Highlights
-
•
Targeting ferroptosis in osteosarcoma.
-
•
A new strategy for the treatment of osteosarcoma.
-
•
A non-traditional mode of regulated cell death.
Keywords: Ferroptosis, Osteosarcoma, Treatment, Iron, Reactive oxygen species
Abstract
Osteosarcoma (OS) is the most common primary bone tumour in children and adolescents, with high degree of malignancy and an extremely poor prognosis. Ferroptosis, a non-traditional mode of regulated cell death (RCD) characterised by iron-dependent accumulation of lipid reactive oxygen species (ROS), is closely associated with a variety of cancers. It has been demonstrated that ferroptosis can regulate OS progression and exert an essential role in the treatment of OS, which is potentially of great value. By targeting ferroptosis in OS, the present review article summarises the relevant mechanisms and therapeutic applications along with discussing current limitations and future directions, which may provide a new strategy for the treatment of OS.
1. Introduction
Osteosarcoma (OS) is a primary musculoskeletal cancer originating from primitive mesenchymal cells and is most common in children and teenagers, accounting for approximately 2.4% of pediatric and adolescent cancers worldwide [1], [2]. Due to its high malignancy, early metastasis and susceptibility to drug resistance, it features a high rate of disability and mortality as well as an extremely poor prognosis [3]. Although lots of attempts have been made to develop novel drugs and therapies for treating OS, the results are unsatisfactory and the 5-year survival rate remains relatively low [4]. The current outlook for OS treatment is not promising, and new treatment strategies are urgently needed.
Ferroptosis is a neoteric form of regulated cell death (RCD) discovered in recent years and caused by iron catalysis and lipid peroxidation which can lead to reactive oxygen species (ROS) deposition [5]. The current view is that although a moderate increase in ROS can promote tumour cell proliferation, excessive ROS accumulation will result in irreversible oxidative damage thereby inducing ferroptosis [6], [7]. And ROS scavenging is the primary responsibility of glutathione peroxidase 4 (GPX4), produced by glutathione (GSH), which is also the only known intracellular enzyme that can reduce lipid hydroperoxides [8]. Ferroptosis intervenes in a wide range of illness processes including cancer, cardiovascular disease and neurological disease, and performs an essential role [9].
In the present review, the revealed mechanisms and applications of ferroptosis in OS are summarised thoroughly, and the relevant limitations and future directions are discussed in depth.
2. Ferroptosis in cancer
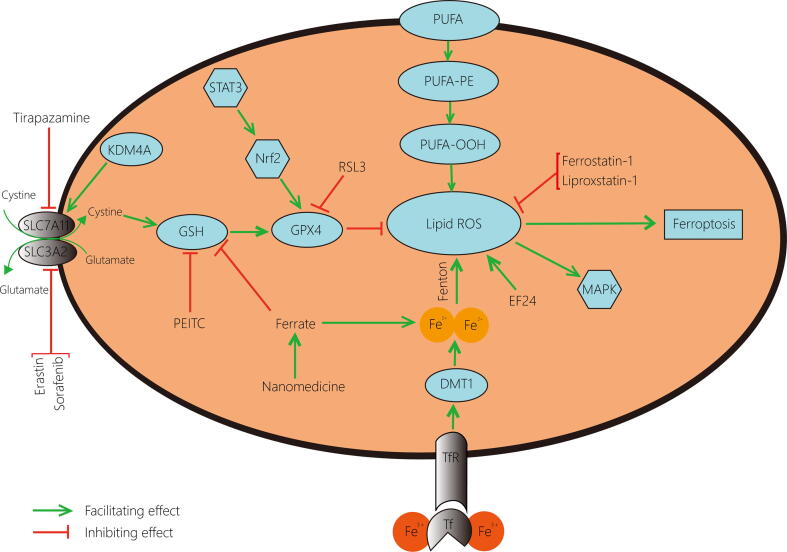
Ferroptosis acts as a non-negligible factor in regulating the growth process of cancer cells [10], and exploring the role of ferroptosis-related regulatory mechanisms in cancer is of great significance for both disease cognition and treatment. Since most of the current therapeutic regimens work by inducing apoptosis in tumour cells, once tumour cells undergo apoptotic escape, they will become resistant, resulting in less sensitive treatment and worse prognosis [11]. Distinct from apoptosis, ferroptosis, a novel type of programmed cell death, possesses unique characteristics and has shown potential value in the study of tumours [10]. In particular, in musculoskeletal cancers, a range of cancers distinguished by their complexity and curative difficulty [12], ferroptosis has been proven to affect target cells by mediating multiple enzymes and pathways [13], [14], [15], [16]. The conventional inducers of ferroptosis, RSL3, Erastin and the inhibitor Ferrostatin-1, have been confirmed effective in certain musculoskeletal cancer cells [17], [18], [19], and several agents can destroy tumour cells through ferroptosis to achieve prominent anti-neoplastic actions in musculoskeletal cancers [20], [21], all of which indicate the importance of ferroptosis in the treatment of this type of cancers (Table 1). As the most common bone-derived tumour [22], OS has also turned out to be tightly linked to ferroptosis, involving a variety of latent mechanisms and therapeutic applications (Fig. 1).
Table 1.
Ferroptosis-related in different types of musculoskeletal cancer.
| Cancer type | Compound | Target | Effect | References |
|---|---|---|---|---|
| Osteosarcoma | PEITC | Consuming GSH | Inducing ferroptosis | [30], [40] |
| Nanomedicine | Accumulating Fe2+ ; consuming GSH | Inducing ferroptosis | [29] | |
| Tirapazamine | Suppressing SLC7A11 | Inducing ferroptosis | [70] | |
| EF24 | Accumulating ROS | Inducing ferroptosis | [25] | |
| KDM4A | Accelerating system Xc‑ | Inhibiting ferroptosis | [52] | |
| STAT3 | Accumulating GPX4 | Inhibiting ferroptosis | [35] | |
| Artemisinin | Affecting Fe2+ levels | Inducing cytotoxicity | [79] | |
| Rhabdomyosarcoma | Fenretinide | Accumulating ROS | Inducing ferroptosis | [20] |
| RSL3 | Consuming GPX4 | Inducing ferroptosis | [17], [19] | |
| Erastin | Suppressing system Xc‑ | Inducing ferroptosis | [17], [18], [19] | |
| NR4A1 | Consuming ROS | Inhibiting ferroptosis | [7] | |
| Ferrostatin-1 | Consuming ROS | Inhibiting ferroptosis | [19] | |
| Fibrosarcoma | HO-1 | Accumulating Fe2+ | Inducing ferroptosis | [13] |
| Serine hydrolase inhibitor | Accumulating ROS | Inducing ferroptosis | [14] | |
| ALOX15 activator | Accelerating lipid oxidation | Inducing ferroptosis | [15] | |
| IDH2 | Accumulating GSH | Inhibiting ferroptosis | [16] | |
| Lysosome inhibitor | Consuming ROS | Inhibiting ferroptosis | [21] |
Fig. 1.
Occurrence and regulation of ferroptosis in osteosarcoma cells. The accumulation of ROS is necessary for the development of ferroptosis. The peroxidation of unsaturated fatty acids and the catalysis of iron are the initiating factors for the production of ROS, and the application of EF24 promotes the formation of ROS while Ferrostatin-1 and Liproxstatin-1 suppress this process. In addition, the GPX4 pathway, which is essential for the inhibition of ferroptosis by ROS scavenging, is regulated by several elements: Erastin and Sorafenib limit cystine transport by acting on System Xc‑; Tirapazamine suppresses SLC7A11; KDMA4 mediates the demethylation of SLC7A11 to promote cystine transit; PEITC down-values GSH level; Ferrate not only depletes GSH but also increases iron content; STAT3/Nrf2 pathway facilitates GPX4 production and RSL3 inhibits GPX4 generation. ROS, reactive oxygen species; EF24, 3,5-bis (2-fluorobenzylidine)-4- pyperidone; GPX4, glutathione peroxidase 4; KDM4A, lysine demethylase 4A; PEITC, β-phenethyl isothiocyanate; GSH, glutathione; STAT3, signal transducer and activator of transcription 3; Nrf2, nuclear factor erythroid 2-related factor 2.
3. Potential mechanisms underlying ferroptosis in osteosarcoma
3.1. RCD
RCD manifests as controlled cell death with unique biochemical features, morphological characteristics and immunological outcomes involving tightly structured molecular regulatory mechanisms and signaling cascade responses [23]. Several types of RCD, including apoptosis, necroptosis and autophagy, have been shown to act as important players in the disease development and clinical prognosis of OS [24]. And the corresponding association of RCD with ferroptosis in OS cells is gradually being clarified. By examining OS cells that induced ferroptosis alone and excluding other forms of cell death such as apoptosis and pyroptosis, it was found that the target cells exhibited significant cytotoxicity, leading to a reduction in cell viability and a substantial increase in cell mortality [25].
In addition to acting alone, ferroptosis has been found to be closely associated with other forms of RCD in molecular studies of cancer [26], [27], [28], which is equally applicable in OS. The novel therapeutic agent for OS developed by Fu et al. was found to inhibit tumour growth by inducing and synergizing ferroptosis and apoptosis [29], suggesting that ferroptosis can co-exist and co-operate with another mode of cell death in OS cells. The study by Lv et al. confirmed that OS cells from K7M2 mice treated with chemotherapeutics triggered various models of RCD including ferroptosis, apoptosis and autophagy, and that when reverse intervention with inhibitors corresponding to the death pathway was used, cell survival was only partially rescued by inhibitors of other pathways, except for inhibitors of ferroptosis which almost completely blocked cell death [30]. Ferroptosis may play a dominant role while collaborating with multiple RCD types.
3.2. STAT3
Signal transducer and activator of transcription 3 (STAT3) is one of seven STAT factors concerned with the management of cell growth, differentiation and survival [31]. As a therapeutic target for OS, activation of STAT3 can, in part, lead to adverse transformations such as cellular resistance, immune escape and distant metastasis, therefore STAT3 inhibitors have been identified as promising clinical candidates for OS [32]. Nuclear factor erythroid 2‐related factor 2 (Nrf2) works as a downstream signaling molecule of STAT3 [33], while GPX4, the crucial enzyme for ferroptosis, has been proven to be modulated by Nrf2 in cancer [34]. Liu et al. noted that STAT3 and Nrf2 were consequently elevated when GPX4 was raised in OS cells, and a series of in vitro experiments revealed that STAT3 inhibitors reactivated ferroptosis of OS in Saos-2 and MG-63 cell lines, which was achieved by the reduction of Nrf2 and GPX4 [35]. STAT3/Nrf2/GPX4 can act as a ferroptosis-related pathway, and when the pathway is blocked, ROS increases and ferroptosis is reinforced. Successive studies on OS have also confirmed that STAT3 suppression leads to an increase in ROS [36], [37].
3.3. MAPK
Mitogen-activated protein kinase (MAPK) pathway carries out specific functions through a cascade of upstream, midstream and downstream kinases operating on substrates [38]. As the key pathway in the mechanism associated with OS, MAPK is decisive for the balanced relationship between tumour cell viability and mortality [39]. The recent study reported significant activation of MAPK followed by high-level phosphorylation of ERK, p38 and JNK in OS cells undergoing ferroptosis, and confirmed that the ROS/MAPK pathway is involved in the ferroptosis mechanism of OS cells with lipid ROS production being the main cause of MAPK activation [40]. Besides, numerous emerging anticancer substances have exhibited varying degrees of cancer inhibition effect in vitro and in vivo OS assays by mobilising the lipid ROS/MAPK pathway [41], [42].
3.4. HIF
Hypoxia can contribute to tumourigenesis and treatment resistance. Hypoxia-inducible factor (HIF) is a major regulator of tumour microenvironment in the setting of low-oxygen to assist cancer cells in adapting to hypoxic conditions [43] and is gaining widespread attention as an emerging biomarker for multiple cancers [44]. Ferroptosis occurs as a result of excessive accumulation of ROS, and the interaction between ROS and HIF in cancer has been reported, but the regulatory mechanisms involved are not yet clear [45]. Several studies have been presented showing that HIF-1α and ROS level in OS are inversely correlated [46], [47], [48]. Sun et al. discovered an amassing of ROS and increased cell mortality in OS cells with low HIF-1α expression level [48]. In the drug experiment where ROS accumulated through inactivation of GPX4, it was found that OS cells induced ferroptosis with decreased level of HIF-1α expression and a significant reduction in the size of the hypoxic zone formed in mice bearing xenogeneic OS [29]. Ferroptosis may synergize with the decline of HIF to stall the progression of OS while restoring its chemotherapeutic drug sensitivity to some extent.
3.5. Demethylation
Lysine demethylase 4 (KDM4) subfamily H3K9 histone demethylases, as epigenetic regulators, are mainly responsible for the demethylation of histones H3K9 and H3K36 to influence chromosome structure and modulate gene expression [49], resulting in a pivotal function in cell differentiation, development and death, and are involved in the study of diverse cancer prognosis and mechanisms including OS [50], [51]. Chen et al. clarified that deletion of KDM4A induced ferroptosis in OS cells by applying different forms of cell death inhibitors. Further, it was demonstrated that this was related to the function of KDM4A mediating the H3K9me3 demethylation modification of SLC7A11 which is the essential component of System Xc- [52]. Disruption of SLC7A11 demethylation owing to knockdown of KDM4A results in loss of System Xc- mobilization, slowing of cystine transport and GSH production, thereby enhancing ferroptosis.
4. Ferroptosis in the treatment of osteosarcoma
4.1. Chemotherapy resistance
Chemotherapy has become an indispensable instrument in the clinical management of OS [53]. Although rapid advances in treatment techniques such as neoadjuvant chemotherapy and surgery have marginally improved the prognosis of patients with non-metastatic OS, approximately one third of patients still fail treatment, with the emergence of chemoresistance significantly reducing the survival period [54]. The high susceptibility to chemoresistance is also responsible for the lack of dramatic improvement of OS survival rates in the last 20 years while there have been various breakthroughs in outcomes for other cancers [55]. It has been reported that OS cells undergoing ferroptosis become more sensitive to doxorubicin by down-regulating the expression of multidrug resistance gene and transporter P-glycoprotein [29]. A growing number of studies suggest that the arrival of ferroptosis may bring about a change in the unfavourable situation of drug resistance against OS.
By examining cisplatin-resistant OS cells, Liu et al. observed that GPX4 level were significantly higher in resistant cells compared to non-resistant ones, and that this resistance occurred through inhibition of ferroptosis, while the survival rate of non-resistant cells in the cisplatin environment increased dramatically with the addition of ferroptosis inhibitor Liproxstatin-1. Further application of the ferroptosis agonist Erastin, RSL3 and the GPX4 pathway inhibitor BP-1–102 showed a reduction in GPX4, an increment in ROS and a corresponding improvement in cisplatin susceptibility [35]. Another in vitro pilot study also showed a substantial reduction in resistance to cisplatin when ferroptosis was activated in OS cells [52]. The enhanced sensitivity to cisplatin when cellular ferroptosis is triggered provides a new mindset and approach to address the drug resistance dilemma in OS therapy.
4.2. Primary metastasis
OS possesses a high degree of aggressiveness and metastatic potential [56] which predisposes to early metastasis, with a 5-year survival rate of less than 30% for patients with metastases [57]. In particular, lung metastases occur frequently, and due to the lack of effective treatment, some patients may develop lung metastases in less than one year, resulting in a serious social and economic burden [58]. By affecting System Xc- in 143B and HOS OS cell lines in vitro to limit GPX4 production, Chen et al. detected a subsequent decrease in the invasive capacity of target cells that underwent ferroptosis. Subsequent experiments in a mouse model evidenced that the lung metastatic effect of OS was significantly impaired when ferroptosis was evoked [52]. In a similar vein, Xu et al. substantiated the enhanced metastatic ability of OS cells by repressing the formation of ROS [46]. These evidences indicate that ferroptosis may influence and inhibit primary metastasis in OS.
4.3. β-Phenethyl isothiocyanate
β-Phenethyl isothiocyanate (PEITC) is widely found in cruciferous vegetables and has anti-cancer potential [59]. It is considered to be of great value in OS treatment owing to its unique biological properties such as low clearance and high bioavailability [60], and its mechanism of action is thought to be linked to ferroptosis [61]. The study of ferroptosis covering HOS, U-2, MG-63 and 143B human OS cell lines revealed that PEITC altered iron metabolism, uplifting the expression of transferrin receptor 1 (TfR1) and elevating the level of reactive iron. Moreover, PEITC induced oxidative stress. Malondialdehyde (MDA) and ROS, products of lipid peroxidation, were raised and GPX4 was diminished to impair intracellular antioxidant defence systems [40]. This was also demonstrated in the syngeneic orthotopic OS mouse model, where PEITC reduced tumour size and weight with upregulating TfR1 expression and downregulating GPX4 level [30]. PEITC can initiate ferroptosis as one of its mechanisms by which it exerts anti-tumour effect in the treatment of OS.
4.4. Nanomedicine
Nanomedicine, which has revolutionised the field of cancer diagnosis and treatment through the precise delivery and targeted distribution of the drugs it carries [62], has also made remarkable progress in its application to OS [63]. A nanomedicine activated by ultrasound displayed outstanding efficacy against OS by binding doxorubicin and ferrate on a silica nanoplatform, with ferrate being released via ultrasound-induced mild hyperthermia. The release of ferrate enhanced the catalytic effect of iron on one hand and inhibited the production of GSH on the other, touching off ferroptosis from multiple routes and consequently effectually restraining the growth of OS together with doxorubicin [29]. In addition, several distinct nanoparticles have shown similar roles in the treatment of OS, leading to the induction of lipid peroxidation and the production of ROS with the aim of accelerating the lethal effect on tumour cells [64], [65]. The combination of superior function and high biocompatibility is the strength of nanomedicine for clinical translation, and ferroptosis is injecting new vigour into OS nanotherapy.
4.5. Tirapazamine
Tirapazamine (TPZ) has been identified for targeting hypoxic tumour cells and previously as an adjuvant for cisplatin to enhance its efficacy [66]. Recently, TPZ is often dependent on nanoplatform and combined with phototherapy for its hypoxia-activated prodrug effect, and a variety of TPZ-dependent photodynamic therapies have been validated for significant efficacy which is closely correlated with the production of ROS [67], [68], [69]. However the specific association of TPZ with ROS and the cell killing mechanisms involved remain elusive. In the latest study, Shi et al. demonstrated for the first time that TPZ inhibited the proliferation of OS cells by mediating ferroptosis through the observation of intracellular Fe2+ staining fluorescence and the detection of lipid peroxidation. This inhibition was achieved by partial down-regulation of SLC7A11 and was reversed by SLC7A11 overexpression [70]. TPZ promotes ferroptosis by reducing GPX4 and increasing ROS through suppression of SLC7A11, which may be one of its tumour killing mechanisms.
4.6. Curcumin and its analogues
Curcumin, a polyphenol derived from Curcuma longa, is an innovative cancer therapeutic agent since it is associated with cancer progression through sophisticated biological mechanisms and has low toxicity [71]. Curcumin has been indicated in numerous studies to exert an antagonistic impact against OS through upregulation of ROS expression [72], [73], [74]. Lin et al. identified that EF24, a synthetic analogue of curcumin, lowered OS cell survival accompanied with elevated levels of iron, MDA and ROS, and followed by the application of corresponding pathway inhibitors clarified that only ferroptosis occurred and no other cell death mechanisms were concerned [25]. Curcumin and its analogues are recognized as possessing immense potential in OS treatment underlying ferroptosis, which is intimately linked to their pharmacological profile, contributing to provide insight into their clinical value.
4.7. Artemisinin
Artemisinin is the main bioactive component of Artemisia annua L. Although artemisinin is widely used as a traditional anti-malarial drug, its anti-cancer properties are also generally known [75] and can modulate relevant signaling pathways in OS to curb proliferation and metastasis [76], [77]. In the study by Hosoya et al., ROS production was detected in D17 canine OS cell lines treated with dihydroartemisinin and increased in a dose-dependent manner [78]. However, Isani et al. discovered that artemisinin-intervened OS cells had reduced iron concentrations compared to untreated ones, which might contribute to the stimulation of the labile redox-active iron pool thereby promoting ROS deposition and ferroptosis [79]. The cytotoxicity of artemisinin on OS is thought to be possibly related to ferroptosis, but more robust evidence is needed to verify this.
5. Discussion and conclusions
As an emerging form of cell death, ferroptosis has presented significant research potential and application value in clinical tumour trials. By targeting ferroptosis in OS, the present article focuses on summarizing, discussing and prospecting the underlying mechanisms and related therapeutic applications, with the aim to elucidate the vital role of ferroptosis in OS. However, there remain some incomplete conclusions and even results that conflict with other studies. The previously reported dual effect of HIF in regulating ferroptosis of cancer cells [80] is not reflected in OS, and artemisinin, which is supposed to increase free iron levels in tumour cells [81], decreased those levels in certain OS assays, all of which need to be further verified. Moreover, according to existing studies, the current ferroptosis research on the treatment of OS is still limited to chemotherapy, while other options such as radiotherapy and immunotherapy, which have been shown to be closely associated with ferroptosis in solid tumours, have not been reported in OS. It has been established that radiotherapy can regulate ferroptosis in various cancer cells by down-regulating SLC7A11 [82], up-regulating ACSL4 [83], activating the cGAS pathway [84] and other approaches. The relevance of immunotherapy to ferroptosis in certain specific tumour cells has been demonstrated [85], [86], and the immunogenicity of ferroptosis in fibrosarcoma, a kind of musculoskeletal cancer as OS, has also been explored [87]. The impact of ferroptosis in the non-pharmacological treatment of OS remains to be confirmed due to the lack of sufficient evidence-based practice.
As it stands, on one hand, future ferroptosis research in OS will maintain a focus on topical cancer mechanisms, with typical cancer genes represented by RAS [88] and TP53 [89] indicating substantial potential in the ferroptosis pathway, while relevant studies in OS have not yet been conclusive. On the other hand, there will be a convergence of application research in the clinical management of OS, including traditional chemotherapeutic agents, novel chemotherapeutic agents, chemotherapy-related nanomedicines and non-chemotherapeutic schemes such as immunotherapy and radiotherapy. Despite the fact that the development of targeting the ferroptosis pathway seems as expanded treatment options for tumours, the current view is that ferroptosis-promoting therapies are only suitable in the combination with other approaches [80] and given the high susceptibility of OS to drug resistance, the synergistic effect of drugs involving ferroptosis in the treatment of OS and the linked molecular mechanisms are undoubtedly of considerable value for both research and clinical practice. In general, targeting ferroptosis offers a new therapeutic strategy for OS.
6. Availability of Data and Materials
Not applicable.
7. Ethics Approval and Consent to Participate
Not applicable.
Funding
This work was supported by the Project of the Natural Science Foundation of Hebei Province [H2019206309].
CRediT authorship contribution statement
Jiazheng Zhao: Conceptualization, Data curation, Investigation, Writing - original draft. Yi Zhao: Data curation, Investigation, Visualization. Xiaowei Ma: Data curation, Investigation. Benzheng Zhang: Data curation, Investigation. Helin Feng: Data curation, Investigation, Writing - review & editing.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
Not applicable.
References
- 1.Lei Y., Junxin C., Yongcan H., Xiaoguang L., Binsheng Y. Role of microRNAs in the crosstalk between osteosarcoma cells and the tumour microenvironment. J Bone Oncol. 2020;25 doi: 10.1016/j.jbo.2020.100322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sadykova L.R., Ntekim A.I., Muyangwa-Semenova M., Rutland C.S., Jeyapalan J.N., Blatt N., Rizvanov A.A. Epidemiology and Risk Factors of Osteosarcoma. Cancer Invest. 2020;38(5):259–269. doi: 10.1080/07357907.2020.1768401. [DOI] [PubMed] [Google Scholar]
- 3.Zhang Y., Yang J., Zhao N., Wang C., Kamar S., Zhou Y., He Z., Yang J., Sun B., Shi X., Han L., Yang Z. Progress in the chemotherapeutic treatment of osteosarcoma. Oncol Lett. 2018;16(5):6228–6237. doi: 10.3892/ol.2018.9434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Z.D. Prudowsky J.T. Yustein Recent Insights into Therapy Resistance in Osteosarcoma 13 2020 Cancers (Basel) [DOI] [PMC free article] [PubMed]
- 5.Dixon S.J., Lemberg K.M., Lamprecht M.R., Skouta R., Zaitsev E.M., Gleason C.E., Patel D.N., Bauer A.J., Cantley A.M., Yang W.S., Morrison B., 3rd, Stockwell B.R. Ferroptosis: an iron-dependent form of nonapoptotic cell death. Cell. 2012;149(5):1060–1072. doi: 10.1016/j.cell.2012.03.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Galadari S., Rahman A., Pallichankandy S., Thayyullathil F. Reactive oxygen species and cancer paradox: To promote or to suppress. Free Radic. Biol. Med. 2017;104:144–164. doi: 10.1016/j.freeradbiomed.2017.01.004. [DOI] [PubMed] [Google Scholar]
- 7.Hedrick E., Mohankumar K., Lacey A., Safe S. Inhibition of NR4A1 Promotes ROS Accumulation and IL24-Dependent Growth Arrest in Rhabdomyosarcoma. Mol. Cancer Res. 2019;17(11):2221–2232. doi: 10.1158/1541-7786.MCR-19-0408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yang W.S., SriRamaratnam R., Welsch M.E., Shimada K., Skouta R., Viswanathan V.S., Cheah J.H., Clemons P.A., Shamji A.F., Clish C.B., Brown L.M., Girotti A.W., Cornish V.W., Schreiber S.L., Stockwell B.R. Regulation of ferroptotic cancer cell death by GPX4. Cell. 2014;156(1–2):317–331. doi: 10.1016/j.cell.2013.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Li J., Cao F., Yin H.L., Huang Z.J., Lin Z.T., Mao N., Sun B., Wang G. Ferroptosis: past, present and future. Cell Death Dis. 2020;11(2):88. doi: 10.1038/s41419-020-2298-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wang H. MicroRNAs and Apoptosis in Colorectal Cancer. Int J Mol Sci. 2020;21(15) doi: 10.3390/ijms21155353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Xu T., Ding W., Ji X., Ao X., Liu Y., Yu W., Wang J. Molecular mechanisms of ferroptosis and its role in cancer therapy. J. Cell. Mol. Med. 2019;23(8):4900–4912. doi: 10.1111/jcmm.14511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ferrari A., Dirksen U., Bielack S. Sarcomas of Soft Tissue and Bone. Prog Tumor Res. 2016;43:128–141. doi: 10.1159/000447083. [DOI] [PubMed] [Google Scholar]
- 13.Kwon M.Y., Park E., Lee S.J., Chung S.W. Heme oxygenase-1 accelerates erastin-induced ferroptotic cell death. Oncotarget. 2015;6(27):24393–24403. doi: 10.18632/oncotarget.5162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kathman S.G., Boshart J., Jing H., Cravatt B.F. Blockade of the Lysophosphatidylserine Lipase ABHD12 Potentiates Ferroptosis in Cancer Cells. ACS Chem. Biol. 2020;15(4):871–877. doi: 10.1021/acschembio.0c00086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Shintoku R., Takigawa Y., Yamada K., Kubota C., Yoshimoto Y., Takeuchi T., Koshiishi I., Torii S. Lipoxygenase-mediated generation of lipid peroxides enhances ferroptosis induced by erastin and RSL3. Cancer Sci. 2017;108(11):2187–2194. doi: 10.1111/cas.13380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kim H., Lee J.H., Park J.W. Down-regulation of IDH2 sensitizes cancer cells to erastin-induced ferroptosis. Biochem. Biophys. Res. Commun. 2020;525(2):366–371. doi: 10.1016/j.bbrc.2020.02.093. [DOI] [PubMed] [Google Scholar]
- 17.Codenotti S., Poli M., Asperti M., Zizioli D., Marampon F., Fanzani A. Cell growth potential drives ferroptosis susceptibility in rhabdomyosarcoma and myoblast cell lines. J. Cancer Res. Clin. Oncol. 2018;144(9):1717–1730. doi: 10.1007/s00432-018-2699-0. [DOI] [PubMed] [Google Scholar]
- 18.Dächert J., Ehrenfeld V., Habermann K., Dolgikh N., Fulda S. Targeting ferroptosis in rhabdomyosarcoma cells. Int. J. Cancer. 2020;146(2):510–520. doi: 10.1002/ijc.32496. [DOI] [PubMed] [Google Scholar]
- 19.Schott C., Graab U., Cuvelier N., Hahn H., Fulda S. Oncogenic RAS Mutants Confer Resistance of RMS13 Rhabdomyosarcoma Cells to Oxidative Stress-Induced Ferroptotic Cell Death. Front Oncol. 2015;5:131. doi: 10.3389/fonc.2015.00131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Brack E., Wachtel M., Wolf A., Kaech A., Ziegler U., Schäfer B.W. Fenretinide induces a new form of dynamin-dependent cell death in pediatric sarcoma. Cell Death Differ. 2020;27(8):2500–2516. doi: 10.1038/s41418-020-0518-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Torii S., Shintoku R., Kubota C., Yaegashi M., Torii R., Sasaki M., Suzuki T., Mori M., Yoshimoto Y., Takeuchi T., Yamada K. An essential role for functional lysosomes in ferroptosis of cancer cells. Biochem. J. 2016;473(6):769–777. doi: 10.1042/BJ20150658. [DOI] [PubMed] [Google Scholar]
- 22.Hameed M., Mandelker D. Tumor Syndromes Predisposing to Osteosarcoma. Adv Anat Pathol. 2018;25(4):217–222. doi: 10.1097/PAP.0000000000000190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tang D., Kang R., Berghe T.V., Vandenabeele P., Kroemer G. The molecular machinery of regulated cell death. Cell Res. 2019;29(5):347–364. doi: 10.1038/s41422-019-0164-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Li J., Yang Z., Li Y., Xia J., Li D., Li H., Ren M., Liao Y., Yu S., Chen Y., Yang Y., Zhang Y. Cell apoptosis, autophagy and necroptosis in osteosarcoma treatment. Oncotarget. 2016;7(28):44763–44778. doi: 10.18632/oncotarget.8206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lin H., Chen X., Zhang C., Yang T., Deng Z., Song Y., Huang L., Li F., Li Q., Lin S., Jin D. EF24 induces ferroptosis in osteosarcoma cells through HMOX1. Biomed. Pharmacother. 2021;136 doi: 10.1016/j.biopha.2020.111202. [DOI] [PubMed] [Google Scholar]
- 26.Koren E., Fuchs Y. Modes of Regulated Cell Death in Cancer. Cancer Discov. 2021;11(2):245–265. doi: 10.1158/2159-8290.CD-20-0789. [DOI] [PubMed] [Google Scholar]
- 27.Woo Y., Lee H.J., Jung Y.M., Jung Y.J. Regulated Necrotic Cell Death in Alternative Tumor Therapeutic Strategies. Cells. 2020;9(12) doi: 10.3390/cells9122709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhou Y., Shen Y., Chen C., Sui X., Yang J., Wang L., Zhou J. The crosstalk between autophagy and ferroptosis: what can we learn to target drug resistance in cancer. Cancer Biol Med. 2019;16(4):630–646. doi: 10.20892/j.issn.2095-3941.2019.0158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fu J., Li T., Yang Y., Jiang L., Wang W., Fu L., Zhu Y., Hao Y. Activatable nanomedicine for overcoming hypoxia-induced resistance to chemotherapy and inhibiting tumor growth by inducing collaborative apoptosis and ferroptosis in solid tumors. Biomaterials. 2021;268 doi: 10.1016/j.biomaterials.2020.120537. [DOI] [PubMed] [Google Scholar]
- 30.Lv H.H., Zhen C.X., Liu J.Y., Shang P. PEITC triggers multiple forms of cell death by GSH-iron-ROS regulation in K7M2 murine osteosarcoma cells. Acta Pharmacol. Sin. 2020;41(8):1119–1132. doi: 10.1038/s41401-020-0376-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Guanizo A.C., Fernando C.D., Garama D.J., Gough D.J. STAT3: a multifaceted oncoprotein. Growth Factors. 2018;36(1–2):1–14. doi: 10.1080/08977194.2018.1473393. [DOI] [PubMed] [Google Scholar]
- 32.Liu Y., Liao S., Bennett S., Tang H., Song D., Wood D., Zhan X., Xu J. STAT3 and its targeting inhibitors in osteosarcoma. Cell Prolif. 2021;54(2) doi: 10.1111/cpr.12974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gao S., Zhan L., Yang Z., Shi R., Li H., Xia Z., Yuan S., Wu Q.P., Wang T., Yao S. Remote Limb Ischaemic Postconditioning Protects Against Myocardial Ischaemia/Reperfusion Injury in Mice: Activation of JAK/STAT3-Mediated Nrf2-Antioxidant Signalling. Cell. Physiol. Biochem. 2017;43(3):1140–1151. doi: 10.1159/000481755. [DOI] [PubMed] [Google Scholar]
- 34.Shin D., Kim E.H., Lee J., Roh J.L. Nrf2 inhibition reverses resistance to GPX4 inhibitor-induced ferroptosis in head and neck cancer. Free Radic. Biol. Med. 2018;129:454–462. doi: 10.1016/j.freeradbiomed.2018.10.426. [DOI] [PubMed] [Google Scholar]
- 35.Liu Q., Wang K. The induction of ferroptosis by impairing STAT3/Nrf2/GPx4 signaling enhances the sensitivity of osteosarcoma cells to cisplatin. Cell Biol. Int. 2019;43(11):1245–1256. doi: 10.1002/cbin.11121. [DOI] [PubMed] [Google Scholar]
- 36.Cai N., Zhou W., Ye L.L., Chen J., Liang Q.N., Chang G., Chen J.J. The STAT3 inhibitor pimozide impedes cell proliferation and induces ROS generation in human osteosarcoma by suppressing catalase expression. American journal of translational research. 2017;9(8):3853–3866. [PMC free article] [PubMed] [Google Scholar]
- 37.Zuo D., Zhou Z., Wang H., Zhang T., Zang J., Yin F., Sun W., Chen J., Duan L., Xu J., Wang Z., Wang C., Lin B., Fu Z., Liao Y., Li S., Sun M., Hua Y., Zheng L., Cai Z. Alternol, a natural compound, exerts an anti-tumour effect on osteosarcoma by modulating of STAT3 and ROS/MAPK signalling pathways. J. Cell. Mol. Med. 2017;21(2):208–221. doi: 10.1111/jcmm.12957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lee S., Rauch J., Kolch W. Targeting MAPK Signaling in Cancer: Mechanisms of Drug Resistance and Sensitivity. Int J Mol Sci. 2020;21(3) doi: 10.3390/ijms21031102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kushlinskii N.E., Fridman M.V., Braga E.A. Molecular Mechanisms and microRNAs in Osteosarcoma Pathogenesis. Biochemistry Mosc. 2016;81(4):315–328. doi: 10.1134/S0006297916040027. [DOI] [PubMed] [Google Scholar]
- 40.Lv H., Zhen C., Liu J., Shang P. β-Phenethyl Isothiocyanate Induces Cell Death in Human Osteosarcoma through Altering Iron Metabolism, Disturbing the Redox Balance, and Activating the MAPK Signaling Pathway. Oxid Med Cell Longev. 2020;2020:5021983. doi: 10.1155/2020/5021983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.L. Ning, S. Wan, Z. Jie, Z. Xie, X. Li, X. Pan, X. Wan, W. Chen, H. Huang, J. Wang, A. Qin, S. Fan, X. Zhao, Lycorine Induces Apoptosis and G1 Phase Arrest Through ROS/p38 MAPK Signaling Pathway in Human Osteosarcoma Cells In Vitro and In Vivo, Spine 45 (3) (2020) E126-126E139. [DOI] [PubMed]
- 42.Zhu J., Yu W., Liu B., Wang Y., Shao J., Wang J., Xia K., Liang C., Fang W., Zhou C., Tao H. Escin induces caspase-dependent apoptosis and autophagy through the ROS/p38 MAPK signalling pathway in human osteosarcoma cells in vitro and in vivo. Cell Death Dis. 2017;8(10) doi: 10.1038/cddis.2017.488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Vaupel P., Multhoff G. Fatal Alliance of Hypoxia-/HIF-1α-Driven Microenvironmental Traits Promoting Cancer Progression. Adv. Exp. Med. Biol. 2020;1232:169–176. doi: 10.1007/978-3-030-34461-0_21. [DOI] [PubMed] [Google Scholar]
- 44.Pezzuto A., Carico E. Role of HIF-1 in Cancer Progression: Novel Insights. A Review, Curr. Mol. Med. 2018;18(6):343–351. doi: 10.2174/1566524018666181109121849. [DOI] [PubMed] [Google Scholar]
- 45.Niecknig H., Tug S., Reyes B.D., Kirsch M., Fandrey J., Berchner-Pfannschmidt U. Role of reactive oxygen species in the regulation of HIF-1 by prolyl hydroxylase 2 under mild hypoxia. Free Radic. Res. 2012;46(6):705–717. doi: 10.3109/10715762.2012.669041. [DOI] [PubMed] [Google Scholar]
- 46.Xu W.N., Yang R.Z., Zheng H.L., Jiang L.S., Jiang S.D. NDUFA4L2 Regulated by HIF-1α Promotes Metastasis and Epithelial-Mesenchymal Transition of Osteosarcoma Cells Through Inhibiting ROS Production. Front Cell Dev Biol. 2020;8 doi: 10.3389/fcell.2020.515051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Huang S.T., Bi K.W., Kuo H.M., Lin T.K., Liao P.L., Wang P.W., Chuang J.H., Liou C.W. Phyllanthus urinaria induces mitochondrial dysfunction in human osteosarcoma 143B cells associated with modulation of mitochondrial fission/fusion proteins. Mitochondrion. 2014;17:22–33. doi: 10.1016/j.mito.2014.05.002. [DOI] [PubMed] [Google Scholar]
- 48.Sun W., Wang B., Qu X.L., Zheng B.Q., Huang W.D., Sun Z.W., Wang C.M., Chen Y. Metabolism of Reactive Oxygen Species in Osteosarcoma and Potential Treatment Applications. Cells. 2019;9(1) doi: 10.3390/cells9010087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lee D.H., Kim G.W., Jeon Y.H., Yoo J., Lee S.W., Kwon S.H. Advances in histone demethylase KDM4 as cancer therapeutic targets. FASEB J. 2020;34(3):3461–3484. doi: 10.1096/fj.201902584R. [DOI] [PubMed] [Google Scholar]
- 50.Liu X., Zhang Q., Zhao Y., Xun J., Wu H., Feng H. Association of JMJD2B and Hypoxia-Inducible Factor 1 Expressions with Poor Prognosis in Osteosarcoma. Anal Cell Pathol (Amst) 2020;2020:2563208. doi: 10.1155/2020/2563208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Berry W.L., Janknecht R. KDM4/JMJD2 histone demethylases: epigenetic regulators in cancer cells. Cancer Res. 2013;73(10):2936–2942. doi: 10.1158/0008-5472.CAN-12-4300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Chen M., Jiang Y., Sun Y. KDM4A-mediated histone demethylation of SLC7A11 inhibits cell ferroptosis in osteosarcoma. Biochem. Biophys. Res. Commun. 2021;550:77–83. doi: 10.1016/j.bbrc.2021.02.137. [DOI] [PubMed] [Google Scholar]
- 53.Heng M., Gupta A., Chung P.W., Healey J.H., Vaynrub M., Rose P.S., Houdek M.T., Lin P.P., Bishop A.J., Hornicek F.J., Chen Y.L., Lozano-Calderon S., Holt G.E., Han I., Biau D., Niu X., Bernthal N.M., Ferguson P.C., Wunder J.S. Japanese Musculoskeletal Oncology Group (JMOG), Soft Tissue Osteosarcoma International Collaborative (STOIC), The role of chemotherapy and radiotherapy in localized extraskeletal osteosarcoma. Eur. J. Cancer. 2020;125:130–141. doi: 10.1016/j.ejca.2019.07.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Nomura M., Rainusso N., Lee Y.C., Dawson B., Coarfa C., Han R., Larson J.L., Shuck R., Kurenbekova L., Yustein J.T. Tegavivint and the β-Catenin/ALDH Axis in Chemotherapy-Resistant and Metastatic Osteosarcoma. J. Natl. Cancer Inst. 2019;111(11):1216–1227. doi: 10.1093/jnci/djz026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Meazza C., Scanagatta P. Metastatic osteosarcoma: a challenging multidisciplinary treatment. Expert Rev Anticancer Ther. 2016;16(5):543–556. doi: 10.1586/14737140.2016.1168697. [DOI] [PubMed] [Google Scholar]
- 56.Wang D., Song Z., Wang Z. Common mechanism of pathogenesis in various types of metastatic osteosarcoma. Oncol Lett. 2017;14(5):6307–6313. doi: 10.3892/ol.2017.6955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Yang C., Tian Y., Zhao F., Chen Z., Su P., Li Y., Qian A. Bone Microenvironment and Osteosarcoma Metastasis. Int J Mol Sci. 2020;21(19) doi: 10.3390/ijms21196985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Siegel R.L., Miller K.D., Jemal A. Cancer statistics, 2020. CA Cancer J Clin. 2020;70(1):7–30. doi: 10.3322/caac.21590. [DOI] [PubMed] [Google Scholar]
- 59.Palliyaguru D.L., Yuan J.M., Kensler T.W., Fahey J.W. Isothiocyanates: Translating the Power of Plants to People. Mol Nutr Food Res. 2018;62(18) doi: 10.1002/mnfr.201700965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Ji Y., Kuo Y., Morris M.E. Pharmacokinetics of dietary phenethyl isothiocyanate in rats. Pharm. Res. 2005;22(10):1658–1666. doi: 10.1007/s11095-005-7097-z. [DOI] [PubMed] [Google Scholar]
- 61.Kasukabe T., Honma Y., Okabe-Kado J., Higuchi Y., Kato N., Kumakura S. Combined treatment with cotylenin A and phenethyl isothiocyanate induces strong antitumor activity mainly through the induction of ferroptotic cell death in human pancreatic cancer cells. Oncol. Rep. 2016;36(2):968–976. doi: 10.3892/or.2016.4867. [DOI] [PubMed] [Google Scholar]
- 62.Haider N., Fatima S., Taha M., Rizwanullah M., Firdous J., Ahmad R., Mazhar F., Khan M.A. Nanomedicines in Diagnosis and Treatment of Cancer: An Update. Curr. Pharm. Des. 2020;26(11):1216–1231. doi: 10.2174/1381612826666200318170716. [DOI] [PubMed] [Google Scholar]
- 63.Pereira-Silva M., Alvarez-Lorenzo C., Concheiro A., Santos A.C., Veiga F., Figueiras A. Nanomedicine in osteosarcoma therapy: Micelleplexes for delivery of nucleic acids and drugs toward osteosarcoma-targeted therapies. Eur J Pharm Biopharm. 2020;148:88–106. doi: 10.1016/j.ejpb.2019.10.013. [DOI] [PubMed] [Google Scholar]
- 64.Gurunathan S., Jeyaraj M., Kang M.H., Kim J.H. Tangeretin-Assisted Platinum Nanoparticles Enhance the Apoptotic Properties of Doxorubicin: Combination Therapy for Osteosarcoma Treatment. Nanomaterials (Basel) 2019;9(8) doi: 10.3390/nano9081089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Chu X., Mao L., Johnson O., Li K., Phan J., Yin Q., Li L., Zhang J., Chen W., Zhang Y. Exploration of TiO2 nanoparticle mediated microdynamic therapy on cancer treatment. Nanomedicine. 2019;18:272–281. doi: 10.1016/j.nano.2019.02.016. [DOI] [PubMed] [Google Scholar]
- 66.Reddy S.B., Williamson S.K. Tirapazamine: a novel agent targeting hypoxic tumor cells. Expert Opin Investig Drugs. 2009;18(1):77–87. doi: 10.1517/13543780802567250. [DOI] [PubMed] [Google Scholar]
- 67.Sun Y., Zhao D., Wang G., Wang Y., Cao L., Sun J., Jiang Q., He Z. Recent progress of hypoxia-modulated multifunctional nanomedicines to enhance photodynamic therapy: opportunities, challenges, and future development. Acta Pharm Sin B. 2020;10(8):1382–1396. doi: 10.1016/j.apsb.2020.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Ma B., Sheng J., Wang P., Jiang Z., Borrathybay E. Combinational phototherapy and hypoxia-activated chemotherapy favoring antitumor immune responses. Int J Nanomedicine. 2019;14:4541–4558. doi: 10.2147/IJN.S203383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Liu H., Jiang W., Wang Q., Hang L., Wang Y., Wang Y. ROS-sensitive biomimetic nanocarriers modulate tumor hypoxia for synergistic photodynamic chemotherapy. Biomater Sci. 2019;7(9):3706–3716. doi: 10.1039/c9bm00634f. [DOI] [PubMed] [Google Scholar]
- 70.Shi Y., Gong M., Deng Z., Liu H., Chang Y., Yang Z., Cai L. Tirapazamine suppress osteosarcoma cells in part through SLC7A11 mediated ferroptosis. Biochem. Biophys. Res. Commun. 2021;567:118–124. doi: 10.1016/j.bbrc.2021.06.036. [DOI] [PubMed] [Google Scholar]
- 71.Tan B.L., Norhaizan M.E. Curcumin Combination Chemotherapy: The Implication and Efficacy in Cancer. Molecules. 2019;24(14) doi: 10.3390/molecules24142527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Chen P., Wang H., Yang F., Chen H., He W., Wang J. Curcumin Promotes Osteosarcoma Cell Death by Activating miR-125a/ERRα Signal Pathway. J. Cell. Biochem. 2017;118(1):74–81. doi: 10.1002/jcb.25612. [DOI] [PubMed] [Google Scholar]
- 73.Zhang M., Zhang J., Chen J., Zeng Y., Zhu Z., Wan Y. Fabrication of Curcumin-Modified TiO2 Nanoarrays via Cyclodextrin Based Polymer Functional Coatings for Osteosarcoma Therapy. Adv Healthc Mater. 2019;8(23) doi: 10.1002/adhm.201901031. [DOI] [PubMed] [Google Scholar]
- 74.Chang Z., Xing J., Yu X. Curcumin induces osteosarcoma MG63 cells apoptosis via ROS/Cyto-C/Caspase-3 pathway. Tumour Biol. 2014;35(1):753–758. doi: 10.1007/s13277-013-1102-7. [DOI] [PubMed] [Google Scholar]
- 75.Bhaw-Luximon A., Jhurry D. Artemisinin and its derivatives in cancer therapy: status of progress, mechanism of action, and future perspectives. Cancer Chemother. Pharmacol. 2017;79(3):451–466. doi: 10.1007/s00280-017-3251-7. [DOI] [PubMed] [Google Scholar]
- 76.Li Z., Ding X., Wu H., Liu C. Artemisinin inhibits angiogenesis by regulating p38 MAPK/CREB/TSP-1 signaling pathway in osteosarcoma. J. Cell. Biochem. 2019 doi: 10.1002/jcb.28424. [DOI] [PubMed] [Google Scholar]
- 77.Tang C., Ao P.Y., Zhao Y.Q., Huang S.Z., Jin Y., Liu J.J., Luo J.P., Zheng J., Shi D.P. Effect and mechanism of dihydroartemisinin on proliferation, metastasis and apoptosis of human osteosarcoma cells. J. Biol. Regul. Homeost. Agents. 2015;29(4):881–887. [PubMed] [Google Scholar]
- 78.Hosoya K., Murahari S., Laio A., London C.A., Couto C.G., Kisseberth W.C. Biological activity of dihydroartemisinin in canine osteosarcoma cell lines. Am. J. Vet. Res. 2008;69(4):519–526. doi: 10.2460/ajvr.69.4.519. [DOI] [PubMed] [Google Scholar]
- 79.Isani G., Bertocchi M., Andreani G., Farruggia G., Cappadone C., Salaroli R., Forni M., Bernardini C. Cytotoxic Effects of Artemisia annua L. and Pure Artemisinin on the D-17 Canine Osteosarcoma Cell Line. Oxid Med Cell Longev. 2019;2019:1615758. doi: 10.1155/2019/1615758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Chen X., Kang R., Kroemer G., Tang D. Broadening horizons: the role of ferroptosis in cancer. Nat Rev Clin Oncol. 2021;18(5):280–296. doi: 10.1038/s41571-020-00462-0. [DOI] [PubMed] [Google Scholar]
- 81.Ooko E., Saeed M.E., Kadioglu O., Sarvi S., Colak M., Elmasaoudi K., Janah R., Greten H.J., Efferth T. Artemisinin derivatives induce iron-dependent cell death (ferroptosis) in tumor cells. Phytomedicine. 2015;22(11):1045–1054. doi: 10.1016/j.phymed.2015.08.002. [DOI] [PubMed] [Google Scholar]
- 82.Lang X., Green M.D., Wang W., Yu J., Choi J.E., Jiang L., Liao P., Zhou J., Zhang Q., Dow A., Saripalli A.L., Kryczek I., Wei S., Szeliga W., Vatan L., Stone E.M., Georgiou G., Cieslik M., Wahl D.R., Morgan M.A., Chinnaiyan A.M., Lawrence T.S., Zou W. Radiotherapy and Immunotherapy Promote Tumoral Lipid Oxidation and Ferroptosis via Synergistic Repression of SLC7A11. Cancer Discov. 2019;9(12):1673–1685. doi: 10.1158/2159-8290.CD-19-0338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Choi M., Kipps T., Kurzrock R. ATM Mutations in Cancer: Therapeutic Implications. Mol. Cancer Ther. 2016;15(8):1781–1791. doi: 10.1158/1535-7163.MCT-15-0945. [DOI] [PubMed] [Google Scholar]
- 84.Li C., Zhang Y., Liu J., Kang R., Klionsky D.J., Tang D. Mitochondrial DNA stress triggers autophagy-dependent ferroptotic death. Autophagy. 2021;17(4):948–960. doi: 10.1080/15548627.2020.1739447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Wang W., Green M., Choi J.E., Gijón M., Kennedy P.D., Johnson J.K., Liao P., Lang X., Kryczek I., Sell A., Xia H., Zhou J., Li G., Li J., Li W., Wei S., Vatan L., Zhang H., Szeliga W., Gu W., Liu R., Lawrence T.S., Lamb C., Tanno Y., Cieslik M., Stone E., Georgiou G., Chan T.A., Chinnaiyan A., Zou W. CD8+ T cells regulate tumour ferroptosis during cancer immunotherapy. Nature. 2019;569(7755):270–274. doi: 10.1038/s41586-019-1170-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Dai E., Han L., Liu J., Xie Y., Kroemer G., Klionsky D.J., Zeh H.J., Kang R., Wang J., Tang D. Autophagy-dependent ferroptosis drives tumor-associated macrophage polarization via release and uptake of oncogenic KRAS protein. Autophagy. 2020;16(11):2069–2083. doi: 10.1080/15548627.2020.1714209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Efimova I., Catanzaro E., Van der Meeren L., Turubanova V.D., Hammad H., Mishchenko T.A., Vedunova M.V., Fimognari C., Bachert C., Coppieters F., Lefever S., Skirtach A.G., Krysko O., Krysko D.V. Vaccination with early ferroptotic cancer cells induces efficient antitumor immunity. J Immunother Cancer. 2020;8(2) doi: 10.1136/jitc-2020-001369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Yang W.S., Stockwell B.R. Synthetic lethal screening identifies compounds activating iron-dependent, nonapoptotic cell death in oncogenic-RAS-harboring cancer cells. Chem. Biol. 2008;15(3):234–245. doi: 10.1016/j.chembiol.2008.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Jiang L., Kon N., Li T., Wang S.J., Su T., Hibshoosh H., Baer R., Gu W. Ferroptosis as a p53-mediated activity during tumour suppression. Nature. 2015;520(7545):57–62. doi: 10.1038/nature14344. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.