cStage, Clinical Stage; HER2, human epidermal growth factor receptor; LumA/B-like, luminal A- or B-like; PI, prediction interval.
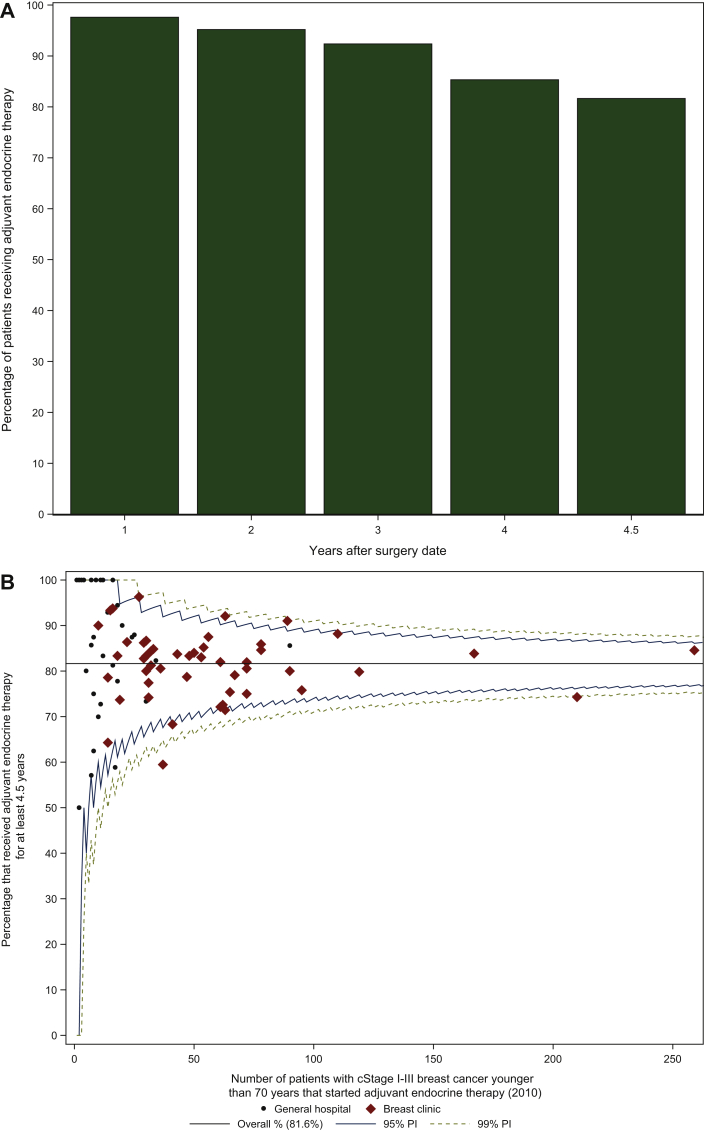
The analysis was based on dates from the IMA database that refer to the delivery dates of endocrine drugs by the pharmacist and not to the actual intake by the patient. The prescribed medication is usually sufficient for at least a 3-month period, which is relevant information for the interpretation of the results. Patients younger than 70 years diagnosed in 2010 with cStage I-III BC who were operated and started adjuvant endocrine therapy were selected (n = 3591). Adjuvant endocrine therapy was delivered by the pharmacy for ≥4.5 years to 81.7% of the patients (n = 2932), which can be interpreted as a continuation of endocrine drug intake for 4.5-5 years (Figure 3A). Adjuvant therapy was prescribed for at least 4 years in 85.3% of cases (n = 3064). Figure 3B depicts the intercentre variability of the duration of adjuvant endocrine treatment for ≥4.5 years; the median centre result was 83.3% (IQR 77.6% to 90.5%).
-
7.Proportion of patients with BC older than 70 years and 75 years, respectively, that received:
-
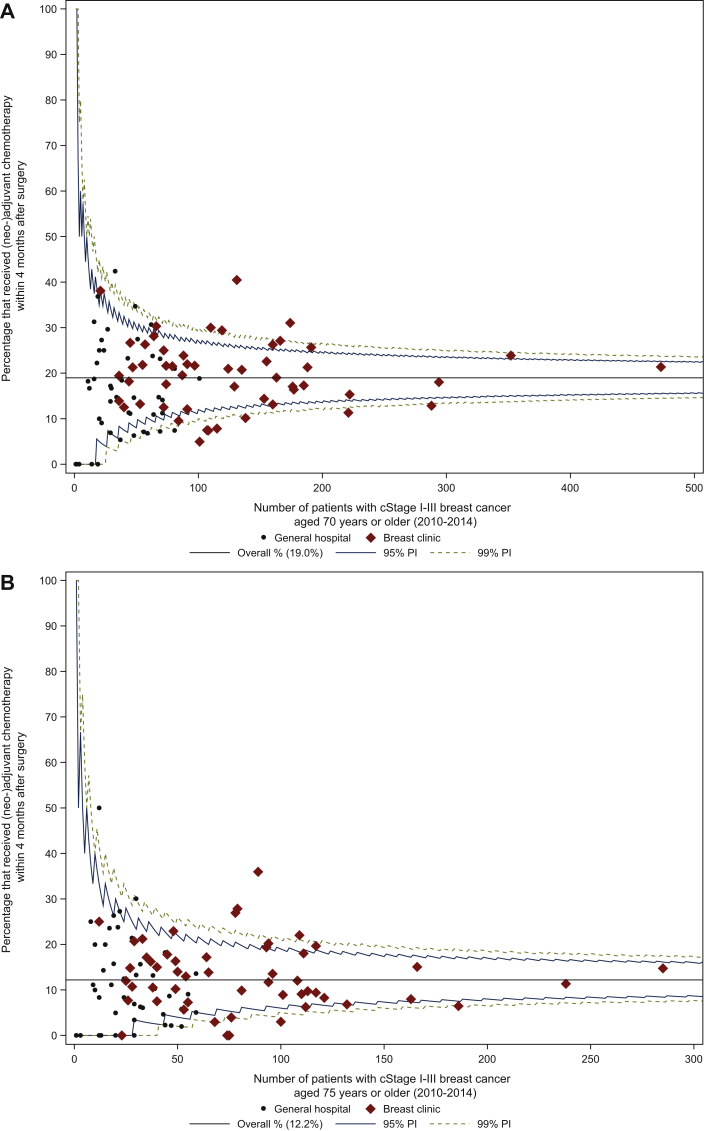
a.(neo)adjuvant chemotherapy (cStage I-III, all subtypes; 2010-2014): in 19.0% (1622/8544 patients) of patients older than 70 years chemotherapy was administered within 4 months after surgery (median centre result 18.2%, IQR 11.4% to 23.9%; Figure 4A). The median centre results and IQRs differed substantially according to treatment centre volume: LVC median centre result 15.7% (IQR 8.3% to 24.4%), MVC median centre result 18.2% (IQR 12.1% to 23.9%), HVC median centre result 21.1% (IQR 16.7% to 24.4%). HVC displayed the highest median result, but also the smallest IQR indicating rather limited variation among HVC. In patients older than 75 years 12.2% received (neo)adjuvant chemotherapy (685/5611 patients; Figure 4B).
-
b.(neo)adjuvant chemotherapy (patients with cStage I-III luminal A/B-like HER2-negative BC; 2014): 13.0% (181/1388) of patients in the population older than 70 years compared with 8.2% (73/891) of patients in the population older than 75 years received (neo)adjuvant chemotherapy. Variability between centres was considerable; in the population older than 70 years the median centre result was 10.0% (IQR 0.0% to 20.0%). In LVC the median centre result was 0.0% (IQR 0.0% to 20.0%), in MVC 11.8% (IQR 6.7% to 17.2%), and in HVC 10.3% (IQR 7.5% to 23.3%).
-
c.endocrine therapy and no surgery (patients with cStage I-II luminal A/B-like HER2-negative BC; 2014): in patients older than 70 years, 11.6% (171/1477 patients) was not operated and received endocrine treatment within the first 12 months after diagnosis; in the population older than 75 years this proportion was 16.2% (162/1000 patients). Variability between centres was considerable; in the population older than 70 years the median centre result was 6.3% (IQR 0.0% to 20.8%). In LVC the median centre result was 0.0% (IQR 0.0% to 21.4%), in MVC 8.0% (IQR 4.6% to 21.4%), and in HVC 7.1% (IQR 3.9% to 18.7%).
-
a.
Figure 3.
Proportion of patients with cStage I-III breast cancer younger than 70 years who were diagnosed in 2010 and started adjuvant endocrine therapy.
(A) Histogram depicting the drop-out among patients receiving adjuvant endocrine therapy after the date of surgery, (B) funnel plot depicting the variability between centres for at least 4.5 years of adjuvant endocrine therapy.
cStage, clinical stage; PI, prediction interval.
Figure 4.
Proportion of patients with cStage I-III breast cancer aged (A) 70 years or older and (B) 75 years or older that received (neo)adjuvant chemotherapy within 4 months after surgery.
cStage, clinical stage; PI, prediction interval.
Discussion
This study demonstrates how population-based data can serve to enable qualitative assessment of quality of care. Based on previous reports on QIs for systemic treatment of BC, we identified 12 process (sub)indicators, with the aim to reveal sensitive aspects of actual (mis)use of resources.12,21 Financial implications, adherence to guidelines, and quality of life were the common threads in the selection process. In general, the overall national results appear within acceptable bounds, but some indicators disclose a substantial variability between centres.
For patients with cStage I BC PET/CT in the diagnostic phase is generally not recommended, although it can be appropriate in selected cases.20 The American Society of Clinical Oncology (ASCO)-led Choosing Wisely Initiative, launched in 2012, was an attempt to avoid unnecessary tests, treatments, and procedures, and promote conversations between patients and clinicians.13,22 Recently published results in the United States indicated a positive influence of this initiative on unnecessary imaging in early-stage BC, albeit patient selection was limited to women above the age of 66.23 Likewise, our national results (applicable to the period 2010-2014) confirmed that PET/CT was infrequently used as staging procedure for patients with cStage I BC.
The European Society for Medical Oncology (ESMO) provides practical guidelines for the management of early BC.20 The EUSOMA recommendations offer QIs for the use of systemic therapy in specific early-stage BC subtypes (e.g. ER-negative invasive BC, HER2-positive invasive BC, inflammatory BC).8 The decision to administer chemotherapy is a multifactorial decision, based on the stage of the disease, the coexistence of comorbidities, the patient's preference, and if available genomic signatures in luminal disease. Nevertheless, it frequently implies a non-negligible repercussion on quality of life.24, 25, 26 Current guidelines do not recommend adjuvant HER2-targeted therapy in pT1aN0 HER2-positive patients; nevertheless, 25.5% of these patients received adjuvant trastuzumab in our population.8,20
For the luminal HER2-negative BC subtype, which is the most common, guidelines for systematic treatment are not always indicating a clear recommendation for specific cases, and there is no consensus among guidelines.20,27,28 Our results show that 19% of patients with cStage I luminal A/B-like HER2-negative BC were administered (neo)adjuvant chemotherapy, with a substantial variability between treatment centres. A tendency towards more administration of chemotherapy in HVC was observed. Access to gene expression profiling tests is another relevant parameter in this context. In Belgium, since 2019 gene expression profiling is reimbursed in breast clinics for a cut-off of 15% of the target population, which may lower variability among centres.29
Our results show that a rather high proportion of upfront metastatic hormone receptor-positive/HER2-negative patients received chemotherapy (regardless of endocrine therapy) within the first 3 months after diagnosis (17%), and in patients younger than 70 years this increases to 25%.
As for the administration of intravenous chemotherapy during the last 2 weeks of life, the results in our cohort (5.4%) appeared reasonable and comparable with other European data.30 Besides, no significant variability was observed between treatment centres in Belgium.
Older patients are particularly important as they represent a large group of the population. However, convincing studies about the added value of systemic treatment, especially chemotherapy, in these patients are scarce.31 In addition, the differences between biological and calendar age and the concept of frailty are extremely relevant in this population, and based on a discussion between treating physician and patient, these factors will decide which strategy will be chosen. In our population, 19% of patients with cStage I-III BC aged older than 70 years received (neo)adjuvant chemotherapy, with a considerable variation between centres. When selecting the patients with hormone receptor-positive/HER2-negative BC, a prognostically favourable subgroup, 13% of older adults were administered chemotherapy. The overall median centre result was 10.0% (IQR 0.0% to 20.0%). In LVC the median centre result was 0.0% (IQR 0.0% to 20.0%), in contrast to higher proportions and smaller IQR in both MVC and HVC [median centre result of 11.8% (IQR 6.7% to 17.2%) and 10.3% (IQR 7.5% to 23.3%), respectively].
The therapeutic option of omitting surgery of the breast in selected older patients with hormone receptor-positive early-stage BC seems not often used, as the proportion of patients with cStage I-II hormone receptor-positive/HER2-negative BC aged older than 70 receiving endocrine therapy and no surgery equalled 12%. Unfortunately, the most important elements that can be considered to prefer this therapeutic strategy (i.e. life expectancy and frailty/comorbidities) are not addressed, and together with the lack of ‘standard’ concerning this proportion hampers the interpretation of this QI.
For adjuvant endocrine therapy, the presented data refer to the delivery of prescribed medication by the pharmacy, which is usually sufficient for at least a 3-month period. Adjuvant endocrine therapy was prescribed for ≥4.5 years in 82% of the patients, which can be interpreted as a continuation of endocrine drug intake for 4.5-5 years. Unfortunately, the analyses did not take into account the various dosages and number of pills of the delivered medication, which could refine the results. Besides, the analyses did not include a requirement of longitudinal continuity with specific time intervals of the prescribed medication, and therefore it is possible that in some cases adjuvant treatment moved towards a secondary metastatic setting, leading to overestimation of the actual adjuvant situation. Meanwhile, patients who received their adjuvant endocrine treatment in the setting of a clinical trial are not included in these analyses, which then again might lead to underestimation. Notwithstanding these limitations, these national results (patients diagnosed in 2010) are reassuring, and are in contrast to a recent analysis of 33 260 women (diagnosed in 2011) with an adherence of 69% after 5 years.32, 33, 34, 35
Some limitations of this study exist: First, although 94% of the operated patients with BC also received their systemic treatment in the same centre, patient allocation remains difficult, becoming even worse with the integration and networking of hospitals. The more patients are followed in one centre, the more the data can be trusted. Second, data of patients treated in clinical trials were not represented, as only treatments reimbursed by the national health insurance are available to the cancer registry. Third, certainly in LVC the absolute numbers were too low to be statistically relevant. Besides, as incidence data on different molecular subtypes were restricted to the year 2014 only, for the QIs selecting a specific subtype numbers were relatively low. Finally, some indicators lack a clear ‘standard’ (e.g. chemotherapy in selected patient groups), leaving a matter of debate. Nevertheless, the generally observed pronounced intercentre differences are interesting findings that warrant discussion and further reflection.
Notwithstanding its limitations, real-world data are increasingly important in medicine and in the study of cancer. This population-based study tries to integrate performed investigations and treatments of BC in Belgium. Today, countries having the capability to calculate indicators using nationwide data are rather exceptional.36,37 In most countries quality improvement programmes, if available, are performed on a voluntary basis.38 Moreover, in Europe EUSOMA offers an active process of monitoring and auditing of breast centre performance, whereas in the United States the National Accreditation Program for Breast Centers also allows participating breast centres to share their data for benchmarking.39,40
To conclude, close contact between cancer registries, caregivers, and researchers improves the quality of the available data and the ability to understand the relevance of the findings. Given the observed disparities between the centres, it is desirable to reflect on the impact that guidelines, education, and awareness can have. The identification of QIs related to appropriateness of care in BC answers the need for transparency. Their use in daily practice should be implemented in all centres treating BC. Besides offering interesting epidemiological data, QIs are part of good clinical practice of all centres and are the starting point of broader team discussions on the provided care.
Acknowledgements
We thank all data registrars for their individual contribution to the national cancer registry database. The work that has been done in this study is in accordance with the main aim of the Innovative Partnership for Action Against Cancer (iPAAC) Joint Action (https://www.ipaac.eu/en/about/).
Funding
This work was supported by The Fonds Pink Ribbon [grant number 2017-J5920810-208700].
Disclosure
The authors have declared no conflicts of interest.
Supplementary data
References
- 1.Ayanian J., Markel H. Donabedian's lasting framework for health care quality. N Engl J Med. 2016;375(3):205–207. doi: 10.1056/NEJMp1605101. [DOI] [PubMed] [Google Scholar]
- 2.Campbell S., Roland M.O., Buetow S.A. Defining quality of care. Soc Sci Med. 2000;51(11):1611–1625. doi: 10.1016/s0277-9536(00)00057-5. [DOI] [PubMed] [Google Scholar]
- 3.Donabedian A. Evaluating the quality of medical care. Milbank Mem Fund Q. 1966;44(3):166–203. [PubMed] [Google Scholar]
- 4.Berwick D.M., Cassel C.K. The NAM and the quality of health care - inflecting a field. N Engl J Med. 2020;383:505–508. doi: 10.1056/NEJMp2005126. [DOI] [PubMed] [Google Scholar]
- 5.McNiff M.P.H. The quality oncology practice initiative, American Society of Clinical Oncology. J Oncol Pract. 2013;2(1):26–30. doi: 10.1200/jop.2006.2.1.26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.del Turco M.R., Ponti A., Bick U. Quality indicators in breast cancer care. Eur J Cancer. 2010;46:2344–2356. doi: 10.1016/j.ejca.2010.06.119. [DOI] [PubMed] [Google Scholar]
- 7.Stordeur S., Vrijens F., Devriese S., Beirens K., van Eycken E., Vlayen J. Developing and measuring a set of process and outcome indicators for breast cancer. Breast. 2011;21(3):253–260. doi: 10.1016/j.breast.2011.10.003. [DOI] [PubMed] [Google Scholar]
- 8.Biganzoli L., Marotti L., Hart C.D. Quality indicators in breast cancer care: an update from the EUSOMA working group. Eur J Cancer. 2017;86:59–81. doi: 10.1016/j.ejca.2017.08.017. [DOI] [PubMed] [Google Scholar]
- 9.Biganzoli L., Cardoso F., Beishon M. The requirements of a specialist breast centre. Breast. 2020;51:65–84. doi: 10.1016/j.breast.2020.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Verhoeven D., Duhoux F.P., de Azambuja E. Quality management of systemic treatment of breast cancer. Belg J Med Oncol. 2018;12(1):16–22. [Google Scholar]
- 11.Verhoeven D., Kaufman C., Mansel R. Systemic Treatment. Oxford University Press; Oxford, UK: 2019. Breast cancer: global quality care. [Google Scholar]
- 12.Verhoeven D., van Walle L., Duhoux F.P. A critical appraisal of quality indicators of breast cancer treatment in Belgium. Ann Oncol. 2019;30(S5):v55–v98. [Google Scholar]
- 13.Born K., Kool T., Levinson W. Reducing overuse in healthcare: advancing choosing wisely. Br Med J. 2019;367:l6317. doi: 10.1136/bmj.l6317. [DOI] [PubMed] [Google Scholar]
- 14.van Bommel A.C., Spronk P.E., Vrancken Peeters M.T. Clinical audit as an instrument for quality improvement in breast cancer care in the Netherlands: the NABON breast cancer audit. J Surg Oncol. 2017;115(3):243–249. doi: 10.1002/jso.24516. [DOI] [PubMed] [Google Scholar]
- 15.Ganz P.A., Levit L.A. Charting a new course for the delivery of high-quality cancer care. J Clin Oncol. 2013;36:4485–4487. doi: 10.1200/JCO.2013.53.7993. [DOI] [PubMed] [Google Scholar]
- 16.Cancer Burden in Belgium, 2004-2017. Belgian Cancer Registry; Brussels: 2020. Available at https://kankerregister.org/media/docs/CancerBurdenfeb2020reduced_new.pdf. Accessed July 5, 2021. [Google Scholar]
- 17.World Health Organization . World Health Organization; Geneva, Switzerland: 2010. International Statistical Classification of Diseases and Related Health Problems 10th Revision. [Google Scholar]
- 18.van Walle L., Vandeven J., Colpaert C. Incidence of breast cancer subtypes in Belgium: a population-based study. Belg J Med Oncol. 2020;14(6):263–273. [Google Scholar]
- 19.Brouckaert O., Laenen A., Vanderhaegen J. Applying the 2011 St Gallen panel of prognostic markers on a large single hospital cohort of consecutively treated primary operable breast cancers. Ann Oncol. 2012;23(10):2578–2584. doi: 10.1093/annonc/mds062. [DOI] [PubMed] [Google Scholar]
- 20.Cardoso F., Kyriakides S., Ohno S. Early breast cancer: clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2019;30:1194–1220. doi: 10.1093/annonc/mdz173. [DOI] [PubMed] [Google Scholar]
- 21.Verhoeven D., Allemani C., Kaufman C., Mansel R., Siesling S., Anderson B. Breast cancer: global quality care optimising care delivery within existing financial and personnel resources. ESMO Open. 2020;4:e000861. doi: 10.1136/esmoopen-2020-000861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Choosing Wisely Initiative. https://www.choosingwisely.org Available at.
- 23.Baltz A., Makhoul I., Siegel E.R. The clinical impact of ASCO “choosing wisely” recommendations on staging imaging for early stage breast cancers: an interrupted time-series analysis utilizing SEER-Medicare data. J Clin Oncol. 2020;38(suppl 15):2078. [Google Scholar]
- 24.Stemmer S.M., Steiner M., Rizel S. Clinical outcomes in patients with node-negative breast cancer treated based on the recurrence score results: evidence from a large prospectively designed registry. NPJ Breast Cancer. 2017;3:33. doi: 10.1038/s41523-017-0034-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sparano J.A., Gray R.J., Makower D.F. Adjuvant chemotherapy guided by a 21-gene expression assay in breast cancer. N Engl J Med. 2018;379:111–121. doi: 10.1056/NEJMoa1804710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kurian A.W., Bondarenko I., Jagsi R. Recent trends in chemotherapy use and oncologists' treatment recommendations for early-stage breast cancer. J Natl Cancer Inst. 2018;110:493–500. doi: 10.1093/jnci/djx239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Denduluri N., Somerfield M.R., Chavez-MacGregor M. Selection of optimal adjuvant chemotherapy and targeted therapy for early breast cancer: ASCO guideline update. J Clin Oncol. 2020;39(6):685–693. doi: 10.1200/JCO.20.02510. [DOI] [PubMed] [Google Scholar]
- 28.Gradishar W.J., Anderson B.O., Abraham J. Breast cancer version 3.2020, NCCN clinical practice guidelines in oncology. J Natl Compr Canc Netw. 2020;18(4):452–478. doi: 10.6004/jnccn.2020.0016. [DOI] [PubMed] [Google Scholar]
- 29.San Miguel L, Dubois C, Gerkens S, et al. Mammaprint test for personalised management of adjuvant chemotherapy decisions in early breast cancer. In: KCE Reports 298. Available at https://kce.fgov.be/sites/default/files/atoms/files/KCE_298_Mammaprint_tests_Report.pdf. Accessed July 5, 2021.
- 30.Martins-Branco D., Lopes S., Canario R. Factors associated with the aggressiveness of care at the end of life for patients with cancer dying in hospital: a nationwide retrospective cohort study in mainland Portugal. ESMO Open. 2020;5:e000953. doi: 10.1136/esmoopen-2020-000953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Extermann M., Aapro M., Audisio R.A. Main priorities for the development of geriatric oncology: a worldwide expert perspective. J Geriatr Oncol. 2011;2(4):270–273. [Google Scholar]
- 32.Barron T.I., Connolly R.M., Bennett K. Early discontinuation of tamoxifen: a lesson for oncologists. Cancer. 2007;109:832–839. doi: 10.1002/cncr.22485. [DOI] [PubMed] [Google Scholar]
- 33.Ruddy K., Mayer E., Partridge A. Patients adherence and persistence with oral anticancer treatment. CA Cancer J Clin. 2009;59:56–66. doi: 10.3322/caac.20004. [DOI] [PubMed] [Google Scholar]
- 34.Friese C.R., Pini T.M., Li Y. Adjuvant endocrine therapy initiation and persistence in a diverse sample of patients with breast cancer. Breast Cancer Res Treat. 2013;138:931–939. doi: 10.1007/s10549-013-2499-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lailler G., Memoli V., Le-Bihan Benjamin C. Five-year adjuvant endocrine therapy adherence among women with breast cancer: a notion-wide French study using administrative data. Clin Breast Cancer. 2021 doi: 10.1016/j.clbc.2021.01.007. In press. [DOI] [PubMed] [Google Scholar]
- 36.Derks M.G.M., Bastiaannet E., Kiderlen M. Variation in treatment and survival of older patients with non-metastatic breast cancer in five European countries: a population-based cohort study from the EURECCA Breast Cancer Group. Br J Cancer. 2018;119:120–129. doi: 10.1038/s41416-018-0090-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Spronk P.E.R., van Bommel A.C.M., Siesling S., Wouters M.W.J.M., Vrancken Peeters M.T.F.D., Smorenburg C.H. Variation in use of neo-adjuvant chemotherapy in patients with stage III breast cancer. Results of the Dutch national breast cancer audit. Breast. 2017;36:34–39. doi: 10.1016/j.breast.2017.08.011. [DOI] [PubMed] [Google Scholar]
- 38.Kowalski C., Ferencz J., Brucker S.Y., Kreienberg R., Wesselmann S. Quality of care in breast cancer centers: results of benchmarking by the German Cancer Society and German Society for Breast Diseases. Breast. 2015;24(2):118–123. doi: 10.1016/j.breast.2014.11.014. [DOI] [PubMed] [Google Scholar]
- 39.van Dam P.A., Tomatis M., Marotti L. The effect of EUSOMA certification on quality of breast cancer care. Eur J Surg Oncol. 2015;41(10):1423–1429. doi: 10.1016/j.ejso.2015.06.006. [DOI] [PubMed] [Google Scholar]
- 40.National Accreditation Program for Breast Centers. https://www.facs.org/quality-programs/napbc Available at.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.